Abstract
Background:
In higher eukaryotes, cell-cycle transitions are regulated by different cyclin-dependent kinases (Cdks) and Cdk inhibitors (CKIs). CKIs include two groups, the Ink4 (p16INK4a, p15INK4b, p18INK4c, and p19INK4d) and the Cip/Kip (p21Cip1, p27Kip1, and p57Kip2) families. The hyperactivity of histone deacetylases (HDACs) is associated with cancer induction. Histone deacetylase inhibitors (HDACIs) such as sodium butyrate (NaBT) can inhibit HDAC activity resulting in apoptosis induction. The present study was designed to investigate the effect of sodium butyrate on p16INK4a, p14ARF, p15INK4b, class I HDACs (HDACs 1, 2, 3), and class II HDACs (HDACs 4, 5, 6), cell growth inhibition, and apoptosis induction in pancreatic cancer AsPC-1 and colon cancer HCT-116 cell lines. In fact, we want to know whether sodium butyrate can reactivate Ink4 and Cip/Kip families by HDACs inhibition.
Materials and Methods:
The AsPC-1 and HCT-116 cells were treated with sodium butyrate at different periods. Then, the MTT assay, cell apoptosis assay, and qRT-PCR were done to determine viability, apoptosis, and the relative expression level of the genes respectively.
Results:
The sodium butyrate increased p16INK4a, p14ARF, and p15INK4b and decreased class I and II HDACs significantly. Besides, HCT-116 cell was more sensitive to sodium butyrate in comparison to AsPC-1 cell. Conclusion: The sodium butyrate can reactivate the p16INK4a, p14ARF, and p15INK4b through inhibition of HDACs in AsPC-1 and HCT-116 cell lines.
Key Words: Sodium butyrate, tumor suppressor genes, cancer
Introduction
In higher eukaryotes, cell-cycle transitions are regulated by different cyclin-dependent kinases (Cdks), the catalytic subunits of a family of mammalian heterodimeric serine/threonine kinases, and their activating cyclin subunits and Cdk inhibitors (CKIs) (Hochegger et al., 2008). Cyclins are accumulated gradually during interphase and are abruptly destroyed during mitosis. Based on the timing of expression in the cell cycle, cyclins are divided into four classes three of which are the G1/S cyclins, S cyclins, and M cyclins, these three groups are directly involved in the cell cycle control. The fourth class, the G1 cyclins, controls the entry into the cell cycle in response to extracellular growth factors or mitogens (Yang et al., 2018). Cdks act as the engine of the cell cycle that drives cell cycle progression whereas cyclins play as the gears. Whereas, CKIs serve as brakes to halt cell cycle progression under abnormal conditions. CKIs include two groups based on their structure and Cdk specificity, the Ink4 and the Cip/Kip families. The Ink4 family members including p16INK4a, p15INK4b, p18INK4c, and p19INK4d which primarily target Cdk4 and Cdk6. Conversely, the Cip/Kip family members including p21Cip1, p27Kip1, and p57Kip2 which interfere with the activities of cyclin D-, E-, A- and B-dependent kinase complexes (Lim et al., 2013). Cellular neoplastic transformation is accompanied with aberrant expression of CDKs and/or cyclins and also the negative regulators, the CDKIs. Of all epigenetic modifications, DNA hypermethylation, which represses tumor suppressor genes (TSGs) such as the Ink4 and the Cip/Kip families leading to gene silencing, has been recognized as a cause of oncogenesis. DNA methylation is achieved by a group of enzymes known as the DNA methyltransferases (DNMTs) which are classified into DNMT1, DNMT1b, DNMT1o, DNMT1p, DNMT2, DNMT3A, DNMT3b with its isoforms, and DNMT3L (Sanaei et al., 2020). In addition to DNA methylation, histone deacetylation is associated with transcriptional repression and tumorigenesis. It has been reported that histone acetyltransferases (HATs) and histone deacetylases (HDACs) are responsible for the acetylation status of histones. Therefore, histone acetylation and deacetylation are dynamic processes induced by HATs and HDACs respectively. The hyperactivity of HDACs is associated with cancer induction (Fortson et al., 2011). Based on sequence homology with yeast proteins, the human HDAC family comprises 18 grouped into four classes: class I (HDAC 1-2-3-8), class II HDACs which can be further divided into two classes: IIa (HDAC 4-5-7-9) and IIb (HDAC 6 and 10), class III HDACs are also termed sirtuins (SIRT1–SIRT7), and class IV contains a single HDAC (HDAC11) with a catalytic domain shared with classes I/II HDACs (Ceccacci et al., 2016). One strategy to inhibit malignant cellular viability and proliferation involves enhancing the function of the CDKI through inhibition of HDACs activity. Histone deacetylase inhibitors (HDACIs) can inhibit HDACs activity. Based on their chemical structure, they can be subdivided into four different classes, including cyclic peptides (e.g. FK-228), hydroxamates (e.g. trichostatin A, TSA), benzamides (e.g. MS-275), and aliphatic acids (e.g. Valproic acid) (Tan et al., 2010). Various in vitro studies have indicated that TSA has a significant effect on pancreatic cancer MiaPaCa-2 and PANC-1 (Cai et al., 2018), SW-1990, BxPC-3, PANC-1, MIA PaCa-2, JHP-1 cell lines (Kitazono et al., 2010). Previously, we reported the effect of histone deacetylase inhibitors trichostatin A on hepatocellular carcinoma (HCC) (Sanaei et al., 2017; Sanaei et al., 2018; Kavoosi et al., 2018), valproic acid on HCC (Sanaei et al., 2018), and also valproic acid on colon cancer (Sanaei et al., 2018). The present study was designed to investigate the effect of sodium butyrate on p16INK4a, p14ARF, p15INK4b, and class I histone deacetylases 1, 2, and 3 genes expression and class II HDACs (HDACs 4, 5, 6), cell growth inhibition, and apoptosis induction in pancreatic cancer AsPC-1 and colon cancer HCT-116 cell lines.
Materials and Methods
Materials
Human pancreatic cancer AsPC-1 and colon cancer HCT-116 cell lines were provided from the National Cell Bank of Iran-Pasteur Institute. Dulbecco’s modified Eagle’s medium (DMEM) and sodium butyrate were obtained from Sigma (St. Louis, MO, USA). Both compounds were dissolved in dimethyl sulfoxide (DMSO) to make a master stock solution. Further concentration was obtained by diluting the provided solution. Several compounds including materials and kits were purchased as provided for previous works (Sanaei et al., 2017; Kavoosi et al., 2018). All other compounds including fetal bovine serum (FBS) (product number f2442), penicillin (CAS number 69 57 8), and streptomycin (CAS number 3810 74 0) obtained from sigma too. Total RNA extraction kit (TRIZOL reagent) and real-time polymerase chain reaction (PCR) kits (qPCR MasterMix Plus for SYBR Green I dNTP) were obtained from Applied Biosystems Inc. (Foster, CA, USA). The cells were maintained in DMEM supplemented with fetal bovine serum 10% and antibiotics in a humidified atmosphere of 5% CO2 in air at 37oC. This is a lab-trial study was approved by the Ethics Committee of Jahrom University of Medical science with a code number of IR.JUMS.REC. 1399.010.
Cell culture and cell viability
Human pancreatic cancer AsPC-1 and colon cancer HCT-116 cell lines were cultured in DMEM supplemented with 10% FBS and antibiotics at 37°C in 5% CO2 overnight. After 24 h of culture, the cells were seeded into 96-well plates (3 × 105 cells per well). After one day, the growth medium was changed with the experimental medium containing various concentrations of sodium butyrate (0, 1, 5, 10, 25, and 50 μM). The control groups received DMSO only, at a concentration of 0.05 %. After 24 and 48h of treatment, the cells were evaluated by MTT assay according to Standard protocols to determine cell viability. In this regard, the MTT solution was added to each well for 4 h at 37oC, the MTT solution was replaced by DMSO and shaken for 10 min to dissolve all of the crystals. Finally, the optical density was detected by a microplate reader at a wavelength of 570 nM. Each experiment was repeated three times (triplicates).
Cell apoptosis assay
To determine apoptotic cells, the pancreatic cancer AsPC-1 and colon cancer HCT-116 cells were cultured at a density of 3 × 105 cells/well and incubated overnight, and then the cells were treated with sodium butyrate (10 μM) for 24 and 48 h. Subsequently, the cells were harvested by trypsinization, washed with cold PBS, and resuspended in Binding buffer (1x). Finally, Annexin-V-(FITC) and PI were used according to the protocol to determine the apoptotic cells by FACScan flow cytometry (Becton Dickinson, Heidelberg, Germany).
Real-time Quantitative Reverse Transcription Polymerase
Chain Reaction (qRT-PCR)
To determine the relative expression level of p16INK4a, p14ARF, p15INK4b, class I histone deacetylases 1, 2, and 3 genes expression and class II HDACs (HDACs 4, 5, 6) genes, qRT-PCR was done. The pancreatic cancer AsPC-1 and colon cancer HCT-116 cells were treated with sodium butyrate (10 μM) for different periods (24 and 48 h). After treatment times, qRT-PCR was done as in our previous works (Sanaei et al., 2020). The primer sequences of the genes are indicated in table 1 (Sakuma. Et al., 2004; Li et al., 2012; Saegusa et al., 2001; He et al., 2013)
Results
Result of cell viability by the MTT assay
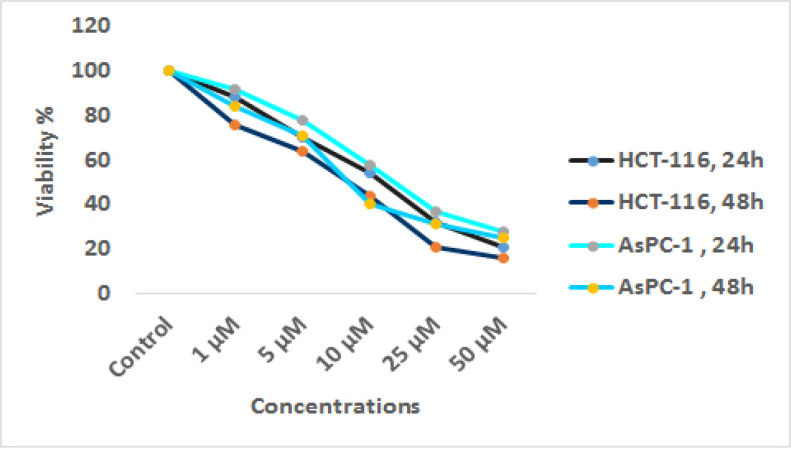
The viability of pancreatic cancer AsPC-1 and colon cancer HCT-116 cells treated with sodium butyrate (0, 1, 5, 10, 25, and 50 μM) was investigated by MTT assay. In this technique, the activity of cellular enzymes produced a dark-blue formazan which is dissolvable in DMSO by which the number of viable AsPC-1 and HCT-116 cells can be demonstrated. As shown in Figure 1, sodium butyrate induced significant cell growth inhibition with a dose- and time-dependent manner (P< 0.004), figure 1. The IC50 value of sodium butyrate was obtained with approximately 10 μM.
Figure1.
In vitro Effects of Sodium Butyrate (0, 1, 5, 10, 25, and 50 μM) on AsPC-1 and HCT-116 Cell Lines Determined by MTT Assay at 24 and 48 h
Result of cell apoptosis assay
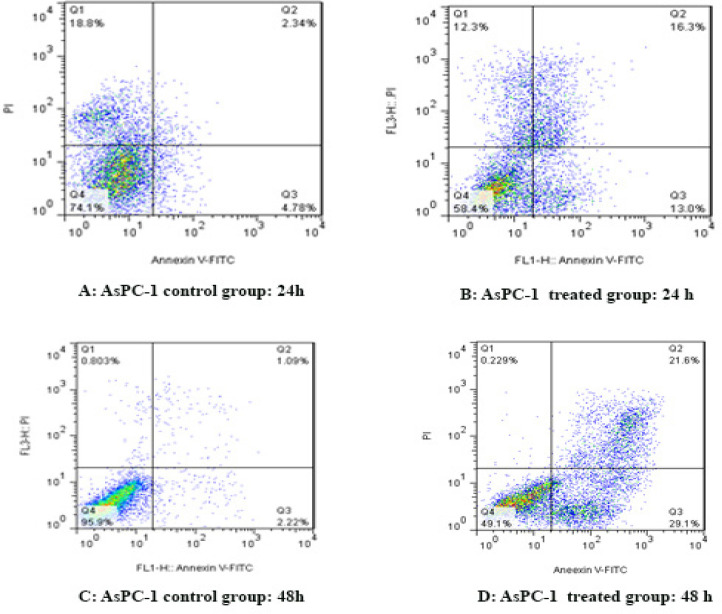
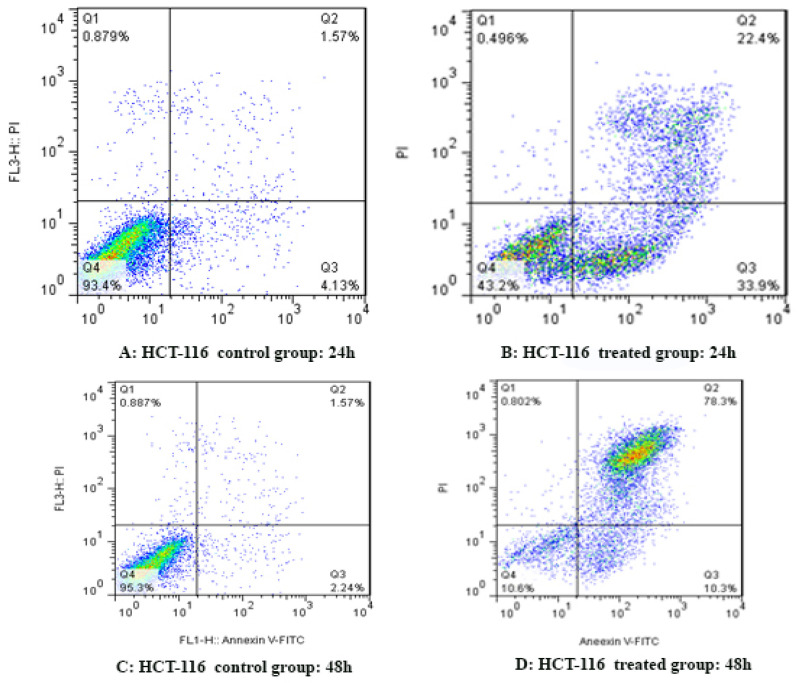
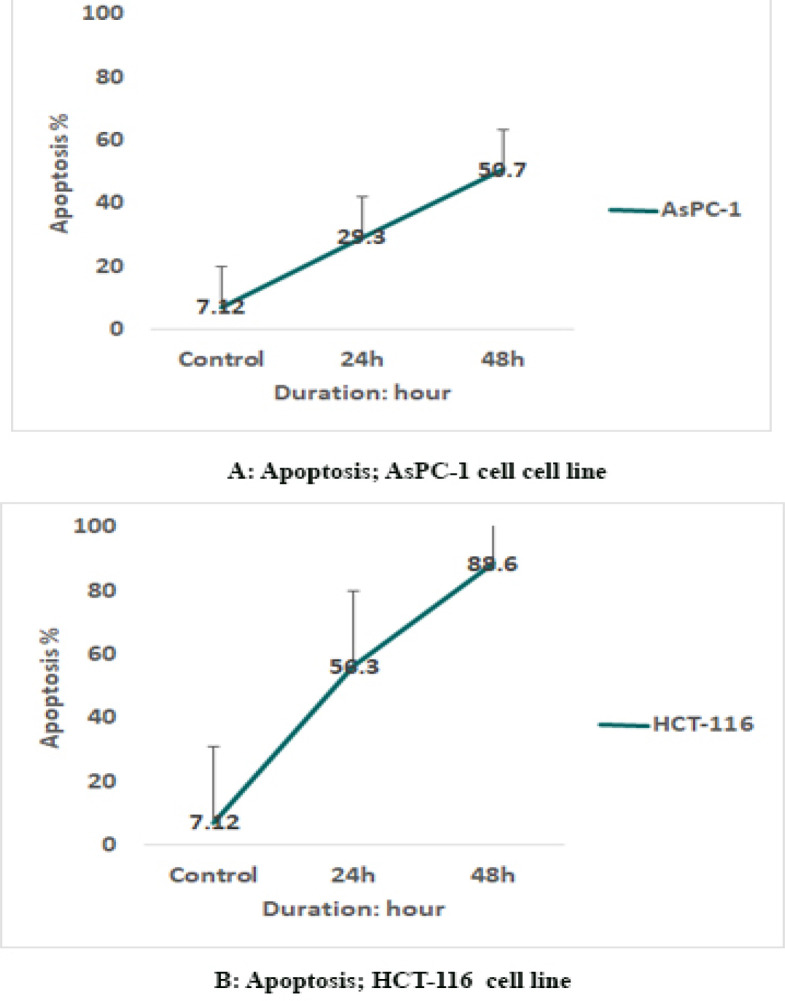
To determine AsPC-1 and HCT-116 cells apoptosis, the cells were treated with sodium butyrate (10 μM) for 24 and 48 h and then stained using annexin-V-(FITC) and PI to determine apoptotic cells in the early and late apoptosis stage. As indicated in Figures 2 and 3, sodium butyrate induced cell apoptosis significantly in a time-dependent manner (P<0.001). The HCT-116 cells were more sensitive to sodium butyrate in comparison to AsPC-1 cells. Maximum apoptosis was seen in HCT-116 cell group after 48 h (Figure 4).
Figure 2.
The Apoptotic Effect of Sodium Butyrate (10 μM) on AsPC-1 Cell versus Control Groups at Different Periods (24 and 48h). The cells were treated with sodium butyrate for 24 and 48h and then the apoptotic effect of sodium butyrate was evaluated by flow cytometric analysis. Results were obtained from three independent experiments and were expressed as mean ± standard error of the mean
Figure 3.
The Apoptotic Effect of Sodium Butyrate (10 μM) on HCT-116 Cells versus Control Groups at Different Periods (24 and 48h). The cells were treated with sodium butyrate for 24 and 48h and then the apoptotic effect was evaluated by flow cytometric analysis. Results were obtained from three independent experiments and were expressed as mean ± standard error of the mean
Figure 4.
Comparative Apoptotic Graph
Result of determination of genes expression
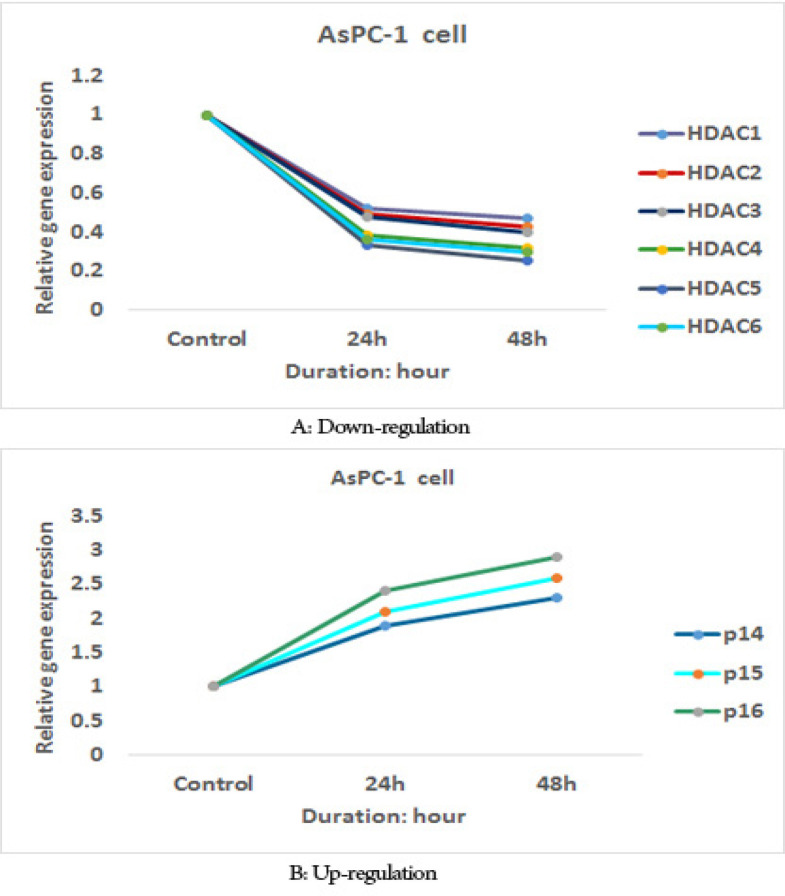
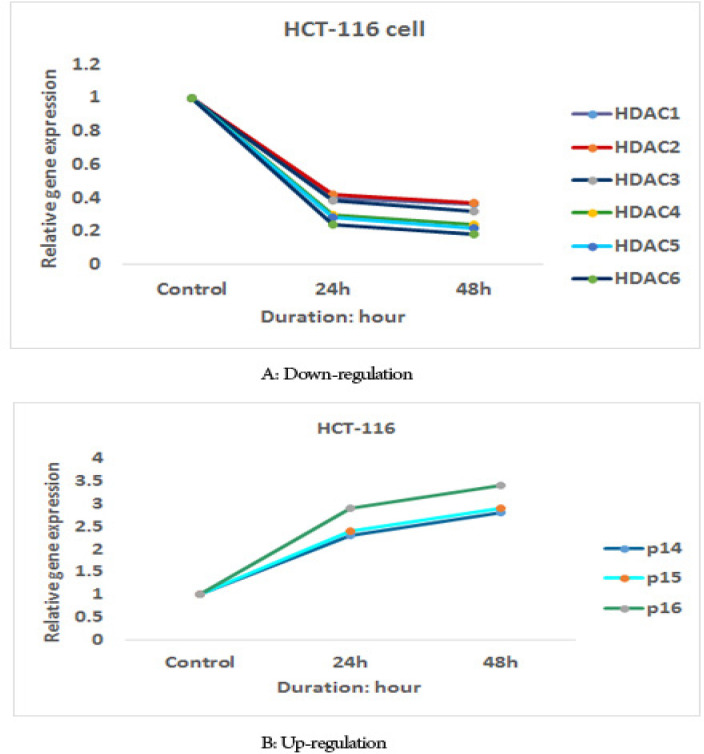
The effect of sodium butyrate (10 μM) on p16INK4a, p14ARF, p15INK4b, class I histone deacetylases 1, 2, and 3 gene expression, and class II HDACs (HDACs 4, 5, 6) genes expression was evaluated by quantitative real-time RT-PCR analysis. The result indicated that treatment with sodium butyrate (10 μM) up-regulated p16INK4a, p14ARF, p15INK4b, and down-regulated class I histone deacetylases 1, 2, and 3 genes expression and class II HDACs (HDACs 4, 5, 6) genes expression significantly in both cell lines, Figures 5 and 6. The sodium butyrate had a significant time-dependent manner (P<0.001).
Figure 5.
The Relative Expression Level of p16INK4a, p14ARF, p15INK4b, Class I Histone Deacetylases 1, 2, and 3 Genes Expression and Class II HDACs (HDACs 4, 5, 6) Genes Expression in the AsPC-1 Cell Line Treated with Sodium Butyrate (10 μM) versus Untreated Control Groups at Different Periods (24 and 48h).
Figure 6.
The Relative Expression Level of p16INK4a, p14ARF, p15INK4b, Class I Histone Deacetylases 1, 2, and 3 Genes Expression and Class II HDACs (HDACs 4, 5, 6) Genes Expression in the HCT-116 Cell Line Treated with Sodium Butyrate (10 μM) versus Untreated Control Groups at Different Periods (24 and 48h).
Discussion
Histone deacetylases catalyze the removal of acetate from modified lysine residues. The activity of these enzymes can induce cancer by deacetylation of TSGs, such as p16INK4a, p14ARF, and p15INK4b, resulting in silenced genes and tumorigenesis. Fortunately, reversible acetylation occurs on specific lysines by histone deacetylase inhibitors such as sodium butyrate leads to reactivation of silenced TSGs and apoptosis induction (Sanaei et al., 2021). In this study, we reported that sodium butyrate down-regulated class I histone deacetylases 1, 2, and 3 gene expression and class II HDACs (HDACs 4, 5, 6) genes, up-regulated p16INK4a, p14ARF, and p15INK4b, inhibited cell growth and induced apoptosis in pancreatic cancer AsPC-1 and colon cancer HCT-116 cell lines. Similarly, it has been shown that sodium butyrate induces cell growth arrest and apoptosis in human HCC cell lines HCC-M and HCC-T (Roberts et al., 2005), and pancreatic ductal adenocarcinoma (PDAC) cell lines IMIM-PC1, IMIM-PC2, and RWP-1 Schneider et al., (2010). As we reported in this study, other researchers have demonstrated that HDACIs activate a member of the INK4 family causing G1 phase arrest in the Jurkat human T cell leukemia cells (Yokota et al., 2004). In vitro studies have demonstrated that HDACIs trichostatin A (TSA) and sodium butyrate activate the p15INK4b gene in human immortalized keratinocyte HaCaT cells and human colorectal carcinoma cell line HCT116 (Hitomi et al., 2003). On the other hand, the analysis of p16INK4a and p19ARF mRNA levels has demonstrated that sodium butyrate and TSA modify the levels of these mRNAs in primary fibroblasts (Matheu et al., 2005). Experimental studies have indicated that sodium butyrate increases p14ARF expression in human lung carcinoma cell line H460, colon carcinoma cell line HCT116, and breast cancer cell line MCF7 (Joseph et al., 2005). In mice, it has been shown that depsipeptide (FK228), a histone deacetylase (HDA) inhibitor, induces the expression of p16INK4a and up-regulated the expression of p21WAF1/Cip1 (Nishida et al., 2004). We observed that butyrate up-regulated p16INK4a, p14ARF, and p15INK4b genes expression. This result encouraged us to evaluate the further molecular mechanism of this agent. Therefore, we investigated the effect of sodium butyrate on class I histone deacetylases (HDAC 1, 2, and 3) and class II HDACs (HDACs 4, 5, 6) gene expression. Our finding demonstrated that sodium butyrate down-regulated class I histone deacetylases (HDAC 1, 2, and 3) and class II HDACs (HDACs 4, 5, 6) gene expression significantly. In line with our result, it has been shown that sodium butyrate inhibits most HDACs, except class III HDAC in pancreatic cancer cell lines (BxPC-3, AsPC-1, MiaPaCa-2, and Panc-1) (Koutsounas et al., 2013).
Our results are consistent with previously published ones reporting that sodium butyrate inhibits HDAC1, HDAC2, and HDAC3 expression in HCT116 and Caco-2 colon cancer cells (Wilson et al., 2006). In addition to the molecular mechanisms mentioned by our groups, other investigators have reported several pathways for sodium butyrate in various cancers. They indicated that this agent decreases anti-apoptosis gene Bcl-xl and Bcl-2 and increases pro-apoptosis gene Bax and Bak in prostate cancer DU145 and PC3 cell lines (Mu et al., 2013). In colon cancer, this compound can increase p21waf1/cip1 expression, block the activity of cdk-cyclin complexes, and cause cell cycle arrest (Coradini et al., 2000). Further, it increases Fas and Fas ligand levels by which induces apoptosis in breast cancer MCF-7, MCF-7ras, T47-D, and BT-20 cells, as well as an arrest in G2/M in MDA-MB-231 cell lines (Chopin et al., 2002). According to our results, sodium butyrate can upregulate p16INK4a, p14ARF, p15INK4b gene expression by inhibition of HDACs activity resulting in apoptosis induction.
In conclusion, our results indicated that sodium butyrate can reactivate tumor suppressor gene p16INK4a, p14ARF, and p15INK4b through down-regulation of class I histone deacetylases 1, 2, and 3 genes expression and class II HDACs (HDACs 4, 5, 6) gene expression in pancreatic cancer AsPC-1 and colon cancer HCT-116 cell lines. The colon cancer HCT-116 cell is more sensitive to sodium butyrate in comparison to the pancreatic cancer AsPC-1 cell. We did not evaluate the protein level of the genes of Ink4 and Cip/Kip families in this study because of budget limitations. Therefore, this evaluation is recommended.
Author Contribution Statement
The authors confirm contribution to the paper as follows: study conception and design: FK, MS; data collection: MS; analysis and interpretation of results: FK, MS; draft manuscript preparation: FK, MS. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
This article was supported by the adjutancy of research of Jahrom University of Medical Sciences, Iran. It was approved by the Ethics Committee of Jahrom University of Medical science with a code number of IR.JUMS.REC. 1399.010. The authors report no conflict of interest. All authors generated the ideas and contributed to the writing of the manuscript, acquisition of data, analysis and interpretation of data, and revised the article.
Conflict of interest
The authors report no conflict of interest.
References
- Joseph J, Wajapeyee N, Somasundaram K. Role of p53 status in chemosensitivity determination of cancer cells against histone deacetylase inhibitor sodium butyrate. Int J Cancer. 2005;115:11–8. doi: 10.1002/ijc.20842. [DOI] [PubMed] [Google Scholar]
- Cai M-H, Xu X-G, Yan S-L, et al. Depletion of HDAC1, 7 and 8 by histone deacetylase inhibition confers elimination of pancreatic cancer stem cells in combination with gemcitabine. Sci Rep. 2018;8:1–11. doi: 10.1038/s41598-018-20004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccacci E, Minucci S. Inhibition of histone deacetylases in cancer therapy: lessons from leukaemia. Br J Cancer. 2016;114:605–11. doi: 10.1038/bjc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin V, Toillon RA, Jouy N, Bourhis XL. Sodium butyrate induces P53-independent, Fas-mediated apoptosis in MCF-7 human breast cancer cells. Br J Pharmacol. 2002;135:79–86. doi: 10.1038/sj.bjp.0704456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coradini D, Pellizzaro C, Marimpietri D, Abolafio G, Daidone M. Sodium butyrate modulates cell cycle-related proteins in HT29 human colonic adenocarcinoma cells. Cell Prolif. 2000;33:139–46. doi: 10.1046/j.1365-2184.2000.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortson WS, Kayarthodi S, Fujimura Y, et al. Histone deacetylase inhibitors, valproic acid and trichostatin-A induce apoptosis and affect acetylation status of p53 in ERG-positive prostate cancer cells. Int J Oncol. 2011;39:111–9. doi: 10.3892/ijo.2011.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Zhang B, Wei X, Wang Z, et al. HDAC 4/5-HMGB 1 signalling mediated by NADPH oxidase activity contributes to cerebral ischaemia/reperfusion injury. J Cell Mol Med. 2013;17:531–42. doi: 10.1111/jcmm.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi T, Matsuzaki Y, Yokota T, Takaoka Y, Sakai T. p15INK4b in HDAC inhibitor-induced growth arrest. FEBS Lett. 2003;554:347–50. doi: 10.1016/s0014-5793(03)01186-4. [DOI] [PubMed] [Google Scholar]
- Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat Rev Mol Cell Biol. 2008;9:910–6. doi: 10.1038/nrm2510. [DOI] [PubMed] [Google Scholar]
- Kavoosi F. Effect of curcumin and trichostatin a on the expression of DNA methyltransfrase 1 in hepatocellular carcinoma cell line hepa 1-6. Iran J Pediat Hematol Oncol. 2018;8:193–201. [Google Scholar]
- Kavoosi F, Sanaei M. Comparative analysis of the effects of valproic acid and tamoxifen on proliferation, and apoptosis of human hepatocellular carcinoma WCH 17 celllin. Iran J Pediat Hematol Oncol. 2018;8:12–20. [Google Scholar]
- Kitazono M, Shinchi H, Ishigami S, Ueno S, Natsugoe S. Effects of a histone deacetylase inhibitor, sodium butyrate, on 53-kDa protein expression and sensitivity to anticancer drugs of pancreatic cancer cells. Curr Ther Res. 2010;71:162–72. doi: 10.1016/j.curtheres.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsounas I, Giaginis C, Patsouris E, Theocharis S. Current evidence for histone deacetylase inhibitors in pancreatic cancer. World J Gastroenterol. 2013;19:813–19. doi: 10.3748/wjg.v19.i6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Ji Y, Liu C, Li J, Zhou Y. Reduced levels of p15INK4b, p16INK4a, p21cip1 and p27kip1 in pancreatic carcinoma. Mol Med Rep. 2012;5:1106–10. doi: 10.3892/mmr.2012.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140:3079–93. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- Matheu A, Klatt P, Serrano M. Regulation of the INK4a/ARF locus by histone deacetylase inhibitors. J Biol Chem. 2005;280:42433–41. doi: 10.1074/jbc.M508270200. [DOI] [PubMed] [Google Scholar]
- Mu D, Gao Z, Guo H, Zhou G, Sun B. Sodium butyrate induces growth inhibition and apoptosis in human prostate cancer DU145 cells by up-regulation of the expression of annexin A1. PLoS One. 2013;8:1–7. doi: 10.1371/journal.pone.0074922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K, Komiyama T, Miyazawa Si, et al. Histone deacetylase inhibitor suppression of autoantibody-mediated arthritis in mice via regulation of p16INK4a and p21WAF1/Cip1 expression. Arthritis Rheum. 2004;50:3365–76. doi: 10.1002/art.20709. [DOI] [PubMed] [Google Scholar]
- Roberts LR, Gores GJ. Hepatocellular carcinoma: molecular pathways and new therapeutic targets. Semin Liver Dis. 2005;25:212–25. doi: 10.1055/s-2005-871200. [DOI] [PubMed] [Google Scholar]
- Saegusa M, Machida BD, Okayasu I. Possible associations among expression of p14ARF, p16INK4a, p21WAF1/CIP1, p27KIP1, and p53 accumulation and the balance of apoptosis and cell proliferation in ovarian carcinomas. Cancer Int J Am Cancer Soc. 2001;92:1177–89. doi: 10.1002/1097-0142(20010901)92:5<1177::aid-cncr1436>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Sakuma K, Chong JM, Sudo M, et al. High-density methylation of p14ARF and p16INK4A in Epstein-Barr virus–associated gastric carcinoma. Int J Cancer. 2004;112:273–8. doi: 10.1002/ijc.20420. [DOI] [PubMed] [Google Scholar]
- Sanaei M, Kavoosi F, Salehi H. Genistein and trichostatin a induction of estrogen receptor alpha gene expression, apoptosis and cell growth inhibition in hepatocellular carcinoma HepG 2 cells. Asian Pac J Cancer Prev. 2017;18:3445. doi: 10.22034/APJCP.2017.18.12.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanaei M, Kavoosi F, Pourahmadi M, Moosavi SN. Effect of genistein and 17-β estradiol on the viability and apoptosis of human hepatocellular carcinoma HepG2 cell line. Adv Biomed Res. 2017;6:163–8. doi: 10.4103/abr.abr_53_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanaei M, Kavoosi F, Roustazadeh A, Golestan F. Effect of genistein in comparison with trichostatin A on reactivation of DNMTs genes in hepatocellular carcinoma. J Clin Transl Hepatol. 2018;6:141–7. doi: 10.14218/JCTH.2018.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanaei M, Kavoosi F, Mansoori O. Effect of valproic acid in comparison with vorinostat on cell growth inhibition and apoptosis induction in the human colon cancer SW48 cells in vitro. Exp Oncol. 2018;40:95–100. [PubMed] [Google Scholar]
- Sanaei M, Kavoosi F, Roustazadeh A, Shahsavani H. In vitro effect of the histone deacetylase inhibitor valproic acid on viability and apoptosis of the PLC/PRF5 human hepatocellular carcinoma cell line. Asian Pac J Cancer Prev. 2018;19:2507. doi: 10.22034/APJCP.2018.19.9.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanaei M, Kavoosi F. Comparative analysis of the effects of valproic acid and tamoxifen on proliferation, and apoptosis of human hepatocellular carcinoma WCH 17 celllin. Iran J Pediat Hematol Oncol. 2018;8:12–20. [Google Scholar]
- Sanaei M, Kavoosi F, Nasiri S. Effect of 5-aza-2ˈ-deoxycytidine on p27Kip1, p21Cip1/Waf1/Sdi1, p57Kip2, and DNA methyltransferase 1 genes expression, cell growth inhibition and apoptosis induction in colon cancer SW 480 and SW 948 cell lines. Galen Med J. 2020;9:e1899. doi: 10.31661/gmj.v9i0.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanaei M, Kavoosi F, Moezzi MA. Effect of 5’-fluoro-2’-deoxycytidine and sodium butyrate on the genes of the intrinsic apoptotic pathway, p21, p53, cell viability, and apoptosis in human hepatocellular carcinoma cell lines. Iran J Pediat Hematol Oncol. 2021 doi: 10.4103/abr.abr_211_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G, Krämer OH, Fritsche P, et al. Targeting histone deacetylases in pancreatic ductal adenocarcinoma. J Cell Mol Med. 2010;14:1255–63. doi: 10.1111/j.1582-4934.2009.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Cang S, Ma Y, Petrillo RL, Liu D. Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. J Hematol Oncol. 2010;3:5–12. doi: 10.1186/1756-8722-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AJ, Byun D-S, Popova N, et al. Histone deacetylase 3 (HDAC3) and other class I HDACs regulate colon cell maturation and p21 expression and are deregulated in human colon cancer. J Biol Chem. 2006;281:13548–58. doi: 10.1074/jbc.M510023200. [DOI] [PubMed] [Google Scholar]
- Yang VW. Elsevier: 2018. The cell cycle. Physiology of the Gastrointestinal Tract; pp. 197–219. [Google Scholar]
- Yokota T, Matsuzaki Y, Miyazawa K, et al. Histone deacetylase inhibitors activate INK4d gene through Sp1 site in its promoter. Oncogene. 2004;23:5340–9. doi: 10.1038/sj.onc.1207689. [DOI] [PubMed] [Google Scholar]