Abstract
Background:
Cannabis legalization is expanding, but there are no established methods for detecting cannabis impairment.
Aim:
Characterize the acute impairing effects of oral and vaporized cannabis using various performance tests.
Methods:
Participants (N = 20, 10 men/10 women) who were infrequent cannabis users ingested cannabis brownies (0, 10, and 25 mg Δ−9-tetrahydrocannabinol, THC) and inhaled vaporized cannabis (0, 5, and 20 mg THC) in six double-blind outpatient sessions. Cognitive/psychomotor impairment was assessed with a battery of computerized tasks sensitive to cannabis effects, a novel test (the DRiving Under the Influence of Drugs, DRUID®), and field sobriety tests. Blood THC concentrations and subjective drug effects were evaluated.
Results:
Low oral/vaporized doses did not impair cognitive/psychomotor performance relative to placebo but produced positive subjective effects. High oral/vaporized doses impaired cognitive/psychomotor performance and increased positive and negative subjective effects. The DRUID® was the most sensitive test to cannabis impairment, as it detected significant differences between placebo and active doses within both routes of administration. Women displayed more impairment on the DRUID® than men at the high vaporized dose only. Field sobriety tests showed little sensitivity to cannabis-induced impairment. Blood THC concentrations were far lower after cannabis ingestion versus inhalation. After inhalation, blood THC concentrations typically returned to baseline well before pharmacodynamic effects subsided.
Conclusions:
Standard approaches for identifying impairment due to cannabis exposure (i.e. blood THC and field sobriety tests) have severe limitations. There is a need to identify novel biomarkers of cannabis exposure and/or behavioral tests like the DRUID® that can reliably and accurately detect cannabis impairment at the roadside and in the workplace.
Keywords: Cannabis, impairment, cannabis edibles, cannabis vaporizers
Introduction
Cannabis is one of the most widely used drugs in the world, and due to recent policy reforms, it is more accessible now than ever before. At the time of this writing, 36 US states and the District of Columbia allow cannabis use for medicinal purposes, and 17 states allow for non-medicinal (“recreational”) use of cannabis. Other developed countries have also legalized cannabis for medicinal (e.g. Australia and many European countries) or non-medicinal use (e.g. Uruguay and Canada). As these policy changes have occurred, perceptions of harm and stigma associated with cannabis use have decreased, whereas the rate of cannabis use among adults has increased (Berg et al., 2015; Carliner et al., 2017).
In controlled laboratory studies, cannabis that contains Δ−9-tetrahydrocannabinol (THC) (the primary psychoactive constituent of the plant) has been reliably shown to acutely impair cognitive/psychomotor functioning (Curran et al., 2016; National Academies of Sciences and Medicine, 2017; Spindle et al., 2018; Volkow et al., 2016) as well as simulated (Arkell et al., 2019; Hartman et al., 2015, 2016) and on-road driving performance (Bosker et al., 2012; Ramaekers et al., 2000). Consequently, a chief concern with the widespread legalization of cannabis is the potential for increased automobile and workplace accidents due to cannabis intoxication. Indeed, recent data from the US has shown an increase in rates of driving under the influence of cannabis (DUIC) (Fink et al., 2020). Moreover, though correlational in nature and thus unable to establish causality, state-level data have shown an increase in automobile accidents in states that have legalized cannabis (Monfort, 2018; Lane and Hall, 2019). Further complicating matters, there is currently no reliable method for detecting cannabis impairment in real-world settings, such as during roadside traffic stops. Standard field sobriety tests, which were designed and validated to detect alcohol impairment (Stuster, 2006), have been shown to lack sensitivity to cannabis (Bosker et al., 2012; Papafotiou et al., 2005b). Further, unlike with alcohol, THC kinetics in blood are not linear and are thus not a reliable proxy for cannabis impairment; see Ginsburg (2019) for a review of issues with measuring cannabis impairment. Developing a comprehensive understanding of the pharmacodynamics (e.g. cognitive, psychomotor, and subjective effects) and pharmacokinetics of cannabis through controlled research is essential for informing appropriate methods to detect impairment. However, prior controlled cannabis studies have predominantly focused on smoked cannabis, meaning much of the extant data regarding acute cannabis effects may not translate entirely to emergent, alternative routes of administration.
Cannabis is best described as a diverse class of products as opposed to a singular product (Spindle et al., 2019a). Although cannabis is most commonly consumed by smoking dried plant material (Borodovsky et al., 2016; Goodman et al., 2020; Knapp et al., 2018), many novel methods of administration have emerged, the two most popular of which are: oral cannabis products (a.k.a., edibles) and vaporized cannabis (Goodman et al., 2020). Oral cannabis products may consist of cannabis-infused baked goods, candies, or drinks (e.g. soda, tea, etc.). Cannabis vaporizers are a broad category of devices that heat cannabis in one of several possible forms (e.g. dried plant material or specially formulated cannabis extracts) to temperatures high enough to produce an aerosol (or “vapor”) for users to inhale, but below temperatures associated with combustion (Spindle et al., 2019a). Devices used to vaporize cannabis or cannabis extracts may include handheld products comparable to electronic cigarettes (often referred to as “vape pens”) or other devices (e.g. “DAB” rigs and larger “desktop” vaporizers). Despite the growing popularity of cannabis edibles and vaporizers, only a handful of studies have characterized the acute effects of these two alternative methods of administration.
Several prior studies have administered cannabis edibles under controlled conditions (Cone et al., 1988; Newmeyer et al., 2017a; Niedbala et al., 2001; Schlienz et al., 2020; Vandrey et al., 2017; Wachtel et al., 2002). Collectively, these studies revealed several important insights. First, the time course of pharmacodynamic effects in these studies differed considerably from studies of inhaled cannabis. Specifically, the onset of effects from cannabis edibles was delayed by approximately 30–45 min following ingestion, peak effects occurred between 1.5 and 4 h, and effects persisted for up to 8 h in some cases; this time course is vastly different from inhaled cannabis (either smoked or vaporized) for which pharmacodynamic effects tend to peak 10–30 min after the use and have a shorter duration (Newmeyer et al., 2017a; Spindle et al., 2018). In addition, several of these studies showed that peak blood concentrations of cannabinoids (e.g. THC and its metabolites) were much lower after cannabis ingestion compared to inhalation (Schlienz et al., 2020; Spindle et al., 2018; Vandrey et al., 2017), even though pharmacodynamic effects may be comparable between these two routes of administration at a given THC dose.
As with edibles, relatively few controlled laboratory studies have been conducted to characterize the pharmacodynamic and pharmacokinetic effects of vaporized cannabis (Abrams et al., 2007; Arkell et al., 2019; Kowal et al., 2015; Newmeyer et al., 2017a; Spindle et al., 2018). These studies have found that the time course of effects from vaporized cannabis is comparable to that of smoked cannabis, and that vaporized cannabis reliably impairs cognitive functioning (Spindle et al., 2018) and driving ability (Arkell et al., 2019) at a range of doses (e.g. 13.75–50 mg THC). In addition, one study found that vaporized cannabis delivered more THC and produced greater pharmacodynamic effects (e.g. subjective drug effects and cognitive impairment) compared to the same dose of smoked cannabis (Spindle et al., 2018). However, in other studies (Abrams et al., 2007; Newmeyer et al., 2017a), the magnitude of effects was similar between smoked and vaporized cannabis; these discordant findings are likely related to variation in the methods used to administer vaporized and smoked cannabis across studies.
Though prior controlled studies of oral or vaporized cannabis have advanced scientific understanding of the acute effects of cannabis, additional research is needed in this area for several reasons. First, only one of these studies, which was published across several papers (Newmeyer et al., 2016, 2017a, 2017b; Swortwood et al., 2017), involved administration of both oral and vaporized cannabis, albeit at one dose of THC (~50 mg). No prior studies have administered oral and vaporized cannabis to the same individuals using multiple doses of THC; this is a critical limitation given that many THC doses are available on the retail market (Steigerwald et al., 2018). Second, many of these studies only included frequent (at least weekly) cannabis users. As a result of expanded legal access to cannabis, infrequent cannabis users make up a growing segment of the cannabis-using population. Importantly, infrequent cannabis users may show heightened sensitivity to cannabis compared with frequent users who are more tolerant to the effects of cannabis (Ramaekers et al., 2009), making it important to further examine acute cannabis effects among these individuals. Third, further understanding of the acute pharmacodynamic effects of these two alternative routes of administration is needed to facilitate the development of novel impairment detection methods and, more broadly, to inform regulatory and clinical decisions involving cannabis. The present study extends these prior acute cannabis administration studies by including multiple doses of both oral and vaporized cannabis, enrolling infrequent cannabis users, and evaluating a novel cannabis impairment detection tool (the DRiving Under the Influence of Drugs (DRUID)® application, see below).
The primary purpose of this study was to characterize the acute pharmacodynamic and pharmacokinetic effects of oral and vaporized cannabis in healthy adult volunteers (N = 20) with current infrequent cannabis use patterns (i.e. no use for ≥30 days at study onset). Infrequent cannabis users were included because this group of individuals has been understudied in controlled cannabis research. Further, infrequent cannabis users were selected to constrain the impact of cannabis/THC tolerance on impairment outcomes and recent cannabis use on the characterization of blood THC pharmacokinetics, both of which could have been influenced if regular cannabis users were enrolled (Odell et al., 2015; Peng et al., 2020; Ramaekers et al., 2009). The primary focus of the pharmacodynamic outcomes was to evaluate a variety of methods for detecting impairment due to acute cannabis exposure. Impairment was assessed via self-report, a battery of computerized cognitive/psychomotor performance tasks (including a novel mobile device application called the DRUID®), and a battery of field sobriety tests commonly used to detect drug/alcohol intoxication in roadside traffic stops. Whole blood THC concentrations, additional subjective drug effects, and vital signs were also measured as secondary outcomes. We hypothesized that, within each route of administration, the high THC dose would produce greater impairment on the various performance measures included relative to placebo, whereas the low THC dose would elicit discriminable drug effects (e.g. increased subjective ratings of “drug effect” relative to placebo) without producing impairment.
Method
Participants
Research participants were recruited via media advertisements and word-of-mouth communication. Potential participants who appeared eligible based on an initial telephone screen completed an in-person screening visit. At this visit, health status was ascertained by medical history review, electrocardiogram (ECG), routine blood testing (chemistry, hematology, and serology), and a physical examination. Recent drug/alcohol use was assessed via the Timeline Follow-Back (Sobell and Sobell, 1992), and participants provided a urine specimen, which was tested (via a rapid enzyme immunoassay test kit) for cannabis, amphetamines, benzodiazepines, cocaine, MDMA, opioids, and phencyclidine and were given an alcohol breathalyzer. A serum pregnancy test was also conducted for female participants. All participants provided written informed consent prior to study participation and received monetary compensation for each completed visit. Experimental procedures were approved by the Institutional Review Board of Johns Hopkins University School of Medicine and were conducted in accordance with the Declaration of Helsinki.
In order to be eligible, participants were required to: (1) be between the ages of 18 and 45; (2) be in good health (determined using the screening visit procedures described above); (3) self-report no cannabis use for at least 1 month prior to their first experimental session; (4) report prior experience inhaling cannabis (either smoking or vaporizing); (5) provide a negative urine test for cannabis and other illicit drugs and a negative breath test for alcohol at the screening visit and before each session; (6) have a body mass index (BMI) between 19 and 36 kg/m2; (6) not be pregnant (assessed via serum at screening and via urine before study sessions) or breast feeding; (7) have no allergies to any of the ingredients used to prepare cannabis brownies (e.g. chocolate, eggs, etc.); and (8) have not donated blood for 30 days prior to screening.
Study design and procedure
This study utilized a placebo-controlled, within-subjects experimental design. All study participants (N = 20; 10 males and 10 females) completed six separate 10-h outpatient drug administration sessions during which they self-administered vaporized cannabis (containing 0, 5, and 20 mg THC) and cannabis brownies (containing 0, 10, and 25 mg THC). All study sessions were conducted at approximately the same time of day. Participants and research staff were blind to THC dose (but not route of administration) for all sessions. Sessions were conducted at the Johns Hopkins Behavioral Pharmacology Research Unit (BPRU) and were separated by at least 1 week to ensure adequate drug washout between doses. For each participant, sessions were clustered by route of administration, meaning they would complete the three oral cannabis sessions first and the three vaporized cannabis sessions second, or vice versa; session clusters were counterbalanced across participants. THC dose order within each session cluster was also counterbalanced across the first 18 participants, whereas dose order was randomly assigned for the final two participants (complete counterbalancing was not possible with a sample size of 20).
Prior to the start of each session, participants self-reported their use of cannabis, alcohol, tobacco, and other drugs since the previous study visit and provided urine and breath samples to test for drug use/pregnancy and alcohol use, respectively. Participants were provided a standardized low-fat breakfast of toast and jam, and an intravenous catheter was placed to allow for repeated blood sampling. Next, a baseline blood sample was collected, baseline vital signs were recorded, and participants completed baseline subjective drug effect questions, computerized performance tasks, and field sobriety tests (see below). Following baseline procedures, participants either consumed a cannabis-containing brownie or inhaled vaporized cannabis. For oral dosing, participants were asked to consume the entire brownie within 5 min and were permitted to drink water as needed. Participants inhaled vaporized cannabis using the Volcano Medic® (Storz and Bickel, Tuttlingen, Germany). The Volcano heated cannabis at 204°C (400°F), and the resulting vapor was captured in a “balloon”; participants were asked to inhale three full balloons ad libitum within a 10-min period. New balloons were used in each session to avoid contamination from prior doses. Balloons were covered with an opaque bag to reduce the visibility of the vapor to participants and study staff. Following drug exposure, study outcomes were completed at regular intervals for 8 h (see section “Outcome measures” below). In-between study session time points, participants engaged in leisure activities of their choosing (e.g. watching television/movies, reading, playing board games, etc.).
Study drug
High THC and placebo cannabis were obtained for this study from the National Institute on Drug Abuse (NIDA) Drug Supply Program. High THC cannabis contained: 10.3% THC, 0.05% cannabidiol (CBD), and 0.85% cannabinol (CBN). The placebo cannabis contained: 0.001% THC, 0.003% CBD, and 0.005% CBN. For vaporized cannabis administration sessions, 194.2 mg of placebo cannabis, high THC cannabis, or a combination of the two were placed into the Volcano Medic® vaporizer to achieve THC doses of 0, 5, or 20 mg. In a similar vein, placebo and active cannabis brownies were prepared by mixing 242.7 mg of placebo cannabis, high THC cannabis, or a combination of the two to achieve THC doses of 0, 10, or 25 mg. The low doses (i.e. 5 mg vaporized THC; 10 mg oral THC) were intended to produce discriminable drug effects without eliciting significant impairment of cognitive/psychomotor functioning, whereas the high doses (i.e. 20 mg vaporized THC; 25 mg oral THC) were intended to produce discriminable drug effects as well as marked cognitive/psychomotor impairment. Our prior controlled cannabis dosing studies (Schlienz et al., 2020; Spindle et al., 2018; Vandrey et al., 2017) informed dose selection for each respective route of administration.
Cannabis brownie preparation consisted of: (1) grinding individually weighed doses of cannabis into a fine powder with a food processor; (2) heating cannabis for 30 min at 250°F (130°C) to facilitate decarboxylation of Δ−9-tetrahydrocannabinolic acid (THC-A) to THC; and (3) mixing the decarboxylated cannabis with brownie batter, along with other required ingredients (e.g. eggs, flour, etc.). Each brownie was prepared separately in an individual baking tray to ensure exact dosing. In prior studies (Schlienz et al., 2020; Vandrey et al., 2017), we have confirmed that these methods result in full conversion from THC-A to THC and reliably yield targeted THC doses. Brownies were prepared 24–48 h prior to each experimental session. For vaporization, a precisely weighed amount of cannabis was placed on the stainless-steel dosing pad of the Volcano Medic®, heated at 204°C (400°F), and the resulting cannabis vapor was self-administered by study participants who inhaled the contents of three fully inflated “balloons” in order to exhaust the entire dose (prestudy testing confirmed that these dosing parameters were sufficient to fully exhaust the highest vaporized dose, 20 mg THC). All cannabis was prepared and dispensed by the Johns Hopkins BPRU Pharmacy.
Outcome measures
All study outcomes described below were assessed at baseline, immediately following drug exposure (i.e. time “0”), and 1, 2, 3, 4, 5, 6, and 8 h after drug exposure. Because the assessment battery was extensive, additional data collection time points could not be included. The order of task administration was the same at all time points and for all participants throughout the experiment.
Computerized cognitive and psychomotor measures.
Cognitive and/or psychomotor performance was assessed using four computerized tasks: (1) the Digit Symbol Substitution Task (DSST), (2) the Divided Attention Task (DAT), (3) the Paced Serial Addition Task (PASAT), and (4) the DRUID® application. The DSST, DAT, and PASAT were completed on a computer, and the DRUID® was completed on an iPad Mini (5th generation, iOS 12.4) (Apple Inc., Cupertino, CA). Participants were trained on these performance tasks during the screening visit until a stable baseline was reached in order to minimize practice effects during sessions.
On the DSST, a measure of psychomotor ability, participants were given 90 s to attempt to replicate the shapes of patterns they saw presented on their screen using their computer keyboard (Jaeger, 2018; McLeod et al., 1982). The primary outcomes were: total attempted and total correct. The DSST has been shown to be sensitive to oral and vaporized cannabis (Arkell et al., 2019; Schlienz et al., 2020; Spindle et al., 2018; Vandrey et al., 2017) as well as alcohol exposure (King and Byars, 2004).
On the DAT, participants performed two tasks simultaneously: they tracked a central stimulus (“target”) using their mouse cursor (which moved side to side at a fixed speed but changed direction at random) while also monitoring a target digit in the center of their screen and peripheral digits that appeared in the four corners of the screen (Kleykamp et al., 2010). Participants were instructed to click the mouse when a peripheral number matched the central number. Primary DAT outcomes were the mean distance (in computer pixels) of the mouse cursor from the central target (i.e. mean distance from central target) and total peripheral numbers correct (out of 24). Performance on the DAT is reliably impaired after oral/vaporized cannabis administration (Arkell et al., 2019; Schlienz et al., 2020; Spindle et al., 2018; Vandrey et al., 2017). In addition, in simulated driving studies, impairment on the DAT has been positively correlated with driving impairment (Jongen et al., 2014, 2015, 2016).
The PASAT is a measure of working memory and executive functioning. In this task, participants viewed a series of single digit numbers and tried to select the sum of the two numbers that were most recently presented on the screen (Gronwall, 1977). Single-digit integers were continually presented, spaced 2.4–2.8 s apart, for a total of 90 trials. The primary outcome was the total number correct.
On the DRUID®, participants performed four 30–45 s tasks, which measured reaction time, decision-making, hand-eye coordination, and time estimation under conditions of divided attention as well as a balance task (Richman and May, 2019). These performance domains are the most reliable predictors of driving impairment caused by cannabis and alcohol (Jongen et al., 2014; Mikulskaya and Martin, 2018). On Task 1, shapes (either a square or circle) flashed on the screen; one shape was designated as the target shape and the other the control shape. Participants were asked to touch the screen where the target shape appeared and to touch the top of the screen when the control shape appeared. Reaction time in touching the screen was assessed along with the number of errors. On Task 2, participants estimated when they felt 30 s had passed by pressing a button on the screen and, while waiting to press the button, they touched the screen where shapes had briefly flashed. Reaction time to stimuli and accuracy of time estimation was measured. On Task 3, participants attempted to keep their finger on a circle that moved randomly around the screen while also counting the number of squares that flashed on the screen. For Task 4, participants stood on one leg for 15 s while holding the iPad in their same hand and then performed this task on the opposite leg (with the iPad in the other hand). The DRUID® application accessed data from an accelerometer located in the iPad to measure stability and balance during this task. Performance data from each of the four tasks was integrated using a statistical algorithm to yield a Global Impairment Score, the primary outcome for the DRUID®.
Field sobriety tests.
A battery of field sobriety tests commonly used by law enforcement to assess impairment from drugs/alcohol at the roadside was administered to participants by research staff. Research staff were trained to administer the field sobriety test battery by a Drug Recognition Expert (DRE) certified by the state of Maryland. In this report, we describe the results of three tests (the One-Leg Stand (OLS), the Walk-and-Turn (WT), and the Modified Romberg Balance (MRB)) that measure aspects of cognitive and/or motor functioning. For the OLS, participants were asked to raise 1 foot 6 in. off the ground and count until they were told to stop; they were stopped by the task administrator after 30 s. For the WT, participants were asked to take nine heel-to-toe steps down a straight line (clearly marked on the ground), starting with the right foot. On the ninth step, participants were to turn around, pivoting on the left foot using a series of steps, and take nine heel-to-toe steps back in the opposite direction. For the MRB, participants were asked to stand with their feet together and arms at their side, close their eyes, tilt their head back, and estimate the passing of 30 s. Exact verbal instructions were given before each test and can be found in the Supplemental material.
While participants performed each test, the task administrator looked for distinctive behavioral indications (or “clues”) that may indicate impairment, which were scored as either present (1) or not present (0). There were four possible clues on the OLS, eight possible clues on the WT, and four possible observations on the MRB (see Supplemental material for list of individual clues/observations). The number of clues/observations detected were summed to produce an overall score for each task (our primary outcomes), with higher scores indicating greater impairment; another outcome was the cumulative number of clues/observations across all three tests. Participants were video recorded performing the field sobriety tests. As with computerized tasks, participants were trained on field sobriety tests during the screening visit until a stable baseline was reached in order to minimize practice effects during sessions. In order to ensure proper technique was followed by task administrators and that clues were scored correctly, the DRE who trained the research staff individually scored a subset of the testing time points (10%, chosen at random) by watching the video recordings. Overall, as shown in Supplemental material, agreement between research staff and the DRE was high for each individual clue/observation from the OLS, WT, and MRB, which provided confidence in the scoring results.
In addition to the WT, OLS, and MRB, other field sobriety tests were administered including the Horizontal Gaze Nystagmus, Lack of Convergence, and Pupillary Response Tests. Upon review of the video recordings, however, the DRE determined that these tests could not be properly scored because task administrators displayed improper technique too often. Of note, participants performed these tasks while their face was placed against a video recording device (the DAX Evidence Recorder®) (Ocular Data Systems, Inc. Pasadena, CA), and the DRE suspected that the presence of this device may have interfered with task administration in some instances (e.g. this device may have made it difficult for some participants to converge their eyes).
Subjective drug effects.
A 21-item Drug Effect Questionnaire (DEQ) was administered, which included items to capture: positive (e.g. “like drug” and “pleasant drug effect”) and negative subjective drug effects (e.g. “unpleasant drug effect” and “anxious/nervous”), behavioral/mood states that are common following acute cannabis exposure (e.g. “paranoid” and “hungry/have munchies”), and participants’ perceived level of impairment (e.g. “trouble with memory” and “difficulty with routine tasks”); see Table 1 for list of all DEQ items. Each item was presented individually on a 100 mm visual analog scale (VAS) with a horizontal line anchored from 0 (“not at all”) on the left to 100 (“extremely”) on the right.
Table 1.
Statistical analyses results for pharmacodynamic outcomes using change-from-baseline data.
| Outcome measures | Dose (D) F | p | Route (R) F | p | Time (T) F | p | D × R F | p | D × T F | p | R × T F | p | D × R × T F | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Subjective measures | ||||||||||||||
| Drug Effect Questionnaire (DEQ) | ||||||||||||||
| Drug effect | 30.79 | <0.001 | 2.51 | ns | 38.89 | <0.001 | 0.89 | ns | 10.54 | <0.001 | 39.02 | <0.001 | 10.86 | <0.001 |
| Unpleasant | 14.25 | <0.001 | 0.45 | ns | 3.25 | 0.007 | 1.28 | ns | 1.77 | ns | 4.29 | <0.001 | 1.64 | ns |
| Pleasant | 27.22 | <0.001 | 0.37 | ns | 24.22 | <0.001 | 0.67 | ns | 4.93 | <0.001 | 14.97 | <0.001 | 3.69 | <0.001 |
| Like drug effect | 17.24 | <0.001 | 1.97 | ns | 21.15 | <0.001 | 0.67 | ns | 3.62 | <0.001 | 12.08 | <0.001 | 2.76 | 0.004 |
| Sick | 8.18 | 0.003 | 0.51 | ns | 2.63 | 0.023 | 2.26 | ns | 1.39 | ns | 1.44 | ns | 2.15 | ns |
| Heart racing | 13.03 | <0.001 | 0.77 | ns | 9.57 | <0.001 | 0.82 | ns | 3.81 | <0.001 | 9.02 | <0.001 | 6.00 | <0.001 |
| Anxious/nervous | 3.05 | ns | 5.89 | ns | 4.30 | <0.001 | 0.75 | ns | 1.94 | ns | 2.89 | 0.032 | 2.54 | 0.012 |
| Relaxed | 2.31 | ns | 2.82 | ns | 2.48 | 0.023 | 0.12 | ns | 1.18 | ns | 1.74 | ns | 1.47 | ns |
| Paranoid | 5.89 | 0.023 | 0.93 | ns | 3.98 | <0.001 | 0.35 | ns | 1.98 | ns | 5.30 | <0.001 | 2.07 | ns |
| Sleepy/tired | 24.93 | <0.001 | 0.25 | ns | 12.19 | <0.001 | 0.10 | ns | 3.56 | <0.001 | 0.58 | ns | 0.43 | ns |
| Alert | 1.04 | ns | 2.92 | ns | 5.39 | <0.001 | 2.22 | ns | 0.85 | ns | 0.87 | ns | 0.67 | ns |
| Irritable | 4.64 | ns | 0.34 | ns | 1.33 | ns | 5.01 | ns | 0.96 | ns | 0.80 | ns | 1.56 | ns |
| Vigorous/motivated | 1.07 | ns | 2.03 | ns | 4.08 | <0.001 | 1.59 | ns | 1.01 | ns | 0.61 | ns | 1.12 | ns |
| Restless | 11.78 | <0.001 | 1.36 | ns | 2.93 | 0.013 | 0.08 | ns | 1.85 | ns | 3.97 | 0.001 | 1.79 | ns |
| Munchies | 13.02 | <0.001 | 7.95 | ns | 13.84 | <0.001 | 0.55 | ns | 2.39 | 0.020 | 5.51 | <0.001 | 1.25 | ns |
| Craving | 1.55 | ns | 1.10 | ns | 4.04 | <0.001 | 0.10 | ns | 1.04 | ns | 0.61 | ns | 0.39 | ns |
| Dry mouth | 26.99 | <0.001 | 1.54 | ns | 8.25 | <0.001 | 1.54 | ns | 3.24 | <0.001 | 8.41 | <0.001 | 4.00 | <0.001 |
| Dry/irritated eyes | 19.89 | <0.001 | 1.10 | ns | 5.01 | <0.001 | 2.12 | ns | 2.94 | 0.001 | 4.46 | <0.001 | 1.88 | ns |
| Trouble with memory | 11.25 | <0.001 | 0.98 | ns | 3.12 | 0.009 | 2.20 | ns | 2.33 | 0.025 | 2.35 | ns | 1.85 | ns |
| Throat irritated | 8.73 | 0.002 | 22.79 | <0.001 | 10.90 | <0.001 | 7.44 | 0.015 | 4.20 | <0.001 | 10.31 | <0.001 | 5.14 | <0.001 |
| Diff. with routine tasks | 9.64 | 0.001 | 0.36 | ns | 5.60 | <0.001 | 2.24 | ns | 3.61 | <0.001 | 5.42 | <0.001 | 2.38 | 0.026 |
| Cognitive measures | ||||||||||||||
| Digit Symbol Substitution Task (DSST) | ||||||||||||||
| Total attempted | 2.54 | ns | 0.52 | ns | 3.08 | 0.002 | 1.37 | ns | 1.42 | ns | 1.69 | ns | 1.06 | ns |
| Total correct | 3.28 | 0.040 | 0.34 | ns | 3.36 | 0.001 | 2.77 | ns | 1.76 | 0.033 | 1.73 | ns | 1.20 | ns |
| Divided Attention Task (DAT) | ||||||||||||||
| Mean distance from central target | 6.93 | 0.001 | 0.13 | ns | 5.46 | <0.001 | 1.01 | ns | 1.61 | ns | 2.34 | 0.017 | 0.71 | ns |
| Total peripheral numbers correct | 5.30 | 0.006 | 0.03 | ns | 3.27 | 0.001 | 0.58 | ns | 1.20 | ns | 0.55 | ns | 1.28 | ns |
| Paced Auditory Serial Addition Task (PASAT) | ||||||||||||||
| Total correct | 6.22 | 0.003 | 2.17 | ns | 5.25 | <0.001 | 0.70 | ns | 1.24 | ns | 1.59 | ns | 0.68 | ns |
| DRiving Under the Influence of Drugs (DRUID) | ||||||||||||||
| Global impairment score | 13.24 | <0.001 | 1.42 | ns | 4.95 | <0.001 | 0.87 | ns | 2.89 | <0.001 | 3.28 | 0.001 | 1.64 | ns |
| Vital signs | ||||||||||||||
| Systolic BP | 0.24 | ns | 0.35 | ns | 2.05 | 0.038 | 0.49 | ns | 0.69 | ns | 1.86 | ns | 1.31 | ns |
| Diastolic BP | 0.29 | ns | 0.50 | ns | 4.28 | <0.001 | 1.79 | ns | 0.81 | ns | 0.42 | ns | 0.94 | ns |
| Heart rate | 10.74 | <0.001 | 1.17 | ns | 19.90 | <0.001 | 0.34 | ns | 3.58 | <0.001 | 9.20 | <0.001 | 5.06 | <0.001 |
Analyses were repeated measures regressions (i.e. linear mixed models). All analyses used a first-order autoregressive (AR1) covariance structure.
ns: not significant.
Statistical significance was set at p < 0.05. For the DEQ items, Holm–Bonferroni alpha corrections were performed within each main effect and interaction (e.g. p-values for dose, p-values for dose × route, etc.); adjusted p-values are presented for all DEQ items.
Physiological measures.
Heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were measured in the seated position with an automated device.
Blood specimens.
Whole blood specimens were collected using 10 ml gray-top vacutainer tubes and concentrations of THC were determined using LC-MS/MS. The limit of quantification (LOQ) for these analyses was 0.5 ng/ml, and upper limit of linearity (ULOL) was 100 ng/ml; further details on the analytical method can be found elsewhere (Coulter et al., 2008; Spindle et al., 2019b; Vandrey et al., 2017). These analyses were performed by Immunalysis Corporation (Pomona, CA, USA). For the first three participants, plasma blood samples were collected as opposed to whole blood. Due to excessive hemolysis, the specimens for these individuals could not be analyzed. Whole blood specimens were collected from the remaining 17 participants and only these results are reported in this manuscript. Additional biospecimens (i.e. urine and oral fluid) were also collected and tested for cannabinoids, but these results are beyond the scope of this paper and will be reported separately.
Data presentation and analysis
Participant demographic characteristics and blood THC data are presented with descriptive statistics (i.e. means and SD). Change-from-baseline data for subjective drug effects, cognitive performance tasks, vital signs, and blood THC were analyzed using repeated-measures regressions (i.e. linear mixed models). Separate regressions were conducted for each outcome and the covariance structure used was a first-order autoregressive (AR1). Each model included three factors: time (9 time points), dose (placebo, low, and high), and route (oral and vaporized). Given the large amount of individual DEQ items, Holm–Bonferroni alpha corrections were performed within each main effect and interaction (e.g. p-values for dose, p-values for dose × route, etc.) to reduce the chances of Type 1 error; alpha corrections were not performed on the main effect and interaction terms for cognitive and psychomotor performance assessments or field sobriety tests given that these respective tests had far fewer unique endpoints (e.g. 1–2) and, as we describe above, each of these assessments taps into different aspects of functioning. Bonferroni-corrected planned comparisons (i.e. Fischer’s LSD tests) were conducted (using marginal means) to compare mean peak change-from-baseline scores across conditions of interest for these outcomes. Specifically, comparisons were made between: (1) placebo and the two active doses within each route of administration (e.g. 0 mg oral vs. 10 mg oral and 0 mg oral vs. 25 mg oral) and (2) both active doses within each route of administration (e.g. 5 mg vs. 20 mg vaporized); the threshold for statistical significance due to the Bonferroni correction was p < 0.016 for these planned comparisons. Peak scores were calculated for each respective route of administration within time frames previously shown to coincide with peak drug effects (i.e. 0–2 h for vaporized conditions and 2–5 h for oral conditions (Newmeyer et al., 2017a, 2017b; Spindle et al., 2018; Vandrey et al., 2017); time points falling outside of these ranges likely would have been impacted by nondrugrelated factors (e.g. boredom and fatigue). For each individual participant, six total peak-change-from-baseline scores were determined (one for each drug condition), and scores from all 20 individual participants were averaged together to produce mean-peak-change-from-baseline scores for each of the six experimental conditions. Additional Bonferroni-corrected planned comparisons were conducted (using raw data only) to compare baseline scores to all other timepoints (i.e. 0, 1, 2, 3, 4, 5, 6, and 8 h post-dosing) within each experimental condition for three subjective items (“drug effect,” “trouble with memory,” and “difficulty with routine tasks”) and primary cognitive and vital sign outcomes; the threshold for statistical significance due to the Bonferroni correction was p < 0.00139 for these planned comparisons.
For field sobriety tests, nonparametric Friedman tests were employed to determine whether there were differences in peak clues observed (on individual tests and cumulatively across all three tests) between dosing conditions; separate Friedman tests were conducted for oral and vaporized dosing conditions. Planned comparisons (i.e. nonparametric Wilcoxon signed-rank tests; threshold for statistical significance: p < 0.016) were used to compare peak clues across individual dosing conditions (the same comparisons were made as with other pharmacodynamic outcomes). Calculation of peak clues was restricted to the same time frames as cognitive tasks for each respective route of administration.
For cognitive and field sobriety tasks, analyses were conducted twice: once using change-from-baseline data and once using raw data (the same Bonferroni corrections were applied in both sets of analyses). This was done to assess the utility of each outcome for measuring impairment both with and without consideration of baseline performance, which ultimately can inform the suitability of using these various tests in environments where a baseline level of performance can be ascertained (e.g. the workplace) versus those where baseline performance is unknown (e.g. roadside traffic stops).
Lastly, the percentage of participants who were “impaired” and “not impaired” based on field sobriety test results and performance on the DRUID® application was compared across dosing conditions using nonparametric Cochran’s Q tests (these analyses were also conducted twice, accounting for and not accounting for baseline performance, and separate tests were conducted within oral and vaporized dosing conditions). Planned comparisons (i.e. nonparametric McNemar’s tests) were used to compare across the aforementioned conditions (e.g. placebo vs. active doses within each route of administration; threshold for statistical significance: p < 0.016). For raw data, participants who exhibited ≥2 clues on a given field sobriety test following drug administration were classified as “impaired” on that test and those who exhibited a DRUID® Global Impairment Score ≥ 57 after drug administration were classified as “impaired.” In the set of analyses accounting for baseline performance, participants who exhibited a peak-change-from-baseline of ≥2 clues on a given field sobriety test or ≥13-point change (from baseline) on the DRUID® Global Impairment Score were considered “impaired.” These particular thresholds for impairment for field sobriety tests and the DRUID® were used because they were associated with a blood–alcohol concentration (BAC) of 0.08% (common per se limit to determine impairment from alcohol) in prior controlled studies (Richman and May, 2019; Stuster, 2006).
Sex differences were explored for the DRUID® and field sobriety tests within each of the six experimental conditions. We focused on these two outcomes only because, in another recent paper (Sholler et al., 2020), we pooled pharmacokinetic and pharmacodynamic data from the present study with data from three other acute cannabis dosing studies to conduct a comprehensive and well-powered set of comparisons between males/females on subjective drug effects (i.e. DEQ), cognitive/psychomotor performance (i.e. DSST, DAT, and PASAT), and THC pharmacokinetics following administration of oral and vaporized cannabis.
All statistical analyses were conducted using the IBM SPSS software (version 25; IBM SPSS, Armonk, NY, USA), and figures were made using the GraphPad Prism (version 8; GraphPad, La Jolla, CA, USA). The choice of statistical tests was based on the respective scales of the data collected. Linear mixed models and Fischer’s LSD tests (SPSS, 2005) were utilized for outcomes with continuous data (i.e. subjective drug effects, cognitive performance tasks, and vital signs), Friedman’s tests and Wilcoxon signed-rank tests (Sheldon et al., 1996; Woolson, 2007) were utilized for outcomes on an ordinal scale (i.e. number of field sobriety clues observed), and Cochran’s Q-tests and McNemar’s tests (McCrum-Gardner, 2008) were used for data on a dichotomous scale (i.e. “impaired” vs. “not impaired” field sobriety and DRUID data).
Results
Table 1 displays results of the linear mixed model analyses (i.e. main effects and interactions) for subjective effects, cognitive outcomes, and vital signs using change-from-baseline data. Table 2 displays mean peak change-from-baseline scores for all pharmacodynamic outcomes as well as the planned comparison results. Statistical results using raw data can be found in Supplemental material. For all data discussed below, we present results in the following order: (1) the omnibus test for that particular outcome (i.e. linear mixed models, Friedman tests, or Cochran’s Q-tests), (2) the first set of planned comparisons (i.e. those comparing mean peak scores across conditions within each route of administration), (3) where applicable, the second set of planned comparisons (i.e. those comparing baseline scores to all post-dosing timepoints within each dosing condition), and (4) where applicable, the same set of results, but when raw data were analyzed.
Table 2.
Mean (SD) peak change-from-baseline scores for pharmacodynamic measures by dosing condition.
| Oral |
Vaporized |
|||||
|---|---|---|---|---|---|---|
| 0 mg |
10 mg |
25 mg |
0 mg |
5 mg |
20 mg |
|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
|
| ||||||
| Subjective measures | ||||||
| Drug Effect Questionnaire (DEQ) | ||||||
| Drug effect | 4.5 (12.5) | 36.9 (31.8)* | 59.5 (36.6)*Δ | 5.6 (15.4) | 58.2 (37.0)* | 84.1 (26.2)*Δ |
| Unpleasant drug effect | −0.1 (0.31) | 4.0 (11.8) | 12.4 (21.8)* | 0.3 (0.7) | 8.0 (22.0) | 20.1 (22.5)*Δ |
| Pleasant drug effect | 9.5 (27.5) | 46.7 (39.8)* | 61.5 (37.1)* | 6.2 (16.6) | 62.1 (37.6)* | 68.1 (32.5)* |
| Like drug effect | 14.2 (34.3) | 44.0 (38.0)* | 53.0 (37.4)* | 9.4 (20.6) | 58.4 (36.1)* | 66.3 (32.0)* |
| Sick | 0.7 (2.9) | 1.7 (3.9) | 7.7 (13.0) | 0.9 (2.9) | 0.2 (0.7) | 13.0 (20.6)*Δ |
| Heart racing | −0.9 (3.8) | 5.3 (10.4) | 17.7 (20.2)*Δ | 0.2 (0.4) | 10.2 (21.7) | 37.8 (35.7)*Δ |
| Anxious/nervous | −1.5 (7.5) | 0.9 (16.0) | 10.2 (23.3) | −0.5 (2.3) | 5.7 (22.1) | 23.0 (25.9)*Δ |
| Relaxed | 12.1 (27.7) | 24.7 (34.3) | 30.3 (39.2) | 8.6 (26.5) | 20.5 (26.7) | 21.8 (35.2) |
| Paranoid | 0.0 (0.0) | 2.9 (7.1) | 8.2 (18.9) | 0.2 (0.7) | 6.8 (19.1) | 17.4 (24.5)*Δ |
| Sleepy | 8.1 (16.4) | 41.1 (26.2)* | 55.6 (37.0)* | 8.3 (20.0) | 27.4 (35.9) | 45.9 (34.1)* |
| Alert | 5.4 (12.0) | −6.3 (25.5) | −3.9 (24.7) | −3.7 (28.5) | 8.5 (29.0) | −1.8 (31.9) |
| Irritable | 0.7 (2.5) | 8.7 (19.6) | 1.5 (5.8) | −1.8 (6.3) | 5.6 (9.5) | 8.7 (21.4)* |
| Vigorous/motivated | 7.2 (23.0) | −6.8 (37.9) | 8.0 (37.0) | 1.8 (30.8) | 5.5 (21.7) | −0.5 (41.1) |
| Restless | 1.8 (5.6) | 9.5 (13.9) | 20.1 (30.6)* | 1.1 (4.5) | 7.7 (20.3) | 23.7 (26.3)*Δ |
| Hungry/have munchies | 10.0 (15.2) | 28.0 (32.4) | 45.4 (36.7)* | 10.0 (18.9) | 46.8 (33.1)* | 51.3 (33.9)* |
| Craving | 1.8 (5.5) | 7.3 (19.5) | 9.4 (22.9) | 0.3 (1.1) | 1.4 (4.9) | 5.9 (17.1) |
| Dry mouth | 0.6 (3.2) | 21.4 (31.1)* | 48.3 (34.2)*Δ | 0.0 (6.0) | 28.3 (29.3)* | 54.7 (33.5)*Δ |
| Dry/red eyes | −1.6 (5.5) | 19.9 (28.3)* | 32.2 (31.1)* | 1.8 (7.2) | 16.6 (22.8) | 41.6 (34.5)*Δ |
| Trouble with memory | 0.5 (8.1) | 10.5 (21.7) | 19.9 (32.0)* | 2.3 (9.4) | 3.1 (10.6) | 26.7 (32.8)*Δ |
| Throat irritated | −2.5 (11.0) | 2.4 (10.5) | 5.5 (12.1) | 0.2 (0.7) | 11.2 (23.4) | 34.5 (32.2)*Δ |
| Difficulty with routine tasks | 0.1 (0.4) | 15.2 (26.4) | 25.3 (29.1)* | 1.2 (4.5) | 9.6 (23.9) | 36.9 (35.1)*Δ |
| Cognitive measures | ||||||
| Digit Symbol Substitution Task (DSST) | ||||||
| Total attempted | 1.1 (6.4) | −4.3 (11.1) | −7.0 (14.3) | −4.5 (7.7) | −3.0 (11.7) | −7.2 (12.7) |
| Total correct | −3.7 (13.3) | −0.6 (11.1) | −6.7 (11.6) | −8.7 (12.1) | −5.0 (9.0) | −6.5 (12.2) |
| Divided Attention Task (DAT) | ||||||
| Mean distance from central target | −0.1 (15.1) | 24.0 (52.7) | 35.5 (48.5)* | 13.5 (26.7) | 13.8 (32.0) | 28.8 (62.8) |
| Total peripheral numbers correct | −0.9 (3.9) | −3.7 (6.0) | −4.5 (7.6) | −0.5 (4.2) | −2.4 (3.2) | −4.2 (7.1) |
| Paced Serial Addition Task (PASAT^ | ||||||
| Total correct | −1.3 (21.1) | −15.2 (25.2) | −21.4 (26.5)* | 0.6 (15.4) | −4.3 (8.4) | −16.4 (19.8)* |
| DRUID | ||||||
| Global impairment score | −0.1 (6.5) | 4.5 (9.3) | 12.9 (8.2)* Δ | 1.3 (6.9) | 4.7 (8.9) | 10.5 (15.2)* |
| Physiological measures | ||||||
| Systolic BP | 1.1 (13.8) | 1.0 (16.1) | 0.5 (15.4) | −2.4 (13.5) | −2.8 (15.3) | −4.8 (15.6) |
| Diastolic BP | −2.3 (11.7) | −0.9 (14.4) | −1.1 (15.2) | 2.8 (12.7) | 2.9 (11.2) | 0.2 (14.0) |
| Heart rate | −0.7 (14.9) | 3.7 (14.8) | 12.2 (14.1)* | −1.8 (11.8) | 8.7 (16.9) | 20.8 (21.4)* |
Peak effects were calculated between 2 and 5 h for oral dosing conditions and between 0 and 2 h for vaporized conditions. Fischer’s LSD tests were used for planned comparisons.
BP: blood pressure.
= Significant difference from placebo condition within that route of administration (Bonferroni-corrected, p < 0.016); Δ = significant difference between low and high dose within that route of administration (Bonferroni-corrected, p < 0.016).
Participants
Thirty-six individuals provided informed consent and were screened for the study. Of these individuals, 23 (13 males and 10 females) were determined to be eligible and were randomized. Of those randomized, 20 (10 males and 10 females) completed the study. Two participants were lost to follow-up during the study participation, and one was discharged from the study due to extremely poor performance on cognitive tasks at baseline. The racial/ethnic breakdown of study completers was: 45% Caucasian, 35% African American, 15% Hispanic, and 5% Asian. Participants were all nontobacco users, and, on average, had not used cannabis for 270 days (SD = 370; range = 30–1278) prior to their first session. Their mean (SD) BMI was 25.1 kg/m2 (4.0), their mean weight was 164.9 lbs (37.5), and their mean age was 28.5 years old (6.2). No participants experienced unanticipated or serious adverse events during the study.
Computerized cognitive and psychomotor measures
Digit symbol substitution test. Linear mixed models detected a significant main effect of dose and a significant dose × time interaction for the DSST total correct (no main effects or interactions were detected for the DSST total attempted). Despite the significant findings on the omnibus test, planned comparisons between dosing conditions did not detect significant differences for peak scores between dose conditions for DSST total correct or total attempted within either route of administration. Further, planned comparisons (between baseline and post-dosing time points) did not detect significant differences between baseline and post-dosing time points within any experimental condition. Results were largely consistent between raw and change score data; the lone exception was that, when raw data were used, there was no longer a significant main effect of dose for DSST total correct (see Supplemental material).
Divided attention test.
Linear mixed models detected a main effect of dose for mean distance from central target and total peripheral numbers correct. Planned comparisons between dosing conditions revealed that peak-change-from-baseline scores for mean distance from central target differed between the 25 mg oral THC and oral placebo conditions. Planned comparisons (between baseline and post-dosing time points) revealed that, relative to baseline, DAT performance (mean distance from central target) was significantly impaired at hours 4 and 5 in the 25 mg oral THC condition and at hour 2 in the 20 mg vaped THC condition (see Figure 1). Linear mixed model results were largely consistent when the raw data were used, except the main effect of dose for peripheral integers correct did not remain significant. When the raw data were used, planned comparisons no longer detected significant differences across dosing conditions within either route of administration.
Figure 1.
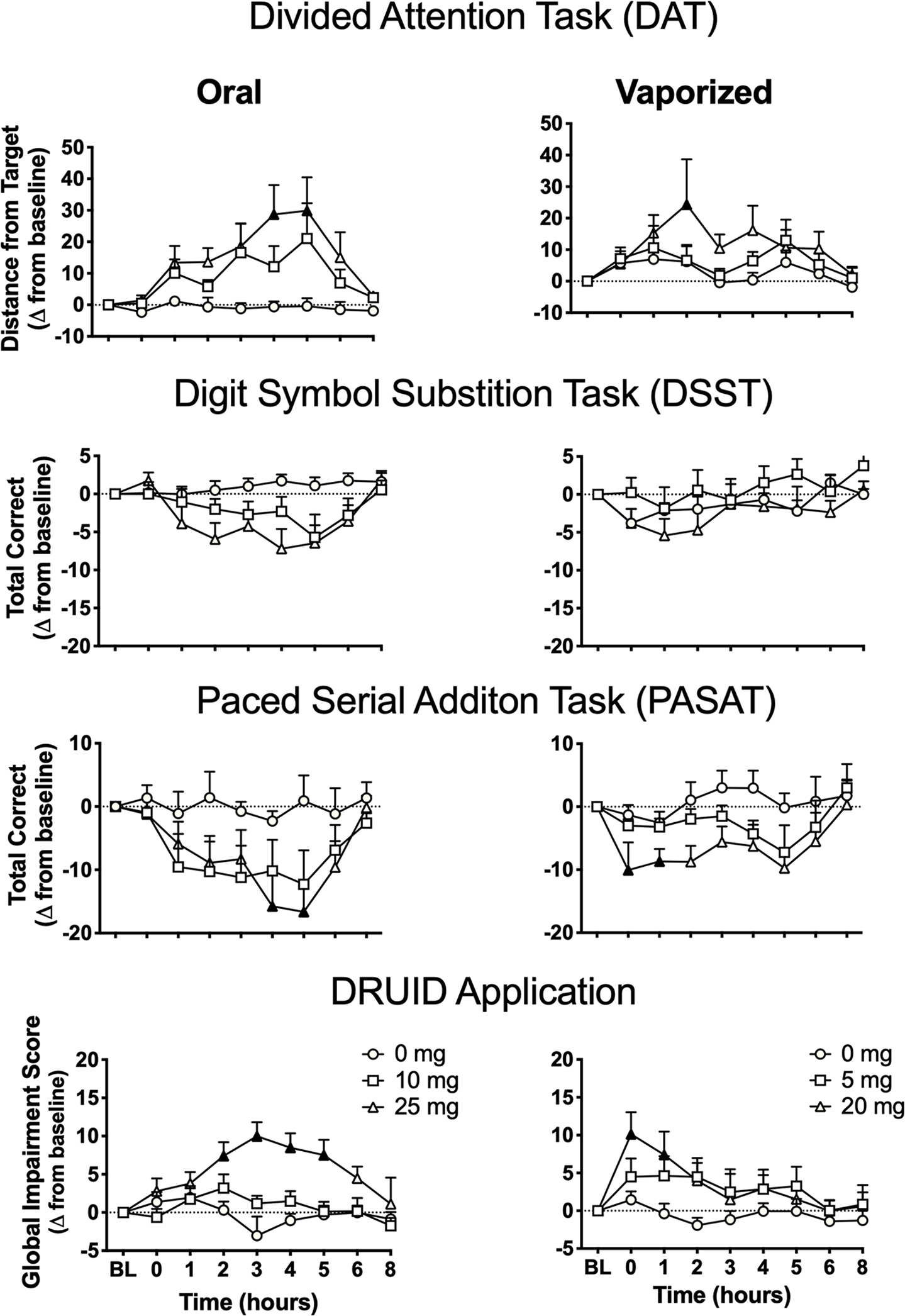
Mean (+SEM) performance on cognitive/psychomotor tasks (change-from-baseline scores) over time in each experimental condition. BL=baseline time point; mg=milligrams THC. Filled symbols indicate significant difference from baseline within that experimental condition. Higher scores on the DAT and DRUID® indicate worse performance (i.e. greater impairment), whereas lower scores on the DSST and PASAT indicate worse performance. Note this exact figure is presented in Supplemental material, but using raw data. Oral and vaporized cannabis dosing was completed immediately prior to “time 0.”
Paced serial addition task.
Linear mixed models detected a main effect of dose for PASAT total correct. Planned comparisons between dosing conditions detected significant differences between the two high doses (i.e. 25 mg oral THC and 20 mg vaped THC) and the respective placebo conditions. Planned comparisons (between baseline and postdosing time points) revealed that, relative to baseline, PASAT performance (i.e. total correct) was significantly impaired at hours 4–5 in the 25 mg oral THC condition and hours 0–1 in the 20 mg vaped THC condition (see Figure 1). Linear mixed model results differed between change-from-baseline and raw data such that, when the raw data was used, the main effect of dose for PASAT total correct was no longer significant. Moreover, planned comparisons with raw peak data did not detect significant differences across conditions for either route of administration for PASAT total correct (see Supplemental material).
DRUID® application.
Figure 2 illustrates performance on the DRUID® (i.e. Global Impairment Scores) for each individual participant at baseline and post-cannabis administration (i.e. raw peak scores). For the DRUID® Global Impairment Score, linear mixed models detected a main effect of dose, a dose × time interaction, and a route × time interaction. Planned comparisons between dosing conditions detected significant differences between the two high doses (i.e. 25 mg oral THC and 20 mg vaped THC) and their respective placebo conditions. In addition, the mean peak score in the 25 mg oral THC condition was significantly greater than that observed in the 10 mg oral THC condition. Planned comparisons between baseline and post-dosing time points revealed that, within the 25 mg oral THC condition, DRUID® performance was significantly impaired relative to baseline at hours 2–5; within the 20 mg vaped THC condition, DRUID® performance was significantly impaired at hours 0–1 (see Figure 1). Linear mixed model results were consistent between change-from-baseline and raw data. Planned comparisons for raw data again revealed significant differences between peak scores in the high dose conditions and each corresponding placebo condition, but there was no longer a significant difference between the two active oral doses. However, when raw data was used, a significant difference emerged between the 20 mg vaped THC and 5 mg vaped THC conditions (see Supplemental material). In the 20 mg vaped THC condition, females exhibited greater peak scores on the DRUID® compared with males (females: mean = 63.2; SD = 13.8; males: mean = 52.5; SD = 6.5; Fischer’s LSD p < 0.05), and sex differences were also observed in this condition when peak-change-from-baseline data were used (females: mean = 16.6; SD = 16.5; males: mean = 4.4; SD = 11.5; Fischer’s LSD p < 0.05); peak DRUID® scores did not differ between males and females in any other condition (using raw and peak-change data).
Figure 2.
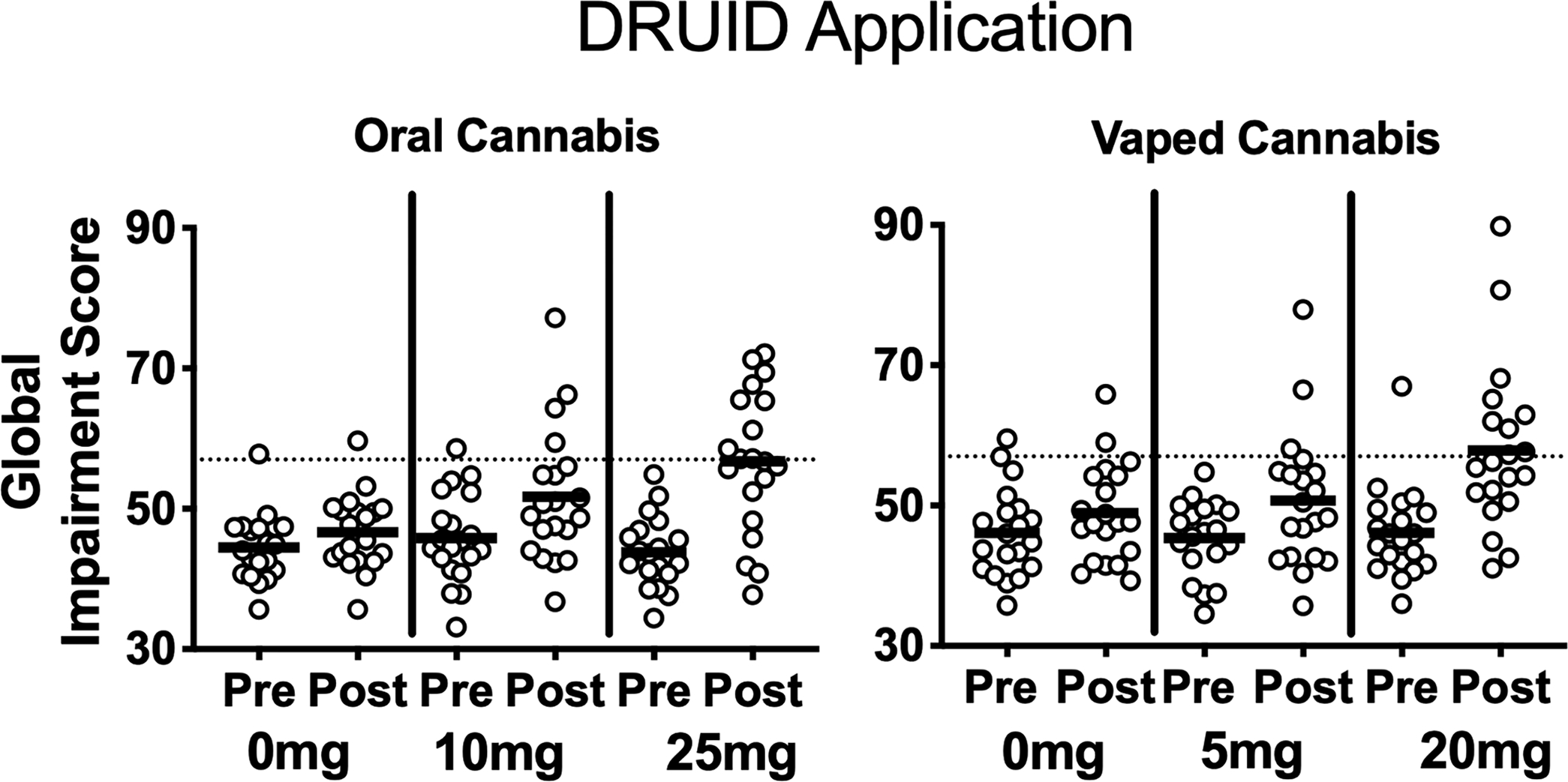
Performance on the DRUID® (i.e. global impairment scores) for each individual participant at baseline and post-cannabis administration (i.e. raw peak scores) in each experimental condition. Higher scores indicate worse performance (i.e. greater impairment). The dotted line (i.e. score of 57) signifies the threshold used to classify participants as “impaired”; this threshold was associated with a blood–alcohol concentration of 0.08% in a prior controlled alcohol dosing study (Richman and May, 2019). Peak scores were calculated for each respective route of administration within time frames previously shown to coincide with peak drug effects (i.e. 0–2h for vaporized conditions and 2–5h for oral conditions).
Field sobriety tests
Figure 3 depicts performance (i.e. peak-change-from-baseline clues for each participant) on the three individual field sobriety tests within each condition; the same figure is presented using raw data (i.e. peak clues observed post-dosing) in Supplemental material.
Figure 3.
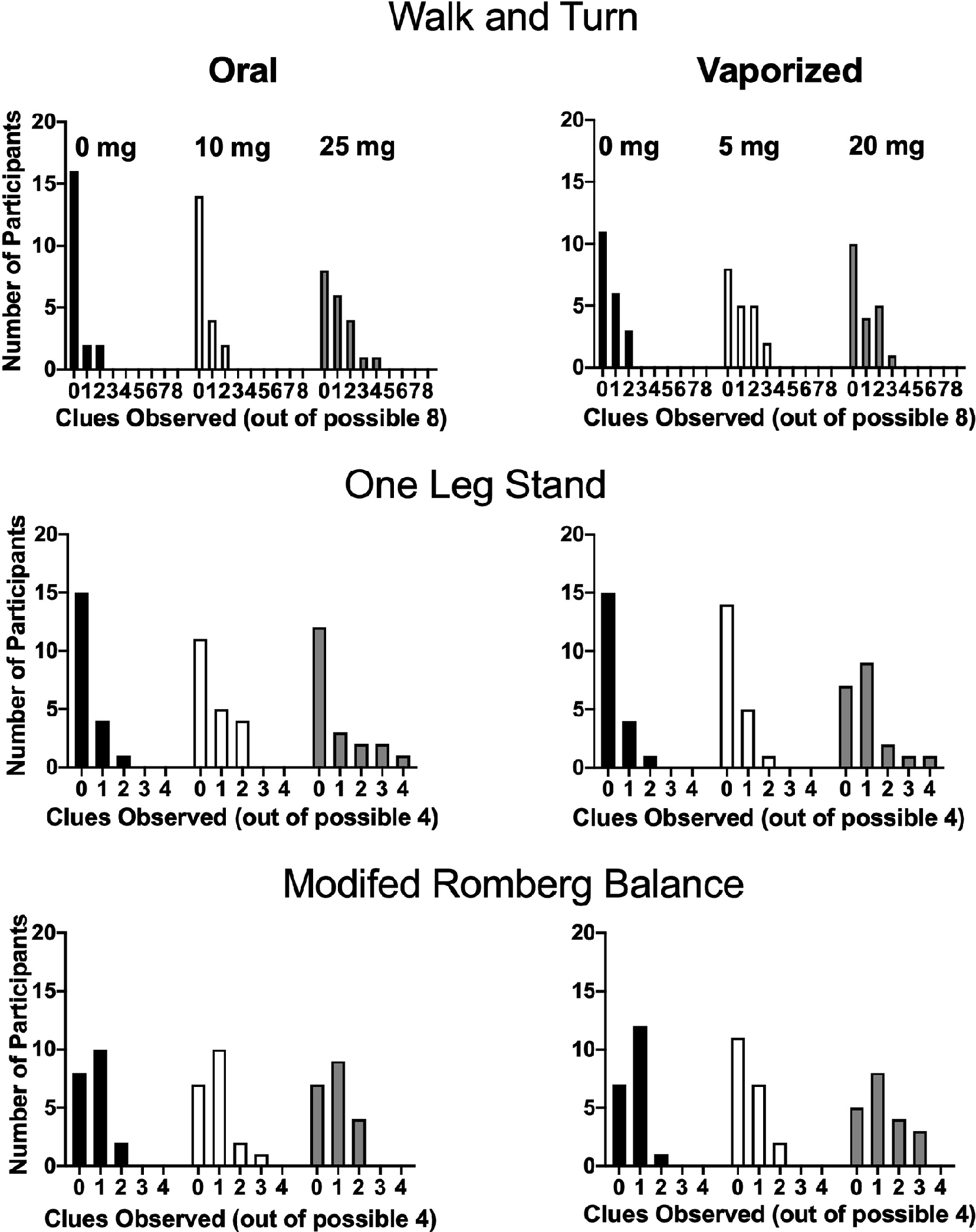
Performance on individual field sobriety tasks (i.e. peak change-from-baseline clues) in each experimental condition. The y-axes indicate the number of participants (out of 20) who displayed a given number of clues, whereas the x-axes display the number of clues observed. More clues indicate worse performance (i.e. greater impairment). Two or more clues on a given test is indicative of impairment. The McNemar’s tests did not detect significant differences across dosing conditions in the number of individuals judged as “impaired” (based on the ≥2 clue criteria) for any of the tests (see Table 3). Note this exact figure is presented in Supplemental material, but using raw data.
Cumulative score.
For oral cannabis conditions, a nonparametric Friedman test revealed a significant main effect of dose (χ2 (2) = 6.66, p = 0.04) for overall field sobriety performance; significant differences were not detected across vaporized conditions (χ2 (2) = 6.03, p: ns). Despite the significant omnibus test result for oral cannabis, planned comparisons between dosing conditions did not detect significant differences between doses of interest (e.g. placebo vs. active oral doses) for either route of administration. When raw data were analyzed, a nonparametric Friedman test revealed significant main effects of dose for both oral (χ2 (2) = 6.30, p = 0.04) and vaporized (χ2 (2) = 10.75, p = 0.01) cannabis conditions. Planned comparisons with raw data indicated that peak cumulative clues observed in the two high dose conditions (i.e. 25 mg oral THC; 20 mg vaped THC) were significantly higher compared to their respective placebo conditions. Sex differences were not observed for cumulative score (using raw and peak-change-from-baseline data) in any of the six experimental conditions.
Walk and turn test.
On the WT test, no differences in peak change-from-baseline clues were detected across oral (χ2 (2) = 5.22, p: ns) or vaporized cannabis conditions (χ2 (2) = 1.97, p: ns) on the nonparametric Friedman tests. Planned comparisons between dosing conditions did not detect significant differences across dosing conditions for either route of administration. Using raw data, nonparametric Friedman tests revealed a significant main effect of dose for oral cannabis conditions (χ2 (2) = 7.56, p = 0.02), but not vaporized (χ2 (2) = 3.93, p: ns). Despite the significant omnibus test for oral cannabis conditions, planned comparisons with raw data did not detect significant differences across dosing conditions for either route of administration. Males exhibited significantly more clues than females in the 25 mg oral THC condition (Mann–Whitney U-test, p < 0.05), but sex differences were not observed in any of the other conditions (results were consistent when raw and peak-change data were used).
One leg stand.
A nonparametric Friedman test detected a significant main effect of dose (χ2 (2) = 9.39, p = 0.01) for vaporized cannabis conditions on the OLS test; significant differences were not detected across oral conditions (χ2 (2) = 3.57, p: ns). Planned comparisons between dosing conditions revealed that peak clues (change from baseline) in the 20 mg vaped THC condition was significantly higher than both the 5 mg vaped THC and vaporized placebo conditions; significant differences were not detected across oral cannabis conditions.
All results were consistent when raw data were used: a significant main effect of dose (χ2 (2) = 10.26, p = 0.01) was found for vaporized but not oral cannabis conditions (χ2 (2) = 4.92, p: ns), and planned comparisons with raw data detected significant differences between 20 mg vaped THC and both 5 mg vaped THC and vaporized placebo. Sex differences were not observed (using raw and peak-change-from-baseline data) in any experimental conditions.
Modified Romberg balance.
Nonparametric Friedman tests did not reveal differences across oral (χ2 (2) = 0.26, p: ns) or vaporized conditions (χ2 (2) = 6.15, p: ns). Further, planned comparisons between dosing conditions did not detect significant differences across conditions for either route of administration. Results (i.e. omnibus Friedman’s tests and planned comparisons) were consistent when change-from-baseline and raw data were used; Friedman’s tests: oral (χ2 (2) = 5.71, p: ns) and vaporized (χ2 (2) = 1.93, p: ns). Sex differences were not observed (using raw and peak-change-from-baseline data) in any experimental conditions.
Percentage of impaired and nonimpaired participants
Using the respective criteria for impairment (i.e. ≥2 clues on a given field sobriety test; DRUID® Global Impairment Score ≥57) the frequency at which participants were considered “impaired” at baseline on these measures was as follows: WT (16/120 sessions, 13%), OLS (10/120 sessions, 8%), MRB (8/120 sessions, 7%), and DRUID (5/120 sessions, 4%).
Table 3 displays the percentage of participants who were classified as “impaired” on the DRUID® and each individual field sobriety test using both change-from-baseline and raw data. On the WT, using the raw data, there were no differences in the percentage of individuals classified as impaired across vaporized (Cochran’s Q (2) = 3.8, p: ns) or oral cannabis conditions (Cochran’s Q (2) = 4.2, p: ns); the same pattern was observed when using change-from-baseline data: oral (Cochran’s Q (2) = 3.2, p: ns); vaporized (Cochran’s Q (2) = 2.2, p: ns). For the OLS, using raw data, the percentage of individuals classified as impaired differed significantly across vaporized (Cochran’s Q (2) = 10.0, p < 0.01) but not oral conditions (Cochran’s Q (2) = 3.7, p: ns); this significant finding did not remain when change-from-baseline data was used: oral (Cochran’s Q (2) = 3.3, p: ns) and vaporized (Cochran’s Q (2) = 6.0, p: ns). On the MRB, there were no differences in the percentage of impaired participants across oral (Cochran’s Q (2) = 1.8, p: ns) or vaporized cannabis conditions (Cochran’s Q (2) = 4.2, p: ns). However, when change-from-baseline data were used, significant differences were detected across vaporized (Cochran’s Q (2) = 7.8, p = 0.02), but not oral (Cochran’s Q (2) = 0.9, p: ns) conditions for the MRB. Despite these significant omnibus Cochran’s Q-tests, planned comparisons between dosing conditions did not detect differences in the percentage of individuals classified as “impaired” on the WT, OLS, or MRB within either oral or vaporized conditions, and these results were the same for both raw and change-from-baseline data. The percentage of participants classified as impaired on the three field sobriety tests did not differ based on sex in any experimental condition (results were consistent using raw and peak-change-from-baseline data).
Table 3.
Percentage of participants (n = 20) classified as “impaired” based on performance on the DRUID® application and field sobriety tests.
| Number (%) | 0 mg oral | 10 mg oral | 25 mg oral | 0 mg vaped | 5 mg vaped | 20 mg vaped |
|---|---|---|---|---|---|---|
|
| ||||||
| Impaired based on raw data scores | ||||||
| Walk and turna | 3 (15%) | 6 (30%) | 8 (40%) | 5 (25%) | 10 (50%) | 9 (45%) |
| One leg standa | 3 (15%) | 7 (35%) | 6 (30%) | 3 (15%) | 3 (15%) | 8 (40%) |
| Modified Romberg balancea | 4 (20%) | 6 (30%) | 7 (35%) | 6 (30%) | 7 (35%) | 11 (55%) |
| DRUIDb | 1 (5%) | 4 (20%) | 10 (50%)* | 2 (10%) | 3 (15%) | 9 (45%) |
| Impaired based on change-from-baseline scores | ||||||
| Walk and turna | 2 (10%) | 2 (10%) | 6 (30%) | 3 (15%) | 7 (35%) | 6 (30%) |
| One leg standa | 1 (5%) | 4 (20%) | 5 (25%) | 1 (5%) | 1 (5%) | 4 (20%) |
| Modified Romberg balancea | 2 (10%) | 3 (15%) | 4 (20%) | 1 (5%) | 2 (10%) | 7 (35%) |
| DRUIDc | 0 (0%) | 2 (10%) | 9 (45%)* | 0 (0%) | 2 (10%) | 7 (35%)* |
Significant difference from placebo within that route of administration (Bonferroni-corrected, p < 0.016); Δ = significant difference between low and high dose within that route of administration (Bonferroni-corrected, p < 0.016). McNemar’s tests were used for planned comparisons.
Impairment criteria:
⩾2 peak (or peak change-from-baseline) clues observed = “impaired”; <2 peak (or peak change-from-baseline) clues = “not impaired.”
⩾57 DRUID® global impairment score = “impaired”; <57 score = “not impaired.”
⩾13-point change (from baseline) on DRUID® global impairment score = “impaired”; <13-point change (from baseline) = “not impaired.”
For the DRUID®, significant differences were detected within both oral and vaporized conditions using both raw (oral (Cochran’s Q (2) = 14.0, p < 0.001) and vaporized (Cochran’s Q (2) = 7.8, p = 0.02)) and change-from-baseline data (oral (Cochran’s Q (2) = 13.4, p < 0.001) and vaporized (Cochran’s Q (2) = 9.3, p = 0.01)). Planned comparisons between dosing conditions revealed that a greater percentage of participants were classified as impaired based on DRUID® performance in both high dose conditions (i.e. 25 mg oral THC; 20 mg vaped THC) compared with the respective placebo conditions, and results were mostly consistent when raw and change-from-baseline data were used (the lone exception was that, when raw data was used, there was not a significant difference between the high vaporized and placebo vaporized conditions). When using raw data, the percentage of participants classified as impaired on the DRUID® differed by sex in the 20 mg vaped THC condition (7 women were classified as impaired in this condition vs. 2 men; Chi-square test, p < 0.05), though this finding did not remain significant when using change-from-baseline data. Sex differences in the percentage of impaired participants were not observed in the other experimental conditions using raw or change-from-baseline data.
Subjective drug effects
Three items (“drug effect,” “trouble with memory,” and “difficulty with routine tasks”) were considered subjective indices of impairment because these questions inquired about overall perceived drug effects or participants’ perceived ability to perform a cognitive or behavioral function (Figure 4 displays mean ratings for each of these items over time for each study condition). Main effects of dose and dose × time interactions (on linear mixed models) were observed for each of these three items, and dose × route × time interactions were also observed for “drug effect” and “difficulty with routine tasks.” For oral cannabis conditions, planned comparisons between dosing conditions revealed significantly higher ratings in the 25 mg oral THC condition compared to placebo for all three items; ratings for “drug effect” also differed between 10 mg oral THC and placebo and between 25 mg oral THC and 10 mg oral THC. For vaporized cannabis conditions, ratings for all three items in the 20 mg vaped THC condition were significantly greater than placebo; ratings for “drug effect” also differed between 5 mg vaped THC and placebo. In addition, ratings for each item differed significantly between the 20 mg vaped THC and 5 mg vaped THC conditions. Planned comparison results between baseline and all subsequent time points within each condition for these three items are illustrated in Figure 4. In the 10 mg oral THC condition, “drug effect” ratings significantly increased at hours 1–4; in the 25 mg oral THC condition, ratings increased significantly at hours 1–6. Ratings for “drug effect” were significantly increased at hours 0–3 in the 5 mg vaped THC condition and at hours 0–5 in 20 mg vaped THC condition. For “trouble with memory,” ratings increased significantly at hours 2–3 in the 25 mg oral THC condition and at hours 0–4 in 20 mg vaped THC condition. For “difficulty with routine tasks,” ratings increased significantly at hours 2–3 in the 25 mg oral THC condition and at hours 0–3 in the 20 mg vaped THC condition.
Figure 4.
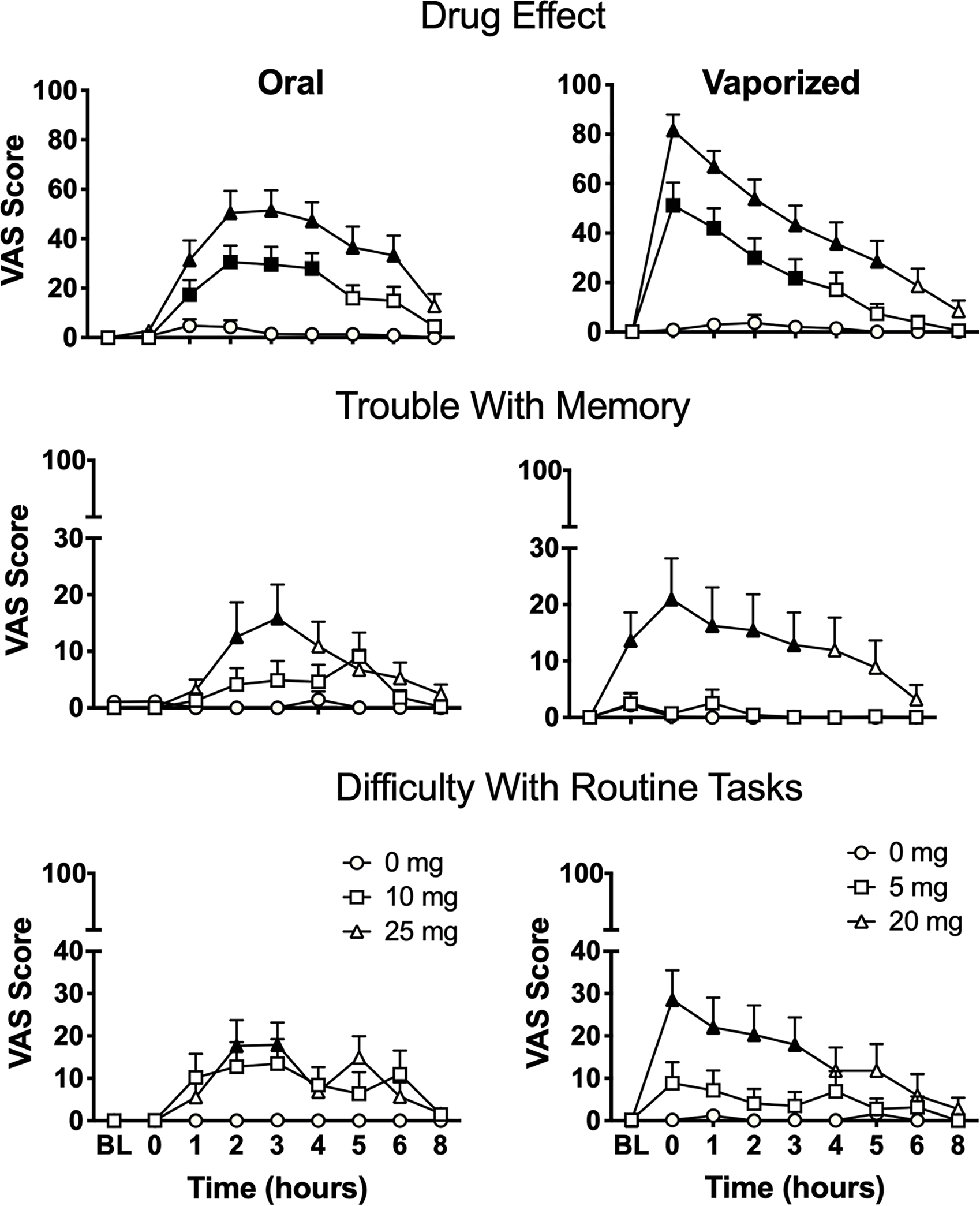
Mean (+ SEM) subjective ratings for visual analog scale (VAS) items “drug effect,” “trouble with memory,” and “difficulty with routine tasks” from the Drug Effect Questionnaire (DEQ) in each experimental condition over time. BL = baseline time point; mg = milligrams THC. Filled symbols indicate significant difference from baseline within that experimental condition. Oral and vaporized cannabis dosing was completed immediately prior to “time 0.”
For other subjective items, main effects of dose and two- or three-way interactions with dose and route/time were often observed on linear mixed models (see Table 1). Planned comparisons between dosing conditions for oral cannabis conditions revealed that mean peak ratings were significantly higher for both active doses (i.e. 10 mg oral THC and 25 mg oral THC) compared with placebo for “pleasant drug effect,” “like drug effect” “sleepy/tired,” “dry mouth,” and “dry/red eyes.” Moreover, ratings in the 25 mg oral THC condition were significantly higher for “unpleasant drug effect,” “heart racing,” “restless,” and “hungry/have munchies” compared with placebo. Peak ratings for “heart racing” and “dry mouth” were also higher for 25 mg oral THC compared to 10 mg oral THC. For vaporized conditions, mean peak ratings were significantly higher for both active doses (i.e. 5 mg vaped THC and 20 mg vaped THC) compared with placebo for “pleasant drug effect,” “like drug effect,” “hungry/have munchies,” and “dry mouth.” Relative to placebo, ratings were also higher in the 20 mg vaped THC condition for “unpleasant drug effect,” “sick,” “heart racing,” “anxious/nervous,” “paranoid,” “sleepy/tired,” “irritable,” “restless,” “dry/red eyes,” and “throat irritated.” Lastly, peak ratings for “unpleasant drug effect,” “sick,” “heart racing,” “anxious/nervous,” “paranoid,” “restless,” “dry mouth,” “dry/red eyes,” and “throat irritated” were also higher in the 20 mg vaped THC compared to 5 mg vaped THC condition (see Table 2).
Physiological outcomes
For HR, a main effect of dose was observed, along with dose × time, route × time, and dose × route × time interactions on the linear mixed model analysis. Planned comparisons between dosing conditions revealed that mean peak beats per minute following administration of both high doses (i.e. 25 mg oral THC and 20 mg vaped THC) was significantly higher compared with the respective placebo conditions. Planned comparisons (between baseline and post-dosing time points) revealed that, within the 25 mg oral THC condition, HR increased significantly at hour 2 relative to baseline (see Figure 5); within the 5 and 20 mg vaped THC conditions, HR was significantly elevated at hours 0–1 relative to baseline.
Figure 5.
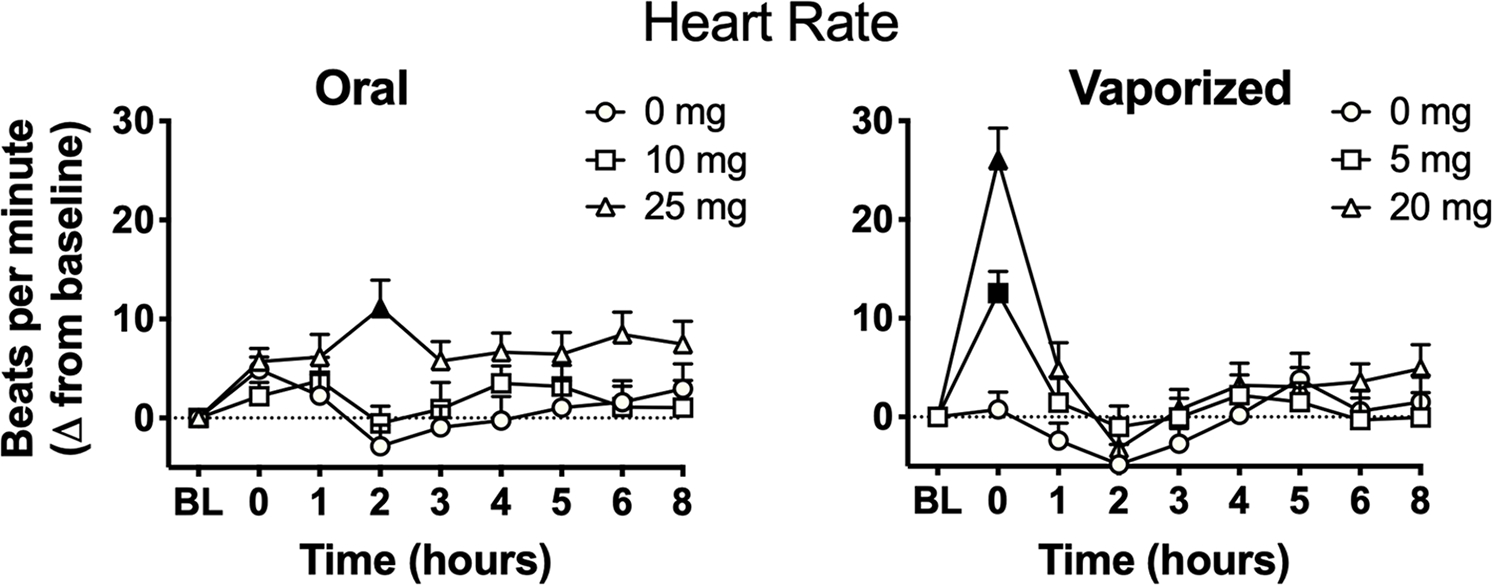
Mean (+SEM) heart rate (beats per minute; raw data) in each experimental condition over time. BL = baseline time point; mg = milligrams THC. Filled symbols indicate significant difference from baseline within that experimental condition. Oral and vaporized cannabis dosing was completed immediately prior to “time 0.”
Whole blood THC concentrations
On average, whole blood THC concentrations peaked 2h after oral cannabis administration (see Figure 6). The mean (SD) whole blood THC concentration (in ng/ml) observed at hour 2 for each oral cannabis condition was: 10 mg: 1.78 (1.93) and 25 mg: 3.06 (2.41). On average, whole blood THC concentrations peaked immediately after inhalation of vaporized cannabis (i.e. at hour “0”; see Figure 6). The mean (SD) whole blood THC concentration (in ng/ml) observed at hour 0 for each vaporized cannabis condition was: 5 mg: 9.19 (10.43) and 20 mg: 37.24 (22.36).
Figure 6.
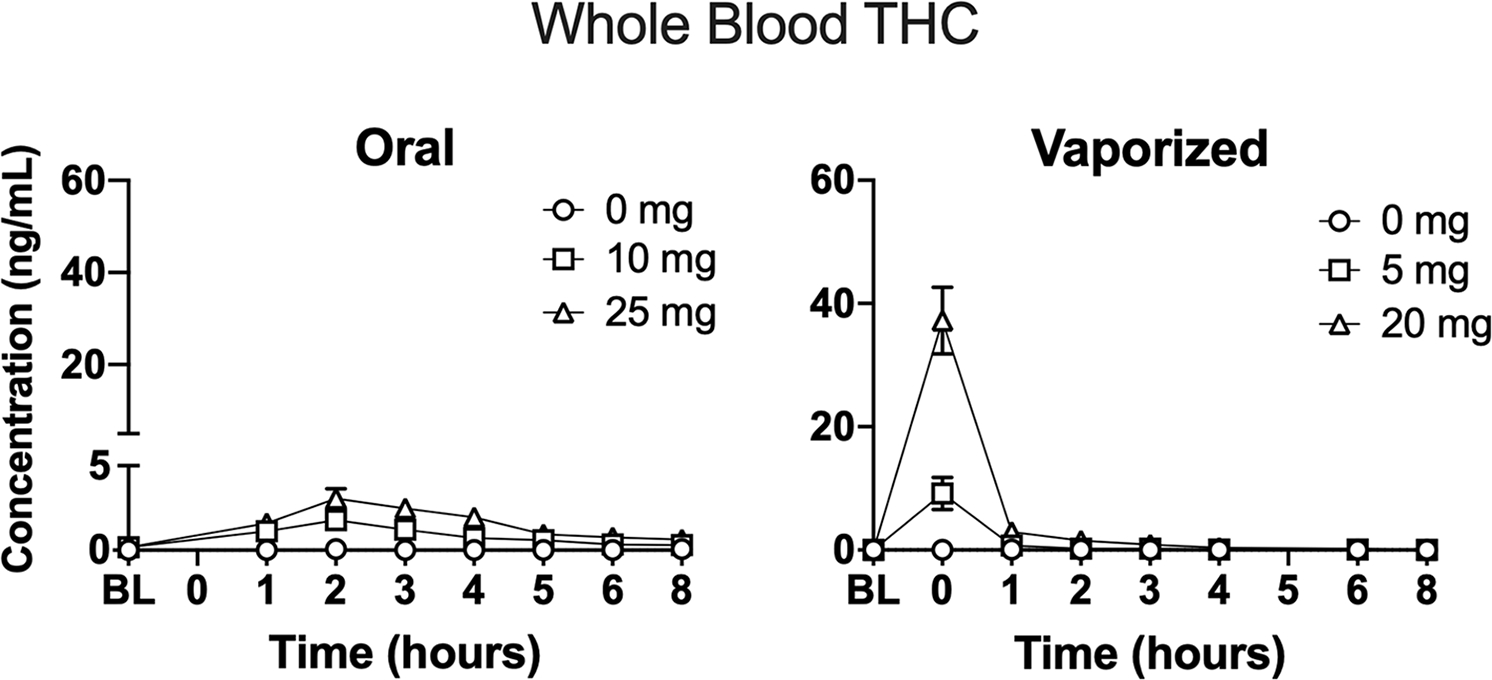
Mean (+SEM) blood THC concentrations (ng/ml) over time in each experimental condition. Note that blood specimens were not collected at hour 0 and hour 5 in oral dosing sessions and vaporized dosing sessions, respectively. Data are displayed for the 17 participants who had whole blood specimens collected. Oral and vaporized cannabis dosing was completed immediately prior to “time 0.”
The linear mixed model analysis detected main effects of dose (F = 81.51, p < 0.001) and time (F = 96.17, p < 0.001), along with a dose × time interaction (F = 51.06, p < 0.001) for blood THC data. Planned comparisons between dosing conditions revealed that mean peak blood THC concentrations were significantly higher following administration of the 5 and 20 mg vaped THC doses compared with vaped placebo. In addition, mean peak blood THC concentrations were significantly higher following administration of the 20 mg THC dose compared with the 5 mg vaped THC dose. Planned comparisons did not detect significant differences in peak blood THC concentrations across the three oral dosing conditions.
Discussion
Given recent policy changes that have expanded the legalization of cannabis, there is a growing need to understand the acute effects of various cannabis products in different cannabis-using populations. In particular, it is increasingly important to characterize the effects of oral cannabis products (or “edibles”) and vaporized cannabis products, as these methods of administration and product types make up a growing segment of the retail cannabis market. However, much of the extant data on acute cannabis effects has come from studies that administered smoked cannabis, and the few prior oral and vaporized cannabis studies have focused mainly on moderate or heavy cannabis users. The present study extends prior research by being the first to evaluate the pharmacodynamic effects of oral and vaporized cannabis (at multiple THC doses) in healthy adults with infrequent cannabis-use patterns, utilizing a rigorous within-subjects and placebo-controlled design.
In general, oral ingestion and vaporization of cannabis increased ratings for a variety of subjective drug effects in a dose-orderly manner. At the low THC doses (i.e. 5 mg vaped THC and 10 mg oral THC), participants reported a perceptible drug effect, which was mostly positive in nature. That is, ratings on items such as “pleasant drug effect” and “like drug effect” were elevated after administration of both low doses, whereas subjective indices of impairment (i.e. “trouble with memory” and “difficulty with routine tasks”) and other negative/aversive subjective effects remained largely unchanged. Conversely, after administration of both high doses (i.e. 20 mg vaped THC and 25 mg oral THC), mean ratings for these subjective measures of impairment and other negative/aversive effects (“unpleasant drug effect,” “heart racing,” and “anxious/nervous”) also increased significantly. These results are in contrast to prior studies that included frequent cannabis users (Cooper and Haney, 2014; Newmeyer et al., 2017a), in which such negative subjective effects were rarely observed after cannabis administration. Thus, even at relatively small doses of THC, infrequent cannabis users may be more susceptible to adverse drug effects than frequent users who are more tolerant to the acute effects of cannabis. Consistent with prior research (Newmeyer et al., 2017a), key differences were observed in the time course of subjective drug effects between oral and vaporized cannabis: on average, peak drug effects occurred 2–3 h after cannabis ingestion, whereas peak effects occurred shortly after inhalation of vaporized cannabis. Collectively, these findings underscore the importance of educating infrequent or novice cannabis users that acute cannabis effects can differ substantially (in terms of time course and magnitude) if the route of administration is changed or if the dose of THC is increased, even modestly.
Cognitive/psychomotor performance was negatively impacted following cannabis administration, and there were key differences in the onset and duration of effects across the two routes of administration that mirrored subjective drug effects (i.e. impairment often occurred immediately after inhalation of vaporized cannabis but was delayed by several hours following oral cannabis ingestion). Notably, however, certain computerized tasks were more sensitive to cannabis exposure than others. For instance, on the DRUID®, significant differences were observed between placebo and the high THC dose for both routes of administration, regardless of whether raw data or change-from-baseline data were used. Conversely, the other cognitive tasks (i.e. PASAT, DSST, and DAT) appeared to be less sensitive to cannabis impairment based on several factors. For example, DSST performance did not differ between active and placebo conditions for either route of administration, and the PASAT only yielded statistically significant differences between dosing conditions when change-from-baseline data were used. One possible explanation for this finding is that by tapping into multiple performance domains simultaneously (e.g. divided attention, reaction time, hand-eye coordination, and balance), the DRUID® may better capture impairment from cannabis relative to tasks, which assess only one or two performance domains. Notably, in our prior acute oral and vaporized cannabis administration studies with infrequent cannabis users and similar doses (i.e. 0, 10, and 25 mg THC), performance on the PASAT, DSST, and DAT were each impaired in a dose-dependent fashion (Schlienz et al., 2020; Spindle et al., 2018). Nevertheless, performance on all tasks varied considerably across participants, with some individuals showing little to no impairment in any drug condition and others showing substantial impairment at the low THC doses (see Figure 2). Such variability is noteworthy considering all participants had not used cannabis recently (meaning tolerance to cannabis was not a contributing factor) and were relatively homogenous in other ways (e.g. age and BMI). Additional research is needed to elucidate the mechanisms underlying the inter-individual variability in pharmacodynamic responses to cannabis/THC exposure, perhaps by determining how much of this variance may be explained by genetic factors that contribute to differences in the rate of drug metabolism. Moreover, additional research is needed on the DRUID® to understand whether it is predictive of driving performance and to elucidate whether various user factors (e.g. age, sex, impulsivity, cannabis experience level, etc.) influence its results.
On each field sobriety test (WT, OLS, and MRB), performance was largely similar across dosing conditions for both routes of administration. That is, individual clues/observations (possible signs of impairment) were often detected at a comparable rate in placebo, low, and high dose THC conditions. Moreover, participants were generally classified as “impaired” on the individual tasks at a similar rate when using an impairment threshold (≥2 clues) that is commonly used by the law enforcement (Downey et al., 2016; Newmeyer et al., 2017b; Papafotiou et al., 2005a). There are several possible explanations for these findings. First, as has been demonstrated in other studies (Bosker et al., 2012; Papafotiou et al., 2005b), common field sobriety tests may simply lack sensitivity to cannabis impairment. The standard field sobriety test battery was explicitly designed and validated to identify impairing effects of alcohol (Stuster, 2006). It is possible that objective signs of impairment from cannabis are more subtle than those from alcohol, meaning extant field sobriety tests may have limited utility for identifying cannabis-impaired individuals. Second, the conditions under which the field sobriety tests were administered may have impacted these results. In this study, participants practiced the field sobriety tests at the pre-study screening visit and also performed the tests numerous times at each experimental session. In a real-world scenario (e.g., a traffic stop), an individual may have little to no prior experience performing field sobriety tests and thus may be more likely to display signs of impairment than participants in this study. Further, the tasks were administered by a member of the research staff in a nonstressful environment. In a non-research setting, a police officer or DRE would presumably administer the tests under more “high-stakes” and stressful circumstances, as inadequate performance may lead to legal consequences. Future studies should consider attempting to more precisely replicate the experience of an actual roadside field sobriety test, perhaps by having police officers administer the tests. Beyond improving ecological validity, such research may also enable systematic evaluation of the role stress plays in false positives during roadside sobriety tests, particularly those conducted in the absence of biological evidence of impairment.
Findings from this study have important implications for the measurement and determination of impairment from cannabis, which is ever-more important given the unprecedented number of individuals with legal access to cannabis. Currently, there are two main strategies used to detect impairment from cannabis: (1) behavioral and (2) biological (Ginsburg, 2019). With behavioral approaches, a person’s degree of impairment is gauged based on their performance on field sobriety tests. Results from this study suggest that novel behavioral tests may need to be designed that are more sensitive to cannabis impairment than conventional field sobriety tests; such novel tests could provide invaluable supplementary information to the field sobriety test administrator, which may be useful for identifying individuals who are impaired from cannabis. Results from this study also suggest that behavioral measures (both computerized tasks and field sobriety tests) almost always benefit from consideration of an individual’s baseline performance (when they are not impaired) because acute pharmacodynamic responses to cannabis vary widely across individuals. This suggests that the utilization of behavioral assessments to determine cannabis impairment may be most effective in environments where a baseline level of performance can be collected (e.g. in the workplace), though, even in such environments it may be important to periodically collect baseline data to account for natural decreases in performance due to age and other factors.
With biological approaches, impairment is inferred based on the concentration of THC in a person’s system. In the US, for example, several states have per se laws, which classify individuals as impaired if they have blood THC concentrations over a certain threshold; individuals with THC levels exceeding this threshold can be automatically charged with an offense if they were operating a motor vehicle. Consistent with several prior studies (Chiang and Barnett, 1984; Cone and Huestis, 1993; Menetrey et al., 2005; Spindle et al., 2018; Vandrey et al., 2017), the present study suggests that blood THC is a poor proxy of cannabis impairment for both oral and vaporized routes of administration. For oral cannabis, peak blood THC concentrations were extremely low (see Figure 6) and often less than per se limits used in some US states (e.g. 5 ng/ml), despite participants displaying marked cognitive/psychomotor impairment, on average. For vaporized cannabis, blood THC concentrations were elevated greatly immediately following cannabis inhalation but decreased rapidly thereafter and often fell below the limit of quantification before cognitive performance deficits and subjective drug effects had subsided. Moreover, there were several participants who exhibited elevated blood THC levels (much higher than any per se limit) directly after vaping without displaying cognitive/psychomotor impairment. Overall, as with current behavioral approaches to cannabis impairment, novel biomarkers of cannabis exposure (aside from THC) are needed in order to effectively identify individuals who are impaired from cannabis.
This study had several limitations. First, only one type of cannabis (THC-dominant), one type of vaporizer (the Volcano Medic®), and one type of cannabis edible (brownie) were used in this study. Future studies should elucidate the pharmacodynamic effects of cannabis using different chemotypes (e.g. CBD-dominant), different types of vaporizers, and different edible products (e.g. drinks and candies). Second, conducting the study in a laboratory setting may have reduced the generality of these results. For example, as noted previously, it is possible that administration of field sobriety tests under these experimental conditions is not sufficiently reflective of real-world circumstances. Third, the study included a relatively homogenous group of participants which limited our ability to examine how individual characteristics (e.g. age, genetics, body fat percentage, and user experience level with cannabis) may impact the pharmacodynamics of cannabis. Fourth, while the DRUID® showed promise in this initial study as well as in another recent study (Karoly et al., 2020) in terms of its sensitivity to cannabis effects, more research is needed on this novel instrument. Lastly, though the sample size (N = 20) in this study was sufficient to achieve the study aims, replication in larger and more diverse samples is encouraged.
Conclusion
In summary, the present study found that oral and vaporized cannabis dose-dependently altered subjective drug effects and impaired cognitive and psychomotor performance in a sample of participants who used cannabis infrequently. The time course of pharmacodynamic effects differed between the two routes of administration, as the onset of effects from oral cannabis were delayed considerably compared to vaporized cannabis. This study also provided further evidence that field sobriety tests and biological concentrations of THC (in blood) may have limited capacity to identify individuals who are intoxicated from cannabis. Lastly, a novel mobile device application (the DRUID®) was the most sensitive measure of impairment when compared to the other cognitive performance tasks administered (i.e. the DSST, DAT, and PASAT) as well as several common field sobriety tests (i.e. the WT, OLS, and MRB). As cannabis legalization continues to expand and more individuals begin using the drug for medicinal or recreational purposes, research to understand the acute effects of emergent cannabis products will be paramount in order to inform product regulation and policies to protect public safety.
Supplementary Material
Acknowledgements
We thank the support staff of the Johns Hopkins University Behavioral Pharmacology Research Unit for outstanding contributions to the implementation of this study. We also thank Dr. Christine Moore and Ms. Cynthia Coulter at Immunalysis Inc., support staff at RTI International, the NIDA Drug Supply Program, and Storz and Bickel for critical services and material support.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the National Institute of Justice (NIJ; Award Number 2016-DN-BX-0193) and the National Institute on Drug Abuse (NIDA; T32DA07209 and R44 DA046272–01A1). The opinions, findings, conclusions, and recommendations expressed in this publication are those of the authors and do not necessarily reflect those of the NIJ or NIDA.
Footnotes
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Spindle has served as a consultant for Canopy Health Innovations Inc. Dr. Vandrey has served as a consultant or received honoraria from Canopy Health Innovations Inc., FSD Pharma, and Present Life Corporation. Michael Milburn is the creator of the DRUID® application and is the Chief Scientific Officer (CSO) and a stockholder of Impairment Science, Inc. The remaining authors have no conflicts of interest to declare.
Supplemental material
Supplemental material for this article is available online.
References
- Abrams DI, Vizoso HP, Shade SB, et al. (2007) Vaporization as a smokeless cannabis delivery system: A pilot study. Clin Pharmacol Ther 82: 572–578. [DOI] [PubMed] [Google Scholar]
- Arkell TR, Lintzeris N, Kevin RC, et al. (2019) Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology (Berl) 236: 2713–2724. DOI: 10.1007/s00213-019-05246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg CJ, Stratton E, Schauer GL, et al. (2015) Perceived harm, addictiveness, and social acceptability of tobacco products and marijuana among young adults: Marijuana, hookah, and electronic cigarettes win. Subst Use Misuse 50: 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodovsky JT, Crosier BS, Lee DC, et al. (2016) Smoking, vaping, eating: Is legalization impacting the way people use cannabis? Int J Drug Policy 36: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosker WM, Kuypers KP, Theunissen EL, et al. (2012) Medicinal Delta(9)-tetrahydrocannabinol (dronabinol) impairs on-the-road driving performance of occasional and heavy cannabis users but is not detected in Standard Field Sobriety Tests. Addiction 107: 1837–1844. [DOI] [PubMed] [Google Scholar]
- Carliner H, Brown QL, Sarvet AL, et al. (2017) Cannabis use, attitudes, and legal status in the U.S.: A review. Prev Med 104: 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang CW and Barnett G (1984) Marijuana effect and delta-9-tetrahydrocannabinol plasma level. Clin Pharmacol Ther 36: 234–238. [DOI] [PubMed] [Google Scholar]
- Cone EJ and Huestis MA (1993) Relating blood concentrations of tetrahydrocannabinol and metabolites to pharmacologic effects and time of marijuana usage. Ther Drug Monit 15: 527–532. [DOI] [PubMed] [Google Scholar]
- Cone EJ, Johnson RE, Paul BD, et al. (1988) Marijuana-laced brownies: Behavioral effects, physiologic effects, and urinalysis in humans following ingestion. J Anal Toxicol 12: 169–175. [DOI] [PubMed] [Google Scholar]
- Cooper ZD and Haney M (2014) Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend 136: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter C, Miller E, Crompton K, et al. (2008) Tetrahydrocannabinol and two of its metabolites in whole blood using liquid chromatography-tandem mass spectrometry. J Anal Toxicol 32: 653–658. [DOI] [PubMed] [Google Scholar]
- Curran HV, Freeman TP, Mokrysz C, et al. (2016) Keep off the grass? Cannabis, cognition and addiction. Nat Rev Neurosci 17: 293–306. [DOI] [PubMed] [Google Scholar]
- Downey LA, Hayley AC, Porath-Waller AJ, et al. (2016) The Standardized Field Sobriety Tests (SFST) and measures of cognitive functioning. Accid Anal Prev 86: 90–98. [DOI] [PubMed] [Google Scholar]
- Fink DS, Stohl M, Sarvet AL, et al. (2020) Medical Marijuana laws and driving under the influence of marijuana and alcohol. Addiction 115: 1944–1953. DOI: 10.1111/add.15031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC (2019) Strengths and limitations of two cannabis-impaired driving detection methods: A review of the literature. Am J Drug Alcohol Abuse 45: 610–622. [DOI] [PubMed] [Google Scholar]
- Goodman S, Wadsworth E, Leos-Toro C, et al. (2020) Prevalence and forms of cannabis use in legal vs. illegal recreational cannabis markets. Int J Drug Policy 76: 102658. [DOI] [PubMed] [Google Scholar]
- Gronwall DM (1977) Paced auditory serial-addition task: A measure of recovery from concussion. Percept Mot Skills 44: 367–373. [DOI] [PubMed] [Google Scholar]
- Hartman RL, Brown TL, Milavetz G, et al. (2015) Cannabis effects on driving lateral control with and without alcohol. Drug Alcohol Depend 154: 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RL, Brown TL, Milavetz G, et al. (2016) Cannabis effects on driving longitudinal control with and without alcohol. J Appl Toxicol 36: 1418–1429. [DOI] [PubMed] [Google Scholar]
- Jaeger J (2018) Digit symbol substitution test: The case for sensitivity over specificity in neuropsychological testing. J Clin Psychopharmacol 38: 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen S, Perrier J, Vuurman EF, et al. (2015) Sensitivity and validity of psychometric tests for assessing driving impairment: Effects of sleep deprivation. PLoS One 10: e0117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen S, Vuurman E, Ramaekers J, et al. (2014) Alcohol calibration of tests measuring skills related to car driving. Psychopharmacology 231: 2435–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen S, Vuurman E, Ramaekers J, et al. (2016) The sensitivity of laboratory tests assessing driving related skills to dose-related impairment of alcohol: A literature review. Accid Anal Prev 89: 31–48. [DOI] [PubMed] [Google Scholar]
- Karoly HC, Milburn MA, Brooks-Russell A, et al. (2020) Effects of high-potency cannabis on psychomotor performance in frequent cannabis users. Cannabis Cannabinoid Res. Epub ahead of print 10 September 2020. DOI: 10.1089/can.2020.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC and Byars JA (2004) Alcohol-induced performance impairment in heavy episodic and light social drinkers. J Stud Alcohol 65: 27–36. [DOI] [PubMed] [Google Scholar]
- Kleykamp BA, Griffiths RR and Mintzer MZ (2010) Dose effects of triazolam and alcohol on cognitive performance in healthy volunteers. Exp Clin Psychopharmacol 18: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp AA, Lee DC, Borodovsky JT, et al. (2018) Emerging trends in cannabis administration among adolescent cannabis users. J Adolesc Health 64: 487–493. DOI: 10.1016/j.jadohealth.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal MA, Hazekamp A, Colzato LS, et al. (2015) Cannabis and creativity: Highly potent cannabis impairs divergent thinking in regular cannabis users. Psychopharmacology (Berl) 232: 1123–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane TJ and Hall W (2019) Traffic fatalities within US states that have legalized recreational cannabis sales and their neighbours. Addiction 114: 847–856. [DOI] [PubMed] [Google Scholar]
- McCrum-Gardner E (2008) Which is the correct statistical test to use? Brit J Oral Maxillofac Surg 46: 38–41. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, et al. (1982) An automated version of the digit symbol substitution test (DSST). Behav Res Methods Instrum 14: 463–466. [Google Scholar]
- Menetrey A, Augsburger M, Favrat B, et al. (2005) Assessment of driving capability through the use of clinical and psychomotor tests in relation to blood cannabinoids levels following oral administration of 20 mg dronabinol or of a cannabis decoction made with 20 or 60 mg Delta9-THC. J Anal Toxicol 29: 327–338. [DOI] [PubMed] [Google Scholar]
- Mikulskaya E and Martin FH (2018) Contrast sensitivity and motion discrimination in cannabis users. Psychopharmacology 235: 2459–2469. [DOI] [PubMed] [Google Scholar]
- Monfort SS (2018) Effect of Recreational Marijuana Sales on Police-Reported Crashes in Colorado, Oregon, and Washington. Arlington, VA: Insurance Institute for Highway Safety. [Google Scholar]
- National Academies of Sciences and Medicine (2017) The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Newmeyer MN, Swortwood MJ, Abulseoud OA, et al. (2017a) Subjective and physiological effects, and expired carbon monoxide concentrations in frequent and occasional cannabis smokers following smoked, vaporized, and oral cannabis administration. Drug Alcohol Depend 175: 67–76. [DOI] [PubMed] [Google Scholar]
- Newmeyer MN, Swortwood MJ, Barnes AJ, et al. (2016) Free and glucuronide whole blood cannabinoids’ pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration in frequent and occasional cannabis users: Identification of recent cannabis intake. Clin Chem 62: 1579–1592. [DOI] [PubMed] [Google Scholar]
- Newmeyer MN, Swortwood MJ, Taylor ME, et al. (2017b) Evaluation of divided attention psychophysical task performance and effects on pupil sizes following smoked, vaporized and oral cannabis administration. J Appl Toxicol 37: 922–932. [DOI] [PubMed] [Google Scholar]
- Niedbala RS, Kardos KW, Fritch DF, et al. (2001) Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. J Anal Toxicol 25: 289–303. [DOI] [PubMed] [Google Scholar]
- Odell MS, Frei MY, Gerostamoulos D, et al. (2015) Residual cannabis levels in blood, urine and oral fluid following heavy cannabis use. Forensic Sci Int 249: 173–180. [DOI] [PubMed] [Google Scholar]
- Papafotiou K, Carter JD and Stough C (2005a) An evaluation of the sensitivity of the Standardised Field Sobriety Tests (SFSTs) to detect impairment due to marijuana intoxication. Psychopharmacology (Berl) 180: 107–114. [DOI] [PubMed] [Google Scholar]
- Papafotiou K, Carter JD and Stough C (2005b) The relationship between performance on the standardised field sobriety tests, driving performance and the level of Delta9-tetrahydrocannabinol (THC) in blood. Forensic Sci Int 155: 172–178. [DOI] [PubMed] [Google Scholar]
- Peng YW, Desapriya E, Chan H, et al. (2020) Residual blood THC levels in frequent cannabis users after over four hours of abstinence: A systematic review. Drug Alcohol Depend 216: 108177. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Theunissen EL, et al. (2009) Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users. J Psychopharmacol 23: 266–277. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Robbe H and O’Hanlon J (2000) Marijuana, alcohol and actual driving performance. Hum Psychopharmacol 15: 551–558. [DOI] [PubMed] [Google Scholar]
- Richman JE and May S (2019) An Investigation of the Druid® smartphone/tablet app as a rapid screening assessment for cognitive and psychomotor impairment associated with alcohol intoxication. Vision Dev Rehab 19: 31–42. [Google Scholar]
- Schlienz NJ, Spindle TR, Cone EJ, et al. (2020) Pharmacodynamic dose effects of oral cannabis ingestion in healthy adults who infrequently use cannabis. Drug Alcohol Depend 211: 107969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon MR, Fillyaw MJ and Thompson WD (1996) The use and interpretation of the Friedman test in the analysis of ordinal-scale data in repeated measures designs. Physiother Res Int 1: 221–228. [DOI] [PubMed] [Google Scholar]
- Sholler DJ, Strickland JC, Spindle TR, et al. (2020) Sex differences in the acute effects of oral and vaporized cannabis among healthy adults. Addict Biol. Epub ahead of print 29 September. DOI: 10.1111/adb.12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC and Sobell MB (1992) Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Allen JP and Litten RZ (eds) Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Human Press, pp.41–72. [Google Scholar]
- Spindle TR, Bonn-Miller MO and Vandrey R (2019a) Changing landscape of cannabis: Novel products, formulations, and methods of administration. Curr Opin Psychol 30: 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Schlienz NJ, et al. (2018) Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: A crossover trial. JAMA Netw Open 1: e184841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Schlienz NJ, et al. (2019b) Acute pharmacokinetic profile of smoked and vaporized cannabis in human blood and oral fluid. J Anal Toxicol 43: 233–258. DOI: 10.1093/jat/bky104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS (2005) Linear Mixed-effects Modeling in SPSS: An Introduction to the MIXED Procedure. Technical Report, pp.1–28. [Google Scholar]
- Steigerwald S, Wong PO, Khorasani A, et al. (2018) The form and content of cannabis products in the United States. J Gen Intern Med 33: 1426–1428. DOI: 10.1007/s11606-018-4480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuster J (2006) Validation of the standardized field sobriety test battery at 0.08% blood alcohol concentration. Human Factors 48: 608–614. [DOI] [PubMed] [Google Scholar]
- Swortwood MJ, Newmeyer MN, Andersson M, et al. (2017) Cannabinoid disposition in oral fluid after controlled smoked, vaporized, and oral cannabis administration. Drug Test Anal 9: 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Herrmann ES, Mitchell JM, et al. (2017) Pharmacokinetic profile of oral cannabis in humans: Blood and oral fluid disposition and relation to pharmacodynamic outcomes. J Anal Toxicol 41: 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, et al. (2016) Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: A review. JAMA Psychiatry 73: 292–297. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, et al. (2002) Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 161: 331–339. [DOI] [PubMed] [Google Scholar]
- Woolson R (2007) Wilcoxon signed-rank test. In: D’Agostino RB, Sullivan LM and Massaro J (eds) Wiley Encyclopedia of Clinical Trials. Hoboken, NJ: John Wiley & Sons, Ltd., pp.1–3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


