Keywords: breathing, cervical, larynx, spinal cord injury, swallow
Abstract
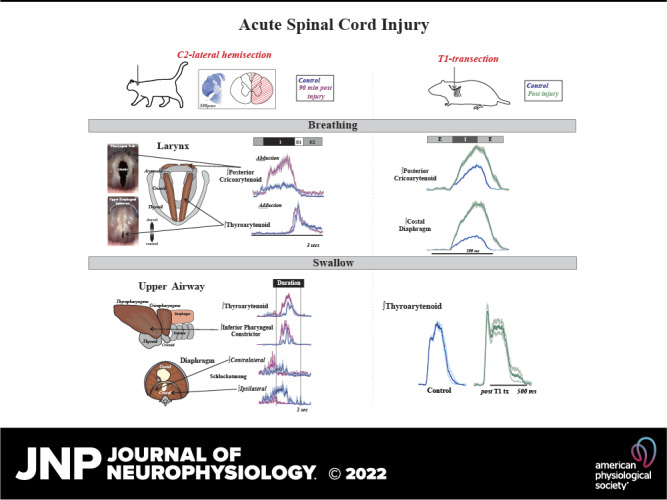
Laryngeal function is vital to airway protection. Although swallow is mediated by the brainstem, the mechanism underlying the increased risk of dysphagia after cervical spinal cord injury (SCI) is unknown. We hypothesized that: 1) loss of descending phrenic drive affects swallow and breathing differently, and 2) loss of ascending spinal afferent information alters swallow regulation. We recorded electromyograms (EMGs) from upper airway and chest wall muscles in freely breathing pentobarbital-anesthetized cats and rats. Laryngeal abductor activity during inspiration increased about twofold following C2 lateral hemisection. Ipsilateral to the injury, the crural diaphragm EMG amplitude was reduced during breathing (62 ± 25% change postinjury), but no animal had complete termination of activity; 75% of animals had increased contralateral diaphragm recruitment, but this did not reach significance. During swallow, laryngeal adductor and pharyngeal constrictor muscles increased activity, and diaphragm activity was bilaterally suppressed. This was unexpected because of the ipsilateral-specific response during breathing. Swallow-breathing coordination was disrupted by injury, and more swallows occurred during early expiration. Finally, to determine if the chest wall is a major source of feedback for laryngeal regulation, we performed T1 total transections in rats. As in the C2 lateral hemisection, inspiratory laryngeal recruitment was the first feature noted after injury. In contrast to the C2 lateral hemisection, diaphragmatic drive increased after T1 transection. Overall, we found that SCI alters laryngeal drive during swallow and breathing, and alters swallow-related diaphragm activity. Our results show behavior-specific effects, suggesting that swallow is affected more than breathing is by SCI, and emphasizing the need for additional studies on the effect of ascending afferents from the spinal cord on laryngeal function.
NEW & NOTEWORTHY This is the first manuscript to determine the impact of cSCI on laryngeal and swallow function, and to describe a possible mechanism for dysphagia and altered airway protection after injury.
INTRODUCTION
The larynx and the upper esophageal sphincter act in concert to control the flow of air and food into the lungs and esophagus, respectively (1). Laryngeal dysfunction, whether central or peripheral in origin, leads to a wealth of behavioral deficits including dysphonia (disorder of voice) (2–5), dystussia (disorder of cough) (6–10), and dysphagia (disorder of swallow) (11–15). Although all these symptoms occur after spinal cord injury, dysphagia has received the least attention.
Swallow has three phases: oral, pharyngeal, and esophageal (16–19). The pharyngeal phase, which is the focus of this manuscript, directs food/liquid past the larynx and through the upper esophageal sphincter, using a highly regulated series of coordinated, bilateral muscle contractions over ∼500 ms (1, 18, 20–23). There are two distinct forces on the bolus to propel movement: positive pressure from oropharyngeal contraction and negative pressure from activation of the diaphragm and other inspiratory chest wall muscles (24, 25). Full closure of the laryngeal opening is required to sustain the pressure necessary for effective movement of the bolus into the esophagus, and a leak in this process significantly increases aspiration risk (26).
Although dysphagia can have a myriad of origins (27–32), the outcome is often the same: penetration (food/liquid entering the larynx) or aspiration (food/liquid below the larynx in the trachea/lungs) (14, 15, 33–35), which significantly increases the risk of pneumonia (36). Although the swallow reflex is brainstem-mediated and the upper airway muscles are controlled by cranial nerves, some studies suggest that spinal cord injury may cause dysphagia (37–40). Clinical work in patients with cervical spinal cord injuries (cSCI) has shown mixed results regarding aspiration risk. Reported incidences vary considerably (16%–75% prevalence) and until recently were only evaluated retrospectively (37, 41–54). In a 2017 study (55) in which 37 patients with cSCI were prospectively evaluated, 74% penetrated or aspirated when evaluated with videofluoroscopy (an X-ray of swallow in which the bolus is laced with barium to observe function). Of the patients with severe dysphagia, 73% had silent aspiration, which means there were no outward signs (cough or throat clear) and suggests the likelihood of significant risk of underdiagnosis. When dysphagia is diagnosed, treatment options are extremely limited. Feeding tube placement is most common. Although this effectively bypasses swallow, decreasing pneumonia risk by ∼40% (56), it negatively affects quality of life and does not promote recovery. Despite recent studies that do report dysphagia in patients with cSCI (52, 55, 57, 58), none identify a mechanism for dysfunction.
For these reasons, we aimed to determine if an acute lateral hemisection at the second cervical level (C2) in cats would disrupt swallow function due to loss of descending efferent connections to the diaphragm and/or loss of the ascending afferent information necessary for laryngeal regulation and appropriate swallow pattern generation. Our first hypothesis was that loss of descending efferent information to the diaphragm would affect swallow and breathing differently. We based this partly on clinical reports that patients with a loss of negative intrathoracic pressure had dysphagia, yet showed no complimentary disorders of breathing (30, 59–61). Our second hypothesis was that loss of ascending spinal afferent information would alter the excitability of the swallow pattern generator, and thus result in changes in motor drive to the upper airway. Although the cat model offers a robust and nuanced study of swallow, the majority of work in the field is completed in rat. We also performed rat T1 transection experiments to complement our hypothesis testing and to offer a cross-species verification of the effects of spinal cord injury on laryngeal drive.
METHODS
Study Design
All experiments were approved by the Institutional Animal Care and Use committee of the University of Louisville and conducted in accordance with the American Physiological Society’s Animal Care Guidelines (62). The cat experiments were performed using four purpose-bred sexually intact adults (1–2 yr of age)—two females (3.3 and 4.0 kg) and two males (4.4 and 4.8 kg). The rat experiments used five adult female Sprague Dawley ex-breeders with an average weight of 0.46 ± 0.05 kg. The number of animals was predetermined before initiation of the experiments and was based on pilot studies not included in this manuscript. Those preliminary studies indicated a robust increase in laryngeal activity after spinal cord injury. An a priori power analysis was conducted to test the difference between two independent group means using a two-tailed test, an effect size (Cohen’s d = 0.80), and an alpha of 0.05. For the cat experiments, the expected mean difference was 75 with a standard deviation of 30, and the result showed a total sample of four with an actual power of 0.92. For the rat experiments, the expected mean difference was 70 with a population SD of 34, and the result showed a total sample of five with an actual power of 0.93.
Our objectives were to determine any changes in 1) motor drive during swallow and breathing following C2 lateral hemisection in the cat, and 2) laryngeal drive during breathing and swallow following T1 complete transection in the rat. The primary outcome measures are amplitudes of laryngeal and inspiratory muscle electromyograms (EMGs). We also collected the following additional measures: EMGs of submental and pharyngeal muscles, arterial blood pressure, arterial blood gas values, end-tidal CO2, body temperature, and heart and respiratory rates. Paired Student t tests or ANOVA were performed when appropriate.
The breathing phases were defined as follows: 1) inspiration was defined as the onset to the peak of diaphragm activity; 2) E1 (i.e., early expiration, post-I, or yield) was defined as the peak of diaphragm activity to the end of the thyroarytenoid burst; 3) E2 was defined as the end of E1 to the beginning of inspiration. Swallow-breathing coordination was assessed by noting the phase of breathing in which swallow was initiated. To quantify the phases in which swallow occurred, we assigned swallows during inspiration, E1, and E2 phases to the numbers 1, 2, and 3 respectively, as we have previously published (23, 63). To assess swallow-breathing coordination, a Wilcoxon signed rank test was used. An assigned coding system was used for the breathing phase in which the swallow occurred: inspiration as “1”; early expiration (i.e., yield or E1) (64) as “2”; and mid/late-expiration as “3”. For all tests, a difference was considered significant if the P value was less than or equal to 0.05.
Electrophysiology Recording
All muscle activity was recorded via electromyography (EMG) using bipolar insulated fine wire electrodes (A-M Systems stainless steel No. 791050) according to the technique of Basmajian and Stecko (65). The signals were amplified (Grass P511 AC Amplifiers, Natus Neurology and band-pass filtered 200–5,000 Hz), recorded at a 10,000 sampling rate (1401 Power3 + ADC16 Expansion, Cambridge Electronic Design) and analyzed using Spike 2 (v7, Cambridge Electronic Design). Moving averages of EMGs were integrated with a 20-ms time constant, and exported to CorelDRAW 2020 (v. 22.1.1.523) for figure creation. As in previous studies, peak EMG amplitude was measured for each muscle during each swallow or breath to determine neural drive (66–70); because swallow EMG amplitude and duration have weak correlations (23, 63, 71–74), amplitude measures that include duration were not performed. For normalization across animals, the raw value was calculated as the percent change in amplitude relative to the median peak EMG amplitude during the control period.
Cat Model
Animals were initially anesthetized with sevoflurane (3%–5%) and then transitioned to sodium pentobarbital (35–40 mg/kg iv); supplementary sodium pentobarbital doses were administered as needed (1–3 mg/kg iv). A dose of atropine sulfate (0.1–0.2 mg/kg iv) was given at the beginning of each experiment to reduce airway secretions. Cannulas were placed in the femoral artery, femoral vein, and trachea. Arterial blood pressure (BPM-832 Pressure Monitor, Charles Ward Electronics) and end-tidal CO2 (Capnomac Ultima, Datex Engstrom) were continuously monitored. Body temperature was monitored and maintained at 37.5 ± 0.5°C (Homeothermic Monitor, Harvard Apparatus). Arterial blood samples were periodically removed for blood gas analysis (VetScan I-STAT & CG4+ cartridges, Abaxis). If needed, Po2 was maintained using air mixtures with enriched oxygen (25%–60%) to achieve values above 100 mmHg (RMA-14-SSV, Dwyer Instruments, Inc. & GSM-3 Gas Mixer, Charles Ward Electronics). Anesthetic level was evaluated using end-tidal and Pco2, as well as reflex activity (jaw tone, pull back, and ocular reflexes).
Swallow was stimulated by infusion of 3 mL of water into the oropharynx and evaluated using the following muscles: mylohyoid, geniohyoid, thyrohyoid, thyropharyngeus, thyroarytenoid, cricopharyngeus, parasternal, and crural diaphragm. These muscles span the actions during the pharyngeal phase of swallow: 1) mylohyoid, geniohyoid, and thyrohyoid for hyolaryngeal elevation; 2) thyropharyngeus for inferior pharyngeal constriction; 3) cricopharyngeus for upper esophageal sphincter regulation; 4) thyroarytenoid for laryngeal adduction; and 5) parasternal, costal diaphragm, and bilateral crural diaphragm Schluckatmung activity (i.e., inspiratory activity during swallow that creates a negative intrathoracic pressure) (1, 23, 25, 74). Breathing motor patterns were evaluated using the posterior cricoarytenoid, thyroarytenoid, and bilateral crural diaphragm. Respiratory phase duration measured with activity from inspiratory and expiratory laryngeal muscles is described in Fig. 1.
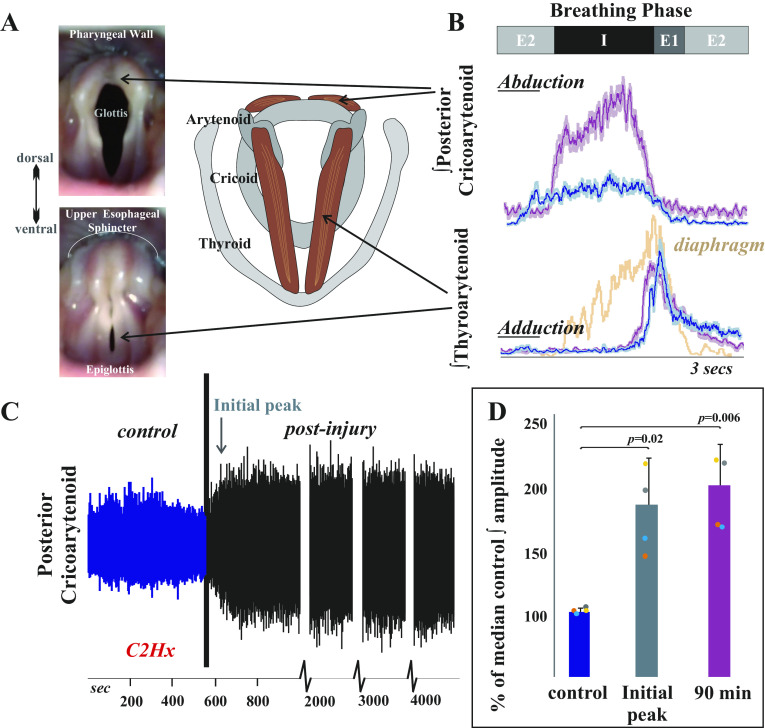
Figure 1.
Inspiratory laryngeal drive increases following cervical spinal cord injury. A: endoscopic laryngeal images of a spontaneously breathing, spinally intact cat (isoflurane anesthesia) show abduction (opening) and adduction (closing) of the vocal folds. Muscular attachments (posterior cricoarytenoid and thyroarytenoid) to cartilages in the larynx (arytenoid, cricoid, and thyroid) are illustrated to the right. The vocal folds are white bands of specialized vibratory tissue that are necessary for voice production, and the space between the vocal folds is the glottis. B: laryngeal valving is regulated across the respiratory cycle, with contraction of the posterior cricoarytenoid opening the glottic space during inspiration, and the thyroarytenoid narrowing the glottic space during early expiration (E1). This narrowing increases early-expiratory subglottic pressure and therefore reduces the initial flow of expired gases. Representative traces of electromyogram (EMG) activity recorded from posterior cricoarytenoid (top) and thyroarytenoid (bottom) muscles during eupnea prior to (blue traces) and 90 min after (purple traces) injury. Diaphragm trace (orange) is underlaid on bottom thyroarytenoid panel for reference. There is a substantial increase in laryngeal inspiratory EMG activity after C2 lateral hemisection in cats. Traces are waveform averages of the rectified and smoothed (50 ms) EMGs across 1 min of stable eupneic activity. C: an unrectified raw trace of the posterior cricoarytenoid EMG shows increased inspiratory motor drive immediately following C2 lateral hemisection in spontaneously breathing cats (intravenous pentobarbital anesthesia), that was sustained >90 min after injury. D: the plotted mean integrated posterior cricoarytenoid EMG amplitudes illustrate a significant increase from control (preinjury) in the periods immediately after the injury [t(3) = −5.1, P = 0.02] and 90 min postinjury [t(3) = −6.3, P = 0.008; paired t tests]. There was no significant change in thyroarytenoid EMG amplitude immediately after injury [t(3) = −0.4, P = 0.68] or at 90 min postinjury [t(3) = 1.98, P = 0.19].
Surgical placement of EMGs proceeded as follows (ipsilateral or contralateral noted relative to lesion side). The digastric muscles were blunt dissected away from the surface of the mylohyoid and electrodes were placed medially in the contralateral mylohyoid. A small horizontal incision was made at the rostral end of the ipsilateral mylohyoid and an elevation of the caudal edge of the incision revealed the geniohyoid muscle. Electrodes were placed 1 cm from the caudal insertion of the geniohyoid muscle. The ipsilateral thyroarytenoid muscle electrodes were inserted through the cricothyroid window into the anterior portion of the vocal folds, which were visually inspected for placement accuracy postmortem. Minor rotation of the larynx and pharynx counterclockwise revealed the superior laryngeal nerve, which facilitated placement of the thyropharyngeus muscle electrodes. The thyropharyngeus is a fan-shaped muscle with the smallest portion attached to the thyroid cartilage; electrodes were placed in the contralateral ventral, caudal portion of the muscle overlaying thyroid cartilage within 5 mm of the rostral insertion of the muscle. To place electrodes within the cricopharyngeus muscle, the larynx and pharynx were rotated counterclockwise to reveal the midline posterior aspect of the larynx. The edge of the cricoid cartilage was located by palpation and electrodes were placed in the cricopharyngeus muscle just cranial to the edge. Contralateral thyrohyoid muscle electrodes were inserted ∼5 mm rostral to the attachment to the thyroid cartilage. The esophagus was blunt dissected from the trachea on the contralateral side, and by elevating the trachea, the dorsal side of the larynx was visualized and the ipsilateral posterior cricoarytenoid EMG placed.
A bilateral auricular block with 1% lidocaine was then performed before placing the animal in the stereotaxic frame. Bilateral crural diaphragm EMGs were placed according to the method detailed by Trelease et al. (75): the 13th rib was manually palpated to locate its junction with the vertebral body, then wire was passed (in a 1.5 in., 22-gauge needle) caudal and lateral to the head of the 13th rib along the sagittal plane (see Fig. 2). Postmortem assessment confirmed placement. The positions of all electrodes were confirmed by visual inspection (both following electrode placement and postmortem) and by EMG activity patterns during breathing and swallow, as we have previously published (1, 23, 40, 74).
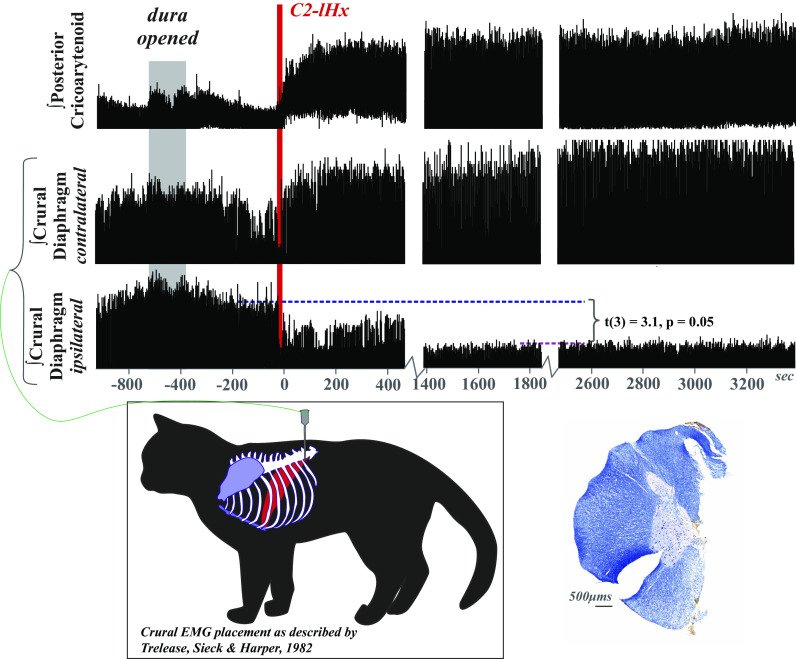
Figure 2.
Respiratory drive is altered following cervical spinal cord injury. Electromyogram (EMG) activity was recorded from posterior cricoarytenoid and bilateral crural diaphragm muscles prior to, during, and after C2 lateral hemisection (C2-lHx) in freely breathing (pentobarbital anesthetized) cats with intact vagi. There was a reduction in peak EMG amplitude, but no evidence of paralysis of the ipsilateral crural diaphragm after lateral hemisection. In three of the four animals, drive of the contralateral diaphragm increased qualitatively, but the effect was not significant as a group t(3) = −1.05, P = 0.3. EMG was rectified and smoothed at 50 ms. One minute of stable eupneic breathing before and at 1 h postinjury was analyzed, and to limit the potential confound of cycle-by-cycle variability, the percent change in peak EMG amplitude was compared with the median peak amplitude during the control period. Crural diaphragm EMG placement is illustrated (postmortem confirmation example in Fig. 3C). A representative example of the C2 lateral hemisection counterstained for Nissl and myelin shows an accurate and complete right side hemisection (left side slit to maintain orientation throughout lesion block during histological processing). Supplemental Table S1 contains additional cardiorespiratory measures.
Rat Model
The animals were initially anesthetized with gaseous isoflurane (1.5%–2% with 100% O2) while a femoral intravenous (iv) cannula was placed for administration of sodium pentobarbital (25 mg/kg iv). Isoflurane was discontinued and supplementary doses of sodium pentobarbital were administered as needed throughout the experiment. A dose of atropine sulfate (0.01 mg/kg iv) was given at the beginning of the experiment to reduce secretions from repeated tracheal stimulation. Following administration of atropine sulfate, a tracheostomy was performed. Body temperature was monitored and maintained at 36.5 ± 0.5°C (Homeothermic Monitor, Harvard Apparatus). Anesthetic level was evaluated by withdrawal reflex of the forelimb and hindlimb and licking in response to oral water administration.
Using the same techniques described earlier, EMGs of the costal diaphragm and posterior cricoarytenoid were used to evaluate breathing. In addition, electrodes were placed bilaterally into the pectoralis muscle to record electrocardiogram (ECG) activity, which was used to remove heart artifact from EMG traces.
Spinal Cord Injury
The phrenic motor neuron population is in the region of C3–C5 in rats and humans (76), and C4–C6 in cats (77). Muscle was separated from the dorsal process and laminae to visualize the entire dorsal aspect of the vertebra overlying the intended spinal lesion level along with the rostral most aspect of the adjacent, caudal vertebra. Bilateral laminectomies were performed at either C2 (cat) or T1 (rat) to expose the spinal cord and the dura mater slit longitudinally along the midline of the entire segment. Following visualization of the dorsal columns and dorsal root entry zones, midline was identified and iridectomy scissors used to cut the left half of the spinal cord (cat) or make a complete spinal transection (rat). Lesion completeness was assessed by visualizing lateral and ventral dural surfaces with magnifiers. Furthermore, with complete transection, the cut ends pull away from each other leaving a gap of a couple of millimeters. Gelfoam, saline, and bovine thrombin (91-010; BioPharm Laboratories, LLC) were used to control local bleeding. Once hemostasis was achieved, gelfoam was left in the lesion site and it was covered with saline-soaked gelfoam and gauze for the remainder of the experiment.
Histological Processing for the C2 Lateral Hemisection
Following the collection of terminal electrophysiology data, animals were perfused transcardially with saline (0.9%) followed by 4% buffered paraformaldehyde (pH 7.4). Spinal cords were removed, lesion level verified, and tissue blocked. Tissue was postfixed for 24–48 h, then cryoprotected in 30% buffered sucrose (pH 7.4) and sectioned on a cryostat (25 μm) at −20 to −22°C. Every 40th section (every 1,000 μm) of the lesion block was processed with Nissl (Cresyl Violet Acetate, Sigma) and myelin (Eriochrome Cyanine R, Merck) stains to allow identification of the injury epicenter and evaluation of the extent of the lesion (78). Sections were dehydrated through serial ethanols (70%–100%) and coverslipped out of xylenes (Fisher) using DPX mounting media (Electron Microscopy Sciences), and photographed using a Zeiss Imager. Z2 and Stereo Investigator (MicroBrighfield).
RESULTS
First, we assessed the effect of C2 lateral hemisection in cats on laryngeal drive during breathing. Figure 1 demonstrates an increase in posterior cricoarytenoid peak EMG activity during inspiration (percent change ∼20-min postinjury 189 ± 35%, 90-min postinjury 173 ± 23%). The posterior cricoarytenoid attaches from the cricoid to the arytenoid cartilages, and its contraction rotates the arytenoid cartilage to move the vocal folds into an abducted (open) position during inspiration. Increased amplitude represents an increased glottic space and decreased laryngeal resistance applied to inspired air. There was no significant change in thyroarytenoid peak EMG amplitude after injury (∼20-min postinjury 106 ± 32%, 90 min 73 ± 30%).
Second, we assessed the effect of C2 lateral hemisection on bilateral crural diaphragm activity during breathing. Ipsilateral to the injury, the crural diaphragm EMG was significantly reduced (Fig. 2), but there was not a significant increase in contralateral recruitment. Obtaining reliable bilateral diaphragm recordings was essential to the assessment, but opening the abdominal cavity affects chest wall activity (79–82) and alters swallow breathing coordination (23). Therefore, we instead used the method described by Trelease et al. (75) (Fig. 2), which produced stable bilateral diaphragm recordings while maintaining normal respiratory mechanics. There was a significant depression of ipsilateral crural diaphragm EMG activity after C2 lateral hemisection (62 ± 25% change postinjury), but no animal had a complete termination of all activity. In addition, 75% of animals had an increase in contralateral diaphragm recruitment, but this did not reach significance. The smallest contralateral effect of injury was at 90% of control, and the largest effect was at 174% of control, which is displayed in Fig. 2. Postmortem tissue examination confirmed that all animals had complete lateral hemisections at C2 and confirmed correct electrode placement in the crural diaphragm. Supplemental Table S1 reports all cardiorespiratory measures before and after injury (heart rate, blood pressure, end-tidal CO2, temperature, respiratory rate, respiratory phase duration, blood gases, and pH). There was only a significant change in heart rate with an overall reduction of 16%.
Third, we determined the effect of C2 lateral hemisection on regulation of the upper airway during swallow (Fig. 3). Measurements from the full complement of muscles recorded are reported in Supplemental Fig. S1. These muscles were chosen because they are vital to the operation of the aerodigestive dual valve mechanism (1). In this scenario, the upper esophageal sphincter and larynx must coordinate their movements to control the flow of air/liquid from the upper airway into either the trachea or esophagus in the thoracic cavity. The primary outcome of the injury was that the thyroarytenoid EMG activity increased acutely (percent change 113 ± 6%, ∼20-min postinjury) and this increase was maintained (90-min postinjury, 118 ± 20%). The thyroarytenoid spans the larynx and constitutes the bulk of the vocal folds. Increased thyroarytenoid contraction ensures that the glottic space is closed during swallow, reducing aspiration risk. However, thyropharyngeus activity (operating as the inferior pharyngeal constrictor) also increased after injury (percent change 90-min postinjury 229 ± 79%). The thyropharyngeus is a fan-shaped muscle that attaches to the lateral aspect on the thyroid cartilage and constitutes the bulk of the pharyngeal wall. Increased thyropharyngeus activity during swallow adds to the pressure exerted on the laryngeal orifice, but is also necessary to maintain a clear pharynx for the subsequent breath. There was also no significant change in the number of swallows stimulated per trial [control 3.5 ± 0.6, ∼20-min postinjury 4.7 ± 2.7 (P = 0.5), and 90-min postinjury 3 ± 0.7 (P = 0.8)].
Figure 3.
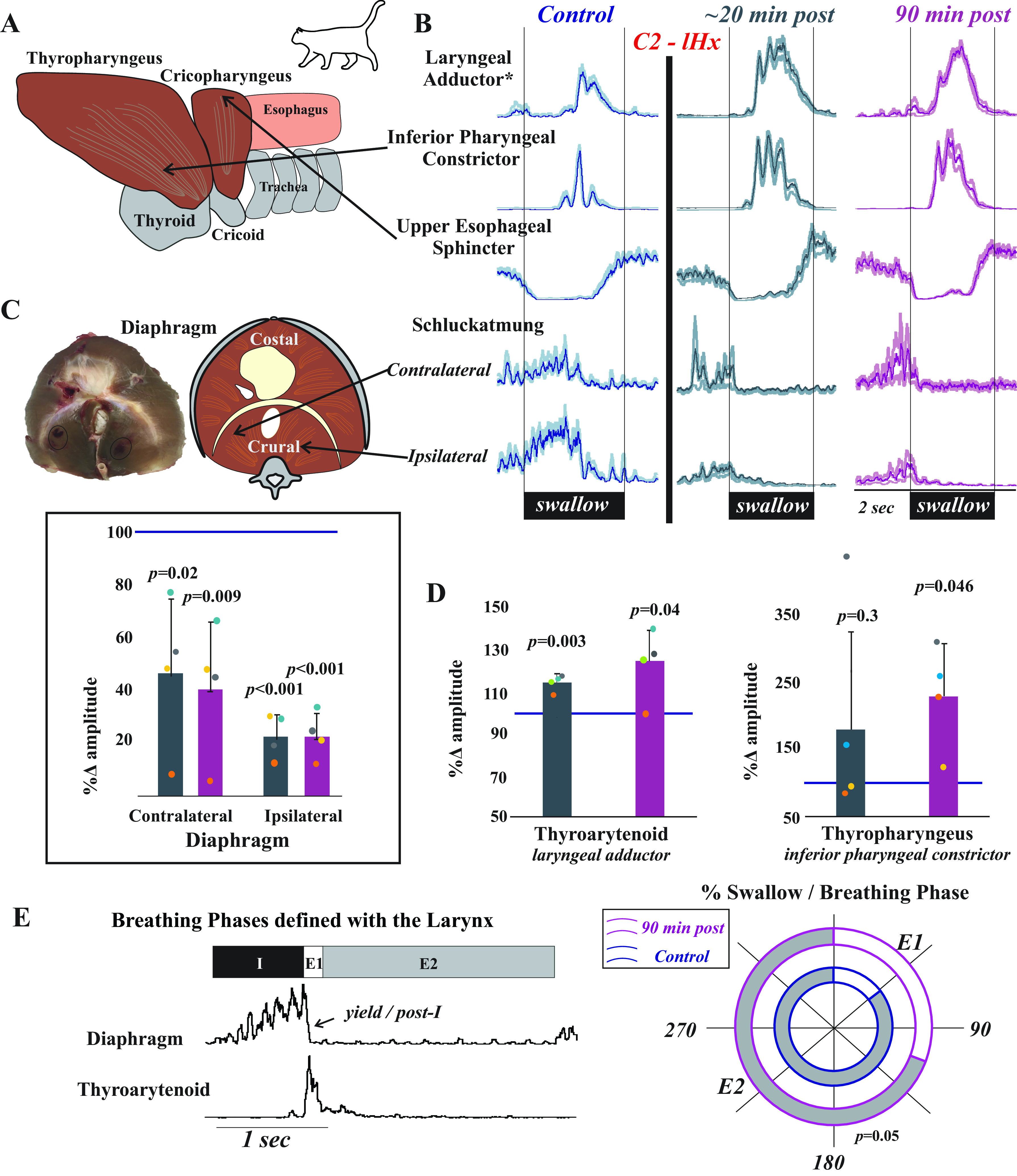
Laryngeal and pharyngeal drive during swallow increases after cervical spinal cord injury. A: while both of the lower pharyngeal muscles (thyropharyngeus and cricopharyngeus) are involved in pharyngeal constriction and maintaining tone in the upper esophageal sphincter, we record from the thyropharyngeus to assess inferior pharyngeal constriction and from the cricopharyngeus to assess upper esophageal sphincter activity. B: representative traces of electromyogram (EMG) activity recorded from muscles during swallow prior to (blue) and ∼20 min (gray) and 90 min (purple) after C2 lateral hemisection (C2-lHx) in cats show changes after injury. The laryngeal adductor is the thyroarytenoid muscle, and traces are waveform averages of the rectified and smoothed (50 ms) EMGs. Swallows were induced with infusion of 3 mL of water into the oropharynx. The control waveform (blue) demonstrates the actuation of the diaphragm during swallow (termed “Schluckatmung”) during expiration. Oral infusion of water reliably elicits swallow, and peak EMG amplitude during swallow for laryngeal closure and pharyngeal constriction significantly increased after injury; swallow-related activity of the crural diaphragm significantly decreased on both sides. C: postmortem and illustrated images of the diaphragm show locations of the EMG placements. The plot shows decreases in waveform average amplitudes for swallow-related diaphragm activity at ∼20 (gray) and 90 (purple) min postinjury as a percent of the control amplitude. Contralateral crural diaphragm amplitude was decreased compared with control at ∼20-min [t(3) = 3.082, P = 0.03] and 90-min postinjury [t(3) = 4.7, P = 0.02]; ipsilateral crural diaphragm amplitude was also decreased at ∼20-min [t(3) = 18.5, P < 0.001] and 90-min postinjury [t(3) = 17.6, P < 0.001] (paired t tests). D: laryngeal adductor (thyroarytenoid) EMG activity during swallow was significantly increased at ∼20-min [t(3) = −6.9, P = 0.003] and 90-min postinjury [t(3) = −2.1, P = 0.04] compared with control. Inferior pharyngeal constrictor (thyropharyngeus) activity during swallow was significantly increased at 90-min postinjury [t(3) = −3.3, P = 0.045]. E: representative EMG example of breathing are shown with breathing phases defined using laryngeal drive. The percentages of swallow in each phase were plotted across a 180° circle plot (white indicates swallows in E1; gray indicates swallows in E2; inner circle with blue outline indicates early time point; outer circle with pink outline indicates 90-min time point). A Wilcoxon signed-rank test detected a significant change in swallow breathing coordination, with significantly greater number of swallows occurring during E1 after injury (Z = −1.9, P = 0.05).
Fourth, we found that diaphragm activity during swallow was bilaterally suppressed after lateral C2 hemisection (percent change 90-min postinjury, 22 ± 9% ipsilateral; 40 ± 25% contralateral). This finding was not anticipated, given that the C2 lateral hemisection only suppressed ipsilateral diaphragm breathing activity, with three of four animals showing increased contralateral EMG activity during breathing.
Fifth, there was a significant change in swallow-breathing coordination. Using the defined breathing phases described in Fig. 3E, each swallow initiation was marked in the corresponding phase of breathing. Following spinal cord injury, there was a significant increase in the percentage of swallows that occurred during E1 (i.e., post-I or yield phase).
Finally, we wanted to determine if our observations in the cat were relevant in another animal model, and to determine if the chest wall is a major source of feedback for laryngeal regulation. Figure 4 illustrates three features of data from rat T1 transections that were similar to the cat data after injury: 1) increased laryngeal inspiratory activity, 2) increased diaphragm activity during breathing, and 3) increased laryngeal adductor activity during swallow. A necessary component of the rat experiments was reliable recording of the posterior cricoarytenoid muscle, which is small and very close to the pharyngeal wall. We used the same EMG technique as in cat, but placed one wire in each of the bilateral muscle bellies. As in the cat C2 lateral hemisection, an increase in inspiratory laryngeal activity (170 ± 34% change) was the first feature noted after rat T1 complete spinal cord transection, and this effect was stable over the recording period. We recorded costal (midline) diaphragm activity in the rat experimental preparations, rather than crural (bilateral) activity, because sided effects were not necessary to the analysis. In contrast to the cat C2 lateral hemisection, diaphragmatic respiratory drive increased after T1 transection in every rat (215 ± 63% change), and this effect was significant (Fig. 4). Similarly, there was a significant increase in thyroarytenoid activity (131 ± 19%) during swallow (Fig. 4, C and D).
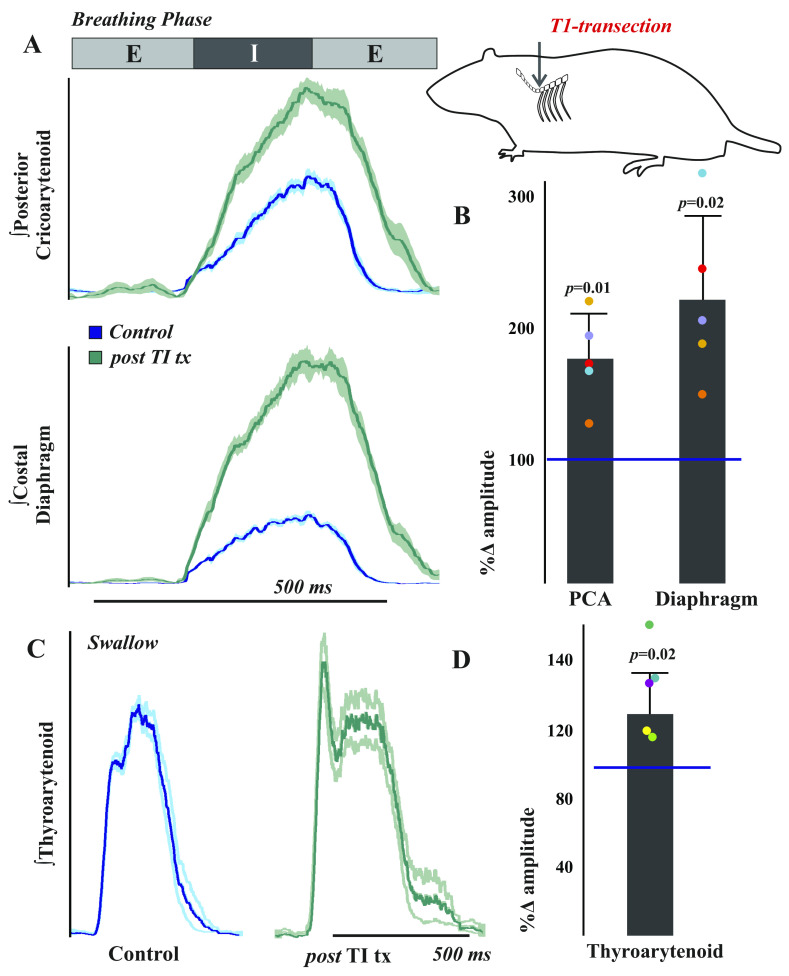
Figure 4.
Respiratory and swallow drive is altered after T1 spinal cord transection in the rat. To test the hypothesis that thoracic afferent feedback may be a significant contributor to laryngeal regulation, we performed T1 total transections in freely breathing (vagi intact) pentobarbital anesthetized female Sprague Dawley rats. A: representative traces of electromyogram (EMG) activity recorded from posterior cricoarytenoid (top) and costal diaphragm (bottom) muscles during eupnea prior to (blue traces) and ∼20 min after (green traces) total transection at the T1 spinal level in rats show increased activity after injury. Traces are waveform averages of the rectified and smoothed (50 ms) EMGs from 1 min of stable eupneic activity. B: EMG amplitudes 20 min after transection were compared with the median amplitude during the control period to control for cycle-by-cycle variability and plotted as a percent of control. As assessed by paired t tests, there were significant increases in posterior cricoarytenoid [t(4) = −4.6, P = 0.01] and costal diaphragm [t(4) = −4.04, P = 0.02] amplitudes posttransection. C: representative waveform averages of thyroarytenoid EMG swallow activity during control and post-T1 total transection (∼20 min) illustrate an increase in thyroarytenoid EMG activity after transection. D: this effect was significant [t(4) = −3.43, P = 0.02].
DISCUSSION
Swallow is a primitive and critical behavior necessary for nutrition and survival (19, 22, 83–86). Some aspects of the swallow motor pattern changed as vertebrates evolved complexity, and it is a common point of disability across a range of human neuro-developmental, traumatic, and degenerative diseases. As the larynx is the central point in regulation of the upper airway, it is vital to regulating airway protective risk. This study demonstrated cross-species effects of spinal cord injury on the upper airway that are indicative of increased risk for aspiration. Moreover, there was behavior-specific facilitation of upper airway motoneurons that are contained entirely within the brainstem. In addition, while we predicted that C2 lateral hemisection would decrease ipsilateral crural diaphragm activity during breathing and swallowing, there was a bilateral suppression during swallow, demonstrating that swallow may be more susceptible to disorder following spinal cord injury.
Intrinsic Laryngeal Muscles
There is limited information regarding the neural circuitry regulating airway protection and other laryngeal behaviors. In contrast, the anatomy and innervation of laryngeal muscles has been well-described. These descriptions, when combined with physiological data such as the current results, aid in elucidating the neural circuitry of laryngeal behaviors. The posterior cricoarytenoid is the intrinsic laryngeal muscle that regulates abduction of the vocal folds during inspiration; it is innervated by the recurrent laryngeal nerve, and it is not active during expiration. In cat, its innervating motoneurons are reported throughout the nucleus ambiguus, with the largest neuron size in the caudal portion (87, 88). In rat, multipolar motoneurons are described in the compact and loose formations of the nucleus ambiguus, mainly in the middle portion of the column, and they have an augmenting discharge pattern during inspiration that is similar to the phrenic nerve output (89, 90). These motoneurons have extensive excitatory and inhibitory synaptic terminals (91), and their connections are found throughout the respiratory and swallow regulatory neuraxis (92). Innervating medullary areas include: several regions of the nucleus tractus solitarius; the midline reticular formation (including the medullary raphe); the pre-Bötzinger complex; and gigantocellular and lateral paragigantocellular reticular nuclei. Innervating pontine areas include: the Kolliker-Fuse nucleus, locus coeruleus, sub-coeruleus, lateral tegmental field, the A5 catecholaminergic cell group, and the medial pontine reticular formation (92).
In contrast, the thyroarytenoid is a primary laryngeal adductor that constitutes the bulk of the vocal folds; it is also innervated by the recurrent laryngeal nerve. In cat, its innervating motoneurons are found in the caudal two-thirds of the nucleus ambiguus continuing past obex (93), and are dorsal to the location of the posterior cricoarytenoid motoneurons (88). They appear as a loose group with a consistent relatively large size (87). In the rat, they are found primarily in the medial nucleus ambiguus in its caudal portion (94). In a pseudorabies tracing study, Van Daele and Cassell (95) found thyroarytenoid connections in areas similar areas to those reported for the posterior cricoarytenoid, and some additional areas. These additional connections were labeled in more regions of the nucleus tractus solitarius and reticular formation (including intermediate and parvocellular formations); the periaqueductal gray; along the ventral respiratory group including the Bötzinger Complex; and in the phrenic nucleus.
Although many brainstem connections to the motoneurons for these muscles have been described, much less is known about the potential anatomical pathways connecting the larynx and spinal cord. In Remmers’ 1973 papers, stimulation of external intercostal nerve afferents evoked laryngeal reflexes that were eliminated by an acute lateral cut (lateral to the ventral horn) at C3. When the stimulation was applied during inspiration, the posterior cricoarytenoid was inhibited, but when stimulated during expiration, the posterior cricoarytenoid and thyroarytenoid were both excited (96, 97). Our use of the T1 transection in the rat underscores the hypothesis that the increase in posterior cricoarytenoid activity after injury may be due to loss of afferent feedback from the chest wall. Information from these afferents travels through several tracts to the brainstem, but the lateral area lesioned by Remmers would have included the spinocerebellar, lateral spinothalamic, and spinoreticular tracts. This is also supported by work from Kirkwood and colleagues showing increased excitation (especially during the E1/post-I phase) of respiratory motoneurons above the lesion in thoracic hemisections which spared the dorsal columns (98), though this may have been due to local interneuron effects. Our recent works studying the effects of acute cerebellectomy on breathing, swallow, and cough suggest that the ascending cerebellar pathways are not responsible (63, 99), leaving the lateral spinothalamic and spinoreticular tracts as possible key pathways.
Dual Valve Hypothesis
A very old hypothesis posits that the diaphragm is active during swallow (100–107). The diaphragm is accepted as the major source of negative pressure that draws air into the lungs during breathing. Our data are consistent with the old but recently neglected hypothesis that the diaphragm acts in a similar aspiration pump role during swallow to draw food/liquid into the esophagus. A reasonable question is, “How can the diaphragm be active during swallow without causing aspiration of material into the lungs?” As published by Pitts et al. (1), the larynx has classically been depicted as a valve. We extended this view to a dual valve system to incorporate the fact that the upper esophageal sphincter works cooperatively with the laryngeal muscles that control the opening to the trachea. In this way, only one “valve” is open at any one time, which ensures effective mechanical movement of air/bolus into the thoracic cavity without a “leak” from the cooperating valve. Common to all behaviors, the diaphragm would function by using negative pressure to draw into the thoracic cavity, including breathing, cough, sneeze, and swallow. Unlike breathing, however, swallow is aided by cooperation from other structures after the bolus passes. This includes closure of the oral cavity and contraction of the tongue and upper pharyngeal muscles, which produces a positive pressure above the food/liquid bolus (108–110).
Although the upper airway is able to exert powerful force on the bolus, dysphagia is common among patients with disordered negative intrathoracic pressure during swallow (24, 109–119). There is extensive literature on how cSCI affects breathing-related phrenic nerve and diaphragm recruitment from the groups of Goshgarian (120, 121), Sieck and Mantilla (122–124), Mitchell and Fuller (125–127), Reier and Lane (128, 129), Alilain and Silver (130–134), and others (135–140); however, we have little knowledge about the effects of cSCI on swallow. The present data demonstrate a bilateral reduction of crural diaphragm activity during swallow, with only an ipsilateral reduction during breathing following C2 lateral hemisection. This provides further evidence of behavior-specific effects, and implies that swallow may be more affected by cervical spinal cord injury than breathing.
Numerous studies of cSCI on breathing have shown that motoneurons and respiration can change throughout the time following injury, even in segments above the lesion (98, 127). There can also be immediate (141) and sustained (142, 143) activation of accessory respiratory muscles to maintain ventilation; their recruitment may play a role in the stability of end-tidal CO2 and respiratory rate after the injury in the present study. The plasticity of laryngeal function observed in a few previous investigations (53, 144–146) warrants further investigation, as evidence of dysphagia worsens health outcomes. For example, Ihalainen et al. (144) showed recovery with decreases in laryngeal penetration and aspiration in the first few months following injury. However, Hamilton et al. (145) postulated that, while symptoms might change after cSCI, dysfunction can be present at all stages including long-term deficits.
We also found that spinal cord injury produced a significant change in swallow breathing coordination, with more postinjury swallows occurring during early expiration (in the E1 phase; also termed post-I or yield), when compared with preinjury swallows. The Kolliker–Fuse nucleus in the pons has been proposed to exert firm control over swallow-breathing coordination, as well as post-I laryngeal adduction (147, 148). However, we observed a permissive effect of swallows occurring during E1, with a behavior-specific change in laryngeal adductor activity after a lesion to the spinal cord rather than the pons. There is a difference in the methodological procedures, as we present EMG activity recorded selectively from the thyroarytenoid muscle, whereas the Dutschmann studies recorded a collection of action potentials from the cervical vagus nerve. However, human studies strongly support pontine modulation of swallow motor activity based on the clinical utility of activating trigeminal nerve c-fibers before/during eating in patients with dysphagia (149–151). Collectively, these findings along with ours strongly suggest that both the spinal cord and pons contribute to swallow breathing coordination, and their precise roles are yet to be determined. Based upon our identification of spinal contributions, swallow likely modulates breathing in a complex rather than straightforward manner, involving multiple central regions.
Dysphagia, especially aspiration of oral secretions, is a significant risk factor for community acquired pneumonia (152) and hospitalization (153). The 2016 annual statistical report from the Spinal Cord Injury Model Systems project says that cervical SCI accounts for 54% of the total population, with high cervical (C1–C4) injury representing 21%. In patients for whom cause of death was known, 65.3% of deaths were due to pneumonia, thus it is the leading known cause. Due to the wide array of functions that involve laryngeal regulation, its dysfunction is of particular concern. The large and unexpected upper airway response to cervical and thoracic lesions demonstrates a need for future key studies investigating the connections between the spinal cord and the larynx. In addition, the behavior-specific effects of the lesions necessitate comprehensive studies to predict the clinical effects of spinal cord injury in humans. There is a strong need for novel therapies or pharmaceutical developments to help these patients at risk for upper airway dysfunction.
SUPPLEMENTAL DATA
Supplemental Fig. S1 and Supplemental Table S1: https://doi.org/10.6084/m9.figshare.20029310.v1.
GRANTS
This study was supported by National Institutes of Health (NIH) Grants NS 110169, HL 103415, HL 155721, and OT2OD023854 (to T.P.); Craig F. Neilsen Foundation Pilot Research Grant 546714 (to T.P.); The Kentucky Spinal Cord and Head Injury Trust (to T.P. and D.R.H.); The Commonwealth of Kentucky Challenge for Excellence (to T.P. and D.R.H.); and the Rebecca F Hammond Endowment (to D.R.H.).
DISCLAIMERS
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.P., K.E.I., A.H., and D.R.H. conceived and designed research; T.P., K.E.I., A.H., M.N.M., M.L.F., K.C.Y., C.L.G., and D.R.H. performed experiments; T.P., K.E.I., M.N.M., and D.R.H. analyzed data; T.P., K.E.I., A.H., M.N.M., M.L.F., K.C.Y., C.L.G., and D.R.H. interpreted results of experiments; T.P., K.E.I., M.N.M., and D.R.H. prepared figures; T.P. and K.E.I. drafted manuscript; T.P., K.E.I., A.H., M.N.M., M.L.F., K.C.Y., C.L.G., and D.R.H. edited and revised manuscript; T.P., K.E.I., A.H., M.N.M., M.L.F., K.C.Y., C.L.G., and D.R.H. approved final version of manuscript.
REFERENCES
- 1. Pitts T, Rose MJ, Mortensen AN, Poliacek I, Sapienza CM, Lindsey BG, Morris KF, Davenport PW, Bolser DC. Coordination of cough and swallow: a meta-behavioral response to aspiration. Respir Physiol Neurobiol 189: 543–551, 2013. doi: 10.1016/j.resp.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cukier-Blaj S, Bewley A, Aviv JE, Murry T. Paradoxical vocal fold motion: a sensory-motor laryngeal disorder. Laryngoscope 118: 367–370, 2008. doi: 10.1097/MLG.0b013e31815988b0. [DOI] [PubMed] [Google Scholar]
- 3. Allegretto M, Morrison M, Rammage L, Lau DP. Selective denervation: reinnervation for the control of adductor spasmodic dysphonia. J Otolaryngol 32: 185–189, 2003. doi: 10.2310/7070.2003.40431. [DOI] [PubMed] [Google Scholar]
- 4. Barton RT. Treatment of spastic dysphonia by recurrent laryngeal nerve section. Laryngoscope 89: 244–249, 1979. doi: 10.1288/00005537-197902000-00007. [DOI] [PubMed] [Google Scholar]
- 5. Neri G, Castiello F, Vitullo F, De Rosa M, Ciammetti G, Croce A. Post-thyroidectomy dysphonia in patients with bilateral resection of the superior laryngeal nerve: a comparative spectrographic study. Acta Otorhinolaryngol Ital 31: 228–234, 2011. [PMC free article] [PubMed] [Google Scholar]
- 6. Fontana GA, Pantaleo T, Lavorini F, Benvenuti F, Gangemi S. Defective motor control of coughing in Parkinson's disease. Am J Respir Crit Care Med 158: 458–464, 1998. doi: 10.1164/ajrccm.158.2.9705094. [DOI] [PubMed] [Google Scholar]
- 7. Fontana GA, Pantaleo T, Lavorini F, Mutolo D, Polli G, Pistolesi M. Coughing in laryngectomized patients. Am J Respir Crit Care Med 160: 1578–1584, 1999. doi: 10.1164/ajrccm.160.5.9901093. [DOI] [PubMed] [Google Scholar]
- 8. Smith Hammond CA, Goldstein LB, Horner RD, Ying J, Gray L, Gonzalez-Rothi L, Bolser DC. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest 135: 769–777, 2009. doi: 10.1378/chest.08-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology 56: 502–506, 2001. doi: 10.1212/wnl.56.4.502. [DOI] [PubMed] [Google Scholar]
- 10. Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson's disease. Dysphagia 23: 297–301, 2008. doi: 10.1007/s00455-007-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ali GN, Wallace KL, Schwartz R, DeCarle DJ, Zagami AS, Cook IJ. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson’s disease. Gastroenterology 110: 383–392, 1996. doi: 10.1053/gast.1996.v110.pm8566584. [DOI] [PubMed] [Google Scholar]
- 12. Bath P, Bath F, Smithard D. Interventions for dysphagia in acute stroke. Cochrane Database Syst Rev 2: CD000323, 1999. doi: 10.1002/14651858.CD000323. [DOI] [PubMed] [Google Scholar]
- 13. Kendall K, Leonard R. Hyoid movements during swallowing in older patients with dysphagia. Arch Otolaryngol Head Neck Surg 127: 1224–1229, 2001. doi: 10.1001/archotol.127.10.1224. [DOI] [PubMed] [Google Scholar]
- 14. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia 11: 93–98, 1996. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 15. Daggett A, Logemann J, Rademaker A, Pauloski B. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia 21: 270–274, 2006. doi: 10.1007/s00455-006-9051-6. [DOI] [PubMed] [Google Scholar]
- 16. Bieger D. Rhombencephalic pathways and neurotransmitters controlling deglutition. Am J Med 111: 85–89, 2001. doi: 10.1016/S0002-9343(01)00824-5. [DOI] [PubMed] [Google Scholar]
- 17. Bosma JF. Deglutition: pharyngeal stage. Physiol Rev 37: 275–300, 1957. doi: 10.1152/physrev.1957.37.3.275. [DOI] [PubMed] [Google Scholar]
- 18. Doty R. Neural organization of deglutition. In Handbook of Physiology. Alimentary Canal, Sect. 6, edited by Code CF. Washington, D.C.: American Physiological Society, 1968, vol. 4. [Google Scholar]
- 19. Miller AJ. Deglutition. Physiol Rev 62: 129–184, 1982. doi: 10.1152/physrev.1982.62.1.129. [DOI] [PubMed] [Google Scholar]
- 20. German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol 102: 1017–1025, 2009. doi: 10.1152/jn.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol (1985) 102: 587–600, 2007. doi: 10.1152/japplphysiol.00456.2006. [DOI] [PubMed] [Google Scholar]
- 22. Doty R, Bosma J. An electromyographic analysis of reflex deglutition. J Neurophysiol 19: 44–60, 1956. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- 23. Pitts T, Rose MJ, Poliacek I, Condrey J, Davenport PW, Bolser DC. Effect of laparotomy on the swallow–breathing relationship in the cat. Lung 193: 129–133, 2015. doi: 10.1007/s00408-014-9662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McConnel FM. Analysis of pressure generation and bolus transit during pharyngeal swallowing. Laryngoscope 98: 71–78, 1988. doi: 10.1288/00005537-198801000-00015. [DOI] [PubMed] [Google Scholar]
- 25. Pitts T, Gayagoy AG, Rose MJ, Poliacek I, Condrey JA, Musselwhite MN, Shen TY, Davenport PW, Bolser DC. Suppression of abdominal motor activity during swallowing in cats and humans. PLoS One 10: e0128245, 2015. [Erratum in PLoS One 13: e0197525, 2018]. doi: 10.1371/journal.pone.0128245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ding P, Fung GS-K, Lin M, Holman SD, German RZ. The effect of bilateral superior laryngeal nerve lesion on swallowing: a novel method to quantitate aspirated volume and pharyngeal threshold in videofluoroscopy. Dysphagia 30: 47–56, 2015. doi: 10.1007/s00455-014-9572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buchholz DW. Neurogenic dysphagia: what is the cause when the cause is not obvious? Dysphagia 9: 245–255, 1994. doi: 10.1007/BF00301918. [DOI] [PubMed] [Google Scholar]
- 28. Coates C, Bakheit AM. Dysphagia in Parkinson’s disease. Eur Neurol 38: 49–52, 1997. doi: 10.1159/000112902. [DOI] [PubMed] [Google Scholar]
- 29. Gordon C, Hewer RL, Wade DT. Dysphagia in acute stroke. Br Med J (Clin Res Ed) 295: 411–414, 1987. doi: 10.1136/bmj.295.6595.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McConnel FM, O'Connor A. Dysphagia associated with pharyngoesophageal segment dysfunction. Acta Otorhinolaryngol Belg 48: 157–163, 1994. [PubMed] [Google Scholar]
- 31. Miller A. The neurobiology of swallowing and dysphagia. Dev Disabil Res Rev 14: 77–86, 2008. doi: 10.1002/ddrr.12. [DOI] [PubMed] [Google Scholar]
- 32. Sue Eisenstadt E. Dysphagia and aspiration pneumonia in older adults. J Am Acad Nurse Pract 22: 17–22, 2010. doi: 10.1111/j.1745-7599.2009.00470.x. [DOI] [PubMed] [Google Scholar]
- 33. Aviv JE, Spitzer J, Cohen M, Ma G, Belafsky P, Close LG. Laryngeal adductor reflex and pharyngeal squeeze as predictors of laryngeal penetration and aspiration. Laryngoscope 112: 338–341, 2002. doi: 10.1097/00005537-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 34. Cvejic L, Harding R, Churchward T, Turton A, Finlay P, Massey D, Bardin PG, Guy P. Laryngeal penetration and aspiration in individuals with stable COPD. Respirology 16: 269–275, 2011. doi: 10.1111/j.1440-1843.2010.01875.x. [DOI] [PubMed] [Google Scholar]
- 35. Robbins J, Coyle J, Rosenbek J, Roecker E, Wood J. Differentiation of normal and abnormal airway protection during swallowing using the penetration-aspiration scale. Dysphagia 14: 228–232, 1999. doi: 10.1007/PL00009610. [DOI] [PubMed] [Google Scholar]
- 36. Mandell LA, Niederman MS. Aspiration pneumonia. N Engl J Med 380: 651–663, 2019. doi: 10.1056/NEJMra1714562. [DOI] [PubMed] [Google Scholar]
- 37. Chaw E, Shem K, Castillo K, Wong S, Chang J. Dysphagia and associated respiratory considerations in cervical spinal cord injury. Top Spinal Cord Inj Rehabil 18: 291–299, 2012. doi: 10.1310/sci1804-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McRae J. The DAISY Project: Identifying Dysphagia in Acute Cervical Spinal Cord Injury. London: Journal of the Intensive Care Society, 2015. [Google Scholar]
- 39. McRae J, Smith C, Beeke S, Emmanuel A. Oropharyngeal dysphagia management in cervical spinal cord injury patients: an exploratory survey of variations to care across specialised and non-specialised units. Spinal Cord Ser Cases 5: 1–10, 2019. doi: 10.1038/s41394-019-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pitts T, Poliacek I, Rose MJ, Reed MD, Condrey JA, Tsai H-W, Zhou G, Davenport PW, Bolser DC. Neurons in the dorsomedial medulla contribute to swallow pattern generation: evidence of inspiratory activity during swallow. PLoS One 13: e0199903, 2018. doi: 10.1371/journal.pone.0199903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kirshblum S, Johnston MV, Brown J, O'Connor KC, Jarosz P. Predictors of dysphagia after spinal cord injury. Arch Phys Med Rehabil 80: 1101–1105, 1999. doi: 10.1016/S0003-9993(99)90068-0. [DOI] [PubMed] [Google Scholar]
- 42. Wolf C, Meiners TH. Dysphagia in patients with acute cervical spinal cord injury. Spinal Cord 41: 347–353, 2003. doi: 10.1038/sj.sc.3101440. [DOI] [PubMed] [Google Scholar]
- 43. Brady S, Miserendino R, Statkus D, Springer T, Hakel M, Stambolis V. Predictors to dysphagia and recovery after cervical spinal cord injury during acute rehabilitation. J Appl Res Clin Exp Ther 4: 1–11, 2004. [Google Scholar]
- 44. Abel R, Ruf S, Spahn B. Cervical spinal cord injury and deglutition disorders. Dysphagia 19: 87–94, 2004. doi: 10.1007/s00455-003-0511-y. [DOI] [PubMed] [Google Scholar]
- 45. Shem K, Castillo K, Naran B. Factors associated with dysphagia in individuals with high tetraplegia. Top Spinal Cord Injury Rehabil 10: 8–18, 2005. doi: 10.1310/HW9N-E1ME-FK6G-00TK. [DOI] [Google Scholar]
- 46. Seidl RO, Nusser-Müller-Busch R, Kurzweil M, Niedeggen A. Dysphagia in acute tetraplegics: a retrospective study. Spinal Cord 48: 197–201, 2010. doi: 10.1038/sc.2009.102. [DOI] [PubMed] [Google Scholar]
- 47. Shin JC, Yoo JH, Lee YS, Goo HR, Kim DH. Dysphagia in cervical spinal cord injury. Spinal Cord 49: 1008–1013, 2011. doi: 10.1038/sc.2011.34. [DOI] [PubMed] [Google Scholar]
- 48. Shem K, Castillo K, Wong S, Chang J. Dysphagia in individuals with tetraplegia: incidence and risk factors. J Spinal Cord Med 34: 85–92, 2011. doi: 10.1179/107902610X12911165974981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shem K, Castillo K, Wong SL, Chang J, Kolakowsky-Hayner S. Dysphagia and respiratory care in individuals with tetraplegia: incidence, associated factors, and preventable complications. Top Spinal Cord Inj Rehabil 18: 15–22, 2012. doi: 10.1310/sci1801-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shem KL, Castillo K, Wong SL, Chang J, Kao M-C, Kolakowsky-Hayner SA. Diagnostic accuracy of bedside swallow evaluation versus videofluoroscopy to assess dysphagia in individuals with tetraplegia. PM R 4: 283–289, 2012. doi: 10.1016/j.pmrj.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 51. Lee JC, Gross BW, Rittenhouse KJ, Vogel AR, Vellucci A, Alzate J, Gillio M, Rogers FB. A bitter pill to swallow: dysphagia in cervical spine injury. J Surg Res 201: 388–393, 2016. doi: 10.1016/j.jss.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 52. Hayashi T, Fujiwara Y, Sakai H, Maeda T, Ueta T, Shiba K. Risk factors for severe dysphagia in acute cervical spinal cord injury. Spinal Cord 55: 940–943, 2017. doi: 10.1038/sc.2017.63. [DOI] [PubMed] [Google Scholar]
- 53. Ihalainen T, Rinta-Kiikka I, Luoto TM, Koskinen EA, Korpijaakko-Huuhka A-M, Ronkainen A. Traumatic cervical spinal cord injury: a prospective clinical study of laryngeal penetration and aspiration. Spinal Cord 55: 979–984, 2017. doi: 10.1038/sc.2017.71. [DOI] [PubMed] [Google Scholar]
- 54. Ihalainen T, Rinta-Kiikka I, Luoto TM, Thesleff T, Helminen M, Korpijaakko-Huuhka A-M, Ronkainen A. Risk factors for laryngeal penetration-aspiration in patients with acute traumatic cervical spinal cord injury. Spine J 18: 81–87, 2017. doi: 10.1016/j.spinee.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 55. Ihalainen T, Rinta-Kiikka I, Luoto TM, Thesleff T, Helminen M, Korpijaakko-Huuhka A-M, Ronkainen A. Risk factors for laryngeal penetration-aspiration in patients with acute traumatic cervical spinal cord injury. Spine J 18: 81–87, 2018. doi: 10.1016/j.spinee.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 56. Ramczykowski T, Grüning S, Gurr A, Muhr G, Horch C, Meindl R, Swol J. [Aspiration pneumonia after spinal cord injury. Placement of PEG tubes as effective prevention]. Unfallchirurg 115: 427–432, 2012. doi: 10.1007/s00113-010-1889-2. [DOI] [PubMed] [Google Scholar]
- 57. Frankel H, Coll J, Charlifue S, Whiteneck G, Gardner B, Jamous M, Krishnan K, Nuseibeh I, Savic G, Sett P. Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord 36: 266–274, 1998. doi: 10.1038/sj.sc.3100638. [DOI] [PubMed] [Google Scholar]
- 58. Richard-Denis A, Erhmann Feldman D, Thompson C, Mac-Thiong J-M. The impact of acute management on the occurrence of medical complications during the specialized spinal cord injury acute hospitalization following motor-complete cervical spinal cord injury. J Spinal Cord Med 41: 388–396, 2018. doi: 10.1080/10790268.2017.1350331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bassotti G, Germani U, Pagliaricci S, Plesa A, Giulietti O, Mannarino E, Morelli A. Esophageal manometric abnormalities in Parkinson’s disease. Dysphagia 13: 28–31, 1998. doi: 10.1007/PL00009546. [DOI] [PubMed] [Google Scholar]
- 60. Bulow M, Olsson R, Ekberg O. Videomanometric analysis of supraglottic swallow, effortful swallow, and chin tuck in patients with pharyngeal dysfunction. Dysphagia 16: 190–195, 2001. doi: 10.1007/s00455-001-0065-9. [DOI] [PubMed] [Google Scholar]
- 61. Hoffman MR, Mielens JD, Ciucci MR, Jones CA, Jiang JJ, McCulloch TM. High-resolution manometry of pharyngeal swallow pressure events associated with effortful swallow and the Mendelsohn maneuver. Dysphagia 27: 418–426, 2012. doi: 10.1007/s00455-011-9385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol 587: 713–719, 2009. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Reed MD, English M, English C, Huff A, Poliacek I, Musselwhite MN, Howland DR, Bolser DC, Pitts T. The role of the cerebellum in control of swallow: evidence of inspiratory activity during swallow. Lung 197: 235–240, 2019. doi: 10.1007/s00408-018-00192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huff A, Reed MD, Iceman KE, Howland DR, Pitts T. Sex-specific vagal and spinal modulation of breathing with chest compression. PLoS One 15: e0234193, 2020. doi: 10.1371/journal.pone.0234193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Basmajian J, Stecko G. A new bipolar electrode for electromyography. J Appl Physiol 17: 849, 1962. doi: 10.1152/jappl.1962.17.5.849. [DOI] [Google Scholar]
- 66. Poliacek I, Stránsky A, Jakus J, Baráni H, Tomori Z, Halasová E. Activity of the laryngeal abductor and adductor muscles during cough, expiration and aspiration reflexes in cats. Physiol Res 52: 749–762, 2003. [PubMed] [Google Scholar]
- 67. Poliacek I, Jakus J, Knocikova J, Barani H, Halasova EVN, Visnovcova N. Medullary raphe midline is involved in production of expulsive expirations in anesthetized rabbits. J Physiol Pharmacol 59: 597–605, 2008. [PubMed] [Google Scholar]
- 68. Poliacek I, Rose MJ, Corrie LWC, Wang C, Jakus J, Barani H, Stransky A, Polacek H, Halasova E, Bolser DC. Short reflex expirations (expiration reflexes) induced by mechanical stimulation of the trachea in anesthetized cats. Cough 4: 1, 2008. doi: 10.1186/1745-9974-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hof AL, Van den Berg J. EMG to force processing. I. An electrical analogue of the Hill muscle model. J Biomech 14: 747–758, 1981. doi: 10.1016/0021-9290(81)90031-2. [DOI] [PubMed] [Google Scholar]
- 70. Gallego JA, Dideriksen JL, Holobar A, Ibáñez J, Pons JL, Louis ED, Rocon E, Farina D. Influence of common synaptic input to motor neurons on the neural drive to muscle in essential tremor. J Neurophysiol 113: 182–191, 2015. doi: 10.1152/jn.00531.2014. [DOI] [PubMed] [Google Scholar]
- 71. Pitts T, Huff A, Reed M, Iceman K, Mellen N. Evidence of intermediate reticular formation involvement in swallow pattern generation, recorded optically in the neonate rat sagittally sectioned hindbrain. J Neurophysiol 125: 993–1005, 2021. doi: 10.1152/jn.00623.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Huff A, Reed MD, Iceman KE, Howland DR, Pitts T. Sex-specific vagal and spinal modulation of swallow and its coordination with breathing. PLoS One 15: e0234194, 2020. doi: 10.1371/journal.pone.0234194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Huff A, Reed MD, Smith BK, Brown EH, Ovechkin AV, Pitts T. Strategies for the integration of cough and swallow to maintain airway protection in humans. Lung 196: 601–608, 2018. doi: 10.1007/s00408-018-0133-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Spearman DG, Poliacek I, Rose MJ, Bolser DC, Pitts T. Variability of the pharyngeal phase of swallow in the cat. PLoS One 9: e106121, 2014. doi: 10.1371/journal.pone.0106121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Trelease R, Sieck G, Harper R. A new technique for acute and chronic recording of crural diaphragm EMG in cats. Electroencephalogr Clin Neurophysiol 53: 459–462, 1982. doi: 10.1016/0013-4694(82)90011-6. [DOI] [PubMed] [Google Scholar]
- 76. Mantilla CB, Sieck GC. Key aspects of phrenic motoneuron and diaphragm muscle development during the perinatal period. J Appl Physiol (1985) 104: 1818–1827, 2008. doi: 10.1152/japplphysiol.01192.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fournier M, Sieck GC. Somatotopy in the segmental innervation of the cat diaphragm. J Appl Physiol (1985) 64: 291–298, 1988. doi: 10.1152/jappl.1988.64.1.291. [DOI] [PubMed] [Google Scholar]
- 78. Mondello S, Jefferson S, Tester N, Howland D. Impact of treatment duration and lesion size on effectiveness of chondroitinase treatment post-SCI. Exp Neurol 267: 64–77, 2015. doi: 10.1016/j.expneurol.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mondal P, Abu‐Hasan M, Saha A, Pitts T, Rose M, Bolser DC, Davenport PW. Effect of laparotomy on respiratory muscle activation pattern. Physiol Rep 4: e12668, 2016. doi: 10.14814/phy2.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Beecher HK. Effect of laparotomy on lung volume. Demonstration of a new type of pulmonary collapse. J Clin Invest 12: 651–658, 1933. doi: 10.1172/JCI100526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Beecher HK. The measured effect of laparotomy on the respiration. J Clin Invest 12: 639–650, 1933. doi: 10.1172/JCI100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Farkas GA, De Troyer A. Effects of midline laparotomy on expiratory muscle activation in anesthetized dogs. J Appl Physiol (1985) 67: 599–605, 1989. doi: 10.1152/jappl.1989.67.2.599. [DOI] [PubMed] [Google Scholar]
- 83. Dullemeijer P. The functional morphology of the head of the common viper. Arch Néerl Zool 11: 387–497, 1956. doi: 10.1163/036551656X00139. [DOI] [Google Scholar]
- 84. Herring SW, Scapino RP. Physiology of feeding in miniature pigs. J Morphol 141: 427–460, 1973. doi: 10.1002/jmor.1051410405. [DOI] [PubMed] [Google Scholar]
- 85. Gupta O. Studies on the morphology, histology and the swallowing mechanism of the digestive tract of a carnivorous fish, Xenentodon cancila (Ham.). Okajimas folia Anat Jpn 48: 29–51, 1971. doi: 10.2535/ofaj1936.48.1_29. [DOI] [PubMed] [Google Scholar]
- 86. Negus V. The mechanism of swallowing: President’s address. Proc R Soc Med 36: 85–92, 1942. [PMC free article] [PubMed] [Google Scholar]
- 87. Pásaro R, Lobera B, Gonzalez-Baron S, Delgado-Garcia J. Cytoarchitectonic organization of laryngeal motoneurons within the nucleus ambiguus of the cat. Exp Neurol 82: 623–634, 1983. doi: 10.1016/0014-4886(83)90085-7. [DOI] [PubMed] [Google Scholar]
- 88. Yoshida Y, Miyazaki T, Hirano M, Shin T, Kanaseki T. Arrangement of motoneurons innervating the intrinsic laryngeal muscles of cats as demonstrated by horseradish peroxidase. Acta Otolaryngol 94: 329–334, 1982. doi: 10.3109/00016488209128920. [DOI] [PubMed] [Google Scholar]
- 89. Berkowitz RG, Chalmers J, Sun Q-J, Pilowsky P. Identification of posterior cricoarytenoid motoneurons in the rat. Ann Otol Rhinol Laryngol 108: 1033–1041, 1999. doi: 10.1177/000348949910801103. [DOI] [PubMed] [Google Scholar]
- 90. Berkowitz RG, Chalmers J, Sun Q-J, Pilowsky P. Intracellular recording from posterior cricoarytenoid motoneurons in the rat. Ann Otol Rhinol Laryngol 108: 1120–1125, 1999. doi: 10.1177/000348949910801205. [DOI] [PubMed] [Google Scholar]
- 91. Hayakawa T, Zheng JQ, Maeda S, Ito H, Seki M, Yajima Y. Synaptology and ultrastructural characteristics of laryngeal cricothyroid and posterior cricoarytenoid motoneurons in the nucleus ambiguus of the rat. Anat Embryol (Berl) 200: 301–311, 1999. doi: 10.1007/s004290050281. [DOI] [PubMed] [Google Scholar]
- 92. Waldbaum S, Hadziefendic S, Erokwu B, Zaidi S, Haxhiu M. CNS innervation of posterior cricoarytenoid muscles: a transneuronal labeling study. Respir Physiol 126: 113–125, 2001. doi: 10.1016/s0034-5687(01)00200-6. [DOI] [PubMed] [Google Scholar]
- 93. Portillo F, Pásaro R. Location of motoneurons supplying the intrinsic laryngeal muscles of rats. Brain Behav Evol 32: 220–225, 1988. doi: 10.1159/000116549. [DOI] [PubMed] [Google Scholar]
- 94. Hernández‐Morato I, Valderrama‐Canales FJ, Berdugo G, Arias G, McHanwell S, Sañudo J, Vázquez T, Pascual‐Font A. Reorganization of laryngeal motoneurons after crush injury in the recurrent laryngeal nerve of the rat. J Anat 222: 451–461, 2013. doi: 10.1111/joa.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Van Daele DJ, Cassell MD. Multiple forebrain systems converge on motor neurons innervating the thyroarytenoid muscle. Neuroscience 162: 501–524, 2009. doi: 10.1016/j.neuroscience.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Remmers JE. Extra‐segmental reflexes derived from intercostal afferents: phrenic and laryngeal responses. J Physiol 233: 45–62, 1973. doi: 10.1113/jphysiol.1973.sp010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Remmers JE, Tsiaras W. Effect of lateral cervical cord lesions on the respiratory rhythm of anaesthetized, decerebrate cats after vagotomy. J Physiol 233: 63–74, 1973. doi: 10.1113/jphysiol.1973.sp010297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ford TW, Anissimova NP, Meehan CF, Kirkwood PA. Functional plasticity in the respiratory drive to thoracic motoneurons in the segment above a chronic lateral spinal cord lesion. J Neurophysiol 115: 554–567, 2016. doi: 10.1152/jn.00614.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Musselwhite MN, Shen TY, Rose MJ, Iceman KE, Poliacek I, Pitts T, Bolser DC. Differential effects of acute cerebellectomy on cough in spontaneously breathing cats. PLoS One 16: e0253060, 2021. doi: 10.1371/journal.pone.0253060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kronecker H, Meltzer S. Die Bedentung des musc. mylohyoid. fur den ersten Act der Schluckbewegung. Du Bois-Reymond’s Archiv f Physiologie 1879–1880: 13, 1880. [Google Scholar]
- 101. Kronecker H, Meltzer S. Uber die Vorgange beim Schlucken. Du Bois-Reymond’s Archiv 1879–1880: 18, 1880. [Google Scholar]
- 102. Kronecker H, Meltzer S. Uber den Schluckakt u. die Rolles des Cardia bei demselben. Du Bois-Reymond’s Archiv f Physiologie 1880–1881: 17–18, 1881. [Google Scholar]
- 103. Rosenthal J. De l’influence du nerf pneumogastrique et du nerf laryngé supérieur sur les movements du diaphragm. Comptes Rendus 52: 764, 1861. [Google Scholar]
- 104. Bidder F. Beiträge zur Kenntniss der Wirkungen des Nervus laryngeus superior. Du Bois-Reymond’s Archiv f Physiologie 1864–1865: 492–507, 1865. [Google Scholar]
- 105. Blumberg J. Untersuchungen uber die Hemmungsfunction des N. laryng. sup. (Inaugural Dissertation). Dorpat, Germany, 1865. [Google Scholar]
- 106. Waller A, Prevost J. Note relative aux nerfs sensitifs qui president aux phenomenes reflexes de la deglutition. Comptes Rendus 16 Aug: 480, 1869. [Google Scholar]
- 107. Meltzer S. Das Schluckcentrum, seine Irradiationen u. die allgemine Bedeutung derselben (Inaugural Dissertation). Physiol Inst. Berlin, Germany. 1882. [Google Scholar]
- 108. McConnel FM, Cerenko D, Jackson RT, Guffin TN Jr.. Timing of major events of pharyngeal swallowing. Arch Otolaryngol Head Neck Surg 114: 1413–1418, 1988. doi: 10.1001/archotol.1988.01860240063025. [DOI] [PubMed] [Google Scholar]
- 109. McConnel FM, Cerenko D, Mendelsohn MS. Manofluorographic analysis of swallowing. Otolaryngol Clin North Am 21: 625–635, 1988. [PubMed] [Google Scholar]
- 110. McConnel FM, Hester TR, Mendelsohn MS, Logemann JA. Manofluorography of deglutition after total laryngopharyngectomy. Plast Reconstr Surg 81: 346–351, 1988. doi: 10.1097/00006534-198803000-00005. [DOI] [PubMed] [Google Scholar]
- 111. Cerenko D, McConnel FM, Jackson RT. Quantitative assessment of pharyngeal bolus driving forces. Otolaryngol Head Neck Surg 100: 57–63, 1989. doi: 10.1177/019459988910000109. [DOI] [PubMed] [Google Scholar]
- 112. Ku DN, Ma PP, McConnel FM, Cerenko D. A kinematic study of the oropharyngeal swallowing of a liquid. Ann Biomed Eng 18: 655–669, 1990. doi: 10.1007/BF02368453. [DOI] [PubMed] [Google Scholar]
- 113. McConnel FM, Cerenko D, Hersh T, Weil LJ. Evaluation of pharyngeal dysphagia with manofluorography. Dysphagia 2: 187–195, 1988. doi: 10.1007/BF02414425. [DOI] [PubMed] [Google Scholar]
- 114. McConnel FM, Cerenko D, Jackson RT, Hersh T. Clinical application of the manofluorogram. Laryngoscope 98: 705–711, 1988. doi: 10.1288/00005537-198807000-00003. [DOI] [PubMed] [Google Scholar]
- 115. McConnel FM, Guffin TN Jr, Cerenko D. The effect of asymmetric pharyngoesophageal pressures on manofluorographic measurements. Laryngoscope 101: 510–515, 1991. doi: 10.1288/00005537-199105000-00012. [DOI] [PubMed] [Google Scholar]
- 116. McConnel FM, Guffin TN Jr, Cerenko D, Ko AS. The effects of bolus flow on vertical pharyngeal pressure measurement in the pharyngoesophageal segment: clinical significance. Otolaryngol Head Neck Surg 106: 169–174, 1992. [PubMed] [Google Scholar]
- 117. McConnel FM, Mendelsohn MS, Logemann JA. Examination of swallowing after total laryngectomy using manofluorography. Head Neck Surg 9: 3–12, 1986. doi: 10.1002/hed.2890090103. [DOI] [PubMed] [Google Scholar]
- 118. McConnel FM, Mendelsohn MS, Logemann JA. Manofluorography of deglutition after supraglottic laryngectomy. Head Neck Surg 9: 142–150, 1987. doi: 10.1002/hed.2890090303. [DOI] [PubMed] [Google Scholar]
- 119. Mendelsohn MS, McConnel FM. Function in the pharyngoesophageal segment. Laryngoscope 97: 483–489, 1987. doi: 10.1288/00005537-198704000-00014. [DOI] [PubMed] [Google Scholar]
- 120. Goshgarian HG. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir Physiol Neurobiol 169: 85–93, 2009. doi: 10.1016/j.resp.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hadley SD, Walker PD, Goshgarian HG. Effects of serotonin inhibition on neuronal and astrocyte plasticity in the phrenic nucleus 4 h following C2 spinal cord hemisection. Exp Neurol 160: 433–445, 1999. doi: 10.1006/exnr.1999.7238. [DOI] [PubMed] [Google Scholar]
- 122. Mantilla CB, Sieck GC. Invited review: mechanisms underlying motor unit plasticity in the respiratory system. J Appl Physiol (1985) 94: 1230–1241, 2003. doi: 10.1152/japplphysiol.01120.2002. [DOI] [PubMed] [Google Scholar]
- 123. Mantilla CB, Bailey JP, Zhan W-Z, Sieck GC. Phrenic motoneuron expression of serotonergic and glutamatergic receptors following upper cervical spinal cord injury. Exp Neurol 234: 191–199, 2012. doi: 10.1016/j.expneurol.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol 179: 57–63, 2011. doi: 10.1016/j.resp.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Fuller DD, Johnson SM, Johnson RA, Mitchell GS. Chronic cervical spinal sensory denervation reveals ineffective spinal pathways to phrenic motoneurons in the rat. Neuroscience Lett 323: 25–28, 2002. doi: 10.1016/S0304-3940(02)00121-0. [DOI] [PubMed] [Google Scholar]
- 126. Fuller DD, Johnson SM, Olson EB, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci 23: 2993–3000, 2003. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mitchell GS, Johnson SM. Plasticity in respiratory motor control: invited review: neuroplasticity in respiratory motor control. J Appl Physiol (1985) 94: 358–374, 2003. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- 128. Lane MA, Lee K-Z, Fuller DD, Reier PJ. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol 169: 123–132, 2009. doi: 10.1016/j.resp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lane MA, Fuller DD, White TE, Reier PJ. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends Neurosci 31: 538–547, 2008. doi: 10.1016/j.tins.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature 475: 196–200, 2011. doi: 10.1038/nature10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Alilain WJ, Li X, Horn KP, Dhingra R, Dick TE, Herlitze S, Silver J. Light-induced rescue of breathing after spinal cord injury. J Neurosci 28: 11862–11870, 2008. doi: 10.1523/JNEUROSCI.3378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Alilain WJ, Silver J. Shedding light on restoring respiratory function after spinal cord injury. Front Mol Neurosci 2: 18, 2009. doi: 10.3389/neuro.02.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sharma H, Alilain WJ, Sadhu A, Silver J. Treatments to restore respiratory function after spinal cord injury and their implications for regeneration, plasticity and adaptation. Exp Neurol 235: 18–25, 2012. doi: 10.1016/j.expneurol.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Awad BI, Warren PM, Steinmetz MP, Alilain WJ. The role of the crossed phrenic pathway after cervical contusion injury and a new model to evaluate therapeutic interventions. Exp Neurol 248: 398–405, 2013. doi: 10.1016/j.expneurol.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 135. DiMarco AF. Phrenic nerve stimulation in patients with spinal cord injury. Respir Physiol Neurobiol 169: 200–209, 2009. doi: 10.1016/j.resp.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 136. Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci 23: 2494–2501, 2003. doi: 10.1523/JNEUROSCI.23-06-02494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ginsborg B, Hirst G. The effect of adenosine on the release of the transmitter from the phrenic nerve of the rat. J Physiol 224: 629–645, 1972. doi: 10.1113/jphysiol.1972.sp009916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Golder FJ, Reier PJ, Bolser DC. Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion. J Neurosci 21: 8680–8689, 2001. doi: 10.1523/JNEUROSCI.21-21-08680.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Polentes J, Stamegna J, Nieto-Sampedro M, Gauthier P. Phrenic rehabilitation and diaphragm recovery after cervical injury and transplantation of olfactory ensheathing cells. Neurobiol Dis 16: 638–653, 2004. doi: 10.1016/j.nbd.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 140. Baussart B, Stamegna J, Polentes J, Tadié M, Gauthier P. A new model of upper cervical spinal contusion inducing a persistent unilateral diaphragmatic deficit in the adult rat. Neurobiol Dis 22: 562–574, 2006. doi: 10.1016/j.nbd.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 141. Jensen VN, Alilain WJ, Crone SA. Role of propriospinal neurons in control of respiratory muscles and recovery of breathing following injury. Front Syst Neurosci 13: 84, 2020. doi: 10.3389/fnsys.2019.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. de Paleville DGT, Sayenko DG, Aslan SC, Folz RJ, McKay WB, Ovechkin AV. Respiratory motor function in seated and supine positions in individuals with chronic spinal cord injury. Respir Physiol Neurobiol 203: 9–14, 2014. doi: 10.1016/j.resp.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yokoba M, Abe T, Katagiri M, Tomita T, Easton PA. Respiratory muscle electromyogram and mouth pressure during isometric contraction. Respir Physiol Neurobiol 137: 51–60, 2003. doi: 10.1016/s1569-9048(03)00092-2. [DOI] [PubMed] [Google Scholar]
- 144. Ihalainen T, Luoto TM, Rinta-Kiikka I, Ronkainen A, Korpijaakko-Huuhka AM. Traumatic cervical spinal cord injury: recovery of penetration/aspiration and functional feeding outcome. Spinal Cord 56: 1000–1007, 2018. doi: 10.1038/s41393-018-0091-1. [DOI] [PubMed] [Google Scholar]
- 145. Hamilton VK, Pitts LL, Walaszek EA, Cherney LR. Videofluoroscopic profiles of swallowing and airway protection post-traumatic cervical spinal cord injury. Dysphagia, 2022. doi: 10.1007/s00455-022-10407-7. [DOI] [PubMed] [Google Scholar]
- 146. Lee SJ, Huh S, Ko S-H, Min JH, Ko H-Y. Utilizing pulmonary function parameters to predict dysphagia in individuals with cervical spinal cord injuries. Ann Rehabil Med 45: 450–458, 2021. doi: 10.5535/arm.21161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Dutschmann M, Herbert H. The Kölliker‐Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off‐switch and upper airway resistance in rat. Eur J Neurosci 24: 1071–1084, 2006. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- 148. Bautista TG, Dutschmann M. Ponto‐medullary nuclei involved in the generation of sequential pharyngeal swallowing and concomitant protective laryngeal adduction in situ. J Physiol 592: 2605–2623, 2014. doi: 10.1113/jphysiol.2014.272468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Nakato R, Manabe N, Shimizu S, Hanayama K, Shiotani A, Hata J, Haruma K. Effects of capsaicin on older patients with oropharyngeal dysphagia: a double-blind, placebo-controlled, crossover study. Digestion 95: 210–220, 2017. [Erratum in Digestion 96: 184, 2017]. doi: 10.1159/000463382. [DOI] [PubMed] [Google Scholar]
- 150. Wang Z, Wu L, Fang Q, Shen M, Zhang L, Liu X. Effects of capsaicin on swallowing function in stroke patients with dysphagia: a randomized controlled trial. J Stroke Cerebrovasc Dis 28: 1744–1751, 2019. doi: 10.1016/j.jstrokecerebrovasdis.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 151. Cui F, Yin Q, Wu C, Shen M, Zhang Y, Ma C, Zhang H, Shen F. Capsaicin combined with ice stimulation improves swallowing function in patients with dysphagia after stroke: a randomised controlled trial. J Oral Rehabil 47: 1297–1303, 2020. doi: 10.1111/joor.13068. [DOI] [PubMed] [Google Scholar]
- 152. Almirall J, Rofes L, Serra-Prat M, Icart R, Palomera E, Arreola V, Clavé P. Oropharyngeal dysphagia is a risk factor for community-acquired pneumonia in the elderly. Eur Respir J 41: 923–928, 2013. doi: 10.1183/09031936.00019012. [DOI] [PubMed] [Google Scholar]
- 153. Komiya K, Ishii H, Kadota J-I. Healthcare-associated pneumonia and aspiration pneumonia. Aging Dis 6: 27–37, 2015. doi: 10.14336/AD.2014.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1 and Supplemental Table S1: https://doi.org/10.6084/m9.figshare.20029310.v1.