Abstract
A Tn5 insertion in tolC eliminated microcin J25 production. The mutation had little effect on the expression of the microcin structural gene and presumably acted by blocking microcin secretion. The tolC mutants carrying multiple copies of the microcin genes were less immune to the microcin. TolC is thus likely a component of a microcin export complex containing the McjD immunity protein, an ABC exporter.
The Escherichia coli peptide antibiotic microcin J25 (MccJ25) is active against gram-negative bacteria, including E. coli itself, Salmonella, and Shigella (17). Penetration of MccJ25 into E. coli cells is mediated by the outer membrane receptor FhuA, the TonB complex, and the SbmA protein (18, 19). Genetic studies have localized a cluster of three genes (mcjA, -B, and -C) necessary for the synthesis of the toxin and a fourth gene (mcjD) that confers immunity to a continuous 4.8-kb region of plasmid DNA (22). The nucleotide sequence of this region revealed four open reading frames corresponding to the four genes previously found (23). Comparison of the predicted McjA polypeptide with the amino acid sequence of MccJ25 indicated that mcjA encodes the primary structure of MccJ25 as a 58-amino-acid precursor. Subsequent removal of a 37-residue leader peptide and cyclization by head-to-tail linkage leads to the mature 21-amino-acid cyclic MccJ25 (3, 23). These processes could be mediated by the mcjB and mcjC gene products. The predicted mcjD gene product, which is highly similar to several membrane translocator proteins of the ABC exporters family, has been shown to be required for MccJ25 secretion, thus explaining its ability to confer MccJ25 immunity to susceptible cells (23).
We are interested in identifying chromosomal genes affecting the production of MccJ25. In this study, we analyze an E. coli null tolC mutant which is impaired in the production of the antibiotic.
Isolation and characterization of a mutation that abolishes MccJ25 production from the low-copy, natural MccJ25-producing plasmid.
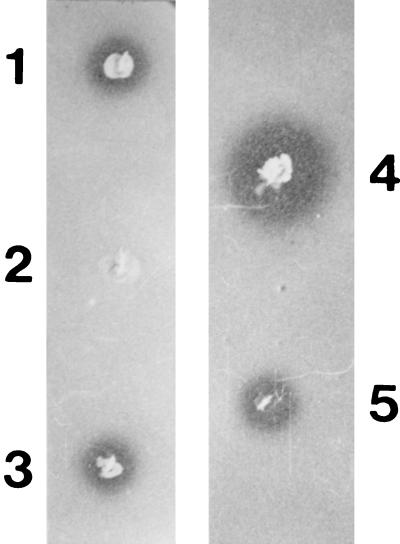
A chromosomal Tn5 insertion that eliminated MccJ25 production from strain MC4100 harboring the wild-type, low-copy-number, MccJ25-producing plasmid pTUC100 (17) was obtained by using λ467 (2) as previously described (4). The transposon was then introduced by P1 transduction (15) into several Hfr strains. Mating experiments using these Tn5 Hfr derivatives suggested a location of the insertion between 62 and 67 min of the E. coli genetic map. Transduction experiments demonstrated that the transposon was 27% cotransducible with the metC locus, which maps at 65 min on the E. coli chromosome (1). This placement raised the possibility that the mutation under study occurred in tolC, which is at min 66 and is cotransducible with metC at about the same frequency (1, 25). Therefore, we tested the mutant for increased sensitivity to detergents, a well-characterized tolC mutant phenotype (25), and it was found to be hypersensitive to 0.05% deoxycholate. Finally, we introduced well-characterized tolC mutations, such as those of strains SC44 (tolC::Tn5) (24) and CAG12184 (tolC::Tn10) (21), by P1 transduction into strain MC4100 (pTUC100). The transductants failed to show inhibition zones when grown on a lawn of sensitive cells (Fig. 1). On the basis of these observations, we concluded that our mutant had an insertion into tolC. Introduction of plasmid pAX629, which carries a cloned copy of the wild-type tolC gene (12), into the mutants rescued the MccJ25+ phenotype (Fig. 1), showing that the effect of the tolC mutation on MccJ25 production is primarily due to the inactivation of the tolC gene itself rather than to a possible polarity of the insertion mutation on the expression of a downstream gene.
FIG. 1.
Inhibition halos produced by tolC mutants and their respective parental strains carrying different microcin plasmids. Tests were carried out by stabbing individual colonies into a lawn of the hypersensitive indicator Salmonella newport. Strains: 1, MC4100 (pTUC100); 2, MC4100 tolC::Tn5 (pTUC100); 3, MC4100 tolC::Tn5 (pTUC100, pAX629); 4, MC4100 (pTUC202); 5, MC4100 tolC::Tn5 (pTUC202).
Cell growth and microcin production are affected in TolC− strains harboring multiple copies of microcin genes.
To test the effect of the tolC mutation on microcin production by a multicopy plasmid, strains MC4100, MC4100 tolC::Tn5, and MC4100 tolC::Tn10 were transformed with pTUC202, a pACYC184 derivative carrying the microcin production and immunity genes cloned in a 6-kb BamHI-SalI fragment (22). Introduction of this plasmid into the tolC mutants resulted in unstable transformants, which grew poorly upon restreaking in Luria-Bertani (LB) medium, forming small and sickly colonies, and frequently lost viability after a few passages. This phenotype was even more severe in minimal medium, where transformants failed to form colonies upon restreaking. This is probably due to the greatest microcin production in this medium (17). In contrast, control MC4100 (pTUC202) transformants gave rise to healthy colonies in both media. The growth-inhibitory phenotype was due to microcin production, because nonproducing derivatives of pTUC202 did not affect the growth of tolC cells.
The size of the antibiosis halo was reduced in strain MC4100 tolC::Tn5 (pTUC202) compared to that produced by the parental strain (Fig. 1). Microcin activity was measured by the critical dilution method (14) in culture supernatants of MC4100 (pTUC202) and MC4100 tolC::Tn5 (pTUC202) grown in LB medium and was expressed as the reciprocal of the last dilution giving a clear inhibition zone. The stationary-phase supernatant of the tolC derivative had a microcin titer 12-fold lower than that of the parent strain. To determine the levels of intracellular microcin, lysates from these strains were prepared and titrated as described previously (22). No difference in the amount of internal microcin of the wild type and the mutant was observed; lysates from both strains had a microcin titer of 32.
The growth-inhibitory phenotype is relieved by mutations that reduce MccJ25 synthesis.
M9 plates supplemented with chloramphenicol were seeded with MC4100 tolC::Tn5 (pTUC202) cells and incubated for 48 h at 37°C. Several mutants that grew well upon restreaking on the same medium were selected for further analysis. They were chloramphenicol resistant and therefore still retained the plasmid. However, they did not secrete microcin. In addition, they remained sensitive to deoxycholate, and thus were not tolC+ revertants (which could arise by precise excision of the Tn5). The apparent suppression of the unhealthy phenotype could result from either a plasmid or a host mutation. If suppression resulted from a mutation in a bacterial gene (e.g., a target mutation or a regulatory mutation which lessened the toxin production), then pTUC202 isolated from the candidate suppressor cells would be expected to display a normal microcin production phenotype when subsequently transformed into fresh MC4100 cells. In this way, we determined that several of our mutants contained host mutations. Additionally, when these mutants were spontaneously cured of the resident plasmid and tested for resistance to exogenous MccJ25, none of the cured derivatives was resistant, suggesting that they were not target mutants but carried regulatory mutations impairing expression of the mcj genes. This was confirmed by introducing plasmid pTUC202 with a mcjA-lacZ translational fusion into one of these mutants as well as into the tolC parental strain. There was a large decrease in mcjA expression in comparison with the control; in fact, the mutant showed a 35-fold reduction in β-galactosidase activity (68 versus 2,415 Miller units [15]). Finally, plasmid mutants impaired in microcin production were also obtained. Altogether, these results indicated that a decrease in MccJ25 production is responsible for the suppression of the unhealthy phenotype.
TolC effect on microcin biosynthesis.
Reduced MccJ25 release from tolC cells might be due to a deficiency in antibiotic biosynthesis or to a decrease in its secretion. To discriminate between these possibilities, plasmid pTUC202 φ(mcjA-lacZ) was transformed into MC4100 and MC4100 tolC::Tn10. The levels of β-galactosidase were not significantly different in both strains throughout exponential growth. For example, at an optical density at 600 nm of 0.7 the wild-type and mutant strains had β-galactosidase readings of 90 and 100 Miller units, respectively. In the stationary phase, after overnight growth, the tolC strain had roughly threefold less β-galactosidase activity than the parent strain (5,000 versus 14,000 units). Although this effect may contribute to the overall decrease in MccJ25 production, it seems too weak to explain by itself the 12-fold reduction in the amount of extracellular microcin seen in mutant cultures. Thus, most of the effect of tolC mutations is likely to be at the secretion level.
The effect of tolC mutations is not a consequence of their effect on major porin expression.
It has been demonstrated that tolC mutations increase the transcription of micF antisense RNA, leading to a concomitant reduction in the expression of OmpF (16). To test whether the effect of the tolC mutation on microcin production was due to the lack of porin, plasmid pTUC202 was introduced into strains MC4100 ompF::Tn5 and MC4100 ompR101. The typical ompR101 mutation normally results in the absence of both OmpF and OmpC proteins (20). The transformants synthesized microcin normally, indicating that the major porins are not involved in microcin production.
The absence of TolC affects MccJ25 immunity negatively.
When the immunity to exogenous microcin of MC4100 (pTUC202) and MC4100 tolC::Tn5 (pTUC202) was titrated by the critical dilution method we found that while the parent strain was fully immune, the microcin preparation used could be diluted 1,280-fold and still inhibit growth of the tolC mutant. This phenomenon was not dependent on the kind of mutation or the genetic background since the same result was observed when another tolC-defective strain, CAG12184 (tolC::Tn10) (21), was used as a host. These results clearly indicated that TolC did affect the expression of immunity. To test the possibility that this deficient immunity phenotype could be alleviated by raising the number of copies of the immunity gene, MC4100 tolC::Tn5 was first transformed with pJS300, a pUC18 derivative carrying only the immunity gene (22), and then with pTUC202. In spite of the supplementary immunity protein, double transformants still exhibited the deficient immunity phenotype and the growth deficiency in minimal medium. We have evidence indicating that endogenously synthesized MccJ25 requires the mcjD gene product to be exported out of the cells (23). Indeed, the McjD protein displays all the structural characteristics common to ABC transporters (7, 23). Thus, the immunity to MccJ25 conferred by McjD seems to be mediated by active extrusion of microcin, which would keep the antibiotic concentration in the cytoplasm below a critical level. Peptide antibiotic systems usually contain a specific immunity gene, not involved in antibiotic production. For example, in the case of the microcin B17 immunity system, although the two proteins McbE-McbF forming the ABC exporter provide a limited immunity by export of the antibiotic, a third component, McbG, is required for full immunity (10). Its counterpart in the MccJ25 system has not yet been found (22, 23). In the present study, we tested the degree of immunity provided by McjD alone by spotting solutions with various MccJ25 concentrations onto an LB plate seeded with MC4100 cells harboring the mcjD gene cloned into low-copy-number plasmids (pACYC184 or pSC101). The transformants were fully immune to added microcin of the highest concentration available (1 mg/ml; 10 μg per spot). Since TolC is implicated in the secretion of several proteins in E. coli (6, 8, 9, 13, 24), it does seem plausible that McjD could form an export complex with TolC for the secretion of MccJ25. If so, the absence of TolC should lead to an impairment of the immunity function. A prediction of this model is that tolC cells harboring pTUC202 should accumulate intracellular active MccJ25. However, as stated before, we have not been able to extract increased amounts of MccJ25 from these cells. It is possible that after transformation with the plasmid there is in fact an accumulation of microcin, which would exert a toxic effect on several metabolic processes, including that of MccJ25 synthesis itself. At the time of lysing the cells, they are so sick that this would lead to a poor recovery of intracellular microcin. In addition, there could be an increased proteolytic degradation of accumulated internal microcin in these cells. A similar situation has been described by Garrido et al. (10) for mcbE and mcbF mutants, which cannot export microcin B17. These authors have been unable to extract microcin B17 from these immune deficient cells, and speculate that this failure is due to the poor growth of the cells and the loss of the microcin-producing plasmid, even under selective pressure. Finally, note that a lack of accumulation following a blockade in export has also been observed for α-hemolysin (24).
Another explanation for the growth-inhibitory phenotype may be proposed: MccJ25 interferes with cell division, leading to filamentous growth (17). One possible reason for filamentation is a defect in nucleoid segregation. On the other hand, tolC mutants are defective in chromosome partitioning, producing anucleate cells (12). It is conceivable that the two effects are additive, that is, the presence of the tolC mutation would potentiate the action of the antibiotic.
Interestingly, the deficient immunity to exogenous microcin was only seen when the cells were producing the antibiotic (i.e., in the presence of endogenous microcin). In fact, MC4100 tolC::Tn5 cells bearing a nonproducing derivative of pTUC202, obtained by insertional inactivation of the mcjA gene with Tn5, were completely resistant to exogenously added microcin. This resistance was due to the immunity gene mcjD, since TolC− cells devoid of the plasmid were completely sensitive to the antibiotic. An attractive hypothesis is that, when absent, TolC may be substituted by another, less efficient, outer membrane translocator. Supporting this is the finding that the tolC null mutations did not completely block MccJ25 secretion from pTUC202 (Fig. 1). This alternative efflux system would be sufficient to provide protection against exogenous microcin. However, in microcin-synthesizing TolC− cells it could become saturated by endogenous microcin, thus leading to high susceptibility to exogenously added antibiotic.
Finally, it is interesting to note that bacterial ABC exporter systems for protein secretion generally require an accessory factor, which is believed to connect the inner membrane transporter with the outer membrane component (5). The gene encoding the accessory factor is always found linked to the gene encoding the ABC protein. However, no accessory factor gene has been detected in the MccJ25 genetic system (23). Similarly, the microcin B17 operon, which also comprises an ABC exporter, does not include an associated accessory factor (10, 11). It may be that in these cases the host provides such factor.
Acknowledgments
We are indebted to Barbara Bachmann (at the E. coli Genetic Stock Center), Cécile Wandersman, Felipe Moreno, and Carol A. Gross for providing strains.
This work was supported by grants from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Consejo de Investigaciones de la Universidad Nacional de Tucumán (CIUNT), and Centro Argentino Brasilero de Biotecnología (CABBIO). J.O.S. and M.J.C. were recipients of CONICET fellowships. M.A.D. was supported by a CIUNT fellowship. R.N.F. was a career investigator of CONICET.
REFERENCES
- 1.Bachmann B J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990;54:130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg D E. Insertion and excision of the transposable kanamycin resistance determinant Tn5. In: Bukari A I, Shapiro J A, Adhya S L, editors. DNA insertion elements, plasmids, and episomes. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1977. pp. 205–212. [Google Scholar]
- 3.Blond, A., J. Péduzzi, C. Goulard, M. J. Chiuchiolo, M. Barthélémy, Y. Prigent, R. A. Salomón, R. N. Farías, F. Moreno, and S. Rebuffat. The cyclic structure of microcin J25, a 21-residue peptide antibiotic from Escherichia coli. Eur. J. Biochem., in press. [DOI] [PubMed]
- 4.De Bruijn F J, Rossbach S. Transposon mutagenesis. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C: ASM Press; 1994. pp. 387–405. [Google Scholar]
- 5.Dinh T, Paulsen I T, Saier M H., Jr A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of gram-negative bacteria. J Bacteriol. 1994;176:3825–3831. doi: 10.1128/jb.176.13.3825-3831.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fath M J, Skvirsky R, Gilson L, Mahanty H K, Kolter R. The secretion of colicin V. In: James R, Lazdunski F, Pattus F, editors. Bacteriocins, microcins, and lantibiotics. Heidelberg, Germany: Springer-Verlag; 1992. pp. 331–348. [Google Scholar]
- 7.Fath M J, Kolter R. ABC transporters: bacterial exporters. Microbiol Rev. 1993;57:995–1017. doi: 10.1128/mr.57.4.995-1017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foreman D T, Martínez Y, Coombs G, Torres A, Kupersztoch Y M. TolC and DsbA are needed for the secretion of STB, a heat-stable enterotoxin of Escherichia coli. Mol Microbiol. 1995;18:237–245. doi: 10.1111/j.1365-2958.1995.mmi_18020237.x. [DOI] [PubMed] [Google Scholar]
- 9.Gaggero C, Moreno F, Laviña M. Genetic analysis of microcin H47 antibiotic system. J Bacteriol. 1993;175:5420–5427. doi: 10.1128/jb.175.17.5420-5427.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrido M C, Herrero M, Kolter R, Moreno F. The export of the DNA replication inhibitor microcin B17 provides immunity for the host cell. EMBO J. 1988;7:1853–1862. doi: 10.1002/j.1460-2075.1988.tb03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genilloud O, Moreno F, Kolter R. DNA sequence, products, and transcriptional pattern of the genes involved in production of the DNA replication inhibitor microcin B17. J Bacteriol. 1989;171:1126–1135. doi: 10.1128/jb.171.2.1126-1135.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiraga S, Niki H, Ogura T, Ichinose C, Mori H, Ezaki B, Jaffé A. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J Bacteriol. 1989;171:1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Létoffé S, Ghigo J-M, Wandersman C. Identification of two components of the Serratia marcescens metalloprotease transporter: protease SM secretion in Escherichia coli is TolC dependent. J Bacteriol. 1993;175:7321–7328. doi: 10.1128/jb.175.22.7321-7328.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayr-Harting A, Hedges A J, Berkeley R C W. Methods for studying bacteriocins. Methods Microbiol. 1972;7A:315–422. [Google Scholar]
- 15.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 16.Misra R, Reeves P. Role of micF in the tolC-mediated regulation of OmpF, a major outer membrane protein of Escherichia coli K-12. J Bacteriol. 1987;169:4722–4730. doi: 10.1128/jb.169.10.4722-4730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salomón R A, Farías R N. Microcin 25, a novel antimicrobial peptide produced by Escherichia coli. J Bacteriol. 1992;174:7428–7435. doi: 10.1128/jb.174.22.7428-7435.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salomón R A, Farías R N. The FhuA protein is involved in microcin 25 uptake. J Bacteriol. 1993;175:7741–7742. doi: 10.1128/jb.175.23.7741-7742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salomón R A, Farías R N. The peptide antibiotic microcin 25 is imported through the TonB pathway and the SbmA protein. J Bacteriol. 1995;177:3323–3325. doi: 10.1128/jb.177.11.3323-3325.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarma V, Reeves P. Genetic locus (ompB) affecting a major outer membrane protein in Escherichia coli K-12. J Bacteriol. 1977;132:23–27. doi: 10.1128/jb.132.1.23-27.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer M, Baker T A, Schnitzler G, Deischel S M, Goel M, Dove W, Jaacks K J, Grossman A D, Erickson J W, Gross C A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989;53:1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solbiati J O, Ciaccio M, Farías R N, Salomón R A. Genetic analysis of plasmid determinants for microcin J25 production and immunity. J Bacteriol. 1996;178:3661–3663. doi: 10.1128/jb.178.12.3661-3663.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solbiati, J. O., M. Ciaccio, R. N. Farías, J. E. González-Pastor, F. Moreno, and R. Salomón. Sequence analysis of the four plasmid genes required to produce the circular peptide antibiotic microcin J25. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 24.Wandersman C, Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci USA. 1990;87:4776–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitney E N. The tolC locus in Escherichia coli K-12. Genetics. 1971;67:39–53. doi: 10.1093/genetics/67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]