Abstract
Background
Interest in shorter antimicrobial regimens and oral treatment for osteoarticular infections is growing. The aim of this study is to assess whether there is an association between the administration of an entirely oral antibiotic therapy (OT) and the clinical outcome of native vertebral osteomyelitis (NVOs).
Methods
We conducted a single-center, retrospective, observational study on consecutive patients with pyogenic NVOs over a 10-year period (2008–2018). We performed multivariate logistic regression analysis to identify risk factors for clinical failure, both in the whole population and in subgroups. The impact of OT versus standard treatment (intravenous induction followed by oral treatment whenever possible) was assessed in patients with a non-multidrug-resistant microorganism (MDRO) etiology, and the impact of a rifampin-containing regimen was assessed in patients affected by NVOs caused by staphylococci or of unknown etiology.
Results
The study population included 249 patients, and 33 (13.3%) experienced clinical failure; the OT group consisted of 54 patients (21.7%). Multivariate regression analysis of the whole population selected Charlson comorbidity index (adjusted odds ratio [aOR], 1.291; 95% confidence interval [CI], 1.114–1.497; P = .001) and MDRO etiology (aOR, 3.301; 95% CI, 1.368–7.964; P = .008) as independent factors for clinical failure. Among patients affected by a non-MDRO NVO, OT was not associated with an increased risk of clinical failure (aOR, 0.487; 95% CI, .133–1.782; P = .271), even after adjustment for the propensity score of receiving OT. In the subgroup of patients with staphylococcal or unknown etiology, NVO rifampin was independently associated with favorable outcome (aOR, 0.315; 95% CI, .105–.949; P = .040).
Conclusions
An entirely oral, highly bioavailable treatment, including rifampin, may be as effective as parenteral treatment in selected patients with NVOs.
Keywords: antibiotic therapy, oral therapy, outcome, vertebral osteomyelitis
An entirely oral antibiotic therapy may be a valuable option for the treatment of adult patients with pyogenic vertebral osteomyelitis caused by non-multidrug-resistant bacteria. The antibiotic regimen should include rifampicin in case of staphylococcal or unknown etiology.
Native vertebral osteomyelitis (NVO) is an infection of the vertebrae and intervertebral discs not related to vertebral surgery. The disease’s course is often complicated by epidural abscess, spinal instability, and neurologic deficits with an overall mortality rate of 2%–20% and a reported relapse rates of 1%–32% [1, 2]. In the past decades, incidence of NVO has steadily increased; in France, it increased from 2/100 000 inhabitants/year in 2002 to 11.3/100 000 inhabitants in 2019 [3, 4]. This increased incidence may be related to ageing of population, higher prevalence of people with chronic diseases (diabetes, chronic renal, and liver failure), immunosuppression, invasive treatments and procedures (dialysis, medical devices, etc), and more effective diagnostic techniques [3].
Management of NVO is based on prolonged antimicrobial treatment (at least 6 weeks) and orthosis protection; surgery is indicated in case of important epidural abscess, progressive neurologic deficits, vertebral instability, or failure of conservative treatment.
Parenteral antibiotics have been historically considered the gold standard for treatment of NVO; concerns about bone penetration and oral bioavailability of antimicrobial may have had a role in this choice [5]. Indeed, Infectious Diseases Society of America (IDSA) guidelines published in 2015 confirmed parenteral therapy as the standard treatment for NVO, whereas oral antibiotics with excellent bioavailability are indicated as a valuable option for early switch. Nevertheless, they do not define patients who may benefit from a parenteral to oral conversion nor the optimal timing for the switch [6].
In a recent study, similar efficacy of oral and intravenous antibiotics has been described for the treatment of osteomyelitis [7]. Considering that intravenous therapy is associated with substantial risks, inconvenience, and higher costs than oral therapy, it seems very rational to investigate whether oral antibiotics may be an effective treatment option for NVO.
The aim of this study was to describe epidemiology and outcome of NVO and to assess risk factors for clinical failure. In particular, we evaluated whether the administration of an entirely oral antibiotic therapy was associated with clinical outcome.
METHODS
Study Design and Setting
We conducted a single-center, observational, retrospective cohort study on all consecutive adult patients treated for NVO at our center from November 2008 to June 2018. The study was carried out at the Infectious Diseases Unit of IRCSS (Istituto di Ricovero e Cura a Carattere Scientifico) Azienda Ospedaliero Universitaria di Bologna, a 1420-bed tertiary hospital in Northern Italy, where a stable Infectious Diseases (ID) consultant team is dedicated to the management of bone and joint infections, for inpatients (more than 2000 ID bedside consultation in 2018) and outpatients (660 ambulatory visits in 2018). Patients with NVO are managed in close collaboration with the Unit of Oncologic and Degenerative Spine Surgery of the IRCCS Istituto Ortopedico Rizzoli, a referral orthopedic hospital.
Study Population
All adult patients (age ≥18 years) with a diagnosis of NVO were screened for inclusion in the study. Exclusion criteria were previous vertebral surgery (with or without instrumentation, independently of timing) involving the vertebrae involved in the infection, NVO due to direct extension (pressure ulcer or penetrating traumas), and mycobacterial, fungal, or brucellar etiology. These conditions were excluded because their management is different from that of pyogenic NVO.
Patient Management
During the study period, standard diagnostic procedures for NVO included the following: basal full blood chemistry, blood cultures, QuantiFERON and Widal-Wright test, contrast-enhanced magnetic resonance (MRI), positron emission tomography with 2-deoxy-2-[fluorine-18]fluoro-d-glucose integrated with computed tomography (18F-FDG PET/CT), and a vertebral biopsy/abscess drainage, when feasible; in case of contraindication to MRI, the patient underwent a contrast-enhanced CT scan.
During antibiotic therapy, weekly assessment of full blood chemistry including inflammatory markers was recommended. After end of treatment (EOT), we planned a monthly assessment of full blood chemistry including inflammatory markers. Clinical and radiological evaluations were scheduled at EOT and 6 months and 12 months after EOT. The choice and the duration of the antimicrobial regimen were at the discretion of the attending ID physician according to clinical characteristics, response to treatment, and culture results when available.
The orthopedic surgeon visited all patients diagnosed with NVO; surgery was usually reserved to patients presenting with neurologic compromise, large epidural abscess, significant vertebral destruction with instability, and uncontrolled pain.
Study Variables and Definitions
We defined NVO as follows: (1) histologically and microbiologically proven NVO - in presence of imaging and clinical and laboratory findings consistent with NVO associated with typical histopathological finding plus a microorganism cultured from the involved vertebra, intervertebral disc space, paravertebral or epidural abscesses drainage (obtained through percutaneous biopsy or open surgery); (2) probable NVO - in presence of imaging and clinical and laboratory findings consistent with NVO and at least 1 blood culture positive for Staphylococcus aureus or imaging and clinical and laboratory findings consistent with NVO associated with typical histopathological findings but negative cultures from involved vertebra, intervertebral disc space, paravertebral, or epidural abscesses (if patients had at least 1 positive blood culture for a pathogen different from S aureus or not); (3) presumptive NVO - in presence of imaging and clinical and laboratory findings consistent with NVO, but histology and culture of spinal tissue were not done. Bacteria were defined as multidrug-resistant microorganism (MDRO) in case of nonsusceptibility to at least 1 agent in 3 or more antimicrobial categories; methicillin-resistant S aureus (MRSA) is always considered multidrug-resistant [8].
The endpoint variable was clinical cure, defined as sustained absence of fever and normal inflammatory markers plus remission of pain and survival at 12 months after the end of the first treatment course (ie, patients with lack of improvement or disease progression during antimicrobials who had their antibiotic therapy discontinued and repeated the diagnostic work-up were considered treatment failures).
Exposure variables were oral and standard parenteral treatment. The oral treatment (OT) group included patients treated exclusively with an oral antimicrobial regimen, based on highly bioavailable molecules for the full course of therapy (<24 hours of parenteral treatment). Standard treatment (ST) was defined as initial parenteral therapy for >24 hours, followed when feasible by oral shift.
Other study variables included the following: demographics (age and sex), comorbidities according to Charlson comorbidity index [9], risk factors for NVO (including invasive procedure in the previous 6 months, recent spinal trauma, injective drug abuse, vascular catheter, hemodialysis, or major infectious events in the previous 12 months), sign and symptoms of NVO, vertebral site of infection, etiology, and medical and surgical treatment.
Statistical Analysis
For descriptive analysis, categorical variables were presented as absolute numbers and their frequencies, and continuous variables were presented as mean ± standard deviation or median and interquartile range (IQR) according to their distribution. Differences between patients’ groups were tested with χ2 tests or Fisher’s exact test when appropriate for categorical variables, and Student t test or Mann-Whitney U test for normally and nonnormally distributed continuous variables, respectively. To analyze the independent risk factors for clinical failure in the whole population, variables with a P ≤ .1 at univariate analysis were entered into a multivariate forward logistic binary regression model.
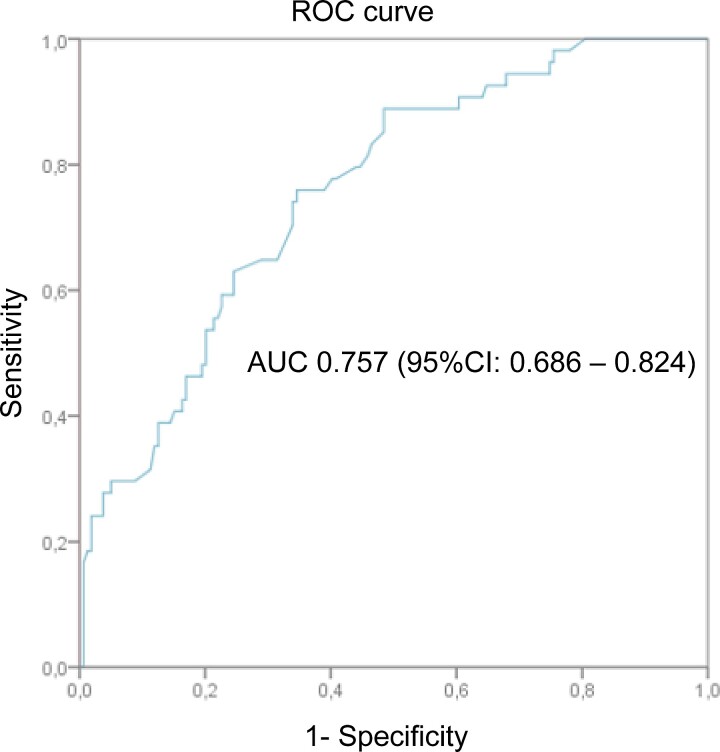
To specifically assess the impact of OT on clinical cure, a further analysis was done including only those patients not affected by MDRO NVO, defined in accordance with the ESCMID definition [8]. Patients included in the OT and ST groups were compared. A propensity score for receiving OT was done. All the of variables with P < .10 at univariate analysis were introduced in a nonparsimonious multivariate logistic regression model, which included the following: proven NVO, Charlson comorbidity index, fever at presentation, cervical site, previous infectious event in the last 12 months, CT-guided biopsy, Staphylococcal etiology (coagulase-negative staphylococci and S aureus), and surgical treatment. The validity of the model was assessed by estimating goodness-of-fit to the data with the Hosmer-Lemeshow test (P = .762) and the receiver operating characteristic curve analysis (Figure 1) with an area under the curve of 0.757 (95% confidence interval [CI], .686–.824; P < .001).
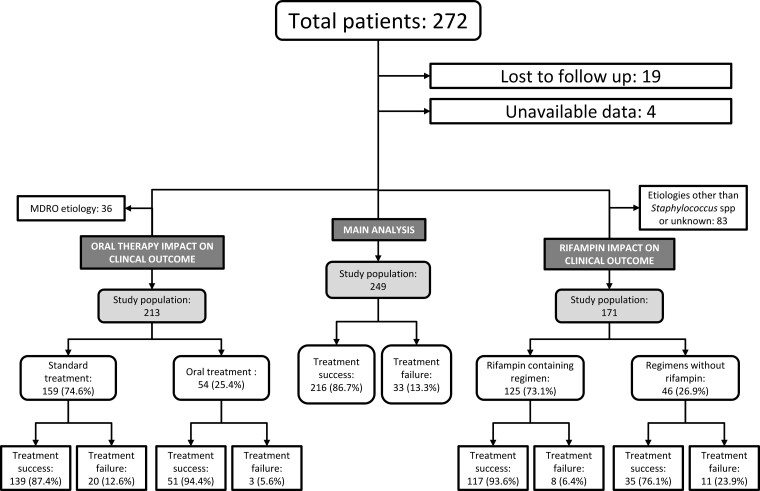
Figure 2.
Flow-chart of the study population. MDRO, multidrug-resistant organism.
Comparison of patients with and without treatment failure was repeated. A multivariate logistic binary regression analysis was done to assess independent risk factors for failure; OT was introduced into the model as the explanatory variable of interest together with the propensity score of receiving OT.
The association with clinical outcome of rifampin-containing regimens on outcome of patients affected by an NVO due to Staphylococcus spp, including methicillin-resistant strains and unknown etiology NVO, was evaluated through a multivariate forward logistic binary regression model including all variables with a P ≤ .1 at univariate analysis.
We used SPSS for Windows, version 20.0 (IBM SPSS, Inc., Chicago, IL) for statistical analysis. All statistical tests were 2-tailed, and P < .05 were considered significant.
Patient Consent Statement
Due to its observational, retrospective design, the study does not include factors necessitating patient consent.
RESULTS
During the study period, 272 patients were treated at our center for pyogenic NVO, and 23 of them were excluded: 19 were lost to follow up before 12 months from EOT and 4 had incomplete data. Thus, the study population consisted of 249 patients (Figure 2).
Figure 1.
Propensity score of receiving oral treatment (covariates included the following: definite native vertebral osteomyelitis, systemic infection in the previous 12 months, Charlson comorbidity index, fever, cervical spine involved, Staphylococcal etiology, surgical treatment, computed tomography-guided biopsy done). AUC, area under the curve; CI, confidence interval; ROC, receiver operating characteristic.
Patient characteristics, demographics, and clinical characteristics of study population are summarized in Table 1. Median age was 69 (IQR, 57–76) years and 81 patients (32.5%) were females; the median Charlson comorbidity index was 5 (IQR, 3–7). The most common risk factor for NVO was the presence of a systemic infection in the past 12 months (35.7%).
Table 1.
Univariate Analysis of Risk Factors for Native Vertebral Osteomyelitis Treatment Failure
| Variable | Treatment Success (n = 216) N (%) |
Treatment Failure (n = 33) N (%) |
Total (N = 249) N (%) |
P Value |
|---|---|---|---|---|
| Demographics | ||||
| Female gender | 67 (31.0) | 14 (42.4) | 81 (32.5) | .231 |
| Age (years, median; IQR) | 68 (55.25–76) | 74 (65–81.50) | 69 (57–76) | .002 |
| Risk Factors | ||||
| Surgical procedure | 39 (18.1) | 5 (15.2) | 44 (17.7) | .810 |
| Invasive procedure | 40 (18.5) | 4 (12.1) | 44 (17.7) | .468 |
| Spinal trauma | 22 (10.2) | 1 (3.0) | 23 (9.2) | .329 |
| Injective drug user | 10 (4.6) | 2 (6.1) | 12 (4.8) | .664 |
| Central venous catheter | 14 (6.5) | 3 (9.1) | 17 (6.8) | .479 |
| Hemodialysis | 3 (1.4) | 6 (18.2) | 9 (3.6) | <.001 |
| Systemic bacterial infection | 78 (36.1) | 11 (33.3) | 89 (35.7) | .847 |
| Comorbidities | ||||
| Myocardial infarction | 25 (11.6) | 10 (30.3) | 35 (14.1) | .008 |
| Congestive heart failure | 43 (19.9) | 11 (33.3) | 54 (21.7) | .110 |
| Peripheral vascular disease | 72 (33.3) | 16 (48.5) | 88 (35.3) | .117 |
| Cerebrovascular disease | 21 (9.7) | 8 (24.2) | 29 (11.6) | .023 |
| Dementia | 6 (2.8) | 2 (6.1) | 8 (3.2) | .287 |
| COPD | 25 (11.6) | 6 (18.2) | 31 (12.4) | .393 |
| Peptic ulcer disease | 8 (3.7) | 2 (6.1) | 10 (4.0) | .626 |
| Mild liver disease | 16 (7.4) | 0 (0.0) | 16 (6.4) | .140 |
| Connective tissue disease | 4 (1.9) | 1 (3.0) | 5 (2.0) | .512 |
| Rheumatologic disease | 21 (9.7) | 2 (6.1) | 23 (9.2) | .748 |
| Diabetes without organ damage | 31 (14.4) | 5 (15.2) | 36 (14.5) | >.999 |
| Diabetes with organ damage | 9 (4.2) | 3 (9.1) | 12 (4.8) | .202 |
| Hemiplegia | 5 (2.3) | 0 (0) | 5 (2.0) | >.999 |
| Moderate/severe renal disease | 28 (13.0) | 15 (45.5) | 43 (17.3) | <.001 |
| Neoplasm (previous 5 years) | 32 (14.8) | 4 (12.1) | 36 (14.5) | .797 |
| Lymphoma | 2 (0.9) | 2 (6.1) | 4 (1.6) | .086 |
| Leukaemia | 1 (0.5) | 1 (3.0) | 2 (0.8) | .248 |
| Moderate/severe liver disease | 15 (6.9) | 7 (21.2) | 22 (8.8) | .015 |
| Metastatic solid tumor | 5 (2.3) | 0 (0.0) | 5 (2.0) | >.999 |
| AIDS | 4 (1.9) | 0 (0.0) | 4 (1.6) | >.999 |
| CCI (median; IQR) | 4 (2–6) | 7 (5–9) | 5 (3–7) | <.001 |
| Clinical Presentation | ||||
| Pain | 210 (97.2) | 31 (93.9) | 241 (96.8) | .287 |
| Fever | 133 (61.6) | 19 (57.6) | 152 (61.0) | .704 |
| Hypostenia | 43 (19.9) | 5 (15.2) | 48 (19.3) | .640 |
| Hypoesthesia | 26 (12.0) | 3 (9.1) | 29 (11.6) | .777 |
| Fecal/urinary incontinence | 10 (4.6) | 0 (0.0) | 10 (4.0) | .367 |
| Vertebral Site | ||||
| Number of vertebral segments involved (median, IQR) | 1 (1–1) | 1 (1–2) | 1 (1–1) | .014 |
| Cervical | 14 (6.5) | 2 (6.1) | 16 (6.4) | >.999 |
| Thoracic | 72 (33.3) | 16 (48.5) | 88 (35.3) | .117 |
| Lumbar | 152 (70.4) | 19 (57.6) | 171 (68.7) | .160 |
| Sacral | 31 (14.4) | 0 (0.0) | 31 (12.4) | .019 |
| Abscessesa | 112 (59.3) | 16 (59.3) | 128 (59.3) | >.999 |
| Diagnosis | ||||
| Diagnostic delay (days, median, IQR)b | 44 (22.25–75.50) | 45 (28.50–105.50) | 44 (23.5–77) | .571 |
| Pre-treatment CRP (mg/dL, median, IQR)c | 5 (2.5–11.0) | 6.50 (4.25–11.75) | 5.0 (3.0–11.0) | .124 |
| CRP normalization time (days, mean ± SD)d | 30 (14–60) | 28 (15–72) | 30 (14–60) | .865 |
| Positive blood culturee | 112 (85.5) | 22 (91.7) | 134 (86.5) | .533 |
| CT-guided biopsy | 112 (51.9) | 15 (45.5) | 127 (51.0) | .576 |
| Positive CT-guided biopsyf | 38 (33.9) | 5 (33.3) | 43 (34.1) | >.999 |
| Infectious endocarditisg | 28 (19.6) | 8 (36.4) | 36 (21.8) | .096 |
| Definitions | ||||
| Definite | 42 (19.4) | 7 (21.2) | 49 (19.7) | .812 |
| Probable | 119 (55.1) | 15 (45.5) | 134 (53.8) | .301 |
| Presumptive | 55 (25.5) | 11 (33.3) | 66 (26.5) | .340 |
| Etiology | ||||
| Staphylococcus spp | 88 (40.7) | 13 (39.4) | 101 (40.6) | >.999 |
| Staphylococcus aureus | 65 (30.1) | 9 (27.3) | 74 (29.7) | .840 |
| CoNS | 23 (10.6) | 4 (12.1) | 27 (10.8) | .766 |
| Streptococcus spp | 23 (10.6) | 5 (15.2) | 28 (11.2) | .446 |
| Enterococcus spp | 9 (4.2) | 3 (9.1) | 12 (4.8) | .202 |
| Gram positive | 122 (56.5) | 21 (63.6) | 143 (57.4) | .439 |
| Enterobacteriaceae | 24 (11.1) | 3 (9.1) | 27 (10.8) | >.999 |
| Pseudomonas aeruginosa | 1 (0.5) | 3 (9.1) | 4 (1.6) | .008 |
| Gram negative | 29 (13.4) | 6 (18.2) | 35 (14.1) | .464 |
| Anaerobes | 2 (0.9) | 0 (0.0) | 2 (0.8) | >.999 |
| MDRO | 26 (12.0) | 10 (30.3) | 36 (14.5) | .005 |
| MRSA | 10 (4.6) | 6 (18.2) | 16 (6.4) | .003 |
| CoNS Oxa-R | 9 (4.2) | 3 (9.1) | 12 (4.8) | .202 |
| Polymicrobial infection | 4 (1.9) | 0 (0.0) | 4 (1.6 | >.999 |
| Unknown etiology | 64 (29.6) | 6 (18.2) | 70 (28.1) | .173 |
| Treatment | ||||
| Surgical treatment | 27 (12.5) | 7 (21.2) | 34 (13.7) | .274 |
| Surgery during antimicrobials | 23 (85.2) | 5 (71.4) | 28 (82.2) | .580 |
| Time from diagnosis to surgery (days, median; IQR) | 29 (13–74) | 25 (13–60) | 23 (16–71) | >.999 |
| Previous antimicrobial treatment | 93 (43.1) | 17 (51.5) | 110 (44.2) | .452 |
| Oral treatment | 51 (23.6) | 3 (9) | 54 (21.7) | .07 |
| Length of first treatment course (days, median; IQR) | 98 (85.25–118.75) | 78 (62–103) | 96 (82–116) | .001 |
| Treatment-related adverse event | 49 (22.7) | 7 (21.2) | 56 (22.5) | >.999 |
| Teicoplanin | 91 (42.1) | 18 (54.5) | 109 (43.7) | .192 |
| Piperacillin/tazobactam | 75 (34.7) | 9 (27.3) | 84 (33.7) | .437 |
| Daptomycin | 20 (9.3) | 5 (15.2) | 25 (10.0) | .346 |
| Levofloxacin | 134 (62.0) | 14 (42.4) | 148 (59.4) | .037 |
| Ciprofloxacin | 9 (4.2) | 2 (6.1) | 11 (4.4) | .644 |
| Rifampicin | 128 (59.3) | 10 (30.3) | 138 (55.4) | .002 |
| Minocycline | 35 (16.2) | 4 (12.1) | 39 (15.7) | .797 |
| Trimethoprim-sulphametoxazole | 6 (2.8) | 0 (0.0) | 6 (2.4) | >.999 |
| Linezolid | 12 (5.6) | 4 (12.1) | 16 (6.4) | .241 |
Abbreviations: AIDS, acquired immune deficiency syndrome; CCI, Charlson comorbidity index; CI, confidence interval; CoNS, coagulase-negative Staphylococcus; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; CT, computed tomography; IQR, interquartile range; MDRO, multidrug-resistant organism; MRSA, methicillin-resistant Staphylococcus aureus; NVO, native vertebral osteomyelitis; OR, odds ratio; SD, standard deviation.
Abscess presence available for 216 patients (189 in favorable outcome group and 27 in clinical failure group).
Diagnostic delay measured from first symptoms appearance and date of definitive diagnosis.
Baseline CRP available in 193 patients (165 in favorable outcome group and 28 in clinical failure group).
CRP normalization time available for 138 patients (131 in favorable outcome group and 7 in clinical failure group).
Blood cultures done in 155 patients (131 in favorable outcome group and 24 in clinical failure group).
Vertebral biopsy done in 127 patients (112 in favorable outcome group and 15 in clinical failure group).
Echocardiography done in 165 patients (143 in favorable outcome group and 22 in clinical failure group).
Median diagnostic delay from symptoms onset was 44 days (IQR, 23.5–77), and back pain was the most common presenting symptom (96.8%), followed by fever (61.0%). Median C-reactive protein (CRP) at baseline was 5.0 mg/dL (IQR, 3.0–11.0) with reference range ≤0.5 mg/dL. Magnetic resonance imaging, computed tomography, and FDG-PET were available for 210 (84.3%), 111 (44.6%), and 221 (88.8%) patients, respectively. Involvement of lumbar tract was predominant (68.7%), with evidence of epidural abscess in 74 patients (34.3%) and paravertebral abscess in 93 cases (43.1%). Concomitant infectious endocarditis was ascertained in 36 patients (21.8%). According to our definition of NVO, there were 49 proven NVO, 134 probable NVO, and 66 presumptive NVO.
Microbiological Findings
Etiologic agent was identified in 179 patients (71.9%). Etiology was identified through blood cultures in 128 patients (51.4%), vertebral biopsy in 37 cases (14.9%), abscess drainage in 8 cases (4.5%), or both blood culture and spinal specimen culture in 6 patients (2.4%).
Microbiological findings for the 179 patients with a microbiological-defined NVO are displayed in Table 1. Overall, 36 patients (20.1% of culture positive NVO) were affected by an NVO due to an MDRO; almost half of them were MRSA (16 patients).
Management
Surgery was performed in 34 patients (13.7%), with a median time from diagnosis of 23 (IQR, 16–71) days. Only 5 patients underwent surgical intervention within 7 days from NVO diagnosis, because of worsening neurological deficits (3 patients) and spinal instability (2 patients). Indication for delayed surgery in the remaining 29 patients were as follows: spinal instability (12 patients), worsening neurological deficits (8 patients), failure of conservative treatment (5 patients), abscess debridement (3 patients), and spinal deformity (1 patient).
Fourteen of the 128 patients with an abscess (11%) underwent percutaneous drainage of abscess and 3 underwent surgical debridement; in the remaining case, abscesses were managed with antibiotics alone.
All patients received an empiric or targeted antimicrobial treatment. The OT group was composed of 54 patients (21.7%), with a median treatment duration of 96.5 days (IQR, 84.5–110.25). The most common antimicrobials used in the OT group were levofloxacin, rifampin, and minocycline, as shown in Supplementary Table 1. The ST group was composed of 195 patients (78.3%), with a total treatment duration median of 96 days (IQR, 81–122).
Overall, 90 patients were treated with a parenteral route of administration for the whole treatment duration, whereas 105 patients initially received a parenteral antimicrobial regimen for a median duration of 22 days (IQR, 14–42) followed by a highly bioavailable oral therapy. Most common antimicrobials used in the ST group belong to glycopeptide and beta lactam + beta lactam inhibitors (BL/BLI), as reported in Supplementary Table 2.
Outcome
Nineteen patients (7.6%) died during the study period: 11 patients died during antimicrobial treatment and 8 died during the follow up. The median time from diagnosis to death was 108 (IQR, 76–156) days. Fourteen patients (5.6%) experienced persistence or relapse of NVO. Overall, 33 patients (13.3%) were considered as having a treatment failure.
In the whole population, multivariate regression analysis selected Charlson comorbidity index (adjusted odds ratio [aOR], 1.291; 95% Conficence Interval [CI], 1.114–1.497; P = .001) and MDRO etiology (aOR, 3.301; [95% CI], 1.368–7.964; P = .008) as independent factors for clinical failure (Table 2).
Table 2.
Multivariable Analysis of Risk Factors for Native Vertebral Osteomyelitis Treatment Failure
| Treatment Failure | OR | 95% CI | P Value |
|---|---|---|---|
| Charlson comorbidity index | 1.291 | 1.114–1.497 | .001 |
| MDRO etiology | 3.301 | 1.368–7.964 | .008 |
| Number of vertebral levels involved | 1.960 | 0.990–3.877 | .053 |
Abbreviations: CI, confidence interval; MDRO, multidrug-resistant organism; OR, odds ratio.
In the subgroup of patients with non-MDRO NVO (213 patients), there were 23 failures: 3 (5.6%) in the OT group and 20 (12.6%) in the ST group (P = .20) (Table 3 and Supplementary Table 3). Multivariate regression analysis for clinical failure showed that OT was not associated with an increased risk of clinical failure (aOR, 0.487; 95% CI, .133–1.782; P = .271). When adjusted for the propensity score of receiving OT instead of ST, the model did not change (Table 4).
Table 3.
Comparison of Patients’ Characteristics Between Oral Treatment and Standard Treatment Groups
| Characteristics | Oral Treatment (n = 54) N (%) |
Standard Treatment (n = 159) N (%) |
Total (n = 213) N (%) |
P Value |
|---|---|---|---|---|
| Demographics | ||||
| Female gender | 17 (31.5) | 51 (32.1) | 68 (31.9) | .936 |
| Age (years, median; IQR) | 68 (55.75–74.25) | 68 (56–77) | 68 (56–77) | .398 |
| Risk Factors for NVO | ||||
| Surgical procedure | 6 (11.1) | 32 (20.1) | 38 (17.8) | .135 |
| Invasive procedure | 13 (24.1) | 25 (15.7) | 38 (17.8) | .166 |
| Spinal trauma | 4 (7.4) | 16 (10.1) | 20 (9.4) | .788 |
| Injective drug user | 5 (9.3) | 7 (4.4) | 12 (5.6) | .181 |
| Central venous catheter | 4 (7.4) | 10 (6.3) | 14 (6.6) | .756 |
| Hemodialysis | 2 (3.7) | 3 (1.9) | 5 (2.3) | .603 |
| Systemic bacterial infection | 24 (44.4) | 50 (31.4) | 74 (34.7) | .083 |
| Comorbidities | ||||
| Myocardial infarction | 9 (16.7) | 22 (13.8) | 31 (14.6) | .610 |
| Congestive heart failure | 9 (16.7) | 37 (23.3) | 46 (21.6) | .308 |
| Peripheral vascular disease | 17 (31.5) | 57 (35.8) | 74 (34.7) | .560 |
| Cerebrovascular disease | 4 (7.4) | 17 (10.7) | 21 (9.9) | .604 |
| Dementia | 0 (0.0) | 7 (4.4) | 7 (3.3) | .195 |
| COPD | 8 (14.8) | 18 (11.3) | 26 (12.2) | .498 |
| Peptic ulcer disease | 1 (1.9) | 8 (5.0) | 9 (4.2) | .454 |
| Mild liver disease | 3 (5.6) | 12 (7.5) | 15 (7.0) | .765 |
| Connective tissue disease | 1 (1.9) | 2 (1.3) | 3 (1.4) | >.999 |
| Rheumatologic disease | 7 (13.0) | 13 (8.2) | 20 (9.4) | .297 |
| Diabetes without organ damage | 10 (18.5) | 20 (12.6) | 30 (14.1) | .278 |
| Diabetes with organ damage | 1 (1.9) | 8 (5.0) | 9 (4.2) | .454 |
| Hemiplegia | 2 (3.7) | 3 (1.9) | 5 (2.3) | .603 |
| Moderate/severe renal disease | 6 (11.1) | 28 (17.6) | 34 (16.0) | .260 |
| Neoplasm (prior 5 years) | 4 (7.4) | 26 (16.4) | 30 (14.1) | .117 |
| Lymphoma | 0 (0.0) | 4 (2.5) | 4 (1.9) | .574 |
| Leukaemia | 0 (0.0) | 1 (0.6) | 1 (0.5) | >.999 |
| Moderate/severe liver disease | 6 (11.1) | 13 (8.2) | 19 (8.9) | .513 |
| Metastatic solid tumor | 0 (0.0) | 4 (2.5) | 4 (1.9) | .574 |
| AIDS | 1 (1.9) | 3 (1.9) | 4 (1.9) | >.999 |
| Charlson comorbidity index (median; IQR) | 4 (2–6) | 5 (2–7) | 5 (2–7) | .125 |
| Symptoms | ||||
| Pain | 53 (98.1) | 153 (96.2) | 206 (96.7) | .682 |
| Fever | 26 (48.1) | 100 (62.9) | 126 (59.2) | .057 |
| Hypostenia | 11 (20.4) | 29 (18.2) | 40 (18.8) | .729 |
| Hypoestesia | 6 (11.1) | 18 (11.3) | 24 (11.3) | .966 |
| Fecal/urinary incontinence | 2 (3.7) | 6 (3.8) | 8 (3.8) | >.999 |
| Vertebral Site | ||||
| Number of vertebral segments involved (median; IQR) | 1 (1–1) | 1 (1–1) | 1 (1–1) | .619 |
| Cervical | 7 (13.0) | 8 (5.0) | 15 (7.0) | .049 |
| Thoracic | 16 (29.6) | 55 (34.6) | 71 (33.3) | .504 |
| Lumbar | 34 (63.0) | 114 (71.7) | 148 (69.5) | .228 |
| Sacral | 7 (13.0) | 19 (11.9) | 26 (12.2) | .844 |
| Abscessa | 31 (62.0) | 76 (55.1) | 107 (56.9) | .397 |
| Infectious endocarditisb | 3 (10.7) | 30 (27.5) | 33 (24.1) | .083 |
| Diagnosis | ||||
| Diagnostic delay (median days, IQR)c | 50 (24.75–83.75) | 42 (24–77) | 45 (24.5–78) | .292 |
| Pretreatment C-reactive protein (mg/dL, median, IQR)d | 4.0 (2.0–9.00) | 6.0 (3.0–11–0) | 5.0 (2.5–11.0) | .069 |
| C-reactive protein negativization timing (days, median; IQR)e | 30.0 (14–46.25) | 30 (10.0–61.0) | 30 (14–58) | .801 |
| Positive blood culturef | 20 (76.9) | 93 (89.4) | 113 (86.9) | .091 |
| CT-guided biopsy | 36 (66.7) | 71 (44.7) | 107 (50.2) | .005 |
| Positive CT-guided biopsyg | 15 (41.7) | 17 (23.9) | 32 (29.9) | .059 |
| Definition | ||||
| Proven | 16 (29.6) | 18 (11.3) | 34 (16.0) | .002 |
| Probable | 28 (51.9) | 90 (56.6) | 118 (55.4) | .554 |
| Presumptive | 10 (18.5) | 51 (32.1) | 61 (28.6) | .057 |
| Etiology | ||||
| Staphylococcus spp | 24 (44.4) | 49 (30.8) | 73 (34.3) | .068 |
| Staphylococcus aureus | 15 (27.8) | 43 (27.0) | 58 (27.2) | .917 |
| CoNS | 9 (16.7) | 6 (3.8) | 15 (7.0) | .001 |
| Streptococcus spp | 4 (7.4) | 24 (15.1) | 28 (13.1) | .170 |
| Enterococcus spp | 0 (0.0) | 11 (6.9) | 11 (5.2) | .069 |
| Gram positive | 28 (51.9) | 87 (54.7) | 115 (54.0) | .715 |
| Enterobacteriaceae | 5 (9.3) | 14 (8.8) | 19 (8.9) | .919 |
| Pseudomonas aeruginosa | 2 (3.7) | 2 (1.3) | 4 (1.9) | .267 |
| Gram negative | 7 (13.0) | 20 (12.6) | 27 (12.7) | .942 |
| Anaerobes | 0 (0.0) | 2 (1.3) | 2 (0.9) | >.999 |
| Polymicrobial infection | 0 (0.0) | 3 (1.9) | 3 (1.4) | .573 |
| Unknown etiology | 19 (35.2) | 51 (32.1) | 70 (32.9) | .674 |
| Treatment | ||||
| Surgical treatment | 2 (3.7) | 21 (13.2) | 23 (10.8) | .073 |
| Surgery during antimicrobials | 0 (0.0) | 6 (3.8) | 6 (2.8) | .341 |
| Delayed surgical treatment | 2 (3.7) | 15 (9.4) | 17 (8.0) | .250 |
| Previous antibiotic treatment | 27 (50.0) | 63 (39.6) | 90 (42.3) | .182 |
| Total treatment length (days, median, IQR) | 96.50 (84.5–110.25) | 97 (81–123) | 97 (82–117) | .980 |
| Treatment-related adverse event | 11 (20.4) | 37 (23.3) | 48 (22.5) | .659 |
| Clostridium difficile colitis | 1 (1.9) | 4 (2.5) | 5 (2.3) | >.999 |
| Tendinopathy | 4 (7.4) | 6 (3.8) | 10 (4.7) | .274 |
| Gastrointestinal intolerance | 1 (1.9) | 6 (3.8) | 7 (3.3) | .682 |
| Hepatotoxicity | 1 (1.9) | 2 (1.3) | 4 (1.4) | >.999 |
| Hematologic toxicity | 2 (3.7) | 9 (5.7) | 11 (5.2) | .734 |
| Skin rash | 2 (3.7) | 5 (3.1) | 7 (3.3) | >.999 |
| CVC-related complications | 0 (0.0) | 3 (1.9) | 3 (1.4) | .573 |
| Unfavorable outcome | 3 (5.6) | 20 (12.6) | 23 (10.8) | .206 |
Abbreviations: AIDS, acquired immune deficiency syndrome; CCI, Charlson comorbidity index; CI, confidence interval; CoNS, coagulase-negative Staphylococcus; COPD, chronic obstructive pulmonary disease; CT, computed tomography; CVC, central venous catheter; IQR, interquartile range; MDRO, multidrug-resistant organism; MRSA, methicillin-resistant Staphylococcus aureus; NVO, native vertebral osteomyelitis; OR, odds ratio; SD, standard deviation.
Abscess presence available for 188 patients (50 in oral treatment [OT] group and 138 in standard treatment [ST] group).
Echocardiography available for 137 patients (28 in OT group and 109 in ST group).
Diagnostic delay measured from first symptoms appearance and date of definitive diagnosis.
Baseline C-reactive protein (CRP) measured in 165 patients (43 in OT group and 122 in ST group).
CRP negativization time available for 119 patients (38 in OT group and 81 in ST group).
Blood culture available for 130 patients (26 in OT group and 104 in ST group).
Vertebral biopsy available for 107 patients (36 in OT group and 71 in ST group).
Table 4.
Multivariable Analysis by Logistic Regression of Risk Factors for Treatment Failure in the Subgroup of Patients With a Non-MDRO Etiology
| Variable | Multivariate Analysis | Multivariable Propensity Score-Balanced Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Charlson comorbidity index | 1.293 (1.109–1.507) | .001 | 1.251 (1.071–1.461) | .005 |
| Number of vertebral levels | 1.968 (0.924–4.194) | .079 | 2.234 (1.020–4.893) | .045 |
| Oral treatment group | 0.487 (0.133–1.782) | .271 | 0.675 (0.173–2.632) | .571 |
| Propensity score | 0.042 (0.001–2.061) | .110 | ||
Abbreviations: CI, confidence interval; MDRO, multidrug-resistant organism; OR, odds ratio.
In the subgroup of patients with staphylococcal NVO or unknown etiology (171 patients), 125 (73.1%) received a rifampin-based antimicrobial regimen. Multivariate regression analysis for clinical failure showed that rifampin was independently associated with favorable outcome (aOR, 0.315; 95% CI, .105–.949; P = .040) (Supplementary Table 4).
DISCUSSION
In this study, we describe the epidemiology and outcome of a large cohort of patients with NVO and managed at an ID referral center. The overall failure rate was relatively low (13%), compared to other published cohorts [2, 10, 11]. A higher Charlson comorbidity index and an MDRO etiology were associated with a worse prognosis. The main finding of our study is that a highly bioavailable oral treatment on the first day (including rifampin for staphylococcal NVO and in case of unknown etiology) may be an effective option for the treatment of NVO not caused by MDROs.
Our study population is comparable to previously published cohorts of NVO in terms of demographics, comorbidities, proportion of etiological diagnosis, and proportion of patients with difficult-to-treat microorganisms. However, we observed a low rate of clinical failure (13.3%). In our opinion, this low failure rate may be related (1) to the clinical management shared between skilled ID specialists and dedicated orthopedic surgeons and (2) to the choice of antibiotics with a good penetration into bone tissue. Moreover, in our center, all patients were supported by an active follow up, consisting of periodic blood tests, regular ambulatory visits, and the possibility of a quick contact if needed. Despite spending an extensive amount of time and resources, we believe that active support is the optimal way of improving adherence to therapy and safety of patients receiving prolonged antibiotic treatments [7, 12].
To date, only a few studies have investigated the efficacy and safety of oral therapy in NVO, and they focused on oral shift after initial parenteral treatment. Flury et al [13] published a small retrospective cohort study showing that switching to an oral antibiotic regimen after 2 weeks of intravenous treatment was safe in patients with decreasing CRP and successful drainage of epidural or paravertebral abscesses. In their randomized controlled trial, Bernard et al [11] reported no differences in treatment failure between patients given protracted intravenous treatment (>1 week) and those given intravenous treatment for less than 1 week. However, low incidence of MRSA (5.5%) and spinal abscesses (19.4%) hampered generalization of their result to other population.
The best evidence supporting oral treatment for bone infection is provided by Oral versus Intravenous Antibiotics for Bone and Joint Infection (OVIVA) trial, which showed that oral antibiotics were noninferior to intravenous antibiotic therapy for complex orthopedic infections. However, in this trial, only 6.8% of patients had a spinal infection, and all patients received parenteral therapy for up to 7 days before randomization [7]. In our cohort, patients included in the OT group received oral antibiotics for the entire treatment course; we did not find significant differences in terms of efficacy nor adverse events between OT and ST.
Another significant finding of our study is the role of rifampin in the treatment of NVO. Several studies on chronic osteomyelitis and orthopedic implant infections have shown that rifampin in addition to an antibiotic regimen improves cure rates in animal models, in retrospective studies in humans, and in randomized clinical trials [14–17]. Previous studies investigating the impact of antimicrobial therapy on NVO outcome generally reported a favorable but nonstatistically significant effect of rifampin combination [2, 11, 18]. In our cohort, no single combination of antimicrobials demonstrated superiority over the others, whereas rifampin-based regimens were independently associated with better outcome among patients with staphylococcal NVO or due to unknown etiology.
Observed median treatment durations in both ST and OT was longer (more than 12 weeks) than now recommended for NVO. This finding can be explained considering that we included cases of NVO diagnosed between 2008 and 2018, and so many of them were managed before 2015, when Bernard et al [11] published their trial showing that 6 weeks of antibiotic treatment for vertebral osteomyelitis was not inferior to 12 weeks of treatment. Moreover, the large number of patients with abscesses managed nonoperatively could have justified several cases of prolonged antibiotic therapy.
The main strength of our study is that it is a real-life study and included heterogeneous patients with NVO. Indeed, we had high prevalence of vertebral abscesses (paravertebral in 42.9% and epidural abscess in 32.3%) and MDRO pathogens (19% of S aureus were MRSA, 50% of coagulase-negative Staphylococcus were methicillin-resistant, and 41.2% of Enterobacteriaceae were resistant to quinolones), and we included patients with infectious endocarditis and/or concomitant bacteremia, reflecting real-life management of NVO.
Our study has several limitations that must be considered when interpreting results. First, it is a single-center study and all patients were managed by the same ID specialistis with great experience in the management of NVO, limiting the possibility to generalize results. Second, some patients have been excluded from the analysis due to loss to follow up or incomplete data, introducing possible biases; however, it is an inherent limitation of retrospective studies. Third, 42.7% of our patients received antibiotics before starting the specific antimicrobial therapy for NVO. This may have introduced some bias in our analysis of OT efficacy; nevertheless, previous antimicrobial treatment rate was equally distributed in OT and ST groups, and it reflects a common condition of the patient presenting with NVO. In real life, it is very common to diagnose NVO in patients with a relevant diagnostic delay who have been previously treated with antibiotics before NVO diagnosis.
Finally, the choice between OT and ST was made at ID specialist’s discretion, without pre-established criteria, which probably introduced multiple bias. We attempted to overcome these confounders with the use of a propensity score, but it is possible that relevant variables may not have been included.
CONCLUSIONS
To conclude, our data suggest that, in patients affected by pyogenic NVO not due to MDRO, an entirely oral highly bioavailable treatment, including rifampin for staphylococcal NVO and in case of unknown etiology, may be as effective as parenteral treatment. Furthermore, prospective studies are needed to investigate this issue and to identify the criteria to select patients suitable for oral therapy.
Supplementary Material
Notes
Financial support. No funding has been received for the present work.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Lorenzo Marconi, Infectious Disease Unit, IRCCS Azienda Ospedaliero Universitaria di Bologna, Bologna, Italy.
Sara Tedeschi, Infectious Disease Unit, IRCCS Azienda Ospedaliero Universitaria di Bologna, Bologna, Italy; Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.
Eleonora Zamparini, Infectious Disease Unit, IRCCS Azienda Ospedaliero Universitaria di Bologna, Bologna, Italy.
Silvia Terzi, Department of Oncological and Degenerative Spine Surgery, IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy.
Nicolò Rossi, Infectious Disease Unit, IRCCS Azienda Ospedaliero Universitaria di Bologna, Bologna, Italy.
Luca Boriani, Department of Oncological and Degenerative Spine Surgery, IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy.
Filippo Trapani, Infectious Disease Unit, IRCCS Azienda Ospedaliero Universitaria di Bologna, Bologna, Italy.
Maddalena Giannella, Infectious Disease Unit, IRCCS Azienda Ospedaliero Universitaria di Bologna, Bologna, Italy; Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.
Damiano Alfio Ruinato, School of Medicine, University of Bologna, Bologna, Italy.
Elisa Marchionni, Infectious Disease Unit, IRCCS Azienda Ospedaliero Universitaria di Bologna, Bologna, Italy.
Alessandro Gasbarrini, Department of Oncological and Degenerative Spine Surgery, IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy.
Pierluigi Viale, Infectious Disease Unit, IRCCS Azienda Ospedaliero Universitaria di Bologna, Bologna, Italy; Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis 2002; 34:1342–50. [DOI] [PubMed] [Google Scholar]
- 2. Park K-H, Cho O-H, Lee JH, et al. . Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis 2016; 62:1262–9. [DOI] [PubMed] [Google Scholar]
- 3. Grammatico L, Baron S, Rusch E, et al. . Epidemiology of vertebral osteomyelitis (VO) in France: analysis of hospital-discharge data 2002–2003. Epidemiol Infect 2008; 136:653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Conan Y, Laurent E, Belin Y, et al. . Large increase of vertebral osteomyelitis in France: a 2010–2019 cross-sectional study. Epidemiol Infect 2021; 149:e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thabit AK, Fatani DF, Bamakhrama MS, Barnawi OA, Basudan LO, Alhejaili SF. Antibiotic penetration into bone and joints: an updated review. Int J Infect Dis 2019; 81:128–36. [DOI] [PubMed] [Google Scholar]
- 6. Berbari EF, Kanj SS, Kowalski TJ, et al. . Executive summary: 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis 2015; 61:859–63. [DOI] [PubMed] [Google Scholar]
- 7. Li H-K, Rombach I, Zambellas R, et al. . Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019; 380:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magiorakos A-P, Srinivasan A, Carey RB, et al. . Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–81. [DOI] [PubMed] [Google Scholar]
- 9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 10. Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum 2009; 39:10–7. [DOI] [PubMed] [Google Scholar]
- 11. Bernard L, Dinh A, Ghout I, et al. . Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015; 385:875–82. [DOI] [PubMed] [Google Scholar]
- 12. Boucher HW. Partial oral therapy for osteomyelitis and endocarditis—is it time? New Eng J Med 2019; 380:487–9. [DOI] [PubMed] [Google Scholar]
- 13. Flury BB, Elzi L, Kolbe M, et al. . Is switching to an oral antibiotic regimen safe after 2 weeks of intravenous treatment for primary bacterial vertebral osteomyelitis? BMC Infect Dis 2014; 14:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van der Auwera P, Klastersky J, Thys JP, Meunier-Carpentier F, Legrand JC. Double-blind, placebo-controlled study of oxacillin combined with rifampin in the treatment of staphylococcal infections. Antimicrob Agents Chemother 1985; 28:467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Norden CW, Bryant R, Palmer D, Montgomerie JZ, Wheat J. Chronic osteomyelitis caused by Staphylococcus aureus: controlled clinical trial of nafcillin therapy and nafcillin-rifampin therapy. South Med J 1986; 79:947–51. [DOI] [PubMed] [Google Scholar]
- 16. Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) study group. JAMA 1998; 279:1537–41. [DOI] [PubMed] [Google Scholar]
- 17. Perlroth J, Kuo M, Tan J, Bayer AS, Miller LG. Adjunctive use of rifampin for the treatment of Staphylococcus aureus infections: A systematic review of the literature. Arch Intern Med 2008; 168:805–19. [DOI] [PubMed] [Google Scholar]
- 18. Li H-K, Rombach I, Zambellas R, et al. . Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med 2019; 380:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.