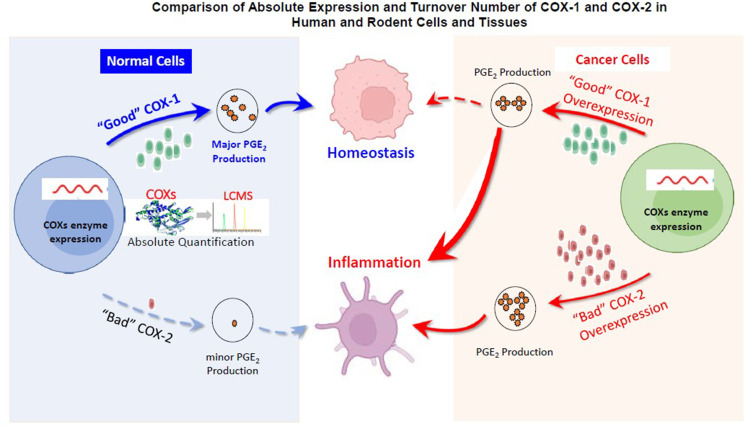
Abstract
Objective
We aim to quantify the absolute protein expression of cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) in various cells and tissues to determine the relative contribution of COX-1 and COX-2 to PGE2 production.
Methods
An LC-MS method was developed and validated, then used for quantifying the absolute amounts of COX-1 and COX-2 in recombinant human COX-1 and COX-2, lysates from different cells, tissue microsomes of rodents and humans, Pirc rat colonic polyps, and biopsy specimens from squamous cell carcinoma (SCC) patients. The COX-1 and COX-2 turnover numbers were subsequently calculated based on apparent formation rates of PGE2.
Results
A robust LC-MS method for quantification of COX-1 and COX-2 was developed and validated and then used to calculate their apparent turnover numbers. The results showed that COX-1 expression levels were much higher than that of COX-2 in all the tested tissues including the colonic epithelium of F344 (28-fold) and Pirc rats (20-fold), colonic polyps of Pirc rats (8-fold), and biopsy specimens of SCC patients (11–17-fold). In addition, both COX-1 and COX-2 were higher in polyps when compared to adjacent mucosa of Pirc rats. The turnover number of recombinant human COX-2 was 14-fold higher than that of recombinant human COX-1. LPS stimulation increased COX-2 protein expression in three cell lines (Raw 264.7, SCC9 and EOMA) as expected but unexpectedly increased COX-1 protein expression (13.8-fold) in EOMA cells.
Conclusion
In human oral cancer tissues and cells as well as Pirc rat colon, COX-1 plays an unexpectedly but more important role than COX-2 in abnormal PGE2 production since COX-1 expression is much higher than COX-2. In addition, COX-1 expression levels are inducible in cells, and higher in polyps than surrounding mucosa in Pirc rat colon. These results indicate that targeted suppression of local COX-1 should be considered to reduce colon-specific PGE2-mediated inflammation.
Keywords: cyclooxygenase-1, cyclooxygenase-2, turnover number, inflammation, absolute quantification, liquid chromatography with tandem mass spectrometry
Graphical Abstract
Background
Cyclooxygenase isoenzymes (COX-1 and COX-2) convert arachidonic acid, which is mainly present in the cell membrane in the form of phospholipids, into prostaglandins (PG) and thromboxane. The inflammatory mediator prostaglandin E2 (PGE2), as one of major products of COX enzymes, exerts multifunctional effects on the processes of inflammation, pain signaling and carcinogenesis.1–3 Physiological levels of PGE2 are mainly maintained by COX-1 in most of the tissues,4–6 and are important for housekeeping functions such as protection of the mucosal integrity, promotion of wound healing, and attenuation of the inflammatory responses during the repair process.7–9 On the contrary, aberrantly elevated level of PGE2, which are usually detected in tissues of proliferative diseases, such as tumors/cancer,10 arthritis11 and fibrosis,12 is presumed to be caused by induced COX-2.4,13 Therefore, it is generally believed that COX-2 is “bad” for our health and COX-1 is “good” for our health, especially in the GI tract.14 Is this really the case?
Evidence in support of COX-2 being “bad” for our health in the GI tract is that COX-2 is upregulated during both inflammation and tumorigenesis. A clinical trial conducted by Pfizer has shown that celecoxib, a selective COX-2 inhibitor, significantly suppressed inflammation and reduced the number of adenomatous colorectal polyps in familial adenomatous polyposis (FAP).15,16 Several studies also found that celecoxib treatment significantly reduced the tumor multiplicity in the colon in Pirc rats (Pirc, F344/NTac-Apcam1137), which has similar biology that closely models human FAP characteristics.17,18 However, it has been proven in humans that systemic inhibition of COX-2 for a prolonged period of time caused significant and sometimes deadly adverse effects because COX-2 is constructively expressed in some tissues including the cardiovascular system.19,20
Evidence in support of COX-1 being “good” for our health is that PGE2 derived from COX-1 is mainly involved in homeostatic functions throughout the body.21 Several studies have detected a stable COX-1 expression level in biopsies of fresh human blood vessels using molecular biology techniques, indicating that COX-1 but not COX-2 is the primary cyclooxygenase isoenzyme in most native endothelial cells.22 However, more and more evidences demonstrated that COX-1 is also inducible during inflammatory response.4,23 PGE2 derived from COX-1 is now thought to drive the initial phase of inflammation, with COX-2 upregulation occurring within several hours.24 The relative levels of each isoform in a particular tissue may be the dominant factor affecting the contribution of COX-1 and -2 to inflammation.25 In addition, preclinical studies have shown that selectively inhibiting COX-1 using COX-1 inhibitors such as SC560 and mofezolac can prevent tumor growth.26,27
To accurately evaluate the role of COX-1 and COX-2 in physiological and pathological conditions, their PGE2 production (ie, enzyme turnover numbers) need to be precisely determined. However, it is impossible to determine the production of PGE2 by COX proteins due to lack to analytical tools. Most of the current semi-quantification methods can only quantify relative expression of COX-1 and COX-2. For example, PCR or RT-PCR can only compare the mRNA (transcription) level of each COX28,29 and Western blot can semi-quantitatively measure the relative protein expressions, but it is done with different antibodies for COX-1 and COX-2. Currently, LC-MS/MS methodology has been widely used in proteomics studies helping with protein characterization, protein absolute quantification, biomarker discovery, etc.30,31 All these lead us to develop a label-free LC-MS method using MRM approach to absolutely quantify COX-1 and COX-2 proteins, which enables accurate comparison across tissues and labs without any additional normalization.32 Furthermore, PGE2 production by COX-1 and COX-2 can be determined by calculating protein turnover numbers.33
The present study was designed to develop and validate a novel isotope-free LC-MS method to absolutely quantify the expression levels of COX-1 and COX-2 in patients’ specimens and different biological matrices, which enables us to calculate the turnover numbers of COX-1 and COX-2 and determine their contribution to inflammation.
Materials and Methods
Chemicals and Reagents
Acetonitrile, ethyl acetate, ammonium hydroxide and water (LC-MS grade) were purchased from EMD (Gibbstown, NJ). Lipopolysaccharide (LPS), ammonium bicarbonate, dithiothreitol, iodoacetamide, trypsin (LCMS grade) and formic acid were bought from Sigma-Aldrich Co. (St. Louis, MO). RIPA buffer was obtained from Cell Signaling (Danvers, MA). Signature peptides shown in Supplementary Table 1 were synthesized by Thermo Scientific (Rockford, IL, purity >95%). Recombinant human COX-1 and COX-2 enzymes were bought from Cayman (Ann Arbor, MI). Pierce™ BCA Protein Assay Kit was supplied by Thermo Scientific (Rockford, IL). Other chemicals were used as received.
Design Surrogate Peptides for Rodent and Human COX Enzymes
The amino acid sequence of the COX enzymes were obtained from National Center for Biotechnology Information. The surrogate peptides were selected using Skyline. The specificity and similarity of each signature peptide were assured by Basic Local Alignment Search Tool (Blast) search in the UniProt database.34 Peptide FDPELLFR (Pep-1), NVPIAVQAVAK (Pep-2), FDPELLFGVQFQYR (Pep-3), and GFWNVVNNIPFLR (Pep-4) used as the surrogate peptides for rodent and human COX-1 and COX-2, respectively (Supplementary Table 1). The three universal tryptic peptides from yeast (Hi3) – NNPVLIGEPGVGK (Pep-5), AIDLIDEAASSIR (Pep-6) and VTDAEIAEVLAR (Pep-7) – were used as internal standards for quantification analysis. For each peptide, four MRM transitions were selected, the average of most sensitive two were used for quantification, and the other two were used for identification assurance.
Instruments and Conditions
All analyses were performed on an UHPLC-MS/MS system consisting of an ExionLC™ UHPLC system and an API 5500 Q-Trap triple quadrupole mass spectrometer (SCIEX, Foster City, CA, USA). The LC conditions were as follows: column, Waters BEH C18, 1.7µm, 50 mm × 2.1 mm (Waters, Milford, MA, USA); mobile phase A, 0.1% aqueous formic acid (FA); mobile phase B, 0.1% FA in acetonitrile; autosampler temperature, 10°C; column temperature, 30°C; injection volume, 10µL; the gradient elution was: 0–7.0min, 10–40%B; 7.0–7.3min, 40–90%B; 7.3–8.0min, 90%B; 8.0–8.5min, 90–10%B; 8.5–10min, 10%B. The peptides were analyzed using multiple reaction monitoring (MRM) scan type in positive mode. The electrospray ionization source conditions were as follows: spray voltage, 5500V; ion source temperature 500°C; curtain gas 30 psi; gas1, 40 psi; and gas 2, 30 psi. The compound-dependent parameters for these peptides and internal standards are listed in Supplementary Table 2. Data were processed using SCIEX OS Software Package (AB SCIEX).
Cell Culture and Lysate Preparation
Cell Culture and Treatment
Raw264.7 cells (macrophages), EOMA cells (endothelial), SCC9 cells (oral cancer) and Caco-2 cells (colon cancer) were selected to investigate the expression of COX enzymes, because oral cancer and colon cancer tissues are known to express high levels of COX-2.35,36 Caco-2 TC7 cells, originally purchased from American Type Culture Collection (ATCC), were kindly provided by Monique Rousset of INSERM U178 (Villejuif, France) and maintained in our lab.37 EOMA cells were kindly provided by Dr Yang Zhang of University of Houston (Houston, TX) and maintained in our lab. Raw264.7 cells were purchased from ATCC and maintained in our lab. SCC9 cells, originally purchased from ATCC, was gifted by Dr Ming You of Medical College of Wisconsin (Milwaukee, WI) and maintained in our lab. The use of various cells in these experiments are approved by University of Houston’s Institutional Biosafety Committee. All cells were cultured in T75-flasks in Dulbecco’s Modified Eagle’s Medium containing 1% L-glutamine, nonessential amino acids and penicillin and 10% fetal bovine serum. For LPS treatment, when cells were reaching 80% confluence, 1 μg/mL LPS was added into the cell culture medium after washing twice with PBS. The incubation time was 8h, 18h and 24h for Raw264.7 cells. The other cells were treated with LPS for 24h. To investigate the inhibition efficacy of celecoxib on Raw264.7 cells, 1 μg/mL LPS and celecoxib at 0.1, 1.0 and 10 μM were added into the cell culture medium and incubated for 8h.
Preparation of Cell Lysate
When subconfluent, cells were washed thrice with PBS before harvest. Cell pellets were lysed with RIPA buffer (20 mM Tris-HCl, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 µg/mL leupeptin) containing protease inhibitor 1mM PMSF immediately before use. Cell suspensions were kept on ice for 10 min followed by briefly sonication. The supernatants were collected and stored after being centrifuged at 14,000 × rpm for 15 minutes at 4°C. The protein concentration was measured by the Pierce BCA Protein Assay Kit using the protocol provided by the vendor.
Animals and Human Samples
Animals
Male F344 rats were originally purchased from Charles River and bred in the animal facility at Universityof Houston (Houston, TX). Pirc rats were kindly gifted by Dr Elizabeth Bryda of University of Missouri (Columbia, MO) and bred in the animal facility at University of Houston (Houston, TX). This study was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Houston, Houston, TX, under protocol number 17–022, and in compliance with the “PHS Policy on Humane Care and Use of Laboratory Animals” established by the National Institutes of Health, Bethesda, MD, USA. Animals were housed in a controlled condition, with temperature of 24 ± 2°C, humidity of 50 ± 5% and a 12-h light/dark cycle.
Biopsy Specimens from Squamous Cell Carcinoma Patients
Patients were consented under the approved Institutional Review Board protocol PRO00017015 at the Medical College of Wisconsin (MCW) for tumor banking in the MCW Institutional Tissue Bank. All patients were diagnosed with upper aerodigestive tract squamous cell carcinoma. Biopsy specimens were collected from cervical metastatic adenopathy of four patients during four separate surgeries. For each case, tumor tissue was embedded in optimal cutting temperature compound and cryopreserved by the MCW Tissue Bank at −80°C. Specimens were shipped with dry ice, using an overnight service. Upon receipt, tissue blocks were stored at −80°C until analysis.
Tissue Microsomes Preparation and Protein Digestion
Preparation of Tissue Microsomes
Tissue microsomes were prepared as described previously. Briefly, tissue samples were isolated, washed with cold saline, solution A (8 mM KH2PO4, 5.6 mM Na2HPO4, 1.5 mM KCl and 96 mM NaCl) and solution B (8 mM KH2PO4, 5.6 mM Na2HPO4,1.5 mM EDTA), and then minced. The minced tissues were homogenized with the ice-cold homogenization buffer (10 mM KPI, 250 mM sucrose, 1 mM EDTA, pH 7.4) followed by centrifugation at 10,400 rpm for 15min 4°C to collect S9 fractions. Then, S9 fractions was ultracentrifuged at 35,000 rpm for 1hr at 4°C. The final microsomes were suspended in 250 mM sucrose solution and stored at −80 °C until use. The protein concentration was measured by Pierce BCA Protein Assay Kit.
Protein Digestion and Sample Preparation
Aliquots of 120 μg proteins from cell lysate or 360 μg tissue microsomes were digested following the previous protocol with minor modification.38 Briefly, 120 μg cell lysate or 360 μg tissue microsomes were denatured and reduced through heating at 95°C for 10 min after mixing with 66 µL of 50 mM ammonium bicarbonate containing 5 mM DTT. Protein alkylation was conducted by spiking 10 µL of 10 mM IAA to the mixture and incubate for 30 min in the dark. The whole mixture was then digested by adding trypsin at a protein-to-trypsin ratio of 50:1 and incubated at 37°C for 2h. The reaction was quenched by adding 0.4%TFA. Samples were followed by centrifugation at 15,000 rpm for 15min. The supernatant was transferred and dried under nitrogen at room temperature. Samples were finally dissolved in 100 µL reconstitution solvent (FA:acetonitrile:water = 0.1:30:70, v/v/v) for LC-MS injection.
Method Validation
The newly developed LC-MS method was fully validated by determining specificity, accuracy, precision, matrix and recovery following the bioanalytical method validation guidelines for pharmaceutical industry released by USFDA. Data for method validation are reported in the Supporting Information.
Western Blot
Protein samples from Raw264.7 cell lysate with or without LPS treatment were subjected to 10% SDS-PAGE and blotted onto the nitrocellulose membrane. The membrane was blocked in 5% non-fat milk in PBST (Phosphate-Buffered Saline, 0.1% Tween 20 Detergent) overnight and probed using primary antibody for COX-2 at a dilution of 1:1000. Subsequently, the band visualization was achieved by incubating with appropriate horseradish peroxidase–conjugated secondary antibody and chemiluminescence agents. The signal was detected by ChemiDoc MP Imaging System from Bio-Rad. Image digitization and quantification were done with Image Lab software.
PGE2 Quantification
The PGE2 was quantitated using the validated method published by us previously with modification.18 Briefly, a 50 μL aliquot of cell medium and 10 µL d4-PGE2 (as the internal standard, final concentration was 20ng/mL) were mixed with 500 μL of ethyl acetate for liquid–liquid extraction. Then, 400 µL supernatant was transferred from the mixture after vortex and centrifugation. The supernatant was dried under the condensed air, and the residue was reconstituted with 100 µL co-solvent (acetonitrile:0.2%ammonium hydroxide water = 2:8, v/v). The supernatant (5 µL) was injected to UHPLC-MS/MS for PGE2 quantification after being vortexed and centrifuged.
Determination of Catalytic Activities of COX-1 and COX-2 Enzymes
The assay was performed as described previously.39 Briefly, Raw264.7 cell lysate was collected after LPS stimulation for 8h and 18h to afford cell lysates. Then, the cell lysates and human recombinant COX-1 and COX-2 enzymes were pre-incubated with 1µM hematin, 5mM l-glutathione, 5mM dopamine hydrochloride and 5 mM EDTA in 50mM potassium phosphate buffer (pH 8.0) at 37°C for 15 min. Then, substrate 10µM arachidonic acid was added into the reaction system and incubated for at 37°C for 10 min. The reaction was stopped by acetonitrile including 1% formic acid and 100 ng/mL d4-PGE2 as the internal standard. The final pH of the reaction system was adjusted using 0.5M NaOH. After centrifugation at 15,000 rpm for 15 min, the supernatant was injected to MS for PGE2 quantification. The turnover number was calculated using the following formula:
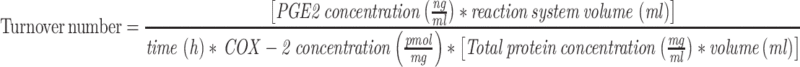
Statistical Analysis
The Pearson’s correlation analyses between absolute COX-2 amount and PGE2 production, absolute COX-2 amount, and WB results were performed on GraphPad Prism 8.0. Pearson’s correlation coefficient (R2) was used to describe how strong the correlation was between two variables.
Results
Development of LC-MS Method for COX-1 and COX-2 Quantification
An isotope-label-free LC-MS method was successfully developed by us for the first time, which provides absolute quantitation of COXs in different biological samples. The data of the method development are reported in Supplementary Data. A representative chromatogram was shown in Supplementary Figure 1 and peptide sequence was listed in Supplementary Table 1. The retention time of Pep-1, Pep-2, Pep-3 and Pep-4 were 4.90, 2.60, 5.80 and 6.50 min, respectively.
Method Validation
Linearity, Specificity, and Lower Limit of the Quantification (LLOQ)
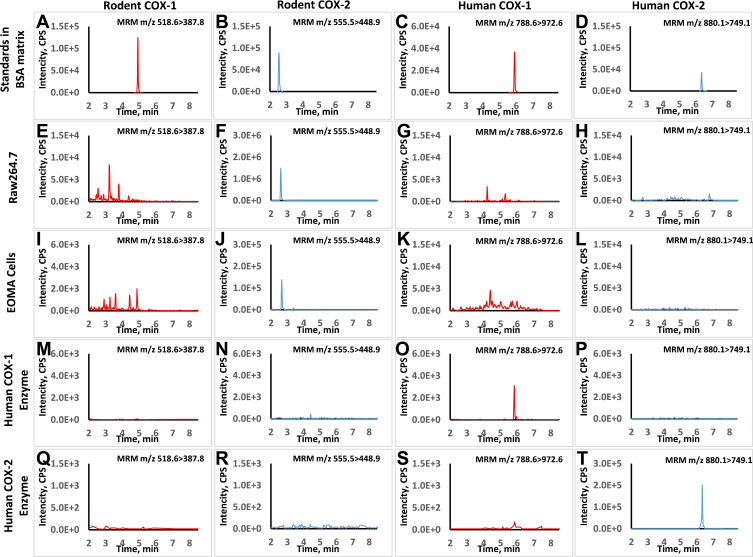
The standard curves of the surrogate peptides were linear from 0.050 to 25.00 nM for Pep-1 and Pep-2, and from 0.20 to 100.00 nM for Pep-3 and Pep-4. The calibration curves presented satisfactory linearity with correlation coefficients (R2) from 0.995 to 0.999 for all selected peptides. There was no interference (S/N >10) observed for these peptides in biological samples as shown in Figure 1. The lower limits of quantification (LLOQ) were 0.05 nM for both Pep-1 and Pep-2 and 0.2 nM for both Pep-3 and Pep-4. The limits of determination (LOD) were 0.024 nM for Pep-1, 0.012 nM for Pep-2, 0.1 nM for Pep-3 and 0.05 nM for Pep-4, indicating high sensitivity for the developed method.
Figure 1.
Typical chromatograms for LC-MS method specificity and selectivity. The retention times of representative peptides for rat COXs and human COXs were at 4.9, 2.6, 5.8 and 6.5 min, respectively (A–D). For Raw264.7 cells with LPS treatment, signal was only detected in rodent COX-2 MRM transition while it was below the detection limit in rodent COX-1 transition (E and F). No interference was found in human COX-1 and COX-2 transition (G and H). For EOMA cells with LPS treatment, signal was detected in rodent COXs’ transitions but not interfered in human COXs’ transitions (I–L). Similarly, the signal was only detected in human COXs’ transitions for the human recombinant enzymes samples and no background interference was found in rodent COXs’ transitions (M–T).
Precision and Accuracy
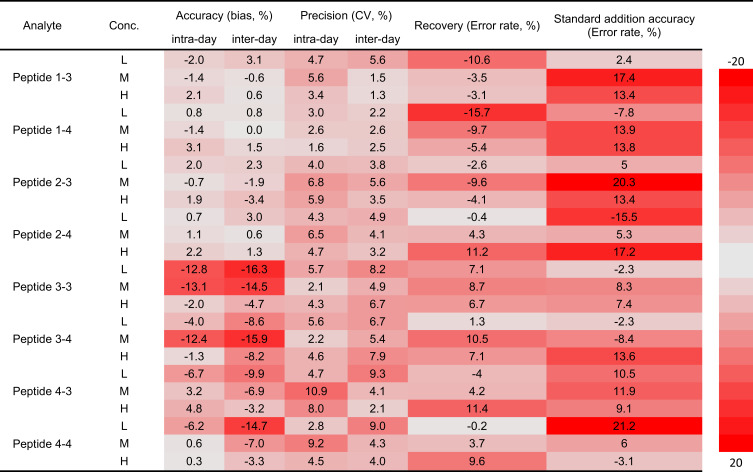
The intra-day and inter-day precision were evaluated by determining the QC samples at low, med and high concentrations of six replicates. The bias for inter-day precisions of four peptides were less than 13.05% at three different concentrations. The intra-day precisions bias was less than 16.30%. The intra-day and inter-day accuracy were with ±20% for all analytes at different QC concentration levels. The results of precision and accuracy are listed in Figure 2 and Supplementary Table 3.
Figure 2.
LC-MS method validation results in the heatmap. The newly developed LC-MS method was fully validated following the bioanalytical method validation guidelines for pharmaceutical industry released by USFDA. All inter- and intra-day accuracy and precision, recovery and standard addition accuracy were within acceptable range. Error rate = Accuracy - 100%.
Recovery and Matrix Effect
The recovery was calculated using QC samples of six replicates at three different concentrations as listed in Figure 2 and Supplementary Table 4. The recoveries of all peptides were within ± 20% range at all concentrations, showing that the recoveries of those peptides were acceptable during the sample preparation. As for the matrix effect, the two sets of standard curves with the BSA and blank raw cell lysate were compared (Supplementary Figure 2), suggesting that matrix effect is in the recommended acceptable range.40
External Standard Addition
The accuracy obtained via external standard addition method of Raw264.7 cell lysate and recombinant human COX-1 and COX-2 enzymes was calculated using three replicates at three different concentrations as listed in Figure 2 and Supplementary Table 5. The accuracy ranged from 79% to 121% of Pep-1, Pep-2, Pep-3 and Pep-4, respectively.
Absolute Quantification of COX-1 and COX-2 Protein Levels in Various Matrices
Without LPS treatment, COX-1 levels were below LLOQ in Raw 264.7, SCC9, EOMA and Caco-2 cells. COX-2 level was only measurable in EOMA cells at 4.15 pmol/mg protein but not in other three cell lines. After induction with LPS at 1 µg/mL for 24h, COX-1 level in EOMA cells was increased to 0.58 pmol/mg, but it was still below LLOQ in other three cell lines. However, COX-2 levels were significantly increased with LPS stimulation. As shown in Table 1, the COX-2 levels were 50.89, 0.09 and 10.91 pmol/mg for Raw264.7, SCC9 and EOMA cells, respectively. Caco-2 cells were not responsive to LPS treatment at 1 µg/mL and 20 µg/mL. For the human recombinant COX-1 or COX-2 enzymes, COX-1 concentration was 356.35 pmol/mg in human recombinant COX-1 enzyme while COX-2 concentration was 3037.68 pmol/mg in human recombinant COX-2 enzyme. For human tissues, both COX-1 and COX-2 levels were below LLOQ in human colon and small intestinal microsomes. COX-2 Level was also below LLOQ in biopsy specimens from the four patients with upper aerodigestive tract squamous cell carcinoma, while COX-1 level was about 0.66–1.01 pmol/mg in those specimens. For animal microsomes, COX-1 level was 0.48 pmol/mg in colon microsomes, while the COX-2 level was 0.023 pmol/mg in Pirc rat colon microsome. COX-1 level was 0.84 pmol/mg in colon microsomes, while the COX-2 level was 0.091 pmol/mg in the Pirc rat polyp microsomes. All these results are listed in Table 1 and Supplementary Figure 3.
Table 1.
COX-1, COX-2 and PGE2 Levels in Different Matrices.
| Biological Samples | Source | LPS | Amount of COX-1 (pmol/mg) | Amount of COX-2 (pmol/mg) | Concentration of PGE2 (ng/mL) |
|---|---|---|---|---|---|
| Human COX-1 enzyme | Human | — | 356.35 ± 25.99 | <0.17 | — |
| Human COX-2 enzyme | — | <0.17 | 3037.68 ± 469.80 | — | |
| SCC Patient Specimen #1 | Human | — | 0.656 | <0.056 | 33.72 |
| SCC Patient Specimen #2 | — | 1.011 | <0.056 | 25.96 | |
| SCC Patient Specimen #3 | — | 0.736 | <0.056 | 32.06 | |
| Human SI microsomes | Human | — | <0.056 | <0.056 | — |
| Human colon microsomes | — | <0.056 | <0.056 | — | |
| SCC9 cells | Human | No | <0.083 | <0.083 | 1.03 ± 0.08 |
| SCC9 cells | Yes | <0.083 | 0.090 ± 0.009 | 5.48 ± 0.21 | |
| Caco-2 | Human | No | <0.083 | <0.083 | <0.050 |
| Caco-2 | Yes | <0.083 | <0.083 | <0.050 | |
| Pirc polyps microsomes | Rat | — | 0.84 ± 0.12 | 0.091 ± 0.075 | — |
| Pirc colon microsomes | — | 0.48 ± 0.22 | 0.023 ± 0.001 | — | |
| F344 colon microsomes | — | 0.41 | <0.014 | — | |
| Raw264.7 cells | Mouse | No | <0.042 | <0.042 | <0.050 |
| Raw264.7 cells | Yes | <0.042 | 50.89 ± 1.23 | 12.50 ± 0.61 | |
| EOMA cells | Mouse | No | <0.042 | 4.15 ± 0.04 | 6.30 ± 0.40 |
| EOMA cells | Yes | 0.58 ± 0.10 | 10.91 ± 0.63 | 14.30 ± 0.17 |
Notes: In cell lysate, LLOQ of rodent and human COXs was 0.042pmol/mg and 0.083pmol/mg, respectively. In tissue microsome, LLOQ of rat and human COXs was 0.014pmol/mg and 0.056pmol/mg, respectively. In recombinant enzyme, LLOQ of human COXs was 0.17pmol/mg. LLOQ of PGE2 in cell culture medium was 0.050 ng/mL.
Catalytic Activity of COXs Enzyme in Raw264.7 Cell Lysates and Recombinant Human Enzymes
In the Raw264.7 cells, only COX-2 was detected after LPS stimulation as shown in Table 1, which allowed us to calculate the apparent COX-2 turnover numbers using the equation described above. After 8h of LPS stimulation, the turnover number was 48.57 ± 2.03 ng·h−1·pmol−1, which equals to 48.57 ng PGE2 production by per picomole COX-2 enzyme in 1h. The relative turnover number was about 30.77 ± 3.96 ng·h−1·pmol−1 for Raw264.7 cells after 18h LPS stimulation, which was approximately 35% decreased when compared to 8h stimulation. For human recombinant enzymes, the relative turnover numbers of COX-1 and COX-2 enzymes were 11.19 ± 1.01 ng·h−1·pmol−1 and 162.26 ± 3.21 ng·h−1·pmol−1, respectively (Table 2).
Table 2.
Relative Turnover Numbers of COX-1 and COX-2 Enzymes.
| Biological Samples | Treatment | Turnover Number (ng·h−1·pmol−1) |
|---|---|---|
| Human COX-2 | – | 162.26 ± 3.21 |
| Human COX-1 | – | 11.19 ± 1.01 |
| Mouse COX-2 | LPS 8h | 48.57 ± 2.03 |
| Mouse COX-2 | LPS18h | 30.77 ± 3.96 |
Correlation Between UHPLC-MS/MS Quantification and Western Blot
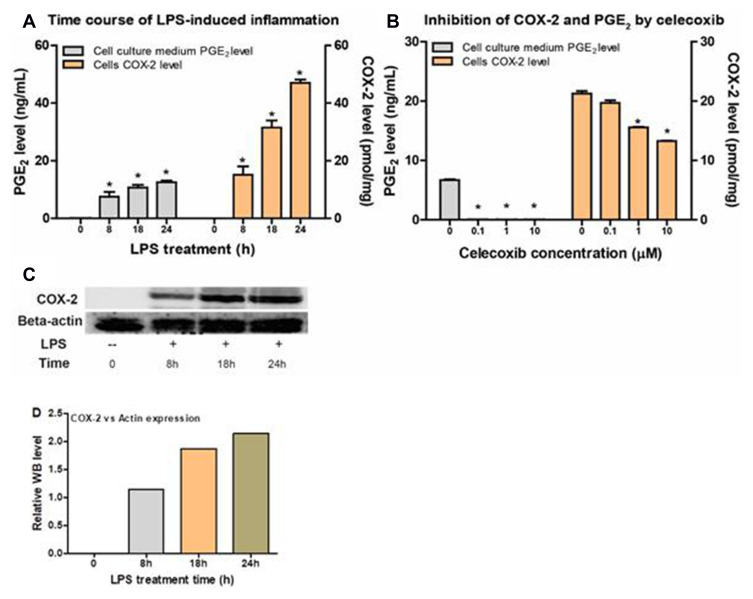
Western blotting was conducted using the Raw264.7 cells with LPS stimulation to correlate the results with those from the newly developed LC-MS method. As shown in Figure 3A, COX-2 expression was below the LLOQ without LPS treatment. Whereas the COX-2 level was significantly elevated with LPS treatment for 8h, and then gradually increased with LPS treatment for 18h and 24h. The basal levels of COX-2 expression were then confirmed by WB analysis as shown in Figure 3C. The Pearson’s correlation analysis between COX-2 expression level from WB and LCMS was performed on GraphPad Prism 8.0. The Pearson’s coefficient (R2) was 0.94, indicating a strong correlation between the two quantification methods of Western blot and LC-MS.
Figure 3.
The correlation between PGE2 levels and COX-2 levels from Raw264.7 cells with or without LPS induction and celecoxib inhibition. (A) PGE2 production and COX-2 expression levels in Raw264.7 cells in the absence or presence of LPS for 8h, 18h and 24h. (B) PGE2 production and COX-2 expression levels in the presence of celecoxib at different concentrations (0, 0.1, 1, and 10 µM). (C) Western blot results of Raw264.7 cells with LPS stimulation at indicated time (8h, 18h and 24h). (D) WB statistical results for relative COX-2 expression level in Raw264.7 cells with LPS treatment.
Correlation Between COX-2 Expression and PGE2 Level
The COX-1 and COX-2 protein amount in the cell lysates and PGE2 level in the cell culture medium of Raw264.7 were used to determine the correlation between proteins and PGE2 levels. As shown in Figure 3A, the highest PGE2 level was found in Raw264.7 cell culture media in the presence of LPS for 24h, while the PGE2 level was less than without LPS treatment. The Pearson’s correlation analysis between PGE2 levels in Raw264.7 cell culture media and the corresponding COX-2 levels from the same batch of cells was performed on GraphPad Prism 8.0. The Pearson’s coefficient (R2) was 0.935, P<0.01, indicating the correlation was significant between PGE2 production and COX-2 protein levels measured via LC-MS method. A linear regression analysis of the same data also generated R2= and p<0.01.
Impact of COX-2 Inhibitors Celecoxib on COX-2 Expression and PGE2 Production
When Raw264.7 cells were incubated with celecoxib at 0.1, 1.0 and 10 µM, both PGE2 production and COX-2 expression levels were significantly decreased. PGE2 production was almost 100% inhibited at 0.1 µM celecoxib, while COX-2 protein expression level was comparable to that in the control group. However, the decrease in COX-2 levels was dose-dependent in the presence of celecoxib at 1.0 µM and 10 µM. As shown in Figure 3B, the COX-2 expression was decreased 29% and 39% in the presence of celecoxib at 1.0 µM and 10 µM.
Discussion
We developed an approach to determine the relative contribution of COX-1 and COX-2 to the production of PGE2 in inflammation in different tissues and cells with the aid of the newly developed and validated LC-MS method that can quantify absolute amount of COX-1 and COX-2 in biological samples. We also found that both COX-1 and COX-2 are inducible in cells and Pirc rats as a result of inflammation.
We found that both COX-1 and COX-2 are inducible during inflammation, which is supported by the results that both COX-1 and COX-2 absolute amounts in acute and chronic inflammation in different cell lines and Pirc rat polyps tissues were upregulated (Table 1). This result is contrasted with the general belief that COX-1 is constructive and a “good” enzyme but COX-2 is induced and a “bad” enzyme. Nevertheless, evidences have shown that non-selective COXs inhibitors, such as sulindac and aspirin, can significantly reduce the number of colon tumors41,42 and this tumor-prevention efficacy may be a benefit of both COX-1 and COX-2 inhibition. Of note, the complex characteristics of COX-1 and COX-2 expression and activity during different physiological and pathological conditions never turn out to be simply regulated.
In the in vitro cell model, a 3- to 50-fold increment in COX-2 expression was found in different cell lines after LPS stimulation while COX-1 expression increment was only found in EOMA cells (Table 1 and Figure 3), suggesting COX-2 was much more sensitive to stimulation than COX-1 in response to acute inflammation. This was consistent with the findings in Pirc rats, where a 75% increment for COX-1 but a 295% increment for COX-2 in colonic inflamed polyps were detected when compared to those in the adjacent mucosa (Table 1).
To understand the contribution for PGE2 production of each enzyme, the in vitro PGE2 production assay was performed. The results showed that the production activity of COX-2 is 14-fold higher than that of COX-1 (Table 2). However, the absolute amount of COX-1 expression was significantly higher (9–20 folds) than that of COX-2 in different matrices (Table 1) and the contribution of COX-1 should not be neglected in inflammation. It is highly possible that both COX-1 and COX-2 play important roles in facilitating polyposis growth in Pirc rats. This conclusion can explain why celecoxib can significantly suppress the mucosal PGE2 production but can only slightly suppress PGE2 production in the polyps, where both COX-1 and COX-2 were upregulated.43 Thus, we can conclude that both COX-1 and COX-2 are involved in the chronic inflammatory process in Pirc rat.
An intriguing question is whether and how we should use selective or nonselective COX-2 inhibitors to gain the maximal benefit without increasing the risk of bleeding due to COX-1 inhibition. Evidences showed that COX-2 may be more essential than COX-1 for PGs production to maintain tissue homeostasis using COX-deficient mice.44 Additionally, it was reported that COX-2 is important for wound healing especially for tissue repair and regeneration facilitated by PEG2.45,46 However, our results suggest that COX-2 could also play an essential role for wound healing as equal nanogram COX-2 protein can generate 14-fold PGE2, the primary mediator of wound healing, as compared to that of the COX-1 enzyme. All this evidence reveals that the roles of COX-1 and COX-2 enzymes are very complex. In this regard, a balance between COX-2 and COX-1 inhibition to maintain PEG2 homeostasis is of great interest and importance in the treatment of inflammatory disease.
Our conclusion was based on the absolute protein measurement using a newly developed and validated LC-MS method. The absolute amount of COX-1 and COX-2 proteins were determined for the first time in the current study, which allowed us to find out that COX-1 expression was significantly higher than that COX-2 in Pirc rat mucosa and polyps. The observation of a higher expression of COX-1 than COX-2 was consistent with those of the mRNA levels in the colon cancer patients’ mucosa and colorectal neoplasia determined by RT-qPCR,23 although RT-qPCR only quantifies mRNA.47 More importantly, the accurate amounts determined using the LC-MS method enable us to calculate the turnover number of each isoenzyme, which allow us to calculate the contribution of COX-1 and COX-2 to PGE2 production.
The relative turnover numbers derived from recombinant human COX-1 and COX-2 enabled the comparison between these two enzymes. However, we were not able to calculate the exact turnover number of rat and mouse COX-1 and COX-2 proteins because the recombinant rat and mouse COXs are not commercially available. To estimate mouse turnover numbers, we used cell lysate prepared from Raw 264.7 cells after LPS stimulation, which is a standard method to selectively induce COX-2. Although mouse COX-2 turnover number was calculated using this model, the relative turnover numbers were apparent rates because COX-2 expression was stimulated by LPS dynamically and mPGES-1 expression also plays an important role in PGE2 production.
Conclusion
In human oral cancer specimen and Pirc rat polyps, COX-1 protein level was much higher than COX-2, when measured using the newly developed absolute quantification methods, affirming the important role of COX-1 in tissue production of PGE2. Furthermore, both COX-1 and COX-2 expression levels and activities were responsive to inflammatory stimulation in human and rodent cells. These results indicate that targeted suppression of local COX-1 should be considered to reduce tissue-specific PGE2 related inflammation.
Acknowledgment
This work is supported by National cancer Institute (R01CA246209) and Cancer Prevention and Research Institute of Texas (RP180863) for Dr Ming Hu, and National Institute of General Medical Sciences (1R15GM126475-01A1) and National cancer Institute (1R03CA212937-01) for Dr. Song Gao.
Abbreviations
COX-1, cyclooxygenase-1; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; FAP, familial adenomatous polyposis; Pirc, polyposis in rat colon; LPS, lipopolysaccharide; Pep-1, FDPELLFR; Pep-2, NVPIAVQAVAK; Pep-3, FDPELLFGVQFQYR; Pep-4, GFWNVVNNIPFLR; Pep-5, NNPVLIGEPGVGK; Pep-6, AIDLIDEAASSIR; Pep-7, VTDAEIAEVLAR; UHPLC-MS, liquid chromatography–mass spectrometry; MRM, multiple reaction monitoring; FA, formic acid; TFA, trifluoroacetic acid; SCC9 cells, human squamous cell carcinomas cells; EOMA cells, mouse hemangioendothelioma endothelial cells; KPI, potassium phosphate buffer; IAA, iodoacetamide; SI, small intestine.
Disclosure
Dr Ming Hu is a co-founder of Sanarentero in 2019 and reports patent for locally bioavailable drugs (US Patent No. 11202773B2). The research work here is not supported by Sanarentero. Other authors report no conflicts of interest in this work.
References
- 1.Wang D, DuBois RN. PPARdelta and PGE2 signaling pathways communicate and connect inflammation to colorectal cancer. Inflamm Cell Signal. 2014;1:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammadi A, Yaghoobi MM, Gholamhoseinian Najar A, Kalantari-Khandani B, Sharifi H, Saravani M. HSP90 Inhibition Suppresses PGE2 Production via Modulating COX-2 and 15-PGDH Expression in HT-29 Colorectal Cancer Cells. Inflammation. 2016;39:1116–1123. [DOI] [PubMed] [Google Scholar]
- 3.Echizen K, Hirose O, Maeda Y, Oshima M. Inflammation in gastric cancer: interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways. Cancer Sci. 2016;107:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zidar N, Odar K, Glavac D, Jerse M, Zupanc T, Stajer D. Cyclooxygenase in normal human tissues–is COX‐1 really a constitutive isoform, and COX‐2 an inducible isoform? J Cell Mol Med. 2009;13:3753–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50:S423–S428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patrono C, Patrignani P, Rodríguez LAG. Cyclooxygenase-selective inhibition of prostanoid formation: transducing biochemical selectivity into clinical read-outs. J Clin Invest. 2001;108:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenson WF. Prostaglandins and epithelial response to injury. Curr Opin Gastroenterol. 2007;23:107–110. [DOI] [PubMed] [Google Scholar]
- 8.Kämpfer H, Schmidt R, Geisslinger G, Pfeilschifter J, Frank S. Wound inflammation in diabetic ob/ob mice: functional coupling of prostaglandin biosynthesis to cyclooxygenase-1 activity in diabetes-impaired wound healing. Diabetes. 2005;54:1543–1551. [DOI] [PubMed] [Google Scholar]
- 9.Shukla SK, Sharma AK, Gupta V, Yashavarddhan M. Pharmacological control of inflammation in wound healing. J Tissue Viability. 2019;28:218–222. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, DuBois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, Gregory SA. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest. 1996;97:2672–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Y, Li H, Yue Z, et al. Cryptotanshinone attenuates cardiac fibrosis via downregulation of COX-2, NOX-2, and NOX-4. J Cardiovasc Pharmacol. 2014;64:28–37. [DOI] [PubMed] [Google Scholar]
- 13.Fujita T, Matsui M, Takaku K, et al. Size-and invasion-dependent increase in cyclooxygenase 2 levels in human colorectal carcinomas. Cancer Res. 1998;58:4823–4826. [PubMed] [Google Scholar]
- 14.Hotz-Behofsits C, Walley M, Simpson R, Bjarnason I. COX-1, COX-2 and the topical effect in NSAID-induced enteropathy. Inflammopharmacology. 2003;11:363–370. [DOI] [PubMed] [Google Scholar]
- 15.Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131:1674–1682. [DOI] [PubMed] [Google Scholar]
- 16.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Eng J Med. 2006;355:873–884. [DOI] [PubMed] [Google Scholar]
- 17.Amos-Landgraf JM, Kwong LN, Kendziorski CM, et al. A target-selected APC-mutant rat kindred enhances the modeling of familial human colon cancer. Proc Natl Acad Sci U S A. 2007;104:4036–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun C, Dashwood W-M, Kwong LN, et al. Accurate quantification of PGE2 in the polyposis in rat colon (Pirc) model by surrogate analyte-based UPLC–MS/MS. J Pharm Biomed Anal. 2018;148:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motsko SP, Rascati KL, Busti AJ, et al. Temporal relationship between use of NSAIDs, including selective COX-2 inhibitors, and cardiovascular risk. Drug Safety. 2006;29:621–632. [DOI] [PubMed] [Google Scholar]
- 20.Trepanier CH, Milgram NW. Neuroinflammation in Alzheimer’s disease: are NSAIDs and selective COX-2 inhibitors the next line of therapy? J Alzheimer’s Dis. 2010;21:1089–1099. [DOI] [PubMed] [Google Scholar]
- 21.Adelizzi RA. COX-1 and COX-2 in health and disease. J Am Osteopath Assoc. 1999;99:S7–12. [PubMed] [Google Scholar]
- 22.Vanhoutte PM. COX-1 and vascular disease. Clin Pharmacol Ther. 2009;86:212–215. [DOI] [PubMed] [Google Scholar]
- 23.Jensen TSR, Mahmood B, Damm MB, et al. Combined activity of COX-1 and COX-2 is increased in non-neoplastic colonic mucosa from colorectal neoplasia patients. BMC Gastroenterol. 2018;18:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang Y, Kim M, Hwang SW. Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. J Neuroinflammation. 2020;17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitamura T, Kawamori T, Uchiya N, et al. Inhibitory effects of mofezolac, a cyclooxygenase-1 selective inhibitor, on intestinal carcinogenesis. Carcinogenesis. 2002;23:1463–1466. [DOI] [PubMed] [Google Scholar]
- 27.Long S, Theiss KL, Mattei A, Loftin CD, Li T. Solid-State Properties of the Cyclooxygenase-1-Selective Inhibitor, SC-560. Aaps Pharmscitech. 2010;11:485–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Said N, Smith S, Sanchez-Carbayo M, Theodorescu D. Tumor endothelin-1 enhances metastatic colonization of the lung in mouse xenograft models of bladder cancer. J Clin Invest. 2011;121:132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong G, Chen H, Shi Y, Jiang C, Yang H. MicroRNA-758 Regulates Oral Squamous Cell Carcinoma via COX-2. Indian J Surgery. 2020;1–7. [Google Scholar]
- 30.Hsu J-L, Chen S-H. Stable isotope dimethyl labelling for quantitative proteomics and beyond. Philosophical Transactions Royal Soc. 2016;374:20150364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen G, Pramanik BN. Application of LC/MS to proteomics studies: current status and future prospects. Drug Discov Today. 2009;14:465–471. [DOI] [PubMed] [Google Scholar]
- 32.Fallon JK, Harbourt DE, Maleki SH, Kessler FK, Ritter JK, Smith PC. Absolute quantification of human uridine-diphosphate glucuronosyl transferase (UGT) enzyme isoforms 1A1 and 1A6 by tandem LC-MS. Drug Metab Lett. 2008;2:210–222. [DOI] [PubMed] [Google Scholar]
- 33.Xu B, Gao S, Wu B, Yin T, Hu M. Absolute quantification of UGT1A1 in various tissues and cell lines using isotope label-free UPLC–MS/MS method determines its turnover number and correlates with its glucuronidation activities. J Pharm Biomed Anal. 2014;88:180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng F, Zou L, Zhang T, et al. Using LC–MS/MS-based targeted proteomics to monitor the pattern of ABC transporters expression in the development of drug resistance. Cancer Manag Res. 2018;10:2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranelletti FO, Almadori G, Rocca B, et al. Prognostic significance of cyclooxygenase‐2 in laryngeal squamous cell carcinoma. Int j Cancer. 2001;95:343–349. [DOI] [PubMed] [Google Scholar]
- 36.Marnett LJ, DuBois RN. COX-2: a target for colon cancer prevention. Annu Rev Pharmacol Toxicol. 2002;42:55–80. [DOI] [PubMed] [Google Scholar]
- 37.Bui D, Li L, Yin T, et al. Pharmacokinetic and Metabolic Profiling of Key Active Components of Dietary Supplement Magnolia officinalis Extract for Prevention against Oral Carcinoma. J Agric Food Chem. 2020;68:6576–6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J, Zheng H, Zeng S, et al. Profiles and Gender-Specifics of UDP-Glucuronosyltransferases and Sulfotransferases Expressions in the Major Metabolic Organs of Wild-Type and Efflux Transporter Knockout FVB Mice. Mol Pharm. 2017;14:2967–2976. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Wang L, Xie L, et al. Design and Synthesis of a Novel NIR Celecoxib-Based Fluorescent Probe for Cyclooxygenase-2 Targeted Bioimaging in Tumor Cells. Molecules. 2020;1:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Resta D, Brambilla F, Arnoldi A. HPLC-Chip-Multiple Reaction Monitoring (MRM) method for the label-free absolute quantification of γ-conglutin in lupin: proteotypic peptides and standard addition method. Food Chem. 2012;131:126–133. [Google Scholar]
- 41.Femia AP, Soares PV, Luceri C, Lodovici M, Giannini A, Caderni G. Sulindac, 3, 3’-diindolylmethane and curcumin reduce carcinogenesis in the Pirc rat, an APC-driven model of colon carcinogenesis. BMC Cancer. 2015;15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu M, Nishihara R, Chen Y, et al. Aspirin exerts high anti-cancer activity in PIK3CA-mutant colon cancer cells. Oncotarget. 2017;8:87379–87389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yun C, Dashwood W-M, Li L, et al. Acute changes in colonic PGE 2 levels as a biomarker of efficacy after treatment of the Pirc (F344/NTac-APC am1137) rat with celecoxib. Inflammation Res. 2020;69:131–137. [DOI] [PubMed] [Google Scholar]
- 44.Loftin CD, Tiano HF, Langenbach R. Phenotypes of the COX-deficient mice indicate physiological and pathophysiological roles for COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68:177–185. [DOI] [PubMed] [Google Scholar]
- 45.Fairweather M, Heit YI, Buie J, et al. Celecoxib inhibits early cutaneous wound healing. J Surg Res. 2015;194:717–724. [DOI] [PubMed] [Google Scholar]
- 46.Gilman KE, Limesand KH. The complex role of prostaglandin E2-EP receptor signaling in wound healing. Am J Physiol Regul Integr Comp Physiol. 2021;320:R287–R296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bustin SA, Nolan T. Pitfalls of quantitative real-time reverse-transcription polymerase chain reaction. J Biomol Tech. 2004;15:155. [PMC free article] [PubMed] [Google Scholar]