Abstract
Avirulent Erwinia carotovora subsp. carotovora CGE234-M403 produces two types of bacteriocin. For the purpose of cloning the bacteriocin genes of strain CGE234M403, a spontaneous rifampin-resistant mutant of this strain, M-rif-11-2, was isolated. By Tn5 insertional mutagenesis using M-rif-11-2, a mutant, TM01A01, which produces the high-molecular-weight bacteriocin but not the low-molecular-weight bacteriocin was obtained. By thermal asymmetric interlaced PCR, the DNA sequence from the Tn5 insertion site and the DNA sequence of a contiguous 1,280-bp region were determined. One complete open reading frame (ORF), designated ORF2, was identified within the sequenced fragment. The 3′ end of another ORF, ORF1, was located upstream of ORF2. A noncoding region and a putative promoter were located between ORF1 and ORF2. Downstream from ORF2, the 5′ end of another ORF (ORF3) was found. Deduction from the nucleotide sequence indicated that ORF2 encodes a protein of 99 amino acids, which showed high homology with Yersinia enterocolitica Yrp, a regulator of enterotoxin (Y-ST) production; Escherichia coli host factor 1, required for Qβ-replicase; and Azorhizobium caulinodans NrfA, required for the expression of nifA. ORF2 was designated brg, bacteriocin regulator gene. A fragment containing ORF2 and its promoter was amplified and cloned into pBR322 and pHSG415r, and the recombinant plasmids, pBYL1 and pHYL1, were transferred into E. coli DH5. Plasmid pBYL1 was reisolated and transferred into the insertion mutant TM01A01. Transformants carrying the plasmid, which was reisolated and designated pBYL1, re-produced the low-molecular-weight bacteriocin.
Erwinia carotovora subsp. carotovora is a phytopathogenic bacterium responsible for the soft-rot disease of many plant species. Despite its economic importance, no efficient method, either chemical or otherwise, has been found to control this worldwide disease. Agrochemicals are generally used for the control of this disease, but in a quest for a more environmentally friendly control methods, biological control is under investigation.
Some bacterial species produce one or more antibacterial substances called bacteriocins, which enhance their competitiveness with other related bacterial species (27). According to the report of Kikumoto et al. (13), the antibacterial activity of two types of bacteriocin produced by biocontrol agents (avirulent E. carotovora subsp. carotovora) may contribute to the suppression of soft-rot disease (18). There is also strong evidence of the effectiveness of biological control of the soft-rot disease of Chinese cabbage (14, 22). A biological control agent with the trade name Biokeeper has therefore been developed for the control of this disease in Japan. In view of these reports, identification and cloning of the gene(s) controlling bacteriocin production may facilitate its use in further control methods, such as the development of resistant cultivars by the cloning of such a gene(s) into Chinese cabbage and tobacco plants.
Gram-negative and gram-positive bacteria commonly harbor plasmid-borne genetic determinants of bacteriocin production and of host cell bacteriocin immunity, but new evidence (4) shows that these genes are located on chromosomal DNA in E. carotovora subsp. carotovora strains.
To date, no genes encoding the low-molecular-weight bacteriocin of E. carotovora subsp. carotovora have been isolated or characterized. Here, we report the cloning and sequencing of DNA containing the bacteriocin regulator gene (brg) from E. carotovora subsp. carotovora CGE234M403’s rifampin-resistant mutant, M-rif-11-2.
Bacterial strains, plasmids, and growth media.
The bacterial strains and plasmids used in this study are listed in Table 1. The putative biocontrol agent produces two types of bacteriocin, low- and high-molecular-weight bacteriocins. E. carotovora subsp. carotovora strains were propagated at 28°C in nutrient agar (NA) containing 1.4% agar or with shaking in Luria-Bertani (LB) medium with 5 g of NaCl per liter substituted for 10 g. Escherichia coli strains were propagated at 37°C in LB medium with shaking. Rifampin, kanamycin, and ampicillin (all at 50 μg per ml) were added to NA and LB agar when necessary.
TABLE 1.
Bacteria and plasmids used in this study
| Bacterium or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Bacteria | ||
| E. coli | ||
| 1830 | Pro− Met− Kmr Nmr; contains transposon Tn5 on the “suicidal” plasmid pJB4JI | Gantotti et al. (8) |
| DH5 | supE44 hsdR17 recA1 endA1 gyrA1 thi-1 relA1 | Hanahan and Reusch et al. (9, 19) |
| E. carotovora subsp. carotovora | ||
| CGE234M403 | Putative biocontrol agent | Laboratory stock (22) |
| M-rif-11-1 | CGE234M403 Rifr | This work |
| M-rif-11-2 | CGE234M403 Rifr | This work |
| M-rif-11-6 | CGE234M403 Rifr | This work |
| T-29 | Wild type | Laboratory stock (13) |
| E108 | Wild type | Laboratory stock (13) |
| TM01A01 | M-rif-11-2 brg::Tn5 Rifr | This work |
| Plasmids | ||
| pBR322 | Ampr Tetr | Bolivar et al. (1) |
| pHSG415r | Ampr Kanr; low copy number | Brady et al. (2) |
| pBDY1 | Ampr Tetr | This work |
| pHDY1 | Ampr Kanr; low copy number | This work |
Ampr indicates ampicillin resistance, Rifr indicates rifampin resistance, and Kanr indicates kanamycin resistance.
Bacterial mating.
Bacterial mating was carried out on NA by the membrane-filter mating method (8), by using 0.22-μm-pore-size membrane filters (Millipore, Inc. Bedford, Mass.). The filters were placed on NA and incubated overnight at 28°C. Appropriate dilutions of the suspension of the progeny of the mating were spread on modified Drigalski’s agar plates (26) containing 50 ppm each of rifampin and kanamycin and were incubated at 28°C for 24 to 48 h before the colonies were counted.
Bacteriocin assays.
Bacteriocin production was examined by the double-layer method of Fredericq (7), but hard and soft IFO-802 media containing, respectively, 1.4 and 0.65% agar were used. Growth inhibition zones around the colonies were considered an indication of bacteriocin production.
Genetic engineering technique.
Plasmids of E. carotovora subsp. carotovora were isolated according to the method of Kado and Liu (11), and E. coli plasmids were isolated by the method of Sambrook et al. (20). Total DNA was isolated as previously described (16).
Oligonucleotide DNA primers were synthesized by the Gibco BRL Co. and Takara Co. (Tokyo, Japan). Reagents were purchased from the Takara Co. The general PCR procedure has been described by Sambrook et al. (20). Thermal asymmetric interlaced PCR (TAIL-PCR) was performed according to the method of Liu and Whittier (15), but the annealing temperature for specific primers was increased from 63 to 65°C for this study. For TAIL-PCR, specific primers that are complementary to the respective sequences of Tn5 were synthesized: AGAGAACACAGATTTAGCCCAGTCGG (PF-1), CCGCACGATGAAGAGCAGAAGTTAT (PF-2), GATCCTGGAAAACGGGAAAGGTTC (PF-3), GCCGAAGAGAACACAGATTTAGCCCA (PR-1), CCGCACGATGAAGAGCAGAAGTT (PR-2), and CAGATCTCTGGAAAACGGGAAAGG (PR-3). In addition, two arbitrary degenerate primers, GTNCGA(C/G)(A/T)CANA(A/T)GTT (N-2) and (A/T)GTGNAG(A/T)ANCANAGA (N-3), were used.
For sequencing of TAIL-PCR products, the ABI PRISM Dye Terminator Cycle Sequencing Ready Reactions Kit was used. Cycle sequencing was carried out on a GeneAmp System 9600 thermocycler. Sequencing using an automated DNA sequencer 373A (ABI) was carried out according to the manufacturer’s protocol.
Southern and colony hybridizations, probe labeling, and detection were performed by using the DIG DNA Labeling and Detection Kit (Boehringer GmbH, Mannheim, Germany) as described by the manufacturer. Hybridization was performed overnight, and the membrane was washed according to the recommendation of the manufacturer.
DNA electrophoresis, restriction digestion, ligation, and transformation for E. coli were carried out as described by Sambrook et al. (20). Plasmid DNA transformation for E. carotovora subsp. carotovora was performed by the methods of Hinton et al. (10) and Hanahan (9).
Computer analysis of sequence data.
The nucleotide sequence and deduced amino acid sequence of Brg were compared by the BLAST and FASTA programs of the National Center for Biotechnology Information server. Sequence data were compiled with DNASIS-Mac software (Hitachi, Tokyo, Japan).
Isolation of transposon insertion mutants.
For the Tn5 mutagenesis, the transmissible plasmid pJB4JI was used. By mating E. coli 1830 with E. carotovora subsp. carotovora M-rif-11-2, 5,500 insertion mutants that could grow on a selective medium containing 50 ppm each of rifampin and kanamycin were isolated. In order to ascertain their antibiotic resistance, their growth on the selective medium was rechecked and found to be a stable property of the isolates. Of these 5,500 isolated mutants, only 1 was defective in the production of low-molecular-weight bacteriocin. This defective mutant was further purified on modified Drigalski’s medium containing 50 ppm each of rifampin and kanamycin. All the mutants defective in low-molecular-weight bacteriocin production were therefore siblings isolated from the same purification plates.
Bacteriocin assay of mutants.
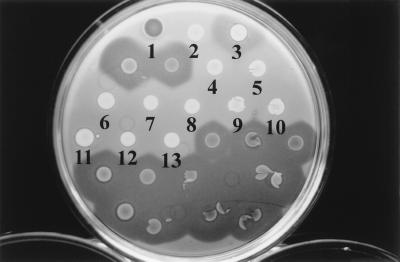
The bacteriocin activity of the test isolates was examined. The parental strain produces two types of bacteriocins: the high-molecular-weight bacteriocin, which is restricted to the immediate surroundings of the colony, and the low-molecular-weight bacteriocin, which diffuses relatively further away from the colony. It was therefore expected that a mutant deficient in the low-molecular-weight bacteriocin would produce a restricted inhibition zone. The inhibition zones of the putative isolates (insertion mutants), typical of the high-molecular-weight bacteriocin, were restricted compared to those of the parent strain (Fig. 1). This indicates the possibility that transposon Tn5 has been successfully inserted into the genes of the low-molecular-weight bacteriocin. The mutants therefore produced only the high-molecular-weight bacteriocin. It was also observed that the insertion mutants were sensitive to UV light, and the duration of the UV light induction (irradiation) in the course of the bacteriocin assay had to be shortened in order to avoid complete killing of the cells. This is also an indication that the gene that is defective in the insertion mutants may not only control low-molecular-weight bacteriocin but also influence the degree of tolerance of this bacterium to UV light.
FIG. 1.
Bacteriocin activity of Tn5 insertion mutants of E. carotovora subsp. carotovora. Numbered strains: 1, Serratia sp. (marker); 2, E. coli 1830/pJB4JI (containing Tn5); 3, M-rif-11-2 (parent); 4 to 13, TM01A01 to TM01A10 (insertion mutants). The unlabeled strains are rifampin-resistant derivatives of strain 89-H-4 which produce both low- and high-molecular-weight bacteriocins.
Detection of Tn5 in the mutants.
To ascertain whether Tn5 had actually been inserted into the putative isolates, nested PCR was used to amplify the nptII gene (25), and two oligo-nucleotides, CTCGACGTTGTCACTGAAGCGGGAAG (P-3) and AAAGCACGAGGAAGCGGTCAGCCCAT (P-4), were synthesized. Almost all the test isolates except M-rif-11-2, which does not harbor the Tn5 gene, produced a short DNA fragment of 500 bp, indicating the presence of the Tn5 insert in the mutants. Southern blot hybridization also confirmed the above results (data not shown).
Amplification of Tn5 insertion junction DNA and sequencing.
After TAIL-PCR was performed three times, two to three bands of different sizes were obtained for each sample. All the fragment products were isolated by electrophoresis and purified, and the sequences of the recovered products were analyzed. Analysis of the respective bands showed a high homology of about 95% or more, indicating a possible similarity in origin. A nucleotide sequence of 1,280 bp was obtained. All the Tn5 insertions characterized were at the same position in the brg gene, as they are all siblings.
Sequence analysis and homology.
The gene structure of the 1,280 bp was determined (GenBank accession no. AF039142). In the direction of transcription indicated by the complementation studies, one complete ORF (ORF2) was present, and Tn5 was located in the same ORF between bp 878 and 879. The 3′ end of another ORF, ORF1, was located upstream of ORF2. A noncoding region and a putative promoter were located between ORF1 and ORF2. Downstream from ORF2, the 5′ end of another ORF (ORF3) was found.
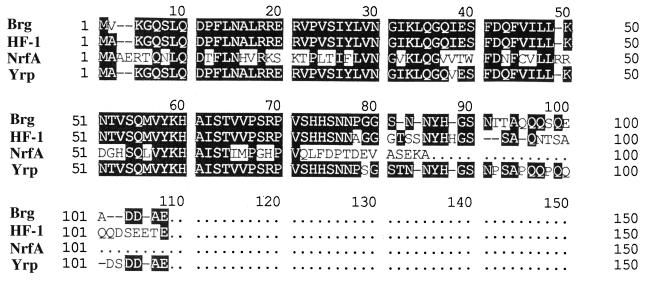
The predicted amino acid sequence of ORF2 was compared with the SwissProt protein sequence data bank. Significant similarities were found between Brg and Yrp (the yrp gene product, regulator of enterotoxin [Y-ST] production in Yersinia enterocolitica [17]), HF-1 (the hfq gene product, a host factor protein required for Qβ-replicase in E. coli K-12 [5, 6]), and NrfA of Azorhizobium caulinodans, which is required for the expression of the nifA gene (12) (Fig. 2). The hfq gene is known to influence the expression of diverse genes in E. coli and other bacteria, including rpoS, hns, and mutS, resulting in pleiotropic phenotypes (3, 21, 24). It was therefore proposed that ORF2 is the bacteriocin regulator gene, and it was subsequently designated brg. Hfq-defective mutants are known to be sensitive to UV light (23). This indirectly supports our earlier assumption that the inactivation of the brg gene may be responsible for the sensitivity of the insertion mutants to UV light.
FIG. 2.
Alignment of the predicted amino acid sequence of the E. carotovora subsp. carotovora brg gene product with the amino acid sequences of Yrp, HF-1, and NrfA. Dashes are inserted to optimize the alignment.
Subcloning of brg DNA.
The brg DNA was amplified by PCR from M-rif-11-2 and subcloned into plasmids pBR322 and pHSG415r by using T4 ligase after PCR amplification of two oligonucleotide primers, DY-R1 (TTCAAGCTTGTGGTGAATTGACAATACGC) and DY-F1 (GGTAGGATCCGTTGTTAGTGCATAGGTTGG), after purification and digestion by restriction enzymes BamHI and HindIII. The new plasmids, designated pHYL1 and pBYL1, used vectors pHSG415r and pBR322, respectively. One hundred colonies were isolated for each plasmid by using a selective LB agar medium containing 50 ppm of ampicillin after the transfer of pHYL1 and pBYL1 into E. coli DH5. The presence of the brg DNA was detected by colony hybridization using brg DNA probes and by electrophoresis after digestion with BamHI and HindIII. The brg band size was certified to be 400 bp (data not shown). The DNA of plasmid pBYL1 was isolated from DH5/pBYL1 and transferred into the insertion mutant TM01A01. One hundred colonies were isolated by selection on modified Drigalski’s medium containing 50 ppm each of kanamycin, rifampin, and ampicillin, and the brg DNA was detected as previously described. The new plasmid was designated pBYL1 after reisolation from the transformed colonies.
Recovery of bacteriocin production and characteristics of the transformed mutants.
The bacteriocin assay for the insertion mutants after transformation indicates a successful recovery of their ability to produce the low-molecular-weight bacteriocin. Their larger inhibition zones were therefore comparable to those of the parent strain, M-rif-11-2 (Table 2). This proves that production of the low-molecular-weight bacteriocin was actually regulated by brg DNA.
TABLE 2.
Bacteriocin activity assay of E. carotovora subsp. carotovora
| Straina | Inhibition zone due to bacteriocin production
|
|
|---|---|---|
| Low-mol-wt bacteriocin | High-mol-wt bacteriocin | |
| M-rif-11-2 | 7 mm | + |
| TM01A01 | None | + |
| TM01A01/pBYL1 | 7 mm | + |
M-rif-11-2 (parent strain), TM01A01 (Tn5 insertion mutant), and pBYL-1/TM01A01/pBYL1 (transformed mutant) were used as bacteriocin producers, and strain T-29 was used as an indicator.
The cloning of the brg DNA and its subsequent transfer into the insertion mutant TM01A01 resulted in the recovery of production of the low-molecular-weight bacteriocin. The homology search and computer analysis for the DNA sequence of the bands obtained by TAIL-PCR also confirmed the above results indicating that brg is required for the production of the low-molecular-weight bacteriocin. The homology search also indicates that Brg may also regulate the expression of genes other than those for bacteriocin production, as observed in the roles of Yrp in Y. enterocolitica (17) and hfq in E. coli K-12 and other bacteria (3, 5, 6, 21, 23, 24).
It has been reported that an hfq-defective mutant (hfq::Ω) exhibited defects in osmosensitivity, cell growth, cell shape and size, plasmid supercoiling, and sensitivity to UV light (23). We also observed that on modified Drigalski’s medium, colonies transformed with pBYL1, containing the brg gene, were green and were larger in diameter (about 13 mm) than the parents and the insertion mutants, which were only 5 mm in diameter and yellow. Further incubation of the brg-defective mutant under this condition results in the death of the cells. The transformed colonies were also found to have a very strong smell compared to those of the parents and the insertion mutants. A possible explanation of this observation is that the utilization of the lactose present in modified Drigalski’s medium results in the formation of lactic acid (lowering the pH), indicated by the yellowish color, which is further utilized as a sole carbon source, leading to an increase in the pH around the colony and the green color of the colony. The differences in color and colony size are therefore due to the differences in the efficiency with which the respective colonies utilize lactic acid and tolerate a low pH. The relative tolerance of the reduced pH and efficiency of lactic-acid utilization by the brg transformant points to a possible control by this gene. The observed sensitivity of the brg-defective mutant to UV light is similar to that previously observed for the E. coli hfq::Ω mutant (23).
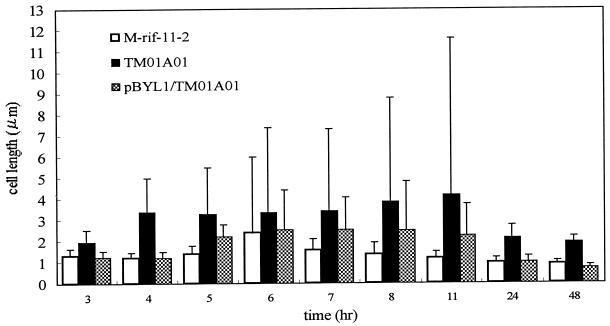
It was also observed that among the insertion mutants, most of the cells, formed a precipitate at the bottom of the test tube when cultured in LB medium with shaking for 24 or 48 h. Further examination showed that, as observed for the hfq::Ω mutant (23), the brg-defective mutants were larger and more elongated than the parent and the transformed cells (Fig. 3). However, among the 160 cells measured for each strain (parent, mutant, and transformant), it was observed that the data for the mutant strain were the least uniform. The nonuniformity seems to stem from impaired or delayed cell division. This could be supported by larger cell size during the 4th and 11th h, followed by a marked reduction in cell size at the 24th h, with a subsequent reduction in standard deviation. It therefore stands to reason that the brg gene affects not only the size of the cells but also, either directly or indirectly, the process of cell division. This may be the reason for the increase in the doubling time observed for the hfq-defective mutant (23).
FIG. 3.
Measured cell lengths of strains M-rif-11-2 (parent), TM01A01 (Tn5 insertion mutant), and TM01A01/pBYL1 (transformed mutant) over a period of 48 h. Bacteria were cultured in LB medium with shaking at 28°C.
All these results show that Brg may be an analogue of Hfq which operates in this strain of E. carotovora subsp. carotovora. Therefore further study of this gene is needed, as it may have pleiotropic effects on other aspects of cell physiology which have not yet been studied.
Nucleotide sequence accession number.
The GenBank accession no. of the sequence of the brg genes is AF039142.
Acknowledgments
We thank M. Sato, National Institute of Sericultural and Entomological Science, Tsukuba, Japan, for donating E. coli 1830 containing pJB4JI, and S. Annda, National Institute of Genetics, Shizuoka, Japan, for donating plasmids pBR322 and pHSG415r. We are also grateful to A. Higashitani, Institute of Genetic Ecology, Tohoku University, Sendai, Japan, for donating E. coli DH5 and for insightful discussion and guidance.
REFERENCES
- 1.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 2.Brady G, Jantzen H M, Bernard H U, Brown R, Schutz G, Hashimoto-Gotoh T. New cosmid vectors developed for eukaryotic DNA cloning. Gene. 1984;27:223–232. doi: 10.1016/0378-1119(84)90143-4. [DOI] [PubMed] [Google Scholar]
- 3.Brown L, Elliott T. Efficient translation of the RpoS sigma factor in Salmonella typhimurium requires host factor I, an RNA-binding protein encoded by the hfq gene. J Bacteriol. 1996;178:3763–3770. doi: 10.1128/jb.178.13.3763-3770.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuang D-Y. M.A. thesis. Sendai, Japan: Tohoku University; 1997. [Google Scholar]
- 5.Franze de Fernandez M T, Eoyang L, August J T. Factor fraction required for the synthesis of bacteriophage Qβ RNA. Nature. 1968;219:588–590. doi: 10.1038/219588a0. [DOI] [PubMed] [Google Scholar]
- 6.Franze de Fernandez M T, Hayward W S, August J T. Bacterial proteins required for replication of phage Qβ ribonucleic acid. Purification and properties of host factor 1, a ribonucleic acid-binding protein. J Biol Chem. 1972;247:824–831. [PubMed] [Google Scholar]
- 7.Fredericq P. Colicins. Annu Rev Microbiol. 1957;11:7–22. doi: 10.1146/annurev.mi.11.100157.000255. [DOI] [PubMed] [Google Scholar]
- 8.Gantotti B V, Kindle K L, Beer S V. Transfer of the drug-resistance transposon Tn5 to Erwinia herbicola and the induction of insertion mutations. Curr Microbiol. 1981;6:417–425. [Google Scholar]
- 9.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 10.Hinton J C D, Perombelon M C M, Salmond G P C. Efficient transformation of Erwinia carotovora subsp. carotovora and E. carotovora subsp. atroseptica. J Bacteriol. 1985;161:786–788. doi: 10.1128/jb.161.2.786-788.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kado C I, Liu S-T. Rapid procedure for detection and isolation of the large and small plasmid. J Bacteriol. 1981;145:1365–1375. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaminski P A, Desnoues N, Elmerich C. The expression of nifA in Azorhizobium caulinodans requires a gene product homologous to Escherichia coli HF-1, and RNA-binding protein involved in the replication of phage Qβ RNA. Proc Natl Acad Sci USA. 1994;91:4663–4667. doi: 10.1073/pnas.91.11.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kikumoto T, Ma S, Takahara Y. Abstracts of the papers presented at the Annual Meeting of the Society, 1993. The Phytopathological Society of Japan, Nara, Japan. (In Japanese.) 1993. Biological control of the soft rot disease of Chinese cabbage. 3. Interactions of avirulent and virulent strains of Erwinia carotovora subsp. carotovora on the petiole of Chinese cabbage, abstr. 195; pp. 315–316. [Google Scholar]
- 14.Kikumoto T, Kyeremeh A G, Chuang D Y, Gunji Y. Biological control of the soft rot disease of Chinese cabbage with avirulent mutant strains of Erwinia carotovora subsp. carotovora. In: Ogoshi A, Kobayashi K, Homma Y, Kodama F, Kondo N, Akino S, editors. Proceedings of the Fourth International Workshop on Plant Growth-Promoting Rhizobacteria. Japan-OECD Joint Workshop. 1997. pp. 118–119. [Google Scholar]
- 15.Liu Y-G, Whittier R F. Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics. 1995;25:674–681. doi: 10.1016/0888-7543(95)80010-j. [DOI] [PubMed] [Google Scholar]
- 16.Miyashita K. Experimental methods in soil microbiology. Soil Microbiological Society of Japan. Youkendou Publishing Co., Tokyo, Japan. (In Japanese.) 1992. DNA probes; pp. 163–172. [Google Scholar]
- 17.Nakao H, Watanabe H, Nakayama S-I, Takeda T. yst gene expression in Yersinia enterocolitica is positively regulated by a chromosomal region that is highly homologous to Escherichia coli host factor 1 gene (hfq) Mol Microbiol. 1995;18:859–865. doi: 10.1111/j.1365-2958.1995.18050859.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakatani F, Tsuyama H. Production of two kinds of antibacterial agents by isolates of Erwinia carotovora. J Fac Agric Iwate Univ. 1973;11:245–253. [Google Scholar]
- 19.Reusch R N, Hiske T W, Sadoff H L. Poly-beta-hydroybutyrate membrane structure and its relationship to genetic transformability in Escherichia coli. J Bacteriol. 1986;168:553–562. doi: 10.1128/jb.168.2.553-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 21.Shi X, Bennett G N. Plasmids bearing hfq and the hns-like gene stpA complement hns mutants in modulating arginine decarboxylase gene expression in Escherichia coli. J Bacteriol. 1994;176:6769–6775. doi: 10.1128/jb.176.21.6769-6775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahara Y. Development of the microbial pesticide for soft-rot disease. PSJ Biocontrol Rep. 1994;4:1–7. . (In Japanese.) [Google Scholar]
- 23.Tsui H C, Leung H C, Winkler M E. Characterization of broadly pleiotropic phenotypes caused by an hfq insertion mutation in Escherichia coli K-12. Mol Microbiol. 1994;13:35–49. doi: 10.1111/j.1365-2958.1994.tb00400.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsui H C, Feng G, Winkler M E. Negative regulation of mutS and mutH repair gene expression by the Hfq and RpoS global regulators of Escherichia coli K-12. J Bacteriol. 1997;179:7476–7487. doi: 10.1128/jb.179.23.7476-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsushima S, Hasebe A, Komoto Y, Charter J P, Miyashita K, Yokoyama K, Pickup R W. Detection of genetically engineered microorganisms in paddy soil using a simple and rapid “nested” polymerase chain reaction method. Soil Biol Biochem. 1995;27:219–227. [Google Scholar]
- 26.Tsuyama H, Sakamoto M. Isolation methods of the soft-rot causing bacteria from the soil. Sci Rep Res Inst Tohoku Univ Ser D. 1952;3:29–34. [Google Scholar]
- 27.Yasunaka K, Amako K. Morphology of bacteriocins. Protein Nucleic Acid Enzyme. 1979;24:719–726. . (In Japanese.) [PubMed] [Google Scholar]