Abstract
Background
Adverse childhood experiences (ACEs) are linked to higher risk of common conditions driving mortality in adulthood, but little evidence exists on whether ACEs are associated with risk of dementia, a leading cause of death in the USA.
Objective
To estimate the relationship between US adults’ reported ACE scores and a positive screen for dementia.
Design
Cross-sectional analysis of a longitudinal, national population-based survey of US older adults.
Participants
Survey respondents aged ≥ 65 years with dementia screening data from the 2017 wave of the Panel Study of Income Dynamics (PSID) and ACE scores from the 2014 PSID Childhood Retrospective Circumstances Survey supplement (1,488 eligible participants unweighted).
Main Measures
Dementia screening data was collected in the 2017 wave of the PSID using the 8-item informant interview to differentiate normal cognition and dementia (AD8). Mean change in AD8 score and probability of a positive dementia screen by ACE score were calculated using adjusted regression models with post-estimation. Analyses were stratified by age group. Measures were analyzed in 2020.
Results
Complete data were available for 1,223 (82%) participants, with a mean age of 73.4 years (SD 7.1, range 65 to 96 years). Adjusted estimated probability of a positive dementia screen increased with each additional adverse childhood experience reported. Older adults with ≥ 4 ACEs had higher rates of a positive dementia screen (AD8 score ≥ 2 points) compared to those with no ACEs (adjusted rate 26.6% versus 16.3%, p = 0.034). Compared to those with no ACE history, respondents with ≥ 4 ACEs had higher odds of a 1-point increase in AD8 score across all intervals of the AD8 scale (aOR 1.79, 95% CI 1.05–3.04). The ACE-positive dementia screen associations were strongest among those aged 65–75.
Conclusions
Greater exposure to ACEs is independently associated with higher probability of a positive dementia screen in older adulthood.
KEY WORDS: dementia, adverse childhood experiences (ACEs), national survey data
INTRODUCTION
Adverse childhood experiences (ACEs) are stressful and potentially traumatic events, including abuse, neglect, and household dysfunction, which occur before age eighteen.1 Numerous studies have linked a higher number of reported ACEs with poorer adult mental and physical health, chronic disease, and premature mortality.2,3 Many leading causes of adult mortality in the USA are associated with ACEs, but little evidence exists regarding ACEs’ association with one of the primary drivers of morbidity and mortality among older adults in the USA—dementia.
Early adversity of various types has been linked prospectively to changes in structure and function of the developing brain during childhood and adolescence, particularly in regions responsible for learning and memory, with consequences for cognitive function throughout the life course.4,5 Previous US studies have demonstrated associations between cognitive impairment and individual categories of childhood adversity, such as child abuse and neglect, poverty, and parental death, most commonly in populations at risk for psychiatric disorders.6–9 It is unclear whether these associations are found in the general population, or whether a composite measure of various forms of childhood adversity better predicts later-life dementia risk in the USA. Conceptually, such a composite measure of early adversity better reflects current evidence regarding the common pathophysiologic pathway—chronic activation and subsequent dysregulation of the hypothalamic–pituitary–adrenal axis—through which various forms of childhood stress have been shown to affect end-organ function and later-life health outcomes.10,11
A single international study in Japan found that a composite measure of ACEs was associated with developing dementia, with those reporting 3 or more ACEs being at highest risk.12 Other international studies have linked childhood trauma with Alzheimer’s disease and other dementias.13
Given the growing awareness of the impact of ACEs, estimates of dementia risk associated with ACEs could increase our understanding of the impact of early adversity throughout the life course, specifically on cognitive function in later life. Such information might also be useful in estimating the benefits to individuals, health systems, and society of upstream investment in ACE prevention and mitigation.
This study is the first to examine the association between ACE scores and a positive dementia screen among a national sample of older adults in the USA, controlling for sociodemographic factors. We explore patterns of association by specific ACEs, stratified by age, and mediated by cardiovascular disease and behavioral risk factors to understand the nature of ACE-dementia relationships.
METHODS
Sample and Data Sources
We used data from the 2017 Panel Study of Income Dynamics (PSID), the longest-running US national panel survey spanning over four generations of American families and containing a host of health and demographic information, composed on main individual and family files plus PSID survey supplements linked to the main data files. Data collected by phone from the 2017 PSID main interview included information on health, education, income, health insurance, family structure, and demographic characteristics for adult heads of household and, if present, their spouses or partners and other household members. Those participants over age 65 were administered the dementia screening measure, the AD8, and this group constituted the sample. English-speaking adult heads of household and their spouses or partners were included in the study if they had previously taken part in the PSID Childhood Retrospective Circumstances Survey (CRCS), a supplementary survey to the PSID collected in 2014–2015 that retrospectively assessed childhood experiences including nine ACEs via its web-based and/or mailed survey.14 Of the 1,409 2014–2015 CRCS respondents 65 years or older who had not passed away by the 2017 PSID interview, all (100%) participated in the 2017 PSID survey and 1,223 (87%) had complete data. The 81 CRCS participants who passed away before 2017 had similar average ACE scores to those who were living (1.12 [SD 1.36] versus 1.19 [SD1.38] ACEs).
Construction of Adverse Childhood Experience Measures
Data on nine categories of ACEs were collected through the CRCS and combined to construct our main composite ACE score measure as described below. Binary indicator measures of parent mental illness, parent substance abuse, parent intimate partner violence, parental divorce or separation, deceased or absent parent, physical abuse, sexual abuse, emotional abuse, and neglect experienced by the respondent were constructed from CRCS items (item language previously published).15 An ACE count variable was constructed as the sum of these nine binary ACE measures for each adult in the PSID sample households and binned into four categories—zero to 1 ACE, 2 ACEs, 3 ACEs, and 4 or more ACEs—to construct our primary predictor variable through an approach similar to prior studies that have validated the use of composite, summative ACE score measures.16–18 This PSID ACE measure has been previously found to predict adult health outcomes and correspond to prospectively collected early life measures of childhood stress among respondents who were PSID participants as children15.
Outcomes
Participants over age 65 were administered the AD8 dementia screen, a pajudgment, orientation, and functionrticipant- or informant-reported, 8-item measure of worsening cognitive impairment used to discriminate dementia from normal cognition. The conventional cut-off used for a positive AD8 dementia screen is 2 or more positive items.19 The AD8 score is the count of the number of “yes” responses to 8 questions about changes in memory, judgment, orientation, and function. Psychometrically, the AD8 has been well-validated, with good internal consistency (Cronbach’s alpha 0.84) and reliability (weighted kappa for test-test reliability 0.67–0.80; intraclass correlation coefficient 0.80 for inter-rater reliability).20,21 The AD8 is correlated with the Clinical Dementia Rating (correlation of 0.65–0.75) and the Mini Mental State Examination (correlation of between − 0.64 and − 0.39).19
Covariates
Covariates were selected based on prior studies to reflect sociodemographic confounders conceptually linked to both childhood adversity and health outcomes.15,22 Covariates for each participant included level of education (categories of less than high school, high school degree or equivalent, and any college or graduate education); continuous years of age; race; Latino/Hispanic ethnicity; gender; marital status; 2013 income level (0–99% of the federal poverty level [FPL], 100–199% FPL, 200–299% FPL, 300–399% FPL, 400% FPL, or more); number of household members and children; and ever having smoked cigarettes.
Statistical Analyses
Our main binomial logistic analytic model regressed the outcome of two or more AD8 “yes” responses (i.e., positive dementia screen) on ACE score categories, adjusted for covariates (Stata, logit; primary predictor of categorical ACE score with categories of 0–1, 2, 3, and 4 + ACEs). To understand how the specific items in the AD8 dementia screener relate to ACE score, we used binomial logistic regression to estimate the odds of an affirmative response to each of the AD8 items associated with an increase in ACE score. Alternate covariate specifications, such as binned age ranges, continuous years of education, and continuous income, tested individually in the model had minimal effect on estimates. Probabilities of a positive screen for dementia were calculated via post-estimation (Stata, margins). Because every 1-point increase in AD8 score indicates greater likelihood of dementia, we also used adjusted ordered logistic regression to examine the association between (1) ACE score and the ordinal AD8 score and (2) each specific ACE and the ordinal AD8 score (Stata, ologit; a series of interval logit models and the Brant test in Stata confirmed the proportional odds assumption held). We also used an adjusted linear regression model to estimate the average difference in AD8 score associated with each additional ACE (Stata, regress). The primary analyses were stratified by participant age (strata 65–74 years old versus 75 years and above) to explore whether associations between ACE score and AD8 score were stronger for a particular age group.
Mediation by chronic cardiovascular conditions (including hypertension, diabetes, a diagnosis of cardiovascular disease, prior stroke, or prior myocardial infarction), high risk of severe mental illness based on the Kessler-6 Emotional Distress Scale (score greater than 12),23,24 or any current alcohol consumption was explored using multiple regression and the difference-between-coefficients approach across models with and without the mediator variables included (one at a time) with y-standardization.25 All models were weighted to accommodate the complex survey design, achieve population representation, and adjust for nonresponse using the PSID 2013 survey base weights further adjusted for CRCS differential nonresponse using age, education, gender, and race/ethnicity variables. All estimates employed survey-robust standard errors.
The data were housed at the University of Michigan’s Institute for Social Research. All analyses were carried out in Stata, version 16 (StataCorp). The UCLA Institutional Review Board approved this study.
RESULTS
The study sample included 1,223 adults with complete data. Roughly 8% reported having experienced four or more ACEs. Their average age was 73.4 years (median 71, range 65–96), approximately half were female, just over 10% were non-White (reflecting demographics of the national sample enrolled in early waves of the PSID), a third had a high school education or less, and just over half had income over 400% of the federal poverty level. Participants whose AD8 dementia screen scores were positive were more likely to be non-white, older, and with lower socioeconomic status (Table 1).
Table 1.
Sample Characteristics, Population-Weighted, Panel Study of Income Dynamics and Childhood Retrospective Circumstances Survey Data, 2013–2017
| Total sample percentage or mean (SD) (n = 1,223) | Sample with positive dementia screen (AD8 Score ≥ 2, n = 213) | p-value for difference | |
|---|---|---|---|
| Adverse Childhood Experiences Score | |||
|
0–1 2 3 4 or more |
69.9 12.4 9.19 8.44 |
65.1 13.2 10.5 11.2 |
0.03 |
| Race | |||
|
White African American Asian/Pacific Islander |
89.2 7.3 2.2 |
86.4 9.2 1.3 |
0.001 |
| Latino/Hispanic Ethnicity | 2.3 | 4.1 | 0.25 |
| Male | 46.1 | 44.3 | 0.80 |
| Education | |||
|
Less than high school High school graduate/GED Any college/vocational/graduate school |
9.9 27.6 62.6 |
16.4 35.6 48.0 |
< 0.001 |
| Age | 73.4 years (SD 7.1) | 76.5 years (SD 8.2) | < 0.001 |
| Income level | |||
|
> 400% FPL 300–400% FPL 200–299% FPL 100–199% FPL < 100% FPL |
53.4 13.9 16.3 12.2 4.3 |
34.0 12.5 29.7 17.5 6.4 |
< 0.001 |
| Household size | 1.85 | 1.73 | < 0.001 |
| Ever smoked cigarettes | 48.4 | 43.5 | 0.19 |
| Any regular alcohol consumption | 59.1 | 49.9 | 0.012 |
| High risk of mental illness | 1.01 | 4.33 | 0.002 |
| Diagnosis of heart disease | 11.3 | 18.3 | 0.004 |
FPL-federal poverty level, GED General education diploma (high school equivalency certificate), SD Standard deviation
p-values <0.05 are bolded above
ACE score was positively associated with AD8 score in the sample overall. Respondents who reported four or more ACEs had a 1.6-fold higher estimated probability of a positive dementia screen (i.e., AD8 score of 2 or higher) compared to respondents reporting one or fewer ACEs (26.6% versus 16.3%, p = 0.034). A dose–response relationship was observed with respect to a positive AD8 dementia screen as ACE score category increased (Table 2, p-value for trend = 0.03).
Table 2.
Associations Between Adverse Childhood Experience (ACE) Score and Adjusted Estimated Probability of Positive AD8 Dementia Screening Score of 2 or More, Overall Sample and Stratified by Age Range (2013–2017 PSID Data)
| Outcome measure |
ACE score category Adjusted estimated probabilities of positive AD8 dementia screen (95% CI) |
|||
|---|---|---|---|---|
| 0–1 ACEs (reference) | 2 ACEs | 3 ACEs | 4 or more ACEs | |
| All respondents (n = 1223) | 16.3% (13.6–19.0) | 18.6% (11.9–25.2) | 21.8% (13.8–29.7) | 26.6%* (16.0–37.2) |
| Respondents ages 65–74 years (n = 820) | 10.6% (7.7–13.4) | 17.4% (9.3–25.7) | 15.4% (6.7–24.0) | 26.8%*** (14.8–38.7) |
| Respondents ages 75 years and over (n = 403) | 24.6% (19.4–29.7) | 19.4% (8.8–30.0) | 32.8% (18.0–47.6) | 24.4% (6.5–42.2) |
Note: Results shown are estimated probabilities from post-estimation from weighted, adjusted binomial logit regression models (Stata logit, post-estimation using margins command), overall adjusted for covariates (including level of education, race, Latino/Hispanic ethnicity, gender, marital status, 2013 income level, number of household members and children, and ever having smoked cigarettes) and stratified by age of participants. Boldfaced values indicate statistical significance of regression model results. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001. Abbreviations: ACE adverse childhood experiences, CI confidence interval
For every single point increase in ACE score, we observed a 0.07-point (95% CI 0.004–0.14, p = 0.036) increase in AD8 score. In our ordered logistic regression model, compared to those reporting 0–1 ACEs, the likelihood of a 1-point increase in AD8 score was 1.3-fold (95% CI 0.84–1.89, NS) higher for those with 2 ACEs, 1.6-fold (95% CI 1.02–2.35, p = 0.039) higher for those with 3 ACEs, and 1.8-fold (95% CI 1.05–3.03, p = 0.031) higher for those with 4 or more ACEs.
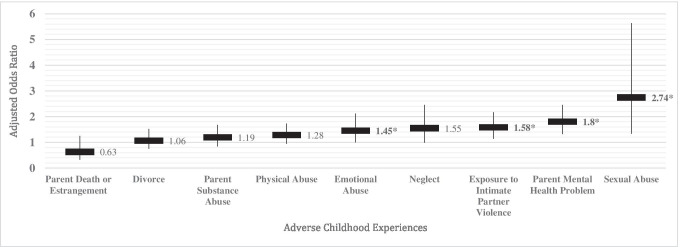
Specific adverse childhood experiences that showed significant positive associations with a positive AD8 dementia screen included history of emotional abuse (aOR 1.45, 95% CI 1.0–2.12, p = 0.049), exposure to intimate partner violence (aOR 1.58, 95% CI 1.14–2.17, p = 0.006), parent mental health problems (aOR 1.8, 95% CI 1.32–2.45, p < 0.001), and sexual abuse (aOR 2.74, 95% CI 1.33–5.64, p = 0.006) (Fig. 1). The AD8 items most strongly associated with increasing ACE score were changes in thinking and memory (aOR 1.88, 95% CI 0.99–3.55, p = 0.052) and changes in remembering appointments (aOR 2.75, 95% CI 1.35–5.59, p = 0.005).
Fig. 1.
Adjusted Odds Ratios for Associations Between Positive AD8 Dementia Screen and Specific Adverse Childhood Experience Variables, Panel Study of Income Dynamics Sample of Older Adults, 2017. Note: Shown are adjusted odds ratios for a unit increase in AD8 dementia screening score associated with each adverse childhood experience, results from ordered logistic regression models, population-weighted and adjusted for covariates, including level of education (categories of less than high school, high school degree or equivalent, and any college or graduate education); continuous years of age; race; Latino/Hispanic ethnicity; gender; marital status; 2013 income level (0–99% of the federal poverty level [FPL], 100–199% FPL, 200–299% FPL, 300–399% FPL, 400% FPL or more); number of household members and children; and ever having smoked cigarettes. Labels with an asterisk * indicate p-value < 0.05.
In models examining the association between a positive AD8 dementia screen and ACE score by age range, the relationship was observed primarily for respondents ages 65–74 years, with little association found between ACES and a positive dementia screen for those 75 years and older (Table 2).
The associations between ACEs and a positive dementia screen were not substantially mediated by chronic cardiovascular conditions (including hypertension, diabetes, a diagnosis of cardiovascular disease, prior stroke, or prior myocardial infarction), high risk of severe mental illness based on the Kessler-6 Emotional Distress Scale (score greater than 12),23,24 or any current alcohol consumption.
DISCUSSION
In our national study of US older adults, a higher number of ACEs was associated with an increased probability of a positive screen for dementia in a dose–response fashion. This association was strongest in adults aged 65–74 years and for those who had 4 or more ACEs. Older adults reporting childhood experiences of emotional abuse, sexual abuse, exposure to intimate partner violence, or parental mental illness had the highest probability of a positive screen for dementia. Increasing ACE scores were most strongly associated with changes in thinking and memory and changes in remembering appointments on the AD8. Differences in chronic conditions, including cardiovascular disease, did not mediate the association between ACEs and a positive screen for dementia.
This is the first US study showing the association between ACE score and dementia risk. However, given the existing literature linking ACEs and chronic disease in adulthood, the finding that ACEs correlate with probability of dementia is not unexpected. Like many chronic diseases linked to ACEs, the risk of dementia is affected by early life stress, as well as sociodemographic and clinical factors and genetic predisposition.26,27 Our findings are consistent with existing studies and expand upon current evidence linking major drivers of US mortality to the life course impacts of trauma, in this case to the late life condition of dementia.
The relationship between ACEs and dementia risk found in our study provides evidence for the significant and enduring deleterious impact on brain development and function associated with childhood adversity.28 Dementia risk in older age associated with higher numbers of ACEs may be a long-term consequence of adaptive neurodevelopment in early childhood in response to trauma.29 For children exposed to adversity, as the brain preferentially activates the amygdala and limbic system in the “flight or fight” neuroendocrine pathway, there may be compensatory reduction in activity and synaptic wiring in the hippocampus and higher cortical regions that are associated with learning and memory, although the extent of this neuroplasticity into older age is not well understood.30 We hypothesize that over time, this pattern of neuroactivation has the potential to impact the formation of neural networks that store and regulate processes of learning and memory.
Our findings have important implications for the health and wellbeing of older adults that extend beyond the likelihood of developing dementia. Greater risk of cognitive impairment in older adults with higher ACEs burden may also make these individuals more vulnerable to elder abuse, financial exploitation, and institutionalization or homelessness. Childhood adversity may impact the likelihood of family formation due to smaller social networks and poorer quality social relationships found among survivors of child maltreatment.31 It has been shown that ACE scores are associated with social isolation and financial stress in later life.32 Social isolation and poor social support has also been associated with cognitive impairment in older adults.33 There may be a domino effect starting with childhood adversity, leading to attachment difficulties and problems with relationships as an adult, and then to increased risk of social isolation and dementia as an older adult.
Our findings suggest that ACEs may lead to earlier onset of cognitive impairment. Adults in the younger age category (65–74 years) had a stronger association between increasing ACE scores and dementia risk. Adults over age 75 years in this sample showed no clear association between ACEs and dementia risk, perhaps demonstrating a “survival bias,” other unmeasured resiliency factors, or a higher incidence of dementia driven by other factors that dilute the ACEs-dementia risk association signal.
The findings may have clinical implications, in addition to their implications for population health. Clinicians assessing patients with cognitive concerns or screening patients for cognitive impairment may wish to consider exposure to childhood adversity as an additional risk factor for cognitive decline. Whether changes in patient management are indicated in cases with a high ACE score is beyond the scope of this single study, but our results raise the question of whether mental, behavioral, and social health interventions for individuals who have experienced ACEs may mitigate dementia risk. As interest in clinical screening for ACEs increases, ACEs information may become more available to clinicians assessing risk and recommending treatment options for their patients.
Our study has limitations. Based on the increased incidence of dementia in adults over 75 years, it is possible that the oldest adults (≥ 75 years) in this sample were less likely to reliably recall their childhood ACEs. This may explain the weaker association between ACEs and dementia risk in the oldest age group. We were unable to validate a positive dementia screen on the AD8 with a clinical diagnosis of dementia for the PSID participants, though the AD8 has a specificity of 86% for the detection of dementia20 and has been previously correlated with the Clinical Dementia Rating.34 The ACE score does not capture nuances such as frequency or severity of exposure to individual ACEs, nor the ages(s) at which the ACEs occurred. ACEs are an imperfect measure of the totality of adversity a person may experience, and resilience factors are not considered in the large existing literature on conventional ACE scores. There are likely protective factors that mediate the association between ACEs and dementia risk, but this data is not available. While our study did not examine protective factors, frequency or severity of ACE exposure, or whether childhood adversity affects mid-life antecedents or later complications of dementia (such as behavioral disturbances, mood disorders, and delirium), these topics are ripe for future research.
CONCLUSION
This is the first US study to find that overall ACE score—a measure of reported exposure to adversity in childhood—is associated with a positive screen for dementia among older adults. The association between ACEs and dementia risk suggests an accelerated neurocognitive decline in learning and memory which may begin with adaptations to early life stress. Childhood adversity and trauma should be considered risk factors for dementia, particularly among adults aged 65–75, and clinicians caring for adults should be aware of the potential impacts of ACEs on neurocognitive function. Further study is needed to explore the possible benefits of screening for ACEs and risk-based cognitive assessment. Preventing and mitigating the occurrence of ACEs and identifying those aging with ACEs may promote brain health, which could benefit patients across the life course and reduce the risk of dementia in older age.
Author Contribution
Dr. A. Schickedanz conceptualized and designed the study, obtained and maintained access to the data, performed the statistical analyses, helped draft the manuscript, and approved the manuscript submitted. Dr. H. Schickedanz contributed substantially to the initial study design, reviewed and revised the analytic approach, drafted the manuscript, critically reviewed and revised the manuscript, and approved the manuscript submitted. Dr. Jennings reviewed and revised the analytic approach, critically reviewed and revised the manuscript, and approved the manuscript submitted.
Funding
The collection of PSID data used in this study by the University of Michigan Institute for Social Research was partly supported by the National Institutes of Health under grant number R01 HD069609 and R01 AG040213, and the National Science Foundation under award numbers SES 1157698 and 1623684. The funders had no role in the design, methods, subject recruitment, data collection, analysis, or preparation of this manuscript.
Declarations
Conflict of Interest
Dr. Adam Schickedanz was funded during this work by sequential career development awards from the National Center for Advancing Translational Sciences (KL2TR001882) and the Eunice Kennedy Shriver National Institute for Child Health and Human Development at NIH (K23HD099308). Drs. Heather Schickedanz and Lee Jennings have no financial disclosures.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control. (Last updated April, 2021). Preventing Adverse Childhood Experiences. Accessed July, 2021: https://www.cdc.gov/violenceprevention/aces/fastfact.html
- 2.Felitti, Vincent J., et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998; 14(4): 245–258. [DOI] [PubMed]
- 3.Brown, David W., et al. Adverse childhood experiences and the risk of premature mortality. Am J Prev Med. 2009; 37(5): 389–396. [DOI] [PubMed]
- 4.Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Frontiers in human neuroscience. 2012;19(6):52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bick J, Nelson CA. Early adverse experiences and the developing brain. Neuropsychopharmacology. 2016;41(1):177–196. doi: 10.1038/npp.2015.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Yang L, Yu L, et al. Childhood physical neglect promotes development of mild cognitive impairment in old age - A case-control study. Psychiatry Res. 2016;242:13–18. doi: 10.1016/j.psychres.2016.04.090. [DOI] [PubMed] [Google Scholar]
- 7.Ritchie K, Jaussent I, Stewart R, et al. Adverse childhood environment and late-life cognitive functioning. Int J Geriatr Psychiatry. 2011;26(5):503–510. doi: 10.1002/gps.2553. [DOI] [PubMed] [Google Scholar]
- 8.Gould F, Clarke J, Heim C, Harvey PD, Majer M, Nemeroff CB. The effects of child abuse and neglect on cognitive functioning in adulthood. J Psychiatr Res. 2012;46(4):500–506. doi: 10.1016/j.jpsychires.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong S, Rhee TG, Piescher KN. Longitudinal association of child maltreatment and cognitive functioning: Implications for child development. Child Abuse Negl. 2018;84:64–73. doi: 10.1016/j.chiabu.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Kalmakis KA, Meyer JS, Chiodo L, Leung K. Adverse childhood experiences and chronic hypothalamic–pituitary–adrenal activity. Stress. 2015;18(4):446–450. doi: 10.3109/10253890.2015.1023791. [DOI] [PubMed] [Google Scholar]
- 11.Kuhlman KR, Geiss EG, Vargas I, Lopez-Duran N. HPA-axis activation as a key moderator of childhood trauma exposure and adolescent mental health. Journal of abnormal child psychology. 2018;46(1):149–157. doi: 10.1007/s10802-017-0282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tani Y, Fujiwara T, Kondo K. Association Between Adverse Childhood Experiences and Dementia in Older Japanese Adults. JAMA Network Open. 2020 Feb 5;3(2):e1920740. [DOI] [PubMed]
- 13.Radford K, Delbaere K, Draper B, et al. Childhood Stress and Adversity is Associated with Late-Life Dementia in Aboriginal Australians. Am J Geriatr Psychiatry. 2017;25(10):1097–1106. doi: 10.1016/j.jagp.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 14.The Panel Study of Income Dynamics’ Childhood Retrospective Circumstances Study (PSID-CRCS) User Guide Final Release 1. Accessed April, 2017. https://psidonline.isr.umich.edu/CRCS/2014UserGuide.pdf
- 15.Schickedanz AB, Escarce JJ, Halfon N, Sastry N, Chung PJ. Adverse Childhood Experiences and Household Out-of-Pocket Healthcare Costs. American journal of preventive medicine. 2019;56(5):698–707. doi: 10.1016/j.amepre.2018.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bethell CD, Carle A, Hudziak J, Gombojav N, Powers K, Wade R, Braveman P. Methods to assess adverse childhood experiences of children and families: toward approaches to promote child well-being in policy and practice. Academic pediatrics. 2017;17(7):S51–S69. doi: 10.1016/j.acap.2017.04.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Randell KA, O’Malley D, Dowd MD. Association of parental adverse childhood experiences and current child adversity. JAMA pediatrics. 2015;169(8):786–787. doi: 10.1001/jamapediatrics.2015.0269. [DOI] [PubMed] [Google Scholar]
- 18.Bethell C, Jones J, Gombojav N, Linkenbach J, Sege R. Positive childhood experiences and adult mental and relational health in a statewide sample: Associations across adverse childhood experiences levels. JAMA pediatrics. 2019 Nov 1;173(11):e193007-. [DOI] [PMC free article] [PubMed]
- 19.Galvin JE, Roe CM, Powlishta KK, et al. The AD8, a brief informant interview to detect dementia. Neurology. 2005;65:559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 20.Galvin JE, Roe CM, Xiong C, Morris JC. Validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942–1948. doi: 10.1212/01.wnl.0000247042.15547.eb. [DOI] [PubMed] [Google Scholar]
- 21.Shaik MA, Xu X, Chan QL, Hui RJY, Chong SST, Chen CLH, Dong Y. The reliability and validity of the informant AD8 by comparison with a series of cognitive assessment tools in primary healthcare. International psychogeriatrics. 2016;28(3):443. doi: 10.1017/S1041610215001702. [DOI] [PubMed] [Google Scholar]
- 22.Schickedanz, A., Halfon, N., Sastry, N. and Chung, P.J., 2018. Parents’ adverse childhood experiences and their children’s behavioral health problems. Pediatrics, 142(2). [DOI] [PMC free article] [PubMed]
- 23.McGinty EE, Presskreischer R, Anderson KE, Han H, Barry CL. Psychological Distress and COVID-19–Related Stressors Reported in a Longitudinal Cohort of US Adults in April and July 2020. JAMA. 2020 Nov 23. [DOI] [PMC free article] [PubMed]
- 24.McGinty EE, Presskreischer R, Han H, Barry CL. Psychological Distress and Loneliness Reported by US Adults in 2018 and April 2020. JAMA. 2020 Jun 3. [DOI] [PMC free article] [PubMed]
- 25.Rijnhart JJ, Twisk JW, Eekhout I, Heymans MW. Comparison of logistic-regression based methods for simple mediation analysis with a dichotomous outcome variable. BMC medical research methodology. 2019;19(1):1. doi: 10.1186/s12874-018-0654-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheikh MA. Coloring of the past via respondent’s current psychological state, mediation, and the association between childhood disadvantage and morbidity in adulthood. J Psychiatr Res. 2018;103:173–181. doi: 10.1016/j.jpsychires.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Lourida I, Hannon E, Littlejohns TJ, Langa KM, Hyppönen E, Kuźma E, Llewellyn DJ. Association of lifestyle and genetic risk with incidence of dementia. Jama. 2019;322(5):430–437. doi: 10.1001/jama.2019.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nature reviews neuroscience. 2016;17(10):652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- 29.Perry BD, Pollard R. Homeostasis, stress, trauma, and adaptation: A neurodevelopmental view of childhood trauma. Child and Adolescent Psychiatric Clinics. 1998;7(1):33–51. doi: 10.1016/S1056-4993(18)30258-X. [DOI] [PubMed] [Google Scholar]
- 30.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10(6):423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 31.Mosley-Johnson, Elise, et al. Assessing the relationship between adverse childhood experiences and life satisfaction, psychological well-being, and social well-being: United States Longitudinal Cohort 1995–2014. Quality of Life Research 28.4 (2019): 907–914. [DOI] [PMC free article] [PubMed]
- 32.Liu Y, Croft JB, Chapman DP, Perry GS, Greenlund KJ, Zhao G, Edwards VJ. Relationship between adverse childhood experiences and unemployment among adults from five US states. Social psychiatry and psychiatric epidemiology. 2013;48(3):357–369. doi: 10.1007/s00127-012-0554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuiper JS, Zuidersma M, Voshaar RC, Zuidema SU, van den Heuvel ER, Stolk RP, Smidt N. Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing research reviews. 2015;1(22):39–57. doi: 10.1016/j.arr.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Chan QL, Xu X, Shaik MA, Chong SS, Hui RJ, Chen CL, Dong Y. Clinical utility of the informant AD8 as a dementia case finding instrument in primary healthcare. Journal of Alzheimer’s Disease. 2016;49(1):121–127. doi: 10.3233/JAD-150390. [DOI] [PubMed] [Google Scholar]