Keywords: aging, baroreflex sensitivity, heart rate variability, inflammation, testosterone
Abstract
Aging is associated with reductions in cardiovagal baroreflex sensitivity (cBRS), which increases cardiovascular disease risk. Preclinical data indicate that low testosterone reduces cBRS. We determined whether low testosterone is associated with greater age-associated reductions in cBRS in healthy men. Twenty-six men categorized as young (N = 6; age = 31 ± 4 yr; testosterone = 535 ± 60 ng/dL), middle-aged/older with normal (N = 10; aged 56 ± 3 yr; testosterone = 493 ± 85 ng/dL) or low (N = 10; age = 57 ± 6 yr; testosterone = 262 ± 31 ng/dL) testosterone underwent recordings of beat-by-beat blood pressure and R-R interval during rest and two Valsalva maneuvers, and measures of carotid artery compliance. IL-6, C-reactive protein (CRP), oxidized LDL cholesterol, and total antioxidant status (TAS) were also measured in blood. Middle-aged/older men had lower cBRS compared with young men (17.0 ± 6.5 ms/mmHg; P = 0.028); middle-age/older men with low testosterone had lower cBRS (5.5 ± 3.2 ms/mmHg; P = 0.039) compared with age-matched men with normal testosterone (10.7 ± 4.0 ms/mmHg). No differences existed between groups during Phase II of the Valsalva maneuver; middle-aged/older men with low testosterone had reduced cBRS (4.7 ± 2.6 ms/mmHg) compared with both young (12.8 ± 2.8 ms/mmHg; P < 0.001) and middle-aged/older men with normal testosterone (8.6 ± 4.4 ms/mmHg; P = 0.046). There were no differences in oxidized LDL (P = 0.882) or TAS across groups (P = 0.633). IL-6 was significantly higher in middle-aged/older men with low testosterone compared with the other groups (P < 0.05 for all) and inversely correlated with cBRS (r = −0.594, P = 0.007). Middle-aged/older men had reduced carotid artery compliance compared with young, regardless of testosterone status (P < 0.001). These observations indicate that low testosterone in middle-aged/older men may contribute to reductions in cBRS. These data suggest that increased inflammation may contribute to reductions in cBRS.
NEW & NOTEWORTHY Middle-aged/older men with low testosterone have accelerated reductions in cardiovagal BRS compared with middle-aged/older men with normal testosterone. Increased concentrations of the proinflammatory cytokine IL-6 appear to contribute to the reductions in cardiovagal BRS in men with low testosterone.
INTRODUCTION
The primary risk factor for cardiovascular (CV) diseases is increasing age and demographic shifts toward older populations are expected to largely increase the number of individuals developing CV diseases (1). Aging induces a number of adverse effects on the CV system, including increased arterial stiffness (2), reduced endothelial function (3, 4), left ventricular diastolic dysfunction (5, 6), and autonomic dysfunction (7, 8). Among these age-related changes in autonomic function is a reduction in cardiovagal baroreflex sensitivity [cBRS; i.e., reflex control of heart rate in response to acute changes in blood pressure (BP)] (9–12). Many CV disease states are linked with reduced cBRS (13, 14), including impaired BP regulation (15) and an increased incidence of sudden cardiac death (16, 17).
In men, aging is also associated with a steady decline in testosterone concentrations at a rate of ∼1% per year starting after the third decade (18, 19). These declines are associated with changes in body composition (20–22), sexual health (22, 23), and psychological well-being (22, 24). Less appreciated are the potential effects of declining testosterone on maintaining normal CV function. For example, our laboratory (25) and others (26–28) have previously published data indicating that low testosterone concentrations in middle-aged/older men is associated with greater age-associated endothelial dysfunction and large artery stiffening (20, 29, 30). Furthermore, preclinical data in orchidectomized rodents indicate that lower testosterone reduces cBRS (31–33) and testosterone supplementation in rodents with low testosterone restores cBRS back to the level of sham operated animals (33). However, the effects of low testosterone on cBRS in humans have yet to be examined.
Therefore, the purpose of this study was to determine if men with low testosterone have greater age-associated reductions in cBRS compared with men with normal testosterone. Based on data in animals (31–33), we hypothesized that cBRS would be reduced in men with low (i.e., <300 ng/dL) testosterone compared with men with normal (i.e., 400–1,000 ng/dL) testosterone. Furthermore, we determined cBRS using the Valsalva maneuver (34), a predictor of future CV death (35), to gain insight into cBRS during periods of increasing and decreasing BP. We hypothesized that cBRS during increasing BP would be reduced in men with low testosterone compared with men with normal testosterone. We also examined indices of heart rate variability (HRV) for additional insight into how the autonomic nervous system is affected by low levels of testosterone. Finally, we sought to determine the mechanisms by which low testosterone may influence cBRS. We (25) and others (36–38) have observed that low testosterone is associated with higher levels of inflammation and oxidative stress, which may affect cBRS via direct mechanisms in the brainstem (14, 39), or indirectly through effects on the vasculature (e.g., reduced large elastic artery compliance) (11, 40). Therefore, we examined indices of oxidative stress, inflammation, and carotid artery compliance as mechanisms that may contribute to greater age-associated reductions in cBRS in men with low testosterone.
METHODS
Study Design
This was a cross-sectional study that was part of an ongoing registered clinical trial (ClincialTrials.gov Identifier NCT02758431). The purpose of the parent clinical trial is to examine the effects of low testosterone on cardiovascular aging in men and, therefore, only men are included. The Colorado Multiple Institution Review Board approved all study protocols and procedures, and they conform to the provisions of the Declaration of Helsinki. All participants provided written and verbal informed consent before participating. All study visits and measurements were performed at the University of Colorado Denver Colorado Clinical and Translational Sciences Institute (CCTSI) Clinical and Translational Research Center (CTRC).
Study Participants
Twenty-six healthy men of all races/ethnic backgrounds aged 50–75 yr (middle-aged/older) and 18–40 yr (young) were recruited from the Denver Metropolitan Area. Middle-aged and older men were categorized into two groups: normal testosterone (serum testosterone 400–1,000 ng/dL) or low testosterone [serum testosterone <300 ng/dL as defined by the American Urology Association (AUA) (41)] at screening. Men were excluded from the study if serum testosterone was 300–399 ng/dL to have discreet groups. Young men also had testosterone levels 400–1,000 ng/dL. All serum testosterone levels were measured in the morning under fasted conditions. Men were included in the study if they met the following inclusion criteria: 1) no use of sex hormones or testosterone boosting supplements for at least 1 yr; 2) body mass index (BMI) <40 kg/m2; 3) nonsmokers; 4) resting blood pressure (BP) <160/90 mmHg; 5) nondiabetic and fasted plasma glucose <126 mg/dL; 6) healthy and free from cardiovascular, cancer, renal, liver, or respiratory disease as assessed by medical history, physical exam, standard blood chemistries (chemistry panel, complete blood count, and circulating thyroid levels), and electrocardiogram at rest and during a graded exercise treadmill test to fatigue; 7) sedentary or recreationally active (< 3 days/wk of vigorous aerobic exercise); 8) no use of medications that might influence cardiovascular function including antihypertensive and lipid lowering medications; and 9) no use of vitamin supplements or anti-inflammatory medications, or willing to stop 1 mo prior and throughout the study.
Participant Characteristics
Screening BP was measured in the morning under fasted conditions (water drinking only) and abstinence of caffeine and exercise. Seated BP was measured via oscillometric assessment (Carescape V100, GE medical systems) following ≥10 min of seated rest. BP was measured in triplicate on both arms and the average of the higher arm is reported here. Total (percent of total mass) body fat was determined using dual X-ray absorptiometry (Hologic Horizon). Hip and waist circumference were measured by a trained technician in triplicate and the average of those measures is reported here. Peak oxygen consumption was determined using an individualized incremental treadmill exercise protocol. A warm-up period was used to determine the walking speed that elicited a heart rate roughly 70% of age-predicted maximum. This speed was maintained during the test whereas the treadmill grade was increased by 2% every 2 min until volitional fatigue. A 12-lead electrocardiogram (Quinton Q4500; Quinton Instruments, Seattle, WA) was used to monitor heart rhythm and rate continuously, whereas BP was measured during each exercise stage. Cardiorespiratory data were collected at 30-s intervals using a ParvoMedics TrueOne 2400 automated metabolic gas analysis system. The highest oxygen uptake (V̇o2) value achieved was recorded as V̇o2peak.
Androgen deficiency symptom questionnaires.
Participants completed the quantitative Androgen Deficiency in the Aging Male (qADAM) questionnaire (42) and the Aging Male’s Symptoms (AMS) scale (43). The qADAM consists of 10 questions with a Likert scale of 1–5, with 5 representing the absence of a given symptom and 1 representing maximal symptoms, with all questions weighted equally. Summation of responses yields a total qADAM score between 10 and 50, with 10 being the most symptomatic and 50 being least symptomatic (42).
The AMS scale is a commonly used scale for measuring health-related quality of life in aging men (43). The AMS scale consists of 17 questions with a Likert scale ranging from 1 to 5 with 5 representing the most symptomatic and 1 representing absence of a given symptom. Symptoms are categorized into three dimensions: 1) psychological (e.g., discouraged, depressed, irritable, anxious, nervous) with a minimum score of 5 and maximum of 25, 2) somato-vegetative (e.g., joint/muscle complaints, hot flushes, increased need for sleep, sleep disturbances, impaired general well-being, decrease in muscle strength, and decreased energy) with a minimum score of 7 and maximum of 35, and 3) sexual (e.g., disturbances of potency, decreased morning erections, decreased libido and sexual activity, and beard growth) with a minimum score of 5 and maximum of 25.
Measurements
Participants were required to fast and abstain from caffeine and alcohol for ≥4 h and exercise for ≥20 h before the start of the experimental study visit, which was conducted in the morning between 0800 and 1200 h. Participants rested supine in a dimly lit, temperature-controlled room for ≥15 min before any data recording.
cBRS and HRV.
Baseline measures of beat-by-beat blood pressure (finger photoplethysmography; Finapres Nova, Finapres Medical Systems) and R-R interval (three-lead electrocardiogram; Finapres Nova, Finapres Medical Systems) were recorded simultaneously during 10 min of quiet rest. Participants then completed 6 min of paced breathing at a rate of 12 breaths/min. Breathing frequency was confirmed using capnography. Following paced breathing, participants completed two Valsalva maneuvers for 20 s each. Data were recorded continuously at 1,000 Hz using data-acquisition software (LabChart 8, ADInstruments) and stored on a computer for offline analysis.
Carotid artery compliance.
Ultrasound imaging of the carotid artery was performed using a multifrequency linear-array transducer (GE Vivid I, GE Healthcare). Maximal systolic and minimum diastolic diameters were analyzed 2–3 cm proximal to the carotid bulb (Vascular Research Tools 6, Medical Imaging Applications, LLC) by a trained investigator (KLM or LED). Carotid systolic and diastolic BP were determined using pulse wave analysis (PWA; Sphygmacor, Atcor Medical). Carotid compliance was calculated using the following equation (44):
where Δd is the change in carotid diameter (end systolic diameter minus end diastolic diameter), d represents the end-diastolic diameter, and ΔP is the carotid pulse pressure.
Blood sampling.
Fasted plasma concentrations of glucose, insulin, total cholesterol (Roche Diagnostic systems, Indianapolis, IN), and high-density lipoprotein cholesterol (Diagnostic Chemical Ltd, Oxford CT) were determined using enzymatic/colorimetric methods. Homeostatic model assessment of insulin resistance (HOMA-IR) was calculated as (glucose × insulin)/405 (45). Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation (46). Oxidized LDL was measured using an enzyme-linked immunosorbent plate assay (Alpco Diagnostics, Windham, NH). Serum total antioxidant status (TAS), a measure of overall antioxidant capacity, was measured using an enzymatic kit (Randox Laboratories, Oceanside, CA). Interleukin-6 (IL-6) was measured using an enzyme-linked immunoassay and high-sensitivity C-reactive protein (CRP) was measured using an immunoturbidimetric method. Total serum testosterone, estradiol, and sex-hormone binding globulin (SHBG) were measured via chemiluminescence using a Beckman Coulter Access II analyzer. Free testosterone was calculated for each participant from concentrations of serum testosterone, SHBG, and albumin using an online algorithm (www.issam.ch) using the Vermeulen equation (47). Testosterone measurements were confirmed by measuring testosterone on at least two occasions. All assays were performed at the University of Colorado Clinical Translational Sciences Institute Clinical Translational Research Center Core laboratory. Intra- and interassay coefficients of variation were as follows: 1) glucose, 0.67% and 1.44%, respectively; 2) insulin, 1.60% and 2.80%; 3) total cholesterol, 0.85% and 1.54%; 4) high-density lipoprotein cholesterol, 0.50% and 1.12%; 5) oxidized LDL, 4.60% and 7.10%; 6) TAS, 2.10% and 3.20%; 7) IL-6, 7.80% and 11.00%; 8) CRP, 1.10% and 1.83%; 9) serum testosterone, 2.10% and 5.10%; 10) estradiol, 4.30% and 8.20%; and 11) SHBG, 3.60% and 5.70%.
Data and Statistical Analysis
Spontaneous cBRS.
Beat-to-beat time series of systolic blood pressure (SBP) and R-R interval were analyzed using the sequence method for estimating spontaneous cBRS (HemoLab version 23.1, Herald Stauss Scientific) (48, 49). Briefly, sequences of three or more consecutive cardiac cycles in which SBP and R-R interval change in the same direction were identified as baroreflex sequences. Sequences were detected only when the variation in SBP was >1 mmHg and changes in R-R interval were >0.5 ms. A linear regression was applied to individual sequences and only those sequences with an R2 > 0.80 were accepted. Values of cBRS were included when there were ≥3 up (increase in both SBP and R-R interval) and ≥3 down (decrease in both SBP and R-R interval) sequences that met these criteria. The slopes of individual linear regressions were calculated and averaged for a measure of spontaneous cBRS. cBRS was determined for all sequences (overall cBRS) and also separately for up and down sequences.
Valsalva maneuver.
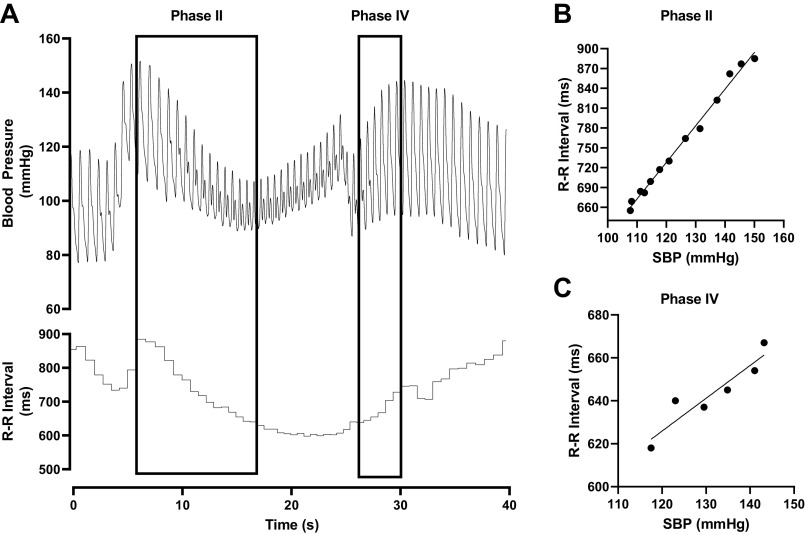
A representative tracing of a Valsalva maneuver and the analysis is presented in Fig. 1. BP and R-R interval are displayed in Fig. 1A with Phase II and Phase IV highlighted. Phase II of the Valsalva maneuver was used to determine cBRS during decreasing BP and Phase IV of the Valsalva maneuver was used to determine cBRS during increasing BP. Linear regression analysis was applied to systolic BP and R-R interval during each phase of the Valsalva maneuver and data from a single participant is demonstrated during Phase II (Fig. 1B) and Phase IV (Fig. 1C). The slope of the linear regression line represents cBRS during each phase and the average of the two Valsalva maneuvers is reported here.
Figure 1.
Representative tracing of blood pressure and R-R interval (A) with regions used for determining down cBRS (Phase II) and up cBRS (Phase IV) identified. Example linear regressions from a single low testosterone participant determining down cBRS (B) and up cBRS (C). cBRS, cardiovagal baroreflex sensitivity.
Heart rate variability.
R-R Intervals during paced breathing were analyzed using Kubios HRV software (v. 3.4.2, Biosignal Analysis and Medical Imaging Group). Time domain HRV was determined by the standard deviation (SD) of normal-to-normal R-R intervals (SDNN) and the root mean square of successive differences in R-R interval (RMSSD). SDNN is considered to be an estimate of overall HRV and RMSSD is an estimate of short-term components of HRV and, therefore, an index of parasympathetic modulation of HR (50). Power spectral analysis using fast Fourier transformation was performed in the low-frequency (LF; 0.04–0.15) and high-frequency (HF; 0.15–0.4 Hz) ranges. The LF/HF ratio was calculated and is a representation of the balance between sympathetic and parasympathetic activity (50, 51).
Statistical analysis.
The statistical approaches were informed by recent guidelines for statistical reporting of cardiovascular research (52). Normality of distribution for continuous variables was determined qualitatively using histograms and quantitatively using the Shapiro–Wilk test. Normally distributed participant characteristics, sex hormones, cBRS, and HRV were examined using Student’s t test or one-way ANOVAs. In the case of a significant effect, Tukey’s post hoc analysis was performed to examine differences between groups. In the case of nonnormally distributed variables (LF Power, HF Power), the Kruskal–Wallis test was performed and Dunn’s multiple comparisons test was used to examine group differences in the case of a significant main effect. Relationships between continuous variables were examined using Pearson’s product-moment correlation coefficient. To gain insight into how inflammation, oxidative stress, and/or carotid artery stiffness affected the relation between testosterone and cBRS, partial correlations were performed between overall cBRS and testosterone controlling for IL-6, CRP, oxidized LDL, TAS, and carotid compliance. Statistical analyses were completed using GraphPad Prism 9.2.0 and IBM SPSS 28.
RESULTS
Descriptive data are presented in Table 1. As expected, age was significantly different between young and middle-age/older men (P < 0.001), but age was similar between the low and normal testosterone middle-age/older groups (P = 0.931). Middle-aged/older men with low testosterone had significantly lower testosterone compared with young men and middle/aged-older men with normal testosterone (P < 0.001). There was a main effect of group on free testosterone (P = 0.045) with middle-aged/older men with low testosterone having lower free testosterone compared with young men (P = 0.041). Estradiol (P = 0.736), follicle stimulating hormone (P = 0.132), and luteinizing hormone (P = 0.206) were similar between groups; however, middle-aged/older men with low testosterone had significantly lower sex hormone binding globulin compared with middle-aged/older men with normal testosterone (P = 0.014). Regardless of testosterone status, middle-age/older men had higher systolic BP (P < 0.05), total cholesterol (P < 0.005), low density lipoprotein cholesterol (P < 0.005), and lower V̇o2max (P < 0.001) compared with young men. Furthermore, middle-aged/older men with low testosterone had higher fasted blood glucose compared with middle-aged/older men with normal testosterone (P = 0.028). There was no difference between groups in symptom scores indicated by the qADAM (P = 0.514); however, there was a main effect on the AMS (P = 0.016), with post hoc testing indicating differences between young and middle-aged men with low testosterone (P = 0.014). There were no differences in subscale data in the sexual (P = 0.351) or psychological (P = 0.802) domains; however, there was a significant main effect in the somato-vegetative domain (P = 0.013) with post hoc testing indicating differences between young and middle-aged/older men with low testosterone (P = 0.014).
Table 1.
Participant characteristics
| Variable | Younger Men | MA/O Men with Low T | MA/O Men with Normal T | P Value |
|---|---|---|---|---|
| N | 6 | 10 | 10 | |
| Race | 3 C, 2 A, 1 AA | 10 C | 9 C, 1 AA | |
| Age, yr | 31 ± 4 | 57 ± 6* | 56 ± 3* | <0.001 |
| Mass, kg | 73.2 ± 12.0 | 93.5 ± 15.4 | 83.9 ± 13.5 | 0.051 |
| BMI, kg/m2 | 23.1 ± 3.4 | 30.1 ± 4.8* | 27.9 ± 3.4 | 0.017 |
| Body fat, % | 20.1 ± 6.0 | 32.4 ± 4.2* | 29.2 ± 5.2* | <0.001 |
| Systolic BP, mmHg | 112 ± 9 | 131 ± 8* | 125 ± 8* | 0.002 |
| Diastolic BP, mmHg | 70 ± 7 | 80 ± 5* | 79 ± 8 | 0.034 |
| V̇o2max, mL/kg/min | 48.6 ± 9.5 | 30.1 ± 4.3* | 31.9 ± 6.0* | <0.001 |
| Total cholesterol, mg/dL | 138.8 ± 29.0 | 193.5 ± 17.0* | 195.0 ± 29.6* | 0.004 |
| LDL cholesterol, mg/dL | 93.8 ± 33.7 | 149.6 ± 15.7* | 151.3 ± 30* | 0.005 |
| Triglycerides, mg/dL | 70.8 ± 27.0 | 150.4 ± 60.9 | 158.9 ± 185.4 | 0.469 |
| Glucose, mg/dL | 86 ± 5 | 98 ± 4* | 86 ± 10† | 0.01 |
| Insulin, µIU/mL | 3 ± 2 | 6 ± 3 | 4 ± 2 | 0.207 |
| HOMA-IR | 0.54 ± 0.45 | 1.11 ± 0.97 | 0.8 ± 0.57 | 0.156 |
| Testosterone, ng/dL | 535 ± 60 | 262 ± 31* | 493 ± 85† | <0.001 |
| Free testosterone, ng/dL | 11.1 ± 2.1 | 6.8 ± 2.5* | 9.2 ± 4.2 | 0.045 |
| Estradiol, pg/mL | 37 ± 18 | 44 ± 18 | 38 ± 14 | 0.736 |
| Follicle stimulating hormone, mIU/mL | 2.4 ± 1.0 | 3.5 ± 1.0 | 3.3 ± 0.8 | 0.132 |
| Luteinizing hormone, mIU/mL | 3.4 ± 1.3 | 2.4 ± 1.0 | 3.5 ± 1.1 | 0.206 |
| Sex hormone binding globulin, nmol/L | 34 ± 9 | 22 ± 11 | 43 ± 5.1† | 0.046 |
| qADAM | 38.2 ± 5.3 | 35.3 ± 4.3 | 35.7 ± 5.4 | 0.514 |
| AMS | 20 ± 3 | 25 ± 4* | 24 ± 3 | 0.016 |
| Sexual domaina | 5.5 [5.0–7.25] | 7.0 [5.75–9.25] | 6.0 [5.0–9.25] | 0.351 |
| Psychological domain | 6.8 ± 2.1 | 7.4 ± 2.7 | 7.7 ± 2.3 | 0.802 |
| Somato-vegetative domain | 8.0 ± 1.5 | 9.3 ± 1.8* | 11.6 ± 3.0 | 0.013 |
Data were examined using one-way ANOVAs and are displayed as means ± SD except in the case of nonnormally distributed data (a), which were examined using the Kruskal-Wallis test and are displayed as median [interquartile range]. A, Asian; AA, African American; AMS, Aging Male Symptom scale; BMI, body mass index; BP, blood pressure; C, Caucasian; HOMA-IR, homeostatic model analysis of insulin resistance; LDL, low-density lipoprotein; MA/O, middle-aged/older; qADAM, quantitative Androgen Deficiency in Aging Male Questionnaire; T, testosterone. Boldface indicates significant main effect. *P < 0.05 compared with younger men, †P < 0.05 compared with MA/O men with low T.
Effects of Age and Testosterone Status on cBRS and HRV
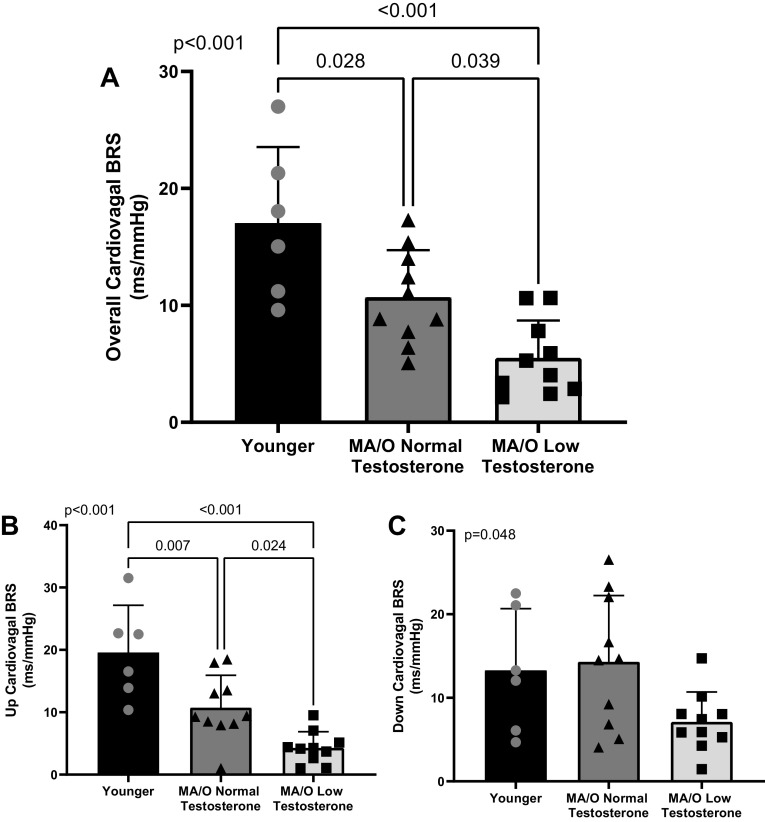
Spontaneous cBRS quantified during 10-min of quiet rest is presented in Fig. 2. Middle-aged/older men with normal testosterone have lower overall cBRS compared with young men (P = 0.028), whereas middle-aged/older men with low testosterone had lower overall cBRS compared with both young (P < 0.001) and middle-aged/older men with normal testosterone (P = 0.039; Fig. 2A). Furthermore, spontaneous cBRS during up sequences (i.e., increasing systolic BP and R-R interval; Fig. 2B) was significantly lower in middle-aged/older men with normal testosterone compared with young men (P = 0.007), and middle-aged/older men with low testosterone had lower up cBRS compared with young (P < 0.001) and middle-aged/older men with normal testosterone (P = 0.024). There was a significant main effect of spontaneous cBRS during down sequences (i.e., decreasing systolic BP and R-R interval; P = 0.048; Fig. 2C); however, post hoc testing revealed no significant differences between groups (young versus middle-aged/older low testosterone, P = 0.176; young versus middle-aged/older normal testosterone, P = 0.950; middle-aged/older low testosterone versus middle-aged/older normal testosterone, P = 0.052).
Figure 2.
Overall cBRS (A) is reduced in middle-aged/older men compared with young men (n = 6) and reduced to a greater extend in middle-aged/older men with low testosterone (n = 10) compared with age-matched men with normal testosterone (n = 10). cBRS during up (B) but not down (C) sequences are reduced in middle-aged/older men with low testosterone. P values reflect the main effect of a one-way ANOVA and post hoc testing. Data are represented as individual data points with means ± SD. cBRS, cardiovagal baroreflex sensitivity; BRS, baroreflex sensitivity; MA/O, middle-aged/older.
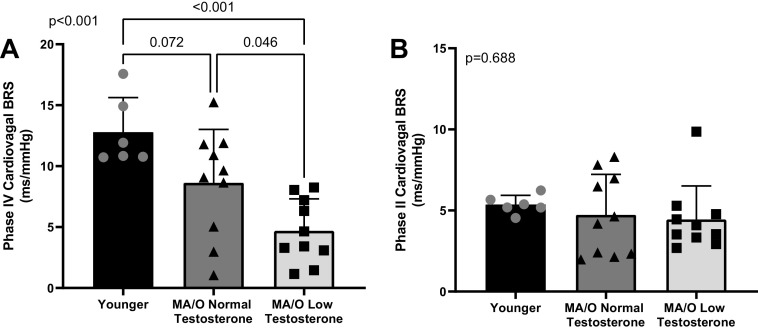
Similarly, cBRS was different between groups during periods of increasing (Phase IV, P < 0.001; Fig. 3A), but not periods of decreasing (Phase II, P = 0.688; Fig. 3B) BP during the Valsalva maneuver. There was a trend for up cBRS determined via the Valsalva maneuver to be lower in middle-aged/older men with normal testosterone compared with young men (P = 0.072) and middle-aged/older with low testosterone had lower up cBRS compared with both young (P < 0.001) and middle-aged/older with normal testosterone (P = 0.046).
Figure 3.
cBRS during Phase IV (increasing BP, A) of the Valsalva maneuver is reduced in middle-aged/older men with low testosterone (n = 10) compared with younger men (n = 6) and middle-aged/older men with normal testosterone (n = 10). There is no difference in cBRS during Phase II (decreasing BP, B) of the Valsalva maneuver between groups. P values reflect the main effect of a one-way ANOVA and post hoc testing. Data are represented as individual data points with means ± SD. BP, blood pressure; BRS, baroreflex sensitivity; cBRS, cardiovagal baroreflex sensitivity; MA/O, middle-aged/older.
Table 2 displays indices of HRV during 6 min of paced breathing at 12 breaths per minute. Variables in the time domain (SDNN, P = 0.111; RMSSD, P = 0.145) were lower in middle-aged/older men with low testosterone; however, these did not reach statistical significance. In the frequency domain, HF and LF power were not normally distributed and thus, statistical testing was performed using the Kruskal–Wallis test. There was a significant main effect for HF (P = 0.041) but not LF power (P = 0.366). HF power was significantly lower in middle-aged/older men with low testosterone compared with young men (P = 0.049), but not different compared with middle-aged/older men with normal testosterone (P = 0.264). There was no significant difference in the LF/HF ratio among groups (P = 0.229).
Table 2.
Heart rate variability
| Variable | Younger Men | MA/O Men with Low T | MA/O Men with Normal T | P Value |
|---|---|---|---|---|
| N | 6 | 10 | 10 | |
| SDNN, ms | 57.6 ± 30.2 | 28.9 ± 8.9 | 43.1 ± 25.2 | 0.067 |
| RMSSD, ms | 59.5 ± 43.3 | 28.7 ± 9.9 | 49.1 ± 31.1 | 0.135 |
| LF power, ms2a | 695 [378–2,529] | 357 [143–611] | 180 [120–1,049] | 0.366 |
| HF power, ms2a | 1,258 [434–3,653] | 165 [128–349]* | 925 [142–2,581] | 0.041 |
| LF/HFa | 0.69 [0.33–1.60] | 1.80 [0.42–1.80] | 0.42 [0.28–1.33] | 0.229 |
Data are means ± SD or median [interquartile range]. HF, high frequency; LF, low frequency; MA/O, middle-aged/older; RMSSD, root mean square of successive differences between normal heartbeats; SDNN, standard deviation of normal-to-normal R-R intervals; T, testosterone. Boldface indicates significant main effect. aNonnormally distributed data. *P < 0.05 compared with younger men.
Potential mechanisms associated with the greater age-associated reduction in cBRS in middle-age/older men with low testosterone.
Table 3 demonstrates biomarkers of oxidative stress, inflammation, and carotid artery stiffness. Oxidized LDL (P = 0.882) and total antioxidant status (P = 0.633) were similar across groups. CRP (P = 0.004) was not normally distributed and therefore tested using the Kruskal–Wallis test. Young men had lower CRP compared with middle-aged/older men with either normal (P = 0.040) or low testosterone (P = 0.008) with no differences between the groups of middle-aged/older men (P < 0.999). IL-6 (P = 0.005) was significantly higher in middle-aged/older men with low testosterone compared with younger men (P = 0.007) and middle-aged/older men with normal testosterone (P = 0.038). Both groups of middle-aged/older men had lower carotid artery compliance compared with young men (both P < 0.005); however, there was no difference between groups of middle-aged/older men (P = 0.753).
Table 3.
Biomarkers of inflammation, oxidative stress, and carotid artery stiffness
| Variable | Younger Men | MA/O Men with Low T | MA/O Men with Normal T | P Value |
|---|---|---|---|---|
| Circulating factors | ||||
| N | 6 | 10 | 10 | |
| IL-6, pg/mL | 0.7 ± 0.44 | 1.98 ± 0.92* | 1.15 ± 0.43† | 0.005 |
| CRP, mg/La | 0.19 [0.06–0.48] | 1.48 [0.82–4.20] | 1.10 [0.71–1.41] | 0.004 |
| Oxidized LDL, U/L | 64.8 ± 33.4 | 65.9 ± 10.1 | 66.7 ± 9.5 | 0.882 |
| TAS, mmol/L | 1.9 ± 0.4 | 1.8 ± 0.5 | 1.7 ± 0.1 | 0.633 |
| Carotid artery measures | ||||
| Carotid artery compliance, mm/mmHg | 0.16 ± 0.05 | 0.09 ± 0.02* | 0.10 ± 0.02* | <0.001 |
Data are means ± SD or median [interquartile range]. CRP, C-reactive protein; IL-6, interleukin-6; LDL, low-density lipoprotein; MA/O, middle-aged/older; T, testosterone; TAS, total antioxidant status. Boldface indicates significant main effect. aNonnormally distributed data. *P < 0.05 compared with younger men, †P < 0.05 compared with MA/O men with low T.
The correlation matrix with all participants is included in Table 4. Age and IL-6 were inversely correlated with overall (age: R2 = 0.465; IL-6: R2 = 0.397) and up cBRS determined using the sequence method (age: R2 = 0.440; IL-6: R2 = 0.326), as well as cBRS during Phase IV of the Valsalva maneuver (i.e., increasing BP; age: R2 = 0.267; IL-6: R2 = 0.235). Total testosterone and carotid artery compliance were directly correlated with overall (total testosterone: R2 = 0.358; carotid artery compliance: R2 = 0.699) and up cBRS determined using the sequence method (total testosterone: R2 = 0.397; carotid artery compliance: R2 = 0.554), as well as cBRS during Phase IV of the Valsalva maneuver (total testosterone: R2 = 0.480; carotid artery compliance: R2 = 0.401). Free testosterone was also correlated with cBRS during Phase IV of the Valsalva maneuver (R2 = 0.198). Total testosterone was correlated with carotid artery compliance (r = 0.445, R2 = 0.198, P = 0.034).
Table 4.
Correlation matrix—all participants
| Variable | Overall BRS |
Up BRS |
Down BRS |
Phase II
|
Phase IV
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| Age, yr | −0.682 | <0.001 | −0.663 | <0.001 | −0.116 | 0.572 | −0.116 | 0.573 | −0.517 | 0.007 |
| Testosterone, ng/dL | 0.598 | 0.001 | 0.630 | <0.001 | 0.354 | 0.076 | 0.162 | 0.430 | 0.693 | <0.001 |
| Free testosterone, ng/dL | 0.260 | 0.210 | 0.378 | 0.063 | 0.321 | 0.118 | 0.192 | 0.358 | 0.445 | 0.026 |
| Estradiol, pg/mL | −0.083 | 0.714 | −0.111 | 0.623 | 0.041 | 0.857 | −0.213 | 0.342 | −0.045 | 0.843 |
| FSH, mIU/mL | −0.109 | 0.637 | −0.088 | 0.704 | −0.045 | 0.846 | −0.066 | 0.775 | −0.206 | 0.369 |
| LH, mIU/mL | 0.329 | 0.145 | 0.431 | 0.051 | 0.210 | 0.360 | −0.045 | 0.847 | 0.326 | 0.149 |
| SHBG, nmol/L | −0.111 | 0.632 | 0.068 | 0.771 | 0.217 | 0.346 | −0.048 | 0.835 | −0.013 | 0.956 |
| IL-6, pg/mL | −0.630 | <0.001 | −0.571 | 0.004 | −0.212 | 0.320 | −0.087 | 0.685 | −0.485 | 0.016 |
| CRP, mg/L | −0.325 | 0.162 | −0.381 | 0.097 | 0.208 | 0.379 | 0.020 | 0.932 | −0.135 | 0.569 |
| Oxidized LDL, U/L | 0.218 | 0.357 | 0.206 | 0.385 | 0.192 | 0.417 | −0.009 | 0.970 | 0.029 | 0.903 |
| TAS, mmol/L | 0.186 | 0.432 | 0.284 | 0.225 | −0.177 | 0.456 | −0.136 | 0.567 | 0.097 | 0.685 |
| Carotid compliance, mm/mmHg | 0.836 | <0.001 | 0.744 | <0.001 | 0.368 | 0.084 | 0.074 | 0.739 | 0.633 | 0.001 |
BRS, baroreflex sensitivity; CRP, C-reactive protein; FSH, follicle stimulating hormone; IL-6, interleukin-6; LDL, low-density lipoprotein; LH, luteinizing hormone; SHBG, sex hormone binding globulin; TAS, total antioxidant status. Boldface indicates significant correlation.
Because cBRS is reduced with increasing age (9), Table 5 demonstrates correlations in just the middle-aged/older men. With younger men excluded, age was no longer correlated with any measure of cBRS. Total and free testosterone was significantly correlated with overall (total testosterone: R2 = 0.241; free testosterone: R2 = 0.207) and up cBRS determined using the sequence method (total testosterone: R2 = 0.272; free testosterone: R2 = 0.482), as well as cBRS during Phase IV of the Valsalva maneuver (total testosterone: R2 = 0.344; free testosterone: R2 = 0.387). IL-6 was inversely correlated with overall (R2 = 0.353) and up cBRS determined using the sequence method (R2 = 0.195), and CRP was inversely correlated with cBRS during Phase IV of the Valsalva maneuver (R2 = 0.341). Carotid artery compliance was correlated with overall cBRS (R2 = 0.361) and cBRS during Phase IV of the Valsalva maneuver (R2 = 0.209); however, there was no correlation between total testosterone and carotid artery compliance (r = 0.228, R2 = 0.052, P = 0.362).
Table 5.
Correlation matrix—MA/O participants only
| Variable | Overall BRS |
Up BRS |
Down BRS |
Phase II
|
Phase IV
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| Age, yr | −0.293 | 0.209 | 0.006 | 0.982 | 0.175 | 0.460 | 0.077 | 0.748 | −0.049 | 0.838 |
| Testosterone, ng/dL | 0.491 | 0.028 | 0.522 | 0.018 | 0.354 | 0.126 | 0.078 | 0.742 | 0.587 | 0.007 |
| Free testosterone, ng/dL | 0.455 | 0.046 | 0.694 | <0.001 | 0.362 | 0.128 | 0.209 | 0.390 | 0.622 | 0.004 |
| Estradiol, pg/mL | −0.227 | 0.397 | −0.223 | 0.407 | −0.110 | 0.685 | −0.318 | 0.320 | −0.135 | 0.617 |
| FSH, mIU/mL | −0.078 | 0.782 | 0.120 | 0.670 | −0.239 | 0.391 | 0.033 | 0.906 | −0.137 | 0.627 |
| LH, mIU/mL | −0.062 | 0.825 | 0.248 | 0.373 | −0.050 | 0.861 | −0.173 | 0.538 | 0.171 | 0.542 |
| SHBG, nmol/L | 0.108 | 0.701 | 0.451 | 0.091 | 0.382 | 0.161 | −0.069 | 0.807 | 0.095 | 0.736 |
| IL-6, pg/mL | −0.594 | 0.007 | −0.442 | 0.048 | −0.253 | 0.296 | −0.033 | 0.893 | −0.364 | 0.126 |
| CRP, mg/L | −0.311 | 0.259 | −0.394 | 0.146 | 0.128 | 0.650 | 0.069 | 0.807 | −0.584 | 0.022 |
| Oxidized LDL, U/L | −0.281 | 0.311 | −0.084 | 0.766 | −0.336 | 0.221 | 0.126 | 0.655 | 0.048 | 0.864 |
| TAS, mmol/L | −0.149 | 0.595 | 0.047 | 0.868 | −0.207 | 0.460 | −0.145 | 0.607 | 0.017 | 0.953 |
| Carotid compliance, mm/mmHg | 0.601 | 0.008 | 0.272 | 0.274 | 0.377 | 0.123 | −0.018 | 0.944 | 0.457 | 0.046 |
BRS, baroreflex sensitivity; CRP, C-reactive protein; FSH, follicle stimulating hormone; IL-6, interleukin-6; LDL, low-density lipoprotein; LH, luteinizing hormone; MA/O, middle-aged/older; SHBG, sex hormone binding globulin; TAS, total antioxidant status. Boldface indicates significant correlation.
Partial correlations between overall cBRS and testosterone were adjusted for carotid compliance and IL-6, which were correlated with testosterone (carotid compliance, r = 0.444, R2 = 0.197, P = 0.034; IL-6, r = −0.632, R2 = 0.399, P < 0.001). After controlling for IL-6, the correlation between testosterone and overall cBRS was no longer significant (r = 0.281, R2 = 0.079, P = 0.194); however, the correlation between testosterone and overall cBRS was still significant when controlling for carotid artery compliance (r = 0.387, R2 = 0.150, P = 0.046).
DISCUSSION
The novel findings of this study are that middle-aged/older men with low testosterone have greater age-associated reductions in cBRS than age-matched men with normal testosterone. Furthermore, we demonstrate that cBRS during periods of increasing BP is reduced in middle-aged/older men with low testosterone compared with middle-aged/older men with normal testosterone, with no differences during periods of decreasing BP. These data indicate that greater age-associated inflammation, possibly related to effects on central artery compliance, is a key mechanism underlying the reduction in cBRS in middle-age/older men with low testosterone.
Aging, Testosterone, and cBRS
The baroreflexes are neurocardiovascular reflexes that operate in negative feedback fashion to maintain circulatory homeostasis. The “arterial” or “high-pressure” baroreceptors, located in the aortic arch and carotid artery, respond to pressure-induced arterial stretch by increasing cardiac parasympathetic outflow (reducing cardiac chronotropy; cBRS) and inhibiting efferent sympathetic activity (reducing vascular tone, cardiac chronotropy, and cardiac inotropy; sympathetic BRS) (53). The importance of the baroreflexes are demonstrated in individuals with autonomic failure, who experience severe persistent hypotension in response to physiological stressors such as meal ingestion (54) or exercise (55).
Aging is associated with impaired reductions in cBRS (9, 11). These observations have been demonstrated repeatedly using a variety of experimental techniques, including the sequence method (56, 57), Valsalva maneuver (10, 58), and the modified Oxford procedure (11, 59). Participant age is a strong predictor of cBRS, with an inverse, linear correlation between cBRS and age (r = −0.65 to 0.69) (10, 59). Consistent with previous findings, we demonstrate that middle-aged/older men have reduced cBRS compared with younger men, determined by both the sequence method and Valsalva maneuver. Moreover, correlations between age and cBRS are similar to previous findings, with a correlation coefficient of r = −0.682 between age and overall cBRS determined via the sequence method.
Postmenopausal women have reduced cBRS compared with younger women (60), an effect that has been attributed to reductions in the primary female sex hormone estrogen (61). The primary male sex hormone, testosterone, decreases with aging as well, although this decline is generally subtler than the loss of estrogen in women, with declines of ∼1% per year staring after the third decade (18, 19). However, the effects of low testosterone concentrations have been underappreciated when it comes to age-related changes in CV function, despite data linking low testosterone with cardiovascular disease (CVD) risk, including hypertension (62, 63) and CV mortality (64, 65). Accordingly, middle-aged/older men in this study were categorized based on serum testosterone as either low (≤300 ng/dL), as defined by the AUA, or normal (>400 ng/dL) testosterone. We found that middle-age/older men with low testosterone had greater age-associated impairments of cBRS compared with men with normal testosterone. cBRS in middle-age/older men with low testosterone was approximately half that of middle-aged/older men with normal testosterone (∼5.5 vs. 10.7 ms/mmHg) despite being matched for age. Moreover, serum testosterone levels were strongly correlated with cBRS, which remained after partialing out the effects of age. In addition to identifying a mechanism by which low testosterone may increase CVD risk, these data highlight the importance of quantifying serum testosterone to separate the effects of aging from the effects of declining sex hormones.
In addition, we demonstrate that baroreflex control of heart rate during periods of increasing BP are particularly affected in middle-aged/older men with low testosterone. This was observed during up sequences (i.e., periods of increasing systolic BP and R-R interval), as well during Phase IV of the Valsalva maneuver, when BP is increasing. In contrast, cBRS was similar between the groups of middle-aged/older men during periods of decreasing BP (down sequences and Phase II of the Valsalva maneuver). These findings are similar to a previous study in aging men that demonstrated reduced cardiac slowing during administration of the vasoconstrictor phenylephrine (66).
We also examined indices of HRV to assess autonomic function. In the time domain, RMSSD is thought to reflect the short-term component of HRV and, therefore, to reflect parasympathetic outflow (50). Similarly, HF power in the frequency domain is also believed to reflect parasympathetic outflow (50). There was a significant main effect of group on HF power and post hoc testing indicated that HF power was lower in middle-aged/older men with low testosterone compared with younger men. Similarly, the mean value in RMSSD was lower in middle-aged/older men with low testosterone; however, this did not reach statistical significance. Together, these data suggest that parasympathetic activity may be reduced in middle-aged/older men with low testosterone; however, these findings require further study in larger cohorts.
Potential mechanisms for reduced cBRS in middle-age/older men with low testosterone.
Consistent with previous descriptions of baroreflex hysteresis (13), we observed lower gain during period of falling pressure (down sequences, Phase II of the Valsalva maneuver) than during periods of rising pressure (up sequences, Phase IV of the Valsalva maneuver). It has been postulated that hysteresis is affected by both mechanical properties of the central arteries where the baroreceptors are located, as well as via neural mechanisms in cardiovascular control areas within the brainstem (67, 68). Accordingly, Hunt and colleagues developed methods to gain insight into the mechanical and neural components of the baroreflex arc (68). Using these methods, it has been demonstrated that older adults, regardless of sex, were able to offset the effects of reduced arterial compliance via higher levels of neural responsiveness during rising BP, such that integrated (i.e., overall) sympathetic BRS was not different between younger and older adults (69). In the present study, we observed differences in cBRS during periods of rising BP in middle-aged/older men with low testosterone, suggesting that reductions in arterial compliance exceeded the ability of the neural components of the baroreflex to preserve baroreflex sensitivity in middle-aged/older men with low testosterone. Without recordings of carotid diameter during baroreflex testing, however, we are not able to determine whether this was due to reductions in arterial compliance or reductions in the neural components of the baroreflex. Future study is warranted to provide more insight into how low testosterone affects cBRS.
Although we did not measure carotid diameter during baroreflex testing, we did quantify carotid compliance separately. Because the baroreceptors are located in the central (aorta and carotid) arteries and respond to arterial stretch, BRS is susceptible to being reduced via reductions in the compliance of these large elastic arteries. Therefore, reduced distension in less compliant (i.e., stiffer) central arteries in response to acute increases in central BP would be expected to result in a correspondingly smaller R-R interval response (i.e., diminished cBRS), a phenomenon that has been observed in aging (10). Therefore, we examined reduced carotid artery compliance as a potential mechanism by which low testosterone affects cBRS. Although carotid artery compliance was strongly correlated with overall and up cBRS, carotid artery compliance was not significantly different between the groups of middle-aged/older men and there was no correlation between testosterone and carotid artery compliance when examined in the middle-aged/older men only. Furthermore, when carotid artery compliance was controlled for using partial correlation analysis, the relation between cBRS and testosterone remained significant. These findings suggest that, although lower cBRS is associated with less compliant carotid arteries, this relation does not appear to be a function of testosterone concentrations.
We also examined indices of inflammation and oxidative stress as mechanisms by which low testosterone influences cBRS. Data from our laboratory (25) and others (36–38) indicate that testosterone is associated with anti-inflammatory and antioxidant effects, and both inflammation and oxidative stress have been linked with reduced cBRS. For example, animal models demonstrate that increased oxidative stress reduces BRS (40, 70). Furthermore, acute systemic infusion of the antioxidant vitamin C improved cBRS in healthy older men, providing experimental support for the concept that oxidative stress contributes mechanistically to reductions in cBRS (11). Similarly, increased inflammation is mechanistically linked with baroreflex dysfunction via effects on the baroreceptor nerve endings and/or on neurons in BP control centers of the brainstem (14, 39), as well as potential indirect effects by reducing central arterial compliance (14, 71).
In this study, we did not observe differences in markers of oxidative stress (oxidized LDL, TAS) between groups, and neither marker of oxidative stress was correlated with cBRS. However, IL-6 was significantly higher in middle-aged/older men with low testosterone compared with middle-age/older men with normal testosterone, consistent with our previous reports (25). Furthermore, IL-6 and CRP were inversely correlated with measures of cBRS in the middle-aged/older men. Together, these data suggest that the greater proinflammatory state of middle-age/older men with low testosterone may contribute to the observed reduced cBRS.
Our study design did not allow us to distinguish the central effects of inflammation on neurons in the brainstem versus peripheral effects of inflammation on carotid artery compliance. However, the relation between testosterone and cBRS remained significant after controlling for carotid artery compliance using partial correlation analysis. We interpret this finding to indicate that inflammation may have direct effects on neurons in the BP control centers. Consistent with this interpretation, Takagishi and colleagues demonstrated that microinjections of IL-6 into the nucleus tractus solitarius inhibited cBRS in rats in a dose-dependent manner (39). In addition, IL-6 attenuated L-glutamate-induced bradycardia, suggesting that IL-6 acts postsynaptically to inhibit neurons in the nucleus tractus solitarius. Furthermore, the authors found that IL-6 microinjections did not affect the baroreflex set point of arterial pressure, suggesting a possible mechanism mediated by γ-aminobutyric acid (GABA)ergic interneurons, as activation of GABA receptors in the nucleus tractus solitarius has been shown to reduce cBRS without affecting the baroreflex set point (72). It should be noted that our sample size was small and thus, these findings should be interpreted cautiously. Further study is needed to confirm these findings in humans and gain further insight into the mechanisms by which low testosterone contributes to reduced cBRS.
It is also worth noting that the classic genomic model of steroid hormone action is centered around gene transcription and protein synthesis; however, an evolving appreciation for the nongenomic actions of hormones has been developed over the past ∼2 decades (73). It is possible that testosterone is therefore acting nongenomically to affect cBRS in the present study. For example, androgen exposure consistently increases intracellular calcium concentrations (73), which could potentially affect the depolarization of postganglionic neurons in the autonomic nervous system. Future studies should directly measure free testosterone to gain additional insight into the genomic versus nongenomic mechanisms by which testosterone affects cBRS.
Role of CVD risk factors contributing to reduced cBRS in middle-aged/older men with low testosterone.
Low testosterone is closely linked with other CVD risk factors that may independently affect cBRS, including obesity (74), insulin resistance (74, 75), and hyperglycemia (76). In the present study; however, the middle-aged/older men participating in this study were well matched for age, BP, body composition, etc. Although there were significant main effects for BMI, BP, total and LDL cholesterol, post hoc testing indicated that these differences were between the young and middle-aged/older men, not between groups based on testosterone status. The middle-aged/older men with low testosterone did have significantly higher fasted blood glucose compared with middle-aged/older men with normal testosterone; however, insulin and HOMA-IR were similar between groups. Despite the lack of significant differences in these CV disease risk factors, it remains possible that increased adiposity, higher blood glucose, or other factors may have contributed to the observed differences in cBRS.
EXPERIMENTAL CONSIDERATIONS AND LIMITATIONS
The present study is not without limitations. Our study was small, and it is possible that it was underpowered to observe statistical differences in some outcomes (e.g., SDNN, RMSSD). We only enrolled men that were healthy, and the results may not be generalizable to men with preexisting CV disease, diabetes, smokers, or those on medications. We recognize that we excluded middle-age/older men with testosterone levels between 300–399 ng/dL, which are still considered within the normal ranges. Our goal was to have distinct categories of testosterone groups, and therefore, we included a value of >400 ng/dL as our beginning value for the normal group and it remains unknown how testosterone values within the 300–399 ng/dL range influence cBRS and warrants further study. Similarly, we were unable to directly measure free testosterone and instead relied on calculations using total testosterone, albumin, and SHBG. Future studies should measure free testosterone to gain insight into genomic versus nongenomic mechanisms by which testosterone affects cBRS.
The aim of this study was to examine the role of low testosterone on age-associated reductions in cBRS in men; therefore, only males were included in this study. These findings are not generalizable to women, and conditions in women in which testosterone concentrations are altered (e.g., polycystic ovary syndrome) may affect cBRS differently. Testosterone also has indirect effects via aromatization to estradiol and some studies have demonstrated associations between low estradiol and CV disease risk factors (77), highlighting the possibility that low estradiol may contribute to lower cBRS in men with low testosterone. However, we did not observe group differences in estradiol in the present study, nor were there correlations between estradiol and cBRS (data not included). Additional study is warranted to examine the potential role in estradiol in maintaining cBRS in aging men. Furthermore, because this is a cross-sectional study, we cannot determine causality, and the effects of testosterone status may not reflect the effects of androgen deprivation therapy or exogenous testosterone treatment. Moreover, we are unable to determine whether the reduced cBRS observed in men with low testosterone was related to some other lifestyle factor (e.g., diet, sleep, physical activity).
Finally, it is important to note that we categorized our men as having low testosterone based on the AUA guidelines of consistently low testosterone levels <300 ng/dL and other organizations, including the Endocrine Society recommend using the standard Center for Disease Control and Prevention (CDC) level for a healthy nonobese young male of 264 ng/dL. Although the mean testosterone value in the low testosterone group in the present study was similar to the CDC standard for the lower end of normal of young men, we observed a twofold age-associated reduction in cBRS in men with low T compared with their age-matched peers indicating that testosterone levels around the standard of normal are associated with severe impairments in cBRS.
Conclusions
Low testosterone has an adverse impact on physical function and quality of life in aging men (78) and is associated with all-cause and CV disease mortality (79). It is estimated that low testosterone will be involved in the development of ∼1.3 million new cases of CV disease and other diseases (e.g., diabetes, osteoporosis) and may be directly responsible for $190–525 billion in US health care expenditures over a 20-yr period (80). However, the effects of declining testosterone concentrations are largely overlooked in studies examining the effects of aging on CV function. The findings from the present study suggest that low testosterone, defined using the AUA guidelines, may contribute to impaired autonomic control of CV function, due in part to increased inflammation. Moreover, this study adds to mounting evidence that normal physiological levels of testosterone may be beneficial to CV health. Future studies should investigate whether maintaining testosterone above a certain level and/or implementing other therapeutic strategies to mitigate inflammation can slow down or reverse the effects of low testosterone on autonomic dysfunction in men.
GRANTS
This research was supported by National Institutes of Health (NIH) Grants R01AG049762 (to K. L. Moreau), U54AG062319 (to W. M. Kohrt), and T32AG000279 (to M. C. Babcock and L. E. DuBose); Colorado Clinical and Translational Sciences Institute NIH Grant UL1TR001082; Colorado Nutrition and Obesity Research Center NIH Grant P30DK048520; and the Eastern Colorado Geriatric Research, Education and Clinical Center of the Veterans Affairs Medical Center (to W. M. Kohrt and K. L. Moreau).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.C.B., K.L.H., B.L.S., W.K.C., W.M.K., and K.L.M. conceived and designed research; M.C.B. performed experiments; M.C.B. analyzed data; M.C.B., L.E.D., K.L.H., B.L.S., W.K.C., W.M.K., and K.L.M. interpreted results of experiments; M.C.B. prepared figures; M.C.B. drafted manuscript; M.C.B., L.E.D., K.L.H., B.L.S., W.K.C., W.M.K., and K.L.M. edited and revised manuscript; M.C.B., L.E.D., K.L.H., B.L.S., W.K.C., W.M.K., and K.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The graphical abstract was created using BioRender.com and is published with permission. We thank the nursing, core laboratory, bionutrition, information systems and administrative staff of the Clinical and Translational Research Center and the Energy Balance Core of the Nutrition and Obesity Research Center for support of the study. We also are grateful to the members of our research group who helped with the initiation of the study and carried out day-to-day activities for the project. Finally, we thank the men who volunteered to participate in the study for time and effort.
REFERENCES
- 1. Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ; American Heart Association Advocacy Coordinating Committee; Stroke Council, Council on Cardiovascular Radiology and Intervention, Council on Clinical Cardiology; Council on Epidemiology and Prevention, Council on Arteriosclerosis; Thrombosis and Vascular Biology, Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Cardiovascular Nursing, Council on the Kidney in Cardiovascular Disease, Council on Cardiovascular Surgery and Anesthesia, and Interdisciplinary Council on Quality of Care and Outcomes Research. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123: 933–944, 2011. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 2. Avolio AP, Deng FQ, Li WQ, Luo YF, Huang ZD, Xing LF, O'Rourke MF. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation 71: 202–210, 1985. doi: 10.1161/01.cir.71.2.202. [DOI] [PubMed] [Google Scholar]
- 3. Babcock MC, DuBose LE, Witten TL, Brubaker A, Stauffer BL, Hildreth KL, Moreau KL. Assessment of macrovascular and microvascular function in aging males. J Appl Physiol (1985) 130: 96–103, 2021. doi: 10.1152/japplphysiol.00616.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seals DR, Moreau KL, Gates PE, Eskurza I. Modulatory influences on ageing of the vasculature in healthy humans. Exp Gerontol 41: 501–507, 2006. doi: 10.1016/j.exger.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6. Hollingsworth KG, Blamire AM, Keavney BD, Macgowan GA. Left ventricular torsion, energetics, and diastolic function in normal human aging. Am J Physiol Heart Circ Physiol 302: H885–H892, 2012. doi: 10.1152/ajpheart.00985.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dinenno FA, Joyner MJ. α-adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation 13: 329–341, 2006. doi: 10.1080/10739680600618843. [DOI] [PubMed] [Google Scholar]
- 8. Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol 274: 621–637, 1978. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Monahan KD. Effect of aging on baroreflex function in humans. Am J Physiol Regul Integr Comp Physiol 293: R3–R12, 2007. doi: 10.1152/ajpregu.00031.2007. [DOI] [PubMed] [Google Scholar]
- 10. Monahan KD, Dinenno FA, Seals DR, Clevenger CM, Desouza CA, Tanaka H. Age-associated changes in cardiovagal baroreflex sensitivity are related to central arterial compliance. Am J Physiol Heart Circ Physiol 281: H284–H289, 2001. doi: 10.1152/ajpheart.2001.281.1.H284. [DOI] [PubMed] [Google Scholar]
- 11. Monahan KD, Eskurza I, Seals DR. Ascorbic acid increases cardiovagal baroreflex sensitivity in healthy older men. Am J Physiol Heart Circ Physiol 286: H2113–H2117, 2004. doi: 10.1152/ajpheart.01054.2003. [DOI] [PubMed] [Google Scholar]
- 12. Kornet L, Hoeks AP, Janssen BJ, Houben AJ, De Leeuw PW, Reneman RS. Neural activity of the cardiac baroreflex decreases with age in normotensive and hypertensive subjects. J Hypertens 23: 815–823, 2005. doi: 10.1097/01.hjh.0000163151.50825.e2. [DOI] [PubMed] [Google Scholar]
- 13. Eckberg DL, Sleight P. Human Baroreflexes in Health and Disease. Oxford, United Kingdom: Oxford University Press, 1992. [Google Scholar]
- 14. Chapleau MW, Li Z, Meyrelles SS, Ma X, Abboud FM. Mechanisms determining sensitivity of baroreceptor afferents in health and disease. Ann N Y Acad Sci 940: 1–19, 2001. doi: 10.1111/j.1749-6632.2001.tb03662.x. [DOI] [PubMed] [Google Scholar]
- 15. Shi X, Wray DW, Formes KJ, Wang H-W, Hayes PM, O-Yurvati AH, Weiss MS, Reese IP. Orthostatic hypotension in aging humans. Am J Physiol Heart Circ Physiol 279: H1548–H1554, 2000. doi: 10.1152/ajpheart.2000.279.4.H1548. [DOI] [PubMed] [Google Scholar]
- 16. La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet 351: 478–484, 1998. doi: 10.1016/S0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 17. La Rovere MT, Specchia G, Mortara A, Schwartz PJ. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. A prospective study. Circulation 78: 816–824, 1988. doi: 10.1161/01.cir.78.4.816. [DOI] [PubMed] [Google Scholar]
- 18. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR; Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 86: 724–731, 2001. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 19. Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci 57: M76–M99, 2002. doi: 10.1093/gerona/57.2.m76. [DOI] [PubMed] [Google Scholar]
- 20. Smith JC, Bennett S, Evans LM, Kynaston HG, Parmar M, Mason MD, Cockcroft JR, Scanlon MF, Davies JS. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab 86: 4261–4267, 2001. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 21. O'Connell MD, Roberts SA, Srinivas-Shankar U, Tajar A, Connolly MJ, Adams JE, Oldham JA, Wu FC. Do the effects of testosterone on muscle strength, physical function, body composition, and quality of life persist six months after treatment in intermediate-frail and frail elderly men? J Clin Endocrinol Metab 96: 454–458, 2011. doi: 10.1210/jc.2010-1167. [DOI] [PubMed] [Google Scholar]
- 22. Zirkin BR, Tenover JL. Aging and declining testosterone: past, present, and hopes for the future. J Androl 33: 1111–1118, 2012. doi: 10.2164/jandrol.112.017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhasin S. The dose-dependent effects of testosterone on sexual function and on muscle mass and function. Mayo Clin Proc 75: S70–S75, 2000. doi: 10.1016/S0025-6196(19)30647-0. [DOI] [PubMed] [Google Scholar]
- 24. Barrett-Connor E, Von Mühlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab 84: 573–577, 1999. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- 25. Babcock MC, DuBose LE, Witten TL, Stauffer BL, Hildreth KL, Schwartz RS, Kohrt WM, Moreau KL. Oxidative stress and inflammation are associated with age-related endothelial dysfunction in men with low testosterone. J Clin Endocrinol Metab 107: e500–e514, 2021. doi: 10.1210/clinem/dgab715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, Eto M, Ouchi Y. Low testosterone level is an independent determinant of endothelial dysfunction in men. Hypertens Res 30: 1029–1034, 2007. doi: 10.1291/hypres.30.1029. [DOI] [PubMed] [Google Scholar]
- 27. Empen K, Lorbeer R, Dörr M, Haring R, Nauck M, Gläser S, Krebs A, Reffelmann T, Ewert R, Völzke H, Wallaschofski H, Felix SB. Association of testosterone levels with endothelial function in men: results from a population-based study. Arterioscler Thromb Vasc Biol 32: 481–486, 2012. doi: 10.1161/ATVBAHA.111.232876. [DOI] [PubMed] [Google Scholar]
- 28. Mäkinen JI, Perheentupa A, Irjala K, Pöllänen P, Mäkinen J, Huhtaniemi I, Raitakari OT. Endogenous testosterone and brachial artery endothelial function in middle-aged men with symptoms of late-onset hypogonadism. Aging Male 14: 237–242, 2011. doi: 10.3109/13685538.2011.593655. [DOI] [PubMed] [Google Scholar]
- 29. Vlachopoulos C, Ioakeimidis N, Miner M, Aggelis A, Pietri P, Terentes-Printzios D, Tsekoura D, Stefanadis C. Testosterone deficiency: a determinant of aortic stiffness in men. Atherosclerosis 233: 278–283, 2014. doi: 10.1016/j.atherosclerosis.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 30. Corrigan FE 3rd, Al Mheid I, Eapen DJ, Hayek SS, Sher S, Martin GS, Quyyumi AA. Low testosterone in men predicts impaired arterial elasticity and microvascular function. Int J Cardiol 194: 94–99, 2015. doi: 10.1016/j.ijcard.2015.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ward GR, Abdel-Rahman AA. Orchiectomy or androgen receptor blockade attenuates baroreflex-mediated bradycardia in conscious rats. BMC Pharmacol 6: 2, 2006. doi: 10.1186/1471-2210-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. El-Mas MM, Afify EA, Mohy El-Din MM, Omar AG, Sharabi FM. Testosterone facilitates the baroreceptor control of reflex bradycardia: role of cardiac sympathetic and parasympathetic components. J Cardiovasc Pharmacol 38: 754–763, 2001. doi: 10.1097/00005344-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 33. Ward GR, Abdel-Rahman AA. Effect of testosterone replacement or duration of castration on baroreflex bradycardia in conscious rats. BMC Pharmacol 5: 9, 2005. doi: 10.1186/1471-2210-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Airaksinen KE, Hartikainen JE, Niemela MJ, Huikuri HV, Mussalo HM, Tahvanainen KU. Valsalva manoeuvre in the assessment of baroreflex sensitivity in patients with coronary artery disease. Eur Heart J 14: 1519–1523, 1993. doi: 10.1093/eurheartj/14.11.1519. [DOI] [PubMed] [Google Scholar]
- 35. Kiviniemi AM, Tulppo MP, Hautala AJ, Perkiömäki JS, Ylitalo A, Kesäniemi YA, Ukkola O, Huikuri HV. Prognostic significance of impaired baroreflex sensitivity assessed from Phase IV of the Valsalva maneuver in a population-based sample of middle-aged subjects. Am J Cardiol 114: 571–576, 2014. doi: 10.1016/j.amjcard.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 36. Maggio M, Basaria S, Ceda GP, Ble A, Ling SM, Bandinelli S, Valenti G, Ferrucci L. The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 28: 116–119, 2005. [PubMed] [Google Scholar]
- 37. Mancini A, Leone E, Festa R, Grande G, Silvestrini A, de Marinis L, Pontecorvi A, Maira G, Littarru GP, Meucci E. Effects of testosterone on antioxidant systems in male secondary hypogonadism. J Androl 29: 622–629, 2008. doi: 10.2164/jandrol.107.004838. [DOI] [PubMed] [Google Scholar]
- 38. Mohamad NV, Wong SK, Wan Hasan WN, Jolly JJ, Nur-Farhana MF, Ima-Nirwana S, Chin KY. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 22: 129–140, 2019. doi: 10.1080/13685538.2018.1482487. [DOI] [PubMed] [Google Scholar]
- 39. Takagishi M, Waki H, Bhuiyan ME, Gouraud SS, Kohsaka A, Cui H, Yamazaki T, Paton JF, Maeda M. IL-6 microinjected in the nucleus tractus solitarii attenuates cardiac baroreceptor reflex function in rats. Am J Physiol Regul Integr Comp Physiol 298: R183–R190, 2010. doi: 10.1152/ajpregu.00176.2009. [DOI] [PubMed] [Google Scholar]
- 40. Nightingale AK, Blackman DJ, Field R, Glover NJ, Pegge N, Mumford C, Schmitt M, Ellis GR, Morris-Thurgood JA, Frenneaux MP. Role of nitric oxide and oxidative stress in baroreceptor dysfunction in patients with chronic heart failure. Clin Sci (Lond) 104: 529–535, 2003. doi: 10.1042/CS20020334. [DOI] [PubMed] [Google Scholar]
- 41. Mulhall JP, Trost LW, Brannigan RE, Kurtz EG, Redmon JB, Chiles KA, Lightner DJ, Miner MM, Murad MH, Nelson CJ, Platz EA, Ramanathan LV, Lewis RW. Evaluation and management of testosterone deficiency: AUA guideline. J Urol 200: 423–432, 2018. doi: 10.1016/j.juro.2018.03.115. [DOI] [PubMed] [Google Scholar]
- 42. Mohamed O, Freundlich RE, Dakik HK, Grober ED, Najari B, Lipshultz LI, Khera M. The quantitative ADAM questionnaire: a new tool in quantifying the severity of hypogonadism. Int J Impot Res 22: 20–24, 2010. doi: 10.1038/ijir.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heinemann L, Zimmermann T, Vermeulen A, Thiel C, Hummel W. A new ‘aging males’ symptoms' rating scale. Aging Male 2: 105–114, 1999. doi: 10.3109/13685539909003173. [DOI] [Google Scholar]
- 44. DuBose LE, Voss MW, Weng TB, Kent JD, Dubishar KM, Lane-Cordova A, Sigurdsson G, Schmid P, Barlow PB, Pierce GL. Carotid β-stiffness index is associated with slower processing speed but not working memory or white matter integrity in healthy middle-aged/older adults. J Appl Physiol (1985) 122: 868–876, 2017. doi: 10.1152/japplphysiol.00769.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 46. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972. [PubMed] [Google Scholar]
- 47. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84: 3666–3672, 1999. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 48. Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl 3: S79–S81, 1985. [PubMed] [Google Scholar]
- 49. Babcock MC, Brian MS, Watso JC, Edwards DG, Stocker SD, Wenner MM, Farquhar WB. Alterations in dietary sodium intake affect cardiovagal baroreflex sensitivity. Am J Physiol Regul Integr Comp Physiol 315: R688–R695, 2018. doi: 10.1152/ajpregu.00002.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Malik M. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93: 1043–1065, 1996. [PubMed] [Google Scholar]
- 51. Malliani A, Lombardi F, Pagani M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br Heart J 71: 1–2, 1994. doi: 10.1136/hrt.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lindsey ML, Gray GA, Wood SK, Curran-Everett D. Statistical considerations in reporting cardiovascular research. Am J Physiol Heart Circ Physiol 315: H303–H313, 2018. doi: 10.1152/ajpheart.00309.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Angell-James JE, Lumley JS. The effects of carotid endarterectomy on the mechanical properties of the carotid sinus and carotid sinus nerve activity in atherosclerotic patients. Br J Surg 61: 805–810, 1974. doi: 10.1002/bjs.1800611014. [DOI] [PubMed] [Google Scholar]
- 54. Mathias CJ, da Costa DF, Fosbraey P, Bannister R, Wood SM, Bloom SR, Christensen NJ. Cardiovascular, biochemical and hormonal changes during food-induced hypotension in chronic autonomic failure. J Neurol Sci 94: 255–269, 1989. doi: 10.1016/0022-510x(89)90235-9. [DOI] [PubMed] [Google Scholar]
- 55. Smith GD, Watson LP, Pavitt DV, Mathias CJ. Abnormal cardiovascular and catecholamine responses to supine exercise in human subjects with sympathetic dysfunction. J Physiol 484: 255–265, 1995. doi: 10.1113/jphysiol.1995.sp020663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Parati G, Frattola A, Di Rienzo M, Castiglioni P, Pedotti A, Mancia G. Effects of aging on 24-h dynamic baroreceptor control of heart rate in ambulant subjects. Am J Physiol Heart Circ Physiol 268: H1606–H1612, 1995. doi: 10.1152/ajpheart.1995.268.4.H1606. [DOI] [PubMed] [Google Scholar]
- 57. Tank J, Baevski RM, Fender A, Baevski AR, Graves KF, Ploewka K, Weck M. Reference values of indices of spontaneous baroreceptor reflex sensitivity. Am J Hypertens 13: 268–275, 2000. doi: 10.1016/S0895-7061(99)00172-7. [DOI] [PubMed] [Google Scholar]
- 58. Monahan KD, Dinenno FA, Tanaka H, Clevenger CM, DeSouza CA, Seals DR. Regular aerobic exercise modulates age-associated declines in cardiovagal baroreflex sensitivity in healthy men. J Physiol 529: 263–271, 2000. doi: 10.1111/j.1469-7793.2000.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Laitinen T, Hartikainen J, Vanninen E, Niskanen L, Geelen G, Länsimies E. Age and gender dependency of baroreflex sensitivity in healthy subjects. J Appl Physiol (1985) 84: 576–583, 1998. doi: 10.1152/jappl.1998.84.2.576. [DOI] [PubMed] [Google Scholar]
- 60. Barnes JN, Matzek LJ, Charkoudian N, Joyner MJ, Curry TB, Hart EC. Association of cardiac baroreflex sensitivity with blood pressure transients: influence of sex and menopausal status. Front Physiol 3: 187, 2012. doi: 10.3389/fphys.2012.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Irigoyen MC, Paulini J, Flores LJ, Flues K, Bertagnolli M, Moreira ED, Consolim-Colombo F, Belló-Klein A, De Angelis K. Exercise training improves baroreflex sensitivity associated with oxidative stress reduction in ovariectomized rats. Hypertension 46: 998–1003, 2005. doi: 10.1161/01.HYP.0000176238.90688.6b. [DOI] [PubMed] [Google Scholar]
- 62. Khaw KT, Barrett-Connor E. Blood pressure and endogenous testosterone in men: an inverse relationship. J Hypertens 6: 329–332, 1988. [PubMed] [Google Scholar]
- 63. Yang Q, Li Z, Li W, Lu L, Wu H, Zhuang Y, Wu K, Sui X. Association of total testosterone, free testosterone, bioavailable testosterone, sex hormone–binding globulin, and hypertension. Medicine (Baltimore) 98: e15628, 2019. doi: 10.1097/MD.0000000000015628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hyde Z, Norman PE, Flicker L, Hankey GJ, Almeida OP, McCaul KA, Chubb SA, Yeap BB. Low free testosterone predicts mortality from cardiovascular disease but not other causes: the Health in Men Study. J Clin Endocrinol Metab 97: 179–189, 2012. doi: 10.1210/jc.2011-1617. [DOI] [PubMed] [Google Scholar]
- 65. Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab 97: 2050–2058, 2012. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- 66. Jones PP, Christou DD, Jordan J, Seals DR. Baroreflex buffering is reduced with age in healthy men. Circulation 107: 1770–1774, 2003. doi: 10.1161/01.CIR.0000057811.86187.88. [DOI] [PubMed] [Google Scholar]
- 67. Studinger P, Goldstein R, Taylor JA. Mechanical and neural contributions to hysteresis in the cardiac vagal limb of the arterial baroreflex. J Physiol 583: 1041–1048, 2007. doi: 10.1113/jphysiol.2007.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hunt BE, Fahy L, Farquhar WB, Taylor JA. Quantification of mechanical and neural components of vagal baroreflex in humans. Hypertension 37: 1362–1368, 2001. doi: 10.1161/01.hyp.37.6.1362. [DOI] [PubMed] [Google Scholar]
- 69. Studinger P, Goldstein R, Taylor JA. Age- and fitness-related alterations in vascular sympathetic control. J Physiol 587: 2049–2057, 2009. doi: 10.1113/jphysiol.2009.170134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mengal V, Silva PH, Tiradentes RV, Santuzzi CH, de Almeida SA, Sena GC, Bissoli NS, Abreu GR, Gouvea SA. Aliskiren and l-arginine treatments restore depressed baroreflex sensitivity and decrease oxidative stress in renovascular hypertension rats. Hypertens Res 39: 769–776, 2016. doi: 10.1038/hr.2016.61. [DOI] [PubMed] [Google Scholar]
- 71. Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, Toutouza M, Stefanadis C. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation 112: 2193–2200, 2005. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
- 72. Callera JC, Bonagamba LG, Nosjean A, Laguzzi R, Machado BH. Activation of GABA receptors in the NTS of awake rats reduces the gain of baroreflex bradycardia. Auton Neurosci 84: 58–67, 2000. doi: 10.1016/S1566-0702(00)00184-3. [DOI] [PubMed] [Google Scholar]
- 73. Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front Neuroendocrinol 29: 169–181, 2008. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 97: 2037–2042, 1998. doi: 10.1161/01.CIR.97.20.2037. [DOI] [PubMed] [Google Scholar]
- 75. Emdin M, Gastaldelli A, Muscelli E, Macerata A, Natali A, Camastra S, Ferrannini E. Hyperinsulinemia and autonomic nervous system dysfunction in obesity: effects of weight loss. Circulation 103: 513–519, 2001. doi: 10.1161/01.cir.103.4.513. [DOI] [PubMed] [Google Scholar]
- 76. Gadegbeku CA, Dhandayuthapani A, Sadler ZE, Egan BM. Raising lipids acutely reduces baroreflex sensitivity. Am J Hypertens 15: 479–485, 2002. doi: 10.1016/S0895-7061(02)02275-6. [DOI] [PubMed] [Google Scholar]
- 77. Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 369: 1011–1022, 2013. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tajar A, Huhtaniemi IT, O'Neill TW, Finn JD, Pye SR, Lee DM, Bartfai G, Boonen S, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Vanderschueren D, Wu FC; EMAS Group. Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS). J Clin Endocrinol Metab 97: 1508–1516, 2012. doi: 10.1210/jc.2011-2513. [DOI] [PubMed] [Google Scholar]
- 79. Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc 90: 224–251, 2015. doi: 10.1016/j.mayocp.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 80. Moskovic DJ, Araujo AB, Lipshultz LI, Khera M. The 20-year public health impact and direct cost of testosterone deficiency in U.S. men. J Sex Med 10: 562–569, 2013. doi: 10.1111/j.1743-6109.2012.02944.x. [DOI] [PubMed] [Google Scholar]