Keywords: chemical biology, glycobiology, glycosaminoglycans, interactome, proteoglycan
Abstract
Proteoglycans are now well regarded as key facilitators of cell biology. Although a majority of their interactions and functions are attributed to the decorating glycosaminoglycan chains, there is a growing appreciation for the roles of the proteoglycan core protein and for considering proteoglycans as replete protein-glycan conjugates. This appreciation, seeded by early work in proteoglycan biology, is now being advanced and exalted by modern approaches in chemical glycobiology. In this review, we discuss up-and-coming methods to unearth the fine-scale architecture of proteoglycans that modulate their functions and interactions. Crucial to these efforts is the production of chemically defined materials, including semisynthetic proteoglycans and the in situ capture of interacting proteins. Together, the integration of chemical biology approaches promises to expedite the dissection of the structural heterogeneity of proteoglycans and deliver refined insight into their functions.
INTRODUCTION
Proteoglycans (PGs) are a unique class of evolutionarily conserved glycoconjugates demarcated by the presence of poly-sulfated, O-linked glycosaminoglycan (GAG) chains covalently attached at one or more sites to a core protein (1). Four major classes of PGs (syndecans, glypicans, lecticans, and small leucine-rich PGs) dominate the study of PG biology. Although they are distinguished by unique core protein sequences, PGs can embody a collection of structural variants due to the polydispersity of GAGs. These linear glycan chains are arranged in domains of sulfated and nonsulfated regions of repeating disaccharides of chondroitin sulfate (CS), heparan sulfate (HS), dermatan sulfate (DS), or keratan sulfate (KS) (Fig. 1A) (1). Differential GAG sulfation patterns result in fine structural variations across different tissues that create binding motifs for various GAG-binding proteins (GAGBPs) (4, 5). Complicating the matter, a given PG can embody an assortment of structural variants across different tissue and even cell types. This is due to differential expression of the plethora of enzymes involved in PG biosynthesis and a lack of template, resulting in changes to the length, number, or identity of GAG chains, down to subtle differences in the extent of GAG sulfation and domain organization (6). Furthermore, the entire PG glycoconjugate can be present as membrane-bound molecules, using either transmembrane or glycophosphatidylinositol (GPI) motifs, or as soluble entities, which are shed by proteases into the extracellular milieu (1).
Figure 1.
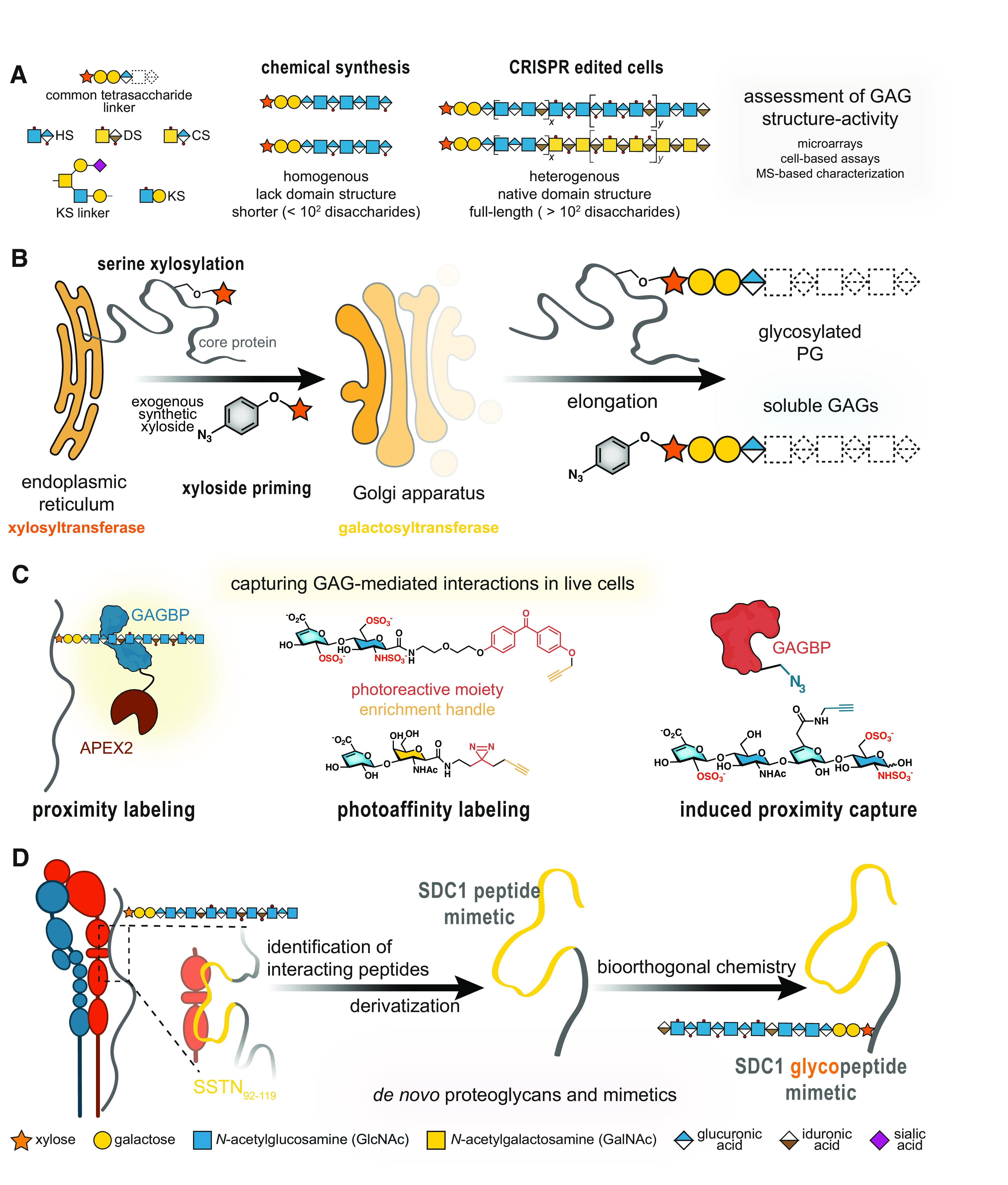
Modern chemical biology approaches to investigate proteoglycan biology. A: chemoenzymatic and total synthesis strategies result in homogenous yet short GAG oligosaccharides, which do not span the lengths or recapitulate the heterogeneity and domain structure of native GAGs. B: synthetic xylosides (bottom) can hijack GAG biosynthesis by mimicking xylosylated core proteins and serving as a substrate for the β4GalT7 galactosyltransferase for elongation. These xylosides can be functionalized with chemical moieties [i.e., azide (2, 3)] for biorthogonal conjugation to surfaces and/or proteins. C: PGs, GAG-binding proteins (GAGBPs), and GAG disaccharides can be modified to enable capture of interaction partners. Proximity labeling with enzymes (e.g., APEX2) tags proteins within a short radius, capturing both direct and indirect interactors. Appending a photoreactive moiety [i.e., benzophenone (top) or diazirine (bottom)] captures interactors of proteins or GAGs (depicted). Induced proximity capture, which requires the modification of both the GAG and associated GAGBP, permits cross linking of GAG to GAGBP complexes. D: polypeptide mimics, such as those derived from the syndecan-1 peptide SSTN92-119 (yellow), permit access to PG glycoconjugates via covalent attachment with GAG oligosaccharides. GAGs, glycosaminoglycans; GAGBPs, GAG-binding proteins; PGs, proteoglycans; SDC1, syndecan-1.
As a consequence of their multivariate nature, PGs can interact with a wide range of proteins to regulate essential functions (7). The diversified roles of PGs are attributed to unique core protein sequences, GAG occupancy (number of GAG modification sites per protein), GAG composition, and membrane localization. As the GAG chains dominate PG size, binding often occurs through charge and orientation-dependent interactions with GAG chains. Indeed, it is estimated that ∼74% of the interactions with syndecans occur via GAG chains (8), and thus GAGs have historically garnered more attention than the core proteins. However, a growing number of studies are commanding greater attention to core protein-dependent interactions, such as those observed with PGs binding CA10, FAM19A, and lacritin, with the latter requiring enzymatic digestion of GAG chains for efficient binding (9–11). For all their biological functions, it is extremely attractive to understand PG interactomes (the complete repertoire of interacting proteins), particularly while dissecting the architectural element responsible (core protein, GAG occupancy/valency, GAG composition and sulfation, membrane localization), and even more so, simultaneously performing such analyses in live cells to preserve the native presentation of ligands and receptors (2). Given their extreme structural diversity, the theoretical PG interactome is vast, and it may hold important clues toward solving PG-mediated diseases, including autoimmunity, cancers, and neurodegeneration (7).
Although attempts have been made to explore the PG interactome, many techniques fall short of appreciating its complexity (12). Indeed, conventional techniques to control PG architecture and identify interactions that rely on overexpression or knockdown can lead to off-target effects, aberrant expression, and/or hypoglycosylation of target proteins (13, 14). Similarly, reductionist approaches may provide only a partial picture of the PG interactome. From this viewpoint, it is evident that multidisciplinary approaches are required to study the effects of PG architecture toward binding and function, and we are witnessing a growing expanse of chemical biology tools to explore these interactomes. Here, we outline strategies for delineating PG interactions and functions. First, we discuss methods for creating chemically defined GAGs and their use in capturing interactions in live cells and highlight the use of high-resolution mass spectrometry (MS) to discover new PGs and dissect GAG occupancy. We also shed light on the lesser-known “part-time” PGs and the seemingly conflicting biological roles of the deglycosylated versus fully glycosylated PG. Finally, we discuss pioneering methods to generate PG mimetics that enable architecture-function relationship studies.
TAKING CHARGE: A DEEPER EXPLORATION OF GAG-MEDIATED INTERACTIONS
It is a longstanding view that most PG interactions occur via interactions with the sulfated, negatively charged GAGs—typically with positively charged biomolecules, such as growth factors (15, 16). As glycosylation is not template-driven, GAG-modifying enzymes generate diverse patterns of sulfation that encode binding sites for proteins (16). Hence, access to well-defined oligosaccharides, complemented with methods to interrogate GAG interactomes, can provide important structural details to define interactions with GAGBPs. Heroic efforts in the chemoenzymatic and total synthesis of sulfation-defined and length-controlled GAG oligosaccharides have been especially impactful in the study of rare GAG modifications (17), and such products have been invaluable tool compounds (18–20) (Fig. 1A). For a deeper delve into GAG synthesis by total or chemoenzymatic synthesis, we recommend the following reviews by Dulaney and Huang (21) and by Gottschalk and Elling (22). Although efforts to control GAG sulfation and extend oligomer length remain prevalent, it is well acknowledged that these analogs do not recapitulate the length, domain arrangement, or heterogeneity of native GAGs. As such, newer chemical biology approaches sacrifice total homogeneity in favor of GAGs with defined disaccharide compositions and polymeric (∼102 disaccharides) lengths by using living cells as factories (Fig. 1A). CRISPR/Cas9 gene editing of sulfotransferases has been used to generate cell-based libraries and sulfation-defined GAGs, which have empowered the discovery of the roles of fine GAG structures (e.g., 3-O-sulfation) (23–25). Inspired by early work from the Esko group describing xyloside inhibitors, new synthetic xylosides consisting of a xylose attached to an aglycone moiety can produce soluble, native GAGs in live cells (Fig. 1B) (26). Crucially, traceable xylosides could inform the localization and clustering of biosynthesis enzymes, as per the GAGosome theory (27). Notably, new MS analyses of such xyloside-primed GAGs have uncovered previously undescribed GAG modifications (2, 3, 28).
Early methods of capturing GAG interactomes utilized heparin affinity chromatography to identify GAGBPs. However, these methods commonly required cell lysis. In such bead-based multivalent displays, interactions with heparin may also be artificially overestimated compared to HS GAGs, due to the increased charge and lack of domain arrangement in heparin. Although such methods continue to reveal new GAGBPs, including the C-type lectin 14a (29), challenges associated with isolating membrane proteins can impede the identification of interactors. Thus, new strategies are permitting the capture of GAG interactors in living cells (30, 31), and enabling the identification of their interactomes (Fig. 1C), especially that of CS that may be missed by traditional heparin affinity methods. For example, using a photoactivatable benzophenone group attached to the CS disaccharide CS-E, the Hsieh-Wilson group identified 54 proteins in live cortical neurons that specifically, and directly, bind this motif (32). Proximity labeling, using engineered enzymes, is another powerful method to identify direct and indirect binders of GAGBPs in a controlled radius (Fig. 1C). Zhen et al. (33) in their study identified 125 interactors of the HS GAGBP FGF1, including CD44. Our group has also utilized this approach to survey the glycoprotein counter-receptors of the glycan-binding proteins galectin-1 and -3 and detected some PGs (30, 31). Importantly, the incorporation of photoreactive moieties or engineered enzymes can overcome challenges associated with membrane proteins and low-affinity glycan-mediated interactions by replacing them with biotin-mediated enrichment.
MORE THAN A SCAFFOLD: IDENTIFYING PG CORE PROTEINS AND THEIR INTERACTIONS
Equally important to the GAG interactome is understanding the interactomes of replete PGs. As core protein sequences are only partially conserved, we expect the resulting interactomes to be vastly different among different PGs. Recent studies unveiling a growing number of part-time PGs and evidence that peptide motifs in PGs can control the sulfation of attached GAGs also elude to this astounding diversity (34). In this section, we will discuss novel methods to identify and analyze PGs at the peptide and glycoconjugate level, including part-time PGs, GAG-independent interactions, and methods to piece apart PG structure-function relationships.
MS-Based Methods to Characterize PGs
Most information regarding PG expression is derived from GAG-targeting antibodies that often cannot discriminate core protein sequences. The fast-growing field of glycoproteomics is rapidly devising new methods to analyze the glycan and protein components of glycoconjugates (35). Although these approaches are typically applied to glycoproteins with N-linked glycans, which are easily identifiable by their consensus sequence (N-X-S/T), there is now an emerging chemical biology toolbox for O-linked glycosylation, including GAGs. Focusing on decorin and hyaluronan-binding PGs, the Zaia group devised an LC-MS and bioinformatics approach to improve the detection of PGs (36), including site-specific N-, mucin O- and GAG-glycosylation. The authors report that complete coverage of a PG can be acquired from a single data acquisition and identified dense regions of mucin-type glycosylation in brevican and neurocan, in addition to novel N- and O-glycosylation on aggrecan. However, this method is unable to distinguish the type of GAG chain occupying the site. Complementarily, Noborn et al. (37) developed a method to characterize hybrid PGs by utilizing slow flow rates in chromatography for the separation and combinatorial analysis of glycan structures and peptides. This system allowed for differentiation of HS and hybrid GAG sites in perlecan, identifying a hybrid-promoting peptide motif in the process. The same group has also identified a novel class of PGs in prohormones, including three previously unidentified PGs (38). Following their characterization of xyloside-primed GAGs, Persson et al. (39) successfully mapped CS/DS chain domains on the PG chromogranin-A.
When assigning PGs by glycoproteomics, one would expect to observe the common tetrasaccharide GlcA-Gal-Gal-Xyl-O- to identify glycopeptides. However, a noncanonical trisaccharide linker (GlcA-Gal-Xyl-O-) was recently observed on bikunin (40). This structure was also observed upon xyloside treatment of two human cell lines. While the biological significance remains unclear, it suggests glucuronosyltransferase GlcAT-1 may exhibit substrate promiscuity.
GAG-Independent Interactions: a Part-Time Job?
Although our knowledge of core protein-dependent interactions is lacking, there are a growing number of reports of part-time PGs, and GAG-independent interactions occurring with PG proteins, some even requiring deglycosylation. For example, the pro-secretory mitogen lacritin recognizes both a peptide motif in the syndecan-1 (SDC1) core protein and proximal stubs generated by heparanase digestion (11). SDC1 has also been identified as a mediator of macropinocytosis in pancreatic ductal adenocarcinoma possessing oncogenic KRas mutations (41). This mechanism critically requires the intracellular C-terminus of SDC1; however, mutagenesis of the ectodomain abolished macropinocytosis while mutating only GAG attachment sites exerted a partial rescue. This suggests that the core protein is essential, whereas HS/CS chains play lesser roles toward extracellular nutrient binding.
Through a structural model of glypican-3 (GPC3), a HSPG known as a co-receptor for the Wnt family of growth factors, Li et al. (42) uncovered an HS-independent binding site for Wnt3A. Consistent with predictions of binding a hydrophobic groove in GPC3, the authors identified a cysteine-rich domain for Wnt3a binding in the N-terminus of GPC3. These findings demonstrate a core protein-dependent Wnt co-receptor function for GPC3 and suggest additional protein-protein interactions may be occurring with GAGBPs. More recently, the same group also identified a novel GPC3 interactor in FAT1; however, the contribution of core protein and GAGs in this interaction is less clear (43). Multiple glypican family proteins function in Wnt signaling, for an extended discussion on the glycan- and core protein-mediated interactions of GPC1, we recommend Ref. 44.
A significant proportion of known PGs are not always decorated with GAG chains, thus dissecting their core protein-mediated interactions is even more crucial to understanding their biology. Although the attachment of GAG chains on many part-time PGs is controlled by splice variants that introduce GAG attachment sites, the HS modifications of the newly discovered PG neurexin have been shown to be regulated by the binding of small proteins to its core. Indeed, binding of CA10 around the site of HS attachment can prevent O-xylosylation of neurexin, without affecting other PGs (9). The expression of this small protein is found only in cells that express neurexin that lacks HS chains. Similarly, FAM19A proteins were recently deorphanized as neurexin ligands (10). Their binding within the secretory pathway occurs at proximal sites of HS, preventing GAG attachment, albeit by an unknown mechanism. The expression of FAM19A family proteins in neurons is thought to be regulated by depolarization, proposing a possible mechanism for temporal control of neurexin glycosylation. The discovery of neurexin-modifying proteins opens a world of possibilities for small proteins, or even small molecules, to regulate glycosylation at the single protein level. Furthermore, the finding that bikunin core protein and glycoconjugate garnered opposing inflammatory responses (20) presents part-time PGs as an exciting, understudied frontier.
Enabling Structure-Function Studies from Chemically Defined Materials
Although GAGs have been a focus for the chemical glycobiology community, there is a growing appreciation for the necessity to study glycoconjugates as replete entities (45). However, simply overexpressing mutants in vitro may not result in the desired architecture as GAG biosynthesis can be outcompeted, resulting in hypoglycosylated proteins (14). To truly explore the structure-function relationships of PGs, a variety of chemical methods have been developed to generate mimetics with defined structures. In particular, the passive incorporation of lipid-functionalized biomolecules has allowed for glycocalyx remodeling, bypassing amenability to transfection (46). The incorporation of lipid-functionalized glycopolymers, consisting of a synthetic backbone with attached defined GAG disaccharides, has facilitated the study of embryonic stem cell fate regulation by HS and CS (47, 48).
Newer methods are incorporating core protein peptides to create glycopeptide mimetics of individual PGs, and to discern the contribution of GAGs and core protein in biological interactions. However, this requires extensive study of the core protein to identify supposed “active sites,” such as synstatin (SSTN92-119), a peptide derived from the αvβ3 binding site in SDC1 at amino acids 92-119 (49) (Fig. 1D). Indeed, Gao et al. (50) in their study conjugated a heparin octasaccharide to SSTN92-119 to produce an SDC1 mimetic. This glycosylated construct engaged integrin αvβ3 faster than SSTN92-119 alone, suggesting a role for HS in this interaction. Although this mimetic uses a peptide backbone, it neither captures the full length nor the structure of SDC1. In particular, the attachment site of the heparin octasaccharide is significantly closer to the SSTN92-119 peptide than GAGs in native SDC1.
Total synthesis has also been used to study SDC4, incorporating amino acids 60-71 and an HS tetrasaccharide, permitting the novel incorporation of O-/N-sulfate groups (51). The authors observed enhanced binding of FGF2 to the glycopeptide than the glycan or peptide alone, confirming modulation of HS biological properties by the SDC4 core protein. Of note, this mimetic incorporated a single pentasaccharide, whereas native SDC4 has two GAG attachment sites in this region (S62, S64), which could further modulate its activity due to divalent effects.
Wang et al. (4) in their study used hydrazine chemistry to incorporate defined GAG oligosaccharides onto the backbone of GPC3. Through site-directed mutagenesis, the authors introduced an aldehyde at sites of GAG attachment for the incorporation of hydrazide-tagged oligosaccharides. Through the introduction of alkyne handles onto GAG chains and azido-modified interactors (Wnt-3A and Shh), the authors used induced proximity capture (Fig. 1C) to demonstrate the impacts of oligosaccharide length and identity in these interactions. Limitations of this method, however, are the requirements of cotransfection and acidic environments (pH 6.0) for hydrazide-functionalized GAG coupling.
Recently, we have introduced a modular platform to produce semisynthetic PGs consisting of full-length core proteins with controlled valency of HS and heparin GAGs (2). Through the assimilation of xyloside priming of native GAGs and the expression of deglycosylated core proteins with unnatural amino acid incorporation, we used copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) to produce chemically defined materials. By performing these steps in vitro and subsequent purification, we circumvent risks of copper toxicity and contaminating free GAGs in our assays. These engineered PGs enabled the elucidation of the effects of GAG valency and core protein identity on syndecan family interactions with known binding proteins. Through further protein engineering, we appended the APEX2 peroxidase to our SDC1 core protein to begin illuminating glycosylation-dependent changes in the interactome.
OUTLOOKS
Here, we have discussed approaches to study both the GAG and protein components of PGs, culminating in methods to produce defined PG mimetics. We foresee that the combination of these chemically defined materials with proximity labeling approaches will permit the identification of PG interactors and allow a deeper understanding of how fine-scale architecture modulates PG biology (Fig. 2A) (2). Indeed, applying these techniques to both PGs and GAGBPs (25, 33) will allow a global survey of PG interactomes in specific environments, such as cellular synapses (52). An APEX2-SDC1 fusion protein has been previously used to study the basolateral surface of three-dimensional human pluripotent stem cell-derived epiblast cysts; however, its status as a PG was not well considered (12). Furthermore, the recent discoveries of neurexin-modifying proteins (9, 10) present the exciting opportunity to uncover other novel means of controlling PG architecture. We envision the advancement of current de novo PG mimetics to include multiple GAG conjugation methods, such that hybrid glycosylated PGs can be produced (Fig. 2B). From these structures and advancements in MS capabilities, we anticipate the ability to identify glycoform-specific PG interactomes.
Figure 2.
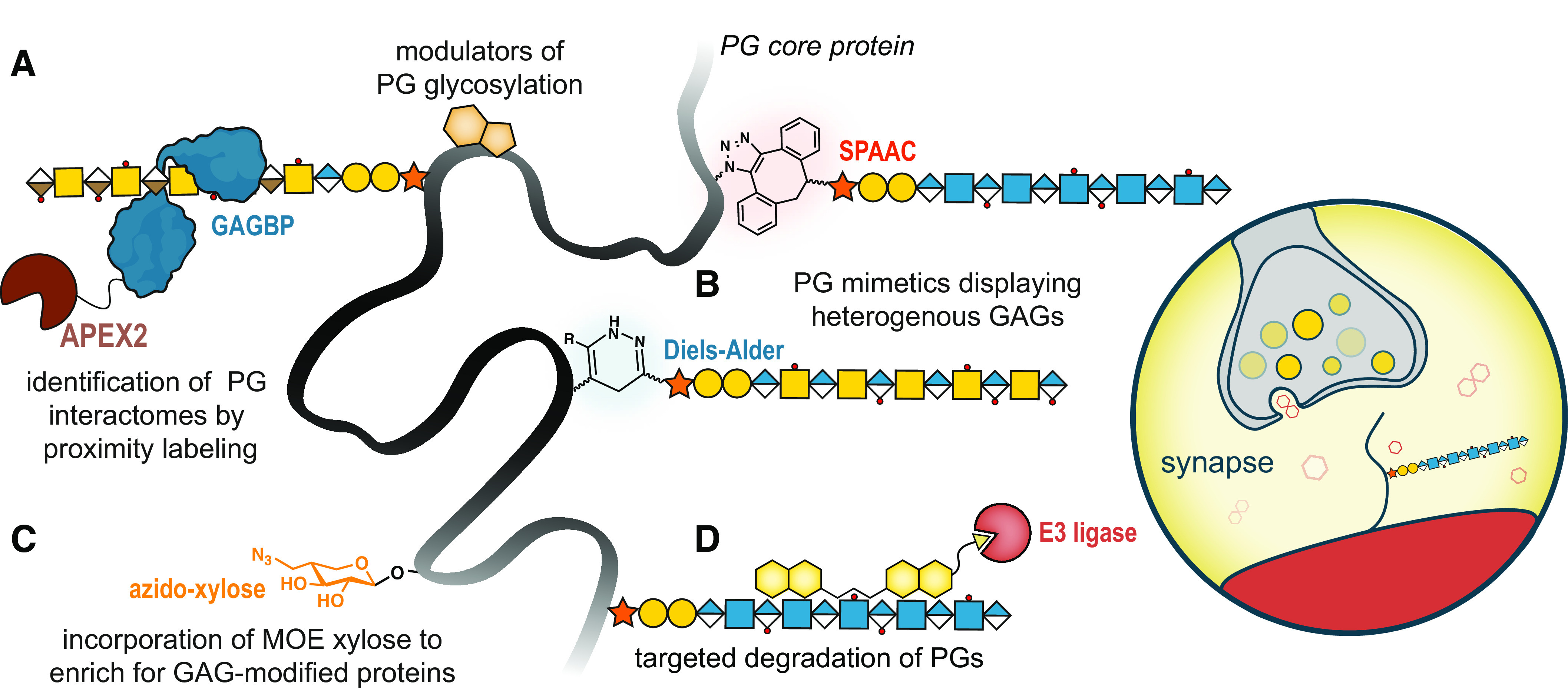
Uncharted opportunities in exploring proteoglycan structure-function relationships. A: the usage of proximity labeling approaches (e.g., APEX2, depicted) permits the identification of the collection of proteoglycans within a cell using GAGBPs. On the contrary, APEX2-PG fusion proteins can allow the identification of both GAG- and core protein-dependent interactions. Furthermore, these interactors can be derivatized to create small molecule modulators of PG glycosylation. B: the expansion of PG mimetics to include both HS and CS/DS GAGs will progress de novo PGs toward replicating native hybrid PGs, further enabling exploration of structure-activity relationships. C: xylose is a unique sugar in mammalian cells, primarily found in GAG core tetrasaccharide structures (GlcA-Gal-Gal-Xyl). The creation of a derivative with a functional handle for enrichment (e.g., azide, depicted) could allow for pull-down of GAG-modified proteins, including crucially, part-time PGs. D: through derivatization of PGs/GAGBPs or small molecules, molecular glue technologies could be used to modulate PG expression or structure in vitro. This could include the targeted degradation of disease-associated PG glycoforms using proteolysis targeting chimeras that recruit E3 ligase (depicted), or modulation of structure by recruiting GAG modifying enzymes (e.g., sulfatases to remove sulfate groups, or heparanase to cleave HS chains). The combination of these approaches would allow for a global view of the complete proteoglycan repertoire and characterization of their interactions and molecular mechanisms within a chosen biological setting, such as synapses. CS/DS, chondroitin sulfate/dermatan sulfate; GAGs, glycosaminoglycans; GAGBPs, GAG-binding proteins; HS, heparan sulfate; MOE, metabolic oligosaccharide engineering; PGs, proteoglycans.
Furthermore, we believe the study of part-time PGs is a largely untouched expanse, with numerous outstanding questions. What triggers the expression of these GAG-modified variants? How can these understudied PGs be identified? We postulate that computational searches for alternative splice variants incorporating SGSG motifs may assist in such endeavors, in combination with site-specific identification of GAGs by mass spectrometry, or the development of selective antibodies. Furthermore, we speculate whether metabolic oligosaccharide engineering, a cornerstone of chemical glycobiology, could be co-opted to introduce a functional handle onto xylosylated proteins (Fig. 2C).
Finally, we foresee the derivatization of PGs or their interactors, for therapeutic gain, enabled by a deeper understanding of structure-function relationships. To date, such endeavors have produced syndecan-derived peptides that inhibit receptor tyrosine kinase activity (49) and Fondaparinux, a synthetic anticoagulant based on the minimal heparin structure required for antithrombin binding (24). In the future, we envision the inclusion of “molecular glues” with small molecules to modulate target glycoforms for selective degradation (53) (Fig. 2D). Similarly, such heterobifunctional molecules could be designed to modulate PG structure through the targeting of modifying enzymes (i.e., sulfatases, heparanases) to modify the interactomes and functions of PGs. It is our view that glycan-protein conjugates must be studied as such, and we believe that the amalgamation of modern chemical biology techniques can elucidate the complete collection of PGs within a cell and their interactomes to further human health.
GRANTS
This work was supported by funding from the National Institutes of Health (NIH) K99/R00 Pathway to Independence Award (R00-HD090292) and the National Institute of General Medical Science (NIGMS) (R35GM142462).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
This article is part of the special collection “Deciphering the Role of Proteoglycans and Glycosaminoglycans in Health and Disease.” Liliana Schaefer, MD, served as Guest Editor of this collection.
AUTHOR CONTRIBUTIONS
M.C. prepared figures; M.C. and M.L.H. drafted and revised manuscript; M.L.H. approved final version of manuscript.
ACKNOWLEDGMENTS
This article is part of the special collection “Deciphering the Role of Proteoglycans and Glycosaminoglycans in Health and Disease.” Liliana Schaefer, MD, served as Guest Editor of this collection.
REFERENCES
- 1.Iozzo RV, Schaefer L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol 42: 11–55, 2015. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Leary TR, Critcher M, Stephenson TN, Yang X, Hassan AA, Bartfield NM, Hawkins R, Huang ML. Chemical editing of proteoglycan architecture. Nat Chem Biol 18: 634–642, 2022. doi: 10.1038/s41589-022-01023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willen D, Mastio R, Soderlund Z, Manner S, Westergren-Thorsson G, Tykesson E, Ellervik U. Azide-functionalized naphthoxyloside as a tool for glycosaminoglycan investigations. Bioconjug Chem 32: 2507–2515, 2021. doi: 10.1021/acs.bioconjchem.1c00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Han N, Xu Y, Zhao Y, Shi L, Filmus J, Li F. Assembling custom side chains on proteoglycans to interrogate their function in living cells. Nat Commun 11: 5915, 2020. doi: 10.1038/s41467-020-19765-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clerc O, Mariethoz J, Rivet A, Lisacek F, Perez S, Ricard-Blum S. A pipeline to translate glycosaminoglycan sequences into 3D models. Application to the exploration of glycosaminoglycan conformational space. Glycobiology 29: 36–44, 2019. doi: 10.1093/glycob/cwy084. [DOI] [PubMed] [Google Scholar]
- 6.Merry CLR, Lindahl U, Couchman JR, Esko JD. Proteoglycans and sulfated glycosaminoglycans. In: Essentials of Glycobiology, edited by Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Mohnen D, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH.. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 2022. [PubMed] [Google Scholar]
- 7.Walimbe T, Panitch A. Proteoglycans in biomedicine: resurgence of an underexploited class of ECM molecules. Front Pharmacol 10: 1661, 2019. doi: 10.3389/fphar.2019.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gondelaud F, Ricard-Blum S. Structures and interactions of syndecans. FEBS J 286: 2994–3007, 2019. doi: 10.1111/febs.14828. [DOI] [PubMed] [Google Scholar]
- 9.Montoliu-Gaya L, Tietze D, Kaminski D, Mirgorodskaya E, Tietze AA, Sterky FH. CA10 regulates neurexin heparan sulfate addition via a direct binding in the secretory pathway. EMBO Rep 22: e51349, 2021. doi: 10.15252/embr.202051349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalaj AJ, Sterky FH, Sclip A, Schwenk J, Brunger AT, Fakler B, Sudhof TC. Deorphanizing FAM19A proteins as pan-neurexin ligands with an unusual biosynthetic binding mechanism. J Cell Biol 219: e202004164, 2020. doi: 10.1083/jcb.202004164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Wang N, Raab RW, McKown RL, Irwin JA, Kwon I, van Kuppevelt TH, Laurie GW. Targeting of heparanase-modified syndecan-1 by prosecretory mitogen lacritin requires conserved core GAGAL plus heparan and chondroitin sulfate as a novel hybrid binding site that enhances selectivity. J Biol Chem 288: 12090–12101, 2013. doi: 10.1074/jbc.M112.422717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Lin C-W, Carleton AE, Cortez CL, Johnson C, Taniguchi LE, Sekulovski N, Townshend RF, Basrur V, Nesvizhskii AI, Zou P, Fu J, Gumucio DL, Duncan MC, Taniguchi K. Spatially resolved cell polarity proteomics of a human epiblast model. Sci Adv 7: eabd8407, 2021. doi: 10.1126/sciadv.abd8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JM, Lee K, Kim MY, Shin HI, Jeong D. Suppressive effect of syndecan ectodomains and N-desulfated heparins on osteoclastogenesis via direct binding to macrophage-colony stimulating factor. Cell Death Dis 9: 1119, 2018. doi: 10.1038/s41419-018-1167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsberg E, Kjellén L. Heparan sulfate: lessons from knockout mice. J Clin Invest 108: 175–180, 2001. doi: 10.1172/JCI13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gómez Toledo A, Sorrentino JT, Sandoval DR, Malmström A, Lewis NE, Esko JD. A systems view of the heparan sulfate interactome. J Histochem Cytochem 69: 105–119, 2021. doi: 10.1369/0022155420988661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vallet SD, Clerc O, Ricard-Blum S. Glycosaminoglycan-protein interactions: the first draft of the glycosaminoglycan interactome. J Histochem Cytochem 69: 92–104, 2021. doi: 10.1369/0022155420946403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain P, Shanthamurthy CD, Leviatan Ben-Arye S, Woods RJ, Kikkeri R, Padler-Karavani V. Discovery of rare sulfated N-unsubstituted glucosamine based heparan sulfate analogs selectively activating chemokines. Chem Sci 12: 3674–3681, 2021. doi: 10.1039/d0sc05862a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zong C, Venot A, Li X, Lu W, Xiao W, Wilkes JL, Salanga CL, Handel TM, Wang L, Wolfert MA, Boons GJ. Heparan sulfate microarray reveals that heparan sulfate-protein binding exhibits different ligand requirements. J Am Chem Soc 139: 9534–9543, 2017. doi: 10.1021/jacs.7b01399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Paz JL, Seeberger PH. Deciphering the glycosaminoglycan code with the help of microarrays. Mol Biosyst 4: 707–711, 2008. doi: 10.1039/b802217h. [DOI] [PubMed] [Google Scholar]
- 20.Ramadan S, Li T, Yang W, Zhang J, Rashidijahanabad Z, Tan Z, Parameswaran N, Huang X. Chemical synthesis and anti-inflammatory activity of bikunin associated chondroitin sulfate 24-mer. ACS Cent Sci 6: 913–920, 2020. doi: 10.1021/acscentsci.9b01199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dulaney SB, Huang X. Strategies in synthesis of heparin/heparan sulfate oligosaccharides: 2000–present. Adv Carbohydr Chem Biochem 67: 95–136, 2012. doi: 10.1016/B978-0-12-396527-1.00003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottschalk J, Elling L. Current state on the enzymatic synthesis of glycosaminoglycans. Curr Opin Chem Biol 61: 71–80, 2021. doi: 10.1016/j.cbpa.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Qiu H, Shi S, Yue J, Xin M, Nairn AV, Lin L, Liu X, Li G, Archer-Hartmann SA, Dela Rosa M, Galizzi M, Wang S, Zhang F, Azadi P, van Kuppevelt TH, Cardoso WV, Kimata K, Ai X, Moremen KW, Esko JD, Linhardt RJ, Wang L. A mutant-cell library for systematic analysis of heparan sulfate structure-function relationships. Nat Methods 15: 889–899, 2018. doi: 10.1038/s41592-018-0189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlsson R, Chopra P, Joshi A, Yang Z, Vakhrushev SY, Clausen TM, Painter CD, Szekeres GP, Chen Y-H, Sandoval DR, Hansen L, Esko JD, Pagel K, Dyer DP, Turnbull JE, Clausen H, Boons GJ, Miller RL. Dissecting structure-function of 3-O-sulfated heparin and engineered heparan sulfates. Sci Adv 7: eabl6026, 2021. doi: 10.1126/sciadv.abl6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss RJ, Spahn PN, Chiang AWT, Liu Q, Li J, Hamill KM, Rother S, Clausen TM, Hoeksema MA, Timm BM, Godula K, Glass CK, Tor Y, Gordts P, Lewis NE, Esko JD. Genome-wide screens uncover KDM2B as a modifier of protein binding to heparan sulfate. Nat Chem Biol 17: 684–692, 2021[Erratum inNat Chem Biol18: 575, 2022]. doi: 10.1038/s41589-021-00776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lugemwa FN, Esko JD. Estradiol beta-D-xyloside, an efficient primer for heparan sulfate biosynthesis. J Biol Chem 266: 6674–6677, 1991. [PubMed] [Google Scholar]
- 27.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem 71: 435–471, 2002. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 28.Persson A, Vorontsov E, Larson G, Nilsson J. Glycosaminoglycan domain mapping of cellular chondroitin/dermatan sulfates. Sci Rep 10: 3506, 2020. doi: 10.1038/s41598-020-60526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandoval DR, Gomez Toledo A, Painter CD, Tota EM, Sheikh MO, West AMV, Frank MM, Wells L, Xu D, Bicknell R, Corbett KD, Esko JD. Proteomics-based screening of the endothelial heparan sulfate interactome reveals that C-type lectin 14a (CLEC14A) is a heparin-binding protein. J Biol Chem 295: 2804–2821, 2020. doi: 10.1074/jbc.RA119.011639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joeh E, O'Leary T, Li W, Hawkins R, Hung JR, Parker CG, Huang ML. Mapping glycan-mediated galectin-3 interactions by live cell proximity labeling. Proc Natl Acad Sci USA 117: 27329–27338, 2020. doi: 10.1073/pnas.2009206117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilen Z, Joeh E, Critcher M, Parker CG, Huang ML. Proximity tagging identifies the glycan-mediated glycoprotein interactors of galectin-1 in muscle stem cells. ACS Chem Biol 16: 1994–2003, 2021. doi: 10.1021/acschembio.1c00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joffrin AM, Hsieh-Wilson LC. Photoaffinity probes for the identification of sequence-specific glycosaminoglycan-binding proteins. J Am Chem Soc 142: 13672–13676, 2020. doi: 10.1021/jacs.0c06046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhen Y, Haugsten EM, Singh SK, Wesche J. Proximity labeling by a recombinant APEX2-FGF1 fusion protein reveals interaction of FGF1 with the proteoglycans CD44 and CSPG4. Biochemistry 57: 3807–3816, 2018. doi: 10.1021/acs.biochem.8b00120. [DOI] [PubMed] [Google Scholar]
- 34.Corti F, Wang Y, Rhodes JM, Atri D, Archer-Hartmann S, Zhang J, Zhuang ZW, Chen D, Wang T, Wang Z, Azadi P, Simons M. N-terminal syndecan-2 domain selectively enhances 6-O heparan sulfate chains sulfation and promotes VEGFA165-dependent neovascularization. Nat Commun 10: 1562, 2019[Erratum inNat Commun10: 2124, 2019]. doi: 10.1038/s41467-019-09605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas DR, Scott NE. Glycoproteomics: growing up fast. Curr Opin Struct Biol 68: 18–25, 2021. doi: 10.1016/j.sbi.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 36.Klein JA, Meng L, Zaia J. Deep sequencing of complex proteoglycans: a novel strategy for high coverage and site-specific identification of glycosaminoglycan-linked peptides. Mol Cell Proteomics 17: 1578–1590, 2018. doi: 10.1074/mcp.RA118.000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noborn F, Gomez Toledo A, Green A, Nasir W, Sihlbom C, Nilsson J, Larson G. Site-specific identification of heparan and chondroitin sulfate glycosaminoglycans in hybrid proteoglycans. Sci Rep 6: 34537, 2016. doi: 10.1038/srep34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikpour M, Nilsson J, Persson A, Noborn F, Vorontsov E, Larson G. Proteoglycan profiling of human, rat and mouse insulin-secreting cells. Glycobiology 31: 916–930, 2021. doi: 10.1093/glycob/cwab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Persson A, Nikpour M, Vorontsov E, Nilsson J, Larson G. Domain mapping of chondroitin/dermatan sulfate glycosaminoglycans enables structural characterization of proteoglycans. Mol Cell Proteomics 20: 100074, 2021. doi: 10.1016/j.mcpro.2021.100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Persson A, Nilsson J, Vorontsov E, Noborn F, Larson G. Identification of a non-canonical chondroitin sulfate linkage region trisaccharide. Glycobiology 29: 366–371, 2019. doi: 10.1093/glycob/cwz014. [DOI] [PubMed] [Google Scholar]
- 41.Yao W, Rose JL, Wang W, Seth S, Jiang H, Taguchi A, et al. Syndecan 1 is a critical mediator of macropinocytosis in pancreatic cancer. Nature 568: 410–414, 2019. doi: 10.1038/s41586-019-1062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li N, Wei L, Liu X, Bai H, Ye Y, Li D, Li N, Baxa U, Wang Q, Lv L, Chen Y, Feng M, Lee B, Gao W, Ho M. A frizzled-like cysteine-rich domain in glypican-3 mediates Wnt binding and regulates hepatocellular carcinoma tumor growth in mice. Hepatology 70: 1231–1245, 2019. doi: 10.1002/hep.30646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng P, Zhang YF, Zhang W, Chen X, Xu T, Hu S, Liang X, Feng M, Yang X, Ho M. Identification of the atypical cadherin FAT1 as a novel glypican-3 interacting protein in liver cancer cells. Sci Rep 11: 40, 2021. doi: 10.1038/s41598-020-79524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pan J, Ho M. Role of glypican-1 in regulating multiple cellular signaling pathways. Am J Physiol Cell Physiol 321: C846–C858, 2021. doi: 10.1152/ajpcell.00290.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Critcher M, Hassan AH, Huang ML. Seeing the forest through the trees: characterizing the glycoproteome. Trends Biochem Sci 47: 492–505, 2022. doi: 10.1016/j.tibs.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabuka D, Forstner MB, Groves JT, Bertozzi CR. Noncovalent cell surface engineering: incorporation of bioactive synthetic glycopolymers into cellular membranes. J Am Chem Soc 130: 5947–5953, 2008. doi: 10.1021/ja710644g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang ML, Smith RA, Trieger GW, Godula K. Glycocalyx remodeling with proteoglycan mimetics promotes neural specification in embryonic stem cells. J Am Chem Soc 136: 10565–10568, 2014. doi: 10.1021/ja505012a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pulsipher A, Griffin ME, Stone SE, Brown JM, Hsieh-Wilson LC. Directing neuronal signaling through cell-surface glycan engineering. J Am Chem Soc 136: 6794–6797, 2014. doi: 10.1021/ja5005174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beauvais DM, Ell BJ, McWhorter AR, Rapraeger AC. Syndecan-1 regulates alphavbeta3 and alphavbeta5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. J Exp Med 206: 691–705, 2009. doi: 10.1084/jem.20081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao J, Xu Y, Liu J, Huang X. Convergent chemoenzymatic synthesis and biological evaluation of a heparan sulfate proteoglycan syndecan-1 mimetic. Chem Commun (Camb) 57: 3407–3410, 2021. doi: 10.1039/d1cc00796c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang W, Eken Y, Zhang J, Cole LE, Ramadan S, Xu Y, Zhang Z, Liu J, Wilson AK, Huang X. Chemical synthesis of human syndecan-4 glycopeptide bearing O-, N-sulfation and multiple aspartic acids for probing impacts of the glycan chain and the core peptide on biological functions. Chem Sci 11: 6393–6404, 2020. doi: 10.1039/d0sc01140a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang P, Lu H, Peixoto RT, Pines MK, Ge Y, Oku S, Siddiqui TJ, Xie Y, Wu W, Archer-Hartmann S, Yoshida K, Tanaka KF, Aricescu AR, Azadi P, Gordon MD, Sabatini BL, Wong ROL, Craig AM. Heparan sulfate organizes neuronal synapses through neurexin partnerships. Cell 174: 1450–1464.e23, 2018. doi: 10.1016/j.cell.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banik SM, Pedram K, Wisnovsky S, Ahn G, Riley NM, Bertozzi CR. Lysosome-targeting chimaeras for degradation of extracellular proteins. Nature 584: 291–297, 2020. doi: 10.1038/s41586-020-2545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


