Abstract
The prevalence of obesity is ∼40% in the United States, and the prepregnancy prevalence of obesity in females is ∼30%. This has in part fueled an increase in metabolic syndrome (MetS) among females who are currently pregnant, have been pregnant, or are planning to become pregnant. Importantly, MetS in pregnancy is associated with increased pregnancy complications. Moreover, MetS in pregnancy may have long-lasting adverse cardiovascular and metabolic health implications for the mother and her offspring. To complicate matters, many adverse pregnancy outcomes seem to increase the risk of MetS in the mother after pregnancy. Herein, we describe the potential mechanisms behind the intersection of MetS, adverse pregnancy outcomes, and subsequent long-term disease in the mother and offspring. Because MetS is a cluster of coexisting conditions, it is challenging to identify mediators that can serve as biomarkers for early diagnosis and targets for MetS prevention and therapy.
Keywords: metabolic syndrome, pregnancy, pre-eclampsia, gestational diabetes, placenta
Metabolic syndrome (MetS) is defined as a cluster of metabolic abnormalities such as obesity, insulin resistance, hypertension, dyslipidemia, and diabetes; it is characterized by chronic low-grade systemic inflammation and directly promotes the development of atherosclerotic cardiovascular disease (CVD) and type 2 diabetes (T2D).1 A significant increase in MetS across the globe and among females who are currently pregnant, have been pregnant, or are planning to become pregnant has been reported,2 and MetS has been associated with adverse pregnancy outcomes such as miscarriages, preterm birth (PTB), pre-eclampsia (PE), gestational diabetes (GDM), and stillbirth.3–6
That MetS has consistently been associated with increased risk for CVD is not surprising; however, pregnancy complications such as PE and GDM, frequently associated with MetS, have also been associated with increased maternal risk for CVD.7,8
Because MetS is a cluster of coexisting conditions, it is challenging to understand its pathophysiology. In addition, the metabolic adaptations that occur during pregnancy complicate even more the identification of mediators that can serve as biomarkers for early diagnosis and targets for MetS prevention and therapy. Importantly, the consequences of MetS in pregnancy affect both the mother and the offspring; a transgenerational inheritance of MetS has also been postulated. Therefore, early diagnosis of MetS may be useful to screen women at higher risk for pregnancy complications, which may additionally associate with future risk of CVD and adverse health outcomes in the offspring.
Metabolic Adaptations During Pregnancy May Increase the Risk of MetS
Pregnancy is associated with important physical, metabolic, hormonal, vascular, and immunological changes leading to a pro-inflammatory, prothrombotic, highly insulin resistant, and hyperlipidemic state.3 These adaptations, necessary for the development of the fetus and to prepare the mother for delivery and lactation, can also increase the susceptibility to develop MetS. In the early stages of pregnancy, the increased levels of estrogen, progesterone, and cortisol favors lipogenesis and accumulation of fat.9 Then, during mid-pregnancy, the diabetogenic effect of pregnancy on metabolism is noticeable when placental-derived hormones lead to an insulin-resistant state.9
Maternal MetS and Adverse Pregnancy Outcomes: A Reciprocal Relationship
It has been widely reported that a higher incidence of pregnancy complications, such as GDM and PE, occur in women with MetS.3–6 In addition, obese pregnant females have an ∼25% higher risk of stillbirth and a higher risk of developing PE compared with nonobese pregnant females.3,5,7,8 Although increased serum glucose, triglyceride, and low-density lipoprotein-cholesterol levels and lower high-density lipoprotein-cholesterol levels have been observed in pre-eclamptic patients with MetS compared with pregnant individuals without MetS, it is not clear whether MetS is secondary to PE or whether PE plays a role in the pathogenesis of MetS. An increased risk of GDM has also been observed among women who are overweight or obese and the risk of developing GDM increases proportionally to the degree of obesity.10
In addition, overweight women are at a significantly higher risk of experiencing recurrent miscarriages compared with those of average weight.4 Similar to those in the nonpregnant state, maternal obesity is related to metabolic and cardiovascular complications of pregnancy, including PE and GDM.3 PE and CVD risk factors (including hypertension) are associated not only by common pathophysiology but also epidemiology.
For example, high blood pressure before pregnancy is a risk factor for PE and PE is in turn a risk factor for hypertension and CVD after pregnancy.11 Moreover, an increased risk of PTB, especially extremely preterm delivery, has been reported among obese and overweight females.12 Furthermore, up to 70% of females with GDM will develop T2D after pregnancy.13 Women with GDM also have a 2.4-fold higher risk of developing MetS after delivery compared with women with a normal pregnancy.13
Although pregnancies affected by MetS are associated with adverse pregnancy outcomes, an increased incidence of MetS has been reported in women and their offspring after a complicated pregnancy; in particular, pregnancies in which an exacerbated pro-inflammatory state has been identified, such as PE and PTB.14,15
MetS in pregnancy is associated with subsequent metabolic and CVD in the mother and offspring.16 Obesity is characterized by an increased inflammatory response, and fetuses of overweight and obese pregnant females also have shown a higher risk of MetS after birth.17
Potential Mechanisms
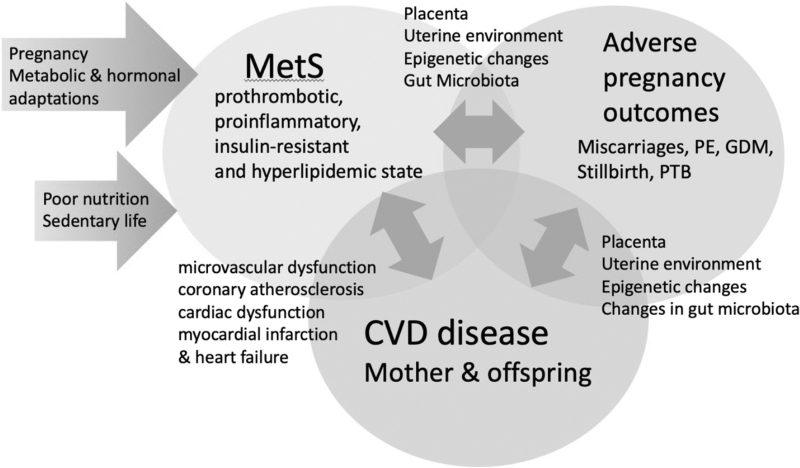
The mechanisms behind the cross-associations between MetS, CVD, and adverse pregnancy outcomes are difficult to differentiate due to the common risk factors. Roles for the intrauterine environment, epigenetic changes, the placenta, and changes in the gut microbiota have been postulated (Fig. 1).
FIG. 1.
Suggested mechanisms in the cross-association between MetS, adverse pregnancy outcomes (miscarriages, PE, GDM, stillbirth and PTB, and CVD). CVD, cardiovascular disease; GDM, gestational diabetes; MetS, metabolic syndrome; PE, pre-eclampsia; PTB, preterm birth.
Although a substantial body of evidence demonstrates that environmental exposures such as poor nutrition and sedentary lifestyle contribute significantly to the risk of MetS, there is growing evidence about the contribution of the intrauterine environment.18 That MetS is more frequently observed in those that were born from complicated pregnancies further supports the importance of exposures in utero to the development of MetS.14,15 Nutritional stress/stimuli during critical periods of early development can also impact metabolic programming19 contributing the etiology of chronic diseases such as obesity, T2D, and CVD later in life. Furthermore, it has been suggested that these effects may also affect subsequent generations, perpetuating MetS and CVD.
An adverse intrauterine environment can result in epigenetic programming, a critical underlying mechanism of fetal metabolic programming.19 Epigenetic variations established in utero can further be passed through multiple generations representing a plausible link between the in utero environment and later disease susceptibility.19
MicroRNAs regulate gene expression and may affect the development and functioning of the cardiovascular and endocrine systems. Interestingly, a postpartum profile of microRNAs associated with diabetes/cardiovascular/cerebrovascular diseases has been identified in women that experienced pregnancy complications such as gestational hypertension, PE, intrauterine growth restriction, and PTB.20,21 MiRNAs also play important regulatory roles in adipocyte differentiation, metabolic integration, insulin resistance, and appetite regulation, suggesting the potential use of miRNAs as novel biomarkers and therapeutic targets for MetS.22
The placenta also plays an important role in the maternal metabolic adaptation of pregnancy and the modulation of the intrauterine environment. A negative effect of maternal obesity on placental development and function leading to impaired offspring programming and adverse health outcomes has been described. Specifically, maternal prepregnancy obesity is associated with a dysregulated placental transcriptome, particularly in pathways relating to inflammation, immune responses, glucocorticoid signaling, and angiogenesis.23 Increased mRNA expression of pro-inflammatory cytokines and increased density of pro-inflammatory macrophages have also been found in placentas of obese females.24
Placental dysfunction is commonly seen in females with obesity, chronic hypertension, diabetes, and dyslipidemia, all major features of MetS.25 Maternal placental syndromes, such as PE and placenta abruption or infarction, are also more prevalent in females with insulin resistance, diabetes, and MetS.25 Furthermore, placental insufficiency observed in PE has been associated with the postpartum development of MetS in females without prepregnancy metabolic disorders.25
Animal models of placental insufficiency have shown an adverse metabolic and cardiovascular phenotype in the offspring.26,27 In one of these models, a role for soluble bioactive factors secreted by the placenta, vascular function modulators, markers of metabolic disease, vasoconstrictors, and pro-inflammatory mediators has been described in the metabolic and CVD observed in the mother and offspring.26
Changes in maternal gut microbiota might also contribute to metabolic diseases, including GDM and obesity. The maternal microbiome during pregnancy may alter not only the developing neonatal microbiota but also intestinal absorption of nutrients and increased lipid deposition, potentially leading to MetS.28 Changes in the gut microbiome composition and the metabolic hormonal environment has been observed in overweight and obese pregnant females and women with GDM.29 Several bacteria have also been correlated with plasma glucose and blood lipid levels in females with GDM and hyperlipidemia.30
Increased gut permeability may explain the role of the microbiota in the pathogenesis of GDM and MetS.31 Specifically, certain mucin-degrading bacteria that increase gut permeability have been detected in GDM,31 facilitating not only the increased absorption of nutrients rich in calories but also the movement of inflammatory mediators from the gut into the maternal circulation, potentially contributing to the development of MetS.
Conclusions
There are connections among maternal MetS, adverse pregnancy outcomes, and future metabolic health for the mother and offspring. Women with MetS during pregnancy are at higher risk for adverse short- and long-term outcomes; conversely, pregnancy complications associated with an increased pro-inflammatory state have been associated with an increased risk of MetS after pregnancy in mother and offspring.
Maternal lifestyle, the intrauterine environment, placental-derived bioactive molecules, microRNAs, the microbiome, and epigenetics may all play a role in the pathogenesis of MetS, the associated long-term health complications to the mother and offspring, and transgenerational inheritance the syndrome. Future studies are required to address the overlapping/common mechanisms among MetS, adverse pregnancy outcomes, and the risk of future MetS and CVD in mother and offspring.
Disclaimer
The contents of this article represent the authors' views and do not constitute an official position of the National Institutes of Health or the U.S. Government.
Author Disclosure Statement
No conflicting financial interests exist.
Funding Information
The authors received no financial support.
References
- 1. Haffner SM. The metabolic syndrome: Inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol 2006;97:3A–11A. [DOI] [PubMed] [Google Scholar]
- 2. Bentley-Lewis R, Koruda K, Seely EW. The metabolic syndrome in women. Nat Clin Pract Endocrinol Metab 2007;3:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grieger JA, Bianco-Miotto T, Grzeskowiak LE, et al. Metabolic syndrome in pregnancy and risk for adverse pregnancy outcomes: A prospective cohort of nulliparous women. PLoS Med 2018;15:e1002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu YH, Bodnar LM, Himes KP, et al. Association of overweight and obesity development between pregnancies with stillbirth and infant mortality in a cohort of multiparous women. Obstet Gynecol 2020;135:634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Su XJ, Huang SJ, Li X, et al. Prepregnancy overweight and obesity are associated with an increased risk of preterm birth in Chinese women. Obes Facts 2020;2:237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paauw ND, Lely AT. Cardiovascular sequels during and after preeclampsia. Adv Exp Med Biol 2018;1065:455–470. [DOI] [PubMed] [Google Scholar]
- 7. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019;62:905–914. [DOI] [PubMed] [Google Scholar]
- 8. Parrettini S, Caroli A, Torlone E. Nutrition and metabolic adaptations in physiological and complicated pregnancy: Focus on obesity and gestational diabetes. Front Endocrinol (Lausanne) 2020;11:611929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chu SY, Callaghan WM, Kim SY, et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 2007;30:2070–2076. [DOI] [PubMed] [Google Scholar]
- 10. Benschop L, Duvekot JJ, Versmissen J, et al. Blood pressure profile 1 year after severe preeclampsia. Hypertension 2018;71:491–498. [DOI] [PubMed] [Google Scholar]
- 11. Cnattingius S, Villamor E, Johansson S, et al. Maternal obesity and risk of preterm delivery. JAMA 2013;309:2362–2370. [DOI] [PubMed] [Google Scholar]
- 12. Vounzoulaki E, Khunti K, Abner SC, et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020;369:m1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vilmi-Kerälä T, Palomäki O, Vainio M, et al. The risk of metabolic syndrome after gestational diabetes mellitus—A hospital-based cohort study. Diabetol Metab Syndr 2015;7:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cho GJ, Jung US, Sim JY, et al. Is preeclampsia itself a risk factor for the development of metabolic syndrome after delivery? Obstet Gynecol Sci 2019;62:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parkinson JR, Hyde MJ, Gale C, et al. Preterm birth and the metabolic syndrome in adult life: A systematic review and meta-analysis. Pediatrics 2013;131:e1240–e1263. [DOI] [PubMed] [Google Scholar]
- 16. Patti AM, Pafili K, Papanas N, et al. Metabolic disorders during pregnancy and postpartum cardiometabolic risk. Endocr Connect 2018;7:E1–E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leddy MA, Power ML, Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol 2008;1:170–178. [PMC free article] [PubMed] [Google Scholar]
- 18. Gluckman PD, Hanson MA, Cooper C, et al. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008;359:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rinaudo P, Wang E. Fetal programming and metabolic syndrome. Annu Rev Physiol 2012;74:107–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hromadnikova I, Kotlabova K, Dvorakova L, et al. Postpartum profiling of microRNAs involved in pathogenesis of cardiovascular/cerebrovascular diseases in women exposed to pregnancy-related complications. Int J Cardiol 2019;291:158–167. [DOI] [PubMed] [Google Scholar]
- 21. Hromadnikova I, Kotlabova K, Krofta L. A history of preterm delivery is associated with aberrant postpartal microRNA expression profiles in mothers with an absence of other pregnancy-related complications. Int J Mol Sci 2021;22:4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heneghan HM, Miller N, Kerin MJ. Role of microRNAs in obesity and the metabolic syndrome. Obes Rev 2010;11:354–361. [DOI] [PubMed] [Google Scholar]
- 23. Altmaë S, Segura MT, Esteban FJ, et al. Maternal pre-pregnancy obesity is associated with altered placental transcriptome. PLoS One 2017;12:e01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roberts KA, Riley SC, Reynolds RM, et al. Placental structure and inflammation in pregnancies associated with obesity. Placenta 2011;32:247–254. [DOI] [PubMed] [Google Scholar]
- 25. Ray JG, Vermeulen MJ, Schull MJ, et al. Metabolic syndrome and the risk of placental dysfunction. J Obstet Gynaecol Can 2005;27:1095–1101. [DOI] [PubMed] [Google Scholar]
- 26. Garrett N, Pombo J, Umpierrez M, et al. Pravastatin therapy during preeclampsia prevents long-term adverse health effects in mice. JCI Insight 2018;3:e120147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sutton EF, Lob HE, Song J, et al. Adverse metabolic phenotype of female offspring exposed to preeclampsia in utero: A characterization of the BPH/5 mouse in postnatal life. Am J Physiol Regul Integr Comp Physiol 2017;312:R485–R491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fasano A. Gut permeability, obesity, and metabolic disorders: Who is the chicken and who is the egg? Am J Clin Nutr 2017;105:32–34. [DOI] [PubMed] [Google Scholar]
- 29. Gomezarango LF, Barrett HL, Mcintyre HD, et al. Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes 2016;65:2214–2223. [DOI] [PubMed] [Google Scholar]
- 30. Hasain Z, Mokhtar NM, Kamaruddin NA, et al. Gut microbiota and gestational diabetes mellitus: A review of host-gut microbiota interactions and their therapeutic potential. Front Cell Infect Microbiol 2020;10:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chakaroun RM, Massier L, Kovacs P. Gut Microbiome, intestinal permeability, and tissue bacteria in metabolic disease: Perpetrators or bystanders? Nutrients 2020;12:1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]