Abstract
Background
Immunotherapy using immune checkpoint inhibitors (ICIs) is the current focus in cancer immunotherapy. However, issues are raised in the area, as the recent studies showed that such therapeutic modality suffers from low durability and low or no efficacy for patients with some tumor types including cases with non‐inflamed or cold cancers. Therefore, efforts have been made to solve the issue using immune combination therapy, such as the use of immunocytokines. The combination of ICI with interleukins (ILs) and IL‐targeting agents is now under consideration in the area of therapy, and the primary results are promising.
Purpose
The focus of this review is to discuss the possibility of using ILs and IL‐targeting drugs in combination with ICI in cancer immunotherapy and describing recent advances in the field using PEGylated ILs and fusion proteins. The key focus in this area is to reduce adverse events and to increase the efficacy and durability of such combination therapy.
Keywords: combination therapy, immune checkpoint inhibitor, interleukin, programmed death‐1 receptor, programmed death‐ligand 1
Exhaustion of CD8+ T cells is promoted by high PD‐L1 expression on immune cells within the TME. Exhaustive CD8+ T cells can retain their effector function when they are under exposure to adequate stimuli. Solo ICI shows low durability and efficacy, but its combination with an appropriate IL‐based therapy is important for reducing resistance and increasing the efficacy of therapy.
1. INTRODUCTION
Immunotherapy is a growing modality in cancer therapy. 1 The efficacy of cytokine therapy is tested in various preclinical and clinical settings. Immunocytokine therapy directly activates cells of the immune system, such as T cells and natural killer (NK) cells. NK cells, for instance, have receptors for interleukins (ILs) 2, 7, 12, 15, 18, and 21, so IL‐based therapy aiming at potentiating the activity of these cytokines may be effective for enhancing the effector function of NK cells. 2 A point is that cytokine therapy in its natural form often renders low responses. Short half‐life related to the use of cytokines hampers their therapeutic exposure and efficacy. Activation of counter‐regulatory pathways and the resultant immunosuppression is another burden reducing the efficacy of such therapy. 3
Another treatment approach is the use of immune checkpoint inhibitor (ICI) therapy, which is a recent focus in cancer immunotherapy. 4 Engagement between programmed death‐1 receptor (PD‐1) with programmed death‐ligand 1 (PD‐L1) promotes a peripheral tolerance and compromises anti‐tumor immunity. 5 Targeting interactions between PD‐1 and PD‐L1 is regarded as a breakthrough of the year 2013 in the Science Journal and is honored by Nobel Prize in Physiology and Medicine in the year 2018. Allison and Honjo started a groundbreaking work in the year 1992 in this area and used the term “brakes on T cell activation” for the activity of checkpoints in the tumor microenvironment (TME). 6 However, only a number of patients develop sustainable responses to the anti‐PD‐1/PD‐L1 therapy. 5 Microsatellite stability subtype of colorectal cancer (CRC), for instance, shows a limited response to anti‐PD‐1 therapy. 7 Pancreatic cancer shows high infiltration of immunosuppressive T cells, while prostate cancer represents limited base‐line T cell infiltration, both of which are low responsive to the ICI therapy. 8 Tumor cells are, in fact, making huge efforts to thwart the efficacy of immunotherapy. 9 The strategy of combination therapy using ILs and IL‐targeting agents along with checkpoint inhibitors is the focus of this review. Here, studies published in the area of checkpoints and immunocytokine therapy are interpreted in order to uncover more facts about the safety and efficacy of such a combination schedule in cancer immunotherapy. Finding an appropriate combination for ICI therapy in cancer patients is an urgent need of the current years. Knowledge in this area is thus being important for solving the current issues related to the ICI, particularly in patients with non‐inflamed or cold cancers.
2. IMMUNE CHECKPOINTS AND CHECKPOINT INHIBITORS
ICI therapy is an approach in cancer immunotherapy that is designed for targeting checkpoint mediators. PD‐1/PD‐L1, cytotoxic T lymphocyte‐associated antigen‐4 (CTLA‐4), and T cell immunoglobulin mucin‐3 (TIM‐3) are known checkpoints used in ICI. 10 , 11 , 12 Pembrolizumab and nivolumab are PD‐1 inhibitors, whereas avelumab, durvalumab, and atezolizumab are PD‐L1 suppressors. Ipilimumab is the inhibitor of CTLA‐4. 13
2.1. The impact of immune checkpoints on cells of tumor immune ecosystem
The immune system plays a vital role in responses from both normal and tumor cells to the therapeutic modalities. 14 Polarization toward macrophage type 2 (M2) cells is linked positively with PD‐L1 expression. 15 There is a positive relation between CD206+ TAMs with high expression of PD‐L1 in gastric cancer. 16 PD‐1 is expressed on the surface of dendritic cells (DCs), CD4+ T cells, CD8+ T cells, and NK cells. 17 Diskin and colleagues reported a link between PD‐L1 engagement on T cells with tumor immune tolerance. PD‐L1+ T cells mediate engagement of PD‐1+ macrophages and induce their polarization into an alternative M2‐like phenotype. 18 CD8+ T cells are the critical effector cells and the known front‐line defensive cells against cancer. 19 The activity of PD‐1 promotes the proliferation of regulatory T cell (Treg) and facilitates apoptosis of CD8+ T cells. 17 PD‐L1 expression on DCs is a key inhibitor of T cell responses, 20 and its deletion on DCs enhances anti‐tumor responses from CD8+ T cells and restricts tumor growth. 21 Exhaustion of CD8+ T cells is promoted by high PD‐L1 expression on myeloid‐derived suppressor cells (MDSCs). 22 , 23 The effector function of CD8+ T cells is also suppressed by PD‐L1 expression on tumor cells. 24 CD8+ T cell dysfunction occurs upon the interaction of PD‐L1 with PD‐1. 25 , 26 , 27 In tumors like hepatocellular carcinoma (HCC) the fraction of PD‐1high CD8+ T cells is increased outstandingly. PD‐1high CD8+ T cells express the inhibitory checkpoints CTLA‐4 and TIM‐3 25 and produce a low amount of IFN‐γ compared with PD‐1− cells. 28 The inhibitory role of PD‐1 on CD8+ T cells occurs not only during chronic inflammation and cancer but also at the time of acute infection 29 (Figure 1).
FIGURE 1.
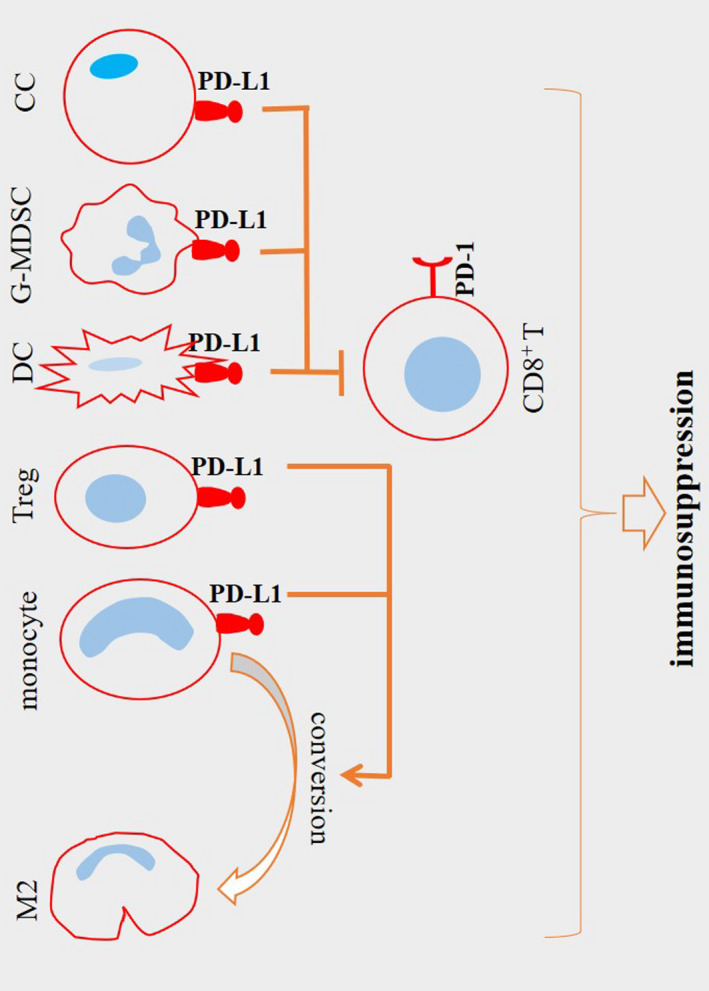
The immunosuppressive activity of programmed death‐ligand 1 (PD‐L1) in tumor microenvironment (TME). Dendritic cells (DCs) express PD‐L1 to suppress responses from CD8+ T cells. PD‐L1+ tumor cells and PD‐L1+ myeloid‐derived suppressor cells (MDSCs) suppress CD8+ T cell effector function and promote their exhaustion. PD‐L1 expression is also related to the macrophage polarization toward the pro‐tumor macrophage type 2 (M2) phenotype. This preferential polarity is stimulated by PD‐L1+ regulatory T cells (Tregs). PD‐L1 acts via interaction with a programmed death‐1 receptor (PD‐1). High expression of this receptor in a PD‐L1high TME stimulates Treg proliferation and promotes CD8+ T cell apoptosis
2.2. Burdens with solo ICI therapy
ICI has revolutionized cancer‐based therapy over the last decade, but there are still a high number of patients who do not respond well to this approach. 30 Responses to the PD‐1 inhibitor therapy are diminished in patients with low expression of checkpoints on tumor‐infiltrating lymphocytes (TILs). In fact, the proportion of checkpoint positive cytotoxic T lymphocytes (CTLs) is lower for tumors placed in the category of cold, compared to that for hot tumors. Thus, cold tumors show low or no responses to the ICI. 31
3. COMBINATION OF ICIs WITH IL THERAPY
ICI combination strategy is a promising approach for improving responses in cancer patients. 32 Cytokines are regulators of the immune system that are active for promoting recruitment of cells of the immune system into TME. They take key roles in cell signaling directed for promoting cellular growth and differentiation, as well as for directing inflammatory or anti‐inflammatory activities. Some cytokines exert potent anti‐tumor activities, 33 while there are cytokines that act for the promotion of cancer growth and tumor aggressive behavior. Due to the urgent need for controlling TME in patients receiving ICI, cytokine combination therapy with either stimulators or inhibitors is suggested in order to have more potent and durable responses. Some ILs are evaluated in serum samples for predicting responses to the ICI. Thus, assessment of ILs and their application in patients receiving ICI have virtues from both diagnostic and therapeutic viewpoints. The impact of combinatory ICI/IL therapy on cellular immunity within TME is summarized in Figure 2.
FIGURE 2.
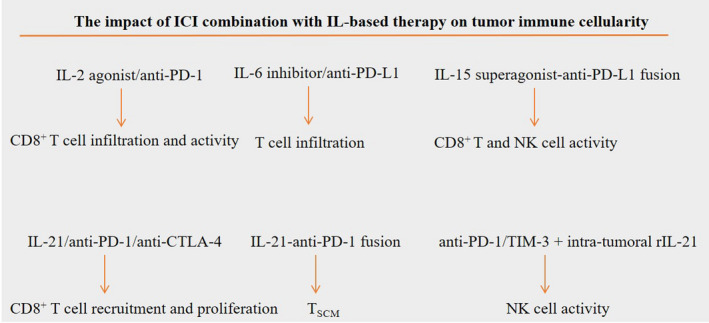
The impact of combinatory immune checkpoint inhibitor (ICI)/interleukin (IL) therapy on cellular immunity within tumor microenvironment (TME). Higher recruitment of CD8+ T cells into TME occurs upon a combination of programmed death‐1 receptor (PD‐1) inhibitors with IL‐2 agonist or IL‐6 inhibitors. Bispecific fusion proteins are promising agents in the current research in cancer immunotherapy. N‐809 is an example of such agents that encompasses a fusion complex for IL‐15 superagonist and anti‐programmed death‐ligand 1 (PD‐L1), and it can be used to promote the activity of both natural killer (NK) and CD8+ T cells. Fusion of IL‐21 to anti‐PD‐1 antibody promotes the formation of memory stem T cells (TSCMs). Effector activity of NK cells is induced by PD‐1/T cell immunoglobulin mucin‐3 (TIM‐3) blockade along with intra‐tumoral rIL‐21. Recruitment and proliferation of CD8+ T cells is also induced by IL‐21 when used in combination with anti‐PD‐1 or anti‐cytotoxic T lymphocyte‐associated antigen‐4 (CTLA‐4) antibodies
3.1. Combination with IL‐2‐based cytokine therapy
IL‐2 is a pleiotropic cytokine that acts on various types of cells of the immune system. 34 , 35 High PD‐1 expression is contributed to the CD8+ T cell anergy through down‐regulation of IL‐2 release from the cells. 36 Combination of IL‐2 with ICI is thus effective for reinvigorating CD8+ T cell responses in patients with chronic infection or cancer. 34 Combination of high‐dose IL‐2 with PD‐1/PD‐L1 blockade therapy displays an objective response rate (ORR) of 22.5% and 24% for metastatic melanoma and metastatic renal cell carcinoma (RCC), respectively. 37 Such effects are considerable when compared with monotherapy of high‐dose IL‐2 for metastatic melanoma (ORR: 13%), 38 but not for metastatic RCC (ORR: 25%). 39 Hsu and colleagues evaluated the impact of proIL‐2 therapy on tumor resistance to ICI. They noticed an increase in the fraction of PD‐L1+CD45+ cells, which helps maximizing the bondage of PD‐L1 inhibitors to PD‐L1+ cells and thereby overcoming ICI resistance. 40 Sharma and colleagues investigated the efficacy of bempegaldesleukin combination with ICI in animal tumor model and noticed the synergizing effects and the benefit of using this IL‐2 prodrug for potentiating the fraction of CD8+ T cells and cancer regression. 41 Bempegaldesleukin (also called NKTR‐214) is an IL‐2 agonist that acts preferentially on CD122 (IL‐2β receptor). The impact of bempegaldesleukin combination with nivolumab on T cells and clinical responses to such therapy has been investigated recently for a number of advanced solid tumors including melanoma, RCC, and non‐small cell lung cancer (NSCLC). Total ORR for such combination therapy was 59.5%, which is outstanding. Cellular analysis showed higher infiltration and effector activity of CD8+ T cells, while no enhancement in the fraction of Tregs was reported for such therapy. 42
3.2. Combination with IL‐6 targeted therapy
IL‐6 is a pleiotropic and multi‐tasking cytokine with a pro‐ or anti‐inflammatory activity that is produced in chronic inflammatory conditions and cancer. In the context of cancer, it is contributed to tumor cell proliferation, immune escape, angiogenesis, and invasion and metastasis. 43 IL‐6 is a predictive biomarker of response to ICI. Kang and colleagues evaluated responses to the PD‐1/PD‐L1 inhibitors in NSCLC patients with different baseline IL‐6 levels. Results showed a higher rate of ORR in patients with low IL‐6 levels (a rate lower than 13.1 pg/ml). Patients with low IL‐6 levels also had longer overall survival (OS) and progression‐free survival (PFS). 44 IL‐6 secretion from macrophages is considered as predictive of weak prognosis in patients with lung cancer. This is due to the inducible effect of IL‐6 on PD‐1 expression from CD8+ T cells and the promotion of M2 polarization. IL‐6 targeting along with anti‐CTLA‐4 therapy has been found to reduce M2 cells and PD‐1+ CD8+ T cells and further improved the survival of tumor‐bearing mice. 45 There is also a link between IL‐6 deficiency with upregulation of major histocompatibility complex class I (MHC‐I) and PD‐L1 expression on tumor cells. Anti‐PD‐L1 therapy is effective for suppressing metastatic colonization of CRC only in mice negative for IL‐6 (but not in IL‐6+/+ animals). 46 Targeting IL‐6 can also be effective for enhancing the efficacy of PD‐L1 blockade therapy of HCC. 47 In addition, changes in plasma IL‐6 levels are correlated with responses from NSCLC to PD‐L1 inhibitors. This is delineated by improved PFS (11 vs. 4 months) in ICI‐treated patients. 48
PD‐L1 expression on melanoma cells is upregulated upon IL‐6 blockade. Induction of IL‐6 is also evoked by PD‐L1 blockade. Anti‐PD‐1/PD‐L1 therapy prompted the expression of IL‐6 from PD‐1+ macrophages. 49 This indicates the therapeutic benefits of using IL‐6 inhibitors in combination with PD‐1/PD‐L1 inhibitor drugs. High IL‐6 expression occurs in the stroma of pancreatic cancer. Combination of IL‐6 inhibitor with PD‐L1 blockade therapy increases T cell infiltration and enhances OS in mice with pancreatic cancer. 50 Glioblastoma is a tumor with cold immunity, thus being insensitive to ICI. Combination with IL‐6 blockade is not effective for sensitizing such tumor to ICI (anti‐PD‐1/anti‐CTLA‐4), but neutralization of IL‐6 along with stimulation of CD40 can cause tumor sensitization to ICI. In fact, the expression of CD40 is induced by IL‐6. Ablation of IL‐6 abrogated CD40 expression. Thus, recovery of CD40 using appropriate stimulators can promote anti‐tumor immunity. 51
IL‐6 inhibition can be an effective approach for managing immune‐related adverse events (irAEs) related to the ICI therapy. 52 Application of the IL‐6 inhibitor tocilizumab can be a choice for modulation of steroid‐refractory irAEs occurring secondary to the ICI therapy in cancer patients. 53 This may confer that tocilizumab therapy can be used as a substitute to the corticosteroid therapy in patients receiving ICI, as shown in the results of the case series. 54 Outcomes of a recent systematic review on multi‐center case series introduced the use of tocilizumab as a therapeutic choice for the management of irAEs occurring following ICI therapy, and the combination of tocilizumab with ICI is proposed as an option for controlling cancer progression as well as for targeting irAEs of ICI. 55
3.3. Combination with IL‐8 targeted therapy
IL‐8 (also called CXCL‐8) is a chemoattractant of myeloid cells that are generated at high levels in many types of solid cancers. IL‐8 promotes the recruitment of immunosuppressive cells, induces angiogenesis, and stimulates epithelial‐mesenchymal transition and tumor metastasis. 56 IL‐8 is considered as a biomarker of response to the ICI. In a study, melanoma and NSCLC patients were administered with pembrolizumab or nivolumab, and serum IL‐8 was evaluated during the course of therapy. It was found a link between early reduction of serum IL‐8 with prolonged OS in such cases, which is indicative of the importance of IL‐8 calculation for monitoring or predicting clinical responses to the ICI. 57 In a large retrospective study, 2000 patients with NSCLC, melanoma, and RCC treated with nivolumab therapy were enrolled, and the results showed a negative relation between elevated IL‐8 serum levels with OS. Further analysis revealed that IL‐8 at 23 pg/ml has its threshold to be considered as a response biomarker in patients who were more benefited from ICI therapy. 58 A link between IL‐8 with poor ICI responses is well‐documented in the recent study by Yuen and colleagues. Here, an inverse relation between high IL‐8 level (in tumor, plasma, or peripheral blood mononuclear cells) with atezolizumab efficacy was identified in patients with metastatic RCC and metastatic urothelial carcinoma. Myeloid cells are the main source of IL‐8, and that high expression of this cytokine is linked with the suppression of antigen presentation machinery. Therefore, outcomes are improved in patients treated with ICI after reversing the negative impact of IL‐8 on myeloid cells and antigen presentation. 59
Castration‐resistant prostate cancer is a type of tumor rarely responsive to the ICI therapy. Castration causes high expression of IL‐8, which further droves intra‐tumoral recruitment of granulocytic MDSCs (G‐MDSCs). Blockade of IL‐8 signaling in combination with ICI has been found to augment the intra‐tumoral density of CD8+ T cells and delayed the occurrence of castration resistance. 60 Results of a recent systematic review also identified IL‐8 as a poor prognostic biomarker of gastric cancer. 61 Mesenchymal stem cells (MSCs) are one of the sources of IL‐8 in tumors like gastric cancer. Sun and colleagues in a study evaluated the mechanism of PD‐L1 regulation in gastric cancer. Results showed the inducible effect of MSC‐derived IL‐8 on PD‐L1 expression from cancer cells, which resulted in tumor cell resistance to the CD8+ T cell cytotoxicity. 62 The outcomes of such a study will rationalize the application of IL‐8 inhibitors in combination with ICI for reawaking the immune system against cancer.
3.4. Combination with IL‐10‐based therapy
Pegilodecakin is the PEGylated recombinant IL‐10 that is able to induce CD8+ T cells and stimulate IFN‐γ, MHC‐I, and MHC‐II. 63 Niang and colleagues in a study evaluated responses from T cells to pegilodecakin in cancer patients. Pegilodecakin promoted CD8+ T cell expansion both within a tumor and in the systemic circulation. It also activated intra‐tumoral CD8+ T cells. Pegilodecakin combination with anti‐PD‐1 therapy resulted in more expansion of LAG‐3+ PD‐1+ CD8+ T cells. 64 The same group in another study evaluated the impact of pegilodecakin combination with nivolumab or pembrolizumab in advanced RCC. Combination therapy resulted in the median PFS of 13.9 months, which is comparable to the median PFS of 1.8 months for mono pegilodecakin therapy. 63 The authors in their third study evaluated the impact of such combination for a number of advanced solid tumors. Results of this multi‐center phase 1b trial showed manageable toxicities, along with promising ORR, particularly in patients with NSCLC (ORR: 43%) and RCC (ORR: 40%). 65 Thus, the combination of the IL‐10 PEGylated form pegilodecakin with anti‐PD‐1 therapy is promising for advanced RCC and NSCLC.
3.5. Combination with IL‐12‐based therapy
Effective anti‐PD‐1 therapy requires DC‐T cell cross‐talking. Anti‐PD‐1 therapy stimulates T cells to release IFN‐γ. IFN‐γ further induces DCs to secrete IL‐12. 66 T cells stimulated with IL‐12 express lower levels of PD‐1 but higher levels of IL‐2 and IFN‐γ. 67 Tavokinogene telsaplasmid is an IL‐12‐based gene therapy in which plasmid DNA is synthesized to encode IL‐12 and is injected via an intra‐tumoral route. Forced expression of IL‐12 using an intra‐tumoral injection of IL‐12 plasmid has found to augmented the number of checkpoint positive CTLs. A combination of IL‐12 plasmid with pembrolizumab (200 mg) is effective for improving ORR (41%) in cold melanoma patients. 31 Avelumab was used in combination with NHS‐muIL‐12 in mice model of mammary tumor. Monotherapy with NHS‐muIL‐12 stimulated infiltration of CD8+ T cells into the tumor area. The combination therapy augmented the proliferation of CD8+ T and NK cells, and it was more effective for hampering tumor growth than solo therapy with either avelumab or NHSmuIL‐12. 68
3.6. Combination with IL‐15‐based therapy
IL‐15 is a cytokine with promising activities against tumors of solid organs. IL‐15 shares the common receptor γ chain with some other ILs and represents a pleiotropic activity on cells of innate and adaptive immunity. IL‐15 preferentially acts on NK and CD8+ T cells 69 in which it is known as a canonical NK cell growth factor and a strong CD8+ T cell agonist. 70 It was found that avelumab therapy considerably enhanced cytotoxic activity of NK cells against PD‐L1+ tumor cells of triple‐negative breast cancer (TNBC), and the lytic activity was augmented upon stimulation of NK cells with IL‐2 and IL‐15. Thus, such types of ILs can be used for increasing the therapeutic activity of avelumab. 71
Superagonists are now being developed for IL‐15. ALT‐803 is a superagonist for IL‐15. The application of ALT‐803 in ovarian cancer is effective for enhancing the cytotoxic activity of NK cells. 72 ALT‐803 is used in combination with the PD‐1 inhibitor nivolumab in metastatic NSCLC patients. Results of this phase 1b trial showed a safety profile and promising clinical efficacy (ORR: 29%) for such therapy. 73 N‐803 is another IL‐15 superagonist that exerts killing activity against ovarian cancer cells through boosting NK cell expansion and functionality. 74 N‐809 is a novel fusion protein and a bispecific agent that encompasses a fusion complex for IL‐15 superagonist and anti‐PD‐L1. This bifunctional fusion protein represents the same capacity to bind to the PD‐L1 as monoclonal antibodies against PD‐L1. Exposure of CD8+ T cells with N‐809 has been found to improved their cytolytic activity against tumor cells and potentiated their proliferation. NK cells also display a higher expression profile for activating receptors on their surface after exposure to the N‐809. 75 The efficacy of N‐809 is surveyed in the murine model of TNBC, and the higher survival rate and lower possibility of spontaneous lung metastasis were the outcomes. 76 However, clinical trials investigating the efficacy of this bispecific agent are rare, prompting a necessity for its application in various solid tumors in order to know more about its safety and efficacy profile in human subjects.
3.7. Combination with IL‐21‐based therapy
IL‐21 is a strong mitogen and survival factor for NK and T cells. The activity of this cytokine also antagonizes the differentiation of Tregs. 3 Combination of ICI with recombinant IL‐21 (rIL‐21) is a strategy for enhancing the tumor suppressor effect of ICI therapy in tumors with low/no expression of MHC‐I. The efficacy of PD‐1/TIM‐3 blockade along with intra‐tumoral rIL‐21 injection is exploited in MHC‐I deficient mouse model and augmented anti‐tumor effects were related to the enhanced effector activity of exhausted NK cells. 2 Combination of IL‐21 with anti‐PD‐1 or anti‐CTLA‐4 therapy in animal tumor models resulted in the augmented intra‐tumoral infiltration of CD8+ T cells, along with their increased proliferation and a higher proportion of effector memory T cells. 77
Protein engineering is a strategy for improving the drug‐like efficacy of natural cytokines. Fusion proteins can be used for this aim. Fusion of IL‐21 cytokine mutein to the PD‐1 antibody can improve the serum half‐life of this cytokine. Such a strategy can also be effective for minimizing the detrimental effects of IL‐21 on local antigen‐presenting cells (APCs). 3 IL‐21/PD‐1 antibody fusion (PD‐1Ab21) promoted the formation of memory stem T cells. 78 PD‐L1 expression is also suppressed by IL‐21/IL‐21R. 79 Contrary to this is a study by Zhao and colleagues who recently identified a positive relation between higher IL‐21‐related inflammation in TME with a possibility of Treg‐mediated immune escape via promoting PD‐1/PD‐L1 activity. 80
4. CONCLUSIONS AND FUTURE DIRECTIONS
Durable responses only for a number of patients are a major burden related to the ICI therapy, and low half‐life, low efficacy, and high toxicity are issues with regard to immunocytokine therapy. The combination of IL‐based therapy can be exploited as a promising strategy for enhancing the efficacy of ICI therapy in tumors representing low or no responses to the immunotherapy, rendering promising objective responses for a number of tumors (Figure 3). Issues, however, still exist with regard to the use of ILs in cancer immunotherapy. One of the challenges in the area is the diverse functionalities reported for some ILs. IL‐10, for instance, can take pro‐ or anti‐tumor activities. Such diverse activities are due to interactions with different types of receptors. A higher IFN‐γ/IL‐10 ratio is reported in melanoma patients who were responsive to PD‐1 inhibitor therapy. 81 By contrast, PEGylated IL‐10 is considered a promising combination with PD‐1 inhibitors, as mentioned above. Diverse functionality related to the different types of ILs requires more attention in future studies particularly in a clinical setting in order to inspect whether the combination of immunocytokines with ICIs is an appropriate regimen for patients with different types of cancers or not. There is also an urgent to closely monitor responses from cells of TME to such combination therapy. This will be helpful for uncovering potential counter‐effects related to the therapy. Responses to the ICIs are affected by a number of factors presented in TME, including the rate of TIL infiltration, PD‐L1 expression profile, and tumor mutational burden. Thus, an appropriate combination to the ICI therapy is a drug that works on these variations in favor of therapy, as well as reducing the rate of AEs evolved by patients receiving such combinatory regimen. In the future, more efforts will be made to use PEGylated ILs and fusion proteins in immune combination therapy. What is understood so far is the promising efficacy and acceptable safety profile of such strategy when is used in combination with ICI. However, this area requires more attention, as there are ambiguities and no sufficient proof for many types of tumors in clinical settings.
FIGURE 3.
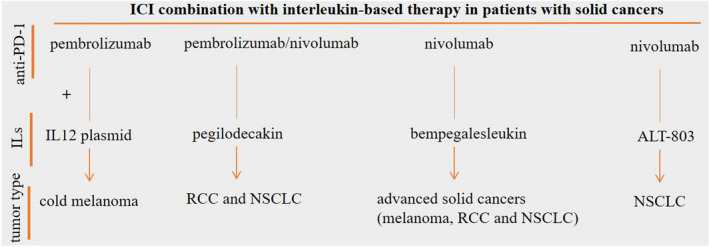
Promising objective response rate (ORR) for combinatory immune checkpoint inhibitor (ICI)/interleukin (IL) therapy in patients with cancer. The combination of programmed death‐1 receptor (PD‐1) inhibitors with IL12 plasmid and PEGylated recombinant IL‐10 (also called pegilodecakin) renders noticeable ORR in cancer patients. ORR is also significant for the IL‐15 superagonist ALT‐803. The most considerable objective response is for the IL‐2 agonist bempegaldesleukin (also called NKTR‐214), which is designed preferentially for CD122 (IL‐2β receptor)
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Collection and revision of information, Keywan Mortezaee and Jamal Majidpoor; Conceptualization, Keywan Mortezaee; writing, original draft preparation, review and editing, Jamal Majidpoor and Keywan Mortezaee. Two authors have read and agreed to publish the manuscript.
CONSENT FOR PUBLICATION
The authors of the paper read, approved, and consent to the final version for the publication.
ETHICS STATEMENT
The manuscript received the Ethical Approval from Kurdistan University of Medical Sciences (Ethical code: IR.MUK.REC.1400.292).
ACKNOWLEDGMENT
This work is supported by Kurdistan University of Medical Sciences.
Mortezaee K, Majidpoor J. Checkpoint inhibitor/interleukin‐based combination therapy of cancer. Cancer Med. 2022;11:2934–2943. doi: 10.1002/cam4.4659
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Mortezaee K, Goradel NH, Amini P, et al. NADPH oxidase as a target for modulation of radiation response; implications to carcinogenesis and radiotherapy. Curr Mol Pharmacol. 2019;12(1):50‐60. [DOI] [PubMed] [Google Scholar]
- 2. Seo H, Kim B‐S, Bae E‐A, et al. IL21 therapy combined with PD‐1 and Tim‐3 blockade provides enhanced NK cell antitumor activity against MHC class I–deficient tumors. Cancer Immunol Res. 2018;6(6):685‐695. [DOI] [PubMed] [Google Scholar]
- 3. Shen S, Sckisel G, Sahoo A, et al. Engineered IL‐21 cytokine muteins fused to anti‐PD‐1 antibodies can improve CD8+ T cell function and anti‐tumor immunity. Front Immunol. 2020;11:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goradel NH, Mohajel N, Malekshahi ZV, et al. Oncolytic adenovirus: a tool for cancer therapy in combination with other therapeutic approaches. J Cell Physiol. 2019;234(6):8636‐8646. [DOI] [PubMed] [Google Scholar]
- 5. Patsoukis N, Wang Q, Strauss L, Boussiotis VA. Revisiting the PD‐1 pathway. Sci Adv. 2020;6(38):eabd2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mortezaee K. Immune escape: a critical hallmark in solid tumors. Life Sci. 2020;258:118110. [DOI] [PubMed] [Google Scholar]
- 7. Liu C, Liu R, Wang B, et al. Blocking IL‐17A enhances tumor response to anti‐PD‐1 immunotherapy in microsatellite stable colorectal cancer. J Immunother Cancer. 2021;9(1). doi: 10.1136/jitc-2020-001895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mortezaee K. Normalization in tumor ecosystem: opportunities and challenges. Cell Biol Int. 2021;45(10):2017‐2030. [DOI] [PubMed] [Google Scholar]
- 9. Mortezaee K. Organ tropism in solid tumor metastasis: an updated review. Future Oncol. 2021;17(15):1943‐1961. [DOI] [PubMed] [Google Scholar]
- 10. Mortezaee K, Majidpoor J. The impact of hypoxia on immune state in cancer. Life Sci. 2021;286:120057. [DOI] [PubMed] [Google Scholar]
- 11. Majidpoor J, Mortezaee K. Angiogenesis as a hallmark of solid tumors‐clinical perspectives. Cell Oncol. 2021;44(4):715‐737. [DOI] [PubMed] [Google Scholar]
- 12. Mortezaee K, Najafi M. Immune system in cancer radiotherapy: resistance mechanisms and therapy perspectives. Crit Rev Oncol Hematol. 2021;157:103180. [DOI] [PubMed] [Google Scholar]
- 13. Majidpoor J, Mortezaee K. The efficacy of PD‐1/PD‐L1 blockade in cold cancers and future perspectives. Clin Immunol. 2021;226:108707. [DOI] [PubMed] [Google Scholar]
- 14. Mortezaee K, Shabeeb D, Musa AE, Najafi M, Farhood B. Metformin as a radiation modifier; implications to normal tissue protection and tumor sensitization. Curr Clin Pharmacol. 2019;14(1):41‐53. [DOI] [PubMed] [Google Scholar]
- 15. Montani MSG, Falcinelli L, Santarelli R, et al. KSHV infection skews macrophage polarisation towards M2‐like/TAM and activates Ire1 α‐XBP1 axis up‐regulating pro‐tumorigenic cytokine release and PD‐L1 expression. Br J Cancer. 2020;123(2):298‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang Y‐K, Wang M, Sun Y, et al. Macrophage spatial heterogeneity in gastric cancer defined by multiplex immunohistochemistry. Nat Commun. 2019;10(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mortezaee K, Parwaie W, Motevaseli E, et al. Targets for improving tumor response to radiotherapy. Int Immunopharmacol. 2019;76:105847. [DOI] [PubMed] [Google Scholar]
- 18. Diskin B, Adam S, Cassini MF, et al. PD‐L1 engagement on T cells promotes self‐tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat Immunol. 2020;21(4):442‐454. [DOI] [PubMed] [Google Scholar]
- 19. Mortezaee K. Redox tolerance and metabolic reprogramming in solid tumors. Cell Biol Int. 2021;45(2):273‐286. [DOI] [PubMed] [Google Scholar]
- 20. Peng Q, Qiu X, Zhang Z, et al. PD‐L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat Commun. 2020;11(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oh SA, Wu D‐C, Cheung J, et al. PD‐L1 expression by dendritic cells is a key regulator of T‐cell immunity in cancer. Nat Cancer. 2020;1(7):681‐691. [DOI] [PubMed] [Google Scholar]
- 22. Weber R, Fleming V, Hu X, et al. Myeloid‐derived suppressor cells hinder the anti‐cancer activity of immune checkpoint inhibitors. Front Immunol. 2018;9:1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mortezaee K. Myeloid‐derived suppressor cells in cancer immunotherapy‐clinical perspectives. Life Sci. 2021;277:119627. [DOI] [PubMed] [Google Scholar]
- 24. Oyer JL, Gitto SB, Altomare DA, Copik AJ. PD‐L1 blockade enhances anti‐tumor efficacy of NK cells. Oncoimmunology. 2018;7(11):e1509819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ma J, Zheng B, Goswami S, et al. PD1 hi CD8+ T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J Immunother Cancer. 2019;7(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mortezaee K, Majidpoor J. Key promoters of tumor hallmarks. Int J Clin Oncol. 2021;27:1‐14. [DOI] [PubMed] [Google Scholar]
- 27. Mortezaee K, Majidpoor J. (Im) maturity in tumor ecosystem. Front Oncol. 2022;11:813897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saito H, Shimizu S, Kono Y, et al. PD‐1 expression on circulating CD8+ T‐cells as a prognostic marker for patients with gastric cancer. Anticancer Res. 2019;39(1):443‐448. [DOI] [PubMed] [Google Scholar]
- 29. Ahn E, Araki K, Hashimoto M, et al. Role of PD‐1 during effector CD8 T cell differentiation. Proc Natl Acad Sci USA. 2018;115(18):4749‐4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cervera‐Carrascon V, Quixabeira DC, Santos JM, et al. Adenovirus armed with TNFa and IL2 added to aPD‐1 regimen mediates antitumor efficacy in tumors refractory to aPD‐1. Front Immunol. 2021;2913. doi: 10.3389/fimmu.2021.706517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Algazi AP, Twitty CG, Tsai KK, et al. Phase II trial of IL‐12 plasmid transfection and PD‐1 blockade in immunologically quiescent melanoma. Clin Cancer Res. 2020;26(12):2827‐2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cheng L, Creasy T, Pilataxi F, et al. Effects of combination treatment with durvalumab plus tremelimumab on the tumor microenvironment in non‐small‐cell lung carcinoma. Cancer Immunol Immunother. 2021;1‐15. doi: 10.1007/s00262-021-03065-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu L, Xie S, Wang L, et al. The ratio of IP10 to IL‐8 in plasma reflects and predicts the response of patients with lung cancer to anti‐PD‐1 immunotherapy combined with chemotherapy. Front Immunol. 2021;12:1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. West EE, Jin H‐T, Rasheed A‐U, et al. PD‐L1 blockade synergizes with IL‐2 therapy in reinvigorating exhausted T cells. J Clin Invest. 2013;123(6):2604‐2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Majidpoor J, Mortezaee K. Interleukin‐2 therapy of cancer‐clinical perspectives. Int Immunopharmacol. 2021;98:107836. [DOI] [PubMed] [Google Scholar]
- 36. Chikuma S, Terawaki S, Hayashi T, et al. PD‐1‐mediated suppression of IL‐2 production induces CD8+ T cell anergy in vivo. J Immunol. 2009;182(11):6682‐6689. [DOI] [PubMed] [Google Scholar]
- 37. Buchbinder EI, Dutcher JP, Daniels GA, et al. Therapy with high‐dose Interleukin‐2 (HD IL‐2) in metastatic melanoma and renal cell carcinoma following PD1 or PDL1 inhibition. J Immunother Cancer. 2019;7(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith FO, Downey SG, Klapper JA, et al. Treatment of metastatic melanoma using interleukin‐2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14(17):5610‐5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McDermott DF, Cheng S‐C, Signoretti S, et al. The high‐dose aldesleukin “select” trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2015;21(3):561‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hsu EJ, Cao X, Moon B, et al. A cytokine receptor‐masked IL2 prodrug selectively activates tumor‐infiltrating lymphocytes for potent antitumor therapy. Nat Commun. 2021;12(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharma M, Khong H, Fa'ak F, et al. Bempegaldesleukin selectively depletes intratumoral Tregs and potentiates T cell‐mediated cancer therapy. Nat Commun. 2020;11(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diab A, Tannir NM, Bentebibel S‐E, et al. Bempegaldesleukin (NKTR‐214) plus nivolumab in patients with advanced solid tumors: phase I dose‐escalation study of safety, efficacy, and immune activation (PIVOT‐02). Cancer Discov. 2020;10(8):1158‐1173. [DOI] [PubMed] [Google Scholar]
- 43. Majidpoor J, Mortezaee K. Interleukin‐6 in SARS‐CoV‐2 induced disease: interactions and therapeutic applications. Biomed Pharmacother. 2022;145:112419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Da Hyun KC‐KP, Chung C, Oh I‐J, et al. Baseline serum interleukin‐6 levels predict the response of patients with advanced non‐small cell lung cancer to PD‐1/PD‐L1 inhibitors. Immune Netw. 2020;20(3). doi: 10.4110/in.2020.20.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuo I‐Y, Yang Y‐E, Yang P‐S, et al. Converged Rab37/IL‐6 trafficking and STAT3/PD‐1 transcription axes elicit an immunosuppressive lung tumor microenvironment. Theranostics. 2021;11(14):7029‐7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toyoshima Y, Kitamura H, Xiang H, et al. IL6 modulates the immune status of the tumor microenvironment to facilitate metastatic colonization of colorectal cancer cells. Cancer Immunol Res. 2019;7(12):1944‐1957. [DOI] [PubMed] [Google Scholar]
- 47. Liu H, Shen J, Lu K. IL‐6 and PD‐L1 blockade combination inhibits hepatocellular carcinoma cancer development in mouse model. Biochem Biophys Res Commun. 2017;486(2):239‐244. [DOI] [PubMed] [Google Scholar]
- 48. Keegan A, Ricciuti B, Garden P, et al. Plasma IL‐6 changes correlate to PD‐1 inhibitor responses in NSCLC. J Immunother Cancer. 2020;8(2):e000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsukamoto H, Fujieda K, Miyashita A, et al. Combined blockade of IL6 and PD‐1/PD‐L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018;78(17):5011‐5022. [DOI] [PubMed] [Google Scholar]
- 50. Mace TA, Shakya R, Pitarresi JR, et al. IL‐6 and PD‐L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018;67(2):320‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang F, He Z, Duan H, et al. Synergistic immunotherapy of glioblastoma by dual targeting of IL‐6 and CD40. Nat Commun. 2021;12(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dimitriou F, Hogan S, Menzies AM, Dummer R, Long GV. Interleukin‐6 blockade for prophylaxis and management of immune‐related adverse events in cancer immunotherapy. Eur J Cancer. 2021;157:214‐224. [DOI] [PubMed] [Google Scholar]
- 53. Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD‐1 blockade. J Oncol Pharm Pract. 2019;25(3):551‐557. [DOI] [PubMed] [Google Scholar]
- 54. Kim ST, Tayar J, Suarez‐Almazor M, et al. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis. 2017;76(12):2061‐2064. [DOI] [PubMed] [Google Scholar]
- 55. Campochiaro C, Nicola F, Alessandro T, et al. Tocilizumab for the treatment of immune‐related adverse events: a systematic literature review and a multicentre case series. Eur J Intern Med. 2021;93:87‐94. [DOI] [PubMed] [Google Scholar]
- 56. Gonzalez‐Aparicio M, Alfaro C. Significance of the IL‐8 pathway for immunotherapy. Hum Vaccin Immunother. 2020;16(10):2312‐2317. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57. Sanmamed M, Perez‐Gracia J, Schalper K, et al. Changes in serum interleukin‐8 (IL‐8) levels reflect and predict response to anti‐PD‐1 treatment in melanoma and non‐small‐cell lung cancer patients. Ann Oncol. 2017;28(8):1988‐1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carleton M, Zhou M, De Henau O, et al. \Serum interleukin 8 (IL‐8) may serve as a biomarker of response to immuno‐oncology (IO) therapy. Am J Clin Oncol. 2018;36:3025‐3025. doi: 10.1200/JCO.2018.36.15_suppl.3025 [DOI] [Google Scholar]
- 59. Yuen KC, Liu L‐F, Gupta V, et al. High systemic and tumor‐associated IL‐8 correlates with reduced clinical benefit of PD‐L1 blockade. Nat Med. 2020;26(5):693‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lopez‐Bujanda ZA, Haffner MC, Chaimowitz MG, et al. Castration‐mediated IL‐8 promotes myeloid infiltration and prostate cancer progression. Nat Cancer. 2021;2(8):803‐818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang Z, Hou Y, Yao Z, Zhan Y, Chen W, Liu Y. Expressivity of Interleukin‐8 and gastric cancer prognosis susceptibility: a systematic review and meta‐analysis. Dose Response. 2021;19(3):15593258211037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sun L, Wang Q, Chen B, et al. Gastric cancer mesenchymal stem cells derived IL‐8 induces PD‐L1 expression in gastric cancer cells via STAT3/mTOR‐c‐Myc signal axis. Cell Death Dis. 2018;9(9):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tannir NM, Papadopoulos KP, Wong DJ, et al. Pegilodecakin as monotherapy or in combination with anti‐PD‐1 or tyrosine kinase inhibitor in heavily pretreated patients with advanced renal cell carcinoma: final results of cohorts A, G, H and I of IVY phase I study. Int J Cancer. 2021;149(2):403‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Naing A, Infante JR, Papadopoulos KP, et al. PEGylated IL‐10 (Pegilodecakin) induces systemic immune activation, CD8+ T cell invigoration and polyclonal T cell expansion in cancer patients. Cancer Cell. 2018;34(5):775‐791.e773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Naing A, Wong DJ, Infante JR, et al. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): a multicentre, multicohort, open‐label, phase 1b trial. Lancet Oncol. 2019;20(11):1544‐1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Garris CS, Arlauckas SP, Kohler RH, et al. Successful anti‐PD‐1 cancer immunotherapy requires T cell‐dendritic cell crosstalk involving the cytokines IFN‐γ and IL‐12. Immunity. 2018;49(6):1148‐1161.e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nguyen KG, Vrabel MR, Mantooth SM, et al. Localized interleukin‐12 for cancer immunotherapy. Front Immunol. 2020;11. doi: 10.3389/fimmu.2020.575597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xu C, Zhang Y, Rolfe PA, et al. Combination therapy with NHS‐muIL12 and avelumab (anti‐PD‐L1) enhances antitumor efficacy in preclinical cancer models. Clin Cancer Res. 2017;23(19):5869‐5880. [DOI] [PubMed] [Google Scholar]
- 69. Knudson KM, Hodge JW, Schlom J, Gameiro SR. Rationale for IL‐15 superagonists in cancer immunotherapy. Expert Opin Biol Ther. 2020;20(7):705‐709. [DOI] [PubMed] [Google Scholar]
- 70. Wrangle J, Velcheti V, Patel M, et al. A37 N‐803 plus nivolumab for advanced or metastatic non‐small cell lung cancer: update on phase II experience of combination PD1 blockade with an IL‐15 superagonist. J Thorac Oncol. 2020;15(2):S24‐S25. [Google Scholar]
- 71. Juliá EP, Amante A, Pampena MB, Mordoh J, Levy EM. Avelumab, an IgG1 anti‐PD‐L1 immune checkpoint inhibitor, triggers NK cell‐mediated cytotoxicity and cytokine production against triple negative breast cancer cells. Front Immunol. 2018;9:2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Felices M, Chu S, Kodal B, et al. IL‐15 super‐agonist (ALT‐803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol Oncol. 2017;145(3):453‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wrangle JM, Velcheti V, Patel MR, et al. ALT‐803, an IL‐15 superagonist, in combination with nivolumab in patients with metastatic non‐small cell lung cancer: a non‐randomised, open‐label, phase 1b trial. Lancet Oncol. 2018;19(5):694‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Van der Meer J, Maas R, Guldevall K, et al. IL‐15 superagonist N‐803 improves IFNγ production and killing of leukemia and ovarian cancer cells by CD34+ progenitor‐derived NK cells. Cancer Immunol Immunother. 2021;70(5):1305‐1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jochems C, Tritsch SR, Knudson KM, et al. The multi‐functionality of N‐809, a novel fusion protein encompassing anti‐PD‐L1 and the IL‐15 superagonist fusion complex. Oncoimmunology. 2019;8(2):e1532764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hicks K, Knudson K, Ozawa Y, Schlom J, Gameiro S. Evaluation of the anti‐tumour efficacy and immune effects of N‐809, a novel IL‐15 superagonist/anti‐PD‐L1 bispecific agent. Ann Oncol. 2019;30:v500. [Google Scholar]
- 77. Lewis KE, Selby MJ, Masters G, et al. Interleukin‐21 combined with PD‐1 or CTLA‐4 blockade enhances antitumor immunity in mouse tumor models. Oncoimmunology. 2018;7(1):e1377873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li Y, Cong Y, Jia M, et al. Targeting IL‐21 to tumor‐reactive T cells enhances memory T cell responses and anti‐PD‐1 antibody therapy. Nat Commun. 2021;12(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xue D, Yang P, Wei Q, Li X, Lin L, Lin T. IL‐21/IL‐21R inhibit tumor growth and invasion in non‐small cell lung cancer cells via suppressing Wnt/β‐catenin signaling and PD‐L1 expression. Int J Mol Med. 2019;44(5):1697‐1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhao Y, Zhang Z, Lei W, et al. IL‐21 is an accomplice of PD‐L1 in the induction of PD‐1‐dependent Treg generation in head and neck cancer. Front Oncol. 2021;11:1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Giunta EF, Barra G, De Falco V, et al. Baseline IFN‐γ and IL‐10 expression in PBMCs could predict response to PD‐1 checkpoint inhibitors in advanced melanoma patients. Sci Rep. 2020;10(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


