Abstract
Objectives:
Cytokeratin (CK) proteins play a vital role in cancer diagnosis, of which,CK-7 is a prominent marker of squamocolumnar junction cells corresponding to the the initiating site of cervical cancer.The current study is aimed to evaluate the expression pattern of CK-7 and to corelate with the clinicopathological features in patients with cervical dysplasia and invasive squamous cell carcinoma.
Methodology:
The hysterectomy and biopsy specimens from women with cervical dysplasia (n=60) and carcinoma (n=60) were evaluated histopathologically and processed for immunohistochemistry (IHC) staining to assess for CK-7 expression. The relationship between CK-7 expression and tumor characteristics like histological type of cervical intraepithelial neoplasia (CIN), tumor type and grade was evaluated. Data was analyzed using the Chi-square test ,wherein the p value ≤ 0.05 were taken for statistical significance.
Results:
Positive CK-7 expression was observed in 25 (41.67%) dysplasia and in 34 (56.67%) carcinoma cases. Majority of the cases were CIN III (n=31, 51.67%), large cell non-keratinizing tumor type (n=54, 90%) and moderately differentiated grade of tumor (n=52, 86.67%), out of which 18 (58.1%), 34 (62.96%) and 30 (57.69%) cases were CK-7 positive, respectively. The difference in clinical diagnosis and tumor characteristics over CK-7 expression was significant (p<0.05). The pattern of CK-7 expression in dysplasia and carcinoma cases were diffuse in 23 (38.33%) and 31 (51.67%) respectively and patchy in 2 (3.33%) and 3 (5%) of them, respectively.
Conclusion:
Significant positive CK-7 expression in cervical dysplasia and carcinoma indicates a good clinical course and its role as a useful predictable marker for cancer progression.
Key Words: Cervical intraepithelial neoplasia, immunohistochemistry, keratins, squamous cell carcinoma
Introduction
Cervical cancer (CC) is the fourth most common cause of cancer deaths amongst women worldwide (Pimple et al., 2016; Arbyn et al., 2020). Majority of the cervical cancers are caused due to the persistent infections of the human papilloma virus (HPV). CC develops from cervical squamous intraepithelial lesions to invasive cancer in a multi-step process (Hashiguchi et al., 2019). HPV are of low-risk types (HPV strains 6, 7, 11, 42, 43, 70, and 90) or high-risk types (HPV strains 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 73, and 82) (Sundström et al., 2015). On the global scale, approximately 570,000 cases of cervical cancer and 311,000 deaths from the disease occurred in 2018. The age-standardised incidence of cervical cancer was estimated to be 13.1 per 100,000 women globally and varied widely among countries, with rates ranging from < 2-75 per 100,000 women. India contributed to more than a third of the global cervical burden, with 97,000 cases and 60,000 deaths (Pimple et al., 2016).
The initiation site of cervical cancer is the squamocolumnar junction (SCJ). The SCJ cells are identified as residual embryonic cells that are vulnerable to neoplastic transformation (Herfs et al., 2013). Cytokeratin (CK) belongs to the family of intermediate filament (IF) proteins that are categorised into type I (CK-9 to CK-23) and type II (CK-1 to CK-8) subclasses (Kanaji et al., 2007). In normal conditions, they are expressed in a tissue-specific manner along with the tumors that arise from them. Hence, they play a vital role in the diagnosis of cancer (Chu et al., 2000). Few known SCJ markers are CK-7, AGR2, MMP7, GDA and CD63 (Herfs et al., 2012; Herfs et al., 2013). The CK-7 is positively expressed in most of the cervical intraepithelial neoplasia’s (CIN) or cervical dysplasia and moreover, positive CK-7 expression in CIN I is more likely to progress to CIN III when compared to a negative CK-7 expression in CIN I. Hence, CK-7 is suggested to be a predictable marker for CIN III progression (Herfs et al., 2012; Paquette et al., 2016; Lee et al., 2017).
In approximately 90% of the undifferentiated malignant tumours, immunohistochemistry (IHC) provides diagnostic guidance. However, identification of the primary site of origin may be a challenge for pathologists when dealing with a small sample size along with increased generation of tumour-specific primary antibodies and the further need for complementary molecular analysis. The intra-tumour heterogeneity can make interpretation more difficult. Therefore, IHC uses an extensive panel and specificity of antibodies for certain tumour types to determine the origin precisely. More so, IHC is employed in evaluating the staining patterns of markers for better diagnosis (Selves et al., 2018).
Studies on the expression of CK-7 with respect to cervical dysplasia are very few and its role as a marker of disease progression is under scrutiny. Therefore, by employing IHC, the aim of this study was to evaluate the expression pattern of CK-7 in patients from our tertiary care centre with dysplasia and invasive squamous cell carcinoma of the uterine cervix. The CK-7 expression was correlated with the clinicopathological features in the spectrum of cervical squamous neoplasms.
Materials and Methods
Study design
This histopathological observational study was conducted at a tertiary care center between July 2016 to June 2018. Approval from the Institutional Ethics Committee (SS-1/EC/05/2016) and written informed consent from patients were acquired before the commencement of the study.
Study subjects
According to previous literature, Paquette et al. reported 46% of the cervical dysplasia and Chu et al. reported 87% of the cervical squamous cell carcinomas were positive for Cytokeratin-7 expression (Chu et al., 2000; Paquette et al., 2016). In the present study, expecting similar results with 95% confidence level and 27% relative precision, a minimum of 60 cases of cervical dysplasia and 60 cases of carcinoma were considered.
The study included all hysterectomy and cervical biopsy specimen from women of all age groups with dysplasia and squamous cell carcinoma of the uterine cervix. Cases with inadequate biopsies along with extensive tumour necrosis and inflammation without sufficient viable tumour cells for accurate evaluation of the IHC results were excluded from the study.
Study procedure
The cervical biopsies (n=115) and hysterectomy (n=5) specimens were received in the Pathology department in 10% formalin for routine histopathological evaluation (HPE) from the departments of Obstetrics and Gynaecology (OBGYN) and Surgical Oncology.
Detailed clinical history and results of relevant investigations (pap smear/colposcopy) were collected from patient’s case files. In every case, the standard protocol for surgical grossing of the specimens was followed. Specimen was kept for fixation in 10% formalin for 24 hours. After a detailed morphological specimen description, multiple sections were taken from the tumour and dysplastic lesions. After conventional processing in a Leica 1020 model Histokinette and embedding in paraffin wax, sections of 5 μm thickness were cut using Leica JUNG RM 2025 model rotator microtome and stained using hematoxylin and eosin (H&E) for histopathological study (Pandey et al., 2014). The H&E stained slides were studied for tumour histology, distribution, grade, differentiation and degree of dysplasia as per the standard reporting protocol.
Processing for Immunohistochemistry
Sections of 4 μm thickness were cut from a paraffin block of tumour tissue and embedded on
glass slides coated with adhesive (poly-L-lysine) for IHC to evaluate for the CK-7 expression. The technique for IHC included antigen retrieval in TRIS buffer in a microwave oven at a temperature 56oC. The heat induced antigen retrieval breaks the formalin induced cross linkage thereby exposing the epitopes to the antibody action. The retrieval solution used was TRIS – EDTA (for 10 minutes for 3 cycles). This was followed by blocking endogenous peroxidase with 3% hydrogen peroxide, incubating with primary mouse monoclonal antibody (Biogenex – anti Cytokeratin 7 – OV-TL 12/30) at a concentration 1:50, linking with rabbit anti-mouse secondary antibody (Biogenex), enzyme labelling with streptavidin-horseradish peroxidase at a concentration 1:50, developing chromogen with deaminobenzidine (DAB) at a concentration 1:100 and counterstaining with hematoxylin. Positive controls were run with each batch of slides. Normal urothelium was used as positive control. The immunostained slides were examined for cytoplasmic staining of CK-7.
The relationship between various parameters such as age, duration of disease presentation, histologic type, grade, differentiation, degree of dysplasia and CK-7 expression were studied.
Method of Reporting by IHC (Paquette et al., 2016; Lee et al., 2017)
CK-7 positive cells were defined as either full epithelial thickness (diffuse or patchy) or surface or basal epithelial diffuse CK-7 stain, whereas all other combinations were considered negative (surface or basal epithelial patchy CK-7 stain).
• Diffuse staining – Area of cells with block strong positivity of approximately 5 to 6 contiguous cells, where staining was observed in >40% of cells.
• Patchy staining – Positive isolated cells with interspersed negative cells in between, where staining was observed in 1-40% cells.
• Negative staining – Staining was observed in <1% of cells.
Statistical analysis
Data was analyzed using R software version 4.0.2 and Excel. Continuous variables were represented as mean± SD. Categorical variables were represented as numbers (%). Dependency between 2 categorical variables was analysed by performing Chi-square test. Level of significance was set at p≤0.05.
Results
The study included 60 patients each with respect to dysplasia and squamous cell carcinoma of the cervix. The mean age of patients in this study was 49.37±11.24 years. With respect to cervical dysplasia, mean age of patients was 49.37±10.08 years, with majority belonging to the age group of 31-55 (n=42, 70%), followed by >55 (n=16, 26.67%) and <30 (n=2, 3.33%) years. With respect to cervical carcinoma, mean age of patients was 50.53±12.26 years, with majority belonging to the age group of 31-55 (n=36, 60%), followed by >55 (n=21, 35%) and <30 (n=3, 5%) years. The difference in the age distribution over clinical diagnosis was insignificant (p=0.5652).
Majority of the women with clinical diagnosis of cervical dysplasia (n=40, 67.67%) and cervical carcinoma (n=35, 58.33%) had a parity of 0-3. whereas the rest had a parity of >3. The difference in the parity over clinical diagnosis was insignificant (p=0.3458).
vaginal discharge (n=20, 33.33%) and vaginal bleeding (n=45, 75%) were seen in those with cervical dysplasia and cervical carcinoma respectively . The high-grade squamous intraepithelial lesions (HSIL) was common diagnosis in the study. (Figure 1). The difference in the distribution of clinical presentation (p=0.0005) and pap smear (p=0.0005) over clinical diagnosis was significant (Table 1).
Figure 1.
(A) Gross image of hysterectomy specimen showing grey-white infiltrating tumor in the cervix, (B) hysterectomy specimen showing grey-white exophytic growth at the posterior lip of the cervix and (C) cone biopsy specimen of the cervix showing grey-white and hemorrhagic areas of CIN III
Table 1.
Distribution of Clinical Parameters Over Clinical Diagnosis
| Parameters | Clinical diagnosis No. of patients (%) |
p value | |
|---|---|---|---|
| Dysplasia (n=60) | Carcinoma (n=60) | ||
| Clinical Presentation | |||
| Vaginal bleeding | 16 (26.67) | 45 (75) | 0.0005* |
| Vaginal discharge | 20 (33.33) | 1 (1.67) | |
| Vaginal mass | 0 | 4 (6.67) | |
| Dyspareunia | 2 (3.33) | 1 (1.67) | |
| Abnormal Uterine Bleeding | 0 | 9 (15) | |
| Asymptomatic/Incidental | 22 (36.67) | 0 | |
| Pap smear | |||
| LSIL | 16 (26.67) | 0 | 0.0005* |
| HSIL | 21 (35) | 17 (28.33) | |
| ASCUS | 3 (5) | 0 | |
| Carcinoma | 0 | 3 (5) | |
| Inflammatory | 2 (3.33) | 0 | |
| Not performed | 18 (30) | 40 (66.67) | |
| Nature of the specimen | |||
| Cervical biopsy | 58 (96.67) | 55 (91.67) | 0.4173 |
| Hysterectomy | 2 (3.33) | 5 (8.33) | |
*Significant (p<0.05); Abbreviations: LSIL, Low-Grade Squamous Intraepithelial Lesions; HSIL, High-Grade Squamous Intraepithelial Lesions; ASCUS, Atypical Squamous Cells of Undetermined Significance
With respect to tumor characteristics, majority were CIN III (n=31, 51.67%), large cell non-keratinizing type (n=54, 90%) and moderately differentiated (n=52, 86.67%) tumors (Table 2).
Table 2.
Representation of Tumor Characteristics
| Characteristic feature | No. of Patients (%) |
|---|---|
| Histological type of CIN (n=60) | |
| CIN I | 17 (28.33) |
| CIN II | 12 (20) |
| CIN III | 31 (51.67) |
| Histological type of tumor (n=60) | |
| Large Cell Non-Keratinizing | 54 (90) |
| Keratinizing | 2 (3.33) |
| Not applicable | 4 (6.67) |
| Histological grade of tumor (n=60) | |
| Well differentiated | 4 (6.67) |
| Moderately differentiated | 52 (86.67) |
| Poorly differentiated | 4 (6.67) |
CIN, Cervical Intraepithelial Neoplasia
Positive CK-7 expression was observed in 25 (41.67%) cervical dysplasia and in 34 (56.67%) cervical carcinoma cases. The difference in tumor characteristics (Histological type of CIN, p=0.01; histological type of tumor, p=0.0034; histological grade of tumor, p=0.009) over CK-7 expression was significant but not with respect to clinical diagnosis (p=0.1003) (Table 3).
Table 3.
Representation of the Clinical Variables with Respect to CK-7 Expression
| Variable | CK-7 expression No. of Patients (Row%) |
p value | |
|---|---|---|---|
| Positive | Negative | ||
| Clinical diagnosis | |||
| Dysplasia (n=60) | 25 (41.67) | 35 (58.33) | 0.1003 |
| Carcinoma (n=60) | 34 (56.67) | 26 (43.33) | |
| Histological type of CIN | |||
| CIN I (n=17) | 2 (11.76) | 15 (88.23) | 0.01* |
| CIN II (n=12) | 5 (41.67) | 7 (58.33) | |
| CIN III (n=31) | 18 (58.1) | 13 (41.93) | |
| Histological type of tumor | |||
| Large Cell Non-Keratinizing (n=54) | 34 (62.96) | 20 (37) | 0.0034* |
| Keratinizing (n=2) | 0 | 2 (100) | |
| Not applicable (n=4) | 0 | 4 (100) | |
| Histological grade of tumor | |||
| Well differentiated (n=4) | 4 (100) | 0 | 0.009* |
| Moderately differentiated (n=52) | 30 (57.69) | 22 (42.3) | |
| Poorly differentiated (n=4) | 0 | 4 (100) | |
*Significant (p<0.05); CK-7, Cytokeratin-7; CIN, Cervical Intraepithelial Neoplasia
The pattern of CK-7 expression in cervical dysplasia and carcinoma is represented in Table 4. The difference in the clinical diagnosis over the pattern of CK-7 expression was insignificant (p=0.2604) (Figures 2 and 3).
Table 4.
Pattern of CK-7 Expression with Respect to Clinical Diagnosis
| Diagnosis | Pattern of CK-7 expression - No. of Patients (Row %) | p value | ||
|---|---|---|---|---|
| Diffuse | Patchy | Absent | ||
| Dysplasia (n=60) | 23 (38.33) | 2 (3.33) | 35 (58.33) | 0.2604 |
| Carcinoma (n=60) | 31 (51.67) | 3 (5) | 26 (43.33) | |
CK-7, Cytokeratin-7
Figure 2.
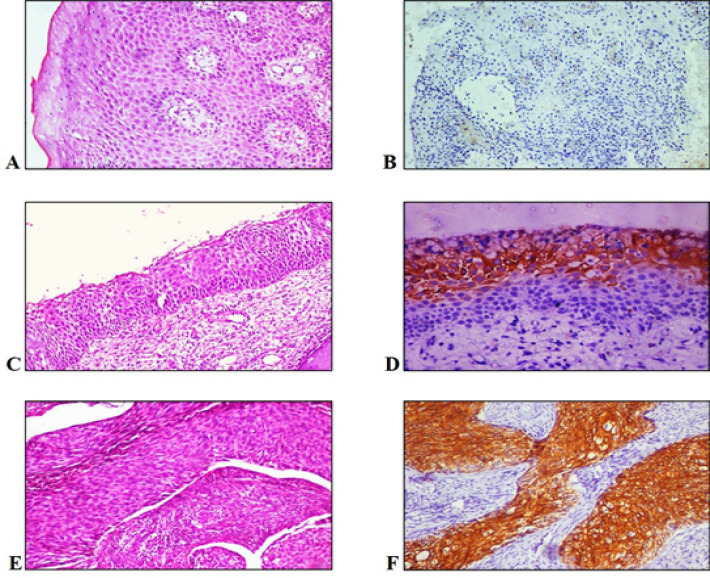
(A) H&E staining of CIN I under 10X, (B) IHC staining of CIN I - dysplastic epithelium showing negative CK-7 expression under 10X, (C) H&E staining of CIN II under 10X, (D) IHC staining of CIN II – superficial epithelial cells showing positive CK-7 expression under 10X, (E) H&E staining of CIN III under 10X and (F) IHC staining of CIN III - dysplastic epithelium showing positive CK-7 expression under 10X
Figure 3.
(A) H&E staining of squamous cell carcinoma under 20X, (B) IHC staining of squamous cell carcinoma showing diffuse membranous and cytoplasmic positivity of CK-7 expression under 20X (C) H&E staining of poorly differentiated carcinoma under 20X, (D) IHC staining of poorly differentiated carcinoma under 20X showing tumor cells negative for CK-7 expression under 20X and (E) positive internal control – endocervical glands showing diffuse membranous positive CK-7 expression under 100X
Out of the 5 hysterectomy specimens with respect to cervical carcinoma received, parametrial invasion was observed in 4 (80%) cases, whereas 3 (75%) cases positively expressed CK-7. Only 1 (20%) case was observed without any parametrial invasion positively expressed CK-7. However, the difference in parametrial invasion over CK-7 expression was insignificant (p=0.576).
Similarly, in the hysterectomy specimens, lymphovascular invasion was observed in 2 (40%) cases, from which only 1 (50%) case positively expressed CK-7. In rest of the 3 (60%) cases without any lymphovascular invasion, all cases expressed CK-7 positively. However, the difference in lymphovascular invasion over CK-7 expression was insignificant (p=0.171).
Discussion
Cervical cancer is on the declining trend in India but is still one of the common cancer-causing deaths in developing countries (Sreedevi et al., 2015). It contributes to approximately 6–29% of all cancers in Indian women (Bobdey et al., 2016). The time taken (approximately 10-20 years) for cervical cancer progression from mild dysplasia to carcinoma, makes it as a relatively early preventable disease, thereby providing the rationale for screening but despite the existence of national guidelines, the screening coverage in India is ghastly low (Srivastava et al., 2018). Therefore, majority of the cancers are detected late contributing to the high mortality rate.
Attempts to identify clinical, molecular and immunohistochemical markers, indicating the disease progression and poor prognosis have been undertaken with respect to all cancer types. The change in the keratin expression pattern from normal in dysplastic and carcinogenic cervical epithelium is particularly subjected to a lot of studies, mainly because of their wider range of expression in carcinomas (Smedts et al., 1992). Therefore, this study was conducted to evaluate the expression of CK-7 and its correlation with clinicopathological factors in dysplasia and carcinoma of the cervix.
Majority of the women in this study belonged to the age group 31-55 years. Similar observations were reported by and Hashiguchi et al., (2019) and Husain et al., (2016), although an epidemiological survey conducted by Sreedevi et al. (2015) revealed that the peak age for cervical cancer in India was common in women aged 55-59 years.
Majority of the patients in this study suffered from vaginal bleeding, which is a common consequence of advanced cervical cancer (Eleje et al., 2015). Pap smear test revealed greater number of patients diagnosed with low-grade squamous intraepithelial lesions (LSIL) and HSIL. The difference between the clinical diagnosis (dysplasia and carcinoma) and CK-7 expression was insignificant in this study (p=0.1003).
Majority of the patients expressed CIN III (51.67%), where 58.1% of them were positive of CK-7 expression. Majority of the CIN I and CIN II cases were negative for CK-7 expression. Hashiguchi et al. and Lee et al. also reported increased CK-7 expression with respect to CIN III (Hashiguchi et al., 2019; Lee et al., 2017). The relationship between keratin expression of SCJ cells and CIN is complex. CK-7 is described as a marker for SCJ progenitor cells and is expressed in mostly in case of CIN II and CIN III. Higher the grade of CIN, higher the CK-7 expression (van der Marel et al., 2014; Umphress et al., 2018; Lima et al. 2018). The site of origin of LSIL (CIN I) plays an important role in its progression. Earlier studies reported that LSILs arising from SCJ had a higher propensity to progress towards HSIL (CIN II and CIN III) when compared to the LSILs from the ectocervix region (Herfs et al., 2013; Paquette et al., 2016; Mills et al., 2017). However, once detected, frequent patient follow-up will determine the primary site of origin.
The CK7 may be more related with viral episomal replication and CK19 (binding partner of CK7) with viral integration, contributing to viral replication and malignant transformation in High risk human papillomavirus (HR HPV) infected cells. In addition, coordinate CK7/CK19 staining may be used as a valuable marker for predicting physical status of HR HPV and E7 oncoprotein level in cervical tumor. ( Lima et al. 2018)The expression in high grade cervical intraepithelial neoplasm and squamous cell carcinoma and their possible association in cervical carcinogenesis were highlighted by the authors.( Lima et al. 2018)
Majority of the tumors were large cell non-keratinizing type (90%), out of which, 62.96% positively expressed CK-7. The difference between the histological type of tumor and CK-7 expression was significant (p=0.0035). Similar observations were reported by Hashiguchi et al., (2019) (p=0.005). Positive CK-7 expression in large cell non-keratinizing type oropharyngeal squamous cell carcinoma was reported by Mehrad et al (2018).
Greater number of tumors in this study were moderately differentiated and 42.3% of them were positive for CK-7 expression. In a study conducted by Husain et al. (2016), 45% of the moderately differentiated tumors were positive for CK expression. Also, positive CK-7 expression was observed in all well differentiated and none of the poorly differentiated tumor cases. Positive CK-7 expression was reported in many low-grade tumors (well and moderately differentiated) when compared to high-grade tumors (poorly differentiated) in a study conducted by Bayrak et al., (2012) in adenocarcinomas. These results are in accordance with our study.
With respect to the expression pattern, the staining pattern varied from diffuse, strong positive to patchy, dim staining, where 38.33% of cervical dysplasia and 51.67% of cervical carcinoma cases exhibited diffuse staining. Staining was strongest at the apex with most cases showing full thickness cytoplasmic staining (tumours having a cord-like and solid growth pattern) while some showed a gradual gradation towards the base (base sparing). The staining was intense at the invasive front of the tumour in this study, with similar observations reported by Lee et al., (2017) With respect to the hysterectomy specimens in this study, the difference in parametrial and lymphovascular invasion over CK-7 expression was insignificant (p>0.05). A study conducted by Fei et al. reported that CK-7 expression was associated with location and differentiation of colorectal cancers, and the same principle could be applied with respect to cervical cancers (Fei et al., 2019).
The combined expression and CK7 and Cyclin-dependent kinase inhibitor (CDKN2A) was associated with a better diagnosis of cervical intraepithelial neoplasia (CIN). Also negative expression in CIN1/2 groups had a greater negative predictive value for patient clinical outcome as per the Lee et al., (2017).
Apart from above mentioned markers, CK5/6, p63 and p40 were also reported useful in diagnosis of cervical neoplasms. A study reported that, the sensitivity of CK5/6, p40, and p63 was 94.06, 85.15, and 89.11%, respectively, and the specificity was 77.78, 97.22, and 93.52%, respectively (Li et al., 2018). Moreover, a combination of these also affects the diagnostic outcome. The specificity of CK5/6 is slightly lower than that of p40 and p63. We also found that a combination of CK5/6 with p40 or p63 slightly decreased the sensitivity (82.18 and 85.15%), and increased the specificity (98.15 and 96.30%), which, in turn, increased the accuracy of diagnosing squamous cell carcinoma (Li et al., 2018).
CK5/6 is a kind of high molecular weight basal cell keratin (58kda and 56kda), existing evidence showed that CK5/6 has high sensitivity and specificity in the diagnosis of SCC. Another marker, p63 (a member of the p53 family, located on chromosome 3q27–29) is also a useful in diagnosis of 92.6% of SCC of cervical region. There is some controveory but in SCC so, p40 is suggested to p63. The sensitivity and specificity of p40 in the diagnosis of squamous cell carcinoma of the lung were 100 and 98%, respectively. (Li et al., 2018)
Small sample size, lack of molecular studies to quantify the CK-7 expression and also to understand the reason for negative expression of CK-7 in few cases are few limitations of this study. Future studies that overcome these limitations would help in understanding the CK-7 expression pattern in a better manner.
In conclusion, in this study, significant positive CK-7 expression over the clinical diagnosis i.e. cervical dysplasia and squamous cell carcinoma indicates a good clinical course. Because of the unpredictable variation in the future outcome of LSILs, it becomes important to identify those which are likely to progress to HSILs. Most LSILs in this study were CK-7 negative. However, its expression in LSIL is indicative of its future progression to HSIL. Importance of employing CK-7 immunomarker in the segregation of high risk LSILs from the low-risk ones were examined and its role as a prognostic marker in cervical dysplasia and carcinoma were also identified.
Author Contribution Statement
Author 1,2: Concepts, Design, Definition of intellectual content, Literature search, Experimental studies, Data acquisition, Data analysis, Manuscript preparation, Manuscript editing. Author 3: Guarantor, Manuscript preparation, Manuscript editing. Authors: 4:Statistical analysis, Manuscript review. Availability of data: Yes.
Acknowledgements
Nil.
Conflict of Interest
The authors declare that there are no conflicts of interest
References
- Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob Health. 2020;8:e191–203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayrak R, Haltas H, Yenidunya S. The value of CDX2 and cytokeratins 7 and 20 expression in differentiating colorectal adenocarcinomas from extraintestinal gastrointestinal adenocarcinomas: cytokeratin 7-/20+ phenotype is more specific than CDX2 antibody. Diagn Pathol. 2012;7:9. doi: 10.1186/1746-1596-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobdey S, Sathwara J, Jain A, Balasubramaniam G. Burden of cervical cancer and role of screening in India. Indian J Med Paediatr Oncol. 2016;37:278–85. doi: 10.4103/0971-5851.195751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: A survey of 435 cases. Mod Pathol. 2000;13:962–72. doi: 10.1038/modpathol.3880175. [DOI] [PubMed] [Google Scholar]
- DE Lima TM, DE Azevedo Focchi GR, DE Almeida BC, et al. Expression of CK7 and CDKN2 in cervical intraepithelial neoplasia and correlation with clinical outcome. Anticancer Res. 2018;38:6673–81. doi: 10.21873/anticanres.13035. [DOI] [PubMed] [Google Scholar]
- Eleje GU, Eke AC, Igberase GO, Igwegbe AO, Eleje LI. Palliative interventions for controlling vaginal bleeding in advanced cervical cancer. Cochrane Database Syst Rev. 2015;5:CD011000. doi: 10.1002/14651858.CD011000.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei F, Li C, Cao Y, et al. CK7 expression associates with the location, differentiation, lymph node metastasis, and the Dukes’ stage of primary colorectal cancers. J Cancer. 2019;10:2510–19. doi: 10.7150/jca.29397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi M, Masuda M, Kai K, et al. Decreased cytokeratin 7 expression correlates with the progression of cervical squamous cell carcinoma and poor patient outcomes. J Obstet Gynaecol Res. 2019;45:2228–36. doi: 10.1111/jog.14108. [DOI] [PubMed] [Google Scholar]
- Herfs M, Parra-Herran C, Howitt BE, et al. Cervical squamocolumnar junction-specific markers define distinct, clinically relevant subsets of low-grade squamous intraepithelial lesions. Am J Surg Pathol. 2013;37:1311–8. doi: 10.1097/PAS.0b013e3182989ee2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herfs M, Vargas SO, Yamamoto Y, et al. A novel blueprint for ‘top down’ differentiation defines the cervical squamocolumnar junction during development, reproductive life, and neoplasia. J Pathol. 2013;229:460–8. doi: 10.1002/path.4110. [DOI] [PubMed] [Google Scholar]
- Herfs M, Yamamoto Y, Laury A, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A. 2012;109:10516–21. doi: 10.1073/pnas.1202684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain NE, Babiker AY, Albutti AS, et al. Clinicopathological significance of vimentin and cytokeratin protein in the genesis of squamous cell carcinoma of cervix. Obstet Gynecol Int. 2016;2016:8790120. doi: 10.1155/2016/8790120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaji N, Bandoh S, Fujita J, et al. Compensation of type I and type II cytokeratin pools in lung cancer. Lung Cancer. 2007;55:295–302. doi: 10.1016/j.lungcan.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Lee H, Lee H, Cho YK. Cytokeratin7 and cytokeratin19 expression in high grade cervical intraepithelial neoplasm and squamous cell carcinoma and their possible association in cervical carcinogenesis. Diagn Pathol. 2017;12:18. doi: 10.1186/s13000-017-0609-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Jing X, Yu J, et al. A combination of cytokeratin 5/6, p63, p40 and MUC5AC are useful for distinguishing squamous cell carcinoma from adenocarcinoma of the cervix. Diagn Pathol. 2018;15:1–9. doi: 10.1186/s13000-020-01018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrad M, Dupont WD, Plummer WD Jr, Lewis JS Jr. Expression and significance of cytokeratin 7, a squamocolumnar junction marker, in head and neck squamous cell carcinoma. Head Neck Pathol. 2018;12:448–54. doi: 10.1007/s12105-017-0874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AM, Paquette C, Terzic T, Castle PE, Stoler MH. CK7 immunohistochemistry as a predictor of CIN1 progression: A retrospective study of patients from the quadrivalent HPV vaccine trials. Am J Surg Pathol. 2017;41:143–52. doi: 10.1097/PAS.0000000000000747. [DOI] [PubMed] [Google Scholar]
- Pandey P, Dixit A, Tanwar A, Sharma A, Mittal S. A comparative study to evaluate liquid dish washing soap as an alternative to xylene and alcohol in deparaffinization and hematoxylin and eosin staining. J Lab Physicians. 2014;6:84–90. doi: 10.4103/0974-2727.141504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette C, Mills AM, Stoler MH. Predictive value of cytokeratin 7 immunohistochemistry in cervical low-grade squamous intraepithelial lesion as a marker for risk of progression to a high-grade lesion. Am J Surg Pathol. 2016;40:236–43. doi: 10.1097/PAS.0000000000000548. [DOI] [PubMed] [Google Scholar]
- Pimple S, Mishra G, Shastri S. Global strategies for cervical cancer prevention. Curr Opin Obstet Gynecol. 2016;28:4–10. doi: 10.1097/GCO.0000000000000241. [DOI] [PubMed] [Google Scholar]
- Selves J, Long-Mira E, Mathieu MC, Rochaix P, Ilié M. Immunohistochemistry for diagnosis of metastatic carcinomas of unknown primary site. Cancers (Basel) 2018;10:108. doi: 10.3390/cancers10040108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedts F, Ramaekers F, Troyanovsky S, et al. Keratin expression in cervical cancer. Am J Pathol . 1992:497–511. [PMC free article] [PubMed] [Google Scholar]
- Sreedevi A, Javed R, Dinesh A. Epidemiology of cervical cancer with special focus on India. Int J Womens Health. 2015;7:405–14. doi: 10.2147/IJWH.S50001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava AN, Misra JS, Srivastava S, Das BC, Gupta S. Cervical cancer screening in rural India: Status and current concepts. Indian J Med Res. 2018;148:687–96. doi: 10.4103/ijmr.IJMR_5_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström K, Ploner A, Arnheim-Dahlström L, et al. Interactions between high- and low-risk hpv types reduce the risk of squamous cervical cancer. J Natl Cancer Inst. 2015;107:djv185. doi: 10.1093/jnci/djv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umphress B, Sanchez B, Paintal A, Nayar R, Maniar KP. 2018) Utility of CK7 versus p16 as a prognostic biomarker in CIN 2. Am J Surg Pathol. 42:479–84. doi: 10.1097/PAS.0000000000001032. [DOI] [PubMed] [Google Scholar]
- Van der Marel J, van Baars R, Alonso I, et al. Oncogenic human papillomavirus-infected immature metaplastic cells and cervical neoplasia. Am J Surg Pathol. 2014;38:470–9. doi: 10.1097/PAS.0000000000000174. [DOI] [PubMed] [Google Scholar]