Abstract
Water fleas of the genus Daphnia have been a model system for hundreds of years and is among the best studied ecological model organisms to date. Daphnia are planktonic crustaceans with a cyclic parthenogenetic life-cycle. They have a nearly worldwide distribution, inhabiting standing fresh- and brackish water bodies, from small temporary pools to large lakes. Their predominantly asexual reproduction allows for the study of phenotypes excluding genetic variation, enabling us to separate genetic from non-genetic effects. Daphnia are often used in studies related to ecotoxicology, predator-induced defence, host–parasite interactions, phenotypic plasticity and, increasingly, in evolutionary genomics. The most commonly studied species are Daphnia magna and D. pulex, for which a rapidly increasing number of genetic and genomic tools are available. Here, I review current research topics, where the Daphnia model system plays a critical role.
Keywords: Daphnia magna, Daphnia pulex, Cladocera, Branchiopoda, Cyclic parthenogenesis
Natural habitat and life cycle
Daphnia is a genus of small planktonic crustaceans with a very wide geographic distribution. Its English name “water flea,” includes other members of the Cladocera order within the class Branchiopoda, united by the morphology of their trunk limbs (Fig. 1). There are over 100 described Daphnia species, each having a rather similar body architecture characterized by a relatively large head with one simple compound eye and a body encased in a bivalve-like shell (Fig. 2). Daphnia are more or less transparent so as to evade visually hunting predators, e.g., planktivorous fish. They moult four to six times before reaching maturity, but continue to moult and grow in regular intervals throughout their life. Newborn Daphnia (Fig. 2) resemble adults, except that they lack the well-developed dorsal brood pouch of adult females or the secondary sexual traits of males (Fig. 3) [1].
Fig. 1.
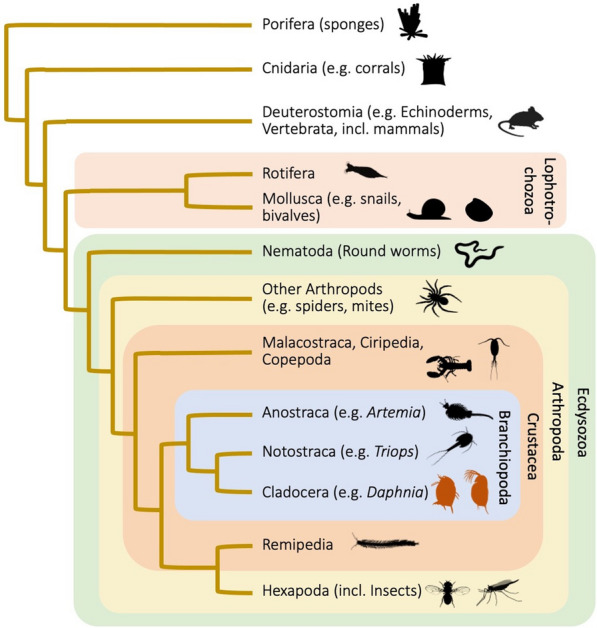
Schematic phylogenetic tree of the animals with a focus on Daphnia (in red). Daphnia are Cladocera, that form together with the Notostraca and Anostraca the Branchiopoda, formerly sometimes referred to as lower Crustaceans. Together with the Malacostraca, Copepoda, Cirripedia and some smaller taxa they form the Crustacea, now believed to be a paraphyletic taxon, because the Hexapoda are part of this clade, but not considered Crustaceans. Thus, Daphnia may be closer related to the model organism Drosophila than to a lobster. All of them are included in the Ecdysozoa, to which also the roundworms (Nematoda) belong. The tree was composed by taking various sources into account [24, 25, 127–130]. For animal pictograms see http://www.phylopic.org
Fig. 2.
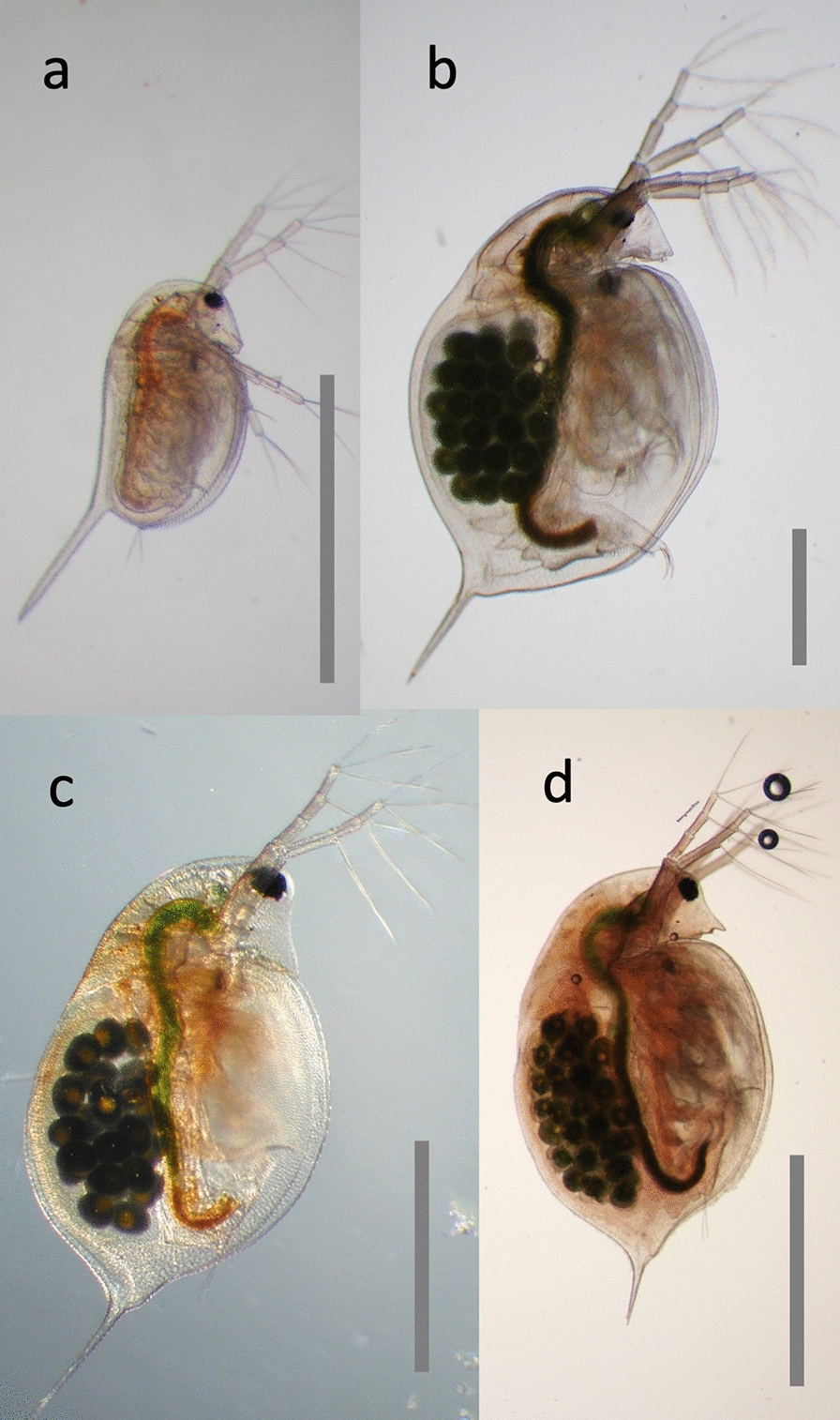
Three Daphnia species often used in biological research, representing three major clades of Daphnia within this large genus. a Newborn D. magna. b Adult D. magna. c Adult D. longispina. d Adult D. pulex. D. magna and D. pulex are predominately pond dwelling species, while D. longispina and related species are often found in large lakes. The three adult females carry parthenogenetic eggs (dark round objects) in their brood chambers. All animals are oriented with their head to the top and the ventral side to the right. The black round object in the head is the single complex eye. The large second antennae are used for swimming. All pictures by D. Ebert. Scale bar = 1 mm
Fig. 3.
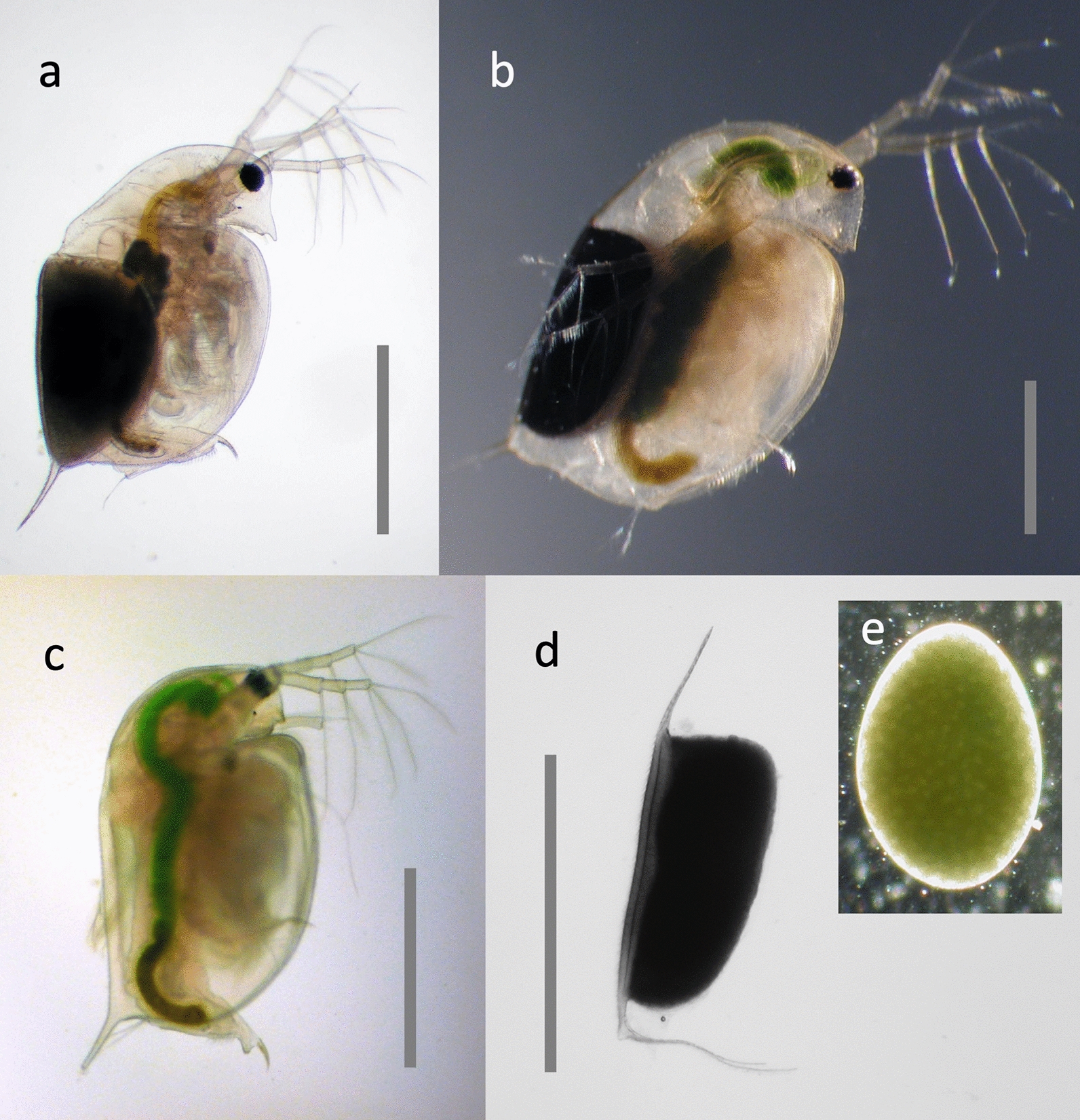
Sexual reproduction in Daphnia. a D. pulex with a sexual resting stage [= ephippium (plural: ephippia), the black structure]. b D. magna with resting stage. The dark mass in the centre part of the body (also visible in the animal in a) is the next asexual clutch being produced in the ovaries. c Male D. magna. d Freshly cast resting stage from D. magna, containing one or two embryos in developmental arrest. The resting stage has still parts of the female’s carapace attached to it (appendages in top and bottom), which is shed together with the resting stage. e Embryo from a resting stage. All pictures by D. Ebert. Scale bar = 1 mm
Daphnia are often found in standing freshwater, from very small pools to very large lakes (Fig. 4), and although they may colonize salt water lakes or estuaries, they do not typically colonize sea water, as some species of related genera do. Daphnia are often a keystone species in ponds and lakes, where they are the main primary consumer, filter-feeding small suspended particles, in particular unicellular algae. As such, they play an important role in aquatic food webs, being themselves prey for fish or diverse invertebrate predators.
Fig. 4.

Daphnia occur in diverse fresh- and brackish-water habitats. a Northernmost located D. magna population so far reported, located on Vardo Island, Norway. b Permanent pond (Aegelsee) in Switzerland. c Shallow rock pool on the Island Granbusken, near Tvärminne in Southern Finland. d Dry rock pool close to the pool in picture c. The sediment surface is covered with Daphnia resting stages. e Temporary rain pond in the Negev Desert, Israel. f Salt water pond in Southern Spain. In the background piles of salt from a salt factory. All pictures by D. Ebert
Daphnia are well known for their ability to reproduce asexually (amictic parthenogenesis, i.e., diploid eggs capable of developing without fertilization) and under favourable conditions they can propagate asexually for many years (Fig. 5). In unstable environments and when environmental conditions deteriorate, Daphnia are able to switch to sexual reproduction. In this case populations produce first males (asexually!) and then haploid eggs that need fertilization. Production of sons and haploid eggs is regulated on the population level, with some genotypes never producing males (so called non-male-producers = NMP) or haploid eggs [2, 3]. Fertilized eggs start development, but then undergo developmental arrest. Resting embryos require a dormant period before they continue development, resulting in only female hatchlings (Fig. 5). The resting stage, also called ephippium (Fig. 3d), refers to the entire resting structure, i.e., the shell and the resting embryos they contain. Because the embryo is still encased in an egg shell and has an egg shape (Fig. 3e), it is often called resting egg, but this can be misleading. Some lines of Daphnia are known that are able to produce resting stages entirely asexually [4].
Fig. 5.
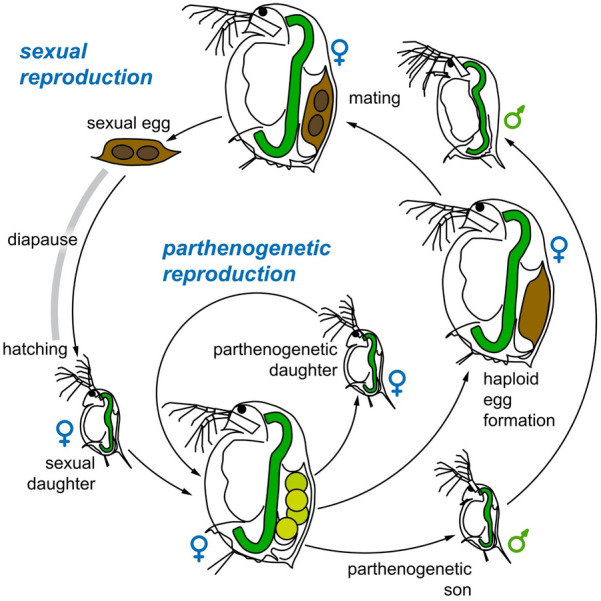
Life cycle of Daphnia. Adult females produce mostly asexual eggs, which develop directly inside their brood chamber (cycle: parthenogenetic reproduction). Mostly daughters hatch from these eggs. Occasionally, asexual eggs develop into males (sons). Some of the adult females in the population may switch to sexual reproduction and produce haploid eggs, which need fertilization by males (outer cycle: sexual reproduction). The female will eventually drop the fertilized eggs in an egg case made from her carapace (brown structure on top left; resting stage = ephippium). The ephippium will sink to the bottom of the water body, where it undergoes diapause. After diapause, one or two sexual offspring will hatch from it and develop into females. Conceptualization by D. Ebert and D. Vizoso. Drawing by D. Vizoso (available on Wikimedia)
In the textbook description of the Daphnia life cycle, sexually produced offspring hatch from resting stages in the spring. These offspring then reproduce asexually across the season and finally terminate the planktonic phase by producing resting stages that endure the harsh winter conditions. However, studies in different habitats have revealed that this is the yearly life-cycle in temperate waterbodies common to Central Europe, where this model system was first developed. Depending on the species and habitat, any ecological aspect of this life-cycle may differ (Fig. 4) [5, 6]. For example, Daphnia may continue in the planktonic form throughout the winter or may persist in short-lived desert rain pools or tiny rock pools. Populations may only be active in winter (rainy season), produce no resting stages or produce resting stages asexually. The commonality among all Daphnia is that asexually reproducing animals are found in habitats, where water temperatures are at least for part of the planktonic season between about 10–30 °C and that they are able to outlive harsh conditions, such as temporary dryness, freezing and periods of low survival probability (e.g., predation, parasitism, toxic water conditions, such as anoxia) in the form of resting stages. Resting stages can survive many years in pond sediment (and laboratory fridges), as they tolerate drying and freezing.
Field collections and laboratory culture
Daphnia can be collected from their natural habitats as either planktonic animals or in the form of their resting stages, which are often found in the sediments. Planktonic females can be cloned naturally, enabling researchers to produce unlimited numbers of clonal offspring and keep genetic lines for many generations under laboratory conditions. Clonal lines are kept in either natural fresh water, commercial mineral water or artificial medium (e.g., [7]) and are mostly fed unicellular green algae. On a commercial scale Daphnia are even farmed as fish food. Fifteen to 22 degrees is a good water temperature for asexual propagation for most species. Resting stages collected from pond and lake sediments or isolated from sliced and dated sediment cores can be hatched by exposure to oxygen-rich water under daylight and in ambient temperatures. Hatching success is, however, often low, especially for old resting stages, for reasons that we do not yet fully understand, although genetic effects (deleterious mutations) certainly play a role [8]. The oldest hatched resting stages was about 700 years old [9]. Each hatchling of sexually produced resting eggs is genetically unique and will start to reproduce clonally. Two hatchlings from one resting stage may be half or full-sibs [10].
Clonal reproduction allows us to produce genetically identical replicates so as to entirely control for genetic background. Sexual reproduction in the lab is more time consuming, because animals (usually small crowded populations) must first be induced to produce male offspring asexually, after which they produce haploid eggs for the males to fertilize [11]. The resulting eggs (actually developmentally arrested embryos) need an obligatory diapause of weeks to months, although methods have been developed to break diapause earlier [12]. Strong differences exist between species regarding the ease of sexual crosses in the laboratory, with D. magna currently being the most often-used species for routine crosses. It is possible to cross females with males from the same clone, resulting in genetically selfed offspring [11, 13]. This combination of sexual reproduction [outcrossing and genetic selfing (mother with clonal sons)] and clonal propagation allows researchers to conduct powerful genetic studies and create genetic panels that can be kept by clonal reproduction for many years [13, 14].
Major interests and research questions
Early beginnings
Daphnia research goes back several hundred years, with many important findings dating back to this old model system. To name just a few: August Weismann established his germ plasm theory working with water fleas [15], and Élie Metchnikoff [16] studied macrophages in Daphnia (Nobel prize 1908). The concept of phenotypic plasticity was also developed in relation to predator-induced defence in Daphnia [17], and the differentiation between prokaryotes and eukaryotes resulted from research on a Daphnia and its symbionts [18]. When advancements in modern biology began to be primarily driven by genetic research, Daphnia fell somewhat out of favour because of the difficulties in doing genetic crosses—a problem that has only recently been solved. Still, Daphnia has remained the prime model in ecological research and Daphnia is one of the best studied organism with regard to their ecology, with past or current focal areas are diel vertical migration [19], resurrection ecology [20], host—parasite interactions [21], community ecology [22] and climate change ecology [6, 23]. Moreover, in the last 20 years as genomics and genetics become incorporated into the tool box of Daphnia research (primarily D. magna and D. pulex), this model system is rising in prominence in the field of environmental genomics.
Phylogenetic position
Daphnia are part of the Branchiopoda, the same group in which the tadpole shrimp Triops (Notostraca) and the brine shrimp Artemia (Anostraca) are placed (Fig. 1). Since the Crustacea are believed to a paraphyletic group (insects are phylogenetically part of the crustacea but typically not considered crustacea), the Branchiopoda are closer to insects than to other typical crustaceans, such as copepods, Malacostraca, Cirripedia and ostracods. Thus, Daphnia may be closer related to Drosophila than to lobster. Recent phylogenomic work has placed the genus Daphnia firmly in this phylogenetic tree and has clarified the evolution of the Cladocera [24] and the family of Daphniidae [25]. Crustacea are part of the Ecdysozoa, characterized by the need to moult their exoskeleton while growing (Fig. 1).
Genome
The Daphnia pulex genome was the first crustacean genome to be sequenced [26, 27], followed by several other species [25, 28, 29]. Using flow cytometry, the genome size of several Daphnia species has been estimated to be about 230 MB, but genome assemblies are currently much smaller, suggesting that about 25% of the genome—likely the centromeric regions—is yet to be discovered. Several genetic maps have been published, allowing contigs to be sorted and oriented into chromosomes [30–32]. Daphnia have 10–12 chromosomes, but polyploid species exist as well.
A major surprise from the first genome was the large number of genes, with early counts around 30,000 [26]. However, a later re-assessment reduced this number to about 18,500 [28]. As both these studies used house-made unpublished bioinformatics scripts, it is difficult to understand the source of this difference and determine the better estimate, although unpublished data from D. magna (P.D. Fields & D. Ebert, unpublished) suggest that the higher estimate is closer to the real number.
Local adaptation
With their habitat limited to standing water bodies, Daphnia populations are strongly subdivided. Gene flow is usually moderate, allowing populations to evolve rapidly in line with local environmental conditions. Animals collected from numerous waterbodies have had their genotypes preserved by clonal culture and then tested in common garden experiments (i.e., all animals being raised and phenotyped under the same environmental conditions) or reciprocal transplant experiments (i.e., transplantation of animals among environments, to work out the conditions to which animals are best adapted to). When genotypes demonstrate superior performance under the environmental conditions the animals have evolved in, it is considered evidence of local adaptation. Daphnia species have been shown to adapt locally to a long and growing list of environmental factors, including heavy metal pollution, predators, high temperature, habitat stability, photoperiod, water salinity, UV light [5, 33–39].
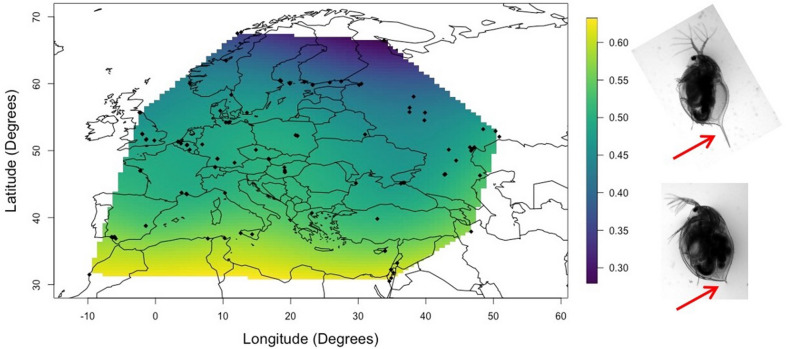
Although most local adaptation studies are based on comparing a few populations with multiple genotypes per population, a novel approach has been to collect single clones from as many populations as possible, instead of collecting multiple genotypes from each of a few populations. This method allows us to map phenotypes on geographic maps to investigate the influence of geography and climatic conditions (see Fig. 6 for an example how the length of the spina maps across Europe). It assumes, however, that a single genotype is representative of the entire population. The Daphnia magna Diversity Panel is a standing collection of hundreds of genotypes, each from a different population used for such studies. Indeed, the strong signal of local adaptation observed across these single genotypes suggests that single genotypes are—at least to some degree—representative of entire populations [6, 33, 39].
Fig. 6.
Example for geographic variation in a phenotypic trait: spina length at maturity across populations of D. magna (indicated as black dots). Phenotypes were generated in a common garden experiment and superimposed on a map according to the site of origin of the Daphnia clone. Scale on the right is in mm. On the right are examples of D. magna with short and long spina (red arrows). All genotypes are part of the Daphnia magna diversity panel a standing collection of hundreds of genotypes each from a different population
Temporal adaptation and resurrection ecology
The long-lasting resting stages of Daphnia provide an even more compelling demonstration of the high evolutionary potential of these populations. While the majority of resting stages usually hatch during favourable conditions for the planktonic phase, some eggs may remain unhatched, though viable. With time, sediment buries these eggs, archiving them in layered sediments of the past. The field of resurrection ecology avails itself of this archive by collecting sediment cores, slicing them into layers and hatching the embryos from resting stages from the different layers [40, 41]. Information about the age of these layers may be obtained using radio isotope analysis. The resurrected Daphnia can then be tested in common garden experiments in response to different environmental variables, preferably those for which a record of past dynamics in the waterbody is present. These studies have revealed the rapid adaptive evolution of different Daphnia species to diverse environmental factors, such as density of predatory fish [42], heavy metal pollution [40], increased density of toxic cyanobacteria [43], and temporal dynamics in eutrophication [20]. One study resurrected, along with D. magna, the bacterial parasite Pasteuria ramosa, testing and confirming the idea that, over time, hosts and parasites coevolve [44]. Once they are combined with genomic methods, resurrection studies will be able to help us understand genetic targets of selection [45], e.g., by pool-sequencing of embryos from resting stages from different layers and studying the change of alleles (SNP-variants) over time.
Host–parasite interaction
In their natural habitat, Daphnia are often infected at high prevalence with various parasites including viruses, bacteria, microsporidia, fungi, nematodes, cestodes and others (Fig. 7) [46, 47]. Many of these parasites can be co-cultured with their hosts, opening the door for experiments that examine host–parasite interactions on the individual and population levels. Compared to many other host–parasite systems, the Daphnia–microparasite system offers a level of experimental control that is unsurpassed, enabling us to test basic models of parasite ecology and evolution, such as the mass action principle [48], evolution of virulence [49], host and parasite evolution and coevolution [50, 51], the effects of ageing in host–parasite interactions [52, 53], infections with multiple parasites [54, 55] and parasitism in the face of predation [56] and other stressors [57]. An advantage of the Daphnia system that comes handy with regard to parasitism is the transparency of the host. Many infections can be diagnosed from outside, without killing the host (Fig. 7). Metchnikov’s [16] observation of phagocytosis was possible in vivo, by studying the fate of individual parasite cells in the living host.
Fig. 7.
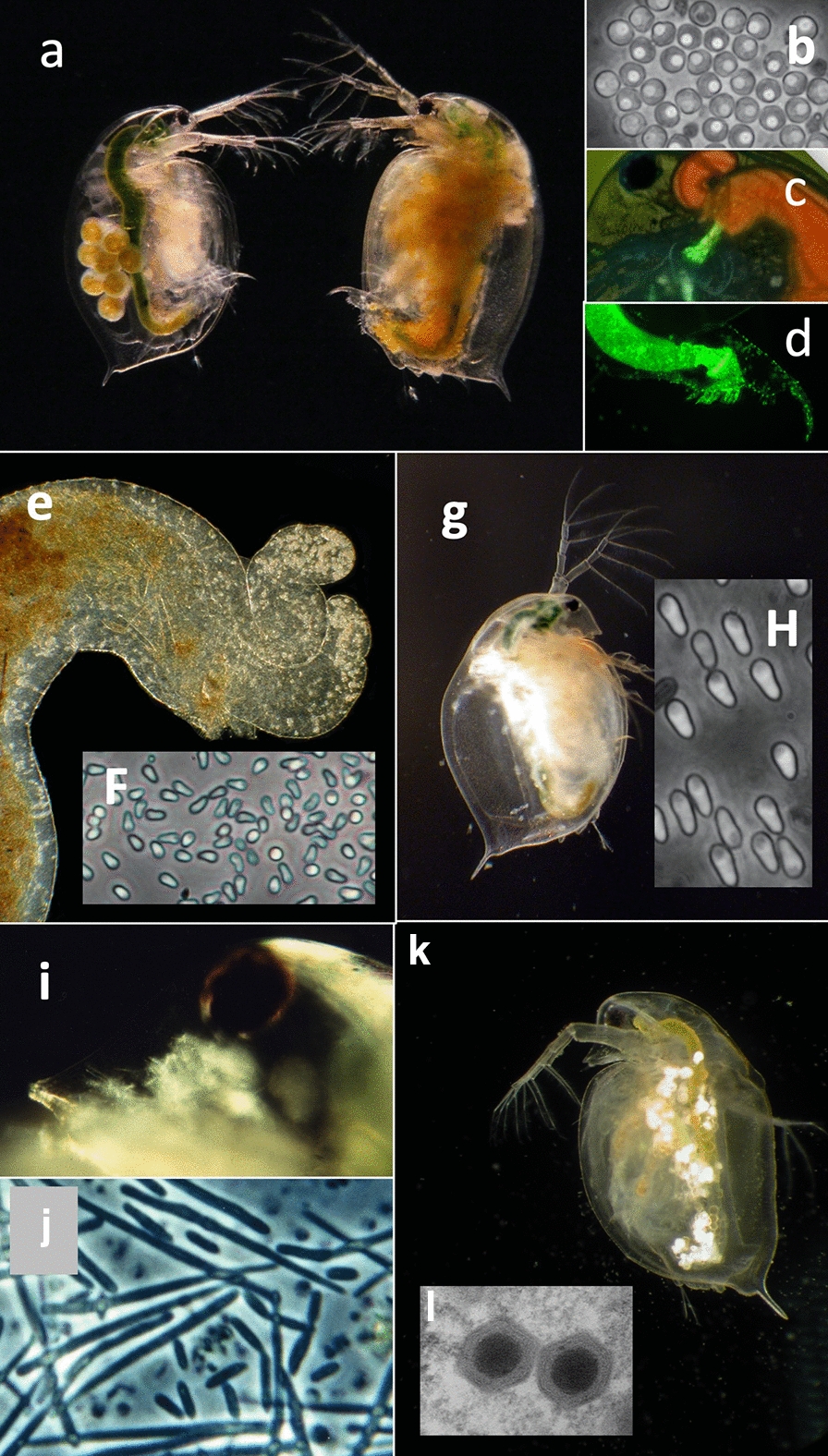
Examples of frequently studied parasites of Daphnia. a–d Bacterium Pasteuria ramosa colonizes the body cavity of the host. a Infected (right) and uninfected (left) D. magna. b Transmission stages (= spores) of P. ramosa. c, d Attachment of green fluorescent labelled P. ramosa spores to the oesophagus (c) and hindgut (d) of the host. Attachment of spores is required for the subsequent infection of the host [72, 131]. e Upper midgut of D. magna with spore clusters of the microsporidium Ordospora colligata in the appendices (upper right corner). The parasite colonized the gut epithelium of the host. f Spores of O. colligata. g D. magna infected with the microsporidian Hamiltosporidium tvaerminnensis. The parasite colonized the ovaries and fat body of the host. h Spores of H. tvaerminnensis. i Head of D. magna infected with Metschnikowia bicuspidata. The needles-like spores are visible through the transparent cuticle. j Spores of M. bicuspidata. k Daphnia pulex infected with the Daphnia Iridovirus (DIV-1), the causative agent of White Fat Cell Disease [68]. l Two DIV-1 particles. Picture taken by Jason Andras (a), David Duneau (c), Benjamin Hüssy (d), Patrick Mucklow (g) and Elena Toenshoff (l). All other pictures by Dieter Ebert
The ability to use entire populations, furthermore, enables us to tackle questions that require replication on the population level, such as studies in experimental evolution [58] and epidemiology [59, 60]. Finally, the ease with which it is possible to study parasitism in natural populations allows us to compare data from natural population with data from laboratory experiments [51, 61] to understand the life cycles, epidemiology and evolution of several natural parasites of Daphnia [61].
While many parasites (and epibionts) of Daphnia have been used in field and lab-work, only a few have been studied intensively: the bacteria Pasteuria ramosa [62, 63], the microsporidia Hamiltosporidium tvaerminnensis [64] and Ordospora colligata [65, 66], the yeast Metschnikowia bicuspidata [21], the chitric fungus Caullerya mesnilli [67] and the Daphnia iridovirus DIV-1 [68]. Current research into the Daphnia parasites is focusing on coevolution and genetic epidemiology [51, 62], with new possibilities opening up as the genomes for most of these parasites becomes available. For example, a GWAS using different genotypes of P. ramosa, recently resulted in the first functional annotation of a parasite gene with regard to Daphnia–parasite interaction: a collagen-like protein was found to be responsible for the attachment of the bacteria to the host cuticle [69].
Cloning of Daphnia genotypes allows separating the effects of nature (genetic effects) and nurture (environmental effects) to a high degree of sophistication. This has been used to map genes involved in phenotypic traits, including resistance to parasites. Strong variation in host resistance has been reported [50, 50] for several parasites of D. magna. Studies that combine QTL-F2 panel mapping and GWAS have identified a number of regions and candidate genes in the D. magna genome that contribute to this variation, with strong variation in the underlying genetic architecture ranging, from single gene effects (major QTLs) to complex multigene effects (several minor QTLs), as they are typical for quantitative traits [71–73].
Phenotypic plasticity
A hallmark of Daphnia biology is its phenotypic plasticity, defined as phenotypic variation expressed by the same genotype in response to environmental cues [74, 75]. Here again, the ability to clone genotypes of Daphnia has been instrumental in the development and research of this field. The most readily observable phenotypic plastic trait is the switch between asexual and sexual reproduction (Figs. 3, 5). This switch is triggered by deteriorating environmental conditions that lower the survival likelihood of asexual offspring and, therefore, make the production of sexually produced resting stages less costly [76]. A photoreceptor gene has been mapped in the genome of D. magna playing a crucial role for the switch from asexual to sexual reproduction [77].
With a well-developed set of gustatory receptors [78], Daphnia are able to sense chemical aspects of their environment, so called “infochemicals” or kairomones [79]. They indicate, for example, the presence of planktivorous fish, invertebrate predators or toxic blue–green algae, allowing the animals to respond in specific ways to reduce the threat (Fig. 8). They may change their body shape (e.g., develop helmets, neck–teeth, elongated tail spines), alter their life-history (e.g., size and age at maturity, offspring size) and change behaviour (phototaxis, swimming parameters) [80–82]. Adaptive phenotypic plasticity also drives responses to environmental conditions. For example, Daphnia produce fewer and larger offspring when food is scarce [83]; and animals produce specific haemoglobin variants tailored to the partial oxygen pressure of the water [84].
Fig. 8.
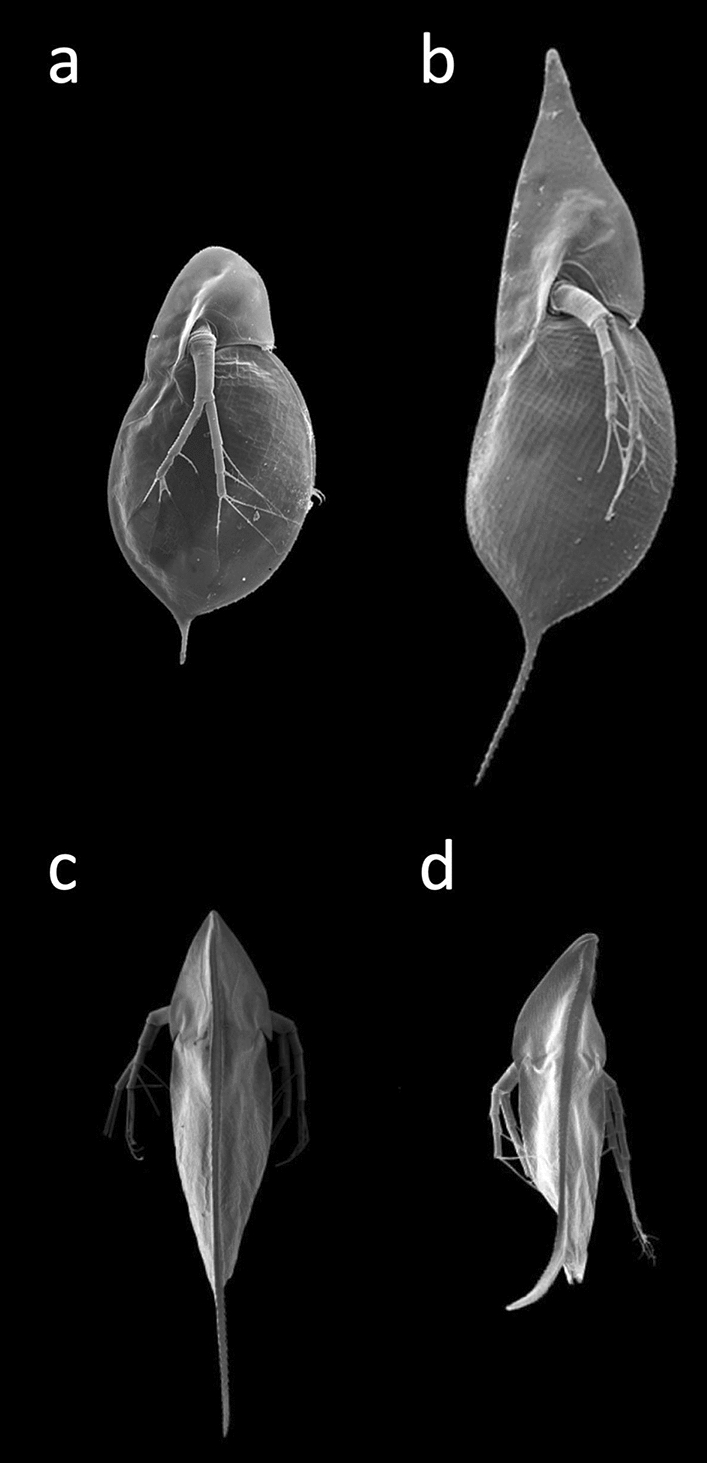
Waterfleas are able to react to cues from the environment, including water turbulences and infochemicals (kairomones) released by different predators, with the formation of highly specific structures, such as protective tail spines, helmets, and neck teeth. a D. cucullata in its normal (uninduced) phenotype (left) and b after induction (right). Helmets can be induced by water turbulence and by kairomones from fish. [81, 132]. c D. barbata: left control, d right induced by kairomones released by the predatory tadpole shrimp Triops cancriformis. The “twist”, a body torsion, induced by the kairomones reduces the likelihood of predation by Triops [75]. Pictures by Christian Laforsch, University of Bayreuth, Germany
Ecotoxicology
Without doubt, the field that spurs the most Daphnia publications is ecotoxicology, which examines the potentially toxic effects of chemical compounds on survival and reproduction. Many countries require that chemical compounds be tested with various organisms before they are produced, released or sold. Daphnia is one of these organisms, mostly D. magna, but also D. pulex, or, more rarely, other species. Guidelines by the Organisation for Economic Cooperation and Development (OECD) require tests, such as the Acute immobilisation test (a short-term or acute toxicity test) and the Daphnia magna Reproduction Test [85, 86]. These tests allow us to produce dose–response curves that can be used to determine parameters, such as the LC50 (lethal concentration at which 50 % animals die) and the lowest observed effect concentration (LOEC), parameters that help determine whether a chemical is potentially harmful for the environment and define any follow-up tests needed to understand the risk.
Moreover, the swimming behaviour of water fleas is increasingly used to quantify the effect of toxins, as well as to monitor the Daphnia response to changes in freshwater supplies for human consumption. Swimming speed, turn frequencies, acceleration and other parameters have all been observed to change as water quality changes. Using video tracking systems, these swimming-related parameters can be estimated in real time, and in the case of flow-through water surveillance systems, ensure that appropriate actions are taken. These systems are much faster than even the acute immobilization test and have become increasingly popular for research and applied aspects [87].
A rarely considered problem with using Daphnia in ecotoxicology is the genetic variation among Daphnia genotypes (clones). Different genotypes of the same species may differ strongly in the estimates of toxicity parameters [88]. Given the commercial interest in the production and sale of chemicals (incl. pharmaceuticals), this variation opens the door for biased reporting. So far, attempts to standardize the genotypes used across the world have not yielded a consensus.
Evolutionary developmental biology
The discovery of the germ plasm by August Weismann in water fleas [15] was a major breakthrough at the time, but in the following hundred years Daphnia was not among the main players in the field of developmental biology. Today, D. pulex and D. magna are frequently used in the evo-devo field, often with a focus on comparative aspects [89], for example concerning the role of Hox genes [90–93], neuro development [94–96] and sex determination [97–100]. Along these lines diverse tool have been developed, for example an atlas for the staging of embryos [101] and diverse tools for genetic manipulation (see next section).
Experimental approaches
RNAi, CRISPR, TALEN
An expanding area of Daphnia research addresses the genetic mechanism underlying phenotypes, with the long-term goal of uncovering gene function in an environmental context. Manipulation of gene expression, as well as gene knock-in and knock-out technology are now possible for Daphnia, with microinjection-based RNA interference (RNAi) used routinely [102–104]. Clustered regularly interspaced short palindromic repeats/CRISPR-associated (CRISPR/Cas) system have been used for gene knock-outs [105, 106], but also for CRISPR/Cas-mediated knock-in via non-homologous end-joining, e.g., for reporter-genes [107]. Likewise, Transcription Activator-Like Effector Nucleases (TALENs) are increasingly used as a versatile genomic manipulation tool in D. magna [108–110]. These methods are primarily carried out via microinjection into the freshly laid asexual eggs of Daphnia, although other avenues such as bacterial feeding are being developed [111]. Mastering microinjection into the asexual eggs of Daphnia is still the main bottleneck for these methods, because the initially very fragile egg membrane breaks easily, but then hardens fast, so that injection is not possible anymore. The time window with the right conditions is short, even so a method has been develop to stretch it (collect eggs immediately after ovulation in ice-cold medium enriched with 80 mM sucrose) [102]. Another limiting factor is the relatively small clutch size of Daphnia, which in the lab is rarely larger than 30, even so in the field D. magna can produce clutches of more than 100 eggs. Thus, often eggs from multiple clutches need to be used. On the other hand, the combination of sexual and asexual reproduction is of great advantage when applying knock-in and knock-out methods. Manipulated genomes can be maintained in stable, clonal culture even in heterozygote state.
Current experimental approaches mainly focus on genes known from other organisms for diverse biological roles, such as development, sex expression (e.g., producing white eyes, knock-in of Green Fluorescent Protein) [97, 106, 107, 112–114]. So far, genes that have a function in the direct interaction of the Daphnia with their environment, for example genes for local adaptation, phenotypic plasticity, predator defence, parasite resistance, perception of environmental factors, such as water quality, photoperiod, and toxins, have hardly been manipulated [115], partly due to the low number of good candidates for genes with such function.
QTL panels and GWAS
The first attempts to map phenotypes to genes in Daphnia was based on an F2 D. magna QTL panel. The position of two deleterious, but naturally segregating mutations were mapped [8]. The same standing QTL panel was later used to map the position of resistance genes to different parasites [71, 116] and genes related to sex induction and breaking of diapause [5, 117]. The mapping of phenotypes to genotypes has become much easier by the use of next generation sequencing for large number of genotypes, conducting genome wide association studies (GWAS). Examples include the mapping of a gene for sex induction, a non-male-producer gene and a parasite resistance super-gene [51, 77, 100].
Transcriptomics
With phenotypic plasticity being a hallmark of Daphnia biology, it is an ideal system to study differential gene expression, as different phenotypes of the same genotype can be contrasted. This has been used intensively for diverse questions related to gene expression differences in different environments or treatments, such as responses to different predators, chemicals, environmental toxins, temperature, salinity, heavy metals, resistance to parasites, sex expression [118–121]. A database, dedicated to gene expression studies in Daphnia (http://www.daphnia-stressordb.uni-hamburg.de/dsdbstart.php) summaries the result of nearly 100 studies published until 2018 [118]. More than 50 % are from D. magna. Only seven studies of the entire data set analyzed whole-transcriptome expression profiles (using RNA-seq). Besides RNA-seq, proteomics is also rapidly developing for Daphnia as a powerful research tool [122].
Karyotyping
Daphnia have rather small and condensed chromosomes, which posed severe difficulties on accurate karyotyping [123, 124]. However, new protocols allowed to move forward [125]. The number of chromosomes for D. magna is worked out to be 10 (N = 1) and is 12 for D. pulex
Research community and resources.
The Daphnia community is rather large with a long tradition. A few hundred papers are published every year that feature Daphnia as the main study organism. The Cladocera community normally holds triannual symposia, but this schedule was interrupted due to the Coronavirus pandemic. A younger meeting series are the “Daphnia Genomics Consortium” meetings, which had convened only three times previously.
Genomic resources
The first genome of D. pulex was already sequenced more than 10 years ago [26] and several genomes of other Daphnia species have since then be sequenced [25, 29] (https://www.ncbi.nlm.nih.gov/genome/?term=Daphnia). Genetic maps exist for D. pulex and D. magna [8, 30, 32, 126]. wFleaBase, the Daphnia Water Flea Genome Database (http://wfleabase.org/) gives an overview, but is not always up-to-date.
Animal resources
Since clonal lines of Daphnia can very easily be produced by collecting females from natural populations and keeping them clonally in the laboratory, a tradition of widely used standard laboratory lines has not come about in the Daphnia community. Even in the field of ecotoxicology, several different genotypes are used by labs around the world. Some of the bigger Daphnia research groups have collection of clones from different species and population. One of the biggest collections is currently in the Ebert-research group at the University of Basel, with The Daphnia magna Diversity panel (fully sequenced clonal lines from more than 230 populations). The same lab also houses more than 300 F2 clones of a D. magna QTL panel [30] and more than 100 sequenced D. magna clones from one single population (the Swisspond panel) [51].
Acknowledgements
I thank Christian Laforsch for the pictures for Fig. 8. I thank the entire Ebert-lab for helpful comments and suggestions for this manuscript. Suzanne Zweizig and Maridel Fredericksen improved the language of the manuscript.
Author contributions
The author read and approved the final manuscript.
Funding
This work is supported by The Swiss National Science Foundation.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares that he has no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ebert D. Ecology, epidemiology and evolution of parasitism in Daphnia. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information: National Center for Biotechnology Information (US) http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Books; 2005.
- 2.Yampolsky LY. Genetic-variation in the sexual reproduction rate within a population of a cyclic parthenogen, Daphnia-magna. Evolution. 1992;46:833–837. doi: 10.2307/2409651. [DOI] [PubMed] [Google Scholar]
- 3.Galimov Y, Walser B, Haag CR. Frequency and inheritance of non-male producing clones in Daphnia magna: evolution towards sex specialization in a cyclical parthenogen? J Evol Biol. 2011;24:1572–1583. doi: 10.1111/j.1420-9101.2011.02288.x. [DOI] [PubMed] [Google Scholar]
- 4.Tucker AE, Ackerman MS, Eads BD, Xu S, Lynch M. Population-genomic insights into the evolutionary origin and fate of obligately asexual Daphnia pulex. Proc Natl Acad Sci USA. 2013;110:15740–15745. doi: 10.1073/pnas.1313388110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roulin AC, Routtu J, Hall MD, Janicke T, Colson I, Haag CR, Ebert D. Local adaptation of sex induction in a facultative sexual crustacean: insights from QTL mapping and natural populations of Daphnia magna. Mol Ecol. 2013;22:3567–3579. doi: 10.1111/Mec.12308. [DOI] [PubMed] [Google Scholar]
- 6.Seefeldt L, Ebert D. Temperature-versus precipitation-limitation shape local temperature tolerance in a Holarctic freshwater crustacean. Proc Biol Sci. 2019 doi: 10.1098/rspb.2019.0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klüttgen B, Dülmer U, Engels M, Ratte HT. ADaM, an artificial freshwater for the culture of zooplankton. Water Res. 1994;28:743–746. doi: 10.1016/0043-1354(94)90157-0. [DOI] [Google Scholar]
- 8.Routtu J, Jansen B, Colson I, De Meester L, Ebert D. The first-generation Daphnia magna linkage map. BMC Genomics. 2010 doi: 10.1186/1471-2164-11-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frisch D, Morton PK, Chowdhury PR, Culver BW, Colbourne JK, Weider LJ, Jeyasingh PD. A millennial-scale chronicle of evolutionary responses to cultural eutrophication in Daphnia. Ecol Lett. 2014;17:360–368. doi: 10.1111/ele.12237. [DOI] [PubMed] [Google Scholar]
- 10.Duneau D, Altermatt F, Ferdy JB, Ben-Ami F, Ebert D. Estimation of the propensity for sexual selection in a cyclical parthenogen. bioRxiv. 2020 doi: 10.1101/2020.02.05.935148. [DOI] [Google Scholar]
- 11.De Meester L. An estimation of the heritability of phototaxis in Daphnia magna Straus. Oecologia. 1989;78:142–144. doi: 10.1007/BF00377210. [DOI] [PubMed] [Google Scholar]
- 12.Retnaningdyah C, Ebert D. Bleach solution requirement for hatching of Daphnia magna resting eggs. J Trop Life Sci. 2016;6:136–141. doi: 10.11594/jtls.06.03.01. [DOI] [Google Scholar]
- 13.Luijckx P, Fienberg H, Duneau D, Ebert D. A matching-allele model explains host resistance to parasites. Curr Biol. 2013;23:1085–1088. doi: 10.1016/J.Cub.2013.04.064. [DOI] [PubMed] [Google Scholar]
- 14.Metzger CMJA, Luijckx P, Bento G, Mariadassou M, Ebert D. The Red Queen lives: epistasis between linked resistance loci. Evolution. 2016;70:480–487. doi: 10.1111/evo.12854. [DOI] [PubMed] [Google Scholar]
- 15.Weismann A. Die Continuität des Keimplasmas als Grundlage einer Theorie der Vererbung. Jena: Gustav Fischer; 1885. [Google Scholar]
- 16.Metchnikoff ME. Über eine Sprosspilzkrankheit der Daphniden. Beitrag zur Lehre der Phagocyten gegen Krankheitserreger. Virchows Arch Path Anat Physiol. 1884;9:177–193. doi: 10.1007/BF02361555. [DOI] [Google Scholar]
- 17.Woltereck R. Weitere experimentelle Untersuchungen über Artveränderung speziell über das Wesen quantitativer Artunterschiede bei Daphniden. Verh deutsch zool Ges. 1909;19:110–172. [Google Scholar]
- 18.Chatton É. Pansporella perplexa amœbien a spores protégées parasite des daphnies. Ann des Sci Nat Zool. 1925;8:5–85. [Google Scholar]
- 19.Ringelberg J. Diel vertical migration of zoplankton in lakes and oceans. Dordrecht: Springer; 2010. [Google Scholar]
- 20.Brede N, Sandrock C, Straile D, Spaak P, Jankowski T, Streit B, Schwenk K. The impact of human-made ecological changes on the genetic architecture of Daphnia species. Proc Natl Acad Sci USA. 2009;106:4758–4763. doi: 10.1073/pnas.0807187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffy MA, Ochs JH, Penczykowski RM, Civitello DJ, Klausmeier CA, Hall SR. Ecological context influences epidemic size and parasite-driven evolution. Science. 2012;335:1636–1638. doi: 10.1126/Science.1215429. [DOI] [PubMed] [Google Scholar]
- 22.Monchamp ME, Enache I, Turko P, Pomati F, Risnoveanu G, Spaak P. Sedimentary and egg-bank DNA from 3 European lakes reveal concurrent changes in the composition and diversity of cyanobacterial and Daphnia communities. Hydrobiol. 2017;800:155–172. doi: 10.1007/s10750-017-3247-7. [DOI] [Google Scholar]
- 23.Brans KI, Jansen M, Vanoverbeke J, Tuzun N, Stoks R, De Meester L. The heat is on: genetic adaptation to urbanization mediated by thermal tolerance and body size. Glob Change Biol. 2017;23:5218–5227. doi: 10.1111/gcb.13784. [DOI] [PubMed] [Google Scholar]
- 24.Van Damme K, Cornetti L, Fields PD, Ebert D. Whole-genome phylogenetic reconstruction as a powerful tool to reveal homoplasy and ancient rapid radiation in waterflea evolution. Syst Biol. 2021 doi: 10.1093/sysbio/syab094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornetti L, Fields PD, Van Damme K, Ebert D. A fossil-calibrated phylogenomic analysis of Daphnia and the Daphniidae. Mol Phylogen Evo. 2019;137:250–262. doi: 10.1016/j.ympev.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Colbourne JK, Pfrender ME, Gilbert D, Thomas WK, Tucker A, Oakley TH, Tokishita S, Aerts A, Arnold GJ, Basu MK, et al. The ecoresponsive genome of Daphnia pulex. Science. 2011;331:555–561. doi: 10.1126/Science.1197761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebert D. A genome for the environment. Science. 2011;331:539–540. doi: 10.1126/science.1202092. [DOI] [PubMed] [Google Scholar]
- 28.Ye ZQ, Xu S, Spitze K, Asselman J, Jiang XQ, Ackerman MS, Lopez J, Harker B, Raborn RT, Thomas WK, et al. A new reference genome assembly for the microcrustacean Daphnia pulex. Genes Genomes Genet. 2017;7:1405–1416. doi: 10.1534/g3.116.038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee BY, Choi BS, Kim MS, Park JC, Jeong CB, Han J, Lee JS. The genome of the freshwater water flea Daphnia magna: a potential use for freshwater molecular ecotoxicology. Aquat Toxicol. 2019;210:69–84. doi: 10.1016/j.aquatox.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Routtu J, Hall MD, Albere B, Beisel C, Bergeron RD, Chaturvedi A, Choi JH, Colbourne J, De Meester L, Stephens MT, et al. An SNP-based second-generation genetic map of Daphnia magna and its application to QTL analysis of phenotypic traits. BMC Genomics. 2014;15:1033. doi: 10.1186/1471-2164-15-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu S, Ackerman MS, Long HA, Bright L, Spitze K, Ramsdell JS, Thomas WK, Lynch M. A male-specific genetic map of the microcrustacean Daphnia pulex based on single-sperm whole-genome sequencing. Genetics. 2015;201:31–38. doi: 10.1534/genetics.115.179028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dukic M, Berner D, Roesti M, Haag CR, Ebert D. A high-density genetic map reveals variation in recombination rate across the genome of Daphnia magna. Bmc Genet. 2016 doi: 10.1186/S12863-016-0445-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yampolsky LY, Schaer TMM, Ebert D. Adaptive phenotypic plasticity and local adaptation for temperature tolerance in freshwater zooplankton. Proc Biol Sci. 2014;281:20132744. doi: 10.1098/rspb.2013.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weider LJ, Hebert PDN. Ecological and physiological differentiation among low-artic clones of Daphnia pulex. Ecology. 1987;68:188–198. doi: 10.2307/1938819. [DOI] [Google Scholar]
- 35.Agra AR, Soares AMVM, Barata C. Life-history consequences of adaptation to pollution: Daphnia longispina clones historically exposed to copper. Ecotoxicology. 2011;20:552–562. doi: 10.1007/s10646-011-0621-5. [DOI] [PubMed] [Google Scholar]
- 36.Fisk DL, Latta LC, Knapp RA, Pfrender ME. Rapid evolution in response to introduced predators I: rates and patterns of morphological and life-history trait divergence. BMC Evol Biol. 2007 doi: 10.1186/1471-2148-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teschner M. Effects of salinity on the life histroy and fitness of Daphnia magna: variability within and between populations. Hydrobiol. 1995;307:33–41. doi: 10.1007/BF00031995. [DOI] [Google Scholar]
- 38.Miner BE, Kerr B. Adaptation to local ultraviolet radiation conditions among neighbouring Daphnia populations. Proc Biol Sci. 2022;278:1306–1313. doi: 10.1098/rspb.2010.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santos JL, Ebert D. Trehalose provisioning in Daphnia resting stages reflects local adaptation to the harshness of diapause conditions. Biol Lett. 2022;18:20210615. doi: 10.1098/rsbl.2021.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerfoot WC, Robbins JA, Weider LJ. A new approach to historical reconstruction: Combining descriptive and experimental paleolimnology. Limnol Oceanogr. 1999;44:1232–1247. doi: 10.4319/lo.1999.44.5.1232. [DOI] [Google Scholar]
- 41.Orsini L, Schwenk K, De Meester L, Colbourne JK, Pfrender ME, Weider LJ. The evolutionary time machine: using dormant propagules to forecast how populations can adapt to changing environments. Trends Ecol Evol. 2013;28:274–282. doi: 10.1016/J.Tree.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cousyn C, De Meester L, Colbourne JK, Brendonck L, Verschuren D, Volckaert F. Rapid, local adaptation of zooplankton behavior to changes in predation pressure in the absence of neutral genetic changes. Proc Natl Acad Sci USA. 2001;98:6256–6260. doi: 10.1073/pnas.111606798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hairston NGJ, Lampert W, Cáceres CE, Holtmeier CL, Weider LJ, Gaedke U, Fischer J, Fox JA, Post DM. Rapid evolution revealed by dormant eggs. Nature. 1999;401:446. doi: 10.1038/46731. [DOI] [Google Scholar]
- 44.Decaestecker E, Gaba S, Raeymaekers JAM, Stoks R, Van Kerckhoven L, Ebert D, De Meester L. Host-parasite ‘Red Queen’ dynamics archived in pond sediment. Nature. 2007;450:870–873. doi: 10.1038/nature06291. [DOI] [PubMed] [Google Scholar]
- 45.Lack JB, Weider LJ, Jeyasingh PD. Whole genome amplification and sequencing of a Daphnia resting egg. Mol Ecol Resour. 2018;18:118–127. doi: 10.1111/1755-0998.12720. [DOI] [PubMed] [Google Scholar]
- 46.Green J. Parasites and epibionts of Cladocera. Trans Zool Soc Lond. 1974;32:417–515. doi: 10.1111/j.1096-3642.1974.tb00031.x. [DOI] [Google Scholar]
- 47.Stirnadel HA, Ebert D. Prevalence, host specificity and impact on host fecundity of microparasites and epibionts in three sympatric Daphnia species. J Anim Ecol. 1997;66:212–222. doi: 10.2307/6023. [DOI] [Google Scholar]
- 48.Regoes RR, Hottinger JW, Sygnarski L, Ebert D. The infection rate of Daphnia magna by Pasteuria ramosa conforms with the mass-action principle. Epidemiol Infect. 2003;131:957–966. doi: 10.1017/S0950268803008793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ben-Ami F, Routtu J. The expression and evolution of virulence in multiple infections: the role of specificity, relative virulence and relative dose. BMC Evol Biol. 2013;13:97. doi: 10.1186/1471-2148-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carius HJ, Little TJ, Ebert D. Genetic variation in a host-parasite association: potential for coevolution and frequency-dependent selection. Evolution. 2001;55:1136–1145. doi: 10.1111/j.0014-3820.2001.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 51.Ameline C, Bourgeois Y, Vogtli F, Savola E, Andras J, Engelstadter J, Ebert D. A two-locus system with strong epistasis underlies rapid parasite-mediated evolution of host resistance. Mol Biol Evol. 2021;38:1512–1528. doi: 10.1093/molbev/msaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Izhar R, Ben-Ami F. Host age modulates parasite infectivity, virulence and reproduction. J Anim Ecol. 2015;84:1018–1028. doi: 10.1111/1365-2656.12352. [DOI] [PubMed] [Google Scholar]
- 53.Izhar R, Routtu J, Ben-Ami F. Host age modulates within-host parasite competition. Biol Lett. 2015;11:20150131. doi: 10.1098/rsbl.2015.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ben-Ami F, Rigaud T, Ebert D. The expression of virulence during double infections by different parasites with conflicting host exploitation and transmission strategies. J Evol Biol. 2011;24:1307–1316. doi: 10.1111/j.1420-9101.2011.02264.x. [DOI] [PubMed] [Google Scholar]
- 55.Manzi F, Halle S, Seemann L, Ben-Ami F, Wolinska J. Sequential infection of Daphnia magna by a gut microsporidium followed by a haemolymph yeast decreases transmission of both parasites. Parasitology. 2021;148:1566–1577. doi: 10.1017/S0031182021001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duffy MA, Hall SR. Selective predation and rapid evolution can jointly dampen effects of virulent parasites on Daphnia Populations. Am Nat. 2008;171:499–510. doi: 10.1086/528998. [DOI] [PubMed] [Google Scholar]
- 57.Hall MD, Vettiger A, Ebert D. Interactions between environmental stressors: the influence of salinity on host-parasite interactions between Daphnia magna and Pasteuria ramosa. Oecologia. 2013;171:789–796. doi: 10.1007/S00442-012-2452-3. [DOI] [PubMed] [Google Scholar]
- 58.Zbinden M, Haag CR, Ebert D. Experimental evolution of field populations of Daphnia magna in response to parasite treatment. J Evol Biol. 2008;21:1088–1078. doi: 10.1111/j.1420-9101.2008.01541.x. [DOI] [PubMed] [Google Scholar]
- 59.Altermatt F, Ebert D. Genetic diversity of Daphnia magna populations enhances resistance to parasites. Ecol Lett. 2008;11:918–928. doi: 10.1111/J.1461-0248.2008.01203.X. [DOI] [PubMed] [Google Scholar]
- 60.Pulkkinen K. Microparasite transmission to Daphnia magna decreases in the presence of conspecifics. Oecologia. 2007;154:45–53. doi: 10.1007/s00442-007-0805-0. [DOI] [PubMed] [Google Scholar]
- 61.Lass S, Ebert D. Apparent seasonality of parasite dynamics: analysis of cyclic prevalence patterns. Proc Biol Sci. 2006;273:199–206. doi: 10.1098/rspb.2005.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fredericksen M, Ameline C, Krebs M, Hussy B, Fields PD, Andras JP, Ebert D. Infection phenotypes of a coevolving parasite are highly diverse, structured, and specific. Evolution. 2021;75:2540–2554. doi: 10.1111/evo.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Auld SKJR, Brand J. Simulated climate change, epidemic size, and host evolution across host-parasite populations. Glob Change Biol. 2017;23:5045–5053. doi: 10.1111/gcb.13769. [DOI] [PubMed] [Google Scholar]
- 64.Haag KL, Larsson JIR, Refardt D, Ebert D. Cytological and molecular description of Hamiltosporidium tvaerminnensis gen. et sp nov., a microsporidian parasite of Daphnia magna, and establishment of Hamiltosporidium magnivora comb. nov. Parasitology. 2011;138:447–462. doi: 10.1017/s0031182010001393. [DOI] [PubMed] [Google Scholar]
- 65.Haag KL, Pombert JF, Sun YK, de Albuquerque NRM, Batliner B, Fields P, Lopes TF, Ebert D. Microsporidia with vertical transmission were likely shaped by nonadaptive processes. Genome Biol Evol. 2020;12:3599–3614. doi: 10.1093/gbe/evz270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirk D, Luijckx P, Stanic A, Krkosek M. Predicting the thermal and allometric dependencies of disease transmission via the metabolic theory of ecology. Am Nat. 2019;193:661–676. doi: 10.1086/702846. [DOI] [PubMed] [Google Scholar]
- 67.Lohr JN, Laforsch C, Koerner H, Wolinska J. A Daphnia parasite (Caullerya mesnili) constitutes a new member of the Ichthyosporea, a group of protists near the animal-fungi divergence. J Eukaryot Microbiol. 2010;57:328–336. doi: 10.1111/j.1550-7408.2010.00479.x. [DOI] [PubMed] [Google Scholar]
- 68.Toenshoff ER, Fields PD, Bourgeois YX, Ebert D. The end of a 60-year riddle: identification and genomic characterization of an iridovirus the causative agent of white fat cell disease in zooplankton. Genes Genomes Genet. 2018;8:1259–1272. doi: 10.1534/g3.117.300429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andras JP, Fields PD, Du Pasquier L, Fredericksen M, Ebert D. Genome-wide association analysis identifies a genetic basis of infectivity in a model bacterial pathogen. Mol Biol Evol. 2020;37:3439–3452. doi: 10.1093/molbev/msaa173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lange B, Kaufmann AP, Ebert D. Genetic, ecological and geographic covariables explaining host range and specificity of a microsporidian parasite. J Anim Ecol. 2015;84:1711–1719. doi: 10.1111/1365-2656.12421. [DOI] [PubMed] [Google Scholar]
- 71.Krebs M, Routtu J, Ebert D. QTL mapping of a natural genetic polymorphism for long-term parasite persistence in Daphnia populations. Parasitology. 2017;144:1686–1694. doi: 10.1017/s0031182017001032. [DOI] [PubMed] [Google Scholar]
- 72.Bento G, Fields PD, Duneau D, Ebert D. An alternative route of bacterial infection associated with a novel resistance locus in the Daphnia-Pasteuria host-parasite system. Heredity. 2020;125:173–183. doi: 10.1038/s41437-020-0332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bento G, Routtu J, Fields PD, Bourgeois Y, Du Pasquier L, Ebert D. The genetic basis of resistance and matching-allele interactions of a host-parasite system: The Daphnia magna-Pasteuria ramosa model. PLoS Genet. 2017 doi: 10.1371/journal.pgen.1006596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petrusek A, Tollrian R, Schwenk K, Haas A, Laforsch C. A “crown of thorns” is an inducible defense that protects Daphnia against an ancient predator. Proc Natl Acad Sci USA. 2009;106:2248–2252. doi: 10.1073/pnas.0808075106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herzog Q, Rabus M, Ribeiro BW, Laforsch C. Inducible defenses with a “Twist”: Daphnia barbata abandons bilateral symmetry in response to an ancient predator. PLoS ONE. 2016;11:e0148556. doi: 10.1371/journal.pone.0148556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerber N, Kokko H, Ebert D, Booksmythe I. Daphnia invest in sexual reproduction when its relative costs are reduced. Proc Biol Sci. 2018;285:20172176. doi: 10.1098/rspb.2017.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roulin AC, Bourgeois Y, Stiefel U, Walser JC, Ebert D. A Photoreceptor contributes to the natural variation of diapause induction in Daphnia magna. Mol Biol Evol. 2016;33:3194–3204. doi: 10.1093/molbev/msw200. [DOI] [PubMed] [Google Scholar]
- 78.Penalva-Arana DC, Lynch M, Robertson HM. The chemoreceptor genes of the waterflea Daphnia pulex: many Grs but no Ors. BMC Evol Biol. 2009 doi: 10.1186/1471-2148-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weiss LC, Albada B, Becker SM, Meckelmann SW, Klein J, Meyer M, Schmitz OJ, Sommer U, Leo M, Zagermann J, et al. Identification of Chaoborus kairomone chemicals that induce defences in Daphnia. Nat Chem Biol. 2018;14:1133. doi: 10.1038/s41589-018-0164-7. [DOI] [PubMed] [Google Scholar]
- 80.Tollrian R. Predator-induced helmet formation in Daphnia cucullata (Sars) Arch Hydrobiol. 1990;119:191–196. doi: 10.1127/archiv-hydrobiol/119/1990/191. [DOI] [Google Scholar]
- 81.Laforsch C, Tollrian R. Inducible defenses in multipredator environments: Cyclomorphosis in Daphnia cucullata. Ecology. 2004;85:2302–2311. doi: 10.1890/03-0286. [DOI] [Google Scholar]
- 82.Horstmann M, Tollrian R, Weiss LC. Thwarting predators? A three-dimensional perspective of morphological alterations in the freshwater crustacean Daphnia. PLoS ONE. 2021 doi: 10.1371/journal.pone.0254263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ebert D. The trade-off between offspring size and number in Daphnia magna: the influence of genetic, environmental and maternal effects. Arch Hydrobiol. 1993;90:453–473. [Google Scholar]
- 84.Moenickes S, Richter O, Pirow R. Approaching the evolutionary advantage of ancillary types of haemoglobin in Daphnia magna by simulation of oxygen supply. J Exp Biol. 2010;213:408–417. doi: 10.1242/jeb.031914. [DOI] [PubMed] [Google Scholar]
- 85.OECD. Test No. 202: Daphnia sp. Acute Immobilisation Test; 2004.
- 86.OECD. Test No. 211: Daphnia magna Reproduction Test; 2012.
- 87.Bownik A, Wlodkowic D. Advances in real-time monitoring of water quality using automated analysis of animal behaviour. Sci Total Environ. 2021;789:147796. doi: 10.1016/j.scitotenv.2021.147796. [DOI] [PubMed] [Google Scholar]
- 88.Baird DJ, Barber I, Bradley M, Calow P, Soares AMVM. The Daphnia Bioassay—a Critique. Hydrobiol. 1989;188:403–406. doi: 10.1007/Bf00027806. [DOI] [Google Scholar]
- 89.Stollewerk A. Evolution of patterning mechanisms. Arthropod Struct Dev. 2010;39:397–398. doi: 10.1016/J.Asd.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 90.Pace RM, Grbic M, Nagy LM. Composition and genomic organization of arthropod Hox clusters. EvoDevo. 2016 doi: 10.1186/s13227-016-0048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Papillon D, Telford MJ. Evolution of Hox3 and ftz in arthropods: insights from the crustacean Daphnia pulex. Develop Genes Evol. 2007;217:315–322. doi: 10.1007/s00427-007-0141-8. [DOI] [PubMed] [Google Scholar]
- 92.Schwarzenberger A, Von Elert E. What makes a man a man? Prenatal antennapedia expression is involved in the formation of the male phenotype in Daphnia. Develop Genes Evol. 2016;226:47–51. doi: 10.1007/s00427-015-0525-0. [DOI] [PubMed] [Google Scholar]
- 93.Shiga Y, Sagawa K, Takai R, Sakaguchi H, Yamagata H, Hayashi S. Transcriptional readthrough of Hox genes Ubx and Antp and their divergent post-transcriptional control during crustacean evolution. Evol Dev. 2006;8:407–414. doi: 10.1111/j.1525-142X.2006.00114.x. [DOI] [PubMed] [Google Scholar]
- 94.Ayyar S, Negre B, Simpson P, Stollewerk A. An arthropod cis-regulatory element functioning in sensory organ precursor development dates back to the Cambrian. BMC Biol. 2010 doi: 10.1186/1741-7007-8-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klann M, Stollewerk A. Evolutionary variation in neural gene expression in the developing sense organs of the crustacean Daphnia magna. Develop Biol. 2017;424:50–61. doi: 10.1016/j.ydbio.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 96.Ungerer P, Eriksson BJ, Stollewerk A. Unravelling the evolution of neural stem cells in arthropods: notch signalling in neural stem cell development in the crustacean Daphnia magna. Develop Biol. 2012;371:302–311. doi: 10.1016/J.Ydbio.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 97.Ishak NSM, Nong QD, Matsuura T, Kato Y, Watanabe H. Co-option of the bZIP transcription factor Vrille as the activator of Doublesex1 in environmental sex determination of the crustacean Daphnia magna. PLoS Genet. 2017 doi: 10.1371/journal.pgen.1006953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kato Y, Kobayashi K, Watanabe H, Iguchi T. Environmental sex determination in the Branchiopod Crustacean Daphnia magna: deep conservation of a doublesex gene in the sex-determining pathway. PLoS Genet. 2011;7:e1001345. doi: 10.1371/Journal.Pgen.1001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nong QD, Matsuura T, Kato Y, Watanabe H. Two Doublesex1 mutants revealed a tunable gene network underlying intersexuality in Daphnia magna. PLoS ONE. 2020;15:e0238256. doi: 10.1371/journal.pone.0238256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ye ZQ, Molinier C, Zhao CX, Haag CR, Lynch M. Genetic control of male production in Daphnia pulex. Proc Natl Acad Sci USA. 2019;116:15602–15609. doi: 10.1073/pnas.1903553116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mittmann B, Ungerer P, Klann M, Stollewerk A, Wolff C. Development and staging of the water flea Daphnia magna (Straus, 1820; Cladocera, Daphniidae) based on morphological landmarks. EvoDevo. 2014 doi: 10.1186/2041-9139-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kato Y, Shiga Y, Kobayashi K, Tokishita S, Yamagata H, Iguchi T, Watanabe H. Development of an RNA interference method in the cladoceran crustacean Daphnia magna. Develop Genes Evol. 2011;220:337–345. doi: 10.1007/S00427-011-0353-9. [DOI] [PubMed] [Google Scholar]
- 103.Toyota K, Miyagawa S, Ogino Y, Iguchi T. Microinjection-based RNA interference method in the Water flea, Daphnia pulex and Daphnia magna. In: Abdurakhmonov IY, editor. RNA interference. London: IntechOpen; 2016. [Google Scholar]
- 104.Adhitama N, Kato Y, Matsuura T, Watanabe H. Roles of and cross-talk between ecdysteroid and sesquiterpenoid pathways in embryogenesis of branchiopod crustacean Daphnia magna. PLoS ONE. 2020 doi: 10.1371/journal.pone.0239893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakanishi T, Kato Y, Matsuura T, Watanabe H. CRISPR/Cas-mediated targeted mutagenesis in Daphnia magna. PLoS ONE. 2014 doi: 10.1371/journal.pone.0098363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Religia P, Nguyen ND, Nong QD, Matsuura T, Kato Y, Watanabe H. Mutation of the Cytochrome P450 CYP360A8 Gene Increases Sensitivity to Paraquat in Daphnia magna. Environ Toxicol Chem. 2021;40:1279–1288. doi: 10.1002/etc.4970. [DOI] [PubMed] [Google Scholar]
- 107.Kumagai H, Nakanishi T, Matsuura T, Kato Y, Watanabe H. CRISPR/Cas-mediated knock-in via non-homologous end-joining in the crustacean Daphnia magna. PLoS ONE. 2017 doi: 10.1371/journal.pone.0186112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Naitou A, Kato Y, Nakanishi T, Matsuura T, Watanabe H. Heterodimeric TALENs induce targeted heritable mutations in the crustacean Daphnia magna. Biol Open. 2015;4:364–369. doi: 10.1242/bio.20149738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakanishi T, Kato Y, Matsuura T, Watanabe H. TALEN-mediated knock-in via non-homologous end joining in the crustacean Daphnia magna. Sci Rep. 2016 doi: 10.1038/srep36252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arao T, Kato Y, Nong QD, Yamamoto H, Watanabe H, Matsuura T, Tatarazako N, Tani K, Okamoto A, Matsumoto T, et al. Production of genome-edited Daphnia for heavy metal detection by fluorescence. Sci Rep. 2020 doi: 10.1038/s41598-020-78572-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schumpert CA, Dudycha JL, Patel RC. Development of an efficient RNA interference method by feeding for the microcrustacean Daphnia. Bmc Biotechnol. 2015 doi: 10.1186/s12896-015-0209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Adhitama N, Matsuura T, Kato Y, Watanabe H. Monitoring ecdysteroid activities using genetically encoded reporter gene in Daphnia magna. Mar Environ Res. 2018;140:375–381. doi: 10.1016/j.marenvres.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 113.Kato Y, Nakanishi T, Watanabe H. Genome editing in the Crustacean Daphnia magna using CRISPR/Cas and TALEN systems. In: Appasani K, Church GM, editors. Genome editing and engineering: from Talens, Zfns and Crisprs to molecular surgery. Cambridge: Cambridge University Press; 2018. [Google Scholar]
- 114.Rivetti C, Campos B, Pina B, Raldua D, Kato Y, Watanabe H, Barata C. Tryptophan hydroxylase (TRH) loss of function mutations induce growth and behavioral defects in Daphnia magna. Sci Rep. 2018 doi: 10.1038/s41598-018-19778-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nguyen ND, Matsuura T, Kato Y, Watanabe H. DNMT3.1 controls trade-offs between growth, reproduction, and life span under starved conditions in Daphnia magna. Sci Rep. 2021 doi: 10.1038/s41598-021-86578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Routtu J, Ebert D. Genetic architecture of resistance in Daphnia hosts against two species of host-specific parasites. Heredity. 2015;114:241–248. doi: 10.1038/hdy.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Czypionka T, Fields PD, Routtu J, van den Berg E, Ebert D, De Meester L. The genetic architecture underlying diapause termination in a planktonic crustacean. Mol Ecol. 2019;28:998–1008. doi: 10.1111/mec.15001. [DOI] [PubMed] [Google Scholar]
- 118.Ravindran SP, Luneburg J, Gottschlich L, Tams V, Cordellier M. Daphnia stressor database: taking advantage of a decade of Daphnia ‘-omics’ data for gene annotation. Sci Rep. 2019 doi: 10.1038/s41598-019-47226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schwarzenberger A, Chen LX, Weiss LC. The expression of circadian clock genes in Daphnia magna diapause. Sci Rep. 2020 doi: 10.1038/s41598-020-77065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brun NR, Fields PD, Horsfield S, Mirbahai L, Ebert D, Colbourne JK, Fent K. Mixtures of aluminum and indium induce more than additive phenotypic and toxicogenomic responses in Daphnia magna. Environ Sci Technol. 2019;53:1639–1649. doi: 10.1021/acs.est.8b05457. [DOI] [PubMed] [Google Scholar]
- 121.Molinier C, Reisser CMO, Fields PD, Segard A, Galimov Y, Haag CR. Evolution of gene expression during a transition from environmental to genetic sex determination. Mol Biol Evol. 2019;36:1551–1564. doi: 10.1093/molbev/msz123. [DOI] [PubMed] [Google Scholar]
- 122.Wilde MV, Brehm J, Schwarzer M, Stockl JB, Laforsch C, Frohlich T. Improving the proteome coverage of Daphnia magna—implications for future ecotoxicoproteomics studies. Proteomics. 2022;22:e2100289. doi: 10.1002/pmic.202100289. [DOI] [PubMed] [Google Scholar]
- 123.Zaffagnini F, Sabelli B. Karyologic observations on the maturation of the summer and winter eggs of Daphnia pulex and Daphnia middendorffiana. Chromosoma. 1972;36:193–203. doi: 10.1007/BF00285213. [DOI] [PubMed] [Google Scholar]
- 124.Zaffagnini F. Reproduction in Daphnia. MemIst Ital Idrobiol (Memorie Dell'Istituto Italiano di Idrobiologia DottMarco De Marchi). 1987;45:245–284.
- 125.Tsuchiya D, Eads BD, Zolan ME. Methods for meiotic chromosome preparation, immunofluorescence, and fluorescence in situ hybridization in Daphnia pulex. In: Keeney S, editor. Meiosis. Heidelberg: Springer; 2009. pp. 235–249. [DOI] [PubMed] [Google Scholar]
- 126.Cristescu MEA, Colbourne JK, Radivojc J, Lynch M. A micro satellite-based genetic linkage map of the waterflea, Daphnia pulex: on the prospect of crustacean genomics. Genomics. 2006;88:415–430. doi: 10.1016/j.ygeno.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 127.Giribet G, Edgecombe GD. Current Understanding of Ecdysozoa and its internal phylogenetic relationships. Integr Comp Biol. 2017;57:455–466. doi: 10.1093/icb/icx072. [DOI] [PubMed] [Google Scholar]
- 128.Martín-Durán JM, Vellutini BC. Introduction: young approaches to animal evolution. In: Martín-Durán Jm, Vellutini BC., editors. Old questions and young approaches to animal evolution. Cham: Springer; 2019. pp. 1–13. [Google Scholar]
- 129.Andrew DR. A new view of insectecrustacean relationships II. Inferences from expressed sequence tags and comparisons with neural cladistics. Arthropod Struct Dev. 2011;40:289–302. doi: 10.1016/j.asd.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 130.von Reumont BM, Jenner RA, Wills MA, Dell’Ampio E, Pass G, Ebersberger I, Meyer B, Koenemann S, Iliffe TM, Stamatakis A, et al. Pancrustacean phylogeny in the light of new phylogenomic data support for remipedia as the possible sister group of hexapoda. Mol Biol Evol. 2012;29:1031–1045. doi: 10.1093/molbev/msr270. [DOI] [PubMed] [Google Scholar]
- 131.Duneau D, Luijckx P, Ben-Ami F, Laforsch C, Ebert D. Resolving the infection process reveals striking differences in the contribution of environment, genetics and phylogeny to host-parasite interactions. BMC Biol. 2011;9:11. doi: 10.1186/1741-7007-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Laforsch C, Tollrian R. Extreme helmet formation in Daphnia cucullata induced by small-scale turbulence. J Plank Res. 2004;26:81–87. doi: 10.1093/plankt/fbg114. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.