Keywords: capsaicin, infant swallowing, kinematics, pediatrics, sensorimotor feedback
Abstract
Sensorimotor feedback is critical to safe and effective swallowing. Because of this, sensory interventions have the potential to treat dysphagia. One such treatment may be found in capsaicin, which activates the internal branch of the superior laryngeal nerve (iSLN). The iSLN initiates the pharyngeal swallow, and a more sensitive iSLN should more readily elicit swallowing and improve swallow safety. We explored the neurophysiological mechanism by which capsaicin improves swallow performance using an infant pig model with a unilateral iSLN lesion. Using high-speed videofluoroscopy, we collected oropharyngeal kinematic data while pigs suckled on bottles, before and after applying capsaicin to the posterior tongue and valleculae. We found that capsaicin application decreased maximal bolus sizes, which improved swallow safety. Furthermore, capsaicin improved performance when infant pigs swallowed more moderately sized boluses. However, capsaicin did not change swallow frequency, the number of sucks prior to each swallow, nor total pharyngeal transit time (TPT). Similarly, excursions of the hyoid, thyroid, and posterior tongue were unchanged. TPT and hyoid and thyroid excursions maintained relationships with bolus size post-capsaicin, suggesting that these variables are less sensitive to sensory intervention. The timing and extent of posterior tongue movement were only correlated with bolus size pre-capsaicin, which could imply that capsaicin fundamentally changes in relationships between tongue movements and bolus size. Our results provide insight into the neural control of swallowing and capsaicin’s mechanism of action, and suggest that capsaicin may be beneficial in treating acute infant dysphagia.
NEW & NOTEWORTHY Chemical sensory interventions alter swallow physiology, which is well-documented in adults but relatively unexplored in infants. Using videofluoroscopy, we found that capsaicin exposure limited infant pigs’ bolus sizes to improve swallow performance without changing swallow frequency. Capsaicin increased the likelihood of safe swallowing with more moderately sized boluses and changed relationships between bolus size and tongue movements, which may impact performance. This work highlights the potential role of capsaicin in treating acute infant dysphagia.
INTRODUCTION
Sensorimotor feedback involves the integration of environmental and proprioceptive cues to modulate motor outputs and alter performance across a wide range of biological processes (1, 2). One important biological process that relies heavily on sensorimotor feedback is feeding, where variation in sensation in the oral cavity, pharynx, and larynx has the capacity to greatly alter swallow performance (3, 4). In fact, some of the most notable causes of oropharyngeal dysphagia (difficulty swallowing) are pathologies linked to decreased sensation, including stroke, nerve damage, neurodegenerative disease, and senescence (5–8). Consequently, enhancing oropharyngeal sensation is a well-established method of altering swallowing to treat dysphagia, and can be accomplished by changing the tactile (i.e., viscosity or carbonation), thermal (i.e., significantly decreased or increased temperature), or chemical (i.e., exposure to substances that activate nociceptors, gustatory receptors, olfactory receptors, or other peripheral chemoreceptors) properties of the substrate (9, 10).
One chemical intervention used to treat dysphagia involves the introduction of capsaicin, a naturally occurring molecule found in chili peppers and other pungent plants (11). When consumed, capsaicin produces oropharyngeal sensations of burning or heat (12–14). Capsaicin heightens oropharyngeal sensation because it is a natural agonist of transient receptor potential ion channels of the subclass vanilloid, specifically member 1 (TRPV1), which has been identified on afferent nerve fibers of the human laryngeal epithelium (15–17). The clinical outcome of oral capsaicin exposure in elderly patients is a decrease in the time to swallow onset (18–20), and application of capsaicin within the ear canal decreases incidence of aspiration pneumonia (21, 22). However, these outcomes are correlative, and we lack an understanding of how capsaicin is acting at the neurophysiologic level to change performance. To develop rehabilitative strategies based on capsaicin’s mechanism of action, we must better understand how capsaicin changes laryngeal sensation and how the structures involved in swallowing subsequently modify their motor activity to alter performance.
An important facet of the neural control of the swallow is found in the internal branch of the superior laryngeal nerve (iSLN). The iSLN is a branch of the vagus nerve (CN X) which provides sensation to the larynx above the vocal folds, the epiglottis, and the base of the tongue (23–25). iSLN laryngeal afferents express TRPV1 channels, and therefore can be activated via the topical application of capsaicin (16, 19). The iSLN has been implicated in triggering the reflexive swallow, as electrical stimulation of the SLN has been shown to elicit a pharyngeal swallow in several models (26–28). During the pharyngeal swallow, the soft palate elevates to protect the nasopharynx and the posterior tongue moves posteriorly and superiorly to propel the bolus through the pharynx and into the esophagus. Simultaneously, the hyolaryngeal apparatus elevates to open the upper esophageal sphincter and protect the opening of the larynx from the incoming bolus. The epiglottis inverts to allow for bolus passage, which also protects the airway (29–32). The capacity of the iSLN to trigger a reflexive swallow is a critical aspect of swallow performance, and populations with either lesioned or anesthetized iSLN afferents experience increased risk of penetration and aspiration (33–37). This is likely due to the iSLN’s role in limiting the volume of a swallowed bolus (33, 35), as reduced bolus sizes have been linked to decreased instances of penetration and aspiration (38, 39). Because impairment of the iSLN results in poor swallow performance, a more sensitive iSLN may function to improve swallow performance in compromised populations—and heightened sensation may be facilitated by activating the TRPV1 channels of the iSLN via the application of capsaicin. This postulation is supported by evidence from previous studies in anesthetized rats and guinea pigs, which found that the topical application of capsaicin to the larynx is sufficient to evoke the pharyngeal swallow (40–42).
This study aimed to evaluate the potential role of capsaicin in increasing the excitability of iSLN afferents. We evaluated the effectiveness of capsaicin in this capacity by applying a capsaicin-containing solution to a validated infant pig animal model of unilateral iSLN damage, and subsequently assessing any changes in swallow properties and swallow safety. Infant populations are excellent models for studying sensorimotor control of swallowing because they exhibit a simplified version of the adult feeding process (43, 44). Furthermore, infants often exhibit dysphagia, especially those born preterm or with neurodevelopmental deficits, yet the role of capsaicin in preventing aspiration has only been studied in adult and elderly populations (18–22). Animal models are ideal for infant swallowing research because they allow for the completion of extensive videofluoroscopic studies without the radiation constraints that are common in human clinical settings (43). The infant pig model is especially useful due to its human-like oropharyngeal anatomy and the well-documented time relationships between human and porcine postnatal development (45). In completing this study, we hypothesized that:
The addition of capsaicin will sensitize the remaining, intact iSLN, which will change the motor output of nerves that innervate the structures involved in swallowing, which will manifest as altered kinematics of oropharyngeal structures.
The addition of capsaicin to the animal model will decrease the size of the liquid bolus.
The addition of capsaicin to the animal model will decrease instances of penetration and aspiration.
MATERIALS AND METHODS
Animal Care and Surgical Procedures
We obtained five infant pigs (Yorkshire/Landrace, Shoup Investments Ltd., Orrville, OH) from the same litter (2 males, 3 females), aged five days upon arrival at the animal care facility. Infant pigs were trained to drink formula (Solustart Pig Milk Replacement, Land o’ Lakes, Arden Mills, MN) from bottles fitted with artificial lamb nipples (NASCO Farm & Ranch, Fort Atkinson, WI) while standing in a transparent, radiolucent box. When the infant pigs were 8 days old, they underwent sterile surgery to implant radiopaque tantalum beads over the hyoid and thyroid using aseptic technique. During this surgery, infant pigs were anesthetized using 2%–5% isoflurane. A bead was sutured over the hyoid bone at the anterior insertion of the sternothyroid muscles, while another bead was sutured into the fascia over the eminence of the thyroid cartilage.
In a separate, nonsterile procedure 12 days after birth, infant pigs had 0.8-mm radiopaque markers placed in their tongue (anterior, middle, and posterior positions), hard palate, soft palate, palatopharyngeal arches, nose, and chin. Oral markers were placed submucosally, whereas the markers in the nose and chin were placed subdermally. Previous studies indicate that the placement of these markers does not alter normal infant feeding (46, 47). A radiopaque microvascular clip was placed on the epiglottal tip to track movements of the epiglottis during each swallow. In a final procedure 14 days after birth, infant pigs underwent sterile surgery to unilaterally transect the SLN on each animal’s right. The right side was chosen as it was more comfortably accessible to the surgeon, and to allow for comparisons with past studies that also lesioned infant pigs’ right SLN (34). After the SLN was identified, two sutures were tied tightly around the nerve. The nerve was then transected between the two sutures, and the ends of the nerve were displaced to prevent reinnervation. SLN lesion was confirmed during postmortem dissections of all animals. After SLN lesion surgery, pigs were allowed to recover for at least 24 h before data collection. For the remainder of the study, animal care followed validated standard care for neonatal pigs (48, 49). A guide to all procedures is given in Supplemental Table S1 (all Supplemental material is available at https://doi.org/10.6084/m9.figshare.19694992.v2).
Data Collection and Capsaicin Preparation
At 15 and 16 days postnatal, infant pigs were recorded during bottle feeding via videofluoroscopy using two C-arm fluoroscopes (GE9400 C-Arm, 65 kV, 5 mA). The fluoroscopes were equipped with digital high-speed cameras (XC1M digital camera; XCitex, Cambridge, MA), which recorded video at 100 frames/s in lateral and dorsoventral views. Pigs were fed formula mixed with barium (E-Z Paque Barium Sulfate, EZ EM Inc., NY), for radiographic visualization of the bolus. During each feeding session, an infant pig was placed in the radiolucent box and suckled freely to satiation. Each video sequence was made up of 12–20 swallows, for a total of 187 swallows across all pigs (n = 95 swallows with capsaicin, 92 without, Supplemental Movie S1). We did not record the swallows from the first 10 s of each feeding session, as past research suggests that these swallows occur at an accelerated rate and are not representative of the feeding sequence as a whole (50). All data collection procedures in this study followed standard X-Ray Reconstruction of Moving Morphology (XROMM) protocols (51).
Infant pigs with unilateral SLN lesions were recorded at 15 days postnatal without capsaicin application, and then at 16 days postnatal immediately following capsaicin application. To apply capsaicin, 0.5 mL of a 10 ppm capsaicin solution was placed on the soft palate and valleculae of each infant pig using a brush with synthetic bristles. Previous studies have shown that the threshold concentration for detection of capsaicin on the human tongue is roughly 0.1–1 ppm, while a burning sensation is elicited on the tongue at concentrations higher than 1 ppm, and pain is experienced at concentrations between 10 and 100 ppm (52). Thus, we chose a concentration of 10 ppm to ensure that the capsaicin created a burning sensation in the infant pigs without causing excessive pain. To formulate this solution, we added capsaicin powder (Supelco, Inc., Bellefonte, PA) to polyethylene glycol 400 (Sigma-Aldrich, Inc., St. Louis, MO). Pigs were sedated with 2%–5% isoflurane during capsaicin application. Feeding sessions began as soon as possible following recovery from anesthesia, which took about 15 min. This allowed the pigs to feed almost immediately after capsaicin was applied, to maximize capsaicin’s effects in sensitizing the nonlesioned iSLN. All procedures in this study were approved by the NEOMED IACUC (Protocol No. 17-04-071). The animals showed no immediate reaction to capsaicin application, and no indications of pain or distress were observed during or after feeding sessions.
Kinematic Data Processing
To isolate each individual swallow for timing and excursion analyses, we observed X-ray video for each feeding sequence and recorded frames for the following swallow events: swallow initiation, pharyngeal clearance of the bolus, and swallow conclusion. Swallow initiation was defined as the frame when the liquid bolus had accumulated in the supraglottic space, and the epiglottis began to move posteriorly. Pharyngeal clearance was defined at the time at which the bolus passed over the tip of the epiglottis, and the epiglottis began to travel anteriorly. Swallow conclusion was defined as the frame when the epiglottis returned to its original resting position. The time interval from swallow initiation to pharyngeal clearance represents the total pharyngeal transit time (TPT time).
To obtain three-dimensional kinematic data from videofluoroscopic recordings, files were exported to XMALAB (53) for data processing. Each tantalum marker was tracked in lateral and dorsoventral views for the duration of the feeding sequence, which allowed for acquisition of marker positions in the form of XYZ coordinates. A 10-Hz low-pass Butterworth filter was applied to the data to eliminate noise. The rate of swallowing in this model is ∼2 Hz, and therefore this filter is appropriate (39, 50, 54, 55). Filtering accuracy was visually inspected for obvious oversmoothing. We created a rigid body of the cranium based on the triangulated nose and hard palate marker coordinates using the “Set from Frame” function. The selected frame was outside of the tracked data set (not during a swallow) and the constituent markers all had intermarker distance standard deviations lower than 0.03 mm.
The transformations of the rigid body and translations of each individual marker were exported from XMALab into Autodesk Maya (Autodesk Inc., San Rafael, CA), along with the undistorted videofluoroscopic recordings. In Maya, we created a plane that was animated with the cranium rigid body transformations, and then placed an anatomical coordinate system parented to transformations of this plane. In this coordinate system, translations about the x-axis represent dorsoventral movement, translations about the y-axis represent mediolateral movements, and translations about the Z axis represent anteroposterior movements. By calculating the three-dimensional excursion of each marker relative to the anatomical coordinate system (oRel, XROMM Maya tools), kinematic data were extracted without the effects of extraneous cranial movements or feeding position. The resulting translational data for each marker was exported from Maya and further processed using a custom MATLAB (v. R2020b, MathWorks, Natick, MA) script.
Following importation of the manually identified swallow frames, the custom MATLAB script extracted the total excursion of the hyoid, thyroid, and posterior tongue during each swallow (collected in millimeter, then scaled to the structure’s maximal excursion in each pig to account for individual variation and slight variations in marker placement). The MATLAB code also identified the timing of oropharyngeal movements as a percentage of swallow duration, specifically the time required for the hyoid and thyroid to reach their peak elevation (i.e., their most superior and anterior positions). The coordination between hyoid and thyroid movements was assessed by considering the difference between peak hyoid elevation and peak thyroid elevation percentages.
The timing of posterior tongue movements was also extracted via MATLAB. The posterior tongue moves posteriorly and superiorly following swallow initiation, and then returns roughly to its initial position upon swallow conclusion—thus, it creates a circular trace in the lateral view. The proportion of the circular trace traveled by the posterior tongue marker upon pharyngeal clearance (i.e., the proportion traveled during the TPT time) was termed the posterior tongue ratio (PTR) and was used to assess the timing of posterior tongue movement relative to the swallow, as described by Gould et al. (49). Further calculations were completed in MATLAB to yield the swallow frequency, the number of sucks completed before each swallow, and the TPT time.
Assessing Bolus Area and Swallow Safety
To measure the size of the liquid bolus formed during each swallow, the swallow initiation frame in the lateral view was isolated and exported to ImageJ (56). Using the free select tool in ImageJ, the outline of the bolus was carefully traced with a stylus on a touchscreen device. The outline was bound posteriorly by the epiglottis, as the amount of milk in the piriform recesses is variable across individual pigs and only accounts for a minimal percentage of total bolus area (33). The area of the selected bolus was collected in pixels and scaled to cm2 using a radiopaque object of known dimensions to account for differences in size and feeding position among infant pigs.
To evaluate swallow performance, we used the Infant Mammalian Penetration-Aspiration Scale (IMPAS), as detailed by Holman et al. (57). Briefly, IMPAS is applicable across infant mammals, and is a modified version of the Penetration-Aspiration Scale (PAS), which is commonly used to assess swallow safety in human adults suffering from dysphagia (58). IMPAS scores range from 1 to 7, whereby a 1 signifies a safe swallow, a 7 signifies silent aspiration, and intermediate values represent varying degrees of penetration and aspiration. For the purposes of this study, IMPAS scores of 1 were considered “Safe,” scores from 2 to 4 were grouped together as “Penetration,” and scores from 5 to 7 were deemed “Aspiration.”
Statistical Analyses
We performed all statistical analyses in R (v. 4.0.3, http://www.r-project.org). To evaluate the effect of capsaicin on bolus size, the kinematics of oropharyngeal structures, and the frequency of both suckling and swallowing, we used linear mixed effects models with capsaicin treatment status as a fixed effect, and individual as a random effect [Response ∼ Treatment + 1|Individual; lmer4 (59)]. The inclusion of individual as a random factor is statistically equivalent to using a repeated-measures design, and ensures that we are testing the response to treatment from each individual’s baseline. Where appropriate, we used a linear regression model to assess relationships between oropharyngeal kinematics and bolus properties. In addition, we calculated Cohen’s d to determine whether effect size was small (d > 0.2), medium (d > 0.5), or large (d > 0.8). We used Levene’s test to assess the equality of variances in the data sets. We used a multinomial logistic regression to evaluate the effect of capsaicin application and bolus size (main effects) on IMPAS score by calculating odds ratios. As previously described, IMPAS scores were categorized into three levels before analysis. Subsequently, we completed Wald Chi-squared analyses to obtain P values and assess significance. To determine which variable was most likely to predict swallow safety, we used Akaike’s information criterion (AIC) to assign the importance of each variable. All mean values are reported ±standard error (SE). All data used in statistical analyses can be provided upon request.
RESULTS
Sucking and Swallowing Properties
The complete descriptive statistics for all linear mixed effects models can be found in Table 1. Swallow frequency was unchanged when animals were exposed to capsaicin (χ2 = 0.926, P = 0.336, Fig. 1). The number of sucks per swallow was also unaffected by the introduction of capsaicin (χ2 = 0.390, P = 0.533). TPT time was reduced with capsaicin (pre-capsaicin mean = 0.139 ± 0.005 s, post-capsaicin mean = 0.131 ± 0.006 s, χ2 = 6.86, P = 0.009, Fig. 2A), though the effect size was small (Cohen’s d = −0.254).
Table 1.
Results from linear mixed effects models, Cohen’s d analyses, and Levene’s tests
| Pre-Capsaicin Means (±SE) | Post-Capsaicin Means (±SE) | χ2, P, d | Levene’s (F, P) | |
|---|---|---|---|---|
| Swallow frequency, swallows/s | 1.58 ± 0.092 | 1.66 ± 0.077 | 0.926, 0.336, NS | 2.66, 0.105 |
| Sucks per swallow | 2.94 ± 0.163 | 2.84 ± 0.172 | 0.390, 0.533, NS | 0.242, 0.624 |
| Total pharyngeal transit time, s | 0.139 ± 0.005 | 0.131 ± 0.006 | 6.86, 0.009, −0.254 | 0.257, 0.613 |
| Hyoid excursion, mm | 3.61 ± 0.274 | 3.11 ± 0.125 | 15.3, <0.001, 0.444 | 0.381, 0.538 |
| Thyroid excursion, mm | 3.94 ± 0.373 | 3.65 ± 0.112 | 5.85, 0.016, 0.245 | 0.0432, 0.836 |
| Timing of hyoid elevation, % | 0.450 ± 0.055 | 0.458 ± 0.007 | 0.042, 0.837, NS | 14.4, <0.001 |
| Timing of thyroid elevation, % | 0.462 ± 0.055 | 0.481 ± 0.007 | 0.508, 0.476, NS | 14.0, <0.001 |
| Posterior tongue excursion, % | 65.6 ± 1.99 | 64.9 ± 4.06 | 0.007, 0.936, NS | 0.303, 0.583 |
| Posterior tongue ratio, % | 72.7 ± 1.88 | 67.6 ± 2.59 | 4.24, 0.040, 0.369 | 0.541, 0.463 |
| Bolus size, cm2 | 1.37 ± 0.125 | 1.11 ± 0.092 | 16.5, <0.001, 0.430 | 10.7, 0.001 |
Bold values indicate a significant difference between pre- and post-capsaicin. Pre- and post-capsaicin are presented as means ± standard error. Values reported from linear mixed effects models are χ2 statistic and P value (<0.05 indicates significance). For reported Cohen’s D values, effects sizes are classified as small (d > 0.2), medium (d > 0.5), or large (d > 0.8). Values reported for Levene’s test are F-statistic and P value (<0.05 indicates significance).
Figure 1.
Frequency of swallowing in infant pigs prior to (left, blue) and following (right, orange) the application of capsaicin to the soft palate and valleculae (pre-capsaicin n = 76 swallows, post-capsaicin n = 85). No significant changes in swallow frequency were observed following capsaicin application (χ2 = 0.926, P = 0.336). Large black circles represent means; box and whisker plots show median and interquartile range; width of each plot indicates the frequency distribution of the data along the y-axis.
Figure 2.
Total pharyngeal transit (TPT) time (A) as it is affected by capsaicin application and (B) as it relates to bolus size in both experimental conditions (pre-capsaicin n = 81 swallows, post-capsaicin n = 90 swallows). In both panels, pre-capsaicin data are shown in blue, and post-capsaicin data are shown in orange. There is a significant decrease in TPT following capsaicin application (χ2 = 6.86, P = 0.009), and TPT is related to bolus size both prior to (R2 = 0.123, P = 7.70 × 10−4) and following (R2 = 0.640, P = 2.20 × 10−16) capsaicin application. In A, large black circles represent means; box and whisker plots show median and interquartile range; width of each plot indicates the frequency distribution of the data along the y-axis; brackets connecting plots show significant differences identified by linear mixed effects models.
Swallow Kinematics
Hyoid excursion was slightly reduced with capsaicin treatment (pre-capsaicin mean = 3.61 ± 0.274 mm, post-capsaicin mean = 3.11 ± 0.125 mm, χ2 = 15.3, P < 0.001, Fig. 3A), though the effect size was small (Cohen’s d = 0.444). Similarly, excursion of the thyroid decreased with capsaicin (pre-capsaicin mean = 3.94 ± 0.373 mm, post-capsaicin mean = 3.65 ± 0.112 mm, χ2 = 5.85, P = 0.016, Fig. 3C), but the effect size was small (Cohen’s d = 0.245).
Figure 3.
The effect of capsaicin on hyoid excursion during the swallow (A), timing of peak hyoid elevation as a percentage of swallow duration (B), thyroid excursion during the swallow (C), and timing of peak thyroid elevation as a percentage of swallow duration (D). In all panels, pre-capsaicin data are shown in blue, while post-capsaicin data are shown in orange (pre-capsaicin n = 81 swallows, post-capsaicin n = 89 swallows). Hyoid excursion and thyroid excursion significantly decreased (χ2 = 15.3, P = <0.001 and χ2 = 5.85, P = 0.016 respectively) following capsaicin application, while the timing of peak hyoid and thyroid elevation were consistent (χ2 = 0.042, P = 0.837 and χ2 = 0.508, P = 0.476 respectively) across treatments. Large black circles represent means; box and whisker plots show median and interquartile range; width of each plot indicates the frequency distribution of the data along the y-axis; and brackets connecting plots show significant differences identified by linear mixed effects models.
The timing of maximal hyoid elevation as a percentage of swallow duration was consistent across conditions (χ2 = 0.042, P = 0.837, Fig. 3B). The timing of maximal thyroid elevation also did not differ after capsaicin application (χ2 = 0.508, P = 0.476, Fig. 3D). As such, it follows that the coordination between hyoid and thyroid movements was unchanged (χ2 = 0.919, P = 0.338, Fig. 3C). Variance in the timing of peak hyoid (F = 14.4, P < 0.001) and thyroid (F = 14.0, P < 0.001) elevation decreased following capsaicin application.
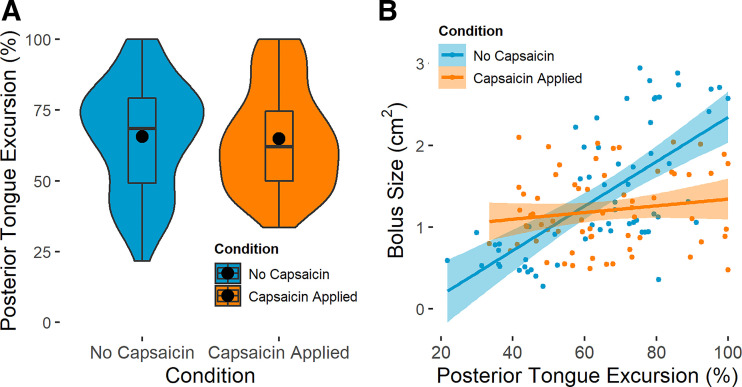
The addition of capsaicin did not affect the total excursion of the posterior tongue during the swallow (χ2 = 0.007, P = 0.936, Fig. 4A). The timing of posterior tongue movement was altered by capsaicin, as the PTR was significantly smaller after the application of capsaicin (pre-capsaicin mean = 72.7 ± 1.88%, post-capsaicin mean = 67.6 ± 2.59%, χ2 = 4.24, P = 0.040, Fig. 5A), though the effect size was small (Cohen’s d = 0.369).
Figure 4.
Excursion of the posterior tongue, given as a percentage of each infant pig’s maximum posterior tongue excursion (pre-capsaicin n = 62 swallows, post-capsaicin n = 67 swallows). A: the effect of capsaicin application on scaled posterior tongue excursion. B: scaled posterior tongue excursion as it relates to bolus size in both experimental conditions. In both panels, pre-capsaicin data are shown in blue, and post-capsaicin data are shown in orange. Scaled posterior tongue excursion did not change (χ2 = 0.007, P = 0.936) following capsaicin application. The relationship between scaled posterior tongue excursion and bolus size was significant prior to (R2 = 0.431, P = 4.25 × 10−9) but not following (R2 = 0.010, P = 0.201) capsaicin application. In A, large black circles represent means; box and whisker plots show median and interquartile range; width of each plot indicates the frequency distribution of the data along the y-axis.
Figure 5.
Posterior tongue ratio (PTR) as it is affected by capsaicin application (A) and as it relates to bolus size in both experimental conditions (B) (pre-capsaicin n = 62 swallows, post-capsaicin n = 67 swallows). In both panels, pre-capsaicin data are shown in blue, and post-capsaicin data are shown in orange. PTR significantly decreased (χ2 = 4.24, P = 0.040) following capsaicin application. The relationship between PTR and bolus size was significant prior to (R2 = 0.218, P = 7.62 × 10−5) but not following (R2 = −0.015, P = 0.966) capsaicin application. In A, large black circles represent means; box and whisker plots show median and interquartile range; width of each plot indicates the frequency distribution of the data along the y-axis; brackets connecting plots show significant differences identified by linear mixed effects models.
Bolus Size and Swallow Performance
Capsaicin decreased bolus size in our animal model (pre-capsaicin mean = 1.37 ± 0.125 cm2, post-capsaicin mean = 1.11 ± 0.092 cm2, χ2 = 16.5, P = 4.98 × 10−5, Fig. 6), though the effect size was small (Cohen’s d = 0.430). Variance in bolus size was significantly decreased following capsaicin application (F = 10.7, P = 0.001). Bolus size and swallow performance were related, such that a 1 cm2 increase in bolus area increased the log odds of penetration (rather than a safe swallow) by 6.43 (P = 8.59 × 10−6) and increased the log odds of aspiration (rather than a safe swallow) by 10.2 (P = 7.89 × 10−11). Changes in swallow performance were also associated with the application of capsaicin, as the multinomial logistic regression models showed that capsaicin decreased the log odds of penetration (rather than a safe swallow) by 3.37 (P = 7.19 × 10−6) and decreased the log odds of aspiration (rather than a safe swallow) by 2.95 (P = 1.02 × 10−3, Table 2, Fig. 6).
Figure 6.
The relationship between capsaicin application and bolus size in cm2 (pre-capsaicin n = 92, post-capsaicin n = 95). The dots shown on the left represent swallows made pre-capsaicin, while those on the right represent swallows made post-capsaicin. The color of each dot indicates the degree of airway compromise during each swallow: safe swallows shown in green, swallows with airway penetration shown in yellow, and swallows with aspiration shown in red.
Table 2.
The log odds of penetration and aspiration as a result of changes in condition (from no capsaicin applied to capsaicin applied) and bolus size (as a result of a 1 cm2 increase in bolus area)
| Condition Odds | Bolus Size Odds | Condition P Value | Bolus Size P Value | |
|---|---|---|---|---|
| Penetration | −3.365 | 6.428 | 7.188 × 10−6 | 8.585 × 10−6 |
| Aspiration | −2.946 | 10.181 | 1.021 × 10−3 | 7.894 ×10−11 |
Negative log odds indicate that the compared group is less likely to experience penetration or aspiration than the reference group. Bolded P values indicate statistical significance.
In determining the best model to predict swallow safety, we evaluated models that used bolus size, capsaicin application status, and a combination of these variables. By comparing AIC values, we found that a model that considered both bolus size and capsaicin status was the best for predicting swallow safety in our animal model (ΔAIC = 0). The model with the next best fit used only bolus size to predict instances of penetration or aspiration, but the ΔAIC for this model was 29.2 relative to the best model, while a model that used only capsaicin status to predict swallow safety had a ΔAIC of 145 relative to the best model (Table 3). This indicates that bolus size and capsaicin status are both predictors of swallow performance, but that a model that considers both of these variables is the best fit for predicting the likelihood of material entering the airway during infant feeding in our animal model.
Table 3.
Identification of the best model to predict swallow safety using Akaike’s information criterion
| Condition (With Or Without Capsaicin) | Bolus Size | ΔAIC | |
|---|---|---|---|
| Model 1 | + | + | 0 |
| Model 2 | + | 29.2301 | |
| Model 3 | + | 144.5859 |
The symbol “+” indicates that a given variable is considered when assessing the model. The model with the lowest AIC value (in this case, Model 1) is the most supportive, and is assigned a ΔAIC of zero. The difference between the best model’s AIC value and each other model’s AIC value gives the ΔAIC values for less supportive models. AIC, Akaike’s information criterion.
Relationships between Swallow Kinematics and Bolus Size
Although the excursion of the posterior tongue was correlated with bolus size in infant pigs without capsaicin (R2 = 0.431, P = 4.25 × 10−9), this relationship was not observed post-capsaicin application (R2 = 0.010, P = 0.201, Fig. 4B). Similarly, PTR was correlated with bolus size before capsaicin application (R2 = 0.218, P = 7.62 × 10−5), but not following capsaicin application (R2 = −0.015, P = 0.966, Fig. 5B). TPT time was correlated with bolus size both before (R2 = 0.123, P = 7.70 × 10−4) and following (R2 = 0.640, P < 2.20 × 10−16) capsaicin application (Fig. 2B). Excursion of the hyoid was not correlated with bolus size either before capsaicin exposure (R2 = 0.035, P = 0.052), or after exposure (R2 = 0.009, P = 0.625). Thyroid excursion was weakly correlated with bolus size before capsaicin exposure (R2 = 0.078, P = 0.007) and after exposure (R2 = 0.123, P < 0.001).
DISCUSSION
The Role of Capsaicin in Reducing Bolus Size and Improving Performance
We found that boluses were reduced to more manageable sizes after exposing our infant pig model to capsaicin. We also found that the application of capsaicin increased the likelihood of safe swallows in boluses of smaller sizes. Reduction in bolus size and improved swallow safety were the primary goals of treatment with capsaicin, and thus our results suggest that heightening oropharyngeal and laryngeal sensation via capsaicin may be a promising means of lowering the likelihood of penetration and aspiration in infants suffering from dysphagia. These results are not unexpected, as many previous studies have identified altered swallow properties and improved swallow performance with laryngeal capsaicin exposure (17, 60–64). There is a slight possibility that the observed reduction in bolus size resulted from a negative response to the capsaicin, such that feeding was less palatable to the infant pigs. However, the pigs appeared enthusiastic to feed following capsaicin exposure, and no individuals hesitated to latch on the nipple or decreased their duration of feeding. As such, we conclude that reduced bolus size was not a direct result of conscious pain perception, though future studies would be beneficial in assessing this conclusion, particularly those that assess sensory thresholds and changes in the extent of innervation before and after capsaicin exposure.
The reduction in mean bolus volume following the application of capsaicin was accompanied by decreased range and variance in bolus volumes, with fewer boluses with areas exceeding 2 cm2 (Fig. 6, Levene’s test F = 10.7, P = 0.001). Because applying capsaicin resulted in pigs generally swallowing boluses less than 2 cm2 in area but did not lead to the formation of smaller boluses than those observed in the nontreated group, the difference in mean bolus size between the two groups was relatively small (an approximate difference of 0.268 cm2) and the calculated effect size was small (Cohen’s d = 0.430).
The mechanism by which capsaicin limited bolus size to areas below 2 cm2 is unclear. Bolus size may be modulated during milk acquisition, as the total volume of milk extracted during each suck depends on the amount of negative pressure generated by the infant (47, 65, 66). Alternatively, bolus size may be affected by the timing of the onset of the pharyngeal swallow—if the pharyngeal swallow is triggered earlier (i.e., swallow frequency across the feeding sequence increases and/or the number of sucks per swallow is reduced), bolus size is limited (35). Because heightened iSLN sensation could imply earlier pharyngeal swallow initiation, we originally hypothesized that altered pharyngeal swallow timing would drive changes in bolus size. However, we did not observe any changes in swallow frequency, nor in the number of sucks per swallow following capsaicin application. This implies that perhaps bolus sizes were reduced by changes in milk acquisition that reduced the volume of milk obtained during each suck, and that future studies may benefit from evaluating the effect of capsaicin on oral aspects of feeding.
The mechanism by which capsaicin improved swallow safety with boluses <2 cm2 also remains elusive. We did not observe any significant changes in the kinematics of the posterior tongue, hyoid, or thyroid—the PTR only decreased by 4% after capsaicin exposure, which may not be biologically relevant, and mean hyoid and thyroid excursions were only reduced by 0.499 mm and 0.291 mm, respectively. Such small differences in excursion may only be discernable using equipment with higher resolution than that used in this study. Thus, the mechanism by which capsaicin improved swallow performance with boluses <2 cm2 remains undetermined and warrants future analysis.
In exploring the potential mechanisms by which capsaicin may have modified feeding behaviors in this study, we consider the neural control of the swallow and the resulting susceptibility of some structures to sensory manipulation. Infant mammalian swallowing is a complex process that depends on the coordinated activity of over 25 bilateral muscle pairs that are innervated by multiple cranial nerves, including CN V, VII, IX, X, and XII (23, 67). All swallowing muscles and the structures they manipulate are subject to sensorimotor control during feeding, and the extent to which they are affected is variable depending on their associated neuroanatomy. Changes in sensitivity of afferent fibers in the larynx, pharynx, and oral cavity will therefore impact different structures to varying degrees. Administering capsaicin theoretically upregulates these afferents, which may affect some aspects of feeding, while having no effect on others. We can assess which of these aspects are susceptible to sensorimotor feedback by comparing their relationship to bolus size before and after capsaicin application to our model system.
Variables That Maintain Their Relationship with Bolus Size Post-Capsaicin
Some variables maintained their relationship with bolus size despite exposure to capsaicin and the reduced range of bolus sizes. For example, TPT time had a positive, linear relationship with bolus size regardless of capsaicin exposure status (Fig. 2B). This suggests that TPT time varies more directly with bolus size than kinematics of the posterior tongue do, and that TPT time can be likely be manipulated in a predictable way via interventions that limit or augment bolus size. Such time differences imply that sensory interventions like capsaicin exposure may not be effective in directly manipulating TPT time via sensorimotor feedback.
Movements of the hyoid were not closely related to bolus size both pre- and post-capsaicin. This is likely because movements of the hyoid are related only to the pharyngeal swallow. Although the pharyngeal swallow is not a true oligoreflex, its electromyographic patterns are highly stereotyped, which explains the consistency in movements of the hyoid (68). Although the excursion of the thyroid did seem to increase as bolus size increased, the correlation between thyroid excursion and bolus size was weak both before and following capsaicin application. The lack of a relationship with bolus size and hyoid or thyroid movements is consistent with previous work (69), although exploring these relationships more explicitly by varying other aspects of sensation may provide valuable insight into the neural control of these structures and how they might vary in their response to sensory feedback.
Variables That Change Their Relationship with Bolus Size Post-Capsaicin
Both posterior tongue excursion and PTR exhibited a linear relationship with bolus size in pigs without capsaicin, but these relationships were not observed after capsaicin exposure (Fig. 4B and Fig. 5B). This may be because boluses in pigs with capsaicin applied were limited to a smaller range, and this range may not be sufficient to extrapolate relationships Alternatively, capsaicin may have been implicated in changing the relationships between posterior tongue kinematics and bolus size, but this hypothesis could not be evaluated without first conducting an in-depth study on the nature of the relationships between the posterior tongue and bolus size in standard infant feeding to establish normative data.
Clinical Implications: The Role of Oropharyngeal Sensation in Treating Dysphagia
In current clinical settings, dysphagia treatments that manipulate oropharyngeal sensation are generally limited to the thickening of liquids. This is because increased viscosity is associated with an altered pharyngeal swallow and improved airway protection (39, 70–72). However, thickened liquids present their own challenges, as they are associated with decreased quality of life and alter sensorimotor integration during feeding (39, 72). Our results indicate that chemical stimulation may be a useful method to pharmacologically reduce aspiration in infants. Chemical treatment of dysphagia does not need to be limited to capsaicin, as piperine, menthol, and other substances can activate various TRP channels to increase sensitivity of laryngeal afferents (15, 17, 73).
Although this study demonstrates the effectiveness of capsaicin in improving feeding performance, TRP channel agonists may not be a viable option for treating long-term dysphagia in infants. Studies show that repeated exposure to capsaicin on the adult human tongue results in desensitization of the afferent fibers of the tongue and airway (52, 74). This implies that chronic exposure to capsaicin will lead to neural habituation, gradually yielding less desirable patient outcomes. However, short-term application of capsaicin may be helpful in reducing acute instances of aspiration in infants, which could be particularly useful in infants with transient dysphagia resulting from neuromuscular immaturity.
Limitations and Future Directions
Although this study illustrates the role of laryngeal agonists in improving swallow performance in infants, a few limitations must be addressed. Capsaicin improved performance by limiting the size of liquid boluses swallowed by infant pigs. However, the overall reduction in bolus size was relatively small, which made evaluating changes in motor responses somewhat difficult. Future research may benefit from applying a solution with an increased concentration of capsaicinoids, as this may elicit a more dramatic reduction in bolus size. Studies in adult humans have safely utilized capsaicinoid solutions with concentrations nearly 20 times more potent than the solution used in this study, and animal models represent a powerful tool for relating capsaicin potency to swallow performance (19, 61).
The nature of capsaicin application used in this study may have allowed migration of the capsaicin solution from the base of the tongue to the anterior oral cavity. This would result in changes in gustatory and tactile information from the tongue and hard palate, which could alter swallow physiology. Future studies should consider a submucosal injection of capsaicin rather than topical application to the mucosa to avoid impacting oral afferents.
This study only assessed the effects of capsaicin over a short period of time and did not take into consideration the longitudinal effects of capsaicin application on infant feeding, which may have clinical relevance because prolonged exposure to capsaicin may induce desensitization. By assessing the effects of capsaicin on infant feeding over longer periods of time, we will be more informed on the time period in which capsaicin is useful in altering feeding performance. Future studies may also consider monitoring the activity of afferent nerve fibers to assess the duration of effectiveness of capsaicin across various exposure durations.
It should be noted that we chose an animal model of SLN damage to highlight capsaicin’s potential role in altering SLN activity and subsequent swallow performance. We do not propose that the benefits of capsaicin exposure would be limited to the treatment of SLN lesions, but that the effects of capsaicin on laryngeal afferents may be a useful tool in treating dysphagia across various etiologies. Future studies that assess the role of capsaicin in treating infant dysphagia should focus on directly monitoring nervous activity in vivo, and on exploring the effects of capsaicin on infant models of dysphagia unrelated to SLN damage. Such studies may clarify the significance of topical capsaicin treatments in perinatal dysphagia.
Conclusions
To our knowledge, this is the first study analyzing the effects of capsaicin on deglutition in infant populations, and certainly the first to collect such high-resolution kinematic data following the administration of capsaicin to a model of infant swallowing. The results of this experiment demonstrate the effectiveness of capsaicin as a means of improving swallowing performance in infants, likely due to capsaicin acting to increase the sensitivity of the iSLN. This sensitivity reduced boluses to a safe and manageable size, but also improved swallow safety in boluses of moderate sizes. By approaching the infant swallow as an integrative, multi-faceted process and thoroughly investigating the changes in physiology associated with capsaicin application, we will gain a better understanding of the neural control of infant feeding. This understanding will inform the manipulation of sensation in clinical settings, which may lead to more effective treatment of infant dysphagia.
SUPPLEMENTAL DATA
Supplemental Table S1 and Supplemental Movie S1: https://doi.org/10.6084/m9.figshare.19694992.v2.
GRANTS
This work was funded by National Institutes of Health Grant R01HD088561 (to R. Z. German).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.E.E., R.Z.G., and C.J.M. conceived and designed research; C.E.E., R.Z.G., L.E.B., and C.J.M. performed experiments; C.E.E. and L.E.B. analyzed data; C.E.E. and C.J.M. interpreted results of experiments; C.E.E. prepared figures; C.E.E. drafted manuscript; C.E.E., R.Z.G., L.E.B., and C.J.M. edited and revised manuscript; C.E.E., R.Z.G., L.E.B., and C.J.M. approved final version of manuscript.
REFERENCES
- 1. Kandel ER, Schwartz JH. The organization and planning of movement. In: Principles of Neural Science, edited by Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. New York: McGraw Hill Medical, 2013, p. 743–768. [Google Scholar]
- 2. Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: the role of the human superior parietal lobe. Nat Neurosci 1: 529–533, 1998. doi: 10.1038/2245. [DOI] [PubMed] [Google Scholar]
- 3. Humbert IA, German RZ. New directions for understanding neural control in swallowing: the potential and promise of motor learning. Dysphagia 28: 1–10, 2013. doi: 10.1007/s00455-012-9432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia 25: 323–333, 2010. doi: 10.1007/s00455-010-9301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altman KW, Richards A, Goldberg L, Frucht S, McCabe DJ. Dysphagia in stroke, neurodegenerative disease, and advanced dementia. Otolaryngol Clin North Am 46: 1137–1149, 2013. doi: 10.1016/j.otc.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 6. Alvarez-Berdugo D, Tomsen N, Clavé P. Sensory stimulation treatments for oropharyngeal dysphagia. In: Dysphagia: Diagnosis and Treatment, edited by Ekberg O. Cham, Switzerland: Springer International Publishing, 2018, p. 763–776. [Google Scholar]
- 7. Gould FDH, Lammers AR, Mayerl CJ, German RZ. Specific vagus nerve lesion have distinctive physiologic mechanisms of dysphagia. Front Neurol 10: 1301, 2019. doi: 10.3389/fneur.2019.01301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walshe M. Oropharyngeal dysphagia in neurodegenerative disease. J Gastroenterol Hepatol Res 3: 1265–1271, 2014. doi: 10.6051/j.issn.2224-3992.2014.03.408-2. [DOI] [Google Scholar]
- 9. Cheng I, Hamdy S. Metaplasticity in the human swallowing system: clinical implications for dysphagia rehabilitation. Neurol Sci 43: 199–209, 2022. doi: 10.1007/s10072-021-05654-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Easterling C. Management and treatment of patients with dysphagia. Curr Phys Med Rehabil Rep 6: 213–219, 2018. doi: 10.1007/s40141-018-0196-7. [DOI] [Google Scholar]
- 11. Cordell GA, Araujo OE. Capsaicin: identification, nomenclature, and pharmacotherapy. Ann Pharmacother 27: 330–336, 1993. doi: 10.1177/106002809302700316. [DOI] [PubMed] [Google Scholar]
- 12. Cichero JAY. Unlocking opportunities in food design for infants, children, and the elderly: Understanding milestones in chewing and swallowing across the lifespan for new innovations. J Texture Stud 48: 271–279, 2017. doi: 10.1111/jtxs.12236. [DOI] [PubMed] [Google Scholar]
- 13. Fattori V, Hohmann MSN, Rossaneis AC, Pinho-Ribeiro FA, Verri WA. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 21: 844, 2016. doi: 10.3390/molecules21070844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okumura Y, Narukawa M, Iwasaki Y, Ishikawa A, Matsuda H, Yoshikawa M, Watanabe T. Activation of TRPV1 and TRPA1 by black pepper components. Biosci Biotechnol Biochem 74: 1068–1072, 2010. doi: 10.1271/bbb.90964. [DOI] [PubMed] [Google Scholar]
- 15. Alvarez-Berdugo D, Rofes L, Farré R, Casamitjana JF, Enrique A, Chamizo J, Padrón A, Navarro X, Clavé P. Localization and expression of TRPV1 and TRPA1 in the human oropharynx and larynx. Neurogastroenterol Motil 28: 91–100, 2016. doi: 10.1111/nmo.12701. [DOI] [PubMed] [Google Scholar]
- 16. Hamamoto T, Takumida M, Hirakawa K, Tatsukawa T, Ishibashi T. Localization of transient receptor potential vanilloid (TRPV) in the human larynx. Acta Otolaryngol 129: 560–568, 2009. doi: 10.1080/00016480802273108. [DOI] [PubMed] [Google Scholar]
- 17. Hossain MZ, Ando H, Unno S, Masuda Y, Kitagawa J. Activation of TRPV1 and TRPM8 channels in the larynx and associated laryngopharyngeal regions facilitates the swallowing reflex. Int J Mol Sci 19: 4113, 2018. doi: 10.3390/ijms19124113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ebihara T, Takahashi H, Ebihara S, Okazaki T, Sasaki T, Watando A, Nemoto M, Sasaki H. Capsaicin troche for swallowing dysfunction in older people. J Am Geriatr Soc 53: 824–828, 2005. doi: 10.1111/j.1532-5415.2005.53261.x. [DOI] [PubMed] [Google Scholar]
- 19. Rofes L, Arreola V, Martin A, Clavé P. Natural capsaicinoids improve swallow response in older patients with oropharyngeal dysphagia. Gut 62: 1280–1287, 2013. doi: 10.1136/gutjnl-2011-300753. [DOI] [PubMed] [Google Scholar]
- 20. Shin S, Shutoh N, Tonai M, Ogata N. The effect of capsaicin-containing food on the swallowing response. Dysphagia 31: 146–153, 2016. doi: 10.1007/s00455-015-9668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jinnouchi O, Ohnishi H, Kondo E, Kawata I, Bando H, Okamoto H, Azuma T, Sato G, Kitamura Y, Abe K, Takeda N. Aural stimulation with capsaicin prevented pneumonia in dementia patients. Auris Nasus Larynx 47: 154–157, 2020. doi: 10.1016/j.anl.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 22. Kondo E, Jinnouchi O, Nakano S, Ohnishi H, Kawata I, Okamoto H, Takeda N. Aural stimulation with capsaicin ointment improved swallowing function in elderly patients with dysphagia: a randomized, placebo-controlled, double-blind, comparative study. Clin Interv Aging 12: 1921–1928, 2017. doi: 10.2147/CIA.S138357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller AJ. Neurophysiological basis of swallowing. Dysphagia 1: 91–100, 1986. doi: 10.1007/BF02407121. [DOI] [Google Scholar]
- 24. Miller AJ. The neurobiology of swallowing and dysphagia. Dev Disabil Res Rev 14: 77–86, 2008. doi: 10.1002/ddrr.12. [DOI] [PubMed] [Google Scholar]
- 25. Sulica L. The superior laryngeal nerve: function and dysfunction. Otolaryngol Clin North Am 37: 183–201, 2004. doi: 10.1016/S0030-6665(03)00175-0. [DOI] [PubMed] [Google Scholar]
- 26. Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol 19: 44–60, 1956. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- 27. Ogura JH, Lam RL. Anatomical and physiological correlations on stimulating the human superior laryngeal nerve. Laryngoscope 63: 947–959, 1953. doi: 10.1288/00005537-195310000-00003. [DOI] [PubMed] [Google Scholar]
- 28. Tsujimura T, Yamada A, Nakamura Y, Fukuhara T, Yamamura K, Inoue M. The digastric muscle is less involved in pharyngeal swallowing in rabbits. Dysphagia 27: 271–276, 2012. doi: 10.1007/s00455-011-9363-z. [DOI] [PubMed] [Google Scholar]
- 29. Larson JE, Herring SW. Movement of the epiglottis in mammals. Am J Phys Anthropol 100: 71–82, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 30. Pearson WG, Langmore SE, Yu LB, Zumwalt AC. Structural analysis of muscles elevating the hyolaryngeal complex. Dysphagia 27: 445–451, 2012. doi: 10.1007/s00455-011-9392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Podvinec S. The physiology and pathology of the soft palate. J Laryngol Otol 66: 452–461, 1952. doi: 10.1017/s0022215100047915. [DOI] [PubMed] [Google Scholar]
- 32. Wall CE, Smith KK. Ingestion in mammals. In: Encyclopedia of Life Sciences. London, UK: Wiley, 2001, p. 1–6. [Google Scholar]
- 33. Ding P, Fung GSK, Lin MD, Holman SD, German RZ. The effect of bilateral superior laryngeal nerve lesion on swallowing: a novel method to quantitate aspirated volume and pharyngeal threshold in videofluoroscopy. Dysphagia 30: 47–56, 2015. doi: 10.1007/s00455-014-9572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ding P, Campbell-Malone R, Holman SD, Lukasik SL, Fukuhara T, Gierbolini-Norat EM, Thexton AJ, German RZ. Unilateral superior laryngeal nerve lesion in an animal model of dysphagia and its effect on sucking and swallowing. Dysphagia 28: 404–412, 2013. doi: 10.1007/s00455-013-9448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ding P, Campbell-Malone R, Holman SD, Lukasik SL, Thexton AJ, German RZ. The effect of unilateral superior laryngeal nerve lesion on swallowing threshold volume. Laryngoscope 123: 1942–1947, 2013. doi: 10.1002/lary.24051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol 550: 287–304, 2003. doi: 10.1113/jphysiol.2003.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lammers AR, Abid S, Ding P, German RZ. Effects of superior laryngeal nerve lesion on kinematics of swallowing and airway protection in an infant pig model. Dysphagia 35: 907–917, 2020. doi: 10.1007/s00455-020-10100-7. [DOI] [PubMed] [Google Scholar]
- 38. Mayerl CJ, Myrla AM, Gould FDH, Bond LE, Stricklen BM, German RZ. Swallow safety is determined by bolus volume during infant feeding in an animal model. Dysphagia 36: 120–129, 2021. doi: 10.1007/s00455-020-10118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mayerl CJ, Edmonds CE, Gould FDH, German RZ. Increased viscosity of milk during infant feeding improves swallow safety through modifying sucking in an animal model. J Texture Stud 52: 603–611, 2021. doi: 10.1111/jtxs.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tsuji K, Tsujimura T, Sakai S, Suzuki T, Yoshihara M, Nagoya K, Magara J, Satoh Y, Inoue M. Involvement of capsaicin-sensitive nerves in the initiation of swallowing evoked by carbonated water in anesthetized rats. Am J Physiol Gastrointest Liver Physiol 319: G564–G572, 2020. doi: 10.1152/AJPGI.00233.2020. [DOI] [PubMed] [Google Scholar]
- 41. Tsujimura T, Udemgba C, Inoue M, Canning BJ. Laryngeal and tracheal afferent nerve stimulation evokes swallowing in anaesthetized guinea pigs. J Physiol 591: 4667–4679, 2013. doi: 10.1113/jphysiol.2013.256024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsujimura T, Sakai S, Suzuki T, Ujihara I, Tsuji K, Magara J, Canning BJ, Inoue M. Central inhibition of initiation of swallowing by systemic administration of diazepam and baclofen in anaesthetized rats. Am J Physiol Gastrointest Liver Physiol 312: G498–G507, 2017. doi: 10.1152/ajpgi.00299.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. German RZ, Crompton AW, Gould FDH, Thexton AJ. Animal models for dysphagia studies: what have we learnt so far. Dysphagia 32: 73–77, 2017. doi: 10.1007/s00455-016-9778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hiiemae KM, Crompton AW. Mastication, food transport, and swallowing. In: Functional Vertebrate Morphology, edited by Hildebrand M, Bramble D, Liem K, Wake D.. Cambridge, MA: Harvard University Press, 1985, p. 262–290. [Google Scholar]
- 45. Eiby YA, Wright LL, Kalanjati VP, Miller SM, Bjorkman ST, Keates HL, Lumbers ER, Colditz PB, Lingwood BE. A pig model of the preterm neonate: anthropometric and physiological characteristics. PLoS One 8: e68763, 2013. doi: 10.1371/journal.pone.0068763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Crompton AW, German RZ, Thexton AJ. Development of the movement of the epiglottis in infant and juvenile pigs. Zoology (Jena) 111: 339–349, 2008. doi: 10.1016/j.zool.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. German RZ, Crompton AW, Levitch LC, Thexton AJ. The mechanism of suckling in two species of infant mammal: miniature pigs and long-tailed macaques. J Exp Zool 261: 322–330, 1992. doi: 10.1002/jez.1402610311. [DOI] [PubMed] [Google Scholar]
- 48. Ballester A, Gould FDH, Bond LE, Stricklen BM, Ohlemacher J, Gross A, DeLozier K, Buddington RK, Buddington K, Danos N, German RZ. Maturation of the coordination between respiration and deglutition with and without recurrent laryngeal nerve lesion in an animal model. Dysphagia 33: 627–635, 2018. doi: 10.1007/s00455-018-9881-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gould FDH, Ohlemacher J, Lammers AR, Gross A, Ballester A, Fraley L, German RZ. Central nervous system integration of sensorimotor signals in oral and pharyngeal structures: Oropharyngeal kinematics response to recurrent laryngeal nerve lesion. J Appl Physiol (1985) 120: 495–502, 2016. doi: 10.1152/japplphysiol.00946.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gierbolini-Norat EM, Holman SD, Ding P, Bakshi S, German RZ. Variation in the timing and frequency of sucking and swallowing over an entire feeding session in the infant pig Sus scrofa. Dysphagia 29: 475–482, 2014. doi: 10.1007/s00455-014-9532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brainerd EL, Baier DB, Gatesy SM, Hedrick TL, Metzger KA, Gilbert SL, Crisco JJ. X-Ray reconstruction of moving morphology (XROMM): Precision, accuracy and applications in comparative biomechanics research. J Exp Zool Part A Ecol Genet Physiol 313: 262–279, 2010. doi: 10.1002/jez.589. [DOI] [PubMed] [Google Scholar]
- 52. Karrer T, Bartoshuk L. Capsaicin desensitization and recovery on the human tongue. Physiol Behav 49: 757–764, 1991. doi: 10.1016/0031-9384(91)90315-F. [DOI] [PubMed] [Google Scholar]
- 53. Knörlein BJ, Baier DB, Gatesy SM, Laurence-Chasen JD, Brainerd EL. Validation of XMALab software for marker-based XROMM. J Exp Biol 219: 3701–3711, 2016. doi: 10.1242/jeb.145383. [DOI] [PubMed] [Google Scholar]
- 54. Mayerl CJ, Gould FDH, Bond LE, Stricklen BM, Buddington RK, German RZ. Preterm birth disrupts the development of feeding and breathing coordination. J Appl Physiol (1985) 126: 1681–1686, 2019. doi: 10.1152/japplphysiol.00101.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Holman SD, Waranch DR, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Lukasik SL, German RZ. Sucking and swallowing rates after palatal anesthesia: an electromyographic study in infant pigs. J Neurophysiol 110: 387–396, 2013. doi: 10.1152/jn.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Holman SD, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Griffioen AM, Inokuchi H, Lukasik SL, German RZ. Development, reliability, and validation of an infant mammalian penetration-aspiration scale. Dysphagia 28: 178–187, 2013. doi: 10.1007/s00455-012-9427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia 11: 93–98, 1996. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 59. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. Neurol Sci 67: 1–48, 2014. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 60. Ortega O, Rofes L, Martin A, Arreola V, López I, Clavé P. A comparative study between two sensory stimulation strategies after two weeks treatment on older patients with oropharyngeal dysphagia. Dysphagia 31: 706–716, 2016. doi: 10.1007/s00455-016-9736-4. [DOI] [PubMed] [Google Scholar]
- 61. Suntrup-Krueger S, Muhle P, Kampe I, Egidi P, Ruck T, Lenze F, Jungheim M, Gminski R, Labeit B, Claus I, Warnecke T, Gross J, Dziewas R. Effect of capsaicinoids on neurophysiological, biochemical, and mechanical parameters of swallowing function. Neurotherapeutics 18: 1360–1370, 2021. doi: 10.1007/s13311-020-00996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nakato R, Manabe N, Shimizu S, Hanayama K, Shiotani A, Hata J, Haruma K. Effects of capsaicin on older patients with oropharyngeal dysphagia: a double-blind, placebo-controlled, crossover study. Digestion 95: 210–220, 2017. [Erratum in Digestion 96: 184, 2017]. doi: 10.1159/000463382. [DOI] [PubMed] [Google Scholar]
- 63. Nascimento W, Tomsen N, Acedo S, Campos-Alcantara C, Cabib C, Alvarez-Larruy M, Clavé P. Effect of aging, gender and sensory stimulation of trpv1 receptors with capsaicin on spontaneous swallowing frequency in patients with oropharyngeal dysphagia: a proof-of-concept study. Diagnostics 11: 461, 2021. doi: 10.3390/diagnostics11030461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pasierski M, Szulczyk B. Beneficial effects of capsaicin in disorders of the central nervous system. Molecules 27: 2484, 2022. doi: 10.3390/molecules27082484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Colley JRT, Creamer B. Sucking and swallowing in infants. Br Med J 2: 422–423, 1958. doi: 10.1136/bmj.2.5093.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Geddes DT, Kent JC, Mitoulas LR, Hartmann PE. Tongue movement and intra-oral vacuum in breastfeeding infants. Early Hum Dev 84: 471–477, 2008. doi: 10.1016/j.earlhumdev.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 67. Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 81: 929–969, 2001. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 68. Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol (1985) 102: 587–600, 2007. doi: 10.1152/japplphysiol.00456.2006. [DOI] [PubMed] [Google Scholar]
- 69. Mayerl CJ, Edmonds CE, Catchpole EA, Myrla AM, Gould FDH, Bond LE, Stricklen BM, German RZ. Sucking versus swallowing coordination, integration, and performance in preterm and term infants. J Appl Physiol (1985) 129: 1383–1392, 2020. [Erratum in J Appl Physiol (1985) 130: 901, 2021]. doi: 10.1152/JAPPLPHYSIOL.00668.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bisch EM, Logemann JA, Rademaker AW, Kahrilas PJ, Lazarus CL. Pharyngeal effects of bolus volume, viscosity, and temperature in patients with dysphagia resulting from neurologic impairment and in normal subjects. J Speech Hear Res 37: 1041–1049, 1994. doi: 10.1044/jshr.3705.1041. [DOI] [PubMed] [Google Scholar]
- 71. Cichero JAY, Nicholson TM, September C. Thickened milk for the management of feeding and swallowing issues in infants: a call for interdisciplinary professional guidelines. J Hum Lact 29: 132–135, 2013. doi: 10.1177/0890334413480561. [DOI] [PubMed] [Google Scholar]
- 72. Newman R, Vilardell N, Clavé P, Speyer R. Effect of bolus viscosity on the safety and efficacy of swallowing and the kinematics of the swallow response in patients with oropharyngeal dysphagia: White Paper by the European Society for Swallowing Disorders (ESSD). Dysphagia 31: 232–249, 2016. [Erratum in Dysphagia 31: 719, 2016]. doi: 10.1007/s00455-016-9696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Legrand C, Merlini JM, de Senarclens-Bezençon C, Michlig S. New natural agonists of the transient receptor potential Ankyrin 1 (TRPA1) channel. Sci Rep 10: 1–10, 2020. doi: 10.1038/s41598-020-68013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lundberg JM, Saria A. Capsaicin-induced desensitization of airway mucosa to cigarette smoke, mechanical and chemical irritants. Nature 302: 251–253, 1983. doi: 10.1038/302251a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1 and Supplemental Movie S1: https://doi.org/10.6084/m9.figshare.19694992.v2.