Abstract
Introduction
Fenofibrate is an agonist of peroxisome proliferator activated receptor alpha (PPAR-α), that possesses anti-inflammatory, antioxidant, and anti-thrombotic properties. Fenofibrate is effective against a variety of viral infections and different inflammatory disorders. Therefore, the aim of critical review was to overview the potential role of fenofibrate in the pathogenesis of SARS-CoV-2 and related complications.
Results
By destabilizing SARS-CoV-2 spike protein and preventing it from binding angiotensin-converting enzyme 2 (ACE2), a receptor for SARS-CoV-2 entry, fenofibrate can reduce SARS-CoV-2 entry in human cells Fenofibrate also suppresses inflammatory signaling pathways, which decreases SARS-CoV-2 infection-related inflammatory alterations. In conclusion, fenofibrate anti-inflammatory, antioxidant, and antithrombotic capabilities may help to minimize the inflammatory and thrombotic consequences associated with SARSCoV-2 infection. Through attenuating the interaction between SARS-CoV-2 and ACE2, fenofibrate can directly reduce the risk of SARS-CoV-2 infection.
Conclusions
As a result, fenofibrate could be a potential treatment approach for COVID-19 control.
Keywords: Fenofibrate, COVID-19, SARS-CoV-2, Angiotensin-converting enzyme 2
Introduction
Fenofibrate, a phenoxy-isobutyric acid derivative (Fig. 1), is metabolized to fenofibric acid, an active metabolite that activates the peroxisome proliferator-activated receptor alpha (PPAR-α) [1].
Fig. 1.
Chemical structure of fenofibrate
Fenofibrate stimulates lipoprotein lipase (LP) through activating PPAR-α and inhibiting apoprotein C-III, which inhibits LP activity [1]. Furthermore, fenofibrate causes lipolysis and lowers triglycerides by converting small low-density lipoprotein (LDL) particles into large particles with a higher affinity for cholesterol receptors, resulting in rapid catabolism of these large particles. The expression of apolipoprotein A-I, A-II, and high-density lipoprotein (HDL) increases when PPAR-α is activated [1, 2]. Overall, fenofibrate lowers cholesterol, triglycerides, LDL, VLDL, and apoprotein B while increasing HDL levels.
In 1974, fenofibrate was synthesized for the first time, and it was first used in medicine in 1975 in France [3]. It can improve glycemic indices in patients with type 2 diabetes mellitus (T2DM) by improving fasting chylomicronemia [4]. It is also used to treat hypertriglyceridemia and mixed dyslipidemia. In T2DM patients, it also lowers diabetic retinopathy and microvascular problems [4]. Fenofibrate also lowers serum uric acid and is used as a supplementary in the treatment of gout [5]. Rhabdomyolysis, pancreatitis, gallstones, and toxic epidermal necrolysis are the most prevalent side effects of fenofibrate [6]. Fenofibrate additionally exhibits pleiotropic effects, such as anti-inflammatory, antioxidant, anti-atherogenic, and antiviral characteristics [7]. As a result, the aim of this critical review was to give an overview of the possible function of fenofibrate in the pathogenesis of SARS-CoV-2 and its consequences.
Fenofibrate and viral infections
Fenofibrate has antiviral activity, and it has been shown to be effective and reduce mortality in a mouse model of Japanese encephalitis [8]. Fenofibrate reduced mortality in mice with Japanese encephalitis by 80% by inhibiting viral-induced microglial activation and the release of pro-inflammatory cytokines [14].
Similarly, fibrate suppresses influenza virus replication and could be an effective antiviral medication alone or in conjunction with antivirals in the scenario of an influenza pandemic [9]. Fenofibrate 45 mg/kg was found to be effective in lowering induced influenza A virus infection in mice, with a 30% reduction in mortality when compared to simvastatin, which had no effect on infection outcome [9]. In HIV-positive patients with hypertriglyceridemia, fenofibrate also inhibits lipid oxidation [10]. Fenofibrate and other PPAR-agonists have been shown to reduce the risk of HIV-1 brain infection by reducing the breakdown of the blood–brain barrier (BBB) caused by elevated matrix metalloproteinase (MMP) levels in HIV-1 infection [11]. Interestingly, PPAR-α inhibits HIV-1-induced neuroinflammation by inhibiting the pro-inflammatory response [12].
In addition, fenofibrate reduces release of hepatitis E virus (HEV) via raising intracellular cholesterol, with subsequent enhancement of lysosomal degradation [13]. Statins, which lower intracellular cholesterol, increase HEV release and infectivity [13]. Activation of PPAR-α, on the other hand, promotes herpes virus replication, suppresses interferon type I production, and causes the creation of reactive oxygen species (ROS) [15]. Following activation of the stimulator of interferon, the activated PPAR-α suppresses the cytoplasmic sensing pathway, impairing the immunological response to herpes virus infection [15]. Herpes virus can also boost PPAR-α expression by encoding a protein that targets these receptors [16]. These findings imply that PPAR-α agonists like fenofibrate suppress the immune system and may increase the risk of herpes virus infection. As a result, there are various debates on whether fenofibrate’s antiviral activity is beneficial or harmful.
Fenofibrate and anti-inflammatory effects
It has been reported that fenofibrate has anti-inflammatory effects through inhibition expression of induced nitric oxide synthase (iNOS), cyclooxygenase 2 (COX2), and matrix metalloproteinase 9 (MMP9) in experiment rats with traumatic brain injury [17]. Fenofibrate reduces the level of inflammatory biomarkers including C-reactive protein (CRP), soluble CD40, IL-6, and monocyte chemoattractant protein 1(MCP-1) independent of changes in lipid profiles [18]. As well, fenofibrate has anti-thrombotic effects by decreasing thrombin-anti-thrombin complex and generated thrombin at site of vascular injury [18]. Fenofibrate promotes anti-inflammatory actions in patients with metabolic syndrome by inducing anti-inflammatory adiponectin, according to a clinical trial involving 50 patients with dyslipidemia treated with the drug [19].
The anti-inflammatory effects of PPAR-α agonists are through induction of an inhibitory protein called inhibitory kappa B (IκB) which inhibits nuclear factor kappa B (NF-κB) [20]. Therefore, fenofibrate through activation of PPAR-α blocks NF-κB-mediated releases of tumor necrosis factor alpha (TNF-α) [21]. Fenofibrate also reduces TNF-expression and release via an adiponectin-dependent mechanism [22]. Fenofibrate improves endothelial dysfunction (ED) in patients with dyslipidemia by stimulating the release of adiponectin, according to Koh et al. [22]. Fenofibrate does, in fact, increase the expression of the anti-inflammatory cytokine IL-10 [23].
Of interest, fenofibrate stimulates PPAR-α in immune cells such as macrophages and lymphocytes, causing the release of IL-10 to be stimulated while IL-17 and interferon gamma (INF-γ) are suppressed [24]. Moreover, fenofibrate also suppresses the expression of the chemokines CCL20, CXCL10, and CCL2 [24]. In virtue of its anti-inflammatory effects, fenofibrate is effective against aggressive rheumatoid arthritis in rats [25]. Likewise, a previous pilot study demonstrated that fenofibrate was effective in the management of erosive osteoarthritis through inhibition of pro-inflammatory cytokines and adipokines [26]. Furthermore, fenofibrate plays a critical role in resolution of neuroinflammation through modulation of pro-inflammatory cytokines and oxylipins from activated astrocytes [27]. These observations indicated that fenofibrate has potent anti-inflammatory effects and could be effective in different inflammatory disorders (Fig. 2).
Fig. 2.
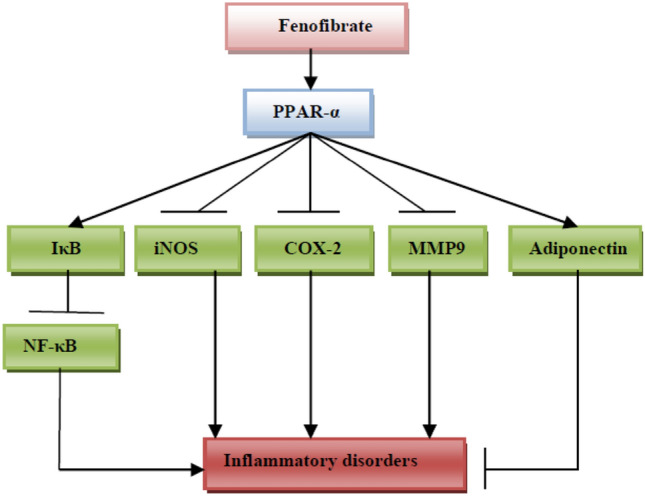
Anti-inflammatory effects of fenofibrate: fenofibrate, through activation of peroxisome proliferator-activated receptor alpha (PPAR-α), inhibits inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX2), matrix metalloproteinase 9 (MMP9), and activates release of adiponectin and inhibitory kappa B (IκB), causing a reduction of inflammatory disorders
Fenofibrate and COVID-19
The existing coronavirus 2019 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has sparked a worldwide pandemic [28]. Uninterrupted SARS-CoV-2 cytopathic injury and release of pro-inflammatory cytokines can induce development of acute respiratory distress syndrome (ARDS) [29]. Due to high expression of angiotensin-converting enzyme 2 (ACE2), a receptor for SARS-CoV-2 entrance, SARS-CoV-2 preferentially damages lung alveolar type II pneumocyte cells in COVID-19 [30]. The majority of COVID-19 patients are asymptomatic or have only minor respiratory symptoms. Despite this, a small percentage of COVID-19 patients experienced severe respiratory problems as a result of the progression of acute lung injury (ALI) and/or ARDS [31].
Hyperinflammation and the development of a cytokine storm as a result of an excessive immune response against SARS-CoV-2 are the main causes of COVID-19-induced complications [32]. As a result, anti-inflammatory and immunosuppressive drugs may be effective in the treatment of SARS-CoV-2-induced immunological dysregulation and over-activation [33, 34]. Fenofibrate’s anti-inflammatory and immuno-regulatory properties may actually be useful in the battle against SARS-CoV-2 infection.
Various in vitro investigations conducted during the COVID-19 era revealed that fenofibrate was beneficial against SARS-CoV-2 infection. Fenofibrate, by destabilizing SARS-CoV-2 spike protein and preventing it from binding ACE2, can lower SARS-CoV-2 in human cells by 70%. Fenofibrate also prevents the accumulation of sphingolipid, which is required for SARS-CoV-2 replication [35, 36]. Fenofibrate, but not fenofibric acid, inhibits ACE2 dimerization and prevents it from engaging the SARS-CoV-2 receptor binding domain in an in vitro investigation [37]. Buschard discovered that the glycosphingolipid sulfatide (3-O-sulfogalactosylceramide), a key component of sphingomyelin, inhibits viral entry across the cell membrane, including SARS-CoV-2 [38]. Sulfatide levels were shown to be low in hypertension and metabolic syndrome patients, but greater in children [39, 40]. Fenofibrate increases sulfatide glycosphingolipid levels, which may reduce SARS-CoV-2 infectivity [38]. Since vitamin K stimulates sulfatide biosynthesis in the brain, the vitamin K antagonist warfarin impairs brain sulfatide [41]. Sulfatide, on the other hand, may have a role in the pathogenesis of autoimmunity by acting as a possible ligand for L and P-selectin and up-regulating the expression of chemokine co-receptors (CXCR4) on neutrophils [42]. In an experimental investigation, sulfatide stimulates the replication of influenza A virus and hepatitis C virus (HCV) [43, 44]. As a result, the role of fenofibrate-mediated sulfatide in the prevention of SARS-CoV-2 should be reconsidered.
Of interest, SARS-CoV-2 triggers expression of cholesterol and lipogenesis in bronchial epithelial cells causing lipotoxicity [45]. Therefore, lipid-lowering activity of fenofibrate can inhibit accumulation of lipid in bronchial epithelial cells and associated inflammation [46]. Remarkably, PPAR-α is highly expressed in alveolar epithelial cells of the lung which conducted in fatty acid oxidation and promoting pulmonary function [47]. It has been shown that expression of alveolar PPAR-α was reduced by 60% in response to lung inflammation and high circulating TNF-α with subsequent disruption of alveolar fatty acid oxidation [48]. Delayer-Orthez et al. reported that mice with deficiency of PPAR-α were susceptible for severe pulmonary inflammation in response to lipopolysaccharide (LPS)-induced inflammation [49]. Therefore, reduction in the expression of PPAR-α may increase for development of pulmonary inflammation and ALI/ARDS in COVID-19 [47].
PPAR-α also controls endothelial function, namely the fate of progenitor cells and colony-forming cells [50]. Disruption of endothelial function by SARS-CoV-2 with production of endothelial dysfunction (ED) may diminish PPAR-α expression in endothelial cells, leading to the establishment of a cytokine storm [51]. In COVID-19, these alterations impair pulmonary vascular responsiveness, resulting in a disruption of the alveolar–capillary barrier, contributing in ventilation–perfusion mismatches and hypoxemia [52]. Furthermore, SARS-CoV-2 can cause ED by inducing apoptosis in endothelial cells, lowering anti-thrombotic activity and causing diffuse pulmonary microthrombosis [53]. As a result, SARS-CoV-2-induced ED impairs expression of endothelial PPA-α with the spread of systemic inflammation. As a result, PPAR-α agonists such as fenofibrate may be beneficial against COVID-19 by reducing ED and pulmonary inflammation while also diminishing metabolic disorders.
Mechanism of fenofibrate against COVID-19
SARS-CoV-2 infection triggers activation of toll-like receptor 4 (TLR4) with release of pro-inflammatory cytokines such as TNF-α and IL-6 mainly in patients with cardio-metabolic disorders [54]. Activation of TLR4 induces activation of NF-κB and release of pro-inflammatory cytokines [54]. Over-activation of TLR4 attenuates expression of PPAR-α with intensification release of pro-inflammatory cytokines [55]. Of interest, oleoylethanolamide, a bioactive lipid, stimulates PPAR-α which initiates a cascade of events which decreases the inflammatory response and reactions [56].
TLR4/NF-κB axis is highly activated in SARS-CoV-2 infection and linked with development of hyperinflammation, cytokine storm, and multi-organ injury [57]. In LPS-stimulated vascular smooth muscle cells, fenofibrate suppresses TLR4 expression while increasing PPAR-α activity [58]. Furthermore, fenofibrate inhibits NF-κB expression and thereby protects the outer blood retinal barrier [59]. In recent studies, activation of the TLR4/NF-κB axis has been linked to lung inflammation and ALI [60]. As a result, fenofibrate may be beneficial against ALI through modulating the TLR4/NF-κB axis. Fenofibrate, according to Zhuo et al., can attenuate ALI in mice caused by intestinal ischemia by ameliorating intestinal and lung damage through anti-ischemic, anti-inflammatory, and antioxidant characteristics [61]. These findings suggested that fenofibrate could be a virtuous candidate for treating COVID-19 patients who have hyperinflammation and are at high risk of developing ALI.
Indeed, SARS-CoV-2 infection triggers activation of nod-like receptor pyrin 3 (NLRP3) inflammasome with subsequent stimulation of immune cells, TLR4/NF-κB axis and pro-inflammatory cytokine release [62]. These changes linked NLRP3 inflammasome activation with development of cytokine storm and sever systemic inflammation. Thus, inhibitors of NLRP3 inflammasome could be beneficial against immune over-activation-induced hyperinflammation and development of cytokine storm in COVID-19 [62]. Of note, fenofibrate had ability to mitigate diabetic retinopathy by inhibiting activation of NLRP3 inflammasome in mice with experimental diabetes [63]. In a similar way, Liu et al. illustrated that fenofibrate enhances expression of nuclear erythroid-related factor 2 (Nrf2) which is the main regulator of oxidative defense, by which it decreases the risk of oxidative stress-induced neuroinflammation and retinal injury [63]. Nrf2 is activated by oxidative stress and plays a critical role in the regulation of oxidative stress and inflammation by antioxidant and anti-inflammatory properties [64]. Nrf2 is inhibited during SARS-CoV-2 infection leading to oxidative and inflammatory disorders [64]. Taken together, fenofibrate through inhibition of NLRP3 inflammasome and activation of Nrf2 may reduce the oxidative and inflammatory disorders in COVID-19.
Furthermore, in SARS-CoV-2 infection, activation of the inflammatory p38 mitogen-activated protein kinase (p38MAPK) signaling pathway is associated with viral replication, ALI, and severe cardiac damage [65]. SARS-CoV-2 infection can induce overexpression of p38MAPK, resulting in ED, vasoconstriction, and thrombosis [65]. In COVID-19, p38MAPK inhibitors are trialed in patients with severe SARS-CoV-2 infection. Concerning the potential effect of fenofibrate on the expression of p38MAPK, Hou et al. demonstrated that PPAR-α agonists like fenofibrate had nephroprotective effect in hypertensive rats via suppression the activity of p38MAPK [66]. Likewise, fenofibrate decreases cardiovascular-associated inflammatory changes by modulation the effect of activated T cells in releasing pro-inflammatory cytokines through inhibition of p38MAPK [67]. In this sense, fenofibrate through suppression of activated p38MAPK could be beneficial against SARS-CoV-2 infection by inhibiting p38MAPK-dependent inflammatory disorders.
Likewise, mechanistic target of rapamycin (mTOR) which is member of protein kinases senses cellular regulatory signals to control autophagy, organelle biogenesis, and expression of inflammatory genes [68]. As well, mTOR pathway is triggered during SARS-CoV-2 infection for transcription and translation of SARS-CoV-2 mRNA [69]. Inhibition of mTOR signaling pathway may reduce viral replication and might be a latent therapeutic option against COVID-19 [69]. It has been shown that fenofibrate inhibits activated mTOR pathway with induction of apoptosis of cancer cells in human prostate [70]. Similarly, fenofibrate can attenuate cardiac hypertrophy via inhibition of mTOR pathway [71]. Taken together, fenofibrate through inhibition of mTOR pathway may decrease severity of SARS-CoV-2 infection.
Furthermore, advanced glycation end product (AGE) is intricate in the pathogenies of SARS-CoV-2 infection; however, soluble RAGE (sRAGE) reflects the underlying AGE in COVID-19 [71]. Lim and colleagues reported that sRAGE serum level is regarded as a biomarker and indicator for COVID-19 severity, mortality, and the need for assist ventilation [72]. An observational-cohort study comprised 164 COVID-19 patients revealed that sRAGE serum level was increased in patients with COVID-19 pneumonia and correlated with disease severity [72]. Suppression formation of AGE in COVID-19 may attenuate the pathogenesis of SARS-CoV-2 infection and associated inflammations. Interestingly, fenofibrate inhibits activity of polyol pathway with subsequent reduction of AGE, oxidative stress, and release of inflammatory cytokines [73]. Fenofibrate also suppress activity of vascular endothelial growth factor (VEGF) in patients with diabetic retinopathy [73]. VEGF is regarded as key factor in the development of ALI and ARDS through induction of vascular permeability. VEGF serum levels were illustrated to be higher in patients with severe COVID-19 compared with the mild one [74, 75]. SARS-CoV-2 infection-induced down-regulation of ACE2 is considered as the main cause for overexpression of VEGF. Thus, fenofibrate can decrease COVID-19 severity via inhibiting the activity of AGE and VEGF.
In addition, lipid-lowering drug statins have pleiotropic effects; however, its outcome on the circulating adiponectin was contentious, and diverse studies accuse statins of decreasing the adiponectin level [76, 77]. Nevertheless, a meta-analysis demonstrated that statins increase adiponectin level through intonation of inflammatory cytokines [77]. Into the bargain, fenofibrate improves circulating adiponectin which has potent anti-inflammatory effects [22]. Of note, ACE2 has perilous role in the regulation of adiponectin production; ACE2 and Ang1-7 reduce epicardial adipose tissue-mediated inflammation via up-regulation expression of adiponectin [78]. As well, fosinopril attenuates liver fibrosis by up-regulating adiponectin through ACE2/Ang1-7 expression [78, 79]. Therefore; dysregulation of renin–angiotensin system (RAS) in COVID-19 could be the possible cause of low adiponectin level. Therefore, fenofibrate through induction production and release of adiponectin could explain its beneficial effect against SARS-CoV-2.
Fascinatingly, fenofibrate improves ED by suppressing production of endothelin-1 (ET-1), increases synthesis of endothelial NO production and inhibition of vascular inflammation [80]. Fenofibrate increases synthesis of tetrahydrobiopterin which is necessary for activation of eNOS and NO production with regulation of vascular reactivity [81]. Besides, ED is regarded as a hallmark of COVID-19 and mainly predominates in the SARS-CoV-2 infection. ED is associated with thrombosis, ALI/ARDS, and multi-organ injury [82]. Lee et al. reported that fenofibrate had anti-platelet and anti-thrombotic properties. The anti-platelet effect is mediated by inhibition of thromboxane A2, COX1, and mobilization of cytosolic calcium [83]. As well, fenofibrate reduces thrombin-activatable fibrinolysis inhibitor thereby decreasing the risk of thrombosis in patients with metabolic syndrome [84]. Thrombosis and platelet hyper-activation are linked with COVID-19 severity [85]. Therefore, fenofibrate through modulation of ED, thrombosis, and platelet hyper-activation can reduce COVID-19 severity.
Overall, fenofibrate seems to be of a great benefit against pathogenesis of SARS-CoV-2 infection and COVID-19 severity by its anti-inflammatory, antioxidant, and anti-thrombotic activities (Fig. 3).
Fig. 3.
Mechanism of fenofibrate against SARS-CoV-2 infection: SARS-CoV-2 induces activation of signaling pathways including toll-like receptor 4 (TLR4), nuclear factor kappa B (NF-κB), mechanistic target of rapamycin (mTOR), nod-like receptor pyrin 3 (NLRP3) inflammasome, advanced glycation end product (AGE), p38 mitogen-activated protein kinase (p38MAPK), and vascular endothelial growth factor (VEGF) with subsequent development of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)
Moreover, Feher et al. illustrated that fenofibrate inhibits replication of SARS-CoV-2 in human lung epithelial cells [86]. As well, fenofibrate attenuates SARS-CoV-2-induced up-regulation of lipogenesis and glycolysis with subsequent suppression of metabolic footprint for viral replication [87]. However, the other drugs such as metformin, rosiglitazone, and empagliflozin which have impacts on the lipogenesis and glycolysis did not affect viral replications [87]. Furthermore, laboratory findings have been suggested as modes of action of fenofibrate against SARS-CoV-2 infection [87]. A cohort-observational study involved 477,803 COVID-19 patients; 596 on fenofibrate therapy for at least 3 months compared to 2980 on non-fibrate therapy showed that there was no difference in 28-day mortality and survival rate [86]. These findings suggest that in vitro positive effects of fenofibrate are not correlated with in vivo effects. Thus, randomized clinical trials are recommended to confirm the modifying effect of fenofibrate on the pathogenesis of SARS-CoV-2 infection and clinical outcomes in COVID-19 patients.
Concerning the risk of drug-drug interactions between fenofibrate and drugs used in the management of COVID-19, fenofibrate interacts with several drugs like statins, bile acid sequestrants, cyclosporine, and warfarin [88]. Interestingly, there is no significant interaction between fenofibrate and remdesivir, which is commonly used in the management of severely affected COVID-19 patients [89]. Thus, fenofibrate seems to be an active drug together with anti-SARS-CoV-2 agents in the management of COVID-19.
The present review had several limitations, including the scarcity of clinical and preclinical studies concerning the role of fenofibrate in the management of COVID-19. However, this critical review discusses the potential role of the anti-inflammatory, antioxidant, and anti-thrombotic properties of fenofibrate against COVID-19.
Conclusion
Fenofibrate is a PPAR-α agonist that has anti-inflammatory, antioxidant, and anti-thrombotic properties through modulation of inflammatory signaling pathway, oxidative stress, and platelet activation. Fenofibrate through these properties may reduce SARS-CoV-2 infection-associated inflammatory and thrombotic complications. It can directly reduce the risk of SARS-CoV-2 infection through attenuation of the interaction between SARS-CoV-2 and ACE2. Therefore, fenofibrate could be a novel therapeutic option in the management of COVID-19. Thus, experimental, preclinical, and clinical studies are recommended to confirm the possible role of fenofibrate against COVID-19.
Author contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by Hayder M. Alkuraishy, Ali I. Al-Gareeb. The first draft of the manuscript was written by Shadi Salem Alkhayyat, Hayder M. Al-kuraishy, Ali I. Al-Gareeb, Maisra M. El-Bouseary, Amal M. AboKamer, Jesus Simal Gandara and Gaber E. Batiha. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received for conducting this study.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shadi Salem Alkhayyat, Email: DR_shadi2002@hotmail.com.
Hayder M. Al-kuraishy, Email: Hayderm36@yahoo.com
Ali I. Al-Gareeb, Email: Dr.alialgareeb78@yahoo.com
Maisra M. El-Bouseary, Email: maysra_mohamed@pharm.tanta.edu.eg
Amal M. AboKamer, Email: amalabokamer@pharm.tanta.edu.eg
Gaber El-Saber Batiha, Email: gaberbatiha@gmail.com.
Jesus Simal-Gandara, Email: jsimal@uvigo.es.
References
- 1.Rasheed HA, Hussien NR, Al-Naimi MS, Al-Kuraishy HM, Al-Gareeb AI. Fenofibrate and Crataegus oxyacantha is an effectual combo for mixed dyslipidemia. Biomed Biotechnol Res J. 2020;4(3):259. doi: 10.4103/bbrj.bbrj_26_20. [DOI] [Google Scholar]
- 2.Goncalves MD, Hwang SK, Pauli C, Murphy CJ, Cheng Z, Hopkins BD, et al. Fenofibrate prevents skeletal muscle loss in mice with lung cancer. Proc Natl Acad Sci USA. 2018;115(4):E743–E752. doi: 10.1073/pnas.1714703115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung JY, Choi Y, Suh CH, Yoon D, Kim HA. Effect of fenofibrate on uric acid level in patients with gout. SciRep. 2018;8(1):16767. doi: 10.1038/s41598-018-35175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.FIELD Study Investigators Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 5.Fischer J, Ganellin CR. Analogue-based drug discovery. Chem Int—Newsmag IUPAC. 2010;32(4):12–15. [Google Scholar]
- 6.Rabasa-Lhoret R, Rasamisoa M, Avignon A, Monnier L. Rare side-effects of fenofibrate. Diabetes Metab. 2001;27(1):66–68. [PubMed] [Google Scholar]
- 7.Tsimihodimos V, Miltiadous G, Daskalopoulou SS, Mikhailidis DP, Elisaf MS. Fenofibrate: metabolic and pleiotropic effects. CurrVascPharmacol. 2005;3(1):87–98. doi: 10.2174/1570161052773942. [DOI] [PubMed] [Google Scholar]
- 8.Sehgal N, Kumawat KL, Basu A, Ravindranath V. Fenofibrate reduces mortality and precludes neurological deficits in survivors in murine model of Japanese encephalitis viral infection. PLOSONE. 2012;7(4):e35427. doi: 10.1371/journal.pone.0035427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alleva LM, Budd AC, Clark IA. Minimising influenza disease with fibrates. Int J InfectDis. 2008;12:e176. doi: 10.1016/j.ijid.2008.05.440. [DOI] [Google Scholar]
- 10.Badiou S, Merle De Boever CM, Dupuy AM, Baillat V, Cristol JP, Reynes J. Fenofibrate improves the atherogenic lipid profile and enhances LDL resistance to oxidation in HIV-positive adults. Atherosclerosis. 2004;172(2):273–279. doi: 10.1016/j.atherosclerosis.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Huang W, Chen L, Zhang B, Park M, Toborek M. PPAR agonist-mediated protection against HIV Tat-induced cerebrovascular toxicity is enhanced in MMP-9-deficient mice. J Cereb Blood Flow Metab. 2014;34(4):646–653. doi: 10.1038/jcbfm.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang W, Rha GB, Han MJ, Eum SY, András IE, Zhong Y, et al. PPARα and PPARγ effectively protect against HIV-induced inflammatory responses in brain endothelial cells. J Neurochem. 2008;107(2):497–509. doi: 10.1111/j.1471-4159.2008.05626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glitscher M, Martín DH, Woytinek K, Schmidt B, Tabari D, Scholl C, et al. Targeting cholesterol metabolism as efficient antiviral strategy against the hepatitis E virus. Cell MolGastroenterol Hepatol. 2021;12(1):159–180. doi: 10.1016/j.jcmgh.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Wang Z, Chen H, Chen Z, Tian Y. Antioxidants: potential antiviral agents for Japanese encephalitis virus infection. IntJ InfectDis. 2014;24:30–36. doi: 10.1016/j.ijid.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Tao L, Lowe A, Wang G, Dozmorov I, Chang T, Yan N, Reese TA. Metabolic control of viral infection through PPAR-α regulation of STING signaling. BioRxiv. 2019;13:17. [Google Scholar]
- 16.Sychev ZE, Hu A, DiMaio TA, Gitter A, Camp ND, Noble WS, et al. Integrated systems biology analysis of KSHV latent infection reveals viral induction and reliance on peroxisome mediated lipid metabolism. PLOSPathog. 2017;13(3):e1006256. doi: 10.1371/journal.ppat.1006256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen XR, Besson VC, Palmier B, Garcia Y, Plotkine M, Marchand-Leroux C. Neurological recovery-promoting, anti-inflammatory, and anti-oxidative effects afforded by fenofibrate, a PPAR alpha agonist, in traumatic brain injury. J Neurotrauma. 2007;24(7):1119–1131. doi: 10.1089/neu.2006.0216. [DOI] [PubMed] [Google Scholar]
- 18.Undas A, Celinska-Löwenhoff M, Domagala TB, Iwaniec T, Dropinski J, Löwenhoff T, Szczeklik A. Early antithrombotic and anti-inflammatory effects of simvastatin versus fenofibrate in patients with hypercholesterolemia. Thromb Haemost. 2005;94(1):193–199. doi: 10.1160/TH05-01-0067. [DOI] [PubMed] [Google Scholar]
- 19.Rosenson RS. Effect of fenofibrate on adiponectin and inflammatory biomarkers in metabolic syndrome patients. Obesity. 2009;17(3):504–509. doi: 10.1038/oby.2008.530. [DOI] [PubMed] [Google Scholar]
- 20.Delerive P, Gervois P, Fruchart JC, Staels B. Induction of IκBα expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-α activators. J BiolChem. 2000;275(47):36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 21.Chen N, Jiang K, Yan GG. Effect of fenofibrate on diabetic retinopathy in rats via SIRT1/NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(19):8630–8636. doi: 10.26355/eurrev_201910_19180. [DOI] [PubMed] [Google Scholar]
- 22.Koh KK, Han SH, Quon MJ, Yeal Ahn JY, Shin EK. Beneficial effects of fenofibrate to improve endothelial dysfunction and raise adiponectin levels in patients with primary hypertriglyceridemia. Diabetes Care. 2005;28(6):1419–1424. doi: 10.2337/diacare.28.6.1419. [DOI] [PubMed] [Google Scholar]
- 23.Rada MJ, Donato M, Penas FN, Alba Soto CD, Cevey ÁC, Pieralisi AV, et al. IL-10-dependent and-independent mechanisms are involved in the cardiac pathology modulation mediated by fenofibrate in an experimental model of Chagas heart disease. Front Immunol. 2020;11:2429. doi: 10.3389/fimmu.2020.572178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JW, Bajwa PJ, Carson MJ, Jeske DR, Cong Y, Elson CO, et al. Fenofibrate represses interleukin-17 and interferon-γ expression and improves colitis in interleukin-10–deficient mice. Gastroenterology. 2007;133(1):108–123. doi: 10.1053/j.gastro.2007.03.113. [DOI] [PubMed] [Google Scholar]
- 25.Wahba MG, Messiha BA, Abo-Saif AA. Protective effects of fenofibrate and resveratrol in an aggressive model of rheumatoid arthritis in rats. PharmBiol. 2016;54(9):1705–1715. doi: 10.3109/13880209.2015.1125931. [DOI] [PubMed] [Google Scholar]
- 26.Shirinsky IV, Shirinsky VS. Treatment of erosive osteoarthritis with peroxisome proliferator-activated receptor alpha agonist fenofibrate: a pilot study. RheumatolInt. 2014;34(5):613–616. doi: 10.1007/s00296-013-2766-4. [DOI] [PubMed] [Google Scholar]
- 27.Chistyakov DV, Astakhova AA, Goriainov SV, Sergeeva MG. Comparison of PPAR ligands as modulators of resolution of inflammation, via their influence on cytokines and oxylipins release in astrocytes. IntJ MolSci. 2020;21(24):9577. doi: 10.3390/ijms21249577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Kuraishy HM, Al-Gareeb AI, Alzahrani KJ, Alexiou A, Batiha GE. Niclosamide for Covid-19: bridging the gap. MolBiolRep. 2021;48(12):8195–8202. doi: 10.1007/s11033-021-06770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onohuean H, Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Batiha GE. Covid-19 and development of heart failure: mystery and truth. Naunyn-SchmiedebergsArch Pharmacol. 2021;394(10):2013–2021. doi: 10.1007/s00210-021-02147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Kuraishy HM, Al-Gareeb AI, Alkazmi L, Alexiou A, Batiha GE. Levamisole therapy in COVID-19. Viral Immunol. 2021;34(10):722–725. doi: 10.1089/vim.2021.0042. [DOI] [PubMed] [Google Scholar]
- 31.Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Gyebi GA, Batiha GE. Covid-19-induced dysautonomia: a menace of sympathetic storm. ASN Neuro. 2021;13:17590914211057635. doi: 10.1177/17590914211057635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Kuraishy HM, Al-Gareeb AI, Qusty N, Cruz-Martins N, El-Saber Batiha GE. Sequential doxycycline and colchicine combination therapy in Covid-19: the salutary effects. PulmPharmacol Ther. 2021;67:102008. doi: 10.1016/j.pupt.2021.102008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Kuraishy HM, Al-Gareeb AI, Alqarni M, Cruz-Martins N, El-Saber BG. Pleiotropic effects of tetracyclines in the management of COVID-19: emerging perspectives. Front Pharmacol. 2021;12:642822. doi: 10.3389/fphar.2021.642822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batiha GE. Cyclosporine attenuates Covid-19: ensnare or victory. Ann Clin Med Case Rep. 2021;7(4):1–8. [Google Scholar]
- 35.Hassan T. Fenofibrate, a drug for COVID-19? All we need to know. J Rawalpindi Med Coll. 2021;25(4):441–442. [Google Scholar]
- 36.Gupta RK, Nwachuku EL, Zusman BE, Jha RM, Puccio AM. Drug repurposing for COVID-19 based on an integrative meta-analysis of SARS-CoV-2 induced gene signature in human airway epithelium. PLOSONE. 2021;16(9):e0257784. doi: 10.1371/journal.pone.0257784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasmin F, Zeeshan MH, Ullah I. The role of fenofibrate in the treatment of COVID-19. Ann Med Surg. 2022;74:102974. doi: 10.1016/j.amsu.2021.102974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buschard K. Fenofibrate increases the amount of sulfatide which seems beneficial against Covid-19. MedHypotheses. 2020;143:110127. doi: 10.1016/j.mehy.2020.110127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo R, Hu X, Yamada Y, Harada M, Nakajima T, Kashihara T, et al. Effects of hypertension and antihypertensive treatments on sulfatide levels in serum and its metabolism. HypertensRes. 2019;42(5):598–609. doi: 10.1038/s41440-018-0160-z. [DOI] [PubMed] [Google Scholar]
- 40.Blomqvist M, Kaas A, Månsson JE, Formby B, Rynmark BM, Buschard K, Fredman P. Developmental expression of the type I diabetes related antigen sulfatide and sulfated lactosylceramide in mammalian pancreas. J Cell Biochem. 2003;89(2):301–310. doi: 10.1002/jcb.10513. [DOI] [PubMed] [Google Scholar]
- 41.Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008;100(4):530–547. doi: 10.1160/TH08-03-0147. [DOI] [PubMed] [Google Scholar]
- 42.Novakova L, Singh AK, Axelsson M, Ståhlman M, Adiels M, Malmeström C, et al. Sulfatide isoform pattern in cerebrospinal fluid discriminates progressive MS from relapsing-remitting MS. J Neurochem. 2018;146(3):322–332. doi: 10.1111/jnc.14452. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi T, Murakami K, Nagakura M, Kishita H, Watanabe S, Honke K, et al. Sulfatide is required for efficient replication of influenza A virus. J Virol. 2008;82(12):5940–5950. doi: 10.1128/JVI.02496-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi T, Suzuki T. Role of sulfatide in virus infection and replication. Trends Glycosci Glycotechnol. 2009;21(121):255–265. doi: 10.4052/tigg.21.255. [DOI] [Google Scholar]
- 45.Ehrlich A, Uhl S, Ioannidis K, Hofree M, tenOever BR, Nahmias Y. The SARS-CoV-2 transcriptional metabolic signature in lung epithelium. SSRN Electron J. 2020 doi: 10.2139/ssrn.3650499. [DOI] [Google Scholar]
- 46.Pawar A, PalA GK, Squitti R, Rongiolettie M. Molecular basis of quercetin as a plausible common denominator of macrophage-cholesterol-fenofibrate dependent potential COVID-19 treatment axis. Results Chem. 2021;3:100148. doi: 10.1016/j.rechem.2021.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui H, Xie N, Banerjee S, Ge J, Guo S, Liu G. Impairment of fatty acid oxidation in alveolar epithelial cells mediates acute lung injury. AmJ RespirCell MolBiol. 2019;60(2):167–178. doi: 10.1165/rcmb.2018-0152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaefer MB, Pose A, Ott J, Hecker M, Behnk A, Schulz R, et al. Peroxisome proliferator-activated receptor-α reduces inflammation and vascular leakage in a murine model of acute lung injury. EurRespirJ. 2008;32(5):1344–1353. doi: 10.1183/09031936.00035808. [DOI] [PubMed] [Google Scholar]
- 49.Delayre-Orthez C, Becker J, Guenon I, Lagente V, Auwerx J, Frossard N, Pons F. PPARα downregulates airway inflammation induced by lipopolysaccharide in the mouse. RespirRes. 2005;6(1):91. doi: 10.1186/1465-9921-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shao Y, Chen J, Dong LJ, He X, Cheng R, Zhou K, et al. A protective effect of PPARα in endothelial progenitor cells through regulating metabolism. Diabetes. 2019;68(11):2131–2142. doi: 10.2337/db18-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heffernan KS, Ranadive SM, Jae SY. Exercise as medicine for COVID-19: on PPAR with emerging pharmacotherapy. MedHypotheses. 2020;143:110197. doi: 10.1016/j.mehy.2020.110197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huertas A, Montani D, Savale L, Pichon J, Tu L, Parent F, et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? EurRespirJ. 2020;56(1):2001634. doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. NatRevImmunol. 2021;21(5):319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.BrandãoSCS RamosJOX, Dompieri LT, GodoiETAM FJL, SarinhoESC,, et al. Is toll-like receptor 4 involved in the severity of COVID-19 pathology in patients with cardiometabolic comorbidities? CytokineGrowth Factor Rev. 2021;58:102–110. doi: 10.1016/j.cytogfr.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Necela BM, Su W, Thompson EA. Toll-like receptor 4 mediates cross-talk between peroxisome proliferator-activated receptor γ and nuclear factor-κB in macrophages. Immunology. 2008;125(3):344–358. doi: 10.1111/j.1365-2567.2008.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tutunchi H, Saghafi-Asl M, Ostadrahimi A. A systematic review of the effects of oleoylethanolamide, a high-affinity endogenous ligand of PPAR-α, on the management and prevention of obesity. Clin ExpPharmacol Physiol. 2020;47(4):543–552. doi: 10.1111/1440-1681.13238. [DOI] [PubMed] [Google Scholar]
- 57.Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708. doi: 10.3389/fimmu.2020.01708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ji Y, Wang Z, Li Z, Liu J. Modulation of LPS-mediated inflammation by fenofibrate via the TRIF-dependent TLR4 signaling pathway in vascular smooth muscle cells. Cell Physiol Biochem. 2010;25(6):631–640. doi: 10.1159/000315082. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Ramírez M, Hernández C, Palomer X, Vázquez-Carrera M, Simó R. Fenofibrate prevents the disruption of the outer blood retinal barrier through downregulation of NF-κB activity. Acta diabetol. 2016;53(1):109–118. doi: 10.1007/s00592-015-0759-3. [DOI] [PubMed] [Google Scholar]
- 60.Jiang Q, Wang S, Shi Y. Posttreatment with LYRM03 protects rats from acute lung inflammation induced by lipopolysaccharide via suppressing the NF-κB/MyD88/TLR4 axis. J SurgRes. 2019;243:316–324. doi: 10.1016/j.jss.2019.05.036. [DOI] [PubMed] [Google Scholar]
- 61.Zhu Q, He G, Wang J, Wang Y, Chen W. Protective effects of fenofibrate against acute lung injury induced by intestinal ischemia/reperfusion in mice. SciRep. 2016;6(1):22044. doi: 10.1038/srep22044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Batiha GE, Al-GareebDAI QS, Alshammari EM, Rotimi D, Adeyemi OS, Al-Kuraishy HM. Common NLRP3 inflammasome inhibitors and Covid-19: divide and Conquer. SciAfr. 2021 doi: 10.1016/j.sciaf.2021.e01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Q, Zhang F, Zhang X, Cheng R, Ma JX, Yi J, Li J. Fenofibrate ameliorates diabetic retinopathy by modulating Nrf2 signaling and NLRP3 inflammasome activation. Mol Cell Biochem. 2018;445(1–2):105–115. doi: 10.1007/s11010-017-3256-x. [DOI] [PubMed] [Google Scholar]
- 64.Zhu DD, Tan XM, Lu LQ, Yu SJ, Jian RL, Liang XF, et al. Interplay between nuclear factor erythroid 2-related factor 2 and inflammatory mediators in COVID-19-related liver injury. World J Gastroenterol. 2021;27(22):2944–2962. doi: 10.3748/wjg.v27.i22.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grimes JM, Grimes KV. p38MAPK inhibition: a promising therapeutic approach for COVID-19. J Mol Cell Cardiol. 2020;144:63–65. doi: 10.1016/j.yjmcc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hou X, Shen YH, Li C, Wang F, Zhang C, Bu P, Zhang Y. PPARα agonist fenofibrate protects the kidney from hypertensive injury in spontaneously hypertensive rats via inhibition of oxidative stress and MAPK activity. Biochem BiophysResCommun. 2010;394(3):653–659. doi: 10.1016/j.bbrc.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 67.Cheng SM, Chu KM, Lai JH. The modulatory mechanisms of fenofibrate on human primary T cells. EurJ PharmSci. 2010;40(4):316–324. doi: 10.1016/j.ejps.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Zheng Y, Li R, Liu S. Immunoregulation with mTOR inhibitors to prevent COVID-19 severity: a novel intervention strategy beyond vaccines and specific antiviral medicines. J MedVirol. 2020;92(9):1495–1500. doi: 10.1002/jmv.26009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Terrazzano G, Rubino V, Palatucci AT, Giovazzino A, Carriero F, Ruggiero G. An open question: is it rational to inhibit the mTor-dependent pathway as COVID-19 therapy? Front Pharmacol. 2020;11:856. doi: 10.3389/fphar.2020.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lian X, Gu J, Gao B, Li Y, Damodaran C, Wei W, et al. Fenofibrate inhibits mTOR-p70s6K signaling and simultaneously induces cell death in human prostate cancer cells. Biochem BiophysResCommun. 2018;496(1):70–75. doi: 10.1016/j.bbrc.2017.12.168. [DOI] [PubMed] [Google Scholar]
- 71.Yalcin Kehribar D, Cihangiroglu M, Sehmen E, Avci B, Capraz A, Yildirim Bilgin A, et al. The receptor for advanced glycation end product (RAGE) pathway in COVID-19. Biomarkers. 2021;26(2):114–118. doi: 10.1080/1354750X.2020.1861099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lim A, Radujkovic A, Weigand MA, Merle U. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COVID-19 disease severity and indicator of the need for mechanical ventilation, ARDS and mortality. Ann IntensiveCare. 2021;11(1):50. doi: 10.1186/s13613-021-00836-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ju HB, Zhang FX, Wang S, Song J, Cui T, Li LF, Zhang HY. Effects of fenofibrate on inflammatory cytokines in diabetic retinopathy patients. Medicine. 2017;96(31):e7671. doi: 10.1097/MD.0000000000007671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Turkia M. COVID-19 as an endothelial disease and its relationship to vascular endothelial growth factor (VEGF) and iodide. SSRN Electron J. 2020 doi: 10.2139/ssrn.3604987. [DOI] [Google Scholar]
- 75.Jonnalagadda VG, Shaik A. Statin on insulin and adiponectin levels: true or false prophecy? Diabetes Metab Syndr Obes. 2018;11:131. doi: 10.2147/DMSO.S160853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh P, Zhang Y, Sharma P, Covassin N, Soucek F, Friedman PA, Somers VK. Statins decrease leptin expression in human white adipocytes. PhysiolRep. 2018;6(2):e13566. doi: 10.14814/phy2.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chruściel P, Sahebkar A, Rembek-Wieliczko M, Serban MC, Ursoniu S, Mikhailidis DP, et al. Impact of statin therapy on plasma adiponectin concentrations: a systematic review and meta-analysis of 43 randomized controlled trial arms. Atherosclerosis. 2016;253:194–208. doi: 10.1016/j.atherosclerosis.2016.07.897. [DOI] [PubMed] [Google Scholar]
- 78.Liu B, Wu R, Zhang W, Zhang F, Zhou H, Wang L, et al. Fosinopril improves liver fibrosis by upregulating ACE2/angiotensin-(1–7) axis activation in rats with nonalcoholic steatohepatitis. LatAmJ Pharm. 2012;31:588–596. [Google Scholar]
- 79.Cheema PS, Nandi D, Nag A. Exploring the therapeutic potential of forkhead box O for outfoxing COVID-19. Open Biol. 2021;11(6):210069. doi: 10.1098/rsob.210069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Newaz M, Blanton A, Fidelis P, Oyekan A. NAD (P) H oxidase/nitric oxide interactions in peroxisome proliferator activated receptor (PPAR) α-mediated cardiovascular effects. Mutat Res. 2005;579(1–2):163–171. doi: 10.1016/j.mrfmmm.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 81.Esenboga K, Çiçek ÖF, Oktay AA, Ayral PA, Gürlek A. Effect of fenofibrate on serum nitric oxide levels in patients with hypertriglyceridemia. Adv Clin ExpMed. 2019;28(7):931–936. doi: 10.17219/acem/94161. [DOI] [PubMed] [Google Scholar]
- 82.Gavriilaki E, Anyfanti P, Gavriilaki M, Lazaridis A, Douma S, Gkaliagkousi E. Endothelial dysfunction in COVID-19: lessons learned from coronaviruses. CurrHypertensRep. 2020;22(9):63. doi: 10.1007/s11906-020-01078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee JJ, Jin YR, Yu JY, Munkhtsetseg T, Park ES, Lim Y, et al. Antithrombotic and antiplatelet activities of fenofibrate, a lipid-lowering drug. Atherosclerosis. 2009;206(2):375–382. doi: 10.1016/j.atherosclerosis.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 84.Kilicarslan A, Yavuz B, Guven GS, Atalar E, Sahiner L, Beyazit Y, et al. Fenofibrate improves endothelial function and decreases thrombin-activatable fibrinolysis inhibitor concentration in metabolic syndrome. Blood Coagul Fibrinolysis. 2008;19(4):310–314. doi: 10.1097/MBC.0b013e3283009c69. [DOI] [PubMed] [Google Scholar]
- 85.Kashi M, Jacquin A, Dakhil B, Zaimi R, Mahé E, Tella E, Bagan P. Severe arterial thrombosis associated with Covid-19 infection. ThrombRes. 2020;192:75–77. doi: 10.1016/j.thromres.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feher M, Joy M, Munro N, Hinton W, Williams J, de Lusignan S. Fenofibrate as a COVID-19 modifying drug: laboratory success versus real-world reality. Atherosclerosis. 2021;1(339):55–56. doi: 10.1016/j.atherosclerosis.2021.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garg H, Khanna P. Covid and cholesterol (C&C): something to worry about or much ado about nothing? Trends Anaesth Crit Care. 2021;36:39–40. doi: 10.1016/j.tacc.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee HW, Kang WY, Jung W, Gwon MR, Cho K, Yang DH, Yoon YR, Seong SJ. Evaluation of the pharmacokinetic drug–drug interaction between micronized fenofibrate and pitavastatin in healthy volunteers. Pharmaceutics. 2020;12(9):869. doi: 10.3390/pharmaceutics12090869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pawar A, Pal A, Goswami K, Squitti R, Rongiolettie M. Molecular basis of quercetin as a plausible common denominator of macrophage-cholesterol-fenofibrate dependent potential COVID-19 treatment axis. Results Chem. 2021;1(3):100148. doi: 10.1016/j.rechem.2021.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.



