Abstract
Background:
Racial disparities in SARS-CoV-2 infection, hospitalization, and multisystem inflammatory syndrome in children (MIS-C) have been reported. However, these reports have been based on incomplete data relying on passive reporting, unknown catchment populations, and unknown infection prevalence. We aimed to characterize population-based incidence of MIS-C and COVID-19 hospitalizations among non-Hispanic Black and White children using active surveillance based on seroprevalence-based cumulative incidence of pediatric SARS-CoV-2 infection in a defined catchment 16-county area of Mississippi.
Methods:
Active, population-based surveillance for MIS-C and acute COVID-19 hospitalizations meeting clinical and laboratory criteria was conducted by adjudicating clinicians at the major pediatric referral hospital for Mississippi, University of Mississippi Medical Center, from March 2020, to February 2021. Race-stratified SARS-CoV-2 seroprevalence was estimated using convenience samples from persons <18 years to calculate cumulative SARS-CoV-2 infections in the population.
Results:
Thirty-eight MIS-C cases and 74 pediatric acute COVID-19 hospitalizations were identified. Cumulative incidence of MIS-C was 4.7 times higher among Black compared with White children (40.7 versus 8.3 cases per 100,000 SARS-CoV-2 infections). Cumulative incidence of COVID-19 hospitalization was 62.3 among Black and 33.1 among White children per 100,000 SARS-CoV-2 infections.
Conclusions:
From the same catchment area, active surveillance, and cumulative incidence of infection estimated by seroprevalence, we show strikingly higher incidence of SARS-CoV-2-hospitalization and MIS-C in non-Hispanic Black children compared with White children before COVID-19 vaccination introduction in children. These disparities in SARS-CoV-2 manifestations cannot be accounted for by differences in exposure or testing. Targeted vaccine interventions will lessen disparities observed with SARS-CoV-2 manifestations in children.
Keywords: SARS-CoV-2, COVID-19, pediatric, multisystem inflammatory syndrome in children, racial disparities, seroprevalence
SARS-CoV-2 infection and COVID-19 disease, and multisystem inflammatory syndrome in children (MIS-C), a rare, hyperinflammatory syndrome occurring after SARS-CoV-2 infection, are more frequent in Black persons.1,2 However, the accuracy of these rates could be influenced by 3 factors: first, they are based on passively reported cases with possible under-reporting; second, catchment population for the reported cases is not always known; and third, SARS-CoV-2 infection rates are not well characterized in the source population of these reporting systems.3,4 We performed active population-based surveillance for MIS-C and acute COVID-19 hospitalizations coupled with serologic testing for SARS-CoV-2 infection to estimate cumulative incidence of infection in a tightly defined catchment area to examine racial disparities in MIS-C and acute COVID-19 requiring hospitalization among persons younger than 18 years.
METHODS
We calculated race-stratified rates of MIS-C and acute COVID-19 hospitalizations per 100,000 SARS-CoV-2 infections in children <18 years old from a 16-county catchment area in central Mississippi. MIS-C and acute COVID-19 hospitalizations cases were identified through active surveillance and SARS-CoV-2 infection rates were estimated using serologic testing of residual clinical specimens of children seeking medical care for any cause during May 2020 to February 2021 (before widespread vaccine introduction in children).
The University of Mississippi Medical Center (UMMC) is the main pediatric referral center for the state of Mississippi and provides clinical and laboratory services for associated clinics throughout the state. For population-based surveillance, the UMMC source population was defined as residents of a 16-county area including the state capitol of Jackson, Mississippi, for whom UMMC is the nearest acute care hospital, based on prior convenience sample surveillance studies.5 According to 2019 bridged US Census estimates, racial and ethnic composition of the 16-county area (population, 1,080,808) was 49% non-Hispanic Black, 46% non-Hispanic White, and 5% Hispanic or other race/ethnicity.6 References to Black and White race herein refer to non-Hispanic Black and White, specifically.
MIS-C and Acute COVID-19 Hospitalization Case Definitions
Active surveillance for hospitalized patients meeting inclusion criteria for investigation of MIS-C and pediatric acute COVID-19 was conducted at UMMC. MIS-C cases included in this analysis were hospitalized patients who met the CDC MIS-C case definition of clinically severe illness with fever, elevation of inflammatory markers, involvement of 2 or more organ systems and evidence of current or recent SARS-CoV-2 infection (positive reverse transcription–polymerase chain reaction [RT-PCR], SARS-CoV-2 rapid antigen test, or SARS-CoV-2 serology), or exposure to a suspected or confirmed COVID-19 case within the 4 weeks before the onset of symptoms, and in the absence of an alternative plausible diagnosis.7 Among hospitalized patients younger than 18 years who did not meet criteria for MIS-C, acute COVID-19 was defined as presence of one or more symptoms consistent with acute COVID-19 (fever, cough, shortness of breath, loss of taste, loss of smell, or gastrointestinal symptoms), respiratory support or new pulmonary findings on chest imaging, with positive RT-PCR or rapid antigen detection.8 Patients who were asymptomatic or had alternative reasons for admission not related to COVID-19 were excluded from this analysis. All MIS-C cases and acute COVID-19 hospitalizations at UMMC who met inclusion criteria were adjudicated by a clinician team of pediatric infectious disease, rheumatologists, and hospitalists (CVH, VAH, AD, RS, AP) to confirm case classification. Data regarding underlying conditions were defined and categorized from electronic health records, and were categorized as chronic lung, chronic metabolic, hematologic, cardiovascular, neurologic, immunocompromised, gastrointestinal, rheumatologic, or renal, consistent with a previously described case series (Table 1).9
TABLE 1.
Characteristics of MIS-C and Acute COVID Cases in 16-county Area for University of Mississippi Medical Center, March 2020 Through February 2021
| MIS-C* (N = 38), No. (%) | Acute COVID-19 Hospitalizations* (N = 74), No. (%) | |
|---|---|---|
| Age group (yrs) | ||
| <1 | 0 (0) | 21 (28) |
| 1–5 | 12 (32) | 10 (14) |
| 6–10 | 11 (29) | 8 (11) |
| 11–17 | 15 (39) | 35 (47) |
| Race/ethnicity † | ||
| Hispanic | 0 (0) | 5 (7) |
| Non-Hispanic | ||
| Black | 31 (82) | 49 (66) |
| Other‖ | 2 (5) | 4 (5) |
| White | 4 (11) | 16 (22) |
| Sex | ||
| Female | 21 (55) | 27 (36) |
| Male | 17 (45) | 47 (64) |
| Month ‡ | ||
| Apr 2020 | 1 (3) | 4 (5) |
| May 2020 | 1 (3) | 6 (8) |
| Jun 2020 | 1 (3) | 5 (7) |
| Jul 2020 | 1 (3) | 11 (15) |
| Aug 2020 | 3 (8) | 4 (5) |
| Sep 2020 | 5 (13) | 2 (3) |
| Oct 2020 | 0 (0) | 2 (3) |
| Nov 2020 | 0 (0) | 11 (15) |
| Dec 2020 | 5 (13) | 12 (16) |
| Jan 2021 | 12 (32) | 12 (16) |
| Feb 2021 | 9 (24) | 5 (7) |
| Underlying medical condition | ||
| ≥1 underlying medical condition‡ | 13 (34) | 43 (58) |
| Chronic lung disease | 4 (11) | 18 (24) |
| Chronic metabolic disease | 2 (5) | 8 (11) |
| Sickle cell disease | 1 (3) | 6 (8) |
| Cardiovascular disease | 3 (8) | 5 (7) |
| Neurologic disorder | 3 (8) | 10 (14) |
| Immunocompromised condition | 0 (0) | 3 (4) |
| Gastrointestinal/liver disease | 0 (0) | 9 (12) |
| Renal disease | 1 (3) | 3 (4) |
| Prematurity | 1 (3) | 3 (4) |
| Other¶ | 0 (0) | 4 (5) |
| Obesity § | 15 (42) | 21 (42) |
| ICU stay | ||
| Admitted to ICU | 19 (50) | 21 (28) |
| Length of stay, median (IQR), d | 8.0 (5.9–9.8) | 4.8 (2.7–9.8) |
BMI indicates body mass index; IQR, interquartile range; MIS-C, multisystem inflammatory syndrome in children.
One participant contributed to both MIS-C and acute COVID-19 hospitalization frequencies, and age groups above were chosen to match Supplementary Table 1 and Mississippi State Department of Health age group for Pediatric COVID-19/MIS-C reporting (https://msdh.ms.gov/msdhsite/_static/14,0,420,873.html#pediatric). By CDC age groups, our frequencies are as follows: age < 1, 0/38 or 0%; age 1–4, 9/38 (24%); age 5–11, 15/38 (39%); age 12–15, 8/38 (21%); age 16 to <18, 6/38 (16%).
Ethnicity for 1 MIS-C case who identifies as Black is missing.
No cases in March.
Obesity defined as BMI equal to or greater than the 95th percentile in age- and sex-specific groups. BMI not calculated for children <2 years—therefore percent expressed accounts for 36 MIS-C (2 were under age 2) and 50 (24 were under age 2) for acute COVID-19 hospitalized children, respectively.
Other includes rheumatologic, autoimmune, and inflammatory conditions.
‖Conditions were categorized using Epic data for encounter diagnoses and problem lists as previously published with the following notations: includes asthma but not wheezing without asthma diagnosis; new onset diabetes mellitus with COVID requiring hospitalization was included (not chronic metabolic); new onset heart murmurs were not categorized as chronic cardiovascular disease unless associated with a diagnosis of abnormality by echocardiogram; hemoglobinopathy included sickle cell disease; malignancy was categorized under “immunocompromised.”
SARS-CoV-2 Antibody Seroprevalence
The University of Mississippi Medical Center provides clinical laboratory services for university hospitals in central Mississippi and associated university network clinics statewide (5). Beginning in May 2020, residual serum and plasma from patient blood specimens submitted to the UMMC central laboratory for diagnostic testing were collected for those <18 years of age. SARS-CoV-2 serologic testing was performed at CDC using a commercial assay for anti–SARS-CoV-2 total antibody (VITROS, Ortho Clinical Diagnostics), or an enzyme linked immunosorbent assay (ELISA) developed by CDC to measure total SARS-CoV-2 antibodies against the extracellular domain of the SARS-CoV-2 spike protein.10 Seropositivity for either assay was defined as a ratio of test sample signal to cutoff value (S/C) ≥1.0.10 Patient information for residual serum specimens was extracted from electronic medical records at UMMC, including patient age, sex, race/ethnicity, result of any SARS-CoV-2 viral testing or serology, COVID-19 vaccination, and date of specimen collection. Monthly seroprevalence and 95% exact confidence bounds were calculated from the proportion of seropositive specimens collected during the month, stratified by patient race/ethnicity (non-Hispanic White, non-Hispanic Black or Hispanic). For individuals with multiple residual specimens available from separate medical encounters, 1 specimen per person was included in the analysis, either the first seropositive specimen or the earliest specimen from persons who tested seronegative in all specimens.
COVID-19 Case Report Data
Numbers of reported COVID-19 cases among persons younger than 18 years in the 16-county area were obtained from the Mississippi State Department of Health (MSDH). COVID-19 case data were categorized using basic variables (age group, sex, race/ethnicity, and calendar month of case report).
Data Analysis
Cumulative numbers of persons younger than 18 years in the source population infected with SARS-CoV-2 since the beginning of the COVID-19 pandemic were estimated by multiplying monthly race-specific seroprevalence by the White or Black population younger than 18 years old in the 16-county source population, according to 2019 US Census bridged data.6 Statistical analyses were conducted using SAS (version 9.4; SAS Institute), and 95% confidence intervals were estimated from Poisson regression accounting for age group and calendar month.
This study was reviewed and approved by UMMC Institutional Review Board and conducted consistent with applicable federal law and policy of the Centers for Disease Control and Prevention.
RESULTS
From March 2020 through February 2021, UMMC admitted 56 patients younger than 18 years diagnosed with MIS-C and 99 patients with acute COVID-19. Of these, 38 (68%) MIS-C cases and 74 (75%) patients with acute COVID-19 resided in the 16-county UMMC catchment population (see Figure, Supplemental Digital Content 1, http://links.lww.com/INF/E747). The proportions of patients with MIS-C and acute COVID-19 by age, sex, race/ethnicity, month, and underlying medical condition, are shown in Table 1, and seroprevalence adjusted for the 16-county radius is shown in Table (Supplemental Digital Content 2, http://links.lww.com/INF/E748). None of the MIS-C case patients were infants, compared with infants comprising 28% of acute COVID-19 hospitalizations; 23 (61%) of MIS-C cases were 1 to <10 years old versus 18 (24%) of acute COVID-19 hospitalizations. A higher percentage of MIS-C cases were female (55%) compared with acute COVID-19 hospitalizations (36%). Non-Hispanic Black children accounted for 31 (82%) of MIS-C cases, and 49 (66%) of acute COVID-19 hospitalizations, versus 4 (11%) and 16 (22%), respectively, among non-Hispanic White children. Underlying medical conditions, excluding obesity, were identified among 13 (34%) of MIS-C case patients and 43 (58%) of acute COVID-19 patients; with most common underlying conditions reported including chronic lung disease. Among 36 MIS-C cases and 50 acute COVID-19 hospitalized children ≥2 years of age, 15 (42%) and 21 (42%) were obese. There were no deaths within this catchment population during this period.
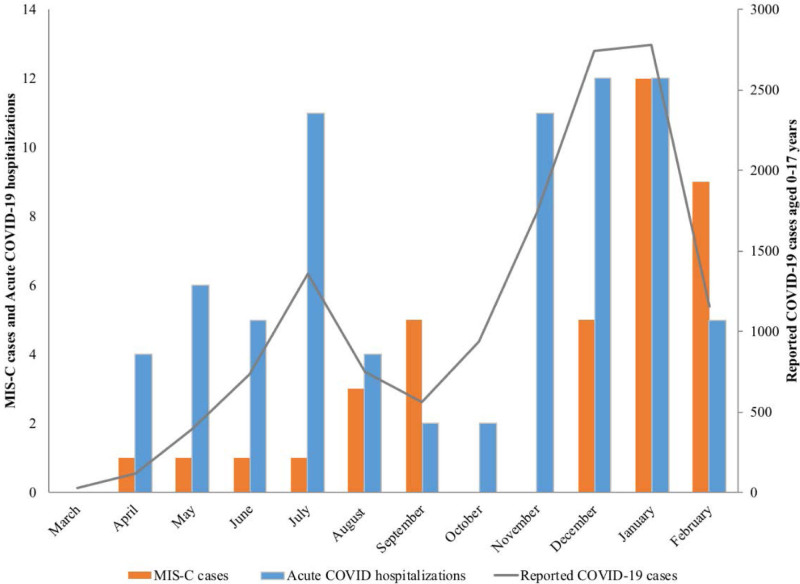
Monthly MIS-C and acute COVID-19 hospitalizations followed trends in reported pediatric COVID-19 cases (Fig. 1), with peak numbers of MIS-C cases lagging 1 month behind peak numbers of COVID-19 hospitalizations and reported cases. From March 2020 through February 2021, 4797 COVID-19 cases among White and 6025 cases among Black residents younger than 18 years were reported to MSDH from the 16-county catchment population (see Tables, Supplemental Digital Content 2 and 3, http://links.lww.com/INF/E748).
FIGURE 1.
Pediatric MIS-C cases and acute COVID-19 hospitalizations by month compared with COVID-19 cases among children and adolescents <18 years of age in Jackson reported to Mississippi State Department of Health from Jackson and 16-county area, Mississippi, March 2020–February 2021.
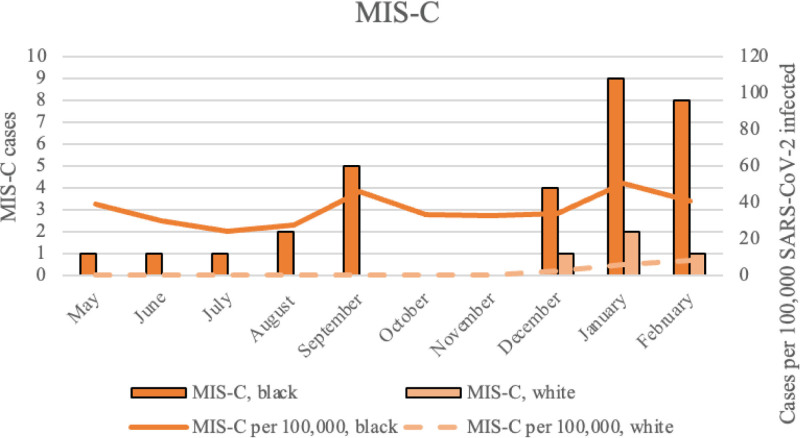
From May 2020 through February 2021, cumulative incidence of MIS-C and acute COVID-19 hospitalizations per 100,000 estimated SARS-CoV-2 infections was higher among Black compared with White children (Fig. 2A,B). During the 10-month period, cumulative incidence of MIS-C was 5 times higher among Black children (40.7 per 100,000 SARS-CoV-2 infections as compared with White children 8.3 per 100,000 infections) (Table 2). Cumulative incidence of acute COVID-19 hospitalizations was 2 times higher among Black children (62.3 per 100,000 infections) versus White children (33.1 per 100,000 infections), with rate differences for the entire study period of 32.4 for MIS-C (for Black versus White children), and 29.2 for COVID-19 hospitalization (Table 2). By February 2021, an estimated 78,661 Black and 48,374 White children in the 16-county area had already been infected with SARS-CoV-2 (Table 1; Tables, Supplemental Digital Content 2 and 3, http://links.lww.com/INF/E748; Figs. 1 and 2A,B).
FIGURE 2.
MIS-C cases per 100,000 (A) acute COVID-19 hospitalizations (B) and estimated SARS-CoV-2 infections in non-Hispanic Black and White children and adolescents <18 years of age in Jackson, Mississippi, and 16 surrounding counties, Mississippi, August 2020–February 2021.
TABLE 2.
Cumulative Estimates of SARS-CoV-2 Infections Based on Seroprevalence, and Cumulative Incidence of MIS-C and Acute COVID-19 Hospitalizations per 100,000 SARS-CoV-2 Infections Among Children and Adolescents Aged <18 years in Jackson, Mississippi, and Surrounding Counties, Mississippi, August 2020–February 2021
| + | Black, Non-Hispanic | White, Non-Hispanic | ||||
|---|---|---|---|---|---|---|
| Estimated SARS-CoV-2 Infections* | MIS-C Cases | Acute COVID-19 Hospitalizations | Estimated SARS-CoV-2 Infections* | MIS-C Cases | Acute COVID-19 Hospitalizations | |
| Month | No. | No. (Rate† per 100,000 Infections) | No. (Rate per 100,000 Infections) | No. | No. (Rate† per 100,000 Infections) | No. (Rate per 100,000 Infections) |
| Mar 2020 | ND | 0 (ND) | 0 (ND) | ND | 0 | 0 (ND) |
| Apr 2020 | ND | 1 (ND) | 3 (ND) | ND | 0 | 1 (ND) |
| May 2020 | 5138 | 1 (38.9) | 5 (155.7) | 0 | 0 | 1 (.) |
| Jun 2020 | 10,031 | 1 (29.9) | 5 (129.6) | 1830 | 0 | 0 (109.3) |
| Jul 2020 | 16,393 | 1 (24.4) | 7 (122.0) | 4053 | 0 | 1 (74.0) |
| Aug 2020 | 21,653 | 2 (27.7) | 3 (106.2) | 14,120 | 0 | 1 (28.3) |
| Sep 2020 | 23,978 | 5 (45.9) | 1 (100.1) | 18,957 | 0 | 1 (26.4) |
| Oct 2020 | 32,786 | 0 (33.6) | 2 (79.3) | 19,872 | 0 | 0 (25.2) |
| Nov 2020 | 33,397 | 0 (32.9) | 6 (95.8) | 27,717 | 0 | 4 (32.5) |
| Dec 2020 | 44,285 | 4 (33.9) | 7 (88.1) | 39,483 | 1 (2.5) | 2 (27.9) |
| Jan 2021 | 47,711 | 9 (50.3) | 6 (94.3) | 54,257 | 2 (5.5) | 5 (29.5) |
| Feb 2021 | 78,661 | 8 (40.7) | 4 (62.3) | 48,374 | 1 (8.3) | 0 (33.1) |
ND indicates not determined; MIS-C, multisystem inflammatory syndrome in children.
Estimated number of persons younger than 18 years previously infected with SARS-CoV-2 based on race-specific seroprevalence among convenience samples of residual pediatric sera collected for routine clinical testing at University of Mississippi Medical Center, multiplied by the non-Hispanic White (N = 122,335) and non-Hispanic Black (N = 130,740) population <18 years old in Jackson, Mississippi, and 16 surrounding counties.
Cumulative rate of MIS-C cases or acute COVID-19 hospitalizations per 100,000 SARS-CoV-2 infected persons younger than 18 years in Jackson, Mississippi, and surrounding counties, by race.
DISCUSSION
Using active population-based surveillance for MIS-C and COVID-19 hospitalizations and SARS-CoV-2 seroprevalence data, we found that non-Hispanic Black children experienced 5-fold higher cumulative incidence of MIS-C and 2-fold higher rates of acute COVID-19 hospitalizations per 100,000 SARS-CoV-2 infections compared with non-Hispanic White children in central Mississippi. Monthly estimates of SARS-CoV-2 antibody seroprevalence increased rapidly over the study period with higher rates of infection among Black compared with White children. By February 2021, more than half of residual clinical serum specimens from persons younger than 18 years obtained from the UMMC central laboratory tested SARS-CoV-2 antibody positive, indicating high burden of infection in this source population even before circulation of the SARS-CoV-2 Delta and Omicron variants. These results provide strong evidence for increased risk of developing MIS-C and COVID-19 requiring hospitalization among Black compared with White children following SARS-CoV-2 infection. Equitable vaccine distribution with high-coverage across populations is critical to eliminate racial disparities and prevent associated complications of COVID-19 in children.
These findings are consistent with studies that have used reported COVID-19 cases, extrapolated numbers of SARS-CoV-2 infections, and census data as denominators to investigate disparities in rates of MIS-C and COVID-19 hospitalizations among children, many of which occur in a preceding or parallel period of time to the timeframe included in this report.2,4,11 MIS-C and COVID-19 hospitalization cases in these studies have been identified through notifiable disease reporting and active surveillance. Racial and ethnic disparities in MIS-C have also been described in the United Kingdom disproportionately affecting children of Afro-Caribbean descent,12 and in an early case series from Paris with MIS-C more commonly seen among children of African ancestry.13 Recent reports and CDC data indicate MIS-C was more common in Blacks, Hispanic or Latino, and Asian or Pacific Islander persons than White persons, with 6431 MIS-C cases nationwide and 55 deaths, with 31.9% Black (non-Hispanic), 27.5% Hispanic/Latino, and 32.5% White.3
Reasons for disparities in MIS-C and COVID-19 hospitalization case incidence are unknown. Social determinants of health contribute to disparities observed during the COVID-19 at all levels of disease severity.14,15 It is also of note that MIS-C cases have not yet commonly been described in East Asian countries (eg, China) that have gone through COVID-19 epidemic peaks.16 It is unclear whether factors such as host genetics, differences of environmental exposures, viral load or mode of transmission, or other coronavirus exposures account for these findings.17 Obesity has been implicated as a descriptive factor associated with MIS-C, as this characteristic shows as one of the more common underlying illnesses for children for those children who have underlying illnesses diagnosed with MIS-C in other cases series.18–21 Interestingly, our data show that 42% of those with MIS-C were noted to have obesity, although our sample size is small to derive conclusions about this characteristic in MIS-C versus acute COVID-19. Also it is of note that obesity is more prevalent in Mississippi overall, but in particular in the African-American population.22 Further studies need to delineate the relationship between risk factors for complications of SARS-CoV-2 infection.
The burden of COVID-19 hospitalizations is also disproportionately borne by non-White populations. However, differences in testing rates23 or positivity rates between Blacks and Whites24 do not account for the disparities seen in our report since we estimate infections based on seroprevalence. We cannot exclude differences in health care seeking for MIS-C and acute COVID-19, as well as serology testing by race/ethnicity. However, because we stratified serology by race, this seems unlikely. It has already been reported that risk ratios for hospitalization and death overall are higher in racial and ethnic minorities when compared with Whites.3 Differences in prevalence of underlying conditions by race may account for some of the racial/ethnic disparities in acute COVID-19 hospitalizations, but our sample size is too small to make any definite conclusions. Age distribution in our MIS-C cohort is in agreement with national surveillance data, with the highest percentage of MIS-C cases occurring in children in the 5–11 age group (see Table 1, footnote), although female sex in our dataset was slightly higher.
This analysis is subject to at least 5 limitations. First, the source population in central Mississippi is not representative of the US population and less diverse with <5% of the population identified as Hispanic or racial and ethnic groups other than Black or non-Hispanic White. Factors that contribute to racial disparities in this population may differ from those in other settings, although we believe that reports from geographic areas with different demographic characteristics are crucial in informing public health practices and policies. Second, numbers of MIS-C cases and acute COVID-19 hospitalizations at this hospital limited examination of racial disparities by age group or sex; differences in age and sex distribution of MIS-C and acute COVID-19 hospitalizations may have affected comparisons of cumulative incidence rates. Third, cases of MIS-C and acute COVID-19 early in the pandemic may have been missed before widespread testing and greater awareness of MIS-C. Fourth, patient race and ethnicity was extracted from medical records, rather than reported by patients or guardians, whereas US Census data are based on self-reported race and ethnicity.25 Finally, monthly race-specific SARS-CoV-2 seroprevalence estimates were based on serologic testing of convenience samples of residual serum specimens from the UMMC central laboratory rather than population-based serosurveys. While multiple serum specimens from the same individual were excluded from seroprevalence estimates, children with chronic conditions requiring routine hematologic tests were likely overrepresented.
However, this study had several strengths. Active surveillance for MIS-C and pediatric COVID-19 hospitalizations was conducted throughout the study period at the main pediatric hospital serving the source population. Hospitalized children were systematically evaluated for MIS-C and acute COVID-19 using established protocols and cases were adjudicated by a panel of experts at UMMC to confirm case classification based on previously reported criteria.7,20 Cumulative incidence was estimated per 100,000 SARS-CoV-2 infections by extrapolating monthly seroprevalence in pediatric serum specimens to calculate numbers of Black and White children in the source population who had been infected with SARS-CoV-2; race-specific seroprevalence was measured using residual sera from pediatric patients in the same geographic area, rather than census data or reported case data alone.26
In conclusion, we have strong evidence for racial disparity in MIS-C risk in our patient population, which reflects national reported trends during this period. Although our data predate the rise of the Delta and Omicron variants, our data also predate the widespread availability of SARS-CoV-2 vaccination in children with limited uptake in those 16 and older, presenting a picture of pandemic dynamics in the state in the absence of vaccine-induced protection. This is vital as recent data show vaccination reduces the risk of MIS-C in patients 12 years and up,27 and reduces acute COVID-19 hospitalization with severe disease in children 5–18 years of age.8,28 The impact of vaccination on younger, vaccine-eligible children should now be followed in the context of both primary and booster vaccination and MIS-C. In Mississippi, overall vaccination rates are below the national average, although the gap in vaccination between Blacks and Whites in Mississippi has narrowed.29,30 Vaccination has the potential to eliminate disparities, as has been seen with other infectious diseases,31 and the impact of vaccination, in those of all races, on severe outcomes as well as disparities for SARS-CoV-2 infection needs to be monitored in the hopes of eliminating disease burden in those disproportionately affected, and for all.
Supplementary Material
Footnotes
Support for this study was provided by the University of Mississippi Medical Center, Vice Chancellor’s Office for Research. Laboratory and epidemiologic support were provided by CDC.
C.V.H. receives funding from CDC for public health-related activities. C.V.H. is a consultant/speaker for BioFire (bioMérieux).
C.V.H. and B.F. conceived and designed the study. C.V.H., S.S.K., P.V., K.I., V.A.H., L.M., L.M.M., G.S., U.A., J.M.W., K.P., T.K., P.B., A.P., R.P.S., A.D., M.S., P.H., L.H., N.T., J.D., and B.F. contributed to acquisition, analysis, and interpretation of data. C.V.H. and B.F. drafted the article and K.I. critically appraised for important intellectual content and oversaw approval of the version to be published. C.V.H., S.S.K., L.M., L.M.M., P.V., K.I., and B.F. oversaw and are responsible for agreement in accountability accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work. All authors contributed to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Sara S. Kim, Email: omy1@cdc.gov.
Preeti Vemula, Email: preetivemula26@gmail.com.
Kengo Inagaki, Email: kinagaki@umc.edu.
Virginia A. Harrison, Email: vharrison@umc.edu.
Lacy Malloch, Email: lmalloch@umc.edu.
Lora M. Martin, Email: lmartin4@umc.edu.
Gurbaksh Singh, Email: gsingh2@umc.edu.
Urita Agana, Email: ua57@msstate.edu.
John M. Williams, Email: jwilliams32@umc.edu.
Kayla Patterson, Email: knpatterson@umc.edu.
Theresa Kittle, Email: theresa.kittle@msdh.ms.gov.
Paul Byers, Email: paul.byers@msdh.ms.gov.
April Palmer, Email: apalmer@umc.edu.
Roberto P. Santos, Email: rsantos@umc.edu.
Meagan Stephenson, Email: qrb2@cdc.gov.
Leroy Hung, Email: lhung@umc.edu.
Phillip Hankins, Email: phankins@umc.edu.
Nathalie Thornburg, Email: nax3@cdc.gov.
Jan Drobeniuc, Email: jqd6@cdc.gov.
Brendan Flannery, Email: bif4@cdc.gov.
REFERENCES
- 1.Havers FP, Whitaker M, Self JL, et al. ; COVID-NET Surveillance Team. Hospitalization of adolescents aged 12-17 years with laboratory-confirmed COVID-19 - COVID-NET, 14 States, March 1, 2020-April 24, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee EH, Kepler KL, Geevarughese A, et al. Race/Ethnicity among children with COVID-19-Associated multisystem inflammatory syndrome. JAMA Netw Open. 2020;3:e2030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Risk for COVID-19 Infection, Hospitalization, and Death By Race/Ethnicity. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html. Accessed 13 January, 2022.
- 4.Payne AB, Gilani Z, Godfred-Cato S, et al. ; MIS-C Incidence Authorship Group. Incidence of multisystem inflammatory syndrome in children among US persons infected with SARS-CoV-2. JAMA Netw Open. 2021;4:e2116420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradbury RS, Lane M, Arguello I, et al. Parasitic disease surveillance, Mississippi, USA. Emerg Infect Dis. 2021;27:2201–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Census Data for Mississippi. Available at: https://wonder.cdc.gov/. Accessed July, 2021.
- 7.CDC. Partner updates: case definition for MISC-C April 7, 2021. Available at: https://www.cdc.gov/mis-c/hcp/.
- 8.Olson SM, Newhams MM, Halasa NB, et al. ; Overcoming COVID-19 Investigators. Effectiveness of BNT162b2 vaccine against critical Covid-19 in adolescents. N Engl J Med. 2022;386:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldstein LR, Tenforde MW, Friedman KG, et al. ; Overcoming COVID-19 Investigators. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23-May 12, 2020. JAMA Intern Med. 2020;180:1576–1586. [DOI] [PubMed] [Google Scholar]
- 11.Stierman B, Abrams JY, Godfred-Cato SE, et al. Racial and ethnic disparities in multisystem inflammatory syndrome in children in the United States, March 2020 to February 2021. Pediatr Infect Dis J. 2021;40:e400–e406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dennis-Heyward EA, Shah SN. Pediatric COVID-19 disparities and prioritizing equity-children are not spared. JAMA Pediatr. 2021;175:898–900. [DOI] [PubMed] [Google Scholar]
- 15.Lopez L, III, Hart LH, III, Katz MH. Racial and ethnic health disparities related to COVID-19. JAMA. 2021;325:719–720. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Tang Y, Shi Y, et al. Why multisystem inflammatory syndrome in children has been less commonly described in Asia? Transl Pediatr. 2020;9:873–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Consiglio CR, Cotugno N, Sardh F, et al. ; CACTUS Study Team. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–981.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bautista-Rodriguez C, Sanchez-de-Toledo J, Clark BC, et al. Multisystem inflammatory syndrome in children: an international survey. Pediatrics. 2021;147:e2020024554. [DOI] [PubMed] [Google Scholar]
- 19.Dufort EM, Koumans EH, Chow EJ, et al. ; New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldstein LR, Rose EB, Horwitz SM, et al. ; Overcoming COVID-19 Investigators; CDC COVID-19 Response Team. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godfred-Cato S, Bryant B, Leung J, et al. ; California MIS-C Response Team. COVID-19-Associated multisystem inflammatory syndrome in children—United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Project RWJF. The State of Childhood Obesity. Available at: https://stateofchildhoodobesity.org/children1017/. Accessed January 14, 2022.
- 23.Saatci D, Ranger TA, Garriga C, et al. Association between race and COVID-19 outcomes among 2.6 million children in England. JAMA Pediatr. 2021;175:928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inagaki K, Garg P, Hobbs CV. SARS-CoV-2 positivity rates among children of racial and ethnic minority groups in mississippi. Pediatrics. 2021;147:e2020024349. [DOI] [PubMed] [Google Scholar]
- 25.Boehmer U, Kressin NR, Berlowitz DR, et al. Self-reported vs administrative race/ethnicity data and study results. Am J Public Health. 2002;92:1471–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hobbs CV, Drobeniuc J, Kittle T, et al. ; CDC COVID-19 Response Team. Estimated SARS-CoV-2 seroprevalence among persons aged <18 years—Mississippi, May-September 2020. MMWR Morb Mortal Wkly Rep. 2021;70:312–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zambrano LD, Newhams MM, Olson SM, et al. ; Overcoming COVID-19 Investigators. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12–18 Years—United States, July–December 2021. Available at: https://www.cdc.gov/mmwr/volumes/71/wr/mm7102e1.htm?s_cid=mm7102e1_w. Accessed January 7, 2022. [DOI] [PMC free article] [PubMed]
- 28.Price AM, Olson SM, Newhams MM, et al. BNT162b2 protection against the omicron variant in children and adolescents. N Engl J Med. 2022. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control. Available at: https://covid.cdc.gov/covid-data-tracker/#county-view?list_select_state=Mississippi&data-type=CommunityLevels. Accessed April 18, 2022.
- 30.Mississippi State Department of Health. Available at: https://msdh.ms.gov/msdhsite/_static/resources/12130.pdf. Accessed April 18, 2022.
- 31.Flannery B, Schrag S, Bennett NM, et al. ; Active Bacterial Core Surveillance/Emerging Infections Program Network. Impact of childhood vaccination on racial disparities in invasive Streptococcus pneumoniae infections. JAMA. 2004;291:2197–2203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.