Abstract
An odontogenic keratocyst (OKC) is a benign but aggressive intraosseous tumor derived from the remains of the original tooth germ or dental lamina. It has a marked ability to recur and become cancerous. However, patients with early-stage OKC often have no symptoms and manifestations. The common clinical manifestation is swelling. Hence, it is critical to precisely diagnose the disease, to use differential diagnosis in combination with auxiliary examination methods, and to select the most appropriate treatment option to reduce the loss of bone tissue and the related damage to patients. In recent years, with the advancement in understanding the molecular basis of this disease and the development of early detection and targeted therapy, the diagnosis and the prognosis of OKC have been improved. The aim of this study was to provide an overview on the clinical features, diagnosis, and treatment of OKC. The molecular and genetic basis of this disease and the characteristics of malignant transformation of OKC were also discussed. Finally, we presented patient cases from our clinical practice to provide some advice on the diagnosis and treatment of OKC.
Keywords: Odontogenic cysts, odontogenic keratocyst, odontogenic tumors, keratocystic odontogenic tumor
Introduction
In 2005, WHO reclassified odontogenic keratocysts (OKC) as tumors by modifying the previous two editions of WHO classification. Keratocystic odontogenic tumor (KCOT) was recommended as the appropriate name [1]. However, in 2017, WHO changed odontogenic keratocystic tumor back to odontogenic keratocyst (OKC) [2], which reflects the extensive and continuous investigation of this disease that has been carried on. OKC is a benign tumor derived from the remains of the original tooth germ or dental lamina. Unlike most other jaw cysts, it has a very high recurrence rate and a tendency to become cancerous [3,4]. OKC can occur in any location of the jaw, but more often in the ascending ramus and molar area. Nevertheless, there are few reports showing OKC occurs in soft tissues, most of which occurs in the Gingiva [5,6], buccal space [7,8], posterior trigone of the molar [9], deep facial area [10], or the temporal muscle [11].
The clinical features of OKC
OKC mostly occurs in adults 30-50 years old, accounting for 14.3% of the total cases of odontogenic tumors [12]. There are generally no obvious symptoms and signs in patients with early-stage OKC, as even if the lesion is large, it still does not cause obvious jaw expansion, making it unnoticeable by facial appearance. Occasionally, patients have symptoms such as swelling, pain, abnormal sensation, pus outflow, and loose teeth [13]. Among these, swelling is the most common clinical manifestation of OKC [14]. If an OKC continues to grow, and the bone gradually expands around, facial deformity will occur. As a result, the surface bone becomes a very thin bone plate, and the palpation can have a ping-pong-like feeling and make the so-called parchment-like brittle sound. In severe cases, pathological fractures can happen, or this layer of bone plate is eventually absorbed, as well as a wave motion when touched. The tumor cells in OKC can also invade the nasal cavity and maxillary sinus. In severe cases, these cells can affect vision and even lead to diplopia [15]. If OKC is adjacent to the teeth, it can cause the adjacent teeth to be compressed, resulting in the teeth to shift, loosen, or tilt. Thus, OKC can be accompanied by tooth loss clinically. It is worth noting that the extraction of moving teeth in OKC patients should be performed with extreme caution. If the loosen tooth is caused by OKC, tooth extraction and injury can rupture the cyst, and sebum-like substances will be visible. If the tumor has secondary infection, it can cause symptoms including swelling, pain, fever, and general discomfort. Importantly, if swelling also happens in other body parts, the patient’s medical history should be examined in detail to prevent from missed diagnosis or misdiagnosis. For example, in our hospital, a 41-year-old female patient presented with painless progressive swelling in the left temporal region. A large curved surgical scar was found in the left neck. The patient had undergone two OKC operations at the age 25 and 38, respectively. Physical examination revealed a prominent elevation of the left temporal region with normal skin, and a mass of about 3.5 cm×3.0 cm in size was palpable. The mass was slightly soft and had boundaries without tenderness and movement. It appeared to be located deeply under the temporal muscle.
Diagnosis of OKC
OKC can be diagnosed according to the medical history and the clinical manifestations. Puncture is a relatively reliable diagnostic method. Yellow and white keratin-like (sebum-like) substances can be seen on the site in most cases. Keratin staining of the extracted material will further validate the diagnosis of OKC. X-ray examination is also very useful for diagnosis. The X-ray image will show a clear round or oval transparent shadow with well-defined edges. There is often an obvious white bone reaction line around, and sometimes the edges can be irregular. B-scan ultrasonography can also be used as an auxiliary examination for diagnosis. As shown in Figure 1C, a CT (computed tomography) scan revealed that there was a low-density mass with a small high-density lesion around the local area deep in the left temporal region above the zygomatic arch. The edge of the mass was clear, and the left mandibular ramus disappeared, but the head with metal joint was in position. Ultrasonography showed a cystic mass in the left temporal region. From the CT scan of the patient before the second operation, we found that the OKC was larger and had affected the coracoid apex (Figure 1B). Based on the patient’s past treatment history and the examination by specialist, the possibility of OKC recurrence in temporal region was considered. In this case, it was not easy to diagnose OKC by the temporal mass alone, as there was a possibility of hemangioma and lipoma [16,17]. Hemangioma is a congenital benign tumor or vascular malformation, and it is positive for movement test [18,19]. On the other hand, lipoma is soft, and its skin can appear “Orange peel”-like if the mass is pressed at the base by hand. The CT image of lipoma will be a lipoid density mass because it contains lipid material [20]. We ruled out the possibility of other tumors, such as hemangioma or lipoma, by analyzing the patient’s entire treatment history and the clinical features. The recurrent OKC in the left temporal region was considered. This diagnosis was further supported by the presence of keratin in the histological staining of the resected tumor sample [21-23]. Pathological section diagnosis is the final gold standard. According to the pathological section after operation, the diagnosis was OKC (Figure 2).
Figure 1.
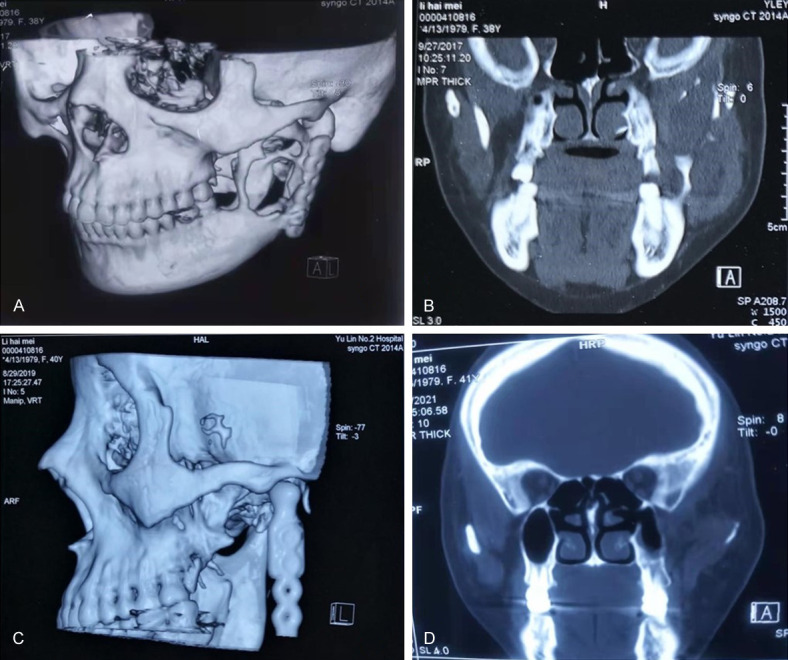
CT imaging examination of patients. A. Before the second operation, bony destruction of the ascending ramus of the mandible was seen. B. Before the second operation, in the coronal view, the bone destruction of the ascending ramus of the mandible was seen. C. Before the third operation, on this preoperative CT scan, bony structures could be seen in the temporal mass. D. Before the third operation, CT scan showed possible tumor mass in the coracoid region: the shadow of a cyst in the temporal part of the deep zygomatic arch.
Figure 2.
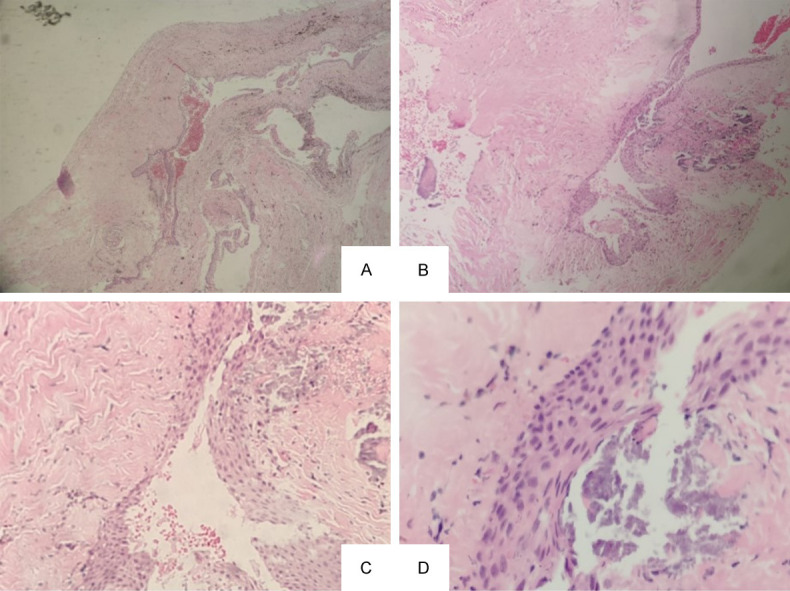
Histopathologic features indicate a keratocystic odontogenic tumor. Images were taken at magnification of Х40 (A), Х100 (B), Х200 (C), and Х400 (D). The outer layer is fibrous tissue, and the inner layer is stratified squamous epithelium with keratosis or incomplete keratosis. There are ascocyst or epithelial island in the fibrous capsule.
Treatment of OKC
One of the unfavorable features of OKC is its high recurrence rate after treatment, which happens in about 25-60% of OKC patients [24]. The cause for this high recurrence rate in OKC is complex. One is due to the aggressive biological nature of OKC, which is prone to recurrence [29,30]. In addition, since the capsule wall is thin and easy to break, it is difficult to remove OKC lesion completely, and the remaining epithelium of the capsule wall has high proliferative ability, which leads to recrudescence. To make matter worse, there are satellite capsules or microcapsules in the connective fibrous capsules to support the growth of OKC.
The recurrence rate in OKC is closely related to the surgical method used too. Appropriate procedures will help reduce the chance of recurrence. It has been reported that during surgery, after completely scraping the capsule wall, smearing the bone wound with corrosive agents such as phenol or silver nitrate or applying cryotherapy to eliminate the ascocyst can prevent recurrence [25]. Enucleation combined with Carnoy’s solution can also significantly reduce the recurrence rate [26,27]. Patients receiving complete resection show the lowest recurrence rate [28]. This procedure requires part of the bone around the cyst to be removed. If the lesion is too large or OKC recurs many times, the jaw should be removed together with the diseased soft tissue, and bone grafting should be performed immediately.
Even so, sometimes recurrence cannot be avoided, as shown in a case treated in our hospital. The patient received the first surgery for OKC 13 years before it recurred. In the second operation, although the patient underwent a radical osteotomy, the tumor remained on the upper end of the deep coracoid process and zygomatic arch, due to insufficient preoperative preparation and poor visual field during operation. As a result, the tumor continued to grow and protruded into the temporal region. In the third operation, the flap was turned down along the superficial layer of deep temporal fascia to the upper edge of zygomatic arch. Thus, the tumor under the temporal muscle above zygomatic arch could be palpable, and after the superficial fascia and temporal muscles were dissected, the tumor could be fully exposed. Furthermore, after a blunt and sharp dissection, the adhesions between the tumor and scar tissue on the deep surface of the zygomatic arch were visible, and the OKC was completely removed. The wound was rinsed with a large amount of saline, placed with negative pressure drainage, and closed layered sutures. Three days after the surgery, the negative pressure drainage was removed. When the tumor capsule was opened in vitro, a large amount of cloudy fluid came out, and a small amount of white keratin was found inside. Postoperative recovery was satisfactory. Up to now, the patient has been followed up for three years without recurrence. However, since the tumor has recurred twice, continuing close monitor is needed. Although the complete resection of the tumor and the surrounding bone is more effective in treating OKC, the accompanying damage is greater to patients; hence, overall evaluation and consideration should be given. In our case, this patient presented with an isolated temporal tumor, and the treatment history and the imaging examination suggested a secondary surgical intervention. Therefore, for the treatment of OKC, reading the scan images carefully before the operation and paying attention to the operation area will be crucial for the complete removal of tumor mass.
Molecular and genetic basis of OKC
Early diagnosis and appropriate surgical resection of OKC can minimize jaw damage before facial swelling occurs [31]. Therefore, many studies focus on investigating the molecular basis of OKC. The detailed analysis of gene interaction networks and signaling pathways have provided valuable information to our understanding of the disease and to identify potential therapeutic targets for OKC treatment. Through gene screening, PCNA and TP53 were found to play critical role in the development of OKC [32]. The expression of PCNA is increased in OKC [33]. OKC may also be the result of the combined effect from dysregulation in multiple tumor suppressor genes or oncogenes. For example, the expression of tumor suppressor gene p53 is down regulated in OKC [34], simultaneously, the tumor suppressor gene PTCH1 is also mutated [35,36]. In addition, three members of the p53 protein family, p53, p63 and p73, are expressed in OKC, and their expression is related to angiogenesis [37]. Furthermore, M2 polarized macrophages are ubiquitously found in OKC to promote angiogenesis. This function of M2 polarized macrophages may be related to the secretion of angiogenic cytokines, such as vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), and matrix metalloprotein-9 (MMP-9) [38]. VEGF can initiate angiogenesis in blood vessels, which supports the lesion growth of OKC [39]. Both CD34 and CD99 are transmembrane glycoproteins and are reported to participate in the progression of OKC. The expression level of CD34 is high in cyst microvessels, which is related to intercellular adhesion and inflammation [40]. CD99 is distributed in the basal layer and upper basal layer of OKC, which may serve as potential target for tumor therapy [41]. The expression of epidermal growth factor receptor (EGFR) is also mainly found in the cytoplasm of the basal layer and upper basal layer of OKC, promoting the proliferation of epithelial cells [42]. Moreover, data from proteomic analysis show that OKC cells and oral keratinocytes share similar proteomic characteristics. Analyses on identifying differentially expressed protein between normal and OKC tissues reveal that the dysregulation of desmosomes and ECM degradation are important changes in the pathobiology of OKC [43].
Malignant transformation of OKC
Although the malignant transformation of OKC occurs with a low frequency, it tends to be misdiagnosed compared with other cancerous lesions in the mouth, which is a major concern to the prognosis of OKC [44]. The most common signs and symptoms of the malignant transformation of OKC are pain and swelling [45,46]. Well differentiated squamous cell carcinoma is the most common type of malignant tumor [48]. Other phenotypic changes and characteristics, listed as follows, are also considered for the possible malignant transformation of OKC [49]. First, the mass is large or increases rapidly, and shows chronic inflammation [50]. Second, without surgery, there exits local numbness in the lower lip or the face, or nosebleed suddenly appears [51]. Third, there is an obvious pain or intermittent pain in the mass area [52]. Fourth, imaging examination shows the bone has increased transmission shadow, unclear boundary, wormhole like and sawtooth like changes in the surrounding bone or obvious bone absence [53]. Fifth, there are multiple recurrences at the same site and abnormally enlarged lymph nodes in the ipsilateral neck of the lesion. If the above pathological changes are observed in patients, the possibility of malignant transformation of OKC should be considered. Pathologists should carefully examine all biopsy tissues or extracted specimens and evaluate whether there are malignant changes in the capsule wall through multiple samples or even continuous sections [54,55]. The treatment of malignant transformation of OKC should be based on the principle of malignant tumor treatment.
Conclusion
OKC has received increasing attention because of its high recurrence rate and the tendency of malignant transformation. The clinical manifestation, diagnosis and treatment option should be evaluated carefully. Before selecting treatment for keratocystic odontogenic tumors, the preoperative image data must be read carefully to make sure that the operative area is clear; the omission of the tumor is avoided, and the tumor is completely removed. All these precautions will significantly help prevent the recurrence or malignant transformation of OKC.
Disclosure of conflict of interest
None.
References
- 1.Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 2.Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg P. WHO classification of Head and Neck Tumours: odontogenic and maxilofacial bone tumours. 4th edition. Lyon: IARC; 2017. pp. 205–260. [Google Scholar]
- 3.Mehdi A, Saeed B, Narges H, Hooman A, Sepehr A, Zahra A. Review on the most important management of keratocystic odontogenic tumor. Klin Onkol. 2022;35:10–19. doi: 10.48095/ccko202210. [DOI] [PubMed] [Google Scholar]
- 4.Moura BS, Cavalcante MA, Hespanhol W. Keratocystic odontogenic tumor. Rev Col Bras Cir. 2016;43:466–471. doi: 10.1590/0100-69912016006013. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues BT, Israel MS, de Moura KL, Pinheiro GL, Carlos R, Pires FR. Peripheral odontogenic keratocyst: report of two new cases and review of the literature. J Clin Exp Dent. 2020;12:e1005–e1010. doi: 10.4317/jced.57653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preston RD, Narayana N. Peripheral odontogenic keratocyst. J Periodontol. 2005;76:2312–2315. doi: 10.1902/jop.2005.76.12.2312. [DOI] [PubMed] [Google Scholar]
- 7.Gröbe A, Hanken H, Blessmann M, Zustin J, Heiland M, Al-Dam A. An odontogenic keratocystic tumor in the buccal space: an unusual site of origin and a review of the literature. In Vivo. 2012;26:847–851. [PubMed] [Google Scholar]
- 8.Yamamoto K, Matsusue Y, Kurihara M, Takahashi Y, Kirita T. A keratocyst in the buccal mucosa with the features of keratocystic odontogenic tumor. Open Dent J. 2013;7:152–156. doi: 10.2174/1874210601307010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shathur A, Patel B, Pitiyage G, Cameron S, Hyde N. Odontogenic keratocyst located in the retromolar trigone. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;132:e82–e85. doi: 10.1016/j.oooo.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, Zheng JW, Yang J. CT and MR imaging features of peripheral keratocystic odontogenic tumors. China Journal of Oral and Maxillofacial Surgery. 2014;12:167–171. [Google Scholar]
- 11.Worrall SF. Recurrent odontogenic keratocyst within the temporalis muscle. Br J Oral Maxillofac Surg. 1992;30:59–62. doi: 10.1016/0266-4356(92)90139-a. [DOI] [PubMed] [Google Scholar]
- 12.Johnson NR, Gannon OM, savage NW, Batstone MD. Frequency of odontogenic cysts and tumors: a systematic review. J Investig Clin Dent. 2014;5:9–14. doi: 10.1111/jicd.12044. [DOI] [PubMed] [Google Scholar]
- 13.Hadziabdic N, Dzinovic E, Udovicic-Gagula D, Sulejmanagic N, Osmanovic A, Halilovic S, Kurtovic-Kozaric A. Nonsyndromic examples of odontogenic keratocysts: presentation of interesting cases with a literature review. Case Rep Dent. 2019;2019:9498202. doi: 10.1155/2019/9498202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monteiro L, Santiago C, Amaral BD, Al-Mossallami A, Albuquerque R, Lopes C. An observational retrospective study of odontogenic cyst’s and tumours over an 18-year period in a Portuguese population according to the new WHO Head and Neck Tumour classification. Med Oral Patol Oral Cir Bucal. 2021;26:e482–e493. doi: 10.4317/medoral.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A, Rai B, Nair MA, Bhut MK. Keratecystic odontogenic tumor with impacted maximal third molar involving the right maximal antrum: an unusual case report. Indian J Dent Res. 2011;22:157–160. doi: 10.4103/0970-9290.79984. [DOI] [PubMed] [Google Scholar]
- 16.Bucci T, De Giulio F, Romano A, Insabato L, Califano L. Cavernous haemangioma of the temporalis muscle: case report and review of the literature. Acta Otorhinolaryngol Ital. 2008;28:83–86. [PMC free article] [PubMed] [Google Scholar]
- 17.Gennaro P, Benedetti S, Cascino F, Gabriele G. Intramuscular lipoma of the temporalis muscle extending to the infratemporal fossa: surgical pitfalls and short literature review. BMJ Case Rep. 2022;15:e245465. doi: 10.1136/bcr-2021-245465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curé JK. Imaging of vascular lesions of the head and neck. Facial Plast Surg Clin North Am. 2001;9:525–549. [PubMed] [Google Scholar]
- 19.Vilanova JC, Barceló J, Villalón M. MR and MR angiography characterization of soft tissue vascular malformations. Curr Probl Diagn Radiol. 2004;33:161–170. doi: 10.1016/j.cpradiol.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Ran RK, Su LJ, Kuang PD. Diagnostic value of CT in benign and malignant soft massed of scalp. J Clin Radiol. 2013;32:327–330. [Google Scholar]
- 21.Sakamoto K, Morita K, Shimada Y, Omura K, Izumo T, Yamaguchi A. Peripheral odontogenic keratocyst associated with nevoid basal cell carcinoma syndrome: acase report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:e19–23. doi: 10.1016/j.oooo.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Grobe A, Hanken H, Blessmann M, Zustin J, Heiland M, Al-Dam A. An odontogenic keratocystictumor in the buccal space: an unusual site of origin and a review of the literature. In Vivo. 2012;26:847–851. [PubMed] [Google Scholar]
- 23.Zhu L, Yang J, Zheng JW. Radiological and clinical features of peripheral keratocystic odontogenic tumor. Int J Clin Exp Med. 2014;7:300–306. [PMC free article] [PubMed] [Google Scholar]
- 24.Pogrel MA. The keratinocystic odontogenic tumor. Oral Maxillofac Surg Clin North Am. 2013;25:21–30. doi: 10.1016/j.coms.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Chrcanovic BR, Gomez RS. Recurrence probability for keratocystic odontogenic tumors: an analysis of 6427 cases. J Craniomaxillofac Surg. 2017;45:244–251. doi: 10.1016/j.jcms.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Gosau M, Draenert FG, Müller S, Frerich B, Bürgers R, Reichert TE, Driemel O. Two modifications in the treatment of keratocystic odontogenic tumors (KCOT) and the use of Carnoy’s solution (CS)--a retrospective study lasting between 2 and 10 years. Clin Oral Investig. 2010;14:27–34. doi: 10.1007/s00784-009-0264-6. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Liu B, Cheng G, Wang SP, Wang YN. Recurrent keratocystic odontogenic tumours: report of 19 cases. Dentomaxillofac Radiol. 2012;41:96–102. doi: 10.1259/dmfr/22891281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanas N, Freund B, Schwartz M. Systematic review of the treatment and diagnosis of the odontogenic keratosystem. Oral Surg oral Med Oral Pathol Oral Radiol Endod. 2000;90:553–558. doi: 10.1067/moe.2000.110814. [DOI] [PubMed] [Google Scholar]
- 29.Borghesi A, Nardi C, Giannitto C, Tironi A, Maroldi R, Di Bartolomeo F, Preda L. Odontogenic keratocyst: imaging features of a benign lesion with an aggressive behaviour. Insights Imaging. 2018;9:883–897. doi: 10.1007/s13244-018-0644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pazdera J, Kolar Z, Zboril V, Tvrdy P, Pink R. Odontogenic keratocysts/keratocystic odontogenic tumours: biological characteristics, clinical manifestation and treatment. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158:170–174. doi: 10.5507/bp.2012.048. [DOI] [PubMed] [Google Scholar]
- 31.Rajendra Santosh AB. Odontogenic cysts. Dent Clin North Am. 2020;64:105–119. doi: 10.1016/j.cden.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Santos EM, Santos HO, Dos Santos Dias I, Santos SH, Batista de Paula AM, Feltenberger JD, Sena Guimarães AL, Farias LC. Bioinformatics analysis reveals genes involved in the pathogenesis of ameloblastoma and keratocystic odontogenic tumor. Int J Mol Cell Med. 2016;5:199–219. [PMC free article] [PubMed] [Google Scholar]
- 33.Ba K, Li X, Wang H, Liu Y, Zheng G, Yang Z, Li M, Shimizutani K, Koseki T. Are all odontogenic keratocysts keratocystic odontogenic tumors? Correlation between imaging features and epithelial cell proliferation. J Clin Imaging Sci. 2013;3:3. [Google Scholar]
- 34.Ghafouri-Fard S, Atarbashi-Moghadam S, Taheri M. Genetic factors in the pathogenesis of ameloblastoma, dentigerous cyst and odontogenic keratocyst. Gene. 2021;771:145369. doi: 10.1016/j.gene.2020.145369. [DOI] [PubMed] [Google Scholar]
- 35.Guo YY, Zhang JY, Li XF, Luo HY, Chen F, Li TJ. PTCH1 gene mutations in Keratocystic odontogenic tumors: a study of 43 Chinese patients and a systematic review. PLoS One. 2013;8:e77305. doi: 10.1371/journal.pone.0077305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adesina OM, Adebiyi KE, Effiom OA, Omoniyi-Esan GO, Owotade FJ, Fatusi OA, Kolude B, Odujoko OO, Ladeji A. Comparative Immunohistochemical Analysis of p53 and a-SMA in Ameloblastoma, AOT and OKC. West Afr J Med. 2022;39:248–255. [PubMed] [Google Scholar]
- 37.Chandrangsu S, Sappayatosok K. p53, p63 and p73 expression and angiogenesis in keratocystic odontogenic tumors. J Clin Exp Dent. 2016;8:e505–e511. doi: 10.4317/jced.52843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong WQ, Chen G, Zhang W, Xiong XP, Zhao Y, Liu B, Zhao YF. M2-polarized macrophages in keratocystic odontogenic tumor: relation to tumor angiogenesis. Sci Rep. 2015;5:15586. doi: 10.1038/srep15586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta B, Chandra S, Singh A, Sah K, Raj V, Gupta V. The role of vascular endothelial growth factor in proliferation of odontogenic cysts and tumors: an immunohistochemical study. Dent Res J (Isfahan) 2016;13:256–63. doi: 10.4103/1735-3327.182187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chacham M, Almoznino G, Zlotogorski-Hurvitz A, Buchner A, Vered M. Expression of stem cell markers in stroma of odontogenic cysts and tumors. J Oral Pathol Med. 2020;49:1068–1077. doi: 10.1111/jop.13102. [DOI] [PubMed] [Google Scholar]
- 41.Mishra H, Gulati N, Jain A, Juneja S, Shetty DC. Neuroectodermal influence of CD 99 immunoexpression correlates with the clinical behavior of odontogenic cysts and tumors. J Oral Maxillofac Pathol. 2021;25:423–429. doi: 10.4103/jomfp.JOMFP_29_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Oliveira MG, Lauxen Ida S, Chaves AC, Rados PV, Sant’Ana Filho M. Odontogenic epithelium: immunolabeling of Ki-67, EGFR and survivin in pericoronal follicles, dentigerous cysts and keratocystic odontogenic tumors. Head Neck Pathol. 2011;5:1–7. doi: 10.1007/s12105-010-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diniz MG, Duarte-Andrade FF, Stussi F, Vitório JG, Fonseca FP, Ramos Domingues R, Paes Leme AF, Gomes CC, Gomez RS. Deregulation of desmosomal proteins and extracellular matrix proteases in odontogenic keratocyst. Oral Dis. 2021;27:952–961. doi: 10.1111/odi.13598. [DOI] [PubMed] [Google Scholar]
- 44.Mahdavi N, Zavarei M, Derakhshan S, Nasab MH. Orthokeratinized odontogenic cyst: report of eight cases and review of literature regarding its malignant transformation. J Oral Maxillofac Pathol. 2021;25:S11–S17. doi: 10.4103/jomfp.JOMFP_1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makowski GJ, McGuff S, van Sickels JE. Squamous cell carcinoma in a maxillary odontogenic keratocyst. J Oral Maxillofac Surg. 2001;59:76–80. doi: 10.1053/joms.2001.19297. [DOI] [PubMed] [Google Scholar]
- 46.Siar CH, Ng KH. Squamous cell carcinoma in an orthokeratinised odontogenic keratocyst. Int J Oral Maxillofac Surg. 1987;16:95–98. doi: 10.1016/s0901-5027(87)80036-x. [DOI] [PubMed] [Google Scholar]
- 47.MacLeod RI, Soames JV. Squamous cell carcinoma arising in an odontogenic keratocyst. Br J Oral Maxillofac Surg. 1988;26:52–57. doi: 10.1016/0266-4356(88)90150-7. [DOI] [PubMed] [Google Scholar]
- 48.Kumchai H, Champion AF, Gates JC. Carcinomatous transformation of odontogenic keratocyst and primary intraosseous carcinoma: a systematic review and report of a case. J Oral Maxillofac Surg. 2021;79:1081.e1–1081.e9. doi: 10.1016/j.joms.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 49.Liu C. Malignant transformation of keratocystic odontogenic tumor: case report and review of literature. J Oral Maxillofac Surg. 2017;27:223–226. [Google Scholar]
- 50.Bereket C, Bekçioğlu B, Koyuncu M, Şener I, Kandemir B, Türer A. Intraosseous carcinoma arising from an odontogenic cyst: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:e445–9. doi: 10.1016/j.oooo.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 51.Mitchell O, Cameron M, Coghill S. Squamous cell carcinoma arising from an odontogenic keratocyst associated with an impacted upper canine. Br J Oral Maxillofac Surg. 2010;48:S25–55. [Google Scholar]
- 52.Borras-Ferreres J, Sanchez-Torres A, Gay-Escoda C. Malignant changes developing from odontogenic cysts: a systematic review. J Clin Exp Dent. 2016;8:e622. doi: 10.4317/jced.53256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chaisuparat R, Coletti D, Kolokythas A, Ord RA, Nikitakis NG. Primary intraosseous odontogenic carcinoma arising in an odontogenic cyst or de novo: a clinicopathologic study of six new cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:194–200. doi: 10.1016/j.tripleo.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 54.Ackermann GL, Altini M, Shear M. The unicystic ameloblastoma: a clinicopathological study of 57 cases. J Oral Pathol. 1988;17:541–546. doi: 10.1111/j.1600-0714.1988.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 55.Li TJ, Wu YT, Yu SF, Yu GY. Unicystic ameloblastoma: a clinicopathologic study of 33 Chinese patients. Am J Surg Pathol. 2000;24:1385–1392. doi: 10.1097/00000478-200010000-00008. [DOI] [PubMed] [Google Scholar]


