Abstract
Ulcerative colitis (UC) is a chronic intestinal inflammatory disease and familial adenomatous polyposis (FAP) is an autosomal dominant inherited disease. Both diseases, despite being different, may require the same surgical procedure: proctocolectomy with ileal pouch-anal anastomosis (IPAA). The main complication after this procedure is pouch inflammation (pouchitis). This inflammatory complication can affect up to 60 percent of patients who receive IPAA for UC, and a very small percentage of the FAP patients. The purpose of this review was to determine the current molecular mechanisms in its pathogenesis and detail the risk factors involved in pouchitis, its diagnosis, and treatment.
Keywords: Ulcerative colitis, familial adenomatous polyposis, ileal pouch-anal anastomosis, pouchitis
Introduction and general aspects of ulcerative colitis and familial adenomatous polyposis
Ulcerative colitis (UC) represents a chronic intestinal inflammatory disease with a non-fully understood etiology. The disease is characterized by periods of relapse and remission, and it usually affects the rectum, and a continuous extension of the colon, often with an abrupt transition between the inflamed and non-inflamed mucosa. Given that the use of biological drugs has provided more efficient management for patients, the rates of UC-related surgeries in the last decades have decreased remarkably. However, UC patients are prone to complications such as toxic colitis, intestinal bleeding, dysplasia, and cancer [1].
Familial adenomatous polyposis (FAP) is an autosomal dominant inherited disease, characterized by the formation of hundreds to thousands of adenomatous polyps in the large intestine and rectum. Generally, FAP arises early in life, which can lead to colon cancer in most patients before the age of 40. The etiology of FAP is based on a genetic mutation in the tumor suppressor gene, called Adenomatous Polyposis Coli (APC), which predisposes to the development of colorectal cancer [2,3].
Despite being different, both diseases may require the same surgical procedure. Proctocolectomy with ileal pouch-anal anastomosis (IPAA) is the elective procedure of choice in the surgical management of refractory UC and FAP with many polyps in the rectum. Twenty-five to thirty percent of UC patients undergo IPAA. The objectives of this surgical approach focus on avoiding malignant degeneration and promoting definitive treatment, as well as maintaining fecal continence [4]. The main IPAA-related complication is pouch inflammation (pouchitis) which can affect up to 60% of UC and a very small percentage of FAP patients [4-6].
IPAA has positively altered surgical management of both diseases, making it possible to avoid a permanent stoma [4-6]. In this review we will address the molecular aspects of pouchitis, showing the differences between UC and FAP. We also will highlight the risk factors involved and the general aspects of treatment.
Surgical technique, clinical aspects, and functionality of the ileoanal pouch procedure
The first report of the ileal reservoir dates back to 1933. Since then, several complex surgical procedures have been attempted without much success. The great evolution in pouch surgery occurred in 1978 when Sir Alan Parks and Mr. John Nicholls from St. Mark’s Hospital in London agreed to carry out a three-limbed ileal reservoir with ileoanal anastomosis, creating the first IPAA. They fashioned an S-shaped pouch for a patient with UC after proctocolectomy [4,6].
After that, the surgical approach to pouch formation advanced rapidly and several types of reservoirs were developed, such as “J”, “W”, and “S” pouches. As described in 1980 by Utsunomiya et al., the J-pouch is the most common type of ileal pouch, and this preference is mainly due to its superior storage and emptying function over the S or W-pouch configurations [5,7].
The surgery may require one, two, or three stages, including the creation of a temporary diverting ileostomy in order to minimize the complications of an ileal pouch fistula [6]. Over the years, there has also been a progression in the way the anastomosis is made. Nowadays, surgeons prefer a stapled anastomosis rather than a hand-sewn one. While handsewn anastomosis permits mucosectomy (the original description of the technique included a mucosectomy followed by a handsewn anastomosis between pouch and anus), that removes a potentially inflammatory rectal mucosa, at the same time it increases the risk of the anal sphincter and anal transition zone (AZT) damage, which is an important sensory-rich area of the anal canal that allows flatus/stool discrimination. A stapled IPAA, on the other hand, avoids a mucosectomy. In addition, it is quicker to perform and can offer a better functional outcome with lower nocturnal seepage and incontinence. As the advancement of technologies progresses, laparoscopic, robotic, single incision laparoscopic surgery (SILS), and transanal proctectomy (TaTME) have also been used for performing IPAA. These new techniques promise to improve surgical accuracy, postoperative recovery, and postoperative pain. However, more studies are needed [4,6,8]. Figure 1 illustrates the IPAA procedure.
Figure 1.
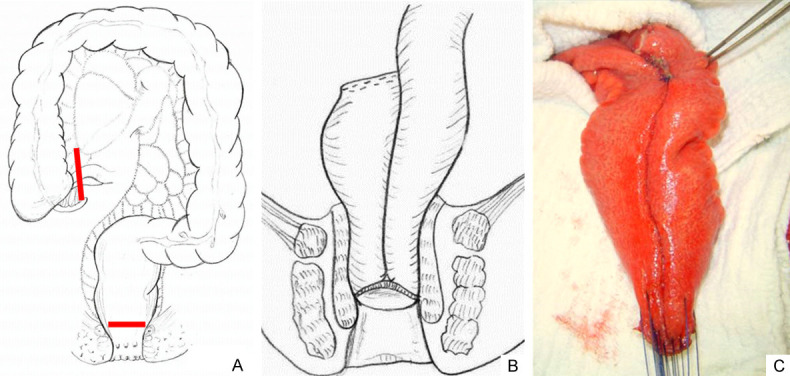
Proctocolectomy and ileal pouch-anal anastomosis (IPAA). A. Illustration showing the extension of the intestinal resection. All large intestine and rectum are removed, as evidenced in the follow-up between the red lines. B. Restorative proctocolectomy with J-shaped pouch and ileal pouch-anal anastomosis. C. Surgical aspect of an ileal J-shaped reservoir during a procedure, before performing a handsewn anastomosis (Colorectal Surgery Unit, Unicamp).
A main advantage of IPAA is the avoidance of a permanent ileostomy. Most UC patients who undergo IPAA report good quality of life after surgery with an average of six bowel movements per day, due to the improvement of the underlying disease. Overall, ileal pouch function is reported to be stable over time [9-11].
The evaluation of these patients must be strict and numerous tests contribute to the assessment and monitoring of expected complications. Pouch endoscopy is useful for assessing inflammatory changes in the pouch (granularity, loss of vascular pattern, edema, friability, mucosal hemorrhage, contact bleeding, and superficial ulcers) and it also allows the assessment of pre-pouch ileum and rectal cuff, if present. Furthermore, this technique also allows biopsies to be performed when necessary [10,11].
In addition to pouch endoscopy, pouchgram is another evaluating exam, which is useful to evaluate strictures, long efferent limb, decreased pouch complacency, and emptying. The use of a pouchogram before ileostomy closure should be indicated for cases of suspected clinical complications. However, pouchogram’s sensitivity to predict complications following ileostomy closure in patients after IPAA is rather low and it rarely changes the management of these patients in clinical practice [6,12]. Figure 2 shows the endoscopic and radiological characteristics of the ileal pouch.
Figure 2.
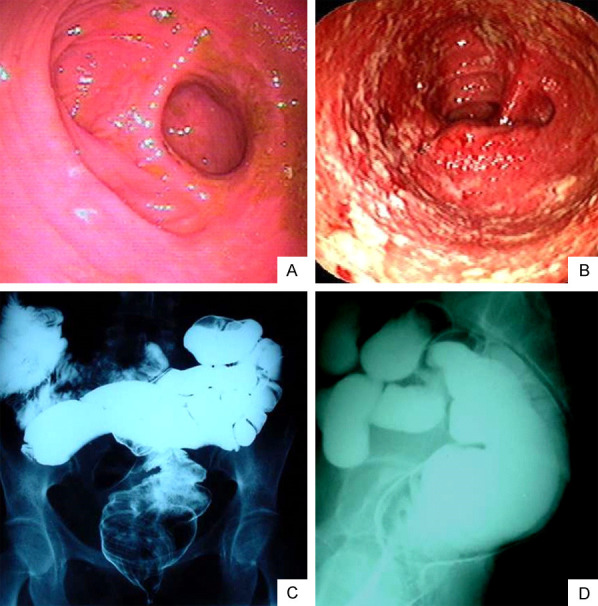
Endoscopic and radiological aspects of the ileal pouch. Endoscopic image of patients showing in (A) Normal mucosal appearance and in (B) Abnormal mucosa suggesting pouchitis. The endoscopic elements of the ileal pouchitis are granularity, loss of vascular pattern, edema, friability, mucosal hemorrhage, and superficial ulcers (Endoscopy Unit, Gastrocenter, Unicamp). Radiological aspects of the ileal pouch in the pouchogram, in (C) Frontal view (posteroanterior) and (D) Lateral view evidencing no abnormalities performed before ileostomy closure (Radiology Unit, Unicamp).
Although the mortality rate for IPAA is low, several pouch-specific complications following this surgery are common, such as hemorrhage, acute pelvic sepsis, anastomotic leak, infected hematoma, portal vein thrombus; as well as late complications, including chronic pelvic sepsis, pouch fistulae, small bowel obstruction, pouch dysfunction, cuffitis, dysplasia/malignancy, infertility and pouchitis [4,6,8].
Theoretically, once the colon and rectum are removed, either FAP or UC patients experience an improvement in the symptoms of the disease. However, about 50% of UC patients who have undergone IPAA develop at least one episode of subsequent pouchitis [6,8]. Pouchitis is an idiopathic nonspecific inflammation confined to the ileal pouch that leads to symptoms similar to that of UC, including increased frequency of bowel movements, abdominal cramps, straining during defecation, incontinence, hematochezia, mucous and/or bloody exudates, fever, abdominal pain and urgency [4,6,8].
In a series of publications and clinical follow-ups of PAF and UC patients, it was observed that both diseases may develop pouchitis after IPAA. Although the first episode of pouchitis can occur following ileostomy closure, the patients can develop it within the first year after the ileoanal reservoir procedure [4,6,8]. The definitive diagnosis is made in combination with clinical, endoscopic, and histopathological findings. The endoscopic appearance of pouchitis resembles the colorectal inflammation of UC. The main score used in the literature is the Pouchitis Disease Activity Index (PDAI), in which demographic data and scored symptoms are collected and graded [13]. Biopsies taken from the posterior wall of the pouch (if the pouch has a normal endoscopic appearance) or from inflamed areas are accessed together with scored symptoms. A total PDAI of seven or more indicates the presence of pouchitis. Symptomatic patients with no endoscopic and histologic evidence of pouchitis, besides a PDAI less than seven points, indicate the absence of pouchitis [9,13-15].
Although pouchitis can also be seen in FAP patients who undergo the same IPAA procedure, they rarely develop this inflammatory condition of the pouch. The etiology of pouchitis remains unclear. However, the difference in the percentage of involvement between UC and FAP points to molecular and immunological mechanisms, which may underlie this inflammation in ileal reservoir, mainly in UC.
Pouchitis: insight into the molecular mechanisms involved in the pathogenesis
Several factors have been suggested to influence the genesis of this complication, such as immune alteration, genetic susceptibility, autophagy-associated epigenetic changes, environmental factors, fecal stasis, bacterial overgrowth, dysbiosis, deprivation of short-chain fatty acids (SCFA), mucosal ischemia-reperfusion, and oxygen radicals in ischemia-induced lesions [8,10,11]. Many of these factors were evidenced not only in UC but also in FAP patients with IPAA, hampering their interpretation as isolated causes of primary pouchitis. In this section, we will discuss such factors. Table 1 illustrates the most relevant studies that deal with the potential factors involved in the primary pouchitis etiology.
Table 1.
Current molecular and clinical aspects involved in the pathogenesis of primary pouchitis
| Factors | Authors | Comments |
|---|---|---|
| Immune alterations | Segal et al., 2010. [18] | -Over-expression of molecules that participate in leukocyte activation and recognition |
| Kusunoki et al., 2006; Leal et al., 2011. [17,19] | -TLR2 and TLR4 expression upregulated | |
| Koltun et al., 2016; Barbara et al., 1994; Keighley et al., 1995; Goes et al., 2008. [129,20,21,26] | -Presence of pro-inflammatory cytokines in ileal pouches of UC patients, such as TNF-α, IL-1β, IL-6, IL-8, and interferon (IFN)-γ might explain higher rates of pouchitis in this group | |
| Desreumaux et al., 2000. [22] | -Decreased expression of IL-10 | |
| Neurath, et al., 2005. [28] | -Increased activation of the pro-inflammatory transcription factor STAT1 | |
| Herzig, et al., 2006. [31] | -Decreased levels of α and β defensins | |
| Kusunoki et al., 2020. [129] | -Increased IFN-γ mRNA expressions in patients who developed pouchitis | |
| Ponsioen et al., 2021. [130] | -Increased MAdCAM-1 expression in active inflammation in the pouch | |
| Genetic susceptibility | Peña et al., 2005. [35] | -Single-nucleotide polymorphisms (SNPs) involved in the susceptibility to pouchitis and its severity |
| Koltun et al., 2012. [34] | -Mutations in the nucleotide-binding and oligomerization domain (NOD) and TNFSF15 | |
| Peña et al., 2005. [35] | -Association of TLR9-1237C and CD14-120T alleles with the development of chronic pouchitis | |
| Autophagy/Apoptosis | Leal et al., 2018. [64] | -Modulation of macroautophagy markers leading to the mucosal inflammation with an increase of p62 in the ileal pouch |
| Heriot et al., 2006. [62] | -Differential expression of Beclin1 in the colon of UC patients | |
| Shen et al., 2012. [134] | -Increase in the deep crypt apoptosis in autoimmune pouchitis | |
| Góes, et al., 2008. [26] | -Increased expression of anti-apoptotic protein (phospho-BAD) in UC patients could explain higher rates of pouchitis in this group | |
| Mucosal ischemia-reperfusion | De Simone et al., 2001. [47] | -Increase of iNOS activity levels in the inflamed pouch compared with uninflamed control pouches |
| Blikslager et al., 2017. [113] | -TLR4-TRAF6 pathway and the effects of SOCS-1 may participate in the regulation of multi-organ damage caused by intestinal ischemia-reperfusion injury | |
| Environmental and clinical factors | Thirlby et al., 2000. [66] | -Greater occurrence of pouchitis in patients with extensive UC |
| van Heerden et al., 1990; Nozawa et al., 2019; Targan et al., 2007. [67,131,78] | -Pouchitis frequently occurred with a higher risk in patients with extraintestinal manifestations | |
| D’Hoore et al., 2008. [79] | -Younger age represents a higher risk of developing pouchitis | |
| Coates et al., 2018. [132] | -Cessation of smoking was associated with an increase in the development of pouchitis | |
| Pardi et al., 2013. [133] | -ANCA-positive patients present a higher risk of developing chronic pouchitis after IPAA | |
| Fukushima et al., 2012; Petrovska et al., 2010; Dotan et al., 2015. [89,93,94] | -The development of pouchitis was associated to a decrease in bacterial diversity in the microbiota of the ileal pouch, which would influence adequate functional performance |
Immune system dysfunction
Toll-like receptors (TLRs) comprise a class of proteins that play a key role in the innate immune system and intestinal epithelium defense. These receptors are responsible for recognizing bacterial lipopolysaccharides from both commensal and pathogenic bacteria. Several studies have shown a variation in gene expression of these receptors in the context of inflammatory bowel diseases (IBD) [16-24]. Toiyama et al. (2006) demonstrated that TLR2 expression was upregulated in patients with pouchitis, whereas TLR4 expression was increased in both normal pouches and pouchitis [17]. Similarly, using in vitro experiments in which macrophages were stimulated with TLR bacteria and ligands, Rahman et al. (2010) demonstrated that the response associated with TLR4 was defective in patients with UC, suggesting an over-expression of molecules that participate in leukocyte activation and recognition [18].
Accordingly, Paiva et al. (2011) evaluated the inflammatory activity in endoscopically normal ileal pouch mucosa and found a higher TLR4 expression in UC when compared with FAP patients and control individuals. These results suggest that pouchitis may be the consequence of positive regulation of intracellular pathways activated by bacterial products, which may contribute to the maintenance of an inflammatory-prone state in UC patients [19]. Malfunctioning TLR signaling can lead to inflammatory disorders, by NF-κB (nuclear transcription factor κB) activation, which plays a role in the transcription of several genes responsible for controlling the innate response, such as interleukin (IL)-1, IL-2, IL-6, IL-12, and tumor necrosis factor (TNF)-α. Several studies have demonstrated the presence of pro-inflammatory cytokines in ileal pouches of UC patients, such as TNF-α, IL-1β, IL-6, IL-8, and interferon (IFN)-γ [8,20-22]. A similar cytokine pattern was shown through the isolation of mononuclear cells from the lamina propria and culture of samples from colonic mucosa of UC patients and ileal mucosa from ileal pouch of UC patients who underwent this procedure. Pouchitis in UC is also characterized by a decreased expression of IL-10. These findings suggest that the inflamed reservoir may reactivate the immunological mechanisms that lead to UC [19-25].
Higher levels of TNF-α, IL-1β, IL-6, and IL-8 were verified in the ileal pouch mucosa of UC patients when compared with FAP. Moreover, both UC and FAP patients had higher levels of TNF-α when compared with the control group (p<0.05). However, there was no difference in NFκB activation among these groups [26]. Signal transducer and activator of transcription (STAT) also play a role in IBD pathophysiology, mainly in UC. Higher activation and expression of STAT1 have already been identified in UC mucosa, whereas in Crohn’s disease (CD) this pathway is not activated. High levels of SOCS-3 (suppressor of cytokine signaling), an inhibitor of STAT activation, were observed in CD patients and normal controls [27-29]. Interestingly, UC patients with non-inflamed ileal pouches after proctocolectomy had higher STAT1 levels when compared to the control group and FAP. These same patients also had higher levels of IFN-γ when compared with controls [30]. Patients with pouchitis also presented the same pattern, showing an increased activation of the pro-inflammatory transcription factor STAT1 in mucosal biopsies from inflamed pouches compared with both uninflamed and normal preoperative ileum [28].
The mechanisms of the innate immune response are essential for primary response in the intestinal mucosa against antigens. Defensins are important components in the innate immune response and play a key role in the homeostasis of the intestinal microbiota [24,31]. Kiehne et al. (2006) found decreased levels of α and β defensin in UC pouchitis when compared with FAP. On the contrary, a low incidence of pouchitis in FAP was correlated with increased levels of hBD-1-β-defensin and low levels of inflammatory cytokines [31].
Genetic polymorphisms associated with pouchitis
Genetic technological advances using genome-wide association study (GWAS) have enabled researchers to identify the correlation between the genome and the occurrence of IBD, providing valuable information for the scientific community. Specific gene signatures can predict pouchitis and likely postoperative complications [32-34].
Andus et al. (1997) found an association between the IL-1RN*2 allele (IL-1ra allele 2 gene) and the reduction of IL-1ra levels in colon mucosa of patients with IBD. This genetic variant can prevent adequate control of inflammation in the intestinal mucosa and it is associated with the risk of developing pouchitis in UC patients [32]. This association was not confirmed by the study carried out by Aisenberg et al. (2004) [33].
Lammers et al. (2005) aimed to investigate the single-nucleotide polymorphisms (SNPs) involved in the susceptibility to pouchitis and its severity. Based on the analyses of 157 IPAA patients, they found an association of TLR9-1237C and CD14-120T alleles with the development of chronic pouchitis [35].
In this context, Sehgal et al. (2012) analyzed SNPs associated with pouchitis after IPAA in patients who developed severe pouchitis for at least 2 years after surgery. The results demonstrated that mutations in the nucleotide-binding and oligomerization domain (NOD) and TNFSF15 locus were correlated with severe cases of pouchitis [34].
In the same way as TLRs-type receptors, NOD2 (CARD15), one of the members of the NOD family, is associated with increased susceptibility to several inflammatory diseases, especially IBD. These studies have demonstrated a potential association of NOD variants (rs2066874, NOD2insC, 1007fsCins) with the development of pouchitis [36-38]. NOD2 is a cytoplasmic molecule involved in the detection of microbial cell wall components and the regulation of inflammatory processes, as well as apoptosis. It recognizes muramyl dipeptide (MD) from Gram-positive and Gram-negative bacteria that stimulate the enteric immune system. Thus, if there is a loss or reduction in NOD2 function, the immune response to bacterial pathogens may be altered [39,40]. Through these studies, Meier et al. (2005) demonstrated that mutations in the NOD2 gene may predispose the development of pouchitis after IPAA in patients with UC. The percentage of NOD2 mutations was significantly higher in patients with pouchitis when compared to individuals without pouchitis (18% and 8%, respectively) [41]. In addition, a multicenter study that enrolled 714 patients, demonstrated that the risk variant NOD2insC was associated with chronic pouchitis with an added ratio of 3.2 when compared with those who did not have pouchitis [42].
Huang et al. (2017) performed the first longitudinal study that analyzed transcriptomic changes in the ileal pouch during the first year after IPAA. UC patients presented a shift in their transcriptional program after functionalization. As a result of reprogramming, many of the genes expressed in the colon became active in the ileal pouch mucosa, while many specific genes in the ileal region became less expressed. This colon-like ileal shift can lead to increased susceptibility to the disease. Mainly, the authors found genetic factors that could explain the colon involvement and its consequent propensity to develop pouchitis in the ileal mucosa of these patients. These changes would then be related to colonic metaplasia, suppression of xenobiotic metabolism, increased immune activation, and P450 signaling pathway [43]. Huang’s group confirmed what was seen by Morgan et al. (2015). The colonic marker CEACAM-7 was one of the main upregulated genes in the UC pouch [44]. The expression of matrix metalloproteinase (MMPs) in the ileal pouch is similar to that seen in inflamed tissues caused by IBD, with increased MMP1, MMP2, and MMP3 in both pouchitis and UC [45-50].
Several studies have analyzed the changes in gene expressions and, consequently, in histological characteristics that occur in both UC and FAP pouches. Paziewska et al. (2015) analyzed the gene array differences between UC and FAP pouches. The authors observed different gene expressions among pouches, suggesting that a UC pouch is more susceptible to developing pouchitis [51]. Similarly, Ben-Shachar et al. (2013) also utilized DNA microarrays to compare the normal terminal ileum of UC patients, healthy pouches, chronically inflamed pouches, and patients with Crohn’s-like pouchitis. The analysis showed that patients with chronic pouchitis and Crohn’s-like pouchitis presented several genetic changes, which are more severe when compared with individuals with normal ileum [52].
Autophagy and apoptosis pathways
Autophagy is responsible for the regulation of cellular processes that involve cell survival and death. When it occurs correctly, it promotes survival through the generation of energy for mitochondrial oxidation [53,54]. However, defects or exacerbations in cellular autophagy processes have been associated with cellular death and the induction of stress signals [55]. The role of autophagy has already been demonstrated in IBD [56-61].
Lovegrove et al. (2006) found a differential expression of Beclin-1 protein, which is involved in the autophagy initiation process, in the colon of UC patients [62]. Similarly, Leal et al. (2010) have demonstrated the correlation between signaling pathways and apoptosis in the ileal pouch mucosa of patients who underwent IPAA [63]. To determine transcriptional changes that occur in the inflammatory process of the ileal pouch, Paiva et al. (2018) evaluated the molecules involved in the autophagy pathway in the ileal pouch mucosa of FAP and UC patients. They found defective autophagy markers in both FAP and UC pouches, even in endoscopically normal mucosa, suggesting a mechanism for mucosa inflammation predisposition [64].
Another study suggests an important role for epithelial apoptosis mediated by Fas-Fas-L predisposing patients to pouchitis. Increased levels of epithelial apoptosis lead to increased villous atrophy when comparing UC patients with and without pouchitis [65]. Taken together, autophagy and apoptosis pathways may partially justify the differences in the molecular mechanisms of pouchitis in UC and FAP.
Environmental and clinical factors
In addition to immunological and genetic aspects, the occurrence of pouchitis after IPAA has been associated with environmental and clinical factors, such as length of UC, involvement of extraintestinal sites, active smoke, age during surgery, and serological markers.
Length of UC lesions
A study carried out by Simchuk et al. (2000) analyzed short- and long-term outcomes of patients who underwent IPAA for 12 years. This data demonstrated a greater occurrence of pouchitis in patients with extensive UC when compared to individuals with only left-sided involvement, which suggests a strong association between extensive UC and risk for pouchitis [66].
Extraintestinal involvement
Besides intestinal manifestations, there is often an IBD extra-intestinal (EIM) occurrence, which can involve the joints, eyes, liver, and skin. Lohmuller et al., (1990) analyzed the association between EIM and the development of pouchitis in 734 patients. The study suggests that pouchitis frequently occurred with a higher risk in patients with EIMs [67]. Primary Sclerosing Cholangitis (PSC) is one of the UC-related EIMs and it is also a chronic autoimmune hepatopathy, characterized by inflammation and fibrosis of intra- and extrahepatic bile ducts. The association between PSC and pouchitis after IPAA has not been fully established. Some researchers investigated this context and suggested a negative correlation. However, other studies indicate that PSC could be a protective factor for CD in the pouch, given its association with inflammation in the afferent limb of the pouch [68,69].
Another very common EIM in IBDs is arthritis, in which the prevalence rates are 14.3% to 44% among patients. Arthritis is a rheumatological autoimmune disease that affects the synovial membranes of multiple joints (hands, wrists, elbows, knees, ankles, feet, shoulders, cervical spine) [70]. As already discussed in the genetic section, mutations in the NOD2 allele are risk factors for the occurrence of pouchitis, but a mutation in this allele is also associated with auto-inflammatory diseases, like arthritis. Seril et al. (2016) described two cases of pouchitis after IPAA in UC patients that also presented EIM. In their study, both patients had mutations in the NOD2 allele and presented symptoms of the arthralgia spectrum (polyarthralgia and migratory oligoarthritis) [71].
Active smoking
A consensus on the consequences of smoking on UC and pouchitis has not been yet reached. Therefore, several studies have attempted to analyze the impact of smoking on predisposition to pouchitis. Initial experimental studies in rats suggested that active smoking reduced the risks of developing both UC and colitis [72,73]. Interestingly, two studies have suggested a protective role for tobacco against pouchitis in active smokers [74,75]. Based on these findings, the use of small doses of tobacco was used in several case reports as a treatment for a patient with severe disease [76,77]. Furthermore, a study by Fleshner et al. (2007) found opposite results to those seen previously, in which active smoking increased the incidence of acute pouchitis [78].
Age as a risk factor
The literature suggests that the age of UC diagnosis or IPAA performance is associated with the risk of developing pouchitis. In this context, Ferrante et al. (2008) analyzed for 6.5 years 173 patients with UC who underwent IPAA. Their results showed that undergoing surgery at a younger age represents a higher risk of developing pouchitis [79]. Similarly, Uchino et al. (2013) showed, in a Japanese cohort, that 44.3% of 149 patients with UC who underwent IPAA developed pouchitis. Of these, the mean age was lower in those who developed pouchitis. Differently from Ferrante’s study, Uchino showed that individuals younger than 26 years had a higher risk of developing chronic pouchitis when compared with patients older than 26 years [80].
Microbial changes and pouchitis
Alterations in luminal microbiota are now regarded as a key element for the development, evolution, and treatment of pouchitis [81]. The use of antibiotics (such as ciprofloxacin, rifaximin, or metronidazole) and probiotics may help control the symptoms and decrease inflammation in many patients with IPAA [8]. Besides, increased serum levels of antibodies against bacterial antigens have been observed in patients with an inflamed pouch. This evidence provides further support to the hypothesis of a relationship between pouchitis and an imbalance of bacterial overgrowth [82].
In addition, several studies compared the intestinal microbiota of UC and FAP patients undergoing IPAA in order to understand the mechanisms that contribute to the development of pouchitis. Tyler et al. (2013) compared the microbiota of patients with FAP and UC at different stages of IPAA. In the first stage of an ileoanal pouch operation (total colectomy), UC patients showed less bacterial diversity in mucosa samples in the colon before colectomy compared to FAP patients presenting lower levels of Clostridium perfringens. In addition, microorganism species, such as Klebsiella and Lactobacillus that are normally found in the formed pouch, were not present in pre-colectomy [83,84].
Almeida et al. (2008) also associated the predominant presence of Veilonnella species in the mucus of the terminal ileum with a persistently abnormal intestinal microbiota in UC patients [85]. Morgan et al. (2015) associated the presence of R. gnavus, B. vulgatus, and C. perfringens genera and the absence of Blautia and Roseburia genera with the predisposition to develop pouchitis [44]. Fecal samples of UC patients had a greater predominance of anaerobic and decreased aerobic bacteria in the ileal pouch when compared with FAP patients [84].
Microbiota and longitudinal follow-up post-surgery IPAA
Changes in microbiome diversity are strongly related to the development of pouchitis in the first-year post-surgery [85-97]. A great evidence of change in microbial constitution immediately after the ileostomy closure and the restitution of fecal flow through the pouch has already been observed [92]. Hinata et al. (2012) noticed changes in microorganism patterns two months after the restoration of intestinal continuity, with an increase in colon-predominant anaerobic bacteria in fecal samples with a decrease in ileum predominant species [89]. When compared to control groups, Almeida et al. (2008) found an increase of Enterobacter sp and Klebsiella sp and a decrease of Enterococcus sp and Fusobacterium sp in the ileal pouch [91].
Maharkashak et al. (2017) investigated for one year after surgery the profile of the microbiota in UC patients. They found a significant decrease in the diversity of microorganisms in those with pouchitis in less than one year when compared to those who did not develop inflammation in the first year. Interestingly, when patients were followed up for 3 years after surgery, there was an increase in microbial diversity, suggesting that microbial diversity may indicate a predictor of local inflammation [87].
Fungal dysbiosis and pouchitis
In a normal pouch, a great diversity of fungi coexists with bacteria in similar intestine niches, being represented mainly by Aspergillus, Candida, Nigrospora, and Rhodotorila [91]. Although fungal variation in pouchitis has been less explored, a recent study confirmed the role of fungal dysbiosis induced by 0.5% Fluconazole in experimental pouchitis and by 5% dextran sulfate sodium for 7 consecutive days in a rat model of IPAA, showing that dysbiosis increased mortality, weight loss and worsened CD4+ cell infiltration and severity of pouchitis [91].
The functional importance of the microbiota and the impact on the development of pouchitis
Gut microbiota is essential for several host physiological processes, including digestion of dietary factors, protection against colonization of pathogenic microorganisms, and development and performance of the gut immune system [92]. The development of pouchitis has been linked to a decrease in bacterial diversity in the microbiota of the ileal pouch, which would influence adequate functional performance [89,93,94].
Ruminococous
(Ruminococcaceae Family), for example, is important for the degradation of polysaccharides in the intestine that are essential for the maintenance of mucosal integrity and other benefits for the host [95,97], and a significant increase in Ruminococous was found in the normal ileal pouch when compared to patients with pouchitis [87].
Bacteroidetes and Firmicutes are the main phyla in the gut (about 90%) and they consist of relevant bacteria in the production of short-chain-acid (SCFA) metabolites. SCFA substrates are important sources of energy for colonic epithelial cells, they also present anti-inflammatory properties, and maintain epithelial barrier integrity [97]. SCFA is the main product of fermentation of dietary carbohydrates and fiber by mandatory anaerobic bacteria. The reduction of SCFAs has been found in cases of pouchitis when compared to an uninflamed ileal pouch [98-100].
Butyrate is an important SCFA that contributes to gut health and plays an important role in maintaining the epithelial barrier function, reducing oxidative stress, improving the immune system, and inducing regulatory T cells [101-104]. Examples of butyrogenic bacteria are Gram-positive Firmicutes belonging to Clostridium clusters XIVa and IV, standing out the species F. Prausnitzii, Roseburia spp, and Eunacterium rectale [105].
Enterocytes utilize butyrate and glutamine as fuel. However, a significant decrease in glutamine oxidation was observed in the ileal pouch mucosa while butyrate oxidation remained constant [104,106]. Thus, the presence of butyrogenic cells was considered beneficial for the ileal pouch offering high concentrations of butyrate even with changes in microbial composition [103]. In UC patients, when compared to non-UC controls, a decrease of butyrate oxidation in the ileum is observed. That could indicate a predisposition to reduce butyrate metabolism when the ileal pouch is performed [106]. Corroborating this hypothesis, a decrease of three bacterial groups, which belonged to the Clostridiales order, was found in the pre-pouch ileum. These bacteria are butyrate-producing and are considered beneficial for a healthy gut [86]. Patients who develop a healthy microbiota after IPAA may have the development of specific butyrate-producing community bacterial profiles, similar to those found in a healthy colon [107].
A higher incidence of sulfate-reducing bacteria in UC pouches compared with FAP pouches may be attributed to the low incidence of pouchitis in FAP patients. This bacterium uses sulfur instead of oxygen for breathing, leading to the production of hydrogen sulfide, which, in turn, inhibits butyrate oxidation and thus reduces the concentration available as nutrition to the intestinal epithelial cells, resulting in damage to the ileal pouch mucosa [86,108].
Colon-like metaplasia of ileal pouch
The colonic microbiota is efficient for the degradation of complex indigestible carbohydrates. On the other hand, the small intestine can metabolize small carbohydrates and adapt rapidly to the fluctuation of nutrient availability in the lumen [109-111].
Even though the pouch originates from the ileum, the pouch bacterial community changes, becoming more similar to the colon [85,88,89]. Fecal stasis may cause greater bacterial load and increase the adhesion of Bacterioidetes and Firmicutes [109-112]. An increase in facultative anaerobic bacteria and a decrease in anaerobes occur after ileostomy closure, becoming similar to the colon’s microbiota [84,88]. Almeida et al. (2008) described that the decrease of typical small bowel microorganisms, such as Enterococcus spp and Lactobacillus spp, occurs in less than 2 months, contributing to a similar colonic microbiota [85].
One of the reasons why fecal stasis in the ileal pouch has been implicated in colon metaplasia and mucin glycosylation transformation is the fact that such changes do not occur in ileostomies before IPAA [110,111]. Another factor that contributes to this bacterial alteration is the adaptation of the pouch due to the loss of glutamine oxidation based on butyrate oxidation, which also resembles the colon and contributes to the development of colonic metaplasia [104]. Even with evidence of colonic-like composition in the ileal pouch, the clinical significance of such aspect remains unclear. A hypothetical explanation for this is that the development of pouchitis would be related to the failure of the ileal pouch to develop a mature bacterial community similar to that found in the healthy colon [90].
Limitations and bias
The study of microbiota and pouchitis has expanded in the last few decades, but it still presents many obstacles. Most of the data come from North American and European countries, with a much smaller number of studies coming from Africa, South America, and Asia, which can lead to important biases [107]. For example, differences among diets can alter the host’s intestinal microbial composition [85].
Another limitation is the temporal dynamics of the composition of the small intestine microbiota, which may change in the morning and afternoon profiles over 9-18 days. Furthermore, certain drugs may influence microbiota, such as antibiotics, proton pump inhibitors, among other medications [87,110,111].
Finally, the heterogeneity of analysis techniques and sampling strategies are also factors that hinder conclusions about the relationship between intestinal microbiota and pouchitis. Moreover, we still do not know whether changes in the intestinal microbiota are the causes or the consequences of changes in the immune system or genetic factors.
Mucosal ischemia-reperfusion and oxygen radicals in ischemia-induced lesions
Intestinal ischemia-reperfusion occurs in a variety of pathophysiological situations. This complication is observed in patients with acute mesenteric ischemia, severe blood loss, and/or hypovolemia, frequently observed in patients undergoing major surgery or in patients with trauma, shock, or sepsis. Besides that, intestinal ischemia-reperfusion may be involved in the development and perpetuation of intestinal inflammation, including in UC and FAP patients who undergo IPAA [4,6].
At a cellular level, intestinal ischemic injury reduces cellular mitochondrial ATP (adenosine triphosphate) generation, activates hydrolases, reduces cell membrane selective permeability, and increases calcium influx into ischemic cells. Reperfusion may exacerbate the extent of injury through the activation of an intense systemic inflammatory response, such as marked proinflammatory cytokine release, production of reactive oxygen species (ROS), increased expression of nitric oxide (NO), TLR-4 signaling, and activation of inflammatory transcription factors, among other pro-inflammatory mechanisms [4,8,10,11].
Nitric oxide has been suggested to play an important role in the physiology and pathogenesis of the gastrointestinal (GI) tract. Indeed, nitric oxide production by the inducible nitric oxide synthase (iNOS) may act as a protective agent at the onset of the inflammatory response in the GI tract. Recent studies have suggested that the overproduction of NO by iNOS is detrimental during chronic inflammation. In this regard, many investigators have shown that IBD is associated with increased mucosal production of NO and increased iNOS expression [4,8,10,11,47]. Similar results were found by Ulisse et al. (2001). They observed a significant increase of iNOS activity levels in the inflamed pouch compared with uninflamed control pouches [47].
In addition, studies involving SOCS-1 and the pro-inflammatory TLR4-TRAF6 signaling pathway have suggested that intestinal ischemia-reperfusion injury affects other vital organs, such as lung, liver, and kidney, through amplification of organ-specific inflammatory stimuli to systemic inflammatory responses. These studies indicate that the TLR4-TRAF6 pathway and the effects of SOCS-1 may participate in the regulation of multi-organ damage caused by intestinal ischemia-reperfusion injury [113].
In this way, although many studies have provided essential information regarding the mechanisms of inflammation and apoptosis regulation during intestinal ischemia-reperfusion injury, further studies are needed to fully understand the relationship between the iNOS activity, TLR4-TRAF6 pathway, and SOCS-1, especially in patients with IBD and in those with pouchitis. Acute ischemia is a topic that requires a lot of attention from the surgeon who treats IBD, mainly because it is a potentially fatal clinical emergency, as well as difficult to manage clinically with consequent high morbidity and mortality. Moreover, when the ischemia is chronic, it can be a silent process that may lead to pouch disfunction and even complete loss of the reservoir [8,10,11,110-113].
Serological markers of developing pouchitis
The measurement of direct antibodies against microbial antigens is often used to distinguish CD and UC. In addition to the diagnostic factors, these antibodies enable us to predict possible complications, responses to medications, and the need for surgical intervention [114-116].
Among the identified antibodies are: Perinuclear anti-neutrophil cytoplasmic antibody (pANCA) and the anti-Saccharomyces cerevisiae antibody (ASCA); the first is usually identified in 41-73% of UC patients and 6-38% in CD patients, and the latter is more specific for CD [114].
Antibody titers are associated with the development of pouchitis. However, a definite consensus is still lacking. Fleshner et al. (2001) aimed to evaluate whether pANCA can be related to acute and/or chronic pouchitis after IPAA. Sixty-three percent of the patients enrolled in the study expressed levels of pANCA. After a 32-month follow-up, 34% of those patients developed pouchitis. In addition to these findings, the cumulative risk of developing chronic pouchitis in patients with high pANCA expression was significantly higher than in those with lower antibody expression [115].
These findings were confirmed by Tyler et al., who studied endoscopic data from 399 patients with UC who underwent IPAA at Mount Sinai Hospital in Toronto, Canada. More than 16% of the patients enrolled developed chronic pouchitis, and 12.5% developed pouchitis and Crohn’s disease Like (CDL), of which 14% were positive for ASCA. These results demonstrated an association between pANCA and inflammatory complications in the pouch, in addition to an association between the CDL phenotype and ASCA expression [83].
Contrarily, some studies have found no association between the presence of antibodies and the occurrence of pouchitis. Yasuda et al. did not find any association of pANCA and UC with pouchitis after IPAA. They concluded that antibody titer should not be used as a pre-surgery marker for predicting pouchitis development in the follow-up [116].
Reumaux et al. found similar data results by analyzing a group with 108 patients, 98 of the patients underwent IPAA (88% with pouchitis, and 12% without). pANCA was determined in those individuals by immunofluorescence assay. The data demonstrated that 52% of the IPAA-patients without pouchitis and 67% of the patients with pouchitis were pANCA positive, concluding that there was no correlation between the serological marker and the disease [117]. Aisenberg et al. (2004), who analyzed a cohort of 102 patients with UC that underwent IPAA, performed another study that confirms this scenario. In the analyzed group, both pANCA and ASCA showed no correlation with pouchitis [41].
Therefore, the relationship between serological markers pANCA and ASCA and the probability of developing pouchitis after IPAA remains questionable.
Crohn’s disease of the ileoanal pouch
Crohn’s disease (CD) in the pouch after IPAA is an increasingly noticed diagnosis and it must be distinguished from the primary pouchitis we had mentioned in the sections above. Its etiology is not yet completely understood. On the one hand, CD may have been misdiagnosed before surgery, or, on the other hand, it may be a de novo CD. The cause of de novo CD in the ileoanal pouch remains largely unknown and has been related to the factors described above, such as a change in microbiome following pouch stasis, environmental factors, genetics, and others. Clinically, these patients present abundant symptoms and should be examined carefully when they develop fistulas, strictures, and chronic refractory pouchitis, as well as granulomas in the histopathological study. The diagnosis is sometimes challenging and takes into account clinical, radiological, endoscopic findings, and personal history. Patients can develop CD at any time after IPAA. Higher rates of pouch failure and the need for pouch excision have been observed [4,118,119].
Clinical management
Microbial-based therapies for pouchitis
With the growing evidence of the impact of dysbiosis on the development of pouchitis, different therapeutic options have also emerged which aim to manipulate microbiota.
Antibiotics
The effectiveness of antibiotics as a treatment in most pouchitis cases (metronidazole, rifaximin, and ciprofloxacin) is an important indication that dysbiosis plays a role in the pathogenesis of the disease. Biopsies from pouchitis patients have shown that antibiotic therapy reduces groups of specific bacteria including Bacteroides, Firmicutes, and Tenericutes [44]. While analyzing fecal samples from sick patients who were using antibiotics, they found a greater number of Firmicutes and lesser Proteobacteria when compared to those who were not taking antibiotics [120]. Ciprofloxacin has been linked to decreasing Clostridium perfringens and Escherichia coli and had less effect on anaerobic bacteria. Metronidazole reduced C. perfringens and anaerobic bacteria, but not Escherichia Coli. Thus, ciprofloxacin appears to be less harmful to non-pathogenic species and more efficient against pathogenic species than metronidazole [121].
Antibiotic treatment has also been linked to restoring SCFA concentrations to normal levels in cases of pouchitis [122]. Even though it is a widely used treatment, the risk of bacterial resistance and uncertainty of toxicity limits its long-term use.
Several other antibiotics have been reported to be effective in uncontrolled series of patients with pouchitis. Most patients with pouchitis are likely to have symptomatic improvement after 1-2 days of therapy with metronidazole or ciprofloxacin [121-123].
Probiotics and prebiotics
The use of probiotics has shown significant induction of remission and prevention of pouchitis recurrence, and also shows effectiveness after antibiotic-induced remission. The most widely used is VSL # 3, which has been shown to increase bacterial diversity within the ileoanal pouch in addition to decreasing fungal diversity when compared to placebo treatment, which restores the balance between fungi and bacteria [124].
VSL # 3 was associated with increased concentration of Lactobacilli spp, Bifidobacterium spp, and E. coli in biopsy samples. However, the microbiota alteration after the use of probiotics was not long-term sustained [94,95]. For pouchitis, probiotics have been used as a therapeutic option in cases of remission, but no data support the use of probiotics as primary therapy [124].
Welters et al. (2002) analyzed the effect of insulin supplementation for two weeks and found significant clinical and histological improvement associated with an increase in butyrate levels and a reduction in pH, B. fragilis, and also an increase in bile acid levels [125]. However, the same protocol had been performed by Meijer HP et al. (2000) without beneficial results [126].
Fecal Microbial Transplantation (FMT)
FMT treatment for pouchitis still shows controversial results as the studies show low clinical remission rates (9-21.5%). Research is still very limited due to the small study size and discordant protocols that include dose, frequency, donor selection, and route of administration of FMT as well as variation of each patient’s microbiota [127].
Other therapies
Oral and rectal corticosteroids may be beneficial in active pouchitis. In patients who require oral steroids for induction of remission, maintenance therapy with immune modulators or low-dose oral budesonide should be considered. In cases of severe and refractory pouchitis, treatment with infliximab may be beneficial. Overall, infliximab appears to have good clinical effectiveness in selected patients achieving up to 80% short-term and around 50% long-term response [6,10,128]. Other therapies have been studied in pouchitis, including bismuth enemas, SCFA enemas, and glutamine suppositories, but the role of these treatments in pouchitis remains to be clarified [8].
Conclusion
This review correlated the development of pouchitis in UC patients with potential genetic, microbial, and immunological causes. Although the pathophysiology of pouchitis remains unknown, many relevant advances occurred in recent decades that allow physicians and researchers to develop better strategies to treat pouchitis in the follow-up after IPAA. After the diagnosis of pouchitis, the patient is usually treated with antibiotics, suggesting that the disease is at least partially mediated by a bacterial component. Increases in the bacterial populations that are commonly considered pathogenic are seen in the uninflamed UC pouch compared with FAP pouches. Therefore, more longitudinal studies in the same patients after IPAA are necessary to evaluate how these multiple factors can influence the gut and pouch microbiota.
Acknowledgements
We thank Dr. Tristan Torriani for the English revision of our manuscript. This work was supported by National Council for Scientific and Technological Development (CNPq) [Grant scholarship number #302557/2021-0 for R.F.L.]. L.B.P. (co-author) received a post-doctoral scholarship from Funding for Education, Research and Extension Support (FAEPEX), University of Campinas. L.M.G. (co-author) was supported by São Paulo Research Foundation (FAPESP) [Grant number #2020/01924-5]. B.L.R. (author) was supported by a post-doctoral scholarship in laboratory management from Funding for Education, Research and Extension Support (FAEPEX), University of Campinas.
Disclosure of conflict of interest
None.
References
- 1.Gajendran M, Loganathan P, Jimenez G, Catinella AP, Ng N, Umapathy C, Ziade N, Hashash JG. A comprehensive review and update on ulcerative colitis. Dis Mon. 2019;65:100851. doi: 10.1016/j.disamonth.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Kanth P, Grimmett J, Champine M, Burt R, Samadder NJ. Hereditary colorectal polyposis and cancer syndromes: a primer on diagnosis and management. Am J Gastroenterol. 2017;112:1509–1525. doi: 10.1038/ajg.2017.212. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Guo L, Li YH, Feng GH, Teng F, Li W, Zhou Q. MicroRNA-494 promotes cancer progression and targets adenomatous polyposis coli in colorectal cancer. Mol Cancer. 2018;17:1. doi: 10.1186/s12943-017-0753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng KS, Gonsalves SJ, Sagar PM. Ileal-anal pouches: a review of its history, indications, and complications. World J Gastroenterol. 2019;25:4320–4342. doi: 10.3748/wjg.v25.i31.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barton JG, Paden MA, Lane M, Postier RG. Comparison of postoperative outcomes in ulcerative colitis and familial polyposis patients after ileoanal pouch operations. Am J Surg. 2001;182:616–620. doi: 10.1016/s0002-9610(01)00795-4. [DOI] [PubMed] [Google Scholar]
- 6.Pardi DS, Sandborn WJ. Systematic review: the management of pouchitis. Aliment Pharmacol Ther. 2006;23:1087–1096. doi: 10.1111/j.1365-2036.2006.02884.x. [DOI] [PubMed] [Google Scholar]
- 7.Utsunomiya J, Iwama T, Imajo M, Matsuo S, Sawai S, Yaegashi K, Hirayama R. Total colectomy, mucosal proctectomy, and ileoanal anastomosis. Dis Colon Rectum. 1980;23:459–466. doi: 10.1007/BF02987076. [DOI] [PubMed] [Google Scholar]
- 8.Schieffer KM, Williams ED, Yochum GS, Koltun WA. Review article: the pathogenesis of pouchitis. Aliment Pharmacol Ther. 2016;44:817–835. doi: 10.1111/apt.13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SN, Shen B, Remzi F. When not to pouch: important considerations for patient selection for ileal pouch-anal anastomosis. Gastroenterol Hepatol (N Y) 2017;13:466–475. [PMC free article] [PubMed] [Google Scholar]
- 10.de Buck van Overstraeten A, Wolthuis AM, Vermeire S, Van Assche G, Laenen A, Ferrante M, Rutgeerts P, D’Hoore A. Long-term functional outcome after ileal pouch anal anastomosis in 191 patients with ulcerative colitis. J Crohns Colitis. 2014;8:1261–1266. doi: 10.1016/j.crohns.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Karlbom U, Lindfors A, Påhlman L. Long-term functional outcome after restorative proctocolectomy in patients with ulcerative colitis. Colorectal Dis. 2012;14:977–984. doi: 10.1111/j.1463-1318.2011.02873.x. [DOI] [PubMed] [Google Scholar]
- 12.Santorelli C, Hollingshead J, Clark SK. Clinical value of pouchogram prior to ileostomy closure after ileal pouch anal anastomosis. Tech Coloproctol. 2018;22:541–544. doi: 10.1007/s10151-018-1823-0. [DOI] [PubMed] [Google Scholar]
- 13.Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Phillips SF. Pouchitis after ileal pouch-anal anastomosis: a pouchitis disease activity index. Mayo Clin Proc. 1994;69:409–415. doi: 10.1016/s0025-6196(12)61634-6. [DOI] [PubMed] [Google Scholar]
- 14.Kohyama M, Takesue Y, Ohge H, Murakami Y, Shimamoto F, Sueda T. Pouchitis disease activity index (PDAI) does not predict patients with symptoms of pouchitis who will respond to antibiotics. Surg Today. 2009;39:962–968. doi: 10.1007/s00595-009-3988-7. [DOI] [PubMed] [Google Scholar]
- 15.Benlice C, Shen B, Steele SR. Prevention and medical treatment of pouchitis in ulcerative colitis. Curr Drug Targets. 2019;20:1399–1408. doi: 10.2174/1389450120666190723130137. [DOI] [PubMed] [Google Scholar]
- 16.Pinho M. Molecular biology of inflammatory bowel diseases. Rev bras. Colo-proctol. 2008;28:119–123. [Google Scholar]
- 17.Toiyama Y, Araki T, Yoshiyama S, Hiro J, Miki C, Kusunoki M. The expression patterns of Toll-like receptors in the ileal pouch mucosa of postoperative ulcerative colitis patients. Surg Today. 2006;36:287–290. doi: 10.1007/s00595-005-3144-y. [DOI] [PubMed] [Google Scholar]
- 18.Rahman FZ, Smith AM, Hayee B, Marks DJ, Bloom SL, Segal AW. Delayed resolution of acute inflammation in ulcerative colitis is associated with elevated cytokine release downstream of TLR4. PLoS One. 2010;5:e9891. doi: 10.1371/journal.pone.0009891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paiva NM, Ayrizono ML, Milanski M, Coope A, Oliveira LM, Fagundes JJ, Velloso LA, Coy CS, Leal RF. Differential expression of TLR2, TLR4 and JNK in mucosa of ileal pouches for ulcerative colitis. Is there a role for bacterial antigen pathway in asymptomatic patients? Int J Clin Exp Med. 2011;4:179–186. [PMC free article] [PubMed] [Google Scholar]
- 20.Gionchetti P, Campieri M, Belluzzi A, Bertinelli E, Ferretti M, Brignola C, Poggioli G, Miglioli M, Barbara L. Mucosal concentrations of interleukin-1 beta, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in pelvic ileal pouches. Dig Dis Sci. 1994;39:1525–1531. doi: 10.1007/BF02088059. [DOI] [PubMed] [Google Scholar]
- 21.Patel RT, Bain I, Youngs D, Keighley MR. Cytokine production in pouchitis is similar to that in ulcerative colitis. Dis Colon Rectum. 1995;38:831–837. doi: 10.1007/BF02049839. [DOI] [PubMed] [Google Scholar]
- 22.Bulois P, Tremaine WJ, Maunoury V, Gambiez L, Hafraoui S, Leteurtre L, Cortot A, Sandborn WJ, Colombel JF, Desreumaux P. Pouchitis is associated with mucosal imbalance between interleukin-8 and interleukin-10. Inflamm Bowel Dis. 2000;6:157–164. doi: 10.1097/00054725-200008000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Lammers KM, Vergopoulos A, Babel N, Gionchetti P, Rizzello F, Morselli C, Caramelli E, Fiorentino M, d’Errico A, Volk HD, Campieri M. Probiotic therapy in the prevention of pouchitis onset: decreased interleukin-1beta, interleukin-8, and interferon-gamma gene expression. Inflamm Bowel Dis. 2005;11:447–454. doi: 10.1097/01.mpa.0000160302.40931.7b. [DOI] [PubMed] [Google Scholar]
- 24.Silva FA, Rodrigues BL, Ayrizono ML, Leal RF. The Immunological basis of inflammatory bowel disease. Gastroenterol Res Pract. 2016;2016:2097274. doi: 10.1155/2016/2097274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evgenikos N, Bartolo DC, Hamer-Hodges DW, Ghosh S. Assessment of ileoanal pouch inflammation by interleukin 1beta and interleukin 8 concentrations in the gut lumen. Dis Colon Rectum. 2002;45:249–255. doi: 10.1007/s10350-004-6156-6. [DOI] [PubMed] [Google Scholar]
- 26.Leal RF, Coy CS, Ayrizono ML, Fagundes JJ, Milanski M, Saad MJ, Velloso LA, Góes JR. Differential expression of pro-inflammatory cytokines and a pro-apoptotic protein in pelvic ileal pouches for ulcerative colitis and familial adenomatous polyposis. Tech Coloproctol. 2008;12:33–38. doi: 10.1007/s10151-008-0395-9. [DOI] [PubMed] [Google Scholar]
- 27.Kühbacher T, Gionchetti P, Hampe J, Helwig U, Rosenstiel P, Campieri M, Buhr HJ, Schreiber S. Activation of signal-transducer and activator of transcription 1 (STAT1) in pouchitis. Clin Exp Immunol. 2001;123:395–401. doi: 10.1046/j.1365-2249.2001.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mudter J, Weigmann B, Bartsch B, Kiesslich R, Strand D, Galle PR, Lehr HA, Schmidt J, Neurath MF. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am J Gastroenterol. 2005;100:64–72. doi: 10.1111/j.1572-0241.2005.40615.x. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber S, Rosenstiel P, Hampe J, Nikolaus S, Groessner B, Schottelius A, Kühbacher T, Hämling J, Fölsch UR, Seegert D. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut. 2002;51:379–385. doi: 10.1136/gut.51.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leal RF, Ayrizono ML, Milanski M, Coope A, Fagundes JJ, Velloso LA, Coy CS. Activation of signal transducer and activator of transcription-1 (STAT-1) and differential expression of interferon-γ and anti-inflammatory proteins in pelvic ileal pouches for ulcerative colitis and familial adenomatous polyposis. Clin Exp Immunol. 2010;160:380–385. doi: 10.1111/j.1365-2249.2009.04088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiehne K, Brunke G, Wegner F, Banasiewicz T, Folsch UR, Herzig KH. Defensin expression in chronic pouchitis in patients with ulcerative or familial adenomatous polyposis coli. World J Gastroenterol. 2006;12:1056–1062. doi: 10.3748/wjg.v12.i7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andus T, Daig R, Vogl D, Aschenbrenner E, Lock G, Hollerbach S, Köllinger M, Schölmerich J, Gross V. Imbalance of the interleukin 1 system in colonic mucosa--association with intestinal inflammation and interleukin 1 receptor antagonist [corrected] genotype 2. Gut. 1997;41:651–657. doi: 10.1136/gut.41.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aisenberg J, Legnani PE, Nilubol N, Cobrin GM, Ellozy SH, Hegazi RA, Yager J, Bodian C, Gorfine SR, Bauer JJ, Plevy SE, Sachar DB. Are pANCA, ASCA, or cytokine gene polymorphisms associated with pouchitis? Long-term follow-up in 102 ulcerative colitis patients. Am J Gastroenterol. 2004;99:432–441. doi: 10.1111/j.1572-0241.2004.04107.x. [DOI] [PubMed] [Google Scholar]
- 34.Sehgal R, Berg A, Polinski JI, Hegarty JP, Lin Z, McKenna KJ, Stewart DB, Poritz LS, Koltun WA. Genetic risk profiling and gene signature modeling to predict risk of complications after IPAA. Dis Colon Rectum. 2012;55:239–248. doi: 10.1097/DCR.0b013e31823e2d18. [DOI] [PubMed] [Google Scholar]
- 35.Lammers KM, Ouburg S, Morre SA, Crusius JB, Gionchett P, Rizzello F, Morselli C, Caramelli E, Conte R, Poggioli G, Campieri M, Peña AS. Combined carriership of TLR9-1237C and CD14-260T alleles enhances the risk of developing chronic relapsing pouchitis. World J Gastroenterol. 2005;11:7323–7329. doi: 10.3748/wjg.v11.i46.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson CA, Boucher G, Lees CW, Franke A, D’Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A, Lagacé C, Scott R, Amininejad L, Bumpstead S, Baidoo L, Baldassano RN, Barclay M, Bayless TM, Brand S, Büning C, Colombel JF, Denson LA, De Vos M, Dubinsky M, Edwards C, Ellinghaus D, Fehrmann RS, Floyd JA, Florin T, Franchimont D, Franke L, Georges M, Glas J, Glazer NL, Guthery SL, Haritunians T, Hayward NK, Hugot JP, Jobin G, Laukens D, Lawrance I, Lémann M, Levine A, Libioulle C, Louis E, McGovern DP, Milla M, Montgomery GW, Morley KI, Mowat C, Ng A, Newman W, Ophoff RA, Papi L, Palmieri O, Peyrin-Biroulet L, Panés J, Phillips A, Prescott NJ, Proctor DD, Roberts R, Russell R, Rutgeerts P, Sanderson J, Sans M, Schumm P, Seibold F, Sharma Y, Simms LA, Seielstad M, Steinhart AH, Targan SR, van den Berg LH, Vatn M, Verspaget H, Walters T, Wijmenga C, Wilson DC, Westra HJ, Xavier RJ, Zhao ZZ, Ponsioen CY, Andersen V, Torkvist L, Gazouli M, Anagnou NP, Karlsen TH, Kupcinskas L, Sventoraityte J, Mansfield JC, Kugathasan S, Silverberg MS, Halfvarson J, Rotter JI, Mathew CG, Griffiths AM, Gearry R, Ahmad T, Brant SR, Chamaillard M, Satsangi J, Cho JH, Schreiber S, Daly MJ, Barrett JC, Parkes M, Annese V, Hakonarson H, Radford-Smith G, Duerr RH, Vermeire S, Weersma RK, Rioux JD. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246–252. doi: 10.1038/ng.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon H, Trier MF, Andersen V, Harbord M. Heritability in inflammatory bowel disease: from the first twin study to genome-wide association studies. Inflamm Bowel Dis. 2015;21:1428–1434. doi: 10.1097/MIB.0000000000000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lécine P, Esmiol S, Métais JY, Nicoletti C, Nourry C, McDonald C, Nunez G, Hugot JP, Borg JP, Ollendorff V. The NOD2-RICK complex signals from the plasma membrane. J Biol Chem. 2007;282:15197–15207. doi: 10.1074/jbc.M606242200. [DOI] [PubMed] [Google Scholar]
- 39.Kabi A, McDonald C. FRMBP2 directs NOD2 to the membrane. Proc Natl Acad Sci U S A. 2012;109:21188–21189. doi: 10.1073/pnas.1219395110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barnich N, Aguirre JE, Reinecker HC, Xavier R, Podolsky DK. Membrane recruitment of NOD2 in intestinal epithelial cells is essential for nuclear factor - KappaB activation in muramyl dipeptide recognition. J Cell Biol. 2005;170:21–26. doi: 10.1083/jcb.200502153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meier CB, Hegazi RA, Aisenberg J, Legnani PE, Nilubol N, Cobrin GM, Duerr RH, Gorfine SR, Bauer JJ, Sachar DB, Plevy SE. Innate immune receptor genetic polymorphisms in pouchitis: is CARD15 a susceptibility factor? Inflamm Bowel Dis. 2005;11:965–971. doi: 10.1097/01.mib.0000186407.25694.cf. [DOI] [PubMed] [Google Scholar]
- 42.Tyler AD, Milgrom R, Stempak JM, Xu W, Brumell JH, Muise AM, Sehgal R, Cohen Z, Koltun W, Shen B, Silverberg MS. The NOD2insC polymorphism is associated with worse outcome following ileal pouch-anal anastomosis for ulcerative colitis. Gut. 2013;62:1433–1439. doi: 10.1136/gutjnl-2011-301957. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y, Dalal S, Antonopoulos D, Hubert N, Raffals LH, Dolan K, Weber C, Messer JS, Jabri B, Bendelac A, Eren AM, Rubin DT, Sogin M, Chang EB. Early transcriptomic changes in the ileal pouch provide insight into the molecular pathogenesis of pouchitis and ulcerative colitis. Inflamm Bowel Dis. 2017;23:366–378. doi: 10.1097/MIB.0000000000001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan XC, Kabakchiev B, Waldron L, Tyler AD, Tickle TL, Milgrom R, Stempak JM, Gevers D, Xavier RJ, Silverberg MS, Huttenhower C. Associations between host gene expression, the mucosal microbiome, and clinical outcome in the pelvic pouch of patients with inflammatory bowel disease. Genome Biol. 2015;16:67. doi: 10.1186/s13059-015-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mäkitalo L, Piekkala M, Ashorn M, Pakarinen M, Koivusalo A, Karikoski R, Natunen J, Saarialho-Kere U, Rintala R, Kolho KL. Matrix metalloproteinases in the restorative proctocolectomy pouch of pediatric ulcerative colitis. World J Gastroenterol. 2012;18:4028–4036. doi: 10.3748/wjg.v18.i30.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stallmach A, Chan CC, Ecker KW, Feifel G, Herbst H, Schuppan D, Zeitz M. Comparable expression of matrix metalloproteinases 1 and 2 in pouchitis and ulcerative colitis. Gut. 2000;47:415–422. doi: 10.1136/gut.47.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ulisse S, Gionchetti P, D’Alò S, Russo FP, Pesce I, Ricci G, Rizzello F, Helwig U, Cifone MG, Campieri M, De Simone C. Expression of cytokines, inducible nitric oxide synthase, and matrix metalloproteinases in pouchitis: effects of probiotic treatment. Am J Gastroenterol. 2001;96:2691–2699. doi: 10.1111/j.1572-0241.2001.04139.x. [DOI] [PubMed] [Google Scholar]
- 48.Bailey CJ, Hembry RM, Alexander A, Irving MH, Grant ME, Shuttleworth CA. Distribution of the matrix metalloproteinases stromelysin, gelatinases A and B, and collagenase in Crohn’s disease and normal intestine. J Clin Pathol. 1994;47:113–116. doi: 10.1136/jcp.47.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matthes H, Stallmach A, Matthes B, Herbst H, Schuppan D, Riecken EO. Indications for different collagen metabolism in Crohn’s disease and ulcerative colitis. Med Klin (Munich) 1993;88:185–192. [PubMed] [Google Scholar]
- 50.Günther U, Matthes H, Herbst H, Stallmach A, Riecken EO, Schuppan D. Phenotype of cells expressing matrix metalloproteinase-3 in ulcerative colitis. Ann N Y Acad Sci. 1998;859:237–240. doi: 10.1111/j.1749-6632.1998.tb11137.x. [DOI] [PubMed] [Google Scholar]
- 51.Paziewska A, Horbacka K, Goryca K, Mikula M, Jarosz D, Dabrowska M, Krokowicz P, Karon J, Ostrowski J. Transcriptional changes between uninflamed ulcerative colitis and familial adenomatous polyposis pouch mucosa can be attributed to an altered immune response. Acta Biochim Pol. 2015;62:69–75. doi: 10.18388/abp.2014_778. [DOI] [PubMed] [Google Scholar]
- 52.Ben-Shachar S, Yanai H, Baram L, Elad H, Meirovithz E, Ofer A, Brazowski E, Tulchinsky H, Pasmanik-Chor M, Dotan I. Gene expression profiles of ileal inflammatory bowel disease correlate with disease phenotype and advance understanding of its immunopathogenesis. Inflamm Bowel Dis. 2013;19:2509–2521. doi: 10.1097/01.MIB.0000437045.26036.00. [DOI] [PubMed] [Google Scholar]
- 53.Eskelinen EL. Maturation of autophagic vacuoles in mammalian cells. Autophagy. 2005;1:1–10. doi: 10.4161/auto.1.1.1270. [DOI] [PubMed] [Google Scholar]
- 54.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 55.Tanida I. Autophagosome formation and molecular mechanism of autophagy. Antioxid Redox Signal. 2011;14:2201–2214. doi: 10.1089/ars.2010.3482. [DOI] [PubMed] [Google Scholar]
- 56.Heatha RJ, Xavier RJ. Autophagy, immunity and human disease. Curr Opin Gastroenterol. 2009;25:512–520. doi: 10.1097/MOG.0b013e32833104f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cummings JR, Cooney R, Pathan S, Anderson CA, Barrett JC, Beckly J, Geremia A, Hancock L, Guo C, Ahmad T, Cardon LR, Jewell DP. Confirmation of the role of ATG16L1 as a Crohn’s disease susceptibility gene. Inflamm Bowel Dis. 2007;13:941–946. doi: 10.1002/ibd.20162. [DOI] [PubMed] [Google Scholar]
- 58.Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J, Günther S, Prescott NJ, Onnie CM, Häsler R, Sipos B, Fölsch UR, Lengauer T, Platzer M, Mathew CG, Krawczak M, Schreiber S. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 59.Parkes M, Barrett JC, Prescott NJ, Tremelling M, Anderson CA, Fisher SA, Roberts RG, Nimmo ER, Cummings FR, Soars D, Drummond H, Lees CW, Khawaja SA, Bagnall R, Burke DA, Todhunter CE, Ahmad T, Onnie CM, McArdle W, Strachan D, Bethel G, Bryan C, Lewis CM, Deloukas P, Forbes A, Sanderson J, Jewell DP, Satsangi J, Mansfield JC Wellcome Trust Case Control Consortium. Cardon L, Mathew CG. Sequence variants in the autophagy gene IRGM and multiple other replicating loci contribute to Crohn’s disease susceptibility. Nat Genet. 2007;39:830–832. doi: 10.1038/ng2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt C, Giese T, Ludwig B, Menges M, Schilling M, Meuer SC, Zeuzem S, Stallmach A. Increased cytokine transcripts in pouchitis reflect the degree of inflammation but not the underlying entity. Int J Colorectal Dis. 2006;21:419–426. doi: 10.1007/s00384-005-0024-2. [DOI] [PubMed] [Google Scholar]
- 61.Haq S, Grondin J, Banskota S, Khan WI. Autophagy: roles in intestinal mucosal homeostasis and inflammation. J Biomed Sci. 2019;26:19. doi: 10.1186/s12929-019-0512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lovegrove RE, Tilney HS, Heriot AG, von Roon AC, Athanasiou T, Church J, Fazio VW, Tekkis PP. A comparison of adverse events and functional outcomes after restorative proctocolectomy for familial adenomatous polyposis and ulcerative colitis. Dis Colon Rectum. 2006;49:1293–1306. doi: 10.1007/s10350-006-0608-0. [DOI] [PubMed] [Google Scholar]
- 63.Leal RF, Ayrizono Mde L, Milanski M, Fagundes JJ, Moraes JC, Meirelles LR, Velloso LA, Coy CS. Detection of epithelial apoptosis in pelvic ileal pouches for ulcerative colitis and familial adenomatous polyposis. J Transl Med. 2010;8:11. doi: 10.1186/1479-5876-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Paiva NM, Pascoal LB, Negreiros LMV, Portovedo M, Coope A, Ayrizono MLS, Coy CSR, Milanski M, Leal RF. Ileal pouch of ulcerative colitis and familial adenomatous polyposis patients exhibit modulation of autophagy markers. Sci Rep. 2018;8:2619. doi: 10.1038/s41598-018-20938-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coffey JC, Bennett MW, Wang JH, O’Connell J, Neary P, Shanahan F, Redmond HP, Kirwan WO. Upregulation of Fas-Fas-L (CD95/CD95L)-mediated epithelial apoptosis--a putative role in pouchitis? J Surg Res. 2001;98:27–32. doi: 10.1006/jsre.2001.6129. [DOI] [PubMed] [Google Scholar]
- 66.Simchuk EJ, Thirlby RC. Risk factors and true incidence of pouchitis in patients after ileal pouch-anal anastomoses. World J Surg. 2000;24:851–856. doi: 10.1007/s002680010136. [DOI] [PubMed] [Google Scholar]
- 67.Lohmuller JL, Pemberton JH, Dozois RR, Ilstrup D, van Heerden J. Pouchitis and extraintestinal manifestations of inflammatory bowel disease after ileal pouch-anal anastomosis. Ann Surg. 1990;211:622–627. [PMC free article] [PubMed] [Google Scholar]
- 68.Shen B, Bennett AE, Navaneethan U, Lian L, Shao Z, Kiran RP, Fazio VW, Remzi FH. Primary sclerosing cholangitis is associated with endoscopic and histologic inflammation of the distal afferent limb in patients with ileal pouch-anal anastomosis. Inflamm Bowel Dis. 2011;17:1890–1900. doi: 10.1002/ibd.21594. [DOI] [PubMed] [Google Scholar]
- 69.Wu XR, Mukewar S, Kiran RP, Hammel JP, Remzi FH, Shen B. The presence of primary sclerosing cholangitis is protective for ileal pouch from Crohn’s disease. Inflamm Bowel Dis. 2013;19:1483–1489. doi: 10.1097/MIB.0b013e318281f410. [DOI] [PubMed] [Google Scholar]
- 70.Palm O, Moum B, Jahnsen J, Gran JT. The prevalence and incidence of peripheral arthritis in patients with inflammatory bowel disease, a prospective population-based study (the IBSEN study) Rheumatology (Oxford) 2001;40:1256–1261. doi: 10.1093/rheumatology/40.11.1256. [DOI] [PubMed] [Google Scholar]
- 71.Seril DN, Yao QP, Shen B. Auto-inflammatory diseases in ileal pouch patients with NOD2/CARD15 mutations. Gastroenterol Rep (Oxf) 2016;4:73–76. doi: 10.1093/gastro/gou069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Regueiro M, Kip KE, Cheung O, Hegazi RA, Plevy S. Cigarette smoking and age at diagnosis of inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:42–47. doi: 10.1097/00054725-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 73.Ko JK, Sham NF, Guo X, Cho CH. Beneficial intervention of experimental colitis by passive cigarette smoking through the modulation of cytokines in rats. J Investig Med. 2001;49:21–29. doi: 10.2310/6650.2001.34087. [DOI] [PubMed] [Google Scholar]
- 74.Merrett MN, Mortensen N, Kettlewell M, Jewell DO. Smoking may prevent pouchitis in patients with restorative proctocolectomy for ulcerative colitis. Gut. 1996;38:362–364. doi: 10.1136/gut.38.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shen B, Fazio VW, Remzi FH, Brzezinski A, Bennett AE, Lopez R, Hammel JP, Achkar JP, Bevins CL, Lavery IC, Strong SA, Delaney CP, Liu W, Bambrick ML, Sherman KK, Lashner BA. Risk factors for diseases of ileal pouch-anal anastomosis after restorative proctocolectomy for ulcerative colitis. Clin Gastroenterol Hepatol. 2006;4:81–89. doi: 10.1016/j.cgh.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 76.Iskandar H, Greer JB, Schraut WH, Regueiro MD, Davis PL, Hartman DJ, Siegel CA, Herfarth HH, Williams ED, Schwartz MB. IBD LIVE case series-case 1: smoking, a controversial but effective treatment for ulcerative colitis. Inflamm Bowel Dis. 2014;20:1696–1701. doi: 10.1097/MIB.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 77.Calabrese E, Yanai H, Shuster D, Rubin DT, Hanauer SB. Low-dose smoking resumption in ex-smokers with refractory ulcerative colitis. J Crohns Colitis. 2012;6:756–762. doi: 10.1016/j.crohns.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 78.Fleshner P, Ippoliti A, Dubinsky M, Ognibene S, Vasiliauskas E, Chelly M, Mei L, Papadakis KA, Landers C, Targan S. A prospective multivariate analysis of clinical factors associated with pouchitis after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2007;5:952–958. doi: 10.1016/j.cgh.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 79.Ferrante M, Declerck S, De Hertogh G, Van Assche G, Geboes K, Rutgeerts P, Penninckx F, Vermeire S, D’Hoore A. Outcome after proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis. Inflamm Bowel Dis. 2008;14:20–28. doi: 10.1002/ibd.20278. [DOI] [PubMed] [Google Scholar]
- 80.Uchino M, Ikeuchi H, Matsuoka H, Bando T, Takesue Y, Tomita N. Clinical features and management of pouchitis in Japanese ulcerative colitis patients. Surg Today. 2013;43:1049–1057. doi: 10.1007/s00595-012-0377-4. [DOI] [PubMed] [Google Scholar]
- 81.Ward MA, Pierre JF, Leal RF, Huang Y, Shogan B, Dalal SR, Weber CR, Leone VA, Musch MW, An GC, Rao MC, Rubin DT, Raffals LE, Antonopoulos DA, Sogin ML, Hyman NH, Alverdy JC, Chang EB. Insights into the pathogenesis of ulcerative colitis from a murine model of stasis-induced dysbiosis, colonic metaplasia, and genetic susceptibility. Am J Physiol Gastrointest Liver Physiol. 2016;310:G973–G988. doi: 10.1152/ajpgi.00017.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen B. Bacteriology in the etiopathogenesis of pouchitis. Dig Dis. 2012;30:351–357. doi: 10.1159/000338125. [DOI] [PubMed] [Google Scholar]
- 83.Tyler AD, Knox N, Kabakchiev B, Milgrom R, Kirsch R, Cohen Z, McLeod RS, Guttman DS, Krause DO, Silverberg MS. Characterization of the gut-associated microbiome in inflammatory pouch complications following ileal pouch-anal anastomosis. PLoS One. 2013;8:e66934. doi: 10.1371/journal.pone.0066934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smith FM, Coffey JC, Kell MR, O’Sullivan M, Redmond HP, Kirwan WO. A characterization of anaerobic colonization and associated mucosal adaptations in the undiseased ileal pouch. Colorectal Dis. 2005;7:563–570. doi: 10.1111/j.1463-1318.2005.00833.x. [DOI] [PubMed] [Google Scholar]
- 85.Almeida MG, Kiss DR, Zilberstein B, Quintanilha AG, Teixeira MG, Habr-Gama A. Intestinal mucosa-associated microflora in ulcerative colitis patients before and after restorative proctocolectomy with an ileoanal pouch. Dis Colon Rectum. 2008;51:1113–1119. doi: 10.1007/s10350-008-9347-8. [DOI] [PubMed] [Google Scholar]
- 86.Duncan SH, Barcenilla A, Stewart CS, Pryde SE, Flint HJ. Acetate utilization and butyryl coenzyme A [CoA]: acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microbiol. 2002;68:5186–5190. doi: 10.1128/AEM.68.10.5186-5190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maharshak N, Cohen NA, Reshef L, Tulchinsky H, Gophna U, Dotan I. Alterations of enteric microbiota in patients with a normal ileal pouch are predictive of pouchitis. J Crohns Colitis. 2017;11:314–320. doi: 10.1093/ecco-jcc/jjw157. [DOI] [PubMed] [Google Scholar]
- 88.Kohyama A, Ogawa H, Funayama Y, Takahashi K, Benno Y, Nagasawa K, Tomita S, Sasaki I, Fukushima K. Bacterial population moves toward a colon-like community in the pouch after total proctocolectomy. Surgery. 2009;145:435–447. doi: 10.1016/j.surg.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 89.Hinata M, Kohyama A, Ogawa H, Haneda S, Watanabe K, Suzuki H, Shibata C, Funayama Y, Takahashi K, Sasaki I, Fukushima K. A shift from colon- to ileum-predominant bacteria in ileal-pouch feces following total proctocolectomy. Dig Dis Sci. 2012;57:2965–2974. doi: 10.1007/s10620-012-2165-9. [DOI] [PubMed] [Google Scholar]
- 90.Young VB, Raffals LH, Huse SM, Vital M, Dai D, Schloss PD, Brulc JM, Antonopoulos DA, Arrieta RL, Kwon JH, Reddy KG, Hubert NA, Grim SL, Vineis JH, Dalal S, Morrison HG, Eren AM, Meyer F, Schmidt TM, Tiedje JM, Chang EB, Sogin ML. Multiphasic analysis of the temporal development of the distal gut microbiota in patients following ileal pouch anal anastomosis. Microbiome. 2013;1:9. doi: 10.1186/2049-2618-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu F, Feng DY, Ding C, Zhang TH, Chen JW, Yu ZQ, Zhao L, Xu Y, Zhu WM, Gong JF. Fungal dysbiosis aggravates pouchitis in a rat model of ileal pouch anal anastomosis. Inflamm Bowel Dis. 2020;26:1831–1842. doi: 10.1093/ibd/izaa111. [DOI] [PubMed] [Google Scholar]
- 92.Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 93.McLaughlin SD, Walker AW, Churcher C, Clark SK, Tekkis PP, Johnson MW, Parkhill J, Ciclitira PJ, Dougan G, Nicholls RJ, Petrovska L. The bacteriology of pouchitis: a molecular phylogenetic analysis using 16S rRNA gene cloning and sequencing. Ann Surg. 2010;252:90–98. doi: 10.1097/SLA.0b013e3181e3dc8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reshef L, Kovacs A, Ofer A, Yahav L, Maharshak N, Keren N, Konikoff FM, Tulchinsky H, Gophna U, Dotan I. Pouch inflammation is associated with a decrease in specific bacterial taxa. Gastroenterology. 2015;149:718–727. doi: 10.1053/j.gastro.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 95.Ze XL, Duncan SH, Louis P, Flint HJ. Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. ISME J. 2012;6:1535–1543. doi: 10.1038/ismej.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 97.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Preter V, Bulteel V, Suenaert P, Geboes KP, De Hertogh G, Luypaerts A, Geboes K, Verbeke K, Rutgeerts P. Pouchitis, similar to active ulcerative colitis, is associated with impaired butyrate oxidation by intestinal mucosa. Inflamm Bowel Dis. 2009;15:335–340. doi: 10.1002/ibd.20768. [DOI] [PubMed] [Google Scholar]
- 99.Clausen MR, Tvede M, Mortensen PB. Short-chain fatty acids in pouch contents from patients with and without pouchitis after ileal pouch-anal anastomosis. Gastroenterology. 1992;103:1144–1153. doi: 10.1016/0016-5085(92)91497-r. [DOI] [PubMed] [Google Scholar]
- 100.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 102.Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 104.Chapman MA, Hutton M, Grahn MF, Williams NS. Metabolic adaptation of terminal ileal mucosa after construction of an ileoanal pouch. Br J Surg. 1997;84:71–73. [PubMed] [Google Scholar]
- 105.McLellan SL, Newton RJ, Vandewalle JL, Shanks OC, Huse SM, Eren AM, Sogin ML. Sewage reflects the distribution of human faecal Lachnospiraceae. Environ Microbiol. 2013;15:2213–2227. doi: 10.1111/1462-2920.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gibson GR, Cummings JH, Macfarlane GT. Growth and activities of sulphate - reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiology Ecology. 2016;86:103–111. [Google Scholar]
- 107.Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM, Thomas LV, Zoetendal EG, Hart A. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zoetendal EG, Raes J, van den Bogert B, Arumugam M, Booijink CC, Troost FJ, Bork P, Wels M, de Vos WM, Kleerebezem M. The human small intestinal microbiota is driven by rapid uptake and conversion of simple carbohydrates. ISME J. 2012;6:1415–1426. doi: 10.1038/ismej.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bambury N, Coffey JC, Burke J, Redmond HP, Kirwan WO. Sulphomucin expression in ileal pouches: emerging differences between ulcerative colitis and familial adenomatous polyposis pouches. Dis Colon Rectum. 2008;51:561–567. doi: 10.1007/s10350-008-9200-0. [DOI] [PubMed] [Google Scholar]
- 110.de Silva HJ, Millard PR, Kettlewell M, Mortensen NJ, Prince C, Jewell DP. Mucosal characteristics of pelvic ileal pouches. Gut. 1991;32:61–65. doi: 10.1136/gut.32.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shepherd NA, Jass JR, Duval I, Moskowitz RL, Nicholls RJ, Morson BC. Restorative proctocolectomy with ileal reservoir: pathological and histochemical study of mucosal biopsy specimens. J Clin Pathol. 1987;40:601–607. doi: 10.1136/jcp.40.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Booijink CC, Zoetendal EG, Kleerebezem M, de Vos WM. Microbial communities in the human small intestine: coupling diversity to metagenomics. Future Microbiol. 2007;2:285–295. doi: 10.2217/17460913.2.3.285. [DOI] [PubMed] [Google Scholar]
- 113.Younggeon J, Blikslager AT. Intestinal ischemia-reperfusion: rooting for the SOCS? Dig Dis Sci. 2017;62:4–6. doi: 10.1007/s10620-016-4328-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prideaux L, Cruz P, Ng SC, Kamm MA. Serological antibodies in inflammatory bowel disease: a systematic review. Inflamm Bowel Dis. 2012;18:1340–1355. doi: 10.1002/ibd.21903. [DOI] [PubMed] [Google Scholar]
- 115.Fleshner PR, Vasiliauskas EA, Kam LY, Fleshner NE, Gaiennie J, Abreu-Martin MT, Targan SR. High level perinuclear antineutrophil cytoplasmic antibody (pANCA) in ulcerative colitis patients before colectomy predicts the development of chronic pouchitis after ileal pouch-anal anastomosis. Gut. 2001;49:671–677. doi: 10.1136/gut.49.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yasuda N, Thomas P, Ellis H, Herbst F, Nicholls J, Ciclitira P. Perinuclear anti-neutrophil cytoplasmic antibodies in ulcerative colitis after restorative proctocolectomy do not correlate with the presence of pouchitis. Scand J Gastroenterol. 1998;33:509–513. doi: 10.1080/00365529850172089. [DOI] [PubMed] [Google Scholar]
- 117.Reumaux D, Colombel JF, Masy E, Duclos B, Heresbach D, Belaïche J, Cortot A, Duthilleul P GETAID. Groupe d’Etude des Affections Inflammatoires du Tube Digestif. Anti-neutrophil cytoplasmic auto-antibodies (ANCA) in ulcerative colitis (UC): no relationship with disease activity. Inflamm Bowel Dis. 2000;6:270–274. doi: 10.1002/ibd.3780060403. [DOI] [PubMed] [Google Scholar]
- 118.Dalal RL, Shen B, Schartz DA. Management of pouchitis and other commom complication of the pouch. Inflamm Bowel Dis. 2018;24:989–996. doi: 10.1093/ibd/izy020. [DOI] [PubMed] [Google Scholar]
- 119.Lightner AL, Pemberton JH, Loftus EJ Jr. Crohn’s diseas of the ileoanal pouch. Inflamm Bowel Dis. 2016;22:1502–1508. doi: 10.1097/MIB.0000000000000712. [DOI] [PubMed] [Google Scholar]
- 120.Imhann F, Bonder MJ, Vila AV, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, Dijkstra G, Franke L, Xavier RJ, Jonkers D, Wijmenga C, Weersma RK, Zhernakova A. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gosselink MP, Schouten WR, van Lieshout LM, Hop WC, Laman JD, Ruseler-van Embden JG. Eradication of pathogenic bacteria and restoration of normal pouch flora: comparison of metronidazole and ciprofloxacin in the treatment of pouchitis. Dis Colon Rectum. 2004;47:1519–1525. doi: 10.1007/s10350-004-0623-y. [DOI] [PubMed] [Google Scholar]
- 122.Dubinsky V, Reshef L, Bar N, Keizer D, Golan N, Rabinowitz K, Godny L, Yadgar K, Zonensain K, Tulchinsky H, Gophna U, Dotan I. Predominantly antibiotic-resistant intestinal microbiome persists in patients with pouchitis who respond to antibiotic therapy. Gastroenterology. 2020;158:610–624. e13. doi: 10.1053/j.gastro.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 123.Sagar PM, Taylor BA, Godwin P, Holdsworth PJ, Johnston D, Lewis W, Miller A, Quirke P, Williamson M. Acute pouchitis and deficiencies of fuel. Dis Colon Rectum. 1995;38:488–493. doi: 10.1007/BF02148848. [DOI] [PubMed] [Google Scholar]
- 124.Ghouri YA, Richards DM, Rahimi EF, Krill JT, Jelinek KA, DuPont AW. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin Exp Gastroenterol. 2014;7:473–487. doi: 10.2147/CEG.S27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Welters CF, Heineman E, Thunnissen FB, van den Bogaard AE, Soeters PB, Baeten CG. Effect of dietary inulin supplementation on inflammation of pouch mucosa in patients with an ileal pouch-anal anastomosis. Dis Colon Rectum. 2002;45:621–627. doi: 10.1007/s10350-004-6257-2. [DOI] [PubMed] [Google Scholar]
- 126.Meijer HP, Welters CF, Heineman E, Salomons GS, Büller HA, Dekker J, Einerhand AW. Enteral inulin does not affect epithelial gene expression and cell turnover within the ileoanal pouch. Dis Colon Rectum. 2000;43:1427–1434. doi: 10.1007/BF02236640. [DOI] [PubMed] [Google Scholar]
- 127.Claytor JD, El-Nachef N. Fecal microbial transplant for inflammatory bowel disease. Curr Opin Clin Nutr Metab Care. 2020;23:355–360. doi: 10.1097/MCO.0000000000000676. [DOI] [PubMed] [Google Scholar]
- 128.Herfarth HH, Long MD, Isaacs KL. Use of biologics in pouchitis: a systematic review. J Clin Gastroenterol. 2015;49:647–654. doi: 10.1097/MCG.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kitajima T, Okita Y, Kawamura M, Kondo S, Toiyama Y, Uchida K, Kusunoki M. The relationship between preoperative T helper cytokines in the ileal mucosa and the pathogenesis of pouchitis. BMC Gastroentero. 2020;20:277. doi: 10.1186/s12876-020-01421-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de Krijger M, Wildenberg ME, Mookhoek A, Verheul S, de Jonge WJ, Ponsioen CY. Expression of MAdCAM-1 and Gut-homing T cells in inflamed pouch mucosa. J Crohns Colitis. 2021;15:1491–1499. doi: 10.1093/ecco-jcc/jjab041. [DOI] [PubMed] [Google Scholar]
- 131.Hata K, Okada S, Shinagawa T, Toshiaki T, Kawai K, Nozawa H. Meta-analysis of the association of extraintestinal manifestations with the development of pouchitis in patients with ulcerative colitis. BJS Open. 2019;3:436–444. doi: 10.1002/bjs5.50149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gorrepati VS, Stuart A, Deiling S, Koltun W, Tinsley A, Williams ED, Coates MD. Smoking and the risk of pouchitis in ulcerative colitis patients with ileal pouch-anal anastomosis. Inflamm Bowel Dis. 2018;24:2027–2032. doi: 10.1093/ibd/izy097. [DOI] [PubMed] [Google Scholar]
- 133.Singh S, Sharma PK, Loftus EV Jr, Pardi DS. Meta-analysis: serological markers and the risk of acute and chronic pouchitis. Aliment Pharmacol Ther. 2013;37:867–875. doi: 10.1111/apt.12274. [DOI] [PubMed] [Google Scholar]
- 134.Jiang W, Goldblum JR, Lopez R, Lian L, Shen B. Increased crypt apoptosis is a feature of autoimmune-associated chronic antibiotic refractory pouchitis. Dis Colon Rectum. 2012;55:549–557. doi: 10.1097/DCR.0b013e31824ab7c6. [DOI] [PubMed] [Google Scholar]


