Abstract
The sn-glycerol-3-phosphate acyltransferase (plsB) catalyzes the first step in membrane phospholipid formation. A conditional Escherichia coli mutant (plsB26) has a single missense mutation (G1045A) predicting the expression of an acyltransferase with an Ala349Thr substitution. The PlsB26 protein had a significantly reduced glycerol-3-phosphate acyltransferase specific activity coupled with an elevated Km for glycerol-3-phosphate.
The sn-glycerol-3-phosphate (G3P) acyltransferase catalyzes the first committed step in membrane phospholipid formation and acylates the 1-position of G3P with either acyl-acyl carrier protein (ACP) or acyl-coenzyme A (CoA) thioesters. The G3P acyltransferase in Escherichia coli has been extensively studied and is the membrane-bound product of the plsB gene (5, 15). Consistent with its position at the start of the phospholipid biosynthetic pathway, the PlsB protein functions as a sensor that monitors the metabolic state of the cell through its allosteric interactions with ATP and guanosine-3′,5′-tetraphosphate to coordinate the rate of G3P acylation with macromolecular biosynthesis and cell growth (5, 7, 11). Mutants defective in PlsB (plsB26) activity were isolated as G3P auxotrophs (1), a growth phenotype attributed to the expression of an acyltransferase with an elevated Km for G3P (1, 2). The identity of the mutation in plsB26 strains is unknown. Thus, the biochemical properties of the mutant protein are unknown, and it is not even clear if an active protein is expressed from the plsB26 allele since the possibility remains that the high Km activity detected in mutant membranes was due to a novel acyltransferase revealed by the absence of the normal PlsB protein. The structural gene for the PlsB acyltransferase is located at 92 min on the linkage map (10); however, moving this region of the chromosome from the plsB26 mutant into another genetic background did not transfer the G3P auxotrophic phenotype. This led to the discovery that a second mutation, called plsX, is required for expression of the G3P auxotrophic phenotype (9). The role of the PlsX protein in phospholipid biosynthesis is unknown, although it probably is not itself an acyltransferase (6). In light of the critical function of the acyltransferase reaction in phospholipid biosynthesis and the central role that the plsB26 mutation has played in studying this pathway (5, 15), we have determined the molecular and biochemical basis for the acyltransferase defect in plsB26 strains.
Identification of the mutation in the plsB26 allele.
The structure of the plsB26 allele in strain BB26 (plsB26 plsX50 glpD3 glpR2 phoA8 Strs relA1 spoT1 tonA22 T2R pit-10 HfrC) (1) was determined by using a PCR-based approach to sequence the entire gene plus about 100 bases of upstream DNA. Oligonucleotides (Table 1) were used in pairs to amplify overlapping 500-bp fragments, which were sequenced with the same primers by the Center for Biotechnology at St Jude Children’s Research Hospital. This procedure gave complete coverage of the 2.5-kbp gene and led to the identification of a single base pair change of G1045A compared to the sequence of the wild-type gene. This missense mutation lies in the first position of codon 349 and changes the predicted amino acid at this position from an Ala to a Thr. The wild-type plsB gene in the parental strain 8 (glpD3 glpR2 phoA8 Strs relA1 spoT1 tonA22 T2R pit-10 HfrC) was also sequenced in the same manner to verify that it was identical to the previously published sequence (10).
TABLE 1.
Primers used to amplify and sequence plsB26
| Primera | Sequence (5′-3′) | bpb |
|---|---|---|
| 1 (+) | TTAGCGCCGCGCGAAACAT | −102 |
| 2 (−) | AACATCACCGACACTGGCA | 386 |
| 3 (+) | AGCTGTTCCACGACTATC | 308 |
| 4 (−) | AGTCGGTTCCAGGTGAAG | 833 |
| 5 (+) | ATGGAAGAGATTGCGGCGAA | 754 |
| 6 (−) | TCGGAATCAGCGTAATCGGA | 1264 |
| 7 (+) | ATTCCGTCGAGTACTTCGTG | 1133 |
| 8 (−) | CACGTTGCGCATCAGATCCA | 1677 |
| 9 (+) | GCATCACGTCAGCGCTCACT | 1600 |
| 10 (−) | CGCATCAATAACGTCCGGCA | 2004 |
| 11 (+) | CCGCGACGTATTGATGGA | 1902 |
| 12 (−) | GTTCTCGAGCCGGCAATTCT | 2450 |
Orientation of primer with respect to plsB gene sequence is indicated in parenthesis.
Position of 5′ base of primer. A of ATG initiation codon is position 1.
The region of plsB26 containing the mutation, between primers 5 and 6 (Table 1), was amplified by PCR from the chromosome of strain BB26 (plsB26), digested with SfiI and MluI, and then ligated into the corresponding sites in plasmid pRJ54 (PlsB), which contains the wild-type plsB gene with a COOH-terminal Flag-tag epitope (8). This construct, pJH3 (PlsB26), expressed the Flag-tag version of the PlsB26 protein. DNA sequencing of the plasmid confirmed that only the A349T mutation was present in the PlsB26 construct.
Expression and activities of the PlsB and PlsB26 proteins.
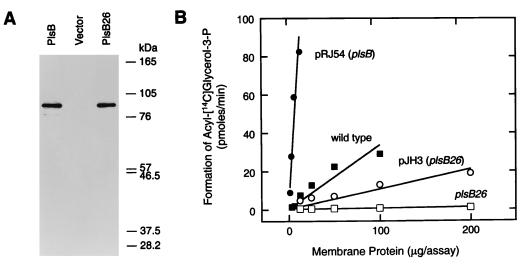
Expression vectors containing Flag-PlsB (pRJ54), Flag-PlsB26 (pJH3), or an empty vector (pACYC177) were transformed into strain SJ22 (plsB26 plsX50 panD2 zac-220::Tn10 glpD3 glpR2 glpKi relA1 spoT1 pit-10 phoA8 ompF627 fluA22) (14). There were the same amounts of Flag-PlsB and Flag-PlsB26 proteins in the membrane preparations assayed by immunoblotting with the M2 anti-Flag antibody (Kodak IBI, Inc) (Fig. 1A). These data demonstrated that both the wild-type and mutant proteins were expressed at the same levels and were assembled into the membrane. Thus, the differences in specific activity and catalytic properties described below were attributed to inherent differences in the biochemical properties of the proteins and not to differences in the level of expression.
FIG. 1.
Expression and activity of the PlsB26 mutant protein. (A) Membranes were prepared from strain SJ22 (plsB26) harboring either plasmid pRJ54 (plsB) or pJH3 (plsB26) or the empty vector (pACYC177), and the proteins were separated by sodium dodecyl sulfate-gel electrophoresis on 10% polyacrylamide gels. The samples were transferred to nitrocellulose membranes and immunoblotted with the M2 anti-Flag antibody to detect the expression of the epitope-tagged acyltransferases. (B) Membranes were prepared from strain 8 (wild type) (■) or strain SJ22 (plsB26) transformed with either the empty vector pACYC177 (□), pRJ54 (plsB) (●), or pJH3 (plsB26) (○). G3P acyltransferase assays were performed with the indicated amounts of membrane protein. The assays contained 0.1 M Tris-HCl (pH 8.6)–1-mg/ml bovine serum albumin–200 μM [14C]G3P (specific activity, 13 Ci/mmol)–50 μM palmitoyl-CoA and were incubated at 37°C for 15 min (12, 13).
The specific activities of the G3P acyltransferases were compared in membranes isolated from the transformants (Fig. 1B). Membranes from strain SJ22/pACYC177 had a low specific activity (0.004 nmol/min/mg), whereas membranes from strain SJ22/pRJ54 (PlsB) had significantly elevated levels of acyltransferase activity (7.2 nmol/min/mg). The specific activity from strain SJ22/pJH3 (PlsB26) was 0.1 nmol/min/mg. This specific activity was 25-fold higher than the background rate in the empty vector control but was 72-fold lower than that of membranes derived from cells transformed with the wild-type PlsB. These experiments were performed with palmitoyl-CoA as the acyl donor; however, the specific activities were also tested with palmitoyl-acyl carrier protein and the same ratio of activities were obtained (not shown).
The transformation of strain SJ22 (plsB26) with pJH3 (PlsB26) eliminated the G3P auxotrophic growth phenotype. This result suggested that the elevated expression of PlsB26 was sufficient to complement the defect in phospholipid synthesis. We compared the specific activity of the G3P acyltransferase in the wild-type parent strain 8 to that in strain SJ22/pJH3 (PlsB26) at 200 μM G3P (Fig. 1B). The specific activity of membranes from strain 8 was 0.33 nmol/min/mg compared to 0.1 nmol/min/mg in strain SJ22/pJH3. The overexpression of PlsB26 in strain SJ22/pJH3 yielded a total G3P acyltransferase activity that was 30% of the activity found in wild-type cells. Thus, the elevation of the PlsB26 G3P acyltransferase level was sufficient to override the G3P auxotrophic phenotype.
Biochemical properties of the PlsB26 protein.
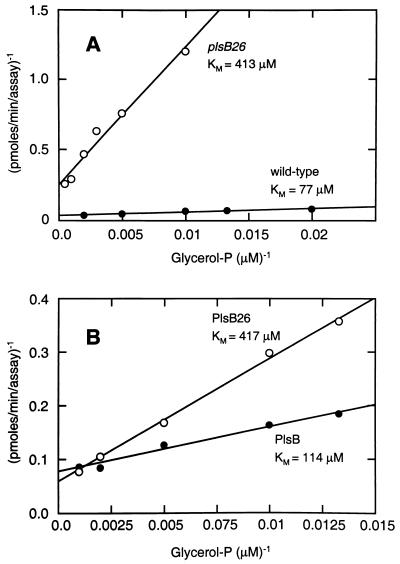
A characteristic feature of the G3P acyltransferase activity in membranes derived from plsB26 mutants is an elevated Km for G3P (1–4, 9). We first confirmed this original observation under our assay conditions (Fig. 2A). Indeed, the apparent Km for G3P in these experiments was about fivefold higher in membranes isolated from plsB26 mutants (425 μM) than in membranes isolated from wild-type cells (75 μM). The Vmax for the wild-type protein was 0.625 nmol/min/mg, whereas the mutant Vmax was reduced 60-fold to 0.01 nmol/min/mg. Both membrane preparations had approximately the same Km for palmitoyl-CoA (100 μM) when assayed at 200 mM G3P (not shown).
FIG. 2.
Kinetic analysis of wild-type and mutant G3P acyltransferase. (A) Membranes were prepared from strain SJ22 (plsB26) (○) and the parental wild type, strain 8 (●), and assayed for activity at 50 μM palmitoyl-CoA between 50 and 1,000 μM G3P. Assays contained 400 μg of protein from SJ22 and 50 μg of protein from strain 8. (B) Membranes were prepared from strain SJ22 (plsB26) harboring either pRJ54 (Flag-PlsB) (2 μg/assay) (●) or pJH3 (Flag-PlsB26) (100 μg/assay) (○) and were assayed for G3P acyltransferase activity at 50 μM palmitoyl-CoA between 50 and 1,000 μM G3P.
The G3P Km in membranes derived from cells that overexpressed either the wild-type or PlsB26 mutant protein was determined to establish whether the PlsB26 protein had an elevated Km as well as reduced specific activity. The G3P Km values calculated in these experiments were 114 μM for PlsB and 417 μM for PlsB26 (Fig. 2B). These data confirmed that the PlsB26 protein had an elevated Km for G3P compared to that of the wild-type protein. The Vmax was 8.77 nmol/min/mg for the overexpressed wild-type protein, and the Vmax was 0.27 nmol/min/mg for PlsB26. This difference of 33-fold was very similar to the difference observed between the wild-type strain 8 and strain SJ22 (plsB26) and indicated that the PlsB26 protein was the high Km acyltransferase previously described in membranes isolated from plsB26 mutants.
Conclusions.
The mutant PlsB(A349T) protein expressed from the plsB26 allele is catalytically defective with a decreased Vmax and increased Km for G3P compared to those of the wild-type PlsB. These findings are consistent with the work of previous investigators who observed an increased Km for G3P in membranes from plsB26 strains and attributed this activity to a defective PlsB protein (1–4, 9). The function that Ala349 plays in the wild-type protein, and why substitution with Thr should lead to a defective enzyme, is not clear. His306 and Asp311 are involved in catalysis (8), and A349 is relatively close to these residues in the primary sequence. However, the location of A349 cannot be determined in the absence of a three-dimensional structure. Thus, A349 may lie in or very close to the G3P binding site on the enzyme, or it may exert a more subtle effect on the overall shape of the active site by perturbing the protein structure.
Nucleotide sequence accession number.
The nucleotide sequence of plsB26 determined in this study has been deposited in the GenBank database under accession no. AF106625.
Acknowledgments
We thank Julie Harris, Jina Wang, and R. Brent Calder for technical assistance and Suzanne Jackowski for helpful discussions.
This work was supported by National Institutes of Health Grant GM 34496, Cancer Center (CORE) Support Grant CA 21765, and the American and Lebanese Syrian Associated Charities.
REFERENCES
- 1.Bell R M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974;117:1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell R M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: properties of wild type and Km defective sn-glycerol-3-phosphate acyltransferase activities. J Biol Chem. 1975;250:7147–7152. [PubMed] [Google Scholar]
- 3.Bell R M, Cronan J E., Jr Mutants of Escherichia coli defective in membrane phospholipid synthesis. Phenotypic suppression of sn-glycerol-3-phosphate acyltransferase Km mutants by loss of feedback inhibition of the biosynthetic sn-glycerol-3-phosphate dehydrogenase. J Biol Chem. 1975;250:7153–7158. [PubMed] [Google Scholar]
- 4.Cronan J E, Jr, Bell R M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: mapping of sn-glycerol 3-phosphate acyltransferase Km mutants. J Bacteriol. 1974;120:227–233. doi: 10.1128/jb.120.1.227-233.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cronan J E, Jr, Rock C O. Biosynthesis of membrane lipids. In: Neidhardt F C, Curtis R, Gross C A, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C: American Society for Microbiology; 1996. pp. 612–636. [Google Scholar]
- 6.Heath R J, Goldfine H, Rock C O. A gene (plsD) from Clostridium butyricum that functionally substitutes for the sn-glycerol-3-phosphate acyltransferase gene (plsB) of Escherichia coli. J Bacteriol. 1997;179:7257–7263. doi: 10.1128/jb.179.23.7257-7263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heath R J, Jackowski S, Rock C O. Guanosine tetraphosphate inhibition of fatty acid and phospholipid synthesis in Escherichia coli is relieved by overexpression of glycerol-3-phosphate acyltransferase (plsB) J Biol Chem. 1994;269:26584–26590. [PubMed] [Google Scholar]
- 8.Heath R J, Rock C O. A conserved histidine is essential for glycerolipid acyltransferase catalysis. J Bacteriol. 1998;180:1425–1430. doi: 10.1128/jb.180.6.1425-1430.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larson T J, Ludtke D N, Bell R M. sn-Glycerol-3-phosphate auxotrophy of plsB strains of Escherichia coli: evidence that a second mutation, plsX, is required. J Bacteriol. 1984;160:711–717. doi: 10.1128/jb.160.2.711-717.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lightner V A, Larson T J, Tailleur P, Kantor G D, Raetz C R H, Bell R M, Modrich P. Membrane phospholipid synthesis in Escherichia coli: cloning of a structural gene (plsB) of the sn-glycerol-3-phosphate acyltransferase. J Biol Chem. 1980;255:9413–9420. [PubMed] [Google Scholar]
- 11.Raetz C R H. Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev. 1978;42:614–659. doi: 10.1128/mr.42.3.614-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rock C O, Goelz S E, Cronan J E., Jr ATP stimulation of the sn-glycerol-3-phosphate acyltransferase of Escherichia coli. Arch Biochem Biophys. 1981;211:113–118. doi: 10.1016/0003-9861(81)90436-7. [DOI] [PubMed] [Google Scholar]
- 13.Rock C O, Goelz S E, Cronan J E., Jr Phospholipid synthesis in Escherichia coli. Characteristics of fatty acid transfer from acyl-acyl carrier protein to sn-glycerol-3-phosphate. J Biol Chem. 1981;256:736–742. [PubMed] [Google Scholar]
- 14.Rock C O, Jackowski S. Regulation of phospholipid synthesis in Escherichia coli. Composition of the acyl-acyl carrier protein pool in vivo. J Biol Chem. 1982;257:10759–10765. [PubMed] [Google Scholar]
- 15.Wilkison W O, Bell R M. sn-Glycerol-3-phosphate acyltransferase from Escherichia coli. Biochim Biophys Acta. 1997;1348:3–9. doi: 10.1016/s0005-2760(97)00099-4. [DOI] [PubMed] [Google Scholar]