Abstract
Background
Despite advances in diagnostic and therapeutic approaches, candidemia remains associated with high mortality rates. This study aimed at identifying predictors of mortality among patients with candidemia, with a focus on early interventions that can improve prognosis.
Methods
This was a single-center retrospective study including all adult patients with at least 1 positive blood culture for Candida species from 2014 to 2021.
Results
A total of 222 episodes of candidemia were included. Most candidemias were of unknown origin (36%) or vascular catheter related (29%). Septic shock developed in 29% episodes. Overall, 14-day mortality rate was 23%. In univariate analyses, septic shock was associated with higher 14-day mortality, whereas catheter-related candidemia and early (<72 hours) interventions, such as appropriate antifungal therapy, source control, and infectious diseases consultation, were associated with improved survival. In a Cox multivariate regression model, septic shock (odds ratio [OR], 3.62 [95% confidence interval {CI}, 2.05–6.38]) was associated with higher mortality. While the impact of early antifungal therapy did not reach statistical significance, early (<72 hours) infectious diseases consultation (OR, 0.46 [95% CI, .23–.91]) and early source control (OR, 0.15 [95% CI, .08–.31]) were associated with better survival. Subanalyses showed that the benefits of early source control, specifically catheter removal, were significant among patients with sepsis or septic shock, but not among those without sepsis. These associations remained significant after exclusion of patients who died prematurely or were in palliative care.
Conclusions
Early source control, in particular catheter removal, was a key determinant of outcome among candidemic patients with sepsis or septic shock.
Keywords: antifungal treatment, catheter removal, sepsis, septic shock, source control
This analysis of the predictors of mortality in candidemia showed that early (<72 hours) source control, in particular vascular catheter removal, was a key determinant of outcome among patients with sepsis or septic shock.
Candidemia remains an important cause of hospital-acquired bloodstream infection (BSI), as shown by a European point prevalence study during 2011–2012, where Candida spp constituted the sixth most common group of pathogens, accounting for 7% of such infections [1]. In a study from 7 Swiss hospitals (including community and nosocomial infections), Candida spp counted for 2% of pathogens recovered from positive BSIs during 2014–2018, being the tenth most common group of pathogens [2].
Despite recent advances in diagnostic and therapeutic procedures, candidemia is still associated with high morbidity and mortality rates (30%–40%), in particular among patients with septic shock (50%–70%) [3–8]. Early start of appropriate antifungal therapy was shown to play an important role in survival [9–14].
Catheter removal among patients with presumed catheter-related candidemia is highly recommended by the Infectious Diseases Society of America (IDSA) [15]. While the benefit of this intervention has been shown in some studies [4, 11, 14, 16, 17], others failed to demonstrate an impact on survival [5, 18]. Other interventions for source control, such as drainage of abscesses in intra-abdominal candidiasis, are also associated with improved outcomes [10, 19, 20].
The aim of the present study was to identify predictors of mortality in candidemic patients and especially the role of early interventional procedures, such as infectious diseases (ID) consultation, appropriate antifungal therapy, and adequate source control.
METHODS
Study Design
This retrospective study was conducted at the Lausanne University Hospital (Lausanne, Switzerland), a 1500-bed tertiary care hospital with 35 intensive care unit (ICU) beds, during an 8-year period (2014–2021). The study was approved by the institutional ethics review board (Swissethics Project 2021-02516) for the retrospective use of clinical data.
Patients
All adult patients (≥18 years old) who had at least 1 positive blood culture bottle for a Candida spp were included, provided that there was no attestation of refusal of general consent. Mortality at day 14 was the primary outcome. Date regarding demographics (age, sex), comorbidities, signs and symptoms of infection, type and severity of infection, laboratory results, antifungal treatment, source control (ie, catheter removal, radiological, or surgical interventional procedures), decisions of care withdrawal and outcomes, were collected in the patients’ electronic health records. Candida spp were identified by matrix-assisted laser desorption/ionization–time of flight mass spectrometry (Bruker, Billerica, Massachusetts) and antifungal susceptibility testing was performed by microbroth dilution method (Sensititre YeastOne, Trek Diagnostics Systems, ThermoFisher Scientific, Cleveland, Ohio). Results of minimum inhibitory concentrations were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) clinical breakpoints [21]. All data were collected, stored, and managed using REDCap (Research Electronic Data Capture) by an ID specialist. REDCap is hosted at Lausanne University Hospital. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies [22, 23].
Definitions
The date of collection of the first positive blood culture was defined as infection onset. According to internal guidelines, an ID consultation was performed on a mandatory basis within the same day of blood culture positivity for Candida spp. A new episode was considered if >30 days had elapsed since the first positive blood culture. Infection was categorized as sepsis or septic shock according to the definition of the Sepsis-3 International Consensus [24]. Catheter-related candidemia was defined according to IDSA guidelines [25]. We used the cutoff of 72 hours to define early interventions, which corresponds to the usual time to positivity of Candida spp in blood cultures. Early appropriate antifungal treatment was defined as initiation of an antifungal agent, for which the pathogenic Candida spp was defined as susceptible or susceptible-dose-dependent according to CLSI criteria [21], within 72 hours from infection onset at an adequate dosage and penetration in the infection site. Source control was considered as warranted in the following scenarios: (1) removal of intravascular catheter (central or peripheral) in patients with catheter-related candidemia or candidemia of unknown origin; and (2) surgical or imaging-guided drainage in the presence of a documented deep infection site (eg, abscess, peritoneal collection, empyema, endocarditis, hydronephrosis). Early source control was defined if the above procedures were performed within 72 hours from candidemia onset. Patients were considered to be on maximal care until a decision of treatment withdrawal or instauration of palliative care was documented in the medical record.
Statistical Analyses
SPSS version 26.0 (SPSS, Chicago, Illinois) software was used for data analysis. Categorical variables were analyzed using the χ2 or Fisher exact test and continuous variables with Mann-Whitney U test. Univariate logistic regression models were assessed with 14-mortality as dependent variable. Covariates were tested for multi-collinearity through variance inflation factor assessment; those clinically relevant and not collinear were used in multivariate analysis. After checking Cox assumptions, a multivariate Cox proportional hazards regression model was performed with 14-day mortality as the time-to-event. For the multivariate analysis, episodes for which no source control was warranted were imputed as appropriate early source control. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of any association. All statistic tests were 2-tailed and P < .05 was considered statistically significant. Kaplan-Meier curves of the survival probability of patients with candidemia according to appropriate early source control and presence of sepsis or septic shock, and of patients with candidemia of unknown origin and catheter-related candidemia according to early source control and presence of sepsis or septic shock, were performed. Since it was previously suggested that source control could be influenced by care withdrawal [3], Kaplan-Meier curves were performed among patients who were alive and in maximal care for 7 days after infection onset in order to assess the role of early source control on survival.
RESULTS
According to the database of the microbiology laboratory, 255 episodes of candidemia were identified, from which 222 episodes of candidemia in 209 patients were included (Figure 1); the 13 subsequent episodes of candidemia occurred at a median of 5 months from the previous episode (range, 2–41 months). A total of 229 Candida spp were isolated (2 different species were isolated in 7 episodes). The median time from blood culture sampling to positivity was 38 hours (range, 4–96 hours) with 68 (31%) episodes for which blood cultures were positive after 72 hours. Time to positivity did not significantly differ according to the source of candidemia. Candida albicans predominated (112 [49%]), followed by Candida glabrata (67 [29%]), Candida tropicalis (19 [8%]), and Candida parapsilosis (11 [5%]). Seventeen isolates (7%) belonged to other Candida spp (C krusei, C dubliniensis, C kefyr, C lusitaniae, C pelliculosa). No significant trend was observed during the study period. According to CLSI criteria, 24 isolates (11%) were resistant to fluconazole and 29 (13%) were resistant or intermediate to at least 1 echinocandin (anidulafungin or micafungin). Most candidemias were of unknown origin (80 [36%]), followed by vascular catheter related (64 [29%]), and secondary to intra-abdominal (54 [24%]) or urinary tract (15 [7%]) infections. Sepsis developed in 122 episodes (55%) and septic shock in 65 (29%). No difference of infection source was observed between patients who developed sepsis and those who did not. Secondary complications of candidemia, such as chorioretinitis or endocarditis, occurred in 5% and 2% of cases, respectively.
Figure 1.
Flowchart of included patents.
ID consultation was provided in 166 (75%) cases within 72 hours from infection onset. Of the remaining 56 patients, 18 had a consultation between 3 and 7 days. Among the 38 who did not have an ID consultation, 11 were deceased or on palliative care when the ID consultant was informed of the positive blood culture (positivity of blood cultures after 72 hours). Antifungal treatment was initiated within 72 hours from infection onset in 174 episodes (78%) and it was appropriate in 154 episodes (69%). Median time to start of antifungal therapy was 1 day (range, 0–5 days). Source control was warranted in 201 episodes (91%); among them it was performed within 72 hours in 110 (55%) cases and beyond 72 hours in 55 (27%) cases, whereas it was not performed in the remaining 36 (18%) cases. Early (<72 hours) source control consisted of intravascular catheter removal in 81 (74%) cases, surgical/radiological procedures of drainage in 25 (23%) cases, and correction of urinary tract obstruction in 4 (4%) cases.
Overall 14-day and 30-day mortality were 23% and 30%, respectively. Seven patients died within 72 hours from candidemia onset. Results of univariate analysis for predictors of 14-day mortality are shown in Table 1. Mortality was significantly higher among patients with more severe baseline conditions (sepsis or septic shock, higher Sequential Organ Failure Assessment score, hospitalization at intermediate care or ICU at the time of candidemia). Intravascular catheter–related candidemia was associated with lower mortality compared to unknown or other sources of infection. Regarding the management of candidemia, early (<72 hours) ID consultation, early appropriate antifungal therapy, and early source control were associated with lower mortality. There was no significant difference regarding the type of antifungal therapy (echinocandins vs other antifungals).
Table 1.
Predictors of 14-Day Mortality of Candidemia Episodes
| Univariate Analysis | Cox Proportional Hazard Multivariate Regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictor | All Episodes (N = 222) | Survivors (n = 171) | Nonsurvivors (n = 51) | P Value | P Value | OR (95% CI) | |||
| Demographics | |||||||||
| Male sex | 146 | (66) | 116 | (68) | 30 | (59) | .234 | … | |
| Age, y, median (Q1–Q3) | 68 | (53–75) | 66 | (51–74) | 70 | (60–76) | .115 | … | |
| Comorbidities | |||||||||
| Congestive heart failure | 26 | (12) | 20 | (12) | 6 | (12) | .989 | … | |
| COPD | 31 | (14) | 25 | (15) | 6 | (12) | .606 | … | |
| Cirrhosis | 34 | (15) | 28 | (16) | 6 | (13) | .613 | … | |
| Diabetes mellitus | 49 | (22) | 38 | (22) | 11 | (22) | .921 | … | |
| CKD (moderate or severe)a | 40 | (18) | 31 | (18) | 9 | (18) | .937 | … | |
| Malignancy (solid organ or hematologic) | 80 | (36) | 58 | (34) | 22 | (43) | .229 | … | |
| Obesity | 49 | (22) | 37 | (22) | 12 | (24) | .775 | … | |
| Immunosuppressionb | 45 | (20) | 37 | (22) | 8 | (16) | .430 | … | |
| Neutropenia | 25 | (11) | 22 | (12) | 3 | (6) | .166 | … | |
| Location at candidemia onset | |||||||||
| Community | 23 | (10) | 18 | (11) | 5 | (10) | … | ||
| Medical or surgical ward | 88 | (40) | 74 | (43) | 14 | (28) | … | ||
| Intermediate or intensive care unit | 111 | (50) | 79 | (46) | 32 | (63) | .038c | … | |
| Microbiological data | |||||||||
| Mixed bacterial/fungal BSI | 46 | (21) | 35 | (21) | 11 | (22) | .651 | … | |
| Multiple Candida spp isolated from blood cultures | 7 | (3) | 7 | (4) | 0 | (0) | .356 | … | |
| Candida spp (n = 229) | … | … | … | … | … | … | … | ||
| C albicans | 112 | (50) | 85 | (50) | 27 | (53) | … | ||
| Non-albicans | 113 | (51) | 89 | (52) | 24 | (47) | .532d | … | |
| C glabrata | 67 | (30) | 52 | (30) | 15 | (29) | … | ||
| C tropicalis | 19 | (9) | 16 | (9) | 3 | (6) | … | ||
| C parapsilosis | 11 | (5) | 10 | (6) | 1 | (2) | … | ||
| Othere | 17 | (8) | 12 | (8) | 5 | (10) | … | ||
| Time to blood culture positivity ≥72 h | 68 | (31) | 51 | (30) | 17 | (33) | .633 | … | |
| Prolonged candidemia (≥48 h) | 55 | (25) | 44 | (26) | 11 | (22) | .546 | … | |
| Nonsusceptibility (resistance or intermediate)f | |||||||||
| Fluconazole | 24 | (11) | 17 | (10) | 7 | (14) | .445 | … | |
| Anidulafungin | 6 | (3) | 5 | (3) | 1 | (2) | 1.000 | … | |
| Micafungin | 28 | (13) | 24 | (14) | 4 | (8) | .242 | … | |
| Infection data | |||||||||
| Fever (≥38°C) | 171 | (77) | 142 | (83) | 29 | (57) | <.001 | … | |
| Sepsis | 122 | (55) | 77 | (45) | 45 | (88) | <.001 | … | |
| Septic shock | 65 | (29) | 36 | (21) | 29 | (58) | <.001 | <.001 | 3.62 (2.05–6.38) |
| SOFA score, median (Q1–Q3) | 4 | (2–9) | 3 | (1–7) | 9 | (4–14) | <.001 | … | |
| Breakthrough infectiong | 28 | (13) | 24 | (14) | 4 | (8) | .242 | … | |
| Infection site | |||||||||
| Unknown origin | 80 | (36) | 56 | (33) | 24 | (47) | … | ||
| Catheter-related (central or peripheral vascular) | 64 | (29) | 56 | (33) | 8 | (16) | .017h | .245 | 0.62 (.29–1.37) |
| Intra-abdominal | 54 | (24) | 39 | (23) | 15 | (29) | … | ||
| Urinary tract infection | 15 | (7) | 14 | (8) | 1 | (2) | … | ||
| Otheri | 9 | (4) | 6 | (4) | 3 | (6) | … | ||
| Complication of candidemia | |||||||||
| Chorioretinitis | 12 | (5) | 12 | (7) | 0 | (0) | .073 | … | |
| Laboratory data | |||||||||
| WBC count, ×109/L, median (Q1–Q3) | 11.8 | (5.9–16.4) | 11.0 | (5.5–14.5) | 15.3 | (11.6–18.8) | <.001 | … | |
| Platelets, ×109/L, median (Q1–Q3) | 210 | (105–343) | 223 | (128–353) | 167 | (71–331) | .078 | … | |
| CRP, mg/L, median (Q1–Q3) (n = 200) | 134 | (72–246) | 118 | (68–223) | 165 | (89–299) | .053 | … | |
| Procalcitonin, μg/L, median (Q1–Q3) (n = 84) | 2.6 | (0.6–13.5) | 1.5 | (0.5–12.5) | 3.2 | (1.8–12.7) | .123 | … | |
| Positive BDG (n = 45) | 39 | (87) | 28 | (82) | 11 | (100) | .134 | … | |
| Management of candidemia | |||||||||
| Antifungal therapy initiated within 72 h | 174 | (78) | 144 | (84) | 30 | (59) | <.001 | … | |
| Echinocandin | 130 | (59) | 105 | (62) | 25 | (49) | .231j | … | |
| Fluconazole | 54 | (24) | 47 | (28) | 7 | (14) | … | ||
| Liposomal amphotericin B | 3 | (1) | 2 | (1) | 1 | (2) | … | ||
| Appropriate antifungal within 72 h | 154 | (69) | 129 | (75) | 25 | (49) | <.001 | .314 | 0.70 (.35–1.40) |
| Source control (n = 201) | 165 | (82) | 147 | (96) | 18 | (38) | <.001 | … | |
| Source control within 72 h (n = 201) | 110 | (55) | 104 | (68) | 6 | (13) | <.001 | <.001 | 0.15 (.08–.31) |
| ID consultation within 72 h | 166 | (75) | 140 | (82) | 26 | (51) | <.001 | .026 | 0.46 (.23–.91) |
Data are depicted as No. (%) unless otherwise indicated.
Abbreviations: BDG, β-d-glucan; BSI, bloodstream infection; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; ID, infectious diseases; OR, odds ratio; Q1, quartile 1; Q3, quartile 3; SOFA, Sequential Organ Failure Assessment; WBC, white blood cell.
Defined as estimated glomerular filtration rate <60 mL/min/1.73 m2.
Immunosuppression was defined as ongoing immunosuppressive treatment at infection onset, intravenous chemotherapy in the 30 days prior to infection onset, AIDS, neutropenia, and asplenia.
Comparison against both community and medical or surgical wards.
Comparison of non-albicans Candida spp vs C albicans.
Eight Candida krusei, 4 Candida dubliniensis, 2 Candida kefyr, 2 Candida lusitaniae, 1 Candida pelliculosa.
According to the Clinical and Laboratory Standards Institute.
Breakthrough infection was defined as the occurrence of candidemia in a patient having received at least 3 consecutive days of systemic antifungal therapy.
Comparison against non-catheter-related candidemia.
Five endocarditis, 3 empyema, 1 deep surgical site infections.
Comparison echinocandins vs other antifungals.
In Cox multivariate regression models (Table 1), 5 clinically relevant variables were used (catheter-related candidemia, septic shock, early ID consultation, early appropriate antifungal treatment, and early source control). Septic shock (OR, 3.62 [95% CI, 2.05–6.38]; P <.001) was associated with 14-day mortality, while early ID consultation (OR, 0.46 [95% CI, .23–.91]; P = .026) and early source control (OR, 0.15 [95% CI, .08–.31]; P < .001) were associated with better survival. Early appropriate antifungal therapy and catheter-related candidemia did not reach statistical significance in this model.
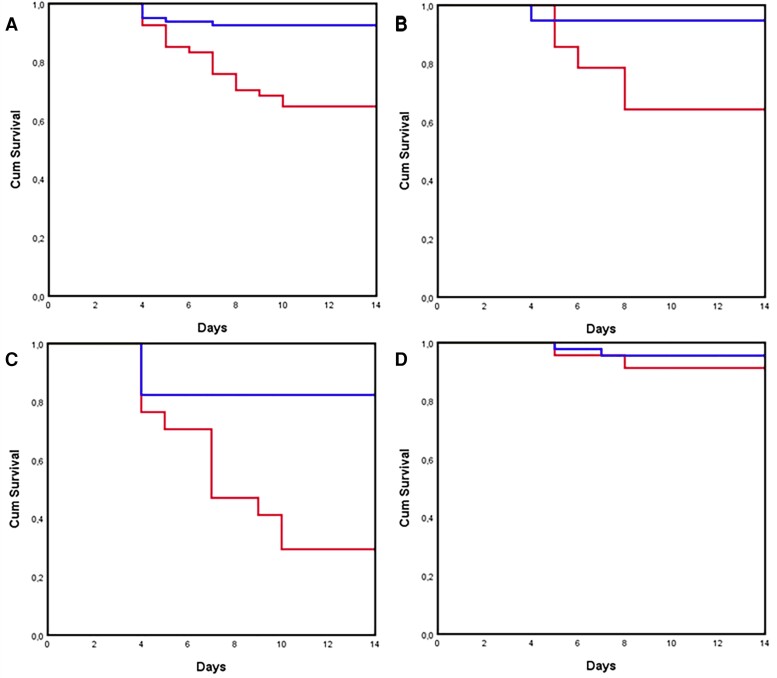
Figure 2 shows Kaplan-Meier curves of the survival probability of patients with candidemia according to early source control in the 201 episodes with survival ≥72 hours for which source control was warranted. Early source control was associated with better outcome in all episodes (Figure 2A, P < .001) and in the subgroups of sepsis (Figure 2B, P = .001) and septic shock (Figure 2C, P < .001), but no association was found in patients without sepsis (Figure 2D, P = .143). The association of early source control and improved survival was still significant in a subanalysis restricted to the 156 patients who were alive and in maximal care for 7 days after candidemia onset and for which source control was warranted (Supplementary Figure 1A, P < .001).
Figure 2.
Kaplan-Meier curves of the survival probability of patients with candidemia according to early source control in the 201 episodes with survival ≥72 hours for which source control was warranted. Early source control was associated with better outcome in all episodes (A) (P < .001) and in the subgroups of sepsis (B) (P = .001) and septic shock (C) (P < .001), but no association was found in patients without sepsis (D) (P = .143). Red line: no early source control, blue line: early source control.
Figure 3 shows Kaplan-Meier curves of the survival probability of patients with candidemia of unknown origin and catheter-related candidemia according to early catheter removal in 136 episodes with survival ≥72 hours. Early catheter removal was associated with better outcome in all episodes (Figure 3A, P < .001) and in the subgroups of sepsis (Figure 3B, P = .020) and septic shock (Figure 3C, P = .040), but no association was found in patients without sepsis (Figure 3D, P = .491). Subanalyses showed that early catheter removal was associated with better outcome in both subgroups: vascular catheter related (Supplementary Figure 2A, P = .020) and candidemia of unknown origin (Supplementary Figure 2B, P = .005). The association of catheter removal and improved survival remained significant in the subanalysis restricted to the 121 patients with catheter-related or primary candidemia who were alive and in maximal care for 7 days after candidemia onset (Supplementary Figure 1B, P < .001).
Figure 3.
Kaplan-Meier curves of the survival probability of patients with candidemia of unknown origin and catheter-related candidaemia according to early catheter removal in 136 episodes with survival ≥72 hours. Early catheter removal was associated with better outcome in all episodes (A) (P < .001) and in the subgroups of sepsis (B) (P = .020) and septic shock (C) (P = .040), but no association was found in patients without sepsis (D) (P = .491). Red line: no early catheter removal, blue line: early catheter removal.
Among the 58 patients with a documented source of candidemia that was not catheter-related (ie, exclusion of catheter-related and primary candidemia) who had an intravascular catheter in place, early catheter removal (53%) had no impact on mortality (P = .829).
DISCUSSION
This study assessing the factors associated with mortality in candidemia highlights the crucial role of early interventions, such as source control and involvement of ID specialists. The benefit of early source control, consisting mainly of intravascular catheter removal, was particularly evident among the most critically ill patients (ie, with sepsis or septic shock).
While some studies have shown the impact of abdominal source control on outcomes of intra-abdominal candidiasis [19, 20], the impact of intravascular catheter removal on improved survival has been suggested by a majority of previous reports but not all [3, 4, 6, 9–11, 14, 16–18, 26]. These retrospective observational studies suffer from many biases [27]. The impact of catheter removal on survival may be affected by many factors, such as the severity of infection (no sepsis vs sepsis or septic shock), the timing of mortality endpoint (early or late), the actual source of infection (intravascular catheter vs another source), and the timing of catheter removal and limitations of therapeutic interventions (eg, maximal care vs palliative approach). Few studies have taken these parameters into account. Some of them suggested a benefit of early vs late catheter removal [6, 14, 16, 26]. However, very heterogeneous practices regarding early catheter removal have been observed, which were influenced by the patients’ underlying conditions: ICU patients with maximal care plans were more prone to have their catheter removed compared to less critically ill patients or those with debilitating underlying conditions and a palliative therapeutic plan [3, 28]. After consideration of these confounding factors, the association of catheter retention and mortality often disappeared [5, 10]. In the present study, we tried to take into consideration these different biases. We analyzed the impact of catheter removal in the subgroup of patients with catheter-related candidemia or in the absence of any other documented source of infection (ie, candidemia of unknown origin). We found a positive association with survival in the subgroup of patients with sepsis or septic shock, but not in the less severe forms of infection. To analyze the possible bias of catheter retention due to care withdrawal among the most debilitated patients, we performed a subanalysis among patients who were still alive and under maximal care for 7 days after infection onset: the association of catheter removal and survival was still significant in this subset. While current guidelines recommend removal of intravascular catheters in candidemia [15, 29], this intervention is not always feasible and safe (eg, in case of severe thrombocytopenia, infusion of vasoactive drugs, continuous renal replacement therapy), as illustrated by a randomized trial of candidemia in which early catheter removal was recommended per protocol, but was actually performed in only 51% of patients [30]. Our results might be helpful in identifying the subset of patients who may really benefit from this intervention, such as those with sepsis and documented catheter-related candidemia or primary candidemia of unknown origin.
As previously shown [31, 32], early ID consultation was associated with better outcome, and this association remained significant in the multivariate analysis. ID consultants may favor guidelines’ adherence by systematic checking for recommended interventions, as established by the European Confederation of Medical Mycology QUALity of Clinical Candidemia Management (EQUAL) score, which was shown to improve prognosis of candidemia [33, 34]. According to our internal procedures, an ID consultation is warranted for each candidemic episode within the same day of blood culture positivity. However, 17% of patients did not have a consultation within the first 72 hours, nor later in the course of infection. It is noteworthy that a substantial proportion (29%) of these patients who did not have an ID consultation were already deceased or in palliative care at the time of blood culture positivity (for all of them positivity of blood cultures after 72 hours), which suggests that the lack of ID consultation may be a consequence rather than a cause of worse outcome. In addition, we could not analyze the actual impact of ID consultation on the management of candidemia, such as source control interventions, adjustment of antifungal therapy or diagnostic procedures to detect complications (eg, chorioretinitis, endocarditis).
The importance of early appropriate antifungal therapy to improve the outcomes of candidemia has been previously demonstrated [11–14]. In the present study, we found that this goal was not achieved in about 30% cases, which was due to delayed start of antifungal therapy (ie, >72 hours after the blood culture sampling) in 70% of them and inappropriate early antifungal therapy in 30% of them. While early appropriate antifungal therapy was associated with improved survival in univariate analysis, it failed to achieve statistical significance in the multivariate Cox regression model.
The present study has several limitations. First, it is a single-center study with a relatively low incidence of candidemia. As previously suggested by a study from the same institution, candidemia is increasingly observed among the most debilitated patients with other chronic or acute conditions that may contribute to overall mortality rates [7]. Some data about parameters that may have influenced outcome were lacking (eg, total parenteral nutrition, adherence of attending physicians to the propositions of ID consultants) and/or were not included in the multivariate analysis to avoid overfitting of the model. Finally, the cutoff of 72 hours for management of candidemia might appear arbitrary. While it represents the median time of candidemia detection by blood cultures, delayed time to positivity (≥72 hours) was observed in a substantial proportion (about 30%) of cases, delay in blood culture positivity may be associated with some confounding factors, such as the type of Candida spp (mainly C glabrata). However, the mortality rates between C glabrata and other Candida spp infections did not differ and we did not observe a difference of time to positivity according to the site of infection. The fact that mortality rates among patients with delayed (>72 hours) or no source control was particularly high highlights the need for faster diagnostic tools, such as the T2Candida Panel [35].
In conclusion, this study supports the key role of early source control including intravascular catheter removal in the management of candidemia among the most severely ill patients (ie, with criteria of sepsis or septic shock).
Supplementary Material
Contributor Information
Matthaios Papadimitriou-Olivgeris, Infectious Diseases Service, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland; Service of Hospital Preventive Medicine, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Julien Battistolo, Infectious Diseases Service, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Julien Poissy, Infectious Diseases Service, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland; Unité de Glycobiologie Structurale et Fonctionnelle, Pôle de réanimation, University of Lille, Centre Hospitalier Universitaire de Lille, Lille, France.
Alix Coste, Institute of Microbiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Pierre-Yves Bochud, Infectious Diseases Service, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Thierry Calandra, Infectious Diseases Service, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Laurence Senn, Infectious Diseases Service, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland; Service of Hospital Preventive Medicine, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Frédéric Lamoth, Infectious Diseases Service, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland; Institute of Microbiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Study design: L. S. and F. L. Data collection and management: M. P.-O., J. B., A. C., and J. P. Data interpretation: M. P.-O., P. -Y. B., T. C., L. S., and F. L. Statistical analyses: M. P.-O. and P.-Y. B. Drafting and writing of manuscript: M. P.-O. and F. L. Review of manuscript: A. C., J. P., P.-Y. B., T. C., and L. S.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. European Centre for Disease Prevention and Control (ECDC) . Point prevalence survey of healthcare associated infections and antimicrobial use in European acute care hospitals. Stockholm: ECDC; 2013. [Google Scholar]
- 2. Adam KM, Osthoff M, Lamoth F, et al. Trends of the epidemiology of candidemia in Switzerland: a 15-year FUNGINOS survey. Open Forum Infect Dis 2021; 8:ofab471. doi: 10.1093/ofid/ofab471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Damonti L, Erard V, Garbino J, et al. Catheter retention as a consequence rather than a cause of unfavorable outcome in candidemia. Intensive Care Med 2017; 43:935–9. doi: 10.1007/s00134-017-4737-9. [DOI] [PubMed] [Google Scholar]
- 4. Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 2012; 54:1110–22. doi: 10.1093/cid/cis021. [DOI] [PubMed] [Google Scholar]
- 5. Nucci M, Braga PR, Nouer SA, Anaissie E. Time of catheter removal in candidemia and mortality. Braz J Infect Dis 2018; 22:455–61. doi: 10.1016/j.bjid.2018.10.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee YM, Kim DY, Kim YJ, Park KH, Lee MS. Clinical impacts of delayed central venous catheter removal according to the severity of comorbidities in patients with candidaemia. J Hosp Infect 2019; 103:420–7. doi: 10.1016/j.jhin.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 7. Battistolo J, Glampedakis E, Damonti L, et al. Increasing morbidity and mortality of candidemia over one decade in a Swiss university hospital. Mycoses 2021; 64:1512–20. doi: 10.1111/myc.13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bassetti M, Righi E, Ansaldi F, et al. A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med 2014; 40:839–45. doi: 10.1007/s00134-014-3310-z. [DOI] [PubMed] [Google Scholar]
- 9. Cortes JA, Montanez AM, Carreno-Gutierrez AM, et al. Risk factors for mortality in Colombian patients with candidemia. J Fungi 2021; 7:442. doi: 10.3390/jof7060442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis 2012; 54:1739–46. doi: 10.1093/cid/cis305. [DOI] [PubMed] [Google Scholar]
- 11. Labelle AJ, Micek ST, Roubinian N, Kollef MH. Treatment-related risk factors for hospital mortality in Candida bloodstream infections. Crit Care Med 2008; 36:2967–72. doi: 10.1097/CCM.0b013e31818b3477. [DOI] [PubMed] [Google Scholar]
- 12. Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 2006; 43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 13. Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother 2005; 49:3640–5. doi: 10.1128/AAC.49.9.3640-3645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garnacho-Montero J, Diaz-Martin A, Garcia-Cabrera E, Ruiz Perez de Pipaon M, Hernandez-Caballero C, Lepe-Jimenez JA. Impact on hospital mortality of catheter removal and adequate antifungal therapy in Candida spp. bloodstream infections. J Antimicrob Chemother 2013; 68:206–13. doi: 10.1093/jac/dks347. [DOI] [PubMed] [Google Scholar]
- 15. Pappas PG, Kauffman CA, Andes DR, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 62:e1–50. doi: 10.1093/cid/civ933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puig-Asensio M, Padilla B, Garnacho-Montero J, et al. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population-based surveillance in Spain. Clin Microbiol Infect 2014; 20:O245–254. doi: 10.1111/1469-0691.12380. [DOI] [PubMed] [Google Scholar]
- 17. Horn DL, Ostrosky-Zeichner L, Morris MI, et al. Factors related to survival and treatment success in invasive candidiasis or candidemia: a pooled analysis of two large, prospective, micafungin trials. Eur J Clin Microbiol Infect Dis 2010; 29:223–9. doi: 10.1007/s10096-009-0843-0. [DOI] [PubMed] [Google Scholar]
- 18. Nucci M, Anaissie E, Betts RF, et al. Early removal of central venous catheter in patients with candidemia does not improve outcome: analysis of 842 patients from 2 randomized clinical trials. Clin Infect Dis 2010; 51:295–303. doi: 10.1086/653935. [DOI] [PubMed] [Google Scholar]
- 19. Vergidis P, Clancy CJ, Shields RK, et al. Intra-abdominal candidiasis: the importance of early source control and antifungal treatment. PLoS One 2016; 11:e0153247. doi: 10.1371/journal.pone.0153247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lagunes L, Rey-Perez A, Martin-Gomez MT, et al. Association between source control and mortality in 258 patients with intra-abdominal candidiasis: a retrospective multi-centric analysis comparing intensive care versus surgical wards in Spain. Eur J Clin Microbiol Infect Dis 2017; 36:95–104. doi: 10.1007/s10096-016-2775-9. [DOI] [PubMed] [Google Scholar]
- 21. Clinical and Laboratory Standards Institute (CLSI) . Reference method for broth dilution antifungal susceptibility testing of yeasts. 4th ed. CLSI document M27. Wayne, PA: CLSI; 2017. [Google Scholar]
- 22. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu CY, Huang LJ, Wang WS, et al. Candidemia in cancer patients: impact of early removal of non-tunneled central venous catheters on outcome. J Infect 2009; 58:154–60. doi: 10.1016/j.jinf.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 27. Janum S, Afshari A. Central venous catheter (CVC) removal for patients of all ages with candidaemia. Cochrane Database Syst Rev 2016; 7:CD011195. doi: 10.1002/14651858.CD011195.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodriguez D, Park BJ, Almirante B, et al. Impact of early central venous catheter removal on outcome in patients with candidaemia. Clin Microbiol Infect 2007; 13:788–93. doi: 10.1111/j.1469-0691.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- 29. Cornely OA, Bassetti M, Calandra T, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 2012; 18(Suppl 7):19–37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 30. Kuse ER, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369:1519–27. doi: 10.1016/S0140-6736(07)60605-9. [DOI] [PubMed] [Google Scholar]
- 31. Kobayashi T, Marra AR, Schweizer ML, et al. Impact of infectious disease consultation in patients with candidemia: a retrospective study, systematic literature review, and meta-analysis. Open Forum Infect Dis 2020; 7:ofaa270. doi: 10.1093/ofid/ofaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee RA, Zurko JC, Camins BC, et al. Impact of infectious disease consultation on clinical management and mortality in patients with candidemia. Clin Infect Dis 2019; 68:1585–7. doi: 10.1093/cid/ciy849. [DOI] [PubMed] [Google Scholar]
- 33. Mellinghoff SC, Hoenigl M, Koehler P, et al. EQUAL Candida score: an ECMM score derived from current guidelines to measure quality of clinical candidaemia management. Mycoses 2018; 61:326–30. doi: 10.1111/myc.12746. [DOI] [PubMed] [Google Scholar]
- 34. Huang HY, Lu PL, Wang YL, Chen TC, Chang K, Lin SY. Usefulness of EQUAL Candida score for predicting outcomes in patients with candidaemia: a retrospective cohort study. Clin Microbiol Infect 2020; 26:1501–6. doi: 10.1016/j.cmi.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 35. Pfaller MA, Wolk DM, Lowery TJ. T2MR and T2Candida: novel technology for the rapid diagnosis of candidemia and invasive candidiasis. Future Microbiol 2016; 11:103–17. doi: 10.2217/fmb.15.111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.