Abstract
A phase 1 open-label, non-randomized clinical trial was conducted to determine feasibility and safety of autologous human Schwann cell (ahSC) transplantation accompanied by rehabilitation in participants with chronic spinal cord injury (SCI). Magnetic resonance imaging (MRI) was used to screen eligible participants to estimate an individualized volume of cell suspension to be implanted. The trial incorporated standardized multi-modal rehabilitation before and after cell delivery. Participants underwent sural nerve harvest, and ahSCs were isolated and propagated in culture. The dose of culture-expanded ahSCs injected into the chronic spinal cord lesion of each individual followed a cavity-filling volume approach. Primary outcome measures for safety and trend-toward efficacy were assessed. Two participants with American Spinal Injury Association Impairment Scale (AIS) A and two participants with incomplete chronic SCI (AIS B, C) were each enrolled in cervical and thoracic SCI cohorts (n = 8 total). All participants completed the study per protocol, and no serious adverse events related to sural nerve harvest or ahSC transplantation were reported. Urinary tract infections and skin abrasions were the most common adverse events reported. One participant experienced a 4-point improvement in motor function, a 6-point improvement in sensory function, and a 1-level improvement in neurological level of injury. Follow-up MRI in the cervical (6 months) and thoracic (24 months) cohorts revealed a reduction in cyst volume after transplantation with reduced effect over time. This phase 1 trial demonstrated the feasibility and safety of ahSC transplantation combined with a multi-modal rehabilitation protocol for participants with chronic SCI.
Keywords: autologous transplantation, chronic, human, paraplegia, Schwann cells, spinal cord injury
Introduction
In chronic spinal cord injury (SCI), there is neuropathological, neurophysiological, and magnetic resonance imaging (MRI) evidence that the remyelination of damaged central axons is incomplete.1–5 Cell transplantation is one approach to assist recovery in chronic SCI. Schwann cells (SCs), which can enter the injury epicenter as part of a natural post-injury process called schwannosis,6 is an excellent candidate for cell transplantation. When mitogen expanded SCs are transplanted into chronic post-SCI cavities in animal models, they engender new tissue formation, bridging the cavity, and can have trophic effects that elicit plasticity of residual damaged axons.7 The improved function in a modest number of axons can have effects that are detectable with standardized animal behavioral tests.7 Thus, it is hypothesized that similar improvements in function may occur in humans with chronic SCI when transplanted with autologous SCs.8
Schwann cells support spinal cord repair after SCI in a number of ways. In rodent SCI models, transplanted SCs have been found to survive in the central nervous system (CNS),7,9–11 prevent secondary tissue damage,9,12,13 and reduce the substantial cavitation that occurs following contusive SCI.9,14 Transplanted SCs provide a physical substrate that supports growing axons,7,9–11 and release growth-promoting molecules15 and favorable components of the extracellular matrix such as collagen and laminin.16 SCs also express cell adhesion molecules that support axon growth, such as neural cell adhesion molecule and L1 on their surfaces.17 Transplanted SCs have been shown to support new growth of sensory and propriospinal axons,10,18–21 ensheath and myelinate regenerating or demyelinated axons,9,11,14,20,21 and restore axonal conduction.22,23 Further, SCs transplants do not exhibit tumorigenicity or enhance behavioral hypersensitivity after SCI.24 Recent studies show that implanted SCs may also be neuroprotective by reducing the pro-inflammatory response after SCI.25 In addition, SC transplantation has been shown to improve functional recovery in pre-clinical subacute9,13 and chronic SCI models.7
Our team published a phase 1 clinical trial evaluating the safety of autologous human SC (ahSC) transplantation into the injury epicenter of six participants with subacute (4-7 weeks post-injury) thoracic complete SCI.26 Autologous SCs were cultured in vitro from an approximately 15-cm segment of sural nerve harvested from each participant. The ahSCs were then transplanted into the epicenter of the spinal lesion using a stereotactic injector anchored to the operating table under apnea in a sequential dose escalation paradigm. At 1 year post-transplantation, there were no serious adverse events related to the cell therapy. There was no detectable evidence of additional spinal cord damage, mass lesion, or syrinx formation seen on post-transplantation MRI. Participants continue to be monitored in yearly visits—with most participants now at over 5 years follow-up. There have been no reported long-term adverse sequelae. We demonstrated longitudinal neurophysiological changes over a 1-year period before and after ahSC transplantation in these participants.27 Observed changes included the emergence of motor evoked potentials (MEPs) and subclinical voluntary electromyography (EMG) activation in the legs and detectable connections to intercostals below the clinically-determined neurological level of injury (NLI). In addition, changes indicating recovery of sudomotor sympathetic activity were documented.
In chronic SCI, there is general agreement that potential repair strategies should be combined with rehabilitation protocols to improve functional recovery.28 Strategies that combine invasive and non-invasive treatments with rehabilitation training can trigger coordinated neural activity leading to neuroplasticity, a key mechanism to promote recovery following SCI.29–34 Indeed, the strongest evidence for efficacy in SCI clinical trials was associated with those that included an exercise component.35,36 However, variations in the conditioned state of the body can confound functional outcome measures in trials that combine rehabilitation with other therapies. For this reason, to assess the impact of therapeutic agents, it is optimal that clinical trial participants with chronic SCI first achieve a standard conditioned state.37 The addition of standardized training protocols pre- and post-transplant for each participant potentially primes the body for the best physical response to the intervention and can allow a more rigorous assessment of transplantation effect.38
The primary purpose of this phase 1 trial was to assess the safety and feasibility of ahSC transplantation using an individualized dosing strategy with a cavity-filling technique in participants with chronic SCI. We included a unique multi-modal physical training protocol and designed the study to evaluate a potential trend towards efficacy assessed by various quantitative and qualitative measures.
Methods
Trial design
This was a phase 1, prospective, open-label, unblinded, non-randomized, U.S. Food and Drug Administration (FDA)–approved clinical trial (NCT02354625) designed to evaluate the safety of transplantation of ahSC in participants with chronic SCI. The trial was specifically designed to test the ahSC transplants in progressively more incomplete neurologic injuries (American Spinal Injury Association Impairment Scale [AIS] A to C), progressing from cavities in the thoracic to the cervical spinal cord—escalating the risk and potential benefit in a step-wise fashion. Thus, each successive participant's transplant was based on the observation of a short-term safety readout from preceding SCI participants within the trial. Written informed consent was obtained in a two-step process ahead of screening and again before transplant from all study participants according to the protocol approved by the Human Subjects Research Office at the University of Miami Miller School of Medicine (eProst #20140846).
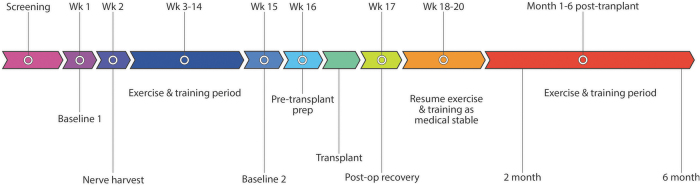
Potential participants underwent a screening process to determine eligibility. Following acceptance to the trial, participants were engaged in on-site study activities for 10 consecutive months (Fig. 1) and have continued to be monitored for 5 years post-transplant. A week of baseline assessments (Baseline 1, Week 1) preceded any study interventions. A sural nerve harvest procedure was then performed (Week 2). Participants then engaged in fitness and rehabilitation training for 12 weeks (Weeks 3-14) at the Miami Project to Cure Paralysis. Baseline assessments were repeated (Baseline 2) to capture any changes resulting from participation in the fitness program. Transplantation of ahSC was performed at the University of Miami Hospital, and participants spent an average of 3-4 inpatient days in a step-down unit. Participants recuperated for 3-4 weeks following surgery, then resumed participation in the fitness and rehabilitation program with the principal investigator's approval. Assessments were repeated at 2 and 6 months post-ahSC transplantation. Participants were then enrolled in a separate, long-term follow-up protocol for 5 years post-transplant (eProst #20160034).
FIG. 1.
Study design and timeline of interventions.
At screening and regularly throughout the trial, all participants met with a neuropsychologist with SCI specialization who engaged them in a continuous informed consent framework that has been recommended when engaging vulnerable populations in research. In addition, the neuropsychologist provided additional education to the participant and their families specific to the trial and evaluated each person's capacity to provide informed consent, including a deep understanding that the primary objectives of the study were safety. Also important were the sense that the participants were engaged in the trial to advance the science and gaining an understanding of other reasons motivating participation. The neuropsychologist directly evaluated whether therapeutic misconception39 was occurring throughout the trial. In addition, potential psychological barriers to participation were assessed. As will be further elucidated upon in a subsequent manuscript, there was no evidence that therapeutic misconception was present in this sample of chronically injured individuals either at baseline or at the 6-month follow up assessment.
Inclusion/exclusion criteria
Individuals between the ages of 18 and 65 with traumatic SCI that occurred a minimum of 12 months prior to enrollment were considered for eligibility. NLI was between cervical-level 5 (C5) and thoracic-level 12 (T12), as defined by the International Standards for the Neurological Classification of Spinal Cord Injury (ISNCSCI), with AIS grade A, B, or C at the time of enrollment. Lesion length was ≤3 cm and lesion volume ≤2 cc, as approximated by MRI. Individuals with penetrating injuries and those who could not safely undergo MRI were excluded from the study. Additional details on eligibility criteria are available in the Supplementary Material.
Sural nerve harvest (Week 2)
In the operating room, after a careful sterile prep and drape, the lateral calf above the lateral malleolus was infiltrated with local anesthetic with 1% epinephrine after positioning the participant in the ¾ prone position. The sural nerve was identified, and an approximately 15cm segment was harvested for ahSC preparation by sharp dissection. The harvested sural nerve was transported in Belzer's transport media (Static Preservation Solution (SPS-1), Organ Recovery Systems, Inc., Chicago IL) at 2-8°C to Cell Processing Facility for further culture and expansion procedures.
The sural nerve was then processed to grow a purified population of SCs. Cell purity and concentration were estimated prior to transplantation. The cell processing protocol was published in a previous publication,26 but a few steps were distinct in this study. After ahSCs were purified and expanded to P1, the cells were cryopreserved. The cell manufacturing process for the cryopreserved cells resumed at 3-5 weeks prior to transplantation. The detailed cell processing protocol is available in the Supplementary Material.
Fitness and rehabilitation protocol
A detailed description of the fitness and multi-modal rehabilitation protocol is presented in the Supplementary Material. In brief, for conditioning of lower extremities, participants underwent functional electrical stimulation cycle training on a cycle ergometer (Restorative Therapies RT300; RTI, Inc., Baltimore, MD) twice a week.37 Participants also underwent bi-weekly body-weight–supported locomotor training. Participants with SCI classified as AIS A or B used a treadmill-based robotic gait orthosis (Lokomat, Hocoma Inc, Zurich, or ReoAmbulator, Motorika Medical, Ltd., Caesarea, Israel). Participants with SCI classified as AIS C underwent overground locomotor skill training to focus on walking-related and functional tasks. Upper extremity circuit resistance training sessions38 were performed in the participants' home three times a week on non-consecutive days.
Schwann cell transplantation
Unique to this study, the volume of the intramedullary cavity at the level of injury was calculated for all participants based on the pre-operative screening MRI and used to estimate the volume of cell product to be transplanted. The maximum volume that we proposed to deliver was 2 mL (i.e., 200 million cells). The first two participants transplanted in cohort 1 received a maximum of 500 μL (50 million cells) as mandated by the FDA as an initial safety step. For all other participants, we injected each cavity until it was filled with cell suspension.
Participants underwent the spinal surgical procedure in the prone position under general anesthesia, with monitoring of sensory and motor evoked potentials. The intramedullary cyst was visualized prior to opening the dura using intra-operative ultrasound imaging (Hitachi HI Vision Ascendus, Hitachi Medical Systems Europe Holding AG, Switzerland/12 MHz linear array transducer on an IU22 scanner (Hitachi Aloka Medical America, Inc, Wallingford, CT). The extent of dural opening was determined based on intraoperative ultrasound and pre-operative MRI findings. The dorsal surface of the injured spinal cord was exposed after the dura was opened and tacked up. In distinction from the prior ahSC study, there was no need for apnea during injection using this method.
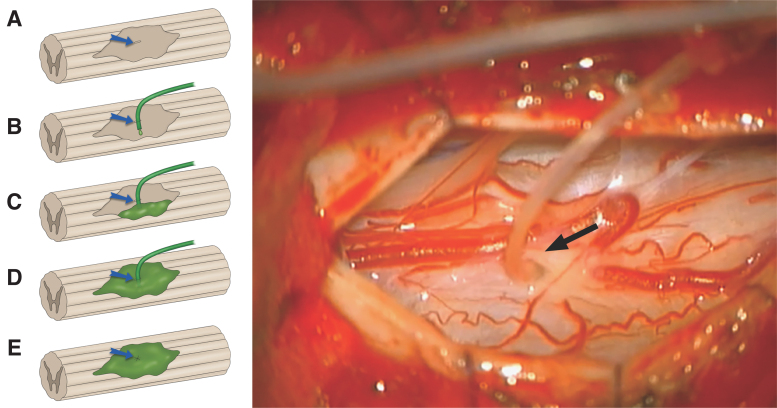
A number 11 blade was used to make a small incision (1-2 mm) parallel to the long axis of the spinal cord. Using a combination of scalpel tip and a blunt probe, a tract was gently defined, which entered the cystic cavity. Soft medical grade tubing (ID 0.31 mm, OD 0.62 mm) was inserted through the myelotomy into the cavity (Fig. 2). The tubing, pre-filled with cells, was connected to an insulin syringe loaded with ahSC at 100,000 cells/μL. Initially, the injection proceeded at less than 50 μL/min until there was visible egress of the cell suspension from the myelotomy, as visualized at high magnification under the surgical microscope. The egress was collected onto cotton surgical patties that encircled the myelotomy site to preclude distribution in the subarachnoid space. This injection continued until further egress occurred, and then a 2-min waiting period was observed. When the injection was complete, a pial purse-string suture was tightened around the delivery catheter as it was removed. When the catheter was outside of the spinal cord, a 9-0 pial purse string was placed and tightened and tied down to prevent cell egress from the myelotomy. Up to three injection attempts were made to fill compartmentalized cavities. The total volume delivered was recorded. The cord surface was observed for 5 min for any evidence of cell leakage. The ultrasound probe was introduced to determine if the injectate was distributed throughout the cavity. The dura was then closed in a watertight fashion with 5-0 prolene sutures and covered with Duragen Plus (Integra, Plainsboro, NJ).
FIG. 2.
Illustration (A-E) showing pial opening (blue arrow), placement of tubing into injured cavity, injection of autologous human Schwann cells (ahSC; green) and closure of pial opening. Intraoperative view (right) with the surgical microscope showing insertion of tubing (black arrow) into the chronic cyst cavity for injection of ahSC.
Outcome measures
Outcomes in this study were classified as primary and secondary outcome measures for safety and trend toward efficacy. The primary outcome measures for safety included protocol compliance, feasibility, stability of neurological function, and adverse events. Secondary outcomes for safety included lack of emergence of sustained clinically significant neuropathic pain or spasticity, and the absence of a detectable mass lesion or cavity enlargement on MRI at 6 months follow-up. Basic blood chemistry for liver and kidney function also was monitored and will be reported in a separate article.
Primary outcome measures for trend-toward efficacy included neurological status assessed using the ISNCSCI exam, along with functional assessments of the upper and lower extremities, and measures of functional independence. Secondary outcome measures for trend-toward efficacy included assessment of bowel and bladder function, along with other parameters of autonomic function, fitness measures, spasticity measures, pain history, and pain-related sensory function, and self-reported function.
Primary outcomes related to safety
Compliance with protocol-specified procedures was monitored, and deviations from the protocol were documented. Due to the lengthy and complex nature of this study, which involved an extensive screening process, rehabilitation interventions over 10 months, two elective surgical procedures, and multiple assessment periods, it was important to assess the feasibility of carrying out this type of study in individuals with chronic SCI. Adverse events and serious adverse events were monitored and documented, and relatedness to study associated procedures was determined. An independent data safety committee reviewed study protocols and progress, including all adverse events at regular intervals.
Secondary outcomes related to safety
Participants were interviewed regarding their pain using the International SCI Pain Basic Dataset,40 which contains basic questions for the worst, second-worst, and third-worst pain problems experienced. No changes to the participant's pre-trial pain medications were made after enrollment. They were also interviewed regarding the severity of neuropathic pain symptoms using the Neuropathic Pain Symptom Inventory (NPSI), which has recently been shown to be a valid and reliable measure for the SCI chronic pain population.41 The quantitative sensory testing (QST) assesses dorsal column-medial lemniscus (DCML; mechanical stimuli) and anterolateral spinothalamic (STT: noxious and thermal stimuli) mediated function/dysfunction and has been shown to be a valid and reliable measure in the SCI chronic pain population. Consistent with our previous protocols,42 sensory thresholds were measured in painful and non-painful areas in dermatomes both at- and below-level of injury (L4) and were converted to Z-scores relative to a normative pain-free sample. Dynamic mechanical allodynia, thermal allodynia, directional cutaneous kinesthesia, and graphesthesia were evaluated if present. Tests for graphesthesia and directional cutaneous kinesthesia have recently been found to be reliable and valid assessments of DCML function in people with SCI.42
The Spinal Cord Assessment Tool for Spastic Reflexes was used to measure clonus, flexor, and extensor spastic reflex activity.43 A composite score was calculated by summing up the bilateral scores (range, 0-18).44 The modified Ashworth Scale was used to measure stretch reflex responses of the hamstring and quadriceps muscles, and composite scores were calculated by summing up the bilateral scores.45 The pendulum test was used to measure spastic activity in the quadriceps muscles, and a 60 Hz, 8-camera, three-dimensional motion capture system (Peak Motus® Software, Peak Performance, Centennial, CO) was used to capture kinematic data as published previously.46 The average of three trials was used to calculate the first swing excursion of the more spastic leg.
Imaging
In addition to the screening MRI, participants underwent MRI (1.5T magnet using metal artifact reduction sequences with and without contrast) of the spine before transplantation (if requested by the investigator), 1 day after transplantation, and at 2 months, 6 months, 2 years, and 5 years after transplantation to assess for unexpected changes in spinal cord structure or potential tumorigenesis. Following transplantation, the presence of edema, hemorrhage, and contrast-enhancement was determined. Lesion volume was determined by using the calibrated freehand measurement tool on serial axial images to calculate the area of signal abnormality multiplied by the cut thickness.
Primary outcomes for trend toward efficacy
For evaluation of neurological function, the ISNCSCI exam was performed by trained examiners at each assessment time point and used to determine AIS grade.47 Testing of non-key muscles for hip extension, hip abduction, and knee flexion, along with wrist flexion, thumb flexion, and thumb extension, were also included. Pre-transplant ISNCSCI-related outcomes were averaged (Baseline 1 and Baseline 2) to account for variability.
Functional mobility and independence were assessed using The Spinal Cord Independence Measure (SCIM) Version III.48 A substantial improvement was predefined as an improvement of 10 points, with an improvement of 4 points representing a “small significant improvement.”49 The spinal cord injury-functional index (SCI-FI) computer adaptive test (CAT) (basic mobility, self-care, fine motor function, wheelchair mobility, and ambulation domains), the International Spinal Cord Injury (ISCI) Quality of Life (QOL) Basic Dataset, and Guy Farrar 7 Subject Global Impression of Change (SGIC) were used to document any changes perceived by the participant.50,51
Secondary outcomes for trend toward efficacy
Bladder and bowel functions were assessed using the ISCI Lower Urinary Tract Function Basic Dataset52 and the ISCI Bowel Function Basic Dataset, respectively.53 The ISCI Male or Female Sexual Function Basic Dataset monitored for changes in sexual function. Orthostatic changes in blood pressure (BP) as an index of autonomic dysfunction were assessed during a progressive head-up tilt challenge (10 min at 0°, 5 min at 20°, 5 min at 40°, 10 min at 60°). Heart rate was recorded continuously, and BP documented during the last 2 min at each position. The physical conditioning state was tested at four study time points: Baseline 1 (BL1; pre-biopsy ±7 days), Baseline 2 (BL2; post–12-week conditioning ±7 days), Month 2 (M2; post-transplant ±7 days), and Month 6 (M6; 6 months post-transplant ±7 days). Fitness markers included cardiorespiratory endurance testing by monitoring cardiorespiratory measures during a graded exercise test on an arm ergometer and one-repetition maximum isoinertial strength testing during upper extremity maneuvers. The testing for fitness markers is detailed in the Supplementary Material.
Neurophysiological testing
Building upon our prior experience, we developed a rigorous neurophysiological protocol to detect changes in conduction, including those below the clinical detection threshold, as previously demonstrated. At the baseline time-point and then following the exercise training prior to transplantation, and then at 2 and 6 months, we assessed for transcranial MEPs, somatosensory-evoked potentials (SSEPs), and the presence of a sympathetic skin reflex (SSR). Intraoperative neurophysiologic monitoring during ahSC transplant surgery, including SSEPs and MEPs, was also performed.
Statistical analysis
Descriptive statistics were used for the analysis of safety data across time and focused on within-participant differences. The primary end-point was set at 6 months post-transplantation. To test the core hypothesis and secondary outcome measures, the analysis for efficacy trends was conducted on the 2- and 6-month data for all participants enrolled. As this is an open-label safety study without a control group, no formal power calculation was performed. We considered the stability of the ISNCSCI examination to be the primary indicator of safety with the assumption of test–retest stability in these chronically injured individuals, thus serving as their own controls. Given that the test-retest properties of the motor score are well established,54,55 we therefore, conducted a within-participants repeated measures paired t-test between the baseline and 6-month post-transplant assessments for the total motor scores with the null hypothesis that the observed differences would not exceed the published test–retest variance.
Results
Demographics
Four participants with chronic cervical SCI and four participants with chronic thoracic SCI were enrolled in this study. All enrolled participants underwent sural nerve harvest and Schwann cell transplantation. Participant demographics and baseline clinical data are presented in Table 1.
Table 1.
Demographic Data for Participants Undergoing ahSC Transplantation
| Subject ID | Sex | Age | Cause of injury | Single neurological level | AIS grade | Race/ethnicity | Education | Employment | Marital status |
|---|---|---|---|---|---|---|---|---|---|
| 102 | M | 46 | Fall | T10 | A | White/Non-Hispanic | Some college | Retired | Married |
| 107 | F | 31 | MVA | T2 | A | Asian/Non-Hispanic | Some college | Unemployed | Single |
| 111 | F | 52 | Fall | T10 | C | White/Non-Hispanic | Some college | Retired | Married |
| 113 | M | 27 | Fall | T11 | B | White/Non-Hispanic | Graduate degree | Employed | Single |
| 115 | M | 20 | MVA | C6 | A | White/Hispanic | GED | Employed | Single |
| 119 | M | 22 | Sports | C5 | A | White/Non-Hispanic | High school | Student | Single |
| 120 | M | 48 | MVA | C6 | B | White/Hispanic | High school | Unemployed | Married |
| 123 | M | 20 | Sports | C6 | C | White/Non-Hispanic | High school | Unemployed | Single |
ahSC, autologous human Schwann cell; AIS, American Spinal Injury Association (ASIA) Impairment Scale; ID, identification; M, male; F, female; MVA, motor vehicle accident, GED, general education development.
ahSC transplantation data
Data for ahSC processing and injections are shown in Table 2. The median dose of cells injected was 39 × 106 (range 17-120 × 106 cells,170-1200 μL), and the median ahSC purity at the time of transplantation was 97.4% (96.5-98.8%). Intra-operative electrophysiology measures were not altered substantially by the surgical procedure.
Table 2.
Sural Nerve Harvest and Cell Transplantation Data for Participants Undergoing ahSC Transplantation
| Subject ID | Time Post-injury (years) | Nerve harvest to transplant (weeks) | Nerve length (cm) | Estimated dose based on imaging ( × 106 cells) | Actual dose ( × 106 cells) | Injections (#) | Duration of injections (min:sec) | Rate (μL/min) | Rate (μL/min) | Cell viability (%) | Endotoxin (EU/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 102 | 15 | 15 | 14.5 | 25 | 23 | 2 | 9:00 | ∼30 | 97.1 | 96.6 | 0.0200 |
| 107 | 1 | 15 | 13.0 | 43 | 50 | 2 | 10:00 | ∼50 | 97.7 | 97.1 | 0.0075 |
| 111 | 10 | 15 | 14.0 | 17 | 20 | 1 | 4:00 | ∼50 | 98.7 | 95.1 | 0.0001 |
| 113 | 2 | 15 | 15.0 | 54 | 20 | 2 | 7:17 | ∼30 | 97.4 | 96.2 | 0.0070 |
| 115 | 7 | 15 | 15.0 | 80 | 31 | 3 | 8:56 | ∼40 | 97.4 | 96.3 | 0.0260 |
| 119 | 5 | 16 | 14.0 | 120 | 75 | 3 | 11:43 | ∼63 | 97.3 | 93.9 | 0.0180 |
| 120 | 8 | 36* | 15.0 | 30 | 30 | 1 | 6:00 | ∼50 | 98.8 | 94.3 | 0.0060 |
| 123 | 2 | 15 | 12.0 | 35 | 34 | 3 | 10:40 | ∼35 | 96.5 | 95.6 | 0.0030 |
Pre-transplant time extended due to modification of injection system.
Protocol compliance (exercise dose, timeline, visit compliance)
All participants completed 12 weeks of pre-transplant training and 6 months post-transplant. One participant (TMP120) completed 30 weeks of pre-transplant training due to a delay in the transplant surgery as a change in device sterilization protocol was completed as an added safety measure. On average, the other participants completed 37 (± 2) circuit resistance training (CRT) sessions pre-transplant and 62 (± 5) sessions post-transplant. Excluding TMP120, functional electrical stimulation (FES), and locomotor training (LT) sessions occurred an average of 24 (± 1) times pre-transplant and 40 (± 1) post-transplant. All participants completed all assessment visits as described by the study protocol.
Imaging
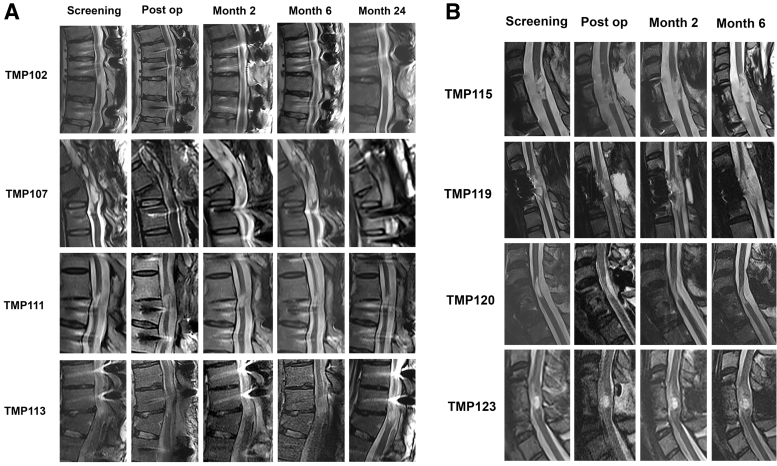
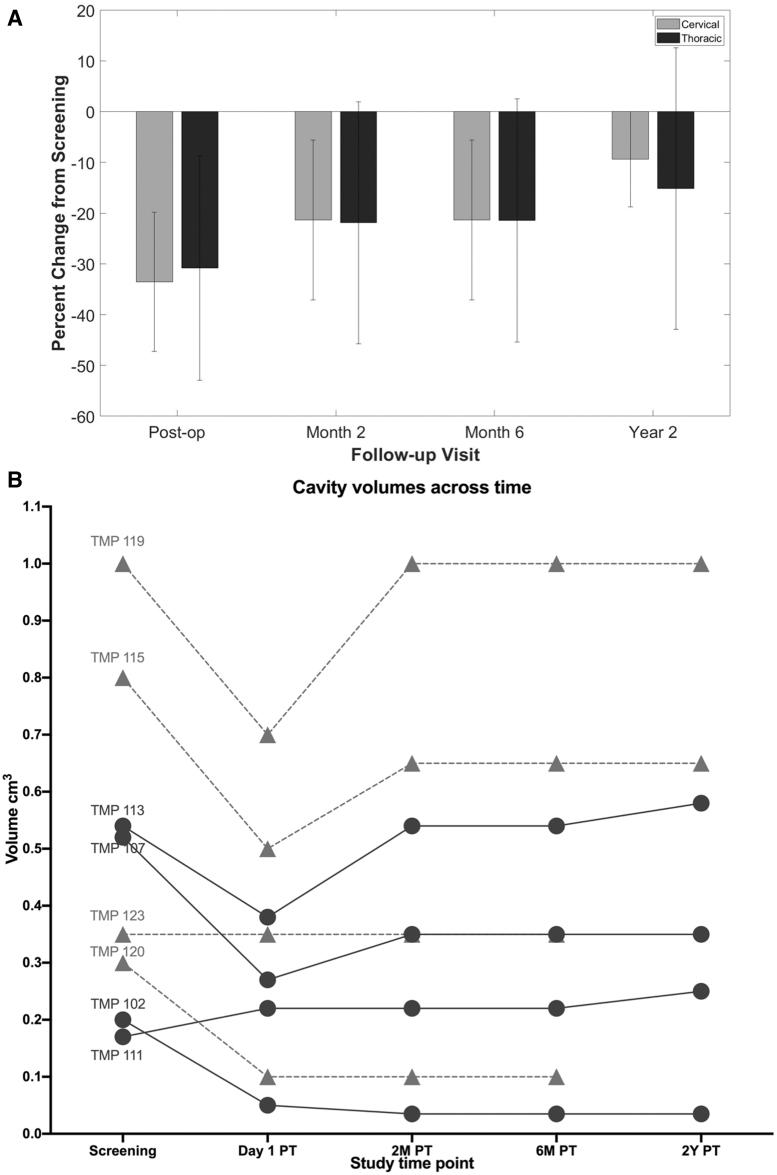
MRI of the spinal cord ahSC injection site post-operatively and at last follow-up demonstrated no evidence of hemorrhage, cyst formation, increased tethering, or tumorigenesis (Fig. 3). The lesion volume for three participants in the thoracic cohort decreased on post-operative Day 1 (baseline: 0.2-0.54 cc; post-operative Day 1: 0.05-0.38, Fig. 4). In one participant with thoracic SCI (TMP111), the lesion increased on post-operative Day 1 from 0.17 cc to 0.22 cc and remained larger than baseline at Year 2. In another participant (TMP113), the lesion volume increased from post-operative Day 1 MRI (0.38 cc) until follow-up at 2 years (0.58 cc).
FIG. 3.
Serial sagittal magnetic resonance images for the cervical (A) and thoracic (B) cohort showing changes in spinal cord cyst geometry before and after autologous human Schwann cell transplantation.
FIG. 4.
(A) Mean (± standard deviation) change in spinal cord cyst volume calculated from MR images for the cervical and thoracic chronic spinal cord injury cohort after autologous human Schwann cells transplantation; (B) The volume of the spinal cord cavities estimated from serial magnetic resonance imaging is shown (PT: post-transplant). Cervical cavities are indicated as triangles and thoracic cavities as circles. The average volume of thoracic cavities was smaller. In 6/8 participants an initial cavity volume reduction was observed that averaged 34.3 ± 12.1%.
In the cervical SCI cohort, the magnitude of change in cyst volume was less than the thoracic cohort, and cyst volumes decreased in three participants (baseline: 0.3-1.0 cc; post-operative Day 1: 0.1-0.7 cc) and remained stable in one participant (baseline: 0.35 cc; post-operative Day 1: 0.35 cc). At 6 months, two participants had cyst volumes that were identical to their baseline, and two participants had persistently lower cyst volumes. A 2-year follow-up MRI was available for two participants in the cervical SCI cohort, which showed stable lesion volumes.
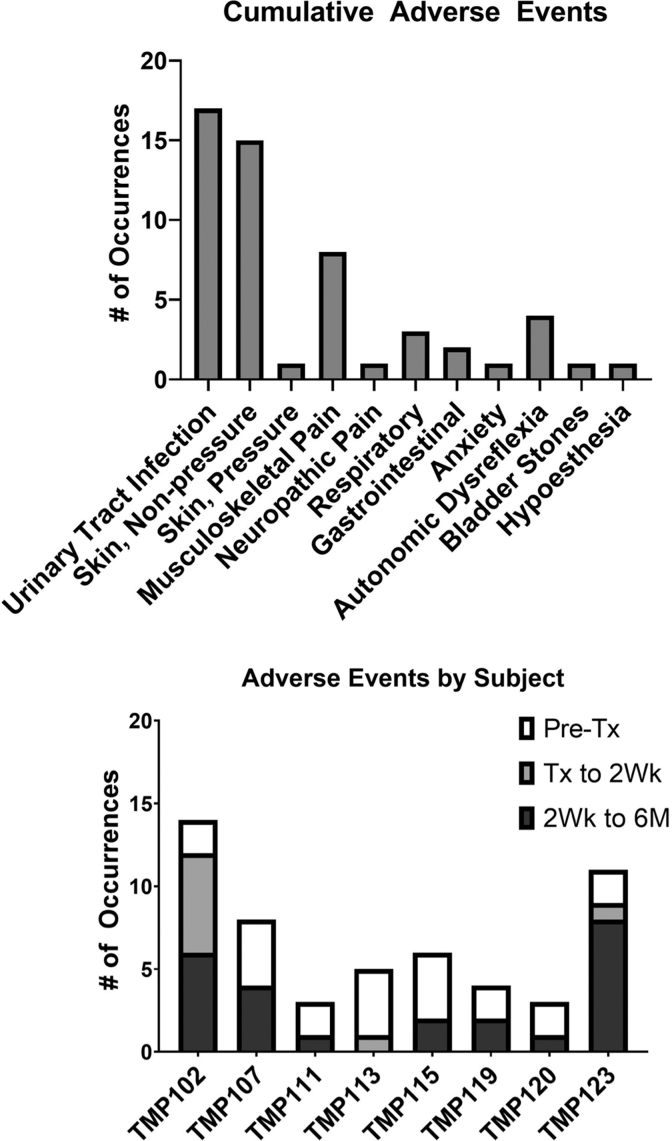
Adverse events and serious adverse events
There were 54 adverse events documented throughout the protocol among the eight participants (Fig. 5). There were no serious adverse events. Fourteen adverse events were definitely or probably related to study procedures and were related to either the exercise and rehabilitation training (skin abrasions and irritation from harness, muscle, or joint pain) or the transplant surgical procedure (headaches, nausea, hypoesthesia). Urinary tract infections and skin injuries were most common, which is consistent with issues experienced by the chronic SCI population.
FIG. 5.
Adverse event profile by type and participant.
Neurological motor and sensory impairment
AIS grade transiently shifted from AIS B to AIS C in one participant (TMP113) due to a short-term change in sensation following transplantation, which caused the neurological level of injury to ascend by one level, and then returned to AIS B at subsequent evaluations. In another participant (TMP 123), AIS grade changed from AIS C to AIS B at Week 1 after the transplantation, due to temporary inability to contract the anal sphincter, but then reverted back to AIS C at subsequent evaluations. In all other participants, AIS grades remained unchanged throughout the course of the study, up to 6 months post-transplantation. The neurological level of injury improved by 1 level (T9 to T10) in one participant (TM102) at a 6-month follow-up. In two participants (TMP 111 and TMP 113), transient improvements in the neurological level of injury by 1-2 levels after exercise and training (TMP113) and transplantation (TMP111) were noted. These returned to the baseline neurological level at 6-month follow-up. No change in the neurological level of injury was noted in the cervical SCI cohort (Table 1).
Changes in motor and sensory scores as assessed by the ISNCSCI for individual participants are shown in Table 3. No changes in upper extremity motor scores were noted in the thoracic cohort. In the cervical cohort, one participant (TMP115) showed a 1-point increase in right triceps (C7) strength from 2 to 3 at Week 1, and this was sustained at Month 6. No change in the left triceps strength was noted. In another participant (TMP120), left triceps strength improved from 3 to 4 at Month 2, and this was sustained at Month 6. There were no changes in lower extremity motor scores for the cervical cohort. As the primary analysis for safety on total motor scores, the paired within-participants assessment resulted in a t-statistic of 1.528, df = 7, and a p value (two-tailed) of 0.17, indicating no significant difference between the motor scores at baseline versus 6 months post-transplant (GraphPad Prism,9.0).
Table 3.
ISNCSCI Data at Baseline and 6 Months Post-Transplant for Participants Undergoing ahSC Transplantation
| Subject ID | Δ NLI | Δ AIS | UEMS (BL) | UEMS (M6) | Δ UEMS | LEMS (BL) | LEMS (M6) | Δ LEMS | Total SS (BL) | Total SS (M6) | Δ Total SS | LT (BL) | LT (M6) | Δ LT | PP (BL) | PP (M6) | Δ PP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 102 | +1 | - | 50 | 50 | - | 0 | 4 | +4 | 132 | 138 | +6 | 67 | 70 | +3 | 65 | 68 | +3 |
| 107 | - | - | 50 | 50 | - | 0 | 0 | - | 74.5 | 77 | +2.5 | 38 | 40 | +2 | 36.5 | 37 | +0.5 |
| 111 | - | - | 50 | 50 | - | 0 | 0 | - | 150 | 153 | +3 | 80.5 | 81 | +0.5 | 69.5 | 72 | +2.5 |
| 113 | - | - | 50 | 50 | - | 0 | 0 | - | 147 | 148 | +1 | 75 | 74 | -1 | 72 | 74 | +2 |
| 115 | - | - | 23 | 24 | +1 | 0 | 0 | - | 52 | 53 | +1 | 28 | 29 | +1 | 24 | 24 | - |
| 119 | - | - | 18 | 18 | - | 0 | 0 | - | 37 | 40 | +3 | 20 | 22 | +2 | 17 | 18 | +1 |
| 120 | - | - | 26 | 27 | +1 | 0 | 0 | - | 77.5 | 70 | -7.5 | 56 | 50 | -6 | 21.5 | 20 | -1.5 |
| 123 | - | - | 23 | 23 | 0 | 0 | 0 | - | 140 | 137 | -3 | 85 | 80 | -5 | 55 | 57 | +2 |
ISNCSCI, International Standards for the Neurological Classification of Spinal Cord Injury; ahSC, autologous human Schwann cell; NLI, neurological level of injury; AIS, American Spinal Injury Association Impairment Scale; UEMS, upper extremity motor score; M6, month 6; BL, averaged baseline (baseline 1 and baseline 2); LEMS, lower extremity motor score; SS, sensory score; LT, light touch; PP, pin prick.
Improvements in total sensory scores were noted in all participants in the thoracic cohort and two participants in the cervical cohort (Table 1). One participant in the thoracic cohort (TMP102) achieved the minimum clinically important difference (> 5.19) in sensory scores.50 Total sensory scores at 6 months declined by 7.5 points in one participant in the cervical cohort (TMP120); light touch sensation declined by 6 points, and pin prick sensation declined by 1.5 points from averaged baseline. For TMP102, equal improvements were seen for light touch and pinprick sensation. For TMP120, light touch sensation declined by 6 points, and pin prick sensation declined by 1.5 points. Sensory scores varied in the other participants, but were below the MCID at 6 months and within the reported intra-rater variability for ISNCSCI.55
Neurophysiology
In those participants in whom intraoperative MEPs and SSEPs could be obtained, no adverse changes in conduction were detected before and after the cell implantation. The detailed neurophysiology assessments at the four study time-points will be published separately.
Pain history and pain-related sensory function
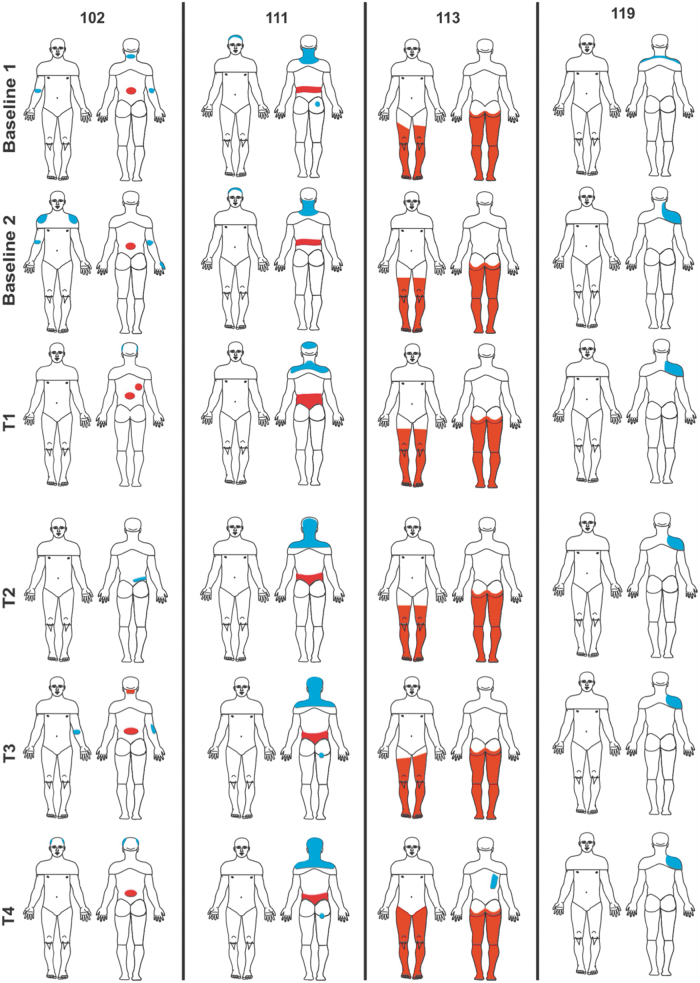
Overall, four participants did not experience any pain at baseline or at study completion, except for some transient musculoskeletal pain that completely resolved at the end of the study. Four participants experienced pain at the onset, during, and at completion of the study, and their data is presented in Figure 6 and in the Supplementary Material. One participant's neuropathic pain increased in severity from baseline, although no increase in neuropathic pain was observed in the other three participants. The QST findings were unremarkable and consistent with injury severity and pain status.
FIG. 6.
Pain diagrams for four participants who experienced chronic pain at Baseline, and the T1 (1 month), T2 (6 month), T3 (12 month), and T4 (2 year) time-points.
Fitness outcomes
Cardiorespiratory conditioning
All participants with tetraplegia were rated in the “good” range when using normative data to assess their BL1 cardiorespiratory conditioning.8 Two participants with paraplegia were classified as “excellent,” one “fair,” and one “poor.” For individual analysis, several participants increased, and several decreased their VO2 peak (Table 4; range: −3.7 to +4.2 mL.kg−1.min−1) The group average reflected an 8.5% improvement at M6. However, the average absolute difference in VO2 was a minimal 1.01 mL.kg−1.min−1.
Table 4.
Cardiorespiratory Conditioning Measured as VO2 Peak during Graded Upper Limb Exercise in Participants
| Subject ID | BL1 | BL2 | M2 | M6 | Δ : BL1 to M6 | Absolute change | Subjective cardiorespiratory fitness at BL1 |
|---|---|---|---|---|---|---|---|
| mL/kg/min | mL/kg/min | mL/kg/min | mL/kg/min | % | mL/kg/min | ||
| 102 | 35 | 34.1 | 28.6 | 31.3 | -10.6 | -3.7 | Excellent |
| 107 | 7.5 | 8.4 | 8.1 | 8.1 | 8.0 | 0.6 | Poor |
| 111 | 14.5 | 12.5 | 14.3 | 17 | 17.2 | 2.5 | Fair |
| 113 | 26.6 | 30.6 | 31.8 | 30.8 | 15.8 | 4.2 | Excellent |
| 115 | 11.4 | 9.1 | 13.4 | 10.9 | -4.4 | -0.5 | Good |
| 119 | 9.5 | 12.2 | 9.3 | 9 | -5.3 | -0.5 | Good |
| 120 | 10 | 10.6 | 11.8 | 12.9 | 29.0 | 2.9 | Good |
| 123 | 14 | 13.8 | 11.8 | 16.6 | 18.6 | 2.6 | Good |
| Mean | 16.1 | 16.4 | 16.1 | 17.1 | 8.5 | 1.01 |
BL1, Baseline 1, pre-biopsy ±7 days; BL2: Baseline 2, post–12-week conditioning ±7 days; M2, Month 2; post-transplant ±7 days; M6, Month 6, 6 months post-transplant ±7 days.
Strength conditioning
Minimal strength gains for the four participants with paraplegia averaged 2.5., 5, and 8.2% greater than the BL1 values at BL2, M2, and M6, respectively. These increases contrast our previous report averaging 21.1% when using a more intense training algorithm for three training months.56 For participants with tetraplegia, negligible changes of -1, -2, and -1% were observed from BL1 at BL2, M2, and M6 time-points, respectively.
Discussion
In this study, we demonstrated feasibility, safety, and an acceptable adverse event profile after ahSC transplantation in a cohort of participants with chronic complete and incomplete cervical and thoracic SCI. We calculated a participant-specific volume of cell suspension to inject and implemented an innovative technique of cavity-filling for ahSC transplantation into the cystic cavity. In addition, a well-defined standardized pre- and post-transplantation rehabilitation protocol was included to optimize the detection of a treatment effect.
The size of the lesion cavity was an important determinant of eligibility for participation in this trial, and many individuals screened (55% of thoracic and 64% of cervical SCI) were excluded due to imaging findings.57 Although MRI screening criteria may limit the number of eligible chronic SCI participants, it enabled identification of individuals with relatively similar lesion morphology and of smaller volumes for whom an intra-lesional cell transplantation appears safe and feasible. The smaller cyst volume will potentially increase the likelihood of ahSC transplant integration and regenerative benefit. In animals, the lesion volumes resulting after carefully graded experimental SCI are reasonably uniform.58–60 Humans have chronic cavities with highly variable dimensions and structure.61,62 This may result from differences in the pattern and severity of injury, differences in inflammation and ischemia, and tissue reorganization and fibrosis. The relatively large observed cavity size and variability is reflected by the large number of people with chronic SCI screened for the current study.57 The upper limit of cavity volume at 2 mL in enrolled participants was based on the feasibility of being able to expand 200 million ahSC in a reasonable time frame at no more than three to four cell passages. The ahSC “dose” we report was calculated based on the volume and concentration of cells injected. However, these doses represent an approximation since it was difficult to determine the volume lost due to cell reflux during surgery. It was necessary to see some cell reflux to indicate cavity-filling.
We call the novel transplantation methodology used in this trial the “cavity-filling delivery approach.”63,64 This technique is distinct from other intramedullary cell transplantation procedures where cells were injected using uniform cell doses and volumes across participants either rostral or caudal to the chronic lesion into residual spared parenchyma.65 Following SCI, tissue is progressively lost at the epicenter resulting in a cavity with a variably gliotic tissue margin,66,67 and the axons close to the cavity have defects in myelination.3,68 This ahSC repair strategy was intended to improve the function in these axons with impaired signaling by ensheathment, myelination, and trophic support. SCs can form myelin around central axons in astroglial depleted areas.69 Animal studies have shown that nearby axons extend new processes and are engaged by Schwann cells.10,70–72 Thus, this new tissue may serve as a bridge for new axonal growth across the injury epicenter, fostering a limited degree of regeneration and structural plasticity.71,73
To optimize ahSC engraftment, we filled the lesion cavity with a suspension of ahSCs. At least acutely, the pre-operatively calculated cavity volume based on pre-surgical T2-weighted MRI could be completely filled with autologous SCs. This theoretically allowed all cavity margin surfaces to undergo potential engraftment by the transplanted ahSC and should provide sufficient cells to support new tissue growth within the cavity space. However, without advanced techniques to trace the ahSC, we could not convincingly observe new tissue on post-transplant MRI, and we cannot yet determine the survival of the transplanted cells—an important knowledge gap in our field.
In performing these transplants, it is important to avoid creating excess pressure within the cavity that could rupture and potentially damage the residual spinal tissue around the cavity.63 Our technique to deliver cells resulted in cavity filling at a low delivery pressure facilitated by the small myelotomy opening. A significant factor affecting the distribution of the transplanted cells were cavity septations that were, to some extent, predictable from pre-transplant MRI but better visualized using intra-operative ultrasound. We found during cell delivery that some septations communicated whereas others did not. This can result in an uneven distribution of transplanted cells.
A standardized pre- and post-transplant multi-modal rehabilitation and training program is considered an important adjunct to cellular therapies for chronic SCI.28 A sedentary lifestyle imposed by SCI typically reduces the conditioning level to the lowest end of the human fitness continuum,74 accompanied by multidimensional challenges to pre-injury health and function.75 Conversely, intense exercise is reported to promote enhanced post-SCI musculoskeletal function by promoting neuroplastic changes in the damaged spinal cord.76 It is desirable to bring untrained, chronically injured individuals to a stable fitness plateau prior to the administration of therapeutics, especially those where cells are delivered at a single time-point.77
In a prior study, we investigated patterns of recovery in people with chronic complete thoracic SCI, who underwent a multi-modal training program for locomotion, upper body conditioning, and functional electrical stimulation.37 Some participants experienced improvements in function, but improvements in pain and spasticity were not consistent across participants. Variability in training programs between SCI participants in clinical trials could confound outcomes of the intervention being tested.78 Therefore, we used a standardized multi-modal low-intensity conditioning program for muscles located both above and below the lesion level. Supra-lesional muscles of the arms and trunk underwent conditioning exercise using a circuit resistance protocol reported in persons with chronic SCI.56 As the circuit training results in high levels of conditioning for both strength and cardiorespiratory endurance, the training protocol used a reduced weekly frequency for muscles spared by SCI. To sustain lower extremity mass and contractile functions that are pathologically altered after SCI,79,80 the participants underwent cycling exercise using low-intensity contractions of lower extremity muscles stimulated by transcutaneous electrical current. We expect that this conditioning maintained mass, circulation, and cardiac functions81,82 but contributed little to upper extremity strength and cardiorespiratory endurance. The detailed assessment of autonomic and metabolic activity, which can be improved after the rehabilitation performed in this study, will be published separately.
Despite using higher cell doses as compared to our prior study in people with subacute SCI,26 we have not identified major safety concerns associated with ahSC transplantation in this chronic SCI participant cohort. A trend toward efficacy was evaluated in this study, and the minimum clinically important improvement was achieved for sensory scores in one person. The observed clinical effects of ahSC transplantation in chronic SCI may be more pronounced when used in combination with agents that modify the glial scar83,84 or provide additional neurotrophic factors to influence the migration and survival of cells.85 This study demonstrates the safety and feasibility of ahSC transplantation in combination with a rehabilitation protocol in this cohort of people with chronic SCI. Imaging follow-up at 5 years is awaited to confirm the long-term safety of ahSC transplantation for chronic SCI.
Conclusion
This phase 1 open-label study demonstrated feasibility and safety of ahSC transplantation in participants with chronic cervical and thoracic SCI. Unique aspects of this study may be applicable to future SCI trials for injecting individualized volume of cells and incorporation of a standard rehabilitation protocol before and after cell transplantation.
Supplementary Material
Funding Information
Bryon Riesch Paralysis Foundation; Katie Samson Foundation; David and Jenni Belford–Belford Charitable Trust; Carnival Foundation; Buoniconti Fund to Cure Paralysis; Miami Project to Cure Paralysis; Christine E. Lynn–E.M. Lynn Foundation; Madeleine and Micky Arison; Joseph R. Coulter III Foundation.
Author Disclosure Statement
Allan D. Levi:Grant support - Department of Defense (DOD), NIH/NINDS – R25 / R21
Teaching Honorariums: AANS, Medtronic.
James D. Guest: Grant support, DOD, NIH-R21/RO1, Scientific advisory board In Vivo therapeutics, consultant to Abbvie.
Mary Bartlett Bunge, W. Dalton Dietrich, James D. Guest, Aisha Khan, Allan D. Levi and Damien Pearse have disclosed a relationship with Aceso Therapeutics that includes equity.
For the other authors, no competing financial interests exist.
Supplementary Material
References
- 1. Bunge, R.P., Puckett, W.R., Becerra, J.L., Marcillo, A., and Quencer, R.M. (1993). Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv. Neurol. 59, 75–89. [PubMed] [Google Scholar]
- 2. Cohen-Adad, J., El Mendili, M.-M., Lehéricy, S., Pradat, P.F., Blancho, S., Rossignol, S., and Benali, H. (2011). Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. NeuroImage 55, 1024–1033. [DOI] [PubMed] [Google Scholar]
- 3. Guest, J.D., Hiester, E.D., and Bunge, R.P. (2005). Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp. Neurol. 192, 384–393. [DOI] [PubMed] [Google Scholar]
- 4. Norenberg, M.D., Smith, J., and Marcillo, A. (2004). The pathology of human spinal cord injury: defining the problems. J. Neurotrauma 21, 429–440. [DOI] [PubMed] [Google Scholar]
- 5. Wolfe, D.L., Hayes, K.C., Hsieh, J.T., and Potter, P.J. (2001). Effects of 4-aminopyridine on motor evoked potentials in patients with spinal cord injury: a double-blinded, placebo-controlled crossover trial. J. Neurotrauma 18, 757–771. [DOI] [PubMed] [Google Scholar]
- 6. Bruce, J.H., Norenberg, M.D., Kraydieh, S., Puckett, W., Marcillo, A., and Dietrich, D. (2000). Schwannosis: role of gliosis and proteoglycan in human spinal cord injury. J. Neurotrauma 17, 781–788. [DOI] [PubMed] [Google Scholar]
- 7. Barakat, D.J., Gaglani, S.M., Neravetla, S.R., Sanchez, A.R., Andrade, C.M., Pressman, Y., Puzis, R., Garg, M.S., Bunge, M.B., and Pearse, D.D. (2005). Survival, integration, and axon growth support of glia transplanted into the chronically contused spinal cord. Cell Transplant. 14, 225–240. [DOI] [PubMed] [Google Scholar]
- 8. Guest, J., Santamaria, A.J., and Benavides, F.D. (2013). Clinical translation of autologous Schwann cell transplantation for the treatment of spinal cord injury. Curr. Opin. Organ Transplant. 18, 682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takami, T., Oudega, M., Bates, M.L., Wood, P.M., Kleitman, N., and Bunge, M.B. (2002). Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J. Neurosci. 22, 6670–6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu, X.M., Chen, A., Guénard, V., Kleitman, N., and Bunge, M.B. (1997). Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J. Neurocytol. 26, 1–16. [DOI] [PubMed] [Google Scholar]
- 11. Pearse, D.D., Sanchez, A.R., Pereira, F.C., Andrade, C.M., Puzis, R., Pressman, Y., Golden, K., Kitay, B.M., Blits, B., Wood, P.M., and Bunge, M.B. (2007). Transplantation of Schwann cells and/or olfactory ensheathing glia into the contused spinal cord: Survival, migration, axon association, and functional recovery. Glia 55, 976–1000. [DOI] [PubMed] [Google Scholar]
- 12. Pearse, D.D., Marcillo, A.E., Oudega, M., Lynch, M.P., Wood, P.M., and Bunge, M.B. (2004). Transplantation of Schwann cells and olfactory ensheathing glia after spinal cord injury: does pretreatment with methylprednisolone and interleukin-10 enhance recovery? J. Neurotrauma 21, 1223–1239. [DOI] [PubMed] [Google Scholar]
- 13. Schaal, S.M., Kitay, B.M., Cho, K.S., Lo, T.P., Barakat, D.J., Marcillo, A.E., Sanchez, A.R., Andrade, C.M., and Pearse, D.D. (2007). Schwann cell transplantation improves reticulospinal axon growth and forelimb strength after severe cervical spinal cord contusion. Cell Transplant. 16, 207–228. [DOI] [PubMed] [Google Scholar]
- 14. Pearse, D.D., Pereira, F.C., Marcillo, A.E., Bates, M.L., Berrocal, Y.A., Filbin, M.T., and Bunge, M.B. (2004). cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 10, 610–616. [DOI] [PubMed] [Google Scholar]
- 15. Taylor, J.S.H., and Bampton, E.T.W. (2004). Factors secreted by Schwann cells stimulate the regeneration of neonatal retinal ganglion cells. J. Anat. 204, 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Toyota, B., Carbonetto, S., and David, S. (1990). A dual laminin/collagen receptor acts in peripheral nerve regeneration. Proc. Natl. Acad. Sci. U. S. A. 87, 1319–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martini, R. and Schachner, M. (1988). Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and myelin-associated glycoprotein) in regenerating adult mouse sciatic nerve. J. Cell. Biol. 106, 1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bamber, N.I., Li, H., Lu, X., Oudega, M., Aebischer, P., and Xu, X.M. (2001). Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur. J. Neurosci. 13, 257–268. [PubMed] [Google Scholar]
- 19. Guest, J.D., Rao, A., Olson, L., Bunge, M.B., and Bunge, R.P. (1997). The ability of human Schwann cell grafts to promote regeneration in the transected nude rat spinal cord. Exp. Neurol. 148, 502–522. [DOI] [PubMed] [Google Scholar]
- 20. Xu, X.M., Guénard, V., Kleitman, N., and Bunge, M.B. (1995). Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J. Comp. Neurol. 351, 145–160. [DOI] [PubMed] [Google Scholar]
- 21. Xu, X.M., Zhang, S.X., Li, H., Aebischer, P., and Bunge, M.B. (1999). Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur. J. Neurosci. 11, 1723–1740. [DOI] [PubMed] [Google Scholar]
- 22. Imaizumi, T., Lankford, K.L., and Kocsis, J.D. (2000). Transplantation of olfactory ensheathing cells or Schwann cells restores rapid and secure conduction across the transected spinal cord. Brain Res 854, 70–78. [DOI] [PubMed] [Google Scholar]
- 23. Pinzon, A., Calancie, B., Oudega, M., and Noga, B.R. (2001). Conduction of impulses by axons regenerated in a Schwann cell graft in the transected adult rat thoracic spinal cord. J. Neurosci. Res. 64, 533–541. [DOI] [PubMed] [Google Scholar]
- 24. Bastidas, J., Athauda, G., De La Cruz, G., Chan, W.-M., Golshani, R., Berrocal, Y., Henao, M., Lalwani, A., Mannoji, C., Assi, M., Otero, P.A., Khan, A., Marcillo, A.E., Norenberg, M., Levi, A.D., Wood, P.M., Guest, J.D., Dietrich, W.D., Bartlett Bunge, M., and Pearse, D.D. (2017). Human Schwann cells exhibit long-term cell survival, are not tumorigenic and promote repair when transplanted into the contused spinal cord. Glia 65, 1278–1301. [DOI] [PubMed] [Google Scholar]
- 25. Pearse, D.D., Bastidas, J., Izabel, S.S., and Ghosh, M. (2018). Schwann cell transplantation subdues the pro-inflammatory innate immune cell response after spinal cord injury. Int. J. Mol. Sci. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anderson, K.D., Guest, J.D., Dietrich, W.D., Bartlett Bunge, M., Curiel, R., Dididze, M., Green, B.A., Khan, A., Pearse, D.D., Saraf-Lavi, E., Widerström-Noga, E., Wood, P., and Levi, A.D. (2017). Safety of autologous human Schwann cell transplantation in subacute thoracic spinal cord injury. J. Neurotrauma 34, 2950–2963. [DOI] [PubMed] [Google Scholar]
- 27. Santamaria, A.J., Benavides, F.D., Saraiva, P.M., Anderson, K.D., Khan, A., Levi, A.D., Dietrich, W.D., and Guest, J.D. (2020). Neurophysiological changes in the first year after cell transplantation in sub-acute complete paraplegia. Front. Neurol. 11, 514181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thuret, S., Moon, L.D.F., and Gage, F.H. (2006). Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 7, 628–643. [DOI] [PubMed] [Google Scholar]
- 29. Fouad, K. and Tetzlaff, W. (2012). Rehabilitative training and plasticity following spinal cord injury. Exp. Neurol. 235, 91–99. [DOI] [PubMed] [Google Scholar]
- 30. van Hedel, H.J.A. and Dietz, V. (2010). Rehabilitation of locomotion after spinal cord injury. Restor. Neurol. Neurosci. 28, 123–134. [DOI] [PubMed] [Google Scholar]
- 31. Angeli, C.A., Boakye, M., Morton, R.A., Vogt, J., Benton, K., Chen, Y., Ferreira, C.K., and Harkema, S.J. (2018). Recovery of over-ground walking after chronic motor complete spinal cord injury. N. Engl. J. Med. 379, 1244–1250. [DOI] [PubMed] [Google Scholar]
- 32. Gad, P., Lee, S., Terrafranca, N., Zhong, H., Turner, A., Gerasimenko, Y., and Edgerton, V.R. (2018). Non-invasive activation of cervical spinal networks after severe paralysis. J. Neurotrauma 35, 2145–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wagner, F.B., Mignardot, J.B., Le Goff-Mignardot, C.G., Demesmaeker, R., Komi, S., Capogrosso, M., Rowald, A., Seáñez, I., Caban, M., Pirondini, E., Vat, M., McCracken, L.A., Heimgartner, R., Fodor, I., Watrin, A., Seguin, P., Paoles, E., Van Den Keybus, K., Eberle, G., Schurch, B., Pralong, E., Becce, F., Prior, J., Buse, N., Buschman, R., Neufeld, E., Kuster, N., Carda, S., von Zitzewitz, J., Delattre, V., Denison, T., Lambert, H., Minassian, K., Bloch, J., and Courtine, G. (2018). Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563, 65–71. [DOI] [PubMed] [Google Scholar]
- 34. Jo, H.J. and Perez, M.A. (2020). Corticospinal-motor neuronal plasticity promotes exercise-mediated recovery in humans with spinal cord injury. Brain 143, 1368–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gomes-Osman, J., Cortes, M., Guest, J., and Pascual-Leone, A. (2016). A systematic review of experimental strategies aimed at improving motor function after acute and chronic spinal cord iInjury. J. Neurotrauma 33, 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Morrison, S.A., Lorenz, D., Eskay, C.P., Forrest, G.F., and Basso, D.M. (2018). Longitudinal recovery and reduced costs after 120 sessions of locomotor training for motor incomplete spinal cord injury. Arch. Phys. Med. Rehabil. 99, 555–562. [DOI] [PubMed] [Google Scholar]
- 37. Gant, K.L., Nagle, K.G., Cowan, R.E., Field-Fote, E.C., Nash, M.S., Kressler, J., Thomas, C.K., Castellanos, M., Widerström-Noga, E., and Anderson, K.D. (2018). Body system effects of a multi-modal training program targeting chronic, motor complete thoracic spinal cord injury. J. Neurotrauma 35, 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maher, J.L., Anderson, K.D., Gant, K.L., and Cowan, R.E. (2021). Development and deployment of an at-home strength and conditioning program to support a phase I trial in persons with chronic spinal cord injury. Spinal Cord 59, 44–54 [DOI] [PubMed] [Google Scholar]
- 39. Henderson, G.E., Churchill, L.R., Davis, A.M., Easter, M.M., Grady, C., Joffe, S., Kass, N., King, N.M.P., Lidz, C.W., Miller, F.G., Nelson, D.K., Peppercorn, J., Rothschild, B.B., Sankar, P., Wilfond, B.S., and Zimmer, C.R. (2007). Clinical trials and medical care: defining the therapeutic misconception. PLoS Med. 4, e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Widerström-Noga, E., Biering-Sørensen, F., Bryce, T., Cardenas, D.D., Finnerup, N.B., Jensen, M.P., Richards, J.S., and Siddall, P.J. (2008). The international spinal cord injury pain basic data set. Spinal Cord 46, 818–823. [DOI] [PubMed] [Google Scholar]
- 41. Wong, M.L., Fleming, L., Robayo, L.E., and Widerström-Noga, E. (2020). Utility of the Neuropathic Pain Symptom Inventory in people with spinal cord injury. Spinal Cord 58, 35–42. [DOI] [PubMed] [Google Scholar]
- 42. Wong, M.L., Tibbett, J., Adedolapo, T., and Widerstrom-Noga, E. (2019). The Graph-DCK Scale: a measure of dorsal column function after spinal cord injury. Spinal Cord 57, 412–418. [DOI] [PubMed] [Google Scholar]
- 43. Benz, E.N., Hornby, T.G., Bode, R.K., Scheidt, R.A., and Schmit, B.D. (2005). A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch. Phys. Med. Rehabil. 86, 52–59. [DOI] [PubMed] [Google Scholar]
- 44. Thompson, C.K. and Hornby, T.G. (2013). Divergent modulation of clinical measures of volitional and reflexive motor behaviors following serotonergic medications in human incomplete spinal cord injury. J. Neurotrauma 30, 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Akpinar, P., Atici, A., Ozkan, F.U., Aktas, I., Kulcu, D.G., Sarı, A., and Durmus, B. (2017). Reliability of the Modified Ashworth Scale and Modified Tardieu Scale in patients with spinal cord injuries. Spinal Cord 55, 944–949. [DOI] [PubMed] [Google Scholar]
- 46. Ness, L.L. and Field-Fote, E.C. (2009). Effect of whole-body vibration on quadriceps spasticity in individuals with spastic hypertonia due to spinal cord injury. Restor. Neurol. Neurosci. 27, 621–631. [DOI] [PubMed] [Google Scholar]
- 47. Kirshblum, S.C., Waring, W., and Biering-Sorensen, F. (2011). Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med 34, 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Itzkovich, M., Gelernter, I., and Biering-Sorensen, F. (2007). The Spinal Cord Independence Measure (SCIM) version III: reliability and validity in a multi-center international study. Disabil. Rehabil. 29, 1926–1933. [DOI] [PubMed] [Google Scholar]
- 49. Scivoletto, G., Tamburella, F., Laurenza, L., and Molinari, M. (2013). The spinal cord independence measure: how much change is clinically significant for spinal cord injury subjects. Disabil. Rehabil. 35, 1808–1813. [DOI] [PubMed] [Google Scholar]
- 50. Charlifue, S., Post, M.W., Biering-Sørensen, F., Catz, A., Dijkers, M., Geyh, S., Horsewell, J., Noonan, V., Noreau, L., Tate, D., and Sinnott, K.A. (2012). International spinal cord injury quality of life basic data set. Spinal Cord 50, 672–675. [DOI] [PubMed] [Google Scholar]
- 51. Tulsky, D.S., Kisala, P.A., Victorson, D., Tate, D.G., Heinemann, A.W., Charlifue, S., Kirshblum, S.C., Fyffe, D., Gershon, R., Spungen, A.M., Bombardier, C.H., Dyson-Hudson, T.A., Amtmann, D., Kalpakjian, C.Z., Choi, S.W., Jette, A.M., Forchheimer, M., and Cella, D. (2015). Overview of the Spinal Cord Injury–Quality of Life (SCI-QOL) measurement system. J. Spinal Cord Med. 38, 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Biering-Sorensen, F., Craggs, M., and Kennelly, M. (2008). International lower urinary tract function basic spinal cord injury data set. Spinal Cord 46, 325–330. [DOI] [PubMed] [Google Scholar]
- 53. Krogh, K., Perkash, I., Stiens, S.A., and Biering-Sørensen, F. (2009). International bowel function basic spinal cord injury data set. Spinal Cord 47, 230–234. [DOI] [PubMed] [Google Scholar]
- 54. Graves, D.E., Frankiewicz, R.G., and Donovan, W.H. (2006). Construct validity and dimensional structure of the ASIA motor scale. J. Spinal Cord Med. 29, 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marino, R.J., Jones, L., Kirshblum, S., Tal, J., and Dasgupta, A. (2008). Reliability and repeatability of the motor and sensory examination of the international standards for neurological classification of spinal cord injury. J. Spinal Cord Med. 31, 166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nash, M.S., van de Ven, I., van Elk, N., and Johnson, B.M. (2007). Effects of circuit resistance training on fitness attributes and upper-extremity pain in middle-aged men with paraplegia. Arch. Phys. Med. Rehabil. 88, 70–75. [DOI] [PubMed] [Google Scholar]
- 57. Burks, J.D., Gant, K.L., Guest, J.D., Jamshidi, A.G., Cox, E.M., Anderson, K.D., Dietrich, W.D., Bunge, M.B., Green, B.A., Khan, A., Pearse, D.D., Saraf-Lavi, E., and Levi, A.D. (2019). Imaging characteristics of chronic spinal cord injury identified during screening for a cell transplantation clinical trial. Neurosurg. Focus 46, E8. [DOI] [PubMed] [Google Scholar]
- 58. Casas, C.E., Herrera, L.P., Prusmack, C., Ruenes, G., Marcillo, A., and Guest, J.D. (2005). Effects of epidural hypothermic saline infusion on locomotor outcome and tissue preservation after moderate thoracic spinal cord contusion in rats. J. Neurosurg. Spine 2, 308–318. [DOI] [PubMed] [Google Scholar]
- 59. Duerstock, B.S. and Borgens, R.B. (2002). Three-dimensional morphometry of spinal cord injury following polyethylene glycol treatment. J. Exp. Biol. 205, 13–24. [DOI] [PubMed] [Google Scholar]
- 60. Nishi, R.A., Liu, H., Chu, Y., Hamamura, M., Su, M.Y., Nalcioglu, O., and Anderson, A.J. (2007). Behavioral, histological, and ex vivo magnetic resonance imaging assessment of graded contusion spinal cord injury in mice. J. Neurotrauma 24, 674–689. [DOI] [PubMed] [Google Scholar]
- 61. Freund, M., Habicht, D., Aschoff, A., Kalvine, K., and Sartor, K. (2002). Posttraumatic syringomyelia: volumetric phantom and patient studies using MR imaging. Eur. Radiol. 12, 2965–2972. [DOI] [PubMed] [Google Scholar]
- 62. Nidecker, A., Kocher, M., Maeder, M., Gratzl, O., Zäch, G.A., Benz, U.F., and Burckhardt, B. (1991). MR-imaging of chronic spinal cord injury. Association with neurologic function. Neurosurg. Rev. 14, 169–179. [DOI] [PubMed] [Google Scholar]
- 63. Guest, J., Benavides, F., Padgett, K., Mendez, E., and Tovar, D. (2011). Technical aspects of spinal cord injections for cell transplantation. Clinical and translational considerations. Brain Res. Bull. 84, 267–279. [DOI] [PubMed] [Google Scholar]
- 64. Santamaría, A.J., Solano, J.P., Benavides, F.D., and Guest, J.D. (2018). Intraspinal delivery of Schwann cells for spinal cord injury. Methods Mol. Biol. 1739, 467–484. [DOI] [PubMed] [Google Scholar]
- 65. Levi, A.D., Anderson, K.D., Okonkwo, D.O., Park, P., Bryce, T.N., Kurpad, S.N., Aarabi, B., Hsieh, J., and Gant, K. (2019). Clinical outcomes from a multi-center study of human neural stem cell transplantation in chronic cervical spinal cord injury. J. Neurotrauma 36, 891–902. [DOI] [PubMed] [Google Scholar]
- 66. Bunge, R.P., Puckett, W.R., and Hiester, E.D. (1997). Observations on the pathology of several types of human spinal cord injury, with emphasis on the astrocyte response to penetrating injuries. Adv. Neurol. 72, 305–315. [PubMed] [Google Scholar]
- 67. Kakulas, B.A. (1999). The applied neuropathology of human spinal cord injury. Spinal Cord 37, 79–88. [DOI] [PubMed] [Google Scholar]
- 68. Bunge, R.P. (1993). Expanding roles for the Schwann cell: ensheathment, myelination, trophism and regeneration. Curr. Opin. Neurobiol. 3, 805–809. [DOI] [PubMed] [Google Scholar]
- 69. Sasaki, M. and Ide, C. (1991). Aberrant remyelination of axons after heat injury in the dorsal funiculus of rat spinal cord. Acta Neuropathol. (Berl.) 81, 557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Guest, J.D., Hesse, D., Schnell, L., Schwab, M.E., Bunge, M.B., and Bunge, R.P. (1997). Influence of IN-1 antibody and acidic FGF-fibrin glue on the response of injured corticospinal tract axons to human Schwann cell grafts. J. Neurosci. Res. 50, 888–905. [DOI] [PubMed] [Google Scholar]
- 71. Keirstead, H.S., Morgan, S.V., Wilby, M.J., and Fawcett, J.W. (1999). Enhanced axonal regeneration following combined demyelination plus schwann cell transplantation therapy in the injured adult spinal cord. Exp. Neurol. 159, 225–236. [DOI] [PubMed] [Google Scholar]
- 72. Tuszynski, M.H., Weidner, N., McCormack, M., Miller, I., Powell, H., and Conner, J. (1998). Grafts of genetically modified Schwann cells to the spinal cord: survival, axon growth, and myelination. Cell Transplant. 7, 187–196. [DOI] [PubMed] [Google Scholar]
- 73. Weidner, N., Blesch, A., Grill, R.J., and Tuszynski, M.H. (1999). Nerve growth factor-hypersecreting Schwann cell grafts augment and guide spinal cord axonal growth and remyelinate central nervous system axons in a phenotypically appropriate manner that correlates with expression of L1. J. Comp. Neurol. 413, 495–506. [DOI] [PubMed] [Google Scholar]
- 74. LaPorte, R.E., Adams, L.L., Savage, D.D., Brenes, G., Dearwater, S., and Cook, T. (1984). The spectrum of physical activity, cardiovascular disease and health: an epidemiologic perspective. Am. J. Epidemiol. 120, 507–517. [DOI] [PubMed] [Google Scholar]
- 75. Nash, M.S. and Gater, D.R. (2020). Cardiometabolic disease and dysfunction following spinal cord injury: origins and guideline-based countermeasures. Phys. Med. Rehabil. Clin. N. Am. 31, 415–436. [DOI] [PubMed] [Google Scholar]
- 76. Loy, K. and Bareyre, F.M. (2019). Rehabilitation following spinal cord injury: how animal models can help our understanding of exercise-induced neuroplasticity. Neural Regen. Res. 14, 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Steeves, J.D., Lammertse, D.P., Kramer, J.L.K., Kleitman, N., Kalsi-Ryan, S., Jones, L., Curt, A., Blight, A.R., and Anderson, K.D. (2012). Outcome measures for acute/subacute cervical sensorimotor complete (AIS-A) spinal cord injury during a phase 2 clinical trial. Top. Spinal Cord Inj. Rehabil. 18, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tuszynski, M.H., Steeves, J.D., Fawcett, J.W., Lammertse, D., Kalichman, M., Rask, C., Curt, A., Ditunno, J.F., Fehlings, M.G., Guest, J.D., Ellaway, P.H., Kleitman, N., Bartlett, P.F., Blight, A.R., Dietz, V., Dobkin, B.H., Grossman, R., andPrivat A., ; International Campaign for Cures of Spinal Cord Injury Paralysis. (2007). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP Panel: clinical trial inclusion/exclusion criteria and ethics. Spinal Cord 45, 222–231. [DOI] [PubMed] [Google Scholar]
- 79. Castro, M.J., Apple, D.F., Hillegass, E.A., and Dudley, G.A. (1999). Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur. J. Appl. Physiol. 80, 373–378. [DOI] [PubMed] [Google Scholar]
- 80. Spungen, A.M., Adkins, R.H., Stewart, C.A., Wang, J., Pierson, R.N., Waters, R.L., and Bauman, W.A. (2003). Factors influencing body composition in persons with spinal cord injury: a cross-sectional study. J. Appl. Physiol. 95, 2398–2407. [DOI] [PubMed] [Google Scholar]
- 81. Nash, M.S., Bilsker, S., Marcillo, A.E., Isaac, S.M., Botelho, L.A., Klose, K.J., Green, B.A., Rountree, M.T., and Shea, J.D. (1991). Reversal of adaptive left ventricular atrophy following electrically-stimulated exercise training in human tetraplegics. Paraplegia 29, 590–599. [DOI] [PubMed] [Google Scholar]
- 82. Ragnarsson, K.T., Pollack, S., O'Daniel, W., Edgar, R., Petrofsky, J., and Nash, M.S. (1988). Clinical evaluation of computerized functional electrical stimulation after spinal cord injury: a multicenter pilot study. Arch. Phys. Med. Rehabil. 69, 672–677. [PubMed] [Google Scholar]
- 83. Bartus, K., Galino, J., James, N.D., Hernandez-Miranda, L.R., Dawes, J.M., Fricker, F.R., Garratt, A.N., McMahon, S.B., Ramer, M.S., Birchmeier, C., Bennett, D.L.H., and Bradbury, E.J. (2016). Neuregulin-1 controls an endogenous repair mechanism after spinal cord injury. Brain J. Neurol. 139, 1394–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Warren, P.M., Andrews, M.R., Smith, M., Bartus, K., Bradbury, E.J., Verhaagen, J., Fawcett, J.W., and Kwok, J.C.F. (2020). Secretion of a mammalian chondroitinase ABC aids glial integration at PNS/CNS boundaries. Sci. Rep. 10, 11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bunge, M.B. (2016). Efficacy of Schwann cell transplantation for spinal cord repair is improved with combinatorial strategies. J. Physiol. 594, 3533–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.