CONSPECTUS:
Cancer immunotherapy, particularly checkpoint blockade immunotherapy (CBI), has revolutionized the treatment of some cancers by reactivating the anti-tumor immunity of hosts with durable response and manageable toxicity. However, many cancer patients with low tumor antigen exposure and immunosuppressive tumor microenvironments do not respond to CBI. A variety of methods are investigated to reverse immunosuppressive tumor microenvironments and turn “cold” tumors “hot” with the goal of extending the therapeutic benefits of CBI to a broader population of cancer patients. Immunostimulatory adjuvant treatments, such as cancer vaccines, photodynamic therapy (PDT), radiotherapy (RT), radiotherapy-radiodynamic therapy (RT-RDT), and chemodynamic therapy (CDT), promote antigen presentation and T cell priming, and when used in combination with CBI, reactivate and sustain systemic anti-tumor immunity. Cancer vaccines directly provide tumor antigens while immunoadjuvant therapies such as PDT, RT, RT-RDT, and CDT kill cancer cells in an immunogenic fashion to release tumor antigens in situ. Direct administration of tumor antigens as cancer vaccine or indirect intratumoral immunoadjuvant therapies as in situ cancer vaccines initiate the immuno-oncology cycle for anti-tumor immune response.
With the rapid growth of cancer nanotechnology in the past two decades, a large number of nanoparticle platforms have been studied and some nanomedicines have been translated into clinical trials. Nanomedicine provides a promising strategy to enhance the efficacy of immunoadjuvant therapies to potentiate cancer immunotherapy. Among these nanoparticle platforms, nanoscale metal-organic frameworks (nMOFs) have emerged as a unique class of porous hybrid nanomaterials with metal cluster secondary building units and organic linkers. With molecular modularity, structural tunability, intrinsic porosity, tunable stability, and biocompatibility, nMOFs are ideally suited for biomedical applications, particularly cancer treatments.
In this account, we present recent breakthroughs in the design of nMOFs as nanocarriers for cancer vaccine delivery and as nanosensitizers for PDT, CDT, RT and RDT. The versatility of nMOFs allows for their fine-tuning to effectively load tumor antigens and immunoadjuvants as cancer vaccines and significantly enhance local anti-tumor efficacy of PDT, RT, RT-RDT, and CDT via generating reactive oxygen species (ROS) for in situ cancer vaccination. These nMOF-based treatments are further combined with cancer immunotherapies to elicit systemic anti-tumor immunity. We discuss novel strategies to enhance light tissue penetration and overcome tumor hypoxia in PDT, to increase energy deposition and ROS diffusion in RT, to combine the advantages of PDT and RT to enable RT-RDT, and to trigger CDT by hijacking aberrant metabolic processes in tumors. Loading nMOFs with small molecule drugs such as indoleamine 2,3-dioxygenase inhibitor, toll-like receptor agonist imiquimod, and biomacromolecules such as CpG oligodeoxynucleotides and anti-CD47 antibody synergizes with nMOF-based radical therapies to enhance their immunotherapeutic effects. Further combination with immune checkpoint inhibitors activates systemic anti-tumor immune responses and elicits abscopal effects. With structural and compositional tunability, nMOFs are poised to provide a new clinically deployable nanotechnology platform to promote immunostimulatory tumor microenvironments by delivering cancer vaccines, mediating PDT, enhancing RT, enabling RT-RDT, and catalyzing CDT and potentiate cancer immunotherapy.
Graphical Abstract
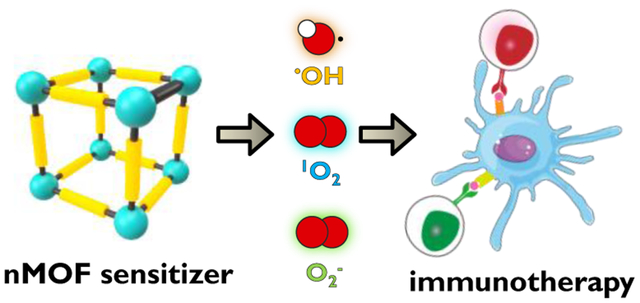
Introduction
Cancer, characterized by traits such as accumulation of dysregulatory metabolism and aberrant growth of cells, is one of the major threats to public health, with approximately 1.8 million new cases and 0.6 million deaths in the United States in 2019.5 Alongside conventional cancer therapies such as surgery, chemotherapy, and radiotherapy, cancer immunotherapies have received intense interest in the past decade due to their ability to elicit durable responses with manageable side effects in a small subset of cancer patients.6
Host immunity is the first line of defense against cancer by identifying and eliminating nascent tumor cells in a process known as immune surveillance. As shown in Figure 1, the process of anti-tumor immune response starts with the release of tumor antigens (TAs).7 Inflammatory tumor microenvironment (TME) and dying tumor cells recruit antigen presenting cells (APCs) such as macrophages and dendritic cells (DCs) to capture and process TAs. Activated APCs travel to the tumor-draining lymph nodes (TDLNs) with antigens captured on major histocompatibility complex (MHC) molecules and present TAs to T cell receptors (TCRs) on T cells, leading to the priming and activation of tumor-specific effector T cells. The primed T cells travel through blood circulation, infiltrate tumor beds, and bind specifically to tumor cells through the interaction between TCRs and TAs on tumor cell surface, leading to apoptosis of tumor cells and the release of more TAs. This immunooncology cycle propagates anti-tumor immune responses in a self-sustained and restricted manner.
Figure 1.
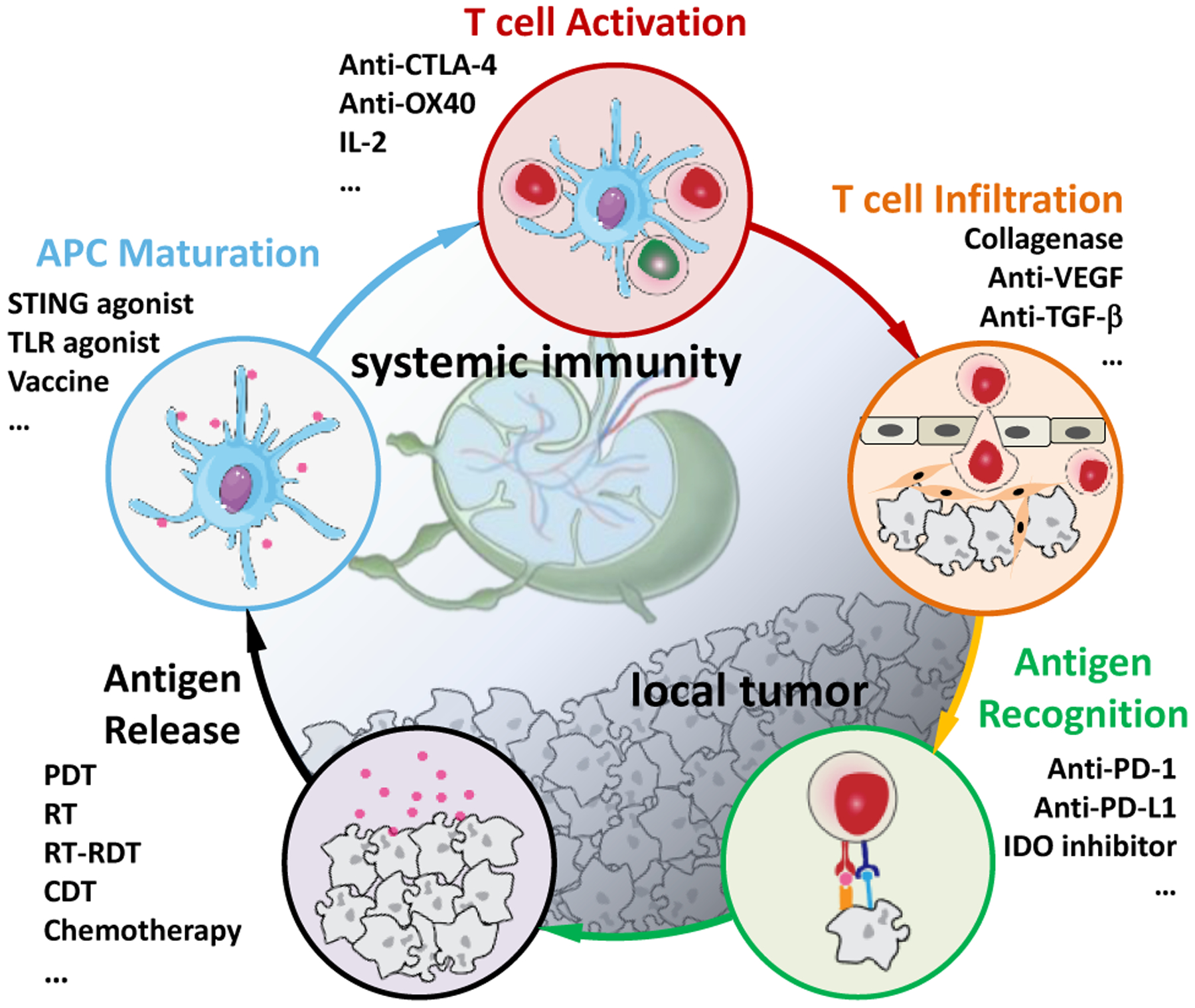
The immuno-oncology cycle and the use of immunotherapeutics to enhance key steps. This cycle starts with TA release from cancer cells and includes multiple steps of antigen presentation by mature APCs, T cell activation in lymph nodes, and T cell trafficking and infiltration into tumor beds, and ends with the recognition of TAs by cytotoxic T lymphocytes (CTLs) to kill cancer cells. Adapted with permission from Ref. 7. Copyright 2013 Elsevier Inc.
Unfortunately, in cancer patients, the immuno-oncology cycle functions abnormally in one or more steps. First, many tumors exhibit low mutational burden or release few TAs due to less immunogenic TMEs.8 Second, advanced tumors dysregulate signaling pathways, hijack immunosuppressive cells/cytokines, and deplete effector cells/molecules to escape from immune surveillance.9 One widely investigated tumor regulatory pathway is programmed cell death protein 1 (PD-1) and its two ligands (PD-L1 and PD-L2) as immune checkpoints, which inhibit kinase signaling pathways responsible for T cell activation, stunting effector T cell activity in tumors.10 Upregulated PD-L1 on tumor cells not only bypasses recognition by effector T cells but also expands regulatory T cells (Tregs), a subset of immunosuppressive T cells, leading to more immunosuppression in TMEs.11 In another regulatory metabolism, indoleamine 2,3-dioxygenase (IDO) overexpressed in TMEs converts tryptophan to kynurenine, causing T cell anergy, and promoting the differentiation of Tregs.12
Cancer immunotherapies including checkpoint blockade immunotherapy (CBI) targeting the PD-1/PD-L1 axis, the cytotoxic T-lymphocyte antigen-4 (CTLA-4) pathway and other immunosuppressive metabolisms have become an important treatment modality for some cancers due to its ability to reactivate and sustain the immuno-oncological cycle.13 However, CBI has failed to provide survival benefits for non-immunogenic tumors (so called “cold” tumors) with immunosuppressive TMEs and inadequate T-cell infiltration. The combination of CBI with immunomodulatory adjuvant treatments are thus being investigated to break immune tolerance and potentiate host anti-tumor immunity in a broader population of cancer patients.14 In immunology, adjuvants refer to substances that can potentiate or regulate immune responses to an antigen.15 Thus, immunoadjuvant therapy focuses on stimulating inflammatory responses to synergize with CBI, reinvigorating and sustaining anti-tumor immunity. For example, several chemotherapy/CBI combinations have been approved for treating lung cancer and triple negative breast cancer while over a hundred ongoing clinical trials are testing the combination of radiation therapy with anti-CTLA-4 or anti-PD-1/PD-L1 antibodies.16–18
Nanotechnology provides a promising strategy to enhance the efficacy of various immunoadjuvant therapies to potentiate cancer immunotherapy.19 Among many nanoformulations, nanoscale metal-organic frameworks (nMOFs) have emerged as a unique class of inorganic-organic hybrid nanomaterials with several favorable attributes for biological applications.20 Built from metal cluster secondary building units (SBUs) and organic linkers, nMOFs integrate crystallinity and porosity. By leveraging reticular chemistry, nMOFs accommodate the orthogonal design of a plethora of topologies and structures. As molecular materials, nMOFs are highly tunable and can possess multi-functionalities with good biocompatibility.
The exploration of nMOFs in biomedical imaging and anticancer drug delivery started approximately 15 years ago.21 The Lin lab pioneered the incorporation of paramagnetic metal ions, such as Gd3+ and Mn2+, into nMOFs for magnetic resonance imaging.22–23 The porosity of nMOFs was leveraged to encapsulate exceptionally high payloads of therapeutic and diagnostic cargoes.24 In the context of immuno-oncology, significant efforts have been focused on expanding the potential of nMOFs as carriers to deliver TAs and immunoadjuvants as cancer vaccines (Figure 2a). nMOFs have also been designed and functionalized as on-demand therapeutics that are activated by either external energy stimuli or endogenous triggers to generate reactive oxygen species (ROS) for immunogenic cell death (ICD) of tumor cells.25 nMOF-induced immunogenic local treatment can be viewed as in situ cancer vaccination to synergize with ICB to elicit systemic anti-tumor immunity (Figure 2b).
Figure 2.
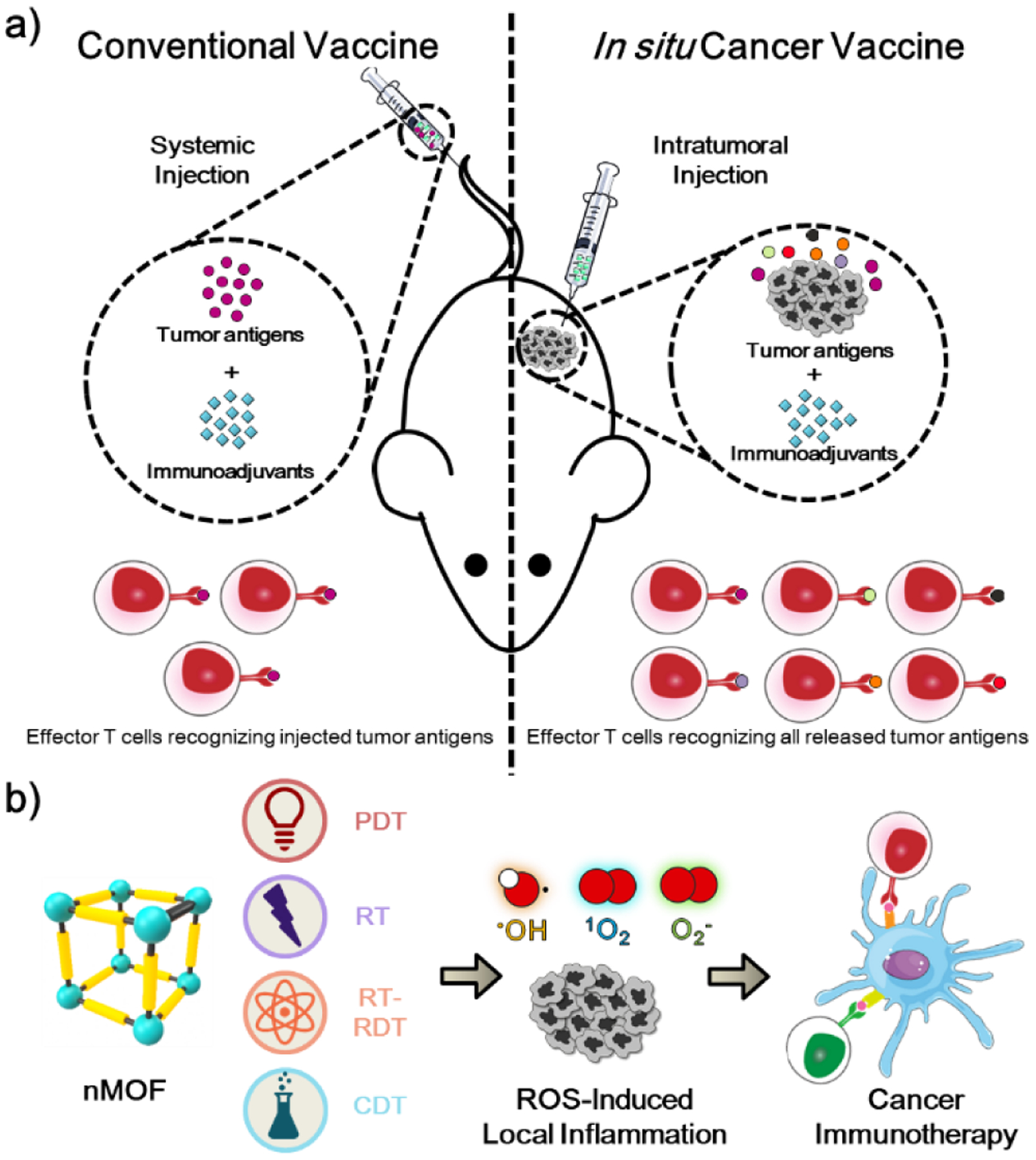
(a) Principle of conventional cancer vaccine by systemically injecting tumor antigens vs in situ cancer vaccine by intratumorally injecting immunoadjuvants to stimulate tumor antigen release. (b) Schematic showing nMOF-mediated local treatment, including PDT, RT, RT-RDT, and CDT, to kill tumor cells via ROS generation and augment innate immunity for synergistic effects with CBI.
ICD is characterized by the release of TAs and danger associated molecular patterns (DAMPs) to promote immunostimulation or/and subvert immunosuppression for APC maturation, antigen presentation, T cell activation and infiltration. During ICD, calreticulin, a chaperone protein translocated from endoplasmic reticulum to the cell membrane, serves as an “eat-me” signal for macrophages and immature DCs to engulf dying tumor cells and apoptotic debris.26 Among DAMPs, high mobility group box-1 (HMGB-1) and heat shock proteins interact with toll-like receptor-4 for DC maturation while adenosine triphosphate (ATP) activates inflammasomes.27
In this account, we summarize recent progress on the use of nMOFs as nanocarriers for cancer vaccines and as nanosensitizers for photodynamic therapy (PDT), radiotherapy (RT), radiotherapy-radiodynamic therapy (RT-RDT), and chemodynamic therapy (CDT) to activate TMEs. nMOF-mediated local inflammation triggers innate immunity to promote antigen presentation and synergize with ICB to systemically reinvigorate host anti-tumor immunity.
Cancer vaccines
Vaccines have been used to immunize hosts against pathogens to fight infectious disease and pandemics for over a century.28 As tumor cells can be recognized and eliminated by functional host immune systems, cancer vaccines have long been explored to immunize hosts with TAs for cancer treatment.29 Vaccination of hosts with TAs can increase the exposure of immune cells to tumor antigens to elicit anti-tumor immunity.30 TAs are typically packed with immunoadjuvants including DAMPs or pathogen-associated molecular patterns (PAMPs) to amplify tumor-specific T cell responses.31 In particular, TAbased cancer vaccines have been widely investigated in the clinic, leading to the approval of the prostatic acid phosphatase based prostate cancer vaccine Sipuleucel T by the FDA.32 However, clinical success of TA-based cancer vaccines is limited by ineffective delivery of peptides to lymph nodes due to rapid renal clearance and enzymatic degradation and inefficient internalization of TAs by APCs.33
In 2016, Qu and coworkers reported the use of ZIF-8 (ZIF denotes zeolitic imidazolate framework) to encapsulate ovalbumin (OVA), a modal antigen, and adsorb CpG oligodeoxynucleotide, an immunoadjuvant that binds toll-like receptor 9 in plasmacytoid DCs and B cells, to activate anti-tumor immune responses.34 OVA@ZIF-8 was first synthesized by sonicating Zn(NO3)2, 2-methylimidazole, and PVP-modified OVA in MeOH, and then mixed with Class B CpG to yield OVA@ZIF-8-CpG. The release of OVA from OVA@ZIF-8-CpG at pH=6.0 was demonstrated in the murine-derived macrophage cell line RAW264.7. OVA@ZIF-8-CpG promoted the secretion of tumor necrosis factor (TNF)-α of RAW264.7 cells in vitro and serum interferon (IFN)-γ in vivo and facilitated CD8+ and CD4+ T cell proliferation more efficiently than a mixture of OVA and CpG. This study suggests that nMOFs may stabilize TAs and immunoadjuvants and enhance their cellular internalization to increase the immunostimulation of cancer vaccines.
In 2019, Zhong et al. used two different OVA@ZIF-8 nanoparticles, ZNPs and ZANPs (doped with Al3+ for adjuvant effect), for cancer vaccination. They performed antigenspecific immune analysis and studied anticancer immune effects.35 ZANPs upregulated the expression of CD40 and CD86 on bone marrow-derived dendritic cells (BMDCs) while CpG/ZANPs further increased IFN-γ and IL-12p70 secretion levels from BMDCs, which was attributed to enhanced cellular uptake of ZANPs via interactions of doped Al3+ and lipids on DC membrane. ZANPs enhanced the signal of H-2Kb-SIINFEKL, an OVA-MHC tetramer on BMDCs, suggesting effective antigen cross-presentation. CpG/ZANPs co-delivered CpG and OVA into lymph node-resident DCs (CD11c+) and macrophages (CD11c−CD11b+) in female C57BL/6 mice to stimulate innate immune responses. CpG/ZANPs triggered the production of IgG2a in sera and increased percentages of TNF-α+ and IFN-γ + CD8+ T cells in splenocytes, indicating the activation of humoral and cellular immunity. CpG/ZANPs showed strong anti-tumor efficacy with long-term survival of EG7-OVA tumor bearing C57BL/6 mice, indicating successful anti-tumor treatment by an nMOF-based cancer vaccine.
Peptide-based antigens as cancer vaccines face several significant hurdles, including heterogeneous somatic mutations and hence varied tumor antigens among patients.36 Personalized vaccines with patient-specific neo-antigens or autologous whole tumor lysates can overcome tumor heterogeneity,37 but their production processes are lengthy, complicated, and expensive.38 A promising strategy to personalized cancer vaccination uses immunostimulatory treatments to generate tumor antigens in situ, which can modulate local TMEs to relieve immunosuppression and elicit systemic anti-tumor immune responses in a personalized fashion.39 For instance, intratumoral injection of oncolytic viruses such as talimogene laherparepvec (T-VEC) inflicts direct cytotoxic effects on cancer cells and recruits DCs for antigen presentation, acting as in situ cancer vaccines with tolerable side effects.40 Non-viral treatments with potent ICD effects, such as phototherapy, radiotherapy, and some chemotherapy-based nanomedicines can also release TAs, generate DAMPs, and deliver PAMPs to generate cancer vaccines in situ for cancer immunotherapy.41
PDT for immunotherapy
PDT uses photosensitizers and light to convert tissue oxygen to cytotoxic ROS, particularly singlet oxygen (1O2), for local cancer treatment.42 In 2014, Lu et al. first reported the use of nMOFs as highly effective photosensitizers for PDT with strong anti-tumor efficacy.43 nMOFs enhance PDT efficacy by delivering high payloads of PSs without self-quenching and facilitating ROS diffusion though open channels. Structural and molecular tunability of nMOFs allows optimization to maximize PDT efficacy and alleviate the adverse impact of hypoxia that is commonly observed in aggressive tumors.44–48
As a highly immunogenic local treatment, PDT is known to induce ICD, recruit neutrophils, and facilitate T cell infiltration.49 In 2016, Lu et al. first reported nMOF-mediated PDT for cancer immunotherapy (Figure 3a).1 A chlorin-based nMOF, Hf-TBC, was loaded with a hydrophobic small molecule inhibitor to IDO to afford IDOi@Hf-TBC. 83.3% of loaded IDOi was released from IDOi@Hf-TBC in 24 h. Upon light irradiation, intratumorally injected Hf-TBC mediated PDT to induce ICD and release TAs. Matured APCs, such as DCs and macrophages, processed and presented TAs to prime naïve T cells. Simultaneously, slowly released IDOi inhibited immunosuppressive metabolism to reactivate tumor-infiltrated CTLs to kill cancer cells. Bilateral murine colorectal tumor model MC38 was employed to assess the abscopal effect induced by IDOi@Hf-TBC.
Figure 3.
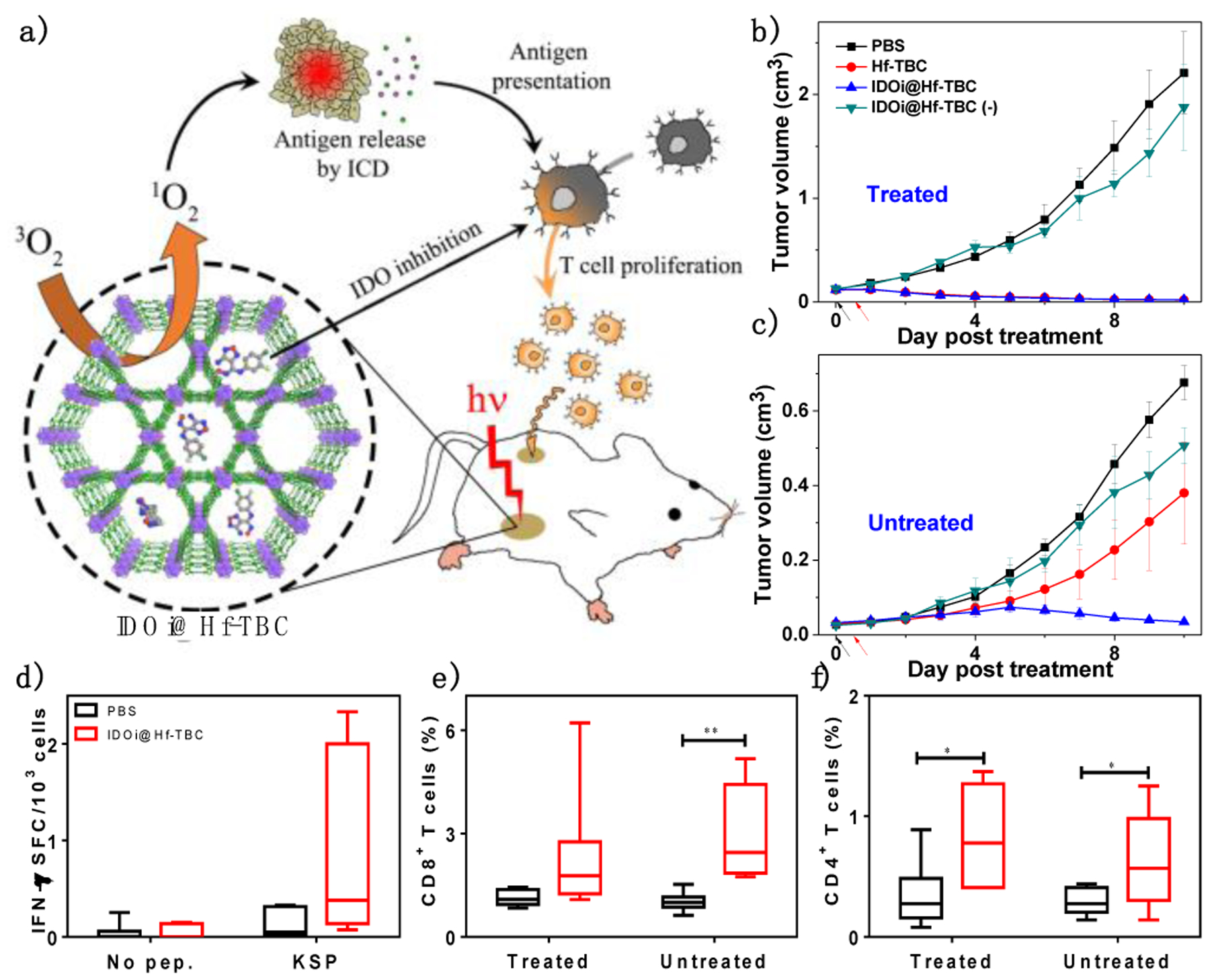
(a) Schematic showing the abscopal effect induced by IDOi@Hf-TBC. Primary treated (b) and distant untreated (c) tumor growth curves of bilateral MC38 tumor-bearing mice treated with IDOi@Hf-TBC plus light irradiation and various controls. Black and red arrows refer to intratumoral injection and light irradiation, respectively. (d) ELISpot assay to detect IFN-γ producing T cells with tumor-specific responses in splenocytes after treatments. The percentage of tumor-infiltrating CD8+ cells (e) and CD4+ T cells (f) with respect to the total cells. *P<0.05 and **P<0.01 from control. Reproduced with permission from Ref. 1. Copyright 2016 American Chemical Society.
As shown in Figure 3b&c, IDOi@Hf-TBC exhibited superior in vivo anti-tumor efficacy over control groups. At treatment endpoint, mice treated with IDOi@Hf-TBC or Hf-TBC with light irradiation at a dose of 90 J/cm2 had tumors only 1% of PBS-treated tumors in volume. However, only IDOi@Hf-TBC plus light treatment regressed distant, untreated tumors, suggesting the PDT and IDOi combination induced systemic anti-tumor immunity. Enzyme-linked immunospot (ELISpot) assay and immune profiling showed that IDOi@Hf-TBC plus light induced ICD and activated innate immunity to stimulate tumor-specific T cell response (Figure 3d) with increased tumor infiltration of CD8+ (Figure 3e) and CD4+ T cells (Figure 3f). This study showed that nMOF-mediated PDT caused local inflammation which synergized with IDO inhibition to elicit systemic anti-tumor immunity with consistent and durable abscopal effects.
In 2018, Zhang and coworkers studied immunotherapeutic effects of nMOF-mediated PDT and anti-PD-1 (αPD-1) CBI.50 Zr-TTBP (TTBP denotes tetrakis(4-carboxy-phenyl)tetrabenzoporphyrin) with PCN-224 topology showed strong cytotoxicity upon light irradiation. The anti-tumor efficacy of Zr-TTBP-mediated PDT plus αPD-1 was evaluated on murine triple-negative breast cancer 4T1 tumor-bearing Balb/c mice. 4T1 tumor-bearing Balb/c mice were intravenously injected with Zr-TTBP and irradiated with light, followed by three intravenous administrations of αPD-1. The combination of nMOF-mediated PDT and αPD-1 not only exhibited superb local anti-tumor efficacy, but also showed strong anti-metastatic effect. Zr-TTBP-mediated PDT potentiated αPD-1 to activate the adaptive anti-tumor immune responses with strong T cell infiltration into the tumors and trigger the secretion of inflammatory cytokines such as IFN-γ and TNF-α.
As type II PDT is highly oxygen-dependent, hypoxic TMEs found in many advanced tumors present another major obstacle for effective PDT. In 2018, Lan et al. reported Fe-TBP [TBP denotes tetra(p-benzoato)porphyrin], a new nMOF assembled from Fe3O SBUs and TBP linkers to overcome tumor hypoxia.51 Under hypoxic conditions, Fe-TBP plus light catalyzed a cascade reaction in which intracellular hydrogen peroxide (H2O2) was decomposed by the Fe3O clusters to produce ground state 3O2 through a Fenton-like reaction whereas the generated 3O2 was converted to cytotoxic 1O2 by photo-excited TBP linkers (Figure 4a). The relief of tumor hypoxia by Fe-TBP was further confirmed by immunofluorescence staining of intratumoral hypoxia-inducible factor-1α (HIF-1α) (Figure 4b). With the enhanced tissue oxygen supply, Fe-TBP-mediated PDT significantly improved the efficacy of anti-PD-L1 antibody (αPD-L1), to elicit abscopal effects in a bilateral CT26 tumor model (Figure 4c). The combination treatment elicited >90% regression of both treated primary tumors and untreated distant tumors upon a low light dose of 45 J/cm2 (Figure 4d). Flow cytometry and immunostaining studies revealed significant infiltration of cytotoxic T cells into tumors.
Figure 4.
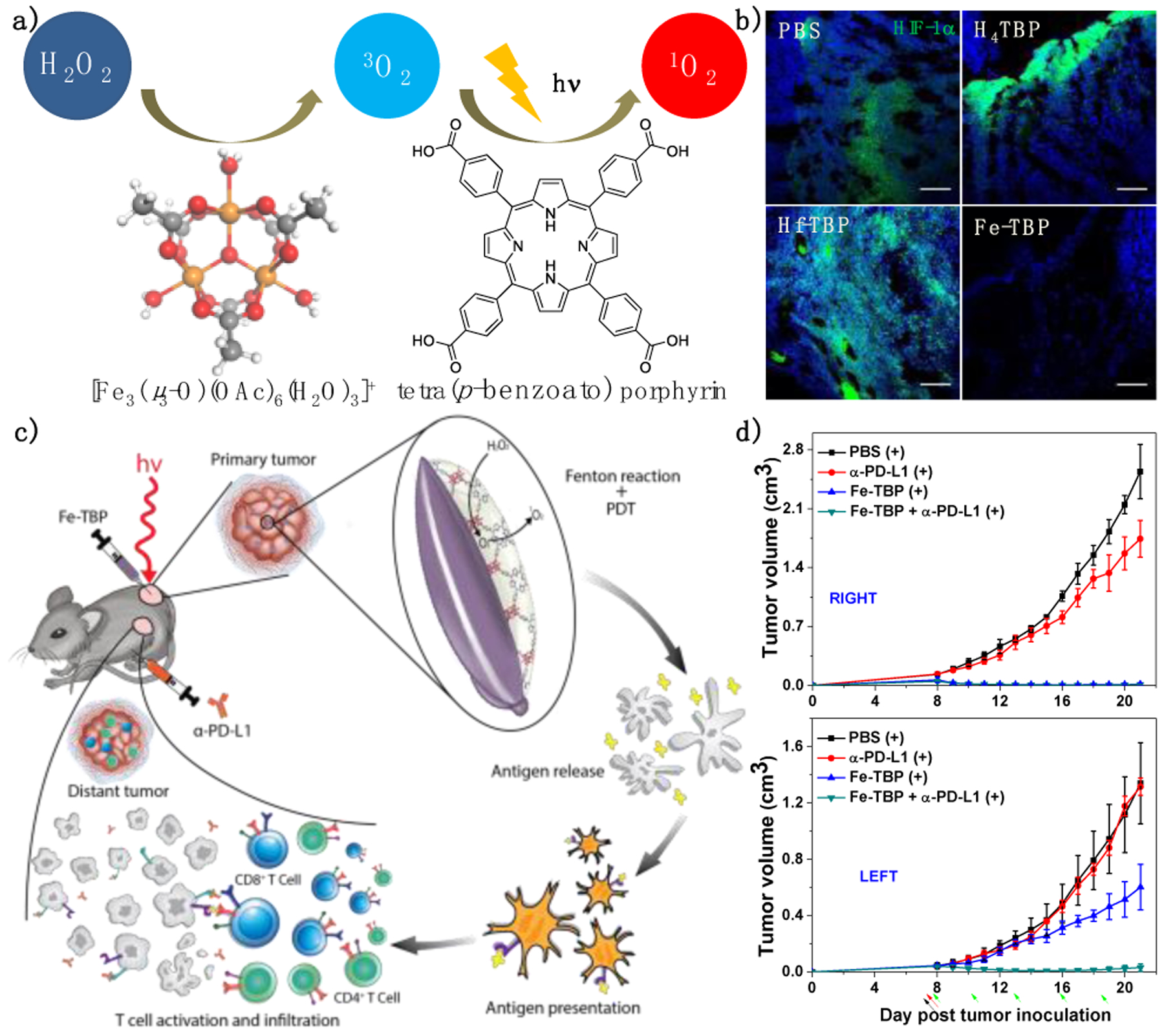
(a) Illustration of the conversion of H2O2 to 1O2 by Fe-TBP and light irradiation. (b) Immunofluorescence staining showing HIF-1α expression on tumors treated with PBS, H4TBP, Hf-TBP, or Fe-TBP plus light irradiation. Scale bar = 50 μm. (c) Schematic figure showing local inflammation induced by Fe-TBP-mediated PDT synergizes with αPD-L1 CBI. (d) Primary right (top) and distant left (bottom) tumor growth curves after treatment of CT26 tumor-bearing mice Fe-TBP with (+) or without (−) light irradiation. Black, red and green arrows refer to intratumoral injection, light irradiation, and intraperitoneal injection, respectively. Reproduced with permission from Ref. 48. Copyright 2016 American Chemical Society.
Similar as nMOF-based cancer vaccine, co-delivery of immunoadjuvants with nMOFs has been used to further enhance the immunotherapeutic effects of nMOF-mediated PDT and CBI. In 2019, Ni et al. designed a cationic nMOF, W-TBP, to promote tumor antigen presentation by DCs via immunogenic PDT and class C CpG delivery.52 Cationic W-TBP allowed for effective CpG loading and CpG internalization by DCs, leading to expression of co-stimulatory molecule CD80 and MHC-II on DCs and upregulation of immunostimulatory biomarkers IFN-α and IL-6. W-TBP-mediated PDT induced ICD to release TAs whereas the delivered CpG enhanced DC maturation. The enhanced antigen presentation synergized with CBI to afford consistent innate immune response and elicit robust adaptive immunity, leading to superb abscopal effect with >97% tumor regression on a bilateral TUBO murine breast cancer model. Cai et. al. reported the use of PCN-224 nMOF to encapsulate acriflavine, a small molecule HIF inhibitor. Acriflavine-loaded PCN-224 was then coated with CpG and hyaluronic acid.53 The PCN-224-based nanoplatform effectively overcame tumor hypoxia for PDT treatment and synergistically activated the innate and adaptive immune responses, which was supported by elevated levels of TNF-α, IL-12p70, and IFN-γ.
RT for immunotherapy
RT has been used to treat cancers for over a century with tissue-penetrating ionizing radiation. Fractionated RT is regarded as immunosuppressive treatment owing to higher sensitivity of T lymphocytes to ionization radiation compared to tumor cells.54Recently, however, high-dose, hypofractionated RT has been studied as an immunomodulatory adjuvant treatment for CBI, with over a hundred ongoing clinical trials evaluating the synergy between hypofractionated RT and CBI.55 However, the therapeutic effects of hypofractionated RT are achieved at the expense of damaging surrounding tissues. Toxicity from high-dose X-ray irradiation and non-synchronous dosing regimens between RT and CBI have limited the potential of this RT and CBI combination.56
The therapeutic ratio of RT can be improved with radiosensitizers by increasing radiation absorption difference between healthy and tumor tissues. In 2018, Ni et al. first reported the use of Hf-based nMOFs, Hf6-DBA and Hf12-DBA, as nanoradiosensitizers for RT.2 With high-Z elements and high specific surface areas, Hf-nMOFs enhanced radiotherapeutic effects of X-rays by increasing energy deposition to generate hydroxyl radical (•OH) and facilitate •OH diffusion through MOF channels to elicit cytotoxic effects (Figure 5a). As a result, Hf-nMOFs were more efficient in •OH generation than HfO2 NPs as probed by aminophenyl fluorescein assay (Figure 5b).
Figure 5.
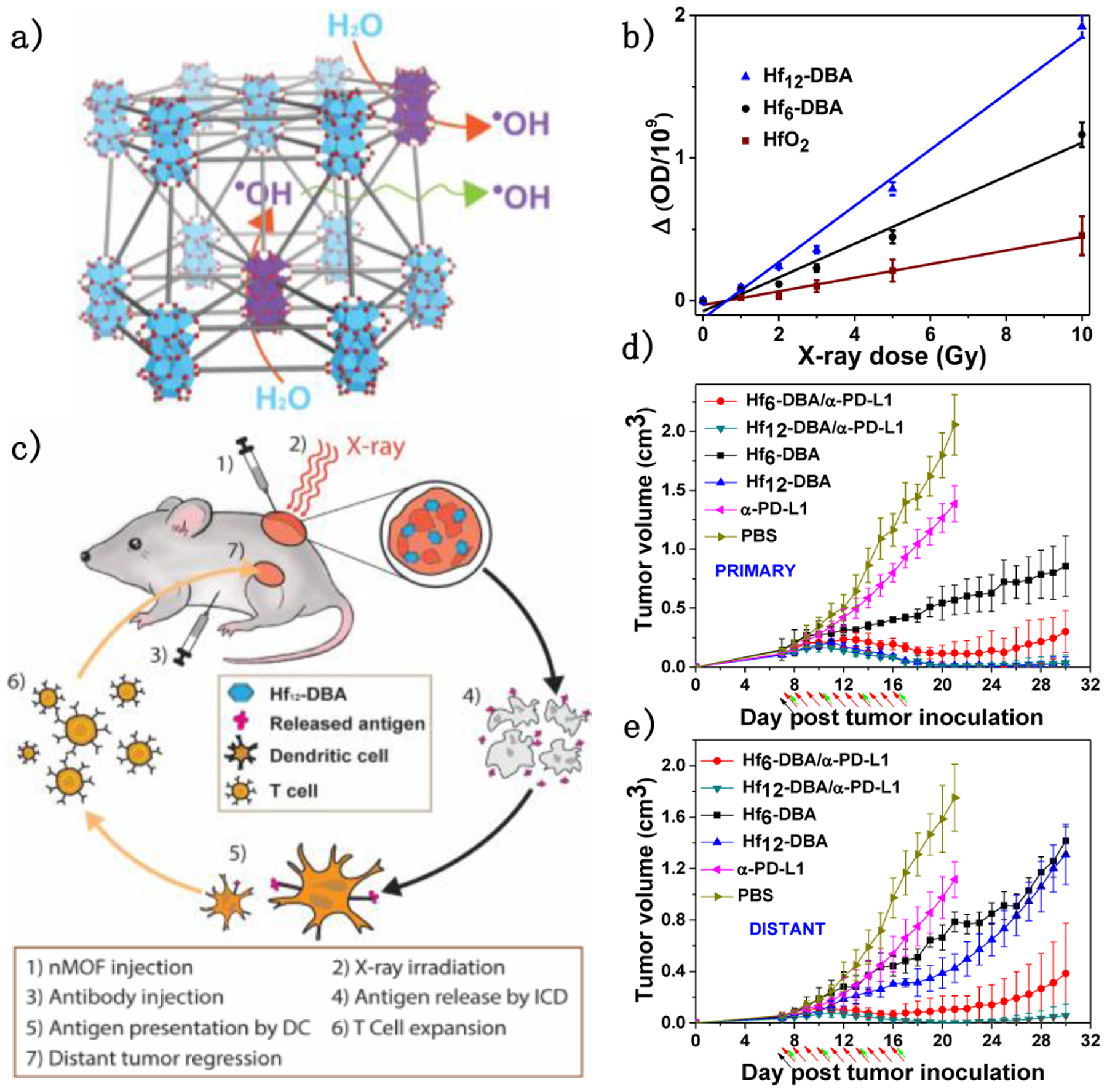
(a) Schematic showing the enhanced radiosensitization of Hf-based nMOFs. (b) •OH generation by HfO2, Hf6-DBA, or Hf12-DBA. (c) Schematic illustration of abscopal effect induced by Hf-nMOF-mediated RT and CBI. Tumor growth curves of treated primary (d) and untreated distant (e) tumors after treatment with different formulations plus X-ray irradiation. Black, red and green arrows refer to intratumoral injection, X-ray irradiation, and intraperitoneal injection, respectively.
Upon low-dose X-ray irradiation, nMOF-enhanced RT activated local inflammation to generate an immunological hotbed to synergize with αPD-L1 CBI (Figure 5c). A bilateral model of CT26 was established to assess the abscopal effect of Hf-nMOF mediated RT synergized with CBI. Hf-nMOFs were intratumorally injected into the primary tumors and then irradiated with 1 Gy/fraction of X-ray for 10 fractions. αPD-L1 antibody was intraperitoneally injected every three days. Hf12-DBA outperformed Hf6-DBA on primary tumor treatment (Figure 5d), indicating the superior radiosensitization effect of Hf12-DBA. In conjunction with αPD-L1 CBI, Hf12-DBA regressed local, irradiated tumors and shrank distant, non-irradiated tumors (Figure 5e).
RT-RDT for immunotherapy
To further improve the local efficacy and immunogenicity of low-dose RT, Lin and coworkers disclosed nMOF-mediated RT-RDT that combines the advantages of both PDT and RT in 2018.57 Upon X-ray irradiation, Hf-oxo cluster SBUs in Hf-DBP (DBP denotes di(p-benzoato)porphyrin) absorb X-rays to enhance •OH generation and transfer energy to photosensitizing DBP linkers to generate 1O2 (Figure 6a&b).3 Intratumoral injection of Hf-DBP efficiently regressed tumors at extremely low doses of X-ray irradiation in multiple mouse models of cancer. The local anti-tumor efficacy of Hf-DBP-mediated RT-RDT was abrogated upon depletion of B cells, CD4+ T cells, or CD8+ T cells (Figure 6f), demonstrating the importance of immune modulation in local tumor destruction. IDOi@Hf-DBP in conjunction with low dose X-ray irradiation regressed both primary treated tumors and distant untreated tumors in bilateral TUBO breast cancer and CT26 colon cancer mouse models (Figure 6c–e). Immune profiling of tumor-infiltrating leukocytes showed the increase of CD4+ and CD8+ T cells in the tumors whereas ELISpot assay demonstrated the presence of tumor antigen-specific CD8+ T cells after IDOi@Hf-DBP plus X-ray treatment. These results suggest the in situ vaccination induced by Hf-DBP plus X-ray treatment and IDOi immunotherapy synergize with each other to generate systemic antitumor immunity.
Figure 6.
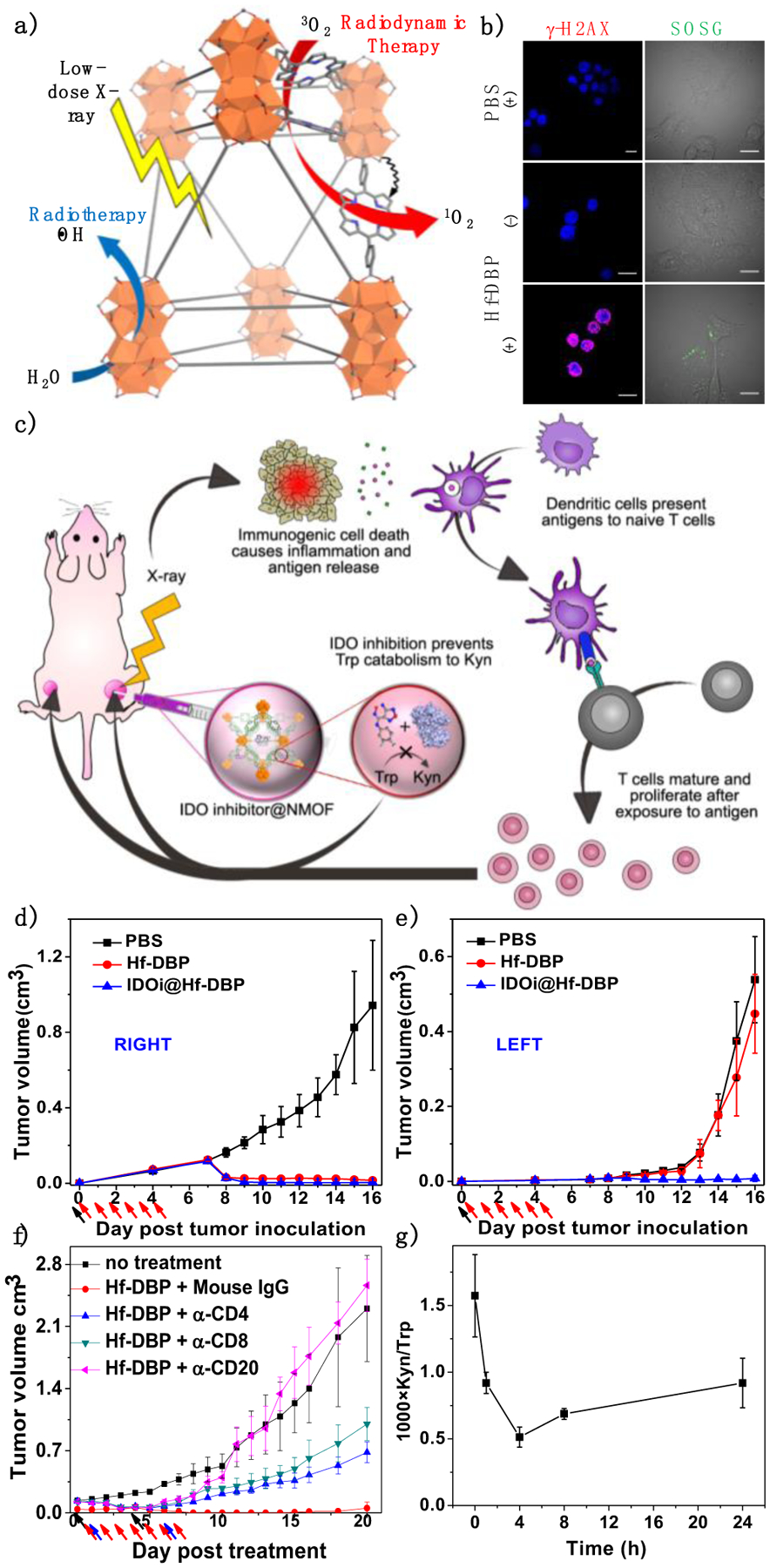
(a) Schematic showing Hf-DBP -enabled RT-RDT to generate both •OH via radiolysis and 1O2 via energy transfer to DBP. (b) in vitro DNA double strand breaks and 1O2 generation probed by γ-H2AX and SOSG, respectively. Scale bar = 10 μm. (c) Schematic illustration of abscopal effect induced by nMOF-enabled RT-RDT and IDOi. Tumor growth curves of treated primary (right, d) and untreated distant (left, e) tumors after treatment with Hf-DBP plus X-ray irradiation. (f) Tumor curves after treatment with Hf-DBP-mediated RT-RDT with depletion of B cell or T cell. (g) Plasma kynurenine concentrations of mice intratumorally injected with IDOi@ Hf-DBP-. N=3. Black, red and blue arrows refer to intratumoral injection, X-ray irradiation and intraperitoneal injection, respectively. Reproduced with permission from Ref. 3. Copyright 2018 Nature Publishing Group.
In 2019, the same lab reported the combination of a monolayer version of Hf-DBP (Hf-MOL) with αPD-L1 CBI for treating orthotopic triple negative breast cancer with lung-metastasis (Figure 7a).58 The lower dimensionality of Hf-MOL further facilitated ROS diffusion upon low-dose X-ray irradiation and the enhanced ICD induced by RT-RDT was systemically characterized by detecting calreticulin exposure, ATP secretion and HMGB1 release. Murine breast cancer cell 4T1 treated with Hf-MOL plus X-ray vaccinated mice with anti-tumor immunity. The combination treatment of Hf-MOL-mediated RT-RDT and αPD-L1 CBI not only eradicated the orthotopic tumors (Figure 7b) but also significantly diminished lung metastasis (Figure 7c), suggesting systemic anti-tumor immunity and anti-metastatic property. Mechanistic studies showed that local RT-RDT treatment plus systemic CBI drastically increased CD8+ T cell infiltration in the orthotopic tumors and the lungs, and effectively depleted myeloid-derived suppressor cells in the spleen (Figure 7e). The combination treatment relieved spleen enlargement (Figure 7d) and prevented lung metastasis by suppressing mesenchymal-to-epithelial transition (Figure 7e).
Figure 7.
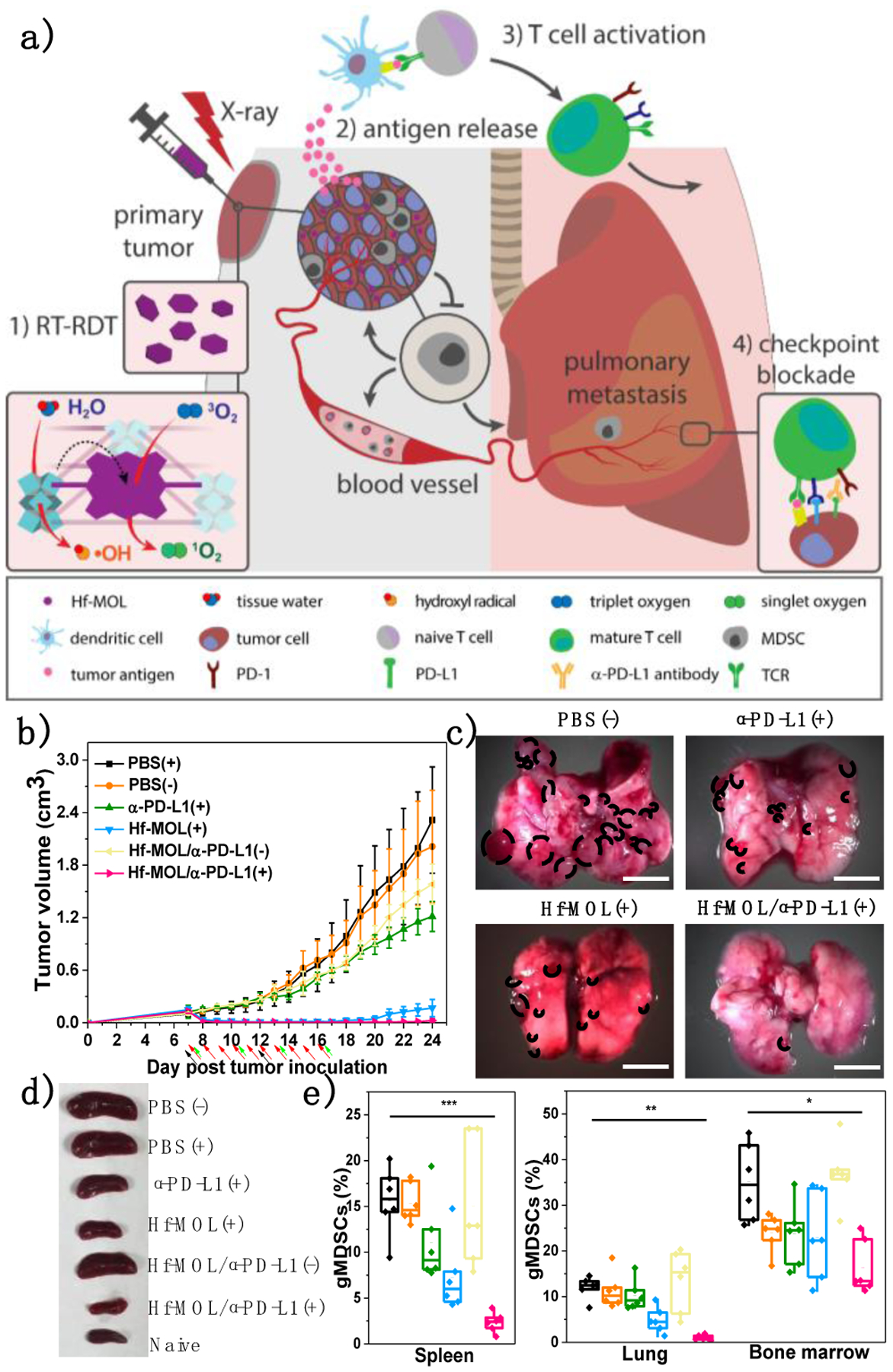
Scheme (a) and efficacy curves (b) of Hf-MOL-mediated local PDT synergized with αPD-L1 CBI to reactivate systemic anti-tumor immunity. (c) Local nMOF-mediated RT-RDT on orthotopic breast tumors potentiated αPD-L1 CBI to suppress lung-metastasis. (d) Photos of mouse spleens after various treatments. (e) gMDSC percentages to total living cells in spleens, lungs, and bone marrows. *P<0.05, **P<0.01, and ***P<0.005 from control. Reproduced with permission from Ref. 57. Copyright 2019 Elsevier Inc.
In 2020, Ni et al. used Hf-DBP to co-deliver hydrophobic small-molecule toll-like receptor 7 agonist, imiquimod (IMD), and a hydrophilic macromolecule, anti-CD47 antibody (αCD47), for cancer immunotherapy.59 As a FDA-proved immunotherapeutic agent, IMD delivered by Hf-DBP repolarizes immunosuppressive M2 macrophages (pro-tumor) to immunostimulatory M1 macrophages (anti-tumor). Clinically investigated αCD47 conjugated to the surface of Hf-DBP blocks CD47 overexpressed on tumor cell surface to promote phagocytosis. Intratumorally administered IMD@Hf-DBP/αCD47 effectively maintained local concentrations of IMD and αCD47 to repolarize immunosuppressive macrophage. Upon X-ray irradiation, the activated TME and innate immunity elicit adaptive immunity when synergized with αPD-L1 ICB, leading to the eradication of both primary and distant tumors on a bilateral colorectal tumor model. nMOFs thus provide a unique platform to co-deliver multiple immunoadjuvants for modulating local innate immunity and inducing systematic immune responses.
Similar to PDT, the efficacy of RT-RDT is adversely impacted by tumor hypoxia owing to its dependence on tissue oxygen. In 2020, the same lab developed a strategy for hypoxia-tolerant RT-RDT.60 By metalating Hf-DBP with FeCl2, the resultant Hf-DBP-FeIII exhibited biomimetic catalase-like property to generate •OH for causing DNA DSBs and convert intratumoral H2O2 to 3O2, which was converted to 1O2 by the RDT process. Thus, upon low-dose X-ray irradiation, Hf-DBP-Fe-enabled RT-RDT exhibited much stronger anti-tumor efficacy with enhanced immunogenicity compared to Hf-DBP, synergizing with αPD-L1 CBI for treating hypoxic tumors.
CDT for immunotherapy
CDT kills tumor cells with ROS generated from endogenous triggers, such as H2O2, hormonal metabolite, and glutathione, presenting a promising strategy to generate a pro-inflammatory TME for cancer immunotherapy. Considering imbalanced glycolytic homeostasis in cancer cells, significant effort has been focused on introducing redox-active elements such as Mn, Fe, and Cu to decompose intratumoral H2O2 to generate cytotoxic •OH through Fenton-like reactions.
Hormonal imbalance, particularly overexpression of estradiol, is a hallmark of many cancers.61 Interestingly, estradiol can form stable adducts with DNA via generating ROS in the downstream metabolic process catalyzed by bioavailable Cu2+ ions.62 In 2019, the Lin lab reported the first nMOF-mediated synergistic CDT and PDT for anti-tumor treatment using Cu-TBP (Figure 8a).4 Acidic TMEs decompose Cu-TBP to activate the PDT effect of released TBP, which is quenched by the paramagnetic Cu2+ ions in intact Cu-TBP. High payload and effective delivery of Cu2+ provides a localized supply of free Cu2+ to hijack the estradiol metabolic pathway and promote cytotoxic ROS generation for effective radical therapy. By screening intracellular estradiol concentrations, B16F10 and SKVO-3 with high estradiol levels were selected to test Cu-TBP-mediated CDT. This novel CDT process was supported by the detection of H2O2, •OH, and O2− in test tubes and in cells (Figure 8b). Cu-TBP-mediated dual-triggered radical therapy combined hormone-induced CDT and porphyrin-based PDT to effectively regress local B16F10 and SKOV-3 tumors, demonstrating the feasibility of using nMOF-mediated CDT on hormonally dysregulated tumors. Efficient ROS generation promoted ICD and phagocytosis, the vital link between innate and adaptive anti-tumor immune response (Figure 8c). Cu-TBP-catalyzed ROS generation plus CBI elicited a strong abscopal effect on the bilateral B16F10 tumor model, leading to a 50% cure rate without recurrence.
Figure 8.
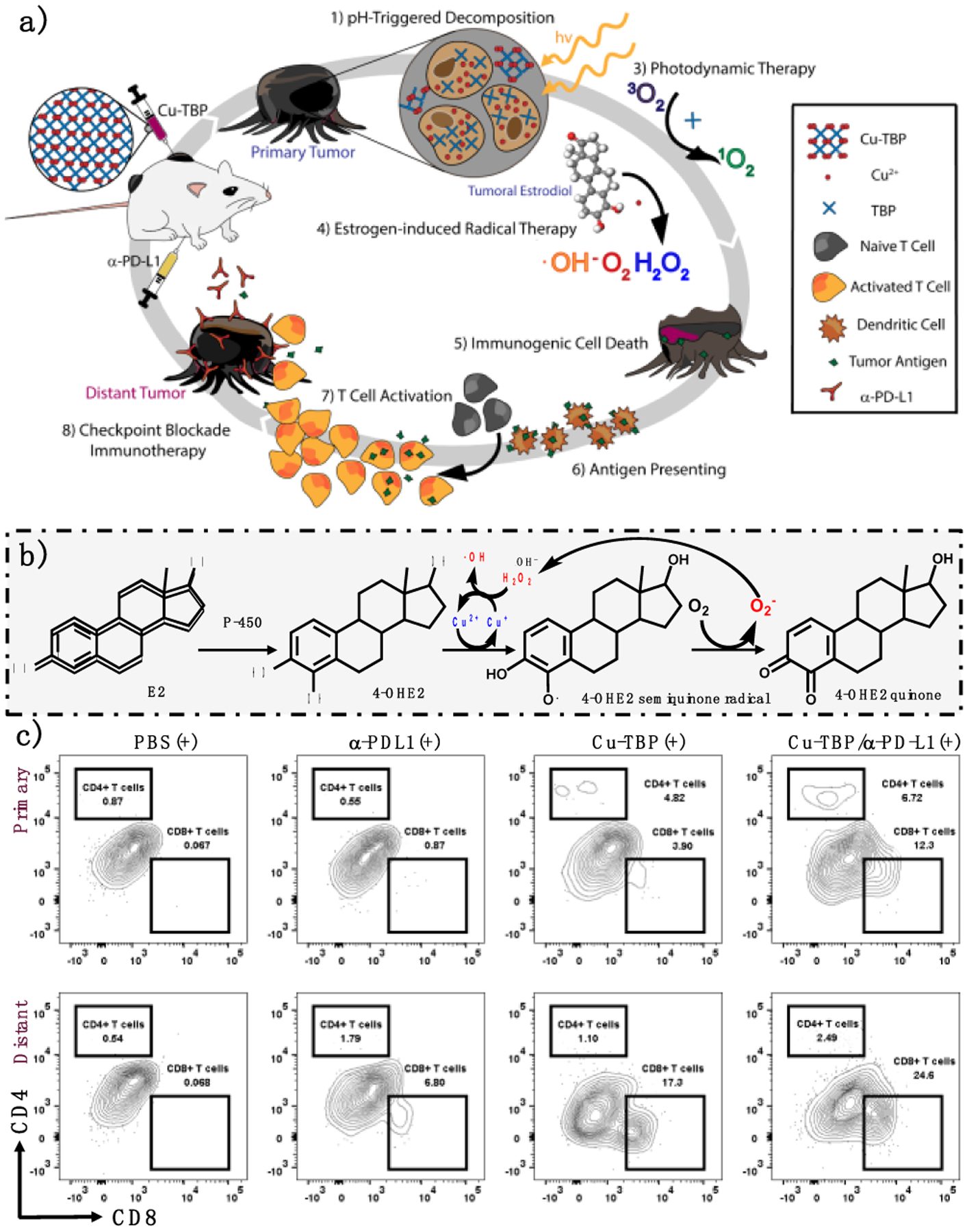
(a) Schematic of Cu-TBP-catalyzed CDT triggered by intratumoral hormone combined with PDT to potentiate CBI. (b) Hormone-induced Cu-mediated ROS generation process. (c) Tumor infiltration of CD4+ and CD8+ T cells owing to activated anti-tumor adaptive immunity. Reproduced with permission from Ref. 4. Copyright 2019 Elsevier Inc.
Summary
In the past few years, nMOFs have been used to enhance cancer immunotherapy via two distinct strategies. The first strategy uses nMOFs as porous nanomaterials to deliver peptide-based antigens, nuclei acid-based immunoadjuvants, and other biomacromolecules as cancer vaccines to directly immunize hosts with anti-tumor immunity. The surface property of nMOFs can be readily modified to adsorb immunoadjuvants via covalent and noncovalent interactions. The second strategy uses nMOFs as nanosensitizers to enhance the generation of ROS by PDT, RT, RDT, and CDT and elicit local anti-tumor effects and generate inflammatory TMEs. nMOF-mediated ICD exposes TAs and DAMPs as an in situ cancer vaccine and stimulates APCs. As a result, nMOF-based local treatments reshape immunosuppressive TMEs to immunostimulatory ones, potentiating cancer immunotherapy with increased tumor-infiltrating T cells. The pros and cons of these strategies are listed in Table 1.
Table 1.
Summary of key advantages and disadvantages of-nMOF-based cancer immunotherapies.
| Strategy | Pros | Cons | |
|---|---|---|---|
| Cancer vaccine | Clinically practiced modality | Limited efficacy with peptide-based vaccine | |
| In situ cancer vaccine | PDT | Highly immunogenic, limited side effect | Tissue penetration of light; tumor hypoxia |
| RT | Highly penetrating; clinically used in broad-spectrum tumors | Limited efficacy; potential longterm genotoxicity | |
| RT-RDT | Highly penetrating; highly immunogenic with low-dose X-ray; | Potential toxicity of heavy metals | |
| CDT | Endogenous trigger for ROS generation | Toxicity of transition metals; cannot be externally controlled | |
Three broad categories of nMOF engineering have been studied to enhance cancer immunotherapy (Figure 9). First, molecular engineering of organic linkers afford diverse nMOFs with novel functions. Second, crystal engineering of nMOFs endows diverse topologies and functions with different metal cluster SBUs. Third, through immunoengineering, a number of immunotherapeutic agents, including small molecule drug, biomacromolecules including oligodeoxynuleotides and antibodies are incorporated into nMOFs for immune modulation and systemic anti-tumor immunity.
Figure 9. Schematic showing three categories of nMOF engineering for cancer immunotherapy.
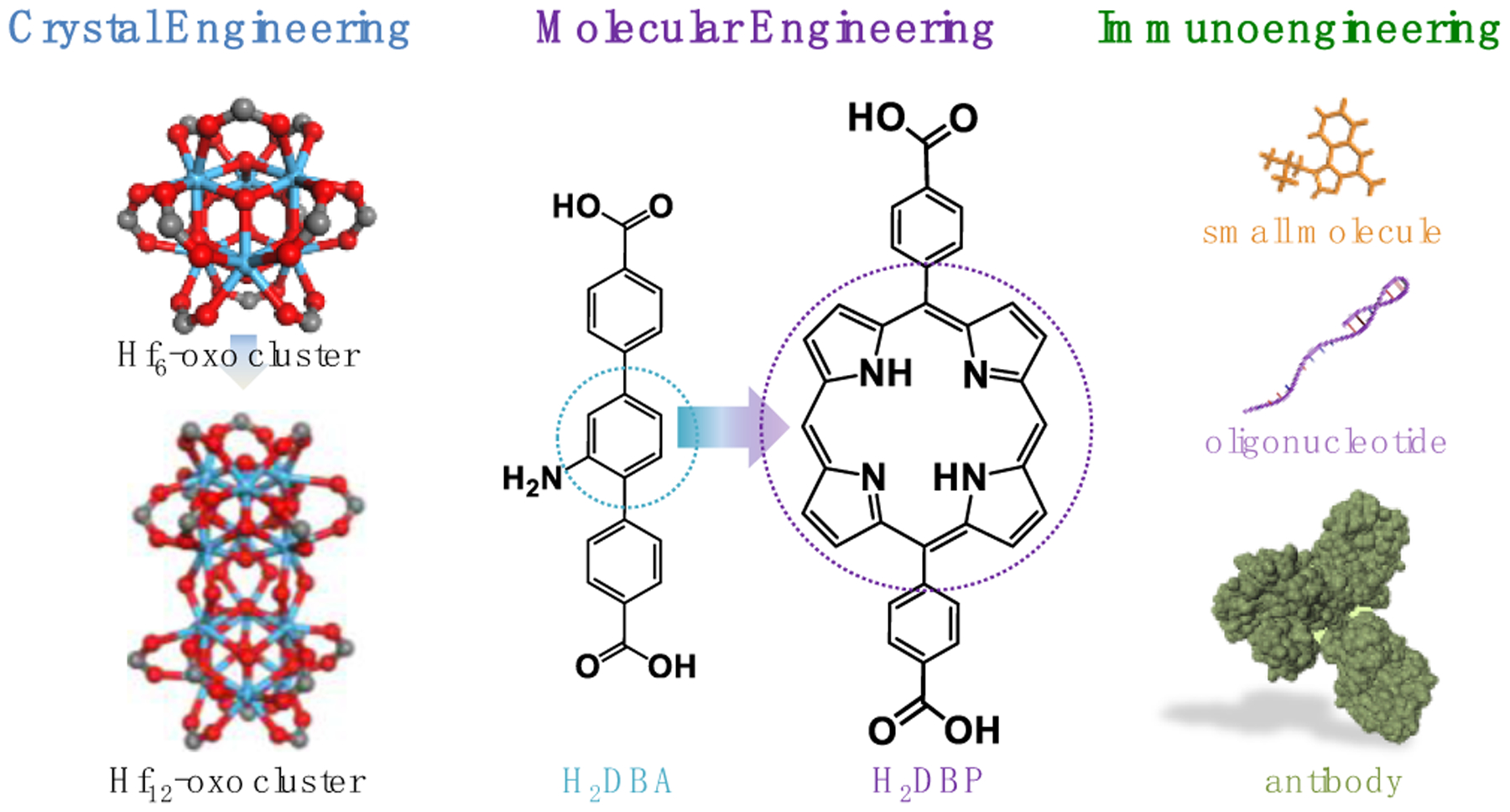
By tuning organic linkers via molecular engineering and metal-oxo cluster SBUs and topologies via crystal engineering and incorporating immunotherapeutics via immunoengineering, nMOF-based treatments amplify local inflammation to potentiate systemic anti-tumor immunity when combined with immune checkpoint blockade.
Metal toxicity is a concern for the use of nMOF-based treatments on human patients. RiMO-301, an nMOF formulation for radioenhancement, has shown no treatment-related adverse events in the ongoing first-in-human clinical trial (NCT03444714). We anticipate to see increased efforts on combining nMOF-mediated local therapies and cancer immunotherapies in the near future. The synthetic tunability of nMOFs will allow fine-tuning of their structures and compositions for enhanced PDT, RT, RDT-RDT, and CDT, which will be synergistically combined with various cancer immunotherapies to elicit superb anti-tumor efficacy. We foresee a bright future for nMOF-based cancer immunotherapy with contributions from multidisciplinary researchers.
Funding Sources
We acknowledge the National Cancer Institute (U01-CA198989, 1R01CA253655), Department of Defense (PC170934P2), the University of Chicago Medicine Comprehensive Cancer Center (NIH CCSG: P30 CA014599), and the Ludwig Institute for Metastasis Research for funding support.
Biographies
Kaiyuan Ni was born in Hefei, Anhui, China in 1993. He graduated from Xiamen University in 2015 with honors. He received his Ph.D. in Prof. Wenbin Lin lab at the University of Chicago. His research interests include nanoscale metal-organic frameworks and layers for cancer therapy and diagnosis.
Taokun Luo was born in Xi’an City, Shaanxi Province, China in 1996. He received his B.S. in Chemistry with a minor in Philosophy from Tsinghua University in 2018. He is currently a Ph.D. candidate in Prof. Wenbin Lin’s group at the University of Chicago. His research interest focuses on the application of nanoscale metal-organic frameworks for cancer therapy and immunotherapy.
Geoffrey T. Nash was born in Chicago, IL in 1995. He received his MChem from the University of Sussex in 2018. He is currently pursuing his Ph.D. in Chemistry at the University of Chicago under the guidance of Prof. Wenbin Lin. His research interests are focused on the application of nanoscale metal-organic frameworks and layers for cancer therapy and renewable energy.
Wenbin Lin is the James Franck Professor of Chemistry, Radiation and Cellular Oncology, and Ludwig Center for Metastasis Research at the University of Chicago. His group has pioneered the applications of MOFs in cancer therapy, bioimaging, Earth-abundant metal catalysis, artificial photosynthesis, asymmetric catalysis, and second-order nonlinear optics. His startup company has three anticancer drug candidates under clinical investigations.
Footnotes
Conflict of Interests
The authors declare the following competing financial interest(s): W.L. is founder of Coordination Pharmaceuticals, which licensed the nMOF technology from the University of Chicago. The remaining authors declare no competing interests.
The authors declare the following competing financial interest(s): W.L. is founder and chairman of Coordination Pharmaceuticals Inc., which licensed the nMOF technologies from the University of Chicago.
REFERENCES
- 1.Lu K; He C; Guo N; Chan C; Ni K; Weichselbaum RR; Lin W, Chlorin-based nanoscale metal–organic framework systemically rejects colorectal cancers via synergistic photodynamic therapy and checkpoint blockade immunotherapy. J. Am. Chem. Soc 2016, 138 (38), 12502–12510. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first work reporting nMOF-mediated PDT for cancer immunotherapy.
- 2.Ni K; Lan G; Chan C; Quigley B; Lu K; Aung T; Guo N; La Riviere P; Weichselbaum RR; Lin W, Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nat. Commun 2018, 9 (1), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first work reporting nMOF-enhanced RT for cancer immunotherapy.
- 3.Lu K; He C; Guo N; Chan C; Ni K; Lan G; Tang H; Pelizzari C; Fu YX; Spiotto MT; Weichselbaum RR; Lin W, Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed. Eng 2018, 2, 600. [DOI] [PubMed] [Google Scholar]; This is the first work reporting nMOF-enabled RT-RDT for cancer immunotherapy.
- 4.Ni K; Aung T; Li S; Fatuzzo N; Liang X; Lin W, Nanoscale metal-Organic framework mediates radical therapy to enhance cancer immunotherapy. Chem 2019, 5 (7), 1892–1913. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; This is the first work reporting nMOF-mediated CDT for cancer immunotherapy.
- 5.Miller KD; Nogueira L; Mariotto AB; Rowland JH; Yabroff KR; Alfano CM; Jemal A; Kramer JL; Siegel RL, Cancer treatment and survivorship statistics, 2019. CA. Cancer J. Clin 2019. [DOI] [PubMed] [Google Scholar]
- 6.Mellman I; Coukos G; Dranoff G, Cancer immunotherapy comes of age. Nature 2011, 480 (7378), 480489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen DS; Mellman I, Oncology meets immunology: the cancer-immunity cycle. Immunity 2013, 39 (1), 1–10. [DOI] [PubMed] [Google Scholar]
- 8.Blankenstein T; Coulie PG; Gilboa E; Jaffee EM, The determinants of tumour immunogenicity. Nature Reviews Cancer 2012, 12 (4), 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn GP; Bruce AT; Ikeda H; Old LJ; Schreiber RD, Cancer immunoediting: from immunosurveillance to tumor escape. Nat. Immunol 2002, 3 (11), 991–998. [DOI] [PubMed] [Google Scholar]
- 10.Ohigashi Y; Sho M; Yamada Y; Tsurui Y; Hamada K; Ikeda N; Mizuno T; Yoriki R; Kashizuka H; Yane K, Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clinical cancer research 2005, 11 (8), 29472953. [DOI] [PubMed] [Google Scholar]
- 11.Blank C; Brown I; Peterson AC; Spiotto M; Iwai Y; Honjo T; Gajewski TF, PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer research 2004, 64 (3), 1140–1145. [DOI] [PubMed] [Google Scholar]
- 12.Puccetti P; Grohmann U, IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-κB activation. Nature Reviews Immunology 2007, 7 (10), 817–823. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y; Zheng W; Yang K; Harris KG; Ni K; Xue L; Lin W; Chang EB; Weichselbaum RR; Fu Y-X, Intratumoral accumulation of gut microbiota facilitates CD47-based immunotherapy via STING signaling. Journal of Experimental Medicine 2020, 217 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma P; Allison JP, The future of immune checkpoint therapy. Science 2015, 348 (6230), 56–61. [DOI] [PubMed] [Google Scholar]
- 15.Guy B, The perfect mix: recent progress in adjuvant research. Nature Reviews Microbiology 2007, 5 (7), 396–397. [DOI] [PubMed] [Google Scholar]
- 16.Lynch TJ; Bondarenko I; Luft A; Serwatowski P; Barlesi F; Chacko R; Sebastian M; Neal J; Lu H; Cuillerot J-M, Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non–small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J. Clin. Oncol 2012, 30 (17), 20462054. [DOI] [PubMed] [Google Scholar]
- 17.Schmid P; Rugo HS; Adams S; Schneeweiss A; Barrios CH; Iwata H; Diéras V; Henschel V; Molinero L; Chui SY, Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21 (1), 44–59. [DOI] [PubMed] [Google Scholar]
- 18.Crittenden M; Kohrt H; Levy R; Jones J; Camphausen K; Dicker A; Demaria S; Formenti S In Current clinical trials testing combinations of immunotherapy and radiation, Semin. Radiat. Oncol, Elsevier: 2015; pp 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nam J; Son S; Park KS; Zou W; Shea LD; Moon JJ, Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater 2019, 4 (6), 398–414. [Google Scholar]
- 20.Furukawa H; Cordova KE; O’Keeffe M; Yaghi OM, The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341 (6149). [DOI] [PubMed] [Google Scholar]
- 21.Della Rocca J; Liu D; Lin W, Nanoscale metal–organic frameworks for biomedical imaging and drug delivery. Acc. Chem. Res 2011, 44 (10), 957–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieter WJ; Taylor KM; An H; Lin W; Lin W, Nanoscale metal− organic frameworks as potential multimodal contrast enhancing agents. J. Am. Chem. Soc 2006, 128 (28), 9024–9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor KM; Rieter WJ; Lin W, Manganese-based nanoscale metal− organic frameworks for magnetic resonance imaging. J. Am. Chem. Soc 2008, 130 (44), 14358–14359. [DOI] [PubMed] [Google Scholar]
- 24.Taylor-Pashow KM; Della Rocca J; Xie Z; Tran S; Lin W, Postsynthetic modifications of iron-carboxylate nanoscale metal− organic frameworks for imaging and drug delivery. J. Am. Chem. Soc 2009, 131 (40), 14261–14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ni K; Lan G; Lin W, Nanoscale Metal–Organic Frameworks Generate Reactive Oxygen Species for Cancer Therapy. ACS Cent. Sci 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimsley C; Ravichandran KS, Cues for apoptotic cell engulfment: eat-me, don’t eat-me and come-get-me signals. Trends Cell Biol. 2003, 13 (12), 648–656. [DOI] [PubMed] [Google Scholar]
- 27.Sims GP; Rowe DC; Rietdijk ST; Herbst R; Coyle AJ, HMGB1 and RAGE in Inflammation and Cancer. Annual Review of Immunology 2010, 28 (1), 367–388. [DOI] [PubMed] [Google Scholar]
- 28.Plotkin SA; Plotkin SL, The development of vaccines: how the past led to the future. Nature Reviews Microbiology 2011, 9 (12), 889–893. [DOI] [PubMed] [Google Scholar]
- 29.Palefsky JM; Giuliano AR; Goldstone S; Moreira ED Jr; Aranda C; Jessen H; Hillman R; Ferris D; Coutlee F; Stoler MH, HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. New England Journal of Medicine 2011, 365 (17), 1576–1585. [DOI] [PubMed] [Google Scholar]
- 30.Ma L; Dichwalkar T; Chang JY; Cossette B; Garafola D; Zhang AQ; Fichter M; Wang C; Liang S; Silva M, Enhanced CAR–T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science 2019, 365 (6449), 162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dane EL; Irvine DJ, Big thinking for adjuvants. Nature biotechnology 2015, 33 (11), 1146. [DOI] [PubMed] [Google Scholar]
- 32.Hu Z; Ott PA; Wu CJ, Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nature Reviews Immunology 2018, 18 (3), 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purcell AW; McCluskey J; Rossjohn J, More than one reason to rethink the use of peptides in vaccine design. Nature reviews Drug discovery 2007, 6 (5), 404. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y; Wang F; Ju E; Liu Z; Chen Z; Ren J; Qu X, Metal - organic - framework - based vaccine platforms for enhanced systemic immune and memory response. Advanced Functional Materials 2016, 26 (35), 6454–6461. [Google Scholar]
- 35.Zhong X; Zhang Y; Tan L; Zheng T; Hou Y; Hong X; Du G; Chen X; Zhang Y; Sun X, An aluminum adjuvant-integrated nano-MOF as antigen delivery system to induce strong humoral and cellular immune responses. Journal of controlled release 2019, 300, 81–92. [DOI] [PubMed] [Google Scholar]
- 36.Sahin U; Türeci Ö, Personalized vaccines for cancer immunotherapy. Science 2018, 359 (6382), 1355–1360. [DOI] [PubMed] [Google Scholar]
- 37.Kuai R; Ochyl LJ; Bahjat KS; Schwendeman A; Moon JJ, Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater 2017, 16 (4), 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheetz L; Park KS; Li Q; Lowenstein PR; Castro MG; Schwendeman A; Moon JJ, Engineering patient-specific cancer immunotherapies. Nat. Biomed. Eng 2019, 3 (10), 768–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H; Mooney DJ, Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nature materials 2018, 17 (9), 761–772. [DOI] [PubMed] [Google Scholar]
- 40.Liu C; Lou Y; Lizée G; Qin H; Liu S; Rabinovich B; Kim GJ; Wang Y-H; Ye Y; Sikora AG, Plasmacytoid dendritic cells induce NK cell–dependent, tumor antigen–specific T cell cross-priming and tumor regression in mice. J. Clin. Investig 2008, 118 (3), 1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kepp O; Marabelle A; Zitvogel L; Kroemer G, Oncolysis without viruses—inducing systemic anticancer immune responses with local therapies. Nature Reviews Clinical Oncology 2019, 1–16. [DOI] [PubMed] [Google Scholar]
- 42.Dolmans DE; Fukumura D; Jain RK, Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3 (5), 380–387. [DOI] [PubMed] [Google Scholar]
- 43.Lu K; He C; Lin W, Nanoscale Metal–Organic Framework for Highly Effective Photodynamic Therapy of Resistant Head and Neck Cancer. J. Am. Chem. Soc 2014, 136 (48), 16712–16715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu K; He C; Lin W, A chlorin-based nanoscale metal–organic framework for photodynamic therapy of colon cancers. Journal of the American Chemical Society 2015, 137 (24), 7600–7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo T; Ni K; Culbert A; Lan G; Li Z; Jiang X; Kaufmann M; Lin W, Nanoscale Metal–Organic Frameworks Stabilize Bacteriochlorins for Type I and Type II Photodynamic Therapy. J. Am. Chem. Soc 2020, 142 (16), 7334–7339. [DOI] [PubMed] [Google Scholar]
- 46.Lan G; Ni K; Veroneau SS; Feng X; Nash GT; Luo T; Xu Z; Lin W, Titanium-Based Nanoscale Metal–Organic Framework for Type I Photodynamic Therapy. J. Am. Chem. Soc 2019, 141 (10), 4204–4208. [DOI] [PubMed] [Google Scholar]
- 47.Liu C; Liu B; Zhao J; Di Z; Chen D; Gu Z; Li L; Zhao Y, Nd3+-Sensitized Upconversion Metal–Organic Frameworks for Mitochondria - Targeted Amplified Photodynamic Therapy. Angew. Chem. Int. Ed 2019. [DOI] [PubMed] [Google Scholar]
- 48.Li Y; Di Z; Gao J; Cheng P; Di C; Zhang G; Liu B; Shi X; Sun L-D; Li L, Heterodimers made of upconversion nanoparticles and metal–organic frameworks. J. Am. Chem. Soc 2017, 139 (39), 13804–13810. [DOI] [PubMed] [Google Scholar]
- 49.Castano AP; Mroz P; Hamblin MR, Photodynamic therapy and anti-tumour immunity. Nature Reviews Cancer 2006, 6 (7), 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng J-Y; Zou M-Z; Zhang M; Wang X-S; Zeng X; Cong H; Zhang X-Z, π-extended benzoporphyrin-based metal–organic framework for inhibition of tumor metastasis. ACS Nano 2018, 12 (5), 4630–4640. [DOI] [PubMed] [Google Scholar]
- 51.Lan G; Ni K; Xu Z; Veroneau SS; Song Y; Lin W, Nanoscale metal–organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J. Am. Chem. Soc 2018, 140 (17), 5670–5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ni K; Luo T; Lan G; Culbert A; Song Y; Wu T; Jiang X; Lin W, A Nanoscale Metal–Organic Framework to Mediate Photodynamic Therapy and Deliver CpG Oligodeoxynucleotides to Enhance Antigen Presentation and Cancer Immunotherapy. Angew. Chem 2020, 132 (3), 1124–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai Z; Xin F; Wei Z; Wu M; Lin X; Du X; Chen G; Zhang D; Zhang Z; Liu X, Photodynamic Therapy Combined with Antihypoxic Signaling and CpG Adjuvant as an In Situ Tumor Vaccine Based on Metal–Organic Framework Nanoparticles to Boost Cancer Immunotherapy. Adv. Healthc. Mater 2020, 9 (1), 1900996. [DOI] [PubMed] [Google Scholar]
- 54.Schaue D; McBride WH, T lymphocytes and normal tissue responses to radiation. Frontiers in oncology 2012, 2, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gajewski TF; Schreiber H; Fu Y-X, Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol 2013, 14 (10), 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaue D; Ratikan JA; Iwamoto KS; McBride WH, Maximizing tumor immunity with fractionated radiation. Int. J. Radiat. Oncol. Biol. Phys 2012, 83 (4), 1306–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin W; He C; Lu K, Nanoparticles for photodynamic therapy, x-ray induced photodynamic therapy, radiotherapy, radiodynamic therapy, chemotherapy, immunotherapy, and any combination thereof. U.S. Patent Application US201462063770P. [Google Scholar]
- 58.Ni K; Lan G; Chan C; Duan X; Guo N; Veroneau SS; Weichselbaum RR; Lin W, Ultrathin Metal-Organic-Layer Mediated Radiotherapy-Radiodynamic Therapy. Matter 2019, 1 (5), 1331–1353. [PMC free article] [PubMed] [Google Scholar]
- 59.Ni K; Luo T; Culbert A; Kaufmann M; Jiang X; Lin W, Nanoscale Metal–Organic Framework Co-delivers TLR-7 Agonists and Anti-CD47 Antibodies to Modulate Macrophages and Orchestrate Cancer Immunotherapy. Journal of the American Chemical Society 2020. [DOI] [PubMed] [Google Scholar]
- 60.Ni K; Lan G; Song Y; Hao Z; Lin W, Biomimetic nanoscale metal–organic framework harnesses hypoxia for effective cancer radiotherapy and immunotherapy. Chemical Science 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grodstein F; Stampfer MJ; Colditz GA; Willett WC; Manson JE; Joffe M; Rosner B; Fuchs C; Hankinson SE; Hunter DJ, Postmenopausal hormone therapy and mortality. New England Journal of Medicine 1997, 336 (25), 1769–1776. [DOI] [PubMed] [Google Scholar]
- 62.Thibodeau PA; Paquette B, DNA damage induced by catecholestrogens in the presence of copper (II): generation of reactive oxygen species and enhancement by NADH. Free Radic. Biol. Med 1999, 27 (11–12), 1367–1377. [DOI] [PubMed] [Google Scholar]


