Abstract
It has been reported that antibiotics (ATBs) have adverse effect on the efficacy of treatment with immune checkpoint inhibitors (ICIs) in cancer patients. Since different classes of ATBs have different antibacterial spectrum, we aimed to study whether all ATBs had similar or different negative effects on the clinical outcomes of ICIs in patients with advanced non-small cell lung cancer (NSCLC). Patients with advanced NSCLC who received ICIs were included in this retrospective study and grouped by the class of ATBs they had used around the ICIs treatment time. The overall survival (OS) and the progression free survival (PFS) of patients among these groups were compared using Kaplan-Meier method and Cox proportional hazards model. A total of 148 eligible patients were enrolled, and 80 patients used ATBs. The results indicated that quinolones had no significant negative consequence on the clinical outcomes, while β-lactams significantly shortened the OS and PFS of patients. Furthermore, patients exposed to the combination of β-lactams and quinolones suffered the worst OS and PFS. Moreover, the subgroup analysis of β-lactams revealed that only penicillins, but not carbapenems and cephalosporins, markedly reduced both OS and PFS. In addition to the class of ATBs used, the time frame of ATBs used also affected the clinical outcomes of ICIs therapy. Patients receiving ATBs within 60 days prior to and 30 days after the initiation of ICI treatment had significantly shorter OS and PFS compared with those who did not use ATBs. This study demonstrated that different classes of ATBs had disparate negative impacts on the clinical outcomes, and the use of β-lactams, especially penicillins, should be avoided in advanced NSCLC patients who are receiving or scheduled to receive ICIs within 60 days.
Keywords: Non-small cell lung cancer, antibiotics, β-lactams, immune checkpoint inhibitor, clinical outcome
Introduction
The clinical application of immune checkpoint inhibitors (ICIs) has improved the poor prognosis of non-small cell lung cancer (NSCLC) in the last ten years, and ICIs have become the standard treatment options for patients with advanced NSCLC [1-3]. The ICIs, particularly the inhibitors of programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) axis, have significantly improved the five-year survival rate of patients with advanced NSCLC from 5% to 16% [4,5]. However, according to the statistics, the efficacies of both PD-1 and PD-L1 inhibitors vary greatly across individual NSCLC patient, and the objective response rate (RR) is only about 20% [6,7]. In recent years, a growing number of studies have revealed that gut microbiota is an important host factor associated with the efficacy of ICIs, and patients who respond to ICIs have significantly higher microbial diversity and abundance than non-responders [8,9].
Emerging evidence demonstrates that gut microbiota enhances the sensitivity of cancer patients to ICIs through multiple mechanisms. For example, Sivan A. et al. found that commensal Bifidobacterium augmented the function of dendritic cell, thereby leading to the enhanced priming and accumulation of CD8+ T cell in the tumor microenvironment [10]. Routy B. et al. reported that oral supplementation with Akkermansia muciniphila restored the efficacy of PD-1 blockade in non-responders in an interleukin-12-dependent manner through improving the recruitment of CCR9+ CXCR3+ CD4+ T lymphocytes into tumor sites [11]. These results indicate that alterations in the microbiome composition of gut may affect the therapeutic effects of ICIs. It is well known that antibiotics (ATBs), especially broad-spectrum ATBs, can affect the abundance of up to 30% of the microbial community in the gut, leading to the dramatic decreases in richness, taxonomic diversity and species evenness [12-14]. More importantly, many studies have indicated that ATBs play a detrimental role in the clinical outcomes of ICIs in cancer patients, and exposure to ATBs lead to worse overall survival (OS), progression free survival (PFS) and RR, as well as an increased risk in the progression of primary disease and in the development of resistance to ICIs [15-17].
For NSCLC, although some studies have demonstrated that ATBs are significantly associated with reduced clinical benefits from ICIs [16-19], these studies did not perform the subgroup analysis of specific ATB classes. In the study by Ahmed J. et al., they found that narrow-spectrum ATBs did not affect the RR, while broad-spectrum ATBs were significantly associated with a lower RR and a trend toward slower response time [20], which suggested that different classes of ATBs had different effects on the prognosis of NSCLC patients treated with ICIs. Therefore, it is essential to study the impact of different class of ATBs on ICIs in detail. In addition, because ATBs impose protracted changes to the gut microbiota, which can last up to several months [21], it is not sufficient to only study the effect of ATBs used concurrently or within 30 days before ICIs treatment on the therapeutic outcomes of ICIs.
In the present study, we enrolled not only NSCLC patients whose interval time between ATB use and ICI therapy was less than 30 days, but also those patients with a longer interval time of 30~60 days. We further conducted a subgroup analysis based on the interval time. More importantly, we investigated the impacts of specific classes of ATBs including quinolones, cephalosporins, penicillins and carbapenems on the efficacy of ICI therapy.
Methods
Enrollment of patients
We conducted a retrospective study of patients diagnosed with advanced NSCLC who received ICI treatments including Camrelizumab, Pembrolizumab, Toripalimab, Sintilimab, Tislelizumab, Nivolumab and Durvalumab between October 2018 and June 2021 in the Affiliated Hospital of Xuzhou Medical University. These patients had received ICIs as monotherapy or in combination with chemotherapy, radiotherapy, or anti-angiogenesis treatments. The following clinical information of enrolled patients was collected from the electronic medical records system, including gender, age, smoking history, histological types, Karnofsky performance status (KPS) score, body mass index (BMI), lines of therapy, therapeutic regimen and the date of disease progression, death or last follow-up.
The follow-up ended on December 2021, and the primary endpoints were OS and PFS. OS was calculated from the start date of the first cycle of ICI therapy to the date of death of any cause or the last follow-up in surviving participants, and PFS was defined from the start date of the first cycle of ICI administration to the date of disease progression assessed by oncologist or death, whichever occurred first.
According to the time of ATB used relative to ICI therapy started, we divided the patients into the following five groups: 30~60 days prior to ICIs treatment, within 30 days prior to ICIs treatment, concurrently, within 30 days after ICIs treatment, and 30~60 days after ICIs treatment group. Other patients were included in the ATB-untreated (non-ATB) group.
Statistical analysis
Chi-square test or Fisher’s exact test was used to analyze the relationships between clinical characteristics, which were quantified by percentages. OS and PFS curves were evaluated by the Kaplan-Meier method and compared by the log-rank test. Cox proportional hazards model was used to calculate hazard ratio (HR) and 95% confidence interval (CI). Statistical analysis was performed using GraphPad Prism software (version 8.3, Inc., San Diego, CA), and P<0.05 was considered statistically significant.
Results
Clinical data of enrolled patients
A total of 148 patients with advanced NSCLC were included in this study, and their clinical data were summarized in Table 1. Among them, 43 cases received Pembrolizumab and 42 cases received Camrelizumab, while 31 cases received Sintilimab. The number of patients who received Nivolumab, Toripalimab or Tislelizumab was 15, 9 and 5, respectively. The remaining 3 patients were treated successively with Camrelizumab and Durvalumab, Camrelizumab and Nivolumab, or Pembrolizumab and Sintilimab, respectively. Among these 148 patients, 71 patients (48.0%) received ICIs as first-line therapy, and others received ICIs as second- (56 patients, 37.8%) or third-line (21 patients, 14.2%) treatment.
Table 1.
Clinical data of enrolled patients
| Variable | Total (n=148) | ATB group (n=80) | Non-ATB group (n=68) | χ2 value | P-value |
|---|---|---|---|---|---|
| Gender, n (%) | |||||
| Male | 116 (78.4) | 68 (85.0) | 48 (70.6) | 4.505 | 0.034 |
| Female | 32 (21.6) | 12 (15.0) | 20 (29.4) | ||
| Age (years), n (%) | |||||
| <65 | 73 (49.3) | 37 (46.3) | 36 (52.9) | 0.658 | 0.417 |
| ≥65 | 75 (50.6) | 43 (53.7) | 32 (47.1) | ||
| Smoking history, n (%) | |||||
| No | 65 (43.9) | 28 (35.0) | 37 (54.4) | 5.623 | 0.018 |
| Yes | 83 (56.1) | 52 (65.0) | 31 (45.6) | ||
| Histological type, n (%) | |||||
| Adenocarcinoma | 76 (51.4) | 39 (48.8) | 37 (54.4) | 0.472 | 0.492 |
| Squamous carcinoma | 72 (48.6) | 41 (51.2) | 31 (45.6) | ||
| KPS score, n (%) | |||||
| <80 | 63 (42.6) | 35 (43.8) | 28 (41.2) | 0.100 | 0.752 |
| ≥80 | 85 (57.4) | 45 (56.2) | 40 (58.8) | ||
| BMI, n (%) | |||||
| <24 | 83 (56.1) | 46 (57.5) | 37 (54.4) | 0.142 | 0.706 |
| ≥24 | 65 43.9) | 34 (42.5) | 31 (45.6) | ||
| Treatment line, n (%) | |||||
| First-line | 71 (48.0) | 48 (60.0) | 23 (33.8) | 10.369 | 0.006 |
| Second-line | 56 (37.8) | 24 (30.0) | 32 (47.1) | ||
| Third-line | 21 (14.2) | 8 (10.0) | 13 (19.1) | ||
| Treatment regimen, n (%) | |||||
| ICI monotherapy | 61 (41.2) | 31 (38.7) | 30 (44.1) | 0.437 | 0.509 |
| ICI combined with other treatments | 87 (58.8) | 49 (61.3) | 38 (55.9) | ||
We first dichotomized the patients according to whether they used ATBs or not. A total of 80 patients (54.1%) received ATBs within 60 days before or after the first administration of ICIs (hereafter referred to as the ATB group), and the most frequently used ATBs were β-lactams and quinolones. The remaining 68 patients (45.9%) who did not receive ATBs were assigned to non-ATB group. As shown in Table 1, most clinical variables were well balanced between the ATB group and the non-ATB group except for gender, smoking history and the lines of ICIs treatment.
ATBs attenuated the efficacy of ICIs in advanced NSCLC patients
We first evaluated the impacts of ATB use on the OS and PFS of patients under ICIs therapy. As shown in Figure 1A, the use of ATBs had a detrimental effect on the OS of advanced NSCLC patients. Compared with patients in non-ATB group, the median OS (mOS) of patients in ATB group was significantly reduced from 24.2 months to 19.6 months (HR=2.285, 95% CI: 1.410~3.701, P<0.001). Additionally, we observed a shorter median PFS (mPFS) in ATB group than in non-ATB group (5.60 months vs. 9.30 months, HR=1.640, 95% CI: 1.156~2.327, P=0.004) (Figure 1B).
Figure 1.
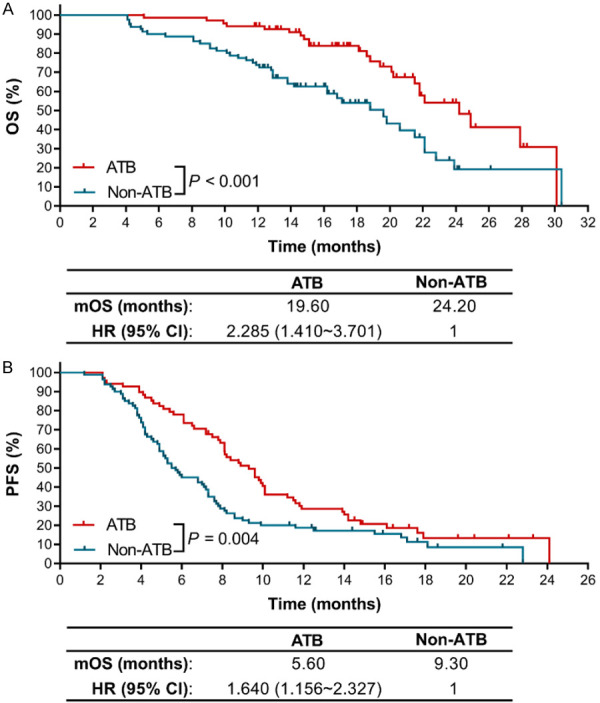
The effects of ATBs use on the clinical outcomes of ICIs therapy in advanced NSCLC patients. A. The Kaplan-Meier survival curves for OS. B. The Kaplan-Meier survival curves for PFS.
Different classes of ATBs exhibited disparate negative impacts on the OS and PFS of advanced NSCLC patients treated with ICIs
Since different classes of ATBs have different antibacterial spectrum, we speculated that not all ATBs had similar negative effects on the clinical outcomes of ICIs. In this study, the ATBs we examined were mainly quinolones, β-lactams, and a combination of the two. After a statistical analysis, we found that quinolones had no obvious harmful effect on OS (HR=1.392, 95% CI: 0.646~2.998, P=0.860) (Figure 2A) and PFS (HR=0.910, 95% CI: 0.548~1.511, P=0.718) (Figure 2B). The mOS (22.10 months) and mPFS (9.00 months) of patients in quinolones use group were similar to patients in the non-ATB group. However, β-lactams were closely associated with the attenuated therapeutic efficacy of ICIs. Patients in β-lactams use group showed a significantly reduced OS (mOS: 18.80 months) compared with the OS of patients in non-ATB group (HR=1.974, 95% CI: 0.901~4.323, P=0.029) (Figure 2A). Similar effect of β-lactams on the PFS of patients was also observed (mPFS: 5.20 months, HR=2.138, 95% CI: 1.215~3.763, P<0.001) (Figure 2B). Furthermore, compared with patients in non-ATB group, patients in the group of using a combination of β-lactams and quinolones suffered the worst OS (mOS: 12.45 months; HR=4.811, 95% CI: 2.040~11.350, P<0.001) (Figure 2A) and PFS (mPFS: 4.55 months; HR=2.713, 95% CI: 1.470~5.007, P<0.001) (Figure 2B).
Figure 2.
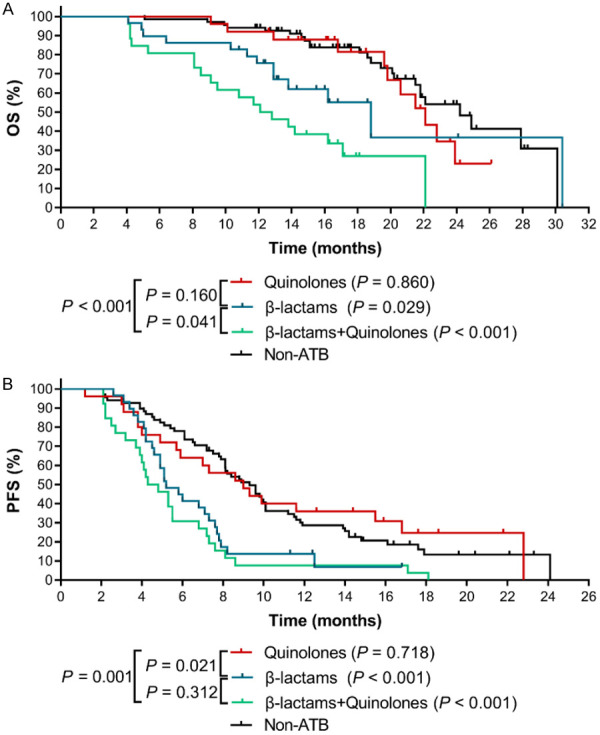
The effects of different classes of ATBs on the clinical outcomes of ICIs therapy in advanced NSCLC patients. A. The Kaplan-Meier survival curves for OS. B. The Kaplan-Meier survival curves for PFS. The P value in bracket represents the statistical difference of the corresponding group compared with the Non-ATB group.
In addition, we found that, compared with quinolones, β-lactams exerted the most remarkable negative impact on PFS (HR=1.919, 95% CI: 1.061~3.471, P=0.021) (Figure 2B) and a marginal effect on OS (HR=1.695, 95% CI: 0.752~3.823, P=0.160) (Figure 2A). Strikingly, the mOS and mPFS of quinolones group were almost doubled compared with those in β-lactams and quinolones combination group, demonstrating a significant adverse effect by the combination of β-lactams and quinolones (OS: HR=0.275, 95% CI: 0.128~0.592, P<0.001; PFS: HR=0.402, 95% CI: 0.218~0.740, P=0.001).
Penicillins use shortened the OS and PFS of advanced NSCLC patients treated with ICIs
We further performed a subgroup analysis of β-lactams group including the most clinically used cephalosporins, penicillins and carbapenems. The results showed that, overall, different classes of β-lactams had various effects on the therapeutic outcomes of ICIs. In general, the mOS of patients using ATBs was shorter than the mOS of patients in non-ATB group: 18.8 months for cephalosporins, 12.9 months for penicillins, and 11.9 months for carbapenems. A statistically significant difference was observed for penicillins (HR=3.076, 95% CI: 0.575~16.470, P=0.027) and carbapenems (HR=8.287, 95% CI: 0.367~187.200, P<0.001). Additionally, the adverse effect of carbapenems on OS was more severe than that of penicillins (HR=5.373, 95% CI: 0.517~55.830, P=0.004) (Figure 3A). Similarly, as presented in Figure 3B, the three types of β-lactams also caused worse PFS (the mPFS for cephalosporins group, penicillins group and carbapenems group was 5.90 months, 5.15 months and 4.90 months, respectively), and the differences were statistically significant between cephalosporins group and non-ATB group (HR=2.263, 95% CI: 1.0637~4.821, P=0.003), and between the penicillins group and non-ATB group (HR=2.434, 95% CI: 0.774~7.657, P=0.019). In sum, our data suggested that the use of β-lactams, especially penicillins, should be avoided in advanced NSCLC patients who are undergoing or will receive ICIs therapy.
Figure 3.
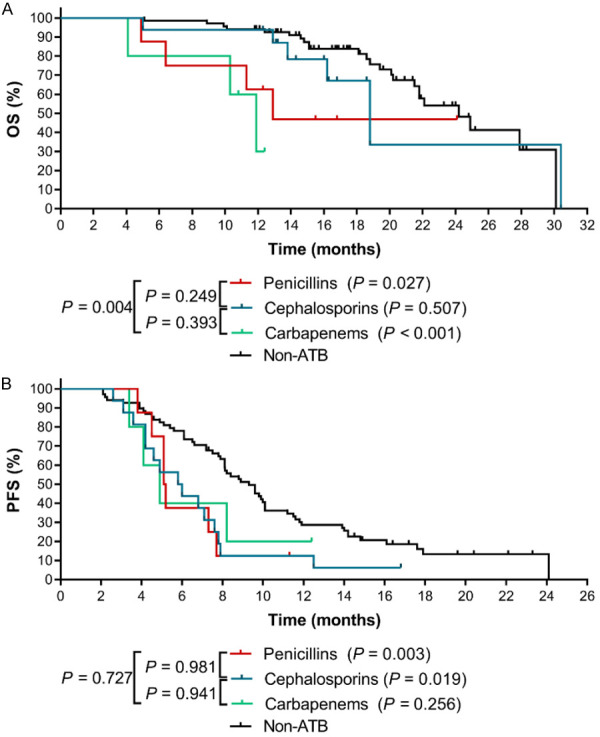
The effects of different types of β-lactams on the clinical outcomes of ICIs therapy in advanced NSCLC patients. A. The Kaplan-Meier survival curves for OS. B. The Kaplan-Meier survival curves for PFS. The P value in bracket represents the statistical difference of the corresponding group compared with the Non-ATB group.
The time frame of exposure to ATBs affected the OS and PFS of advanced NSCLC patients
Furthermore, we analyzed whether the negative association between the use of ATBs and clinical outcomes of patients under ICIs treatment was significant across all the treatment time frames (30~60 days before and after the use of ICIs, within 30 days before and after the use of ICIs, concurrent use of ICIs). Our results indicated that the mOS and mPFS of patients in concurrent ICIs group reduced by 50%, 12.9 months and 4.85 months, respectively, to those of non-ATB group (OS: HR=2.140, 95% CI: 0.897~5.103, P=0.019; PFS: HR=1.933, 95% CI: 0.990~3.777, P=0.014) (Figure 4). In addition, the mOS of patients using ATBs either 30~60 days or within 30 days before ICIs treatment was only 18.8 months and 17.1 months, respectively, which were significantly shorter than that in non-ATB group (30~60 days before ICIs group vs. non-ATB group: HR=3.523, 95% CI: 0.937~13.250, P=0.002; within 30 days before ICIs group vs. non-ATB group: HR=3.203, 95% CI: 1.327~7.727, P<0.001). The same statistical significances were also observed for PFS between 30~60 days before ICIs group (mPFS: 3.95 months) and non-ATB group (HR=2.224, 95% CI: 0.843~5.865, P=0.020), within 30 days before ICIs group (mPFS: 6.35 months) and non-ATB group (HR=1.657, 95% CI: 0.982~2.796, P=0.028).
Figure 4.
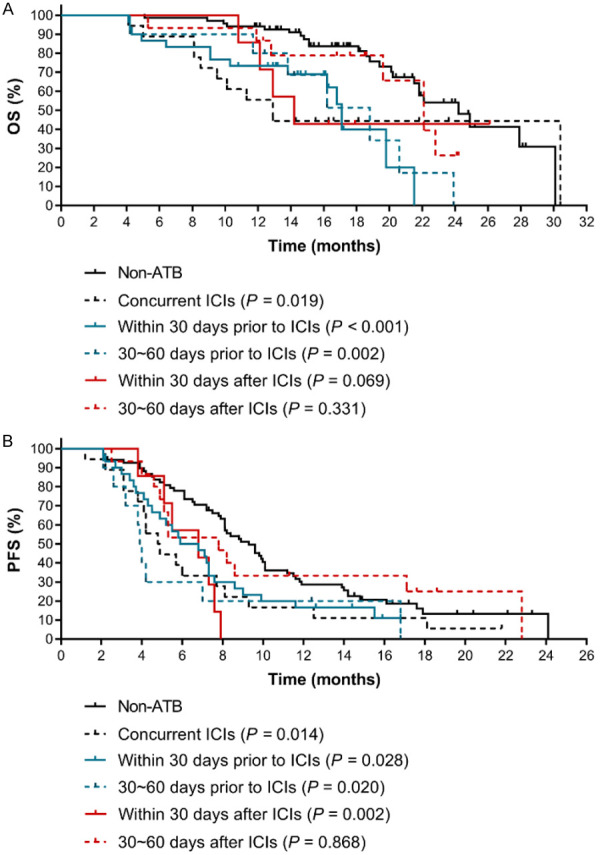
The effects of time frame of exposure to ATBs on the clinical outcomes of ICIs therapy in advanced NSCLC patients. A. The Kaplan-Meier survival curves for OS. B. The Kaplan-Meier survival curves for PFS. The P value in bracket represents the statistical difference of the corresponding group compared with the Non-ATB group.
In contrast, compared with non-ATB group, the use of ATBs between 30 and 60 days after ICIs therapy had no significant effect on the patients’ OS and PFS (mOS: 22.1 months, HR=1.500, 95% CI: 0.578~3.893, P=0.331; mPFS: 7.80 months, HR=1.053, 95% CI: 0.559~1.984, P=0.868). The mOS of patients using ATBs within 30 days after ICIs treatment was also shorter (mOS: 14.2 months), but it didn’t reach significant difference (P=0.069). However, there was a significant difference in PFS (mPFS: 6.80 months, HR=3.133, 95% CI: 0.866~11.330, P=0.002). Collectively, these results indicated that caution should be taken when using ATBs within 60 days before and 30 days after ICIs therapy to prevent from the adverse effect of ATBs on the therapeutic efficacy of ICIs.
Discussion
The clinical application of ICIs has radically changed the therapeutic paradigm for NSCLC [22]. Meanwhile, the impact of ATBs on ICIs is an emerging area that attracts special attention. Cancer patients, especially those with advanced malignant tumors and weakened immune system, are susceptible to infections and need ATB treatment. Unfortunately, it has been found that ATBs could further accelerate disease development and aggravation [23-25]. Not only did the retrospective studies based on either single-arm studies or populations of patients with advanced NSCLC treated within a single center have shown this adverse effect [17,26,27], but the pooled data from two randomized trials (NCT01903993 and NCT02008227) also suggested that the use of ATBs dramatically decreased the efficacy of ICIs [28]. However, few studies have explored whether different classes of broad-spectrum ATBs can reduce the clinical benefits of ICIs similarly or differently. This is particularly important, given that infections are contraindications to antitumor therapies, and most infections can only be cured by ATBs. It is not feasible to completely avoid ATBs in clinical practice; therefore, it is critical to use amicable and suitable ATBs to minimize the negative impacts of ATBs on ICIs’ efficacy. In this present study, we discovered that different classes of ATBs did exhibit disparate negative impacts on the OS and PFS, which provided the basis for clinical decision making when choosing ATBs to patients with advanced NSCLC treated with ICIs. In particular, we found that quinolones had little effect on OS and PFS, whereas β-lactams, especially penicillins, were significantly associated with the impaired therapeutic efficacy of ICIs. The most adverse effect was observed in patients who simultaneously used both β-lactams and quinolones, probably due to the fact that these two classes of ATBs have different antibacterial spectrum and antimicrobial activity. For instance, piperacillin/tazobactam affects enterobacteria, enterococci, bifidobacteria, eubacteria, lactobacilli, clostridia and Gram-positive cocci, but have little effect on anaerobic Gram-negative cocci and bacteroides [29]. On the other hand, quinolones deplete the Enterobacteriaceae and anaerobic bacteria including clostridia, bifidobacterial, and bacteroides species [30,31]. Mechanistically, the altered microbial community presents a substantially different repertoire of microbial-associated molecular patterns to the receptors located in immune cells [14], which leads to an altered stimulation of signal receptors, such as the Toll-like receptors that participates in the regulation of T cell differentiation, neutrophil priming, and cytokine release [32]. Furthermore, Hernandez E. et al. found that the microbiota of patients received β-lactams possessed the enzymatic activities for carbohydrate degradation, leading to the imbalance of glucose metabolism [33], which played a fundamental role in remodeling the immunosuppressive tumor microenvironment and resulting in the immune evasion [34-36]. Moreover, microbiota is known to play crucial roles in the priming and maturation of the adaptive immune system [37]. Schumann A. et al. have reported that the microbiota changes caused by Amoxicillin, a frequently used penicillin, markedly reduced the expression of major histocompatibility complex class I and II genes [38], which participates in the adaptive immune system and promotes the immune evasion of caners [39,40].
Although individual study has demonstrated that the abundance of microflora returns to their pre-antibiotic level 14 days after the ATB withdrawal [41], whether the effect of ATBs on the microbiota composition is short-term or lasts persistently for a prolonged time remains undefined [42]. Consequently, it is difficult to determine how long the use of ATBs will compromise the effect of ICIs therapy, which is a serious issue in clinical treatment of cancer patients. Combined with our findings, it is recommended that clinicians should be cautious when prescribing penicillins to patients within the time window of 60 days before and 30 days after ICIs. Therefore, our findings provide insights into using ATBs to patients under ICIs treatment.
There are several limitations in this study. First, the different routes of ATB administration (oral or intravenous), dosage and duration were important factors but were difficult to adjust in our analysis. Second, some discrepancies in clinical characteristics such as gender, smoking history and lines of therapy were observed, which might also confound our analysis. In addition, this study was a single-center, retrospective study, and the selection bias was inevitable. Furthermore, the small sample size of some subgroups would also affect the analysis results to some extent. Hence, the findings of this study need to be further validated in additional larger, multicenter and prospective clinical trials.
Conclusions
This study provided more evidence to support the view that different classes of ATBs exhibit differential effect on the clinical outcomes of ICIs therapy, and the use of β-lactams, especially penicillins, should be avoided in advanced NSCLC patients who are about to receive or undergoing ICIs therapy.
Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grant 81972845; Introduction of Specialist Team in Clinical Medicine of Xuzhou under Grant 2019TD003; Xuzhou Key Research and Development Program undere Grant KC21168.
Disclosure of conflict of interest
None.
References
- 1.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus Docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 3.Ninomiya K, Hotta K. Pembrolizumab for the first-line treatment of non-small cell lung cancer. Expert Opin Biol Ther. 2018;18:1015–1021. doi: 10.1080/14712598.2018.1522300. [DOI] [PubMed] [Google Scholar]
- 4.Banna GL, Passiglia F, Colonese F, Canova S, Menis J, Addeo A, Russo A, Cortinovis DL. Immune-checkpoint inhibitors in non-small cell lung cancer: a tool to improve patients’ selection. Crit Rev Oncol Hematol. 2018;129:27–39. doi: 10.1016/j.critrevonc.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, Powderly J, Heist R, Sequist LV, Smith DC, Leming P, Geese WJ, Yoon D, Li A, Brahmer J. Five-year follow-up of Nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J. Clin. Oncol. 2018;36:1675–1684. doi: 10.1200/JCO.2017.77.0412. [DOI] [PubMed] [Google Scholar]
- 6.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukuya T, Carbone DP. Predictive markers for the efficacy of anti-PD-1/PD-L1 antibodies in lung cancer. J Thorac Oncol. 2016;11:976–988. doi: 10.1016/j.jtho.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, Boselli L, Routier E, Cassard L, Collins M, Vaysse T, Marthey L, Eggermont A, Asvatourian V, Lanoy E, Mateus C, Robert C, Carbonnel F. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28:1368–1379. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 10.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragon L, Jacquelot N, Qu B, Ferrere G, Clemenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 12.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6:1543. doi: 10.3389/fmicb.2015.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinato DJ, Gramenitskaya D, Altmann DM, Boyton RJ, Mullish BH, Marchesi JR, Bower M. Antibiotic therapy and outcome from immune-checkpoint inhibitors. J Immunother Cancer. 2019;7:287. doi: 10.1186/s40425-019-0775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, Brock C, Power D, Hatcher O, Falconer A, Ingle M, Brown A, Gujral D, Partridge S, Sarwar N, Gonzalez M, Bendle M, Lewanski C, Newsom-Davis T, Allara E, Bower M. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5:1774–1778. doi: 10.1001/jamaoncol.2019.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC, Chaft JE, Rouche JA, Zitvogel L, Zalcman G, Albiges L, Escudier B, Routy B. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29:1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouaknine Krief J, Helly de Tauriers P, Dumenil C, Neveux N, Dumoulin J, Giraud V, Labrune S, Tisserand J, Julie C, Emile JF, Chinet T, Giroux Leprieur E. Role of antibiotic use, plasma citrulline and blood microbiome in advanced non-small cell lung cancer patients treated with nivolumab. J Immunother Cancer. 2019;7:176. doi: 10.1186/s40425-019-0658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao S, Gao GH, Li W, Li XF, Zhao C, Jiang T, Jia YJ, He YY, Li AW, Su CX, Ren SX, Chen XX, Zhou CC. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2019;130:10–17. doi: 10.1016/j.lungcan.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed J, Kumar A, Parikh K, Anwar A, Knoll BM, Puccio C, Chun H, Fanucchi M, Lim SH. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. Oncoimmunology. 2018;7:e1507670. doi: 10.1080/2162402X.2018.1507670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon MY, Yoon SS. Disruption of the gut ecosystem by antibiotics. Yonsei Med J. 2018;59:4–12. doi: 10.3349/ymj.2018.59.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, D’Amico TA, DeCamp MM, Dilling TJ, Dobelbower M, Doebele RC, Govindan R, Gubens MA, Hennon M, Horn L, Komaki R, Lackner RP, Lanuti M, Leal TA, Leisch LJ, Lilenbaum R, Lin J, Loo BW Jr, Martins R, Otterson GA, Reckamp K, Riely GJ, Schild SE, Shapiro TA, Stevenson J, Swanson SJ, Tauer K, Yang SC, Gregory K, Hughes M. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 23.Lange K, Buerger M, Stallmach A, Bruns T. Effects of antibiotics on gut microbiota. Dig Dis. 2016;34:260–268. doi: 10.1159/000443360. [DOI] [PubMed] [Google Scholar]
- 24.Meng CT, Bai CM, Brown TD, Hood LE, Tian Q. Human gut microbiota and gastrointestinal cancer. Genomics Proteomics Bioinformatics. 2018;16:33–49. doi: 10.1016/j.gpb.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lurienne L, Cervesi J, Duhalde L, de Gunzburg J, Andremont A, Zalcman G, Buffet R, Bandinelli PA. NSCLC immunotherapy efficacy and antibiotic use: a systematic review and meta-analysis. J Thorac Oncol. 2020;15:1147–1159. doi: 10.1016/j.jtho.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Huemer F, Rinnerthaler G, Lang D, Hackl H, Lamprecht B, Greil R. Association between antibiotics use and outcome in patients with NSCLC treated with immunotherapeutics. Ann Oncol. 2019;30:652–653. doi: 10.1093/annonc/mdz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalabi M, Cardona A, Nagarkar DR, Dhawahir Scala A, Gandara DR, Rittmeyer A, Albert ML, Powles T, Kok M, Herrera FG imCORE working group of early career investigators. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol. 2020;31:525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Nord CE, Brismar B, Kasholm-Tengve B, Tunevall G. Effect of piperacillin/tazobactam treatment on human bowel microflora. J Antimicrob Chemother. 1993;31(Suppl A):61–65. doi: 10.1093/jac/31.suppl_a.61. [DOI] [PubMed] [Google Scholar]
- 30.de Lastours V, Fantin B. Impact of fluoroquinolones on human microbiota. Focus on the emergence of antibiotic resistance. Future Microbiol. 2015;10:1241–1255. doi: 10.2217/fmb.15.40. [DOI] [PubMed] [Google Scholar]
- 31.Bhalodi AA, van Engelen TSR, Virk HS, Wiersinga WJ. Impact of antimicrobial therapy on the gut microbiome. J Antimicrob Chemother. 2019;74:i6–i15. doi: 10.1093/jac/dky530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ubeda C, Pamer EG. Antibiotics, microbiota, and immune defense. Trends Immunol. 2012;33:459–466. doi: 10.1016/j.it.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernandez E, Bargiela R, Diez MS, Friedrichs A, Perez-Cobas AE, Gosalbes MJ, Knecht H, Martinez-Martinez M, Seifert J, von Bergen M, Artacho A, Ruiz A, Campoy C, Latorre A, Ott SJ, Moya A, Suarez A, Martins dos Santos VA, Ferrer M. Functional consequences of microbial shifts in the human gastrointestinal tract linked to antibiotic treatment and obesity. Gut Microbes. 2013;4:306–315. doi: 10.4161/gmic.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic instruction of immunity. Cell. 2017;169:570–586. doi: 10.1016/j.cell.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganapathy-Kanniappan S. Linking tumor glycolysis and immune evasion in cancer: emerging concepts and therapeutic opportunities. Biochim Biophys Acta Rev Cancer. 2017;1868:212–220. doi: 10.1016/j.bbcan.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H. Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol. 2020;10:572912. doi: 10.3389/fcimb.2020.572912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schumann A, Nutten S, Donnicola D, Comelli EM, Mansourian R, Cherbut C, Corthesy-Theulaz I, Garcia-Rodenas C. Neonatal antibiotic treatment alters gastrointestinal tract developmental gene expression and intestinal barrier transcriptome. Physiol Genomics. 2005;23:235–245. doi: 10.1152/physiolgenomics.00057.2005. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto K, Venida A, Yano J, Biancur DE, Kakiuchi M, Gupta S, Sohn ASW, Mukhopadhyay S, Lin EY, Parker SJ, Banh RS, Paulo JA, Wen KW, Debnath J, Kim GE, Mancias JD, Fearon DT, Perera RM, Kimmelman AC. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature. 2020;581:100–105. doi: 10.1038/s41586-020-2229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhatchinamoorthy K, Colbert JD, Rock KL. Cancer immune evasion through loss of MHC class I antigen presentation. Front Immunol. 2021;12:636568. doi: 10.3389/fimmu.2021.636568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adamsson I, Edlund C, Sjostedt S, Nord CE. Comparative effects of cefadroxil and phenoxymethylpenicillin on the normal oropharyngeal and intestinal microflora. Infection. 1997;25:154–158. doi: 10.1007/BF02113603. [DOI] [PubMed] [Google Scholar]
- 42.Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - a systematic review. J Infect. 2019;79:471–489. doi: 10.1016/j.jinf.2019.10.008. [DOI] [PubMed] [Google Scholar]


