Abstract
Once the World Health Organization (WHO) declared the COVID-19 (Coronavirus Infectious Disease-19) outbreak to be pandemic, massive efforts have been launched by researchers around the globe to combat this emerging infectious disease. Strategies that must be investigated such as expanding testing capabilities, developing effective medicines, as well as developing safe and effective vaccines for COVID-19 disease that produce long-lasting immunity to human system. Now-a-days, bio-sensing, medication delivery, imaging, and antimicrobial treatment are just a few of the medical applications for nanoparticles (NPs). Since the early 1990s, nanoparticle drug delivery methods have been employed in clinical trials. Since then, the discipline of nanomedicine has evolved in tandem with expanding technological demands to better medicinal delivery. Newer generations of NPs have emerged in recent decades that are capable of performing additional delivery tasks, allowing for therapy via novel therapeutic modalities. Many of these next generation NPs and associated products have entered clinical trials and have been approved for diverse indications in the present clinical environment. For systemic applications, NPs or nanomedicine-based drug delivery systems have substantial benefits over their non-formulated and free drug counterparts. Nanoparticle systems, for example, are capable of delivering medicines and treating parts of the body that are inaccessible to existing delivery systems. As a result, NPs medication delivery is one of the most studied preclinical and clinical systems. NPs-based vaccines delivering SARS-CoV-2 antigens will play an increasingly important role in prolonging or improving COVID-19 vaccination outcomes. This review provides insights about employing NPs-based drug delivery systems for the treatment of COVID-19 to increase the bioavailability of current drugs, reducing their toxicity, and to increase their efficiency. This article also exhibits their capability and efficacy, and highlighting the future aspects and challenges on nanoparticle products in clinical trials of COVID-19.
Keywords: COVID-19, Nanoparticles, Pandemic, Vaccines, Drug delivery, Immunotherapy
1. Introduction of nanomedicine: An update
The Chinese COVID-19 outbreak was deemed a Public Health Emergency of International Concern by the World Health Organization (WHO) on January 30, 2020, posing a significant danger to nations with weak health systems [1]. Globally, the COVID-19 pandemic has caused more than 572,239,451 confirmed cases and more than 6,390,401 fatalities as per the WHO Coronavirus (COVID-19) Dashboard (https://covid19.who.int/) on 29th July 2022 at 5:30 p.m. While, a total of 12,248,795,623 vaccine doses have been administered as on 25th July 2022. The COVID-19 epidemic has a negative impact on the world's healthcare systems and has repercussions for many facets of modern life [2]. Globally rising instances have brought attention to the need for revised management recommendations [3]. In this context, nanotechnology and nano-based vaccines played an important role in the health care system.
Nanomedicine involves nanotechnology, biomedical and pharmaceutical sciences. The field has evolved rapidly with the formation of new nanoparticle-based formulations for theragnostic uses, therapeutic applications and others [4]. Food and Drug Administration (FDA) defined NPs-based formulations as products integrated with NPs (ranging from 1 to 100 nm) [5,6]. These formulations show several merits compared to free drug agents by exhibiting an elevated solubility and enhanced efficacy, pharmacokinetics, and minimal detrimental effect. Over 50 nano-pharmaceuticals are currently available in the market and they include wide range of nano-formulations with lipid NPs being the predominant [[5], [6], [7], [8], [9]].
Lipid particles are lipid system with several components, mainly consisting of a phospholipid, cholesterol, an ionizable lipid and a PEGylated lipid. Liposome is the major type of lipid NPs (LNPs) and was initially documented in 1961 by Alec D Bangham (a British haematologist). The NPs were observed under an electron microscope when adding a negative stain to dry phospholipids that aligned into spherical structure via a lipid bilayer [5,10,11]. Subsequently, the first liposomes of interest which was modified by active targeting ligands were designed and this led to markedly enhanced liposome action by elevating accumulation at the target cells, tissues or organs with liberating the carried drug to other sites [12]. Thus, nano-formulations have improved the pharmacokinetic, biopharmaceutical and pharmacodynamics features of drugs.
The emergence of coronavirus has brought about the use of nanomaterials in the management of COVID-19. This involves the mechanistic actions that inhibit the virus entry into the host cells, resulting to their inactivation [13]. Metal NPs have indicated the potential to inhibit viral attachment to the host cell surface, resulting to the inhibition of viral internalization and viral inactivation. Vaccines and therapeutic antibodies are the most effective approaches for preventing and treating COVID-19 [14]. As of June 25, 2022, Food and Drug Administration approved 30 vaccines whilst 212 vaccines with 734 trials are still under process. Among these, 52 vaccines are in Phase I, 71 vaccines in Phase 2, and 80 vaccines in Phase 3 (https://covid19.trackvaccines.org/vaccines/).
The mode of action in these vaccines is based on the antibodies neutralization of the spike protein that would prevent its uptake by human cells through human angiotensin-converting enzyme-2 (ACE2) receptor [15]. Apart from conventional vaccines like live vaccines, inactivated vaccines, recombinant vaccines, vector based and DNA vaccines, the use of NPs-based vaccines opened the door to the new era of advanced vaccines. It has directed a new, promising and unique approach in the crucial times of pandemic. NPs are small particles ranging in size from 1 nm to 100 nm in diameter [16]. Their small size offers great surface area to volume ratio and therefore these particles possess greater potency, tunability and promising platforms for next generation vaccine development [17]. They are capable of broad antibody-based immune response and can create stronger NPs-Antibody responses. This tendency can be used to determine evolution and variations of pathogenic viruses [18]. Currently, 26 NPs-based vaccines are being tested in human clinical trials. Apart from theses, nearly 60 are passing through their preclinical stage of development [19]. These nanoparticle-vaccines are available in a variety of formats such as virus-like particles (VLPs), micelles, protein NPs and LNPs. NPs-vaccines can be divided into two groups based on the type of antigen loading strategies. (a) NPs that present vaccine antigens on their surface and (b) NPs encapsulating vaccine antigens or nucleic acids within their core [19]. Since NPs with vaccine antigens on their surface are capable of engaging antigen presenting cells (APCs), so they have the tendency to efficiently promote B-cell receptor (BCR) crosslinking those results in immunogenicity. While in the other strategy, the antigen is already encapsulated in the NPs, so this characteristic offers protection and controlled cargo release after immunization [20,21]. This review provides an update on NPs-based drugs in viral infections and insights about employing NPs-based drug delivery systems for the treatment of COVID-19 in clinical trials. Further, this review provides an overview on nanoparticle vaccines against emerging COVID-19 variants, highlights the future aspects and challenges on nanoparticle products in clinical trials of COVID-19.
2. NPs-based drugs in viral infections
Effective antiviral drug development is important for alleviating many manifestations and inhibiting death in persons infected by viruses. Timely antiviral management is a vital measure to decrease the effect on health. Metallic nanoparticles-based medicines have reported to inhibit the replication of viruses owing to cell-virus blocking mechanism. A variety of silver, zinc, and gold based NPs have shown significant therapeutic effect against herpes simplex virus (HSV), influenza A, HIV, Human parainfluenza 3 (HPIV-3), zika virus, monkeypox virus and gastroenteritis virus [22]. One example includes the inhibition of gp120 to CD4 by gold nanoparticles (AuNPs). While silver nanoparticles (AgNPs) are capable of virion protein degradation to prevent Kaposi's sarcoma-associated herpesvirus primary infection. Binding of AgNPs to nuclei or membrane interfere with the virion capacity to attach itself to the host. Also, AgNPs are effective against influenza A, polio type 1 and coxsackievirus B3. CD4-dependent cellular binding/pathogenesis or covalent linking with the sulfhydryl group at the virus level is the mechanism behind the AgNPs action [[23], [24], [25], [26]]. AgNPs possess characteristic antiviral activity and their mechanism of action suggests their physical binding with the glycoprotein of viruses. In this manner, viruses are no longer available to penetrate the host cells. However, agglomeration can be avoided by combining the AgNPs with graphene oxide (GO-). It imparts negative charge to AgNPs. This GO-AgNPs combination has been used to treat deadly feline coronavirus infection. Moreover, this combo inhibits cell proliferation by increasing the production of IFN-stimulating genes (ISGs), and interferon-α (IFN-α) [27].
In another study, a peptide was found that was capable of mimicking heptad repeat 2 (HR2) in MERS virus S2 protein. As, a result, the process of cell fusion is stopped by the interference of HR1 [28]. This inhibitory effect of a peptide was enhanced ten folds when it was immobilized on the surface of gold nanorods. Thus, gold nanorods coupled with this peptide showed brilliant biocompatibility by blocking the cell fusion reaction. Similarly, silicon nano-particles (SiNPs) also proved to be excellent scavengers of viruses. Therefore, it can be a probable treatment of COVID-19 [29]. Iron oxide NPs can be incorporated with a receptor used by SARS-CoV-2 to infect host cell; called S1-RBD protein receptor domain. This can serve as a potent COVID-19 therapy [30]. Selenium NPs have shown characteristic antiviral effects at higher concentrations. A combination of selenium NPs with the antiviral drug, Arabidol (ARB) significantly inhibited the cell entry of influenza virus. Also, viral entry can be blocked by using cationic chitosan NPs by targeting the dendritic cells [31]. Likewise, grapheme quantum dots can inhibit the cell binding tendency of HIV [32]. Also, viral infections can be prevented by graphene and its derivatives via confrontational damage of viral proteins ad graphene and its derivative tend to compete for cell receptors. Another combination of nano-particles, such as AuNPs combined with peptide triazole conjugates were found effective against HIV-1. This results by the binding of envelope spike glycoprotein of virus with receptor proteins from host. Similarly, an effective treatment was found against Influenza A virus by conjugating AuNPs and AgNPs with antiviral peptide (Flupep) [33,34].
Another mode of transportation of antiviral drugs in nanotechnology is called nanocarriers. Organic NPs can be used to deliver antiviral drugs like acyclovir and zidovudine. This method reduces risk of toxicity and promotes improved drug delivery and bioavailability [35]. Another important aspect of this treatment is that the longer the circulation time a nanocarrier takes, the more is its efficacy against prophylaxis. This could prove beneficial for healthcare workers who are at high risk of COVID-19 [36]. Acyclovir has low bioavailability and short life cycle (below 3 h) in oral administration. So, its encapsulation into poly (lactic-co-glycolic acid) (PLGA)-based NPs can enhance its effectiveness by 2.6 folds [37]. A group of natural and synthetic antiviral molecules have been designed and these include peptides, chemical compounds and essential oils. These molecules show antiviral actions on different forms of virus [38,39].
Other agents approved by Food and Drug Administration include zanamivir, oseltamivir and abacavir and they utilized to managing HIV and influenza infection [40]. Recently, remdesivir is utilized for alleviating COVID-19 and its utilization is linked to remarked decrease in the death rate of infected individuals [41]. In spite of the contributions of antiviral molecules, there are many limitations linked with their application, and these include limited effect as a result of poor solubility, toxicity and minimal biostability. In addition to this, enhancement of the antiviral molecule and this involves the blocking of viral protein and cellular receptors associated with viral infection cascades. Therefore, effective delivery of antiviral molecules is necessitated [38,39,42].
In order to rectify the limitations mentioned above, nanoparticle delivery systems have been used in the development of antiviral agents. The nanoparticle delivery systems enable constant systemic flow and maintained the liberation of antiviral molecules, therefore maximizing their therapeutic actions [38,43]. Different types of NPs have been employed in the development of nanoscale delivery systems and characteristics of these nanoparticles can be changed by regulating the size of the system, conjugating needed groups to the surface and controlling its surface charges [38]. A broad range of NPs, including lipid nanoparticles, AuNPs, polymeric NPs and AgNPs are often used as carriers for embedded antiviral molecules [38,44,45]. These transporters can be functionalized with targeting groups, such as ligands, antibodies and receptors. A delivery molecule utilizing bilayer polymeric vesicles functionalized together with phenylboronic acid, has been shown for the management of influenza A virus [38,46]. The optimal ratio of the phenylboronic acid and carrier was determined through in vitro studies, which led to elevated cellular uptake of the antiviral molecules favipiravir and mir-323a that were co-entrapped in carrier [38]. Polymeric vesicles functionalized with phenylboronic acid elevates the therapeutic action of the antiviral molecules and maintained cell viability when compared to free drug [38]. This showed the synergistic therapeutic actions and remarkably improved biocompatibility of these systems. The synergistic therapeutic actions linked to dual-delivery were further used to inhibit HIV-1 entry into host cells [47].
Liposomes designed with 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy-(poly (ethylene glycol))-2000] and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine successfully entrapped two important entry inhibitors, which are protoporphyrin IX and enfuvirtide. Liposome with dual-loaded delivery transporters ensured improved antiviral action on HIV-1 than carriers with single load. These outcomes show the merits of using effective delivery systems that produce synergistic actions to abrogate HIV-1. The transportation of dual-loaded inhibitors in one carrier with high efficacy was in this aspect [38]. Table 1 shows antiviral agents with corresponding carrier NPs. Abrogation of viral replication is essential in antiviral therapeutics and several antiviral molecules have been designed in this way. Nanoparticulate systems are majorly employed to complement antiviral molecules as carriers, to enhance curing property [38,48]. Utilizing the knowledge of the basics of NPs, nanoparticulate systems can serve as potential inhibitors of some viral replication phases, including binding to receptor, cellular entry and formation of viral proteins in host cells [49].
Table 1.
Some antiviral drugs with their nanoparticle carriers.
| Drugs | Nanomaterial | Target virus | Advantage of nanomaterial | Reference |
|---|---|---|---|---|
| Chloroquine | Poly (lactic) acid | Herpes simplex virus-1 | Enhanced release and targeted transportation | [57] |
| Zidovudine | Cellulose poly (ethylene glycol) | Human immunodeficiency virus | Enhanced release, enhanced encapsulation and targeted transportation | [58] |
| Zidovudine | Poly (vinyl pyrrolidone/sialic acid, poly (ethylene glycol) | Human immunodeficiency virus | Enhanced cellular internalization | [59] |
| Latency-reversing molecules | Polylactide-co-glycolide | Human immunodeficiency virus | Reduced toxicity | [60] |
| Glutathione | Silver sulfide | Porcine epidemic diarrhea virus | Decreased viral titer | [61] |
| Oseltamivir | Zinc oxide | Influenza A virus | Enhanced the viability of infected cells | [62] |
| Zanamivir | Selenium | Influenza A virus | Enhanced the viability of infected cells | [63] |
The first phase of a virus life cycle is the receptor binding and this is a potential target for nanomedicine. Recently, studies indicated that NPs are formed through facile surface modulation revealed potent binding with viruses. This blocked the binding of viruses to host cell receptor, leading to inhibition of viral replication. For instance, spiky nanostructures with geometry-matching topography abrogate the influenza A virus [38,50,51]. These spiky nanostructures have silica NPs and are synthesized to match the topography and size of influenza A virus. In addition, they were covered with an erythrocyte membrane to match the hemagglutinin of the virus. The aforementioned nanoparticulate system allow for potent interaction with influenza A virus and inhibit viral entry into the host cells, thus decreasing about 84% of cellular disease. This work disclosed a potential characteristic, nanoparticle topography, to consider in the process of viral inhibitors development. Furthermore, mesoporous silica NPs antiviral action grafted with unique organic moieties such as glycidyloxypropyl, aminopropyl or phenylethyl groups was estimated against human immunodeficiency virus [38]. Also, silica NPs have several cytotoxicity profiles and virucidal action, depending on the chemicals present on their surfaces [38]. Peptide-polymer NPs with high binding potential for influenza virus was estimated for potent receptor inhibiting by modifying the density of the peptide [52]. The above results indicate that altering the surface of features of NPs may potentially aid in regulating the transduction of a virus. Also, these NPs can be employed as antiviral molecules and as delivery transporters in the treatment of viruses [38]. The synergistic antiviral activities of curcumin-modified silver NPs were determined for the potent abrogation of respiratory syncytial virus disease [53]. The toxic effect of silver NPs and poor water solubility of curcumin were successfully ameliorated by combining the two systems. In addition to the virucidal actions of the two agents, their combination enables significant antiviral action on respiratory syncytial virus diseases with less detrimental effects. Biocompatible cell-mimicking nano-decoys formed from cells have been reported to show viral inhibition [38,54]. Rao and co-workers designed nano-decoys consisting of genetically engineered human embryonic kidney 293T/ACE2 cells for managing coronavirus disease. The nano-decoys consisting of cytokine and ACE2 receptors remarkably abrogated the replication of virus by preventing cytokine and virus binding [54]. In another study, ACE2 nano-decoys designed form human lung spheroid cells inhibited coronavirus disease and ameliorated lung injury. In addition to this, human lung spheroid cell-nano-decoys decreased lung injury and enhanced viral clearance through inhalation [38,55].
In this sense, NPs exhibit antiviral action and enable novel strategies for designing antiviral systems using the nanoparticle dual functionality. Nano-systems with virucidal action can be used as antiviral barriers to cover surfaces that are frequently contacted, including public areas, masks and healthcare facilities [56]. The use of antiviral NPs such as zinc oxide, copper, grapheme oxide and silver has revealed novel targets in inhibiting the spread of viruses via contact transmission and they can serve an important role in the establishing practical strategies to prevent outbreaks in future [38].
3. NPs-based drugs in clinical trials against COVID-19
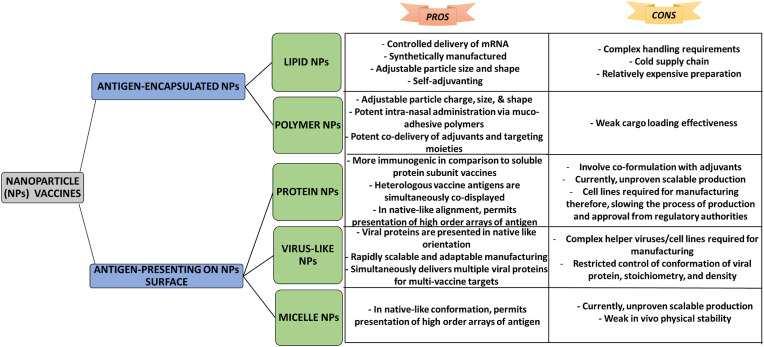
During last decade, role of nanotechnology in diagnosis, prevention, treatment, and production of vaccine against disease has gained explicit attention of researchers [64]. FDA has authenticated the use of nanocrystal formulations, polymer-based NPs, non-polymer NPs, and lipid-based NPs [19,65]. Further, some other NPs like metallic, inorganic, and protein-based NPs are in process of getting approvals to be used as an effective tool in drug delivery [8]. Currently, vaccines have attained an increased attention of researchers due to their effective results in combating several infections, low cost of production, and decreased death rate around the world [66]. Recently, vaccinations against COVID-19 infection have revealed that purposely designed vaccines are helpful in management of public health and social safety. Coronavirus vaccines have aided community by preserving them from infection owing to their therapeutic effects. Nanoparticle vaccines are broadly divided into two subtypes depending upon their antigen loading strategies: Firstly, vaccine antigen presenting (attached on surface) NPs and secondly, NPs encapsulated on vaccine antigen. A schematic illustration showing pros and cons of different vaccines classified depending upon the antigen loading strategies are shown in Fig. 1 [19].
Fig. 1.
A schematic illustration showing pros and cons of different vaccines classified depending upon the antigen loading strategies.
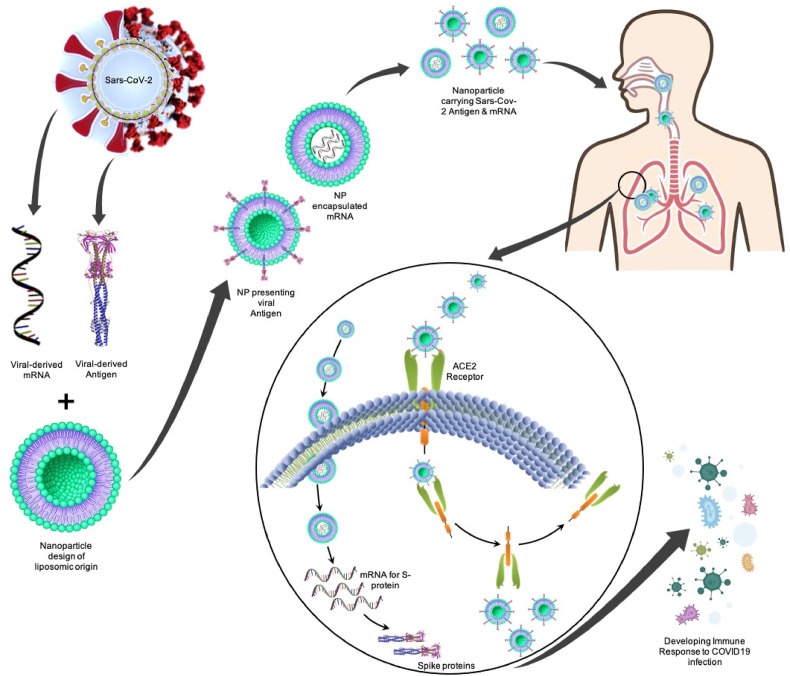
Therefore, various platforms are being investigated for the development of COVID-19 vaccine, as it is need of time for the safety of world community [67,68].However, a main hurdle and concern in designing and developing COVID-19 vaccines is due to mutation of virus original genome structure, which is termed as VOC (variants of concern) [69]. Initially developed COVID-19 vaccines (first generation) were designed keeping in view the original genome of SARS-CoV-2 isolate (Wuhan-Hu-1). On the other hand, various novel strains of SARS-CoV-2 are circulating globally having several mutations in spike protein and are of great concern owing to their probable escape from vaccine produced immunity [69,70]. Vaccines developed using nano-technological methodologies are considered as an innovative approach in advanced vaccine science and ensures the provision of controlled administration in present pandemic situations. NPs are classified as nano-scale and adjustable particles, which features structural mimics of naturally occurring viruses. Adjustable and tunable design characterize them as a potential candidate for development of next generation vaccines, provision of platforms for driving promising natural antibody responses, and therapeutic antibody-based immunity, which may aid in evolution of viruses [19]. Fig. 2 depicts the delivery of nanoparticles carrying SARS-CoV-2 derived antigens to pulmonary system.
Fig. 2.
Delivery of nanoparticles carrying embedded mRNA for S-protein and nanoparticles presenting SARS-CoV-2 derived antigens to a pulmonary system: The specific mRNA that encodes for spike protein is being embedded in a liposomic nanoparticle where another type of nanoparticles is presenting the viral derived antigen. These nanoparticles will work as an immune booster that can either be delivered orally or intravenously into the COVID-19 patient. The mRNA-carrying NPs enter the cells where it releases the specific mRNA that will go on to translate spike protein. The antigen-presenting nanoparticles attaches to the ACE2 receptors in alveoli where it gets endocytosed and the nanoparticle is released from the receptor. As a result of the antigen exposure, the immune response is developed against SARS-CoV-2 infection.
To date, according to WHO, nearly 166 vaccines are in clinical development while 198 are in preclinical phases (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines; assessed on June 25, 2022). World Health Organization has granted 11 vaccines in Emergency Use Listing (EUL) (https://www.who.int/news/item/19-05-2022-who-validates-11th-vaccine-for-covid-19; assessed on June 25, 2022). These include Spikevax, Nuvaxoid, Covovax, Comirnaty, Janssen, Vaxzevria, Covishield, Covaxin, Covilo, CoronaVac, and Convidecia. Coronavirus vaccines are designed depending upon diverse platforms including protein subunits, viral vectors (non-replicating), viral vectors (replicating), DNA-based, RNA-based, virus like particles, inactivated virus, live attenuated virus, viral vector (replicating) + Antigen presenting cell, viral vector (non-replicating) + Antigen presenting cell, and Bacterial antigen spore expression vectors. A total of 189 vaccine candidates are registered in 650 clinical trials across 72 countries worldwide [70]. According to Vu et al. [19], approximately 26 NPs-based COVID-19 vaccines are in human clinical trials while nearly sixty candidates are in different stages of pre-clinical stages.
In the last two years, the field of nanomedicine has gained interest rapidly as a result of global demand for new strategies to enable preventive and therapeutic measures against COVID-19 [[71], [72], [73]] which is induced by the virus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [74]. Among the available strategies in fighting COVID-19, LNPs are the delivery molecule employed in the Moderna and Pfizer-BioNTech coronavirus infection vaccines. In 2020, these two companies were given Emergency Use Authorization in USA [75]. On November 9th, 2021, Pfizer (New York) and BioNTech (Germany) reported that their designed COVID-19 vaccine has an efficacy of nearly 90%. Later, FDA permitted its emergency use in United States. Back in January 2020, BioNtech's scientists started the work on potential COVID-19 vaccine that was based on mRNA (messenger RNA). Basically, this vaccine encompasses genetic instruction for development of spike protein. This vaccine aid cells in producing spike proteins that on release into the circulation provokes immunity response. Lately in March, BioNTech joined with Pfizer to expand the study for which a clinical trial was registered and started in May [76,77]. Generic name of this vaccine is tozinameran and branded under name Comirnaty. This vaccine contains PEGylated liposomes (PEGLips) or LNPs encapsulated Tozinameran [78]. In Comirnaty, LNPs formulation allows mRNA to be stable [79,80]. LNPs-formulated mRNA-based vaccine helps provision of correct genetic information to antigen-presenting cells together with an adjuvant [81]. Preclinical trials revealed prophylactic effectiveness of this technique against different viral targets [[82], [83], [84]]. Clinical trial showed 94.1% effectiveness at suppressing the illness induced by COVID-19 and this study involves 30,420 participants [85]. BNT162b2, a vaccine from Pfizer-BioNTech, is a lipid nanoparticle and has cationic lipid ALC-0315 ((4-hydroxybutyl) azanediyl) bis(hexane-6,1-diyl) bis(2-hexyldecanoate), cholesterol, DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine), PEGDMA (2 [(polyethylene glycol)-2000]-N, N-ditetradecylacetamide) [86]. On December 11th, 2020, U.S. FDA granted BNT162b2 emergency use authorization based on a number of clinical investigations, and this include the one that showed both immunogenicity and safety of BNT162b2 [87]. Also, another clinical investigation including 43,548 individuals showed that BNT162b2 has 95% efficacy in preventing coronavirus disease [88]. Vaccines consisting of LNPs are administered (intramuscular route) in two different doses and are employed to encapsulate mRNA that encodes for spike glycoprotein found on SARS-CoV-2, which regulates the entry of the virus into the host cell [89]. After the spike glycoprotein encoding, the host organism initiates an immune response to the available virus antigenic proteins. Subsequently, the formation of host antibody against the coronavirus is initiated [90]. mRNA molecules transfer into the cytoplasm are enable by lipid nanoparticle, thus, this NPs has rectify the challenges of translation in mRNA technologies [91]. After using the LNPs to overcome the limitations of intracellular delivery, another possibility is antigen presentation and antibody stimulus neutralization against COVID-19. Both BNT162b2 and mRNA-1273 use employed in more than 35 countries with an estimated 1 billion for mRNA-1273 and two billion for BNT162b2 doses to be produced for the whole of 2021 [[92], [93], [94]].
Nearly after a week BioNTech and Pfizer developed their COVID-19 vaccine-Comirnaty, FDA permitted the use of Spikevax, another COVID-19 vaccine, produced by Moderna on 18th December. Spikevax was the second successful vaccine that was approved by FDA for use in emergency. Moderna's Spikevax is also an mRNA based COVID-19 vaccine similar to that produced by BioNTech& Pfizer. Moderna started production of COVID-19 vaccine in January 2020 [78,95]. Like Comirnaty, Moderna's vaccine also contains LNPs or PEGLips loaded mRNA as this assist in preserving mRNA and conferring its lability [79]. The Moderna vaccine (mRNA-1273) is a nanoparticle in liquid form made up of ionizable cationic lipid SM-102 (heptadecan-9-yl 8 ((2 hydroxyethyl) (6 oxo 6-(undecyloxy) hexyl) amino) octanoate), cholesterol, DSPC (1,2-distearoyl-snglycero-3 phosphocholine), and PEG-DMG (1 monomethoxypolyethyleneglycol-2,3-dimyristylglycerol containing polyethylene glycol) [86]. On December 18th, 2020, USA food and drug administration gave emergency use of authorization on mRNA-1273, in respect to number of clinical trials. This includes one that displayed anti-SARS-CoV-2 immune stimuli in individuals without trial-limiting safety concerns [89].
Another COVID-19 vaccine known as Vaxzevria is manufactured jointly by collaboration of University of Oxford and AstraZeneca. Results of clinical trials demonstrated that administration of 2-doses of Vaxzevria twelve weeks apart had 82.4% effectiveness. In December 2020, United Kingdom approved the use of Vaxzervia for emergency in spite of certain misperceptions. Later in January 2021, Serum Institute of India started manufacturing and supplying this vaccine having brand name as Covoshield under the sub-license agreement with AstraZeneca. Vaxzervia carries genetic instruction for development of spike protein (SARS-CoV-2 protein) encapsulated in another non-replicating virus. Purposely, scientists of Oxford-AstraZeneca team attached gene encoding SARS-CoV-2 spike protein with a modified chimpanzee adenovirus (ChAdOx1). Generally, Adenoviruses are naturally occurring viruses causing flu like symptoms. In this vaccine, adenovirus aids in protecting the genetic material via its protective protein coating. Therefore, Vaxzervia needs no frozen storage and can last up to 6 months if refrigerated at 2–8 °C [[96], [97], [98], [99], [100]]. An America biotechnology company named Novavax manufactures and supplies a COVID-19 vaccine named Nuvaxoid. It is a protein subunit-based vaccine and contains part of SARS-CoV-2 spike protein. This vaccine was developed using recombinant nanoparticle technology and contains an ingredient known as Matrix-M™ adjuvant, which aids in creating an effective immune response to the vaccine. Currently this protein-based vaccine is being manufactured and supplied by Serum Institute of India under a trade name Covovax [101].
Ad26.COV2, a COVID-19 vaccine, which is being manufactured by Janssen Pharmaceutical (Johnson & Johnson) with the collaboration of Beth Israel Deaconess Medical Center. Clinical trials have shown efficacy of single dose of this vaccine up to 72%. Like Oxford-AstraZeneca vaccine (Vaxzevria), this is also a non-replicating viral vector type of vaccine. In this vaccine, scientists have genetically modified an adenovirus by placing a gene for making of SARS-CoV-2 spike protein. WHO has permitted its use in case of emergency use [102]. By the end of 2020, Beijing Institute of Biological Products developed an inactivated type of COVID-19 vaccine. This vaccine is also known as BIBP-CorV or by its trade name i.e. Covilo. Clinical trials conducted by Sinopharm have reported an effective of 79% against COVID-19. This vaccine comprises of an inactivated form of SARS-CoV-2, which is not capable of replicating. However, the spike protein remains intact in this inactivated virus that results in triggering the immune response for the production of respective antibodies so that it cannot replicate, but it keeps the surface spike protein intact to trigger the body's immune system to create antibodies for protection against the live virus [[103], [104], [105], [106], [107]]. Some other vaccines namely Covaxin and Coronavac being produced by Bharat Biotech and Sinovac, respectively are also inactivated vaccines. Table 2 shows the details of some nanoparticle-based vaccines for COVID-19 that are in phase-4, and phase-3 of clinical trials or have qualified for WHO Emergency use authorization.
Table 2.
Some nanoparticle-based SARS-CoV-2 vaccines that are in phase-4, phase-3 of clinical trials or have qualified for WHO Emergency use authorization [94,108].
| Sr. No. |
Vaccine Name |
Type of vaccine |
Developer |
Registered Trial Number (s) |
|---|---|---|---|---|
| WHO-EUA qualified COVID-19 vaccines | ||||
| 1 | Covilo | Inactivated Virus | Sinopharm | NCT05204589; NCT04984408; NCT04560881; NCT04917523; NCT04612972; ChiCTR2000034780; IRCT20201214049709N3; IRCT20210206050259N3 |
| 2 | CoronaVac | Sinovac | NCT04800133; NCT04582344; NCT04651790; NCT04992260; NCT04456595; NCT05156632; PHRR210210-003308; NCT05077176; NCT05137418; NCT05225285; NCT04617483; NCT04508075, 669/UN6.KEP/EC/2020; NCT05204589 | |
| 3 | Covaxin | Bharat Biotech | CTRI/2020/11/028976, NCT04641481; NCT04918797 | |
| 4 | Ad26.COV2.S | Non-replicating viral vector | Janssen (Johnson & Johnson) | NCT05220397; NCT05148845; NCT05048940; NCT05047640; NCT05091307; NCT04505722; NCT04838795, PACTR202102855526180; NCT04614948, ISRCTN14722499 |
| 5 | Vaxzevria | Oxford/AstraZeneca | NCT04540393; ISRCTN89951424, NCT04536051; NCT04864561; NCT05007951; NCT04516746; EUCTR2020-001228-32, NCT04400838; CTRI/2020/08/027170; NCT05059106; NCT05236491; NCT04756271; NCT05011526; NCT04885764; NCT05198596; NCT04800133; NCT05017792 | |
| 6 | Covishield | Serum Institute of India | CTRI/2020/08/027170 | |
| 7 | Comirnaty | mRNA | Pfizer/BioNTech | NCT05124171, EUCTR2021-004550-33; jRCT2071210106; NCT05022329; NCT04805125; NCT05142319; NCT05228730; NCT04368728, EUCTR2020-002641-42; NCT05225285; NCT05047640; NCT04754594; NCT04816669; EUCTR2020-005442-42; NCT04951323; NCT04713553; NCT05029245; NCT04800133 |
| 8 | Spikevax | Moderna | NCT04806113; NCT04860297; NCT04811664; NCT05168813; NCT05236491; NCT05048940; NCT04470427; NCT04927065; NCT05249829; NCT04805125; jRCT2071210106; NCT05230953; NCT05228730; NCT05022329; NCT05119855; NCT05142319; NCT05158140; NCT04649151; NCT04796896 | |
| 9 | Nuvaxovid | Protein subunit | Novavax | EUCTR2020-004123-16, NCT04583995; CTRI/2021/02/031554; NCT05236491; NCT04611802 |
| 10 | Covovax | Serum Institute of India: | CTRI/2021/02/031554 | |
| Phase 4 | ||||
| 11 | Covilo | Inactivated Virus | Sinopharm | NCT04863638; NCT05105295; NCT05104216 |
| 12 | CoronaVac | Sinovac | NCT04756830; NCT04747821; NCT04775069; NCT04789356; NCT04754698; NCT04801888; NCT04911790; NCT04953325; NCT04962308; NCT05057169; | |
| 13 | Ad5-nCoV-IH | Non-Replicating Viral Vector | CanSino | NCT04892459 |
| 14 | Ad26.COV2.S | Janssen (Johnson & Johnson) | NCT05057169; NCT05037266; NCT05030974 | |
| 15 | Vaxzevria | Oxford/AstraZeneca | NCT04775069; NCT04760132; NCT04914832 | |
| 16 | Comirnaty | mRNA | Pfizer/BioNTech | NCT04775069; NCT04760132; NCT04969250; NCT04780659; NCT05057182; NCT05047718; NCT04955626; NCT04952766 |
| 17 | Spikevax | Moderna | NCT04760132; NCT05030974; NCT04792567; NCT05047718; NCT04952402; NCT04885907; NCT04969250 | |
| Phase 3 | ||||
| 18 | Nanocovax | Protein subunit | Nanogen Pharma | NCT04922788 |
| 19 | Zifivax | Anhui ZhifeiLongcom | ChiCTR2100050849; NCT05107375; ChiCTR2000040153, NCT04646590; NCT05091411; NCT05128643 | |
| 20 | MVC-COV1901 | Medigen Vaccine Biologics Corporation | NCT05198596; NCT05011526 | |
| 21 | V-01 | Livzon Mabpharm Inc | NCT05096845; NCT05096832 | |
| 22 | ReCOV | Jiangsu Rec-Biotechnology Co Ltd: | NCT05084989 | |
| 23 | RaziCov Pars | Razi Vaccine and Serum Research Institute | IRCT20201214049709N3 | |
| 24 | Recombinant Protein | Sanofi/GSK | NCT04904549; PACTR202011523101903 | |
| 25 | Soberana Plus | Instituto Finlay de Vacunas Cuba | IFV/COR/09 | |
| 26 | SP/GSK subunit B.1.351 vaccine | Sanofi/GSK | NCT05124171, EUCTR2021-004550-33 | |
| 27 | SCTV01C | Sinocelltech | NCT05043285; NCT05043311 | |
| 28 | S-268019 | Shionogi | NCT05212948; jRCT2031210383 | |
| 29 | GBP510 | SK Bioscience Co Ltd | NCT05007951 | |
| 30 | UB-612 | Vaxxinity/DASA | NCT04683224 | |
| 31 | SCB-2019 | Clover Biopharmaceuticals | NCT04672395; NCT05188677; PHRR210209-003334; NCT05193279 | |
| 32 | AKS-452 | University Medical Center Groningen | CTRI/2021/10/037269 | |
| 33 | Abdala | Center for Genetic Engineering and Biotechnology (CIGB) | RPCEC00000359 | |
| 34 | SpikoGen | Vaxine/CinnaGen Co. | NCT05175625, IRCT20150303021315N26; NCT05148871; NCT05005559, IRCT20150303021315N24 | |
| 35 | EpiVacCorona | Vector State Research Center of Virology and Biotechnology | NCT05021016; NCT04780035 | |
| 36 | Corbevax | Biological E Limited | CTRI/2021/10/037066; CTRI/2021/08/036074; CTRI/2021/06/034014 | |
| 37 | Recombinant (Sf9 cell) | West China Hospital | NCT04904471; NCT04887207 | |
| 38 | Noora vaccine | Bagheiat-allah University of Medical Sciences | IRCT20210620051639N3 | |
| 39 | Recombinant SARS-CoV-2 Vaccine (CHO Cell) | National Vaccine and Serum Institute | NCT05069129 | |
| 40 | Covifenz | Virus like particles | Medicago | NCT05040789; NCT04636697 |
| 41 | LYB001 | Yantai Patronus Biotech Co Ltd | NCT05137444 | |
| 42 | AG0302-COVID19 | DNA | AnGes | NCT04655625 |
| 43 | ZyCoV-D | Zydus Cadila | CTRI/2021/01/030416 | |
| 44 | INO-4800 | Inovio | NCT04642638; PACTR202110626944896 | |
| 45 | GX-19 | Genexine | NCT05067946 | |
| 46 | BNT162b2s01 | mRNA | Pfizer/BioNTech | NCT04368728, EUCTR2020-002641-42 |
| 47 | ARCT-154 | Arcturus Therapeutics Inc | NCT05012943 | |
| 48 | DS-5670a | Daiichi Sankyo Co Ltd | jRCT2071210106 | |
| 49 | mRNA-1273.529 | Moderna | NCT05249829 | |
| 50 | GRAd-COV2 | Non-Replicating Viral Vector | ReiThera | NCT04791423; EUCTR2020-005915-39 |
| 51 | AZD2816 | Oxford/AstraZeneca | NCT04973449 | |
| 52 | Sputnik Light | Gamaleya | NCT04741061 | |
| 53 | Convidecia | CanSino | NCT04540419; NCT05169008; NCT04526990 | |
| 54 | Sputnik V | Gamaleya | NCT04656613; NCT04530396; NCT04640233; NCT04642339; NCT04564716; NCT04954092 | |
| 55 | Ad5-nCoV-IH | CanSino | NCT05204589; NCT05169008; NCT05124561 | |
| 56 | Brilife | Replicating Viral Vector | Israel Institute for Biological Research (IIBR) | NCT04990466 |
| 57 | DelNS1-2019-nCoV-RBD-OPT1 | WantaiBioPharm | ChiCTR2100051391; PACTR202110872285345 | |
| 58 | Turkovac | Inactivated virus | Health Institutes of Turkey | NCT04942405; NCT05077176 |
| 59 | VLA2001 | Valneva SE | NCT04956224; NCT04864561 | |
| 60 | COVIranBarekat | Shifa Pharmed Industrial Co | IRCT20201202049567N3 | |
| 61 | KCONVAC | Minhai Biotechnology Co | NCT05204589; NCT04852705 | |
Inorganic and LNPs are the mainly NPs recommended clinically. Anselmo and Mitragotri [94] stated that the first clinically approved particle in 1989 and the most recent clinically recommended NPs show how NPs that are lipid in nature enable regulated interactions between complex microenvironments and encapsulated therapeutics in patients. NPs that are lipid base are used for protecting and carrying sensitive molecules such as mRNA after their production, during their storage and during their use as intramuscular injection. This is in addition to their use as clinically approved intravenous applications [94] and based on this report, the use of NPs for managing COVID-19 is gaining more attentions. There is an increment in the number of clinical trials for recommended nanoparticle from 1220 (2016) to 1935 (2021). They revealed the success recorded in the use of NPs against COVID-19 and continuous progress made on other NPs for their use in clinics [94], though more investigations are required in wake of emerging strains of COVID-19 [109].
4. Nanoparticle vaccines against emerging SARS-CoV-2variants
Around the globe, the vaccines against COVID-19 have been effectively being formulated but threat of SARS-CoV-2 and its variants still persists [110]. Scientists around the world have identified polymorphic changes in code sequences of COVID-19 genome [111]. These mutations in genome sequence of SARS-CoV-2 have affected the effectiveness of viral transmission, vaccine sensitivity, and pathogenicity and therefore known as VOCs. Emergence of VOCs has been a significant threat and hurdle in development of effective SARS-CoV-2 vaccines. Various VOCs have been identified and reported till now that are commonly known as Alpha, Beta, Gamma, Delta, and Omicron variants [[112], [113], [114], [115]]. In spite of significant success in development of SARS-CoV-2 vaccines, it is need of time for development of vaccines having broad spectrum mechanism of action to overcome infections caused by emerging variants of SARS-CoV-2 and reduced immunity.
For this purpose, Li et al. [116] revealed that immunization of SARS-CoV-2 RBD-scNP (receptor binding domain sortase A-conjugated ferritin nanoparticle) induced potential nAbs (neutralizing antibodies) in NHPs (non-human primates) against 8 variants of SARS-CoV-2such as Omicron, Delta, and Beta. As compared to SARS-CoV-2 D614G, the RBD-scNP-induced antibodies neutralized Omicron variant as ID50 titers reduced on an average of 10.6-folds. RBD-scNPs immunization helped in protection non-human primates against SARS-CoV-2 Beta, WA-1, and Delta variants, further mice were also protected from SARS-CoV-2 Beta variant. They were of the view that RBD-scNPs induces neutralizing antibodies against different variants of SARS-CoV-2and protected non-human primates &mice from SARS-CoV-2 variants. This type of vaccine may be helpful in providing immunity against different variants (Omicron & Delta) of SARS-CoV-2 (116).
In 2021, a variant of SARS-CoV-2 known as Delta variant emerged and became a variant of concern. As compared to its original strain, Delta variant have twice the transmission capacity, short incubation time, and elevated viral content. Thus, development of vaccine against Delta variant of SARS-CoV-2 was an urgent need. Purposely, Chen et al. [117] reported that RBD-conjugated NP vaccines, when administrated according to single dose and prime boost approach, induced ample amount of NAbs (neutralizing antibodies) as they protected mice (hACE2) against Delta variant. Moreover, in rhesus macaques, third re-boost of trivalent vaccine resulted in production of broader cross-protective neutralizing antibodies. They concluded that RBD-conjugated NP vaccines are potent 2nd generation vaccines against VOCs of SARS-CoV-2 [117]. Third dose of Walvax COVID-19 vaccine (ARCoV) lead to robust elevation in NAbs against wild type SARSD-CoV-2 and Omicron variant. Administration of homologous booster vaccination of ARCoV might be a significant rational approach against current emergence of Omicron [118].
Owing to the emergence of VOCs of SARS-CoV-2, the need of next generation vaccines having broad spectrum protective effect against COIVD-19 has escalated around the world. In non-human primates, adjuvant SpFN (SARS-CoV-2 spike ferritin nanoparticle) vaccine have been manufactured and evaluated. SpFN (50 μg) administrated twice 28 days apart resulted in induction of TH1 (T helper cell 1)-biased CD4 TH response and evoked NAbs against wild type and VOCs [119]. Both alone and heterologous booster of wild type mRNA vaccine along with Omicron-specific LNP mRNA-based vaccine candidate have been experimented in animals. In vaccination-naive mice, developed Omicron-specific LNP mRNA vaccine evoked significant and specific Ab response. Double dose wild type mRNA vaccinated mice receiving a booster single dose of either homologous booster shot of wild type LNP mRNA or heterologous booster shot of Omicron LNP mRNA helped in restoring diminishing Abs response against Omicron variant. Results showed 40-fold elevation in Abs post two weeks of administration of booster doses. Furthermore, heterologous administration of booster dose of LNP mRNA resulted in 10-to-20-fold increase in NAbs as compared to homologous administration of wild type booster against the Omicron variant [120]. On the other hand, Glycosite-deleted SARS-CoV-2 spike protein mRNA vaccine specifically in S2 (subunit 2) domain for exposing more conserved epitopes evoked strong Abs and CD8+ T cell response thus resulting in broad-spectrum protection against VOCs including alpha variant, beta variant, gamma variant, and omicron variants [121,122].
5. Conclusion and future prospects
The crucial times of pandemic and hard situations calls for innovative strategies. Therefore, nanotechnology emerged as a promising field in the cure of COVID-19. This article not only highlighted the facts, mechanisms and stable delivery of nanoparticle products to human system but has also demonstrated their efficacy and capability. Apart from, absorption, digestion, circulation and functioning of NPs, excretion of nanomedicines is also a subject of great interest. Thus, nano-toxicological studies are a pre-requisite in the process of nano-products formulation and design. Apart from this, the size, morphology and surface area of NPs also play a key role in design and manufacture of NPs along with their commercial production on large scale.
Nanotechnology have played a vital role in diagnosis, treatment, prevention, and vaccination related to various diseases including SARS-CoV-2. Nanotechnology may be consolidated as a significant tool for ensuring safety of healthcare personals and general public. To date, various vaccine platforms including protein subunits, viral vectors (non-replicating), viral vectors (replicating), DNA-based, RNA-based, Virus like particles, inactivated virus, live attenuated virus, viral vector (replicating) + Antigen presenting cell, viral vector (non-replicating) + Antigen presenting cell, and Bacterial antigen spore expression vectors have been developed. To enhance the effectiveness of vaccines, nano-systems like lipid, polymeric, micelles, and metallic NPs are in practice. Synthetic NPs having close morphology and physiochemical similarities of SARS-CoV-2 are an efficacious interventional technique. NPs are functionalized using various functional groups and polymers to perform specific functions.
Owing to extensive research in development of different vaccine platforms, it was possible today that NPs based clinical studies initiated within 2 months post public availability of genome sequence of SARS-CoV-2. Practically, the swift response in production and effectiveness of developed vaccines against SARS-CoV-2 helped in saving countless lives. Nonetheless, time will tell how these NPs based vaccines will evolve during the next phase of COVID-19 and/or in combating upcoming pandemic situations. Different NPs vaccines have their own advantages and disadvantages like mRNA-based vaccines are considered to be more expensive as compared to whole-inactivated virus vaccines as they require an additional ultra-cold supply chain facility too. Further, mRNA and protein NPs vaccines require highly equipped production facilities and therefore is an area of concern in low-income countries. Protein NPs have their own challenges like complex purification, stability issues, and rigid GMP-grade requirements of cell line are hurdles in attaining regulatory approvals. There is dire need for technology transfer to scale up production and associated benefits of NPs vaccine for ensuring ample supply of manufactured vaccines around the globe. NPs vaccines results in offering controlled antigen release, and viral entry inference for treatment and prevention against COVID-19. The safety, stability, and effectiveness of NPs vaccine may be evaluated through clinical outcomes. Ongoing trials to further authenticate the safety and effectiveness of NPs vaccines must be continued.
Ethical approval
Not applicable/Not required. This submission is a critical review. It is not a clinical or experimental study and does not involve human participants and/or research studies.
Funding
This research received no external funding.
Author contribution
Conceptualization and writing-original manuscript, A.R.; Data collection, T.A., A.A.K., N.H., A.O., and M.M.R.; Editing and proof reading, R.S., P.S., Y.S.A., O.S.B., I.N.K., and M.A.K. All authors approved submission of the final manuscript.
Research registration Unique Identifying number (UIN)
1. Name of the registry: Not applicable/Not required [Please note that this submission is a critical review article which discusses the Nanoparticles in clinical trials of COVID-19. It is not a research study/clinical trial/systematic review, and therefore does not require any Registry number.]
2. Unique Identifying number or registration ID: Not applicable/Not required.
3. Hyperlink to your specific registration (must be publicly accessible and will be checked): Not applicable/Not required.
Guarantor
Rohit Sharma.
(Corresponding author).
Provenance and peer review
Not commissioned, externally peer-reviewed.
Data statement
We have shared the data in tabular form with the review manuscript file.
Declaration of competing interest
The authors declare that they have no conflicts of interest.
References
- 1.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A., et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicola M., Alsafi Z., Sohrabi C., Kerwan A., Al-Jabir A., Iosifidis C., et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int. J. Surg. 2020;78:185–193. doi: 10.1016/j.ijsu.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicola M., O'Neill N., Sohrabi C., Khan M., Agha M., Agha R. Evidence based management guideline for the COVID-19 pandemic - review article. Int. J. Surg. 2020;77:206–216. doi: 10.1016/j.ijsu.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma R., Prajapati P. Nanotechnology in medicine: leads from ayurveda. J. Pharm. BioAllied Sci. 2016;8(1):80–81. doi: 10.4103/0975-7406.171730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thi T.T.H., Suys E.J., Lee J.S., Nguyen D.H., Park K.D., Truong N.P. Lipid-based nanoparticles in the clinic and clinical trials: from cancer nanomedicine to COVID-19 vaccines. Vaccines. 2021;9(4):359. doi: 10.3390/vaccines9040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ventola C.L. Progress in nanomedicine: approved and investigational nanodrugs. P T. 2017;42(12):742–755. [PMC free article] [PubMed] [Google Scholar]
- 7.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: an update. Bioeng. Transl. Med. 2019;4(3) doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobo D., Robinson K.J., Islam J., Thurecht K.J., Corrie S.R. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharmaceut. Res. 2016;33(10):2373–2387. doi: 10.1007/s11095-016-1958-5. [DOI] [PubMed] [Google Scholar]
- 9.Germain M., Caputo F., Metcalfe S., Tosi G., Spring K., Åslund A.K., et al. Delivering the power of nanomedicine to patients today. J. Contr. Release. 2020;326:164–171. doi: 10.1016/j.jconrel.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabnis S., Kumarasinghe E.S., Salerno T., Mihai C., Ketova T., Senn J.J., et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 2018;26(6):1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah S., Dhawan V., Holm R., Nagarsenker M.S., Perrie Y. Liposomes: advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020;154-155:102–122. doi: 10.1016/j.addr.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Lu W., Yao J., Zhu X., Qi Y. Nanomedicines: redefining traditional medicine. Biomed. Pharmacother. 2021;134 doi: 10.1016/j.biopha.2020.111103. [DOI] [PubMed] [Google Scholar]
- 13.Kerry R.G., Malik S., Redda Y.T., Sahoo S., Patra J.K., Majhi S. Nano-based approach to combat emerging viral (NIPAH virus) infection. Nanomed. Nanotechnol. Biol. Med. 2019;18:196–220. doi: 10.1016/j.nano.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du L., Yang Y., Zhang X., Li F. Recent advances in nanotechnology-based COVID-19 vaccines and therapeutic antibodies. Nanoscale. 2022;14(4):1054–1074. doi: 10.1039/d1nr03831a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shih H.-I., Wu C.-J., Tu Y.-F., Chi C.-Y. Fighting COVID-19: a quick review of diagnoses, therapies, and vaccines. Biomed. J. 2020;43(4):341–354. doi: 10.1016/j.bj.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbas Z., Labbez C., Nordholm S., Ahlberg E. Size-dependent surface charging of nanoparticles. J. Phys. Chem. C. 2008;112(15):5715–5723. [Google Scholar]
- 17.Zhao L., Seth A., Wibowo N., Zhao C.-X., Mitter N., Yu C., et al. Nanoparticle vaccines. Vaccine. 2014;32(3):327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 18.Diaz-Arévalo D., Zeng M. Nanoparticle-based vaccines: opportunities and limitations. Nanopharmaceuticals. 2020:135–150. [Google Scholar]
- 19.Vu M.N., Kelly H.G., Kent S.J., Wheatley A.K. Current and future nanoparticle vaccines for COVID-19. EBioMedicine. 2021;74 doi: 10.1016/j.ebiom.2021.103699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bale S., Goebrecht G., Stano A., Wilson R., Ota T., Tran K., et al. Covalent linkage of HIV-1 trimers to synthetic liposomes elicits improved B cell and antibody responses. J. Virol. 2017;91(16) doi: 10.1128/JVI.00443-17. e00443-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 22.Kupferschmidt K., Cohen J. Race to find COVID-19 treatments accelerates. Science. 2020;367(6485):1412–1413. doi: 10.1126/science.367.6485.1412. [DOI] [PubMed] [Google Scholar]
- 23.El-Sheekh M.M., Shabaan M.T., Hassan L., Morsi H.H. Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int. J. Environ. Health Res. 2020:1–12. doi: 10.1080/09603123.2020.1789946. [DOI] [PubMed] [Google Scholar]
- 24.Lara H.H., Ayala-Nuñez N.V., Ixtepan-Turrent L., Rodriguez-Padilla C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010;8(1):1–10. doi: 10.1186/1477-3155-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu L., Sun R.W.-Y., Chen R., Hui C.-K., Ho C.-M., Luk J.M., et al. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008;13(2):253–262. [PubMed] [Google Scholar]
- 26.Neogi U., Hill K.J., Ambikan A.T., Heng X., Quinn T.P., Byrareddy S.N., et al. Feasibility of known RNA polymerase inhibitors as anti-SARS-CoV-2 drugs. Pathogens. 2020;9(5):320. doi: 10.3390/pathogens9050320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mainardes R.M., Diedrich C. Future Science; 2020. The Potential Role of Nanomedicine on COVID-19 Therapeutics; pp. 411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang X., Li M., Xu Y., Zhang J., Meng X., An X., et al. Novel gold nanorod-based HR1 peptide inhibitor for Middle East respiratory syndrome coronavirus. ACS Appl. Mater. Interfaces. 2019;11(22):19799–19807. doi: 10.1021/acsami.9b04240. [DOI] [PubMed] [Google Scholar]
- 29.Osminkina L., Timoshenko V.Y., Shilovsky I., Kornilaeva G., Shevchenko S., Gongalsky M., et al. Porous silicon nanoparticles as scavengers of hazardous viruses. J. Nanoparticle Res. 2014;16(6):1–10. [Google Scholar]
- 30.Abo-Zeid Y., Ismail N.S., McLean G.R., Hamdy N.M. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur. J. Pharmaceut. Sci. 2020;153 doi: 10.1016/j.ejps.2020.105465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghuwanshi D., Mishra V., Das D., Kaur K., Suresh M.R. Dendritic cell targeted chitosan nanoparticles for nasal DNA immunization against SARS CoV nucleocapsid protein. Mol. Pharm. 2012;9(4):946–956. doi: 10.1021/mp200553x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iannazzo D., Pistone A., Ferro S., De Luca L., Monforte A.M., Romeo R., et al. Graphene quantum dots based systems as HIV inhibitors. Bioconjugate Chem. 2018;29(9):3084–3093. doi: 10.1021/acs.bioconjchem.8b00448. [DOI] [PubMed] [Google Scholar]
- 33.Alghrair Z.K., Fernig D.G., Ebrahimi B. Enhanced inhibition of influenza virus infection by peptide–noble-metal nanoparticle conjugates. Beilstein J. Nanotechnol. 2019;10(1):1038–1047. doi: 10.3762/bjnano.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emileh A., Tuzer F., Yeh H., Umashankara M., Moreira D.R., LaLonde J.M., et al. A model of peptide triazole entry inhibitor binding to HIV-1 gp120 and the mechanism of bridging sheet disruption. Biochemistry. 2013;52(13):2245–2261. doi: 10.1021/bi400166b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Milovanovic M., Arsenijevic A., Milovanovic J., Kanjevac T., Arsenijevic N. 2017. Nanoparticles in antiviral therapy; pp. 383–410. (Antimicrobial Nanoarchitectonics). Elsevier. [Google Scholar]
- 36.Fredriksen B.N., Grip J. PLGA/PLA micro-and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.) Vaccine. 2012;30(3):656–667. doi: 10.1016/j.vaccine.2011.10.105. [DOI] [PubMed] [Google Scholar]
- 37.Bhosale U.V., Devi K., Choudhary S. Development and in vitro-in vivo evaluation of oral drug delivery system of acyclovir loaded PLGA nanoparticles. Int. J. Drug Deliv. 2013;5(3):331. [Google Scholar]
- 38.Lim J.-W., Ahn Y.-R., Park G., Kim H.-O., Haam S. Application of nanomaterials as an advanced strategy for the diagnosis, prevention, and treatment of viral diseases. Pharmaceutics. 2021;13(10):1570. doi: 10.3390/pharmaceutics13101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lou Z., Sun Y., Rao Z. Current progress in antiviral strategies. Trends Pharmacol. Sci. 2014;35(2):86–102. doi: 10.1016/j.tips.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Clercq E. Antiviral agents active against influenza A viruses. Nat. Rev. Drug Discov. 2006;5(12):1015–1025. doi: 10.1038/nrd2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riva L., Yuan S., Yin X., Martin-Sancho L., Matsunaga N., Pache L., et al. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020;586(7827):113–119. doi: 10.1038/s41586-020-2577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medhi R., Srinoi P., Ngo N., Tran H.-V., Lee T.R. Nanoparticle-based strategies to combat COVID-19. ACS Appl. Nano Mater. 2020;3(9):8557–8580. doi: 10.1021/acsanm.0c01978. [DOI] [PubMed] [Google Scholar]
- 43.Derudas M., McGuigan C., Brancale A., Bugert J.J., Andrei G., Snoeck R., et al. Design, synthesis and biological evaluation of novel acyclovir ProTides. Antivir. Res. 2008;78(2):A55–A56. [Google Scholar]
- 44.Javan F., Vatanara A., Azadmanesh K., Nabi-Meibodi M., Shakouri M. Encapsulation of ritonavir in solid lipid nanoparticles: in-vitro anti-HIV-1 activity using lentiviral particles. J. Pharm. Pharmacol. 2017;69(8):1002–1009. doi: 10.1111/jphp.12737. [DOI] [PubMed] [Google Scholar]
- 45.Mehranfar A., Izadyar M. Theoretical design of functionalized gold nanoparticles as antiviral agents against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) J. Phys. Chem. Lett. 2020;11(24):10284–10289. doi: 10.1021/acs.jpclett.0c02677. [DOI] [PubMed] [Google Scholar]
- 46.Chun H., Yeom M., Kim H.-O., Lim J.-W., Na W., Park G., et al. Efficient antiviral co-delivery using polymersomes by controlling the surface density of cell-targeting groups for influenza A virus treatment. Polym. Chem. 2018;9(16):2116–2123. [Google Scholar]
- 47.Figueira T.N., Domingues M.M., Fo Illien, Cadima-Couto I., Todorovski T., Andreu D., et al. Enfuvirtide-protoporphyrin IX dual-loaded liposomes: in vitro evidence of synergy against HIV-1 entry into cells. ACS Infect. Dis. 2019;6(2):224–236. doi: 10.1021/acsinfecdis.9b00285. [DOI] [PubMed] [Google Scholar]
- 48.Chakravarty M., Vora A. Nanotechnology-based antiviral therapeutics. Drug Deliv. Transl. Res. 2021;11(3):748–787. doi: 10.1007/s13346-020-00818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cagno V., Andreozzi P., D'Alicarnasso M., Silva P.J., Mueller M., Galloux M., et al. Broad-spectrum non-toxic antiviral nanoparticles with a virucidal inhibition mechanism. Nat. Mater. 2018;17(2):195–203. doi: 10.1038/nmat5053. [DOI] [PubMed] [Google Scholar]
- 50.de Souza e Silva J.M., Hanchuk T.D.M., Santos M.I., Kobarg, Bajgelman M.C., Cardoso M.B. Viral inhibition mechanism mediated by surface-modified silica nanoparticles. ACS Appl. Mater. Interfaces. 2016;8(26):16564–16572. doi: 10.1021/acsami.6b03342. [DOI] [PubMed] [Google Scholar]
- 51.Nie C., Stadtmuller M., Yang H., Xia Y., Wolff T., Cheng C., et al. Spiky nanostructures with geometry-matching topography for virus inhibition. Nano Lett. 2020;20(7):5367–5375. doi: 10.1021/acs.nanolett.0c01723. [DOI] [PubMed] [Google Scholar]
- 52.Lauster D., Glanz M., Bardua M., Ludwig K., Hellmund M., Hoffmann U., et al. Multivalent peptide–nanoparticle conjugates for influenza‐virus inhibition. Angew. Chem. Int. Ed. 2017;56(21):5931–5936. doi: 10.1002/anie.201702005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X.X., Li C.M., Huang C.Z. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale. 2016;8(5):3040–3048. doi: 10.1039/c5nr07918g. [DOI] [PubMed] [Google Scholar]
- 54.Rao L., Xia S., Xu W., Tian R., Yu G., Gu C., et al. Decoy nanoparticles protect against COVID-19 by concurrently adsorbing viruses and inflammatory cytokines. Proc. Natl. Acad. Sci. USA. 2020;117(44):27141–27147. doi: 10.1073/pnas.2014352117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z., Wang Z., Dinh P.-U.C., Zhu D., Popowski K.D., Lutz H., et al. Cell-mimicking nanodecoys neutralize SARS-CoV-2 and mitigate lung injury in a non-human primate model of COVID-19. Nat. Nanotechnol. 2021:1–10. doi: 10.1038/s41565-021-00923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rakowska P.D., Tiddia M., Faruqui N., Bankier C., Pei Y., Pollard A.J., et al. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021;2(1):1–19. [Google Scholar]
- 57.Lima T.L.C., Feitosa RdC., Santos-Silva D., Santos-Silva D., Maria A., Siqueira EMdS., et al. Improving encapsulation of hydrophilic chloroquine diphosphate into biodegradable nanoparticles: a promising approach against herpes virus simplex-1 infection. Pharmaceutics. 2018;10(4):255. doi: 10.3390/pharmaceutics10040255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joshy K., Snigdha S., George A., Kalarikkal N., Pothen L.A., Thomas S. Core–shell nanoparticles of carboxy methyl cellulose and compritol-PEG for antiretroviral drug delivery. Cellulose. 2017;24(11):4759–4771. [Google Scholar]
- 59.Joshy K., Snigdha S., Anne G., Nandakumar K., Sabu T. Poly (vinyl pyrrolidone)-lipid based hybrid nanoparticles for anti viral drug delivery. Chem. Phys. Lipids. 2018;210:82–89. doi: 10.1016/j.chemphyslip.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Cao S., Slack S.D., Levy C.N., Hughes S.M., Jiang Y., Yogodzinski C., et al. Hybrid nanocarriers incorporating mechanistically distinct drugs for lymphatic CD4+ T cell activation and HIV-1 latency reversal. Sci. Adv. 2019;5(3) doi: 10.1126/sciadv.aav6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du T., Liang J., Dong N., Lu J., Fu Y., Fang L., et al. Glutathione-capped Ag2S nanoclusters inhibit coronavirus proliferation through blockage of viral RNA synthesis and budding. ACS Appl. Mater. Interfaces. 2018;10(5):4369–4378. doi: 10.1021/acsami.7b13811. [DOI] [PubMed] [Google Scholar]
- 62.Ghaffari H., Tavakoli A., Moradi A., Tabarraei A., Bokharaei-Salim F., Zahmatkeshan M., et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J. Biomed. Sci. 2019;26(1):1–10. doi: 10.1186/s12929-019-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin Z., Li Y., Guo M., Xiao M., Wang C., Zhao M., et al. Inhibition of H1N1 influenza virus by selenium nanoparticles loaded with zanamivir through p38 and JNK signaling pathways. RSC Adv. 2017;7(56):35290–35296. [Google Scholar]
- 64.El-Sayed A., Kamel M. Advances in nanomedical applications: diagnostic, therapeutic, immunization, and vaccine production. Environ. Sci. Pollut. Control Ser. 2020;27(16):19200–19213. doi: 10.1007/s11356-019-06459-2. [DOI] [PubMed] [Google Scholar]
- 65.Tang Z., Zhang X., Shu Y., Guo M., Zhang H., Tao W. Insights from nanotechnology in COVID-19 treatment. Nano Today. 2021;36 doi: 10.1016/j.nantod.2020.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coll P.P., Costello V.W., Kuchel G.A., Bartley J., McElhaney J.E. The prevention of infections in older adults: vaccination. J. Am. Geriatr. Soc. 2020;68(1):207–214. doi: 10.1111/jgs.16205. [DOI] [PubMed] [Google Scholar]
- 67.Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A., et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020;15(8):646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 68.McKay P.F., Hu K., Blakney A.K., Samnuan K., Brown J.C., Penn R., et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020;11(1):1–7. doi: 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang R., Chen J., Gao K., Wei G.-W. Vaccine-escape and fast-growing mutations in the United Kingdom, the United States, Singapore, Spain, India, and other COVID-19-devastated countries. Genomics. 2021;113(4):2158–2170. doi: 10.1016/j.ygeno.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B. 1.351 variant. N. Engl. J. Med. 2021;384(20):1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Le T.T., Cramer J.P., Chen R., Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19(10):667–668. doi: 10.1038/d41573-020-00151-8. [DOI] [PubMed] [Google Scholar]
- 72.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., et al. ACS Publications; 2020. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y., et al. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14(4):3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 74.Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haynes B.F. Mass Medical Soc; 2021. A New Vaccine to Battle Covid-19; pp. 470–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdellatif A.A., Tawfeek H.M., Abdelfattah A., Batiha G.E.-S., Hetta H.F. Recent updates in COVID-19 with emphasis on inhalation therapeutics: nanostructured and targeting systems. J. Drug Deliv. Sci. Technol. 2021;63 doi: 10.1016/j.jddst.2021.102435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bouazzaoui A., Abdellatif A.A., Al-Allaf F.A., Bogari N.M., Al-Dehlawi S., Qari S.H. Strategies for vaccination: conventional vaccine approaches versus new-generation strategies in combination with adjuvants. Pharmaceutics. 2021;13(2):140. doi: 10.3390/pharmaceutics13020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zamboni W.C. Concept and clinical evaluation of carrier-mediated anticancer agents. Oncol. 2008;13(3):248–260. doi: 10.1634/theoncologist.2007-0180. [DOI] [PubMed] [Google Scholar]
- 79.Kim H., Park Y., Lee J.B. Self-assembled messenger RNA nanoparticles (mRNA-NPs) for efficient gene expression. Sci. Rep. 2015;5(1):1–9. doi: 10.1038/srep12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caballero M., Quirce S. Excipients as potential agents of anaphylaxis in vaccines: analyzing the formulations of currently authorized COVID-19 vaccines. J Investig. Allergol. Clin. Immunol. 2021;31(1):92–93. doi: 10.18176/jiaci.0667. [DOI] [PubMed] [Google Scholar]
- 81.Pardi N., Hogan M.J., Naradikian M.S., Parkhouse K., Cain D.W., Jones L., et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018;215(6):1571–1588. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13(10):759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 83.Pardi N., Hogan M.J., Pelc R.S., Muramatsu H., Andersen H., DeMaso C.R., et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543(7644):248–251. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pardi N., LaBranche C.C., Ferrari G., Cain D.W., Tombácz I., Parks R.J., et al. Characterization of HIV-1 nucleoside-modified mRNA vaccines in rabbits and rhesus macaques. Mol. Ther. Nucleic Acids. 2019;15:36–47. doi: 10.1016/j.omtn.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verbeke R., Lentacker I., De Smedt S.C., Dewitte H. The dawn of mRNA vaccines: the COVID-19 case. J. Contr. Release. 2021;333:511–520. doi: 10.1016/j.jconrel.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Walsh E.E., Frenck R.W., Jr., Falsey A.R., Kitchin N., Absalon J., Gurtman A., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586(7830):567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reichmuth A.M., Oberli M.A., Jaklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016;7(5):319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Milane L., Amiji M. Clinical approval of nanotechnology-based SARS-CoV-2 mRNA vaccines: impact on translational nanomedicine. Drug Deliv. Transl. Res. 2021:1–7. doi: 10.1007/s13346-021-00911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baraniuk C. How to vaccinate the world against covid-19. BMJ. 2021:372. doi: 10.1136/bmj.n211. [DOI] [PubMed] [Google Scholar]
- 94.Anselmo A.C., Mitragotri S. Nanoparticles in the clinic: an update post COVID‐19 vaccines. Bioeng. Transl. Med. 2021;6(3) doi: 10.1002/btm2.10246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 96.COVID E. 2021. Vaccine AstraZeneca. [Google Scholar]
- 97.Medicines Agency HpR. 2020. Regulatory Approval of Pfizer/BioNTech Vaccine for COVID-19. [Google Scholar]
- 98.UK Health Security Agency. COVID-19 vaccination guidance for healthcare practitioners V 4.2 2022. https://www.gov.uk/government/publications/covid-19-vaccination-programme-guidance-for-healthcare-practitioners (accessed on 13 March 2022).
- 99.UK Health Security Agency. COVID-19 vaccination guidance for healthcare practitioners V 4.2 2022. https://www.gov.uk/government/publications/covid-19-vaccination-programme-guidance-for-healthcare-practitioner. (accessed on 13 March 2022).
- 100.Medicines & Healthcare products Regulatory Agency (MHRA) Managing allergic reactions with Pfizer/BioNTech. Vaccine. 2020;1836(1):5. [Google Scholar]
- 101.Novavax. Clinical Stage Pipeline – Novavax – Creating Tomorrow's Vaccines Today. Novavax.com (https://www.novavax.com/our-pipeline). (Accessed on 13 March 2022).
- 102.Orders M. FDA authorizes Johnson & Johnson COVID-19 vaccine. Med. Lett. Drugs Ther. 2021;63(1620):41–42. [PubMed] [Google Scholar]
- 103.Drulovic J., Ivanovic J., Martinovic V., Tamas O., Veselinovic N., Cujic D., et al. Humoral response to SARS-CoV-2 COVID-19 vaccines in patients with multiple sclerosis treated with immune reconstitution therapies. Mult. Scler. Relat. Disord. 2021;54 doi: 10.1016/j.msard.2021.103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Al Khames Aga Q.A., Alkhaffaf W.H., Hatem T.H., Nassir K.F., Batineh Y., Dahham A.T., et al. Safety of COVID‐19 vaccines. J. Med. Virol. 2021;93(12):6588–6594. doi: 10.1002/jmv.27214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Abu-Hammad O., Alduraidi H., Abu-Hammad S., Alnazzawi A., Babkair H., Abu-Hammad A., et al. Side effects reported by Jordanian healthcare workers who received COVID-19 vaccines. Vaccines. 2021;9(6):577. doi: 10.3390/vaccines9060577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hatmal MmM., Al-Hatamleh M.A., Olaimat A.N., Hatmal M., Alhaj-Qasem D.M., Olaimat T.M., et al. Side effects and perceptions following COVID-19 vaccination in Jordan: a randomized, cross-sectional study implementing machine learning for predicting severity of side effects. Vaccines. 2021;9(6):556. doi: 10.3390/vaccines9060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doroftei B., Ciobica A., Ilie O.-D., Maftei R., Ilea C. Mini-review discussing the reliability and efficiency of COVID-19 vaccines. Diagnostics. 2021;11(4):579. doi: 10.3390/diagnostics11040579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Papi M., Pozzi D., Palmieri V., Caracciolo G. Principles for optimization and validation of mRNA lipid nanoparticle vaccines against COVID-19 using 3D bioprinting. Nano Today. 2022 doi: 10.1016/j.nantod.2022.101403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharma V., Rai H., Gautam D.N., Prajapati P.K., Sharma R. Emerging evidence on omicron (B. 1.1. 529) SARS‐CoV‐2 variant. J. Med. Virol. 2022;94(5):1876–1885. doi: 10.1002/jmv.27626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ortiz J.R., Neuzil K.M. The value of vaccine programme impact monitoring during the COVID-19 pandemic. Lancet. 2022;399(10320):119–121. doi: 10.1016/S0140-6736(21)02322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Volz E., Hill V., McCrone J.T., Price A., Jorgensen D., O'Toole Á., et al. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell. 2021;184(1):64–75. doi: 10.1016/j.cell.2020.11.020. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B. 1.1. 7 in England. Science. 2021;(6538):372. doi: 10.1126/science.abg3055. eabg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398(10317):2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cherian S., Potdar V., Jadhav S., Yadav P., Gupta N., Das M., et al. SARS-CoV-2 spike mutations, L452R, T478K, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. Microorganisms. 2021;9(7):1542. doi: 10.3390/microorganisms9071542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., et al. MedRxiv; 2020. Emergence and Rapid Spread of a New Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-CoV-2) Lineage with Multiple Spike Mutations in South Africa. [Google Scholar]
- 116.Li D., Martinez D.R., Schäfer A., Chen H., Barr M., Sutherland L.L., et al. bioRxiv; 2022. Breadth of SARS-CoV-2 Neutralization and Protection Induced by a Nanoparticle Vaccine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen R., Zhang X., Yuan Y., Deng X., Wu B., Xi Z., et al. Development of receptor binding domain (RBD)‐Conjugated nanoparticle vaccines with broad neutralization against SARS‐CoV‐2 Delta and other variants. Adv. Sci. 2022;9(11) doi: 10.1002/advs.202105378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang N.-N., Zhang R.-R., Zhang Y.-F., Ji K., Xiong X.-C., Qin Q.-S., et al. Rapid development of an updated mRNA vaccine against the SARS-CoV-2 Omicron variant. Cell Res. 2022;32(4):401–403. doi: 10.1038/s41422-022-00626-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Joyce M.G., King H.A., Elakhal-Naouar I., Ahmed A., Peachman K.K., Macedo Cincotta C., et al. A SARS-CoV-2 ferritin nanoparticle vaccine elicits protective immune responses in nonhuman primates. Sci. Transl. Med. 2021;14(632) doi: 10.1126/scitranslmed.abi5735. eabi5735. [DOI] [PubMed] [Google Scholar]
- 120.Fang Z., Peng L., Filler R., Suzuki K., McNamara A., Lin Q., et al. Omicron-specific mRNA vaccination alone and as a heterologous booster against SARS-CoV-2. Nat. Commun. 2022;13(1):1–12. doi: 10.1038/s41467-022-30878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu C.-Y., Cheng C.-W., Kung C.-C., Liao K.-S., Jan J.-T., Ma C., et al. Glycosite-deleted mRNA of SARS-CoV-2 spike protein as a broad-spectrum vaccine. Proc. Natl. Acad. Sci. USA. 2022;119(9) doi: 10.1073/pnas.2119995119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sharma R., Kumar P., Rauf A., Chaudhary A., Prajapati P.K., Emran T.B., Gonçalves Lima C.M., Conte-Junior C.A. Mucormycosis in the COVID-19 environment: a multifaceted complication. Front. Cell. Infect. Microbiol. 2022:964. doi: 10.3389/fcimb.2022.937481. [DOI] [PMC free article] [PubMed] [Google Scholar]