Abstract
Purpose
The absence of specific treatments for COVID-19 leads to an intense global effort in the search for new therapeutic interventions and better clinical outcomes for patients. This review aimed to present a selection of accepted studies that reported the activity of antidepressant drugs belonging to the selective serotonin receptor inhibitor (SSRI) class for treating the novel coronavirus.
Methods
A search was performed in PubMed and SciELO databases using the following search strategies: [(coronavirus) OR (COVID) OR (SARS-CoV-2) AND (antidepressant) OR (serotonin) OR (selective serotonin receptor inhibitors)]. In the end, eleven articles were included. We also covered information obtained from ClinicalTrials.gov in our research.
Results
Although several clinical trials are ongoing, only a few drugs have been officially approved to treat the infection. Remdesivir, an antiviral drug, despite favorable preliminary results, has restricted the use due to the risk of toxicity and methodological flaws. Antidepressant drugs were able to reduce the risk of intubation or death related to COVID-19, decrease the need for intensive medical care, and severely inhibit viral titers by up to 99%. Among the SSRIs studied so far, fluoxetine and fluvoxamine have shown to be the most promising against SARS-CoV-2.
Conclusion
If successful, these drugs can substantially reduce hospitalization and mortality rates, as well as allow for fully outpatient treatment for mild-to-moderate infections. Thus, repositioning SSRIs can provide benefits when faced with a rapidly evolving pandemic such as COVID-19.
Keywords: Repositioning, Redirection, Antidepressants, Coronavirus, COVID-19, Pandemic
Introduction
With a global Toll of more than 450 million cases and 6 million deaths by March 2022, severe acute respiratory syndrome (SARS) caused by the new coronavirus remains a challenge for health care providers. The absence of specific treatment for COVID-19 leads to an intense global effort in the search for new therapeutic interventions and better clinical outcomes for patients [1].
Drug repositioning (or repurposing) is based on accelerating the traditional drug discovery process by identifying new clinical uses for drugs that have already been proven to be safe and effective in humans. The rationale for drug reuse is that the same molecular pathways may be involved in different diseases [2]. This therapeutic strategy can reduce the costs required for new drug development, with notable savings in preclinical phases I and II [3].
Active substances that have had a well-established clinical use in the European Community for at least 10 years, with recognized efficacy and an acceptable level of safety, are known as well-established use medicines. In such cases, marketing authorization may be based on the requirement of one or more clinical trials for the new indication, unless it is an exceptional situation. One such exceptional situation concerns extremely rare conditions, such as anthrax and Ebola. Thus, during a pandemic, drugs may be licensed with less information available than would normally be acceptable [4].
Although more than 7000 clinical trials (ClinicTrials.gov) are underway to identify drugs that could seriously be considered for reuse against COVID-19, only a few drugs have been officially approved to treat the infection. Despite favorable preliminary results from certain antiviral drugs, research is constrained by the risk of toxicity and methodological flaws [4].
We report in this review the repositioning of antidepressant drugs belonging to the selective serotonin receptor inhibitors (SSRI) class as a suggestion for treating patients infected with the new coronavirus, to reduce the complications caused by the disease and reduce the losses linked to the pandemic.
Methodology
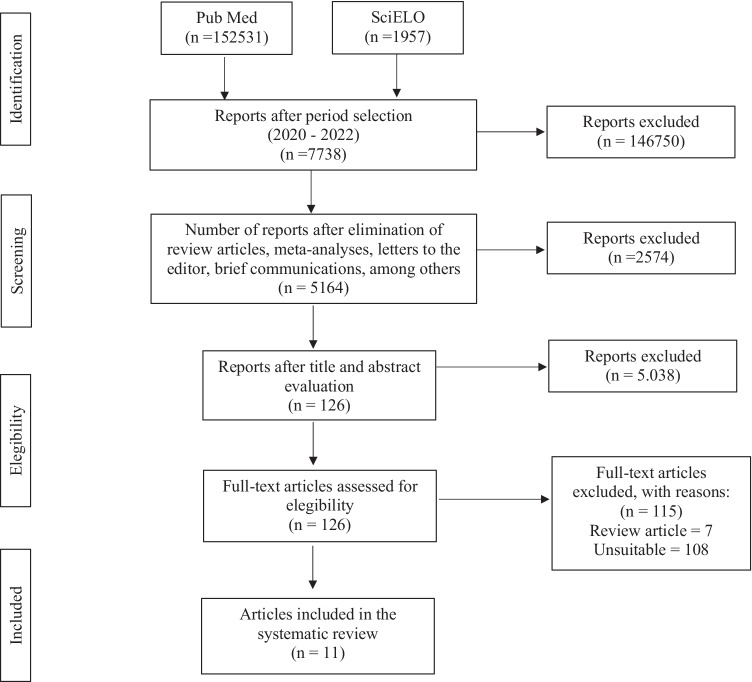
This review was conducted in accordance with the recommendations of the Key Items for Reporting Systematic Reviews and Meta-analyses (PRISMA) [5]. Data collection was conducted through PubMed and SciELO databases, using the following search strategy: [(coronavirus) OR (COVID) OR (SARS-CoV-2) AND (antidepressant) OR (serotonin) OR (Selective Serotonin Receptor Inhibitors)]. The survey was conducted until July 27, 2022. Inclusion criteria were studies accepted since 2020, which evaluated the in vitro and in vivo activity of SSRI drugs in COVID-19, published in English. The systematic search, as illustrated in Fig. 1, returned 154,488 articles that matched the search criteria. The full text of 126 articles was evaluated to verify eligibility for inclusion in our analysis. Of these, 115 articles were excluded for the following reasons: review articles (n = 7) and inappropriate articles (n = 108), that is, articles that did not exactly address the research topic. Finally, eleven studies were included in our review. We also addressed information obtained from clinicaltrials.gov in our study.
Fig. 1.
PRISMA flow diagram
COVID-19 and its clinical consequences
SARS-CoV-2 infection is associated with an amplified immune and inflammatory response. The uncontrolled release of pro-inflammatory cytokines, known as cytokine storm, is triggered by the overexpression of interleukin-6 (IL-6) around the 5 to 7th day of infection. Macrophages and mononuclear cells infiltrate the alveolar tissue and induce the formation of edema, which explains the respiratory symptoms caused by COVID-19, such as cough and difficulty breathing [6, 7].
Increased plasma concentrations of inflammatory mediators in patients with COVID-19 may also promote elevated levels of tumor necrosis factor-alpha (TNF-α), catecholamines, and histamine. At the cellular level, SARS-CoV-2 decreases TCD4 + and TCD8 + lymphocytes, lymphocyte, monocyte, eosinophil, and basophil counts [8–10]. This excessive inflammatory response in COVID-19 is the reason why some anti-inflammatory and immunomodulatory drugs are being evaluated. These drugs can reduce systemic inflammatory symptoms and counteract the effects of the cytokine storm [4, 11, 12].
In this sense, patients with metabolic disorders, such as obesity, are at a greater risk of developing the severe form of the disease [13, 14]. It is suggested that pre-existing underlying inflammation in obese patients may result in an uncontrolled immune response during COVID-19 with additional cytokine production and impaired cellular immunity, predisposing to a more severe disease condition [15].
Obesity is often associated with type 2 diabetes. The authors have shown that uncontrolled glucose levels promote virus replication, increasing cytokine release and inducing T cell dysfunction [14, 15], which is translated by the high prevalence in mortality rates in diabetic patients with coronavírus [16].
Furthermore, one of the most common complications in SARS-CoV-2 infection are cerebrovascular and thrombotic events [17, 18], caused due to cytokine release and consequent activation of the coagulation cascade [19]. Thrombotic events may also be related to clotting alterations caused due to the high concentrations of fibrinogen and D-dimer in COVID-19 [20]. Thus, inadequate blood supply along with impaired lung functions can critically decrease brain oxygenation and have harmful effects on brain function [21].
Increased cytokine release during COVID-19 may also induce the onset of cerebrovascular and neurological changes or aggravate pre-existing conditions, since these disorders are associated with the production of inflammatory mediators [22].
In addition to neurological disorders, neuropsychiatric complications are also a frequent concern in SARS-CoV-2 infection. A surveillance study identified altered mental status in 31% of COVID-19 patients, including a diagnosis of encephalitis and primary psychiatric disorders such as psychosis, dementia, and mania [23]. Additionally, individual reports described changes such as mild cognitive delirium and mood swings.
The restrictions imposed by COVID-19, including quarantine and social isolation, have had devastating adverse effects on the mental health of the world’s population [24]. COVID-19 has been found to significantly increase suicide rates, as well as levels of insomnia, fear, distress, psychological distress, mental health issues, worries, sadness, depression, anxiety, panic disorder, obsessive–compulsive disorder (OCD), and post-traumatic stress disorder (PTSD) [25, 26]. According to Dinakaran et al. (2020), in order for these rates to decrease during the COVID-19 crisis, it is imperative to reduce stress, anxiety, fear, and loneliness [27].
The onset of psychological disorders is also associated with low body immunity and weakened immune response, resulting in severe COVID-19 infections [28, 29]. A study conducted on coronavirus patients reported that the severity of cases occurs due to malfunction or deficiency of the immune system [30]. Immune dysregulation is a consequence of elevated cortisol, the stress hormone, and reduced serotonin [31].
SSRI drugs, their known activities, and possible contributions in the fight against COVID-19
In addition to its classic role as a neurotransmitter (NT), establishing communication between nerve cells and promoting mood enhancement and feelings of happiness, serotonin (5-hydroxytryptamine or 5-HT) expresses its actions in human macrophages and dendritic cells that, by suppressing the production of inflammatory cytokines and chemokines during a stimulus [32], could help reduce the harmful effects of COVID-19 [33].
In addition to treating depression and decreasing susceptibility to suicide [34], 5-HT promotes increased natural killer (NK) cell cytotoxicity and reduced TNF-α production [35]. Recent studies have also shown that exogenous administration of 5-HT provided a decrease in TNF-α, IL-6, and IL-1β, in addition to reversing lipopolysaccharide (LPS)-induced neuroinflammation [36, 37]. On the other hand, circulating serotonin has the potential to modulate numerous functions not only in the respiratory, cardiovascular, and gastrointestinal systems, but also in coagulation, reproduction, and nociception [38].
The role of 5-HT in the immune response in specific viral infections has also been addressed in the literature. In studies by Maneglier et al. (2008), 5-HT decreased HIV infection in human macrophages by downregulating the expression of CCR5, a receptor essential for the entry of the virus into the cell. Serotonin was also able to inhibit the replication of HIV-1/Ba-L, a strain of HIV with macrophage tropism, and ameliorate thrombocytopenia caused by the dengue virus, revealing a potential therapeutic target in infectious viral diseases [39, 40].
In this scenario, the use of antidepressants of the selective serotonin reuptake inhibitors (SSRI) class, which act by inhibiting presynaptic serotonin reuptake and increasing the availability of NT in the synaptic cleft [41], may be beneficial and considered adjuvants in the pharmacological therapy of COVID-19. This class of drugs is commonly used in the treatment of depression, anxiety, PTSD, among other disorders [42]. It was launched in the clinic more than three decades ago and has well-described pharmacodynamic and pharmacokinetic properties, making it a safe option for a possible redirection against the coronavirus.
In patients with COVID-19, SSRIs may help to prevent the release of inflammatory cytokines, which are responsible for worsening disease progression and the subsequent increase in TNF-α [43]. Studies have shown that fluoxetine was able to block IL-6 and the cytokine storm in animal models of infection and in human diseases, such as rheumatoid arthritis [44, 45]. Another SSRI, sertraline has been shown to elicit major anti-inflammatory effects through the reduction and regulation of pro-inflammatory cytokines [46, 47]. Fluvoxamine showed significant immunomodulatory properties, being able to modulate the inflammatory response and cytokine production in monocytes through its high affinity for the sigma-1 receptor (S1R), being able to attenuate tissue damage [48].
One study reported the effectiveness of SSRIs in severe chronic obstructive pulmonary disease (COPD), with a significant increase in oxygen saturation observed in patients receiving these drugs [49].
In metabolic disorders, brain serotonin acts by reducing weight gain, regulating food intake, and the activity of brown adipose tissue, which is responsible for regulating body temperature. Pharmacological interventions that restore central serotonergic levels in obese people may be useful in regulating the immune response during a COVID-19 infection [50].
Recently, it has been revealed that patients recovering from COVID-19 have a higher risk of developing psychiatric disorders, especially anxiety and depression. Thus, SSRIs may have a protective effect on mental health in a post-COVID-19 recovery setting, as they promote anxiolytic and antidepressant action [51], as well as enhance the role of immunity against infections [52–54].
Halperin and collaborators (2007) attested to the anticoagulant properties of SSRIs, which makes this class of drugs promising in patients with COVID-19 who present with cases of venous thrombosis or embolism, responsible for the main causes of disease-related mortality. Fluvoxamine has been shown to possess activity on platelet serotonin concentrations, making it useful in the hypercoagulable state in patients with COVID-19 [55, 56].
Disease severity is also linked to bodily immunity [57]. Antidepressant drugs have the function of increasing the immune response through the inhibition of pro-inflammatory factors, in particular TNF-α, IL-ϒ, IL-1β, and IL-6 [58, 59].
Zafir and colleagues (2007) reported the antioxidant properties of fluoxetine. Chronic administration of this drug for 21 days prevented oxidative damage in animals induced by restraint stress. This fact was evidenced by the significant increase in the main components of antioxidant defense, comprising free radical scavenging enzymes, hydrogen superoxide, and glutathione S-transferase [60].
Studies evaluating the antiviral properties of SSRIs have shown that these drugs decrease Ebola virus activity [61], reduce viral replication of the Coxsackievirus B4 virus [62], and provide dysregulation in HIV coreceptors [44].
Fluoxetine inhibits dengue virus (DENV) and hepatitis C virus (HCV), two members of the Flaviviridae family [63, 64]. It could also inhibit viral replication [65, 66] and increase NK cell activity in patients with HIV [67, 68].
Kristiansen and colleagues (2000) demonstrated that paroxetine reduced HIV p24 antigen levels in an in vitro cell culture system [69]. Sertraline also showed antiviral activity when used effectively in reducing influenza-induced lung inflammation [70]. Fluvoxamine is be able to protect mice from lethal septic shock and reduce the inflammatory response in human blood leukocytes [48].
Buspirone, a serotonin receptor agonist, was able to decrease T-CD8 cell counts and increase CD4/CD8 rates in HIV-infected patients [71], as well as inhibit Chikungunya [72].
Therapeutic perspectives
According to the perspective presented above and in view of the rapid viral spread and the absence of immediate therapeutic interventions to effectively treat SARS-CoV-2 infection, the medical and scientific community has been considering the reuse of drugs already available in the clinic to improve the clinical outcomes of COVID-19.
We present in Table 1 a summary of studies that have evaluated the activity of SSRI drugs in COVID-19. We are aware that to date eleven studies have been accepted addressing this subject.
Table 1.
Accepted studies that evaluated the activity of SSRI and COVID-19 antidepressants
| Reference | Type of study | Study design | Sample characteristics | Main results | Study limitations |
|---|---|---|---|---|---|
| Hoertel et al. [73] | Multicenter observational retrospective cohort study | 7345 adults hospitalized with COVID-19: 460 patients (6.3%) received some antidepressant during hospitalization (257 patients received SSRIs, 71 received SNRIs, 59 received tricyclic antidepressants, 94 tetracyclic antidepressants, 44 α2-antagonist antidepressants) and 6885 did not received treatment with antidepressants | All adults aged 18 years and older who were hospitalized with COVID-19 at the evaluated medical centers were included | Significant association between the use of any antidepressant and reduced risk of intubation or death, especially when there was exposure to escitalopram, fluoxetine and venlafaxine | Missing data for some characteristics (clinical and biological severity of COVID-19); inaccuracies in electronic records (lack of documentation of diseases or medications); incorrect identification of treatment administration method, dosage and frequency |
| Lenze et al. [74] | Randomized, double-blind clinical trial | 80 patients received 100 mg fluvoxamine 3 times daily for 15 days after PCR-confirmed SARS-CoV-2 infection, while 72 patients received placebo. This was a fully remote clinical trial (contact occurred electronically) | Mean age of 46 years; 38 participants (25%) were black adults | Clinical deterioration (defined as onset or hospitalization for dyspnea or pneumonia or decrease in oxygen saturation levels below 92%) occurred in 0 of 80 patients in the fluvoxamine group and in 6 of 72 (8.0%) patients in the placebo group; the fluvoxamine group had 1 serious adverse event and 11 other adverse events, while the placebo group had 6 serious adverse events and 12 other adverse events | Small number of samples; short duration of follow-up (the effect of fluvoxamine on persistent or late symptoms has not been evaluated); the study was conducted within a single geographic area; 20% of participants stopped taking the survey during the 15-day trial |
| Schloer et al. [75] | In vitro | Fluoxetine was tested against human bronchioepithelial cell line (Calu-3) and Vero E6 cell line for 16 h before infection or 1 h after infection | - | Fluoxetine inhibited SARS-CoV-2 infection in Calu-3 epithelial cells and Vero E6 cells, with an EC50 value below 1 μM; application of 10 μM fluoxetine severely reduced viral titers up to 99% | - |
| Eugene [76] | In sílico and in vitro | A population of 1000 subjects was simulated with standard doses of fluoxetine (20 mg/day, 40 mg/day and 60 mg/day) over 10 days of treatment facing SARS-CoV-2 in Calu-3 human lung cells | - | Standard doses of the antidepressant fluoxetine resulted in a range of 79% to 97% of the patient population achieving a minimum plasma concentration of 25.1 ng/ml | CYP2D6 poor or intermediate metabolizers were not monitored; not all medications used by patients and their clinical status were evaluated |
| Seftel and Boulwar [77] | Prospective cohort study | 113 infected workers: 65 opted to receive fluvoxamine (attack dose of 50 to 100 mg, then 50 mg twice daily for 14 days) and 48 opted for observation only without drug therapy. All patients were followed-up in person on day 7 and day 14 of infection | Mean age of 42 years; 75% of participants were men; 84% were latinos; 14% were white | Incidence of hospitalization of 0% with fluvoxamine and 12.5% of patients under observation only; at 14 days, residual symptoms persisted in 0% of patients on fluvoxamine and 60% of patients on observation; the incidence of subsequent hospitalization was 0% in patients on fluvoxamine and 12.5% in observation | Quasi-randomized nature of the comparison |
| Dechaumes et al. [78] | In vitro | Fluoxetine was tested against Vero E6 cells infected by a positive respiratory sample of SARS-CoV-2 | - | The cell viability index was reduced to 86 ± 4% and 98 ± 6% 48 h post-inoculation when treated with 5 and 10 M fluoxetine, respectively, while the value was less than 20% when infected cells were not treated | - |
| Zimniak et al. [79] | In vitro | A concentration of 1.6 μg/ml (~ 5.17 μM) of fluoxetine was tested for 72 h against SARS-CoV-2-infected Vero cells | - | Fluoxetine significantly inhibited SARS-CoV-2 at a concentration of 0.8 μg/ml and the EC50 was determined at 387 ng/ml | - |
| Schloer et al. [80] | In vitro | Fluoxetine/remdesivir combination was tested against human bronchioepithelial cell line (Calu-3) and Vero E6 cell line for 48 h | - | The combination of fluoxetine/remdesivir showed synergistic effects and inhibited the production of SARS-CoV-2 infectious particles > 90% | - |
| Reis et al. [81] | Randomized trial | 9803 participants were selected, of whom 741 received fluvoxamine 100 mg twice a day for 10 days and 756 participants received placebo | Patients hospitalized with COVID-19 are over 18 years old; mean age of 50 years and 58% were women | Fluvoxamine reduced the need for hospitalization and decreased the transfer of critically ill patients to a tertiary hospital compared to placebo; also decreased COVID-19-related deaths by 90% and the need for intensive medical care by about 65% | Tests with a disease that is not well characterized, with no standard of care for early treatment; uncertainty whether the treatment would be applicable in an environment outside Brazil |
| Calusic et al. [82] | Prospective cohort study | 51 hospitalized COVID-19 ICU patients were treated with fluvoxamine 100 mg three times daily for 15 days in addition to standard therapy | 66.7% of the participants were men; 100% were Caucasian | Mortality was lower in the fluvoxamine group (58.8%) compared to the control group (76.5%) | Small sample size, lack of randomization, open design and selection bias. All Caucasian patients (lack of racial diversity) |
| Fred et al. [83] | In vitro | Fluoxetine, citalopram, paroxetine, and fluvoxamine in doses ranging from 0.01 to 20 uM were tested against human embryonic kidney cells expressing ACE2/TMPRSS2 and Calu-1 human lung epithelial cells | - | The drugs inhibited the infection with minimal effects on cell viability; antiviral action was verified in Calu-1 cells against the SARS-CoV-2 lineage | - |
(-) Information not provided by the authors, EC50 effective concentration 50, PCR C-reactive protein, ACE2 angiotensin-2 converting enzyme, SSRIs selective serotonin reuptake inhibitors, SNRIs selective serotonin and noradrenaline receptor inhibitors
Regarding the antiviral properties reported for SSRIs against the coronavirus, the high replicative potential of SARS-CoV-2 promotes an increase in viral load in the lung and, consequently, a large inflammatory process, which can often be fatal. A patent study found that SSRIs have an antiviral effect capable of reducing the expression of chemokines and cytokines in infected cells and therefore play an important role in fighting infections [84].
In Brazil, the study by Reis et al. (2021) demonstrated that fluvoxamine 100 mg administered twice daily for 10 days was able to reduce the need for hospitalization and the transfer of patients with COVID-19 to a tertiary hospital. According to the authors, the low cost of fluvoxamine may make it affordable worldwide, as 10 days of treatment would only cost about $4. This alternative becomes advantageous since the early-stage treatments recommended by the US National Institutes of Health are monoclonal antibodies, which are expensive and difficult to administer in outpatient settings. Additionally, the authors suggest the association of fluvoxamine with drugs that interfere with viral replication. However, it should be noted that this study was carried out in the face of a disease that has not yet been fully characterized, in addition to the uncertainty as to whether the evaluated treatment could be used in different countries [81].
In addition to the accepted studies, a clinical trial (NCT04377308) is currently evaluating the effect of fluoxetine in preventing the severe consequences caused by COVID-19, as well as investigating the relationship of this drug with a decrease in pro-inflammatory cytokines. Another study (NCT04570449) investigates the early use of fluoxetine and its relationship to reduced severity and hospitalization in patients with the disease.
Fluvoxamine, an SSRI approved by the Food and Drug Administration (FDA) fortreating obsessive–compulsive disorder (OCD), is also being evaluated in patients with COVID-19 in the USA (NCT04342663). The initial results of this research group were the reduction of respiratory complications in patients who used fluvoxamine compared with the group that received only placebo. In a double-blind randomized trial, NCT04718480 has been testing 200 mg of fluvoxamine as a daily therapy adjunct to the standard of care in patients with moderate-severity COVID-19.
Trial NCT04727424, conducted in Canada, evaluates drug repositioning against SARS-CoV2, including fluvoxamine 100 mg. NCT04510194 tests the combination of fluvoxamine with metformin for treating non-hospitalized adults with COVID-19, to see if the combination prevents disease progression. NCT04885530 evaluates the effectiveness of repurposing fluvoxamine in reducing symptoms of non-hospitalized participants with mild-to-moderate COVID-19.
Fluvoxamine is also being investigated in a remote survey conducted by videoconference with approximately 400 participants at community treatment centers (NCT04711863). In trial NCT04668950, the aim is to determine whether fluvoxamine could be used early in SARS-CoV-2 infection to prevent more serious complications such as shortness of breath.
Another study (NCT05047952) is evaluating the antidepressant vortioxetine for treating cognitive deficits that develop during or after an infection consistent with COVID-19. Vortioxetine is a multimodal antidepressant that acts on different neurotransmission systems, such as serotonin, norepinephrine, dopamine, GABA, histamine, and acetylcholine [85].
Remdesivir, the first drug to receive emergency use authorization by the FDA for treating severe cases of COVID-19, has its clinical use still controversial due to its short half-life (on average 1 h). The authors suggest that combination therapy of remdesivir with SSRIs would provide beneficial treatment in hospitalized patients with COVID-19, demonstrating that the association between antidepressants and other drugs may be an advantageous option [80, 86].
Among the SSRIs studied so far, fluoxetine and fluvoxamine show to be the most promising drugs against COVID-19. Their actions may occur due to the reduction of the secretion of pro-inflammatory cytokines, such as IL-6, TNF-α, and CCL-2, through modulation of responsiveness to the immune system, interaction with angiotensin-converting enzymes (ACE-2), immunomodulatory actions, and activities directly on T lymphocytes [87]. In addition, as has been shown, these drugs have proven in vitro and in vivo properties against several other viral strains.
Regarding drugs that do not belong to the SSRI class, tricyclic antidepressants have also gained prominence. Clomipramine has been shown to be useful in preventing psychiatric complications occurring after COVID-19 infection. Its anti-inflammatory action and ease of crossing the blood–brain barrier were verified in vitro and in vivo in human and animal studies [88].
The pressure on national health care systems due to large economic losses, failing health care organizations, high intensive care unit admissions, and limited clinically available therapeutic options when faced with COVID-19, makes safe and efficient treatment strategies urgently needed. However, low patient recruitment and small study population size may limit searches for drugs to be repositioned, as well as interfere with their results. Also, most clinical trials present as eligibility criteria patients over 18 years old and belonging to a specific country, increasing the gaps in the research carried out. Thus, more evidence is needed in order to justify the use of this class of drugs in COVID-19.
Final considerations
In light of the current literature and the discussion above, the favorable outlook for the investigation of SSRI drug repositioning in the face of a rapidly evolving pandemic such as COVID-19 is highlighted. Encouraged by preliminary evidence, SSRIs can reduce inflammation caused by the SARS-CoV-2 and prevent organ damage, as they are safe drugs with few side effects. The low cost of these drugs, as well as their wide availability on the market, can promote benefits in the face of the current pandemic since if successful, and these drugs could substantially reduce hospitalization and mortality rates, in addition to allowing a fully outpatient treatment for mild-to-moderate infections. The reuse of antidepressant drugs belonging to the SSRI class deserves deep investigations for treating patients with COVID-19.
Authors’ contributions
The authors contributed significantly to the writing of the review. V.S.F. and R.H. conceived and designed the study. V.S.F. performed the acquisition, analysis, and interpretation of the data and wrote the first version of the manuscript. T.F.R. and M.B.S. reviewed the manuscript for intellectual content. R.H. contributed to the data interpretation and review of the manuscript for intellectual content. The authors have read and approved the final manuscript.
Funding
The authors declare no funding for this study.
Availability of data and materials
All data collected and analyzed are available in this article.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maurya SK, Bhattacharya A, Shukla P, et al. Insights on epidemiology, pathogenesis, diagnosis and possible treatment of COVID-19 infection. Proc Natl Acad Sci India Sect B Biol Sci. 2022 doi: 10.1007/s40011-021-01319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oprea TI, Bauman JE, Bologa CG, et al. Drug repurposing from an academic perspective. Drug Discov Today Ther Strateg. 2011;8:61–69. doi: 10.1016/j.ddstr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 4.Sultana J, Crisafulli S, Gabbay F, et al. Challenges for drug repurposing in the COVID-19 pandemic era. Front Pharmacol. 2020;11:588654. doi: 10.3389/fphar.2020.588654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J Am Med Assoc. 2020;25:324–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 7.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crisafulli S, Isgrò V, La Corte L, et al. Potential role of anti-interleukin (IL)-6 drugs in the treatment of COVID-19: rationale, clinical evidence and risks. BioDrugs. 2020;34:415–422. doi: 10.1007/s40259-020-00430-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7:66–75. doi: 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Codo AC, Davanzo GG, Monteiro LB, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1 alpha/glycolysis-dependent axis. Cell Metabol. 2020;32:498–499. doi: 10.1016/j.cmet.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seidu S, Gillies C, Zaccardi F, et al. The impact of obesity on severe disease and mortality in people with SARS-CoV-2: a systematic review and meta-analysis. Endocrinol Diabetes Metab. 2020;4:e00176. doi: 10.1002/edm2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan M, Khan N, Mustagir G, et al. Effects of underlying morbidities on the occurrence of deaths in COVID-19 patients: a systematic review and meta-analysis. J Glob Health. 2020;10:020503. doi: 10.7189/jogh.10.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bridwell R, Long B, Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med. 2020;38:1549.e3–1549.e7. doi: 10.1016/j.ajem.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colantuoni A, Martini R, Caprari P, et al. COVID-19 sepsis and microcirculation dysfunction. Front Physiol. 2020;11:747. doi: 10.3389/fphys.2020.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu F, McCullough LD. Inflammatory responses in hypoxic ischemic encephalopathy. Acta Pharmacol Sin. 2013;34:1121–1130. doi: 10.1038/aps.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellul M, Varatharaj A, Nicholson TR, et al. Defining causality in COVID-19 and neurological disorders. J Neurol Neurosurg Psychiatry. 2020;91:811–812. doi: 10.1136/jnnp-2020-323667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain A, Bodicherla KP, Raza Q, et al. Impact on mental health by “Living in Isolation and Quarantine” during COVID-19 pandemic. J Family Med Prim Care. 2020;9:5415–5418. doi: 10.4103/jfmpc.jfmpc_1572_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolly TS, Pandian GSDB, Batchelder E et al (2020) Posttraumatic stress disorder exacerbation as a result of public masking in times of COVID-19. Prim Care Companion CNS Disord 22:20l02828. 10.4088/PCC.20l02828 [DOI] [PubMed]
- 26.Jain A, Bodicherla KP, Bashir A et al (2021) COVID-19 and obsessive-compulsive disorder: the nightmare just got real. Prim Care Companion CNS Disord 23:20l02877. 10.4088/PCC.20l02877 [DOI] [PubMed]
- 27.Dinakaran D, Manjunatha N, Naveen Kumar C, et al. Neuropsychiatric aspects of COVID-19 pandemic: a selective review. Asian J Psychiatr. 2020;53:102188. doi: 10.1016/j.ajp.2020.102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao W, Fang Z, Hou G, et al. The psychological impact of the COVID-19 epidemic on college students in China. J Psychiatr Res. 2020;287:112934. doi: 10.1016/j.psychres.2020.112934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozdin S, Bayrak OS. Levels and predictors of anxiety, depression and health anxiety during COVID-19 pandemic in Turkish society: the importance of gender. Int J Soc Psychiatry. 2020;66:504–511. doi: 10.1177/0020764020927051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:e1003. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aan ht Rot M, Mathew SJ, Charney DS, Neurobiological mechanisms in major depressive disorder. CMAJ. 2009;180:305–313. doi: 10.1503/cmaj.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szabo A, Gogolak P, Koncz G. Immunomodulatory capacity of the serotonin receptor 5-HT2B in a subset of human dendritic cells. Sci Rep. 2018;8:1765. doi: 10.1038/s41598-018-20173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herr N, Bode C, Duerschmied D. The effects of serotonin in immune cells. Front Cardiovasc Med. 2017;4:48. doi: 10.3389/fcvm.2017.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li D, He L. Meta-analysis supports association between serotonin transporter (5-HTT) and suicidal behavior. Mol Psychiatr. 2007;12:47–54. doi: 10.1038/sj.mp.4001890. [DOI] [PubMed] [Google Scholar]
- 35.Lu B, Kwan K, Levine YA, et al. α7 nicotinic acetylcholine receptor signaling inhibits inflammasome activation by preventing mitochondrial DNA release. Mol Med. 2014;20:350–358. doi: 10.2119/molmed.2013.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mota CMD, Borges GS, Amorim MR, et al. Central serotonin prevents hypotension and hypothermia and reduces plasma and spleen cytokine levels during systemic inflammation. Brain Behav Immun. 2019;80:255–265. doi: 10.1016/j.bbi.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Voronova IP, Khramova GM, Kulikova EA, et al. 5-HT2A receptors control body temperature in mice during LPSinduced inflammation via regulation of NO production. Pharmacol Res. 2016;103:123–131. doi: 10.1016/j.phrs.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 38.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maneglier B, Guillemin GJ, Clayette P, et al. Serotonin decreases HIV-1 replication in primary cultures of human macrophages through 5-HT(1A) receptors. Br J Pharmacol. 2008;154:174–182. doi: 10.1038/bjp.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Masri MFB, Mantri CK, Rathore APS et al (2019) Peripheral serotonin causes dengue virus-induced thrombocytopenia through 5HT 2 receptors Blood 133:2325–2337. 10.1182/blood-2018-08-869156 [DOI] [PubMed]
- 41.Lochmann D, Richardson T. Selective serotonin reuptake inhibitors. Handb Exp Pharmacol. 2019;250:135–144. doi: 10.1007/164_2018_172. [DOI] [PubMed] [Google Scholar]
- 42.Fluyan D, Mitra P, Jain A, et al. Selective serotonin reuptake inhibitors in the treatment of depression, anxiety, and post-traumatic stress disorder in substance use disorders: a Bayesian meta-analysis. Eur J Clin Pharmacol. 2022;78:931–942. doi: 10.1007/s00228-022-03303-4. [DOI] [PubMed] [Google Scholar]
- 43.Roumestan C, Michel A, Bichon F, et al. Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;8:35. doi: 10.1186/1465-9921-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greeson JM, Gettes DR, Spitsin S. The selective serotonin reuptake inhibitor citalopram decreases human immunodeficiency virus receptor and coreceptor expression in immune cells. Biol Psychiatr. 2016;80:33–39. doi: 10.1016/j.biopsych.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang HY, Deng M, Zhang YH, et al. Specific serotonin reuptake inhibitors prevent interferon-alpha-induced depression in patients with hepatitis C: a meta-analysis. Clin Gastroenterol Hepatol. 2014;12:1452–1460. doi: 10.1016/j.cgh.2013.04.035e1453. [DOI] [PubMed] [Google Scholar]
- 46.Lu Y, Xu X, Jiang T, et al. Sertraline ameliorates inflammation in CUMS mice and inhibits TNF-alpha-induced inflammation in microglia cells. Int Immunopharmacol. 2019;67:119–128. doi: 10.1126/10.1016/j.intimp.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 47.Tynan RJ, Weidenhofer J, Hinwood M, et al. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav Immun. 2012;26:469–479. doi: 10.1016/j.bbi.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Rosen DA, Seki SM, Fernandez-Castaneda A et al (2019) Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med 11:eaau5266. 10.1126/scitranslmed.aau5266 [DOI] [PMC free article] [PubMed]
- 49.Perna G, Cogo R, Bellodi L. Selective serotonin re-uptake inhibitors beyond psychiatry: Are they useful in the treatment of severe, chronic, obstructive pulmonar disease? Depress Anxiety. 2004;20:203–204. doi: 10.1002/da.20041. [DOI] [PubMed] [Google Scholar]
- 50.Kesic M, Bakovic P, Horvaticek M, et al. Constitutionally high serotonin tone favors obesity: study on rat sublines with altered serotonin homeostasis. Front Neurosci. 2020;14:219. doi: 10.3389/fnins.2020.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taquet M, Luciano S, Geddes JR, et al. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dorman-Ilan S, Hertz-Palmor N, Brand-Gothelf A, et al. Anxiety and depression symptoms in COVID-19 isolated patients and in their relatives. Front Psychiatry. 2020;11:581598. doi: 10.3389/fpsyt.2020.581598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sher L. The impact of the COVID-19 pandemic on suicide rates. QJM. 2020;113:707–712. doi: 10.1093/qjmed/hcaa202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei N, Huang B, Lu S, et al. Efficacy of internet-based integrated intervention on depression and anxiety symptoms in patients with COVID-19. J Zhejiang Univ Sci B. 2020;21:400–404. doi: 10.1631/jzus.B2010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Halperin D, Reber G (2007) Influence of antidepressants on hemostasis. Dialogues Clin Neurosci 9:47–59. 10.31887/DCNS.2007.9.1/dhalperin [DOI] [PMC free article] [PubMed]
- 56.Celada P, Dolera M, Alvarez E, et al. Effects of acute and chronis treatmente with fluvoxamine on extracelular and platelet serotonina in the blood of major depressive patients. Relashionship to clinical improvement. J Affect Disord. 1992;25:243–250. doi: 10.1016/0165-0327(92)90082-h. [DOI] [PubMed] [Google Scholar]
- 57.Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. China Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eyre HA, Lavretsky H, Kartika J, et al. Modulatory effects of antidepressant classes on the innate and adaptive immune system in depression. Pharmacopsychiatry. 2016;49:85–96. doi: 10.1055/s-0042-103159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hannestad J, DellaGioia N, Ortiz N, et al. Behavior, and immunity. Citalopram reduces endotoxin-induced fatigue. Brain Behav Immun. 2011;25:256–259. doi: 10.1016/j.bbi.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zafir A, Banu N. Antioxidant potential of fluoxetine in comparison to Curcuma longa in restraint-stressed rats. Eur J Pharmacol. 2007;572:23–31. doi: 10.1016/j.ejphar.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 61.Johansen LM, DeWald LE, Shoemaker CJ et al (2015) A screen of approved drugs and molecular probes identifies therapeutics with anti–Ebola virus activity. Sci Transl Med 7:290ra289–290ra289. 10.1126/scitranslmed.aaa5597 [DOI] [PubMed]
- 62.Alidjinou EK, Sané F, Bertin A, et al. Persistent infection of human pancreatic cells with Coxsackievirus B4 is cured by fluoxetine. Antiviral Res. 2015;116:51–54. doi: 10.1016/j.antiviral.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 63.Medigeshi GR, Kumar R, Dhamija E, et al. N-Desmethylclozapine, fluoxetine, and salmeterol inhibit postentry stages of the dengue virus life cycle. Antimicrob Agents Chemother. 2016;60:6709–6718. doi: 10.1128/AAC.01367-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young K-C, Bai C-H, Su H-C, et al. Fluoxetine a novel anti-hepatitis C virus agent via ROS-, JNK-, and PPARβ/γ-dependent pathways. Antiviral Res. 2014;110:158–167. doi: 10.1016/j.antiviral.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 65.Bauer L, Manganaro R, Zonsics B, et al. Fluoxetine inhibits enterovirus replication by targeting the viral 2C protein in a stereospecific manner. ACS Infect Dis. 2019;5:1609–1623. doi: 10.1021/acsinfecdis.9b00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zuo J, Quinn KK, Kye S, et al. Fluoxetine is a potent inhibitor of coxsackievirus replication. Antimicrob Agents Chemother. 2012;56:4838–4844. doi: 10.1128/AAC.00983-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans DL, Lynch KG, Benton T, et al. Selective serotonin reuptake inhibitor and substance P antagonist enhancement of natural killer cell innate immunity in human immunodeficiency virus/acquired immunodeficiency syndrome. Biol Psychiatr. 2008;63:899–905. doi: 10.1016/j.biopsych.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Frank MG, Hendricks SE, Johnson DR, et al. Antidepressants augment natural killer cell activity: in vivo and in vitro. Neuropsychobiology. 1999;39:18–24. doi: 10.1159/000026555. [DOI] [PubMed] [Google Scholar]
- 69.Kristiansen JE, Hansen JB. Inhibition of HIV replication by neuroleptic agents and their potential use in HIV infected patients with AIDS related dementia. Int J Antimicrob Agents. 2000;14:209–213. doi: 10.1016/s0924-8579(99)00157-0. [DOI] [PubMed] [Google Scholar]
- 70.Kouznetsova J, Sun W, Martinez-Romero C, et al. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg Microbes Infect. 2014;3:e84. doi: 10.1038/emi.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eugen-Olsen J, Benfield T, Axen TE, et al. Effect of the serotonin receptor agonist, buspirone, on immune function in HIV-infected individuals: a six-month randomized, double-blind, placebo-controlled trial. HIV Clin Trials. 2000;1:20–26. doi: 10.1310/UFDA-PU6H-2B0U-K9E6. [DOI] [PubMed] [Google Scholar]
- 72.Bouma EM, van de Pol DPI, Sanders ID, et al. Serotonergic drugs inhibit chikungunya virus infection at different stages of the cell entry pathway. J Virol. 2020;94:13. doi: 10.1128/JVI.00274-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoertel N, Sánchez-Rico M, Vernet R, et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol Psychiatry. 2020;26:5199–5212. doi: 10.1038/s41380-021-01021-4. [DOI] [PubMed] [Google Scholar]
- 74.Lenze EJ, Mattar C, Zorumski C, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324:2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schloer S, Brunotte L, Goretzko J, et al. Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine. Emerg Microbes Infect. 2020;9:2245–2255. doi: 10.1080/22221751.2020.1829082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eugene AR (2020) Fluoxetine pharmacokinetics and tissue distribution suggest a possible role in reducing SARS-CoV-2 titers. MedRxiv. 10.1101/2020.12.17.20248442 [Ahead of print]
- 77.Seftel D, Boulware DR (2021) Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open Forum Infect Dis 8:ofab050. 10.1093/ofid/ofab050 [DOI] [PMC free article] [PubMed]
- 78.Dechaumes A, Nekoua MP, Belouzard S, et al. Fluoxetine can inhibit SARS-CoV-2 in vitro. Microorganisms. 2021;9:339. doi: 10.3390/microorganisms9020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zimniak M, Kirschner L, Hilpert H, et al. The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. Sci Rep. 2021;11:5890. doi: 10.1038/s41598-021-85049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schloer S, Brunotte L, Mecate-Zambrano A, et al. Drug synergy of combinatory treatment with remdesivir and the repurposed drugs fluoxetine and itraconazole effectively impairs SARS-CoV-2 infection in vitro. Br J Pharmacol. 2021;178:2339–2350. doi: 10.1111/bph.15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reis G, Moreira-Silva EAS, Silva DCM, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10:e42–e51. doi: 10.1016/S2214-109X(21)00448-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.M Calusic Marcec R2, Luksa L, et al 2021 Safety and efficacy of fluvoxamine in COVID-19 ICU patients: An open label, prospective cohort trial with matched controls Br J Clin Pharmacol 10.1111/bcp.15126 [DOI] [PMC free article] [PubMed]
- 83.Fred SM, Kuivanen S, Ugurlu H et al (2022) Casarotto PC, Levanov L, Saksela K, Vapalahti O, Castrén E. Antidepressant and antipsychotic drugs reduce viral infection by SARS-CoV-2 and fluoxetine show antiviral activity against the novel variants in vitro. Front Pharmacol 12:755600. 10.1101/2021.03.22.436379 [DOI] [PMC free article] [PubMed]
- 84.Sharma G, Altmeyer R, Pendharker V et al (2010) Compositions and methods for treating viral infections, Google Patents
- 85.Gonda X, Sharma SR, Tarazi FI. Vortioxetine: a novel antidepressant for the treatment of major depressive disorder. Expert Opin Drug Discov. 2019;14:81–89. doi: 10.1080/17460441.2019.1546691. [DOI] [PubMed] [Google Scholar]
- 86.Tempestilli M, Caputi P, Avataneo V, et al. Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19. J Antimicrob Chemother. 2020;75:2977–2980. doi: 10.1093/jac/dkaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sheikhpour M. The current recommended drugs and strategies for the treatment of coronavirus disease (COVID-19) Ther Clin Risk Manag. 2020;16:933–946. doi: 10.2147/TCRM.S262936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nobile B, Durand M, Olié E, et al. The anti-inflammatory effect of the tricyclic antidepressant clomipramine and its high penetration in the brain might be useful to prevent the psychiatric consequences of SARS-CoV-2 infection. Front Pharmacol. 2021;12:615695. doi: 10.3389/fphar.2021.615695. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data collected and analyzed are available in this article.