Abstract
Objective:
To measure the influence of exogenous insulin-like growth factor 1 (IGF1) on follicle growth and maturation in human ovarian cortical xenografts.
Design:
Xenotransplantation model.
Setting:
University-based research laboratory.
Patients/Animals:
Ovarian tissue was donated with consent and institutional review board approval by brain-dead organ donors or patients undergoing ovarian tissue cryopreservation for fertility preservation. Cortical fragments were transplanted into immunocompromised mice.
Interventions:
Cryopreserved ovarian cortical fragments from four women (aged 19, 25, 33, and 46 years) were transplanted into the gluteus muscle of immunocompromised mice in a fibrin matrix containing endothelial cells that were transduced with lentiviral particles encoding secreted IGF1. Xenografts were recovered after 3, 8, and 14 weeks. In addition, C57/Bl6 mice underwent intraovarian injection of saline or recombinant IGF1 (60 μg), followed by superovulation, analysis of ethynyl-deoxyuridine incorporation, and ribonucleic acid sequencing of the whole ovaries.
Main Outcome Measures:
For xenografts: follicle count and distribution; antral follicle count; and corpora lutea/albicans count. For mice: follicle count and distribution; oocyte yield, ethynyl-deoxyuridine incorporation (granulosa cell proliferation); and ovarian transcriptomic signature.
Results:
At 3 weeks, xenografts in the IGF1 condition revealed a decreased percentage of primary follicles and increased percentage of secondary follicles that were concentrated in the preantral subtype; at 8 weeks, an increase in secondary follicles was concentrated in the simple subtype; after 14 weeks, primordial follicles were reduced, and while the number of advanced follicles did not power the experiment to demonstrate significance, antral follicles reduced and corpora lutea increased. Supporting experiments in mice revealed an increase in normal oocytes following intraovarian injection of recombinant IGF1 (60 μg) as well as increased proliferative index among follicles of secondary and preantral stages. Ribonucleic acid sequencing analysis of the whole ovaries following injection of recombinant IGF1 (25 μg) revealed an acute (24 hours) upregulation of transcripts related to steroidogenesis and luteinization.
Conclusions:
Exogenous IGF1 advances the pace of growth among primordial, primary, and secondary stage follicles but results in near absence of antral stage follicles in long-term (14 weeks) xenografts. In mice, acute administration of IGF1 promotes follicle advance and increased oocyte yield. The results suggest that while superphysiological IGF1 alone advances the pace of growth among early/preantral follicles, a sustained and/or later-stage influence undermines antral follicle growth/survival or promotes premature luteinization. These findings provide a temporal framework for interpreting follicle growth/mobilization and may be useful in understanding the clinical application of human growth hormone in the context of assisted reproduction.
Keywords: Insulin-like growth factor 1, human cortical xenograft, growth hormone, endothelial cell cotransplantation, folliculogenesis
The insulin-like growth factor (IGF) signaling pathway plays a prominent role in the survival and expansion of many organ-specific cell types. Insulin-like growth factor 1 (IGF1)was identified in 1957 by Salmon and Daughaday (1) and later recognized as a factor that mediates the effects of growth hormone (GH) (2). Ultimately, due to their resemblance to insulin, two isoforms of IGF isolated from human serum were renamed “insulin-like growth factors 1 and 2” (IGF1 and IGF2) (3), and many other components of the pathway have since been identified (4). The IGF pathway is comprised of three receptors (insulin-like growth factor 1 receptor [IGF1R], insulin-like growth factor 2 receptor, and insulin receptor), three ligands (insulin, IGF1, and IGF2), and six IGF-binding proteins that modulate the bioavailability of IGF ligands to tightly regulate activity. Insulin-like growth factor 1 is a small single-chain polypeptide secreted by the liver in response to GH generated by the pituitary and further transported to target tissues where it performs its function. Insulin-like growth factor 1 is ubiquitously present in most tissues, particularly during postnatal development, and mediates the anabolic and mitogenic activity of GH, primarily through IGF1R and downstream effectors of the Pi3K/Akt pathway.
Numerous studies have established the link between IGF signaling, nutrition, and lifespan: reduced activation of IGF1-mediated signaling increases longevity in nematodes (5), drosophila (6), and mouse (7), and patients with Laron’s syndrome, characterized by loss-of-function mutation of GH receptor and undetectable IGF1 levels, frequently have long lifespans (8). Functional mutations in IGF1R that result in diminished response to IGF1 are more prevalent in the descendants of centenarians (9) and moderate, but significant, increases in longevity were observed prospectively in women with lower serum IGF1 levels (10). Caloric restriction, acting via reduced serum IGF1 levels (11, 12), has been associated with longer lifespan and reduced neoplasia, dyslipidemia, and cardiovascular disease (13).
In addition to the association with longevity and disease burden, perturbations in IGF signaling are also characterized by a reproductive phenotype. Insulin-like growth factor 1 knockout mice are subfertile or infertile, and histologic analysis of the ovaries revealed an increased volume of total follicles but arrest of follicular growth at early antral stages without the presence of corpora lutea (14). Insulin-like growth factor 1 receptor heterozygous knockout mice have smaller litter sizes but a longer reproductive lifespan (7). In humans, patients with Laron’s syndrome tend to be anovulatory and infertile (15), and female members of the African Pygmy tribe, whose IGF1R mutation renders partial resistance to IGF1, were found to be subfertile and oligo-ovulatory in comparison to the members of other local hunter-gatherer tribes exposed to the same harsh environmental factors (16, 17). Insulin-like growth factor 1 receptor expression in the human ovary has been observed at the primordial follicle stage (18), and IGF1 is present in follicular fluid at levels that correspond to serum (19). In vitro and in vivo studies have demonstrated the synergy between follicle-stimulating hormone (FSH) and IGF1 in guiding normal granulosa cell function, follicular growth, and luteinization (20–22), and at the hypothalamic/pituitary level, IGF1 has been shown to regulate gonadotropin-releasing hormone, luteinizing hormone, and prolactin secretion (23, 24). Despite strong evidence for the importance of IGF signaling in governing reproductive function, the effect of IGF1 on human folliculogenesis along the hierarchy of developmental stages leading up to ovulation remains unclear.
Here, we used cortical xenografts with cotransplantation of engineered endothelial cells (ECs) to test the effect of chronic paracrine IGF1 stimulus on human folliculogenesis. We showed that while short-term xenografts in the presence of exogenous IGF1 exhibited an increased growth rate of primary and secondary stage follicles, chronic exposure to IGF1 at long-term endpoints resulted in near absence of antral follicles and increased presence of corpora lutea. Further experiments in mice revealed that advance in cell proliferation among preantral follicles drove increased oocyte yield following direct injection of the ovary with IGF1, and ribonucleic acid (RNA) sequencing suggested accelerated follicle maturation as a potential mechanism. These data isolate the influence of exogenous paracrine IGF1 on human folliculogenesis and suggest that while it may promote increased growth among early-stage follicles, it may also accelerate maturation/luteinization at later stages.
MATERIALS AND METHODS
Procurement of Ovarian Tissue
The ovarian cortex was isolated from whole bilateral ovaries obtained from two brain-dead organ donors, aged 25 and 33 years, as a part of a collaboration with the International Institute for the Advancement of Medicine, and from three other patients, aged 19, 19, and 46 years, following informed consent. The two 19 year-old patients were undergoing cryopreservation of ovarian tissue for fertility preservation due to malignancy, and the 46-year-old patient is a BRCA carrier who underwent prophylactic bilateral salpingo-oophorectomy, from whom a cortical fragment was received following pathological examination. Ovary donors and patients had no history of chemotherapy and no apparent history of endocrine or reproductive abnormalities. The ovaries were resected and placed in sterile Leibovitz medium (Gibco) on ice for transport to the research laboratory for processing following a cold ischemic interval of <4 hours. Cortical fragments from two patients (aged 19 and 46 years) and two donors (aged 25 and 33 years) were used for comparison of control and IGF1-expressing ECs in xenografts. A cortical fragment from the other 19-year-old patient was used to generate the xenograft-resident follicle immunolabeled in Figure 1A to C. A small piece of this fragment was isolated before cryopreservation for histologic examination, revealing significant activity and reserve of primordial follicles. Following this validation, tissue was thawed and xenotransplanted as described.
FIGURE 1.
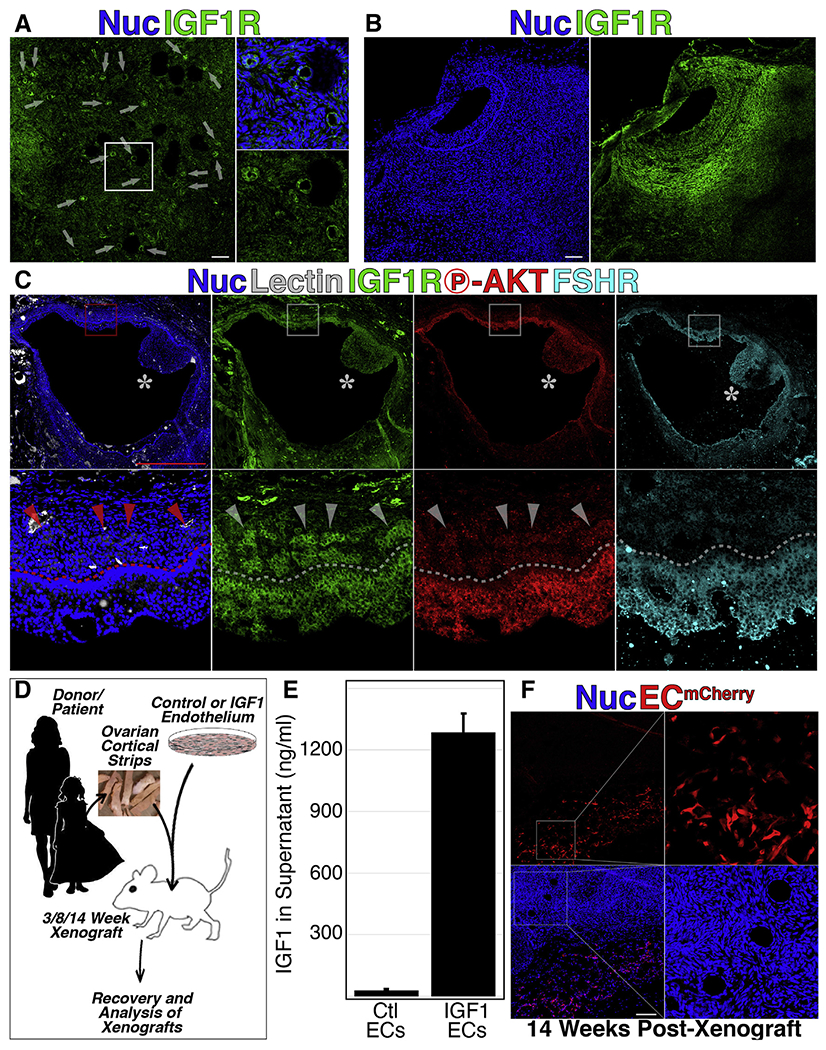
Sustained paracrine stimulus of xenograft-resident follicles with exogenous insulin-like growth factor 1 (IGF1). (A to C) Cryosections from xenografts of human ovarian cortical tissue were labeled with an antibody specific for IGF1 receptor; primary (A), secondary (A), and antral (B and C) stage follicles were positive for IGF1 receptor on the surface of granulosa (A to C) and theca (B and C) cells. The antral follicle in (c) was colabeled with the fluorescently conjugated lectin (Griffonia simplicifolia) and antibodies against phosphorylated Akt and follicle-stimulating hormone receptor. (D) Schematic representation of the experimental framework for cotransplantation of ovarian cortical strip along with either control or IGF1-expressing endothelial cells (ECs) in immune-compromised mice. (E) Cell culture supernatant from ECs transduced with lentiviral particles encoding IGF1 cDNA shows an approximately 100-fold increased presence of IGF1 in the supernatant of transduced vs. control ECs. (F) ECs, labeled by mCherry fluorescent protein persist in the margins of long-term xenografts. Error bars in (E) represent standard deviation between three replicate samples. Scale bars: 100 μm in A to B and F and 1 mm in C.
Ovarian Cortical Xenografts
All procedures were approved, and experiments were performed in accordance with the guidelines and regulations of the Institutional Animal Care and Use Committee (IACUC) of Weill Cornell Medicine (IACUC Protocol #2014-0008–Assessment of angiogenic and hematopoietic tissue in mouse). Ovarian cortical tissue was xenotransplanted into immunocompromised mice as previously described (25, 26). Briefly, cryopreserved tissue was thawed rapidly, washed of cryoprotectant, and encapsulated in fibrin that was premixed with a fluorescently labeled single-cell suspension of ECs. Fibrin-embedded tissue was then transplanted into oophorectomized mice bilaterally under the fascia of the gluteus maximus and the fascia, and the dorsal wall skin was closed with sutures. Xenografted animals were maintained in sterile conditions until the animals were euthanized and xenografts recovered for fixation, cryosectioning, and immunohistochemical staining. Follicles of all stages were quantified with secondary follicles designated as either simple (two layers of granulosa cells) or preantral (more than two layers of granulosa cells but before the emergence of an antral cavity).
Immunofluorescence
Cryosections were labeled as previously described (25, 26). Samples were permeabilized in phosphate-buffered saline (PBS)/0.1% TWEEN with 5% donkey serum (Millipore) and incubated in 2–5-μg/mL concentrations of specified antibodies, washed, and counterstained with 4,6-diamidino-2-phenylindole (DAPI) and mounted in ProLong Gold (Gibco). Images were captured using a Zeiss 710 confocal microscope.
Endothelial Cells
Human ECs were isolated from neonatal umbilical vein (human umbilical vein endothelial cells) as described (27). Cells were provided by Angiocrine Biosciences.
Lentiviral Vectors and Transduction of Cells
Lentiviral particles (GeneCopoeia) were added to cultured ECs and incubated for 48 hours. Subsequently, cells were expanded in culture and frozen using fetal bovine serum containing 10% dimethyl sulfoxide.
Mouse Ovarian Hyperstimulation
Seven days after direct ovary injection with recombinant mouse IGF1 (R&D, Cat. No. 791-MG, 60 μg) or an equal volume of PBS, female mice were superovulated with 0.1-mL CARD HyperOva (Cosmo Bio Co., Cat. No. KYD-010-EX) and 10-IU human chorionic gonadotropin (hCG, Sigma-Aldrich) at intervals of 48 hours. MII oocytes from superovulated female mice were recovered 14– 16 hours after hCG injection. Cumulus cells were dispersed by EmbryoMax Hyaluronidase M2 Medium (MR-0510F, Millipore) for 5 minutes; oocytes were picked and washed in EmbryoMax Advanced KSOM Embryo medium (MR-101, Millipore-Sigma) three times before microscopy and counting.
Mouse Ovarian Injections of Recombinant IGF1
C57Bl/6J 8–10-week-old female mice (Jackson Laboratory, USA) were subjected to intraovarian injections, one in each ovary, of either 60 μg of recombinant IGF1 or an equal volume of PBS. Briefly, under anesthesia using a dorsal approach, a middorsal incision and opening of the peritoneum were performed. The ovaries were identified and mobilized outside of the abdominal cavity with care. Using a 31-gauge insulin syringe, either recombinant IGF1 or saline was injected directly into the ovary. After injections, the ovaries were returned to the abdominal cavity, and the peritoneum and dorsal wall were closed with sutures. For bulk RNA sequencing experiments, female mice received intraovarian injections of recombinant IGF1 (25 μg) or PBS. After 24 or 72 hours, mice were euthanized, and the ovaries (n = 2) were processed for RNA isolation and bulk sequencing. For ethynyl-deoxyuridine (EdU) incorporation assay, female mice received intraovarian injections of either recombinant IGF1 or an equal volume of PBS. Shortly after the ovarian injections, mice were intraperitoneally injected with EdU (Sigma-Aldrich) at a concentration of 50 mg/kg. Forty-eight hours after the initial EdU injection, the ovaries were recovered and fixed overnight in 4% paraformaldehyde, followed by processing for frozen immunohistochemistry.
EdU Staining and Quantification
Cryosections were stained for EdU incorporation using the Click-iT EdU Cell Proliferation Kit for Imaging (Invitrogen) following the manufacturer’s instructions, followed by washing with PBS, and a second Click chemistry reaction with 2-mM azidomethyl phenyl sulfide (Sigma-Aldrich). For quantification of EdU incorporation, images were captured using a Zeiss 710 confocal microscope and analyzed using the Zeiss ZEN software. A total of three nonadjacent slides per ovary, each containing three consecutive sections, were used for quantification purposes. Masking colocalization was used to measure the area of overlap between EdU+ regions and the nuclear stain Hoescht, which was then divided by the total Hoescht+ area to obtain the area of proliferative nuclei in each individual follicle.
RNA Isolation
The whole mouse ovaries were isolated, homogenized, and, following removal of cellular debris, processed using the Arcturus PicoPure RNA Isolation Kit (Thermo Fisher Scientific), with treatment of each sample using RNAse-Free DNAse. RNA quality was checked by Agilent Technologies 2100 Bioanalyzer.
Bulk RNA Sequencing and Analysis
At least 100 ng of high-quality total RNA was used as input to convert the messenger RNA (mRNA) into a library of template molecules for subsequent cluster generation and sequencing using the reagents provided in the Illumina TruSeq RNA Sample Preparation Kit. Following purification of the poly-A containing mRNA molecules using poly-T oligo-attached magnetic beads, the mRNA was fragmented into small pieces using divalent cations under elevated temperature. The cleaved RNA fragments were copied into first-strand complementary DNA (cDNA) using reverse transcriptase and random primers. This was followed by second-strand cDNA synthesis using DNA polymerase I and RNase H. These cDNA fragments then went through an end repair process, the addition of a single “A” base, and then ligation of the adapters. The products were then purified and enriched with polymerase chain reaction to create the final cDNA library. After quantifying and checking the size and purity of the product, multiplexed DNA libraries were normalized to 10 nM, and then two sample libraries were pooled together in equal volumes. Subsequently, 7 pM of each pooled DNA library templates was amplified on an Illumina cBot instrument involving immobilization and 3′ extension, bridge amplification, linearization, and hybridization and then sequenced on one lane of the Illumina HiSeq2000 sequencer using the pair end module and generating 2 × 58-bp-long reads. The samples were aligned to the human GRCh38 reference assembly using the STAR aligner and subsequently, genes were counted in htseq.
Statistical Analyses
For the comparisons in Figures 1 and 4, treated and control conditions were compared using two-tailed Student’s t test with significance defined as P≤.05.
FIGURE 4.
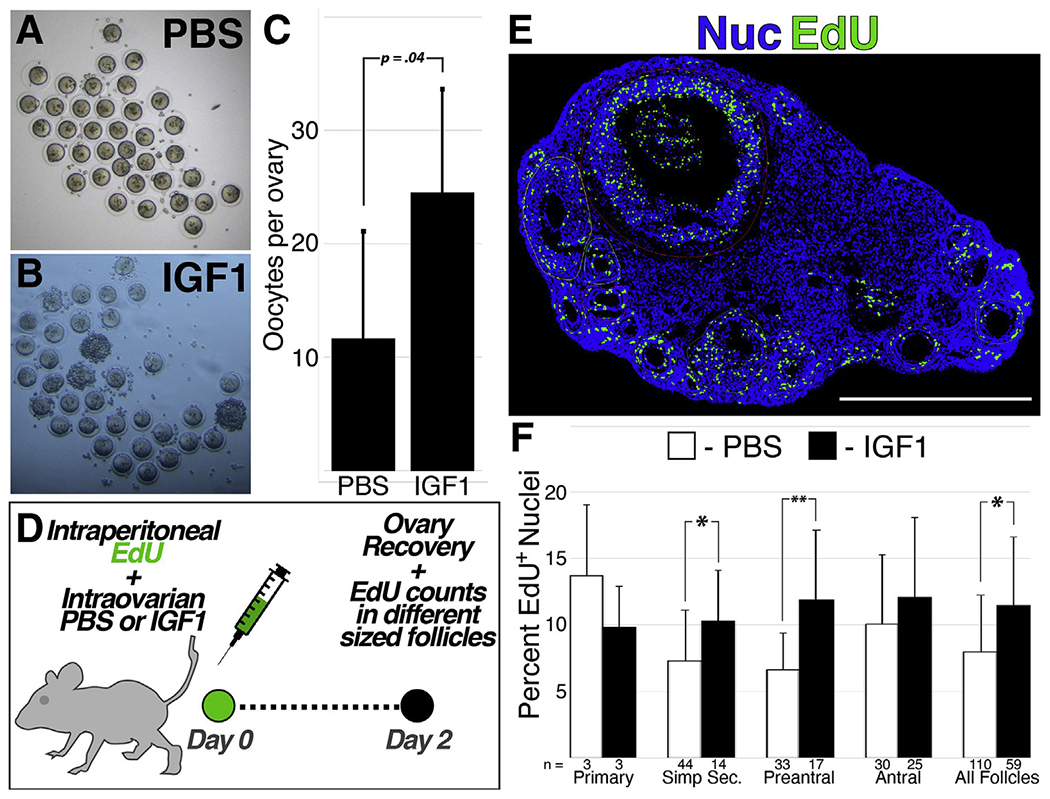
Direct injection of recombinant insulin-like growth factor 1 (IGF1) into the mouse ovary promotes follicle advance and increased oocyte yield. (A to C) Oocytes were recovered from mice and quantified (C) following bilateral ovarian injection of PBS (A) or IGF1 (B)and subsequent hyperstimulation (D to F). Mice were injected intraperitoneally with ethynyl-deoxyuridine at the time of ovary injection with IGF1/PBS (d), with the ovaries harvested for immunofluorescent quantification of proliferating cells 2 days later (E); the percentage of follicle-resident nuclei that were positive for ethynyl-deoxyuridine at across follicles stages is shown in (F) with the number of follicles represented below each bar. Error bars in (C) and (F) represent standard deviation between replicate samples; P value shown in (C) and asterisks in (F) denote a P value of <.05 (*) or <.01 (**). Scale bar: 1 mm.
Data from treated and untreated xenografts (Fig. 2) were compared using the nonparametric Mann–Whitney U test with significance defined as P≤.05.
FIGURE 2.
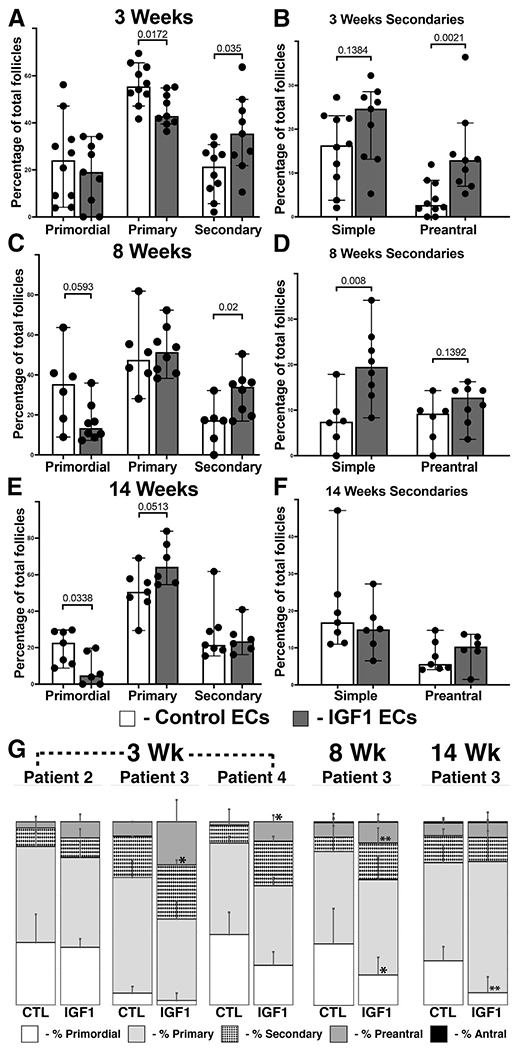
Paracrine insulin-like growth factor 1 (IGF1) stimulus in xenografts promotes follicular advance. (A to F) Xenografts were recovered at 3 (A and B), 8 (C and D), and 14 (E and F) weeks after cotransplantation with control or IGF1-expressing ECs. (G) Intrapatient subanalysis of follicle distribution, where sufficient replicates are available. The percentage of primordial, primary, and secondary follicles was quantified for each xenograft (shown in A, C, and E), with secondary follicles separated into simple and preantral subtypes (shown in B, D, and F). The bars in (A to F) represent the median percentages of follicle subtypes with 95% confidence interval and with each dot representing one replicate; numerical P values are shown. Error bars in (G) represent standard deviation, with asterisks denoting a P value of <.05 (*) or <.01 (**).
RESULTS
Paracrine Delivery of Exogenous IGF1 to Xenografted Ovarian Cortical Tissue
We have previously developed a xenograft platform in which cotransplantation of ECs provides a survival benefit to thawed ovarian cortex in immunocompromised mice (25,26). Examination of short- and long-term xenografts revealed positivity for IGF1R beginning at early primary stages and persisting through antral stages (Fig. 1A and B). Notably, antral follicles exhibited IGF1R+ cells throughout the granulosa cell layer and colocalized with phosphorylated Akt and FSH receptor; however, in larger-sized antral follicles, IGF1R+ cells were distributed in patches throughout the theca layer and were negative for phosphorylated Akt and FSH receptor (Fig. 1C). This expression pattern is consistent with our previous single-cell RNA sequencing study of granulosa, theca, and stroma cells from antral follicles, in which IGF1R expression was noted in both granulosa and theca cells (28). We also previously used cotransplanted ECs as a vector to convey a continuous paracrine source of antimüllerian hormone to improve retention of PrFs in short-term xenografts (25, 26). Here, we applied a similar approach in short-term (3 weeks) and long-term (8 and 14 weeks) xenografts using ECs that constitutively express and secrete IGF1 (Fig. 1D and E) and were retained in the periphery of xenografts at long-term endpoints (14 weeks, Fig. 1F).
Cotransplantation of IGF1 ECs Promotes Advance in Follicle Growth
Xenografts cotransplanted with control or IGF1-expressing ECs were recovered at 3, 8, and 14 weeks, and the distribution of follicle stage was determined for each graft (Supplementary Table 1 and Fig. 2A to F). At 3 weeks after transplant, xenografts in the context of IGF1 showed a decrease in primary follicles and an increase in secondary follicles that were concentrated in the preantral subset (Fig. 2A and B). At 8 weeks after transplant, IGF1 ECs also promoted an increase in secondary follicles, although at this time point, the difference was evident in the simple subset (Fig. 2C and D). Although a trend toward decreased primordial stage follicles was evident at 8 weeks, this reached significance by 14 weeks after transplant, and this coincided with a near-significant increase in primary follicles (Fig. 2E and F). Subanalysis of cortical fragments derived from individual patients was performed where sufficient replicates were present (Fig. 2G), revealing trends that supported the aggregate analysis in both younger (patient 3, 25 years) and older (patient 4, 46 years) patients.
Antral stage follicles were observed beginning at 8 weeks in xenografts cotransplanted with both control (n = 2) and IGF1 (n = 2) ECs (Fig. 3A). Surprisingly, while antral follicles were present in the control condition at 14 weeks, they were near absent from the IGF1 condition at this time point, with only one antral follicle of aberrant morphology observed (Fig. 3B and C). Notably, multiple structures displaying histologic character (Fig. 3D) and molecular markers (TUNEL+HABP1+CD44+, Fig. 3E) of corpora lutea were evident in xenografts, and these structures increased in the IGF1 condition (Fig. 3F).
FIGURE 3.
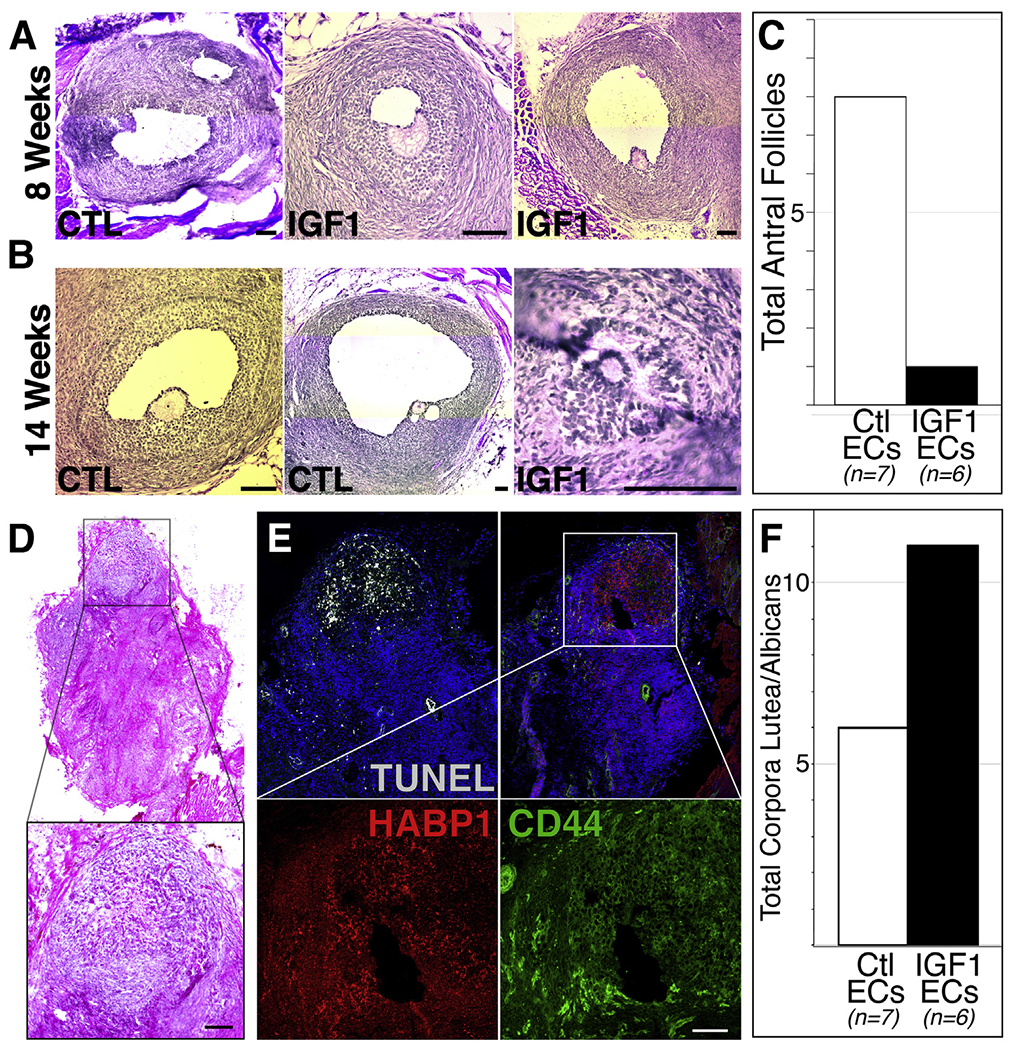
Paracrine insulin-like growth factor 1 (IGF1) stimulus affects the distribution of antral follicles and corpora lutea in long-term xenografts. (A to C) Histologic examples (A and B) and absolute numbers (C) of antral follicles identified in xenografts cotransplanted with control or IGF1 endothelial cells after 8 (A) or 14 (B and C) weeks. (D to F) Histologic (D) and molecular (E) evidence of corpora lutea-like structures that increased (f) in xenografts cotransplanted with IGF1-expressing endothelial cells. The insets in (D) and (E) are magnified in the panels below. Scale bars: 100 μm.
Injection of Recombinant IGF1 into the Mouse Ovary Promotes Follicle Advance and Increased Oocyte Yield
The increased prevalence of corpora lutea-like structures (Fig. 3D to F) suggested that chronic stimulation of xenograft-resident follicles with IGF1 over long-term intervals results in premature luteinization or atresia. To determine whether IGF1 promotes follicle growth in a more robust experimental system, we utilized hormonal stimulation in reproductively mature (12 weeks) C57/Bl6 mice. First, we directly injected the mouse ovaries with recombinant IGF1 (60 μg each) and then induced ovulation via HyperOva and hCG, resulting in a significant increase in the number of recovered oocytes (Fig. 4A to C). Separately, we assessed cell proliferation in the window following IGF1 injection using EdU (Fig. 4D to F). Although the time interval was much shorter than that used for xenografts (Fig. 2), the mouse ovaries similarly displayed significant increases in EdU+ cells that were concentrated among secondary and preantral follicles (Fig. 4F).
RNA Sequencing of the Ovaries Following IGF1 Injection Reveals an Increase in Luteal Transcripts
Although IGF1 promoted advanced growth among primary and secondary follicles in xenografts and mouse ovaries, antral follicles were nearly absent in long-term xenografts (Fig. 3C), but in the mouse ovaries, proliferation in antral follicles was not significantly altered (Fig. 4F), and oocyte yield was increased (Fig. 4C). To reconcile these competing observations and identify molecular targets that may mediate the effects of IGF1, we performed RNA sequencing of the ovaries that were injected PBS or IGF1 at 24 and 72 hours after injection (Fig. 5A and B). Subsequently, 53 and 52 genes were differentially expressed between PBS- and IGF1-injected ovaries at 24 and 72 hours, respectively (Fig. 5C and D). Importantly, numerous genes (highlighted in Fig. 5E and F) that are related to sex steroid metabolism (Cyp19a1, Star, Akr1c18, Hsd17b2) and luteinization/ovulation (Lgals1/3, Alas1, Sfrp4, Abcb1b, etc.) increased at 24 hours and reduced at 72 hours after injection.
FIGURE 5.
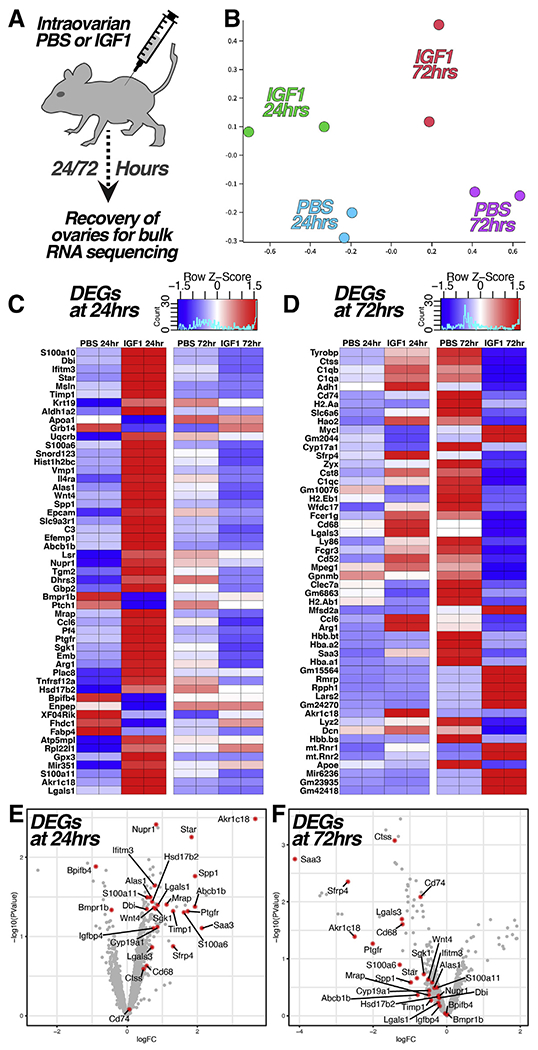
Ribonucleic acid sequencing reveals acute elevation of transcripts evident at later follicle stages. (A to B) The ovaries were recovered from mice for ribonucleic acid sequencing at 24 and 72 hours following direct injection with phosphate-buffered saline (PBS) or recombinant IGF1 (A); a multidimensional scale plot of the relative similarity between samples is shown in (B). (C to D) Heatmaps showing the 53 (C) and 52 (D) transcripts that were differentially expressed (P<.05) between the PBS and IGF1 conditions at 24 (C ) and 72 (D) hours after injection. (E to F) Volcano plots showing the distribution of differentially expressed transcripts between PBS and IGF1 conditions at 24 (E ) and 72 (F) hours after injection; transcripts with relevance to sex steroid biosynthesis/metabolism and/or follicle maturation/luteinization are highlighted in red in (E to F).
DISCUSSION
Many studies have suggested a role for IGF signaling in the regulation of follicle growth; however, the specific influence of IGF1 along the spectrum of growth stages that encompass human folliculogenesis remains unclear. Here, we used a xenograft platform to interrogate the influence of sustained paracrine IGF1 on follicle growth in human ovarian tissue and applied complementary experiments in mice to identify cellular and molecular mechanisms that drive the IGF1-induced phenotype. While these methods are not practical for the mobilization of ovarian follicles in patients, the results confirm that IGF1 signaling is a significant modulator of follicle advance and maturation in the human ovary and suggest both the therapeutic and pathological potential of IGF1 signal modulation in the context of assisted reproduction.
In the ovary, IGF1/IGF1R signaling is required in granulosa cells for FSH-mediated stimulation of Akt and downstream steroidogenesis, and murine studies have suggested a role for IGF1 in both the recruitment and maturation of follicles (20–22). Hence, it is not surprising that IGF1R was observed in xenograft-resident follicles of primary through antral stages (Fig. 1A to C). However, in large antral follicles, IGF1R+ regions within the theca layer were not positive for phosphorylated Akt to the same degree as neighboring granulosa cells (Fig. 1C). We previously performed transcriptomic analysis of granulosa and theca cells from human antral follicles (28) and observed a dynamic expression pattern of IGF-binding proteins that are known to modulate bioactivity of IGF1. Indeed, circulating levels of IGF1 can shift dynamically in response to environmental (food intake) or physiological (age) influences (29), thereby necessitating a multitiered mechanism for controlling signal integration at the level of the ovary. While chronic paracrine stimulation with IGF1 in xenografts may have increased the growth rate of early follicles (Fig. 2A to F), the near absence of antral follicles (Fig. 3C) and presence of corpora lutea-like structures (Fig. 3D to F) in IGF1-stimulated xenografts may have resulted from overcoming controls on IGF signaling in theca-resident populations that typically play a role in follicle maturation/luteinization.
Complimentary experiments in mice (Fig. 4) aligned with results from xenografts indicating an increased pace of follicles at the primary and secondary stages, but unlike xenografts, the mouse ovaries yielded a significantly increased number of oocytes when hyperstimulated (Fig. 4A to C). While it is difficult to draw comparisons between the two experimental models, given the divergence in reproductive biology and differing modes of IGF1 delivery, RNA sequencing analysis (Fig. 5) suggested that the absence of antral follicles in IGF1-stimulated xenografts has resulted from precocious maturation/luteinization. Of 53 transcripts that were differentially expressed between the ovaries 24 hours after injection with PBS or IGF1, many, including Nupr1 (30, 31), Star (32), Bpifb4 (33), Spp1 (34), S100a11 (35), Alas1 (36), Lgals1 (37, 38), Abcb1 (39), Wnt4 (40, 41), Sgk1 (42), Dbi (43), Timp1 (44), Ptgfr (45), and S100a6 (46), are enriched in late antral, luteal, or periovulatory follicles. This is not surprising given the increase in oocyte yield in IGF1-stimulated ovaries following hyper-stimulation, but it suggests that depleted antral follicles in long-term IGF1-stimulated xenografts have resulted from accelerated or precocious luteinization. While these results corroborate the growth and maturation-promoting functions of IGF1 in human ovarian tissue, they also underscore the challenges to applying IGF1 therapeutically to enhance in vitro fertilization (IVF) outcome.
While many lines of evidence support the rationale for clinical application of GH (acting via IGF1 at the follicle level) in IVF treatment, its use remains controversial. The follicular fluid concentration of IGF1 at oocyte retrieval is correlated with follicle numbers and inversely correlated with the amount of ovarian stimulation required (19), and a randomized controlled trial of women undergoing ovulation induction demonstrated that the requirement for gonadotropins was reduced in women who were coadministered GH during ovarian stimulation (47). However, the mechanism by which GH and IGF1 confer a benefit and the optimal mode of administration remain open to debate. Although GH has been applied in a broad selection of patient types, it is most commonly used as an adjuvant in women who have shown a poor response to ovarian stimulation in one or more preceding IVF cycles (48). In the largest randomized study performed, to date, the administration of GH beginning with the commencement of a “long downregulation protocol” resulted in improved stimulation and increased oocytes, fertilization, and embryo development but did not significantly improve cumulative birth rate (49). A more recent study that implemented GH at the outset of ovarian stimulation resulted in more oocytes but did not improve live birth rate (50). Given the extended period of follicle development that occurs before the gonadotropin-dependent phase, commencing GH treatment in poor responders weeks (or months) in advance of ovarian hyperstimulation may be more effective in amplifying the reservoir of responsive follicles. However, GH is not approved by the US Food and Drug Administration for use in patients with IVF outside of GH deficiency, and long-term use can have unwelcome side effects (51). The present study is limited in that the delivery of IGF1 is sustained for the life of the graft and at levels that are not well defined, but augmented growth evident among early-stage follicles (Fig. 2A to F) suggests that transient GH (IGF1) stimulus could be applied clinically to amplify a cohort of early-stage follicles that could be recruited by traditional hyperstimulation in a successive cycle.
We applied a xenograft platform to examine the influence of exogenous IGF1 on the growth and development of human follicles and define the longitudinal effect of chronic IGF1 stimulus as the follicular cohort is mobilized within the xenografted ovarian cortex. While they are a robust experimental tool that enables direct interrogation of human reproductive biology, xenografts provide a relatively poor facsimile of the native ovary, as they undergo surgical resection/processing, cryopreservation, thaw, and the acute inflammatory and hypoxic stress of the posttransplant phase. Moreover, the hypothalamic/pituitary axis and endogenous IGF signaling status of the mouse/host are not evolutionarily aligned with human ovarian tissue. Nevertheless, these experiments, with supporting results from mouse, isolate the influence of IGF1 on human folliculogenesis and corroborate its growth-promoting function.
While these results do not justify the application of augmented ECs and/or recombinant IGF1 in a clinical setting, they shed light on the purview of IGF signaling across human folliculogenesis and may provide a framework for developing compound protocols that amplify the pool of gonadotropin-dependent candidate follicles in poor responders by priming with GH.
Supplementary Material
Acknowledgments
The work was funded in part by a Research Grant to Daylon James from ASRM in 2016.
Footnotes
L.M. has nothing to disclose. N.L.G. has nothing to disclose. E.K. has nothing to disclose. L.P. has nothing to disclose. B.C. has nothing to disclose. M.K. has nothing to disclose. Z.-Y.L. has nothing to disclose. R.P. has nothing to disclose. C.T. has nothing to disclose. J.L. has nothing to disclose. L.Z. has nothing to disclose. R.B. has nothing to disclose. D.W. has nothing to disclose. N.Z. has nothing to disclose. G.S. has nothing to disclose. Z.R. has nothing to disclose. D.J. has nothing to disclose.
REFERENCES
- 1.Salmon WD Jr, Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med 1957;49: 825–36. [PubMed] [Google Scholar]
- 2.Daughaday WH, Hall K, Raben MS, Salmon WD Jr, van den Brande JL, van Wyk JJ. Somatomedin: proposed designation for sulphation factor. Nature 1972;235:107. [DOI] [PubMed] [Google Scholar]
- 3.Rinderknecht E, Humbel RE. Polypeptides with nonsuppressible insulin-like and cell-growth promoting activities in human serum: isolation, chemical characterization, and some biological properties of forms I and II. Proc Natl Acad Sci U S A 1976;73:2365–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hakuno F, Takahashi SI. IGF1 receptor signaling pathways. J Mol Endocrinol 2018;61:T69–86. [DOI] [PubMed] [Google Scholar]
- 5.Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature 2000;408:255–62. [DOI] [PubMed] [Google Scholar]
- 6.Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science 2001;292:107–10. [DOI] [PubMed] [Google Scholar]
- 7.Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 2003;421:182–7. [DOI] [PubMed] [Google Scholar]
- 8.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 2011;3:70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A 2008;105:3438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milman S, Atzmon G, Huffman DM, Wan J, Crandall JP, Cohen P, et al. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell 2014;13:769–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn SE, Kari FW, French J, Leininger JR, Travlos G, Wilson R, et al. Dietary restriction reduces insulin-like growth factor I levels, which modulates apoptosis, cell proliferation, and tumor progression in p53-deficient mice. Cancer Res 1997;57:4667–72. [PubMed] [Google Scholar]
- 12.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell 2008;7:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun 2014;5:3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellve AR, et al. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol 1996;10:903–18. [DOI] [PubMed] [Google Scholar]
- 15.Laron Z, Klinger B. Laron syndrome: clinical features, molecular pathology and treatment. Horm Res 1994;42:198–202. [DOI] [PubMed] [Google Scholar]
- 16.Bailey RC. The comparative growth of Efe pygmies and African farmers from birth to age 5 years. Ann Hum Biol 1991;18:113–20. [DOI] [PubMed] [Google Scholar]
- 17.Geffner ME, Bersch N, Bailey RC, Golde DW. Insulin-like growth factor I resistance in immortalized T cell lines from African Efe Pygmies. J Clin Endocrinol Metab 1995;80:3732–8. [DOI] [PubMed] [Google Scholar]
- 18.Qu J, Godin PA, Nisolle M, Donnez J. Expression of receptors for insulin-like growth factor-I and transforming growth factor-beta in human follicles. Mol Hum Reprod 2000;6:137–45. [DOI] [PubMed] [Google Scholar]
- 19.Oosterhuis GJ, Vermes I, Lambalk CB, Michgelsen HW, Schoemaker J. Insulin-like growth factor (IGF)-I and IGF binding protein-3 concentrations in fluid from human stimulated follicles. Hum Reprod 1998;13:285–9. [DOI] [PubMed] [Google Scholar]
- 20.Baumgarten SC, Armouti M, Ko C, Stocco C. IGF1R expression in ovarian granulosa cells is essential for steroidogenesis, follicle survival, and fertility in female mice. Endocrinology 2017;158:2309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumgarten SC, Convissar SM, Fierro MA, Winston NJ, Scoccia B, Stocco C. IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human Cumulus granulosa cells. J Clin Endocrinol Metab 2014;99:2995–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou P, Baumgarten SC, Wu Y, Bennett J, Winston N, Hirshfeld-Cytron J, et al. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol Endocrinol 2013;27:511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hara N, Takizawa I, Isahaya E, Nishiyama T, Hoshii T, Ishizaki F, et al. Insulin-like growth factor-1 is associated with regulation of the luteinizing hormone production in men receiving androgen deprivation therapy with gonadotropin-releasing hormone analogues for localized prostate cancer. Urol Oncol 2012;30:596–601. [DOI] [PubMed] [Google Scholar]
- 24.Hikake T, Hayashi S, Iguchi T, Sato T. The role of IGF1 on the differentiation of prolactin secreting cells in the mouse anterior pituitary. J Endocrinol 2009;203:231–40. [DOI] [PubMed] [Google Scholar]
- 25.Man L, Park L, Bodine R, Ginsberg M, Zaninovic N, Man OA, et al. Engineered endothelium provides angiogenic and paracrine stimulus to grafted human ovarian tissue. Sci Rep 2017;7:8203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Man L, Park L, Bodine R, Ginsberg M, Zaninovic N, Schattman G, et al. Co-transplantation of human ovarian tissue with engineered endothelial cells: a cell-based strategy combining accelerated perfusion with direct paracrine delivery. J Vis Exp 2018:57472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seandel M, Butler JM, Kobayashi H, Hooper AT, White IA, Zhang F, et al. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc Natl Acad Sci U S A 2008;105:19288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Man L, Lustgarten-Guahmich N, Kallinos E, Redhead-Laconte Z, Liu S, Schattman B, et al. Comparison of human antral follicles of xenograft versus ovarian origin reveals disparate molecular signatures. Cell Rep 2020;32:108027. [DOI] [PubMed] [Google Scholar]
- 29.Hawkes CP, Grimberg A. Insulin-like growth factor-I is a marker for the nutritional state. Pediatr Endocrinol Rev 2015;13:499–511. [PMC free article] [PubMed] [Google Scholar]
- 30.Christenson LK, Gunewardena S, Hong X, Spitschak M, Baufeld A, Vanselow J. Research resource: preovulatory LH surge effects on follicular theca and granulosa transcriptomes. Mol Endocrinol 2013;27:1153–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Million Passe CM, White CR, King MW, Quirk PL, Iovanna JL, Quirk CC. Loss of the protein NUPR1 (p8) leads to delayed LHB expression, delayed ovarian maturation, and testicular development of a sertoli-cell-only syndrome-like phenotype in mice. Biol Reprod 2008;79:598–607. [DOI] [PubMed] [Google Scholar]
- 32.Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem 1994;269:28314–22. [PubMed] [Google Scholar]
- 33.Toda K, Hayashi Y, Ono M, Saibara T. Characterization of ovarian responses to equine chorionic gonadotropin of aromatase-deficient mice with or without 17β-estradiol supplementation. Endocrinology 2016;157:2093–103. [DOI] [PubMed] [Google Scholar]
- 34.Poole DH, Ndiaye K, Pate JL. Expression and regulation of secreted phosphoprotein 1 in the bovine corpus luteum and effects on T lymphocyte chemotaxis. Reproduction 2013;146:527–37. [DOI] [PubMed] [Google Scholar]
- 35.Hanaue M, Miwa N, Uebi T, Fukuda Y, Katagiri Y, Takamatsu K. Characterization of S100A11, a suppressive factor of fertilization, in the mouse female reproductive tract. Mol Reprod Dev 2011;78:91–103. [DOI] [PubMed] [Google Scholar]
- 36.Liu LQ, Li FE, Deng CY. Short communication: molecular cloning and expression pattern of the porcine 5-aminolevulinate synthase 1 (ALAS1) gene and its association with reproductive traits. Genet Mol Res 2016;15. [DOI] [PubMed] [Google Scholar]
- 37.Nio-Kobayashi J, Boswell L, Amano M, Iwanaga T, Duncan WC. The loss of luteal progesterone production in women is associated with a galectin switch via α2,6-sialylation of glycoconjugates. J Clin Endocrinol Metab 2014;99:4616–24. [DOI] [PubMed] [Google Scholar]
- 38.Nio-Kobayashi J, Iwanaga T. Galectin-1 and galectin-3 in the corpus luteum of mice are differentially regulated by prolactin and prostaglandin F2α. Reproduction 2012;144:617–24. [DOI] [PubMed] [Google Scholar]
- 39.Fan HY, Liu Z, Johnson PF, Richards JS. CCAAT/enhancer-binding proteins (C/EBP)-α and -β are essential for ovulation, luteinization, and the expression of key target genes. Mol Endocrinol 2011;25:253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boyer A, Lapointe E, Zheng X, Cowan RG, Li H, Quirk SM, et al. WNT4 is required for normal ovarian follicle development and female fertility. FASEB J 2010;24:3010–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh M, Johnson MA, Greenberg NM, Richards JS. Regulated expression of Wnts and Frizzleds at specific stages of follicular development in the rodent ovary. Endocrinology 2002;143:898–908. [DOI] [PubMed] [Google Scholar]
- 42.Alliston TN, Gonzalez-Robayna IJ, Buse P, Firestone GL, Richards JS. Expression and localization of serum/glucocorticoid-induced kinase in the rat ovary: relation to follicular growth and differentiation. Endocrinology 2000;141:385–95. [DOI] [PubMed] [Google Scholar]
- 43.Li Q, Jimenez-Krassel F, Ireland JJ, Smith GW. Gene expression profiling of bovine preovulatory follicles: gonadotropin surge and prostanoid-dependent up-regulation of genes potentially linked to the ovulatory process. Reproduction 2009;137:297–307. [DOI] [PubMed] [Google Scholar]
- 44.Zhang B, Moses MA, Tsang PC. Temporal and spatial expression of tissue inhibitors of metalloproteinases 1 and 2 (TIMP-1 and -2) in the bovine corpus luteum. Reprod Biol Endocrinol 2003;1:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berisha B, Rodler D, Schams D, Sinowatz F, Pfaffl MW. Prostaglandins in superovulation induced bovine follicles during the preovulatory period and early corpus luteum. Front Endocrinol (Lausanne) 2019;10:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanaue M, Miwa N, Takamatsu K. Immunohistochemical characterization of S100A6 in the murine ovary. Acta Histochem Cytochem 2012;45:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Homburg R, West C, Torresani T, Jacobs HS. Cotreatment with human growth hormone and gonadotropins for induction of ovulation: a controlled clinical trial. Fertil Steril 1990;53:254–60. [DOI] [PubMed] [Google Scholar]
- 48.Hart RJ. Use of growth hormone in the IVF treatment of women with poor ovarian reserve. Front Endocrinol (Lausanne) 2019;10:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dakhly DMR, Bassiouny YA, Bayoumi YA, Hassan MA, Gouda HM, Hassan AA. The addition of growth hormone adjuvant therapy to the long down regulation protocol in poor responders undergoing in vitro fertilization: randomized control trial. Eur J Obstet Gynecol Reprod Biol 2018;228:161–5. [DOI] [PubMed] [Google Scholar]
- 50.Norman RJ, Alvino H, Hull LM, Mol BW, Hart RJ, Kelly TL, et al. Human growth hormone for poor responders: a randomized placebo-controlled trial provides no evidence for improved live birth rate. Reprod Biomed Online 2019;38:908–15. [DOI] [PubMed] [Google Scholar]
- 51.van Bunderen CC, Glad C, Johannsson G, Olsson DS. Personalized approach to growth hormone replacement in adults. Arch Endocrinol Metab 2019;63:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


