Abstract
Due to the increasing population of individualswith cardiovascular diseases and related comorbidities, there is an increasing need for development of synergistic therapeutics. Monocytes are implicated in a broad spectrum of diseases and can serve as a focal point for therapeutic targeting. This review discusses the role of monocytes in cardiovascular diseases and highlights trends in monocyte targets nanoparticles in three cardiovascular-related diseases: Diabetes, Atherosclerosis, and HIV. Finally, the review offers perspectives on how to develop nanoparticle monocyte targeting strategies that can be beneficial for treating co-morbidities.
1. Introduction
There are many reasons to consider monocytes as convergences point for therapeutic intervention. First, monocytes are a circulatory, immune cell population, and their circulatory nature makes them easier targets for therapeutic modulation compared to targeting tissue-resident immune cells. Like other innate immune cells, monocytes have surface pattern recognition receptors (PRRs) that can bind to glycoproteins, reactive oxygen species, chemokine receptors, adhesion molecules, and immunoglobulins [1]. Using these motifs, the surface of nanoparticles can be modified to target specific subsets of monocytes.
Monocytes and macrophages are members of the reticuloendothelial system (RES), a multi-cellular system designed to clear particles within the circulation and tissues. Many nanoparticle strategies attempt to evade monocytes [2,3]. However, with this barrier out of the way, monocyte delivery becomes even more advantageous. Nanoparticles (NPs) have tunable physical and chemical characteristics, making them flexible to design monocyte-targeted drug delivery systems. Conventional NPs range from 1 to 300 nm in size [4] and can affect tissue permeability and cellular endocytosis mechanisms. NPs have an increased ability to travel through the endothelium in inflammatory sites, epithelium, and penetrate microcapillaries and can be functionalized to target specific tissues or cell populations. The physical and chemical characteristics of NPs also affect the pharmacokinetics and pharmacodynamics of drug delivery by enhancing biodistribution, bioavailability, or increasing circulation times [5]. Changing the nanoparticle composition creates opportunities for controlled drug release and NP surface functionalization. In particular, polymeric formulations have been used for their controlled delivery because the polymer degradation can be tightly controlled and thus linked to drug release [6–8].
Furthermore, NPs facilitate combinational therapy, which is beneficial for synchronizing the biodistribution of drugs designed to have a synergistic effect. By modulating NPs composition, it is possible to combine hydrophobic and hydrophilic drugs [9] or therapeutics of different classes such as oligonucleotides, small molecules, and proteins [10,11]. NP encapsulation allows the administration of higher therapeutic doses without increasing systemic toxicity [12]. Drug insolubility is a significant pharmaceutical barrier that nanoparticles addresses. In 2010, an estimated 40% of drugs were insoluble according to the biopharmaceutical classification system (BCS) [13]. Through encapsulation into NPs, previously insoluble, hydrophobic drugs can overcome these in vivo delivery challenges [14]. Genexol, an FDA- approved polymer micelle formulation of paclitaxel, is an excellent example of a successful nanoparticle formulation [15,16].
2. Monocytes and macrophages as a convergence point for nanoparticle-mediated therapeutics
Monocytes comprise 2%-10% of the total white blood cell population and yet are one of the most potent innate immune cells in orchestrating systemic inflammatory responses. Monocytes originate in the bone marrow and circulate within the bloodstream. Upon activation, monocytes can migrate into inflamed tissue and do so as early as two hours after receiving an inflammatory signal [17]. The nearly arrived monocytes then perform antigen presentation, phagocytosis, and cytokine production. Monocytes are myeloid lineage cells closely related to dendritic cells and macrophages and can differentiate into either subtype, depending on the external environmental stimuli. In this manner, monocytes can shape the quality and magnitude of innate and adaptive immune responses.
Monocytes participate in inflammatory processes from wound-healing/regeneration to viral or bacterial clearance. To perform these heterogeneous tasks, monocytes modulate their phenotype through a process called polarization. Polarization can be measured by aggregate changes at the genetic, proteomic, and/or functional level in response to engagement with extracellular stimuli. Monocytes can simultaneously respond to multiple stimuli, resulting in a highly heterogeneous, often contradictory polarization response. While this plasticity is better described as a continuous spectrum, it is useful to superficially categorize them within three discrete, anchoring subsets, classical or pro-inflammatory, intermediate, and non-classical or anti-inflammatory [18]. In humans, the subsets are identified by LPS coreceptor CD14 and FCγIII receptor CD16 membrane surface expression. CD14 is expressed on all monocytes but is highest in the pro-inflammatory (CD14highCD16low) subset responsible for bacterial recognition and induction of pro-inflammatory cytokines [3,19]. Anti-inflammatory monocytes are characterized by low CD14 but high expression of CD16, an immunoglobulin involved in facilitating phagocytosis, signal transduction, and degranulation [20]. Intermediate monocytes (CD14intCD16int) express a relatively equal amount of CD14 and CD16, are high sources of pro-inflammatory cytokines, and are involved in MHCII antigen presentation [21,22]. In mice, monocytes are distinguished by the differential expression of Ly6C [23]. Ly6Chigh expression denotes pro-inflammatory, classical activation while low expression indicates an anti-inflammatory, non-classical phenotype [24].
Monocytes have access to nearly every organ in the human body and chemokine receptors govern their trafficking The major monocyte/macrophage trafficking players are CCR2, CCR5, and CX3CR1. CCR2 is a monocyte receptor for CCL2 ligand whose high expression is associated with pro-inflammatory monocytes [25–27]. CCR2 is required for Ly6Chigh monocyte emigration from the bone marrow and entry into distal tissues [28,29].
Monocyte subset dynamics are implicated in the progression and resolution of many diseases. These phenotypes are relevant because they appear in different quantities and at different times during disease pathogenesis. CCR5 is primarily implicated in lymphocyte migration, but it is upregulated on monocytes under inflammatory conditions like sepsis and viral/bacterial infection [30,31]. CX3CR1 is present on all blood monocytes but has differential expression depending on steady-state and inflammatory conditions [32,33]. CX3CR1hi expression, in contrast, is associated with Ly6Clo non-classical monocytes [27,34]. CX3CR1hi Ly6Clo(CD16+; human) have a wound-healing phenotype and are particularly valuable following injury [35]. Ly6Chigh monocytes migrate to the injury site after a skeletal muscle injury and participate in pathogen clearance and antigen presentation [36,37]. This is followed several days later by LyClo monocytes which facilitate angiogenesis and recruitment of other anti-inflammatory cell phenotypes [38]. Because monocytes serve supporting roles in multiple inflammatory sites, there is potential to approach monocytes as a single target.
In a scientific era where we can design nanoparticle strategies to modulate immune function, we should also consider that a monocyte’s a priori polarization may impact the capability to uptake nanoparticles. when better understood and incorporated into the immunomodulating design, this reciprocal relationship can improve short-term therapeutic efficacy and long-term outcomes. This review explores the current state of monocyte targeted drug delivery with a focus on polarization and/or differentiation modulating nanotechnology. We will focus on monocytes; however, since monocyte function is interlinked with that of macrophages and dendritic cells, the latter populations will also be discussed. We will highlight the role of monocytes in the context of individual diseases and discuss how therapeutics impact them. Finally, we offer a perspective on how nanoparticle targeting can be beneficial as a mechanism for comorbidity targeting.
3. Nanoparticle strategies for treating chronic inflammatory diseases at high risk of developing cardiovascular diseases.
Cardiovascular Disease (CVD) is a leading cause of death in developed countries, accounting for nearly 50% of mortalities [53,54]. CVD is not one ailment but rather a spectrum of diseases affecting the heart structure and function as well as the vasculature systems. CVD can manifest in nearly every tissue; stroke affects blood vessels in the brain, retinopathy, blood vessels in the eye, nephropathy, damage to blood vessels within the kidney. Independent of lifestyle factors such as diet, several chronic inflammatory diseases are risk factors for CVD development [53–55]. An example is Type 1 Diabetes, where individuals have an increased risk of developing CVD. Moreover, CVD is the predominant cause of mortality for individuals with diabetes [56]. There are similar trends in Atherosclerosis [58], HIV [59–61], sickle cell, and SARS-CoV-2 [55,62]. From a cellular standpoint, the cardiovascular system is heavily affected by immune cells such as monocytes and macrophages, endothelial cells, and hematopoietic systems. Monocyte polarization differentiation affect the progression of CVDs [63,64]. Developing nanoparticles that interact with monocytes in advantageous ways could be an avenue to improved diseased outcomes. In the diseases outlined in this paper there are a variety of monocyte receptors that are somehow impacted by disease in monocytes and offer potential therapeutic targets for a range of nanoparticle strategies (Fig. 1).
Fig. 1. Diagram of using nanoparticles to therapeutically targets monocytes and macrophages in common CVDs.
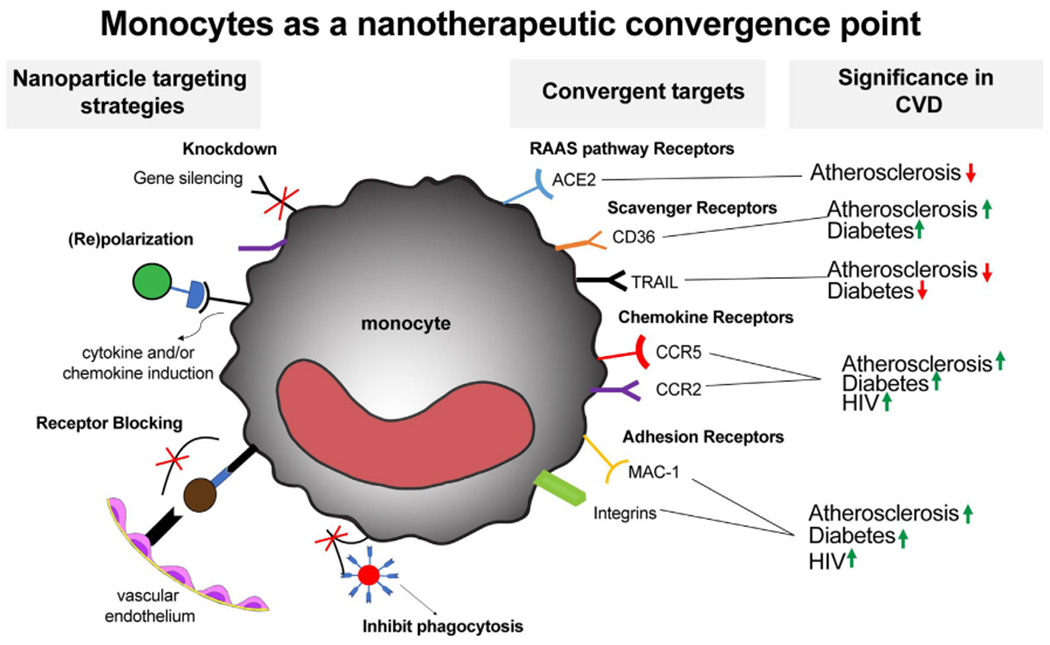
Nanoparticle targeting strategies highlight several themes in which nanoparticles can be used to change monocyte and macrophage function. (Knockdown) siRNA Delivery can used to decrease knockdown functionally relevant proteins. Re(polarization) Nanoparticles be used to change and/or maintain a given phenotype via cytokines or chemokines delivery. (Receptor Blocking) This tactic can be used to block receptor binding thereby inhibiting monocyte adhesion or transmigration into inflamed tissue. In addition, nanoparticles can be used to modulate phagocytosis to prevent bacterial or viral infection. These delivery strategies can be applied to various surface receptor classes on monocytes and macrophages. These receptors are often upregulated in CVDs and as such demonstrate the potential for these cells as convergent therapeutic targets. The arrows indicate whether the receptor is upregulated or downregulated in the disease.
For the purposes of this review, we conducted a systematic PubMed literature search for monocyte or macrophage nanoparticle mediated treatment in select CVDs in comparison to non-nanoformulated therapies. We included papers that either specifically targeted monocytes and/or macrophages as well as those that provided adequate characterization of their behavior. Roughly 4% of the nanoparticle drug delivery related papers targeted monocytes and/or macrophages. (Fig. 2A). This is a reasonable finding given that most CVD related therapies do not use nanoparticle formations but rather are already FDA-approved, small molecule or protein therapeutics (i.e., ACE inhibitors, ARBs, anti-retroviral drugs, insulin). For example, less than 3% of the papers found were actively targeting monocyte or macrophage involvement in Diabetes (Fig. 2B). Most Diabetes-related nanoparticle drug delivery studies were related to insulin delivery. However, a temporal assessment of literature trends show that nanoparticle drug delivery approaches are increasing in popularity, likely because of their ability to address challenges of bioavailability, toxicity, sustained release, and targeted delivery. Because there are numerous individual diseases within the CVD spectrum, we highlighted three CVDs that have a unique pathogenesis but have can some overlap in comorbidity occurrence. Our PubMed literature search revealed that of the cardiovascular diseases we investigated, atherosclerosis had the largest number of papers with drugs modulating monocyte or macrophage function, although only around 4% used nanoparticles as drug carriers (Fig. 2C). Surprising, HIV showed the highest percentage of monocyte or macrophage targeted articles (Fig. 2D). This could be indicative of the major role that monocytes and macrophages play in disease progression. Although this disease prominently associated with CD4 + T-cells, monocytes and macrophages a key viral reservoir and are a growing therapeutic target in the field.
Fig. 2. Literature analysis of monocyte and/or macrophage targeted nanoparticles in CVDs.
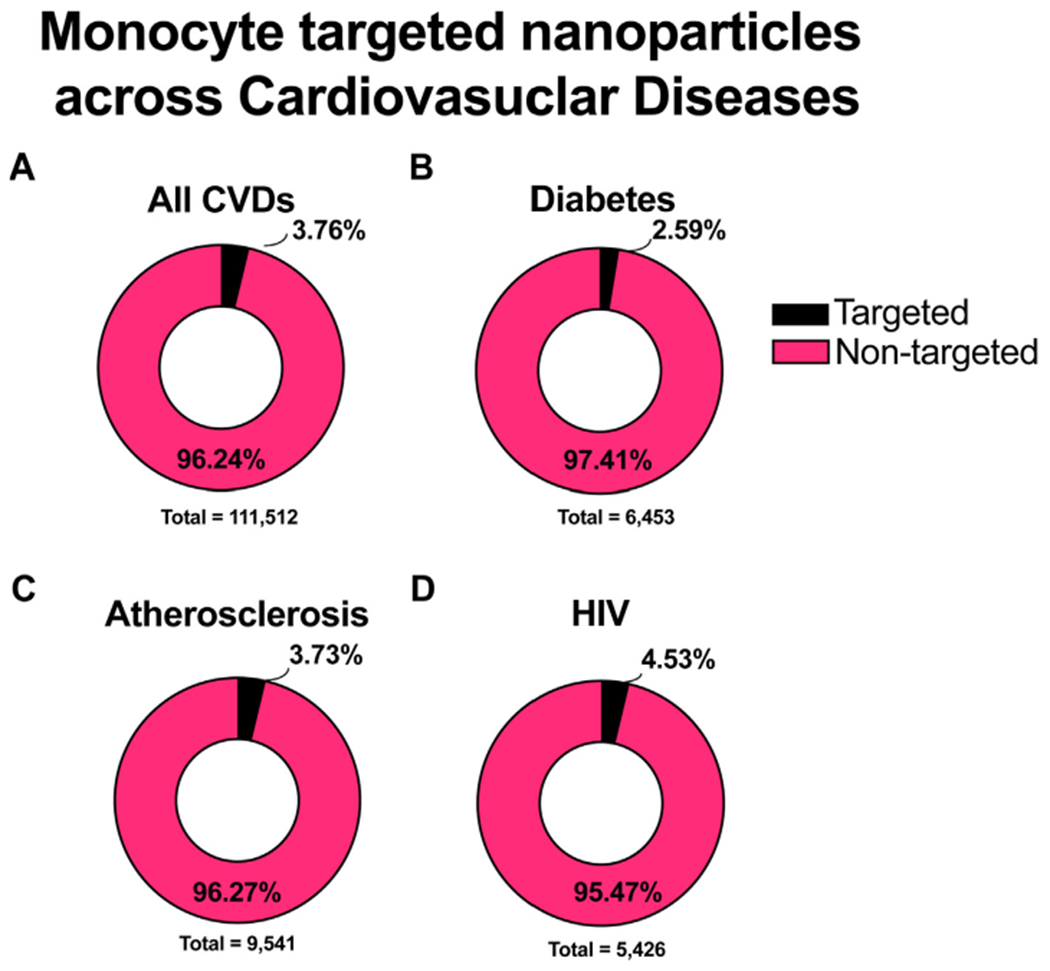
Using Pubmed advanced search methods, papers were compiled, screened, and categorized to quantify the percentage of nanoparticle targeting monocyte papers (Targeted) compared to papers involving any nanoparticle treatment (Non-targeted). The literature was searched with respect to the following categories A) All CVDs, B) Atherosclerosis, C) HIV, and D) Diabetes. Review papers were excluded from this analysis.
Moreover, we used the PubMed literature search to assess the nanoparticle carrier types variation. We found that across All CVDs (Fig. 3A) there was a relatively equal distribution between the three large groups (i.e. polymers, lipids, and metal/semiconductor). However, amongst Atherosclerosis, Diabetes, and HIV the distributions were more skewed, suggesting an interesting design preference phenomena. This section of the review will highlight the role of monocytes in specific CVDs and related monocyte-targeted nanoparticle delivery strategies.
Fig. 3. Literature analysis of monocyte and/or macrophage targeted nanoparticles in CVDs.
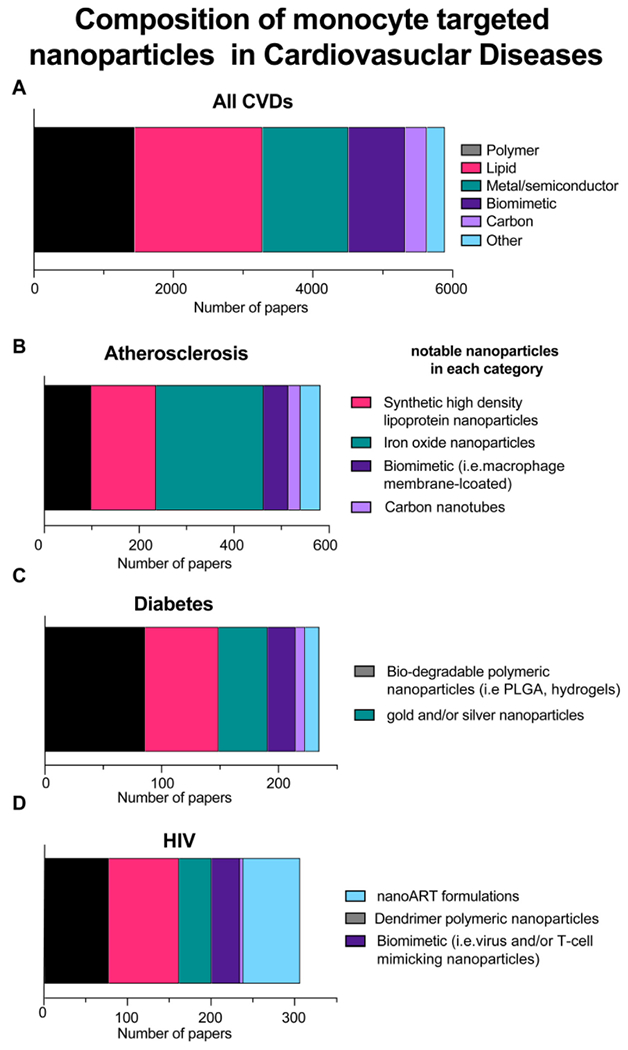
Using the search parameters from the monocyte targeting group shown in (Fig. 2), the studies were further categorized by nanoparticle composition types. The literature was searched with respect to the following categories A) All CVDs, B) Atherosclerosis, C) Diabetes, and D) HIV. Notable nanoparticle delivery schemes were listed for each disease scheme to denote unique trends found in the literature. Review papers were excluded from this analysis.
3.1. Nanoparticles to treat Atherosclerosis
3.1.1. Role of monocytes in Atherosclerosis
Atherosclerosis is a chronic CVD characterized by the inflammation of the arterial wall, which can lead to many complications and other heart diseases. The risk of Atherosclerosis development is increased due to various factors, including high cholesterol, smoking, and high blood pressure. Atherosclerosis typically affects older adults and currently falls within the leading cause of death, heart disease [39]. Current treatments only work to stop or slow the progression of Atherosclerosis but do not effectively reverse the progression of the atherosclerotic lesions [40].
Atherosclerotic plaques are initiated by the expansion of lipoproteins in the subendothelial space, which prompts an immune response [41,42]. As the subendothelial accumulation continues, the lesion blocks the arterial pathways disrupting normal blood flow to the brain, kidneys, heart, and the lower-body extremities. Monocytes are recruited to atherosclerotic lesions early in the plaque formation process. Animal models suggest that Ly6Chi pro-inflammatory monocytes enter plaques at higher numbers and induce pro-inflammatory cytokine-mediated activation of endothelial cells [34,43,44]. Once inside the lesion, monocytes can differentiate into macrophages, further exacerbating the pro-inflammatory imbalance. Atherosclerotic plaques contain a high density of foam macrophages, macrophages that have endocytosed an uncontrolled amount of lipoproteins, and cholesterol ester [45,46].
3.1.2. Nanoparticle strategies
Nanoparticle encapsulation with therapeutics that can repolarize monocytes worked well in Atherosclerosis models. Statins are potent anti-inflammatory agents that benefit from nanoparticle encapsulation due to their low system bioavailability. Statin-loaded nanoparticles can reduce macrophage plaque load, reduced expression of pro-inflammatory and monocyte recruitment genes [47–49]. Interestingly, some studies show statin-loaded high-density lipoprotein nanoparticles can preferentially accumulate in monocyte-derived macrophages rather than monocytes atherosclerotic plaques [48]. This finding suggests that nanoparticle targeting can select for even closely related cell types Pioglitazone-incorporated PLGA nanoparticles showed similar uptake in atherosclerotic resident monocytes and/or macrophages but had the added benefit of modulating monocytes to an Ly6Clo, anti-inflammatory phenotype [50]. The shift to an anti-inflammatory state was correlated with decreased plaque ruptures which clinically is associated with 60% of acute myocardial infarctions [50,51]. Ca2+/calmodulin-dependent protein kinase γ (CaMKIIγ) siRNA nanoparticles predictably result in a decrease in CaMKIIγ but also increase MerTK expression in macrophages [52], effectively polarizing atherosclerotic macrophages into an anti-inflammatory phenotype [53]. While CaMKIIγ is not a canonical anti-inflammatory agent, it altered macrophage function in a desired anti-inflammatory manner. Employing such strategies could be meaningful for identifying novel drug targets.
Around a third of the monocyte or macrophage targeted nanoparticle studies in atherosclerosis used lipid-based nanoparticles (Fig. 3B). This feature could also be attributed to the role of lipid uptake in pro-atherogenic monocytes and macrophages. Oxidized low-density lipoprotein (oxLDL) receptors have been used to target intimal macrophages, the vascular wall resident macrophages in atherosclerotic plaques. Intimal macrophages have increased CD36 expression, a scavenger receptor associated with oxLDL uptake and is correlated with progressive Atherosclerosis [54]. Nanoparticles formulated with phosphatidylcholines and epigallocatechin gallate, L-Enano, show increased binding affinity to mouse macrophages in vitro [55]. In vivo L-Enano administration resulted in smaller atherosclerotic lesion surface areas compared to control. During in vitro studies, L-Enano decreased pro-inflammatory cytokine secretion in peritoneal macrophages. The difference was not seen in vivo; however, the authors cite this may be due to LDLr −/− mouse model associated dermatitis that may mask the L-Enano therapeutic effect [56]. In other studies, the phosphatidylcholine-coated liposome-like nanoparticles demonstrated preferential uptake in intimal macrophages within aortic lesions, highlighting a theranostic targeting technique [57,58].
Another sizable portion of the surveyed literature involved magnetic nanoparticles (MNPs) (Fig. 3B). MNPs have been prominent in Atherosclerosis treatments because of their uptake in monocytes and macrophages as well as for their MRI diagnostic imaging potential. Monocyte chemoattractant protein-1 (MCP-1) conjugated to iron oxide MNPs helped identify regions of high monocyte accumulation in during different stages of Atherosclerosis pathogenesis[59,60]. MNPs combined with a therapeutic agent have also been used. Superparamagnetic iron oxide nanoparticles (SPION) conjugated with dexamethasone, a corticosteroid (SPION-DEXA), achieved an 8.5-fold increase in anti-inflammatory scavenger receptor CD163 expression when incubated with peripheral blood mononuclear cells (PMBCs) in comparison to a 7.5-fold increase when using free DEXA [61,62]. However, reporting of SPION effects on macrophages polarization vary upon dose, disease model and disease stage, yielding inconsistent results [63]. Contrary to their expectations, intra-arterial SPION-DEXA administration increased macrophage presence within early-stage plaque formation and ultimately manifested a pro-inflammatory effect [61,64]. A separate study using pegylated-DNA coated SPIONs exhibited preferential uptake in M2 (F4/80+/CD301+) macrophages though whether there was an increase in the total number of macrophages was not investigated [65].
Inhibition strategies can have anti-atherogenic effect by blocking monocyte interaction with downstream partners. CCR2 is perhaps the most used targets because of its role in monocyte recruit to inflammation sites. Calin et al. used CCR2-antagonist PEGylated liposomes to reduce endothelial cell expressions of vascular cell adhesion molecule-1 (VCAM −1) [66]. This decreased monocyte adhesion and transmigration by approximately 28% and 53%, respectively, in their Boyden chamber atherosclerotic model. CCR2-shRNA nanoparticles are expansive in the literature, though the predominant formulations are variations of polymeric [67,68], lipid [69] structures. Other blocking strategies target lipoprotein uptake or other relevant pro-atherogenic pathways. Self-assembled mucic acid, lauryl chloride, and PEG containing nanoparticles were used to block oxLDL uptake, decrease oxLDL induced TNF-α expression, and reduced foam cell formation [70,71].
3.2. NPs in Diabetes
3.2.1. Role of monocytes in Diabetes
Diabetes Mellitus (DM) is a family of metabolic diseases characterized by high blood sugar levels caused by deficiencies in insulin production or insulin resistance [72]. The two most common classifications of Diabetes are Type 1 and Type 2, although others, gestational Diabetes, fit this spectrum. Type 1 Diabetes (T1D) is linked to the autoimmune targeted destruction of beta cells of the pancreas. In contrast, Type 2 Diabetes (T2D) is attributed to insulin resistance and strongly correlates with obesity and lifestyle. DM contributes directly to increased risk of cardiovascular diseases, including hypertension, cardiovascular autonomic neuropathy, nephropathy, cardiomyopathy, and myocardial infarction [73].
The phenotype of monocytes is altered in the diabetic environment and play a considerable role in the pathogenesis of DM and the subsequent cardiovascular disease [74–76]. The two main types of Diabetes have different mechanisms in which the disease progresses. T1D is an autoimmune disease in which the immune system attacks insulin-producing cells. T2D is typically characterized by insulin resistance or dysregulated insulin production. However, both presentations exhibit increased expression of adhesion molecules and pro-inflammatory cytokines. Moreover, when stimulated, monocytes exhibit a higher pro-inflammatory response in comparison to non-diabetic donor-derived monocytes. Monocytes from individuals with T2D released higher pro-inflammatory cytokines (TNFα, IL6, IL1, IL8) and adhesion molecules when compared to controls and T1D individuals [77]. Blood samples taken from DM patients both T1D and T2D revealed higher monocyte counts and higher pro-inflammatory CD14+CD16− expression in comparison to non-diabetic patients [78].
3.2.2. Nanoparticle strategies
Within the category of monocyte or macrophage targeting studies in Diabetes, there was a common use of relatively even distribution of nanoparticle types employed (Fig. 3C). Nanoparticle targeting across all subsets were used to suppress pro-inflammatory monocytes and macrophages thereby decreasing insulin resistance and beta-cell destruction. Yong et al. developed a TNF-α converting RNA silencing enzyme nanocomplex to target adipose tissue macrophages to decrease TNF-α secretion [79]. The complex saw 90% cellular uptake reduced soluble TNF-α by 50% in LPS-stimulated macrophages. In vivo models in mice saw lower blood glucose levels and higher glucose sensitivity. Similar success in decreasing T2DM insulin resistance and overall pro-inflammatory cytokine expression, was observed when delivering with curcumin-loaded liposomes (curcusomes) [80]. Some of this effect can be contributed to the macrophage targeted effects. Curcumin encapsulated nanoparticles have been shown to produce anti-inflammatory responses in macrophages [81–83]. The direct connection was made in a study by Yekullo et al., which used a leptin deficient mouse model of insulin resistance to demonstrate curcusomes ability to as blocked in vivo LPS-induced IL6 production in macrophages [82]. The same study also showed that curcosomes inhibited an oxidative burst in in vitro peritoneal macrophages as well as dendritic cells.
However, there was an interesting preference for using biodegradable polymers particularly in the context of modulate monocyte behavior in Diabetic, wound healing environments. Polymers like hydrogels are frequently used in wound applications because of their ability to maintain shape and capability for controlled drug release. In the studies surveyed, drug loaded hydrogels were also used to promote anti-inflammatory polarization and angiogenesis [84,85]. One such example is a carboxymethyl cellulose, carbomer, and P. americana composite hydrogel that induced anti-inflammatory polarization and promoted wound closure [86].
Moreover, in contrast to the Atherosclerosis studies where metal nanoparticles were strongly correlated with diagnostic imaging, metal and/or semiconductor nanoparticles found in the Diabetic studies were employed to repolarize monocytes and macrophages [87,88]. In one study, mannose-modified, silicon oxide nanoparticles were used to differentiate macrophages into an anti-inflammatory M2 phenotype to promote angiogenesis in a diabetic mice model skin injury[89].
As mentioned in the first section, monocytes and macrophages can be convergent targets for nanoparticle therapies because of their complimentary roles in CVD disease progression. A promising area is the development of TNF related apoptosis inducing ligand (TRAIL) nanoparticles for T1D and T2D and for Diabetes induced Atherosclerosis. TRAIL is naturally found on circulating monocytes and possess anti-atherogenic properties [90–92]. In addition, loss of TRAIL in an ApoE−/− mouse models led to increased diabetic neuropathy evidenced by increase pro-inflammatory gene expression and macrophage infiltration [93]. Nanoparticle strategies that increased TRAIL surface expression[94,95] could be a meaningful strategy for treating diabetes and diabetic induced complications like Atherosclerosis.
3.3. HIV
3.3.1. Role of monocytes in HIV
HIV Although Human Immunodeficiency Virus Type 1 (HIV) is classically known for its immunosuppression caused by CD4 + T cell depletion, monocytes and macrophages play a huge role in its pathogenesis. Macrophages are infected in the initial stages of HIV infection and persist as viral reservoirs [96]. Data on circulating monocytes is currently under debate because of HIV DNA measurement challenges [97]. However, mounting evidence suggests that monocytes can ultimately become reservoirs that can disseminate the virus to distal organs such as the central nervous system [98,99]. CD14++CD16+ (intermediate) and CD14+CD16++ (non-classical) monocytes subsets are more permissive to HIV-1 infection, a feature attributable to their higher expression levels of CCR5 [100]. CCR5 facilitates viral entry, and higher levels of CCR5 are correlated with higher infection rates in monocytes [101]. CXCR4 is also an effective coreceptor for HIV viral entry in normal and CCR5null monocytes and monocyte-derived macrophages [102].
3.3.2. Nanoparticle strategies to treat HIV
Nano-formulated Antiretroviral treatments (NanoART) comprised the most subpopulation of HIV monocyte/macrophage targeting studies found in our review (Fig. 3D). NanoART has enhanced tissue biodistribution and targeting abilities leading to better outcomes [103,104]. Some ARTs have a short biological half-life and poor pharmacokinetic profiles. Liposomes enhanced transdermal delivery of indinavir and mPEG-PCL encapsulated Indinavir oral delivery both elicited a sustained release profile [105–107]. These advancements were further tested in different in nanoparticle composition including dendrimers, micelles, although the solid drug conjugate was the predominant nanotechnology employed (Fig. 3D).
NanoART formulations have not only helped increase the antiretroviral global bioavailability, but they have enhanced the ability to target reservoir cells like macrophages and monocytes. Dendrimer formulations augmented the uptake of efavirenz by monocytes and micelle formulations increased drug aqueous solubility and bioavailability [108,109]. Monocyte or macrophage targeted nanoparticle therapies have shown increased uptake and viral suppression in monocyte and monocyte-derived macrophages [110,111]. A poly(ethylene oxide)–modified Poly (epsilon-caprolactone) nanoparticle was used to encapsulate the protease inhibitor saquinavir, increasing intracellular concentration in monocytes from 0.54 nM/mg to 4 nM/mg compared to the free drug analog [112]. Nanoparticle encapsulation can also increase ART bioavailability in monocytes/macrophages by bypassing drug transporters and metabolic enzymes [113]. Such is the case for PLGA-based elvitegravir which demonstrated doubled uptake in HIV-infected macrophages, resulting in a 24% decrease in viral replication from day 2 to day six compared to control macrophages [110]. Moreover, nanoparticle complexing enhanced macrophage cellular uptake and the growth inhibition efficiency of gallium during HIV, mycobacterium tuberculosis, or M. smegmatis co-infection [114,115]. These studies indicate the benefit of producing nano-formulations of already successful drugs.
A small, but intriguing subset of papers employed biomimetic nanoparticles, delivery systems that use cell membranes, cell-derived products like exosomes or synthesized biological membranes, as their drug delivery tool (Fig. 3D). One group developed CD4 + T cell membrane-coated nanoparticles to selectively reduced viral replication and induce death via autophagy in HIV-1-infected CD4 + T cells and macrophages [116,117]. Notably, the core of these nanoparticles is PLGA polymers, but the targeting efficacy is highly related to the biomimetic membranes. Similarly, another paper developed immunoliposomes that facilitated macrophage phagocytosis of HIV-1 virus-like particles [118]. By mimicking immune cell and virus membrane interactions, the immunoliposomes elicited phagocytosis in a manner that triggered intracellular trafficking to the cellular phago-lysosome. All the aforementioned strategies demonstrate the power of synergizing nanoparticle design with monocyte and macrophage function.
4. Conclusions and outlook
Monocytes have a significant role cardiovascular disease progression, either by their direct contribution or indirect, accrued side effects. Because of this, nanotechnology can be used to modulate monocyte function, leading to enhanced therapeutic efficacy. Our literature search demonstrated that monocyte or macrophage targeted nanoparticles represent a minority of the total nanoparticle related papers in the CVD arena. However, this is likely to increase over time as the field matures and more nanoparticle versions of existing therapies are developed.
Given the rise in individuals living with CVD comorbidities [106,119], there will be a need to develop therapeutics that can synergistically meet those needs. Monocytes should be considered as a convergent nanoparticle target. Nanoparticles present an opportunity to combine multiple therapies and facilitate cellular or tissue-specific therapeutic targeting. A great example is Metformin, an FDA-approved drug that can treat T2D. Metformin is delivered orally and has little toxicity; however, it is susceptible to cellular efflux through organic cation transporters [120]. Metformin reduces pro-inflammatory cytokine production, foam cell formation, and alter responses to oxidative stress and migration [121,122]. Similarly, Metformin promoted an anti-atherogenic profile in macrophages in an in vivo Normoglycaemic Ldlr−/− hyperlipidaemic mice model [123]. This drug is typically delivered as a small molecule; however, nanoparticle encapsulation could help increase its bioavailability as well as targeting to desired monocyte/macrophage populations.
The rise in comorbidities presents another therapeutic challenge. The existence of pre-existing chronic inflammatory conditions may affect future immune responses. This became evident during the COVID19 pandemic when it was observed that individuals with Diabetes, respiratory illness, and hypertension had worse post-infection outcomes [124]. Individuals with CVD comorbidities are likely to have more opportunistic bacterial and viral infections, often with the monocyte as a vital feature in its progression [29,124,125]. By limiting systemic, off target effects, nanoparticle technology may become beneficial in reducing comorbidity development.
There are several strategic themes in the monocyte drug delivery systems mentioned. Passive targeting (using the cells as drug delivery carriers) worked well for penetrating difficult-to-reach tissues. More direct strategies include depletion, receptor inhibition, thereby preventing monocytes functions like adhesion and phagocytosis. However, true active targeting has been achieved with modulating monocyte and macrophage function. This process will likely become more sophisticated with the improvements in genomic and proteomic disease-related monocyte/macrophage data. In addition, they use of literature meta-analysis will facilitate deeper understanding of emerging drug delivery trends. As we further decipher the role of monocyte heterogeneity, it becomes easier to identify novel targets. Combined with improved nanoparticle formulations, the combinatorial space for monocyte drug targeting is endless.
Acknowledgements
The authors would like to thank the Editors for their support. In addition, we send a special thanks to Colette Bilynsky, Hannah Yankello, and Natasha Vinod for their support with technical editing, formatting, and assistance with graphics.
Funding Sources
Research reported in this publication was supported by National Institute of General Medical Sciences of the National Institutes of Health under award numbers 1 R35 GM142957-01, 5T32GM133353-02, and the GEM Fellowship.
Abbreviations:
- ARV
antiretroviral
- ART
antiretroviral treatment
- BCS
biopharmaceutical classification system
- CaMKIIγ
Ca2+/calmodulin-dependent protein kinase γ
- CVD
cardiovascular disease
- CCL
C-C motif chemokine ligand
- CCR
C-C motif chemokine receptor
- CD
cluster of differentiation
- COVID19
Coronavirus Disease 2019
- CX3CR1
C-X3-C motif chemokine receptor 1
- DNA
deoxyribonucleic acid
- DEXA
dexamethasone
- DM
Diabetes Mellitus
- FCγIII
Fc γ Receptor III
- FDA
Food and Drug Administration
- GP
glycoprotein
- HIV
human immunodeficiency virus
- ICAM-1
intercellular adhesion molecule 1
- IL
Interleukin
- LPS
Lipopolysaccharide
- LDLr
low-density lipoprotein receptor
- Ly6C
lymphocyte antigen 6 complex
- Mac-1
macrophage-1 antigen
- MNP
magnetic nanoparticle
- MHC
Major Histocompatibility Complex
- MerTK
Mer Proto-Oncogene, Tyrosine Kinase
- mPEG-PCL
Methoxypoly(ethylene glycol) Poly(caprolactone)
- MCP-1
monocyte chemoattractant protein-1
- NanoART
nano-formulated antiretroviral treatment
- NP
nanoparticle
- NF-kB
Nuclear Factor κ B
- NRTI
nucleoside reverse transcriptase inhibitors
- oxLDL
oxidized low-density lipoprotein
- PRR
pattern recognition receptors
- PLHIV
people living with HIV
- PEG
poly(ethylene glycol)
- PLGA
poly(lactic-co-glycolic acid)
- Akt
protein kinase B
- RES
reticuloendothelial system
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- shRNA
short hairpin ribonucleic acid
- siRNA
short interfering ribonucleic acid
- SPION
Superparamagnetic iron oxide nanoparticles
- TRAIL
TNF related apoptosis inducing ligand
- TNF-α
tumor necrosis factor α
- T1DM
Type 1 Diabetes Mellitus
- T2DM
Type 2 Diabetes Mellitus
- VCAM-1
vascular cell adhesion molecule-1
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Taylor PR, Martinez-Pomares L, Stacey M, Lin H-H, Brown GD, Gordon S, Macrophage receptors and immune recognition, Annu. Rev. Immunol 23 (1) (2005) 901–944, 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- [2].Tang Y, Wang X, Li J, Nie Y.u., Liao G, Yu Y, Li C, Overcoming the Reticuloendothelial System Barrier to Drug Delivery with a “Don’t-Eat-Us” Strategy, ACS Nano 13 (11) (2019) 13015–13026, 10.1021/acsnano.9b05679. [DOI] [PubMed] [Google Scholar]
- [3].Nie S, Understanding and overcoming major barriers in cancer nanomedicine, Nanomedicine (Lond) 5 (4) (2010) 523–528, 10.2217/nnm.10.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hoshyar N, Gray S, Han H, Bao G, The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction, Nanomedicine (Lond). 11 (6) (2016) 673–692, 10.2217/nnm.16.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Petschauer JS, Madden AJ, Kirschbrown WP, Song G, Zamboni WC, The effects of nanoparticle drug loading on the pharmacokinetics of anticancer agents, Nanomedicine (Lond). 10 (3) (2015) 447–463, 10.2217/nnm.14.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jia L, Wang R, Fan Y, Encapsulation and release of drug nanoparticles in functional polymeric vesicles, Soft Matter 16 (12) (2020) 3088–3095, 10.1039/D0SM00069H. [DOI] [PubMed] [Google Scholar]
- [7].He Z, Wan X, Schulz A, Bludau H, Dobrovolskaia MA, Stern ST, Montgomery SA, Yuan H, Li Z, Alakhova D, Sokolsky M, Darr DB, Perou CM, Jordan R, Luxenhofer R, Kabanov AV, A high capacity polymeric micelle of paclitaxel: Implication of high dose drug therapy to safety and in vivo anti-cancer activity, Biomaterials 101 (2016) 296–309, 10.1016/j.biomaterials.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vinod N, Hwang D, Azam SH, Swearingen AEDV, Wayne E, Fussell SC, Sokolsky-Papkov M, Pecot CV, Kabanov AV, High-capacity poly(2-oxazoline) formulation of TLR 7/8 agonist extends survival in a chemo-insensitive, metastatic model of lung adenocarcinoma, Science, Advances. 6 (2020) eaba5542, 10.1126/sciadv.aba5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Español L, Larrea A, Andreu V, Mendoza G, Arruebo M, Sebastian V, Aurora-Prado MS, Kedor-Hackmann ERM, Santoro MIRM, Santamaria J, Dual encapsulation of hydrophobic and hydrophilic drugs in PLGA nanoparticles by a single-step method: drug delivery and cytotoxicity assays, RSC Adv. 6 (112) (2016) 111060–111069, 10.1039/C6RA23620K. [DOI] [Google Scholar]
- [10].Ebrahimian M, Hashemi M, Maleki M, Hashemitabar G, Abnous K, Ramezani M, Haghparast A, Co-delivery of dual toll-like receptor agonists and antigen in Poly(Lactic-Co-Glycolic) acid/polyethylenimine cationic hybrid nanoparticles promote efficient in vivo immune responses, Front. Immunol 8 (2017) 1–12, 10.3389/fimmu.2017.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jang M, Han HD, Ahn HJ, A RNA nanotechnology platform for a simultaneous two-in-one siRNA delivery and its application in synergistic RNAi therapy, Sci. Rep 6 (2016) 32363, 10.1038/srep32363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Konoeda H, Takizawa H, Gower A, Zhao M, Adeyi OA, Liu M, Pharmacokinetics, tissue distribution and safety of gold nanoparticle/PKC Delta inhibitor peptide hybrid in rats, Nanotoxicology. 14 (3) (2020) 341–354, 10.1080/17435390.2019.1702731. [DOI] [PubMed] [Google Scholar]
- [13].Loftsson T, Brewster ME, Pharmaceutical applications of cyclodextrins: basic science and product development, J. Pharm. Pharmacol 62 (2010) 1607–1621, 10.1111/j.2042-7158.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- [14].Ben Yehuda Greenwald M, Ben Sasson S, Bianco-Peled H, A new method for encapsulating hydrophobic compounds within cationic polymeric nanoparticles, J. Microencapsul 30 (6) (2013) 580–588, 10.3109/02652048.2013.764940. [DOI] [PubMed] [Google Scholar]
- [15].Hennenfent KL, Govindan R, Novel formulations of taxanes: a review. Old wine in a new bottle?, Ann Oncol. 17 (5) (2006) 735–749, 10.1093/annonc/mdj100. [DOI] [PubMed] [Google Scholar]
- [16].Kalepu S, Nekkanti V, Insoluble drug delivery strategies: review of recent advances and business prospects, Acta Pharmaceutica Sinica B. 5 (5) (2015) 442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Auffray C, Fogg D, Garfa M, Elain G, Join-lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F, Behavior Linked references are available on JSTOR for this article : Monitoring of Blood Vessels and Tissues by a Population of Monocytes with Patrolling Behavior, 317 (2017) 666–670. [DOI] [PubMed] [Google Scholar]
- [18].Cignarella A, Tedesco S, Cappellari R, Fadini GP, The continuum of monocyte phenotypes: Experimental evidence and prognostic utility in assessing cardiovascular risk, J. Leukoc. Biol 103 (2018) 1021–1028, 10.1002/JLB.5RU1217-477RR. [DOI] [PubMed] [Google Scholar]
- [19].Zamani F, Zare Shahneh F, Aghebati-Maleki L, Baradaran B, Induction of CD14 Expression and Differentiation to Monocytes or Mature Macrophages in Promyelocytic Cell Lines: New Approach, Adv, Pharm Bull 3 (2013) 329–332, 10.5681/apb.2013.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee SJ, Yoon BR, Kim HY, Yoo S-J, Kang SW, Lee W-W, Activated Platelets Convert CD14+CD16− Into CD14+CD16+ Monocytes With Enhanced FcγR-Mediated Phagocytosis and Skewed M2 Polarization, Front Immunol 11 (2020), 10.3389/fimmu.2020.611133 611133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Monocytes and macrophages in flow: an ESCCA initiative on advanced analyses of monocyte lineage using flow cytometry - Lambert - 2017 - Cytometry Part B: Clinical Cytometry - Wiley Online Library, (n.d.). https://onlinelibrary-wiley-com.proxy.library.cmu.edu/doi/full/10.1002/cyto.b.21280 (accessed March 16, 2021). [DOI] [PubMed] [Google Scholar]
- [22].Gómez-Olarte S, Bolaños NI, Echeverry M, Rodríguez AN, Cuéllar A, Puerta CJ, Mariño A, González JM, Intermediate Monocytes and Cytokine Production Associated With Severe Forms of Chagas Disease, Front. Immunol 10 (2019) 1671, 10.3389/fimmu.2019.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jakubzick CV, Randolph GJ, Henson PM, Monocyte differentiation and antigen-presenting functions, Nat Rev Immunol 17 (6) (2017) 349–362, 10.1038/nri.2017.28. [DOI] [PubMed] [Google Scholar]
- [24].Sunderkötter C, Nikolic T, Dillon MJ, van Rooijen N, Stehling M, Drevets DA, Leenen PJM, Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response, J Immunol. 172 (7) (2004) 4410–4417, 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- [25].Fujimura N, CCR2 inhibition sequesters multiple subsets of leukocytes in the bone marrow, Scientific Reports. (n.d.) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zlotnik A, Yoshie O, The Chemokine Superfamily Revisited, Immunity 36 (5) (2012) 705–716, 10.1016/j.immuni.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Drechsler M, Duchene J, Soehnlein O, Chemokines Control Mobilization, Recruitment, and Fate of Monocytes in Atherosclerosis, Arterioscler. Thromb. Vasc. Biol 35 (5) (2015) 1050–1055, 10.1161/ATVBAHA.114.304649. [DOI] [PubMed] [Google Scholar]
- [28].Yang Q, Wang Y, Pei G, Deng X, Jiang H, Wu J, Zhou C, Guo Y.i., Yao Y, Zeng R, Xu G, Bone marrow-derived Ly6C– macrophages promote ischemia-induced chronic kidney disease, Cell Death Dis. 10 (4) (2019), 10.1038/s41419-019-1531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Serbina NV, Pamer EG, Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2, Nat. Immunol 7 (3) (2006) 311–317, 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- [30].Castanheira F.V.e.S., de Lima KA, Cebinelli GCM, Sônego F, Kanashiro A, Colon D-F, Borges V, Czaikoski PG, Mota JM, Cunha TM, Alves-Filho JC, Liew FY, Cunha FQ, CCR5-Positive Inflammatory Monocytes are Crucial for Control of Sepsis, Shock. 52 (5) (2019) e100–e106, 10.1097/SHK.0000000000001301. [DOI] [PubMed] [Google Scholar]
- [31].Ubogu EE, Callahan MK, Tucky BH, Ransohoff RM, CCR5 expression on monocytes and T cells: modulation by transmigration across the blood-brain barrier in vitro, Cell Immunol. 243 (1) (2006) 19–29, 10.1016/j.cellimm.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Landsman L, Bar-On L, Zernecke A, Kim K-W, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S, CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival, Blood 113 (2009) 963–972, 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- [33].Geissmann F, Jung S, Littman DR, Blood Monocytes Consist of Two Principal Subsets with Distinct Migratory Properties, Immunity 19 (1) (2003) 71–82, 10.1016/S1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- [34].Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ, Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques, J Clin Invest. 117 (1) (2007) 185–194, 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Taylor RA, Hammond MD, Ai Y, Sansing LH, Keep R, CX3CR1 Signaling on Monocytes Is Dispensable after Intracerebral Hemorrhage, PLoS ONE 9 (12) (2014) e114472, 10.1371/journal.pone.0114472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nahrendorf M, Pittet MJ, Swirski FK, Monocytes: protagonists of infarct inflammation and repair, Circulation 121 (2010) 2437–2445, 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Crane MJ, Daley JM, van Houtte O, Brancato SK, Henry WL, Albina JE, Coers J, The Monocyte to Macrophage Transition in the Murine Sterile Wound, PLoS ONE 9 (1) (2014) e86660, 10.1371/journal.pone.0086660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B, Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis, J Exp Med. 204 (2007) 1057–1069, 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Miyawaki NB, Lester PE, Chapter 13: Vascular Disease in the Elderly, (n.d.) 5. [Google Scholar]
- [40].Costopoulos C, Liew TV, Bennett M, Ageing and atherosclerosis: Mechanisms and therapeutic options, Biochem. Pharmacol 75 (6) (2008) 1251–1261, 10.1016/j.bcp.2007.10.006. [DOI] [PubMed] [Google Scholar]
- [41].Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han X, Tang D, Chen R, Research Progress on the Relationship between Atherosclerosis and Inflammation, Biomolecules. 8 (3) (2018) 80, 10.3390/biom8030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH, Role of Endothelial Shear Stress in the Natural History of Coronary Atherosclerosis and Vascular Remodeling: Molecular, Cellular, and Vascular Behavior, J. Am. Coll. Cardiol 49 (25) (2007) 2379–2393, 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- [43].Moroni F, Ammirati E, Norata GD, Magnoni M, Camici PG, The Role of Monocytes and Macrophages in Human Atherosclerosis, Plaque Neoangiogenesis, and Atherothrombosis, Mediators Inflamm. 2019 (2019) 1–11, 10.1155/2019/7434376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Herbin O, Regelmann AG, Ramkhelawon B, Weinstein EG, Moore KJ, Alexandropoulos K, Monocyte Adhesion and Plaque Recruitment During Atherosclerosis Development Is Regulated by the Adapter Protein Chat-H/SHEP1, Arterioscler. Thromb. Vasc. Biol 36 (9) (2016) 1791–1801, 10.1161/ATVBAHA.116.308014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Babaev VR, Fazio S, Gleaves LA, Carter KJ, Semenkovich CF, Linton MF, Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in vivo, J Clin Invest. 103 (12) (1999) 1697–1705, 10.1172/JCI6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yu X-H, Fu Y-C, Zhang D-W, Yin K, Tang C-K, Foam cells in atherosclerosis, Clin. Chim. Acta 424 (2013) 245–252, 10.1016/j.cca.2013.06.006. [DOI] [PubMed] [Google Scholar]
- [47].Hossaini Nasr S, Rashidijahanabad Z, Ramadan S, Kauffman N, Parameswaran N, Zinn KR, Qian C, Arora R, Agnew D, Huang X, Effective atherosclerotic plaque inflammation inhibition with targeted drug delivery by hyaluronan conjugated atorvastatin nanoparticles, Nanoscale. 12 (17) (2020) 9541–9556, 10.1039/D0NR00308E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Duivenvoorden R, Tang J, Cormode DP, Mieszawska AJ, Izquierdo-Garcia D, Ozcan C, Otten MJ, Zaidi N, Lobatto ME, van Rijs SM, Priem B, Kuan EL, Martel C, Hewing B, Sager H, Nahrendorf M, Randolph GJ, Stroes ESG, Fuster V, Fisher EA, Fayad ZA, Mulder WJM, A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation, Nat Commun. 5 (2014) 3065, 10.1038/ncomms4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gao C, Huang Q, Liu C, Kwong CHT, Yue L, Wan J-B, Lee SMY, Wang R, Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines, Nat Commun. 11 (2020) 2622, 10.1038/s41467-020-16439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nakashiro S, Matoba T, Umezu R, Koga J-I, Tokutome M, Katsuki S, Nakano K, Sunagawa K, Egashira K, Pioglitazone-Incorporated Nanoparticles Prevent Plaque Destabilization and Rupture by Regulating Monocyte/Macrophage Differentiation in ApoE−/− Mice, Arterioscler. Thromb. Vasc. Biol 36 (3) (2016) 491–500, 10.1161/ATVBAHA.115.307057. [DOI] [PubMed] [Google Scholar]
- [51].Macchia A, Romero M, D’Ettorre A, Mariani J, Tognoni G, Temporal trends of the gaps in post-myocardial infarction secondary prevention strategies of co-morbid and elderly populations vs. younger counterparts: an analysis of three successive cohorts between 2003 and 2008, Eur. Heart J 33 (4) (2012) 515–522. [DOI] [PubMed] [Google Scholar]
- [52].Tao W, Yurdagul A, Kong N.a., Li W, Wang X, Doran AC, Feng C, Wang J, Islam MA, Farokhzad OC, Tabas I, Shi J, siRNA nanoparticles targeting CaMKIIγ in lesional macrophages improve atherosclerotic plaque stability in mice, Sci. Transl. Med 12 (553) (2020), 10.1126/scitranslmed.aay1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pastore M, Grimaudo S, Pipitone RM, Lori G, Raggi C, Petta S, Marra F, Role of Myeloid-Epithelial-Reproductive Tyrosine Kinase and Macrophage Polarization in the Progression of Atherosclerotic Lesions Associated With Nonalcoholic Fatty Liver Disease, Front. Pharmacol 10 (2019) 604, 10.3389/fphar.2019.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Murray PJ, Wynn TA, Protective and pathogenic functions of macrophage subsets, Nat Rev Immunol. 11 (11) (2011) 723–737, 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang J, Nie S, Zu Y, Abbasi M, Cao J, Li C, Wu D, Labib S, Brackee G, Shen C-L, Wang S, Anti-atherogenic effects of CD36-targeted epigallocatechin gallate-loaded nanoparticles, J. Control. Release 303 (2019) 263–273, 10.1016/j.jconrel.2019.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zabalawi M, Bharadwaj M, Horton H, Cline M, Willingham M, Thomas MJ, Sorci-Thomas MG, Inflammation and skin cholesterol in LDLr−/−, apoA-I−/− mice: link between cholesterol homeostasis and self-tolerance?, J Lipid Res. 48 (1) (2007) 52–65, 10.1194/jlr.M600370-JLR200. [DOI] [PubMed] [Google Scholar]
- [57].Dhanasekara CS, Zhang J, Nie S, Li G, Fan Z, Wang S, Nanoparticles target intimal macrophages in atherosclerotic lesions, Nanomedicine: Nanotechnology, Biology and Medicine. 32 (2021) 102346, 10.1016/j.nano.2020.102346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nie S, Zhang J, Martinez-Zaguilan R, Sennoune S, Hossen MN, Lichtenstein AH, Cao J, Meyerrose GE, Paone R, Soontrapa S, Fan Z, Wang S, Detection of atherosclerotic lesions and intimal macrophages using CD36-targeted nanovesicles, J. Control. Release 220 (2015) 61–70, 10.1016/j.jconrel.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kao C-W, Wu P-T, Liao M-Y, Chung I-J, Yang K-C, Tseng W-Y-I, Yu J, Magnetic Nanoparticles Conjugated with Peptides Derived from Monocyte Chemoattractant Protein-1 as a Tool for Targeting Atherosclerosis, Pharmaceutics. 10 (2018) 62, 10.3390/pharmaceutics10020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Vazquez-Prada KX, Lam J, Kamato D, Xu ZP, Little PJ, Ta HT, Targeted Molecular Imaging of Cardiovascular Diseases by Iron Oxide Nanoparticles, Arterioscler. Thromb. Vasc. Biol 41 (2) (2021) 601–613, 10.1161/ATVBAHA.120.315404. [DOI] [PubMed] [Google Scholar]
- [61].Matuszak J, Lutz B, Sekita A, Zaloga J, Alexiou C, Lyer S, Cicha I, Drug delivery to atherosclerotic plaques using superparamagnetic iron oxide nanoparticles, IJN. 13 (2018) 8443–8460, 10.2147/IJN.S179273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Matute-Blanch C, Montalban X, Comabella M, Chapter 5 - Multiple sclerosis, and other demyelinating and autoimmune inflammatory diseases of the central nervous system, in: Deisenhammer F, Teunissen CE, Tumani H (Eds.), Handbook of Clinical Neurology, Elsevier, 2018: pp. 67–84. 10.1016/B978-0-12-804279-3.00005-8. [DOI] [PubMed] [Google Scholar]
- [63].Figueiredo Borgognoni C, Kim JH, Zucolotto V, Fuchs H, Riehemann K, Human macrophage responses to metal-oxide nanoparticles: a review, Artif Cells Nanomed, Biotechnol 46 (sup2) (2018) 694–703, 10.1080/21691401.2018.1468767. [DOI] [PubMed] [Google Scholar]
- [64].Yang L.i., Yang JB,Chen J, Yu GY, Zhou P, Lei L, Wang ZZ, Chang CCY, Yang XY, Chang TY, Li BL, Enhancement of human ACAT1 gene expression to promote the macrophage-derived foam cell formation by dexamethasone, Cell Res. 14 (4) (2004) 315–323, 10.1038/sj.cr.7290231. [DOI] [PubMed] [Google Scholar]
- [65].Zhang L, Tian XY, Chan CKW, Bai Q, Cheng CK, Chen FM, Cheung MSH, Yin B, Yang H, Yung W-Y, Chen Z, Ding F, Leung K-F, Zhang C, Huang Y.u., Lau JYW, Choi CHJ, Promoting the Delivery of Nanoparticles to Atherosclerotic Plaques by DNA Coating, ACS Appl. Mater. Interfaces 11 (15) (2019) 13888–13904, 10.1021/acsami.8b17928. [DOI] [PubMed] [Google Scholar]
- [66].Calin M, Stan D, Schlesinger M, Simion V, Deleanu M, Constantinescu CA, Gan A-M, Pirvulescu MM, Butoi E, Manduteanu I, Bota M, Enachescu M, Borsig L, Bendas G, Simionescu M, VCAM-1 directed target-sensitive liposomes carrying CCR2 antagonists bind to activated endothelium and reduce adhesion and transmigration of monocytes, Eur. J. Pharm. Biopharm 89 (2015) 18–29, 10.1016/j.ejpb.2014.11.016. [DOI] [PubMed] [Google Scholar]
- [67].Wu Z, Chen C, Luo J, Davis JRJ, Zhang B, Tang L, Shi W, Liao D, EGFP-EGF1-conjugated poly (lactic-co-glycolic acid) nanoparticles as a carrier for the delivery of CCR2– shRNA to atherosclerotic macrophage in vitro, Sci Rep 10 (2020) 19636, 10.1038/s41598-020-76416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Katsuki S, Matoba T, Nakashiro S, Sato K, Koga J-I, Nakano K, Nakano Y, Egusa S, Sunagawa K, Egashira K, Nanoparticle-Mediated Delivery of Pitavastatin Inhibits Atherosclerotic Plaque Destabilization/Rupture in Mice by Regulating the Recruitment of Inflammatory Monocytes, Circulation 129 (8) (2014) 896–906, 10.1161/CIRCULATIONAHA.113.002870. [DOI] [PubMed] [Google Scholar]
- [69].Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, Gorbatov R, Iwamoto Y, Dutta P, Wojtkiewicz G, Courties G, Sebas M, Borodovsky A, Fitzgerald K, Nolte MW, Dickneite G, Chen JW, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M, Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice, Circulation 127 (20) (2013) 2038–2046, 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Chnari E, Nikitczuk JS, Wang J, Uhrich KE, Moghe PV, Engineered Polymeric Nanoparticles for Receptor-Targeted Blockage of Oxidized Low Density Lipoprotein Uptake and Atherogenesis in Macrophages, Biomacromolecules 7 (6) (2006) 1796–1805, 10.1021/bm0600872. [DOI] [PubMed] [Google Scholar]
- [71].Petersen LK, York AW, Lewis DR, Ahuja S, Uhrich KE, Prud’homme RK, Moghe PV, Amphiphilic Nanoparticles Repress Macrophage Atherogenesis: Novel Core/Shell Designs for Scavenger Receptor Targeting and Down-Regulation, Mol. Pharmaceutics 11 (8) (2014) 2815–2824, 10.1021/mp500188g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Kharroubi AT, Diabetes mellitus: The epidemic of the century, World Journal of Diabetes. 6 (6) (2015) 850, 10.4239/wjd.v6.i6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Leon BM, Maddox TM, Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research, World, J Diabetes. 6 (2015) 1246–1258, 10.4239/wjd.v6.i13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kanter JE, Hsu C-C, Bornfeldt KE, Monocytes and Macrophages as Protagonists in Vascular Complications of Diabetes, Front. Cardiovasc. Med 7 (2020) 10, 10.3389/fcvm.2020.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gonzalez Y, Herrera MT, Soldevila G, Garcia-Garcia L, Fabián G, Pérez-Armendariz EM, Bobadilla K, Guzmán-Beltrán S, Sada E, Torres M, High glucose concentrations induce TNF-α production through the down-regulation of CD33 in primary human monocytes, BMC Immunol. 13 (2012) 19, 10.1186/1471-2172-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Seidler S, Zimmermann HW, Bartneck M, Trautwein C, Tacke F, Age-dependent alterations of monocyte subsets and monocyte-related chemokine pathways in healthy adults, BMC Immunol. 11 (2010) 30, 10.1186/1471-2172-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Giulietti A, van Etten E, Overbergh L, Stoffels K, Bouillon R, Mathieu C, Monocytes from type 2 diabetic patients have a pro-inflammatory profile. 1,25-Dihydroxyvitamin D(3) works as anti-inflammatory, Diabetes Res Clin Pract. 77 (1) (2007) 47–57, 10.1016/j.diabres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- [78].Min D, Brooks B, Wong J, Salomon R, Bao W, Harrisberg B, Twigg SM, Yue DK, McLennan SV, Alterations in Monocyte CD16 in Association with Diabetes Complications, Mediators Inflamm. 2012 (2012) 1–10, 10.1155/2012/649083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yong S-B, Song Y, Kim Y-H, Visceral adipose tissue macrophage-targeted TACE silencing to treat obesity-induced type 2 diabetes, Biomaterials 148 (2017) 81–89, 10.1016/j.biomaterials.2017.09.023. [DOI] [PubMed] [Google Scholar]
- [80].Bulboacă AE, Boarescu PM, Bolboacă SD, Blidaru M, Feştilă D, Dogaru G, Nicula CA, Comparative Effect Of Curcumin Versus Liposomal Curcumin On Systemic Pro-Inflammatory Cytokines Profile, MCP-1 And RANTES In Experimental Diabetes Mellitus, Int J Nanomedicine. 14 (2019) 8961–8972, 10.2147/IJN.S226790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang H-G, A Novel Nanoparticle Drug Delivery System: The Anti-inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes, Mol Ther. 18 (9) (2010) 1606–1614, 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yekollu SK, Thomas R, Targeting Curcusomes to Inflammatory Dendritic Cells Inhibits NF-kB and Improves Insulin Resistance in Obese Mice, (n.d.) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Amano C, Minematsu H, Fujita K, Iwashita S, Adachi M, Igarashi K, Hinuma S, Seno M, Nanoparticles Containing Curcumin Useful for Suppressing Macrophages In Vivo in Mice, PLoS ONE 10 (9) (2015) e0137207, 10.1371/journal.pone.0137207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Schirmer L, Atallah P, Werner C, Freudenberg U, StarPEG-Heparin Hydrogels to Protect and Sustainably Deliver IL-4, Adv Healthc Mater. 5 (24) (2016) 3157–3164, 10.1002/adhm.201600797. [DOI] [PubMed] [Google Scholar]
- [85].He M, Sun L, Fu X, McDonough SP, Chu C-C, Biodegradable amino acid-based poly(ester amine) with tunable immunomodulating properties and their in vitro and in vivo wound healing studies in diabetic rats’ wounds, Acta Biomater. 84 (2019) 114–132, 10.1016/j.actbio.2018.11.053. [DOI] [PubMed] [Google Scholar]
- [86].Wang T, Liao Q, Wu Y, Wang X, Fu C, Geng F, Qu Y, Zhang J, A composite hydrogel loading natural polysaccharides derived from Periplaneta americana herbal residue for diabetic wound healing, Int. J. Biol. Macromol 164 (2020) 3846–3857, 10.1016/j.ijbiomac.2020.08.156. [DOI] [PubMed] [Google Scholar]
- [87].Dukhinova MS, Prilepskii Artur.Y., Shtil AA, Vinogradov VV, Metal Oxide Nanoparticles in Therapeutic Regulation of Macrophage Functions, Nanomaterials (Basel). 9 (2019) 1631. 10.3390/nano9111631 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].You C, Li Q, Wang X, Wu P, Ho JK, Jin R, Zhang L, Shao H, Han C, Silver nanoparticle loaded collagen/chitosan scaffolds promote wound healing via regulating fibroblast migration and macrophage activation, Sci Rep. 7 (2017) 10489, 10.1038/s41598-017-10481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gan J, Liu C, Li H, Wang S, Wang Z, Kang Z, Huang Z, Zhang J, Wang C, Lv D, Dong L, Accelerated wound healing in diabetes by reprogramming the macrophages with particle-induced clustering of the mannose receptors, Biomaterials 219 (2019) 119340, 10.1016/j.biomaterials.2019.119340. [DOI] [PubMed] [Google Scholar]
- [90].Cartland SP, Genner SW, Martínez GJ, Robertson S, Kockx M, Lin RCY, O’Sullivan JF, Koay YC, Manuneedhi Cholan P, Kebede MA, Murphy AJ, Masters S, Bennett MR, Jessup W, Kritharides L, Geczy C, Patel S, Kavurma MM, TRAIL-Expressing Monocyte/Macrophages Are Critical for Reducing Inflammation and Atherosclerosis, IScience. 12 (2019) 41–52, 10.1016/j.isci.2018.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Liguori M, Buracchi C, Pasqualini F, Bergomas F, Pesce S, Sironi M, Grizzi F, Mantovani A, Belgiovine C, Allavena P, Functional TRAIL receptors in monocytes and tumor-associated macrophages: A possible targeting pathway in the tumor microenvironment, Oncotarget. 7 (2016) 41662–41676. 10.18632/oncotarget.9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Watt V, Chamberlain J, Steiner T, Francis S, Crossman D, TRAIL attenuates the development of atherosclerosis in apolipoprotein E deficient mice, Atherosclerosis. 215 (2) (2011) 348–354, 10.1016/j.atherosclerosis.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Cartland SP, Erlich JH, Kavurma MM, Schönbach C, TRAIL Deficiency Contributes to Diabetic Nephropathy in Fat-Fed ApoE−/− Mice, PLoS ONE 9 (3) (2014) e92952, 10.1371/journal.pone.0092952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mitchell MJ, Wayne EC, Rana K, Schaffer CB, King MR, Unnatural killer cells: TRAIL-coated leukocytes that kill cancer cells in the circulation, Proceedings of the IEEE Annual Northeast Bioengineering Conference, NEBEC. 2014-Decem (2014) 1–6. 10.1109/NEBEC.2014.6972880 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wayne EC, Chandrasekaran S, Mitchell MJ, Chan MF, Lee RE, Schaffer CB, King MR, TRAIL-coated leukocytes that prevent the bloodborne metastasis of prostate cancer, J Control Release. 223 (2016) 215–223, 10.1016/j.jconrel.2015.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kedzierska K, Crowe SM, The role of monocytes and macrophages in the pathogenesis of HIV-1 infection, Curr Med Chem. 9 (2002) 1893–1903, 10.2174/0929867023368935. [DOI] [PubMed] [Google Scholar]
- [97].Massanella M, Bakeman W, Sithinamsuwan P, Fletcher JLK, Chomchey N, Tipsuk S, Chalermchai T, Routy J-P, Ananworanich J, Valcour VG, Chomont N, Silvestri G, Infrequent HIV Infection of Circulating Monocytes during Antiretroviral Therapy, J. Virol 94 (1) (2019), 10.1128/JVI.01174-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S, van Griensven F, de Souza M, Kim J, Ananworanich J, on behalf of the RV254/SEARCH 010 Study Group, Central Nervous System Viral Invasion and Inflammation During Acute HIV Infection, J. Infect. Dis 206 (2012) 275–282, 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Wong ME, Jaworowski A, Hearps AC, The HIV Reservoir in Monocytes and Macrophages, Front. Immunol 10 (2019), 10.3389/fimmu.2019.01435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Ellery PJ, Tippett E, Chiu Y-L, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM, The CD16 + monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo, J Immunol. 178 (10) (2007) 6581–6589, 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- [101].Tuttle DL, Harrison JK, Anders C, Sleasman JW, Goodenow MM, Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1, J Virol. 72 (6) (1998) 4962–4969, 10.1128/JVI.72.6.4962-4969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Verani A, Pesenti E, Polo S, Tresoldi E, Scarlatti G, Lusso P, Siccardi AG, Vercelli D, Cutting Edge: CXCR4 Is a Functional Coreceptor for Infection of Human Macrophages by CXCR4-Dependent Primary HIV-1 Isolates, The Journal of Immunology. (n.d.) 6. [PubMed] [Google Scholar]
- [103].Jiang Y, Cao S, Bright DK, Bever AM, Blakney AK, Suydam IT, Woodrow KA, Nanoparticle-Based ARV Drug Combinations for Synergistic Inhibition of Cell-Free and Cell-Cell HIV Transmission, Mol. Pharmaceutics 12 (12) (2015) 4363–4374, 10.1021/acs.molpharmaceut.5b00544. [DOI] [PubMed] [Google Scholar]
- [104].Dou H, Morehead J, Destache CJ, Kingsley JD, Shlyakhtenko L, Zhou Y, Chaubal M, Werling J, Kipp J, Rabinow BE, Gendelman HE, Laboratory investigations for the morphologic, pharmacokinetic, and anti-retroviral properties of indinavir nanoparticles in human monocyte-derived macrophages, Virology 358 (1) (2007) 148–158, 10.1016/j.virol.2006.08.012. [DOI] [PubMed] [Google Scholar]
- [105].Dubey V, Mishra D, Nahar M, Jain V, Jain NK, Enhanced transdermal delivery of an anti-HIV agent via ethanolic liposomes, Nanomedicine: Nanotechnology, Biology and Medicine. 6 (4) (2010) 590–596, 10.1016/j.nano.2010.01.002. [DOI] [PubMed] [Google Scholar]
- [106].Arunkumar N, Deecaraman M, Rani C, Nanosuspension technology and its applications in drug delivery, Asian Journal of Pharmaceutics (AJP): Free Full Text Articles from Asian J Pharm. 3 (2014). 10.22377/ajp.v3i3.261. [DOI] [Google Scholar]
- [107].Kurd M, Sadegh Malvajerd S, Rezaee S, Hamidi M, Derakhshandeh K, Oral delivery of indinavir using mPEG-PCL nanoparticles: preparation, optimization, cellular uptake, transport and pharmacokinetic evaluation, Artif Cells Nanomed, Biotechnol. 47 (1) (2019) 2123–2133, 10.1080/21691401.2019.1616553. [DOI] [PubMed] [Google Scholar]
- [108].Dutta T, Agashe HB, Garg M, Balasubramanium P, Kabra M, Jain NK, Poly (propyleneimine) dendrimer based nanocontainers for targeting of efavirenz to human monocytes/macrophages in vitro: Research Paper, J. Drug Target 15 (1) (2007) 89–98, 10.1080/10611860600965914. [DOI] [PubMed] [Google Scholar]
- [109].Chiappetta DA, Hocht C, Taira C, Sosnik A, Efavirenz-loaded polymeric micelles for pediatric anti-HIV pharmacotherapy with significantly higher oral bioavailability, Nanomedicine. 5 (1) (2010) 11–23, 10.2217/nnm.09.90. [DOI] [PubMed] [Google Scholar]
- [110].Gong Y, Chowdhury P, Midde NM, Rahman MA, Yallapu MM, Kumar S, Novel elvitegravir nanoformulation approach to suppress the viral load in HIV-infected macrophages, Biochem. Biophys. Rep 12 (2017) 214–219, 10.1016/j.bbrep.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Borgmann K, Rao KS, Labhasetwar V, Ghorpade A, Efficacy of Tat-Conjugated Ritonavir-Loaded Nanoparticles in Reducing HIV-1 Replication in Monocyte-Derived Macrophages and Cytocompatibility with Macrophages and Human Neurons, AIDS Res. Hum. Retroviruses 27 (8) (2011) 853–862, 10.1089/aid.2010.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Shah LK, Amiji MM, Intracellular Delivery of Saquinavir in Biodegradable Polymeric Nanoparticles for HIV/AIDS, Pharm Res. 23 (11) (2006) 2638–2645, 10.1007/s11095-006-9101-7. [DOI] [PubMed] [Google Scholar]
- [113].Joshi G, Kumar A, Sawant K, Bioavailability enhancement, Caco-2 cells uptake and intestinal transport of orally administered lopinavir-loaded PLGA nanoparticles, Drug Delivery 23 (9) (2016) 3492–3504, 10.1080/10717544.2016.1199605. [DOI] [PubMed] [Google Scholar]
- [114].Narayanasamy P, Switzer BL, Britigan BE, Prolonged-acting, Multi-targeting Gallium Nanoparticles Potently Inhibit Growth of Both HIV and Mycobacteria in Co-Infected Human Macrophages, Sci Rep. 5 (2015) 8824. 10.1038/srep08824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Choi S-R, Britigan BE, Narayanasamy P, Ga(III) Nanoparticles Inhibit Growth of both Mycobacterium tuberculosis and HIV and Release of Interleukin-6 (IL-6) and IL-8 in Coinfected Macrophages, Antimicrob Agents Chemother 61 (4) (2017), 10.1128/AAC.02505-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Zhang G, Campbell GR, Zhang Q, Maule E, Hanna J, Gao W, Zhang L, Spector SA, Chomont N, Pujol N, CD4+ T Cell-Mimicking Nanoparticles Broadly Neutralize HIV-1 and Suppress Viral Replication through Autophagy, MBio. 11 (5) (2020), 10.1128/mBio.00903-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Campbell GR, Zhuang J, Zhang G, Landa I, Kubiatowicz LJ, Dehaini D, Fang RH, Zhang L, Spector SA, CD4+ T cell-mimicking nanoparticles encapsulating DIABLO/SMAC mimetics broadly neutralize HIV-1 and selectively kill HIV-1-infected cells, Theranostics. 11 (18) (2021) 9009–9021, 10.7150/thno.59728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Gramatica A, Petazzi RA, Lehmann MJ, Ziomkowska J, Herrmann A, Chiantia S, αEnv-decorated phosphatidylserine liposomes trigger phagocytosis of HIV-virus-like particles in macrophages, Nanomedicine. 10 (5) (2014) e981–e989, 10.1016/j.nano.2014.02.008. [DOI] [PubMed] [Google Scholar]
- [119].Kauppi P, Linna M, Jantunen J, Martikainen JE, Haahtela T, Pelkonen A, Mäkelä M, Chronic Comorbidities Contribute to the Burden and Costs of Persistent Asthma, Mediators Inflamm. 2015 (2015) 1–7, 10.1155/2015/819194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Chen Y, Shan X, Luo C, He Z, Emerging nanoparticulate drug delivery systemsof metformin, J. Pharm. Investig 50 (3) (2020) 219–230, 10.1007/s40005-020-00480-1. [DOI] [Google Scholar]
- [121].Feng X, Chen W, Ni X, Little PJ, Xu S, Tang L, Weng J, Metformin, Macrophage Dysfunction and Atherosclerosis, Front Immunol. 12 (2021), 10.3389/fimmu.2021.682853 682853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Vasamsetti SB, Karnewar S, Kanugula AK, Thatipalli AR, Kumar JM, Kotamraju S, Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis, Diabetes 64 (2015) 2028–2041, 10.2337/db14-1225. [DOI] [PubMed] [Google Scholar]
- [123].Seneviratne A, Cave L, Hyde G, Moestrup SK, Carling D, Mason JC, Haskard DO, Boyle JJ, Metformin directly suppresses atherosclerosis in normoglycaemic mice via haematopoietic adenosine monophosphate-activated protein kinase, Cardiovasc. Res 117 (2021) 1295–1308, 10.1093/cvr/cvaa171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Merad M, Martin JC, Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages, Nat. Rev. Immunol 20 (6) (2020) 355–362, 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Devaraj S, Chen X, Adams-Huet B, Jialal I, Increased Expression of Fc-γ Receptors on Monocytes in Patients With Nascent Metabolic Syndrome, The Journal of Clinical Endocrinology & Metabolism. 98 (9) (2013) E1510–E1515, 10.1210/jc.2013-2112. [DOI] [PubMed] [Google Scholar]


