Abstract
The aim of this systematic review and meta-analysis was to estimate the relationship between cumulative adverse childhood experiences (ACEs) and myocardial infarction (MI) in adulthood and to examine the role of potential confounding factors that may have contributed to the association. Studies examining the association of cumulative ACEs with MI among adults were identified by searching PubMed/MEDLINE, PsycINFO, ScienceDirect, and ProQuest Dissertations and Thesis. Individual estimates of odds ratios were pooled using random effects meta-analysis. Articles were pooled separately according to whether findings were adjusted for sociodemographic factors, cardiovascular disease (CVD) risk factors, and psychological factors. Several moderators were also examined: age, gender, race/ethnicity, type of MI assessment, type of cumulative ACEs assessment, and quality assessment of included studies. A total of 10 eligible studies met our inclusion criteria. The pooled ORs for the magnitude of the relationship between ACEs and MI were OR = 1.88; 95% CI, 1.40–2.53, before adjustment for CVD risk factors, and OR = 1.78; 95% CI, 1.24–2.57, after adjustment for CVD risk factors. The association between ACEs and MI was OR = 2.09; 95% CI, 1.43–3.06, after further adjustment for psychological factors. Effect sizes were larger when studies included participants predominantly over 55 years of age than younger participants. Cumulative ACEs is associated with an increased risk of MI in adulthood. However, further prospective studies are needed to better understand potential moderators that attenuate or amplify observed relations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40653-021-00404-7.
Keywords: Cardiac disease, Childhood trauma, Cumulative trauma, Meta-analysis, Myocardial infarction, Systematic review
ACEs are some of the most intensive stressors and traumatic events that occur during the first 18 years of a child’s life. They may be defined and characterized as recurring childhood maltreatment (physical, emotional, and sexual abuse, and neglect), household dysfunction (witnessing violence between parents or caregivers, parental separation, incarceration or substance abuse of a household member, mental illness, and attempted or complete suicide by a family member), and community violence that deprive the sense of stability, safety and development of a child (World Health Organization [WHO], 2018). In 1998, Felitti and his colleagues pioneered new thinking in an emerging area of research with their landmark study in which they investigated the impact of adverse childhood experiences (ACEs) on later-life health and well-being (Felitti et al., 1998). The seminal work would pave the way for a series of subsequent studies that have each contributed to our understanding of the harmful effects of ACEs on mental and physical health in adulthood, including the relationship between these experiences and increased risk for poor cardiovascular health outcomes in adulthood. Despite the well-documented associations between the individual contribution of some form of ACEs (physical abuse, emotional abuse, and neglect) and cardiovascular diseases (CVD) in adulthood (Norman et al., 2012), there are no systematic reviews summarizing specifically the relationships between cumulative ACEs and the onset of MI (or heart attack); a coronary heart disease that affects nearly 790.000 people each year, in the United States (Benjamin et al., 2017).
Given ample evidence demonstrating that multiple form of maltreatment frequently co-occur (Dong et al., 2004a, b), a better understanding of how cumulative ACEs influence MI risk is of high importance as it could potentially play a critical role in assessing cardiovascular disease risk. Thus, the primary aim of the present review is to estimate the association between cumulative ACEs and the risk of onset of MI in adulthood. A secondary aim is to evaluate factors that may modify this association, including participants’ demographic characteristics (age, gender, race/ethnicity), type of ACEs measurement, type of assessment of MI, and adjustment or not for CVD risk factors or mental health factors. Finally, we conclude by discussing identified limitations of the data collected and suggest future research directions.
Method
Protocol and Registration
This systematic review was registered with PROSPERO (Number: CRD42020184362) in an effort to avoid unnecessary replication of this project.
Study Identification and Selection
A literature search for all articles pertaining to these two issues was performed according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines. Three electronic bibliographic databases (PubMed/Medline, PsycINFO and ScienceDirect) were consulted using the following keywords/phrases in various combinations: ‘adverse childhood experiences’ OR ‘cumulative ACEs’ OR ‘early trauma’ OR ‘childhood abuse’ AND ‘myocardial infarction’ OR ‘heart attack’ OR ‘MI’ OR ‘acute coronary syndrome’, and all equivalent terms in the French language. ProQuest Dissertations and Theses will also be investigated for unpublished trials (a form of grey literature).
Study Inclusion Criteria and Evaluation
The articles selected for this review included cross-sectional and prospective studies, published in English or French between January 1, 1988 and August 31, 2019. Publications on adult myocardial infarction (MI) involving measures for cumulative ACEs (i.e., an index that included at last three adverse childhood experiences) were included. Investigations on MI in children or adolescents were excluded, as well as studies on other forms of cardiovascular disease such as stroke, congenital heart disease or high blood pressure. We additionally excluded investigations on relatives of people who have suffered from MI or those studies that used animal models. We excluded also studies that measured single items of adversity (e.g., sexual abuse). It should be noted that some studies refer to MI as acute coronary syndrome (ACS) or ischemic heart disease (IHD). For the purposes of the present review, these terms are used interchangeably.
Data Extraction and Analysis
All articles identified by the initial search strategy were evaluated by two authors (MJS and MJB) to ensure that inclusion criteria were met. Data extraction for the included studies was then performed by the first author (MJS). The following aspects of each study were then identified, abstracted, and analyzed: 1) first author, year of publication, country of publication, 2) sample size; sample demographics (age, sex [i.e., % of women], race/ethnicity [i.e., % of white/no Hispanic]) 3) study design (prospective vs. retrospective report of MI); 4) methods of assessment performed to measure cumulative ACEs and whether cumulative adversity measure included abuse household dysfunction (defined as domestic violence, substance use, mental illness, and incarceration), 5) measurement of MI (independently-assessed disease [i.e., outcomes assessed in a laboratory and/or medical setting or diagnosis made by a healthcare provider and indexed in national registers, hospital registers, or medical records]) vs. self-report of a previous diagnosis made by a physician or other healthcare provider); and 6) type of covariates included in analytic models, including traditional risk factors for CVD (e.g., body mass index (BMI), smoking, alcohol use, diet, physical activity, diabetes), and psychological risk factors for CVD (e.g., depression). A list of study characteristics for investigations conducted on the relationship between cumulative ACEs and subsequent MI are presented in two tables (Supplementary Tables S1 and S2).
Statistical Methods
To examine the association between cumulative ACEs and MI, a meta-analysis was carried out when there were more than three studies; otherwise, findings were described narratively. Effect sizes were extracted and reported as odds ratios (OR), or as hazard ratios (HR) and the related 95% confidence intervals (95% CIs) were extracted from or calculated for each of the included studies. OR was used because 9 of the available studies used it to estimate associations and HR was used because 1 study used HR in their results. Given differences in the interpretation of OR versus HR and the inability to harmonize these effect sizes statistically, these effects were pooled separately, as has been the case in another meta-analysis (Bhattacharjee et al., 2013). The direction of effects was consistently coded such that values indicate a relationship between increased exposure to cumulative ACEs and greater risk of MI.
It is common for studies examining cumulative ACEs to test multiple comparisons within the same group (e.g., 0 adversities vs. 1–2 adversities vs. 3–4 adversities), then the most extreme comparison was retained. Eight studies used an extreme groups approach with a reference group of 0 adversities (OR studies: k = 7; HR studies: k = 1). Only two studies did not use the extreme group approach. Instead, these studies analysed dose–response associations between a total ACEs severity score from an adversity scale without a reference group and MI.
We generated forest plots showing the ORs and 95% CIs for each study, and the overall random-effects pooled estimate. We used visual inspection of forest plots and conducted the Cochran Q test and the I2 statistic to evaluate heterogeneity among studies. The values of I2 metric 25%, 50%, and 75% were considered as low, medium, and high heterogeneity. If there were significant heterogeneity among studies (≥ 50%), then moderators were examined as a potential explanation for variability of effect size. To test for sources of heterogeneity for our primary outcome, we performed mixed-effects subgroup analyses, in which random-effects models were used to combine studies within subgroups, that compared pooled ORs separately for articles by quality assessment (poor, fair, or good quality), types of patients (e.g., age, gender, race/ethnicity), ACEs measurement, method of MI measurement, and inclusion of possible confounders in their adjusted analyses. More specifically, we pooled studies according to inclusion of 1) sociodemographic factors, 2) sociodemographic factors and risk factors for CVD, and 3) all this factors and psychological factors as a covariate.
We explored risk of publication bias using visual inspection of funnel plots (plots of effect estimates against its standard error) when sufficient studies (at least four samples) can be included in the meta-analysis. Publication bias may lead to asymmetrical funnel plots. Inn addition, we will be assessing the potential for publication bias to have influenced the results of this meta-analysis by calculating the 'Fail-Safe N'. The ‘Fail-Safe N’ quantifies the number of null studies (i.e. studies without statistically significant results) that are needed to alter a meta-analytical result from statistically significant to statistically non-significant. The Fail-Safe N functions as a measure of robustness as it quantifies the number of studies that are needed to change results, with a low number being indicative for low robustness. If the fail-safe number exceeds the critical value of 5 k + 10 (k = number of studies), the meta-analytic finding is considered robust. Conversely, if the fail-safe number falls below this critical value, a publication bias problem may exist (Orwin, 1983; Rosenthal, 1995).
Pooled effect sizes, moderator and publication bias analyses were conducted using Comprehensive Meta-Analysis Software, Version 3.0 (Borenstein et al., 2013).
Quality Assessment
The tools used to evaluate each study were the National Heart, Lung and Blood Institute (NHLBI) Quality Assessment Tool for Observational Cohort and Cross-sectional Studies (NHLBI, 2014). The methodological criteria for evaluate cohort and cross-sectional studies included: research question, population definition, participation rate, recruitment, sample size, analysis, timeframe, exposure levels, measures and assessment, outcome measures and blinding, loss to follow-up, and confounding variables. Each study was rated as either good (i.e., most criteria met with a low risk of bias), fair (i.e., some criteria met with a moderate risk of bias), or poor (i.e., few criteria met and with a high risk of bias). Rating of studies was independently performed by two reviewers (MJS and CLT), and inconsistencies were resolved by consensus or by consulting a third researcher (MJB).
Results
The initial search identified 667 potential studies, of which 166 duplicates and publications not written in either English or French were excluded, and 461 articles were excluded after screening based on the title and abstract for eligibility. At the end of this first stage, 38 studies were retained and read in full. Nine articles, identified from the bibliographic indexes, were included. As a result of these readings, 37 articles were excluded. A total of 10 articles met the inclusion criteria and were included in the present literature review. The search strategy and reasons for excluding articles are presented in Fig. 1.
Fig. 1.
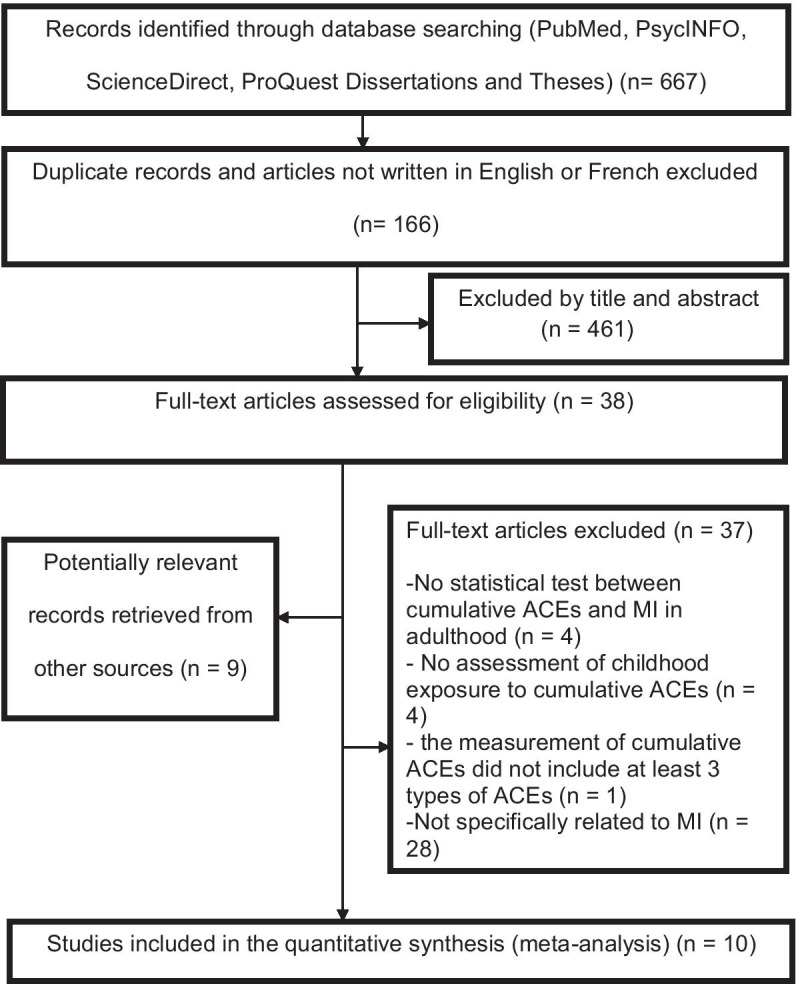
Flow diagram of the literature search strategy following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines
Characteristics of the Included Studies
Detailed characteristics of the included studies and the participants are summarized in Table 1. Ten studies included in this review examined relationship between exposure to cumulative potentially traumatic events before the age of 18 and risk of MI in adulthood: 9 OR studies (total N = 244.175), and 1 HR studies (total N = 3.882). Nine studies were conducted in the United States, and one study was conducted in United Kingdom. The samples ranged from 394 to 79.810 participants and were largely made up of women. Four studies included more than 50% of participants over 55 years old. Of the 9 studies with racial information, 8 studies included more than 60% of white, no-Hispanic participants.
Table 1.
Principal findings of the systematic literature review—Cumulative ACEs and myocardial infarction
| Study | Sample size (N), F (%), A (years range), Race/E (White/No-Hispanic) (%) | Study design, country |
Adverse experiences (in childhood or adolescence) considered Evaluation method (prospective vs. retrospective) |
Assessment instrument, prevalence (%) | Outcome, prevalence (%), measure (self-reported vs. objective) | Main findings |
|---|---|---|---|---|---|---|
| Bellis et al. (2015) |
N = 3.885 F = 54% A = 18–69 Race/E = 86% |
Cross-sectional study, representative population sample from 10 regions, United Kingdom |
Physical, sexual or verbal abuse; exposure to domestic violence, parental separation/divorce, living with a family member with mental illness, who abuses substances (alcohol, drugs) or who has been incarcerated Retrospective evaluation |
ACE questionnaire (11-item version), ACEs ≥ 4: 8.3% Self-administered measure or face-to-face home interview |
MI and coronary heart disease medically diagnosed, Self-reported measure | ACEs ≥ 4 (vs no ACEs) associated with increased risk of MI and coronary heart disease (AHR: 3.11, 95% CI:1.56–6.24, p = .001), after adjusting for demographic (age, gender, ethnicity), and socio-economic factors (economic, social and housing issues |
| Campbell et al. (2016) |
N = 48.526 F = 50% A = 18–64 Race/E = 85% |
Cross-sectional study, telephone survey conducted in 5 American states, representative sample, US |
Physical, sexual or verbal abuse; parental separation/divorce, household dysfunction (exposure to domestic violence, living with a family member with mental illness, who abuses substances or who has been incarcerated) Retrospective evaluation |
ACE questionnaire (11-item version), ACE ≥ 1: 55.4%; ACEs ≥ 4: 13.7% Telephone interview |
MI: 3.9% medically diagnosed Self-reported measure |
ACEs ≥ 4 (vs no ACEs) associated with increased incidence rate of MI (AOR: 1.86, 95% CI: 1.33–2.60; p < .001), after adjusting for socio-economic variables (age, gender, marital status, education, employment, region of U.S., and income) |
| Chanlongbutra et al. (2018) |
N = 79.810 F = 47% A = 18- ≥ 65 Race/E = 85% |
Cross-sectional study based on BRFSS, 9 American states, US | Physical, sexual or verbal abuse, parental separation/divorce, household dysfunction (exposure to domestic violence, living with a family member with mental illness, who abuses substances or who has been incarcerated) |
ACE questionnaire (11- item version), ACEs ≥ 4: 15% Self-administered measure |
MI medical diagnosis Self-reported measure |
ACEs ≥ 4 (vs no ACEs) associated with increased risk of MI in urban areas (AOR: 1.39, 95% CI: 1.08–1.79) and in rural areas (AOR: 1.35, 95% CI: 1.01–1.82), after adjusting for socio-demographic factors (age, gender, race/ethnicity, marital status, education, and family income) |
| Chou and Koenen (2019) |
N = 34.653 F = 52% A ≥ 20 Race/E = 58% |
Cross-sectional study based on NERSARC, representative sample, US |
Physical, sexual or emotional abuse; physical or emotional neglect; household dysfunction (battered mother, parental substance abuse) Retrospective evaluation |
ACE questionnaire Face-to-face computer-assisted interview |
MI medical diagnosis Self-reported measure |
ACEs ≥ 5 (vs no ACEs) associated with increased risk of MI associated with an increased risk of MI (AOR: 3.05, 95% CI 1.10–8.48), after adjusting for demographic, sociodemographic factors (age, gender, economic status), and any household dysfunction |
| Dong et al. (2004a, b) |
N = 17.337 F = 54% A (mean) = 56 Race/E = 75% |
Cross-sectional study, members of Kaiser Permanente's Health Appraisal Center, San Diego, CA, US |
Physical, sexual or emotional abuse; physical or emotional neglect; household dysfunction (exposure to domestic violence, living with a family member with mental illness, who abuses substances or who has been incarcerated) Retrospective evaluation |
ACE questionnaire ACEs ≥ 7: 20.1% Self-administered measure |
Ischemic heart disease (MI, chest pain, severe chest pain during exercise, taking nitroglycerin): 10.6% Self-reported measure |
ACEs ≥ 7 (vs no ACEs) associated with increased risk of IHD, after adjusting for sociodemographic covariates (age, gender, race, and education) (AOR: 3.6, 95% CI 2.4–5.3), after adjusting for socio-demographic factors and traditional CVD risk factors (smoking, physical inactivity, BMI, diabetes, HTA) (AOR: 3.1, 95% CI 2.0–4.6), and after further adjustment for psychological factors (anger and depressive affects) (AOR: 2.6, 95% CI 1.7–3.9) |
| Felitti et al. (1998) |
N = 8.056 F = 52% A (mean) = 56 Race/E = 79% |
Cross-sectional study, members of the Kaiser Permanente's Health Appraisal Center Clinic, San Diego, US |
Physical, sexual or psychological abuse; household dysfunction (exposure to domestic violence, living with a family member with mental illness, who abuses substances or who has been incarcerated) Retrospective evaluation |
ACE questionnaire, ACEs ≥ 4: 6.2% Self-administered measure |
Ischemic heart disease (MI, chest pain, severe chest pain during exercise, taking nitroglycerin): 3.8% Self-reported measure |
ACEs ≥ 4 (vs no ACEs) associated with an increased risk of CVD (AOR: 2.2, 95% CI: 1.3–3.7; p < .05), after adjusting for socio-demographic factors (age, gender, race, and educational attainment) |
| Gilbert et al. (2015) |
N = 53.988 F = 60% A ≥ 18 Race/E = 80% |
Cross-sectional study, telephone survey conducted in 5 American states, representative population sample, US |
Physical, sexual or emotional abuse; household dysfunction (parental separation/divorce, exposure to domestic violence, living with a family member with mental illness, who abuses substances (alcohol, drugs) or who has been incarcerated Retrospective evaluation |
ACE questionnaire ACEs ≥ 4: 15.3% Telephone interview |
MI medical diagnosis Self-reported measure |
ACEs ≥ 7 (vs no ACEs) associated with an increased risk of MI (AOR: 1.9, 95% CI: 1.1–3.4), after adjusting for socio-demographic factors (age, race/ethnicity, income, and educational level) |
| Iniguez and Stankowski (2016) |
N = 800 F = 58% A = 18–65 Race/E = 98% |
Cross-sectional study, telephone survey, Marshfield Clinic patients, WI, US |
Physical, sexual or emotional abuse; physical or emotional neglect; household with violence, mental illness, alcohol or drug abuse, incarceration, parental separation or divorce Retrospective evaluation |
ACE questionnaire ACEs ≥ 4: 15% Telephone interview |
Patient medical records show medical diagnosis of MI Objective measure |
ACEs ≥ 4 (vs no ACE) are associated with lower risk of MI (OR: 0.62, 95% CI 0.29–1.35) |
| Rooks et al. (2015) |
N = 562 F = 0% A (mean) = 55 Race/E = 98% |
Cross-sectional study, data from Emory Twin Study, USA |
Physical, sexual, or emotional abuse; exposure to other types of trauma (i.e., natural disasters), before age 18 Retrospective evaluation |
ETI questionnaire Self-administered measure |
MI: 10.8%, coronary revascularization: 12.5% Objective measure |
Comparing across twin pairs, each increasing ETI quartile level is associated with higher odds of MI and coronary revascularization (AOR: 1.76, 95% CI: 1.26–2.45; p < .05), before and after adjustment for socio-demographic factors, substance abuse (tobacco, alcohol, drugs), physical inactivity, BMI, depression, and PTSD |
| Roy et al. (2010) |
N = 443 F = 60% A (mean) = 29 Race/E = 0% |
Prospective study, African-American patients with type 1 diabetes, USA |
Physical, sexual or emotional abuse; physical or emotional neglect, before age 18 Retrospective evaluation |
CTQ questionnaire, Self-administered measure |
Occurrence of MI and angina pectoris (chest pain) over a 6-year period: 12.9% Objective measure: medical record review |
• Each additional trauma is associated with an increased risk of MI and angina pectoris (AOR: 1.35; 95% CI: 1.08–1.70; p < .008), after adjusting for age, BMI, and proteinuria |
A Age, ACE Adverse Childhood Experiences, AHR Adjusted hazard ratio, AOR Adjusted odds ratio, BRFSS Behavioral Risk Factor Surveillance Survey, BMI Body Mass Index, CI Confidence interval, CTQ Childhood Trauma Questionnaire, CTS Conflict Tactics Scale, CVD Cardiovascular disease, ETI Early Trauma Inventory, F female, HTA Arterial hypertension, IHD Ischemic heart disease, M male, MI Myocardial infarction, N Total sample size, NERSAC National Epidemiologic Study of Alcohol and Related Conditions, PTSD Posttraumatic stress disorder, Race/E Race/Ethnicity, R-CTS Revised Conflict Tactics Scale
Evaluation of Measures
All studies evaluated cumulative ACEs retrospectively using validated assessment instruments. These included the Adverse Childhood Experiences (ACEs) developed by Kaiser Permanent and the Centers for Disease (Centers for Disease Control and Prevention, 2020), the Childhood Trauma questionnaire (CTQ), and the Early Trauma Inventory – Self Report (ETI-SR) for measuring childhood abuse (Bremner et al., 2000). Cumulative ACE were systematically studied in relation to the assessment of subsequent occurrence of MI. Assessments of this cardiovascular event largely rely on patient self-reporting (k = 8) and less frequently medically confirmed on the basis of physician reports, included in the patient's medical record (k = 2). A number of studies additionally included assessments of CVD risk factors (k = 3), and assessments of psychological factors such as depression or posttraumatic stress disorder (PTSD) (k = 2) most likely to be involved in the association between exposure to potentially traumatic events and increased risk of subsequent MI. Nine of these studies were cross-sectional and only one measured MI prospectively (see Supplementary Table S2).
Evaluation of Quality of Studies and Risk of Bias
Results from evaluation of quality of studies are presented in Supplementary Table S3. Most of the studies were of intermediate quality (90%; 9/10), and 10% (1/10) were of high quality. The main limitations of the studies were the lack of objective outcome measures (cardiac events were self-reported by participants in 8 studies), and the retrospective assessment of the ACEs in all studies, and of MI in 9 of 10 studies with respect to the risk of recall. Only one study reported that the outcome assessors blinded to the exposure status of participants. Considering ACEs as the exposure status of participants, and MI as a consequence of exposure status, blinding of outcome assessors to the exposure status of participants was not possible in most studies because participants are themselves outcome assessors (they provide self-reported outcome data and assess whether or not the cardiac event occurred) and are aware of their exposure status (they provide self-reported ACEs). Common strengths included accurate description of objectives, large sample sizes, and inclusion of several covariates in the analytic models.
Principal Findings
The principal findings of studies that examined the association between cumulative ACEs and MI in adulthood are shown in Table 1. These results are presented in several statistical formats such as hazard ratio (HR), or odd ratio (OR). Only one study reported HR findings (Bellis et al., 2015). In this study conducted on a sample of 3.882 participants representative of the UK population, including 54% women, a statistically significant association was observed between cumulative ACEs and MI, after adjustment for demographic and socioeconomic factors (HR = 2.11; 95% CI, 1.59–6.24). Nine studies reported results in the form of odd ratios (ORs). Eight of these studies reported results unadjusted or adjusted for sociodemographic variables (age, gender, ethnicity, education level, etc.). Three studies reported findings adjusted for sociodemographic variables and traditional risk factors for CVD (smoking, high blood pressure, diabetes, etc.), and two studies reported results adjusted for both sociodemographic variables, CVD risk factors, and psychological factors such as depression.
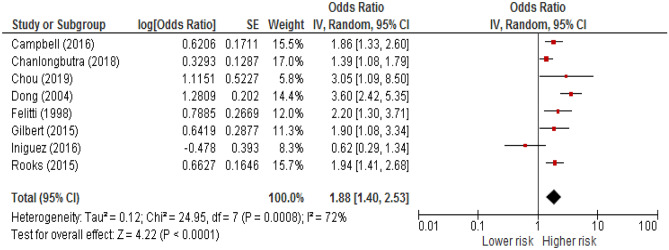
A meta-analysis was performed on 9 studies for which an OR and its precision estimates were available or calculable. The results revealed that exposure to cumulative ACEs were associated with increased risk for MI, before adjustment for CVD risk factors. The random-effects model yielded an aggregated OR of 1.88 (95% CI, 1.40–2.53) (a forest plot is available as Fig. 2). Heterogeneity between estimates was high (Q = 24.95; P = .0008; I2 = 72).
Fig. 2.
Forest plot of the association between cumulative adverse childhood experiences (ACEs) and myocardial infarction in adulthood, before adjustment for cardiovascular disease risk factors. CI = Confidence interval. Diamond represents the summary estimate and associated 95% CI
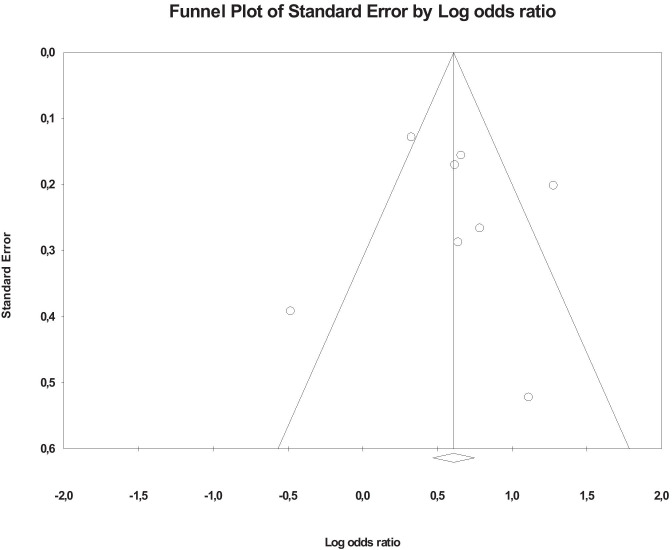
Publication Bias
Visual inspection of the funnel plot of studies focusing on cumulative ACEs included in the meta‐analysis indicated that publication bias was probable (see funnel plot, Fig. 3). However, there was no statistical evidence of publication bias, as the fail-safe N (number of studies with null findings that would be needed to increase the p-value of the meta-analysis to > .05) was 129. Moreover, Orwin’s fail-safe N suggested that 117 studies with null findings would yield a trivial odd ratio of less than 1.04.
Fig. 3.
Funnel plot of Odds Ratio of studies that investigated the association between cumulative adverses childhood experiences (ACEs) and risk of myocardial infarction in adulthood
Possible Moderators
Gender
There are sex differences in exposure to childhood maltreatment and in cardio metabolic outcomes. The incidence of MI was higher in men than in women (Millet et al., 2018) and women have significantly higher ACEs than men (Giano et al., 2020). We conducted an exploratory meta-regression analysis to determine whether an association between the gender compositions of each study sample and its estimate of the association between cumulative ACEs and MI by coding each study for the proportion of women. Studies ranged from 0 to 60% women. In the included studies, the OR in those included more than 50% of women (k = 7) was 1.81 (95% CI, 1.13–2.91) whereas the estimate for those that included 50% or less (k = 2) was 1.62 (95% CI, 1.42–1.84). Although underpowered to detect a significant difference, a mixed effects analysis suggested no difference in the association of ACEs to incident MI according to the gender composition of the studies (Q = .21; P = .65).
Age
The prevalence of myocardial infarction has been shown to increase with age, in both men and women (Rodgers et al., 2019). We conducted an exploratory meta-regression analysis to determine whether an association between the age compositions of each study sample and its estimate of the association between cumulative ACEs and MI by coding each study for the proportion of participants who were more than 55 years old. In the included studies, the OR in those with more than 50% of participants over the age of 55 (k = 4) was 2.34 (95% CI, 1.70–3.23) whereas the estimate for those that included less than 50% of them (k = 5) was 1.43 (95% CI 1.10–1.88). Although underpowered to detect a significant difference, a mixed effects analysis suggested no difference in the association of ACEs to incident MI when the composition of the studies with respect to the age of the participants was taken into account. (Q = 5.42; P = .02).
Race/ethnicity
Children of different races and ethnicities do not experience ACEs equally. In the United-States, 61% of black non-Hispanic children and 51% of Hispanic children have experienced at least one ACE, compared with 40% of white non-Hispanic children and only 23% of Asian non-Hispanic children (Sacks & Murphey, 2018). Moreover, some studies suggest that minority race/ethnicity status is associated with increased risk for MI (Chi et al., 2020; Simon & Ho, 2020). We conducted an exploratory meta-regression analysis to determine whether an association between the racial compositions of each study sample and its estimate of the association between cumulative ACEs and MI by coding each study for the proportion of its participants who identified as non-Hispanic White. Studies ranged from 2–100% non-Hispanic white. In the included studies, the OR in those included more than 70% of non-Hispanic white (k = 6) was 1.81 (95% CI, 1.13–2.91) whereas the estimate for those that included 70% or less (k = 2) was 1.62 (95% CI, 1.42–1.84). Although underpowered to detect a significant difference, a mixed effects analysis suggested no difference in the association of ACEs to incident MI according to the racial composition of the studies (Q = 0.21; P = .65).
Measurement of Cumulative ACEs
Cumulative ACEs were not consistently considered in all studies. In some studies, household dysfunctions were considered among cumulative ACEs, while in other studies they were not. In the included studies, the OR in those included household dysfunctions among cumulative ACEs (k = 7) was 1.84 (95% CI, 1.10–2.60) whereas the estimate for those who did not (k = 2) was 1.59 (95% CI, 1.16–2.26). Although underpowered to detect a significant difference, a mixed effects analysis suggested no difference in the association of cumulative ACEs to incident MI according to the inclusion or exclusion of household dysfunctions among the cumulative ACEs (Q = 0.35; P = .55).
Type of MI Assessment
It may be that the method for determining a MI “case” (i.e., independently assessed disease [i.e., outcomes assessed in a laboratory and/or medical setting or diagnosis made by a healthcare provider and indexed in national registers, hospital registers, or medical records]) vs. self-report of a previous diagnosis made by a physician or other healthcare providers) influences the estimate of MI prevalence. In the included studies, the OR in those that assessed MI independently (k = 3) was 1.33 (95% CI, 0.86–2.07) whereas the estimate for those that used self-reported assessment (k = 6) was 2.10 (95% CI, 1.51–2.93). Although underpowered to detect a significant difference, a mixed effects analysis suggested no significant difference in the association of cumulative ACEs to incident MI by type of MI assessment (Q = 2.65; P = .10).
Quality Assessment
In the intermediate quality studies (k = 8), the OR was 1.88 (95% CI 1.40–2.53), while in the good quality study, the OR was 1.53 (95% CI 1.27–1.83). A mixed effects analysis suggested no significant difference in the association of cumulative ACEs to incident MI by quality studies (Q = 3.10; P = .07).
Subgroup Analyses
Adjustment for CVD Risk
Across 3 studies (N = 18.342), results revealed that exposure to cumulative ACEs was significantly associated with increased risk for MI, after adjustment for health behavioural factors. The random-effects model yielded an aggregated OR of 1.78 (95% CI, 1.24–2.57, p = .002). There was also high significant heterogeneity in the CVD risk-adjusted estimates (Q = 8.313; P = .016; I2 = 75.943).
Adjustment for Psychological Risk Factors
There are well established associations between childhood adversity and the increases the risk of mood disorders (Green et al., 2010), which are widely recognized as increasing the risk for the subsequent onset of heart disease and other chronic conditions (Goldstein et al., 2015; Nicholson et al., 2006). The aggregate OR for the 2 psychological risk-adjusted estimates (N = 17.899) in a random effects model was 2.09 (95% CI, 1.43–3.06). There was also significant heterogeneity in the psychological risk-adjusted estimates (Q = 2.067, P = .15; I2 = 51.628). All studies that adjusted for psychological did so after adjustment for clinical and traditional CVD risk factors. The method used for assessing psychological factors, such as depression are given in Supplementary Table S2.
Discussion
On the basis of the pooled analysis of OR, it was found that cumulative ACEs is associated with an 88% increase in risk for incident MI before adjustment for CVD risk factors across 8 studies comprising more than 240.000 participants. After further adjustment for CVD risk factors, and further adjustment psychological factors, we found that associations between cumulative ACEs and MI were also positive and statistically significant. In the only study reporting HR results, the magnitude of the relation between cumulative ACEs and MI was HR = 2.11; 95% CI, 1.59–6.24, after adjustment for demographic and socioeconomic factors. Although these findings and conclusions seem to be relatively consistent and robust, they should be interpreted considering a number of limitations of our analysis.
This meta-analysis may be subject to publication bias because non-significant findings are less likely to be published. This problem is increased when statistical models are employed because often only significant estimates are reported in many studies. This may result in the association between cumulative ACEs and MI being overstated.
The analysis also suffers from inconsistencies in how cumulative ACEs are defined across the studies, as shown in Table 1. Indeed, cumulative ACEs in the CTQ-based study comprised sexual, physical and emotional abuse, as well as physical and emotional neglect (Roy et al., 2010), whereas in other studies, cumulative ACEs also included various forms of household dysfunction such as parental divorce/separation or incarceration of a family member. Cumulative ACEs could therefore refer to a variety of situations that differ between studies and thus may not have the same potential effects on cardiovascular health. The harmful nature of some of the events included in the cumulative ACEs could be clarified by considering each of them individually to determine whether they adversely affected cardiac health or, conversely, whether they were protective or had little or no impact at all. It should also be noted that measuring the degree of exposure to maltreatment or other potentially traumatic experiences is commonly based on dichotomous questions (i.e., “yes” or “no” questions), without taking into account the duration or chronic nature of the exposure in question, the severity of the events to which the child has been exposed, the specific role of his or her potential abuser (e.g., a family member) and the child’s age when the exposure occurred. However, these variables may have a specific impact on the development of ischemic heart disease. Another aspect to consider is the fact since most data on cumulative ACEs are retrospective and self-reported, participants may not be able to recall such events (traumatic amnesia) or may not wish to report having been victimized for various reasons (shame, embarrassment). These recall biases could be correlated with an underestimation of the frequency of trauma exposure and perhaps explains some conflicting results (Iniguez & Stankowski, 2016). Similarly, data on coronary heart disease, also retrospective and self-reported, are likely to be underestimated or overestimated. We dealt with this issue in the meta-analysis by performing subgroup analyses (independent assessment versus self-reported MI). Circumventing these methodological biases could be achieved by integrating into the research assessments aimed an in-depth objectification of coronary heart disease based on specific tests or reliable diagnostic data. On this basis alone, further epidemiological research is still needed to better understand the possible association between cumulative ACEs and ischemic heart disease. Possible future investigations might include, for example, a thorough assessment of both the nature and duration of exposure to traumatic events during childhood and adolescence based on self-reported data and from other sources (medical, social, legal), in conjunction with a joint prospective assessment of psychological, behavioral and clinical risk factors for MI. An additional line of questioning might examine if the degree of risk of developing MI is different according to age, for men and women exposed to cumulative ACEs, and whether minority race/ethnicity modifies the association of cumulative ACEs with incident MI. Although our study showed a significant evidence of age in the cumulative ACEs/MI association, but no significant evidence of gender, race/ethnicity differences in this association, the lack of age, gender or racial/ethnic diversity both across and within studies highlights the need for future research in this area. Such future investigations would allow for a more detailed understanding of the complex nature of the mechanisms underlying the link between early potentially traumatic events and heart disease, and ultimately would bring us closer to identifying at-risk populations with the view of providing appropriate and timely care. Another limitation of our meta-analysis is that the influence of confounding variables cannot be fully evaluated. While most studies presented multivariable adjusted ORs controlling for a range of socio-demographic and study design variables, very few studies presented associations between cumulative ACEs and MI controlling for age and sex only, or for CVD risk factors, and psychological factors. We again dealt with this issue in our meta-analysis by carrying out separate analyses depending on data availability. Furthermore, we cannot exclude that residual confounding or unmeasured potential confounders may still remain. In this regard, research indicates that several protective factors can prevent or ameliorate the negative effects of ACEs (Moore & Ramirez, 2016). A positive, supportive relationship with one or more adults is of primary importance. Studies show that children with secure attachment relationships with their caregiver(s) are better able to regulate their responses to upsetting situations, compared to children with less secure caregiver attachments (Shonkoff et al., 2005). In addition to supportive relationships, a child’s own intrapersonal skills can be a buffer to the effects of ACEs. Children who have experienced ACEs but demonstrate adaptive behaviours, such as managing their emotions, are more likely to have positive outcomes (Bethell et al., 2014). In view of these features, it might be interesting to identify factors that may contribute to reducing or protecting against the negative effects of cumulative ACEs on cardiac health. Despite evidence of associations between cumulative ACEs and MI, further studies are needed that ensure adequate adjustment for lifetime confounders, and to better understand the burden that would be attributable to them.
Conclusion
Taken together, the studies examined in the present systematic review support the fact that early life exposure to cumulative ACEs is a risk factor for subsequent development of ischemic heart disease, such as MI. Further research, nonetheless, is still needed to identify in more precise terms the mechanisms involved in the direct association between exposure to cumulative potentially traumatic situations during childhood or adolescence and the subsequent occurrence of MI as well as protective factors of the effects of early adverse experiences. These complementary studies can provide a fruitful area of research that could effectively inform interventions.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
MJS developed the search strategy, conducted the data collection, analysed papers against inclusion/exclusion criteria, assessed all final studies for risk of bias, performed the analysis, and drafted the manuscript. MJB contributed to the data collection, and analysed papers against inclusion/exclusion criteria. CLT assessed all final studies for risk of bias. All authors contributed to data analysis and interpretation. All authors have approved of this version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Ethics Approval
As the research did not involve collection of data from human subjects, it did not need to be approved by an organizational unit responsible for the protection of human participants.
Conflict of Interest
The authors declare they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bellis MA, Hughes K, Leckenby N, Hardcastle KA, Perkins C, Lowey H. Measuring mortality and burden of adult disease with adverse childhood experiences in England: A national survey. Journal of Public Health. 2015;37(3):445–454. doi: 10.1093/pubmed/fdu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin, E. J., Blaha, M. J., Chuive, S. E., Cushman, M., Das, S. R., Deo, R., de Ferranti, S. D., Floyd, J., Fornage, M., Gillespie, C., Isasi, C. R., Jiménez, M. C., Jordan, L. C., Judd, S. E., Lackland, D., Lichtman, J. H., Lisabeth, L., Liu, S., Longenecker, C. T., Mackey, R. H., Matsushita, K., Mozaffarian, D., Mussolino, M. E., Nasir, K., Neumar, R. W., Palaniappan, L., Pandey, D. K., Thiagarajan, R. R., Reeves, M. J., Ritchey, M., Rodriguez, C. J., Roth, G.A., Rosamond, W. D., Sasson, C., Towfighi, A., Tsao, C. W., Turner, M. B., Virani, S. S., Voeks, J. H., Willey, J. Z., Wilkins, J. T., Wu, J. H., Alger H. M., Wong, S. S., Muntner, P.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethell CD, Newacheck P, Hawes E, Halfon N. Adverse childhood experiences: Assessing the impact on health and school engagement and the mitigating role of resilience. Health Affairs (project Hope) 2014;33(12):2106–2115. doi: 10.1377/hlthaff.2014.0914. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S, Bhattacharya R, Kelley GA, Sambamoorthi U. Antidepressant use and new-onset diabetes: A systematic review and meta-analysis. Diabetes/metabolism Research and Reviews. 2013;29(4):273–284. doi: 10.1002/dmrr.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein, M., Hedges, L., Higgins, J., & Rothstein, H. (2013). Comprehensive meta-analysis version, 3. Englewood: Biostat.
- Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: The Early Trauma Inventory. Depression and Anxiety. 2000;12(1):1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Campbell JA, Walker RJ, Egede LE. Associations between adverse childhood experiences, high-risk behaviors, and morbidity in adulthood. American Journal of Preventive Medicine. 2016;50(3):344–352. doi: 10.1016/j.amepre.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. https://www.cdc.gov (14 February 2020, date last accessed).
- Chanlongbutra A, Singh GK, Mueller CD. Adverse childhood experiences, health-related quality of life, and chronic disease risks in rural areas of the United States. Journal of Environmental and Public Health. 2018;8:1–15. doi: 10.1155/2018/7151297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou PH, Koenen K. Associations between childhood maltreatment and risk of myocardial infarction in adulthood: Results from the National Epidemiologic Survey on alcohol and Related Conditions. Journal of Psychiatric Research. 2019;116:172–177. doi: 10.1016/j.jpsychires.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Chi, G. C., Kanter, M. H., Li, B. H., Qian, L., Reading, S. R., Harrison, T. N., Jacobsen, S. J., Scott, R. D., Cavendish, J. J., Lawrence, J. M., Tartof, S. Y., & Reynolds, K. (2020). Trends in Acute Myocardial Infarction by Race and Ethnicity. Journal of the American Heart Association,9(5), e013542. 10.1161/JAHA.119.013542 [DOI] [PMC free article] [PubMed]
- Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, Loo CM, Giles WH. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse & Neglect. 2004;28(7):771–784. doi: 10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: Adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz A, Edwards V, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) study. American Journal of Preventive Medicine. 1998;14:245–258. doi: 10.1016/S0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Giano, Z., Wheeler, D. L., & Hubach, R. D. (2020). The frequencies and disparities of adverse childhood experiences in the U.S. BMC public health, 20(1), 1327. 10.1186/s12889-020-09411-z [DOI] [PMC free article] [PubMed]
- Gilbert, L. K., Breiding, M. J., Merrick, M. T., Thompson, W. W., Ford, D. C., Dhingra, S. S., & Parks, S.E. (2015). Childhood adversity and adult chronic disease. An update from ten states and the district of Columbia, 2010. American Journal of Preventive Medicine, 48(3), 345–49. 10.1016/j.amepre.2014.09.006 [DOI] [PubMed]
- Goldstein, B. I., Carnethon, M. R., Matthews, K. A., McIntyre, R. S., Miller, G. E., Raghuveer, G., Stoney, C. M., Wasiak, H., & McCrindle, B. W.; on behalf of the American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2015;132:965–986. doi: 10.1161/CIR.0000000000000229. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication I: Associations with first onset of DSM-IV disorders. Archives General Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez KC, Stankowski RV. Adverse childhood experiences and health in adulthood in a rural population-based sample. Clinical Medicine and Research. 2016;14(3–4):126–137. doi: 10.3121/cmr.2016.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet ER, Peters S, Woodward M. Sex differences in risk factors for myocardial infarction: Cohort study of UK Biobank participants. BMJ. 2018;363:k4247. doi: 10.1136/bmj.k4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Ramirez AN. Adverse childhood experience and adolescent well-being: Do protective factors matter? Child Indicators Research. 2016;9(2):299–316. doi: 10.1007/s12187-015-9324-4. [DOI] [Google Scholar]
- National Heart, Lung, and Blood Institute [NHLBI]. (2014). Quality assesment tool for observational cohort and crosssectional studies. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Nicholson A, Kuper H, Hemingway H. Depression as an aetiological and prognostic factor in coronary heart disease:A meta-analysis of 6362 events among 146 538 participants in 54 observational studies. European Heart Journal. 2006;27:2763–2774. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: A systematic review and meta-analysis. PLoS Medicine. 2012;9:e1001349. doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwin RG. A fail-safe N for effect size in meta-analysis. Journal of Educational Statistics. 1983;8:157–159. [Google Scholar]
- Rodgers JL, Jones J, Bolleddu SI, Vanthenapalli S, Rodgers LE, Shah K, Karia K, Panguluri SK. Cardiovascular Risks Associated with Gender and Aging. Journal of Cardiovascular Development and Disease. 2019;6(2):19. doi: 10.3390/jcdd6020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks C, Veledar E, Goldberg J, Votaw J, Shah A, Bremner JD, Vaccarino V. Long-term consequences of early trauma on coronary heart disease: Role of familial factors. Journal of Traumatic Stress. 2015;28(5):456–459. doi: 10.1002/jts.22044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R. Writing meta-analytical reviews. Psychological Bulletin. 1995;118:183–192. doi: 10.1037/0033-2909.118.2.183. [DOI] [Google Scholar]
- Roy A, Janal MN, Roy M. Childhood trauma and prevalence of cardiovascular disease in patients with type 1 diabetes. Psychosomatic Medicine. 2010;72(8):833–838. doi: 10.1097/PSY.0b013e3181eafc2d. [DOI] [PubMed] [Google Scholar]
- Sacks, V. & Murphey, D. (2018). The Prevalence of Adverse Childhood Experiences, Nationally, by State, and by Race or Ethnicity. Child Trends. Available at: https://www.childtrends.org/publications/prevalence-adverse-childhood-experiences-nationally-state-race-ethnicity
- Shonkoff, J. P., Boyce, W. T., Cameron, J., Duncan, G. J., Fox, N. A., Gunnar, M. R., & Thompson, R. A. (2005). Excessive stress disrupts the architecture of the developing brain (Working Paper, 3), National Scientific Council on the Developing Child.
- Simon S, Ho PM. Ethnic and Racial Disparities in Acute Myocardial Infarction. Current Cardiology Reports. 2020;22:88. doi: 10.1007/s11886-020-01351-9. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2018). Adverse Childhood Experiences International Questionnaire. Available at: https://www.who.int/violence_injury_prevention/violence/activities/adverse_childhood_experiences/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.