Abstract
Intramolecular cyclization of nitrogen-containing molecules onto pendant alkenes is an efficient strategy for the construction of N-heterocycles, which are of paramount importance in, for example, pharmaceuticals and materials. Similar intermolecular cyclization reactions, however, are scarcer for nitrogen building blocks, including N-centred radicals, and divergent and modular versions are not established. Here we report the use of sulfilimines as bifunctional N-radical precursors for cyclization reactions with alkenes to produce N-unprotected heterocycles in a single step through photoredox catalysis. Structurally diverse sulfilimines can be synthesized in a single step, and subsequently engage with alkenes to afford synthetically valuable five-, six- and seven-membered heterocycles. The broad and diverse scope is achievable by a radical-polar crossover annulation enabled by the bifunctional character of the reagents, which distinguishes itself from all other N-centred-radical-based reactions. The modular synthesis of the sulfilimines allows for larger structural diversity of N-heterocycle products than is currently achievable with other single cyclization methods.

Subject terms: Synthetic chemistry methodology, Synthetic chemistry methodology, Photocatalysis, Photocatalysis
Intermolecular cyclization reactions using nitrogen-containing building blocks are scarce. Now, bifunctional sulfilimines have been shown to enable the modular construction of a diverse range of N-heterocycles by reacting with alkenes in a single photocatalysed step. Both sulfilimines and alkenes are easily accessible, providing access to a wide range of N-heterocycles with different ring types, ring sizes and substituents on the skeleton.
Main
Partially and fully saturated N-heterocycles are of high synthetic value, and can for example be accessed by cyclization onto vinyl sulfonium reagents1–4, yet their direct synthesis by cyclization reactions to olefins is not generally established. The development of SnAP reagents is an excellent example of how bifunctional reagents5 can quickly generate useful N-heterocycle diversity through cyclization onto aldehydes6, but similar reactivity via a single-step-reaction with alkenes has not been developed. In 2001, Oshima and co-workers reported a radical chain process using N-allyl-N-chlorotosylamide as a nitrogen-radical precursor for reaction with alkenes to generate N-tosylpyrrolidines7. Shi and co-workers developed diaziridinones as nitrogen-centred radical (NCR) precursors for ring expansion with alkenes to generate N-tert-butyl-protected imidazolidinones under copper catalysis8. Xu and co-workers reported the use of functionalized hydroxylamines to generate carbamate-based NCRs for the construction of oxazolidinones under iron catalysis9. Another example uses N-fluorobenzenesulfonimide specifically for the construction of sultams under copper catalysis10. Despite the large synthetic utility, these methods can only generate a single, specific N-heterocycle, typically with an electron-withdrawing nitrogen-protecting group that may be challenging to remove. No single method appears to be available that can generate several different types of N-heterocycles from olefins11. Here we fill this conceptual void and demonstrate a modular approach to access a large variety of different, synthetically valuable heterocycles that are not currently accessible via other NCRs or polar reactions from simple alkenes in a single step (Fig. 1)12. For example, while morpholine syntheses are well known, their one-step synthesis from olefins has not been reported13.
Fig. 1. Bifunctional sulfilimines for synthesis of various N-heterocycles.
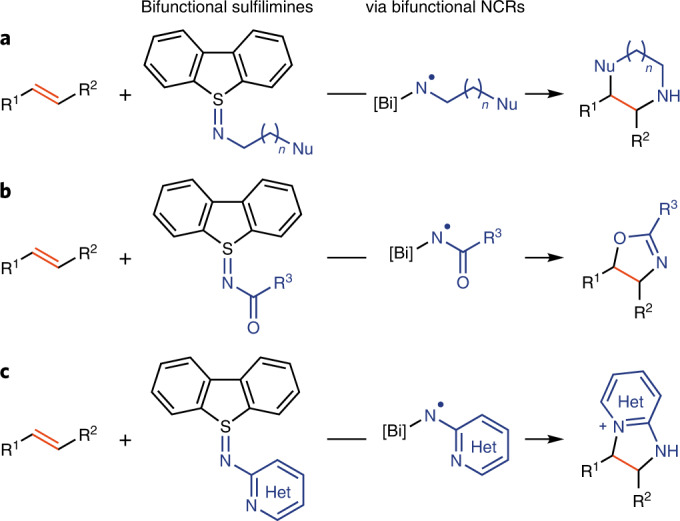
a–c, In this article, bifunctional sulfilimines that feature both nitrogen-radical and polar reactivity have been developed to react with alkenes, giving a divergent and modular approach to versatile N-heterocycles including morpholines, piperazines and oxazepanes (a), dihydrooxazoles (b) and dihydroimidazoles (c). Nu, nucleophilic group; Het, heteroaryl.
NCRs are important intermediates in C–N bond formation reactions14–19. Due to the high bond dissociation energy of the N–H bond (107 kcal mol−1 for ammonia)20, NCRs can function as reactive species for intramolecular hydrogen-atom transfer, for example to generate pyrrolidine derivatives21, as in the Hofmann–Löffler–Freytag reaction22,23. NCRs can also function as electrophilic radicals24, adding to electron-rich π systems in alkenes or arenes to form alkyl or aryl amines. Synthetic applications of such reactivity have resulted in successful hydroamination25,26, aminooxygenation27–30, aminofluorination31–33, carboamination34,35 and aminoazidation36,37, all of which introduce two different functional groups to an alkene in a single step. Despite the advance and scope of difunctionalization reactions with NCRs, intermolecular cyclization reactions to furnish N-heterocycles are still challenging because most nitrogen-radical precursors contain sulfonyl or similar groups on the nitrogen to enhance the electrophilicity of the respective NCRs15,24.
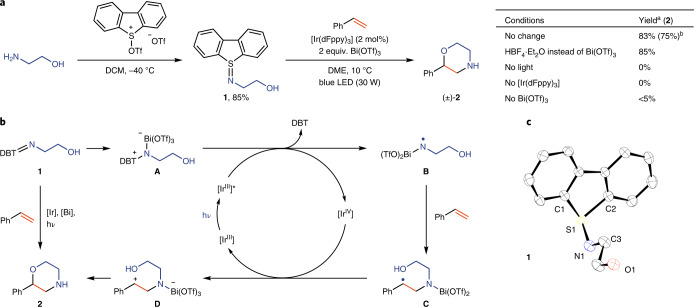
Results and discussion
Our goal was to generate a stable reagent that could be transformed into an electrophilic NCR under conditions that would tolerate the presence and reactivity of a pendant nucleophile for subsequent ring closure. While, a priori, several NCR precursors could meet such a goal, one reason why such a compound class has not yet been disclosed may be the synthetic challenge to make these reagents due to undesired cross-reactivity of the pendant nucleophile or undesired reactivity of the nitrogen-activating group. For example, the chloride released upon NCR formation from N-chloroamonium salts may outcompete a pendant hydroxyl group for addition38. Here we report the use of sulfilimines, a substrate class that has previously been used in other transformations39, to address this synthetic challenge. The bifunctional sulfilimine 1 was obtained directly from commercially available reagents in a reaction of aminoethanol and dibenzothiophene-S-oxide activated by triflic anhydride (Fig. 2a). Irradiation of a photoredox catalyst in the presence of sulfilimine 1, acid and styrene results in phenylmorpholine formation (Fig. 2a). Light and photoredox catalyst are essential for the reactivity (Supplementary Tables 1–6). Both Brønsted acids and Lewis acids accelerate cyclization, possibly due to more efficient single electron transfer (SET) from the excited photoredox catalyst to the sulfilimine coordinated to acid (Fig. 2b and Supplementary Table 1). According to the recorded cyclic voltammogram of sulfilimine 1 (Supplementary Fig. 7), no reduction peak was observed within the evaluated potential, which indicates that mesolytic cleavage of the S=N bond in sulfilimine 1 by initial SET to 1 to the corresponding NCR is slow with standard photocatalysts. In contrast, the Bi(OTf)3-coordinated sulfilimine 1 (A) exhibits a high reduction potential (Ep = −0.4 V versus Ag/AgCl, Supplementary Fig. 7), so that fast SET with the excited iridium photocatalyst (E1/2(IrIII*/IrIV) = −1.28 V vs saturated calomel electrode)40 can be observed, to form B (Fig. 2b). Bi(OTf)3 was identified as optimal because Brønsted acids could result in cationic polymerization of activated olefins such as electron-rich styrenes (Supplementary Table 2)41. In addition, both Lewis and Brønsted acids could be responsible for rendering the amine radical electrophilic for polarity-matched addition to the electron-rich π system of the olefin42–44. A stoichiometric amount of Bi(OTf)3 is required due to the basicity of the products. A conceptual advantage of the sulfilimines over other NCR precursors is their ability to enable easy introduction of pendant nucleophilic functional groups on NCRs, and directly afford unprotected N–H nitrogen heterocycles in a single step without the need for covalent activating groups or a deprotection step. Upon addition to the π system, the oxidized photoredox catalyst can oxidize the resulting carbon radical C for subsequent intramolecular nucleophilic attack (D) of the pendant nucleophile and regeneration of the photoredox catalyst resting state (Fig. 2b).
Fig. 2. Synthesis of sulfilimine 1, reaction optimization and proposed mechanism of the cyclization reaction.
a, The sulfilimine 1 was obtained from the reaction of aminoethanol and triflic-anhydride-activated dibenzothiophene-S-oxide in a single step. The reaction optimization shows that both photocatalyst and acid additive are essential for the reactivity; aYield determined from1H NMR with CH2Br2 as an internal standard. bIsolated yield in parenthesis. DCM, dichloromethane; DME, 1,2-dimethoxyethane. b. A radical-polar-crossover annulation process was proposed. The acid additive plays a crucial role in activation of sulfilimine 1 to generate bifunctional NCR B as a key intermediate. DBT, dibenzothiophene. c. X-ray crystal structure of 1 (Supplementary Tables 7 and 8; hydrogen atoms are omitted for clarity). Selected bond distances and angles: S(1)–N(1), 1.591(2) Å; C(1)–S(1)–C(2), 88.85(9)°, C(3)–N(1)–S(1), 116.97(14)°.
Various bifunctional sulfilimine reagents can be synthesized in a single step (Tables 1 and 2). Primary amines including aminoalcohols, diamines and aminopyridines react to produce iminodibenzothiophenes with pendant hydroxyl (3–6), amide (7, 8) or pyridinyl groups (9, 10). The diversity of the suitable sulfilimines for heterocycle synthesis was extended by reaction of the parent iminodibenzothiophene (11) with acylating reagents, which gives access to other classes of sulfilimines, such as those derived from amides, pyrimidines and triazines from acid chlorides (12–15), chloropyrimidines (16) and chlorotriazines (17), respectively. All sulfilimines shown, with the exception of 6, are easily handled solids and are stable in ambient atmosphere without detectable decomposition for at least three months; while also stable, 6 was isolated as an oil.
Table 1.
Synthesis of bifunctional sulfilimines from amines

Reaction conditions: 1.0 equiv. of dibenzothiophene-S-oxide, 1.1 equiv. of triflic anhydride and 2.5 equiv. of amine in DCM (0.1 M) at −40 °C.
Table 2.
Synthesis of bifunctional sulfilimines from dibenzothiophene-S-imine
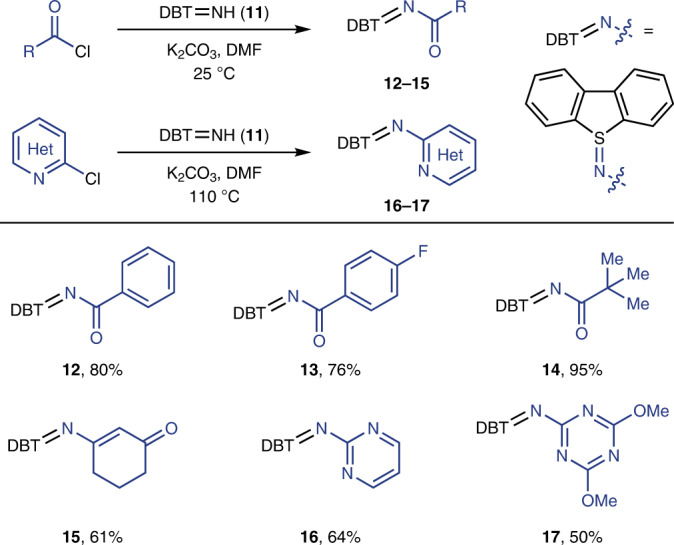
Reaction conditions: 1.0 equiv. of dibenzothiophene-S-imine (11), 2.0 equiv. of K2CO3 and 1.5 equiv. of acid chloride in DMF (0.1 M) at 25 °C for 3 h, or 2.0 equiv. of chloroazine in DMF (0.1 M) at 110 °C for 6 h.
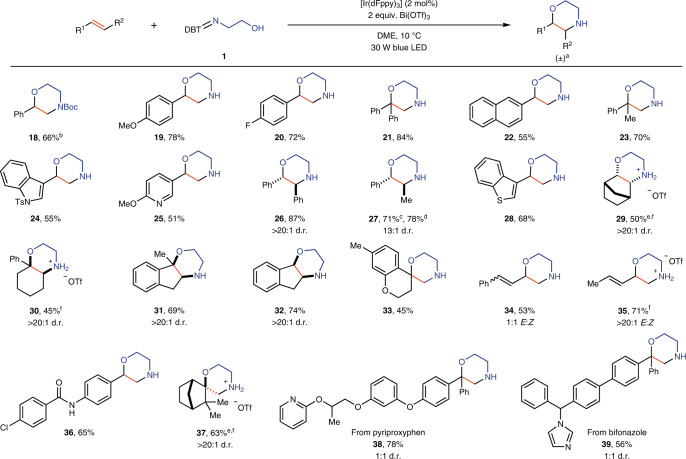
Cyclization of a variety of sulfilimines with a variety of electron-rich olefins gives access to a large family of diverse, synthetically valuable heterocycles in a single step (Table 3 and Fig. 3). The products can be isolated without protecting groups on nitrogen, but in situ protection of nitrogen with a tert-butyloxycarbonyl (Boc) group is facile, if desired, as shown for 18. Styrene derivatives that are prone to cationic polymerization under acidic conditions45 such as 19 and 22 can selectively react with the bifunctional sulfilimine 1 to produce morpholines in 78% and 55% yield, respectively. Olefins with heteroaryl substituents, such as indoles (24), pyridines (25) and benzothiophenes (28), are well tolerated. The putative bismuth(III)-coordinated amine radicals undergo addition reactions chemoselectively to alkenes in the presence of electron-rich arenes (19), allylic hydrogens (23, 27, 30–33, 35) and even ether (DME) as solvent. Such functional groups are often reactive with other NCRs either via radical addition46,47 or via hydrogen-atom transfer processes48–50. In addition to α-styrenes, 1,1-disubstituted alkenes (37) can also undergo cyclization with sulfilimine 1 to produce morpholine heterocycles with a quaternary centre, although for most other investigated alkyl-substituted alkenes that feature allylic hydrogen atoms, allylic amination was observed44. For 1,2-disubstituted alkenes that bear a group that can stabilize a positive charge, high diastereoselectivity is observed for cyclization (26, 27), providing 2,3-disubsituted morpholines. Both trans- and cis-propenylbenzenes produce the same product with the same diastereoselectivity, which supports the proposed intermediacy of NCRs. High regioselectivity is also obtained in reactions with dienes, such as 34 and 35, as cyclization reaction only occurs at the terminal alkene of the diene, producing alkenyl-substituted morpholines. When alkyl-substituted dienes are used, the thermodynamically more stable trans-olefin is obtained as major product (35). In the case of styrene-derived dienes (34), a known photocatalysed isomerization51 occurs to produce cis- and trans-styrenylmorpholines. Cyclic alkenes afford bicyclic and tricyclic morpholines. Highly diastereoselective formation of morpholine derivatives with fused rings is achieved when endocyclic olefins such as norbornene (29), 1-phenyl-1-cyclohexene (30) and indenes (31, 32) are used. Exocyclic olefins such as 7-methyl-4-methylenechromane (33) and camphene (37) are also suitable reaction partners, producing spirocyclic morpholines. These polycyclic heterocycles can be constructed selectively in a single step from the corresponding alkenes, and are not readily accessible via other synthetic methods. Olefins that would afford cations upon radical addition and oxidation that are not sufficiently stabilized, such as in α olefins and 1,2-disubstituted alkenes without stabilizing groups, such as an aryl or vinyl substituent, cannot participate in the reaction (Supplementary Fig. 1), whereas styrene-like, 1,1-disubstituted and diene-based olefins participate successfully. The method can be used for late-stage diversification (38, 39). To further demonstrate the synthetic value of the method, we accomplished a concise synthesis of H1 receptor antagonist 41 (Fig. 3). The modular approach allows us to construct the key morpholine structure directly from alkene 40, which substantially increased the total yield and reduced the step count compared to the previously published procedure52. The reaction requires the use of a stoichiometric amount of dibenzothiophene heterocycle, which, however, can be recycled after successful cyclization; for example, 92% of dibenzothiophene was reisolated after formation of 2.
Table 3.
Scope of alkenes for synthesis of morpholine derivatives
Reaction conditions: alkene (0.2 mmol), 1 (0.4 mmol), Bi(OTf)3 (0.4 mmol), [Ir(dFppy)3] (2 mol%) in DME (1 ml) at 10 °C under a 30 W blue light-emitting diode (LED) for 6 h. aAll chiral products are obtained as racemic mixtures. bIn situ Boc protection of the morpholine product after irradiation of the reaction mixture for 6 h by addition of 6 equiv. of Et3N and 3 equiv. of Boc2O. c(E)-Propenylbenzene is used. d(Z)-Propenylbenzene is used. eAlkene (0.8 mmol), 1 (0.2 mmol), Bi(OTf)3 (0.2 mmol). fBase workup is not applied due to easier purification of these products in protonated form.
Fig. 3. Scope of sulfilimines and synthetic application.
Reaction conditions: alkene (0.2 mmol), sulfilimine (0.4 mmol), Bi(OTf)3 (0.4 mmol), [Ir(dFppy)3] (2 mol%) in DME (1 ml) at 10 °C under a 30 W blue LED. aMethyl methoxyacetate (1 ml) as solvent instead of DME, and base workup is not applied due to easier purification of these products in ionic form. Other types of N-heterocycles, including oxazepanes, piperazines, dihydrooxazoles, dihydroimidazopyridiniums and dihydroimidazotriazinones, are accessible with the modular cyclization approach. A concise route to H1 receptor antagonist 41 has been developed based on the cyclization method.
The modular approach and the accessibility of various bifunctional sulfilimines enables the synthesis of other types of N-heterocycles under the same reaction conditions by simply changing the substituents on the sulfilimines (Fig. 3). Disubstituted morpholines are obtained when sulfilimines such as 3 or 4 react with alkenes. Although low diastereoselectivity (2:1) was observed when the stereocentre is α to the hydroxy substituent, high diastereoselectivity (>20:1) is obtained when the stereocentre is α to the nitrogen substituent. Other sulfilimine reagents (5–8) enable the construction of oxazepanes (44, 45) and piperazines (46, 47) in synthetically useful yields. We subsequently explored the generality of our modular approach to N-heterocycles with sulfilimines derived from amines other than alkyl amines. Acyl-amine-derived sulfilimines (12–14) can also function as bifunctional reagents, which undergo cyclization with alkenes under the same reaction conditions to produce dihydrooxazoles (50–52)53,54. An intriguing reactivity was discovered when using sulfilimine 15 as substrate, providing tetrahydrobenzofuran 53 exclusively in 89% yield instead of an N-heterocycle. A plausible rationale is that the generated enamine NCR reacts to the more stable carbon-centred radical, which is then involved in annulation with 1,1-diphenylethylene (Supplementary Fig. 13). The photocatalytic annulation strategy can also be applied to heteroaryl-amine-derived sulfilimines (9, 10, 16, 17), generating electrophilic arylamine radicals that are reactive for cyclization with alkenes to form dihydroimidazole derivatives (48, 49, 54, 55). In the case of sulfilimine 17, the initially formed triazinium underwent hydrolysis under basic conditions to form triazinone 55. NCRs with alkyl, aryl and acyl substituents on nitrogen can be accessed via the same photocatalytic method, which is difficult to achieve with other NCR precursors55,56. The scope of heterocycles presented herein exceeds that of other reported single methods12.
Preliminary mechanistic experiments are in agreement with the proposed strategy shown in Fig. 2b (Supplementary Figs. 2–12). A 1:1 mixture of sulfilimine 1 and Bi(OTf)3 results in a new peak potential that is absent in both 1 and Bi(OTf)3 alone, which we assign to the 1–Bi(OTf)3 adduct A as observed in the cyclic voltammogram (Fig. 4a). The high reduction potential (Ep = −0.4 V versus Ag/AgCl) may be responsible for a fast SET from the excited iridium photocatalyst, while reduction of 1 by itself was not observed. The existence of adduct A is further substantiated by ultraviolet–visible spectroscopy through a new absorption maximum at 326 nm (Fig. 4b). Radical clock experiments with 2-vinylcyclopropylbenzene and a 1,6-diene under optimized reaction conditions with sulfilimine 1 produce ring-opened product 56 and cyclization product 57, respectively, in agreement with NCRs (Fig. 4c).
Fig. 4. Mechanistic investigations.
a, Cyclic voltammetry for Bi(OTf)3, sulfilimine 1 and a 1:1 mixture of Bi(OTf)3 and 1 in acetonitrile under a scan rate of 100 mV s–1. A new reduction peak at Ep = −0.4 V versus Ag/AgCl was observed when using a 1:1 mixture of Bi(OTf)3 and 1. b, Ultraviolet–visible spectra of Bi(OTf)3, sulfilimine 1 and a 1:1 mixture of Bi(OTf)3 and 1 in DME (2.5 × 10–5 M). A new absorption peak at 326 nm was observed when using a 1:1 mixture of Bi(OTf)3 and 1. Both cyclic voltammetry and ultraviolet–visible spectra indicate a direct interaction of Bi(OTf)3 with 1, which plays a key role in activation of sulfilimine 1 for the generation of the corresponding NCR. c, A radical clock experiment using both 2-vinylcyclopropylbenzene and 1,6-diene shows that the reactions proceed via generation of NCRs.
Conclusion
Photocatalysed modular synthesis has enabled the construction of various N-heterocycles with different ring types, ring sizes and substituents on the skeleton in a single step by reaction of easily available bifunctional sulfilimines and alkenes. The scope of heterocycles provided here is broader than that of other reported single methods for N-heterocycle synthesis from olefins.
Methods
General procedure for cyclization
Under a nitrogen atmosphere, to a 4 ml borosilicate vial equipped with a magnetic stir bar were added alkene (if solid) (0.200 mmol, 1.00 equiv.), sulfilimine (0.400 mmol, 2.00 equiv.), [Ir(dFppy)3] (3.0 mg, 4.0 µmol, 2.0 mol%), Bi(OTf)3 (262 mg, 0.400 mmol, 2.00 equiv.), DME (1 ml, c = 0.2 M), and alkene (if liquid) (0.200 mmol, 1.00 equiv.). The vial was sealed with a septum cap and irradiated for 6 h at 10 °C using a photoreactor equipped with a blue LED module (KT-Elektronik, ‘100 W Power LED blau 450 nm Aquarium’, 450 nm, 30 W), cooled with two Peltier elements (TEC1-12706). Then, the reaction mixture was concentrated to dryness. The residue was dissolved in DCM (5 ml) and washed with saturated aqueous sodium carbonate solution (5 ml). The aqueous phase was extracted with DCM (2 × 5 ml). The organic phase was dried over Na2SO4 and filtered, and the solvent was removed under reduced pressure. The residue was purified by chromatography on silica gel eluting with CH2Cl2/MeOH (50/1–10/1 v/v) to afford the cyclization product.
Note: The reaction is air sensitive. The Schlenk technique was used to avoid air. For simplicity, in our research, we have opted to execute the transformation for most compounds in a glovebox. Control experiments showed that yields were within the error of measurement if the reaction was carried out using a glovebox or the Schlenk technique.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41557-022-00997-y.
Supplementary information
Experimental procedures, product characterization, mechanistic studies, Supplementary Figs. 1–13 and Tables 1–8.
Crystallographic data for compound 1; CCDC reference 2101016
Acknowledgements
We thank N. Haupt, D. Kampen, F. Kohler and D. Margold for mass spectrometry analysis, M. Leutzsch, M. Kochius, S. Tobegen, C. Wirtz and P. Philipps for NMR spectroscopy analysis, J. Rust for X-ray analysis and Y. Wang for the help with Stern–Volmer quenching experiments (all from MPI für Kohlenforschung). We thank B. Lansbergen, E. M. Alvarez, Y. Cai and F. Juliá (all from MPI für Kohlenforschung) for helpful discussions. We thank S. Lin (MPI für Kohlenforschung) for help with revision. We also thank the MPI für Kohlenforschung for funding. Q.C. acknowledges the Alexander von Humboldt Foundation for a Humboldt Research Fellowship.
Author contributions
Q.C. developed the chemistry and optimized the reaction conditions. Q.C., Z.B. and S.T. explored the substrate scope for cyclization. Q.C. and T.R. wrote the manuscript. T.R. directed the project.
Peer review
Peer review information
Nature Chemistry thanks Jia-Rong Chen, A. Stephen K. Hashmi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Funding
Open access funding provided by Max Planck Society.
Data availability
All the data generated or analysed during this study are included in this article and its Supplementary Information. Crystallographic data for the structure reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers 2101016 (1). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41557-022-00997-y.
References
- 1.Wang Y, Zhang W, Colandrea VJ, Jimenez LS. Reactivity and rearrangements of dialkyl- and diarylvinylsulfonium salts with indole-2- and pyrrole-2-carboxaldehydes. Tetrahedron. 1999;55:10659–10672. doi: 10.1016/S0040-4020(99)00605-5. [DOI] [Google Scholar]
- 2.Yamanaka H, Yamane Y, Mukaiyama T. A new method for the preparation of nitrogen-containing heterocycles using diphenylsulfonium triflates. Heterocycles. 2004;63:2813–2826. doi: 10.3987/COM-04-10232. [DOI] [Google Scholar]
- 3.Yar M, McGarrigle EM, Aggarwal VK. An annulation reaction for the synthesis of morpholines, thiomorpholines, and piperazines from β-heteroatom amino compounds and vinyl sulfonium salts. Angew. Chem. Int. Ed. 2008;47:3784–3786. doi: 10.1002/anie.200800373. [DOI] [PubMed] [Google Scholar]
- 4.Juliá F, Yan J, Paulus F, Ritter T. Vinyl thianthrenium tetrafluoroborate: a practical and versatile vinylating reagent made from ethylene. J. Am. Chem. Soc. 2021;143:12992–12998. doi: 10.1021/jacs.1c06632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang H-M, Bellotti P, Ma J, Dalton T, Glorius F. Bifunctional reagents in organic synthesis. Nat. Rev. Chem. 2021;5:301–321. doi: 10.1038/s41570-021-00266-5. [DOI] [PubMed] [Google Scholar]
- 6.Vo C-VT, Mikutis G, Bode JW. SnAP reagents for the transformation of aldehydes into substituted thiomorpholines—an alternative to cross-coupling with saturated heterocycles. Angew. Chem. Int. Ed. 2013;52:1705–1708. doi: 10.1002/anie.201208064. [DOI] [PubMed] [Google Scholar]
- 7.Tsuritani T, Shinokubo H, Oshima K. Radical [3 + 2] annulation of N-allyl-N-chlorotosylamide with alkenes via atom-transfer process. Org. Lett. 2001;3:2709–2711. doi: 10.1021/ol016310j. [DOI] [PubMed] [Google Scholar]
- 8.Yuan W, Du H, Zhao B, Shi Y. A mild Cu(I)-catalyzed regioselective diamination of conjugated dienes. Org. Lett. 2007;9:2589–2591. doi: 10.1021/ol071105a. [DOI] [PubMed] [Google Scholar]
- 9.Lu D-F, Zhu C-L, Jia Z-X, Xu H. Iron(II)-catalyzed intermolecular amino-oxygenation of olefins through the N–O bond cleavage of functionalized hydroxylamines. J. Am. Chem. Soc. 2014;136:13186–13189. doi: 10.1021/ja508057u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaneko K, Yoshino T, Matsunaga S, Kanai M. Sultam synthesis via Cu-catalyzed intermolecular carboamination of alkenes with N-fluorobenzenesulfonimide. Org. Lett. 2013;15:2502–2505. doi: 10.1021/ol4009848. [DOI] [PubMed] [Google Scholar]
- 11.Kaur N, et al. Intermolecular alkene difunctionalizations for the synthesis of saturated heterocycles. Org. Biomol. Chem. 2019;17:1643–1654. doi: 10.1039/C8OB02443J. [DOI] [PubMed] [Google Scholar]
- 12.Vo C-VT, Bode JW. Synthesis of saturated N-heterocycles. J. Org. Chem. 2014;79:2809–2815. doi: 10.1021/jo5001252. [DOI] [PubMed] [Google Scholar]
- 13.Tzara A, Xanthopoulos D, Kourounakis AP. Morpholine as a scaffold in medicinal chemistry: an update on synthetic strategies. ChemMedChem. 2020;15:392–403. doi: 10.1002/cmdc.201900682. [DOI] [PubMed] [Google Scholar]
- 14.Zard SZ. Recent progress in the generation and use of nitrogen-centred radicals. Chem. Soc. Rev. 2008;37:1603–1608. doi: 10.1039/b613443m. [DOI] [PubMed] [Google Scholar]
- 15.Xiong T, Zhang Q. New amination strategies based on nitrogen-centered radical chemistry. Chem. Soc. Rev. 2016;45:3069–3087. doi: 10.1039/C5CS00852B. [DOI] [PubMed] [Google Scholar]
- 16.Chen J-R, Hu X-Q, Lu L-Q, Xiao W-J. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016;45:2044–2056. doi: 10.1039/C5CS00655D. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Xia W. Recent advances in radical-based C–N bond formation via photo-/electrochemistry. Chem. Soc. Rev. 2018;47:2591–2608. doi: 10.1039/C7CS00572E. [DOI] [PubMed] [Google Scholar]
- 18.Wang P, Zhao Q, Xiao W, Chen J. Recent advances in visible-light photoredox-catalyzed nitrogen radical cyclization. Green Synth. Catal. 2020;1:42–51. doi: 10.1016/j.gresc.2020.05.003. [DOI] [Google Scholar]
- 19.Kwon K, Simons RT, Nandakumar M, Roizen JL. Strategies to generate nitrogen-centered radicals that may rely on photoredox catalysis: development in reaction methodology and applications in organic synthesis. Chem. Rev. 2022;122:2353–2428. doi: 10.1021/acs.chemrev.1c00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bordwell FG, Harrelson JA, Jr., Lynch T-Y. Homolytic bond dissociation energies for the cleavage of α-N–H bonds in carboxamides, sulfonamides, and their derivatives. the question of synergism in nitrogen-centered radicals. J. Org. Chem. 1990;55:3337–3341. doi: 10.1021/jo00297a064. [DOI] [Google Scholar]
- 21.Zhang J, Pérez-Temprano MH. Intramolecular C(sp3)–H bond amination strategies for the synthesis of saturated N-containing heterocycles. Chimia. 2020;74:895–903. doi: 10.2533/chimia.2020.895. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann AW. Ueber die Einwirkung des Broms in alkalischer Lösung auf die Amine. Ber. Dtsch. Chem. Ges. 1883;16:558–560. doi: 10.1002/cber.188301601120. [DOI] [Google Scholar]
- 23.Löffler K, Freytag C. Über eine neue Bildungsweise von N-alkylierten Pyrrolidinen. Ber. Dtsch. Chem. Ges. 1909;42:3427–3431. doi: 10.1002/cber.19090420377. [DOI] [Google Scholar]
- 24.Parsaee F, et al. Radical philicity and its role in selective organic transformations. Nat. Rev. Chem. 2021;5:486–499. doi: 10.1038/s41570-021-00284-3. [DOI] [PubMed] [Google Scholar]
- 25.Stella L. Homolytic cyclizations of N-chloroalkenylamines. Angew. Chem. Int. Ed. 1983;22:337–350. doi: 10.1002/anie.198303373. [DOI] [Google Scholar]
- 26.Musacchio AJ, et al. Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines. Science. 2017;355:727–730. doi: 10.1126/science.aal3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyazawa K, Koike T, Akita M. Regiospecific intermolecular aminohydroxylation of olefins by photoredox catalysis. Chem. Eur. J. 2015;21:11677–11680. doi: 10.1002/chem.201501590. [DOI] [PubMed] [Google Scholar]
- 28.Michaelis DJ, Shaffer CJ, Yoon TP. Copper(II)- J. Am. Chem. Soc. 2007;129:1866–1867. doi: 10.1021/ja067894t. [DOI] [PubMed] [Google Scholar]
- 29.Williamson KS, Yoon TP. Iron-catalyzed aminohydroxylation of olefins. J. Am. Chem. Soc. 2010;132:4570–4571. doi: 10.1021/ja1013536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patra T, Das M, Daniliuc CG, Glorius F. Metal-free photosensitized oxyimination of unactivated alkenes with bifunctional oxime carbonates. Nat. Catal. 2021;4:54–61. doi: 10.1038/s41929-020-00553-2. [DOI] [Google Scholar]
- 31.Zhang H, Song Y, Zhao J, Zhang J, Zhang Q. Regioselective radical aminofluorination of styrenes. Angew. Chem. Int. Ed. 2014;53:11079–11083. doi: 10.1002/anie.201406797. [DOI] [PubMed] [Google Scholar]
- 32.Lu D-F, Zhu C-L, Sears JD, Xu H. Iron(II)-catalyzed intermolecular aminofluorination of unfunctionalized olefins using fluoride ion. J. Am. Chem. Soc. 2016;138:11360–11367. doi: 10.1021/jacs.6b07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang H, Studer A. Amidyl radicals by oxidation of α-amido-oxy acids: transition-metal-free amidofluorination of unactivated alkenes. Angew. Chem. Int. Ed. 2018;57:10707–10711. doi: 10.1002/anie.201804966. [DOI] [PubMed] [Google Scholar]
- 34.Jiang H, Studer A. Intermolecular radical carboamination of alkenes. Chem. Soc. Rev. 2020;49:1790–1811. doi: 10.1039/C9CS00692C. [DOI] [PubMed] [Google Scholar]
- 35.McAtee RC, Noten EA, Stephenson CRJ. Arene dearomatization through a catalytic N-centered radical cascade reaction. Nat. Commun. 2020;11:2528. doi: 10.1038/s41467-020-16369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang B, Studer A. Copper-catalyzed intermolecular aminoazidation of alkenes. Org. Lett. 2014;16:1790–1793. doi: 10.1021/ol500513b. [DOI] [PubMed] [Google Scholar]
- 37.Fu N, Sauer GS, Saha A, Loo A, Lin S. Metal-catalyzed electrochemical diazidation of alkenes. Science. 2017;357:575–579. doi: 10.1126/science.aan6206. [DOI] [PubMed] [Google Scholar]
- 38.Govaerts S, et al. Photoinduced olefin diamination with alkylamines. Angew. Chem. Int. Ed. 2020;59:15021–15028. doi: 10.1002/anie.202005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian X, Song L, Hashmi ASK. α-Imino gold carbene intermediates from readily accessible sulfilimines: intermolecular access to structural diversity. Chem. Eur. J. 2020;26:3197–3204. doi: 10.1002/chem.201904869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teegardin K, Day JI, Chan J, Weaver J. Advances in photocatalysis: a microreview of visible light mediated ruthenium and iridium catalyzed organic transformations. Org. Process Res. Dev. 2016;20:1156–1163. doi: 10.1021/acs.oprd.6b00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Throssell JJ, Sood SP, Szwarc M, Stannett V. The instantaneous polymerization of styrene by trifluoroacetic acid. J. Am. Chem. Soc. 1956;78:1122–1125. doi: 10.1021/ja01587a010. [DOI] [Google Scholar]
- 42.Ganley JM, Murray PRD, Knowles RR. Photocatalytic generation of aminium radical cations for C–N bond formation. ACS Catal. 2020;10:11712–11738. doi: 10.1021/acscatal.0c03567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svejstrup TD, Ruffoni A, Juliá F, Aubert VM, Leonori D. Synthesis of arylamines via aminium radicals. Angew. Chem. Int. Ed. 2017;56:14948–14952. doi: 10.1002/anie.201708693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng Q, Chen J, Lin S, Ritter T. Allylic amination of alkenes with iminothianthrenes to afford alkyl allylamines. J. Am. Chem. Soc. 2020;142:17287–17293. doi: 10.1021/jacs.0c08248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aoshima S, Kanaoka S. A renaissance in living cationic polymerization. Chem. Rev. 2009;109:5245–5287. doi: 10.1021/cr900225g. [DOI] [PubMed] [Google Scholar]
- 46.Allen LJ, Cabrera PJ, Lee M, Sanford MS. N-Acyloxyphthalimides as nitrogen radical precursors in the visible light photocatalyzed room temperature C–H amination of arenes and heteroarenes. J. Am. Chem. Soc. 2014;136:5607–5610. doi: 10.1021/ja501906x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruffoni A, et al. Practical and regioselective amination of arenes using alkyl amines. Nat. Chem. 2019;11:426–433. doi: 10.1038/s41557-019-0254-5. [DOI] [PubMed] [Google Scholar]
- 48.Li J, et al. Site-specific allylic C–H bond functionalization with a copper-bound N-centred radical. Nature. 2019;574:516–521. doi: 10.1038/s41586-019-1655-8. [DOI] [PubMed] [Google Scholar]
- 49.Shaw MH, Shurtleff VW, Terrett JA, Cuthbertson JD, MacMillan DWC. Native functionality in triple catalytic cross-coupling: sp3 C–H bonds as latent nucleophiles. Science. 2016;352:1304–1308. doi: 10.1126/science.aaf6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choi G, Zhu Q, Miller D, Gu CJ, Knowles RR. Catalytic alkylation of remote C–H bonds enabled by proton-coupled electron transfer. Nature. 2016;539:268–271. doi: 10.1038/nature19811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metternich JB, et al. J. Org. Chem. 2017;82:9955–9977. doi: 10.1021/acs.joc.7b01281. [DOI] [PubMed] [Google Scholar]
- 52.Botta, M., Castiglioni, E., Di Fabio, R., Spinosa, R. & Togninelli, A. Spiro compounds useful as antagonists of the H1 receptor and their asymmetric preparation, pharmaceutical compositions and use in the treatment of sleep disorders. WO patent 2009016085 (2009).
- 53.Wu F, Kaur N, Alom N, Li W. Chiral hypervalent iodine catalysis enables an unusual regiodivergent intermolecular olefin aminooxygenation. JACS Au. 2021;1:734–741. doi: 10.1021/jacsau.1c00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mumford EM, Hemric BN, Denmark SE. Catalytic, enantioselective syn-oxyamination of alkenes. J. Am. Chem. Soc. 2021;143:13408–13417. doi: 10.1021/jacs.1c06750. [DOI] [PubMed] [Google Scholar]
- 55.Kärkäs MD. Photochemical generation of nitrogen-centered amidyl, hydrazonyl, and imidyl radicals: methodology developments and catalytic applications. ACS Catal. 2017;7:4999–5022. doi: 10.1021/acscatal.7b01385. [DOI] [Google Scholar]
- 56.Jiang H, Studer A. Chemistry with N-centered radicals generated by single-electron transfer-oxidation using photoredox catalysis. CCS Chem. 2019;1:38–49. doi: 10.31635/ccschem.019.20180026. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, product characterization, mechanistic studies, Supplementary Figs. 1–13 and Tables 1–8.
Crystallographic data for compound 1; CCDC reference 2101016
Data Availability Statement
All the data generated or analysed during this study are included in this article and its Supplementary Information. Crystallographic data for the structure reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers 2101016 (1). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.