Abstract
Background
Several studies report that sexuality is often affected by inflammatory bowel diseases (IBD). The aim of this meta-analysis was to investigate the association between IBD and sexual function.
Methods
A literature search was conducted in PubMed, Web of Science, and EMBASE databases (up to September 1, 2020). Scores of sexual functions with a standard deviation and odds ratio (OR) or relative risk (RR) with a 95% CI were used to analysis the association between IBD and sexual function.
Results
Eleven studies with 7,018 male IBD cases and 1,803 female IBD cases were included in the meta-analysis. In male individuals, the pooled results revealed that IBD was significantly associated with impaired erectile function and poor sexual satisfaction (RR for erectile function =1.50, 95% CI: 1.22 to 1.84, P<0.0001; standard mean difference for sexual satisfaction =−0.24, 95% CI: −0.33 to −0.15, P<0.0001). And among female individuals, IBD had impact on most sub-domains of sexual function, except pains.
Conclusions
IBD is associated with worse sexual function. It has significant impact on erectile function and satisfaction for male individuals and has impact on most sub-domains of sexual function for female individuals.
Keywords: Inflammatory bowel disease (IBD), sexual function, erectile function, satisfaction in sexual, systematic review and meta-analysis
Introduction
Inflammatory bowel diseases (IBD) are a group of idiopathic chronic inflammatory intestinal ailments that are divided into two primary types: ulcerative colitis (UC) and Crohn’s disease (CD) (1). IBD is usually diagnosed in young individuals between the age of 15 and 30 (2). Commonly, it is more frequent in Western countries than in other areas of the world, with a prevalence of over 0.3% and affecting over 3.5 million individuals, according to the most recent statistics available (3,4). As a lifelong chronic disorder, IBD may impact health in several ways such as abdominal pain, diarrhea, and fatigue during the active phase of IBD. Also, many studies showed that IBD can cause severe depression and declines in health-related quality of life (5-7). Sexual quality is an important determinant of quality of life and depression is known as an important risk factor of sexual dysfunction. Thus, an association between IBD and sexual dysfunction may exist.
In fact, Gazzard et al. had reported decreased sexual activities in IBD patients 42 years ago (8). And several studies in recent years report that sexual problems are more common among IBD patients compared to the general population of similar age (9). The proportion of sexual dysfunction (reported by Knowles et al. and Mahmood et al.) (10,11) ranges between 44% to 53.9% and 40% to 66% in male patients and female patients, respectively. Besides, there are evidences indicating that IBD do not affect every specific aspect of sexual function (12). The number of relevant studies is still limited without a systematic review focused on specific sub-domains of sexual function. Therefore, the aim of current meta-analysis was to investigate the impact of IBD on overall and specific sub-domains of sexual function. We present the following article in accordance with the MOOSE reporting checklist (available at https://tau.amegroups.com/article/view/10.21037/tau-22-190/rc).
Methods
Literature search
A literature search was performed using the PubMed, Web of Science, and EMBASE databases with restrictions to the English language and publication time between January 1, 1990 and September 1, 2020. It was also registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42020204951) before screening studies for inclusion. The main search terms were sexual function, sexual dysfunction, sexual desire disorders, sexual arousal disorders, erectile dysfunction, premature ejaculation, orgasm disorders, sexual pain disorders, IBD, Ulcerative colon, and Crohn’s disease. The literature search was performed independently by 2 authors and discrepancies were resolved by consultation with a third author.
Study selection criteria
Any available epidemiologic evidence reporting the association between IBD and risk of sexual dysfunction in individuals was included in the present study. According to the patient, intervention, comparison, outcome, and study design (PICOS), the question that guided this meta-analysis was “Does IBD lead to a worse sexual function?”. The PICOS evidence was comprised of the following combinations: adult IBD patients (P); a history of sexual dysfunction or decreased sexual function consciously (I); compared with the general or non-IBD population (C); the assessment of sexual function (O); all study designs were accepted (S). Moreover, we also include studies that provided adequate data to calculate relative risk (RR) or odds ratios (OR) with 95% CI. The excluded criteria were as follows: (I) missing population data literatures; (II) review articles, comments, editorials, or case reports, etc.; (III) animal experiments; (IV) duplicated data; (V) IBD patients with relevant surgery history.
Measurement of IBD and sexual function
IBD and sexual function were defined according to the international classification of diseases codes (13). IBD were diagnosed according to the clinical criteria. Sexual function was measured by any existing and validated scales, such as the International Index of Erectile Function (IIEF), Female Sexual Function Index (FSFI), Brief Index of Sexual Functioning in Women (BISF-W), Arizona Sexual Experience Scale (ASEX), and Sexual Quality of Life Questionnaire (SQoL).
The BISF-W is a validated self-reported, 22-item inventory of sexual desire, activity, arousal, orgasm, and satisfaction (14). This scale is initially developed in response to the lack of a brief, standardized self-reported assessment of women’s overall sexual function that could assess both the qualitative and quantitative domains. It’s considered a useful and established instrument by several studies, although its scoring algorithm is still confronted with questions (15-17).
The ASEX is one of the most commonly used scales to measure sexual function. Its five items quantify sexual impulse, arousal, vaginal lubrication or penis hardening, orgasm reaching capacity, and orgasmic endurance satisfaction (18). This scale has been utilized in a variety of cross-sectional prevalence studies and randomized controlled trials of pharmacological treatment. It has been proven reliable and valid in the assessment of sexual function, as well as being easy-to-use in various clinical settings (19-21). Low scores indicate that the sexual response is strong, easy, and satisfactory, whereas high scores indicate the presence of sexual dysfunction. To obtain a more efficient correlation between IBD and worse sexual function, we picked a negative value of ASEX scores, since the outcome of the ASEX is different from all the other instruments.
Data extraction
Electronic extraction forms were used to extract the data. The extracted data included the authors, study titles, publication year, country, study design, age, number of participants, assessment of sexual function, scores, and OR/RRs for each sub-domain of sexual dysfunction. Discrepancies were resolved by consensus or by consultation with a third reviewer. Data extraction was performed independently by 2 authors and discrepancies were resolved by consultation with a third author.
Quality assessment
The Newcastle–Ottawa Quality Assessment Scale (NOS) was used for quality assessment of the case-control/cohort studies (22). Cross-sectional studies were assessed by using the Agency for Healthcare Research and Quality (AHRQ) checklist, which is an eleven-score system (23). The detail of quality assessment is provided in the supplementary.
Statistical analyses
The impact of IBD on specific sub-domains of sexual function was assessed by using the scores of various sexual function scales and standard deviation (SD). The association between IBD and sexual dysfunction was assessed by using OR/RR and its 95% CI. A random-effects model was used if significant heterogeneity existed (P<0.10, or P>0.10 but I2>50%), otherwise a fixed-effects model was used (P>0.10, or P<0.10 but I2<50%).
Heterogeneity among the studies was assessed using the Cochrane Q test (P<0.10) and I2 statistic. I2 values above 50% were considered high degrees of heterogeneity. Subgroup analyses and sensitivity analyses were conducted to further investigate the potential source of heterogeneity. Publication bias analyses were not conducted because of the limited number of studies in each analysis. Data analyses were performed using RevMan (Version5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
Results
Literature search
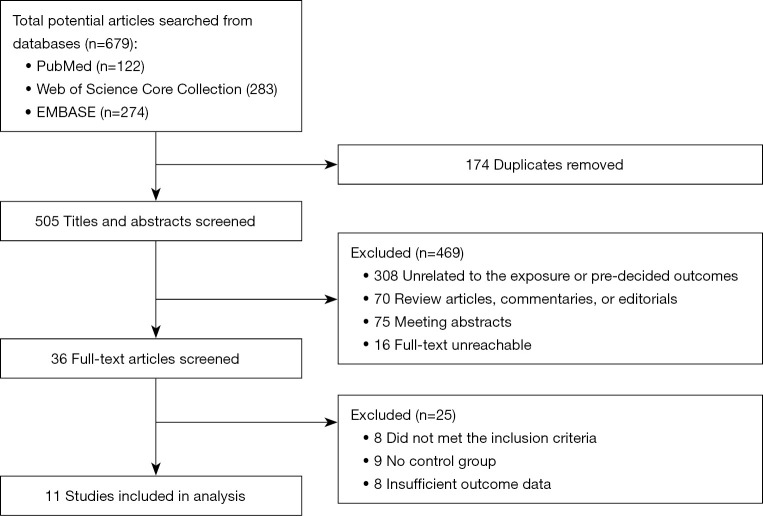
Eleven studies (24-34) with 8,821 IBD patients (7,018 males and 1,803 females) were included in this meta-analysis. Figure 1 shows a flow chart of the study selection process.
Figure 1.
Flow chart of studies selection.
Study characteristics
Among these 11 studies, five studies are cohort studies, two are case-control studies, and three are cross-sectional studies. The mean age of male individuals and female individuals ranged from 31.3 to 52.4 years and 33.6 to 43.8 years, respectively. The detailed characteristics of the 11 included studies are presented in Table 1.
Table 1. Characteristics of 11 included studies in this meta-analysis.
| Study | Design | Gender | Specific diseases | Study Group | Control Group | Diagnosis of IBD | Assessment of sexual dysfunction | Adjustments for potential confounders | Score* | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean age | No. | Mean age | No. | |||||||||
| Bel 2015 (24), The Netherlands | Retrospective cohort | M/F | IBD | M: 51.1±12.8; F: 42.9±12.9 | M: 119; F: 168 | M: 52.4±12.7; F: 43.8±12.8 | M: 91; F: 106 | Registered of Gastroenterology departments of a tertiary referral center and a general hospital | M: IIEF; F: FSFI | Age, depression, diseases activity | 7 | |
| Ateş Bulut 2019 (25), Turkey | Cross-sectional | M/F | IBD | 41±13 | M: 69; F: 53 | 43±13 | M: 20; F: 22 | Diagnosed by the gastroenterology outpatient or inpatient clinics with UC or CD | ASEX | Age, depression and anxiety, diseases activity | 5 | |
| Kao 2016 (26), China | Prospective cohort | M | IBD | 50.1±16.6 | 1,845 | 49.7±16.8 | 7,380 | The Longitudinal Health Insurance Database 2000 (LHID2000) of the NHIRD, provided by the NHRI of Taiwan | IIEF-5 | Age, sex, and comorbidities of CAD, PAD, stroke, CKD, hypertension, diabetes, hyperlipidemia, depression, and anxiety, and medications of steroids, opiates, antidepressants, and anxiolytics | 9 | |
| Marín 2013 (28), Spain | Case-Control | M/F | IBD | M: 46.5±10.2; F: 42.7±9.4 | M: 153; F: 201 | M: 46.1±10.6; F: 41.1±8.9 | M: 73; F: 127 | Identified from the IBD databases of Hospital Universitari Germans Trias i Pujol (Badalona) and Hospital de la Santa Creu i Sant Pau (Barcelona) | M: IIEF; F: FSFI | Age, corticosteroids, biological agents, perianal disease, depression, arterial hypertension, diabetes | 6 | |
| Moody 1992 (29), UK | Retrospective cohort | F | CD | 34.7±6.1 | 50 | 33.6±6.0 | 50 | Identified from the epidemiological data bases in Cardiff and Leicestershire | The modified structured questionnaire developed during actinological studies of risk factors in cervical adenocarcinoma | Age | 5 | |
| Moody 1993 (30), UK | Retrospective cohort | M | UC | 40.2±7.9 | 40 | 38.6±9.4 | 38 | The community data base for IBD in Leicestershire | A modified version of a previously validated questionnaire on sexual problems | NA | 5 | |
| Rivière 2017 (31), France | Cross-sectional | M/F | IBD | M: 39; F: 38 | M: 166; F: 192 | M: 39; F: 36 | M: 56; F: 53 | IBD units of two French tertiary care university hospitals [Bordeaux and Nancy] | M: IIEF; F: FSFI | Age | 7 | |
| Roseira 2020 (32), Portugal | Cross-sectional | M/F | IBD | NA | M: 389; F:458 | NA | M: 206; F: 192 | Confirmed diagnoses of CD or UC in 6 centers in Portugal | SQoL | Age, diagnosis, marital status, depression disorders, and perianal disease | 5 | |
| Timmer 2007 (33), German | Retrospective cohort | M/F | IBD | M: 40; F: 38 | M: 153; F: 181 | M:40.5; F: 39 | M: 153; F: 181 | The national patients organization and the German Crohn’s and Colitis Association (Deutsche Morbus Crohn/Colitis ulcerosa Vereinigung, DCCV e.V) | M: IIEF; F: FSFI | Age, SES, comorbidity, diabetes, hypertensive therapy, confession of faith, type of residency, depression, anxiety | 6 | |
| Valer 2017 (34), Spain | Cross-sectional | M | IBD | 32.0±38.8 | 52 | 31.3±5.1 | 22 | Patients attending outpatient IBD clinics of the 4 participating university hospitals | IIEF | Age, smoking, PRL | 7 | |
*, scores of quality assessment. IBD, inflammatory bowel disease; IIEF, International Index of Erectile Function; FSFI, Female Sexual Function Index; UC, ulcerative colitis; CD, Crohn’s Disease; ASEX, Arizona Sexual Experience Scale; NHRI, National Health Research Institute; CAD, coronary artery disease; PAD, peripheral arterial disease; CKD, chronic kidney disease; NA, not available; SQoL, Sexual Quality of Life Questionnaire; SES, socioeconomic status; PRL, prolactin.
Study quality
The results of the quality assessment of the included studies were presented in Tables S1-S3. Two studies (24,26) were considered high quality, eight (25,28-34) were considered moderate quality, and one (27) were considered low quality due to the lack of information about potential confounders and follow-up years.
Main meta-analysis results
The main results are shown in Figures 2-5. Other results (For male: orgasm, desire, and overall satisfaction; For female: pains) are shown in Figures S1,S2. Tables S4-S11 show the results of sensitivity analyses in males. Tables S12-S19 show the results of sensitivity analyses in females.
Figure 2.
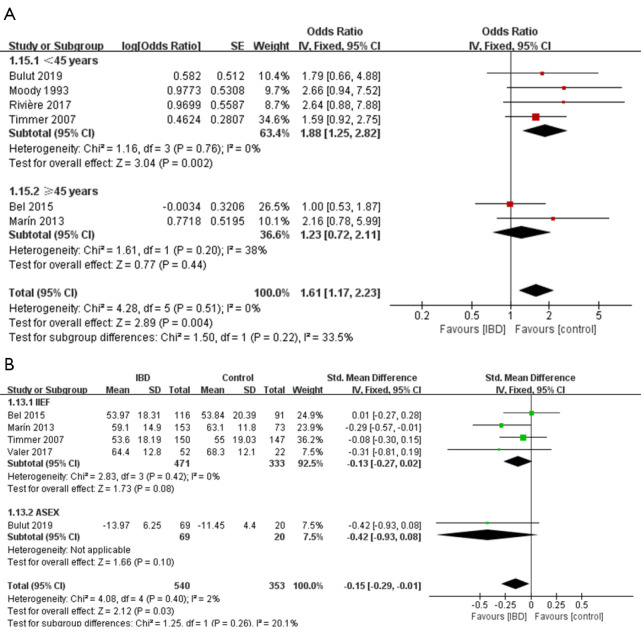
Forest plot showing subgroup analysis results of the prevalence of sexual dysfunction (A) and the total sexual function scores (B) for males. Controls represent male individuals without inflammatory Bowel diseases. SD, standard deviation; IIEF, International Index of Erectile Function; ASEX, Arizona Sexual Experience Scale.
Figure 3.
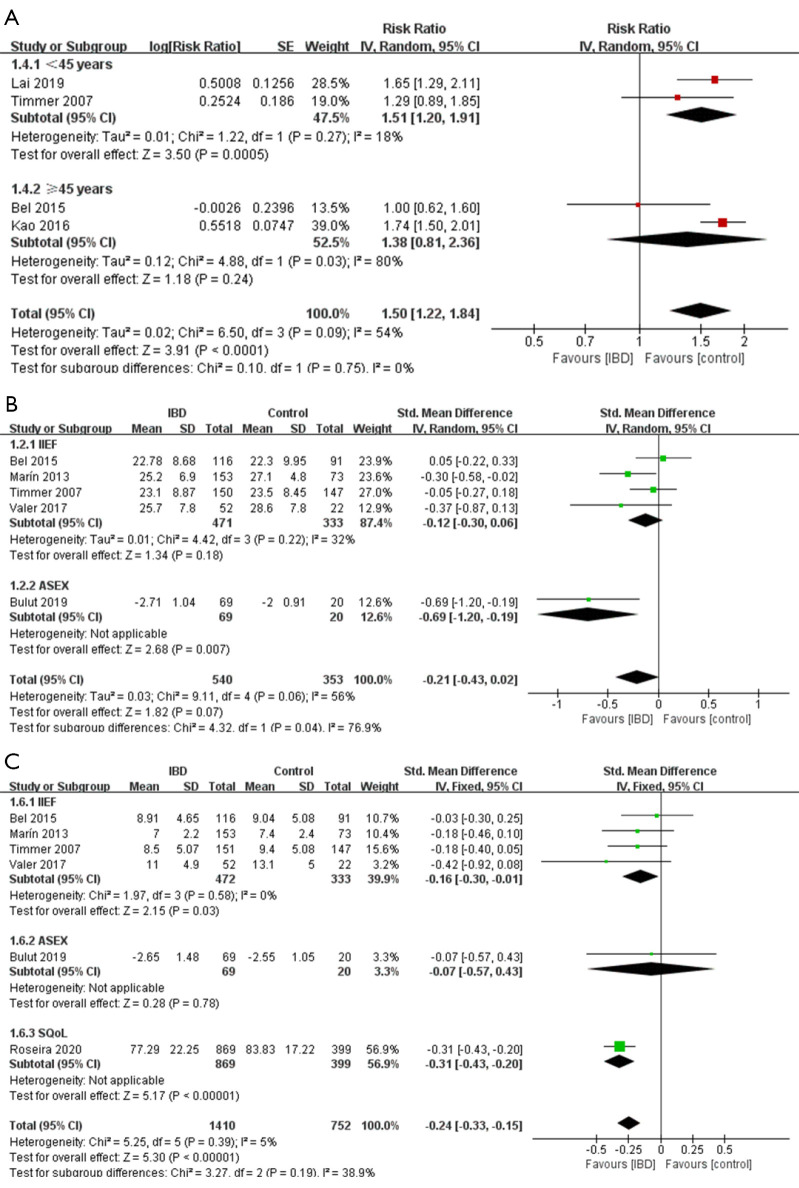
Forest plot showing subgroup analysis results of the prevalence of ED (A), the erectile function scores (B), and the “satisfaction and quality” scores (C) for male. Controls represent male individuals without inflammatory bowel diseases. SD, standard deviation; IIEF, International Index of Erectile Function; ASEX, Arizona Sexual Experience Scale; SQoL, Sexual Quality of Life Questionnaire; ED, erectile dysfunction.
Figure 4.
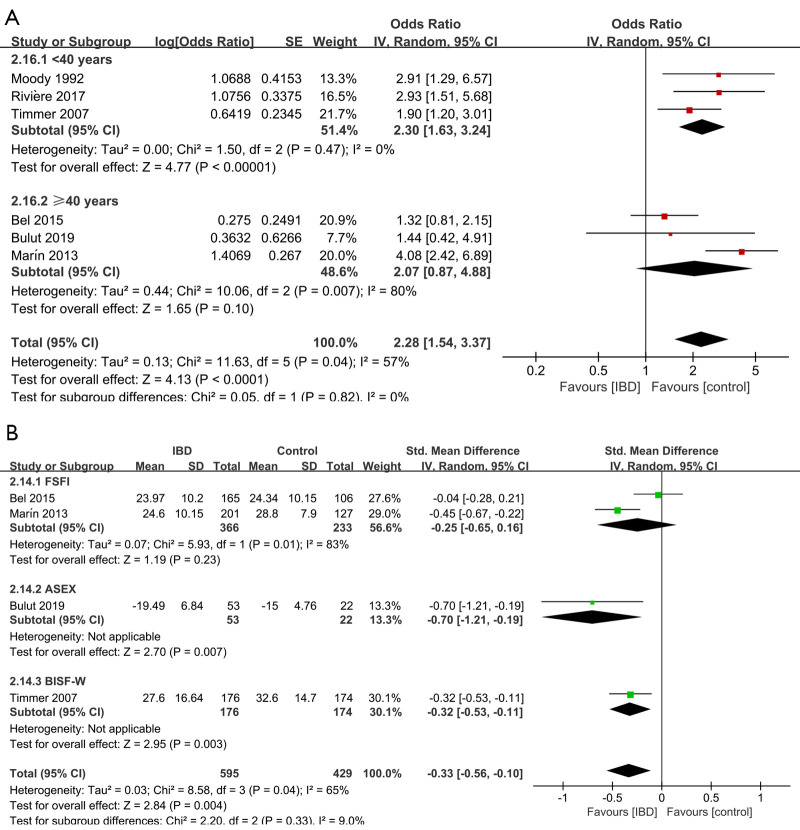
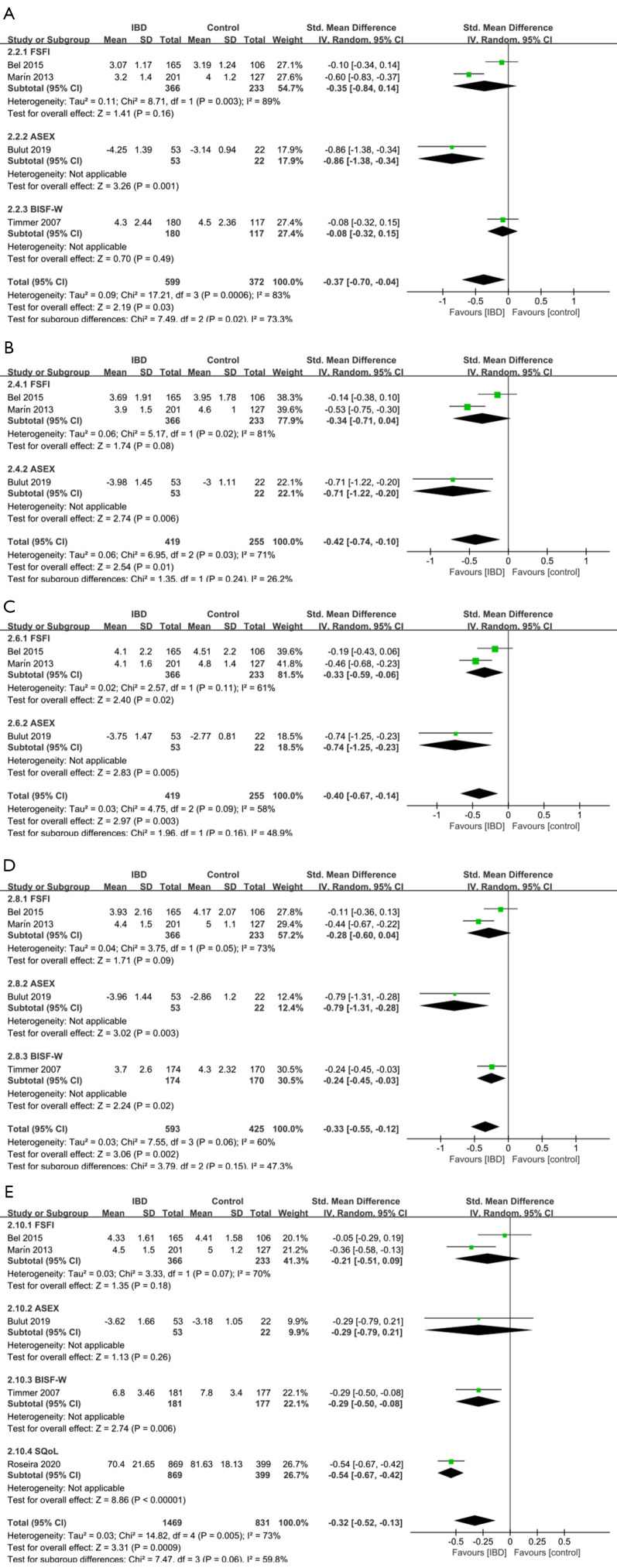
Forest plot showing subgroup analysis results of the prevalence of sexual dysfunction (A) and the total sexual function scores (B) for female. Controls represent female individuals without inflammatory bowel diseases. SD, standard deviation; FSFI, Female Sexual Function Index; ASEX, Arizona Sexual Experience Scale; BISF-W, Brief Index of Sexual Functioning in Women.
Figure 5.
Forest plots showing subgroup analysis results of the specific domains scores for females according to different sexual function assessment scales. (A) Desire, (B) Arousal, (C) Lubrication, (D) Orgasm, (E) Satisfaction and quality. Controls represent male individuals without Inflammatory Bowel Diseases. CI, Confidence interval; SD, Standard deviation; FSFI, Female Sexual Function Index; ASEX, Arizona Sexual Experience Scale; BISF-W, Brief Index of Sexual Functioning in Women. SQoL, Sexual Quality of Life Questionnaire.
Association between IBD and sexual function in male
Overall sexual function
Seven studies (24,25,28,30,31,33,34) evaluated the impact of IBD on overall sexual function. Figure 2 indicates that IBD patients had worse overall sexual function than individuals without IBD (OR =1.61, 95% CI: 1.17 to 2.23, P=0.004). Also, Figure 2 shows that there was a statistically significant association between IBD and low overall sexual function scores (SMD =−0.15, 95% CI: −0.29 to −0.01, P=0.03). Subgroup analyses regarding age showed that (Figure 2), IBD patients under 45 years old exhibited a significantly increased risk of sexual dysfunction compared to those without IBD, while no significant association was found in the group older than 45 years old (OR for <45 years =1.88, 95% CI: 1.25 to 2.82, P=0.002; OR for ≥45 years =1.23, 95% CI: 0.72 to 2.11, P=0.44).
The sensitivity analysis for the overall scores (Table S11) indicated that substantial changes took place on the pooled SMD of analysis for total sexual function when the Ateş Bulut 2019 (25) (SMD =−0.13, 95% CI: −0.27 to 0.02), the Marín 2013 (28) (SMD =−0.11, 95% CI: −0.26 to 0.05) or the Valer 2017 (34) (SMD =−0.14, 95% CI: −0.28 to 0.01) was omitted respectively.
Sub-domains of sexual function
(I). Erectile function
Seven studies (24-28,33,34) evaluated the impact of IBD on erectile dysfunction (ED). As shown in Figure 3, the presence of IBD was significantly associated with an increased risk of ED (RR =1.50, 95% CI: 1.22 to 1.84, P<0.0001). However, no statistical difference in the erectile function scores was found between individuals with or without IBD (SMD =−0.21, 95% CI: −0.43 to 0.02, P=0.07; Figure 3).
As subgroup analyses showed, when using the scale ASEX, IBD was significantly associated with the decreased erectile function scores, while using the scale IIEF did not (SMD for ASEX =−0.69, 95% CI: −1.20 to −0.19, P=0.007; SMD for IIEF =−0.12, 95% CI: −0.30 to 0.06, P=0.18; Figure 3). Interestingly, IBD patients under 45 years old exhibited a significantly increased risk of ED compared to those without IBD (Figure 3) (RR =1.51, 95% CI: 1.20 to 1.91, P=0.0005), while no significant association was found in the group older than 45 years old (RR =1.38, 95% CI: 0.81 to 2.36, P=0.24).
In sensitivity analysis (Tables S5), a substantial change was detected on the new pooled SMD, after omitting the Bel 2015 (24) in the analysis of erectile function scores (SMD =−0.29, 95% CI: −0.54 to −0.03), which meant that the synthetic results of this analysis might be influenced by Bel 2015. As shown in Tables S4, in the sensitivity analysis for the ED prevalence, heterogeneity changed significantly after eliminating the Bel 2015 (24) (I2=11%, P=0.33), which indicated that this study might be the potential sources of the heterogeneity.
(II). Satisfaction & quality
Six studies (24,25,28,32-34) evaluated the satisfaction & quality scores in sexual activities. A significant association between IBD and low sexual satisfaction was found (SMD =−0.24, 95% CI: −0.33 to −0.15, P<0.00001; Figure 3).
Subgroup analyses showed (Figure 3) no significant difference was found between individuals with or without IBD when using the scale ASEX, while significant difference was found using other scales (SMD for ASEX =−0.07, 95% CI: −0.57 to 0.43, P=0.78; SMD for SQoL =−0.31, 95% CI: −0.43 to −0.20, P<0.0001; SMD for IIEF =−0.16, 95% CI: −0.30 to −0.01, P=0.03). The result of sensitivity was shown in Tables S6.
(III). Other sub-domains of sexual function
Five studies (24,25,28,33,34) evaluated the impact of IBD on other sub-domains of sexual function in male. Poor performance on orgasm (SMD =−0.07, 95% CI: −0.20 to 0.07, P=0.28), desire (SMD =0.03, 95% CI: −0.11 to 0.16, P=0.70), and overall satisfaction (SMD =−0.32, 95% CI: −0.65 to 0.02, P=0.06) were not significant associated with IBD (Figure S1). The results of sensitivity analysis were shown in Tables S7-S9.
Association between IBD and sexual function in female
Total sexual function
Six studies (24,25,28,29,31,33) evaluated the impact on total sexual function. Figure 4 shows the pooled OR result, which revealed that IBD was significantly associated with an increased risk of sexual dysfunction (OR =2.28, 95% CI: 1.54 to 3.37, P<0.0001), with high heterogeneity (I2=57%, P=0.04). There was also a significant statistical difference in the total sexual function scores between individuals with or without IBD (SMD =−3.44, 95% CI: −5.52 to −1.37, P=0.001).
Subgroup analyses regarding age showed that (Figure 4) female IBD patients younger than 40 years old exhibited a significantly increased risk of sexual dysfunction compared to those without IBD, while no significant association was found in the group older than 40 years old. (OR for <40 years =2.30, 95% CI: 1.63 to 3.24, P<0.00001; OR for ≥40 years =2.07, 95% CI: 0.87 to 4.88, P=0.10). As regarding the scale used to score sexual function, shown in Figure 4, no significant difference in total sexual function was found between individuals with or without IBD when using the scale FSFI (SMD =−0.25, 95% CI: −0.65 to 0.16, P=0.23). And when using the scale ASEX and the scale BISF-W, the difference between individuals with or without IBD was significant (SMD for ASEX =−0.70, 95% CI: −1.21 to −0.19, P=0.007; SMD for BISF-W =−0.32, 95% CI: −0.53 to −0.11, P=0.003; Figure 4).
Sensitivity analysis (Tables S18) of the prevalence of sexual dysfunction showed that the heterogeneity changed significantly after eliminating the Bel 2015 (24) (I2=30%, P=0.22) and Marín 2013 (28) (I2=21%, P=0.28), which indicated that the two studies might be the potential sources of the heterogeneity. Tables S19 also showed that, in the analysis of total scores, a substantial change was detected on the new pooled SMD, after omitting Bel 2015 (24) (I2=5.7%, P=0.35) and Ateş Bulut 2019 (25) (I2=0%, P=0.76), which indicated that the two studies might be the potential sources of the heterogeneity.
Sub-domains of sexual function
Five studies (24,25,28,32,33) evaluated the impact of IBD on Sub-domains of sexual function in female. IBD patients seemed to have significant worse performance on desire (SMD =−0.37, 95% CI: −0.70 to −0.04, P=0.03), arousal (SMD =−0.42, 95% CI: −0.74 to −0.10, P=0.01), lubrication (SMD =−0.40, 95% CI: −0.67 to −0.14, P=0.003), orgasm (SMD =−0.33, 95% CI: −0.55 to −0.12, P=0.002), and satisfaction & quality (SMD =−0.32, 95% CI: −0.52 to −0.13, P=0.0009) (Figure 5). Surprisingly, the poor performance on pain (SMD =−0.16, 95% CI: −0.65 to 0.33, P=0.52) of IBD patients was not significantly associated with IBD (Figure S2).
Subgroup analysis showed that, when using the ASEX scale to measure the sub-domains of sexual function, it’s usually easier to find a significant difference between the general population and female IBD patients (Figure 5A-5E). While using the FSFI scale, the association was not significant between poor performance on sub-domains (except lubrication) of sexual function and IBD (Figure 5A-5E). The results of sensitivity analysis were shown in Tables S12-S17.
Discussion
Sexual health is defined as a condition of physical, emotional, mental, and social well-being associated with sexuality that is not merely the absence of disease, malfunction, or infirmity (35). Just like body image, it is a critical part of psychosocial functioning that has a major impact on overall Quality of Life (10). Thus, sexual health is a vital issue in IBD since IBD usually strikes adolescent or younger individuals who are at the stage of self-development and reproducing (36). Assessing sexual function is a very valuable approach toward sexual health.
Almost two decades ago, Giese and Terrell (37) had recognized that sexual health issues in IBD patients (include growth and development, body image, intimacy, sexual functioning, fertility, and pregnancy) may be influenced by the disease itself or by the medical and surgical interventions used for treatment. However, the correlation between IBD and sexual function is still controversial because of the significant heterogeneity in study aims, the definition of sexual dysfunction, design, and samples between the studies. Within this context, we conducted this meta-analysis aiming to examine the correlation between IBD and each sub-domain of sexual dysfunction. 36 studies were identified after systematically searching and 11 studies (24-34) were included in the end. The results of this meta-analysis indicated that IBD was significantly associated with worse sexual function in both sexes. The prevalence of sexual dysfunction in males and females was higher compared to the individuals without IBD. And IBD patients had worse performance on specific sub-domains of sexual function compared with individuals without IBD.
Specifically, there were significant decreases in the satisfaction & quality and total sexual function, indicating that male individuals’ sexual function in these two sub-domains may be impacted by IBD. It is worth noting that, even the scores for several sub-domains of sexual function had no difference between patients with or without IBD, it did not absolutely mean that sexual function in these sub-domains did not get worse, for example, erectile function. A significant association between IBD and erectile dysfunction was found in the analysis of RRs of ED. However, as for the erectile function scores, there was no statistical difference between individuals with or without IBD. It might be because the cut-off for ED was different in included studies. Additionally, the impact on sexual function of female patients caused by IBD was much greater than that of male patients (almost all sub-domains scores decreased). These results were consistent with several studies (10-12,38). Additionally, the subgroup analysis was conducted regarding age and scales for sexual function, which indicated that IBD patients at older age seemed to have less impact on sexual function compared to patients at a younger age. One possible explanation for this result is that IBD mainly occurs in adolescence or early aged adults. Elderly patients usually have a longer disease duration. And sexual function is relatively less impacted by IBD with a longer disease duration and is not affected by the long-term severity of the disease (39).
Individuals suffering from IBD may develop hypogonadism as a result of chronic inflammation. Proinflammatory cytokines are known in pediatric studies to disrupt sex steroid synthesis at the testes and ovaries, thereby affecting normal growth. Tumor necrosis factor-α (TNF-α) and interleukins 1 (IL-1) and 6 (IL-6) have been shown to inhibit testosterone generation in Leydig cells and steroidogenesis production in ovarian cells, respectively (40,41). Furthermore, evidence suggests that serum TNF-α levels have a negative correlation with erectile function (42). Additionally, zinc absorption occurs throughout the small intestine, both in the proximal tract (duodenum and jejunum) and the distal tract (ileum). Certain symptoms of inflammatory bowel injury, such as zinc deficiency, have been linked to hypogonadism, which impairs sexual function (43).
Depression is commonly reported to be associated with decreased quality of life and considered as an independent or inter-related factor for chronic conditions (44,45). It is reported as frequent psychological comorbidity in IBD and the major factor for sexual dysfunction (24,28,31,46). The rate of depression is found up to 20–30% in IBD patients in remission disease activity (6). Therefore, comprehensive management of the quality of life and psychological factors is important in IBD patients. And it is essential to assess psychological state when IBD patients report a poor performance on sexual function.
Furthermore, medical treatment and lifestyle (such as alcohol intake) are considered as the factors affecting sexual function. Marín et al. (28) reported that corticosteroids are independently associated with sexual dysfunction, which may be because they could affect body image and then sexual function. Importantly, evidence has shown that immunosuppressive drugs can affect male sexual function and reproduction through multiple mechanisms, such as altering reproductive hormone secretion and/or action, which results in sexual dysfunction, including libido decreases, erectile dysfunction, more difficulty achieving an orgasm or poor performance on orgasm, and sexual satisfaction decreases (47). Also, alcohol intake in IBD patients could cause relapse or deterioration by an inactive state (48), which leads to sexual dysfunction.
Previous meta-analysis conducted by Zhao et al. (49) has investigated the association between IBD and sexual dysfunction. However, only overall sexual dysfunction and subgroup analyses based on age were evaluated. Nevertheless, our meta-analysis is more up-to-date, incorporating 11 studies, and is the first report of the association between IBD and sexual function in specific sub-domains, including the analyses of erectile function, satisfaction and quality, orgasm and desire performance in male, and pain, desire, arousal, lubrication, orgasm and satisfaction and quality in female. Evidence provided by this study enables more precise therapy planning for IBD patients with sexual dysfunctions based on sub-domain analyses.
And several limitations of the current study should also be acknowledged. First, the number of included studies was limited. Compared to other medical diseases, IBD has received less attention in sexual medication and few studies on this topic were conducted. And the information in those studies provided was also limited. Thus, it’s difficult for us to conduct a stratification analysis based on the activity, severity, duration of IBD or specific comorbidities since limited studies provided this information. Zhang’s study (50), which conducted an analysis between medical comorbidities and sexual dysfunction in IBD patients, also raised the same concern about limited data on pertinent risk factors. Second, the majority of the included studies were retrospective cohort studies, which were considered moderate quality with insufficient strength of evidence. Third, even though we tried to minimize heterogeneity by clearly defining the exclusion and inclusion criteria, substantial heterogeneity still existed between those studies for IBD individuals, in spite of the fact that confounding factors were partially adjusted in most of the included studies. The differences in sample size, age, geographical area, comorbidities, medication usage, and varied characteristics of subjects may be responsible for the heterogeneity. Fourth, the subgroup analysis revealed that there was no obvious association between IBD and sexual dysfunction in the elderly population. It’s well-known that gonadal function would decline gradually with age, which might lead to worse sexual function. However, it does not mean that elderly IBD patients must show significantly worse sexual function than those without IBD. The impact of IBD on sexual function was weakened by age, and it might be one reason for the formation of heterogeneity, especially in studies whose participants were in older age. Moreover, the scales used for sexual function assessment vary among different studies. IIEF and FSFI were used to assess sexual function in most studies, while ASEX, SQoL, BISF-W, and the modified structured questionnaire were used in the other studies. O’Toole et al. (51) and de Silva et al. (52), have established and validated a new IBD-specific psychometric tool to assess sexual function for IBD patients for both sexes. Further studies should be conducted basing on these two scales.
Conclusions
IBD is associated with worse sexual function. It has significant impact on erectile function and satisfaction for male individuals and has impact on most sub-domains of sexual function for female individuals. Physicians should assess these specific sub-domains in the sexual functions of IBD patients, and give precision treatment for these areas.
Supplementary
The article’s supplementary files as
Acknowledgments
We would like to thank all the study participants for their efforts.
Funding: This work was supported by National Natural Science Foundation of China (Grant Number: 81702518, 82072838) and Huazhong University of Science and Technology (Grant Number: 2019kfyXKJC06).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Reporting Checklist: The authors have completed the MOOSE reporting checklist. Available at https://tau.amegroups.com/article/view/10.21037/tau-22-190/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tau.amegroups.com/article/view/10.21037/tau-22-190/coif). The authors have no conflicts of interest to declare.
References
- 1.Ronchetti C, Cirillo F, Di Segni N, et al. Inflammatory Bowel Disease and Reproductive Health: From Fertility to Pregnancy-A Narrative Review. Nutrients 2022;14:1591. 10.3390/nu14081591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertani L, Ribaldone DG, Bellini M, et al. Inflammatory Bowel Diseases: Is There a Role for Nutritional Suggestions? Nutrients 2021;13:1387. 10.3390/nu13041387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ananthakrishnan AN, Kaplan GG, Bernstein CN, et al. Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an International Organization for Study of Inflammatory Bowel Diseases consensus. Lancet Gastroenterol Hepatol 2022;7:666-78. 10.1016/S2468-1253(22)00021-8 [DOI] [PubMed] [Google Scholar]
- 4.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390:2769-78. 10.1016/S0140-6736(17)32448-0 [DOI] [PubMed] [Google Scholar]
- 5.Allocca M, Gilardi D, Fiorino G, et al. Sexual and reproductive issues and inflammatory bowel disease: a neglected topic in men. Eur J Gastroenterol Hepatol 2018;30:316-22. 10.1097/MEG.0000000000001074 [DOI] [PubMed] [Google Scholar]
- 6.Iglesias-Rey M, Barreiro-de Acosta M, Caamaño-Isorna F, et al. Psychological factors are associated with changes in the health-related quality of life in inflammatory bowel disease. Inflamm Bowel Dis 2014;20:92-102. [DOI] [PubMed] [Google Scholar]
- 7.Chen LM, Bao CH, Wu Y, et al. Tryptophan-kynurenine metabolism: a link between the gut and brain for depression in inflammatory bowel disease. J Neuroinflammation 2021;18:135. 10.1186/s12974-021-02175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazzard BG, Price HL, Libby GW, et al. The social toll of Crohn's disease. Br Med J 1978;2:1117-9. 10.1136/bmj.2.6145.1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantzouranis G, Fafliora E, Glanztounis G, et al. Inflammatory Bowel Disease and Sexual Function in Male and Female Patients: An Update on Evidence in the Past Ten Years. J Crohns Colitis 2015;9:1160-8. 10.1093/ecco-jcc/jjv140 [DOI] [PubMed] [Google Scholar]
- 10.Knowles SR, Gass C, Macrae F. Illness perceptions in IBD influence psychological status, sexual health and satisfaction, body image and relational functioning: A preliminary exploration using Structural Equation Modeling. J Crohns Colitis 2013;7:e344-50. 10.1016/j.crohns.2013.01.018 [DOI] [PubMed] [Google Scholar]
- 11.Mahmood S, Nusrat S, Crosby A, et al. Assessment of sexual function among inflammatory bowel disease patients. Am J Gastroenterol 2015;110:601-3. 10.1038/ajg.2015.53 [DOI] [PubMed] [Google Scholar]
- 12.Eluri S, Cross RK, Martin C, et al. Inflammatory Bowel Diseases Can Adversely Impact Domains of Sexual Function Such as Satisfaction with Sex Life. Dig Dis Sci 2018;63:1572-82. 10.1007/s10620-018-5021-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CDC. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM). Available online: https://www.cdc.gov/nchs/icd/icd-10-cm.htm
- 14.Taylor JF, Rosen RC, Leiblum SR. Self-report assessment of female sexual function: psychometric evaluation of the Brief Index of Sexual Functioning for Women. Arch Sex Behav 1994;23:627-43. 10.1007/BF01541816 [DOI] [PubMed] [Google Scholar]
- 15.Audier-Bourgain M, Baubet T, Pham-Scottez A, et al. Eating disorders and sexuality: A quantitative study in a French medically assisted procreation course. Brain Behav 2021;11:e02196. 10.1002/brb3.2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blouet A, Zinger M, Capitain O, et al. Sexual quality of life evaluation after treatment among women with breast cancer under 35 years old. Support Care Cancer 2019;27:879-85. 10.1007/s00520-018-4374-z [DOI] [PubMed] [Google Scholar]
- 17.Han Z, Gan Z, Han H, et al. Validity of the Chinese version of the brief index of sexual functioning for women with a new scoring algorithm and comparison of normative and recurrently depressed Han Chinese population. J Sex Med 2014;11:439-46. 10.1111/jsm.12385 [DOI] [PubMed] [Google Scholar]
- 18.McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther 2000;26:25-40. 10.1080/009262300278623 [DOI] [PubMed] [Google Scholar]
- 19.Jannini TB, Rossi R, Sconci V, et al. Italian validation of Arizona Sexual Experience Scale (ASEX) on patients suffering from psychotic spectrum disorders. Riv Psichiatr 2022;57:18-22. [DOI] [PubMed] [Google Scholar]
- 20.Laforgue ÉJ, Busnel G, Lauzeille D, et al. Evolution of sexual functioning of men through treated and untreated depression. Encephale 2021. [Epub ahead of print]. 10.1016/j.encep.2021.06.008 [DOI] [PubMed] [Google Scholar]
- 21.Elnazer HY, Baldwin DS. Structured review of the use of the Arizona sexual experiences scale in clinical settings. Hum Psychopharmacol 2020;35:e2730. 10.1002/hup.2730 [DOI] [PubMed] [Google Scholar]
- 22.GA Wells BS, D O’Connell, J Peterson, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 23.Rostom A, Dubé C, Cranney A, et al. Celiac Disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004. (Evidence Reports/Technology Assessments, No. 104.) Appendix D. Quality Assessment Forms. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK35156/
- 24.Bel LG, Vollebregt AM, Van der Meulen-de Jong AE, et al. Sexual Dysfunctions in Men and Women with Inflammatory Bowel Disease: The Influence of IBD-Related Clinical Factors and Depression on Sexual Function. J Sex Med 2015;12:1557-67. 10.1111/jsm.12913 [DOI] [PubMed] [Google Scholar]
- 25.Ateş Bulut E, Törüner M. The influence of disease type and activity to sexual life and health quality in inflammatory bowel disease. Turk J Gastroenterol 2019;30:33-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kao CC, Lin CL, Huang WY, et al. Association Between Inflammatory Bowel Disease and Erectile Dysfunction: A Nationwide Population-Based Study. Inflamm Bowel Dis 2016;22:1065-70. 10.1097/MIB.0000000000000695 [DOI] [PubMed] [Google Scholar]
- 27.Lai SW, Kuo YH, Fang CW, et al. Inflammatory Bowel Disease and the Risk of Erectile Dysfunction. Inflamm Bowel Dis 2019;25:e164-5. 10.1093/ibd/izz237 [DOI] [PubMed] [Google Scholar]
- 28.Marín L, Mañosa M, Garcia-Planella E, et al. Sexual function and patients' perceptions in inflammatory bowel disease: a case-control survey. J Gastroenterol 2013;48:713-20. 10.1007/s00535-012-0700-2 [DOI] [PubMed] [Google Scholar]
- 29.Moody G, Probert CS, Srivastava EM, et al. Sexual dysfunction amongst women with Crohn's disease: a hidden problem. Digestion 1992;52:179-83. 10.1159/000200951 [DOI] [PubMed] [Google Scholar]
- 30.Moody GA, Mayberry JF. Perceived sexual dysfunction amongst patients with inflammatory bowel disease. Digestion 1993;54:256-60. 10.1159/000201046 [DOI] [PubMed] [Google Scholar]
- 31.Rivière P, Zallot C, Desobry P, et al. Frequency of and Factors Associated With Sexual Dysfunction in Patients With Inflammatory Bowel Disease. J Crohns Colitis 2017;11:1347-52. 10.1093/ecco-jcc/jjx100 [DOI] [PubMed] [Google Scholar]
- 32.Roseira J, Magro F, Fernandes S, et al. Sexual Quality of Life in Inflammatory Bowel Disease: A Multicenter, National-Level Study. Inflamm Bowel Dis 2020;26:746-55. 10.1093/ibd/izz185 [DOI] [PubMed] [Google Scholar]
- 33.Timmer A, Bauer A, Dignass A, et al. Sexual function in persons with inflammatory bowel disease: a survey with matched controls. Clin Gastroenterol Hepatol 2007;5:87-94. 10.1016/j.cgh.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 34.Valer P, Algaba A, Santos D, et al. Evaluation of the Quality of Semen and Sexual Function in Men with Inflammatory Bowel Disease. Inflamm Bowel Dis 2017;23:1144-53. 10.1097/MIB.0000000000001081 [DOI] [PubMed] [Google Scholar]
- 35.Mitchell KR, Lewis R, O'Sullivan LF, et al. What is sexual wellbeing and why does it matter for public health? Lancet Public Health 2021;6:e608-13. 10.1016/S2468-2667(21)00099-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez de Arce E, Quera R, Ribeiro Barros J, et al. Sexual Dysfunction in Inflammatory Bowel Disease: What the Specialist Should Know and Ask. Int J Gen Med 2021;14:2003-15. 10.2147/IJGM.S308214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giese LA, Terrell L. Sexual health issues in inflammatory bowel disease. Gastroenterol Nurs 1996;19:12-7. 10.1097/00001610-199601000-00004 [DOI] [PubMed] [Google Scholar]
- 38.Nøhr EA, Nielsen J, Nørgård BM, et al. Sexual Health in Women with Inflammatory Bowel Disease in the Danish National Birth Cohort. J Crohns Colitis 2020;14:1082-9. 10.1093/ecco-jcc/jjaa038 [DOI] [PubMed] [Google Scholar]
- 39.Timmer A, Bauer A, Kemptner D, et al. Determinants of male sexual function in inflammatory bowel disease: a survey-based cross-sectional analysis in 280 men. Inflamm Bowel Dis 2007;13:1236-43. 10.1002/ibd.20182 [DOI] [PubMed] [Google Scholar]
- 40.Burney BO, Hayes TG, Smiechowska J, et al. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab 2012;97:E700-9. 10.1210/jc.2011-2387 [DOI] [PubMed] [Google Scholar]
- 41.Samir M, Glister C, Mattar D, et al. Follicular expression of pro-inflammatory cytokines tumour necrosis factor-α (TNFα), interleukin 6 (IL6) and their receptors in cattle: TNFα, IL6 and macrophages suppress thecal androgen production in vitro. Reproduction 2017;154:35-49. 10.1530/REP-17-0053 [DOI] [PubMed] [Google Scholar]
- 42.Zhou B, Chen Y, Yuan H, et al. NOX1/4 Inhibitor GKT-137831 Improves Erectile Function in Diabetic Rats by ROS Reduction and Endothelial Nitric Oxide Synthase Reconstitution. J Sex Med 2021;18:1970-83. 10.1016/j.jsxm.2021.09.007 [DOI] [PubMed] [Google Scholar]
- 43.Vaghari-Tabari M, Jafari-Gharabaghlou D, Sadeghsoltani F, et al. Zinc and Selenium in Inflammatory Bowel Disease: Trace Elements with Key Roles? Biol Trace Elem Res 2021;199:3190-204. 10.1007/s12011-020-02444-w [DOI] [PubMed] [Google Scholar]
- 44.DeJean D, Giacomini M, Vanstone M, et al. Patient experiences of depression and anxiety with chronic disease: a systematic review and qualitative meta-synthesis. Ont Health Technol Assess Ser 2013;13:1-33. [PMC free article] [PubMed] [Google Scholar]
- 45.Graff LA, Walker JR, Bernstein CN. Depression and anxiety in inflammatory bowel disease: a review of comorbidity and management. Inflamm Bowel Dis 2009;15:1105-18. 10.1002/ibd.20873 [DOI] [PubMed] [Google Scholar]
- 46.Muller KR, Prosser R, Bampton P, et al. Female gender and surgery impair relationships, body image, and sexuality in inflammatory bowel disease: patient perceptions. Inflamm Bowel Dis 2010;16:657-63. 10.1002/ibd.21090 [DOI] [PubMed] [Google Scholar]
- 47.Perez-Garcia LF, Dolhain RJEM, Vorstenbosch S, et al. The effect of paternal exposure to immunosuppressive drugs on sexual function, reproductive hormones, fertility, pregnancy and offspring outcomes: a systematic review. Hum Reprod Update 2020;26:961-1001. 10.1093/humupd/dmaa022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jussila A, Virta LJ, Pukkala E, et al. Mortality and causes of death in patients with inflammatory bowel disease: a nationwide register study in Finland. J Crohns Colitis 2014;8:1088-96. 10.1016/j.crohns.2014.02.015 [DOI] [PubMed] [Google Scholar]
- 49.Zhao S, Wang J, Liu Y, et al. Inflammatory Bowel Diseases Were Associated With Risk of Sexual Dysfunction in Both Sexes: A Meta-analysis. Inflamm Bowel Dis 2019;25:699-707. 10.1093/ibd/izy345 [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, Wei S, Zeng Q, et al. Prevalence and risk factors of sexual dysfunction in patients with inflammatory bowel disease: systematic review and meta-analysis. Int J Colorectal Dis 2021;36:2027-38. 10.1007/s00384-021-03958-y [DOI] [PubMed] [Google Scholar]
- 51.O'Toole A, de Silva PS, Marc LG, et al. Sexual Dysfunction in Men With Inflammatory Bowel Disease: A New IBD-Specific Scale. Inflamm Bowel Dis 2018;24:310-6. 10.1093/ibd/izx053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Silva PS, O'Toole A, Marc LG, et al. Development of a Sexual Dysfunction Scale for Women With Inflammatory Bowel Disease. Inflamm Bowel Dis 2018;24:2350-9. 10.1093/ibd/izy202 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as