Rituximab is an anti-CD20 monoclonal antibody medication that targets B cells and is part of the current recommended regimen for posttransplant lymphoproliferative disease (PTLD) (1). A randomized placebo-controlled trial demonstrated that B-cell depletion after rituximab induction treatment had no significant effect on ulcerative colitis (UC) (2). However, there have been reports that rituximab may exacerbate UC by depleting CD20-positive mucosal B cells associated with suppression of local IL-10 production (3,4). Figures 1 and 2 demonstrate exacerbation of UC after rituximab induction treatment for PTLD.
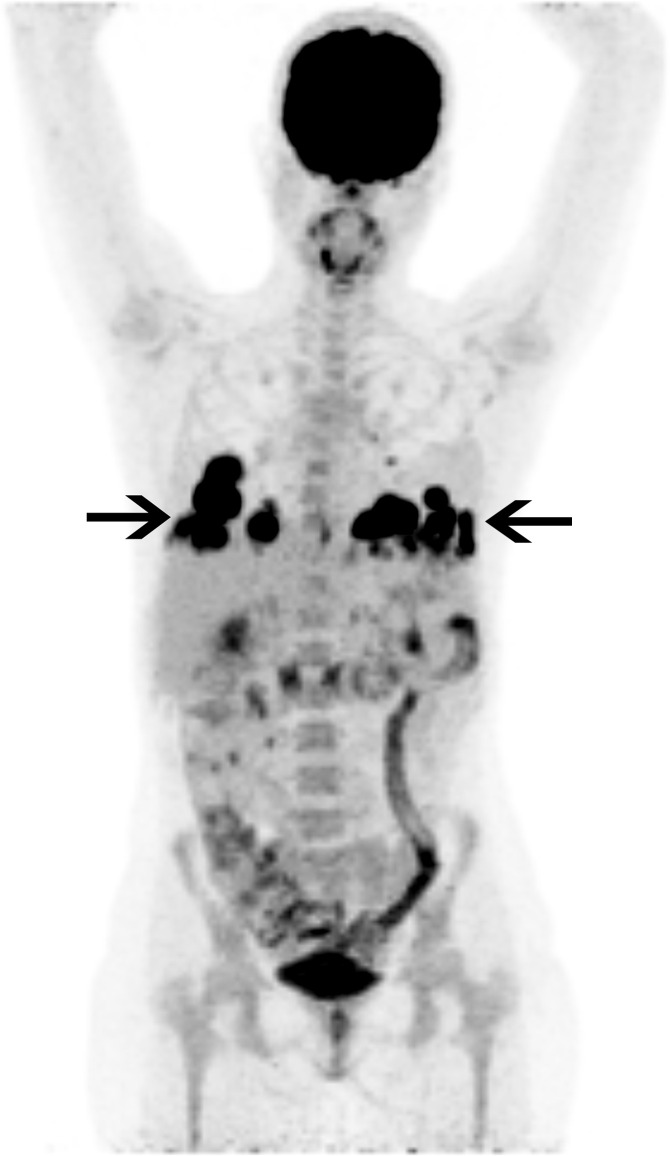
Figure 1:
Fluorodeoxyglucose (FDG) PET/CT image in a 27-year-old woman with ulcerative colitis and sclerosing cholangitis who presented with fever and fatigue. She underwent liver transplantation 3 years prior and was receiving immunosuppressive treatment with tacrolimus. Her chest radiograph (not shown) showed basilar pulmonary masses, which were FDG avid on PET/CT image (black arrows). Histologic confirmation following a CT-guided core biopsy indicated pulmonary posttransplant lymphoproliferative disease.
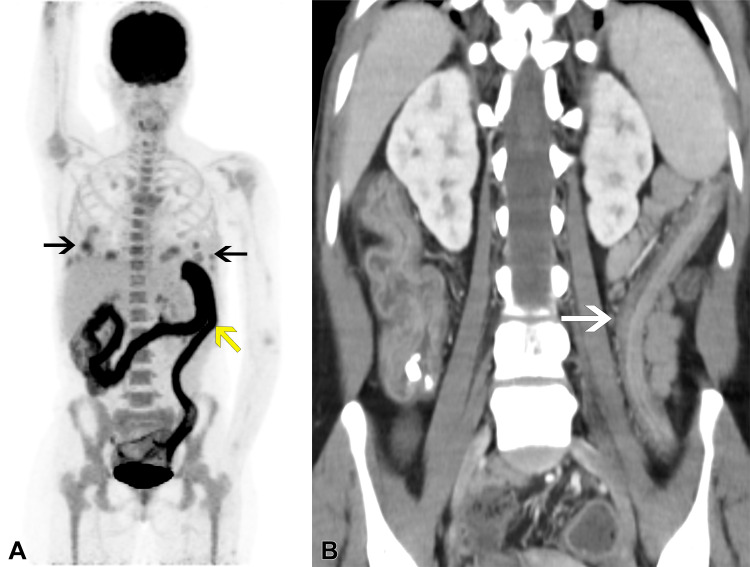
Figure 2:
Follow-up imaging in the same patient after tacrolimus withdrawal and initiation of rituximab induction therapy. Despite initial improvement, the patient presented with fever, abdominal pain, and bloody diarrhea after 2 months, before starting chemotherapy. (A) PET/CT image reveals improved uptake levels of the pulmonary masses (black arrows), but intense, diffuse, pancolonic fluorodeoxyglucose uptake was detected, compatible with active colitis (yellow arrow). (B) Corresponding coronal CT image reveals characteristic “lead-pipe” colon (white arrow), indicating clinical suspicion of ulcerative colitis exacerbation secondary to rituximab. Laboratory test results ruled out infection. Low-dose corticosteroid treatment was started with immediate clinical improvement.
PTLD treatment is complex, involving withdrawal of immunosuppression and administration of rituximab and chemotherapy. When patients are also experiencing UC, the condition may be challenging to treat, and patients should be followed up periodically. Although fluorodeoxyglucose colonic uptake may appear as a physiologic phenomenon or in patients treated with metformin (5), a diffuse pattern with intense uptake favors diagnosis of UC exacerbation secondary to rituximab, requiring treatment adaptation.
Footnotes
Authors declared no funding for this work.
Disclosures of conflicts of interest: M.K. Trainee editorial board member of Radiology: Imaging Cancer. S.A. No relevant relationships. Y.E. No relevant relationships. E.M.M. Honorarium for giving a lecture from Boehringer Ingelheim and Merck Sharp & Dohme.
Keywords: PET/CT, Lymphatic, Thorax, Abdomen/GI, Monoclonal Antibodies
References
- 1. Trappe R , Oertel S , Leblond V , et al . Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial . Lancet Oncol 2012. ; 13 ( 2 ): 196 – 206 . [DOI] [PubMed] [Google Scholar]
- 2. Leiper K , Martin K , Ellis A , et al . Randomised placebo-controlled trial of rituximab (anti-CD20) in active ulcerative colitis . Gut 2011. ; 60 ( 11 ): 1520 – 1526 . [DOI] [PubMed] [Google Scholar]
- 3. Goetz M , Atreya R , Ghalibafian M , Galle PR , Neurath MF . Exacerbation of ulcerative colitis after rituximab salvage therapy . Inflamm Bowel Dis 2007. ; 13 ( 11 ): 1365 – 1368 . [DOI] [PubMed] [Google Scholar]
- 4. El Fassi D , Nielsen CH , Kjeldsen J , Clemmensen O , Hegedüs L . Ulcerative colitis following B lymphocyte depletion with rituximab in a patient with Graves’ disease . Gut 2008. ; 57 ( 5 ): 714 – 715 . [DOI] [PubMed] [Google Scholar]
- 5. Gontier E , Fourme E , Wartski M , et al . High and typical 18F-FDG bowel uptake in patients treated with metformin . Eur J Nucl Med Mol Imaging 2008. ; 35 ( 1 ): 95 – 99 . [DOI] [PubMed] [Google Scholar]