Summary
The emergence of the SARS-CoV-2 Omicron BA.1 (B.1.1.529) variant has raised questions regarding resistance to neutralizing antibodies elicited by natural infection or immunization. We examined the neutralization activity of sera collected from previously SARS-CoV-2-infected individuals and SARS-CoV-2 naive individuals who received BBIBP-CorV or CoronaVac to BA.1 and the earlier variants Alpha, Beta, and Delta. Both sera from convalescent patients over three months after infection and two-dose BBIBP-CorV or CoronaVac vaccine recipients barely inhibited BA.1, less effectively neutralized Beta and Delta, and moderately neutralized Alpha. However, administering a single dose of BBIBP-CorV or CoronaVac in previously infected individuals or a third dose booster vaccination of BBIBP-CorV or CoronaVac in previously vaccinated individuals enhances neutralizing activity against BA.1 and other variants, albeit with a lower antibody titer for BA.1. Our data suggest that a booster vaccination is important to broaden neutralizing antibody responses against the variants.
Subject areas: Immune response and virology
Graphical abstract
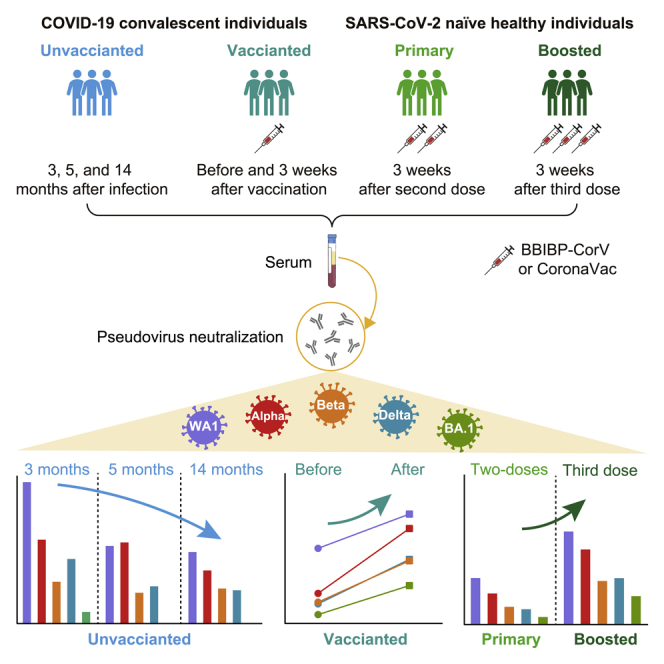
Highlights
-
•
Limited duration of antibody response against BA.1 in convalescent individuals
-
•
Infection before BBIBP-CorV or CoronaVac vaccination boosts neutralization
-
•
Two doses of BBIBP-CorV or CoronaVac elicit limited neutralizing activity against VOCs
-
•
Neutralization breadth for BA.1 is boosted by a third dose of BBIBP-CorV or CoronaVac
immune response” and virology.
Introduction
Several severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VOCs), including B.1.1.7 (Alpha)—was first detected in the United Kingdom, B.1.351 (Beta)—was first detected in South Africa, P.1 (Gamma)—was first detected in Brazil, and B.1.617.2 (Delta)—was first detected in India, have successively emerged with multiple substitutions in the spike glycoprotein. The variant Beta showed significant immune evasion from serum neutralizing antibodies (Wang et al., 2021a) and compromised the efficacy of vaccines (Abu-Raddad et al., 2021; Madhi et al., 2021; Sadoff et al., 2021), whereas the variant Delta quickly outcompeted all other VOCs since it was first identified in India in October 2020 (WHO, 2021) and partially evaded neutralization (Mlcochova et al., 2021). The Omicron variant BA.1 (B.1.1.529) was first identified in South Africa in November 2021 and has spread rapidly worldwide, outcompeting Delta to become the dominant circulating variant (Viana et al., 2021). Given a large number of over 30 mutations in the BA.1 spike protein, it has substantially decreased the neutralization activity of monoclonal and serum polyclonal antibodies elicited by infection or vaccination (Cao et al., 2021; Garcia-Beltran et al., 2022; Henning et al., 2022; Hoffmann et al., 2021; Liu et al., 2021; Planas et al., 2021).
However, studies have shown that a single dose of administered mRNA vaccine booster in previously infected individuals or vaccinated individuals significantly increased the antibody response to neutralize the Omicron variant (Anichini et al., 2021; Bradley et al., 2021; Carreño et al., 2021; Garcia-Beltran et al., 2022; Henning et al., 2022; Hoffmann et al., 2021; Lustig et al., 2021; Planas et al., 2021; Stamatatos et al., 2021; Wang et al., 2021b). The two inactivated SARS-CoV-2 vaccines, BBIBP-CorV by Sinopharm and CoronaVac by Sinovac developed in China, have been widely used in China and many countries, but their immunogenicity (Xia et al., 2020; Zhang et al., 2021) seems low compared to individuals who received an mRNA SARS-CoV-2 vaccine (Anderson et al., 2020; Lim et al., 2021; Walsh et al., 2020). Therefore, whether a single dose of the inactivated vaccines in previously infected individuals or a third dose booster vaccination in vaccinated individuals boosts an enhanced neutralization activity against BA.1 and the earlier variants needs to be determined. To address this question, we determined the sensitivity of variants Alpha, Beta, Delta, and BA.1 to serum neutralizing antibodies in convalescent individuals after natural infection, in previously infected individuals after one dose of the BBIBP-CorV or CoronaVac vaccines, in individuals after a primary vaccine series, and in two-dose BBIBP-CorV- or CoronaVac-vaccinated individuals after a third dose booster.
Results
Substantial escape of BA.1 to convalescent sera
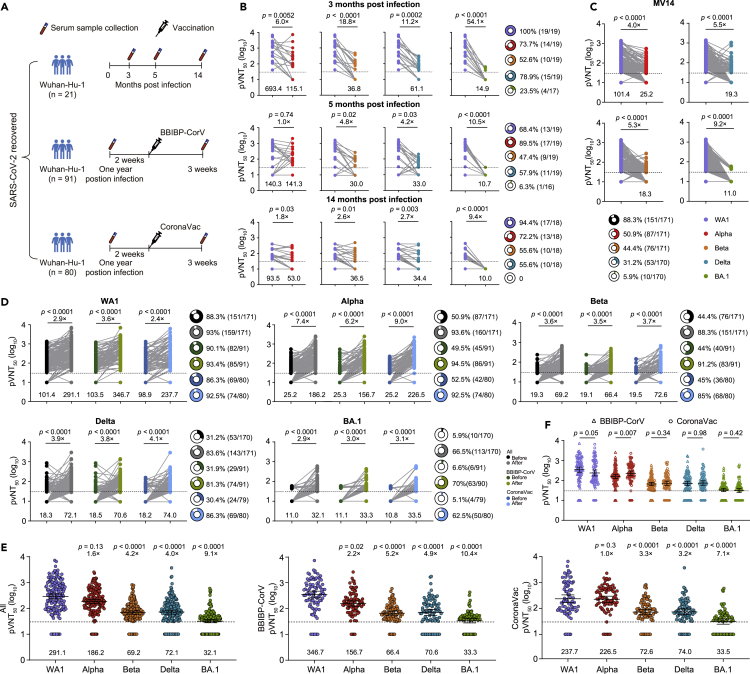
We first examined the dynamic neutralization activity of available longitudinal serum samples (at least two follow-up points) from 21 patients with COVID-19 in our cohort study (Jiang et al., 2021) to Alpha, Beta, Delta, and BA.1 (Figure 1A and Table 1). All these patients were diagnosed with SARS-CoV-2 infection by RT-PCR. They were not vaccinated at each sampling time. We analyzed available serum samples collected at a median of 107 (IQR 103–109, n = 19), 151 (IQR: 146–152, n = 19), and 424 (IQR: 420–426, n = 18) days after infection, referred to as months 3, 5, and 14 (M3, M5, and M14, respectively). The results obtained in pseudovirus assays typically correlate well with those obtained against the authentic virus (Sholukh et al., 2021). We measured the pseudovirus neutralization titer (pVNT50) for each combination of serum and viruses pseudotyped with the spike protein of Alpha, Beta, Delta, and BA.1 variants. With BA.1, the geometric mean titers (GMT) were significantly decreased by ∼9- to 54-fold from M3 to M14 compared to wild-type SARS-CoV-2 (Wuhan-Hu-1, WA1) (Figure 1B). This reduction in neutralizing titers was similar to that of Beta (∼3- to 19-fold) and Delta (∼3- to 11-fold) (Figure 1B). We then classified the individuals as neutralizers (titer ≥30) and nonneutralizers (titer <30) for the variants. Between 52.6% and 100% of the individuals neutralized WA1, Alpha, and Beta at M3, whereas only 23.5% of individuals neutralized BA.1 (Figure 1B). The fraction of neutralizers declined over time, and a few individuals neutralized BA.1, and approximately 50% of individuals at each time point neutralized Beta and Delta (Figure 1B). In contrast, most individuals can neutralize WA1 and Alpha. These results indicate that BA.1 displays extensive resistance to neutralization by sera from convalescent individuals even three months after infection.
Figure 1.
Serum neutralizing sensitivity to SARS-CoV-2 variants in previously infected individuals before and after a single-dose vaccination
(A) Schematic representation of cohorts and sample collection of convalescent individuals after SARS-CoV-2 Wuhan-Hu-1 (WA1) infection and previously WA1-infected individuals before and after vaccination.
(B) Neutralizing activity of sera from convalescent individuals at approximately 3 (n = 19), 5 (n = 19), and 14 (n = 18) months after infection against Alpha, Beta, Delta, and BA.1 compared with SARS-CoV-2 Wuhan-Hu-1 (WA1) virus.
(C) Neutralizing activity of Alpha, Beta, Delta, and BA.1 by sera from 171 previously infected individuals 14 months after infection compared with WA1.
(D) Serum neutralizing antibody to WA1 and variants in 171 previously infected individuals before and after one immunization with the BBIBP-CorV (n = 91) or CoronaVac (n = 80) vaccines.
(E) Comparison of the geometric mean titer (GMT) between WA1 and variants in all 171 previously infected individuals after vaccination, 91 (90 for BA.1 variant due to limited serum volume) previously infected individuals after vaccination with BBIBP-CorV, and 80 previously infected individuals after vaccination with CoronaVac.
(F) Comparison of GMT against WA1 and variants between previously infected individuals vaccinated with BBIBP-CorV and CoronaVac. The fold-change in the GMT is displayed in (B–E), and GMTs are shown below each column. The pie charts in (B–D) show the percentage of samples with neutralization activity. The bar represents the GMT and 95% confidence interval in (E) and (F). The 50% pseudovirus neutralization titer (pVNT50) was measured in serum samples as a 50% inhibitory dilution of pseudovirus infection. The horizontal dashed line represents the limit of detection (titer = 30); this limit was assigned a value of 10 for geometric mean calculations and was considered seronegative. Each symbol of dots or triangles represents one serum specimen. Data are the average of two duplicates. Statistical significance was determined using the two-tailed Friedman test with a false discovery rate for multiple comparisons in (B), (C), and (E), and WA1 was used as the reference, two-tailed Wilcoxon matched-pairs signed-rank test between groups in (D), and two-tailed Wilcoxon Mann–Whitney test between groups in (F).
Table 1.
Characteristics of the cohort of convalescent individuals
| Characteristics | N = 21 |
|---|---|
| Sex | |
| Male | 13 |
| Female | 8 |
| Age (median; IQR) | 46 (37–54) |
| Severity | |
| Severe | 3 |
| Moderate | 16 |
| Asymptomatic | 2 |
| Samples/sampling points after infection (median; IQR) | |
| 3 months | 19/107 (103–109) |
| 5 months | 19/151 (146–152) |
| 14 months | 18/424 (420–426) |
IQR, interquartile range.
Pre-infection followed by immunization with inactivated vaccines elicits potent variant cross-neutralization
Because of the significantly reduced neutralization activity of convalescent sera three months after infection against BA.1, we asked whether previously infected individuals could boost neutralizing activity after receiving a single-dose immunization inactivated vaccine. We analyzed sera from 171 previously infected individuals before and after a single-dose vaccination with BBIBP-CorV (n = 91) or CoronaVac (n = 80) (Table 2). The serum samples were collected before vaccination, with a median day of 433 (IQR: 433–436, referred to as MV14) and three weeks after receiving a dose of BBIBP-CorV or CoronaVac, with a median of 19 (IQR 19–19) days for BBIBP-CorV and 19 (IQR 19–19) days for CoronaVac. Their ages ranged from 19 to 63 for the BBIBP-CorV vaccinee and 21 to 64 for the CoronaVac vaccinee. In line with the results at M14, the greatest loss of neutralizing activity against BA.1 was observed with a 9.2-fold reduction in GMT compared to WA1, and no individuals had neutralizing antibody titers above 100 (Figure 1C). Three weeks after a single immunization, neutralizing antibody titers against WA1, Alpha, Beta, Delta, and BA.1 significantly increased by an overall of 2.9-, 7.4-, 3.6-, 3.9-, and 2.9-fold (GMT of 291.1, 186.2, 69.2, 72.1, and 32.1, respectively) when compared to the neutralizing antibody titers before vaccination (Figure 1C). Although there was a significant increase in the neutralization titer against BA.1 after vaccination and 62.5% of sera neutralized BA.1, a low GMT of 34 was observed, and most serum samples had an antibody titer less than 100. The neutralization titers were, however, reduced by 1.6- (2.2-fold for BBIBP-CorV and 1.0-fold for CoronaVac), 4.2- (5.2-fold for BBIBP-CorV and 3.3-fold for CoronaVac), 4.0- (4.9-fold for BBIBP-CorV and 3.2-fold for CoronaVac), and 9.1 (10.4-fold for BBIBP-CorV and 7.1-fold for CoronaVac)-fold against Alpha, Beta, Delta, and BA.1, respectively, when compared to WA1 (Figure 1E). No significant differences in antibody titers against WA1 and the tested VOCs were observed between the two inactivated vaccines except for a higher antibody titer against Alpha in CoronaVac-vaccinated individuals than in BBIBP-CorV-vaccinated individuals (Figure 1F). Therefore, a single dose of inactivated vaccines boosts efficient neutralizing activity to BA.1 and earlier VOCs, albeit with a lower antibody titer for BA.1.
Table 2.
Characteristics of previously infected individuals with a single dose of BBIBP-CorV or CoronaVac vaccination
| Characteristics | Previously infected + 1-dose BBIBP-CorV | Previously infected + 1-dose CoronaVac |
|---|---|---|
| No. of participants | 91 | 80 |
| Age (median, range) | 42 (19–63) | 40 (21–64) |
| Sex | ||
| Male (%) | 86 (94.5) | 72 (90.0) |
| Female (%) | 5 (5.5) | 8 (10.0) |
| Time interval to blood sampling (Median, IQR) | ||
| Infection to blood sampling | 433.0 (433.0–435.0) | 433.0 (433.0–437.8) |
| One-dose to blood sampling | 19.0 (19.0–19.0) | 19.0 (19.0–19.0) |
IQR, interquartile range.
Two doses of inactivated vaccines elicit poor neutralization of BA.1
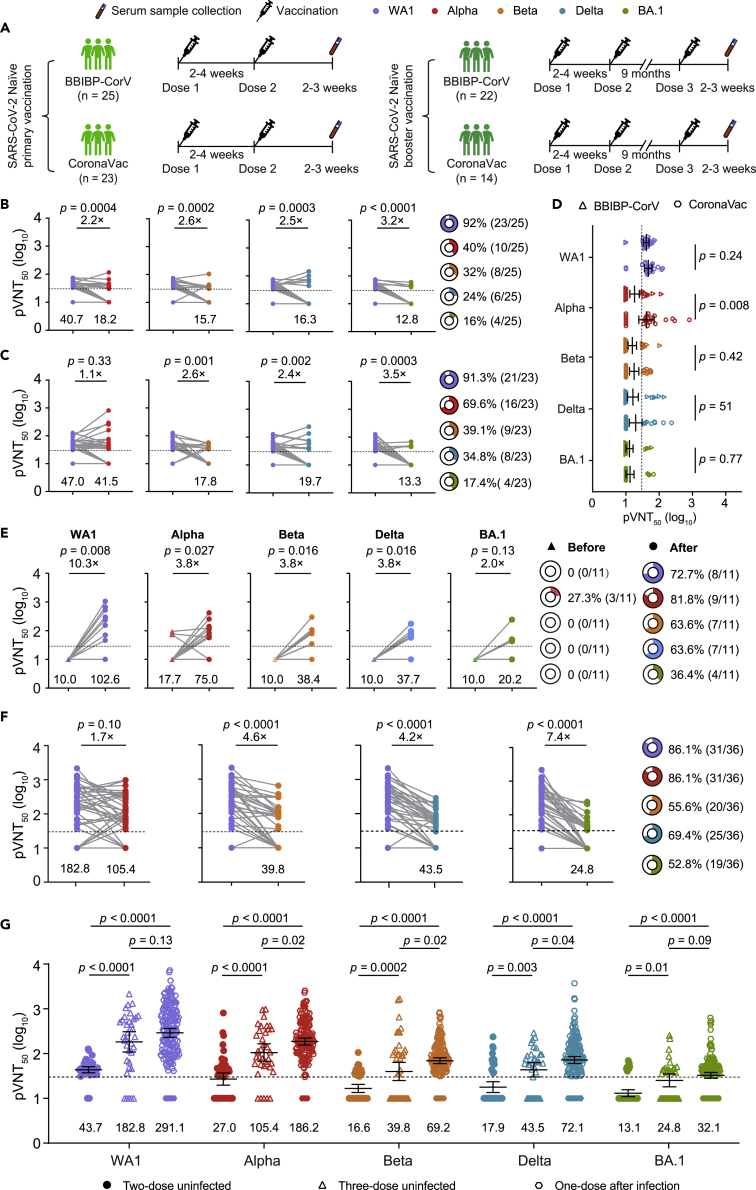
To assess the neutralizing activity of sera collected from SARS-CoV-2 naive individuals after two-dose immunization of inactivated vaccine against the variants, 48 SARS-CoV-2 naive individuals who received two-dose BBIBP-CorV (n = 25) or CoronaVac vaccines (n = 23) were enrolled, and their blood samples were collected 2–3 weeks after the second dose (Figure 2A). Their ages ranged from 28 to 59 for the BBIBP-CorV vaccinee and 34 to 59 for the CoronaVac vaccinee, with a median of 46 and 50, respectively. Eight were male, 17 were female for BBIBP-CorV, seven were male, and 16 were female for the CoronaVac vaccinee (Table 3). We found that 91.7% (44/48) of naive individuals ∼3 weeks after the second dose had a neutralizing antibody titer >30 with a GMT of 43.7 against WA1 (Figures 2B and 2C). However, we observed that more than 60% of serum samples lost neutralizing activity against Beta and Delta variants, although there was an approximately 2.5-fold reduction in GMT in BBIBP-CorV- or CoronaVac-elicited serum neutralizing activity against Beta and Delta (Figures 2B and 2C). Moreover, BA.1 displayed extensive escape of serum neutralization among the tested VOCs, and >80% of the serum samples had no antiviral activity. Similar to the antibody titers against Alpha in the previously infected individuals after vaccination, CoronaVac vaccinees had higher antibody titers against Alpha than BBIBP-CorV vaccinees, whereas no significant differences in antibody titers against WA1 and other tested VOCs were observed between the two groups (Figure 2D). This finding indicates that BA.1 is poorly or not neutralized by serum from SARS-CoV-2 naive individuals after the second vaccination, whereas other variants were less or moderately neutralized.
Figure 2.
Neutralizing antibody response following two-dose and three-dose vaccination
(A) Vaccination and blood sample draw timelines of individuals with primary vaccine series or booster vaccination.
(B and C) Serum neutralization of Alpha, Beta, Delta, and BA.1 by pseudovirus neutralization test in 25 BBIBP-CorV vaccine recipients (B) and 23 CoronaVac vaccine recipients (C) 3 weeks after the second dose.
(D) Comparison of the geometric mean titer (GMT) against SARS-CoV-2 Wuhan-Hu-1 (WA1) and Alpha, Beta, Delta, and BA.1 between naive individuals vaccinated with BBIBP-CorV and CoronaVac.
(E) Serum neutralization of Alpha, Beta, Delta, and BA.1 variants in 11 HCWs with paired serum before and after the third dose vaccination.
(F) Neutralizing activity against variants in the serum of all 36 HCWs after the third dose vaccination.
(G) Comparison of pseudovirus neutralizing GMT to WA1 and variants among 48 naive individuals after two-dose vaccination with BBIBP-CorV and CoronaVac and 36 naive individuals after third-dose vaccination with BBIBP-CorV or CoronaVac and 171 (170 for Delta and BA.1 variant due to limited serum volume) previously infected individuals after a single dose of BBIBP-CorV and CoronaVac. The fold-change of GMT is displayed in (B), (C), (E), and (F), and GMT is shown below each column. The pie charts in (B), (C), (E), and (F) show the percentage of samples with neutralization activity. The bar represents the GMT and 95% confidence interval in (G). The 50% pseudovirus neutralization titer (pVNT50) was measured in serum samples as a 50% inhibitory dilution of pseudovirus infection. The horizontal dashed line represents the limit of detection (titer = 30); this limit was assigned a value of 10 for geometric mean calculations and was considered seronegative. Each symbol of dots, triangles, or hexagons represents one serum specimen. Data are the average of two duplicates. Statistical significance was determined using the two-tailed Friedman test with a false discovery rate for multiple comparisons in (B), (C), and (G), the two-tailed Wilcoxon Mann–Whitney test between groups in (D), and WA1 was used as the reference. The two-tailed Wilcoxon matched-pairs signed-rank test between groups in (E) and the two-tailed Kruskal–Wallis with false discovery rate method in (G) were used.
Table 3.
Characteristics of naive individuals with two doses of BBIBP-CorV or CoronaVac vaccination
| Characteristics | Naive individuals + 2-dose BBIBP-CorV | Naive individuals + 2-dose CoronaVac |
|---|---|---|
| No. of participants | 25 | 23 |
| Age (median, range) | 46 (28–59) | 50 (34–59) |
| Sex | ||
| Male (%) | 8 (32) | 7 (30.4) |
| Female (%) | 17 (68) | 16 (69.6) |
| Time interval to blood sampling (Median, IQR) | ||
| One-dose to blood sampling | 58.0 (53.0–67.0) | 50.0 (48.0–57.0) |
| Two-dose to blood sampling | 21.0 (19.0–24.0) | 16.0 (16.0–17.0) |
IQR, interquartile range.
Three inactivated vaccine doses elicit potent variant cross-neutralization
We then asked whether a third-dose boost vaccination in previously vaccinated individuals would induce equal or even stronger immune responses to neutralize BA.1 and earlier VOCs. Thirty-six healthcare workers (HCWs) who received a third boost dose of BBIBP-CorV or CoronaVac vaccines were enrolled, with a median day of 269 (IQR, 264–288.8) and 259 (IQR, 234.0–259.0) after the second dose vaccination (Figure 2A and Table 4). For 11 HCWs with paired serum samples before and after the third vaccination, we observed that serum samples collected before the third dose, approximately nine months after the second dose vaccination, had no neutralizing activity against WA1, Beta, Delta, and BA.1 except for three serum samples that had less neutralizing activity against Alpha (Figure 2E). However, serum samples after booster immunization with BBIBP-CorV or CoronaVac significantly enhanced neutralizing antibody responses, and most of them had neutralizing antibodies against WA1, Alpha, Beta, Delta, and BA.1 variants, with a 10.3-, 3.8-, 3.8-, and 3.8-, and 2.0-fold increase in GMTs compared to the GMTs before the third vaccination (Figure 2E).
Table 4.
Characteristics of vaccinated individuals with a third dose of BBIBP-CorV or CoronaVac vaccination
| Characteristics | Third dose vaccination |
|
|---|---|---|
| BBIBP-CorV | CoronaVac | |
| No. of participants | 22 | 14 |
| Age (median, range) | 47.0 (30.0–59.0) | 41.0 (35.0–55.0) |
| Sex | ||
| Male (%) | 13 (59.1) | 8 (57.1) |
| Female (%) | 9 (40.9) | 6 (42.9) |
| Interval between 2nd and before 3rd (median, IQR) | 269.0 (264.0–288.8) | 259.0 (234.0–259.0) |
| Interval between 3rd and sampling (median, IQR) | 21.5 (21.0–27.0) | 23.0 (18.0–23.0) |
IQR, interquartile range.
We then compared the neutralizing activity of sera from all 36 HCWs after the third dose to VOCs compared to WA1. Similar to the results from the 11 HCWs, over half of them had neutralizing antibodies against WA1, Alpha, Beta, Delta, and BA.1 variants. However, the neutralization titers were significantly reduced by 4.6- and 4.2-fold against Beta and Delta, respectively, when compared to WA1, and the greatest reduction of 7.4-fold was observed for BA.1 (Figure 2F). Further analysis showed that neutralizing antibody titers against WA1, Alpha, Beta, Delta, and BA.1 were significantly higher in both previously infected individuals with a single-dose booster vaccination and vaccinated individuals with the third booster vaccination when compared to antibody titers in uninfected individuals with two immunizations (Figure 2G). In addition, a single-dose vaccination in previously infected individuals elicited higher antibody titers against Alpha, Beta, and Delta, whereas equal antibody titers against WA1 and BA.1 were observed. These data indicate that the booster vaccination increased the magnitude and breadth of neutralizing antibodies in previously vaccinated individuals and boosted a certain degree of neutralizing activity to BA.1 and other tested variants.
Discussion
This study indicates that sera from both previously infected individuals and SARS-CoV-2 naive vaccinees with a two-dose vaccination course of BBIBP-CorV or CoronaVac displayed extensive loss of neutralizing activity to BA.1. However, a single-dose vaccination of BBIBP-CorV or CoronaVac in previously infected individuals and a third booster vaccination in SARS-CoV-2 naive individuals with a two-dose vaccination course could significantly enhance the neutralization activity of BA.1 and earlier VOCs. However, the neutralization of BA.1 was significantly lower than that of other VOCs.
In contrast to a boost in vaccine-matched neutralizing antibodies in previously infected individuals who received a single mRNA vaccine dose compared to those without prior infection (Anichini et al., 2021; Blain et al., 2021; Bradley et al., 2021; Dimeglio et al., 2021; Lustig et al., 2021; Manisty et al., 2021; Prendecki et al., 2021; Saadat et al., 2021; Stamatatos et al., 2021; Wang et al., 2021b), a single-dose vaccination of other types of vaccines in previously infected individuals, including inactivated vaccine BBIBP-CorV or CoronaVac, has not been studied. This study first documented the antibody response in individuals with prior infection during the first wave of the COVID-19 outbreak in China in early 2020 after a single dose of the BBIBP-CorV or CoronaVac vaccines. Consistent with previous studies (Li et al., 2021; Wang et al., 2021b), we found that approximately 88.3% of 171 previously infected individuals maintained neutralizing antibodies against WA1 14 months after infection. However, the antibody titers against Alpha, Beta, Delta, and BA.1 were significantly decreased, and most of the serum samples had lost the ability to neutralize the earlier variants Beta and Delta. A dramatic loss of neutralizing activity against BA.1 was observed. These findings suggest that most previously infected individuals over one year after infection may be at a higher risk of reinfection with Beta, Delta, and BA.1.
Importantly, after receiving a single immunization with either BBIBP-CorV or CoronaVac, previously infected individuals generated a potent antibody response against vaccine-matched WA1 and the variants Alpha, Beta, and Delta, with an ∼2- to 10-fold increase in GMT compared to WA1, which is in line with previous studies showing that a single dose of mRNA in previously infected individuals enhanced neutralizing activity (Garcia-Beltran et al., 2022; Lustig et al., 2021; Stamatatos et al., 2021; Wang et al., 2021b). However, although approximately 66% of serum samples could neutralize the BA.1 pseudovirus after a single immunization, most of the serum samples had an antibody titer less than 100, indicating that a single immunization with either BBIBP-CorV or CoronaVac in previously infected individuals remains at higher risk of infection with BA.1 compared to other tested variants (Garcia-Beltran et al., 2022). However, whether a second dose of BBIBP-CorV or CoronaVac could offer additional benefit against BA.1 remains to be determined because previous studies have revealed that a second dose mRNA vaccine administration did not induce a substantially greater benefit over a single dose in antibody neutralizing potential in previously infected individuals (Ebinger et al., 2021; Saadat et al., 2021; Stamatatos et al., 2021). Collectively, a single dose of BBIBP-CorV or CoronaVac in previously infected individuals enhances neutralizing breadth across the tested VOCs, including BA.1, albeit at a lower antibody titer.
Neutralizing antibodies are also believed to be crucial for protection against COVID-19 by vaccination (Feng et al., 2021; Gilbert et al., 2021). In this study, we found that the antibody response induced by two doses of BBIBP-CorV or CoronaVac was not only less effective in neutralizing earlier VOCs but also extensively ineffective in neutralizing BA.1, which is in accordance with recent results showing that BA.1 has extensively escaped the serum from individuals with primary vaccination of mRNA, Ad26.COV2.S, ChAdOx1-nCoV-19, and BBIBP-CorV or CoronaVac-inactivated vaccine (Ai et al., 2022; Ai et al., 2021; Chen et al., 2022; Dejnirattisai et al., 2021; Garcia-Beltran et al., 2022; Hoffmann et al., 2021; Wang et al., 2022). While in line with recent studies that either homologous or heterologous booster vaccination enhanced neutralizing activity against earlier VOCs and BA.1 (Ai et al., 2022; Ai et al., 2021; Chen et al., 2022; Garcia-Beltran et al., 2022; Hoffmann et al., 2021; Wang et al., 2022), we also observed that a third dose boost vaccination of BBIBP-CorV or CoronaVac enhanced antibody responses against WA1 in most vaccine recipients and neutralization activity to all tested VOCs. However, ∼52% of serum samples from boosted individuals neutralized BA.1, and a similar result was also observed in one study by Chen et al. in which ∼55% of individuals who received a third dose of CoronaVac had neutralizing antibodies to neutralize BA.1 (Chen et al., 2022). In contrast, two other studies by Ai et al. (2022) and Wang et al. (2022) showed that 70%–85% of individuals with a third dose booster of BBIBP-CorV produced neutralizing antibodies against BA.1. Such differences may be affected by sample size and different pseudovirus systems in these and our studies. Collectively, these data suggest that it is essential to develop boost vaccination strategies for inactivated vaccines to enhance herd immunity to prevent the transmission of SARS-CoV-2 and infection or severe disease.
In summary, our findings show that BA.1 significantly escapes neutralizing antibodies elicited by previous SARS-CoV-2 infection or vaccination, but a single dose of BBIBP-CorV or CoronaVac could partly enhance the immune response against BA.1. Although we did not test serum neutralizing activity against BA.2, BA.3, BA.2.12.1, and BA.4/5, our results would be similar to recent studies that the neutralization data of BA.1, BA.2, and BA.3 could be comparable and that BA.2.12.1 and BA.4/BA.5 could be more substantially evade (Ai et al., 2022; Arora et al., 2022; Cao et al., 2022; Evans et al., 2022; Qu et al., 2022; Yu et al., 2022). Collectively, our findings support the need for the rapid development of additional inactivated vaccine doses as a significant public health measure to reduce the spread of highly mutated SARS-CoV-2 variants.
Limitations of the study
Limitations of our study include that the neutralizing antibodies were measured ∼3 weeks after one single-dose vaccination in previously infected individuals and ∼3 weeks after the third dose in previously vaccinated individuals. It remains to be determined whether the antibody response against BA.1 is long persistent. In addition, despite the extensive escape of BA.1 to antibodies elicited by natural infection and booster vaccination, recent studies have shown that the majority of T cell responses elicited by infection or vaccination remain capable of recognizing BA.1 and early emerged variants (Keeton et al., 2022; Tarke et al., 2022). Whether cellular immunity will be effective as a second-level defense in preventing severe disease after BA.1 infection in the absence of a potent neutralizing antibody response remains to be determined (Goel et al., 2021; Sette and Crotty, 2021). Therefore, measures of T and B cell responses can shed further light on whether a single-dose vaccination of these two inactivated vaccines might be sufficient for augmenting T and B cell memory in previously infected individuals.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| SARS-CoV-2 pseudovirus for WA1 | This study | N/A |
| SARS-CoV-2 pseudovirus for Alpha | This study | N/A |
| SARS-CoV-2 pseudovirus for Beta | This study | N/A |
| SARS-CoV-2 pseudovirus for Gamma | This study | N/A |
| SARS-CoV-2 pseudovirus for Delta | This study | N/A |
| SARS-CoV-2 pseudovirus for BA.1 | This study | N/A |
| SARS-CoV-1 pseudovirus | This study | N/A |
| E.coli DH5α Competent Cells | TaKaRa | Cat# 9057 |
| Biological samples | ||
| Sera from convalescent patients | This study | N/A |
| Sera samples from previously infected individuals before and after vaccination | This study | N/A |
| Sera from vaccinees with two doses of inactivated vaccine | This study | N/A |
| Sera from vaccinees with the third dose of inactivated vaccine | This study | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Phosphate buffered saline (PBS) | Gibco | Cat# C10010500BT |
| Dulbecco’s modified eagle medium (DMEM) | Gibco | Cat# C111995500BT |
| Trypsin-EDTA (0.25%) | Solarbio | Cat# T1300 |
| HEPES | Gibco | Cat# 15630-080 |
| Fetal bovine serum (FBS) | Gibco | Cat# 10099-141C |
| Penicillin/streptomycin | Gibco | Cat# 15140-122 |
| PEI MAX (MW 40000) | Polysciences | Cat# 24765-1 |
| Luciferase Assay Reagent | Vazyme | Cat# DD1201-01 |
| Experimental models: Cell lines | ||
| HeLa-hACE2 cells | Tsinghua University | N/A |
| HEK-293T cells | ATCC | Cat# CRL-3216 |
| Recombinant DNA | ||
| pcDNA3.1-SARS2-Spike | Addgene | Cat# 145032 |
| pCDNA3.3_CoV2_B.1.1.7 | Addgene | Cat# 170451 |
| pcDNA3.3_CoV2_501V2 | Addgene | Cat# 170499 |
| pcDNA3.3-SARS2-B.1.617.2 | Addgene | Cat# 172320 |
| Omicron BA.1 spike plasmid | This study | N/A |
| Firefly luciferase encoding lentivirus backbone plasmid | Tsinghua University | N/A |
| Software and algorithms | ||
| GraphPad Prism | GraphPad Prism | https://www.graphpad.com/scientific-software/prism/ |
| Adobe Illustrator | Version 23.0.1 | https://www.adobe.com/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Mai-Juan Ma (mjma@163.com).
Materials availability
All unique reagents generated during this study are available from the lead contact with a completed materials transfer agreement.
Experimental model and subject details
Ethics statement
The study was approved by the Institutional Review Board of the Beijing Institute of Microbiology and Epidemiology (IRB number: AF/SC-08/02.60 and AF/SC-08/02.124). All participants provided written informed consent.
Sera from convalescent patients
Since February 2020, we have conducted a longitudinal cohort study enrolling 25 Wuhan-Hu-1 SARS-CoV-2-infected individuals to measure one year’s humoral and cellular response maintenance. The partial results of the study regarding antibody and T-cell response in these COVID-19 patients 3–4 months after infection have been published (Jiang et al., 2021). The cohort study is ongoing, the patients were followed up at 5 and 14 months after infection, and blood samples were collected at each follow-up visit. The available longitudinal sera from 21 (8 females/13 males with a median age of 46 years, interquartile range [IQR] 37–54) of 25 COVID-19 patients at 3 (n = 19), 5 (n = 19), and 14 (n = 18) months after infection were included for analysis to evaluate the dynamic changes of convalescent sera in neutralizing activity to variants of concern.
Sera from previously infected individuals before and after vaccination
Between March and April 2021, 264 recovered COVID-19 patients were enrolled over one year after infection to analyze the antibody response, and blood samples were collected. In May 2021, 171 (13 females/158 males with a median age of 42 years; IQR, 33–50) of these 264 patients received a single dose vaccination of BBIBP-CorV or CoronaVac vaccines, and their blood samples were collected ∼3 weeks after vaccination.
Sera from vaccinees with two doses of inactivated vaccine
Twenty-five (17 females/8 males with a median age of 46 years, IQR 28–59) sera for the BBIBP-CorV vaccinees were obtained from the staff who received two doses of immunization administered 14–28 days apart at the local health service center in Dezhou City, Shandong Province, China. Twenty-three (16 females/7 males with a median age of 50 years, IQR 34–59) sera for CoronaVac vaccinees were obtained from healthcare workers who had no prior SARS-CoV-2 infection and received two-dose immunization administered 14–28 days apart at the Ningjin County Community Health Servers Center in Dezhou City, Shandong Province, China. Sera for BBIBP-CorV vaccinees were collected approximately 58 days after the first dose immunization and approximately 21 days after the second dose immunization. Sera for CoronaVac vaccinees were collected approximately 50 days after the first dose immunization and16 days after the second dose immunization.
Sera from vaccinees with the third dose of inactivated vaccine
Sera for BBIBP-CorV and CoronaVac recipients with two doses prior to vaccination were obtained from 36 (15 females/21 males with a median age of 47.0 years, IQR 40.0–52.8) HCWs at a local healthcare center and the Jining Center for Disease Control and Prevention in Jining City of Shandong Province, China. These HCWs were frontline medical staff and received two-dose vaccination with the two inactivated vaccines, BBIBP-CorV by Sinopharm or CoronaVac by Sinovac, between July 2020 and February 2021. In April 2021, serum samples from 11 of 36 HCWs were collected before the third dose vaccination. They received a third dose vaccine between June and July 2021, and serum samples from all of them were collected ∼3 weeks after the third dose vaccination.
Cell lines
Human embryonic kidney HEK-293T cells were cultured at 37°C and 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) containing 10% (v/v) heat-inactivated fetal bovine serum (FBS, Gibco) and supplemented with 1% penicillin–streptomycin (Gibco). Cells were disrupted at confluence with 0.25% trypsin in 1 mM EDTA (Solarbio) every 48–72 h. HeLa-hACE2 cells were provided by Prof. Lin-Qi Zhang from Tsinghua University and were cultured under the same conditions.
Method details
Pseudovirus production and neutralization assay
Pseudovirus particles were produced (Nie et al., 2020). SARS-CoV-2 pseudoviruses were generated by co-transfecting HEK-293T cells (ATCC) with human immunodeficiency virus backbones expressing firefly luciferase (pNL4-3-R-E-luciferase) and pcDNA3.1 vector encoding either wild-type (Wuhan-Hu-1), Alpha (Δ69-70, Δ144, N501Y, A570D, D614G, P681H, T716I, S982A, D1118H), Beta (L18F, D80A, D215G, R246I, K417N, E484K, N501Y, D614G, A701V), Delta (T19R, G142D, Δ156–157, R158G, L452R, T478K, D614G, P681R and D950N), BA.1 (A67V, Δ69–70, T95I, G142D, Δ143-145, Δ211, L212I, 214EPE, G339D, S371L, S373P, S375F, K417N, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, Y505H, T547K, D614G, H655Y, N679K, P681H, N764K, D796Y, N856K, Q954H, N969K, L981F) S proteins. The medium was replaced with fresh medium at 24 hours, and the supernatants were harvested at 48 hours post-transfection and clarified by centrifugation at 300 × g for 10 minutes before being aliquoted and stored at −80°C until use. The SARS-CoV-2 pseudovirus neutralization assay was performed (Li et al., 2020) with the target cell line HeLa cells expressing ACE2 orthologs (HeLa-ACE2). Duplicated threefold serially diluted serum (starting at 1:30) from COVID-19-convalescent individuals and vaccinees was incubated with SARS-CoV-2 pseudotyped virus for 1 hour at 37°C. HeLa-ACE2 cells (200,000 cells/well) were subsequently added to the mixture and incubated for approximately 48 hours at 37°C with 5% CO2. The supernatant was then removed, and luciferase substrate was added to each well, followed by incubation for 2 minutes in darkness at room temperature. Luciferase activity was then measured using a GloMax 96 Microplate Luminometer (Promega). The half-maximal neutralization titers were determined by luciferase activity 48 hours after exposure to the virus-serum mixture with a four-parameter nonlinear regression inhibitor curve in GraphPad Prism 8.4.1 (GraphPad Software). Neutralization titers are reported as the reciprocal of serum dilution that inhibited 50% of pseudovirus infection (pVNT50) compared to control wells with virus alone. pVNT50 values reported as below 30 (the detectable limit) were considered to be negative results and were assigned a value of 10 for the geometric mean calculation.
Quantification and statistical analysis
The Kruskal–Wallis or Friedman test with the false discovery rate method was used for multiple group comparisons. The Wilcoxon Mann–Whitney U test or Wilcoxon matched-pairs signed-rank test was used to compare the difference between the two groups. All statistical analyses were performed using GraphPad Prism (version 8.4.2, La Jolla, California USA), and all statistical tests were 2-sided with a significance level of 0.05. Details are additionally provided in the Figure legends.
Acknowledgments
We thank all patients and vaccine recipients for providing blood samples. This research was funded by the Beijing Natural Science Foundation (L202038) and the Natural Science Foundation of China (81773494 and 81621005).
Author contributions
M.-J.M. conceived the study. D.-L.J., W.-G.J., Z.-Y.W., B.-S.W., L.-B.-L., L.Y., K.-L.Z., Q.-C.M., and G.-L.W. collected serum samples. D.-L.J., L.Y., K.-L.Z., and G.-L.W. performed the experiments; D.-L.J., L.Y., and K.-L.Z. analyzed the data; M.-J.M. drafted the manuscript. All authors reviewed and approved the final manuscript.
Declaration of interests
We declare no competing interests.
Published: September 16, 2022
Data and code availability
This study did not generate sequence data or codes. Data generated in the current study (including neutralization and antibody measurements) have not been deposited in a public repository but are available from the lead contact upon request.
-
•
All data reported in this paper are available within the main manuscript and the supplementary files.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Abu-Raddad L.J., Chemaitelly H., Butt A.A. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N. Engl. J. Med. 2021;385:187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai J., Wang X., He X., Zhao X., Zhang Y., Jiang Y., Li M., Cui Y., Chen Y., Qiao R., et al. Antibody evasion of SARS-CoV-2 Omicron BA.1, BA.1.1, BA.2, and BA.3 sub-lineages. Cell Host Microbe. 2022 doi: 10.1016/j.chom.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai J., Zhang H., Zhang Y., Lin K., Zhang Y., Wu J., Wan Y., Huang Y., Song J., Fu Z., et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg. Microbes Infect. 2021;11:337–343. doi: 10.1080/22221751.2021.2022440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anichini G., Terrosi C., Gandolfo C., Gori Savellini G., Fabrizi S., Miceli G.B., Cusi M.G. SARS-CoV-2 antibody response in persons with past natural infection. N. Engl. J. Med. 2021;385:90–92. doi: 10.1056/NEJMc2103825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P., Zhang L., Rocha C., Sidarovich A., Kempf A., Schulz S., Cossmann A., Manger B., Baier E., Tampe B., et al. Comparable neutralisation evasion of SARS-CoV-2 omicron subvariants BA.1, BA.2, and BA.3. Lancet Infect. Dis. 2022;22:766–767. doi: 10.1016/s1473-3099(22)00224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain H., Tuaillon E., Gamon L., Pisoni A., Miot S., Picot M.C., Bousquet J. Spike antibody levels of nursing home residents with or without prior COVID-19 3 Weeks after a single BNT162b2 vaccine dose. JAMA. 2021;325:1898–1899. doi: 10.1001/jama.2021.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T., Grundberg E., Selvarangan R., LeMaster C., Fraley E., Banerjee D., Belden B., Louiselle D., Nolte N., Biswell R., et al. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N. Engl. J. Med. 2021;384:1959–1961. doi: 10.1056/NEJMc2102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Wang J., Jian F., Xiao T., Song W., Yisimayi A., Huang W., Li Q., Wang P., An R., et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2021;602:657–663. doi: 10.1038/d41586-021-03796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Yisimayi A., Jian F., Song W., Xiao T., Wang L., Du S., Wang J., Li Q., Chen X., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022 doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreño J.M., Alshammary H., Tcheou J., Singh G., Raskin A., Kawabata H., Sominsky L., Clark J., Adelsberg D.C., Bielak D., et al. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2021;602:682–688. doi: 10.1038/d41586-021-03846-z. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen L., Yin S., Tao Y., Zhu L., Tong X., Mao M., Li M., Wan Y., Ni J., et al. The Third dose of CoronVac vaccination induces broad and potent adaptive immune responses that recognize SARS-CoV-2 Delta and Omicron variants. Emerg. Microbes Infect. 2022;11:1524–1536. doi: 10.1080/22221751.2022.2081614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejnirattisai W., Shaw R.H., Supasa P., Liu C., Stuart A.S., Pollard A.J., Liu X., Lambe T., Crook D., Stuart D.I., et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet. 2021;399:234–236. doi: 10.1016/s0140-6736(21)02844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimeglio C., Herin F., Da-Silva I., Porcheron M., Martin-Blondel G., Miedougé M., Izopet J. Strong SARS-CoV-2-neutralizing antibody response of previously-infected healthcare workers given one dose of mRNA vaccine. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab573. [DOI] [PubMed] [Google Scholar]
- Ebinger J.E., Fert-Bober J., Printsev I., Wu M., Sun N., Prostko J.C., Frias E.C., Stewart J.L., Van Eyk J.E., Braun J.G., et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.P., Qu P., Zeng C., Zheng Y.-M., Carlin C., Bednash J.S., Lozanski G., Mallampalli R.K., Saif L.J., Oltz E.M., et al. Neutralization of the SARS-CoV-2 Deltacron and BA.3 variants. N. Engl. J. Med. 2022;386:2340–2342. doi: 10.1056/NEJMc2205019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., Dold C., Fuskova M., Gilbert S.C., Hirsch I., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Beltran W.F., St Denis K.J., Hoelzemer A., Lam E.C., Nitido A.D., Sheehan M.L., Berrios C., Ofoman O., Chang C.C., Hauser B.M., et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell. 2022;185:457–466.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P.B., Montefiori D.C., McDermott A.B., Fong Y., Benkeser D., Deng W., Zhou H., Houchens C.R., Martins K., Jayashankar L., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2021;375:43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel R.R., Painter M.M., Apostolidis S.A., Mathew D., Meng W., Rosenfeld A.M., Lundgreen K.A., Reynaldi A., Khoury D.S., Pattekar A., et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science. 2021;374:abm0829. doi: 10.1126/science.abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning G., Kanika V., Pinkus T.-L., David H., Philipp S., Clara L., Florian K., Leif S., Florian K. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat. Med. 2022 doi: 10.21203/rs.3.rs-1168453/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Krüger N., Schulz S., Cossmann A., Rocha C., Kempf A., Nehlmeier I., Graichen L., Moldenhauer A.-S., Winkler M.S., et al. The Omicron variant is highly resistant against antibody-mediated neutralization – implications for control of the COVID-19 pandemic. Cell. 2021;185:447–456.e11. doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X.L., Wang G.L., Zhao X.N., Yan F.H., Yao L., Kou Z.Q., Ji S.X., Zhang X.L., Li C.B., Duan L.J., et al. Lasting antibody and T cell responses to SARS-CoV-2 in COVID-19 patients three months after infection. Nat. Commun. 2021;12:897. doi: 10.1038/s41467-021-21155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeton R., Tincho M.B., Ngomti A., Baguma R., Benede N., Suzuki A., Khan K., Cele S., Bernstein M., Karim F., et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. 2022;603:488–492. doi: 10.1038/s41586-022-04460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Yu D., Wu X., Liang H., Zhou Z., Xie Y., Li T., Wu J., Lu F., Feng L., et al. Twelve-month specific IgG response to SARS-CoV-2 receptor-binding domain among COVID-19 convalescent plasma donors in Wuhan. Nat. Commun. 2021;12:4144. doi: 10.1038/s41467-021-24230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wu J., Nie J., Zhang L., Hao H., Liu S., Zhao C., Zhang Q., Liu H., Nie L., et al. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell. 2020;182:1284–1294.e9. doi: 10.1016/j.cell.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W.W., Mak L., Leung G.M., Cowling B.J., Peiris M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe. 2021 doi: 10.1016/S2666-5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Iketani S., Guo Y., Chan J.F.-W., Wang M., Liu L., Luo Y., Chu H., Huang Y., Nair M.S., et al. Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Nature. 2021;602:676–681. doi: 10.1038/d41586-021-03796-6. [DOI] [PubMed] [Google Scholar]
- Lustig Y., Nemet I., Kliker L., Zuckerman N., Yishai R., Alroy-Preis S., Mendelson E., Mandelboim M. Neutralizing response against variants after SARS-CoV-2 infection and one dose of BNT162b2. N. Engl. J. Med. 2021;384:2453–2454. doi: 10.1056/NEJMc2104036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., Padayachee S.D., Dheda K., Barnabas S.L., Bhorat Q.E., et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N. Engl. J. Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manisty C., Otter A.D., Treibel T.A., McKnight Á., Altmann D.M., Brooks T., Noursadeghi M., Boyton R.J., Semper A., Moon J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/s0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlcochova P., Kemp S.A., Dhar M.S., Papa G., Meng B., Ferreira I.A.T.M., Datir R., Collier D.A., Albecka A., Singh S., et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021;599:114–119. doi: 10.1038/s41586-021-03944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Li Q., Wu J., Zhao C., Hao H., Liu H., Zhang L., Nie L., Qin H., Wang M., et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Protoc. 2020;15:3699–3715. doi: 10.1038/s41596-020-0394-5. [DOI] [PubMed] [Google Scholar]
- Planas D., Saunders N., Maes P., Guivel-Benhassine F., Planchais C., Buchrieser J., Bolland W.-H., Porrot F., Staropoli I., Lemoine F., et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2021;602:671–675. doi: 10.1038/d41586-021-03827-2. [DOI] [PubMed] [Google Scholar]
- Prendecki M., Clarke C., Brown J., Cox A., Gleeson S., Guckian M., Randell P., Pria A.D., Lightstone L., Xu X.N., et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–1181. doi: 10.1016/s0140-6736(21)00502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu P., Faraone J., Evans J.P., Zou X., Zheng Y.-M., Carlin C., Bednash J.S., Lozanski G., Mallampalli R.K., Saif L.J., et al. Neutralization of the SARS-CoV-2 omicron BA.4/5 and BA.2.12.1 subvariants. N. Engl. J. Med. 2022;386:2526–2528. doi: 10.1056/NEJMc2206725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat S., Rikhtegaran Tehrani Z., Logue J., Newman M., Frieman M.B., Harris A.D., Sajadi M.M. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021;325:1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoff J., Gray G., Vandebosch A., Cárdenas V., Shukarev G., Grinsztejn B., Goepfert P.A., Truyers C., Fennema H., Spiessens B., et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholukh A.M., Fiore-Gartland A., Ford E.S., Miner M.D., Hou Y.J., Tse L.V., Kaiser H., Zhu H., Lu J., Madarampalli B., et al. Evaluation of cell-based and surrogate SARS-CoV-2 neutralization assays. J. Clin. Microbiol. 2021;59:e0052721. doi: 10.1128/jcm.00527-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatatos L., Czartoski J., Wan Y.H., Homad L.J., Rubin V., Glantz H., Neradilek M., Seydoux E., Jennewein M.F., MacCamy A.J., et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021;372:1413–1418. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarke A., Coelho C.H., Zhang Z., Dan J.M., Yu E.D., Methot N., Bloom N.I., Goodwin B., Phillips E., Mallal S., et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 2022;185:847–859.e11. doi: 10.1016/j.cell.2022.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana R., Moyo S., Amoako D.G., Tegally H., Scheepers C., Althaus C.L., Anyaneji U.J., Bester P.A., Boni M.F., Chand M., et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2021;603:679–686. doi: 10.1038/d41586-021-03832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Nair M.S., Liu L., Iketani S., Luo Y., Guo Y., Wang M., Yu J., Zhang B., Kwong P.D., et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature. 2021;593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhao X., Song J., Wu J., Zhu Y., Li M., Cui Y., Chen Y., Yang L., Liu J., et al. Homologous or heterologous booster of inactivated vaccine reduces SARS-CoV-2 Omicron variant escape from neutralizing antibodies. Emerg. Microbes Infect. 2022;11:477–481. doi: 10.1080/22221751.2022.2030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Muecksch F., Schaefer-Babajew D., Finkin S., Viant C., Gaebler C., Hoffmann H.H., Barnes C.O., Cipolla M., Ramos V., et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . 2021. Weekly Epidemiological Update on COVID-19.https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---11-may-2021 [Google Scholar]
- Xia S., Duan K., Zhang Y., Zhao D., Zhang H., Xie Z., Li X., Peng C., Zhang Y., Zhang W., et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Collier A.-r.Y., Rowe M., Mardas F., Ventura J.D., Wan H., Miller J., Powers O., Chung B., Siamatu M., et al. Neutralization of the SARS-CoV-2 omicron BA.1 and BA.2 variants. N. Engl. J. Med. 2022;386:1579–1580. doi: 10.1056/NEJMc2201849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21:181–192. doi: 10.1016/s1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate sequence data or codes. Data generated in the current study (including neutralization and antibody measurements) have not been deposited in a public repository but are available from the lead contact upon request.
-
•
All data reported in this paper are available within the main manuscript and the supplementary files.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.