Abstract
Purpose
Organoids are three-dimensional cultures of stem cells in an environment similar to the body’s extracellular matrix. This is also a novel development in the realm of regenerative medicine. Stem cells can begin to develop into 3D structures by modifying signaling pathways. To form organoids, stem cells are transplanted into the extracellular matrix. Organoids have provided the required technologies to reproduce human tissues. As a result, it might be used in place of animal models in scientific study. The key goals of these investigations are research into viral and genetic illnesses, malignancies, and extracellular vesicles, pharmaceutical discovery, and organ transplantation. Organoids can help pave the road for precision medicine through genetic editing, pharmaceutical development, and cell therapy.
Methods
PubMed, Google Scholar, and Scopus were used to search for all relevant papers written in English (1907–2021). The study abstracts were scrutinized. Studies on the use of stem-cell-derived organoids in regenerative medicine, organoids as 3D culture models for EVs analysis, and organoids for precision medicine were included. Articles with other irrelevant aims, meetings, letters, commentaries, congress and conference abstracts, and articles with no available full texts were excluded.
Results
According to the included studies, organoids have various origins, types, and applications in regenerative and precision medicine, as well as an important role in studying extracellular vesicles.
Conclusion
Organoids are considered a bridge that connects preclinical studies to clinical ones. However, the lack of a standardized protocol and other barriers addressed in this review, hinder the vast use of this technology.
Lay Summary
Organoids are 3D stem cell propagations in biological or synthetic scaffolds that mimic ECM to allow intercellular or matrix-cellular crosstalk. Because these structures are similar to organs in the body, they can be used as research models. Organoids are medicine’s future hope for organ transplantation, tumor biobank formation, and the development of precision medicine. Organoid models can be used to study cell-to-cell interactions as well as effective factors like inflammation and aging. Bioengineering technologies are also used to define the size, shape, and composition of organoids before transforming them into precise structures. Finally, the importance of organoid applications in regenerative medicine has opened a new window for a better understanding of biological research, as discussed in this study.
Keywords: Organoid, Regenerative medicine, Stem cells, Precision medicine
Introduction
A three-dimensional micro-physiological platform consisting of clusters of cells called organoids was obtained with the efforts of scientists in the fields of biotechnology and regenerative medicine [1]. Organoids could imitate a series of pathophysiological and structural features of human tissues and organs [2]. They are also already in use as disease models. Their new therapeutic ways are done through the support of regenerative medicine techniques [1–5]. They are differentiated from human pluripotent stem cells (PSCs), embryonic and adult-tissue stem cells (ESCs and ASCs) [6]. In fact, stem cells in the form of organoids can produce a variety of organ models. The range of organ models is wide, from the stomach and intestine to the brain [6, 7]. Embryonic stem cell differentiated organoids could play a role in research about organ development like what is done in the uterus [6, 8]. Interestingly, these cells could be able to develop into the parts of the brain in the in vitro environment. This process occurs without going through the natural cycle of evolution [6, 9]. Adult stem cells (ASCs) are more specific in that they differentiate into precise tissue depending on their location [6, 10]. In this way, they can replace the destroyed cells [6, 11]. Organoid models of patient-derived induced pluripotent stem cells (iPSC) can be used in the field of personalized medicine [9, 12, 13]. Literally, these are stem cells that grow in a special culture protocol. Also, they could mimic the characteristics of an organ [6, 14]. The results of animal models such as monkeys and mice cannot always be generalized to humans. That is why organoid models are projected to be superior to other in vivo models [3, 6–11, 14, 15]. 3D cell culture is another important advantage of organoids. With the ability to conduct more in-depth research than other cell culture protocols [9, 15]. The use of scaffold as a biological or synthetic hydrogel that can mimic the properties of the extracellular matrix (ECM) has been useful in the preparation of organoid culture protocols [10, 11, 15]. Actually, the signaling pathways and connections between cells and the extracellular matrix in the 3D culture method allow cells to function when they are in the body [10, 16]. The use of organoids derived from human tissues allows us to study the mechanisms within the human body more accurately than other culture methods [8, 10, 14–17]. Despite all the advantages mentioned, organoids have disadvantages, including the absence of all the cell types found in human organs. Also, organoid models are without vascular structure [7]. Although ASCs can be useful in repairing cells and prolonging human life, they also have harmful effects because they have the potential to change into cancerous cells more than other cells [11]. The goal of this study is to discuss the history of organoids, their origin, types, and applications in regenerative and precision medicine. The relationship between stem cells and organoid production was explained. The future of organoids in organ transplantation was considered a novel science. Also, organoid roles in studying extracellular vesicles (Evs) were discussed here. Ultimately, the various uses of current organoid models and the related challenges are discussed in detail.
Organoids: a Novel Insight to Regenerative Medicine
The knowledge of using different cell lines as animal models to achieve goals such as the discovery of new drugs for incurable diseases belongs to the twentieth century [18, 19]. Primarily, genetic studies helped the development of biological processes in order to find the mechanisms of disorders [18–20]. This trend first began in more rudimentary models such as the invertebrate model and progressed to mammalian models and finally human model systems [1]. All of this progress has greatly contributed to the drug design process [1, 16]. At the moment, human models with three-dimensional culture systems that use different stem cells from various organs have overcome the limitations [1, 17]. Also, organoids with their new model have achieved a revolution in biomedical science [1, 17]. Also, the revolution that happened with the advent of organoids had a great impact on the future of cell therapy [21, 22]. Organoids can play a role in therapeutic techniques for diseases with unknown etiology, such as type 1 diabetes [21, 22]. Because of their complex architecture [21, 22], organoid products are significantly different from all other medical field products. Organoids can be effective in translational research as they have a role in studies about human pathophysiology and organ development [23]. But nowadays, it should be noted that the findings of organoid studies are not generalizable to be used in the field of cell therapy for the reasons stated above [21, 22]. For example, the methods could not match with standards in pharmaceutics correctly [21, 22]. As we know, one way to treat cancer is radiation therapy, which affects the cellular response [24]. Organoids can be useful in investigating this cellular process in radiotherapy [24]. In fact, scientists need to understand all the molecular and biological events in cells and organs during radiation therapy so they can determine the maximum dose of radiation [24]. The study of the radiobiological process in the form of organoid models allows us to consider cell-to-cell interactions in more detail [24]. As a result, the results will be closer to clinical observations [24]. Another point is the ability to produce enzymes in the organoid culture protocol, which is used for defective phenotypes [24, 25]. For example, in the article by Anusha S. Shankar et al., the renin was produced by the use of kidney organoids that were differentiated from induced pluripotent stem cells (iPSCs) [24, 25]. Furthermore, organoid technology may be used in the field of organ transplantation by producing the desired organ [11, 16, 26]. Also, organoids derived from patient-derived iPSCs or other cells have been used to test gene therapy [11, 16, 26]. Identifying the wide utilization of organoids from drug mechanisms and enzyme deficiencies to gene therapy and organ transplantation, we find that their emergence is a new innovation in biotechnology with a significant history.
Historical Background of Organoids
The history of organ regeneration in an in vitro environment date back to 1907, when Henry Van Peters Wilson was able to explain how to produce an organ using separate sponge cells [27]. An important point in organoid models has been shown in this experimental research, where adult cells of an organism could produce multicellular complexes without external commands [27, 28]. This theory of cell isolation and organ regeneration has long been used by laboratories in the 1950s [27, 28]. Also, the history of the construction of the first mammalian organoids goes back more than 60 years ago, and they helped biology in order to achieve great advancement [28, 29]. Scientists are investigating the possibility of transforming the body cells of more advanced animals, including vertebrates, into organs after separation [28, 29]. In subsequent experiments on chick embryos, researchers tried to produce organs from different cell types from the kidney, skin, and liver [28, 30]. In fact, this type of research was the first step in testing the cell isolation and regeneration of organs in more advanced animals [28, 30]. The emergence of PSCs has greatly influenced the progress of research in the field of organoids [31]. These cells were first extracted from mouse embryos [31]. Later on, embryonic stem cells were isolated from human blastocyst cells, and this knowledge was further developed when iPSCs were obtained [17, 32]. Biological researchers attempted to control these stem cells’ behavior, including spontaneous regeneration [32]. Regenerative medicine researchers attempted to repair damaged organs by using special stem cells that could differentiate into the target cell [32]. All of these advances helped scientists think about producing organs in a laboratory environment in the form of organoids [2, 32]. Finally, the history of organoids goes back more than 40 years ago, when Howard Green tried to spread human epidermal keratinocytes by co-culture with special mouse embryonic fibroblasts [2, 32]. Mouse embryonic fibroblasts can mimic the human epidermis in the laboratory [2, 17, 32]. Considering the history of organoids, we find that they attracted attention in the twenty-first century, when scientists tried to supersede the use of animal models with new models [28]. Also, during this period, the use of stem cell derivatives greatly improved [28]. We can also mention the history of their application in various fields such as toxicology, organ transplantation, cancer, and the knowledge of embryonic development [28]. Research on the growth of epithelial breast cells on the Engelbreth-Holm-Swarm (EHS) ECM platform was then performed, resulting in three-dimensional duct production with the ability of protein synthesis in order to milk secretion. These important findings had not previously been obtained in two-dimensional culture medium [17, 33]. Furthermore, organoids aided in the development of models for studying breast cancer and the formation of new drugs in target therapy [17, 33, 34]. Interestingly, type II alveolar cells, which lose their different properties in a 2D culture in a plastic dish, are able to maintain them in an EHS matrix environment [35]. This phenomenon shows that intercellular interactions in three-dimensional culture medium with ECM respond better than in two-dimensional culture medium [35]. Intestinal organoids derived from PSCs contain mesenchymal tissue in addition to epithelial tissue with the ability to mimic intestinal function [36, 37]. These investigations progressed to make CNS models for studying the processes in the brains of human bodies [38]. Also, CNS models can be used to study neurobiological phenomena in the course of neurological diseases [38].
Current Types of Organoids and Their Application Scope
Conventionally, 2D tissue cultures and animal models have long been used to study human diseases. However, as organs are three-dimensional, 2D cell cultures lack the exact tissue architecture of the body, and although animal models have been widely used to study diseases, the gap between species hinders the full discovery of diseases [39]. For example, the use of pigs to study gastrointestinal and infectious diseases is widespread and considerable. Nevertheless, due to differences in the gastrointestinal tract of pigs and human beings, pigs serve as asymptomatic carriers of Yersinia infection while humans manifest severe diarrhea [39]. As another example, 2D cell culture systems can be used to study Mycobacterium tuberculosis infection due to the absence of confounding factors and easy infection. However, because they lack real tissue structure, they are unable to accurately predict clinical outcomes [40]. All these concerns led to the invention of 3D organoids using embryonic stem cells (ESCs) and ASCs [1, 17, 41, 42]. Organoids provide micro-environments to study infectious diseases. These 3D tissue cultures allow us to recapitulate interactions between the host and pathogens like viruses, bacteria, and parasites, thus helping the development of drugs for current diseases. [17, 41] The proof of the presented explanation is the provision of lung organoids to investigate SARS-CoV-2 infection. They are used to study the infectivity and effect of viruses on cell functions and, consequently, are used to test possible treatments like Imatinib and mycophenolic acid [43]. Organoids are used to model genetic diseases and investigate the role of genes in their pathogenesis. In order to achieve this goal, organoids are prepared by culturing cells expressing mutations obtained from affected patients or by inducing mutations in wild-type organoids [17, 41]. In the same way as infectious diseases, organoids are used to create treatments for genetic diseases [17, 41]. As an example, cystic fibrosis is a genetic disease caused by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR). Organoids can be used to screen for drugs that can compensate for and regulate these mutant channels [44]. Organoids used for cancer, which are also called tumoroids, are established by using cancer cells obtained via biopsies or independently via induction of mutations. These organoids serve as the technology of bio banking, which is the collection of different subtypes of cancer in an in vitro environment with the aim of studying mutations and genotype–phenotype correlations as well as the molecular basis and pathways involved in tumorigenesis. Besides, biobanks can serve as platforms on which the responses of cancer cells to possible treatments are investigated [17, 41, 45, 46]. Tumoroids are preclinical models to elaborate on personalized drugs and screen them before applying them to patients [17, 41, 45]. In this regard, a study performed by Calandrini et al. can be mentioned in which tumoroids and matching normal kidneys of 50 children with different kidney cancers, including Wilms tumors, renal cell carcinomas, malignant rhabdoid tumors, and congenital mesoblastic nephromas, were stablished [47]. For this purpose, normal and cancerous tissues were obtained from kidneys that underwent nephrectomy or biopsy. Stem cells were then isolated by enzymatic and mechanical methods and used to construct organoids. Later, the constructed organoids underwent histological examinations to study phenotypic characteristics of cancerous tissues like cell marker presentations [47]. By using methods like whole-genome sequencing, genetic and epigenetic features such as mutation signature and DNA methylation profile were surveyed. Later, the current tumoroids were used as platforms to screen the efficacy of chemotherapeutic agents like actinomycin D and vincristine [47]. Moreover, organoids are used to study genome editing, the signaling pathways and mechanisms of cell and organ development [41]. Organoids can be isolated from patients with genetic disease. Then the mutation responsible for the disease can be corrected using CRISPR/Cas9 technology and, later, these edited organoids serve as sources for autologous cell therapies and can be transplanted to the appropriate site [41]. An example of this approach is the use of RPGR gene edited organoids for the treatment of retinitis pigmentosa, which leads to the gain of defects caused by the disease like photoreceptor reduction [41]. Another advantage of organoids is the use of these cell cultures as micro labs in which different signaling pathways can be manipulated to discover their roles in the maintenance or differentiation of cells. For instance, the same method was applied to study the function of Notch, Wnt, or EGFR signaling pathways in the development of enteroendocrine cells [41]. Another application of organoids is that in these microenvironments, toxic and effective doses of drugs, combinant therapies like immunotherapy, and clinical outcomes of the treatments can be predicted. Organoids cultured by isolating iPSCs from a particular patient are safe and reproducible models in which different drugs can be examined and the most effective one is identified. They are also considered a hope for cell therapy and organ transplantation [9, 41, 45, 48]. Some of the most common organoids and their clinical applications are described in Table 1.
Table 1.
Some of the most common organoids and their clinical applications
| Organoid type | Preclinical application | Clinical application | Ref |
|---|---|---|---|
| Kidney | Glomerular related disease- chronic kidney disease- polycystic kidney disease- acute kidney injury | Biobanks for Wilms tumors- malignant rhabdoid tumors- renal cell carcinomas- congenital mesoblastic nephromas | [47, 49, 50] |
| Intestine | Necrotizing enterocolitis- Hirschsprung’s disease- diverticular disease- cystic fibrosis- Short bowel syndrome—Celiac disease- | Cystic fibrosis—celiac disease | [51–56] |
| Retina | Usher syndrome – retinitis pigmentosa—microphtalmia | [57–60] | |
| Prostate | Prostate cancer | Biobanks for prostate cancer | [61–63] |
| Brain |
Microcephaly- zika virus infection- autism. Schizophrenia—neurodegenerative diseases - Miller–Dieker syndrome - Timothy syndrome - |
Biobanks for glioblastoma | [64–67] |
| Liver |
Hepatocellular carcinoma and non-alcoholic fatty liver disease – biliary atresia- inflammatory bowel disease- cystic fibrosis-alpha-1- anti-trypsin (A1AT) deficiency-Wilson’s disease-Alagille syndrome-viral hepatitis- malaria infection |
Cholangiopathies | [16, 68–70] |
| Breast | Breast cancer | [71] | |
| Organoid type | Preclinical application | Clinical application | Ref |
| Kidney | Glomerular related disease- chronic kidney disease- polycystic kidney disease- acute kidney injury | Biobanks for Wilms tumors- malignant rhabdoid tumors- renal cell carcinomas- congenital mesoblastic nephromas | [47, 49, 50, 71] |
| Intestine | Necrotizing enterocolitis- Hirschsprung’s disease- diverticular disease- cystic fibrosis- Short bowel syndrome—Celiac disease- | Cystic fibrosis—celiac disease | [51–56] |
| Retina | Usher syndrome – retinitis pigmentosa—microphtalmia | [57–60] | |
| Prostate | Prostate cancer | Biobanks for prostate cancer | [61–63] |
| Brain |
Microcephaly- zika virus infection- autism. Schizophrenia—neurodegenerative diseases - Miller–Dieker syndrome - Timothy syndrome - |
Biobanks for glioblastoma | [64–67] |
| Liver |
Hepatocellular carcinoma and non-alcoholic fatty liver disease – biliary atresia- inflammatory bowel disease- cystic fibrosis-alpha-1- anti-trypsin (A1AT) deficiency-Wilson’s disease-Alagille syndrome-viral hepatitis- malaria infection |
Cholangiopathies | [16, 68–70] |
| Breast | Breast cancer | [71] |
Organoids and Regenerative Medicine
Regenerative medicine is the science of constructing biological tissues in an in vitro environment using stem cells, biocompatible materials, and biochemical factors, and these tissues are similar to those in the body [72]. These manufactured tissues are used to study the mechanisms and diagnostic processes as well as probable treatments of the diseases [72–76]. Moreover, they are utilized to develop extracorporeal life support systems or to be implanted into the body to replace a damaged organ or repair injuries [72]. One of the recent accomplishments of regenerative medicine is the construction of organoids. Organoids are the 3D assemblage of organ-specific cells derived from stem cells [23]. The cells are cultured in a biologic (decellularized) or synthetic scaffold which mimics the ECM and provides proteins and growth factors necessary for the growth and differentiation of cells [17, 77–80]. Compared to previous efforts in regenerative medicine (cell transplantation, 2D cell cultures, etc.), organoids are structures that sustain their functions and features for a long time. They have a high degree of genetic stability, expansion and differentiation capacity, and self-organization. By providing an optimal chemico-physical and mechanical environment, the level of functionality and self-organization reaches an appropriate state [17, 78, 81, 82]. Moreover, they contain several cell types and thus can replicate cell–cell interactions [83]. In addition to other applications of organoids explained in the previous section, one of the anticipated uses of regenerative medicine is the usage of organoids for transplantation and substitution of damaged organs. For multiple diseases, organ transplantation is the best and most certain treatment. However, allogenic transplantation is restricted due to the limited number of matched donors and long-term immunosuppression [17, 84–86]. As the alternatives like cell transplantation and artificial organs are temporary answers, the emergence of organoids as a source of organ transplantation is considered a hopeful remedy [83]. Since organoids are able to combine with other novel technologies like gene editing and nanotechnology, they are substitutable treatments for certain diseases like chronic kidney disease (CKD), biliary atresia, inflammatory bowel disease (IBD) and metabolic hepatic disease [17, 23, 77, 78, 84, 87–90]. As proof of the current statement, a study by Yui et al. can be used. In this study, Lgr5 + stem cells were taken from the colon epithelium and used to grow colon epithelium organoids that were identical to the real tissue. In order to investigate the functionality of the organoids, the group induced acute colitis in recipient mice and later transplanted the organoids to the injured area using enema. Shortly afterwards, the transplanted cells covered the submucosa of the injured tissue, and the mice started to gain weight. Subsequently, the graft epithelium recovered the injured tissue and manifested colon crypts which were morphologically indistinguishable from the original epithelium around [77, 91]. However, to pass from preclinical studies to clinical application, many concerns regarding safety, ethical, and legal matters still remain [17]. Lack of an optimized differentiation protocol, incomplete maturation, contamination with unwanted cells and pathogens are some of the challenges that should be addressed in this method [23, 84, 88, 89].
A New Model for Improving Regenerative Medicine
In spite of being a hopeful treatment for many diseases, regenerative medicine should undergo different preclinical studies to be qualified for clinical administration. Before a treatment is used on people, these preclinical studies try to figure out all of its risks, safety, and effectiveness [73, 92]. Animals play an important role as models in preclinical studies. Due to physiological and anatomical similarities between animals and humans, they are frequently used to study the pathophysiology of diseases [39]. Animals, as multi-organ creatures, recapitulate the complexity and inter-organ signaling pathways [70]. They are useful for discovering human-pathogen interactions and can be genetically modified [40]. A variety of animal models are used in regenerative medicine [1]. Danio rerio, zebrafish, for example, is an appropriate candidate for genome screening and manipulation. As zebrafish are complex enough, they can recapitulate human physiology [1]. Moreover, high reproducibility at a manageable price and a transparent embryo for developmental studies are the benefits of this model. However, it manifests huge genome complexity and limited biological similarity with the human system [1]. Caenorhabditis elegans is an easily established and maintained system, which is cost-effective and enables retail developmental biology. It is widely used to analyse cell lineage and cell death pathways. Yet, its metabolism, lifespan, and immune system are different from those of human beings [1]. Genetically engineered mouse models are the most preferred models, but they are expensive and compared to the egg-laying animals, the early-stage development of the mouse is inaccessible [1]. However, although general biologic processes were initially studied in animals, some human-specific mechanisms still exist that cannot be modelled by animals [1]. Using animal models is expensive and needs skilled personnel [40]. Ethical issues, anatomical differences, and difficulty in housing for large animals are some of the other obstacles. In addition, due to anatomical and physiological differences between humans and animals, the results obtained from animal studies cannot fully predict the clinical outcomes [40, 70]. Thus, some successful therapies in animal models cannot effectively be translated into humans [9]. Given the limitations listed above, the development of 2D cell architectures represented a promising avenue for disease modeling at the time. While replacing the need for animal models, this architecture has led to many successful understandings of cellular signaling pathways and drug discovery. And it has provided deeper insight into molecular mechanisms [1, 39]. As a result, the first human-specific laboratory model has emerged [1]. 2D cell cultures are cost-effective and time-saving. They are easy to maintain and can be widely manipulated in genetic studies [1]. They can be used for genome editing and biobanking. Studies based on 2D cultures are not hampered by confounding factors and impediments like the effect of the immune system, compared to animal models [40, 93]. Nevertheless, 2D cell cultures have notable limitations since they lack the spatial 3D organization of the body organs which is necessary for their function [42, 70]. They are thought to be non-physiological because of the absence of tissue complexity and because they are immortal [17]. Furthermore, they cannot stimulate the effect of stromal factors, microenvironment, and some of the organ-specific functions. [45] As a result, 2D cell cultures failed to accurately predict clinical outcomes [40]. The emergence of 3D cell cultures, or organoids, is a recent revolution in disease modeling. Organoids are a promising laboratory tool for genome editing, drug discovery, and transplantation. They circumvent limitations on the way toward personalized medicine [70]. Organoids manifest organ-specific functions and enable the understanding of cell movement, differentiation, and tissue development. They pave the way for understanding disease pathophysiologic mechanisms [42]. These 3D cultures are able to mimic cell–cell interactions and the microenvironment present in the body [83]. One of the key factors causing all these benefits gained from organoids is the 3D structure of the ECM around cells, which leads to a gradient of nutrients and signaling pathways. Thus, cells are exposed to different levels of nutrients and growth factors [42, 83, 94]. Another key is the possibility of administration of different exogenous growth factors which guide cells to differentiate into certain cell lines [42, 83, 94]. Moreover, organoids contain different types of cells which are capable of self-organization and allow the system to mimic cell–cell and cell-ECM interactions [42, 83, 94]. Organoids are relatively cost-effective and capable of self-renewal and self-organization [1, 40]. On this basis, it is concluded that organoids maintain the benefits of the previous models, as well as bypass the majority of their limitations, opening up a new possibility in advancing regenerative medicine.
Stem Cell-Derived Organoids
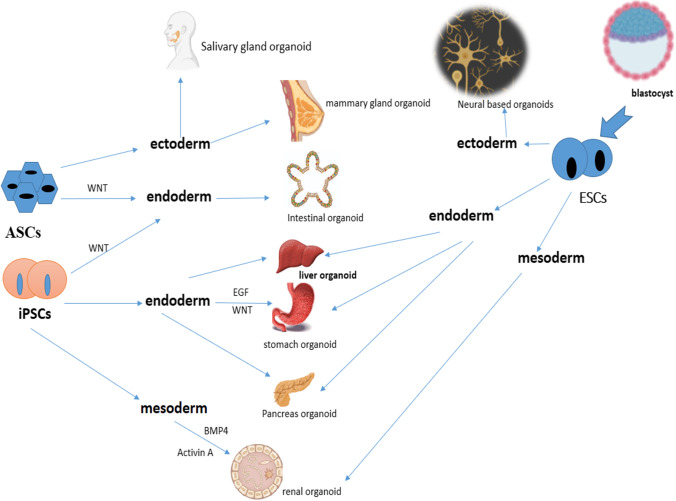
As mentioned previously, organoid technology benefits from the unique properties of stem cells, which can include multi-capabilities and the power of self-renewability [95]. When we consider this ability of stem cells, we find that by providing a suitable culture method, each type of stem cell can differentiate and produce organ structures [95]. In this section we discuss different types of stem cells, including ASCs and PSCs, which consist of both ESCs and iPSCs (induced pluripotent stem cells), that can produce organoids during programmed processes [1, 95, 96]. Adult cell-derived stem cells can differentiate into specific cells of the same tissue or organ in a suitable environment with the same conditions as in the body [95, 97]. One of the advantages of these organoids is their genetic stability over a long time and the preservation of the unique characteristics of each organ [95, 97]. At the same time, ASC-derived organoids can be used to study human biology and genetics with gene modification technology [98]. Using this genetic manipulation, the cell-to-cell connections and cell differentiation can be modified [98]. Gene modification and transgenic organization concepts can also be applied to other stem cell-derived organoids, such as iPSCs [98, 99]. These conditions can be met by observing the appropriate transgenic environment and regular and advanced protocols [98–100]. In fact, the first organoids derived from ASCs could differentiate into the small intestine of mice [2]. Thus, intestinal organoids have the ability to form from intestinal ASCs [2]. Also, two types of tissue of ectodermal origin are obtained from ASCs, which include mammary glands and salivary glands [95]. In this way, the Wnt signaling pathway plays an important role in the propagation of organoids for the long-term [95]. In general, the Wnt signaling pathway can be considered a crucial way to increase the lifespan of many organoid environments that are derived from ASCs, iPSCs, and ESCs [95]. iPSCs-derived organoids can also play a role in the production of endoderm-differentiated organs such as the small intestine, stomach, and pancreas [101]. It should be noted that the organoid environment raised from iPSCs can reconstruct the characteristics of the human gastric corpus [95, 101]. The researchers first found that they needed a retinoic acid signaling pathway to produce gastric organoid. They were able to obtain gastric organoids from the intestinal one with retinoic acid admission [95, 102]. However, in this pathway to make the gastric organoid the desired one, epidermal growth factor (EGF) and Matrigel were also needed [102, 103]. With all these measures, the end of the stomach, which is the antrum and is most similar to the intestinal tissue, was obtained [95, 103]. The WNT signaling pathway is needed to form the proximal part of the stomach, which includes the corpus with parietal cells for acid production [95, 102, 103]. Also, the human intestinal organoids (HIO) made from iPSCs can do things like contract the intestines like the nervous system of the intestines [104, 105]. This procedure necessitates the involvement of iPSC-derived neural crest [105], so the intestinal nervous system model can be simulated by merging neural crests derived from iPSCs and HIOs [105]. This results in a model that can study molecular and cellular processes in diseases such as Hirschsprung and the effects of new drugs on intestinal diseases [95, 104, 105]. iPSCs are also able to produce tissues of mesodermal origin, such as the kidneys [106]. Some factors are needed to construct the intermediate mesoderm of kidney tissue in order to produce ureteric bud and metanephric mesenchyme at the same time [106]. These factors include Activin A, Bone morphogenetic protein 4 (BMP4), and fibroblast growth factor 9 (FGF9) [95, 106, 107]. One subclass of PSC, including ESCs, can produce cerebellum, optic cup, and hippocampal-like organoids in different environmental conditions, and all of these organoids originate in ectodermal tissue [103]. Also, ESC differentiation can yield organoids that consist of neuroepithelial tissue that mimic cortical features [103]. In fact, by inhibiting TGF and BMP signaling pathways, neuroectodermal structures can be generated spontaneously from ESCs [95, 102, 108]. Hepatic organoids were also obtained with a new method from ESCs that can be converted to both hepatocytes and cholangiocytes [109]. These hepatic organoids can proliferate and replace damaged cells with mature hepatocytes after grafting in FRG mice with liver dysfunction [95, 110–112]. The creative point is that it is possible to study drug toxicity on the liver using ESC-derived organoids and apply it to screening models [109, 113]. Thus, in a study by Shinozawa et al. in 2021, the toxicity of 238 drugs was tested on an organoid model of the liver [113]. It should be noted that the widespread use of stem cells and the communication of cells through free RNAs has greatly helped to increase the clinical application of organoids [114, 115]. This issue can be used in organ transplantation [115]. However, these connections in the intercellular environment can be formed through EVs, including exosomes, which could be produced by stem cells. [114, 115]. Figure 1 summarizes how differentiated types of organoids differ from stem cells.
Fig. 1.
Stem cells and differentiated organoids. Stem cells have the ability to transform into any types of tissues and different types of them can produce endoderm, ectoderm, and mesoderm in different conditions [104]
Organoids as 3D Culture Models for EVs Analysis
As mentioned previously, one of the most desirable applications of organoids is their use in cancer studies. In this regard, EVs are developing tools which can increase the importance of organoids in oncologic studies [116, 117]. EVs are stable lipid bi-layered structures that contain nucleic acids, lipids, proteins, and metabolites. These vesicles are secreted by different cells under physiologic or pathologic conditions and can be considered biomarkers of diseases. Also, EVs are mediators of cell–cell crosstalk which impact the target cells through autocrine or paracrine mechanisms [116, 117]. It has been shown that tumoral cells release EVs in order to establish intercellular and matrix-cellular connections, which can later cause autocrine or paracrine oncogenesis and metastasis [118, 119]. EVs also contribute to angiogenesis and immune system modulation and regulate cancer progression. Meanwhile, as the concentration of EVs is reported to be increased in many malignancies, they play the role of a biomarker for these diseases, carrying important information about these malignancies [118, 119]. The discovery of tumoroids allowed us to study the production of EVs as well as other characteristics of cancer disease in a 3D model [120, 121]. Organoids derived from tumors (tumoids) are helpful platforms on which EVs are produced and studied [120]. One example in this regard is a study in cervical cancer tumors, which revealed that tumoroid-derived EV small RNA profiles display a high similarity to circulating EVs in the bodies of patients with cervical cancer. Also, it suggests that following EV molecular changes in interstitial fluids can be a noninvasive method to investigate tissue behaviors on a molecular scale [121]. As another example, in a study by Szvicsek et al. on colorectal cancer (CRC) organoids, it has been shown that EVs play as transmission vehicles for proteins and miRNAs. They also found that the composition of ECM is a critical factor that influences the production of EVs since the accumulation of collagen type 1 is seen to be correlated with an increased level of EVs in CRC organoids [122]. The same interaction between collagen type 1 and EVs is reported by Franchi et al. in their study on breast cancer organoids and Zeöld et al. in a study of ductal pancreatic adenocarcinoma organoids [120, 123]. Meanwhile, Szvicsek et al. reported that fibroblast-derived EVs have a promotive role in the tumorigenesis of CRCs. Finally, the effect of genetics in the production of EVs is suggested by studying the role of Apc mutation in activation of the Wnt pathway and EV production as the downstream result [122]. Similarly, in a study by Sándor et al. on lung adenocarcinoma organoids, the role of the Wnt pathway on cell proliferation and EV secretion has been indicated [124]. In a recent study by Taha et al., it has been indicated that matrix metalloproteinase 3 (MMP3) has a crucial role in maintaining the physical integrities of EVs and any reduction in MMP3 results in disorganization of EV structures, inhibition of their protumorigenic properties, and finally a reduction in tumor proliferation and progression [116]. One more study by Namba et al. on colon cancer organoids demonstrated that the ATP-binding cassette transporter G1 (ABCG1) pump (a cholesterol lipid efflux pump) has a crucial role in tumor progression via altering EV secretion. These tumoroids suggest that depletion of ABCG1 causes intracellular accumulation of EVs and, subsequently, reduces tumor progression and metastasis. Thus, this study offers ABCG1 as a potential therapeutic target for colon cancer [125]. Ke et al. exposed gastric organoids to esophageal adenocarcinoma (EAC)-derived EVs and reported that these EVs induce neoplastic characteristics in gastric organoids, probably due to their role in transferring miR-25 and miR-210. They also successfully lowered the cell proliferation and neoplastic phenotypes in gastroids by inhibition of miR-25 and miR-210 [117]. Besides, EVs can help in providing therapy for cancers in other ways too. For instance, Murgoci et al. used microglia-derived EVs to inhibit tumor invasion in a spheroid model of glioma, suggesting these EVs as nanotherapeutic agents for glioma [126]. In another method, EVs are loaded with certain proteins, lipids, or nucleic acids that are supposed to be carried to target cells and have an inhibitory effect on cancers. An example in this regard is the investigation by Jeong et al. into non-small cell lung cancer. They used EVs as carriers of miRNA-497 in order to suppress tumor growth and genes associated with this kind of cancer [127]. Also, miRNA-497 has an inhibitory role in angiogenesis by decreasing VEGF-A-mediated angiogenic sprouting. All together, they proposed this combination of EV-miRNA as a possible therapy for non-small cell lung cancer [127]. Despite all these benefits, the use of spheroids for EV studies has some challenges. For instance, spheroids used to model cancers have a necrotic core which secretes apoptotic bodies, interfering with EV studies. Thus, to handle this problem, spheroids should be dissociated to determine the viability of cells, the obstacle that prevents further use of the organoid. This problem, along with the other challenges, remains to be addressed in order to gain complete knowledge about EVs [128].
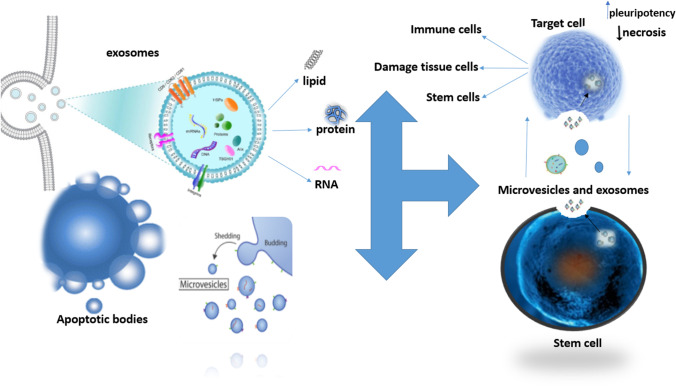
Figure 2 shows an overview of EVs and their role in cell-to-cell communication in the organoid model.
Fig. 2.
Stem cell effects via EVs in an organoid model. As shown in the picture, the three major subgroups of Evs include microvesicles, exosomes, and apoptotic bodies. Since the stem cells are very dynamic, they can obtain Evs such as exosomes [129]. According to the mentioned applications of EVs, they can be used as nanoparticles in organoid models to make them more efficient [130, 131]. EVs: extracellular vesicles
Human Organoids for Precision Medicine
In 1999, Francis Collins took the first step toward precision medicine by publishing the document “Medical and Societal Consequences of the Human Genome Project,” in which he mentions the role of the human genome in prediction, treatment, and prevention of diseases [132]. The recently established concept of “precision medicine,” which is also called personalized medicine, takes advantage of new technologies such as DNA sequencing to refer to inter-individual differences as dominant factors in the evaluation and treatment of diseases [132–134]. Originally, precision medicine used to focus on DNA sequencing and genomic material as the main causes of heterogeneity in disease manifestations and management. However, nowadays, precision medicine implies the incorporation of a wide range of human features like lifestyle, socioeconomic circumstances, and family history as well as genetic factors in diseases [132–134]. In conventional approaches, patients with a certain type of disease were treated with a similar therapy [135]. Nonetheless, recently, it has been shown that while a particular therapy fits some patients, it does not have the expected result in others. This phenomenon is due to inter-personal variation and declares the need for personalized medicine as a “one patient, one treatment” method [135]. Despite this, therapeutic methods offered by precision medicine are applied to preclinical models in the first step to predict the treatment in humans. In this regard, organoids are suitable candidates as they can recapitulate the in vivo environment [136]. One of the wide applications of organoids in precision medicine is in the field of precision oncology. Precision oncology is an attempt to find the most effective therapeutic strategy for every single patient that has the maximum benefit and minimum risk for the patient. This attempt is rooted in the fact that cancer cells are genetically unstable and undergo several mutations while proliferating [137, 138]. Thus, the created cell lines manifest higher genome abnormalities than the primary cells, which leads to intra-tumor heterogeneity. It has been shown that intra-tumor heterogeneity is the main cause of cancer progression and drug resistance [137, 138]. In order to overcome this barrier, several investigations used DNA sequencing of the primary and metastatic tumor cells to identify mutations responsible for drug sensitivity and to discover the optimum treatment for patients [137, 138]. However, even when genetic alterations were figured out, a significant number of the patients failed to respond to the drugs as expected. To address this challenge, organoids derived from patients’ tumor samples were established and called “patient-derived organoids” (PDOs) [137, 138]. The PDOs are the avatars of the primary tumors but in an ex vivo condition as they mimic the phenotypic and genotypic features of the original tumors [17, 139]. PDO obtained from a single patient can serve as a cancer biobank and fulfill different aims of precision medicine. In this biobank, genetic material is studied, genome and drug sensitivity are integrated, and multiple tests of drug screening are performed [137–140]. Moreover, cancer responses to various therapeutic strategies like chemotherapy, radiotherapy, and combination therapy are examined. As a result, the most effective treatment for the patient is selected, and as shown by clinical trials, once this selected therapy is applied to the patient, the results match those from PDOs [17]. Also, a database is created which matches drug sensitivity and tumor genetics. On the other hand, this biobank can offer biomarkers predicting the effectiveness of drugs. These biomarkers can be molecules within tumors that are used as targets for interventions or can be environmental factors that stimulate tumor progression or augment treatment response [17, 137–142]. Another benefit of PDOs is the discovery of carcinogenic mutations and molecular subtypes of tumors in order to categorize them [17, 143]. In addition to precision oncology, personalized medicine has taken initial steps toward other territories of medicine in recent years [1, 17]. As an example, cystic fibrosis is a genetic disease mainly caused by the loss of function of the CFTR gene. Human organoids developed to mimic this disease can be used to screen the efficacy of the current drugs for patients or to produce and test new drugs [1, 17]. Another example is the use of organoids to study inflammatory diseases. Inflammatory bowel disease is an autoimmune disorder that has impaired epithelium-microbe interaction as a main feature [144]. To confront this malfunction, intestinal organoids with the ability to mimic this disease have been developed to investigate epithelium-microbe crosstalk like ROS production and inflammatory cytokine release [144]. However, despite the current achievements, precision medicine has a long way to go to take down all the challenges and turn them into an applicable therapeutic approach [144].
Conclusion and Future Perspectives
Organoids has the ability to fulfill many of the research objectives and can replace the previous models like 2D cultures and animal models, filing the gap between preclinical studies, and clinical application of their results. Thus, in this paper, we aim to study the advancement of this technology and its application in regenerative medicine. Organoids are used to study a wide range of diseases including infectious diseases, genetic diseases, and cancers. They can be used to study the role of EVs as important aspect of cancers. Moreover, organoids are considered platforms to practice genome editing and drug screening and to investigate cell development pathways [44, 145]. However, to achieve the goals aimed for organoid technology, it has to overcome some limitations. Once an organoid is transplanted to human body, host immune system activates and rejects the transplanted tissue [87]. In order to address this challenge, an integration of bioengineering and nanotechnology has developed organoids encapsulated with natural ECM. This structure has a high immune escape ability and can prevent the rejection [87]. Another challenge is the incomplete maturation of the organoids after transplantation; as the results, organoids would not be able to perform all the expected functions [42, 84]. While transplanting an organoid, it is important to determine its capacity of maldifferentiation. According to the researches, organoids contaminated with undifferentiated pluripotent stem cells or other unrelated cells can later differentiate into unwanted and even hazardous tissues with genetic abnormalities. This matter rises concerns about safety and tumor formation of organoids [78, 84, 87]. To address this challenge, cells used to provide organoids should be purified from their surrounding stroma by microdissection methods. Identification of biomarkers to purify subpopulation of stem cells is another solution. Adjusting the composition of the microenvironment around especially by manipulating growth factors can direct organoids to the appropriate differentiation. Genetic stability of the organoid cells should be checked regularly as well. Yet, the need for an effective differentiation protocol remains of concern [44, 78, 84, 146]. A further limitation is that organoids fail to grow a proper vascularization after engraftment. At the present time, the most encouraging solution is to depend on transplanted tissues to stimulate the growth of host vasculature. However, combining the transplanted organoids with blood vessel organoids is considered a future solution to support long-term survival of the culture [42, 145]. As mentioned above, despite all the discoveries made in the field of organoid technology, many challenges like definition of growth factors and metabolites needed for their long-term self-renewal, detection of the inter-cellular crosstalk and many other limitations remain to be answered and that is when organoid can appear as the effective treatment for the diseases and perform expected functions in human body [44].
Abbreviations
- ASCs
Adult stem cells
- BMP
Bone morphogenetic protein
- CF
Cystic fibrosis
- CFTR
Cystic fibrosis transmembrane conductance regulator
- CKD
Chronic kidney disease
- CRC
Colorectal cancer
- ECM
Extracellular matrix
- EHS
Engelbreth-Holm-Swarm
- ESCs
Embryonic stem cells
- EGF
Epidermal growth factor
- EVs
Extracellular vesicles
- FGF
Fibroblast growth factors
- iPSCs
Induced pluripotent stem cells
- IBD
Inflammatory bowel disease
- PDOs
Patient-derived organoids
- PSCs
Pluripotent stem cells
- TGF
Transforming growth factor
- CRC
Colorectal cancer
- MMP3
Matrix metalloproteinase 3
- EAC
Eophageal adenocarcinoma
- HIO
Human intestinal organoid
Declarations
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Babak Arjmand, Email: barjmand@sina.tums.ac.ir.
Zahra Rabbani, Email: z.rabbani1@gmail.com.
Faezeh Soveyzi, Email: F-soveyzi@student.tums.ac.ir.
Akram Tayanloo-Beik, Email: a.tayanloo@gmail.com.
Mostafa Rezaei-Tavirani, Email: Tavirany@yahoo.com.
Mahmood Biglar, Email: mbiglar@tums.ac.ir.
Hossein Adibi, Email: adibi@tums.ac.ir.
Bagher Larijani, Email: emrc@tums.ac.ir.
References
- 1.Kim J, Koo B-K, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21(10):571–584. doi: 10.1038/s41580-020-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet. 2018;19(11):671–687. doi: 10.1038/s41576-018-0051-9. [DOI] [PubMed] [Google Scholar]
- 3.Kratochvil MJ, et al. Engineered materials for organoid systems. Nat Rev Mater. 2019;4(9):606–622. doi: 10.1038/s41578-019-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simian M, Bissell MJ. Organoids: a historical perspective of thinking in three dimensions. J Cell Biol. 2017;216(1):31–40. doi: 10.1083/jcb.201610056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leach TS, et al. et al. Chapter 83 - Tissue organoid models and applications. In: Lanza R, et al.et al., editors. Principles of Tissue Engineering. 5. Academic Press; 2020. pp. 1537–1549. [Google Scholar]
- 6.Sato T, Clevers H. SnapShot: Growing Organoids from Stem Cells. Cell. 2015;161(7):1700–1700.e1. doi: 10.1016/j.cell.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Foley KE. Organoids: a better in vitro model. Nat Methods. 2017;14(6):559–562. doi: 10.1038/nmeth.4307. [DOI] [PubMed] [Google Scholar]
- 8.Aghayan HR, et al. Mesenchymal stem cells’ seeded amniotic membrane as a tissue-engineered dressing for wound healing. Drug Deliv Transl Res. 2021;12(3):538–549. doi: 10.1007/s13346-021-00952-3. [DOI] [PubMed] [Google Scholar]
- 9.Huch M, et al. The hope and the hype of organoid research. Development. 2017;144(6):938–941. doi: 10.1242/dev.150201. [DOI] [PubMed] [Google Scholar]
- 10.Lancaster MA, Huch M. Disease modelling in human organoids. Dis Model Mech. 2019;12(7):dmm039347. doi: 10.1242/dmm.039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clevers HC. Organoids: avatars for personalized medicine. Keio J Med. 2019;68(4):95. doi: 10.2302/kjm.68-006-ABST. [DOI] [PubMed] [Google Scholar]
- 12.Arjmand B, Larijani B. Personalized medicine: a new era in endocrinology. Acta Med Iran. 2017;55(3):142–143. [PubMed] [Google Scholar]
- 13.Arjmand B, et al. Zebrafish for personalized regenerative medicine; a more predictive humanized model of endocrine disease. Front Endocrinol (Lausanne) 2020;11:396. doi: 10.3389/fendo.2020.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi T. Organoids for drug discovery and personalized medicine. Annu Rev Pharmacol Toxicol. 2019;59(1):447–462. doi: 10.1146/annurev-pharmtox-010818-021108. [DOI] [PubMed] [Google Scholar]
- 15.Goodarzi P, et al. Development and validation of Alzheimer’s disease animal model for the purpose of regenerative medicine. Cell Tissue Bank. 2019;20(2):141–151. doi: 10.1007/s10561-019-09773-8. [DOI] [PubMed] [Google Scholar]
- 16.Prior N, Inacio P, Huch M. Liver organoids: from basic research to therapeutic applications. Gut. 2019;68(12):2228–2237. doi: 10.1136/gutjnl-2019-319256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corrò C, Novellasdemunt L, Li VSW. A brief history of organoids. Am J Physiol Cell Physiol. 2020;319(1):C151–C165. doi: 10.1152/ajpcell.00120.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Payab M, et al. Brown adipose tissue transplantation as a novel alternative to obesity treatment: a systematic review. Int J Obes (Lond) 2021;45(1):109–121. doi: 10.1038/s41366-020-0616-5. [DOI] [PubMed] [Google Scholar]
- 19.Payab M, et al. Development of a novel anti-obesity compound with inhibiting properties on the lipid accumulation in 3T3-L1 adipocytes. Iran Biomed J. 2020;24(3):155–163. doi: 10.29252/ibj.24.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tebyanian H, et al. A comparative study of rat lung decellularization by chemical detergents for lung tissue engineering. Open Access Maced J Med Sci. 2017;5(7):859–865. doi: 10.3889/oamjms.2017.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vives J, Batlle-Morera L. The challenge of developing human 3D organoids into medicines. Stem Cell Res Ther. 2020;11(1):72. doi: 10.1186/s13287-020-1586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hohwieler M, et al. Pancreatic progenitors and organoids as a prerequisite to model pancreatic diseases and cancer. Stem Cells Int. 2019;2019:9301382. doi: 10.1155/2019/9301382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyoshi T, et al. Kidney organoids in translational medicine: disease modeling and regenerative medicine. Dev Dyn. 2020;249(1):34–45. doi: 10.1002/dvdy.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagle PW, Coppes RP. Current and future perspectives of the use of organoids in radiobiology. Cells. 2020;9(12):2649. doi: 10.3390/cells9122649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shankar AS, et al. Human kidney organoids produce functional renin. Kidney Int. 2021;99(1):134–147. doi: 10.1016/j.kint.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Sheikh-Hosseini M, Larijani B, Gholipoor Kakroodi Z, et al. Gene Therapy as an Emerging Therapeutic Approach to Breast Cancer: new developments and challenges. Hum Gene Ther. 2021;32(21–22):1330–1345. doi: 10.1089/hum.2020.199. [DOI] [PubMed] [Google Scholar]
- 27.Wilson HV. A new method by which sponges may be artificially reared. Science. 1907;25(649):912–915. doi: 10.1126/science.25.649.912. [DOI] [PubMed] [Google Scholar]
- 28.Davies JA. Chapter 1 - Organoids and mini-organs: introduction, history, and potential. In: Davies JA, Lawrence ML, editors. Organoids and mini-organs. Academic Press; 2018. pp. 3–23. [Google Scholar]
- 29.Johnson MB, March AR, Morsut L. Engineering multicellular systems: using synthetic biology to control tissue self-organization. Curr Opin Biomed Eng. 2017;4:163–173. doi: 10.1016/j.cobme.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss P, Taylor AC. Reconstitution of complete organs from single-cell suspensions of chick embryos in advanced stages of differentiation. Proc Natl Acad Sci U S A. 1960;46(9):1177–1185. doi: 10.1073/pnas.46.9.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans M. Origin of mouse embryonal carcinoma cells and the possibility of their direct isolation into tissue culture. Reproduction. 1981;62(2):625–631. doi: 10.1530/jrf.0.0620625. [DOI] [PubMed] [Google Scholar]
- 32.Hynds RE, Bonfanti P, Janes SM. Regenerating human epithelia with cultured stem cells: feeder cells, organoids and beyond. EMBO Mol Med. 2018;10(2):139–150. doi: 10.15252/emmm.201708213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weeber F, et al. Tumor organoids as a pre-clinical cancer model for drug discovery. Cell Chem Biol. 2017;24(9):1092–1100. doi: 10.1016/j.chembiol.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Chan JL, et al. Intramyocardial bone marrow stem cells in patients undergoing cardiac surgical revascularization. Ann Thorac Surg. 2020;109(4):1142–1149. doi: 10.1016/j.athoracsur.2019.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shannon JM, Mason RJ, Jennings SD. Functional differentiation of alveolar type II epithelial cells in vitro: effects of cell shape, cell-matrix interactions and cell-cell interactions. Biochim Biophys Acta. 1987;931(2):143–156. doi: 10.1016/0167-4889(87)90200-X. [DOI] [PubMed] [Google Scholar]
- 36.Tsuruta S, Uchida H, Akutsu H. Intestinal Organoids generated from human Pluripotent stem cells. JMA J. 2020;3(1):9–19. doi: 10.31662/jmaj.2019-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Min S, Kim S, Cho S-W. Gastrointestinal tract modeling using organoids engineered with cellular and microbiota niches. Exp Mol Med. 2020;52(2):227–237. doi: 10.1038/s12276-020-0386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onuwaje I, Phillips JB. Chapter 16 - Three-dimensional culture systems in central nervous system research. In: Salgado AJ, editor. Handbook of Innovations in Central Nervous System Regenerative Medicine. Elsevier; 2020. pp. 571–601. [Google Scholar]
- 39.Seeger B. Farm animal-derived models of the intestinal epithelium: recent advances and future applications of intestinal organoids. Altern Lab Anim. 2020;48(5–6):215–233. doi: 10.1177/0261192920974026. [DOI] [PubMed] [Google Scholar]
- 40.Fonseca KL, et al. Experimental study of tuberculosis: from animal models to complex cell systems and organoids. PLoS Pathog. 2017;13(8):e1006421. doi: 10.1371/journal.ppat.1006421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Artegiani B, Clevers H. Use and application of 3D-organoid technology. Hum Mol Genet. 2018;27(R2):R99–R107. doi: 10.1093/hmg/ddy187. [DOI] [PubMed] [Google Scholar]
- 42.Xinaris C, Brizi V, Remuzzi G. Organoid models and applications in biomedical research. Nephron. 2015;130(3):191–199. doi: 10.1159/000433566. [DOI] [PubMed] [Google Scholar]
- 43.Johansen MD, et al. Animal and translational models of SARS-CoV-2 infection and COVID-19. Mucosal Immunol. 2020;13(6):877–891. doi: 10.1038/s41385-020-00340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barkauskas CE, et al. Lung organoids: current uses and future promise. Development. 2017;144(6):986–997. doi: 10.1242/dev.140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu H, et al. Organoid technology and applications in cancer research. J Hematol Oncol. 2018;11(1):116. doi: 10.1186/s13045-018-0662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson J, et al. Characterization of a novel breast cancer cell line derived from a metastatic bone lesion of a breast cancer patient. Breast Cancer Res Treat. 2018;170(1):179–188. doi: 10.1007/s10549-018-4719-9. [DOI] [PubMed] [Google Scholar]
- 47.Calandrini C, Schutgens F, Oka R, et al. An organoid biobank for childhood kidney cancers that captures disease and tissue heterogeneity. Nat Commun. 2020;11(1):1310. doi: 10.1038/s41467-020-15155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huch M, Koo BK. Modeling mouse and human development using organoid cultures. Development. 2015;142(18):3113–3125. doi: 10.1242/dev.118570. [DOI] [PubMed] [Google Scholar]
- 49.Morizane R, Bonventre JV. Kidney organoids: a translational journey. Trends Mol Med. 2017;23(3):246–263. doi: 10.1016/j.molmed.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Przepiorski A, et al. The utility of human kidney organoids in modeling kidney disease. Semin Nephrol. 2020;40(2):188–198. doi: 10.1016/j.semnephrol.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Almeqdadi M, et al. Gut organoids: mini-tissues in culture to study intestinal physiology and disease. Am J Physiol Cell Physiol. 2019;317(3):C405–c419. doi: 10.1152/ajpcell.00300.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chusilp S, et al. Intestinal organoids in infants and children. Pediatr Surg Int. 2020;36(1):1–10. doi: 10.1007/s00383-019-04581-3. [DOI] [PubMed] [Google Scholar]
- 53.Yoo JH, Donowitz M. Intestinal enteroids/organoids: a novel platform for drug discovery in inflammatory bowel diseases. World J Gastroenterol. 2019;25(30):4125–4147. doi: 10.3748/wjg.v25.i30.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fair KL, Colquhoun J, Hannan NRF. Intestinal organoids for modelling intestinal development and disease. Philos Trans R Soc Lond B Biol Sci. 2018;373(1750):20170217. doi: 10.1098/rstb.2017.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dieterich W, Neurath MF, Zopf Y. Intestinal ex vivo organoid culture reveals altered programmed crypt stem cells in patients with celiac disease. Sci Rep. 2020;10(1):3535. doi: 10.1038/s41598-020-60521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vijftigschild LA, et al. β2-Adrenergic receptor agonists activate CFTR in intestinal organoids and subjects with cystic fibrosis. Eur Respir J. 2016;48(3):768–779. doi: 10.1183/13993003.01661-2015. [DOI] [PubMed] [Google Scholar]
- 57.Artero Castro A, et al. Deciphering retinal diseases through the generation of three dimensional stem cell-derived organoids: concise review. Stem Cells. 2019;37(12):1496–1504. doi: 10.1002/stem.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kruczek K, Swaroop A. Pluripotent stem cell-derived retinal organoids for disease modeling and development of therapies. Stem Cells. 2020;38(10):1206–1215. doi: 10.1002/stem.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goureau O, Reichman S, Orieux G. Retinal organoids as a new tool for understanding and treating retinal diseases. Med Sci (Paris) 2020;36(6–7):626–632. doi: 10.1051/medsci/2020098. [DOI] [PubMed] [Google Scholar]
- 60.Guo Y, et al. Modeling retinitis pigmentosa: retinal organoids generated from the iPSCs of a patient with the USH2A mutation show early developmental abnormalities. Front Cell Neurosci. 2019;13:361. doi: 10.3389/fncel.2019.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Puca L, et al. Patient derived organoids to model rare prostate cancer phenotypes. Nat Commun. 2018;9(1):2404. doi: 10.1038/s41467-018-04495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gleave AM, et al. A synopsis of prostate organoid methodologies, applications, and limitations. Prostate. 2020;80(6):518–526. doi: 10.1002/pros.23966. [DOI] [PubMed] [Google Scholar]
- 63.Beshiri ML, et al. A PDX/organoid biobank of advanced prostate cancers captures genomic and phenotypic heterogeneity for disease modeling and therapeutic screening. Clin Cancer Res. 2018;24(17):4332–4345. doi: 10.1158/1078-0432.CCR-18-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chuye LB, et al. Brain organoids: expanding our understanding of human development and disease. Results Probl Cell Differ. 2018;66:183–206. doi: 10.1007/978-3-319-93485-3_8. [DOI] [PubMed] [Google Scholar]
- 65.Di Lullo E, Kriegstein AR. The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci. 2017;18(10):573–584. doi: 10.1038/nrn.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jacob F, et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell. 2020;180(1):188–204.e22. doi: 10.1016/j.cell.2019.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lancaster MA, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501(7467):373–379. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeng F, et al. Liver buds and liver organoids: new tools for liver development, disease and medical application. Stem Cell Rev Rep. 2019;15(6):774–784. doi: 10.1007/s12015-019-09909-z. [DOI] [PubMed] [Google Scholar]
- 69.Hindley CJ, Cordero-Espinoza L, Huch M. Organoids from adult liver and pancreas: Stem cell biology and biomedical utility. Dev Biol. 2016;420(2):251–261. doi: 10.1016/j.ydbio.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 70.Semertzidou A, et al. Organoid models in gynaecological oncology research. Cancer Treat Rev. 2020;90:102103. doi: 10.1016/j.ctrv.2020.102103. [DOI] [PubMed] [Google Scholar]
- 71.Roelofs C, et al. Breast tumour organoids: promising models for the genomic and functional characterisation of breast cancer. Biochem Soc Trans. 2019;47(1):109–117. doi: 10.1042/BST20180375. [DOI] [PubMed] [Google Scholar]
- 72.Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annu Rev Chem Biomol Eng. 2011;2:403–430. doi: 10.1146/annurev-chembioeng-061010-114257. [DOI] [PubMed] [Google Scholar]
- 73.Tayanloo-Beik A, et al. Cellular therapy for treatment of spinal cord injury in Zebrafish model. Mol Biol Rep. 2021;48(2):1787–1800. doi: 10.1007/s11033-020-06126-7. [DOI] [PubMed] [Google Scholar]
- 74.Sheikh Hosseini M, et al. Cellular dust as a novel hope for regenerative cancer medicine. Adv Exp Med Biol. 2020;1288:139–160. doi: 10.1007/5584_2020_537. [DOI] [PubMed] [Google Scholar]
- 75.Goodarzi P, et al. Tissue engineered skin substitutes. Adv Exp Med Biol. 2018;1107:143–188. doi: 10.1007/5584_2018_226. [DOI] [PubMed] [Google Scholar]
- 76.Arjmand B, et al. Regenerative medicine perspectives in polycystic ovary syndrome. Adv Exp Med Biol. 2021;1341:125–141. doi: 10.1007/5584_2021_623. [DOI] [PubMed] [Google Scholar]
- 77.Nakamura T, Sato T. Advancing intestinal organoid technology toward regenerative medicine. Cell Mol Gastroenterol Hepatol. 2018;5(1):51–60. doi: 10.1016/j.jcmgh.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Messina A, et al. Pluripotent-stem-cell-derived hepatic cells: hepatocytes and organoids for liver therapy and regeneration. Cells. 2020;9(2):420. doi: 10.3390/cells9020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aghayan HR, et al. Bacterial contamination of amniotic membrane in a tissue bank from Iran. Cell Tissue Bank. 2013;14(3):401–406. doi: 10.1007/s10561-012-9345-x. [DOI] [PubMed] [Google Scholar]
- 80.Aghayan HR, et al. GMP-compliant production of human placenta-derived mesenchymal stem cells. Methods Mol Biol. 2021;2286:213–225. doi: 10.1007/7651_2020_282. [DOI] [PubMed] [Google Scholar]
- 81.Huch M, Boj SF, Clevers H. Lgr5(+) liver stem cells, hepatic organoids and regenerative medicine. Regen Med. 2013;8(4):385–387. doi: 10.2217/rme.13.39. [DOI] [PubMed] [Google Scholar]
- 82.Blutt SE, et al. Use of organoids to study regenerative responses to intestinal damage. Am J Physiol Gastrointest Liver Physiol. 2019;317(6):G845–g852. doi: 10.1152/ajpgi.00346.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olgasi C, Cucci A, Follenzi A. iPSC-derived liver organoids: a journey from drug screening, to disease modeling, arriving to regenerative medicine. Int J Mol Sci. 2020;21(17):6215. doi: 10.3390/ijms21176215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nam SA, et al. Graft immaturity and safety concerns in transplanted human kidney organoids. Exp Mol Med. 2019;51(11):1–13. doi: 10.1038/s12276-019-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aghayan HR, et al. Organ donation workshop - a survey on nurses’ knowledge and attitudes toward organ and tissue donation in Iran. Int J Artif Organs. 2009;32(10):739–744. doi: 10.1177/039139880903201005. [DOI] [PubMed] [Google Scholar]
- 86.Arjmand B, et al. Co-transplantation of human fetal mesenchymal and hematopoietic stem cells in type 1 diabetic mice model. Front Endocrinol (Lausanne) 2019;10:761. doi: 10.3389/fendo.2019.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Navarro-Tableros V, et al. Generation of human stem cell-derived pancreatic organoids (POs) for regenerative medicine. Adv Exp Med Biol. 2020;1212:179–220. doi: 10.1007/5584_2019_340. [DOI] [PubMed] [Google Scholar]
- 88.Geuens T, van Blitterswijk CA, LaPointe VLS. Overcoming kidney organoid challenges for regenerative medicine. NPJ Regen Med. 2020;5:8. doi: 10.1038/s41536-020-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Okamoto R, et al. Organoid-based regenerative medicine for inflammatory bowel disease. Regen Ther. 2020;13:1–6. doi: 10.1016/j.reth.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Naganuma H, Nishinakamura R. From organoids to transplantable artificial kidneys. Transpl Int. 2019;32(6):563–570. doi: 10.1111/tri.13404. [DOI] [PubMed] [Google Scholar]
- 91.Yui S, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med. 2012;18(4):618–623. doi: 10.1038/nm.2695. [DOI] [PubMed] [Google Scholar]
- 92.Payab M, et al. Stem cell and obesity: current state and future perspective. Adv Exp Med Biol. 2018;1089:1–22. doi: 10.1007/5584_2018_227. [DOI] [PubMed] [Google Scholar]
- 93.Li M, Izpisua Belmonte JC. Organoids - preclinical models of human disease. N Engl J Med. 2019;380(6):569–579. doi: 10.1056/NEJMra1806175. [DOI] [PubMed] [Google Scholar]
- 94.Duval K, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology (Bethesda) 2017;32(4):266–277. doi: 10.1152/physiol.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 95.Bartfeld S, Clevers H. Stem cell-derived organoids and their application for medical research and patient treatment. J Mol Med (Berl) 2017;95(7):729–738. doi: 10.1007/s00109-017-1531-7. [DOI] [PubMed] [Google Scholar]
- 96.Sahu S, Sharan SK. Translating embryogenesis to generate organoids: novel approaches to personalized medicine. iScience. 2020;23(9):101485. doi: 10.1016/j.isci.2020.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Drost J, Clevers H. Translational applications of adult stem cell-derived organoids. Development. 2017;144(6):968–975. doi: 10.1242/dev.140566. [DOI] [PubMed] [Google Scholar]
- 98.Menche C, Farin HF. Strategies for genetic manipulation of adult stem cell-derived organoids. Exp Mol Med. 2021;53(10):1483–1494. doi: 10.1038/s12276-021-00609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hofer M, Lutolf MP. Engineering organoids. Nat Rev Mater. 2021;6(5):402–420. doi: 10.1038/s41578-021-00279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hsia GSP, et al. Clinical application of human induced pluripotent stem cell-derived organoids as an alternative to organ transplantation. Stem Cells Int. 2021;2021:6632160. doi: 10.1155/2021/6632160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang M, Liu Y, Chen Y-G. Generation of 3D human gastrointestinal organoids: principle and applications. Cell Regen. 2020;9(1):6. doi: 10.1186/s13619-020-00040-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clevers H. Modeling development and disease with organoids. Cell. 2016;165(7):1586–1597. doi: 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 103.Lewis A, et al. Self-organization of organoids from endoderm-derived cells. J Mol Med (Berl) 2021;99(4):449–462. doi: 10.1007/s00109-020-02010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Azar J, et al. The use of stem cell-derived organoids in disease modeling: an update. Int J Mol Sci. 2021;22(14):7667. doi: 10.3390/ijms22147667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Workman MJ, et al. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med. 2017;23(1):49–59. doi: 10.1038/nm.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Little MH. Growing kidney tissue from stem cells: how far from “party trick” to medical application? Cell Stem Cell. 2016;18(6):695–698. doi: 10.1016/j.stem.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shankaran A, et al. Advances in development and application of human organoids. 3 Biotech. 2021;11(6):257. doi: 10.1007/s13205-021-02815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lee WJ, et al. Generation of brain organoids from mouse ESCs via teratoma formation. Stem Cell Res. 2020;49:102100. doi: 10.1016/j.scr.2020.102100. [DOI] [PubMed] [Google Scholar]
- 109.Wang S, et al. Human ESC-derived expandable hepatic organoids enable therapeutic liver repopulation and pathophysiological modeling of alcoholic liver injury. Cell Res. 2019;29(12):1009–1026. doi: 10.1038/s41422-019-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sui J, et al. Sphingolipid metabolism in type 2 diabetes and associated cardiovascular complications. Exp Ther Med. 2019;18(5):3603–3614. doi: 10.3892/etm.2019.7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rippon HJ, Bishop AE. Embryonic stem cells. Cell Prolif. 2004;37(1):23–34. doi: 10.1111/j.1365-2184.2004.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huang Y, et al. Regenerative medicine for the hepatobiliary system: A review. J Hepatobiliary Pancreat Sci. 2020;28(11):913–930. doi: 10.1002/jhbp.882. [DOI] [PubMed] [Google Scholar]
- 113.Shinozawa T, et al. High-fidelity drug-induced liver injury screen using human pluripotent stem cell–derived organoids. Gastroenterology. 2021;160(3):831–846.e10. doi: 10.1053/j.gastro.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fujii M, Sato T. Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat Mater. 2021;20(2):156–169. doi: 10.1038/s41563-020-0754-0. [DOI] [PubMed] [Google Scholar]
- 115.Yáñez-Mó M, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Taha EA, et al. Knockout of MMP3 weakens solid tumor organoids and cancer extracellular vesicles. Cancers (Basel) 2020;12(5):1260. doi: 10.3390/cancers12051260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ke X, et al. Esophageal adenocarcinoma-derived extracellular vesicle MicroRNAs induce a neoplastic phenotype in gastric organoids. Neoplasia. 2017;19(11):941–949. doi: 10.1016/j.neo.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brassard-Jollive N, et al. In vitro 3D systems to model tumor angiogenesis and interactions with stromal cells. Front Cell Dev Biol. 2020;8:594903. doi: 10.3389/fcell.2020.594903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bordanaba-Florit G, et al. 3D Cell cultures as prospective models to study extracellular vesicles in cancer. Cancers (Basel) 2021;13(2):307. doi: 10.3390/cancers13020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zeold A, et al. Shared extracellular vesicle miRNA profiles of matched ductal pancreatic adenocarcinoma organoids and blood plasma samples show the power of organoid technology. Cell Mol Life Sci. 2021;78(6):3005–3020. doi: 10.1007/s00018-020-03703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thippabhotla S, Zhong C, He M. 3D cell culture stimulates the secretion of in vivo like extracellular vesicles. Sci Rep. 2019;9(1):13012. doi: 10.1038/s41598-019-49671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Szvicsek Z, et al. Extracellular vesicle release from intestinal organoids is modulated by Apc mutation and other colorectal cancer progression factors. Cell Mol Life Sci. 2019;76(12):2463–2476. doi: 10.1007/s00018-019-03052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Franchi M, et al. Extracellular matrix-mediated breast cancer cells morphological alterations, invasiveness, and microvesicles/exosomes release. Cells. 2020;9(9):2031. doi: 10.3390/cells9092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sandor GO, et al. Wnt activity and cell proliferation are coupled to extracellular vesicle release in multiple organoid models. Front Cell Dev Biol. 2021;9:670825. doi: 10.3389/fcell.2021.670825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Namba Y, et al. Depletion of lipid efflux pump ABCG1 triggers the intracellular accumulation of extracellular vesicles and reduces aggregation and tumorigenesis of metastatic cancer cells. Front Oncol. 2018;8:376. doi: 10.3389/fonc.2018.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Murgoci AN, et al. Brain-cortex microglia-derived exosomes: nanoparticles for glioma therapy. ChemPhysChem. 2018;19(10):1205–1214. doi: 10.1002/cphc.201701198. [DOI] [PubMed] [Google Scholar]
- 127.Jeong K, et al. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab Chip. 2020;20(3):548–557. doi: 10.1039/C9LC00958B. [DOI] [PubMed] [Google Scholar]
- 128.Abdollahi S. Extracellular vesicles from organoids and 3D culture systems. Biotechnol Bioeng. 2021;118(3):1029–1049. doi: 10.1002/bit.27606. [DOI] [PubMed] [Google Scholar]
- 129.Xie M, Xiong W, She Z, et al. Immunoregulatory effects of stem cell-derived extracellular vesicles on immune cells. Front Immunol. 2020;11:13. doi: 10.3389/fimmu.2020.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kraińska MM, et al. Extracellular vesicles derived from mesenchymal stem cells as a potential therapeutic agent in acute kidney injury (AKI) in felines: review and perspectives. Stem Cell Res Ther. 2021;12(1):504. doi: 10.1186/s13287-021-02573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meldolesi J. Extracellular vesicles, news about their role in immune cells: physiology, pathology and diseases. Clin Exp Immunol. 2019;196(3):318–327. doi: 10.1111/cei.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Joyner MJ, Paneth N. Promises, promises, and precision medicine. J Clin Invest. 2019;129(3):946–948. doi: 10.1172/JCI126119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Goetz LH, Schork NJ. Personalized medicine: motivation, challenges, and progress. Fertil Steril. 2018;109(6):952–963. doi: 10.1016/j.fertnstert.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.König IR, et al. What is precision medicine? Eur Respir J. 2017;50(4):1700391. doi: 10.1183/13993003.00391-2017. [DOI] [PubMed] [Google Scholar]
- 135.Aboulkheyr Es H, et al. Personalized cancer medicine: an organoid approach. Trends Biotechnol. 2018;36(4):358–371. doi: 10.1016/j.tibtech.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 136.Homicsko K. Organoid technology and applications in cancer immunotherapy and precision medicine. Curr Opin Biotechnol. 2020;65:242–247. doi: 10.1016/j.copbio.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 137.Pauli C, et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov. 2017;7(5):462–477. doi: 10.1158/2159-8290.CD-16-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu R, et al. Tumor organoid models in precision medicine and investigating cancer-stromal interactions. Pharmacol Ther. 2021;218:107668. doi: 10.1016/j.pharmthera.2020.107668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee TW, et al. Patient-derived xenograft and organoid models for precision medicine targeting of the tumour microenvironment in head and neck cancer. Cancers (Basel) 2020;12(12):3743. doi: 10.3390/cancers12123743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kondo J, Inoue M. Application of cancer organoid model for drug screening and personalized therapy. Cells. 2019;8(5):470. doi: 10.3390/cells8050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tayanloo-Beik A, et al. OMICS insights into cancer histology; metabolomics and proteomics approach. Clin Biochem. 2020;84:13–20. doi: 10.1016/j.clinbiochem.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 142.Larijani B, et al. Metabolomics and cell therapy in diabetes mellitus. Int J Mol Cell Med. 2019;8(Suppl1):41–48. doi: 10.22088/IJMCM.BUMS.8.2.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Praharaj PP, et al. Circulating tumor cell-derived organoids: current challenges and promises in medical research and precision medicine. Biochim Biophys Acta Rev Cancer. 2018;1869(2):117–127. doi: 10.1016/j.bbcan.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tran F, et al. Stem cells and organoid technology in precision medicine in inflammation: are we there yet? Front Immunol. 2020;11:573562. doi: 10.3389/fimmu.2020.573562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gkatzis K, et al. Use of three-dimensional organoids and lung-on-a-chip methods to study lung development, regeneration and disease. Eur Respir J. 2018;52(5):1800876. doi: 10.1183/13993003.00876-2018. [DOI] [PubMed] [Google Scholar]
- 146.Grassi L, et al. Organoids as a new model for improving regenerative medicine and cancer personalized therapy in renal diseases. Cell Death Dis. 2019;10(3):201. doi: 10.1038/s41419-019-1453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]