Abstract
Background:
Human papillomavirus (HPV) and Epstein-Barr virus (EBV) are associated with head and neck cancer, including tonsil cancer (TC) in the oropharyngeal area. Increasing incidence of HPV and EBV infection in different cancer tissues of oropharynx in both epithelial and lymphoid tissues, have been reported. However, little is known about association of these tumor viruses with TC in the Thai population. Here, we investigated the prevalence of HPV and EBV infection in different histology of TC and their association with TC from Thai patients.
Methods:
Eighty-three exfoliated tonsil cells from non-cancer controls (NCC) and 65 formalin-fixed paraffin-embedded TC tissues (TC) that were histologically classified as tonsillar squamous-cell carcinoma (TSCC) or diffuse large B-cell lymphoma (DLBCL) were studied. Prevalence of HPV and EBV infection was determined by real-time PCR. HPV genotyping was performed by reverse line blot hybridization and HPV genome status was investigated by multiplex qPCR. Localization of EBV infection was determined by EBER in situ hybridization.
Results:
Infection of HPV and EBV in TC cases was 16.9% and 30.8%, whereas in exfoliated tonsil cells was 1.2% and 66.3% respectively. HPV infection was significantly higher in TSCC (30.6%) than DLBCL samples (13.8%). HPV58 was commonly detected and presented as an integrated form in TSCC, whereas only episomal form was found in DLBCL. EBV infection was significantly higher in DLBCL (44.8%) than TSCC samples (19.4%), and detected in both lower than among exfoliated tonsil cell samples (66.3%). By EBER in situ hybridization in TSCC, EBV infection localized both in epithelial cells and infiltrating lymphocytes. The co-occurrence of HPV and EBV infection was 11.11% and 13.79% of TSCC and DLBCL, respectively, was associated with well-differentiated TSCC.
Conclusion:
HPV and EBV infection was significantly involved in a specific TC tissue, and associated with a good clinical outcome in TSCC.
Key Words: Human papillomavirus, Epstein-Barr virus, tonsillar squamous cell carcinoma, diffuse large B cell lymphoma
Introduction
Tonsillar squamous cell carcinoma (TSCC) is the common form of oropharyngeal squamous cell carcinoma (OPSCC), representing 23.1% of all malignant tumors in oropharynx (Weatherspoon et al., 2015). The incidence of TSCC has significantly increased in the last two decades, from 0.6 to 1.45 cases per 100,000 persons, especially in men and adults aged between 40 and 59 years of age (Turculeanu et al., 2015). The common risk factors of TSCC are use of tobacco, alcohol and betel-quid chewing as well as infections by oncogenic viruses, such as HPV and EBV (Pannone et al., 2011; Zaravinos, 2014). High prevalence of HPV infection is found in TSCC (Klussmann et al., 2003; Nordfors et al., 2014; Nasman et al., 2015), whereas prevalence of EBV infection in TSCC is less common. Infection of EBV has been mostly reported in OPSCC cases, with different prevalence in the oropharynx subset in the range of 57.5% to 79.7% (Deng et al., 2014; Polz-Gruszka et al., 2015). However, little is known about oncogenic processes of HPV and EBV infection to promote TSCC carcinogenesis.
Diffuse large B-cell lymphoma (DLBCL) originated from B lymphocytes is the most common subtype of aggressive non-Hodgkin lymphoma (NHL) and the most common histological type of tonsil lymphomas (Yamanaka et al., 1985). DLBCL can develop in the lymph nodes or in “extranodal sites” (areas outside the lymph nodes) such as thyroid, the gastrointestinal tract, testes, etc., and can occur in childhood, or generally increases with age. Approximately 10% of patients with NHL present with extranodal disease in the head and neck region. Furthermore, more than half of these head and neck lymphomas occur in the Waldeyer ring (WR) that is increasing in Asia (Jacobs and Hoppe, 1985; Jacobs et al., 1986). The WR includes the pharyngeal (adenoids), tubal, palatine and lingual tonsils (base of the tongue), and WR lymphoma most frequently occurs in the palatine tonsil that was found 40% to 50% (Banfi et al., 1970; Aviles et al., 1996). Most patients are over the age of 60 at diagnosis and accounting for 30% to 40% of all newly diagnosed lymphomas (Friedberg and Fisher, 2008; Korkolopoulou et al., 2016). Risk factors for DLBCL remain unknown, although various chemical substances and medical drugs have been suggested. However, there is an association between DLBCL and immunologic deficiency diseases (Martelli et al., 2013). More recently, virus infection, particularly by EBV, has been reported as an etiological factor for DLBCL. EBV-positive DLBCL of the elderly is a tumor that develops in patients who are older than 50 years and without any history of immunosuppression or prior lymphoma (Swerdlow et al., 2008). The prevalence of EBV in DLBCL varies geographically. In Western countries, the infection of EBV in DLBCL is rare (<5%), but is higher in Asia and Latin America (3.8% to 11.4%; (Pan et al., 2013). However, the prevalence of HPV in DLBCL has not been studied yet. The prevalence of HPV and EBV infections in TSCC and DLBCL patients in the Thai population has also not been studied yet. Therefore, we aimed to investigate the prevalence of HPV and EBV infections in TC, in specific histologic types and their association with different histology grades of TSCC patients.
Materials and Methods
Specimens
Sixty-five formalin-fixed paraffin-embedded TC tissues (FFPE-TC), including 36 TSCC and 29 of DLBCL, were retrieved from archived material in the Department of Pathology, Faculty of Medicine, Khon Kaen University during 2011 to 2015. In each case, histological confirmation of the diagnosis had been made from FFPE tissue sections.
Exfoliated tonsil cells were collected from 83 subjects without cancer or any chronic infection in the head and neck to use as the normal control (non-cancer control, NCC) who were initially evaluated and diagnosed by an otorhinolaryngologist at the Department of Otorhinolaryngology, Srinagarind Hospital, Faculty of Medicine, Khon Kaen University. The use of exfoliated tonsil cells and FFPE tissue archived specimens was approved by the Khon Kaen University Ethics Committee for Human Research (No. HE561407 and HE581159, respectively).
HPV and EBV DNA detection by real-time PCR
DNA was extracted from FFPE-TC samples using a DNeasy Blood & Tissue Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instruction. DNA was extracted from exfoliated tonsil cells by using the lysis buffer-proteinase K method (Sambrook et al., 1989)). The quality of extracted DNA was assessed by detecting the presence of the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene (Teeratakulpisarn et al., 2007). The extracted DNA was further investigated for HPV and EBV DNA by Applied Biosystems®7500 fast instrument (Applied Biosystems, Foster City, CA, USA). Consensus primers for the conserved L1 ORF of HPV; GP5+/GP6+, were used for HPV detection (van den Brule et al., 2002). For the detection of EBV DNA, primers targeting EBV DNA polymerase (BALF5) were used (Pozo and Tenorio, 1999). The extracted DNA from HeLa and SiHa cells were used as a positive control for HPV detection and from B95-8 cells was used as a positive control for EBV detection. The primers used in this study were listed in Table 1.
Table 1.
Primer Sequences
| Primer name | Forward (5'-3') | Reward (5'-3') | Reference |
|---|---|---|---|
| GP5+/GP6+ | TTTGTTACTGTGGTAGATACTAC | GAAAAATAAACTGTAAATCATATTC | van den Brule et al., 2002 |
| BALF5 | CGAGTCATCTACGGGGACACGGA | AGCACCCCCACATATCTCTTCTT | Pozo and Tenorio, 1999 |
HPV genotyping by reverse line blot hybridization (RLBH)
HPV-positive samples were subjected to RLBH for HPV genotyping. The protocol is described in a previous study (van den Brule et al., 2002; Aromseree et al., 2014). For chemiluminescence detection, membrane was incubated with SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL USA). The signal was detected by Image Quant LAS 4000mini (GE Healthcare, Upsala, Sweden). The 37 different HPV genotype probes used in this study were listed in Supplementary Table 1.
HPV58 physical status investigation by multiplex qPCR
HPV-positive samples were further investigated for the physical status of their genome by multiplex qPCR method. The protocol is described in a previous study (Prasongdee et al., 2018 and patent no. 1501001199).
EBV localization by Epstein–Barr virus-encoded small RNA (EBER) in situ hybridization (ISH)
EBV-positive FFPE-TC samples were used to detect the localization of EBV infection by using ISH. The protocol is described in a previous study (Peferoen et al., 2010). Briefly, FFPE tissues were cut at 5 μm thickness and probed with EBER peptide nucleic acid (PNA) fluorescein probe (Y5200; Dako Glostrup, Denmark). FFPE nasopharyngeal squamous cell carcinoma (NPC) tissue was used as positive control.
Statistical analysis
The software packages Stata 12.0 and Graphpad Prism were used for all data analysis. Chi-square and Mann- Whitney U tests were used to compare the rates of HPV, EBV and co-occurrence of HPV and EBV in TSCC and DLBCL. P values less than 0.05 were considered statistically significant.
Results
Patient characteristics
Table 2 shows the characteristics of NCC and TC patients with histological classification as TSCC and DLBCL. The mean ages of TSCC and DLBCL and NCC samples were 62, 54 and 50 years, respectively. Females were in the majority among NCC but male were in the majority in both TSCC and DLBCL cases. Well-differentiated TSCC cases were the most common histological grade, followed by moderately and poorly differentiated.
Table 2.
Demographic Data of Normal Control (NCC) and Tonsil Cancer Patients (TC) with Histological Difference of TSCC and DLBCL
| Demographic | NCCa (n=83) | TSCCb (n=36) | DLBCLc (n=29) |
|---|---|---|---|
| Age range (years) | 24-84 | 27-81 | 7-83 |
| Age (years, mean ± SD) | 61.70 ± 14.15 | 54.03 ± 12.35 | 50.48 ± 18.53 |
| Sex | |||
| Male | 32 (38.55%) | 31 (86.11%) | 15 (51.72%) |
| Female | 51 (64.45%) | 5 (13.19%) | 14 (48.28%) |
| Histology | |||
| Well differentiated | - | 23 (63.89%) | - |
| Moderately differentiated | - | 8 (22.22%) | - |
| Poorly differentiated | - | 4 (11.11%) | - |
| Undifferentiated | - | 1 (2.78%) | - |
Prevalence of HPV and EBV infection
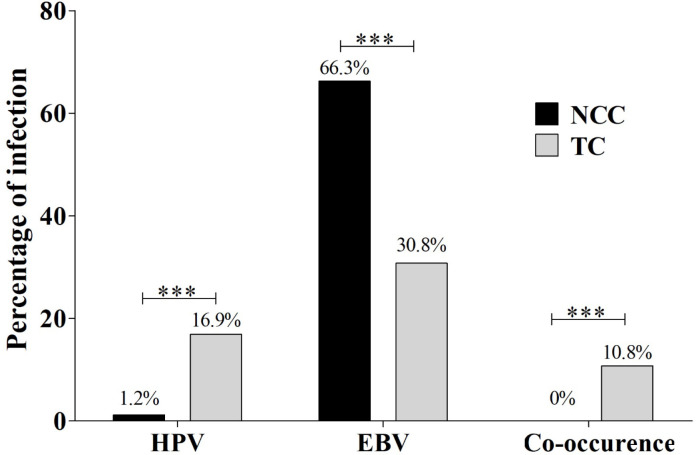
The prevalence of HPV and EBV infection of TC samples was 16.9% and 30.8%, respectively. HPV infection was significantly higher in TC samples (16.9%) than that of exfoliated tonsil cells from NCC samples (1.2%, p<0.001), while higher prevalence of EBV infection in NCC samples (66.3%) than in TC samples (30.8%) was detected. The co-occurrence of these two viruses was detected in 10.8% of TC samples, but could not be detected in exfoliated tonsil cells from NCC samples (Figure 1).
Figure 1.
The Prevalence of HPV, EBV and Co-Occurrence Infection in NCC and TC Samples. Chi-square test was used to compare the prevalence of HPV, EBV and co-occurrence infection in NCC and TC cases (*: p < 0.05; **: p < 0.01; ***: p < 0.001)
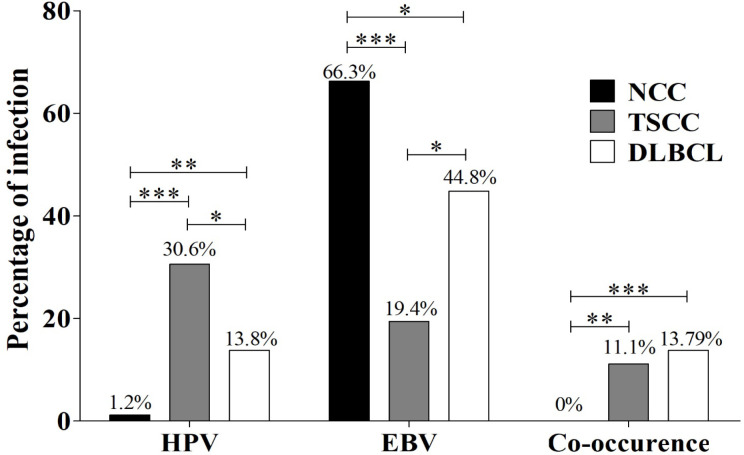
Furthermore, we analyzed the association of HPV and EBV infection in the context of cancer tissue types, TSCC and DLBCL. HPV infection was significantly higher in TSCC (30.6%) than in DLBCL (13.8%, p<0.05). However, the prevalence of HPV in NCC was 1.2%, which was significantly lower than in TSCC and DLBCL samples (p<0.01). The prevalence of EBV was significantly higher in DLBCL (44.8%) than in TSCC (19.4%, p<0.05). Interestingly, the co-occurrence of HPV and EBV was significantly higher in both TSCC (11.1%) and DLBCL (13.8%) than in NCC samples (0%, p<0.001, Figure 2). These results show an association of HPV and EBV in TC specific tissue type and suggest that HPV may play a role in the development of TSCC rather than DLBCL. In addition, EBV may play a role in the carcinogenesis of DLBCL rather than TSCC.
Figure 2.
The Prevalence of HPV, EBV and Their Co-Occurrence in NCC, TSCC and DLBCL. Chi-square test was used to analyze the prevalence of HPV, EBV and their co-occurrence in NCC, TSCC and DLBCL (*: p < 0.05; **: p < 0.01; ***: p < 0.001).
HPV genotypes and their physical status
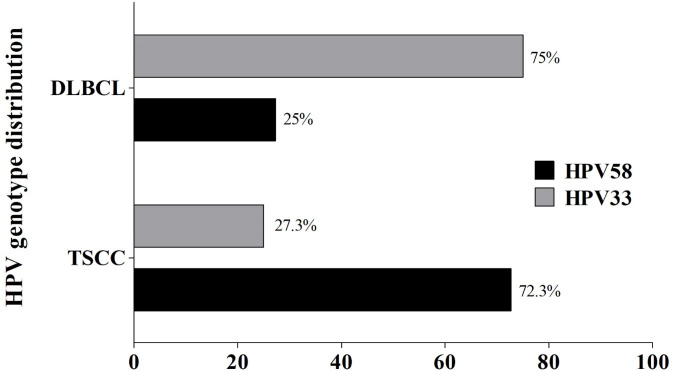
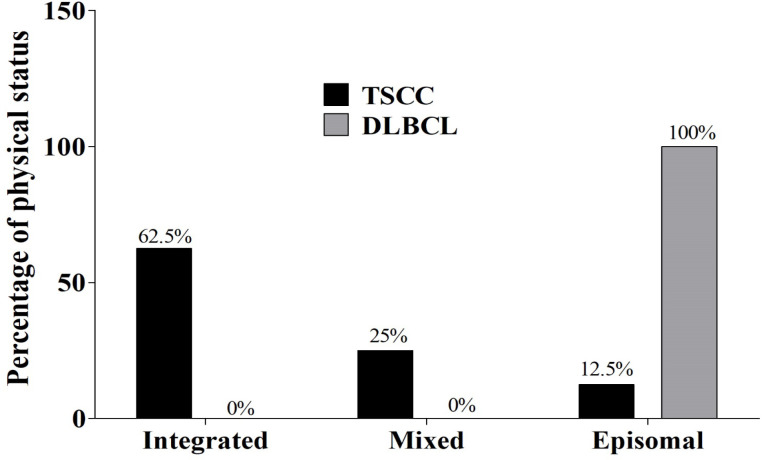
Here, HPV58 was the most frequently detected genotype in TSCC samples (72.7 %), whereas HPV33 was found in the majority of DLBCL cases (75.0%, Figure 3). Among NCC samples, only one case of HPV infection was detected: the genotype was HPV16. Therefore, we further examined the physical status of HPV58 infection by multiplex qPCR. Among TSCC samples, pure integrated form of HPV58 genome (5/8, 62.5%) was the most common, followed by mixed form (2/8, 25%) and pure episomal form (1/8, 12.5%), respectively (Figure 4). Conversely, in DLBCL, genomes in the only sample infected with HPV58 were episomal (Figure 4). This result might confirm that HR-HPV, especially HPV58, play a role in the carcinogenesis of TSCC rather than of DLBCL.
Figure 3.
Distribution of HPV Genotypes in TSCC and DLBCL Using RLBH Method
Figure 4.
HPV58 Genome Physical Status in TSCC and DLBCL Using Multiplex qPCR
Localization of EBV infection in tonsil tissues
To further determine the localization of EBV infection in TC, EBER ISH, a gold-standard method for detecting latent EBV infection, was performed. Figure 5 shows the EBER-positive signals that were found predominantly in the nuclei of infiltrating lymphocytes. In DLBCL samples, positive reactivity for EBER was found only in infiltrating lymphocytes. Interestingly, TSCC tissue samples, the positive signals of EBER were also found in both epithelial cells and in infiltrating lymphocytes. This result might suggest that EBV infection plays an important role in both TSCC by establishing latent infection of tonsil squamous epithelial cells and in DLBCL by direct effect on lymphocytes.
Correlation of HPV and EBV infection with histological grade of TSCC
In the present study, TSCC was well correlated with HPV and EBV infection. Therefore, an association of HPV and/or EBV infection with the histological grade of TSCC was investigated. The result showed mostly HPV infection was detected in low-grade tumor tissues (well differentiated, 54.5%) followed by intermediate-grade (moderately differentiated, 27.3%) and high-grade (poorly differentiated, 18.2%) tumors. The same pattern was found in EBV infection and in the combined infections of HPV and EBV (Figure 6). These correlations suggest that TSCC patients even with HPV or EBV or HPV and EBV infections should experience good clinical outcomes.
Discussion
The prevalence of HPV and EBV infections was investigated in patients with specific histology types of TC including TSCC and DLBCL in northeastern Thailand. HPV genotype and the physical status of HPV genome, as well as localization of EBV infection, were also determined to evaluate their association with development of TC. The high prevalence of HPV infection was found in TC cases than exfoliated tonsil cell samples from NCC. Prevalence of HPV infection differed significantly between TSCC and DLBCL cases (Figure 2). Previous studies have shown a low prevalence of HPV in cancer-free tonsils (Rusan et al., 2015; Herberhold et al., 2016). On the other hand, a high prevalence of HPV was reported in TSCC cases. In South Korea, HPV infection in TSCC increased from 5.9 % in 1991 to 31.6% in 2009 (No et al., 2015). In Western countries, the incidence of TC and proportion of HPV-positive cases increased 2.8- and 2.9-fold between 1970 and 2002, respectively, suggesting an important role of HPV in TSCC (Hammarstedt et al., 2006). Similarly, the overall prevalence of HPV in TSCC from 2000-2010 in Denmark was 58% and the incidence of TSCC and HPV-positive TSCC cases increased to 2.7% and 4.9% per year, respectively (Garnaes et al., 2015). The prevalence of HPV in DLBCL cases has not been reported yet. However, there is a study that reported that HPV infection is associated with an increased risk of lymphoma and suggested that the persistent HPV infection may induce chronic immune activation and/or failure of the immune system to clear HPV infection and to control lymphoma development (Intaraphet et al., 2015). Collectively, these findings suggest that HPV, and especially the integrated form of HPV58, is associated with TSCC. The results agree with previous studies showing that integration of HPV genome was mostly detected in TSCC cases (Badaracco et al., 2007; Hassani et al., 2015). In addition, integration of the HPV16 genome is significantly associated with the amplification of c-myc and up-regulation of HIF-1α that may directly influence carcinogenesis of the tonsils (Kim et al., 2007).
The prevalence of EBV infection in TC was significantly lower than in exfoliated tonsil cell samples from NCC. In addition, among TC samples, the prevalence of EBV infection was significantly higher among DLCBL than TSCC. This is consistent with a previous study in Japan which found the prevalence of EBV infection of 74% and 71% in adenoids and tonsils of an adult group, respectively, and 66% and 63% in adenoids and tonsils of a child group (Seishima et al., 2017). A survey among nationals of the United Arab Emirates using EBER ISH reported the prevalence of EBV infection in tonsils and adenoids was 43% and 15%, respectively, and EBV was detected in B lymphocytes (Al-Salam e al., 2011). EBV is therefore very common in Waldeyer’s ring, particular the palatine tonsils, and may have a role in carcinogenesis. However, the prevalence of EBV infections in TC is still low, for example, only 1.9% in a study of pharyngeal tonsillar carcinomas (Khabie et al., 2001). On the other hand, a high prevalence of EBV DNA (86%) was reported in palatine tonsil carcinoma patients, suggesting that this carcinoma may be associated with EBV (Szkaradkiewicz et al., 2002).
In contrast to TSCC, several studies have investigated the association of EBV infection with DLBCL. For instance, EBV DNA was detected in 26.5% of non-Hodgkin’s lymphomas in Khorasan, Iran. Of these, 50% of DLBCL cases were positive for EBV, suggesting that EBV may be an etiological factor in some subtypes of non-Hodgkin’s lymphomas, especially DLBCL (Abadi et al., 2013). In South Korea, the prevalence of EBV in DLCBL cases, as determined using EBER ISH, was 6.7% (Hong et al., 2017). Furthermore, LMP1 and EBNA2 were expressed in 92% and 15% of EBV-positive DLBCL cases, respectively (Hofscheier et al., 2011). Ok et al (2014) reported that the prevalence of EBV in DLBCL cases in Western countries was 4%, as evidenced by EBER ISH. Of these, 66.7% were positive for LMP1 and 26.1% were positive for EBNA2. Based on expression of EBER, LMP1, and other genes, 34.8%, 39.1% and 26.1% of cases were classified as EBV latency I, II and III, respectively (Ok et al., 2014). In addition, EBV infection in DLBCL cases was suggested to be associated with worse outcomes or poor prognosis (Uner et al., 2011; Hong et al., 2017). EBV was mostly found in infiltrating cells. However, the epithelial cells also showed a positive signal for EBER. Possibly, infection of epithelial cells of tonsil tissues by EBV may play a role in progression of tonsil cancer. Consistent with this, in pharyngeal tonsils, almost 60% of EBER-positive cells were found in T-cell-rich interfollicular regions, 27% and 15% were present within the tonsillar crypts and B-cell-rich germinal centers of secondary follicles, respectively (Hudnall et al., 2005; Gotoh et al., 2011; Kaneko et al., 2014). Taken together, the infection of EBV may be a risk factor promoting the tumorigenesis of DLBCL.
In conclusion, HPV and EBV infection are significantly associated with TC development, but are prominent in different histological types. Infection by HPV, in particular HPV58, was significantly associated with TSCC, while EBV was significantly associated with DLBCL. Combined infections of HPV and EBV also influence carcinogenesis of both TSCC and DLBCL. TSCC patients with infection of HPV and EBV seem to have better outcomes than those without infection. However, the mechanisms behind the oncogenic role of HPV and EBV in TSCC and DLBCL need to be studied further.
Author Contribution Statement
Chukkris Heawchaiyaphum: conceptualization, methodology, validation, formal analysis, investigation, data curation, writing—original draft preparation, visualization, writing—review and editing, visualization; Tipaya Ekalaksananan: conceptualization, data curation, writing—review and editing, supervision, funding acquisition; Natcha Patarapadungkit: resources; Patravoot Vatanasapt: resources; Chamsai Pientong: Conceptualization, data curation, writing—review and editing, supervision, project administration, funding acquisition.
Acknowledgements
We would like thank to all participants enrolled in this study and all medical staff of Srinagarind Hospital, Khon Kaen, Thailand for the collection of specimens. We would like to acknowledge Prof. David Blair, for editing the MS via Publication Clinic KKU, Thailand.
Funding Statement
This study was financially supported by the Thailand Research Fund through the Royal Golden Jubilee Ph.D. Program (Grant No. PHD/0141/2554) and by Khon Kaen University, Khon Kaen, Thailand (Grant No. 581203).
Ethical Statement
The use of exfoliated tonsil cells and FFPE tissue archived specimens was approved by the Khon Kaen University Ethics Committee for Human Research (No. HE561407 and HE581159, respectively).
Data Availability Statement
Data are provided within the article.
Declaration of Competing Interest
The authors declare no potential conflicts of interest.
References
- Abadi RZM, Sistani NS, Mohtasham N, et al. The prevalence of Epstein-Barr virus infection in head and neck non-Hodgkin’s lymphomas in Khorasan, northeast of Iran. J Pak Med Assoc. 2013;63:882–7. [PubMed] [Google Scholar]
- Al-Salam S, Al Dhaheri S, Awwad A, et al. Prevalence of Epstein–Barr virus in tonsils and adenoids of United Arab Emirates nationals. Int J Pediatr Otorhinolaryngol. 2011;75:1160–6. doi: 10.1016/j.ijporl.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aromseree S, Chaiwongkot A, Ekalaksananan T, et al. The three most common human papillomavirus oncogenic types and their integration state in Thai women with cervical precancerous lesions and carcinomas. J Med Virol. 2014;86:1911–9. doi: 10.1002/jmv.24034. [DOI] [PubMed] [Google Scholar]
- Aviles A, Delgado S, Ruiz H, et al. Treatment of non-Hodgkin’s lymphoma of Waldeyer’s ring: radiotherapy versus chemotherapy versus combined therapy. Eur J Cancer B Oral Oncol. 1996;32:19–23. doi: 10.1016/0964-1955(95)00058-5. [DOI] [PubMed] [Google Scholar]
- Badaracco G, Rizzo C, Mafera B, et al. Molecular analyses and prognostic relevance of HPV in head and neck tumours. Oncol Rep. 2007;17:931–40. [PubMed] [Google Scholar]
- Banfi A, Bonadonna G, Carnevali G, et al. Lymphoreticular sarcomas with primary involvement of Waldeyer’s ring Clinical evaluation of 225 cases. Cancer. 1970;26:341–51. doi: 10.1002/1097-0142(197008)26:2<341::aid-cncr2820260216>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Deng Z, Uehara T, Maeda H, et al. Epstein-Barr virus and human papillomavirus infections and genotype distribution in head and neck cancers. PLoS One. 2014;9 doi: 10.1371/journal.pone.0113702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg JW, Fisher RI. Diffuse large B-cell lymphoma. Hematol Oncol Clin North Am. 2008;22:941–52. doi: 10.1016/j.hoc.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnaes E, Kiss K, Andersen L, et al. A high and increasing HPV prevalence in tonsillar cancers in Eastern Denmark, 2000–2010: The largest registry-based study to date. Int J Cancer. 2015;136:2196–203. doi: 10.1002/ijc.29254. [DOI] [PubMed] [Google Scholar]
- Gotoh K, Ito Y, Maruo S, et al. Replication of Epstein-Barr virus primary infection in human tonsil tissue explants. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarstedt L, Lindquist D, Dahlstrand H, et al. Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer. 2006;119:2620–3. doi: 10.1002/ijc.22177. [DOI] [PubMed] [Google Scholar]
- Hassani S, Castillo A, Ohori J-I, et al. Molecular pathogenesis of human papillomavirus type 16 in Tonsillar squamous Cell Carcinoma. Anticancer Res. 2015;35:6633–8. [PubMed] [Google Scholar]
- Herberhold S, Hellmich M, Panning M, et al. Human polyomavirus and human papillomavirus prevalence and viral load in non-malignant tonsillar tissue and tonsillar carcinoma. Med Microbiol Immunol. 2016;206:93–103. doi: 10.1007/s00430-016-0486-6. [DOI] [PubMed] [Google Scholar]
- Hofscheier A, Ponciano A, Bonzheim I, et al. Geographic variation in the prevalence of Epstein–Barr virus-positive diffuse large B-cell lymphoma of the elderly: a comparative analysis of a Mexican and a German population. Mod Pathol. 2011;24:1046–54. doi: 10.1038/modpathol.2011.62. [DOI] [PubMed] [Google Scholar]
- Hong JY, Ryu KJ, Park C, et al. Clinical impact of serum survivin positivity and tissue expression of EBV-encoded RNA in diffuse large B-cell lymphoma patients treated with rituximab–CHOP. Oncotarget. 2017;8:13782. doi: 10.18632/oncotarget.14636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudnall SD, Ge Y, Wei L, et al. Distribution and phenotype of Epstein–Barr virus-infected cells in human pharyngeal tonsils. Mod Pathol. 2005;18:519–27. doi: 10.1038/modpathol.3800369. [DOI] [PubMed] [Google Scholar]
- Intaraphet S, Farkas DK, Johannesdottir Schmidt SA, et al. Human papillomavirus infection and lymphoma incidence using cervical conization as a surrogate marker: a Danish nationwide cohort study. Hematol Oncol. 2015;35:172–176. doi: 10.1002/hon.2270. [DOI] [PubMed] [Google Scholar]
- Jacobs C, Hoppe RT. Non-Hodgkin’s lymphomas of head and neck extranodal sites. Int J Radiat Oncol Biol Phys. 1985;11:357–64. doi: 10.1016/0360-3016(85)90158-0. [DOI] [PubMed] [Google Scholar]
- Jacobs C, Weiss L, Hoppe RT. The management of extranodal head and neck lymphomas. Arch Otolaryngol Head Neck Surg. 1986;112:654–8. doi: 10.1001/archotol.1986.03780060066010. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Kojima M, Suzuki S, et al. Atypical interfollicular hyperplasia of tonsils resembling mucosa-associated lymphoid tissue lymphoma: a clinicopathological, immunohistochemical study and Epstein-Barr virus findings in 12 cases. J Clin Exp Hematop. 2014;54:111–6. doi: 10.3960/jslrt.54.111. [DOI] [PubMed] [Google Scholar]
- Khabie N, Savva A, Kasperbauer JL, et al. Epstein-Barr Virus DNA is not increased in Tonsillar Carcinoma. Laryngoscope. 2001;111:811–4. doi: 10.1097/00005537-200105000-00011. [DOI] [PubMed] [Google Scholar]
- Kim SH, Koo BS, Kang S, et al. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int J Cancer. 2007;120:1418–25. doi: 10.1002/ijc.22464. [DOI] [PubMed] [Google Scholar]
- Klussmann JP, Weissenborn SJ, Wieland U, et al. Human papillomavirus-positive tonsillar carcinomas: a different tumor entity? Med Microbiol Immunol. 2003;192:129–32. doi: 10.1007/s00430-002-0126-1. [DOI] [PubMed] [Google Scholar]
- Korkolopoulou P, Vassilakopoulos T, Milionis V, et al. Recent advances in aggressive large B-cell lymphomas: A Comprehensive Review. Adv Anat Pathol. 2016;23:202–43. doi: 10.1097/PAP.0000000000000117. [DOI] [PubMed] [Google Scholar]
- Martelli M, Ferreri AJ, Agostinelli C, et al. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87:146–71. doi: 10.1016/j.critrevonc.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Nasman A, Nordfors C, Holzhauser S, et al. Incidence of human papillomavirus positive tonsillar and base of tongue carcinoma: a stabilisation of an epidemic of viral induced carcinoma? Eur J Cancer. 2015;51:55–61. doi: 10.1016/j.ejca.2014.10.016. [DOI] [PubMed] [Google Scholar]
- No JH, Sung MW, Hah JH, et al. Prevalence and prognostic value of human papillomavirus genotypes in tonsillar squamous cell carcinoma: A Korean multicenter study. Cancer. 2015;121:535–44. doi: 10.1002/cncr.29086. [DOI] [PubMed] [Google Scholar]
- Nordfors C, Vlastos A, Du J, et al. Human papillomavirus prevalence is high in oral samples of patients with tonsillar and base of tongue cancer. Oral Oncol. 2014;50:491–7. doi: 10.1016/j.oraloncology.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Ok CY, Li L, Xu-Monette ZY, et al. Prevalence and clinical implications of Epstein–Barr virus infection in de novo diffuse large B-cell lymphoma in Western countries. Clin Cancer Res. 2014;20:2338–49. doi: 10.1158/1078-0432.CCR-13-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Meng B, Zhang H, et al. Low incidence of Epstein–Barr virus-positive diffuse large B-cell lymphoma of the elderly in Tianjin, northern China. Leuk Lymphoma. 2013;54:298–303. doi: 10.3109/10428194.2012.715347. [DOI] [PubMed] [Google Scholar]
- Pannone G, Santoro A, Papagerakis S, et al. The role of human papillomavirus in the pathogenesis of head & neck squamous cell carcinoma: an overview. Infect Agent Cancer. 2011;6:4. doi: 10.1186/1750-9378-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peferoen LA, Lamers F, Lodder LN, et al. Epstein Barr virus is not a characteristic feature in the central nervous system in established multiple sclerosis. Brain. 2010;133:e137–e. doi: 10.1093/brain/awp296. [DOI] [PubMed] [Google Scholar]
- Polz-Gruszka D, Stec A, Dworzanski J, et al. EBV, HSV, CMV and HPV in laryngeal and oropharyngeal carcinoma in Polish patients. Anticancer Res. 2015;35:1657–61. [PubMed] [Google Scholar]
- Pozo F, Tenorio A. Detection and typing of lymphotropic herpesviruses by multiplex polymerase chain reaction. J Virol Methods. 1999;79:9–19. doi: 10.1016/s0166-0934(98)00164-5. [DOI] [PubMed] [Google Scholar]
- Prasongdee P, Tippayawat P, Limpaiboon T, et al. The development of simultaneous measurement of viral load and physical status for human papillomavirus 16 and 18 coinfection using multiplex quantitative polymerase chain reaction. Oncol Lett. 2018;16:6977–87. doi: 10.3892/ol.2018.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusan M, Klug TE, Henriksen J, et al. Prevalence of tonsillar human papillomavirus infections in Denmark. Eur Arch Otorhinolaryngol. 2015;272:2505–12. doi: 10.1007/s00405-014-3225-x. [DOI] [PubMed] [Google Scholar]
- Seishima N, Kondo S, Wakisaka N, et al. EBV infection is prevalent in the adenoid and palatine tonsils in adults. J Med Virol. 2017;89:1088–95. doi: 10.1002/jmv.24737. [DOI] [PubMed] [Google Scholar]
- Szkaradkiewicz A, Kruk-Zagajewska A, Wal M, et al. Epstein-Barr virus and human papillomavirus infections and oropharyngeal squamous cell carcinomas. Clin Exp Med. 2002;2:137–41. doi: 10.1007/s102380200019. [DOI] [PubMed] [Google Scholar]
- Teeratakulpisarn J, Ekalaksananan T, Pientong C, et al. Human metapneumovirus and respiratory syncytial virus detection in young children with acute bronchiolitis. Asian Pac J Allergy Immunol. 2007;25:139–45. [PubMed] [Google Scholar]
- Turculeanu A, Mogoanta CA, IoniTa E, et al. TNF-alpha evaluation in tonsil cancer. Rom J Morphol Embryol. 2015;56:101–6. [PubMed] [Google Scholar]
- Uner A, Akyurek N, Saglam A, et al. The presence of Epstein–Barr virus (EBV) in diffuse large B-cell lymphomas (DLBCLs) in Turkey: special emphasis on ‘EBV-positive DLBCL of the elderly’. APMIS. 2011;119:309–16. doi: 10.1111/j.1600-0463.2011.02736.x. [DOI] [PubMed] [Google Scholar]
- van den Brule AJ, Pol R, Fransen-Daalmeijer N, et al. GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J Clin Microbiol. 2002;40:779–87. doi: 10.1128/JCM.40.3.779-787.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherspoon DJ, Chattopadhyay A, Boroumand S, et al. Oral cavity and oropharyngeal cancer incidence trends and disparities in the United States: 2000–2010. Cancer Epidemiol. 2015;39:497–504. doi: 10.1016/j.canep.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka N, Harabuchi Y, Sambe S, et al. Non-Hodgkin’s lymphoma of Waldeyer’s ring and nasal cavity. Clinical and immunologic aspects. Cancer. 1985;56:768–76. doi: 10.1002/1097-0142(19850815)56:4<768::aid-cncr2820560412>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Zaravinos A. An updated overview of HPV-associated head and neck carcinomas. Oncotarget. 2014;5:3956–69. doi: 10.18632/oncotarget.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are provided within the article.