Abstract
The population of patients with end‐stage renal disease is rapidly growing and hemodialysis remains the most common treatment option. We present a case of a young patient with arteriovenous fistula (AVF)‐related heart failure, and a review of the main hemodynamic changes after AVF formation and ligation procedures.
Keywords: arteriovenous fistula, dialysis, heart failure
Short abstract
AVF is the gold standard vascular access for hemodialysis. It has the longest potency, is cost‐effective, and carries low risk of infections. We present a review of hemodynamic changes after AVF formation and ligation procedure. A decision for vascular access, as well as dialysis modality, should be individualized.
1. INTRODUCTION
Despite the fact that current prevalence of heart failure (HF) worldwide does not exceed 1% of general population, the incidence of this condition dramatically changes in patients with end‐stage renal disease (ESRD). 1 According to Kottgen et al., 2 ESRD is accompanied by HF in up to 21% of cases. The global hemodialysis (HD) population is rapidly growing and arteriovenous fistula (AVF) remain the gold standard vascular access for dialysis. 3 We report a case of a patient with AVF‐related HF, and a review of the main hemodynamic changes after AVF formation and ligation procedures.
2. CASE PRESENTATION
A 31‐year‐old woman developed ESRD due to diabetic nephropathy. A left brachiocephalic AVF was created and shortly after fistula maturation, HD was initiated.
One year later, the patient was referred to our clinic for general evaluation before renal transplantation. However, she complained of tachycardia, dyspnea on exercise, and general weakness. These symptoms progressed during the last 3 months. Ultrasound investigation revealed increase of the mean volume flow from 1300 to 2200 ml/min in 6 months period. Cardiovascular instability during dialysis was not seen. However, episodes of hypertension at the end of the session were observed. Upon cardiologic evaluation, high levels of BNP (4054.4 ng/L) and NT pro –BNP (>100,000 ng/L) were found. According to echocardiography, all parts of the heart were dilated, signs of left ventricular diastolic and systolic dysfunction were observed, and moderate increase in pulmonary artery pressures was recorded (Table 1). Although dobutamine stress test was negative, it showed severely damaged systolic function upon rest. The patient was assigned to NYHA III functional class according to the performance on spiroergometry. Factors such as chronotropic failure (heart rate increased to 100 beats per minute), left ventricular failure, anemia (hemoglobin 110 g/L) and aneurysmal dilatation of AVF were responsible for that.
TABLE 1.
Echocardiogram parameters before and after the ligation of aneurysmal fistula
| Measurement | Before arteriovenous fistula (AVF) closure | After AVF closure |
|---|---|---|
| LV EF (%) | 25 | 34 |
| LV end‐diastolic diameter (cm) | 5.5 | 5 |
| LV end‐systolic diameter (cm) | 4.8 | 4.3 |
| E/A | 3.47 | 1.53 |
| LVMI (g/m2) | 142 | 138 |
| LV length × Width (cm) | 6.6 × 4.7 | 6.7 × 4.7 |
| PA pressure (mmHg) | 51 | 43 |
| RV length × Width (cm) | 6 × 3 | 6.0 × 3.9 |
Abbreviations: E/A, peak velocity in early/late diastole; LV EF, left ventricular ejection fraction; LV, left ventricular; LVMI, left ventricular mass index; PA, pulmonary artery; RV, right ventricular.
We made the decision to liquidate AVF. AVF ligation surgery was successful, the patient was shortly discharged (Figure 1). Tunneled central venous catheter was inserted to ensure vascular access for HD.
FIGURE 1.
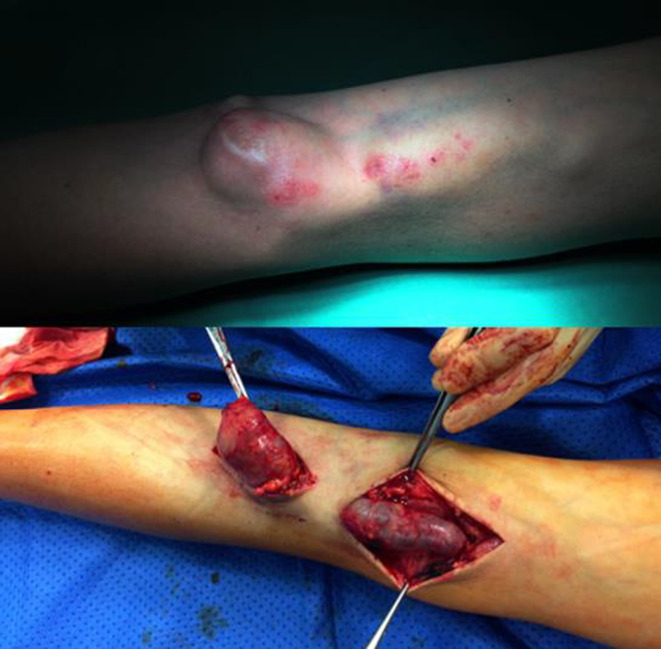
Left brachiocephalic arteriovenous fistula before (upper photo) and during (lower photo) surgery
Upon control visit, 1 month later, the obtained echocardiography results showed improvement, compared with initial investigation (Table 1). The patient was listed on the transplant waitlist and transplanted after 12 months.
3. DISCUSSION
The risk of worsened cardiac decompensation in patients with ESRD after the formation of AVF has been discussed in previous studies. 4 , 5 However, de novo developed HF after HD via AVF access is less common in clinical practise. We discuss a case of a young patient who did not show any signs of cardiac decompensation before kidney replacement therapy. Nevertheless, shortly after the start of HD, typical symptoms of HF emerged. Cardiac evaluation revealed high level of BNP and NT‐proBNP, which are prognostic markers of HF in patients with ESRD. 6 , 7 Furthermore, echocardiographic evaluation revealed LV hypertrophy, reduced LVEF. Zanib et al. 8 observed the frequency of LV hypertrophy among HD patients based on Framingham, Sokolow‐Lyon and Cornell criteria and the results showed the incidence rate of 40%, 32% and 25% respectively. Kuwahara et al. 9 findings suggest that type II diabetes has a significant impact for LV dysfunction and thus causes HF in pre‐dialysis patients. In our case, the patient developed ESRD due to diabetic nephropathy. However, no symptoms of HF pre‐dialysis were noted. After comprehensive clinical examination, the decision to ligate the AVF was made to prevent further complications related to dialysis. One month after successful surgery, control echocardiography was performed showing the improvement in LV parameters compared with initial investigation (Table 1).
4. CONCLUSION
Newly formed AVF causes significant hemodynamic alterations among HD patients. These patients already have structural and functional heart and circulatory system changes because of uremia, anemia, hyperphosphatemia, and volume overload. Therefore, a decision for vascular access, as well as dialysis modality, must be individualized.
AUTHOR CONTRIBUTIONS
All authors contributed equally to the manuscript and read and approved the final version of the manuscript.
FUNDING INFORMATION
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST
The authors have no conflict of interest in the subject matter or materials discussed in this manuscript.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor‐in‐Chief of this journal upon request.
ACKNOWLEDGEMENT
None.
Samsone VG, Rimsevicius L, Kantauskaite M, et al. Improved heart failure after closure of arteriovenous fistula. Clin Case Rep. 2022;10:e06184. doi: 10.1002/ccr3.6184
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Lippi G, Sanchis‐Gomar F. Global epidemiology and future trends of heart failure. AME Med J. 2020;5:15. [Google Scholar]
- 2. Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol. 2007;18(4):1307‐1315. [DOI] [PubMed] [Google Scholar]
- 3. Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10):573‐585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banerjee D, Rosano G, Herzog CA. Management of heart failure patient with CKD. Clin J Am Soc Nephrol. 2021;16(7):1131‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel RB, Fonarow GC, Greene SJ, et al. Kidney function and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol. 2021;78(4):330‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fisher C, Berry C, Blue L, Morton JJ, McMurray J. N‐terminal pro B type natriuretic peptide, but not the new putative cardiac hormone relaxin, predicts prognosis in patients with chronic heart failure. Heart. 2003;89(8):879‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Masson S, Latini R, Anand IS, et al. Direct comparison of B‐type natriuretic peptide (BNP) and amino‐terminal proBNP in a large population of patients with chronic and symptomatic heart failure: The Valsartan Heart Failure (Val‐HeFT) Data. Clin Chem. 2006;52(8):1528‐1538. [DOI] [PubMed] [Google Scholar]
- 8. Zanib A, Anwar S, Saleem K, Khan HMW, Zafar S. Frequency of left ventricular hypertrophy among patients on maintenance hemodialysis by voltage criteria and its relationship with biophysical‐chemical parameters. Cureus. 2020;12(3):e7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuwahara M, Ishigami J, Shikuma S, et al. Type II diabetes mellitus is a risk factor for heart failure in pre‐dialysis patients. Ther Apher Dial. 2012;16(6):541‐547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.


