Abstract
Background:
During the coronavirus disease 2019 (COVID-19) pandemic, the world has a large number of reported COVID-19 cases and deaths. Information on characteristics and mortality rate of pediatric intensive care unit (PICU) cases with COVID-19 remains limited. This study aims to identify the risk factors for mortality related to COVID-19 in children admitted to PICU.
Methods:
A retrospective multicenter cohort study was conducted between March 2020 and April 2021 at 44 PICUs in Turkey. Children who were 1 month–18-year of age with confirmed COVID-19 admitted to PICU were included in the study. Children with multisystem inflammatory syndrome and asymptomatic for COVID-19 were excluded.
Results:
Of 335 patients with COVID-19, the median age was 6.8 years (IQR: 1.2–14) and 180 (53.7 %) were male, 215 (64.2 %) had at least one comorbidity. Age and gender were not related to mortality. Among 335 patients, 166 (49.5%) received mechanical ventilation, 17 (5.1%) received renal replacement therapy and 44 (13.1 %) died. Children with medical complexity, congenital heart disease, immunosuppression and malignancy had significantly higher mortality. On multivariable logistic regression analysis, organ failure index [odds ratio (OR): 2.1, 95 confidence interval (CI): 1.55–2.85], and having congenital heart disease (OR: 2.65, 95 CI: 1.03–6.80), were associated with mortality.
Conclusions:
This study presents detailed data on clinical characteristics and outcomes of patients with COVID-19 admitted to PICU in the first pandemic year in Turkey. Our study shows that having congenital heart disease is associated with mortality. In addition, the high organ failure score in follow-up predict mortality.
Keywords: Child, COVID-19, mortality, PICU
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported first on March 11, 2020, by the Turkish Ministry of Health. There are over 128 million confirmed cases and over 2.8 million deaths worldwide, approximately 3.3 million confirmed cases, and over 31 thousand deaths in Turkey as of April 20211–2. According to the American Academy of Pediatrics, of nearly 25 million cases reported, children constitute 13.4% (3.4 million) of all cases3. Children generally have a milder disease course than adults, with typically better prognosis. A small number of children with COVID-19 required pediatric intensive care unit (PICU) admission4. Studies including adult patients report that in-hospital mortality is associated with male sex, immunosuppression, renal disease, chronic lung disease, cardiovascular disease, neurologic disorders and diabetes5,6. Children were thought to be mildly affected but as the pandemic progressed, severe cases and new studies on mortality have emerged. A study discussing outcomes of children with COVID-19 in US and Canadian intensive care units reveals that prehospital comorbidities play an important role in severe disease4.
Our aim in this study was to evaluate the independent risk factors associated with the mortality of children with COVID-19 requiring treatment in PICUs in Turkey.
METHODS
Study Population
We performed a retrospective cohort study including pediatric patients with COVID-19 infection who were admitted to PICU between March 1, 2020, and April 1, 2021. This study includes patient data from 44 pediatric intensive care units in 23 cities across Turkey (see list of Study Centers, Supplemental Digital Content 1; http://links.lww.com/INF/E744). All children less than 18 years old who required PICU care and had laboratory-confirmed COVID-19 diagnoses were included in the study. COVID-19 diagnosis was confirmed either by a positive RT-polymerase chain reaction (RT-PCR) on a nasopharyngeal specimen or antibody positivity developed during the disease course when the first RT-PCR and antibody testing were both negative. Only laboratory-confirmed cases were included and analyzed. We identified these cases using the International Classification of Diseases (ICD-9) code for COVID-19. Children who died within 12 h of PICU admission or suspected cases who had radiological or clinical findings that were compatible with COVID-19 but had negative PCR test results or the patients who were hospitalized for non-COVID reasons (such as trauma or surgery) but had incidentally positive COVID-19 PCR results were excluded from the study. The study was approved by the University of Health Sciences Turkey Bakirkoy Dr. Sadi Konuk Research and Training Hospital Ethics Committee (approval number 2021/77) and the Ministry of Health Scientific Research Platform of Turkey (approval number 2021-01-13T14_19_40). Institutional review board approval was obtained from each participating hospital in accordance with local ethical regulations. The study has been performed in accordance with the ethical standards laid down in the Declaration of Helsinki.
Data Collection
Each center recorded and collected data using preprinted case report forms. The collected variables included age, gender, comorbidities, mode of presentation (ie, asymptomatic, respiratory, gastrointestinal, neurological, hematological or circulatory), Pediatric Risk of Mortality (PRISM) IV7, organ failure index (OFI)8–9, pediatric acute respiratory distress syndrome (pARDS) classifications10, pediatric COVID-19 severity score4, contact history, presenting symptoms, laboratory parameters on admission, medical treatment, respiratory support, prone positioning, renal replacement therapy (RRT), extracorporeal membrane oxygenation (ECMO), PICU length of stay and outcome. Information on comorbidities was collected from previous medical records, chronic medications and parents. The severity of pARDS was defined as mild, moderate or severe according to the Pediatric Acute Lung Injury Consensus Conference10. Mild pARDS is defined as the oxygenation index (OI) of 4–8 [oxygen saturation index (OSI) = 5–7.5], moderate as an OI of 8–16 (OSI = 7.5–12.3) and severe as an OI >16 (OSI >12.3). The respiratory support modalities for which data was available were oxygen support (nasal cannula and oxygen mask), the high-flow nasal cannula (HFNC), noninvasive mechanic ventilation (NIV), invasive mechanical ventilation (IMV), high-frequency oscillatory ventilation (HFOV), prone positioning, surfactant therapy and recruitment maneuvers. Medical treatments such as favipiravir, oseltamivir, lopinavir/ritonavir, remdesivir, hydroxychloroquine, glucocorticoid therapy, intravenous immunoglobulin (IVIG), convalescent plasma transfusion, anakinra, colchicine, tocilizumab, anticoagulation and inotrope treatment and outcome data related COVID-19 were collected.
Statistics
Statistical analyzes in this study were performed using the Number Cruncher Statistical System 2007 Statistical Software (Utah, USA) package program. The Shapiro-Wilk normality test, as well as descriptive statistical methods (mean, standard deviation, median and interquartile range), were used to evaluate the data. Shapiro-Wilk test was used to test for normality. Then Levene’s test was conducted to test the homogeneity of variance for the independent t-test. The independent t-test was used for the comparison of the normally distributed variables in paired groups, and the Mann Whitney U test was used for the comparison of the non-normally distributed variables and pairwise groups. According to Levene’s test, the Welch t-test was used instead of the independent t-test if the equal variance was not assumed. We used univariable logistic regression to analyze the contributing factors for mortality. Then, those statistically significant factors in the univariable logistic regression analysis were enrolled into the binary logistic multivariable regression analysis to identify the independent risk factors for mortality in children with COVID-19. To avoid the interaction effect, we choose OFI rather than PRISM-IV, max vasoactive-inotropic score (VIS) and cardiac involvement. The results were evaluated at the significance level of P < 0.05.
RESULTS
There were 20,892 consecutive all-cause admissions at the 44 PICUs and a total of 1506 deaths, for an overall all-cause mortality rate of 7.21% during the study period. Of 20,892 patients, 353 were diagnosed with proven SARS-CoV-2 infection. Two patients >18 years old, a patient who died within 12 hours, and 15 children with a positive PCR test whose primary diagnoses were trauma, poisoning, etc. were excluded from the final analysis of mortality as they were asymptomatic and had no radiologic and laboratory evidence for COVID-19 (see Figure, Supplemental Digital Content 2; http://links.lww.com/INF/E745). In PICUs, the calculated COVID-19 rate was 1.6% (335/20892). Of 335 children, 180 were male (53.7 %); the median age was 6.8 years (IQR: 1.2–14). Table 1 shows baseline characteristics, presenting signs, symptoms and presenting system involvement of all children with COVID-19 admitted to PICUs. Overall, 215 (64.2 %) children had known comorbidity. In all children, 162 (48.4%) were diagnosed with pARDS during the study period; 123 (36.7%) received IMV [median duration of 10.5 days (IQR 5–27)], 43 (12.8%) received NIV, 82 (24.5%) received HFNC oxygen therapy and 49 (12.8%) received only oxygen therapy. Five children (1.5%) who underwent IMV required HFOV. Of 335 children with COVID-19, 44 children died, 37 (11.0%) were discharged with sequelae and 254 (75.8%) were discharged with their baseline status before hospitalization. The calculated COVID-19 PICU mortality rate was 13.1% (44/335). The demographics, comorbidities, treatment and maximum respiratory support were compared between survivors and nonsurvivors and are shown in Table 2. The mean age was 6.78 years (IQR 1.2–13.9) in the survival group and 6.84 years (IQR 1.2–14.9) in the nonsurvival group. Age and gender were not significantly different. Nonsurvivors had more comorbid conditions 39 (88.64 %) compared to survivors 176 (60.48%). Children with medical complexity, congenital heart disease, immunosuppression, and malignancy had significantly higher mortality (P < 0.05). The OFI, PRISM scores, the severity of illness and the use of favipiravir, glucocorticoid, IVIG, and inotropes, the severity of pARDS, the utilization of maximum respiratory support, prone positioning, recruitment and surfactant therapy were significantly higher in nonsurvivors (P < 0.05). Continuous renal replacement therapy, plasma exchange and ECMO were more performed in nonsurvivors (P < 0.05). The laboratory findings on admission comparing survivors to nonsurvivors are also shown in Table 2. Nonsurvivors had significantly higher levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea, creatinine, sodium, lactate dehydrogenase (LDH), lipase, C-reactive protein (CRP), procalcitonin, d-dimer, ferritin, pro-brain natriuretic peptide, lactate and lower levels of albumin than survivors (P < 0.05). Antiviral treatments were used in 267 patients (80%). The most common of these was favipiravir. Glucocorticoid therapy was used in 162 (48%), IVIG in 75 (22%), convalescent plasma in 8 (2%), anakinra in 3 (0.9%) tocilizumab in 2 (0.6%), anticoagulation in 177 (53%), inotrope in 84 (25%), renal replacement therapy in 17 (5%), plasma exchange in 22 (7%) and hemadsorption therapy in 2 (0.6%) children as a single agent or in combination. Median PICU length of stay for all patients until the study endpoint was 7 days (IQR: 4–15), and significantly higher in nonsurvivors (P < 0.05, Table 2).
TABLE 1.
Baseline Characteristics of COVID-19 Patients at Admission
| Characteristics | n (%) (total n = 335) |
|---|---|
| Age, month | |
| ≤12 | 76 (22.69) |
| 13–60 | 76 (22.69) |
| 61–120 | 45 (13.43) |
| ≥121 | 138 (41.19) |
| Male gender | 180 (53.73) |
| Contact history | 220 (65.67) |
| Household | 188 (56.12) |
| Nonhousehold | 32 (9.55) |
| Comorbidity | 215 (64.18) |
| ≥2 Comorbidity | 117 (34.93) |
| Signs and symptoms | |
| Fever | 241 (71.94) |
| Respiratory distress | 225 (67.16) |
| Cough | 212 (63.28) |
| Fatigue | 132 (39.40) |
| Nausea or vomiting | 62 (18.51) |
| Myalgia | 57 (17.01) |
| Altered consciousness | 57 (17.01) |
| Convulsion | 46 (13.73) |
| Sore throat | 45 (13.43) |
| Diarrhea | 38 (11.34) |
| Abdominal pain | 20 (5.97) |
| Headache | 19 (5.67) |
| Rash | 15 (4.48) |
| Loss of taste | 13 (3.89) |
| Chest pain | 12 (3.58) |
| Loss of smell | 9 (2.69) |
| Presentation | |
| Respiratory | 281 (83.88) |
| Neurological | 79 (23.58) |
| Gastrointestinal | 74 (22.09) |
| Circulatory | 51 (15.22) |
| Hematological | 28 (8.36) |
| Other | 28 (8.36) |
TABLE 2.
Comparisons of Survivors’ vs Nonsurvivors in Children with COVID-19
| Parameters | All Patients (n = 335) | Survivors (n = 291) | Nonsurvivors (n = 44) | P |
|---|---|---|---|---|
| Age, month n (%) | ||||
| ≤12, n (%) | 76 (22.69) | 66 (22.68) | 10 (22.73) | 0.96 |
| 13–60, n (%) | 76 (22.69) | 66 (22.68) | 10 (22.73) | |
| 61–120, n (%) | 45 (13.43) | 38 (13.06) | 7 (15.91) | |
| ≥121, n (%) | 138 (41.19) | 121 (41.58) | 17 (38.64) | |
| Male, n (%) | 180 (53.73) | 155 (53.26) | 25 (56.82) | 0.66 |
| Comorbidities n (%) | 215 (64.18) | 176 (60.48) | 39 (88.64) | 0.00 |
| ≥2 Comorbidities n (%) | 117 (34.93) | 92 (31.62) | 25 (56.82) | 0.00 |
| Epilepsy, n (%) | 62 (18.51) | 54 (18.56) | 8 (18.18) | 0.95 |
| Developmental delay, n (%) | 54 (16.12) | 45 (15.46) | 9 (20.45) | 0.40 |
| Medical complexitya, n (%) | 47 (14.13) | 35 (12.03) | 12 (27.27) | 0.01 |
| Congenital heart disease, n (%) | 27 (8.06) | 20 (6.87) | 7 (15.91) | 0.04 |
| Chronic lung disease, n (%) | 25 (7.46) | 21 (7.22) | 4 (9.09) | 0.66 |
| Immune suppression, n (%) | 21 (6.27) | 14 (4.81) | 7 (15.92) | 0.01 |
| Obesity, n (%) | 21 (6.27) | 19 (6.53) | 2 (4.55) | 0.61 |
| Malignancy, n (%) | 21 (6.27) | 12 (4.12) | 9 (20.45) | 0.00 |
| Diabetes, n (%) | 16 (4.78) | 14 (4.81) | 2 (4.55) | 0.94 |
| Asthma, n (%) | 14 (4.18) | 12 (4.12) | 2 (4.55) | 0.90 |
| Endocrine disease, n (%) | 17 (5.07) | 14 (4.81) | 3 (6.82) | 0.58 |
| Chronic kidney disease, n (%) | 11 (3.28) | 9 (3.09) | 2 (4.55) | 0.61 |
| Metabolic disease, n (%) | 11 (3.28) | 9 (3.09) | 2 (4.55) | 0.61 |
| Down syndrome, n (%) | 9 (2.69) | 6 (2.06) | 3 (6.82) | 0.07 |
| Hematologic disease, n (%) | 3 (0.90) | 3 (1.03) | 0 (0.00) | 1.00 |
| Psychiatric disease, n (%) | 2 (0.60) | 1 (0.34) | 1 (2.27) | 0.12 |
| Post-transplantation, n (%) | 2 (0.60) | 2 (0.69) | 0 (0.00) | 0.58 |
| PRISM-IV, median (IQR) | 7 (2–16) | 6 (2–12) | 31.5 (13–63) | 0.00 |
| OFI n (%) | 0.00 | |||
| 1–2 organ failure n (%) | 160 (47.76) | 148 (50.86) | 12 (27.27) | |
| 3–4 organ failure n (%) | 63 (18.81) | 44 (15.12) | 19 (43.18) | |
| 5–6 organ failure n (%) | 19 (5.67) | 6 (2.06) | 13 (29.55) | |
| Severity of illness n (%) | ||||
| Mild n (%) | 72 (21.49) | 72 (24.74) | 0 (0.00) | 0.00 |
| Moderate n (%) | 54 (16.12) | 54 (18.56) | 0 (0.00) | |
| Severe n (%) | 104 (31.04) | 96 (32.99) | 8 (18.18) | |
| Critical n (%) | 105 (31.34) | 69 (23.71) | 36 (81.82) | |
| Vital signs, mean ± SDS | ||||
| Heart rate (beats/min), mean ± SD | 128.65 ± 30.8 | 127.28 ± 29.94 | 137.81 ± 35.04 | 0.04 |
| Respiratory rate (breaths/min) mean ± SD | 38.79 ± 14.1 | 38.49 ± 13.69 | 41.00 ± 16.85 | 0.30 |
| SBP mm Hg mean ± SD | 98.92 ± 20.47 | 99.74 ± 19.31 | 93.57 ± 26.54 | 0.14 |
| DBP mm Hg mean ± SD | 61.21 ± 15.37 | 61.90 ± 14.77 | 56.70 ± 18.42 | 0.04 |
| SPO2, mean ± SD | 91.41 ± 8.83 | 91.61 ± 9.04 | 90.14 ± 7.30 | 0.31 |
| Laboratory finding | ||||
| White blood cell count, × 109/L, mean ± SD | 10887.73 ± 7847.97 | 10829.42 ± 7774.51 | 11273.39 ± 8401.63 | 0.73 |
| Lymphocyte count, × 109/L median (IQR) | 1680 (950–3300) | 1750 (1012–3400) | 1200 (656–2300) | 0.00 |
| Neutrophil count, × 109/L, median (IQR) | 5240 (2750–9700) | 5430 (3000–10287) | 3450 (1090–7790) | 0.00 |
| Platelet count, × 109/L, mean ± SD | 267.40 ± 147.48 | 273.55 ± 140.09 | 226.88 ± 185.99 | 0.12 |
| Hemoglobin, mean ±SD | 11.17 ± 2.25 | 11.25 ± 2.20 | 10.64 ± 2.54 | 0.09 |
| Hematocrit, mean ±SD | 33.69 ± 6.76 | 33.87 ± 6.57 | 32.50 ± 7.85 | 0.21 |
| Aspartate aminotransferase, U/L, median (IQR) | 36 (25–65) | 35 (25–58) | 69.5 (35–150) | 0.00 |
| Alanine aminotransferase, U/L, median (IQR) | 25 (15–45) | 23.5 (15–41) | 33.5 (22–115) | 0.00 |
| Urea, mg/dl, median (IQR) | 21 (15–32) | 21 (15–30) | 30 (16–47) | 0.01 |
| Creatinine, mg/dl, median (IQR) | 0.47 (0.3–0.68) | 0.47 (0.29–0.67) | 0.49 (0.37–1.08) | 0.04 |
| Sodium mEq/L, mean ±SD | 137.65 ± 5.41 | 137.41 ± 5.25 | 139.27 ± 6.20 | 0.03 |
| Potassium mEq/L, mean ±SD | 4.18 ± 0.74 | 4.20 ± 0.73 | 4.06 ± 0.82 | 0.24 |
| Albumin, mg/dl, mean ±SD | 3.65 ± 0.62 | 3.70 ± 0.60 | 3.31 ± 0.64 | 0.00 |
| Creatine kinase, U/L, median (IQR) | 103 (48–221) | 101 (50–221) | 120 (46–264) | 0.79 |
| Lactate dehydrogenase, U/L, median (IQR) | 353 (275–526) | 337 (272–500) | 475 (356–789) | 0.00 |
| Amylase, U/L, median (IQR) | 45 (28–77) | 42 (27–72) | 61 (31–129) | 0.06 |
| Lipase, U/L, median (IQR) | 28 (13–48) | 24 (11–41) | 45 (21–99) | 0.01 |
| C-reactive protein, mg/dl, median (IQR) | 2.1 (0.42–8.19) | 1.88 (0.39–7.6) | 4.93 (1.1–16.03) | 0.00 |
| Procalcitonin, ng/mL, median (IQR) | 0.32 (0.1–2.3) | 0.28 (0.1–1.35) | 2.28 (0.31–16.8) | 0.00 |
| D-dimer, mg/dl, median (IQR) | 1 (0.54–2.26) | 0.97 (0.5–1.97) | 2.08 (1–3.63) | 0.00 |
| Troponin, median (IQR) | 3.3 (0.13–13) | 3.9 (0.15–13.3) | 1.65 (0.1–11.85) | 0.51 |
| Ferritin, mg/dl, median (IQR) | 224 (67–555) | 194.5 (65.5–487.25) | 565 (129.5–1413) | 0.00 |
| Fibrinogen, mg/dl, median (IQR) | 321 (234–447) | 329 (241–440) | 305.5 (156–466) | 0.24 |
| Prothrombin time, s, mean ± SDS | 14.69 ± 6.01 | 14.51 ± 4.73 | 15.90 ± 11.48 | 0.47 |
| APTT, s, mean ± SD | 30.36 ± 10.43 | 30.09 ± 9.45 | 32.10 ± 15.40 | 0.25 |
| Pro-BNP, median (IQR) | 550 (116–2520) | 456 (112–1782) | 3126 (296–7444) | 0.02 |
| Creatine kinase–MB, U/L, median (IQR) | 3.7 (0.94–20.2) | 3.36 (0.95–19) | 4.63 (0.87–27) | 0.60 |
| IL-6, median (IQR) | 34.4 (11.3–72.28) | 29.7 (9–66) | 55.5 (28.5–1234) | 0.05 |
| Baseline blood gases | ||||
| pH, mean ±SD | 7.33 ± 0.12 | 7.33 ± 0.12 | 7.32 ± 0.13 | 0.34 |
| pCO2, mm Hg, mean ± SD | 44.51 ± 17.1 | 44.27 ± 16.66 | 36.12 ± 19.88 | 0.51 |
| HCO3, mm Hg, mean ± SD | 22.38 ± 6.19 | 22.36 ± 5.97 | 22.55 ± 7.60 | 0.85 |
| Lactate, mmol/L, median (IQR) | 1.8 (1.3–2.74) | 1.8 (1.3–2.6) | 2.55 (1.5–4.1) | 0.00 |
| pARDS n (%) | 0.00 | |||
| Mild, n (%) | 67 (20.00) | 63 (21.65) | 4 (9.09) | |
| Moderate, n (%) | 38 (11.34) | 33 (11.34) | 5 (11.36) | |
| Severe, n (%) | 57 (17.01) | 26 (8.93) | 31 (70.45) | |
| Superinfections | ||||
| Gram-positive,n (%) | 37 (11.04) | 29 (9.97) | 8 (18.18) | 0.11 0.010.11 |
| Gram-negative,n (%) | 37 (11.04) | 27 (9.28) | 10 (22.73) | |
| Fungi,n (%) | 20 (5.97) | 15 (5.15) | 5 (11.36) | |
| Treatment | ||||
| Favipiravir, n (%) | 164 (48.96) | 134 (46.05) | 30 (68.18) | 0.01 |
| Oseltamivir, n (%) | 44 (13.13) | 41 (14.09) | 3 (6.82) | 0.18 |
| Lopinavir/ritonavir, n (%) | 32 (9.55) | 28 (9.62) | 4 (9.09) | 0.91 |
| Remdesivir, n (%) | 27 (8.08) | 24 (8.28) | 3 (6.82) | 0.74 |
| Hydroxychloroquine, n (%) | 61 (18.21) | 54 (18.56) | 7 (15.91) | 0.67 |
| Glucocorticoid therapy, n (%) | 162 (48.36) | 134 (46.05) | 28 (63.6) | 0.03 |
| IVIG, n (%) | 75 (22.39) | 57 (19.59) | 18 (40.91) | 0.00 |
| Convalescent plasma transfusion, n (%) | 8 (2.39) | 6 (2.06) | 2 (4.55) | 0.32 |
| Anakinra, n (%) | 3 (0.9) | 3 (1.03) | 0 (0.00) | 0.50 |
| Colchicine, n (%) | 3 (0.9) | 3 (1.03) | 0 (0.00) | 0.50 |
| Tocilizumab, n (%) | 2 (0.6) | 2 (0.69) | 0 (0.00) | 0.58 |
| Anticoagulation, n (%) | 177 (52.84) | 148 (50.86) | 29 (65.91) | 0.06 |
| Inotrope treatment, n (%) | 84 (25.07) | 42 (14.43) | 42 (95.45) | 0.00 |
| Maximum Vasoactive inotrope score, median (IQR) |
30 (15–50) | 18.75 (10–30) | 50 (26.25–205) | 0.00 |
| Maximum respiratory support | ||||
| None, n (%) | 38 (11.34) | 38 (13.06) | 0 (0.00) | 0.00 |
| Oxygen only, n (%) | 49 (14.63) | 49 (16.84) | 0 (0.00) | |
| High-flow oxygen, n (%) | 82 (24.48) | 81 (27.84) | 1 (2.27) | |
| Noninvasive ventilation, n (%) | 43 (12.84) | 43 (14.78) | 0 (0.00) | |
| Invasive ventilation, n (%) | 118 (35.22) | 79 (27.15) | 39 (88.64) | |
| HFOV, n (%) | 5 (1.49) | 1 (0.34) | 4 (9.09) | |
| Other respiratory support | ||||
| Prone position, n (%) | 80 (23.88) | 60 (20.62) | 20 (45.45) | 0.00 |
| Recruitment, n (%) | 34 (10.15) | 21 (7.22) | 13 (29.55) | 0.00 |
| Surfactant, n (%) | 5 (1.49) | 2 (0.69) | 3 (6.82) | 0.00 |
| Extracorporeal treatment | ||||
| Renal replacement therapy, n (%) | 17 (5.07) | 3 (1.03) | 14 (31.82) | 0.00 |
| Plasma exchange, n (%) | 22 (6.57) | 14 (4.81) | 8 (18.18) | 0.00 |
| Hemadsorption, n (%) | 2 (0.60) | 1 (0.34) | 1 (2.27) | 0.12 |
| ECMO, n (%) | 5 (1.49) | 2(0.69) | 3 (6.82) | 0.00 |
| PICU stay, median (IQR) | 7 (4–15) | 7 (4–14) | 11 (6–19) | 0.01 |
| Hospital stay,median (IQR) | 14 (8–24) | 14 (8–23) | 15 (10–37) | 0.31 |
APTT indicates Activated Partial Thromboplastin Time; BNP, brain natriuretic peptide; DBP, diastolic blood pressure; ECMO, extracorporeal membrane oxygenation; HFOV, High-frequency oscillatory ventilation; IQR, interquartile range; OFI, organ failure Index; IVIG, intravenous immunoglobulin; SBP, systolic blood pressure; SD, standard deviation; pARDS, pediatric acute respiratory distress syndrome; PRISM-IV, Pediatric Risk of Mortality IV.
Medical complexity: Defined as children who had a long-term dependence on technological support (including tracheostomy, etc).
Based on the PRISM score, all patients were divided into 3 categories. While the majority of the cases (211 patients) had a PRISM score lower than 10 points, 58 patients had a PRISM score between 11 and 20 points, and 66 patients had a PRISM score higher than 20 points (Table 3). The need for inotrope was higher in patients with mortality in all PRISM score groups. Renal replacement and plasma exchange therapy were associated with higher mortality in patients with a PRISM score of 11–20. Renal replacement and IVIG therapy were associated with higher mortality in patients with a PRISM score higher than 20.
TABLE 3.
Treatment and its Effect on Mortality in Different PRISM Score Categories
| PRISM Score <10 (n = 211) | PRISM Score 11-20 (n = 58) | PRISM Score >20 (n = 66) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Survivors (n = 204) | Nonsurvivors (n = 7) | P value* | Survivors (n = 49) | Nonsurvivors (n = 9) | P value* | Survivors (n = 38) | Nonsurvivors (n = 28) | P value* | |
| Favipiravir, n (%) | 84 (41.2) | 4 (57.1) | 0.45 | 29 (59.2) | 7 (77.8) | 0.46 | 21 (55.3) | 19 (67.9) | 0.30** |
| Oseltamivir, n (%) | 29 (14.2) | 0 (0) | 0.60 | 5 (10.2) | 1 (11.1) | 0.94 | 7 (18.4) | 2 (7.1) | 0.28 |
| Lopinavir/ritonavir, n (%) | 18 (8.8) | 1 (14.3) | 0.49 | 7 (14.3) | 0 (0) | 0.59 | 3 (7.9) | 3 (10.7) | 0.69 |
| Remdesivir, n (%) | 16 (7.8) | 0 (0) | 0.46 | 6 (12.2) | 1 (11.1) | 0.92 | 2 (5.4) | 2 (7.1) | 0.58 |
| Hydroxychloroquine, n (%) | 34 (16.7) | 0 (0) | 0.60 | 10 (20.4) | 1(11.1) | 0.51 | 10 (26.3) | 6 (21.4) | 0.65† |
| Glucocorticoid therapy, n (%) | 83 (40.7) | 3 (42.9) | 0.91 | 28 (57.1) | 7 (77.8) | 0.30 | 23 (60.5) | 18 (64.3) | 0.76† |
| IVIG, n (%) | 35 (19.5) | 1 (14.3) | 0.82 | 13 (26.5) | 4 (44.4) | 0.43 | 8 (21.1) | 13 (46.4) | 0.03 † |
| Convalescent plasma transfusion, n (%) | 5 (2.5) | 1 (14.3) | 0.19 | 1 (2.0) | 1 (11.1) | 0.29 | 0 (0) | 0 (0) | – |
| Anakinra, n (%) | 2 (1.0) | 0 (0) | 0.79 | 0 (0) | 0 (0) | – | 1 (2.6) | 0 (0) | 0.58 |
| Colchicine, n (%) | 3 (1.5) | 0 (0) | 0.75 | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| Tocilizumab, n (%) | 1 (0.5) | 0 (0) | 0.85 | 0 (0) | 0 (0) | – | 1 (2.6) | 0 (0) | 0.58 |
| Anticoagulation, n (%) | 96 (47.1) | 3 (42.9) | 0.83 | 28 (57.1) | 7 (77.8) | 0.30 | 24 (63.2) | 19 (67.9) | 0.69† |
| Inotrope treatment, n (%) | 14 (6.9) | 6 (85.7) | 0.00 | 12 (24.5) | 9 (100) | 0.00 | 16 (42.1) | 27 (96.4) | 0,00 † |
| Renal replacement therapy, n (%) | 1 (0.6) | 1 (14.3) | 0.07 | 1 (2.0) | 6 (66.7) | 0.00 | 1 (2.6) | 7 (25.0) | 0.01 |
| Plasma exchange, n (%) | 7 (3.9) | 0 (0) | 0.62 | 5 (10.2) | 4 (44.4) | 0.03 | 2 (5.3) | 4 (14.3) | 0.39 |
| Hemadsorption, n (%) | 0 (0) | 0 (0) | - | 0 (0) | 0 (0) | - | 1 (2.6) | 1 (3.6) | 0.67 |
| ECMO, n (%) | 0 (0) | 0 (0) | - | 1 (2.0) | 0 (0) | 0.67 | 1 (2.6) | 3 (10.7) | 0.30 |
ECMO indicates extracorporeal membrane oxygenation; IVIG, intravenous immunoglobulin.
Fisher’s Exact Test.
Chi-Square test
On multivariable logistic regression analysis, OFI score [odds ratio (OR): 2.1; 95%) confidence interval (CI): 1.55–2.85], and congenital heart disease (OR: 2.65; 95% CI: 1.04–6.80) were independently associated with mortality. We observed no independent association of lactate, lymphocyte or CRP with mortality (Table 4).
TABLE 4.
Variables Predictive of COVID-19 Mortality According to Logistic Regression
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| PRISM-IV score | 1.05 | 1.04–1.07 | ||
| Organ failure Index | 2.65 | 2.05–3.44 | 2.1 | 1.55–2.85 |
| Congenital heart disease | 2.56 | 1.02–6.47 | 2.65 | 1.04–6.80 |
| Cardiac involvement | 9.03 | 4.46–18.2 | ||
| Maximum VIS | 1.02 | 1.01–1.04 | ||
| Lactate | 1.19 | 1.07–1.33 | 1.19 | 0.94–1.5 |
| Lymphocyte (x103/mm3) | 0.57 | 0.39–0.85 | 0.77 | 0.47–1.27 |
| C-reactive protein | 1.05 | 1.02–1.08 | 1.01 | 0.96–1.06 |
| D-dimer | 1.08 | 1.03–1.14 | 1.05 | 1.00–1.10 |
CI indicates confidence interval; OD, odds ratio; PRISM-IV, pediatric risk of mortality IV; VIS, vasoactive-inotropic score.
DISSCUSSION
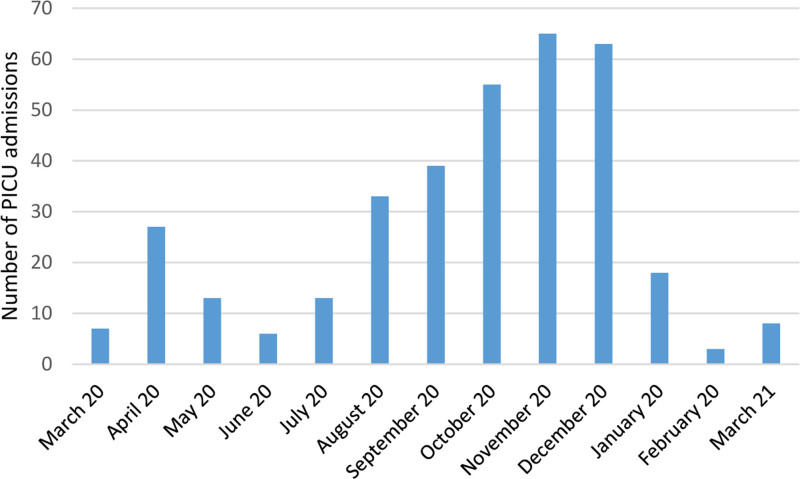
To our knowledge, this study is one of the largest severe pediatric COVID-19 outcomes to date. In this multicenter retrospective cohort study, we report the characteristics, clinical course and mortality-related risk factors of critically ill children with confirmed COVID-19 at 44 PICUs in Turkey during the first year of the pandemic. The 2 peaks of the COVID-19 disease seen in children were similar to the whole world’s disease peaks (Fig. 1)11.
FIGURE 1.
Monthly number of new PICU admissions with COVID-19.
In our study, 64.2% of patients had comorbidities and among them, the most common comorbidities were epilepsy, developmental delay and medical complexity. Furthermore, malignancy, immune suppression, medical complexity and congenital heart disease were significantly higher in nonsurvivors compared to survivors. According to studies in the early days of the pandemic, adults with COVID-19 indicated that comorbidities, such as diabetes, hypertension, malignancies, chronic respiratory disease and obesity are significant risk factors for severe infection12–16. Shekerdemian et al4 reported that in children with COVID-19 admitted to PICU, common comorbidities were medical complexity, immune suppression/malignancy and obesity. A recent meta-analysis including 275,661 children without comorbidities and 9353 children with comorbidities showed that children with comorbidities had a higher risk of severe COVID-19 and associated mortality than children without comorbidity. In addition, the study notes that childhood obesity probably leads to a worse COVID-19 prognosis12. In our study, obesity was not associated with mortality, which may be due to the fact that there was the small number of obese patients in the nonsurvivor group. A study from India reported that 94 children with any form of heart disease had a higher risk of mortality when they acquired COVID-19 infection17. The majority of deaths occurred in unoperated patients. Similar to this report, in multivariable logistic regression analysis, having congenital heart disease is independently associated with mortality in our study. These findings suggest that preventive measures are needed for improving the outcomes in children with congenital heart disease.
Scoring measurements such as PRISM-IV, OFI and VIS were used for mortality prediction and disease severity in our study. As expected, all these scores were statistically higher in nonsurvivors. In multivariable logistic regression analysis, the OFI score was significantly associated with higher mortality.
In this report, we also compared the treatment modalities between survivors and nonsurvivors. The respiratory support modalities, prone positioning, recruitment and surfactant therapy were significantly more used in nonsurvivors. Likewise, continuous RRT, plasma exchange and ECMO were significantly more performed in nonsurvivors. Not surprisingly, more ill children required more treatments, and many of those who died got maximum treatment. We analyzed treatments and their effect on mortality in different PRISM Score categories to describe the effect of giving or not giving specific treatment within each group on mortality. We think that larger patient series are needed in each severity group for a more accurate conclusion.
The prognostic significance of laboratory tests was also evaluated between survivors and nonsurvivors. Nonsurvivors had significantly higher levels of AST, ALT, urea, creatinine, sodium, LDH, lipase, CRP, procalcitonin, d-dimer, ferritin, pro-brain natriuretic peptide, lactate and lower levels of albumin, lymphocyte and neutrophil count than survivors at admission (Table 2). Yang et al18 reported that lymphopenia was detected in 80% of critically ill adults with COVID-19. However, there is an inconsistency between existing studies regarding the correlation of hematological findings with disease severity in children. The association between lymphopenia and COVID-19 severity in children was documented in a few studies19–20. Although lymphocyte count is significantly lower in nonsurvivors, no relationship was found between lymphopenia and mortality in multivariable analysis in our study.
In adults, hospitalization status and increased oxygen support requirements with elevated d-dimer levels are associated with worse outcomes, including thrombosis13. Additionally, an elevated d-dimer level at admission was an independent risk factor of mortality in adults21. In children with COVID-19, elevated d-dimer levels also have been reported, but much less is known about the relationship with mortality. Similar to adults, Mitchell et al22 reported that coagulopathy, elevated D-dimer, and increased thrombotic events were observed in 27 children with severe respiratory complications related to COVID-19. In our cohort, d-dimer levels were significantly higher in nonsurvivors. Our study suggests that; severe pediatric patients particularly those with respiratory failure, d-dimer levels should be closely monitored and early initiation of antithrombotic therapy should be considered.
In our study, of 335 children with critical COVID-19, 49.5% of them were mechanically ventilated, 25% of them needed inotrope treatment, 48% of them had 1–2 organ failures, and 24.5% of them had 3 or more organ failures (Table 2). In a study from North America, of the 48 children with COVID-19 admitted to 14 PICUs in a 20-day study period, 46% of them were mechanically ventilated, 25% of them needed inotrope support, 8% of them had 3 or more organ failure and the mortality rate was 4.2% up to the time of the report. Twenty percent of patients were still being treated with the severe condition at the study endpoint. Compared with this report, the higher mortality rate may be due to the fact that disease severity and the number of organ failure was higher in our study, and also the early termination of the North American study without providing all critical patients outcome4. In a study from Indonesia that included 50 critically ill children with confirmed COVID-19, the observed mortality rate was 40% which is higher than our study23. In an Egyptian study, 103 severe COVID-19 patients were admitted to PICU, 41.7% of patients were mechanically ventilated with a mortality rate of 20%24. In a Spanish study conducted in 76 hospitals including 666 hospitalized children, 123 of them required PICU admission. Seventy-six of 123 patients (62%) diagnosed with Multisystem Inflammatory Syndrome in Children (MIS-C), 26% of patients were mechanically ventilated with a mortality rate of 7%. In this study, the lower percentage of the patients who underwent mechanical ventilation indicates less disease severity that may explain the lower rate of mortality25. In our study, we did not include MIS-C patients in our cohort because it has a different clinical course and prognosis from the severe acute COVID-19 illness in children. The difference in mortality rates in different countries might be explained by different disease severity of the patients who were admitted to the PICUs and also differences in accessibility to PICU level of care.
There is a wide variety of publications on the relationship between age and mortality. In some studies, it was reported that children under 1 year were at risk, whereas in others, only neonates had a greater probability of developing the severe disease26–28 An adult study showed that a 10-year increase in age and male sex were significantly associated with mortality6. In our cohort, although boys were more admitted to PICU, mortality was not related to age groups or gender.
A recent meta-analysis showed that the most common clinical manifestations were fever 51%, cough 41%, sore throat 16%, nasal congestion 17% and rhinorrhea 14% in children29. In our study, although fever was the most common symptom in children at admission, it was followed by respiratory distress and cough. The most common system presentation was respiratory (84%) followed by neurological (23.6%), gastrointestinal (22%), circulatory (15.2%), and hematological (8.4%).
This study has limitations. First, the fact that the study is retrospective thus, the data may be subject to incomplete reporting. Second, we could not obtain the total number of outpatient and hospitalized cases with COVID-19 from the 44 participating centers, so we could not calculate the case fatality rate, hospitalization rate and PICU admission rate in children. We could only calculate the mortality rate of critically ill COVID-19 patients admitted to the PICU. Third, the unequal sample size between survivors and nonsurvivors which may have impacted the results. Therefore, the nonassociation between age and mortality may be due to the small sample size of the mortality group.
In conclusion, a high organ failure index, and having congenital heart disease are associated with higher mortality in pediatric severe COVID-19. Our study suggests that strategies to improve outcomes are needed in children with congenital heart disease such as priority of vaccination, prioritization of surgery, etc. and d-dimer levels should be closely monitored in critically ill children with COVID-19. Another study comparing PICU data in between the 1st year and 2nd years of the pandemic is at the data collection stage. While the 2nd year pandemic mortality data are presented, a mortality analysis will also be performed for the variants.
ACKNOWLEDGMENTS
The members of the TuPCOM study Group are as follows:
Fatih Varol, MD, dr_fvarol@yahoo.com, 0000-0002-2424-6887, Sancaktepe Þehit Prof. Dr. Ýlhan Varank Training and Research Hospital, Department of Pediatric Critical Care, Istanbul, Turkey; Emel Uyar, MD, uyaremel@yahoo.com, 0000-0002-8265-0618, Ankara City Hospital, Division of Pediatric Critical Care, Ankara, Turkey; Gökçen Özçifçi, MD, gkcnozcifci@gmail.com, 0000-0001-5245-9786, University of Health Sciences Turkey, Van Training and Research Hospital, Division of Pediatric Critical Care, Van, Turkey; Güntülü Þýk, MD, Associate Prof, drguntulu@hotmail.com, 0000-0002-4526-0485, Acýbadem Mehmet Ali Aydýnlar University School of Medicine, Department of Pediatric Critical Care, Istanbul, Turkey; Kývanç Terzi, MD, kvnctrz@gmail.com, 0000-0003-4545-0294, Department of Pediatric Critical Care Medicine, Life Support Practice and Research Center, Hacettepe University, Ankara, Turkey; Osman Yesilbaþ, MD, Associate Prof, drosmanyesilbas@gmail.com, 0000-0002-4290-0491, Karadeniz Technical University Faculty of Medicine, Division of Pediatric Critical Care, Trabzon, Turkey; Dinçer Yýldýzdaþ, MD, Prof, dyildizdas@gmail.com, 0000-0003-0739-5108, Cukurova University Faculty of Medicine, Department of Pediatric Intensive and Critical Care, Adana, Turkey; Baþak Akyýldýz, MD, Prof, basaknurbesra@gmail.com, 0000-0001-8540-0625, Erciyes University Faculty of Medicine, Department of Pediatric Critical Care, Kayseri, Turkey; Makbule Nilüfer Yalýndað Öztürk, MD, Associate Prof, nilufer.ozturk@gmail.com, 0000-0001-7040-2812, Marmara University Istanbul Pendik Education and Research Hospital, Department of Pediatric Critical Care Unit, Istanbul, Turkey; Abdullah Yazar, MD, Associate Prof, drabdullahyazar@hotmail.com, 0000-0003-1243-9830, Necmettin Erbakan University, Meram Medical School, Division of Pediatric Critical Care, Konya, Turkey; Nazik Yener, MD, Prof, nazika@omu.edu.tr, 0000-0003-2469-0598, Ondokuz Mayýs University School of Medicine, Department of Pediatric Critical Care, Samsun, Turkey; Mey Talip Petmezci, MD, meytalip@gmail.com, 0000-0002-6409-3854, Prof. Dr. Cemil Tascioglu City Hospital, Division of Pediatric Critical Care, Istanbul, Turkey; Süleyman Bayraktar, MD, bsuleyman@hotmail.com, 0000-0002-8080-2438, University of Health Sciences Turkey, Haseki Training and Research Hospital, Division of Pediatric Critical Care, Istanbul, Turkey; Arzu Oto, MD, arzuhoto@gmail.com, 0000-0003-0229-2759, University of Health Sciences Turkey, Bursa Yuksek Ihtisas Training and Research Hospital, Division of Pediatric Critical Care, Bursa, Turkey; Muhterem Duyu, MD, Associate Prof, drmuhteremduyu@gmail.com, 0000-0001-7892-2927, Istanbul Medeniyet University, Goztepe Prof. Dr. Suleyman Yalcin City Hospital, Department of Pediatrics, Pediatric Intensive Care Unit, Istanbul, Turkey; Ufuk Yükselmiþ, MD, ufuk810@gmail.com, 0000-0003-1150-2586, University of Health Sciences Turkey, Dr. Lutfi Kirdar Kartal Training and Research Hospital, Division of Pediatric Critical Care, Istanbul, Turkey; Nurettin Onur Kutlu, MD, Prof, onurkutlu@hotmail.com, 0000-0002-3306-6570, Istanbul Basaksehir Çam and Sakura City Hospital, Department of Pediatric Critical Care, Istanbul, Turkey; Ümüt Altuð, MD, drumitaltug@gmail.com, 0000-0002-6864-377X, Sanliurfa Education and Research Hospital, Department of Pediatric Critical Care, Sanliurfa, Turkey; Sevgi Topal, MD, sevgi_topal86@hotmail.com, 0000-0002-7725-5509, Erzurum Regional Training and Research Hospital, Division of Pediatric Critical Care, Erzurum, Turkey; Zeynelabidin Öztürk, MD, zeynelabidin_ozturk@hotmail.com, 0000-0001-8548-4144, University of Health Sciences Turkey, Dr. Sami Ulus Maternity Child Health and Diseases Training and Research Hospital, Division of Pediatric Critical Care, Ankara, Turkey; Emine Akkuzu, MD, eminemencek@hotmail.com, 0000-0001-8698-5928, Isparta City Hospital Department of Pediatric Critical Care, Isparta, Turkey; Tolga Besci, MD, tolgabes@gmail.com, 0000-0003-0104-2272, Dokuz Eylul University, Department of Pediatrics, Division of Pediatric Intensive Care, Izmir, Turkey; Pýnar Yazýcý Özkaya, MD, dryazicipinar@gmail.com, 0000-0002-1209-2534, Ege University Faculty of Medicine, Department of Pediatric Critical Care, Izmir, Turkey; Fatih Durak, MD, fatihdurak44@hotmail.com, 0000-0002-3209-2697, Gaziantep Cengiz Gokcek Women’s Health and Children Hospital, Department of Pediatric Critical Care, Gaziantep, Turkey; Nagehan Aslan, MD, Associate Prof, nagehan_aslan@hotmail.com, 0000-0002-6140-8873, Malatya Training and Research Hospital, Division of Pediatric Critical Care, Malatya, Turkey; Özlem Saraç Sandal, MD, drozlemsarac@hotmail.com, 0000-0003-2684-0625, University of Health Sciences Turkey, Dr. Behcet Uz Children’s Hospital, Department of Pediatric Critical Care, Izmir, Turkey; Çaðlar Ödek, MD, Associate Prof, caglar_odek@hotmail.com, 0000-0002-2521-3411, Bursa Uludag University Faculty of Medicine, Division of Pediatric Intensive Care Unit, Bursa, Turkey; Ülkem Kocoðlu Barlas, MD, ulkemkocoglu@yahoo.com, 0000-0001-7445-5858, University of Health Sciences Turkey, Istanbul Bagcilar Research and Training Hospital, Division of Pediatric Critical Care, Istanbul, Turkey; Mehmet Çeleðen, MD, mcelegen@hotmail.com, 0000-0002-6841-3675, Afyonkarahisar Health Sciences University, Department of Pediatrics, Afyonkarahisar, Turkey; Murat Özkale, MD, drmuratozkale@gmail.com, 0000-0003-0625-1057, Dr. Turgut Noyan Teaching and Medical Research Center, Baþkent University School of Medicine, Division of Pediatric Critical Care, Adana, Turkey; Murat Kangýn, MD, Associate Prof, m_kangin@hotmail.com, 0000-0003-0042-0569, University of Health Sciences Turkey, Gazi Yasargil Training and Research Hospital, Department of Pediatric Critical Care, Diyarbakir, Turkey; Gürkan Atay, MD, drgurkanatay@yahoo.com, 0000-0002-0317-5872, University of Health Sciences Turkey, Ümraniye Research and Training Hospital, Department of Pediatric Critical Care, Istanbul, Turkey; Yasemin Çoban, MD, yasemincoban83@gmail.com, 0000-0002-5283-239x, Mugla Sitki Kocaman University Faculty of Medicine, Department of Pediatric Critical Care, Mugla, Turkey; Nuri Alaçakýr, MD, alacakir@yahoo.com, 0000-0002-8327-1070, Republic of Turkey Trakya University School of Medicine, Division of Pediatric Critical Care, Edirne, Turkey; Fulya Kamit, MD, Associate Prof, fulyakamit@yahoo.co.uk, 0000-0003-1078-9781, Istanbul Gaziosmanpasa Hospital, Yeni Yuzyil University, Division of Pediatric Critical Care, Istanbul, Turkey; Ayhan Yaman, MD, dryamanayhan@yahoo.com.tr, 0000-0002-5651-1286, Istinye University School of Medicine, Bahcesehir Liv Hospital, Division of Pediatric Critical Care, Istanbul, Turkey; Ayþe Filiz Yetimakman, MD, Associate Prof filizyetimakman@hotmail.com, 0000-0002-9334-4464, Kocaeli University Faculty of Medicine, Division of Pediatric Critical Care, Kocaeli, Turkey; Ayþe Ýrem Sofuoðlu, MD, ayseiremsf@gmail.com, 0000-0002-3757-912X, University of Health Sciences Turkey, Bakirkoy Dr. Sadi Konuk Training and Research Hospital, Department of Pediatric Critical Care Medicine; Fatih Aygün, MD, Associate Prof, faygun9@hotmail.com, 0000-0001-6519-6583, Istanbul University-Cerrahpaþa, Cerrahpaþa Medical Faculty, Department of Pediatric Critical Care Medicine, Istanbul, Turkey; Anar Gurbanov, MD, gurbanov. anar1986@gmail.com, 0000-0003-1383-5130, Ankara University Faculty of Medicine, Department of Pediatric Critical Care, Ankara, Turkey; Murat Ersoy, MD, ersoymurat33@yahoo.com, 0000-0002-4407-1244, Mersin City Training and Research Hospital Department of Pediatrics, Mersin, Turkey; Þefika Elmas Bozdemir, MD, drsefika@hotmail.com, 0000-0003-1058-0320, Dortcelik Children’s Hospital, Division of Pediatric Infectious Disease, Bursa, Turkey; Eylem Kýral, MD, dr_eylem@hotmail.com, 0000-0003-2245-5340, Eskisehir Osmangazi University, Faculty of Medicine, Department of Pediatric Critical Care, Eskisehir, Turkey; Ebru Þahin, MD, ebruguneysahin@hotmail.com, 0000-0002-2680-2437, Sancaktepe Þehit Prof. Dr. Ýlhan Varank Training and Research Hospital, Department of Pediatric Critical Care, Istanbul, Turkey; Oktay Perk, MD, droktayperk@hotmail.com, 0000-0002-2586-5954, Ankara City Hospital, Division of Pediatric Critical Care, Ankara, Turkey; Agop Çýtak, MD, Prof, agopcitak@hotmail.com, 0000-0002-5108-3913, Acýbadem Mehmet Ali Aydýnlar University School of Medicine, Department of Pediatric Critical Care, Istanbul, Turkey; Benan Bayrakçý, MD, Prof, benan@hacettepe.edu.tr, 0000-0003-3307-0948, Department of Pediatric Critical Care Medicine, Life Support Practice and Research Center, Hacettepe University, Ankara, Turkey; Zeynep Gökçe Gayretli Aydýn, Associate Prof MD, zggayretli@gmail.com, 0000-0003-4291-1067, Karadeniz Technical University Faculty of Medicine, Department of Pediatrics, Trabzon, Turkey; Faruk Ekinci, MD, farukekinci83@gmail.com, 0000-0001-6675-3150, Cukurova University Faculty of Medicine, Department of Pediatric Intensive and Critical Care, Adana, Turkey; Benhur Þirvan Çetin, MD, benhurcetin@gmail.com, 0000-0002-8470-4907, Erciyes University Faculty of Medicine, Department of Pediatric Infectious Disease, Kayseri, Turkey; Feyza Ýnceköy Girgin, MD, feyzagirgin@hotmail.com, 0000-0003-4324-0488, Marmara University Istanbul Pendik Education and Research Hospital, Department of Pediatric Critical Care Unit, Istanbul, Turkey; Fatih Akýn, MD, drfatihakin@gmail.com, 0000-0001-5725-3867, Necmettin Erbakan University, Meram Medical School, Division of Pediatric Critical Care, Konya, Turkey; Hatice Albayrak, MD, hatice_albayrak52@hotmail.com, 0000-0003-3054-8870, Ondokuz Mayýs University School of Medicine, Department of Pediatric Critical Care, Samsun, Turkey; Merve Boyraz, MD, Boyrazmerve87@gmail.com, 0000-0002-4158-0270, Istanbul Medeniyet University, Goztepe Prof. Dr. Suleyman Yalcin City Hospital, Department of Pediatrics, Istanbul, Turkey; Hatice Sinav Utku, MD, utkuhtc@gmail.com, 0000-0002-8648-2359, Istanbul Basaksehir Çam and Sakura City Hospital, Department of Pediatric Critical Care, Istanbul, Turkey; Mutlu Uysal Yazýcý, MD, Associate Prof, mutluuysal@yahoo.com, 0000-0001-7377-4718, University of Health Sciences Turkey, Dr. Sami Ulus Maternity Child Health and Diseases Training and Research Hospital, Division of Pediatric Critical Care, Ankara, Turkey; Gazi Arslan, MD, gaziarslan@gmail.com, 0000-0002-8616-3761, Dokuz Eylul University,Department of Pediatrics, Division of Pediatric Intensive Care, Izmir, Turkey; Ýrem Ersayoðlu, MD, irem_e@hotmail.com, 0000-0001-6965-0886, Ege University Faculty of Medicine, Department of Pediatric Critical Care, Izmir, Turkey; Ahmet Ziya Birbilen, MD, abirbilen@hotmail.com, 0000-0001-7131-3993, Gaziantep Cengiz Gokcek Women’s Health and Children Hospital, Department of Pediatric Critical Care, Gaziantep, Turkey; Ekin Soydan, MD, dr-ekinsoydan@hotmail.com, 0000-0003-2626-5499, University of Health Sciences Turkey, Dr Behcet Uz Children’s Hospital, Department of Pediatric Critical Care, Izmir, Turkey; Beyhan Bülbül, MD, beyhanbulbul@uludag.edu.tr, 0000-0002-5720-1212, Bursa Uludag University Faculty of Medicine, Division of Pediatric Infectious disease, Bursa, Turkey; Ebru Atike Ongun, MD, Associate Prof, ebru_temel@yahoo.com, 0000-0002-1248-8635, University of Health Sciences Turkey, Antalya Training and Research Hospital, Department of Pediatric Critical Care Medicine, Antalya, Turkey; Yasemin Özkale, MD, dryaseminozkale@gmail.com, 0000-0003-3009-336x, Dr. Turgut Noyan Teaching and Medical Research Center, Baþkent University School of Medicine, Division of Pediatric Critical Care, Adana, Turkey; Mehmet Nur Talay, MD, Mntalay70@gmail.com, 0000-0002-7361-3823, Gazi Yasargil Training and Research Hospital, Department of Pediatric Critical Care, Diyarbakir, Turkey; Seher Erdoðan, MD, Associate Prof, seher70@gmail.com, 0000-0002-3393-3363, University of Health Sciences Turkey, Ümraniye Research and Training Hospital, Department of Pediatric Critical Care, Istanbul, Turkey; Mehmet Emin Menentoðlu, MD, menentoglu@hotmail.com, 0000-0003-3839-4722, Mustafa Oður, MD, mustafaonogur@gmail.com, 0000-0003-2731-4723, University of Health Sciences Turkey, Bakirkoy Dr. Sadi Konuk Training and Research Hospital, Department of Pediatric Critical Care Medicine, Murat Duman, MD, Prof, mduman@deu.edu.tr, 0000-0001-6767-5748, Dokuz Eylul University, Department of Pediatrics, Division of Pediatric Intensive Care, Izmir, Turkey.
Supplementary Material
Footnotes
The authors have no funding or conflicts of interest to disclose.
The study was approved by the University of Health Sciences Turkey Bakirkoy Dr Sadi Konuk Training and Research Hospital Ethics Committee (approval number 2021/77) and the Ministry of Health Scientific Research Platform of Turkey (approval number 2021-01-13T14_19_40).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Nihal Akcay, Email: drnihalakcay@gmail.com.
Hasan Serdar Kihtir, Email: serdar.kihtir@gmail.com.
Cansu Durak, Email: bzmrt@hotmail.com.
Tanil Kendirli, Email: tanilkendirli@hotmail.com.
Merve Havan, Email: merve.havan@gmail.com.
Esra Kockuzu, Email: caglaes@yahoo.com.
Gurkan Bozan, Email: drgurkanbozan@gmail.com.
Collaborators: Fatih Varol, Emel Uyar, Gökçen Özçifçi, Güntülü Şik, Kivanç Terzi, Osman Yesilbaş, Dinçer Yildizdaş, Başak Akyildiz, Makbule Nilüfer Yalindağ Öztürk, Abdullah Yazar, Nazik Yener, Mey Talip Petmezci, Süleyman Bayraktar, Arzu Oto, Muhterem Duyu, Ufuk Yükselmiş, Nurettin Onur Kutlu, Ümüt Altuğ, Sevgi Topal, Zeynelabidin Öztürk, Emine Akkuzu, Tolga Besci, Pinar Yazici Özkaya, Fatih Durak, Nagehan Aslan, Özlem Saraç Sandal, Çağlar Ödek, Ülkem Kocoğlu Barlas, Mehmet Çeleğen, Murat Özkale, Murat Kangin, Gürkan Atay, Yasemin Çoban, Nuri Alaçakir, Fulya Kamit, Ayhan Yaman, Ayşe Filiz Yetimakman, Ayşe İrem Sofuoğlu, Fatih Aygün, Anar Gurbanov, Murat Ersoy, Şefika Elmas Bozdemir, Eylem Kiral, Ebru Şahin, Oktay Perk, Agop Çitak, Benan Bayrakçi, Zeynep Gökçe Gayretli Aydin, Faruk Ekinci, Benhur Şirvan Çetin, Feyza İnceköy Girgin, Fatih Akin, Hatice Albayrak, Merve Boyraz, Hatice Sinav Utku, Mutlu Uysal Yazici, Gazi Arslan, İrem Ersayoğlu, Ahmet Ziya Birbilen, Ekin Soydan, Beyhan Bülbül, Ebru Atike Ongun, Yasemin Özkale, Mehmet Nur Talay, Seher Erdoğan, Mehmet Emin Menentoğlu, Mustafa Oğur, and Murat Duman
References
- 1.World Health Organization (WHO). Coronavirus disease (COVID-19) dashboard. [Internet]. [cited 2021 Apr 1]. Available at: https://covid19.who.int/
- 2.T.C Sağlik Bakanliği COVID-19 bilgilendirme platformu. [Internet]. [cited 2021 Apr 1]. Available at: https://covid19.saglik.gov.tr/
- 3.The American Academy of Pediatrics. [Internet]. [cited 2021 Apr 29]. Available at: https://downloads.aap.org/AAP/PDF/AAP%20and%20CHA%20-%20Children%20and%20COVID-19%20State%20Data%20Report%204.29.21%20FINAL.pdf
- 4.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. ; International COVID-19 PICU Collaborative. Characteristics and outcomes of children with Coronavirus Disease 2019 (COVID-19) infection admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. 2020;174:868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parohan M, Yaghoubi S, Seraji A, et al. Risk factors for mortality in patients with Coronavirus Disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Aging Male. 2020;23:1416–1424. [DOI] [PubMed] [Google Scholar]
- 6.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in Intensive Care Units in Lombardy, Italy [published correction appears in JAMA Intern Med. 2021 Jul 1;181(7):1021]. JAMA Intern Med. 2020;180:1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollack MM, Dean JM, Butler J, et al. The ideal time interval for critical care severity-of-illness assessment. Pediatr Crit Care Med. 2013;14:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkinson JD, Pollack MM, Ruttimann UE, et al. Outcome of pediatric patients with multiple organ system failure. Crit Care Med. 1986;14:271–274. [DOI] [PubMed] [Google Scholar]
- 9.Proulx F, Fayon M, Farrell CA, et al. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996;109:1033–1037. [DOI] [PubMed] [Google Scholar]
- 10.Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Worldometer’s COVID-19 data. [Internet]. [cited 2021 Apr 01] Available at: https://www.worldometers.info/coronavirus/
- 12.Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caussy C, Wallet F, Laville M, et al. Obesity is associated with severe forms of COVID-19. Obesity (Silver Spring). 2020;28:1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan W, Liang W, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sachdeva S, Ramakrishnan S, Choubey M, et al. ; PCSI-COVID-19 study group. Outcome of COVID-19-positive children with heart disease and grown-ups with congenital heart disease: a multicentric study from India. Ann Pediatr Cardiol. 2021;14:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu X, Zhang L, Du H, et al. ; Chinese Pediatric Novel Coronavirus Study Team. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu H, Wu J, Hong L, et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colling ME, Kanthi Y. COVID-19-associated coagulopathy: an exploration of mechanisms. Vasc Med. 2020;25:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell WB, Davila J, Keenan J, et al. Children and young adults hospitalized for severe COVID-19 exhibit thrombotic coagulopathy. Pediatr Blood Cancer. 2021;68:e28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewi R, Kaswandani N, Karyanti MR, et al. Mortality in children with positive SARS-CoV-2 polymerase chain reaction test: lessons learned from a tertiary referral hospital in Indonesia. Int J Infect Dis. 2021;107:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saleh NY, Aboelghar HM, Salem SS, et al. The severity and atypical presentations of COVID-19 infection in pediatrics. BMC Pediatr. 2021;21:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tagarro A, Cobos-Carrascosa E, Villaverde S, et al. ; EPICO-AEP Working Group. Clinical spectrum of COVID-19 and risk factors associated with severity in Spanish children. Eur J Pediatr. 2022;181:1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Götzinger F, Santiago-García B, Noguera-Julián A, et al. ; ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garazzino S, Lo Vecchio A, Pierantoni L, et al. ; Italian SITIP-SIP Pediatric Infection Study Group. Epidemiology, clinical features and prognostic factors of pediatric SARS-CoV-2 infection: results from an Italian Multicenter Study. Front Pediatr. 2021;9:649358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esposito S, Marchetti F, Lanari M, et al. ; Working Group on COVID-19 in Pediatrics of the Emilia-Romagna Region (RE-CO-Ped). COVID-19 management in the pediatric age: consensus document of the COVID-19 Working Group in Paediatrics of the Emilia-Romagna Region (RE-CO-Ped), Italy. Int J Environ Res Public Health. 2021;18:3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui X, Zhao Z, Zhang T, et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19). J Med Virol. 2021;93:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.