Abstract
Background: Long-term stress and chronic stress events play an important role in the etiology of depression. The study aimed to investigate the antidepressant-like effect of freshly prepared crude ethanolic extract of Saraca asoca flower (ESAF) in a mice model of acute restraint stress. Methodology: Rhamnazin, Myricetin and Quercetin were analytically characterized through liquid chromatography-mass spectrometry and high-performance liquid chromatography from Saraca asoca flower in a 0.1% acetic acid fraction of ethanol. The antidepressant effect was tested by repeated administration of freshly prepared ESAF on mice subjected to repeated and different forms of stress induction for 2 hours every day in the morning and night for seven consecutive days. The antidepressant activity was measured by known behavioral animal models: forced swim test (FST) and tail suspension test (TST). At the end of the experiment, each group of mice was sacrificed by cervical dislocation, followed by an estimation of the biochemical data. Results: The oral administration of ESAF in doses of 50, 100, & 250 mg/kg for seven consecutive days gave a significant decrease in the time of immobility (P<0.05) and reversed the depression-like behavior induced by acute restraint methods and behavioral models. ESAF treated groups showed a significant increase in glutathione peroxidase (GSH-PX) activity in the hippocampus of the acutely restrained mice. In addition, ESAF 250 mg/kg reduced plasma corticosterone levels in mice subjected to different forms of acute restraint stress compared to other groups, comparable to the standard imipramine. Conclusion: Our study showed the antidepressant activity of the ESAF. This effect may be attributed to the presence of antioxidant bioflavonoids namely, Rhamnazin, Myricetin and quercetin. Reduction in the plasma corticosterone levels along with an increase in the antioxidant enzymatic activity such as GSP-PX and SOD in the mice’s hippocampus is the proposed molecular hypothesis for its neuroprotective mechanism.
Keywords: Saraca asoca flower, liquid chromatography mass spectrometry, high performance liquid chromatography, acute restraint stress, force swim test, tail suspension test, immobility, hippocampus, corticosterone
Introduction
Stress is a crucial element of the existing healthcare system and of various ailments, including depression [1]. Acute and chronic stress may lead to a series of dysfunctions in the CNS, such as impaired cognition, anxiety, depression, and amnesia, along with an alteration in the free radical scavenging enzymes in discrete regions of the brain [2]. Although stress is mainly due to impaired antioxidant defense mechanisms, it has also been shown to play a significant role in the pathogenesis of neuropsychiatric disorders in both animals and humans [1]. Therefore, exposure to acute restraint stress in rodents has altered some behavioral features and induced depression-like behavior in mice [3].
Depression is a common and possibly life-threatening complication that increasingly affects the world population. In addition, it has emerged as one of the most common comorbid conditions along with diseases like cancer, diabetes, stroke, and cardiovascular disorders. [4-6]. Depression is mainly treated by pharmacologic treatments, i.e., blockade of neurotransmitters (Monoamine hypothesis) [7].
Currently available antidepressants are probably acting through different targets. Although approximately two-thirds of depressed patients benefit from available drugs, the long-term effects are still disappointing. In addition, psychoactive drugs do not meet their required therapeutic plasma concentration/demands for patients especially with other comorbid illnesses, causing unwanted adverse effects, which is a major drawback [8].
Herbal medicines can be good sources to explore novel approaches to find new therapies for central nervous system disorders. Presently, evidence suggests the effectiveness of many herbal sources for the treatment of depression due to their neuroprotective and antioxidant properties. In addition, many studies have shown that plant-derived active compounds can significantly reverse depression-like behavior in animals [9]. Chronic administration of Baicalein, a flavonoid from the root of Scutellaria baicalensis, has shown a significant reversal of the hopeless behaviors in mice subjected to restraint stress [10]. Resveratrol is a powerhouse of antioxidants; a study conducted on Resveratrol has displayed an antidepressant activity in animal screening models of depression by renovating the expression of BDNF (brain-derived neurotrophic factor) in the hippocampus and frontal cortex [11]. Moreover, the study demonstrated that even a crude extract of Pilea microphylla rich in flavonoids decrease hopeless behaviors among restrained mice [12].
Saraca asoca (S. asoca) belonging to the “Fabaceae” family, is a known small to medium-sized handsome evergreen tree. In Hinduism, ‘Ashoka’ means “one of that relieves pain and grief” and is considered a sacred tree. It has been widely used in folk medicine as an anticancer, antioxidant, antibacterial, anti-aging, anti-fertility, anti-arthritic, cardioprotective, larvicidal, antimutagenic/genoprotective, antidepressant, and anti-inflammatory, and is extensively used in Ayurveda, Unani, and Homeopathy practices [13-15]. The ethanolic extract of S. asoca leaves and bark have been demonstrated to possess antidepressant effects in animal screening models, namely forced swim test (FST) and tail suspension test (TST) [13,16]. Therefore, the present investigation is for phytochemical screening and characterization, followed by evaluation of effects of the ethanolic extract of S. asoca flower (Roxb.) Wilde on depression through two known animal screening models.
Materials and methods
Chemicals
These included: Ethanol, normal saline, Imipramine hydrochloride (Sun pharmaceuticals), DMSO, diluted in 0.9% NaCl solution, Antibody. All chemicals were of analytical grade.
Plant material and extractions
The fresh flower of S. asoca was collected in the months of April and May (2016) from West Bengal and taxonomically authenticated in the Department of Pharmacognosy, JSS Pharmacy College, Mysuru, and we preserved the flower in the herbarium for the future references (Specimen Voucher No. SA-10601/Pharma). The collected flower was eroded with distilled water and dried under a shaded environment at room temperature for 6-8 days. The dehydrated part of the flower was coarsely powdered and the fine powder was separated. The coarse powder of the flower (800 g) was subjected to extraction with ethanol by soxhlet apparatus, and the extract was concentrated to dry by a vacuum extractor. The extract was subjected to weighing to calculate the percentage of yield in terms of air-dried crude material. The result of ethanolic extract of S. asoca flowers (ESAF) was kept in a refrigerator for further use. Before administration, the ESAF was freshly prepared with normal saline, and three doses (50, 100, and 250 mg/kg) were selected in accordance with the therapeutic index.
Experimental animals
After approval from the Institutional animal ethics committee (1075/ac/07/CPCSEA), the Guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) were strictly followed for the animal treatment. Female mice weighing between 25-30 gm were randomly selected from the breeding stock at Central Animal Facility of JSS Medical College, JSS Academy of Higher Education and Research, Mysore. Animals were housed in propylene cages under known standard conditions (25±3)°C, humidity 45%-55%, 12/12 hr light/dark cycle with free access of food and water ad libitum. The animals were acclimatized for a period of 7 days before the study. Experimental animals were maintained under the guidance of the Indian National Science Academy (INSA). The experimental protocols were approved, and complete efforts were made to reduce the numbers and suffering of the animals used in the experiment.
Phytochemical analysis
The freshly prepared S. asoca flower extract was initially subjected to phytochemical screening to determine the secondary active metabolites using known standard tests [8,9]. This was followed by the characterization of secondary active metabolites using an analytical tool such as High-Performance Liquid Chromatography (HPLC).
Phytochemical characterization of secondary metabolite using HPLC analysis
A Shimadzu HPLC (Japan) equipped with LC-10 ATVP separation module and SPD-10 AVP UV-detector was used for the chromatographic study. The column Eclipse XDB C18 (250 mm × 4.6 mm, 5 µm) thermostated at 30°C, was used for the separation. Mobile phase A was 0.1% of acetic acid- prepared by dissolving 0.25 mL of acetic acid in 250 mL of water and filtered through 0.45 µm membrane filter, while Mobile phase B was Acetonitrile. The analysis was carried out under gradient conditions. The wavelength of the chromatographic study was 325 nm.
Acute oral toxicity study
Eighteen young, healthy adult female Swiss albino mice (25-30 g. b.wt.) were divided into six groups of 3 animals each. All animals in the six groups were subjected to acute oral toxicity tests to determine the therapeutic dose as per the OECD 423-Acute toxicity guidelines [10]. ESAF extracts were administrated orally up to a maximum dose of 2500 mg/kg. The body weight was recorded before and after the experiment. With a dose of 2500 mg/kg, mice did not show any abnormal clinical signs during all 13 days of experimentation. However, some pathological changes were observed at the end of the experiment.
Assessment of antidepressant activity in acute restraint stress (ARS) induced mice
Animal grouping and treatments
Swiss albino mice (25-30 g) were randomly allocated to seven groups of 6 mice in each group. Each group of animals was treated with the respective drug orally once daily for 7 days, followed by different forms of restraining stress. Dose and routes of administration as mentioned below.
Normal Control Group: sham animals received normal saline (10 ml/kg).
Stress Control Group: animals were exposed to ARS with normal saline.
Standard Group: animals were exposed to ARS with Imipramine (15 mg/kg p.o).
Test group 1: animals were exposed to ARS and treated with ESAF (50 mg/kg. p.o).
Test group 2, animals were exposed to ARS and treated with ESAF (100 mg/kg p.o).
Test group 3, animals were exposed to ARS and treated with ESAF (250 mg/kg p.o).
Test group 4, animals were exposed to ARS and treated with ESAF (50 mg/kg + Imipramine 0.5 mg/kg p.o).
Acute restraint stress methods
Acute restraint stress was induced as per the previous study procedures as follows: cold water swimming (4°C for 5 min); food or water deprivation (23 hr: 1 hr); soiled cage (100 ml of water spilled onto the bedding: 23 hr); empty water bottles (23 hr: 12 hr); continuous overnight illumination (24 hr); tilting of the cage (45° with 23 hr: 1 hr); intermittent illumination (light on and off every 2 hr); and also, animals were was inserted into acute restrainer. Stressor methods were employed each day individually for seven days.
Behavioral testing
After the last dose of drug administration, animals were subjected to known antidepressant screening models like Forced swim test (FST) and the Tail suspension test (TST). The experiment was conducted in dark experimental laboratories with minimal noise. A blinded observer noted the behavioral testing score.
Force swim test (FST)
The antidepressant activity was assessed by known behavioral animal models as previously described by Porsolt et al., 1977, with slight modifications. The mouse was placed individually into a beaker (25 cm height × 10 cm diameter) containing water (23±2°C) up to the mark so that mouse could not be escape from it or deep enough to touch the bottom of the container. The immobility score was recorded during the last 6 min swimming period, and the time of immobility was recorded during the last 4 min of the test.
Tail suspension test (TST)
This is a known mouse screening model to evaluate drugs for their antidepressant activity described by Steru et al., 1985. All the seven groups of animals were subjected to the TST test after the last dose administration. Individual mouse tail was suspended about 58 cm above the tabletop by its tail using an adhesive plaster placed 1 cm proximal from the tail tip for 6 min. A hopeless behavior was considered only when it hung passively and completely motionless. Motionless sessions during the last minutes of the test were measured [13].
Biochemical analysis of hippocampal homogenate
After completion of the experiment, acute restraint stress-induced mice were sacrificed by the amputation method. Once the mice brain was removed, the hippocampus was quickly dissected out on an ice-cold surface. Short tissues were taken from the brain in cold 0.9% NaCl solution toward stretch 10% of homogenate (w/v). Further, nuclei and debris were removed from tissues by centrifugation at 250 rpm/min for 10 min at 4°C, and the supernatant was collected for further investigation.
Total superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX) activity were measured as per the standard assay protocol (Sigma-Aldrich). The estimation of one unit of SOD activity is calculated by the amount of enzyme in each ml of solution at 50% of inhibition at 37°C. Similarly, one unit of GSH-PX activity is calculated through the net quantity of enzyme accomplished for hydrolyzing 1 μmol of glutathione (GSH) each min at 37°C. Bovine serum albumin was kept as a standard, and protein concentration was determined in hippocampal homogenates.
Corticosterone assay
All the mice of different groups were sacrificed by amputation on the last day of the experiment and mice blood was collected in an EDTA containing tube from the trunk of the mouse. The blood was centrifuged at 1000 rpm/min for 15 min at 4°C, and the plasma was stored at -70°C until used. Plasma corticosterone levels were determined by an enzyme-linked immunosorbent assay (ELISA).
Statistical analysis
The data were analyzed using GRAPH PRISM PAD version-7. Observations were expressed as mean ± SD/S.E.M. followed by data analysis by One-way ANOVA followed by Dunnet’s multiple compression test. The difference of means was considered to be significant at P<0.05, and results are displayed below in a graphical format.
Results
High-performance liquid chromatography determination of Rhamnazin, Myricetin, and Quercetin in acetic acid fractions of ESAF
Scheduled solicitation of the developed HPLC method yielded separated peaks of Rhamnazin, Myricetin and quercetin in acetic acid fraction of ESAF (Figure 1 and Table 1). Quantitative analysis showed the presence of Rhamnazin, Myricetin and quercetin in 0.1% of acetic acid fraction of ESAF.
Figure 1.
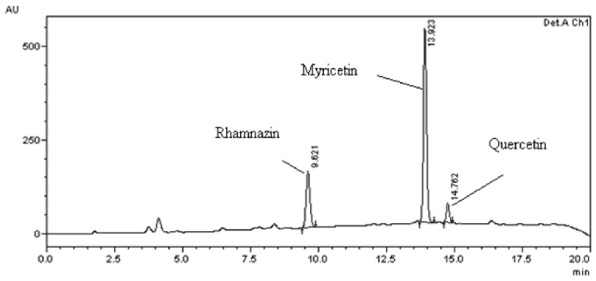
HPLC Chromatogram of Rhamnazin, Myricetin and Quercetin in 0.1% acetic acid fraction of ethanol extract of Saraca asoca flower.
Table 1.
Phytochemical characterization of ethanolic extract of Saraca asoca flower using High-Performance Liquid Chromatography
| Time (min) | Pump-A Cons | Pump-B Cons |
|---|---|---|
| 0.01 | 30 | 70 |
| 5.00 | 60 | 40 |
| 10.00 | 60 | 40 |
| 20.0 | 30 | 70 |
| 25 | 30 | 70 |
Acute oral toxicity study
The acute oral toxicity study was done to identify the therapeutic index, i.e. the ratio between actual pharmacological dose and fatal dose in the selected Swiss albino mice. Oral administration of ESAF up to 2500 mg/kg (p.o) body weight was safe up to 2000 mg/kg BW and did not show any mortality. Behaviors of the animals were monitored for the first 2 h and 30 min and next to every 4 h in subsequent 13 days. The results are expressed in Table 2. Results revealed no mortality or toxic symptoms during and after the experiments. In addition, all the mice equally accessed the food and water provided. Hence, as per the oral toxicity study results, the dose for further experimentation was optimized up to 250 mg/kg [11].
Table 2.
Illustrations oral acute toxicity studies of ethanolic extract of Saraca asoca flower
| Group | Dose treated (mg/kg) | No of animals per group | Dose difference (d) | Mortality (m) |
|---|---|---|---|---|
| 1 | 50 | 3 | Nil | |
| 2 | 100 | 3 | 50 | Nil |
| 3 | 500 | 3 | 400 | Nil |
| 4 | 1000 | 3 | 500 | Nil |
| 5 | 1500 | 3 | 500 | Nil |
| 6 | 2000 | 3 | 500 | Nil |
| 7 | 2500 | 3 | 500 | Death was occurred |
Lethal dose (LD50) = higher dose -Σ (dx m)/n.
n = No. of animals in each group.
LD50 = 2500 - 0 = 2500 mg/kg.
Effective dose (ED50) = Lethal dose (LD50)/10 = 2500/10 = 250 mg/kg.
Effect of the repeated administration of ESAF on immobility time in the FST and TST
The result depicted in Figures 2 and 3 shows that drugs given by oral route for seven days decreased the immobility time in FST and TST models, a behavioral profile characteristic of antidepressant-like effect. One-way ANOVA revealed a significant effect of the ESAF extract in FST [F (6, 35) = 13.7, (P<0.0001) and TST [F (6, 35) = 94.34, P<0.0001]. The corresponding Dunnet’s multiple comparison analysis indicated a significant decrease in the immobility time in FST by ESAF alone in doses of 50 mg/kg, (P<0.046), 100 mg/kg (P<0.018), 250 mg/kg (P<0.0033) and also in the low dose combination group (ESAF 50 mg/kg and imipramine 10 mg/kg) (P<0.0001) when compared to the stress control group. In case of TST also, ESAF elicited significant reduction in immobility at the doses of 50 mg/kg (P<0.04), 100 mg/kg (P<0.02), 250 mg/kg (P<0.0018) and the combined test group (ESAF - 10 mg/kg and imipramine - 5 mg/kg) (P<0.0001) as compared to the stress control group (Figure 4).
Figure 2.
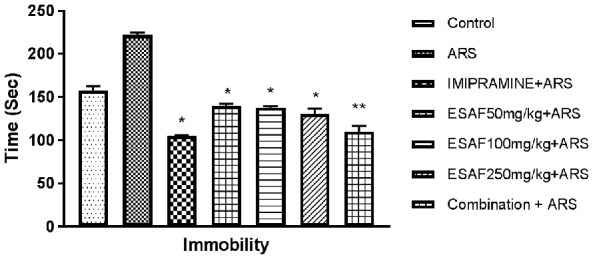
Effect of repeated (7 days) administration of ESAF with ARS [50, 100, 250 mg/kg and combination group p.o. (EASF 50 mg/kg+Imipramine 5 mg/kg) with ARS] in the FST model. Each column represents the mean ± S.E.M (n = 6). Statistical analysis was performed by One-way ANOVA, followed by Dunnet’s multiple comparison test. *P<0.05, **P<0.01, **P<0.001 as compared with the Stress control group. ARS: Acute restraint stress; ESAF: Ethanolic Extract of S. asoca Flowers.
Figure 3.
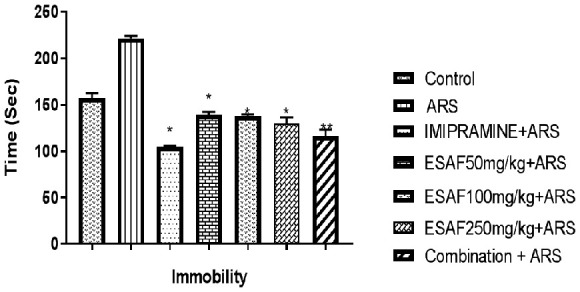
Effect of repeated (7 days) administration of ESAF with ARS [(50, 100, 250 mg/kg and combination group p.o (EASF 50 mg/kg+Imipramine 5 mg/kg) with ARS] in the TST model. Each column represents the mean ± S.E.M (n = 6). Statistical analysis was performed by one-way ANOVA, followed by Dunnet’s multiple comparison test. *P<0.05, **P<0.01, **P<0.001 as compared with the vehicle-treated group. ARS: Acute restraint stress; ESAF: Ethanolic Extract of S. asoca Flowers.
Figure 4.
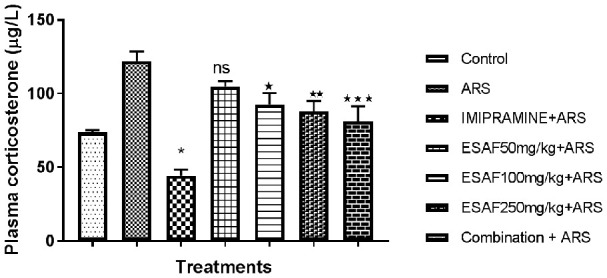
Effect of repeated (7 days) administration of ESAF with ARS [(50, 100, 250 mg/kg and combination group p.o (EASF 50 mg/kg+Imipramine 5 mg/kg)+ARS] on plasma corticosterone levels. Each column represents the mean ± S.E.M. Statistical analysis was performed by one-way ANOVA, followed by Dunnet’s multiple comparison test. *P<0.05, **P<0.01, **P<0.001 as compared to ARS control group. Plasma corticosterone levels of acute restrained mice with ESAF treatments for 7 consecutive days reverse significantly [F (6, 35) = 15.05, P<0.0001] the increased plasma corticosterone levels. ESAF 50 mg/kg showed not significant (P<0.2400) effect compared to ARS control group but was significant when compared to the control group. While EASF 100 & 250 mg/kg showed significant (P<0.0116 & 0.0029) effect when compared to ARS control, the combination group showed highly significant effect (P<0.0003) comparable to the ARS control group. ARS: Acute restraint stress; ESAF: Ethanolic Extract of S. asoca Flowers.
Assessment of plasma corticosterone levels in mice subjected to acute restraint stress for seven consecutive days with ESAF treatment
Acute restraint stress in mice may lead to biochemical changes in the brain and body, and especially it may elevate the corticosterone levels acting through the hypothalamic-pituitary-adrenal (HPA) axis. Our results also showed that repeated exposure of mice to acute restraint stress leads to significant increase in the plasma corticosterone level compared to unstressed control group mice. Repeated administration of ESAF (100 and 250 mg/kg) to mice exposed to ARS significantly [F (6, 35) = 15.05, P<0.0001] reversed the plasma corticosterone levels compared to control. In addition, the combination group showed highly significant activity and reversal of the plasma corticosterone levels comparable to the standard.
Assessment of GSH-PX and SOD enzyme activities on EASF treated acute restraint-induced mice
Many studies have shown that mice involved in acute restraint techniques may have a direct increase in basal levels of oxidative stress. We found that mice exposed to acute restraint stress exhibit had much reduced hippocampal GSP-PX activity compared to the ARS Stress control group (Figure 5). Repeated oral administration of ESAF significantly reversed, i.e., decreased oxidative stress by increasing the hippocampal GSP-PX activity as shown in the graph. Similarly, the SOD activity in the hippocampus of mice treated with ESAF and repeated restraint exhibited decreased activity compared to the ARS control group (Figure 6).
Figure 5.
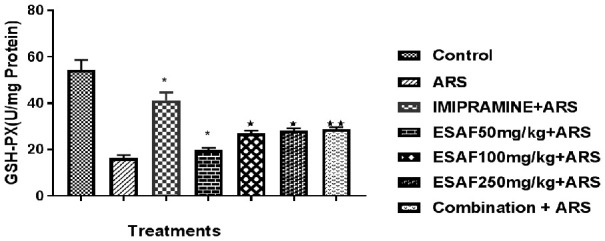
Effect of repeated (7 days) administration of ESAF with ARS [(50, 100, 250 mg/kg and combination group p.o (EASF 50 mg/kg+Imipramine 5 mg/kg) + ARS] on GSH-PX levels: Each column represents the mean ± S.E.M. Statistical analysis was performed by one-way ANOVA, followed by Dunnet’s multiple comparison test. *P<0.05, **P<0.01, **P<0.001 as compared to ARS control group. EASF 50 mg/kg for 7 days significantly increased the activity of GSH-PX enzyme in the hippocampus of mice subjected to ARS; F [(6, 35) = 32.33, P<0.001)]. Dunnet’s comparison test also revealed that ESAF 100 mg/kg (P<0.0115) and 250 mg/kg (P<0.0044) significantly increased the activity of GSH-PX and the combination group showed an even more highly significant effect (P<0.0025) when compared to other two doses. ARS: Acute restraint stress; ESAF: Ethanolic Extract of S. asoca Flowers.
Figure 6.
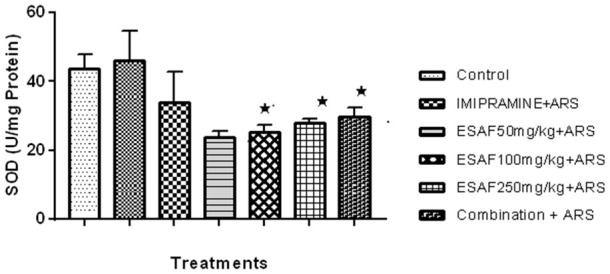
Effect of repeated (7 days) administration of ESAF with ARS [(50, 100, 250 mg/kg and combination group p.o. (EASF 50 mg/kg+Imipramine 5 mg/kg) + ARS]. Each column represents the mean ± S.E.M. Statistical analysis was performed by one-way ANOVA, followed by Dunnet’s multiple comparison test. *P<0.05, **P<0.01, **P<0.001 as compared to ARS. EASF for seven days significantly increased the activity of SOD enzyme in the hippocampus of mice subjected to ARS F [(6, 35) = 32.33, P<0.001)] in corresponds to Dunnet’s comparison test revealed ESAF 100 mg/kg (P<0.025) and 250 mg/kg P<0.040) significantly increased the activity of SOD. The combination group showed even more significance (P<0.001) when compared to the other two doses. ARS: Acute restraint stress; ESAF: Ethanolic Extract of S. asoca Flowers.
Discussion
S. asoca is a traditionally used medicinal plant for various conditions like diabetes, ulcer, larvicidal, or gynecological disorders. Data from the literature have shown that S. asoca flower extract exhibits antibacterial activity against enterobacteria [14,15]. In addition, dried powders of flowers taken twice daily alone or mixed with milk or honey are used to treat diabetes, and even the bark decoction was also used [17]. Recently, research has been undertaken to screen natural sources and their secondary metabolites for the treatment of neurological disorders. Therefore, we focused on screening secondary metabolites of S. asoca flower for its antidepressant role in mice subjected to acute restraint stress.
Minor stress is usually thought to be most valuable to screen antidepressant activity of drugs in known animal depression models. Initially, mice were subjected to a different kind of stress for seven consecutive days to mimic human depressive symptoms followed by oral administration of ethanolic extract of S. asoca flower (ESAF) twice daily for seven days. Our results showed that repeated doses of ESAF could reverse the depression like-behavior in animals using FST and TST animal models. These behavioral models are known paradigms to screen antidepressant activity and predict the treatment’s efficacy. In ARS groups, the immobility time significantly increased in rodents exposed to different ways of stress; it replicates a helpless situation and decreased state of mood and those ARS methods directly mimic the depressed behavior of humans [18]. In the current study, we found that repeated oral administration of ESAF in doses of 50,100,250 mg/kg and in combination treatments exhibited an antidepressant behavior compared to controls, but except for 50 mg/kg of EASF, the rest of the administrated doses significantly reversed the depression-like behavior in mice subjected to acute restraint stress. In addition, ESAF at 250 mg/kg dose exhibited a significantly high response compared to 100 mg/kg. The combination group showed more significant reduction of immobility time, almost comparable to the standard group in both FST and TST models.
Numerous studies strongly suggest plants containing flavonoids, tannins, triterpenoids, and saponins have a strong antidepressant role [17]. The phytochemical tests of ESAF indicate the presence of many biologically active ingredients. Further, HPLC peaks showed the presence of three known flavonoids, namely Rhamnazin, Myricetin and quercetin in the 0.1% acetic acid fraction. Flavonoids are basically the plant’s phenolic compounds that are found in many sources and have a wide variety of biological activities, including antioxidant, anticancer, cardioprotective, anti-inflammatory, antianxiety, antidepressant, and neuroprotection. Many researchers have shown that consumption of flavonoid sources may decrease certain human diseases [19].
Many studies have been reported that stress is a major factor that can damage the brain by increased production of reactive oxygen species (ROS) like OH-, O2 -, and H2O2 at the cellular level. This is one factor that directly links to the pathogenesis of depression in many ways, including the HPA-axis [20,21]. Our results indicate that administration of crude extracts made up of many biologically active ingredients, like flavonoids including myricetin, can chelate the metal ions intracellularly and stop ROS production. Earlier studies also concluded that natural sources with myricetin can scavenge the ROS [22] although they also enhance the other antioxidants. Further, a study in both in vitro and in vivo has reported that myricetin significantly enhances the activity of Glutathione S-acetyl transferase (GST), an enzyme responsible for protecting the cell against oxidative stress by the generation of free radicals [12]. Even rhamnazin also acts as an antioxidant and prevents oxidative stress [23].
Recently, a study demonstrated four flavonoids isolated from standardized Ginkgo biloba by HPLC method, specifically kaempferol, Quercetin, API, and Chrysin were identified as MAO inhibitors. They significantly increased the levels of monoamines, 5HT and NE which is beneficial in neurological disorders like depression [24]. Our study is focused on the HPA axis and GSH-PX, and SOD enzyme activity. Correspondingly, HPA-axis hyperactivation leads to an increase in the basal plasma corticosterone levels; therefore it is considered to be a major target for the treatment of depression and other neuropsychiatric disorders. Earlier studies have shown that mice subjected to repeated acute stress probably have higher plasma corticosterone levels and may develop depression [25]. So far, studies have investigated the modulatory effect of HPA axis using herbal sources. Studies have shown significant reversal of the depression-like behavior in animals, but the mechanism is not clear. Our results suggest that ESAF treated mice subjected to acute restraint stress significantly/partially reversed the plasma corticosterone, which might slightly contribute to the antidepressant activity.
Stress also disrupts the coordination between mind and body due to altered biochemical reactions, including enzyme activity. Many studies are showing repeated exposure to stress in animal models may increase basal oxidative stress. Some studies have also found that chronic administration of corticosterone can also induce oxidative damage comparable to that induced by restraint stress [26], which implicated oxidative stress as one of the major pathologic mechanisms underlying chronic stress exposure and depression. Our study assessed hippocampal GSH-PX and SOD activity. Repeated oral administration of ESAF for seven consecutive days restored the GSH-PX activities to normal compared to ARS groups. However, ESAF 50 mg/kg did not show any significance compared to the ARS group. EASF (100, 250 mg/kg) restored both the GSH-PX and SOD activities. In addition, the combination group showed slightly more significance than the other two doses and comparable to the ARS group. An overall summary of our findings indicates that repeated administration of ESAF strongly elicits neuroprotective effects due to the presence of three known flavonoids and others in extracts. The underlying mechanism might be partially mediated by the balance in the hippocampus’s plasma corticosterone and anti-oxidative stress.
Conclusion
Saraca asoca flower ethanolic extract in varying doses has exhibited significant antidepressant like activity in the tested animal behavioral models. This is attributed to the presence of antioxidant bioflavonoids namely, Rhamnazin, Myricetin and quercetin. Reduction in the plasma corticosterone levels along with an increase in the antioxidant enzymatic activity like GSP-PX and SOD in the mice hippocampus may be the proposed molecular hypothesis for its neuroprotective mechanism. Other neuroprotective mechanisms of Saraca asoca flower need to be explored.
Acknowledgements
We acknowledge the help of all the authors and the institution for the smooth conduction of this study.
Disclosure of conflict of interest
None.
References
- 1.Hritcu L, Noumedem JA, Cioanca O, Hancianu M, Kuete V, Mihasan M. Methanolic extract of Piper nigrum fruits improves memory impairment by decreasing brain oxidative stress in amyloid beta(1-42) rat model of Alzheimer’s disease. Cell Mol Neurobiol. 2014;34:437–449. doi: 10.1007/s10571-014-0028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanni C, Govoni S, Lucchelli A, Boselli C. Depression and antidepressants: molecular and cellular aspects. Cell Mol Life Sci. 2009;66:2985–3008. doi: 10.1007/s00018-009-0055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaji KS, Arun Kishore NR, Lal KP, Prince M. Revealing a hidden problem. An evaluation of a community dementia case-finding program from the Indian 10/66 dementia research network. Int J Geriatr Psychiatry. 2002;17:222–225. doi: 10.1002/gps.553. [DOI] [PubMed] [Google Scholar]
- 5.Sushith S, Krishnamurthy HN, Reshma S, Janice D, Madan G, Ashok KJ, Prathima MB, Kalal BS. Serum ischemia-modified albumin, fibrinogen, high sensitivity C- reactive proteins in type-2 diabetes mellitus without hypertension and diabetes mellitus with hypertension: a case-control study. Rep Biochem Mol Biol. 2020;9:241–249. doi: 10.29252/rbmb.9.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shetty R, Rai M, Chandrashekar R, Kalal BS. Diabetogenic effect of gluten in Wistar albino rats: a preliminary preclinical screening. Med Pharm Rep. 2020;93:47–52. doi: 10.15386/mpr-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado PL. Depression: the case for a monoamine deficiency. J Clin Psychiatry. 2000;61(Suppl 6):7–11. [PubMed] [Google Scholar]
- 8.Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–388. doi: 10.2147/PPA.S29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perviz S, Khan H, Pervaiz A. Plant alkaloids as an emerging therapeutic alternative for the treatment of depression. Front Pharmacol. 2016;7:28. doi: 10.3389/fphar.2016.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee B, Sur B, Park J, Kim SH, Kwon S, Yeom M, Shim I, Lee H, Hahm DH. Chronic administration of baicalein decreases depression-like behavior induced by repeated restraint stress in rats. Korean J Physiol Pharmacol. 2013;17:393–403. doi: 10.4196/kjpp.2013.17.5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurley LL, Akinfiresoye L, Kalejaiye O, Tizabi Y. Antidepressant effects of resveratrol in an animal model of depression. Behav Brain Res. 2014;268:1–7. doi: 10.1016/j.bbr.2014.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma Z, Wang G, Cui L, Wang Q. Myricetin attenuates depressant-like behavior in mice subjected to repeated restraint stress. Int J Mol Sci. 2015;16:28377–28385. doi: 10.3390/ijms161226102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill M, Kinra M, Rai A, Chamallamudi MR, Kumar N. Evaluation of antidepressant activity of methanolic extract of Saraca asoca bark in a chronic unpredictable mild stress model. Neuroreport. 2018;29:134–140. doi: 10.1097/WNR.0000000000000944. [DOI] [PubMed] [Google Scholar]
- 14.Hegde S, Saini A, Hegde HV, Kholkute SD, Roy S. Molecular identification of Saraca asoca from its substituents and adulterants. 3 Biotech. 2018;8:161. doi: 10.1007/s13205-018-1175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahid M, Shahzad A, Malik A, Anis M. Antibacterial activity of aerial parts as well as in vitro raised calli of the medicinal plant Saraca asoca (Roxb.) de Wilde. Can J Microbiol. 2007;53:75–81. doi: 10.1139/w06-107. [DOI] [PubMed] [Google Scholar]
- 16.Ali MA, Kim SY, Pan TK, Al-Hemaid F, Elshikh MS, Elangbam M, Lee J, Farah MA, Al-Anazi KM. Complete chloroplast genome of vulnerable medicinal plant Saraca asoca (Fabaceae) Mitochondrial DNA B Resour. 2020;5:754–755. doi: 10.1080/23802359.2020.1715300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh S, Krishna THA, Kamalraj S, Kuriakose GC, Valayil JM, Jayabaskaran C. Phytomedicinal importance of Saraca asoca (Ashoka): an exciting past, an emerging present and a promising future. Curr Sci. 2015;109:760–1801. [Google Scholar]
- 18.Yankelevitch-Yahav R, Franko M, Huly A, Doron R. The forced swim test as a model of depressive-like behavior. J Vis Exp. 2015:52587. doi: 10.3791/52587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatt S, Nagappa AN, Patil CR. Role of oxidative stress in depression. Drug Discov Today. 2020;25:1270–1276. doi: 10.1016/j.drudis.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Kalal BS, Pai VR, Upadhya D. Valproic acid reduces tumor cell survival and proliferation with inhibitors of downstream molecules of epidermal growth factor receptor pathway. J Pharmacol Pharmacother. 2018;9:11–16. [Google Scholar]
- 22.Kalal BS, Modi PK, Najar MA, Behera SK, Upadhya D, Prasad TSK, Pai VR. Hyperphosphorylation of HDAC2 promotes drug resistance in a novel dual drug resistant mouse melanoma cell line model: an in vitro study. Am J Cancer Res. 2021;11:5881–5901. [PMC free article] [PubMed] [Google Scholar]
- 23.Wu G, Dai X, Li X, Jiang H. Antioxidant and anti-inflammatory effects of rhamnazin on lipopolysaccharide-induced acute lung injury and inflammation in rats. Afr J Tradit Complement Altern Med. 2017;14:201–212. doi: 10.21010/ajtcam.v14i4.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah ZA, Sharma P, Vohora SB. Ginkgo biloba normalises stress-elevated alterations in brain catecholamines, serotonin and plasma corticosterone levels. Eur Neuropsychopharmacol. 2003;13:321–325. doi: 10.1016/s0924-977x(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 25.Qin DD, Rizak J, Feng XL, Yang SC, Lu LB, Pan L, Yin Y, Hu XT. Prolonged secretion of cortisol as a possible mechanism underlying stress and depressive behaviour. Sci Rep. 2016;6:30187. doi: 10.1038/srep30187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zafir A, Banu N. Induction of oxidative stress by restraint stress and corticosterone treatments in rats. Indian J Biochem Biophys. 2009;46:53–58. [PubMed] [Google Scholar]


