Abstract
The activation of the PI3K signaling pathway resulting from genetic alterations induces carcinogenesis and resistance to anticancer therapies. Breast cancer is a major malignancy that is associated with dysregulation of the PI3K signaling pathway. PIK3CA mutations and PTEN loss occur in every subtype of breast cancer. PI3K inhibitors are being evaluated in breast cancer after the success of an alpha isoform-specific PI3K inhibitor in estrogen receptor (ER)-positive/HER2-negative metastatic breast cancer. Some preclinical data indicate the potential for PI3K/mTOR targeting in combination with trastuzumab for HER2-positive breast cancer with or without expression of the estrogen receptor. However, the role of this therapy in HER2-positive breast cancer with PIK3CA mutations and/or PTEN loss remains unclear. We examined three HER2-positive, ER-negative breast cancer cell lines to determine the efficacy of a novel alpha isoform-specific PI3K inhibitor in combination with trastuzumab. The results indicated that this combination was effective in PIK3CA-mutant or PTEN-deficient breast cancer cells by inducing apoptosis and inhibiting the expression of downstream proteins. PTEN loss by siRNA modulation in parental HER2-positive cancer cells with PI3K signaling pathway alterations could not confer resistance to alpelisib or GDC-0077 plus trastuzumab. We selected the CK-MB-1 cell line without alterations in the PI3K pathway to demonstrate that PI3K inhibitors plus trastuzumab represented a biomarker-specific treatment. In vivo effects of alpelisib plus trastuzumab were tested and confirmed in a mouse model, showing the combination strategy offered the best opportunity to achieve tumor volume reduction. With known safety profiles, this cytotoxic chemotherapy-free regimen warrants further attention as a biomarker-driven strategy for treating HER2-positive breast cancer.
Keywords: PI3K inhibitor, alpha-isoform, PIK3CA, PTEN, breast cancer, trastuzumab
Introduction
Human epidermal growth factor receptor 2 (HER2)-positive breast cancer accounts for 20% of the entire breast cancer population [1]. Patients with this subtype had a poor outcome until the development of anti-HER2 agents [2-8]. Anti-HER2 monoclonal antibodies, tyrosine kinase inhibitors, and antibody-drug conjugates have markedly improved the outcomes of affected patients. However, resistance remains a major issue that hampers the efficacy of anti-HER2 strategies. The major mechanism of resistance to these agents is alterations of the phosphatase and tensin homolog (PTEN)/phosphoinositide 3-kinase (PI3K)/v-akt murine thymoma viral oncogene homolog (AKT) pathway [9,10]. Patients harboring these gene mutations have a poor prognosis [10,11]. Studies showed that resistance may be overcome by PI3K, mammalian target of rapamycin (mTOR) or PI3K/mTOR dual inhibitors [12]. Although efforts have been made to include everolimus, an mTOR inhibitor, in the treatment regimens for HER2-positive metastatic breast cancer, efficacy is modest and does not contribute significantly, based on results of the BOLERO-1 and BOLERO-3 studies [13,14].
Another concern regarding mTOR inhibition is that it induces feedback activation of AKT [15], thus PI3K inhibition or PI3K/mTOR dual inhibition may represent a better strategy to block this pathway. Nevertheless, dactolisib, buparlisib, and taselisib have not been effective in human diseases primarily because of intolerable side effects [16-19]. Thus, the use of pan-class PI3K inhibitors results in greater toxicity than efficacy in breast cancer. Alpelisib, an alpha isoform-specific PI3K inhibitor, was investigated in the SOLAR-1 study that targeted hormone receptor (HR)-positive/HER2-negative breast cancer [20]. With an acceptable toxicity profile, alpelisib plus fulvestrant resulted in better progression-free survival compared with fulvestrant monotherapy in patients harboring PIK3CA mutations that failed previous endocrine therapy. Besides alpelisib, GDC-0077 is another alpha isoform-specific PI3K inhibitor exhibiting potent PI3K inhibition [21], which has been evaluated in HR-positive/HER2-negative breast cancer.
Therefore, we are still looking for an effective and safe biomarker-driven strategy to treat patients with HER2-positive metastatic breast cancer with notorious PIK3CA mutations. As more knowledge of novel alpha isoform-specific PI3K inhibitors becomes available, we can evaluate their efficacy in PIK3CA-mutant HER2-positive breast cancer. In the present study, we examined the efficacy of alpelisib and GDC-0077 together with trastuzumab in three trastuzumab-resistant HER2-positive breast cancer cell lines in vitro and in vivo. Notably, PTEN loss in cancer cells has been reported to cause resistance to PI3K inhibitors and contribute to worse outcomes in patients [22,23]. The issue of PTEN loss with or without concomitant PIK3CA mutation was also addressed. By introducing a combination with an alpha isoform-specific PI3K inhibitor and trastuzumab, we aim to provide an effective cytotoxic chemotherapy-free regimen that may be further evaluated in human clinical trials.
Materials and methods
Cell lines, cell culture, and reagents
BT-474 cells (RRID: CVCL_0179) were obtained from the Bioresource Collection and Research Center (BCRC, Taiwan) and maintained in Hybri-Care medium [American Type Culture Collection (ATCC), Manassas, VA, USA] supplemented with 10% fetal bovine serum (FBS) (Gibco by Life Technologies, Waltham, MA, USA). HCC1954 (RRID: CVCL_1259) and HCC1569 (RRID: CVCL_1255) cells were obtained from ATCC and maintained in RPMI-1640 medium (Gibco by Life Technologies) supplemented with 10% FBS. UACC893 (RRID: CVCL_1782) cells were obtained from the ATCC and maintained in Leibovitz’s L-15 medium (Merck KGaA, Darmstadt, Germany) supplemented with 10% FBS. CK-MB-1 cells were collected and established from a patient with breast cancer-associated malignant ascites and maintained in RPMI-1640 medium supplemented with 10% FBS [24]. All cell lines were maintained at 37°C in an atmosphere of 5% CO2, except for UACC893 cells, which were maintained at 37°C without CO2. All cell lines have been authenticated by short tandem repeat profiling within the last two years. Trastuzumab was purchased from the pharmacy at the National Cheng Kung University Hospital and manufactured by Genentech (San Francisco, CA, USA). Trastuzumab was diluted with phosphate-buffered saline (PBS). Alpelisib and GDC-0077 were purchased from Selleck Chemicals (Houston, TX, USA) dissolved in dimethyl sulfoxide (DMSO, Merck KGaA) for in vitro experiments.
Western blot analysis
For the preparation of cell lysates, the harvested samples were incubated on ice in whole cell extract lysis buffer for 30 min. Lysates were centrifuged at 12,000 rpm for 10 min and the protein concentration was measured using the Bradford assay (Bio-Rad, Hercules, CA, USA). For western blot analysis, 15-100 μg of lysates (depending on the target proteins assayed) were boiled for 10 min in sample buffer and then separated on sodium dodecyl sulfate-polyacrylamide gels. Proteins were transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA) and blocked with 5% nonfat milk in 20X tris-buffered saline with 2% Tween® 20 detergent. The primary antibodies used were as follows: HER2, beta-actin (Merck KGaA), ERα (Santa Cruz Biotechnology, Santa Cruz, CA, USA), PTEN, phospho-S473AKT, AKT, phospho-S244/S240S6, S6, cleaved-PARP, PARP, cleaved-caspase 3, and caspase 3 (Cell Signaling Technology, Beverly, MA, USA). Anti-rabbit and anti-mouse secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA, USA). ImageJ software (National Institutes of Health, USA) was used to determine the intensity of each band relative to the control after normalizing to the intensity of beta-actin.
Colony formation assay
Breast cancer cells (n=1000) were seeded into 100 mm2 petri dishes for 24 h and treated with the indicated agents for another 24 h. After treatment, cells were cultured with medium and changed every seven days. After 21 days of maintenance, the colonies were rinsed with PBS then fixed with 25% acetic acid in methanol and stained with 0.05% crystal violet dye in water. A qualified colony was defined as consisting of at least 50 cells and numbers were counted by ImageJ software.
In vitro antiproliferation activity analysis
Cells were seeded at concentrations of 1.5×104-3×104 cells/200 μl/well in 96-well plates for 24 h and treated with the indicated agents for 72 h. After treatment, the MTT proliferation assay was performed according to the manufacturer’s instructions. Briefly, 20 µl of MTT reagent (5 mg/ml, Merck KGaA) were added to each well and incubated for 3 h. Because of the mixed (adherence and suspension) growth properties of HCC1569 cells, the WST-1 assay (Takara Bio Inc., Shiga, Japan) was selected and performed according to the manufacturer’s instructions. After the required incubation period, 20 μL of WST-1 reagent was added to each well and incubated for 30 min and 1 h. The results were determined by measuring the absorbance of the solution at a wavelength of 490 nm using a spectrophotometer.
In vitro apoptosis analysis
Annexin V cell staining was used to detect apoptosis. Cells were collected and resuspended in annexin V binding buffer and stained with annexin V-FITC and propidium iodide (PI) (BD, Franklin Lakes, NJ, USA) for 15 min at room temperature according to the manufacturer’s instructions. Cells were then analyzed using flow cytometry (FACSCalibur, BD).
Combination index (CI)
The combination index of drugs was simulated by CompuSyn software [25]. Data were obtained from the antiproliferative effects of the indicated drugs and the combination 72 h after treatment. Synergy is defined as CI<1, an additive effect as CI=1, and antagonism as CI>1.
Small interfering RNA (siRNA) knockdown of PTEN (siPTEN)
Cells were transiently transfected by siRNA (assay ID: s61222) for 48 h with Lipofectamine 3000 (Thermo Fisher, Waltham, MA, USA). Lipofectamine 3000 reagent was mixed in serum-free medium at 17X dilution. For the other tube, 1 μg PTEN siRNA was mixed in serum-free medium, then P3000 reagent was added. Diluted Lipofectamine 3000 reagent was subsequently added to the siRNA-P3000 containing tube. Transfection solution was incubated at room temperature for 10-15 min. The mixture was incubated with cells and gently swirled to distribute evenly.
Caspase 3 and 7 activity
Magic Red reagents are substrates for caspase 3 and 7, and fluorescence is emitted as a result of enzyme activity. For the detection of caspase 3 and 7 activity, 30X-diluted Magic Red reagents were added and incubated for 1 h at 37°C after the cells were treated with the indicated agents for 18 hours. Stained cells were subsequently evaluated by fluorescence microscopy.
Orthotopic xenograft mouse model and in situ TUNEL assays
Five- to six-week-old BALB/cAnN.Cg-Foxn1nu /CrlNarl female mice were bred and cared for by following the institutional guidelines. HCC1954 cells (3×106) in 100 μL PBS were inoculated into the mammary gland of each mouse to establish a xenograft mouse tumor model. Tumor size was determined by measuring the length and width. Tumor volume was calculated using the equation: (length × width2)/2. Mice were randomly allocated into three groups with each treatment group containing six mice once tumors reached an average size of 150-200 mm3. The solvent for alpelisib consisted of 10% DMSO, 40% PEG 300, 5% Tween 80 (Merck KGaA), and 45% saline (Taiwan Biotech CO., LTD., Taiwan), whereas trastuzumab was prepared with PBS. Oral administration was performed with feeding needles, ST-F173 (Shineteh Instruments CO., LTD., Taiwan). The control group received oral solvent 5 days per week, whereas the other two groups received oral alpelisib at 30 mg/kg/day five days per week or oral alpelisib five days per week plus intraperitoneal injection of trastuzumab at 30 mg/kg/day twice per week. Tumor volume was measured twice weekly and the body weight of mice was recorded daily. Mice were sacrificed after 29 days of treatment. Tumors were harvested and formalin-fixed/paraffin-embedded tissues were prepared. Tissue sections were stained with TUNEL reagent using the Fluorescein In Situ Apoptosis Detection Kit (green, Roche Diagnostics, Rotkreuz, Switzerland) according to the manufacturer’s instructions, then counterstained with Hoechst 33342 dye (blue, staining nuclei, Merck KGaA) and HER2 antibody/PE-Texas Red-conjugated secondary antibody (red, cell membrane, Merck KGaA). Tissue sections were examined using ECLIPSE Ti (Nikon, Tokyo, Japan) fluorescence microscopy at a magnification of 200X. The images collected were then analyzed by ImageJ software and the numbers of TUNEL positive cells per field were calculated. The animal protocol discussed previously has been reviewed and approved by the Institutional Animal Care and Use Committee, National Cheng Kung University (110012).
Statistical analysis
Each in vitro experiment was repeated at least three times to confirm the replicability of results. The data were presented as the mean ± standard deviation and analyzed using a two-tailed Student’s t-test. The growth curves of the tumor xenografts show the tumor volume at each measured time points. Each plot represents the mean tumor volume, whereas error bars represented the standard error. The curves for body weight present the daily mean weight ± the standard error for each group. The data for mean tumor volume and body weight in three groups were examined using a two-way ANOVA with GraphPad Prism version 9.1.2 for Windows (GraphPad Software, La Jolla CA, USA, www.graphpad.com). The positive tissue TUNEL signals were statistically analyzed by the Kruskal-Wallis test embedded in GraphPad Prism. P-values less than 0.05 were considered statistically significant and are indicated by asterisks as follows: *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Results
Effects of PI3K inhibitors in HER2-positive trastuzumab-resistant breast cancer cells
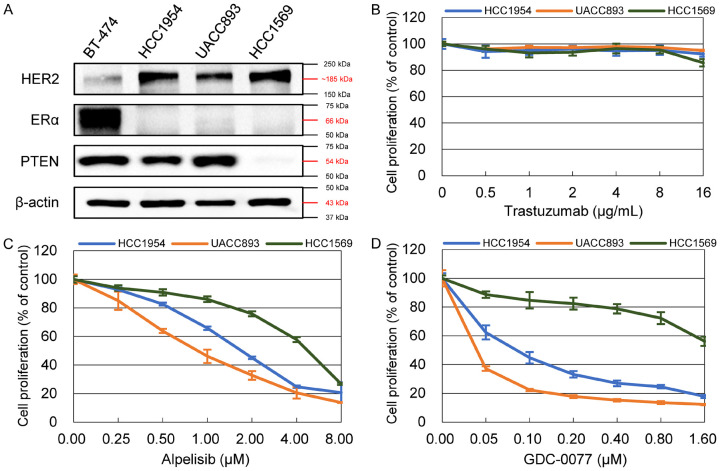
HCC1954 and UACC893 are ER-negative/HER2-positive breast cancer cell lines bearing PIK3CA mutations with intact PTEN protein expression (Figure 1A). HCC1569 is an ER-negative/HER2-positive breast cancer cell line without PIK3CA mutation, but with lost PTEN protein expression. These cell lines exhibit resistance to the trastuzumab anti-HER2 monoclonal antibody (Figure 1B) and the maximal concentration of trastuzumab used was 50-fold higher than the IC50 of the trastuzumab-sensitive BT-474 cell line [12]. In contrast, alpelisib and GDC-0077, known as alpha isoform-specific PI3K inhibitors, showed marked antiproliferative effects in these cells (Figure 1C, 1D). The IC50 value of alpelisib in HCC1954, UACC893, and HCC1569 cells was 1.950±0.184 μM, 0.870±0.171 μM, and 4.073±0.429 μM, respectively. The IC50 value for GDC-0077 in HCC1954, UACC893, and HCC1569 cells was 0.082±0.016 μM, 0.007±0.001 μM, and 3.853±0.865 μM, respectively. Although HCC1569 cells were relatively less sensitive to PI3K inhibition compared with the other two cell lines, the antiproliferative effect of PI3K inhibitors in HCC1569 cells was concentration-dependent. In addition, a soft agar colony formation assay was also done to alternatively evaluate the effects of PI3K inhibitors on the resistant cell lines. Cells were treated with alpelisib at a concentration of 0.5 and 8 μM, and with GDC-0077 at a concentration of 0.3 and 4.8 μM. Both drugs inhibited colony formation in a concentration-dependent manner (Figure S1).
Figure 1.
Characteristics of resistant HER2-positive cell lines. A. HER2 expression and lack of estrogen receptor expression was confirmed through protein analysis in three resistant cell lines. HCC1569 cells, unlike two other cell lines, had no PTEN expression. B. All three cell lines showed resistance to trastuzumab within the range of 0.5-16 μg/mL. C, D, Prominent antiproliferative effects are shown after resistant cells were treated with different concentrations of alpelisib and GDC-0077. At the same concentration of PI3K inhibitor, HCC1569 cells exhibited relative resistance compared with HCC1954 and UACC893 cells. Cell viability was determined by MTT assay 72 hours after incubation with the indicated drugs and normalized that of non-treated cells. Each plot indicates the mean value of at least triplicate measurements and error bars indicate the SD.
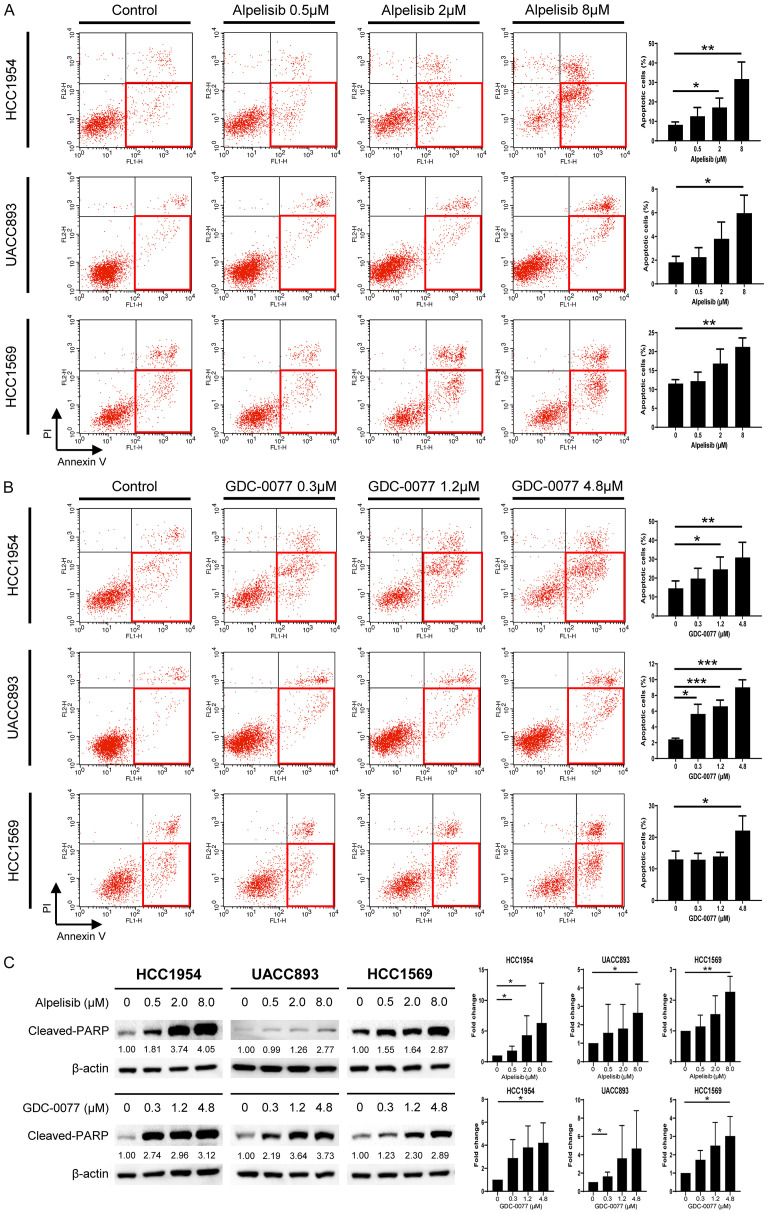
Activation of the PI3K/AKT signaling pathway inhibits apoptosis and promotes the survival of cancer cells [26]. Thus, PI3K inhibitors are expected increase apoptosis. We examined PI3K inhibitors to determine whether there were enhanced apoptosis in HER2-positive resistant breast cancer cells. HCC1954, UACC893, and HCC1569 cells were treated with alpelisib and GDC-0077 separately. Alpelisib was used at concentrations of 0.5, 2, and 8 μM. GDC-0077 was used at concentrations of 0.3, 1.2, and 4.8 μM. Cells were stained with annexin V/PI and evaluated by flow cytometry. After treatment with PI3K inhibitors, we observed increased apoptosis in cells with PIK3CA mutations or PTEN loss (Figure 2A, 2B). Because of the longer doubling time of UACC893 cells [27,28], apoptosis was more pronounced after 72 hours of treatment rather than 48 hours (Figure 2A, 2B; Figure S2). We found that targeting the PI3K pathway could induce apoptosis. These results were confirmed by western blot analysis. Using the identical dosage as that in the flow cytometry experiments, the up-regulation of cleaved-PARP expression in cell lysates was clearly observed after exposure to the PI3K inhibitors (Figure 2C). Therefore, dysregulation of apoptosis in these resistant cells could be ameliorated by these two alpha isoform-specific PI3K inhibitors.
Figure 2.
PI3K inhibitors induce apoptosis in trastuzumab-resistant breast cancer cells with PIK3CA mutations or PTEN loss. A. Three cells lines were treated with alpelisib at the indicated concentrations. Cells were stained with annexin V/PI and analyzed by flow cytometry to determine the proportion of apoptotic cells. HCC1954 and HCC1569 cells were collected 48 hours after treatment, whereas UACC893 cells were analyzed at 72 hours. The x-axis indicates the intensity of annexin V, whereas the y-axis indicates PI. B. Cells lines were treated with GDC-0077 at the indicated concentrations and the proportion of apoptotic cells were analyzed. C. Lysates were collected 24 hours after alpelisib or GDC-0077 treatment and subjected to immunoblotting for detection of cleaved-PARP antibody. Equal loading of proteins was verified using beta-actin. The numbers under each blot represent the intensity of cleaved-PARP relative to that of the control. Experiments were repeated at least three times and the results are summarized as bar charts. Each bar represents the mean ± SD. P-value: *P<0.05, **P<0.01, and ***P<0.001.
Synergistic effects of PI3K inhibitors plus trastuzumab are specific for HER2-positive, PIK3CA mutated, and/or PTEN loss breast cancer cells
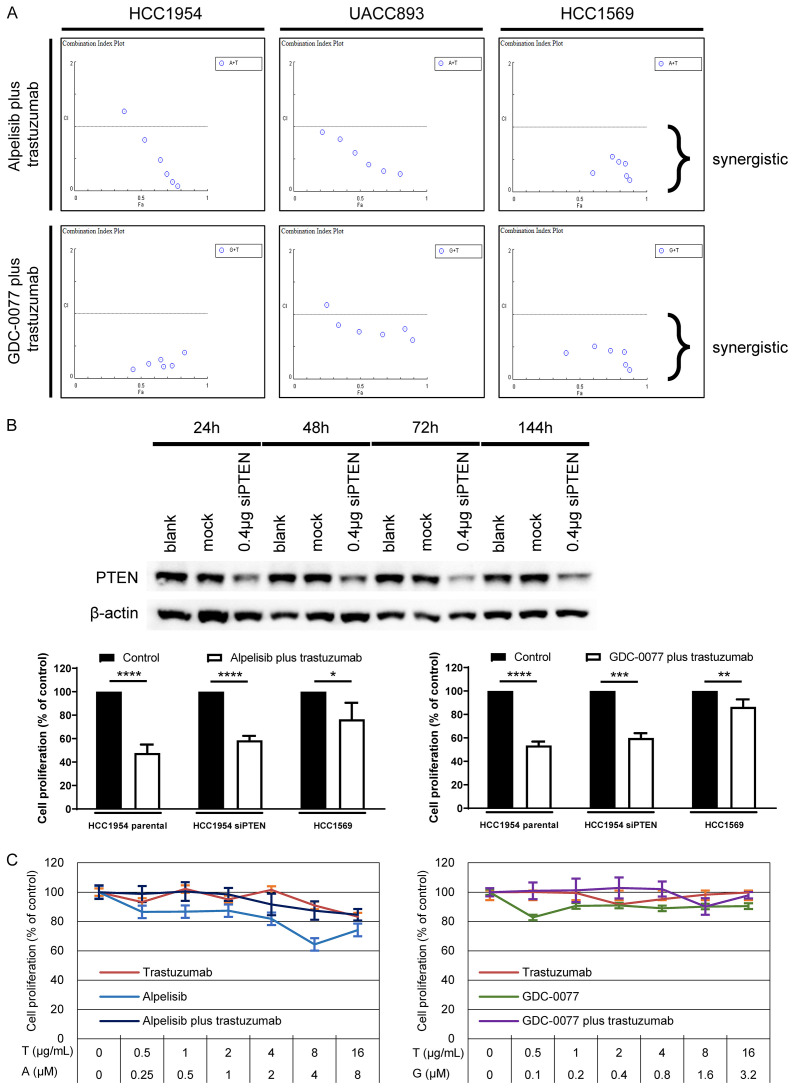
Continuous HER2 blockage is the primary strategy to manage HER2-positive breast cancer even if resistance has developed [29]. Since it is beneficial to suppress the HER2 pathway, we evaluated the efficacy of PI3K inhibitors in combination with trastuzumab in vitro. We used the software, CompuSyn [25], to calculate the combination index of alpelisib plus trastuzumab and GDC-0077 plus trastuzumab. The range of concentration was 0.125-4 μM, 0.0125-3.2 μM, and 0.5-16 μg/mL for alpelisib, GDC-0077, and trastuzumab, respectively. A combination index <1 indicates synergy and all results were <1 except for alpelisib at 4 μM plus trastuzumab at 16 μg/mL in HCC1954 cells and GDC-0077 at 0.4 μM plus trastuzumab at 16 μg/mL in UACC893 cells (Figure 3A; Tables S1, S2). These two exceptions may have been caused by prominent antiproliferative effects from monotherapy with alpelisib or GDC-0077 at a concentration higher than the IC50, thus further synergistic effects from the combination could not be demonstrated. These results suggest that PI3K inhibitors in combination with trastuzumab result in synergistic effects against PIK3CA mutated or PTEN-deficient breast cancer cells. To determine the effects on cells with concurrent PIK3CA mutations and loss of PTEN function, we evaluated the PI3K inhibitors in combination with trastuzumab in HCC1954 cells that were pretreated with siRNA to induce down-regulation of PTEN protein. The effect of siPTEN on decreasing the production of PTEN protein lasted over 144 hours following its application (Figure 3B). We selected the concentrations of alpelisib, GDC-0077, and trastuzumab based on the results of the synergy experiments and the IC50 values obtained for the HCC1954 parental cells. Alpelisib at a concentration of 2 μM was administered with 8 μg/mL trastuzumab, whereas GDC-0077 was tested at 0.1 μM in combination with 1 μg/mL trastuzumab. Although knocking down the function of PTEN could disrupt PI3K pathway signaling independent of PIK3CA mutation, the combination treatment with PI3K inhibitors and trastuzumab still exhibited efficacy in these cells with concurrent PIK3CA mutations and decreased PTEN protein (Figure 3B).
Figure 3.
Synergy of PI3K inhibitors plus trastuzumab is specific for PI3K pathway alteration-related resistance. A. Alpelisib plus trastuzumab and GDC-0077 plus trastuzumab both exhibit synergistic effects (CI <1) in resistant breast cancer cells. B. HCC1954 cells were transiently transfected with siRNA resulting in PTEN knockdown. The combination strategy with one PI3K inhibitor and trastuzumab still results in prominent antiproliferative effects in HCC1954 cells exhibiting a partial loss of PTEN protein. Experiments were repeated in triplcate and the results are summarized as bar charts representing the mean ± SD. P-value: *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001. C. CK-MB-1 cells are neither PIK3CA mutant nor PTEN deficient. PI3K inhibitors or trastuzumab showed no efficacy in CK-MB-1 cells either as monotherapy or in combination.
The selection of biomarkers with predictive values is important for the development of novel agents or combination strategies. To strengthen the positive predictive values of PIK3CA mutations and loss of PTEN protein in cells treated with PI3K inhibitors plus trastuzumab, we selected a cell line, CK-MB-1, known as HER2-positive trastuzumab-resistant breast cancer cells without PIK3CA mutation and with intact expression of PTEN [24] to test its response to PI3K inhibitors plus trastuzumab. Trastuzumab alone, PI3K inhibitors alone, or the combination exhibited little antiproliferative effects against CK-MB-1 cells (Figure 3C). This poor response was not altered by increasing the alpelisib and GDC-0077 concentrations higher than the IC50 values obtained for HCC1954, UACC893, and HCC1569 cells. These findings suggest a strategy for using PI3K inhibitors plus trastuzumab in cells with an active PI3K signaling pathway.
By suppressing the PI3K downstream signaling pathway, combination treatment with PI3K inhibitors and trastuzumab induces marked apoptosis in resistant breast cancer cells
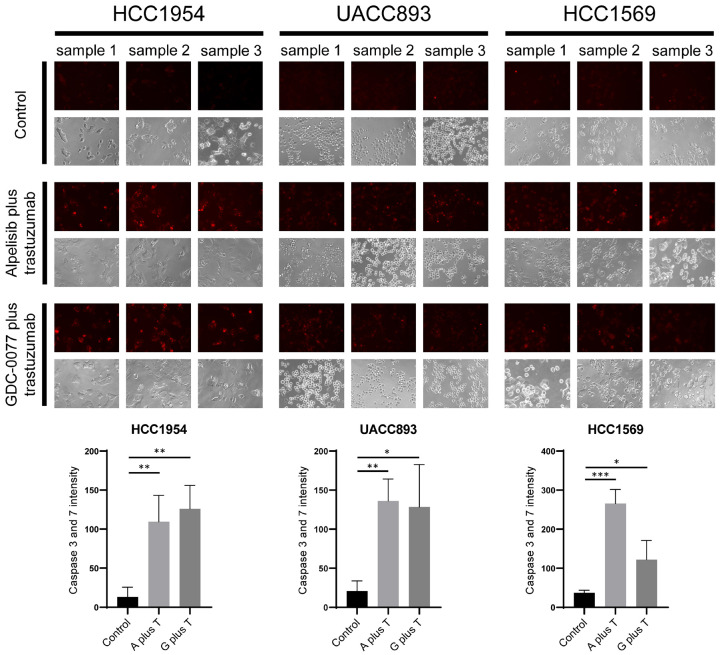
The unchanged expression of phospho-AKT and phospho-S6 compared with the control indicated that the downstream signaling pathway was not suppressed after exposure to trastuzumab in HCC1954, UACC893, and HCC1569 cells (Figures 4A, 4B, S3, S4). In contrast, at IC50 values, down-regulation of phospho-AKT and -S6 was observed once PI3K inhibitors were administered. The combination treatment of alpelisib plus trastuzumab or GDC-0077 plus trastuzumab elicited similar effects. We further evaluated the relationship between the suppressive effects and apoptosis of tumor cells. PI3K inhibitors with or without trastuzumab contribute to prominent apoptosis as evidenced by up-regulation of cleaved-caspase 3 and cleaved-PARP. Moreover, the combination of alpelisib or GDC-0077 with trastuzumab exhibited the potential to induce more apoptosis-associated proteins compared with PI3K inhibitors alone (Figures S3, S4). We confirmed these apoptotic events using the Magic Red Caspase 3/7 Assay Kit after resistant cancer cells were treated with PI3K inhibitors plus trastuzumab. A considerably redder fluorescent signal was observed in cells treated with alpelisib or GDC-0077 plus trastuzumab compared with the control groups (Figure 5). This suggests that apoptosis of resistant breast cancer cells is triggered once the HER2-PI3K pathway has been adequately blocked. Since PI3K inhibitors and trastuzumab have synergistic effects in trastuzumab-resistant breast cancer cells and the combination strategy induces more prominent apoptosis, the differences between PI3K inhibitors with or without trastuzumab should be further examined in vivo.
Figure 4.
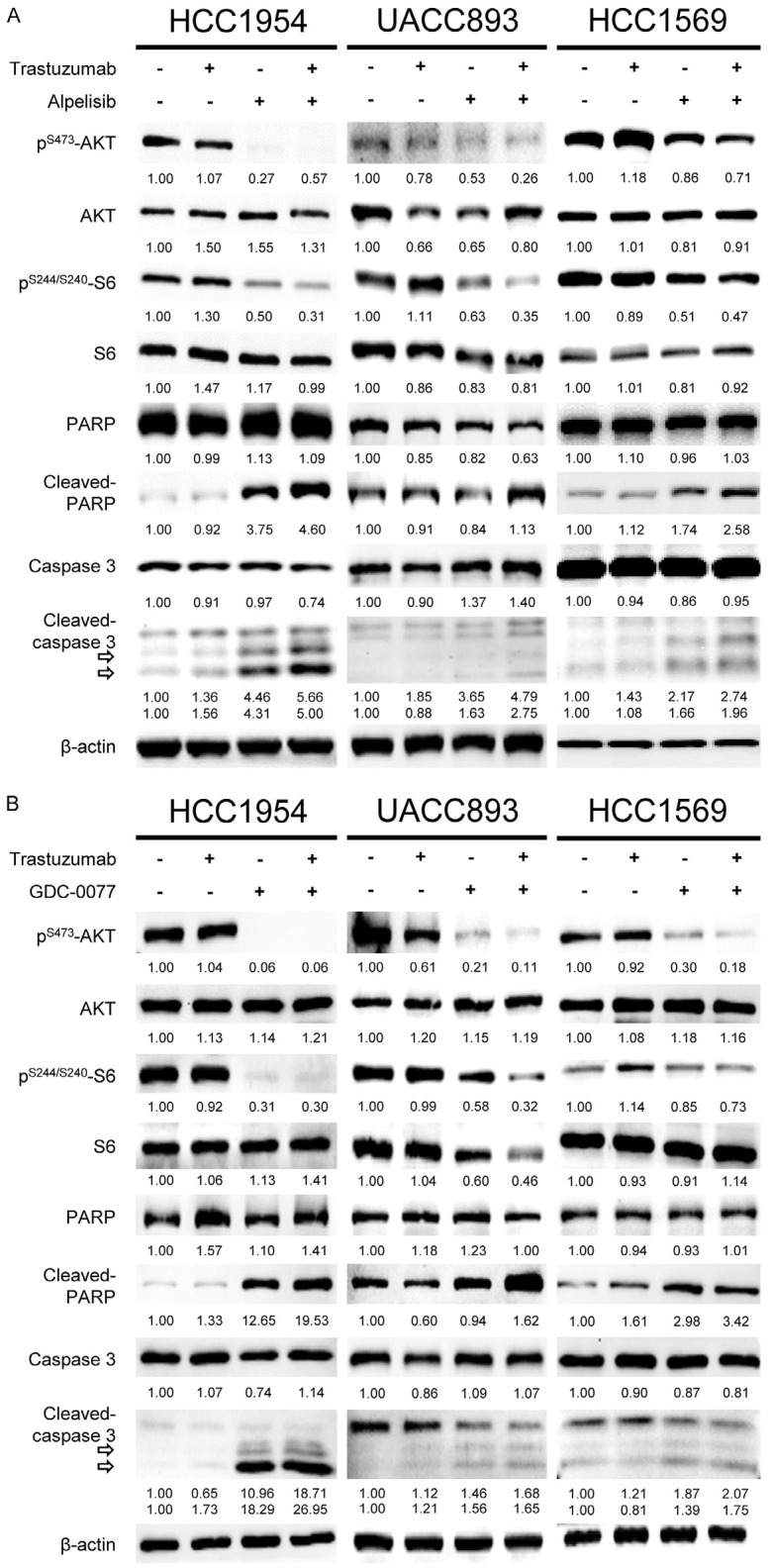
PI3K inhibitors with or without trastuzumab induce down-regulation of phospho-AKT, phospho-S6, and apoptosis. A. HCC1954, UACC893, and HCC1569 cells were treated with the indicated agents. Lysates were collected 24 hours after treatment. The cells treated with alpelisib and alpelisib plus trastuzumab showed down-regulation of phospho-AKT and phospho-S6. Within the same groups, overexpression of cleaved-PARP and cleaved-caspase 3 were observed. The control and trastuzumab groups did not show the above protein cleavage. B. Three breast cancer cell lines were treated with the indicated agents. GDC-0077 with or without trastuzumab contributed to down-regulation of phospho-AKT and phospho-S6, and up-regulation of cleaved-PARP and cleaved-caspase 3, whereas the other two groups did not. The trastuzumab concentration was 2 μg/mL. The alpelisib concentrations were 2, 0.5, and 4 μM for HCC1954, UACC893, and HCC1569 cells, respectively. The GDC-0077 concentrations were 0.8, 0.4, and 3.2 μM for HCC1954, UACC893, and HCC1569 cells, respectively. Numbers under each western blot represent the intensity of the protein relative to the control.
Figure 5.
Increased caspase 3 and 7 activities in trastuzumab-resistant breast cancer cells treated with PI3K inhibitors plus trastuzumab. Red fluorescence representes the activities of caspase 3 and 7 in treated cells. The concentration of drug was the same as that in the combination protocols used for western blot analysis. After treatment with the indicated agents, more red signal was observed in the combination groups compared with the control groups. The differences in caspase 3 and 7 intensity were statistically significant between the controls and PI3K inhibitors plus trastuzumab. The bar charts represent the mean caspase 3 and 7 intensity ± SD. P-value: *P<0.05, **P<0.01, and ***P<0.001. A plus T: alpelisib plus trastuzumab; G plus T: GDC-0077 plus trastuzumab.
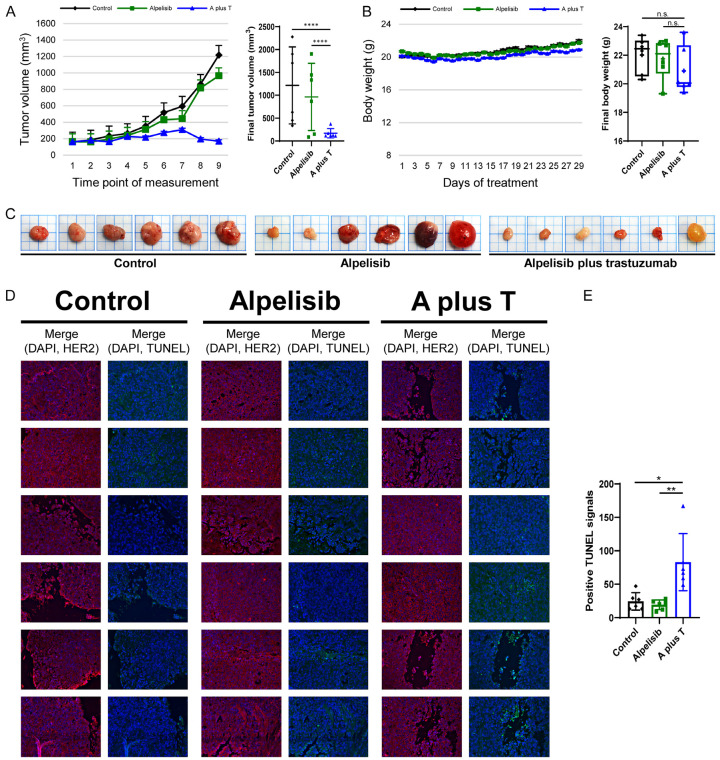
The treatment of alpelisib plus trastuzumab inhibits the growth of tumor xenografts bearing PIK3CA mutations
We examined the response of HCC1954 xenografts to a control agent (solvent for alpelisib), alpelisib, and alpelisib plus trastuzumab. The control and alpelisib were administered orally five days a week. Trastuzumab was given as an intraperitoneal injection twice a week. These treatments lasted for four weeks and the mice were sacrificed 24 hours after the last treatment. Growth curves for the xenografts in the different treatment groups separated after the second week (Figure 6A). Alpelisib plus trastuzumab exhibited the best response and contributed to a prominent reduction of tumor volume. The differences in final tumor volume between the combination group and the other two groups were statistically significant with P-values <0.0001. In contrast, the alpelisib only group did not exhibit a significant tumor volume reduction compared with the control group. Mice in the combination group appeared to have less body weight compared with the other two groups (Figure 6B). Considering different xenograft volumes (Figure 6A, 6C), the difference in final body weight between the alpelisib plus trastuzumab group and the other two groups may be explained, but was not statistically significant. No deaths occurred in the three treatment groups by the end of the experiment.
Figure 6.
Alpelisib plus trastuzumab causes prominent inhibition in the growth of HCC1954 xenografts and significantly increases apoptosis. A. Each plot indicates the mean value of the tumor volume. Final tumor volume was summarized and statistically analyzed. B. Each plot represents the mean value of body weight. Box charts show the distribution of final body weight. C. Images of the xenografts were taken immediately after harvesting. D. DAPI, HER2, and TUNEL signals were captured in each xenograft. Blue, red, and green fluorescence indicates DAPI, HER2, and TUNEL signals, respectively. E. Bar charts represent the mean value of positive TUNEL signals in the xenografts. Error bars represent the standard error in tumor volume and body weight, and SD in TUNEL signals. P-value: *P<0.05, **P<0.01, and ****P<0.0001. A plus T: alpelisib plus trastuzumab; n.s.: not significant.
Slides were prepared from the harvested xenografts and stained with DAPI, HER2, and TUNEL. We used the expression of HER2 for outlining cell structure and differentiating cells without nuclei from the empty space. We selected representative fields then calculated positive TUNEL signals, which were observed within cells and not in the blank area. Strong HER2 expression was observed in the three groups after treatment (Figure 6D). Positive TUNEL signals were evident in the alpelisib plus trastuzumab group compared with the control group and the alpelisib only group. The number of positive TUNEL signals in each group were statistically analyzed and the results indicated that tumor cells treated with alpelisib plus trastuzumab exhibited more apoptotic events compared with those in the other two groups (Figure 6E).
Discussion
Considering patient quality of life, more cytotoxic chemotherapy-free, biomarker-driven regimens have been developed for breast cancer. However, chemotherapy is still needed as part of treatment for HER2-positive tumors and overcome the diverse resistant mechanisms of the HER2-positive subtype. Thus, antibody-drug conjugates (ADCs) are being developed to lower the systemic cytotoxicity compared with that of cytotoxic drugs [30]. T-DM1 and T-DXd are ADCs approved for HER2-positive breast cancer. Although T-DM1 has demonstrated efficacy for PIK3CA-mutated tumors, based on the EMILIA and T3RESA trials, this benefit may not be achieved in the first-line phase 3 MARIANNE trial [31-33]. On the other hand, more clinical data is needed for T-DXd, as a promising ADC, to demonstrate efficacy in patients with PIK3CA mutations [6]. Moreover, concerns regarding the toxicity of ADCs or shedding payloads in the circulation [30]. Therefore, there is still a need for a biomarker-driven strategy for HER2-positive breast cancer exhibiting alterations in the PI3K/AKT/mTOR pathway.
Our study revealed promising effects of PI3K inhibitors in combination with trastuzumab in PIK3CA-mutant and/or PTEN deficient, HER2-positive breast cancer cells. Cancer cell growth was hindered by blocking signaling pathways and there was also increased apoptosis induced by the combination strategy. Trastuzumab alone had essentially no effect on HCC1954, UACC893, and HCC1569 cells; however, the resistance phenotype of these cells could be circumvented by adding PI3K inhibitors, such as alpelisib and GDC-0077. Synergistic effects were achieved by combining PI3K inhibitors with trastuzumab. The reason why this combination works may originate from the constitutive blockage of the HER2 signal, together with the inhibition of downstream signaling. This theory has been proven before, but most combinations are too toxic to be administered to patients with breast cancer [12,17-19]. Finally, alpelisib together with fulvestrant contributed to better progression-free survival in patients with HR-positive/HER2-negative breast cancer [20], which indicates that alpelisib may be useful for other indications. The dose of alpelisib in the mouse model was selected based on published studies [34,35]. To avoid intolerable toxicities from the combination, we selected a dose of alpelisib that was lower than historical reports and may be the reason why xenografts responded poorly to alpelisib monotherapy. However, we demonstrated the effects of alpelisib, which may be potentiated by the combination strategy with trastuzumab. There were significant apoptotic events in tumors treated with alpelisib plus trastuzumab, which complement the results of western blot analysis, flow cytometry, and the activities of caspase 3 and 7. This combination treatment was well-tolerated based on the fact that no mice died during the treatment period and no differences in body weight were observed among the three groups after considering the weight of the xenografts.
Tumor cells may develop resistance to PI3K inhibition through loss of PTEN [22,23]. We simulated this condition using siRNA to knock down the expression of PTEN. The growth of siPTEN-treated HCC1954 cells was still affected by PI3K inhibitors plus trastuzumab. This indicates that the combination strategy still works despite resistance via PIK3CA mutation and/or PTEN loss. In contrast, cancer cells without alteration of PI3K or PTEN do not respond to the combination treatment. We found that the proliferation of CK-MB-1 was barely inhibited by trastuzumab or PI3K inhibitors. This poor response was not circumvented by combining PI3K inhibitors and trastuzumab together in CK-MB-1 cells. Thus, it was unlike the synergism observed with PI3K inhibitors and trastuzumab in HCC1954, UACC893, and HCC1569 cells. These biomarker-specific results are consistent with the response of PIK3CA mutation-dependent tumors in the SOLAR-1 trial. Similarly, the AKT inhibitor, capivasertib, showed activity in HR-positive/HER2-negative breast cancer [36] and should be further evaluated in HER2-positive breast cancer cells with PIK3CA mutations or loss of PTEN. After treatment with PI3K inhibitors or AKT inhibitors, possible mechanisms of resistance, such as impairment of BRCA1/2 expression, may emerge as issues requiring further elucidation [37,38].
Although ADCs are now the standard of care, as a second-line treatment or beyond, for patients with HER2-positive metastatic breast cancer, their efficacy in patients harboring PIK3CA mutations remains inconclusive. Therefore, alpelisib plus T-DM1 had been tested in a phase I trial to address this issue [39]. Although some responders were observed in this trial, it is uncertain whether the combination had synergistic effects at a cost of more toxicity. Out of all patients, 41% had grade 3/4 maculopapular rashes and 18% had grade 3/4 thrombocytopenia with the regimen of alpelisib plus T-DM1. A clear benefit has not yet emerged for combining tyrosine kinase inhibitors with ADCs for HER2-positive breast cancer. The efficacy of neratinib plus T-DM1, tucatinib plus T-DM1, and tucatinib plus T-DXd are still under investigation in clinical trials [40-42]. Trastuzumab in combination with chemotherapy, other monoclonal antibodies, endocrine therapy, or small molecule drugs have been studied and shown efficacy with tolerable side effects in the HER2-positive subtype of breast cancer [4,7,43,44]. As the backbone of anti-HER2 therapy, trastuzumab is an important drug that can not only inhibit the HER2 signaling pathway but can also induce cell-mediated cytotoxicity. Moreover, its safety profile has been established and allowing it to be combined with other novel drugs. Therefore, our research has confirmed synergistic effects of PI3K inhibitors and trastuzumab both in vitro and in vivo. This biomarker-oriented treatment strategy may provide insight for designing clinical trials for patients with HER2-positive metastatic breast cancer who fail anti-HER2 treatments. The combination with alpelisib, trastuzumab, and pertuzumab is now under investigation as maintenance therapy in a first-line setting for metastatic HER2-positive breast cancer [45]. However, it is too early to judge whether this combination can be applied to first-line maintenance therapy. As quite a few patients bear PIK3CA and/or PTEN mutations after HER2-directed treatments [46], the biomarker-driven combination with alpelisib plus trastuzumab could be a strategy for later-line patients, which is worth further investigation in human studies.
Acknowledgements
The authors would like to thank Enago (www.enago.com) for the English language review. This work was funded by the Center of Applied Nanomedicine, National Cheng Kung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education and National Cheng Kung University Hospital, Taiwan (NCKUH-11006007). All animal handling was reviewed and approved by the Institutional Animal Care and Use Committee, National Cheng Kung University (110012).
Disclosure of conflict of interest
None.
Abbreviations
- ADC
antibody-drug conjugate
- AKT
v-akt murine thymoma viral oncogene homolog
- CI
combination index
- DMSO
dimethyl sulfoxide
- ER
estrogen receptor
- FBS
fetal bovine serum
- HER2
Human epidermal growth factor receptor 2
- HR
hormone receptor
- mTOR
mammalian target of rapamycin
- PBS
phosphate-buffered saline
- PI3K
phosphoinositide 3-kinase
- PTEN
phosphatase and tensin homolog
- siRNA
small interfering RNA
Supporting Information
References
- 1.Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF American Society of Clinical Oncology/College of American Pathologists. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 4.Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM CLEOPATRA Study Group. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Dieras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K EMILIA Study Group. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, Sohn J, Denduluri N, Perrin C, Aogi K, Tokunaga E, Im SA, Lee KS, Hurvitz SA, Cortes J, Lee C, Chen S, Zhang L, Shahidi J, Yver A, Krop I DESTINY-Breast01 Investigators. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382:610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murthy RK, Loi S, Okines A, Paplomata E, Hamilton E, Hurvitz SA, Lin NU, Borges V, Abramson V, Anders C, Bedard PL, Oliveira M, Jakobsen E, Bachelot T, Shachar SS, Muller V, Braga S, Duhoux FP, Greil R, Cameron D, Carey LA, Curigliano G, Gelmon K, Hortobagyi G, Krop I, Loibl S, Pegram M, Slamon D, Palanca-Wessels MC, Walker L, Feng W, Winer EP. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382:597–609. doi: 10.1056/NEJMoa1914609. [DOI] [PubMed] [Google Scholar]
- 8.Cortes J, Kim SB, Chung WP, Im SA, Park YH, Hegg R, Kim MH, Tseng LM, Petry V, Chung CF, Iwata H, Hamilton E, Curigliano G, Xu B, Huang CS, Kim JH, Chiu JWY, Pedrini JL, Lee C, Liu Y, Cathcart J, Bako E, Verma S, Hurvitz SA DESTINY-Breast03 Trial Investigators. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386:1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 9.Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest. 2011;121:3797–3803. doi: 10.1172/JCI57152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, Linn SC, Gonzalez-Angulo AM, Stemke-Hale K, Hauptmann M, Beijersbergen RL, Mills GB, van de Vijver MJ, Bernards R. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Baselga J, Cortes J, Im SA, Clark E, Ross G, Kiermaier A, Swain SM. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J. Clin. Oncol. 2014;32:3753–3761. doi: 10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien NA, McDonald K, Tong L, von Euw E, Kalous O, Conklin D, Hurvitz SA, di Tomaso E, Schnell C, Linnartz R, Finn RS, Hirawat S, Slamon DJ. Targeting PI3K/mTOR overcomes resistance to HER2-targeted therapy independent of feedback activation of AKT. Clin Cancer Res. 2014;20:3507–3520. doi: 10.1158/1078-0432.CCR-13-2769. [DOI] [PubMed] [Google Scholar]
- 13.Andre F, O’Regan R, Ozguroglu M, Toi M, Xu B, Jerusalem G, Masuda N, Wilks S, Arena F, Isaacs C, Yap YS, Papai Z, Lang I, Armstrong A, Lerzo G, White M, Shen K, Litton J, Chen D, Zhang Y, Ali S, Taran T, Gianni L. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15:580–591. doi: 10.1016/S1470-2045(14)70138-X. [DOI] [PubMed] [Google Scholar]
- 14.Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano MS, Neciosup SP, Tseng LM, Zhang Q, Shen K, Liu D, Dreosti LM, Burris HA, Toi M, Buyse ME, Cabaribere D, Lindsay MA, Rao S, Pacaud LB, Taran T, Slamon D. Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial. Lancet Oncol. 2015;16:816–829. doi: 10.1016/S1470-2045(15)00051-0. [DOI] [PubMed] [Google Scholar]
- 15.Yori JL, Lozada KL, Seachrist DD, Mosley JD, Abdul-Karim FW, Booth CN, Flask CA, Keri RA. Combined SFK/mTOR inhibition prevents rapamycin-induced feedback activation of AKT and elicits efficient tumor regression. Cancer Res. 2014;74:4762–4771. doi: 10.1158/0008-5472.CAN-13-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlo MI, Molina AM, Lakhman Y, Patil S, Woo K, DeLuca J, Lee CH, Hsieh JJ, Feldman DR, Motzer RJ, Voss MH. A phase Ib study of BEZ235, a dual inhibitor of Phosphatidylinositol 3-Kinase (PI3K) and Mammalian Target of Rapamycin (mTOR), in patients with advanced renal cell carcinoma. Oncologist. 2016;21:787–788. doi: 10.1634/theoncologist.2016-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baselga J, Im SA, Iwata H, Cortes J, De Laurentiis M, Jiang Z, Arteaga CL, Jonat W, Clemons M, Ito Y, Awada A, Chia S, Jagiello-Gruszfeld A, Pistilli B, Tseng LM, Hurvitz S, Masuda N, Takahashi M, Vuylsteke P, Hachemi S, Dharan B, Di Tomaso E, Urban P, Massacesi C, Campone M. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:904–916. doi: 10.1016/S1470-2045(17)30376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baselga J, Dent SF, Cortés J, Im YH, Diéras V, Harbeck N, Krop IE, Verma S, Wilson TR, Jin HX. Phase III study of taselisib (GDC-0032)+ fulvestrant (FULV) v FULV in patients (pts) with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): primary analysis from SANDPIPER. Am Soc Clin Oncol. 2018 [Google Scholar]
- 19.Di Leo A, Johnston S, Lee KS, Ciruelos E, Lonning PE, Janni W, O’Regan R, Mouret-Reynier MA, Kalev D, Egle D, Csoszi T, Bordonaro R, Decker T, Tjan-Heijnen VCG, Blau S, Schirone A, Weber D, El-Hashimy M, Dharan B, Sellami D, Bachelot T. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:87–100. doi: 10.1016/S1470-2045(17)30688-5. [DOI] [PubMed] [Google Scholar]
- 20.Andre F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, Iwata H, Conte P, Mayer IA, Kaufman B, Yamashita T, Lu YS, Inoue K, Takahashi M, Papai Z, Longin AS, Mills D, Wilke C, Hirawat S, Juric D SOLAR-1 Study Group. Alpelisib for PIK3CA-mutated, hormone receptor-positive advanced breast cancer. N Engl J Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 21.Hong R, Edgar K, Song K, Steven S, Young A, Hamilton P, Arrazate A, De La Cruz C, Chan C, Pang J. Abstract PD4-14: GDC-0077 is a selective PI3Kalpha inhibitor that demonstrates robust efficacy in PIK3CA mutant breast cancer models as a single agent and in combination with standard of care therapies. AACR. 2018 [Google Scholar]
- 22.Juric D, Castel P, Griffith M, Griffith OL, Won HH, Ellis H, Ebbesen SH, Ainscough BJ, Ramu A, Iyer G, Shah RH, Huynh T, Mino-Kenudson M, Sgroi D, Isakoff S, Thabet A, Elamine L, Solit DB, Lowe SW, Quadt C, Peters M, Derti A, Schegel R, Huang A, Mardis ER, Berger MF, Baselga J, Scaltriti M. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kalpha inhibitor. Nature. 2015;518:240–244. doi: 10.1038/nature13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welt A, Wiesweg M, Theurer S, Abenhardt W, Groschek M, Muller L, Schroder J, Tewes M, Chiabudini M, Potthoff K, Bankfalvi A, Marschner N, Schuler M, Breitenbucher F. Buparlisib in combination with tamoxifen in pretreated patients with hormone receptor-positive, HER2-negative advanced breast cancer molecularly stratified for PIK3CA mutations and loss of PTEN expression. Cancer Med. 2020;9:4527–4539. doi: 10.1002/cam4.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung WP, Huang WL, Liao WA, Huang WL, Liu YY, Su WC. Development of the CK-MB-1 trastuzumab-resistant HER2-positive breast cancer cell line and xenograft animal models. Cancer Med. 2021;10:2370–2379. doi: 10.1002/cam4.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou TC, Martin N. Paramus (NJ): ComboSyn Inc; 2005. CompuSyn for drug combinations: PC software and user’s guide: a computer program for quantitation of synergism and antagonism in drug combinations, and the determination of IC50 and ED50 and LD50 Values. [Google Scholar]
- 26.Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 27.Meltzer P, Leibovitz A, Dalton W, Villar H, Kute T, Davis J, Nagle R, Trent J. Establishment of two new cell lines derived from human breast carcinomas with HER-2/neu amplification. Br J Cancer. 1991;63:727–735. doi: 10.1038/bjc.1991.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cowley GS, Weir BA, Vazquez F, Tamayo P, Scott JA, Rusin S, East-Seletsky A, Ali LD, Gerath WF, Pantel SE, Lizotte PH, Jiang G, Hsiao J, Tsherniak A, Dwinell E, Aoyama S, Okamoto M, Harrington W, Gelfand E, Green TM, Tomko MJ, Gopal S, Wong TC, Li H, Howell S, Stransky N, Liefeld T, Jang D, Bistline J, Hill Meyers B, Armstrong SA, Anderson KC, Stegmaier K, Reich M, Pellman D, Boehm JS, Mesirov JP, Golub TR, Root DE, Hahn WC. Parallel genome-scale loss of function screens in 216 cancer cell lines for the identification of context-specific genetic dependencies. Sci Data. 2014;1:140035. doi: 10.1038/sdata.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giordano SH, Temin S, Chandarlapaty S, Crews JR, Esteva FJ, Kirshner JJ, Krop IE, Levinson J, Lin NU, Modi S, Patt DA, Perlmutter J, Ramakrishna N, Winer EP, Davidson NE. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO clinical practice guideline update. J. Clin. Oncol. 2018;36:2736–2740. doi: 10.1200/JCO.2018.79.2697. [DOI] [PubMed] [Google Scholar]
- 30.Drago JZ, Modi S, Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021;18:327–344. doi: 10.1038/s41571-021-00470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baselga J, Lewis Phillips GD, Verma S, Ro J, Huober J, Guardino AE, Samant MK, Olsen S, de Haas SL, Pegram MD. Relationship between Tumor biomarkers and efficacy in EMILIA, a phase III study of trastuzumab emtansine in HER2-positive metastatic breast cancer. Clin Cancer Res. 2016;22:3755–3763. doi: 10.1158/1078-0432.CCR-15-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SB, Wildiers H, Krop IE, Smitt M, Yu R, Lysbet de Haas S, Gonzalez-Martin A. Relationship between tumor biomarkers and efficacy in TH3RESA, a phase III study of trastuzumab emtansine (T-DM1) vs. treatment of physician’s choice in previously treated HER2-positive advanced breast cancer. Int J Cancer. 2016;139:2336–2342. doi: 10.1002/ijc.30276. [DOI] [PubMed] [Google Scholar]
- 33.Perez EA, de Haas SL, Eiermann W, Barrios CH, Toi M, Im YH, Conte PF, Martin M, Pienkowski T, Pivot XB, Burris HA 3rd, Stanzel S, Patre M, Ellis PA. Relationship between tumor biomarkers and efficacy in MARIANNE, a phase III study of trastuzumab emtansine +/- pertuzumab versus trastuzumab plus taxane in HER2-positive advanced breast cancer. BMC Cancer. 2019;19:517. doi: 10.1186/s12885-019-5687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong CH, Ma BBY, Hui CWC, Lo KW, Hui EP, Chan ATC. Preclinical evaluation of ribociclib and its synergistic effect in combination with alpelisib in non-keratinizing nasopharyngeal carcinoma. Sci Rep. 2018;8:8010. doi: 10.1038/s41598-018-26201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan Y, Wen W, Yost SE, Xing QH, Yan J, Han ES, Mortimer J, Yim JH. Combination therapy with BYL719 and LEE011 is synergistic and causes a greater suppression of p-S6 in triple negative breast cancer. Sci Rep. 2019;9:7509. doi: 10.1038/s41598-019-43429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones RH, Casbard A, Carucci M, Cox C, Butler R, Alchami F, Madden TA, Bale C, Bezecny P, Joffe J, Moon S, Twelves C, Venkitaraman R, Waters S, Foxley A, Howell SJ. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020;21:345–357. doi: 10.1016/S1470-2045(19)30817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibrahim YH, Garcia-Garcia C, Serra V, He L, Torres-Lockhart K, Prat A, Anton P, Cozar P, Guzman M, Grueso J, Rodriguez O, Calvo MT, Aura C, Diez O, Rubio IT, Perez J, Rodon J, Cortes J, Ellisen LW, Scaltriti M, Baselga J. PI3K inhibition impairs BRCA1/2 expression and sensitizes BRCA-proficient triple-negative breast cancer to PARP inhibition. Cancer Discov. 2012;2:1036–1047. doi: 10.1158/2159-8290.CD-11-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yap TA, Kristeleit R, Michalarea V, Pettitt SJ, Lim JSJ, Carreira S, Roda D, Miller R, Riisnaes R, Miranda S, Figueiredo I, Rodrigues DN, Ward S, Matthews R, Parmar M, Turner A, Tunariu N, Chopra N, Gevensleben H, Turner NC, Ruddle R, Raynaud FI, Decordova S, Swales KE, Finneran L, Hall E, Rugman P, Lindemann JPO, Foxley A, Lord CJ, Banerji U, Plummer R, Basu B, Lopez JS, Drew Y, de Bono JS. Phase I trial of the PARP Inhibitor olaparib and AKT inhibitor capivasertib in patients with BRCA1/2- and non-BRCA1/2-mutant cancers. Cancer Discov. 2020;10:1528–1543. doi: 10.1158/2159-8290.CD-20-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jain S, Shah AN, Santa-Maria CA, Siziopikou K, Rademaker A, Helenowski I, Cristofanilli M, Gradishar WJ. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res Treat. 2018;171:371–381. doi: 10.1007/s10549-018-4792-0. [DOI] [PubMed] [Google Scholar]
- 40.Borges VF, Ferrario C, Aucoin N, Falkson C, Khan Q, Krop I, Welch S, Conlin A, Chaves J, Bedard PL, Chamberlain M, Gray T, Vo A, Hamilton E. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a phase 1b clinical trial. JAMA Oncol. 2018;4:1214–1220. doi: 10.1001/jamaoncol.2018.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abraham J, Montero AJ, Jankowitz RC, Salkeni MA, Beumer JH, Kiesel BF, Piette F, Adamson LM, Nagy RJ, Lanman RB, Sperinde J, Huang W, Allegra CJ, Srinivasan A, Wang Y, Pogue-Geile KL, Lucas PC, Jacobs SA. Safety and efficacy of T-DM1 plus neratinib in patients with metastatic HER2-positive breast cancer: NSABP foundation trial FB-10. J. Clin. Oncol. 2019;37:2601–2609. doi: 10.1200/JCO.19.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fabrice A, Erika PH, Sherene L, Peter S, Carey KA, Tinghui Y, Sarice B, Celina MDC, Pia H, Komal LJ. Trastuzumab deruxtecan (T-DXd) combinations in patients with HER2-positive advanced or metastatic breast cancer: a phase 1b/2, open-label, multicenter, dose-finding and dose-expansion study (DESTINY-Breast07) J. Clin. Oncol. 2021;39:TPS1096–TPS1096. [Google Scholar]
- 43.Tolaney SM, Wardley AM, Zambelli S, Hilton JF, Troso-Sandoval TA, Ricci F, Im SA, Kim SB, Johnston SR, Chan A, Goel S, Catron K, Chapman SC, Price GL, Yang Z, Gainford MC, Andre F. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21:763–775. doi: 10.1016/S1470-2045(20)30112-1. [DOI] [PubMed] [Google Scholar]
- 44.Rugo HS, Im SA, Cardoso F, Cortes J, Curigliano G, Musolino A, Pegram MD, Wright GS, Saura C, Escriva-de-Romani S, De Laurentiis M, Levy C, Brown-Glaberman U, Ferrero JM, de Boer M, Kim SB, Petrakova K, Yardley DA, Freedman O, Jakobsen EH, Kaufman B, Yerushalmi R, Fasching PA, Nordstrom JL, Bonvini E, Koenig S, Edlich S, Hong S, Rock EP, Gradishar WJ SOPHIA Study Group. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7:573–584. doi: 10.1001/jamaoncol.2020.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hurvitz SA, Chia SKL, Ciruelos EM, Hu X, Im SA, Janni W, Jerusalem G, Lacouture M, O’Regan R, Rugo HS, Yap YS, Ghaznawi F, Han Y, Su F, Chandarlapaty S. 352TiP EPIK-B2: a phase III study of alpelisib (ALP) as maintenance therapy with trastuzumab (T) and pertuzumab (P) in patients (pts) with PIK3CA-mutated (mut) human epidermal growth factor receptor-2-positive (HER2+) advanced breast cancer (ABC) Ann Oncol. 2020;31:S389–S390. [Google Scholar]
- 46.Modi S, Andre F, Krop IE, Saura C, Yamashita T, Kim SB, Tamura K, Chen S, Suto F, Kuwahara Y. Trastuzumab deruxtecan for HER2-positive metastatic breast cancer: DESTINY-Breast01 subgroup analysis. Am Soc Clinl Oncol. 2020 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.