Abstract
Background
Medicaid expansion under the Affordable Care Act (ACA) is associated with increased insurance coverage among patients with cancer. Whether these gains translate to improved survival is largely unknown. This study examines changes in 2-year survival among patients newly diagnosed with cancer following the ACA Medicaid expansion.
Methods
Patients aged 18-62 years from 42 states’ population-based cancer registries diagnosed pre (2010-2012) and post (2014-2016) ACA Medicaid expansion were followed through September 30, 2013, and December 31, 2017, respectively. Difference-in-differences (DD) analysis of 2-year overall survival was stratified by sex, race and ethnicity, census tract–level poverty, and rurality.
Results
A total of 2 555 302 patients diagnosed with cancer were included from Medicaid expansion (n = 1 523 585) and nonexpansion (n = 1 031 717) states. The 2-year overall survival increased from 80.58% pre-ACA to 82.23% post-ACA in expansion states and from 78.71% to 80.04% in nonexpansion states, resulting in a net increase of 0.44 percentage points (ppt) (95% confidence interval [CI] = 0.24ppt to 0.64ppt) in expansion states after adjusting for sociodemographic factors. By cancer site, the net increase was greater for colorectal cancer (DD = 0.90ppt, 95% CI = 0.19ppt to 1.60ppt), lung cancer (DD = 1.29ppt, 95% CI = 0.50ppt to 2.08ppt), non-Hodgkin lymphoma (DD = 1.07ppt, 95% CI = 0.14ppt to 1.99ppt), pancreatic cancer (DD = 1.80ppt, 95% CI = 0.40ppt to 3.21ppt), and liver cancer (DD = 2.57ppt, 95% CI = 1.00ppt to 4.15ppt). The improvement in 2-year overall survival was larger among non-Hispanic Black patients (DD = 0.72ppt, 95% CI = 0.12ppt to 1.31ppt) and patients residing in rural areas (DD = 1.48ppt, 95% CI= -0.26ppt to 3.23ppt), leading to narrowing survival disparities by race and rurality.
Conclusions
Medicaid expansion was associated with greater increase in 2-year overall survival, and the increase was prominent among non-Hispanic Blacks and in rural areas, highlighting the role of Medicaid expansion in reducing health disparities. Future studies should monitor changes in longer-term health outcomes following the ACA.
A major component of the Patient Protection and Affordable Care Act (ACA) provides states financial incentives to increase Medicaid eligibility to adults aged 18-64 years with household income up to 138% federal poverty line regardless of parental status. Upon Medicaid expansion becoming effective in January 2014, 24 states and the District of Columbia expanded Medicaid eligibility, with more states opting in during later years. To date, 38 states and the District of Columbia have adopted Medicaid expansion and 12 states have not (1).
Previous studies have found that Medicaid expansion was associated with reduced uninsured rate, increases in cancer screening, shifts to early-stage cancers at diagnosis, and declines in problems affording health care among cancer survivors (2-5). Moreover, Medicaid expansion narrowed socioeconomic disparities in insurance coverage among cancer patients and survivors (2-6). Whether these gains translate to improved survival, however, is largely unknown. A few studies that examined the benefit of Medicaid expansion on cancer survival only included a limited number of states or selected cancer sites, and they reported mixed results (7-10). Herein, using a recently available population-based cancer registry dataset from 42 states, we examined the changes in 2-year survival rates among newly diagnosed cancer patients following the ACA Medicaid expansion by cancer site and key socioeconomic factors.
Methods
Data and Sample
We used the Cancer Incidence in North America (CiNA) Survival dataset compiled by the North American Association of Central Cancer Registries (NAACCR) (11). The dataset includes population-based central cancer registries that meet quality standards and completeness requirements (12). Deaths were ascertained through either passive or active patient follow-up and linkages with state death data and the National Death Index (12). This CiNA Survival dataset provides vital status through December 31, 2017, for cancer patients from the majority of states and through December 31, 2016, for patients from the District of Columbia, Michigan, Nevada, and Virginia. We identified patients aged 18-62 years newly diagnosed with first primary cancers in 2010-2012 and 2014-2016 residing in 24 states (AZ, AR, CA, CO, CT, DE, HI, IL, IA, KY, MD, MI, MN, NV, NH, NJ, NM, NY, ND, OH, OR, RI, WA, WV) and the District of Columbia that expanded Medicaid eligibility under the ACA in 2014 and 17 states (AL, FL, GA, ID, ME, MS, MO, NE, NC, OK, SD, TN, TX, UT, VA, WI, WY) that had not expanded Medicaid eligibility by the end of 2017. Patients from 5 states that expanded Medicaid in 2015 or later (AK, IN, LA, MT, PA) were not included. Also, patients from expansion states Massachusetts and Vermont as well as patients from nonexpansion states Kansas and South Carolina were not included in this study because these state registries declined participation or their data were not usable for survival analysis. As in conventional cancer surveillance research (13,14), patients identified from only death certificate or autopsy and in situ patients except for bladder cancer were excluded (cancer registries in the United States routinely report in situ and invasive bladder cancers combined because of difficulties in differentiating the 2 groups of lesions). Patients diagnosed in 2013 (n = 497 356) were excluded as 2013 was considered a phase-in period for the ACA (5); patients aged 63-64 years (n = 353 210) were excluded because they could become age-eligible for Medicare during follow-up; patients with unknown or invalid follow-up month (n = 37 906) or missing key sociodemographic characteristics (race and ethnicity, census tract poverty, rurality) (n = 85 491) were also excluded (Supplementary Figure 1).
Patients were characterized by age at diagnosis, sex, race and ethnicity, geographic region, census tract poverty, and rurality based on the residence at the time of diagnosis. Cancer type was coded according to the International Classification of Diseases for Oncology–3 and World Health Organization 2008 definitions, and cancer stage at diagnosis was coded according to the Surveillance, Epidemiology, and End Results (SEER) Program Summary Stage (15). Cause of death was coded according to the SEER cause-specific death classification definitions to identify deaths attributable to the index cancer (15). The study was based on deidentified data and was deemed exempt by the NAACCR institutional review board.
Statistical Analyses
Two-year overall survival was our primary outcome because it is clinically meaningful and represents the maximum follow-up time currently available. We used a difference-in-differences (DD) approach to examine the association of Medicaid expansion with changes in survival, where patients diagnosed in 2010-2012 were pre-ACA and patients diagnosed in 2014-2016 were post-ACA; patients from 24 expansion states and the District of Columbia were the treatment group, and those from 17 nonexpansion states were the control group. Patients were followed through September 30, 2013, if diagnosed in 2010-2012 (because patients could become eligible for Medicaid or self-purchased private insurance from Marketplace under the ACA in January 2014 during their follow-up) and December 31, 2017, if diagnosed in 2014-2016, or until death from any cause, 2 years after diagnosis, or loss to follow-up if earlier. Alive patients from the District of Columbia, Michigan, Nevada, and Virginia were considered lost to follow-up after December 31, 2016. Survival curves were drawn by pre- and post-ACA status and Medicaid expansion status. Unadjusted and multivariable flexible parametric survival models (16,17) were fitted to calculate the DD for 2-year overall survival, with state Medicaid expansion status, pre- and post-ACA, and their interaction term as the main independent variables and adjusting for age group, sex, race and ethnicity, geographic region, census tract–level poverty, and rurality. Insurance status and stage at diagnosis were not included in multivariable models as they are considered to be in the causal pathway. Compared with the semiparametric Cox proportional hazards models, the flexible parametric survival models allow direct estimation of the survival rates and differences in survival rates at a given follow-up time and are not constrained by the proportional hazard assumption (18). Analyses were conducted for all cancers combined and for 19 specific common cancer types as shown in Table 1 (19). Stratified analyses were conducted by sex, race and ethnicity, census tract–level poverty, and rurality to examine changes in disparities. To examine the role of stage at diagnosis in the causal pathway, we first conducted a supplementary DD analysis for diagnosis at local stage as the outcome in linear probability models to examine the association of Medicaid expansion and changes in stage diagnosis and then included stage as a covariate in adjusted flexible parametric models to examine if the observed associations of Medicaid expansion with 2-year overall survival attenuated. Furthermore, supplementary analyses were conducted for patients diagnosed with advanced stage (regional or distant) cancers to characterize the potential lead-time bias. Supplementary analyses were also conducted using cause-specific survival for patients whose cause of death information was usable for survival analysis, where death from the first primary cancer was the event. Two sensitivity analyses were conducted: 1) patients diagnosed pre-ACA (2010-2012) were followed through the end of the study instead of censoring on September 30, 2013; 2) patients from District of Columbia, Michigan, Nevada, and Virginia were excluded, as their follow-up time was available only through December 31, 2016, which was 1 year less than patients from other states.
Table 1.
Characteristics of newly diagnosed cancer patients, data compiled by the North American Association of Central Cancer Registriesa
| Characteristic | Total No. (%) |
Medicaid expansion states, No. (%) |
Nonexpansion states, No. (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Deaths | Cause-specific deaths | Cases | Deaths | Cause-specific deaths | Cases | Deaths | Cause-specific deaths | |
| (N = 2 555 302) | (N = 453 487) | (N = 332 520) | (n = 1 523 585) | (n = 257 950) | (n = 177 765) | (n = 1 031 717) | (n = 195 537) | (n = 154 755) | |
| Year of diagnosis | |||||||||
| 2010-2012 | 1 239 110 (48.5) | 226 685 (50.0) | 164 762 (49.5) | 744 802 (48.9) | 131 053 (50.8) | 89 606 (50.4) | 494 308 (47.9) | 95 632 (48.9) | 75 156 (48.6) |
| 2014-2016 | 1 316 192 (51.5) | 226 802 (50.0) | 167 758 (50.5) | 778 783 (51.1) | 126 897 (49.2) | 88 159 (49.6) | 537 409 (52.1) | 99 905 (51.1) | 79 599 (51.4) |
| Sex | |||||||||
| Male | 1 198 446 (46.9) | 263 781 (58.2) | 192 306 (57.8) | 709 125 (46.5) | 149 373 (57.9) | 102 255 (57.5) | 489 321 (47.4) | 114 408 (58.5) | 90 051 (58.2) |
| Female | 1 356 856 (53.1) | 189 706 (41.8) | 140 214 (42.2) | 814 460 (53.5) | 108 577 (42.1) | 75 510 (42.5) | 542 396 (52.6) | 81 129 (41.5) | 64 704 (41.8) |
| Race and ethnicity | |||||||||
| Hispanic | 295 501 (11.6) | 48 586 (10.7) | 39 451 (11.9) | 183 073 (12.0) | 29 859 (11.6) | 23 421 (13.2) | 112 428 (10.9) | 18 727 (9.6) | 16 030 (10.4) |
| Non-Hispanic Black | 340 173 (13.3) | 76 020 (16.8) | 50 482 (15.2) | 165 396 (10.9) | 36 164 (14.0) | 20 751 (11.7) | 174 777 (16.9) | 39 856 (20.4) | 29 731 (19.2) |
| Non-Hispanic Otherb | 129 006 (5.0) | 20 646 (4.6) | 16 270 (4.9) | 100 661 (6.6) | 15 685 (6.1) | 12 284 (6.9) | 28 345 (2.7) | 4961 (2.5) | 3986 (2.6) |
| Non-Hispanic White | 1 790 622 (70.1) | 308 235 (68.0) | 226 317 (68.1) | 1 074 455 (70.5) | 176 242 (68.3) | 121 309 (68.2) | 716 167 (69.4) | 131 993 (67.5) | 105 008 (67.9) |
| Age group, y | |||||||||
| 18-44 | 534 393 (20.9) | 50 109 (11.0) | 37 506 (11.3) | 319 829 (21.0) | 28 774 (11.2) | 20 405 (11.5) | 214 564 (20.8) | 21 335 (10.9) | 17 101 (11.1) |
| 45-54 | 848 092 (33.2) | 144 222 (31.8) | 106 767 (32.1) | 505 423 (33.2) | 81 384 (31.6) | 56 713 (31.9) | 342 669 (33.2) | 62 838 (32.1) | 50 054 (32.3) |
| 55-62 | 1 172 817 (45.9) | 259 156 (57.1) | 188 247 (56.6) | 698 333 (45.8) | 147 792 (57.3) | 100 647 (56.6) | 474 484 (46.0) | 111 364 (57.0) | 87 600 (56.6) |
| Rural/urban status | |||||||||
| Metropolitan | 2 176 758 (85.2) | 372 833 (82.2) | 272 350 (81.9) | 1 331 108 (87.4) | 218 761 (84.8) | 149 939 (84.3) | 845 650 (82.0) | 154 072 (78.8) | 122 411 (79.1) |
| Nonmetropolitan urban | 338 607 (13.3) | 71 666 (15.8) | 53 527 (16.1) | 174 987 (11.5) | 35 345 (13.7) | 24 928 (14.0) | 163 620 (15.9) | 36 321 (18.6) | 28 599 (18.5) |
| Nonmetropolitan rural | 39 937 (1.6) | 8988 (2.0) | 6643 (2.0) | 17 490 (1.1) | 3844 (1.5) | 2898 (1.6) | 22 447 (2.2) | 5144 (2.6) | 3745 (2.4) |
| Census tract % persons below poverty | |||||||||
| <5.0% | 466 766 (18.3) | 54 725 (12.1) | 39 248 (11.8) | 315 412 (20.7) | 36 878 (14.3) | 25 754 (14.5) | 151 354 (14.7) | 17 847 (9.1) | 13 494 (8.7) |
| 5.0%-9.99% | 636 805 (24.9) | 95 062 (21.0) | 69 248 (20.8) | 403 453 (26.5) | 59 000 (22.9) | 40 556 (22.8) | 233 352 (22.6) | 36 062 (18.4) | 28 692 (18.5) |
| 10.0%-19.99% | 824 246 (32.3) | 155 029 (34.2) | 114 465 (34.4) | 463 752 (30.4) | 83 669 (32.4) | 57 546 (32.4) | 360 494 (34.9) | 71 360 (36.5) | 56 919 (36.8) |
| ≥20.0% or more | 627 485 (24.6) | 148 671 (32.8) | 109 559 (32.9) | 340 968 (22.4) | 78 403 (30.4) | 53 909 (30.3) | 286 517 (27.8) | 70 268 (35.9) | 55 650 (36.0) |
| Geographic region | |||||||||
| Pacific region | 427 456 (16.7) | 70 720 (15.6) | 57 996 (17.4) | 427 456 (28.1) | 70 720 (27.4) | 57 996 (32.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mountain states | 172 484 (6.8) | 28 176 (6.2) | 21 202 (6.4) | 134 067 (8.8) | 22 758 (8.8) | 16 740 (9.4) | 38 417 (3.7) | 5418 (2.8) | 4462 (2.9) |
| East North Central region | 394 420 (15.4) | 68 995 (15.2) | 29 351 (8.8) | 335 775 (22.0) | 59 801 (23.2) | 20 991 (11.8) | 58 645 (5.7) | 9194 (4.7) | 8360 (5.4) |
| West North Central region | 167 139 (6.5) | 28 853 (6.4) | 26 185 (7.9) | 90 941 (6.0) | 14 175 (5.5) | 12 887 (7.2) | 76 198 (7.4) | 14 678 (7.5) | 13 298 (8.6) |
| New England | 76 604 (3.0) | 11 661 (2.6) | 8 938 (2.7) | 63 358 (4.2) | 9262 (3.6) | 6727 (3.8) | 13 246 (1.3) | 2399 (1.2) | 2211 (1.4) |
| Mid-Atlantic region | 461 134 (18.0) | 74 667 (16.5) | 46 665 (14.0) | 392 789 (25.8) | 63 506 (24.6) | 46 665 (26.3) | 68 345 (6.6) | 11 161 (5.7) | 0 (0.0) |
| South Atlantic region | 376 663 (14.7) | 72 040 (15.9) | 65 003 (19.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 376 663 (36.5) | 72 040 (36.8) | 65 003 (42.0) |
| East South Central states | 186 518 (7.3) | 40 709 (9.0) | 26 522 (8.0) | 50 058 (3.3) | 11 212 (4.3) | 9971 (5.6) | 136 460 (13.2) | 29 497 (15.1) | 16 551 (10.7) |
| West South Central states | 292 884 (11.5) | 57 666 (12.7) | 50 658 (15.2) | 29 141 (1.9) | 6516 (2.5) | 5788 (3.3) | 263 743 (25.6) | 51 150 (26.2) | 44 870 (29.0) |
| Cancer type | |||||||||
| Female breast | 469 917 (18.4) | 19 516 (4.3) | 13 707 (4.1) | 281 914 (18.5) | 10 747 (4.2) | 7070 (4.0) | 188 003 (18.2) | 8769 (4.5) | 6637 (4.3) |
| Prostate | 295 275 (11.6) | 6512 (1.4) | 3 270 (1.0) | 176 474 (11.6) | 3 837 (1.5) | 1778 (1.0) | 118 801 (11.5) | 2675 (1.4) | 1492 (1.0) |
| Colon and rectum | 213 405 (8.4) | 35 597 (7.8) | 26 244 (7.9) | 124 383 (8.2) | 19 938 (7.7) | 13 848 (7.8) | 89 022 (8.6) | 15 659 (8.0) | 12 396 (8.0) |
| Lung and bronchus | 209 800 (8.2) | 127 177 (28.0) | 95 844 (28.8) | 118 351 (7.8) | 69 925 (27.1) | 49 188 (27.7) | 91 449 (8.9) | 57 252 (29.3) | 46 656 (30.1) |
| Uterine corpus | 108 016 (4.2) | 8805 (1.9) | 6 351 (1.9) | 68 431 (4.5) | 5346 (2.1) | 3622 (2.0) | 39 585 (3.8) | 3459 (1.8) | 2729 (1.8) |
| Oral cavity and pharynx | 79 527 (3.1) | 15 088 (3.3) | 10 511 (3.2) | 46 051 (3.0) | 8172 (3.2) | 5264 (3.0) | 33 476 (3.2) | 6916 (3.5) | 5247 (3.4) |
| Kidney and renal pelvis | 100 759 (3.9) | 12 424 (2.7) | 8 703 (2.6) | 58 277 (3.8) | 7071 (2.7) | 4653 (2.6) | 42 482 (4.1) | 5353 (2.7) | 4050 (2.6) |
| Melanoma | 126 222 (4.9) | 5781 (1.3) | 4 134 (1.2) | 76 107 (5.0) | 3329 (1.3) | 2259 (1.3) | 50 115 (4.9) | 2452 (1.3) | 1875 (1.2) |
| Non-Hodgkin lymphoma | 98 584 (3.9) | 13 636 (3.0) | 9 490 (2.9) | 60 128 (3.9) | 7929 (3.1) | 5187 (2.9) | 38 456 (3.7) | 5707 (2.9) | 4303 (2.8) |
| Thyroid | 150 539 (5.9) | 1 399 (0.3) | 735 (0.2) | 96 575 (6.3) | 816 (0.3) | 396 (0.2) | 53 964 (5.2) | 583 (0.3) | 339 (0.2) |
| Pancreas | 52 749 (2.1) | 35 953 (7.9) | 28 051 (8.4) | 31 215 (2.0) | 20 987 (8.1) | 15 459 (8.7) | 21 534 (2.1) | 14 966 (7.7) | 12 592 (8.1) |
| Liver and intrahepatic bile duct | 54 862 (2.1) | 33 164 (7.3) | 24 529 (7.4) | 31 881 (2.1) | 18 719 (7.3) | 12 927 (7.3) | 22 981 (2.2) | 14 445 (7.4) | 11 602 (7.5) |
| Ovary | 40 430 (1.6) | 7236 (1.6) | 5480 (1.6) | 25 026 (1.6) | 4413 (1.7) | 3155 (1.8) | 15 404 (1.5) | 2823 (1.4) | 2325 (1.5) |
| Bladder | 57 664 (2.3) | 6989 (1.5) | 4713 (1.4) | 35 521 (2.3) | 4071 (1.6) | 2572 (1.4) | 22 143 (2.1) | 2918 (1.5) | 2141 (1.4) |
| Esophagus | 22 310 (0.9) | 13 778 (3.0) | 10 370 (3.1) | 13 097 (0.9) | 7962 (3.1) | 5594 (3.1) | 9213 (0.9) | 5816 (3.0) | 4776 (3.1) |
| Leukemia | 62 618 (2.5) | 11 876 (2.6) | 8390 (2.5) | 37 167 (2.4) | 7043 (2.7) | 4793 (2.7) | 25 451 (2.5) | 4833 (2.5) | 3597 (2.3) |
| Brain and other nervous system | 45 123 (1.8) | 18 236 (4.0) | 14 126 (4.2) | 27 267 (1.8) | 10 862 (4.2) | 7978 (4.5) | 17 856 (1.7) | 7374 (3.8) | 6148 (4.0) |
| Cervix | 44 182 (1.7) | 7696 (1.7) | 5943 (1.8) | 24 745 (1.6) | 4143 (1.6) | 3016 (1.7) | 19 437 (1.9) | 3553 (1.8) | 2927 (1.9) |
| Stomach | 33 815 (1.3) | 16 382 (3.6) | 12 690 (3.8) | 20 845 (1.4) | 10 044 (3.9) | 7445 (4.2) | 12 970 (1.3) | 6338 (3.2) | 5245 (3.4) |
| Other sites | 289 505 (11.3) | 56 242 (12.4) | 39 239 (11.8) | 170 130 (11.2) | 32 596 (12.6) | 21 561 (12.1) | 119 375 (11.6) | 23 646 (12.1) | 17 678 (11.4) |
The percentages for cause-specific deaths were based on 2 077 319 cases with usable cause of death information. Medicaid expansion states included 24 states (AZ, AR, CA, CO, CT, DE, HI, IL, IA, KY, MD, MI, MN, NV, NH, NJ, NM, NY, ND, OH, OR, RI, WA, WV) and the District of Columbia that expanded Medicaid eligibility under the Affordable Care Act in 2014; nonexpansion states included 17 states (AL, FL, GA, ID, ME, MS, MO, NE, NC, OK, SD, TN, TX, UT, VA, WI, WY) that had not expanded Medicaid eligibility by the end of 2017.
Non-Hispanic Other included non-Hispanic American Indian or Alaska Native, Asian or Pacific Islander, and unknown.
The parallel assumption of the DD methods pre-ACA was tested by grouping the pre-ACA diagnoses into quarter intervals and testing the statistical significance of an expansion-by-quarter interaction term in the flexible parametric survival models. P values for the interaction term were more than .05 for nearly all cancer types except bladder cancer and leukemia (Supplementary Table 1, available online), supporting the parallel trend assumption generally.
SEER*Stat version 8.3.8 (National Cancer Institute and Information Management Services, Inc) and SAS statistical software version 9.4 (SAS Institute Inc, Cary, NC, USA) were used in data preparation and descriptive analyses. The rstpm2 package (17) in R Studio version 1.1.419 (2009-2018 RStudio, Inc, Boston, MA, USA) was used to fit flexible parametric survival models. A 2-sided P value less than .05 was considered statistically significant. With sample size of more than 2.5 million, the study had greater than 90% power for detecting an increase in death risk as small as 1% (20).
Results
A total of 2 555 302 patients diagnosed with cancer were identified from Medicaid expansion (n = 1 523 585) and nonexpansion (n = 1 031 717) states. Non-Hispanic Black patients and patients from high poverty areas and nonmetropolitan areas were disproportionately represented in nonexpansion states (Table 1). During 2-year follow-up, 453 487 deaths occurred, including 257 950 in expansion states and 195 537 in nonexpansion states.
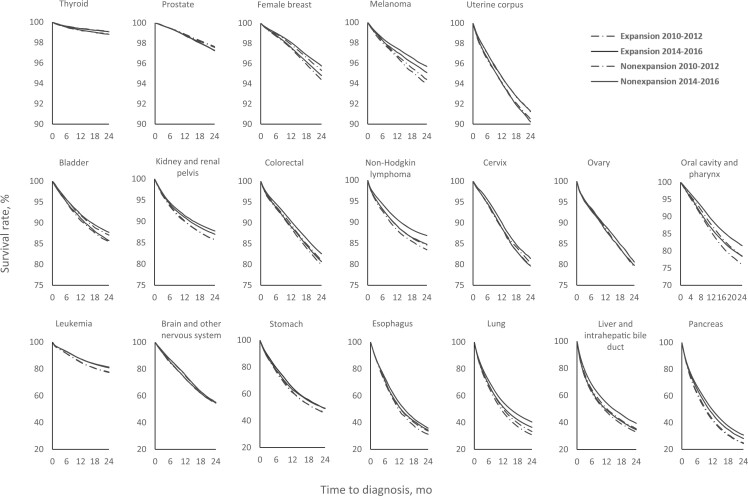
As shown in the survival curves (Figure 1), patients in expansion states generally had better survival than those in nonexpansion states. For most cancer types, overall survival improved after the ACA in both expansion and nonexpansion states, with the improvements for certain cancer types such as the cancers of lung, liver, and pancreas larger in the expansion states.
Figure 1.
Overall survival curves of patients newly diagnosed with cancer aged 18-62 years by Medicaid expansion status pre- and post–Affordable Care Act, in 24 months following diagnosis, North American Association of Central Cancer Registries 2010-2016. Patients diagnosed in 2011-2012 were followed up through September 30, 2013, and patients diagnosed in 2014-2016 were followed up through December 31, 2017.
As shown in Table 2, the 2-year overall survival rate increased from 80.58% pre-ACA to 82.23% post-ACA in Medicaid expansion states and from 78.71% to 80.04% in nonexpansion states for both sexes combined, resulting in a net increase of 0.44ppt (95% confidence interval [CI] = 0.24ppt to 0.64ppt) in expansion states after adjusting for sociodemographic factors. By cancer site and for both sexes combined, the net increase was greater for cancers of the colon and rectum (DD = 0.90ppt, 95% CI = 0.19ppt to 1.60ppt), lung (DD = 1.29ppt, 95% CI = 0.50ppt to 2.08ppt), non-Hodgkin lymphoma (DD = 1.07ppt, 95% CI = 0.14ppt to 1.99ppt), pancreas (DD = 1.80ppt, 95% CI = 0.40ppt to 3.21ppt), and liver (DD = 2.57ppt, 95% CI = 1.00ppt to 4.15ppt) (Table 2). When stratified by sex, the net increase in adjusted 2-year overall survival associated with Medicaid expansion was statistically significant for overall cancer combined (DD = 0.42ppt, 95% CI = 0.17ppt to 0.68ppt), colorectal cancer (DD = 0.66ppt, 95% CI = 0.07ppt to 1.25ppt), kidney cancer (DD = 1.34ppt, 95% CI = 0.36ppt to 2.31ppt), non-Hodgkin lymphoma (DD = 1.17ppt, 95% CI = 0.26ppt to 2.08ppt), and pancreatic cancer (DD = 2.55ppt, 95% CI = 0.33ppt to 4.76ppt) among women and for overall cancer combined (DD = 0.48ppt, 95% CI = 0.16ppt to 0.79ppt), lung cancer (DD = 1.34ppt, 95% CI = 0.32ppt to 2.37ppt), and liver cancer (DD = 3.58ppt, 95% CI = 1.82ppt to 5.34ppt) among men (Supplementary Table 2, available online). The exception to these patterns is a net decrease in 2-year overall survival for brain cancer (DD = −2.23ppt, 95% CI = −4.11ppt to −0.35ppt) in expansion vs nonexpansion states among women.
Table 2.
Changes in 2-year overall survival among newly diagnosed cancer patients, data compiled by the North American Association of Central Cancer Registriesa
| Expansion states |
Nonexpansion states |
Unadjusted model |
Adjusted modelb |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer type | 2010-2012 (%) | 2014-2016 (%) | Absolute difference (ppt) (95% CI) | 2010-2012 | 2014-2016 | Absolute difference (95% CI) (ppt) | DD (95% CI) (ppt) | P | DD (95% CI) (ppt) | P |
| All cancer combined | 80.58 | 82.23 | 1.66 (1.53 to 1.79) | 78.71 | 80.04 | 1.33 (1.17 to 1.49) | 0.33 (0.12 to 0.53) | .002 | 0.44 (0.24 to 0.64) | <.001 |
| Female breast | 95.30 | 95.73 | 0.43 (0.27 to 0.60) | 94.38 | 94.78 | 0.40 (0.18 to 0.62) | 0.03 (−0.24 to 0.31) | .81 | 0.09 (−0.17 to 0.36) | .49 |
| Prostate | 97.62 | 97.29 | −0.34 (−0.49 to -0.18) | 97.55 | 97.25 | −0.30 (−0.50 to -0.11) | −0.03 (−0.29 to 0.22) | .80 | −0.10 (−0.35 to 0.14) | .41 |
| Colon and rectum | 80.99 | 82.55 | 1.56 (1.10 to 2.02) | 79.87 | 80.64 | 0.77 (0.21 to 1.32) | 0.79 (0.07 to 1.51) | .03 | 0.90 (0.19 to 1.60) | .01 |
| Lung cancer and bronchus | 33.56 | 39.63 | 6.08 (5.53 to 6.62) | 31.00 | 35.60 | 4.60 (4.00 to 5.20) | 1.48 (0.67 to 2.29) | <.001 | 1.29 (0.50 to 2.08) | .001 |
| Uterine corpus | 91.21 | 91.25 | 0.04 (−0.41 to 0.49) | 90.42 | 90.20 | −0.22 (−0.83 to 0.40) | 0.26 (−0.51 to 1.02) | .51 | 0.21 (−0.53 to 0.95) | .57 |
| Oral cavity and pharynx | 78.40 | 81.37 | 2.97 (2.19 to 3.75) | 75.61 | 78.00 | 2.38 (1.42 to 3.34) | 0.59 (−0.65 to 1.82) | .35 | 0.62 (−0.58 to 1.81) | .31 |
| Kidney and renal pelvis | 85.64 | 87.68 | 2.04 (1.46 to 2.62) | 85.61 | 86.93 | 1.32 (0.63 to 2.01) | 0.72 (−0.18 to 1.62) | .12 | 0.74 (−0.15 to 1.62) | .10 |
| Melanoma | 94.40 | 95.66 | 1.26 (0.93 to 1.59) | 93.96 | 95.06 | 1.10 (0.68 to 1.52) | 0.16 (−0.38 to 0.69) | .57 | 0.31 (−0.21 to 0.83) | .24 |
| Non-Hodgkin lymphoma | 84.44 | 86.70 | 2.26 (1.67 to 2.84) | 83.32 | 84.61 | 1.29 (0.53 to 2.05) | 0.96 (0 to 1.93) | .049 | 1.07 (0.14 to 1.99) | .02 |
| Thyroid | 99.09 | 99.05 | −0.04 (−0.17 to 0.09) | 98.83 | 98.82 | −0.01 (−0.20 to 0.18) | −0.03 (−0.26 to 0.20) | .82 | −0.02 (−0.24 to 0.20) | .85 |
| Pancreas | 24.57 | 30.71 | 6.13 (5.17 to 7.09) | 23.92 | 27.98 | 4.06 (2.94 to 5.18) | 2.07 (0.60 to 3.54) | .006 | 1.80 (0.40 to 3.21) | .01 |
| Liver and intrahepatic bile duct | 35.60 | 39.84 | 4.24 (3.19 to 5.30) | 32.85 | 34.47 | 1.62 (0.42 to 2.81) | 2.63 (1.03 to 4.22) | .001 | 2.57 (1.00 to 4.15) | .001 |
| Ovary | 79.50 | 80.70 | 1.20 (0.15 to 2.25) | 79.45 | 79.71 | 0.26 (−1.08 to 1.61) | 0.94 (−0.77 to 2.64) | .28 | 0.99 (−0.66 to 2.65) | .24 |
| Bladder | 86.94 | 87.57 | 0.63 (−0.10 to 1.36) | 85.32 | 85.70 | 0.38 (−0.60 to 1.35) | 0.26 (−0.96 to 1.47) | .68 | 0.28 (−0.90 to 1.46) | .64 |
| Esophagus | 32.84 | 35.74 | 2.90 (1.29 to 4.51) | 30.82 | 33.72 | 2.90 (1.03 to 4.78) | −0.01 (−2.48 to 2.46) | .99 | 0.24 (−2.20 to 2.68) | .85 |
| Leukemia | 77.28 | 80.64 | 3.36 (2.48 to 4.23) | 77.12 | 81.09 | 3.97 (2.92 to 5.02) | −0.61 (−1.97 to 0.75) | .38 | −0.55 (−1.90 to 0.80) | .42 |
| Brain and other nervous system | 55.25 | 55.23 | −0.02 (−1.26 to 1.21) | 53.57 | 53.84 | 0.27 (−1.26 to 1.79) | −0.29 (−2.25 to 1.67) | .77 | −1.26 (−2.96 to 0.44) | .15 |
| Cervix | 80.07 | 81.24 | 1.18 (0.12 to 2.23) | 79.33 | 79.35 | 0.03 (−1.19 to 1.24) | 1.15 (−0.46 to 2.76) | .16 | 1.04 (−0.53 to 2.60) | .19 |
| Stomach | 45.26 | 48.95 | 3.68 (2.30 to 5.07) | 45.17 | 48.35 | 3.19 (1.44 to 4.94) | 0.50 (−1.74 to 2.73) | .66 | 0.45 (−1.75 to 2.65) | .69 |
Medicaid expansion states included 24 states (AZ, AR, CA, CO, CT, DE, HI, IL, IA, KY, MD, MI, MN, NV, NH, NJ, NM, NY, ND, OH, OR, RI, WA, WV) and the District of Columbia that expanded Medicaid eligibility under the Affordable Care Act in 2014; nonexpansion states included 17 states (AL, FL, GA, ID, ME, MS, MO, NE, NC, OK, SD, TN, TX, UT, VA, WI, WY) that had not expanded Medicaid eligibility by the end of 2017. CI = confidence interval; DD = difference-in-differences.
Flexible parametric survival models adjusted for age group, sex, race and ethnicity, census tract–level poverty, rurality, and region.
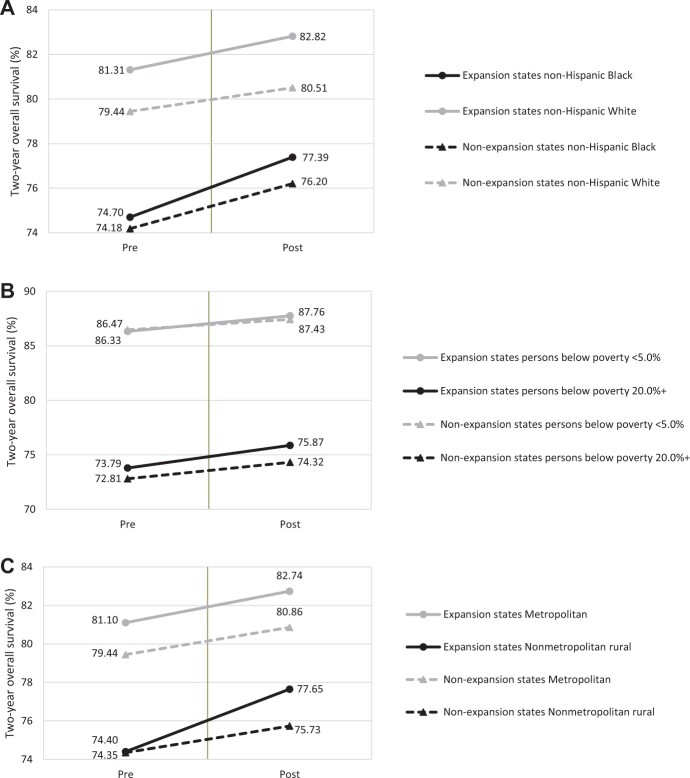
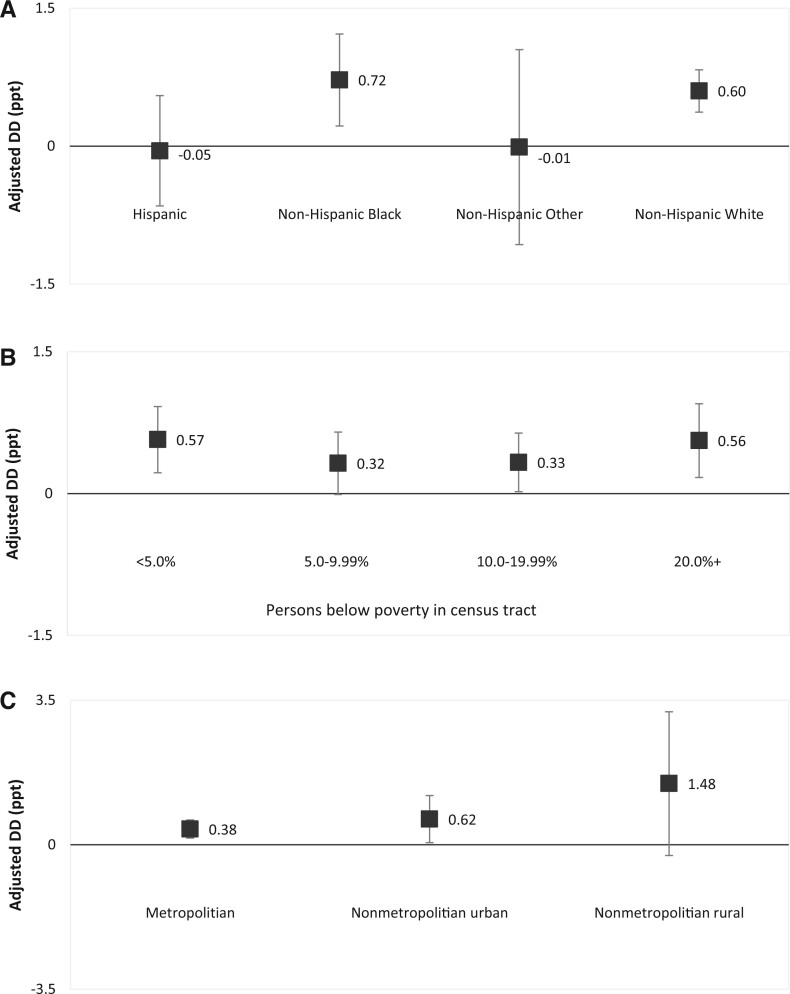
By sociodemographic characteristics (Figure 2;Supplementary Tables 3-5, available online), the largest improvement in 2-year overall survival rate following the ACA occurred in non-Hispanic Black patients (difference = 2.70ppt, 95% CI = 2.27ppt to 3.12ppt), in those residing in the poorest areas (difference = 2.08ppt, 95% CI = 1.77ppt to 2.38ppt), and in nonmetropolitan rural areas (difference = 3.25ppt, 95% CI = 1.93ppt to 4.57ppt) in expansion states, resulting in narrowing disparities by race, area-level poverty, and rurality in the expansion states but widening disparities between states that did vs did not expand Medicaid eligibility. The adjusted net increase in 2-year survival rate associated with Medicaid expansion was most prominent among non-Hispanic Black patients (DD = 0.72ppt, 95% CI = 0.12ppt to 1.31ppt) and patients living in the poorest areas (DD = 0.56ppt, 95% CI = 0.12ppt to 1.00ppt), richest areas (DD = 0.57ppt, 95% CI = 0.14ppt to 0.99ppt), and in rural areas (DD = 1.48ppt, 95% CI = −0.26ppt to 3.23ppt) (Figure 3;Supplementary Tables 3-5, available online). Two-year survival increased in Hispanic patients similarly in expansion and nonexpansion states, leading to little net change associated with Medicaid expansion.
Figure 2.
Changes in 2-year overall survival by Medicaid expansion status and sociodemographic factors among newly diagnosed cancer patients, North American Association of Central Cancer Registries 2010-2016. Two-year overall survival rates are presented by (A) race and ethnicity, (B) census tract–level poverty, and (C) rurality.
Figure 3.
Associations of Medicaid expansion status with increases in 2-year overall survival by sociodemographic factors among newly diagnosed cancer patients, North American Association of Central Cancer Registries 2010-2016. DD and their 95% confidence intervals are presented by (A) race and ethnicity, (B) census tract–level poverty, and (C) rurality. Non-Hispanic Other included American Indian or Alaska Native, Asian or Pacific Islander, and unknown. Adjusted DD was calculated with flexible parametric survival models adjusting for age group, sex, race and ethnicity, rurality, and region when applicable. DD = difference-in-differences, measuring the association between Medicaid expansion and changes in 2-year overall survival; ppt = percentage point.
The supplementary analyses for stage at diagnoses showed that Medicaid expansion was associated with a shift to local stage at diagnosis (Supplementary Table 6, available online), and including stage at diagnosis in the survival models attenuated the observed associations between Medicaid expansion and changes in 2-year overall survival (Supplementary Table 7, available online), demonstrating the role of stage at diagnosis in the causal pathway.
When limiting the analyses to patients diagnosed with regional and distant stages, patterns in 2-year overall survival were generally similar to the findings for all-stages combined (Supplementary Table 8, available online). Increases in 2-year cause-specific survival also had a similar pattern with those in 2-year overall survival although the magnitude of the increases in cause-specific survival were smaller (Supplementary Table 9, available online).
The sensitivity analysis without censoring pre-ACA patients on September 30, 2013 (Supplementary Table 10, available online) and the sensitivity analysis excluding patients from the District of Columbia, Michigan, Nevada, and Virginia (Supplementary Table 11, available online) both generated similar estimates as our main analyses and did not change the conclusions.
Discussion
Using recently released nationwide population-based data, this study provides evidence that Medicaid expansion under the ACA was associated with improved survival among newly diagnosed cancer patients. The evidence was strongest for cancers with poor prognosis such as cancers of the lung, pancreas, and liver, as well as for colorectal cancer, which can be detected through screening services. Moreover, the survival benefit associated with Medicaid expansion was greater in non-Hispanic Black patients and in people residing in rural areas, leading to narrowing disparities in cancer survival by race and rurality in the expansion states. It also led to widening of survival disparities between expansion and nonexpansion states. As such, our findings of the positive effects of Medicaid expansion on cancer survival provide further evidence for the importance of expanding Medicaid eligibility in all states, particularly considering the economic crisis and health-care disruptions caused by the COVID-19 pandemic. The American Rescue Plan Act of 2021 provides new incentives for Medicaid expansion in states that have yet to expand eligibility (21).
Since its implementation in 2014, Medicaid expansion under the ACA has increased insurance coverage in the general population and among cancer patients and survivors, particularly among low-income and minority populations (2-6). Such gains can lead to improved cancer survival through multiple pathways in the cancer care continuum. First, insurance coverage can increase access to screening services and other preventive services so that cancer can be detected at early stage, which is strongly associated with better cancer prognosis and survival. Several studies (4,22,23) have reported shifts to early stage cancer diagnosis associated with Medicaid expansion, which is also confirmed in our supplementary analyses. Our supplementary analyses also showed that the improved survival associated with Medicaid expansion could partly be explained by the shift to local stage diagnosis. Using lung cancer as an example, since annual screening was first recommended to high-risk individuals in 2013, uptake has been slowly increasing, and insurance coverage has been shown to be a strong correlate (24). Our data suggest that for the 1.29ppt net improvement in 2-year survival associated with Medicaid expansion among lung cancer patients, part of it can be explained by increase in local-stage diagnosis (Supplementary Table 7, available online). Lead-time bias may also account for a small portion of the improvement (Supplementary Table 8, available online).
Second, insurance coverage may improve timely receipt, adherence to, and/or completion of standard cancer treatments. The cancers with improved survival associated with Medicaid expansion in our study—lung cancer, liver cancer, and pancreatic cancer—are cancers with poor prognosis for which timely treatment is a strong determinant of short-term survival. Moreover, the availability of novel treatments, such as immunotherapy and targeted therapy, has increased for these cancers in recent years (25-27), and access to these new therapies, including access to clinical trials, may also have contributed to the improved survival. Previous studies that examined the association between Medicaid expansion and receipt of treatment for various cancer types found mixed results (6,28,29), suggesting the need for further examination. Third, insurance coverage can improve access to cancer surveillance care and care for other comorbid conditions among cancer survivors. Medicaid expansion has been shown to be associated with reduced care unaffordability among cancer survivors, with the largest reductions among people with low incomes and multiple comorbid conditions and who were unemployed (3). This improvement can be reflected in overall survival, which captures deaths from other diseases in addition to cancer. Last, insurance coverage may also increase access to palliative and end-of-life care. Although not designed to prolong life, studies have shown that cancer patients who received palliative care and hospice care not only reported improved quality of life but also lived longer (30). The effects of Medicaid expansion on receipt of end-of-life care and quality of life among cancer patients represents an important area for additional research.
Unexpectedly, we found that the 2-year survival after diagnosis of brain or other nervous system tumors among women improved more in nonexpansion states than in expansion states. The classification and reporting for brain and other central nervous system tumors have rapidly changed in recent years, and the adoption of such changes may vary by states’ cancer registration procedures (32,33). There were also expanding molecular understanding and advances in detection and diagnosis of these tumors in recent years (31,32). Future research is warranted to elucidate whether these changes affected expansion and nonexpansion states differently and its implication to cancer survival.
The survival improvements associated with Medicaid expansion vary substantially by sociodemographic factors. Particularly, larger improvements among cancer patients who were non-Hispanic Black and living in rural areas suggest Medicaid expansion plays a role in mitigating health disparities for socioeconomically disadvantaged populations. An unexpected finding is that the adjusted association of Medicaid expansion and survival improvement was found to be similarly high for patients in the poorest areas and richest areas. It could mean that Medicaid-eligible low-income patients who were living in the richest areas might have better resources and access to acquire Medicaid and health care afterward (eg, more treatment options); however, we did not have individual-level income information to gauge the possibility, which merits further investigation with adequate individual-level data. Nevertheless, expanding access to care for socioeconomically disadvantaged populations is exceptionally important now, because racial minorities and low-income Americans are disproportionately experiencing adverse effects of the COVID-19 pandemic (33-35). Expanding Medicaid to all low-income individuals in those states that have yet expanded Medicaid would mitigate the health disparities from the pandemic in these states. Further, Medicaid expansion was shown to have spillover effects on reducing adverse social determinants of health, such as housing evictions and recidivism (36,37), which may be challenges faced by the socioeconomically disadvantaged populations during and after the economic downturn from the pandemic.
This study presents the first evidence on the benefit of Medicaid expansion on cancer survival for a wide range of common cancer types with population-based nationwide data on more than 2.5 million newly diagnosed cancer patients. The findings on lung cancer are generally similar to 2 prior studies conducted in a limited number of states and selected cancers (7,8). Compared with previous studies using data from either the 13 states participating in the SEER program or the hospital-based National Cancer Database and examining single type or group of cancers (9,10), our sample has the least selection bias. Another strength of the current study is the power to conduct stratified analyses by sex, race and ethnicity, area-level income, and rurality to further investigate the effect of Medicaid expansion on cancer survival disparities. Moreover, the flexible parametric survival models were used to directly estimate the difference in 2-year survival, a more interpretable survival outcome compared with differences in hazard ratios in previous studies. Given the cause-of-death information provided in NAACCR CiNA data, we were also able to examine changes in both overall and cancer-specific survival associated with Medicaid expansion. As we enter the era where COVID-19 is a new competing cause of death, patterns in both overall survival and cause-specific survival among cancer patients merit continued monitoring.
The main limitation of our study is the relatively short follow-up time after the ACA Medicaid expansion implementation. With data 3 years postimplementation, we were able to examine only the changes in 2-year survival, with which differences for cancer types with better prognosis may not be detectable. For example, the adjusted DD were positive for 15 of the 19 investigated cancer types, indicating a tendency of improved survival associated with Medicaid expansion but statistically significant for only a few cancers with poor prognosis or possible early detection. The relatively short follow-up time also prevented us from including states that expanded Medicaid after 2015 in the study; examining the effects of duration of expansion with all states warrants future research. Improvements in 2-year survival may be partly explained by shortened lead time for some cancers because of increased screening or symptom surveillance services associated with gains in insurance coverage. However, improvements in 2-year survival were robust when we limited the sample to patients diagnosed with regional and distant stage diseases, suggesting that any lead-time bias has minimal effect on our findings. Moreover, we were unable to include a small percentage (<1.5%) of patients whose follow-up time was unknown or invalid; these patients tended to be male, non-Hispanic Black, and from rural and poor areas. Excluding them could result in slight overestimation of the survival rates and underestimation of disparities in our analyses. Last, the parallel assumption in DD analyses appeared to not hold for bladder cancer and leukemia; thus, findings for these cancers should be interpreted with caution.
In conclusion, using a population-based dataset capturing nationwide cancer patients, this study found that Medicaid expansion was associated with greater increase in 2-year overall survival, largely driven by the improvements in survival for cancer types with poor prognosis, suggesting improved access to timely and effective treatments. Improved survival associated with Medicaid expansion was also found for cancers amenable to screening services among females, suggesting increased access to screening and preventive services for women. Furthermore, the increase was largest among people who were non-Hispanic Black and living in rural areas, highlighting the promising role of Medicaid expansion in reducing health disparities. Future studies should monitor changes in longer-term health outcomes following the ACA.
Funding
Not applicable.
Notes
Role of the funder: Not applicable.
Disclosures: XH and JZ have received a grant from AstraZeneca for research outside of the current study. KRY serves on the Flatiron Health Equity Advisory Board. The other authors have no disclosures. KRY, who is a JNCI Associate Editor and coauthor on this article, was not involved in the editorial review or decision to publish the manuscript.
Author contributions: XH: project administration and visualization. XH and JZ: formal analysis and writing—original draft. XH, JZ, KRY, CJJ, AJ: conceptualization, investigation, methodology, writing—review & editing.
Prior presentations: An abstract containing main findings of the manuscript has been presented as an Oral Presentation at the American Society of Clinical Oncology Annual Meeting in June 4-8, 2021 (Virtual).
Acknowledgements: We gratefully acknowledge the contributions of the state and regional cancer registry staff for their work in collecting the North American Association of Central Cancer Registries CiNA data used in this study.
Data Availability
The data underlying this article were provided by the American Association of Central Cancer Registries (NAACCR) by permission. The data cannot be shared publicly per the Data User Agreement. The NAACCR CiNA Public Use Data Set with limited number of variables is available through application at https://www.naaccr.org/cina-public-use-data-set/.
Supplementary Material
Contributor Information
Xuesong Han, Surveillance & Health Equity Science, American Cancer Society, Atlanta, GA, USA.
Jingxuan Zhao, Surveillance & Health Equity Science, American Cancer Society, Atlanta, GA, USA.
K Robin Yabroff, Surveillance & Health Equity Science, American Cancer Society, Atlanta, GA, USA.
Christopher J Johnson, Cancer Data Registry of Idaho, Boise, ID, USA.
Ahmedin Jemal, Surveillance & Health Equity Science, American Cancer Society, Atlanta, GA, USA.
References
- 1.Kaiser Family Foundation. Status of state action on the Medicaid expansion decisions: interactive map. Kaiser Family Foundation; 2022. https://www.kff.org/medicaid/issue-brief/status-of-state-medicaid-expansion-decisions-interactive-map/. Accessed April 26, 2022.
- 2. Zhao J, Mao Z, Fedewa SA, et al. The Affordable Care Act and access to care across the cancer control continuum: a review at 10 years. CA Cancer J Clin. 2020;70(3):165–181. [DOI] [PubMed] [Google Scholar]
- 3. Han X, Jemal A, Zheng Z, et al. Changes in noninsurance and care unaffordability among cancer survivors following the affordable care act. J Natl Cancer Inst. 2020;112(7):688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Han X, Yabroff KR, Ward E, et al. Comparison of insurance status and diagnosis stage among patients with newly diagnosed cancer before vs after implementation of the Patient Protection and Affordable Care Act. JAMA Oncol. 2018;4(12):1713–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jemal A, Lin CC, Davidoff AJ, et al. Changes in insurance coverage and stage at diagnosis among nonelderly patients with cancer after the Affordable Care Act. J Clin Oncol. 2017;35(35):3906–3915. [DOI] [PubMed] [Google Scholar]
- 6. Moss HA, Wu J, Kaplan SJ, et al. The Affordable Care Act’s Medicaid expansion and impact along the cancer-care continuum: a systematic review. J Natl Cancer Inst. 2020;112(8):779–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu Y, Colditz GA, Kozower BD, et al. Association of Medicaid expansion under the Patient Protection and Affordable Care Act with non-small cell lung cancer survival. JAMA Oncol. 2020;6(8):1289–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lam MB, Phelan J, Orav EJ, et al. Medicaid expansion and mortality among patients with breast, lung, and colorectal cancer. JAMA Netw Open. 2020;3(11):e2024366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moghavem N, Oh DL, Santiago-Rodriguez EJ, et al. Impact of the Patient Protection and Affordable Care Act on 1-year survival in glioblastoma patients. Neurooncol Adv. 2020;2(1):vdaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barrington DA, Sinnott JA, Calo C, et al. Where you live matters: a National Cancer Database study of Medicaid expansion and endometrial cancer outcomes. Gynecol Oncol. 2020;158(2):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.North American Association of Central Cancer Registries. CiNA survival. https://www.naaccr.org/cina-survival/. Accessed February 23, 2021.
- 12. Johnson C, Wilson R, Sherman R, et al., eds. Cancer in North America: 2013-2017 Volume Four: Cancer Survival in the United States and Canada 2010-2016. Springfield, IL: North American Association of Central Cancer Registries, Inc.; 2020., , [Google Scholar]
- 13. Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222–231. [DOI] [PubMed] [Google Scholar]
- 14. Han X, Zhu S, Tian Y, et al. Insurance status and cancer stage at diagnosis prior to the Affordable Care Act in the United States. J Registry Manag. 2016;41(3):143–151. [PubMed] [Google Scholar]
- 15.National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer.gov/. Accessed February 23, 2021.
- 16. Royston P, Parmar MK.. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21(15):2175–2197. [DOI] [PubMed] [Google Scholar]
- 17. Clements M. Introduction to the rstpm2 package. The Comprehensive R Archive Network. https://cran.r-project.org/web/packages/rstpm2/vignettes/Introduction.pdf. Accessed March 18, 2021.
- 18. Burke K, Jones M, Noufaily A.. A flexible parametric modelling framework for survival analysis. J Roy Stat Soc Ser C (Appl Stat). 2020;69(2):429–457. [Google Scholar]
- 19. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA A Cancer J Clin. 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 20.Power and Sample Size. Calculator. http://powerandsamplesize.com/Calculators/Test-Time-To-Event-Data/Cox-PH-2-Sided-Equality. Accessed February 25, 2022.
- 21. Musumeci M. Medicaid provisions in the American Rescue Plan Act. Kaiser Family Foundation; 2021. https://www.kff.org/medicaid/issue-brief/medicaid-provisions-in-the-american-rescue-plan-act/ Accessed March 30, 2021.
- 22. Soni A, Simon K, Cawley J, et al. Effect of Medicaid expansions of 2014 on overall and early-stage cancer diagnoses. Am J Public Health. 2018;108(2):216–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ajkay N, Bhutiani N, Huang B, et al. Early impact of Medicaid expansion and quality of breast cancer care in Kentucky. J Am Coll Surg. 2018;226(4):498–504. [DOI] [PubMed] [Google Scholar]
- 24. Fedewa SA, Kazerooni EA, Studts JL, et al. State variation in low-dose computed tomography scanning for lung cancer screening in the United States. J Natl Cancer Inst. 2021;113(8):1044–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foley K, Kim V, Jaffee E, et al. Current progress in immunotherapy for pancreatic cancer. Cancer Lett. 2016;381(1):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gambardella V, Tarazona N, Cejalvo JM, et al. Personalized medicine: recent progress in cancer therapy. Cancers (Basel). 2020;12(4):1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van den Bulk J, Verdegaal EM, de Miranda NF.. Cancer immunotherapy: broadening the scope of targetable tumours. Open Biol. 2018;8(6):180037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ermer T, Walters SL, Canavan ME, et al. Understanding the implications of Medicaid expansion for cancer care in the US: a review. JAMA Oncol. 2022;8(1):139–148. [DOI] [PubMed] [Google Scholar]
- 29. Nathan NH, Bakhsheshian J, Ding L, et al. Evaluating Medicaid expansion benefits for patients with cancer: National Cancer Database analysis and systematic review. J Cancer Policy. 2021;29:100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 31. Miller KD, Ostrom QT, Kruchko C, et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin. 2021;71(5):381–406. [DOI] [PubMed] [Google Scholar]
- 32. Ostrom QT, Cioffi G, Waite K, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018. Neuro Oncol. 2021;23(12 suppl 2):iii1–iii105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaglioti AH, Li C, Douglas MD, et al. Population-level disparities in COVID-19: measuring the independent association of the proportion of Black population on COVID-19 cases and deaths in US counties. J Public Health Manag Pract. 2021;27(3):268–277. [DOI] [PubMed] [Google Scholar]
- 34. Neelon B, Mutiso F, Mueller NT, et al. Spatial and temporal trends in social vulnerability and COVID-19 incidence and death rates in the United States. PLoS One. 2021;16(3):e0248702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garcia E, Eckel SP, Chen Z, et al. COVID-19 mortality in California based on death certificates: disproportionate impacts across racial/ethnic groups and nativity. Ann Epidemiol. 2021;58:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Allen HL, Eliason E, Zewde N, et al. Can Medicaid expansion prevent housing evictions? Health Aff (Millwood). 2019;38(9):1451–1457. [DOI] [PubMed] [Google Scholar]
- 37. Fry CE, McGuire TG, Frank RG.. Medicaid expansion’s spillover to the criminal justice system: evidence from six urban counties. RSF. 2020;6(2):244–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by the American Association of Central Cancer Registries (NAACCR) by permission. The data cannot be shared publicly per the Data User Agreement. The NAACCR CiNA Public Use Data Set with limited number of variables is available through application at https://www.naaccr.org/cina-public-use-data-set/.