Abstract
PURPOSE:
To evaluate the expression of programmed death ligand 1 (PD-L1) and programmed death ligand 2 (PD-L2) in ocular adnexal sebaceous carcinoma (OASC), and to appraise these findings within the context of recent comparable studies.
DESIGNS:
Retrospective case series.
METHODS:
Twenty cases of primary OASC were immunostained for PD-L1, PD-L2 and CD8. PD-L1 and PD-L2 expression were graded with both the combined positive score (CPS) and the tumor proportion score (TPS). Both raw CPS and TPS were reported, as well as positivity with TPS and CPS ≥1. CD8 expression was graded on a 0–3 scale. Charts were reviewed for clinical correlations. The results of the current study were compared with results of similar recent investigations.
RESULTS:
For the 20 cases, mean expression of PD-L1 with CPS was 29.7 (range 0–101.5) and with TPS was 12.2 (range 0–95.8); mean expression of PD-L2 with CPS was 7.9 (range 0–37.3) and with TPS was 1.9 (range 0–12.9). PD-L1 CPS ≥1 was detected in 95% of OASC, while PD-L1 TPS ≥1 was found in 75%. PD-L2 CPS ‡1 was present in 60%, while only 20% had PD-L2 TPS ≥1. Immune cells appeared to contribute to a substantial proportion of PD-L1 and PD-L2 positivity, and a conspicuous CD8-positive T-lymphocytic infiltrate was present in most tumors. Significant correlations were identified between tissue expression of PD-L1, PD-L2, and CD8. Tissues with greater levels of PD-L1 tended to express higher levels of PD-L2 and CD8. The degree of PD-L1 and PD-L2 expression was also associated with the area in millimeters squared of the immunostained tumor, suggesting that tumor sampling may influence interpretation of PD-L1 and PD-L2 expression in ocular adnexal tumors.
CONCLUSIONS:
The current and preceding studies confirm that PD-L1 and PD-L2 are expressed in a high percentage of OASCs. These results support the premise that checkpoint inhibitor drugs hold considerable therapeutic promise for patients with OASC and stimulate the institution of clinical trials.
Drugs targeting the programmed death 1 (PD-1) receptor and its 2 ligands, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2), have been accorded an essential role in the treatment of many nonophthalmic neoplasms.1 By subverting the normal PD-1/PD-L1/PD-L2 system, tumor cells can evade the body’s immune response. Blockade of PD-1, PD-L1 or PD-L2 with a class of antibody-based drugs called immune checkpoint inhibitors (ICI) allows the body’s immune system to become reactivated so that it once again recognizes and destroys malignant cells.1 In nonophthalmic malignancies, expression of PD-L1 and PD-L2 has been found to correlate with tumor response to ICI drugs.2 Furthermore, expression levels of PD-L1 can serve as a prognostic biomarker of response for certain tumors.3,4 Expression of PD-L1 within the tumor microenvironment helps guide decisions toward patients receiving ICI drugs and helps to determine whether they are eligible to enter clinical trials.5
ICI drugs are not yet routinely used for ocular adnexal malignancies. In fact, ICI drugs are recognized by ophthalmologists today for their ophthalmic inflammatory side effects.6–11 Despite such side effects, checkpoint inhibitor drugs should not be disregarded by the ophthalmic community because there exists tremendous potential for their use in the treatment of selected ocular adnexal malignancies, particularly for those where there are limited therapeutic options. Eyelid sebaceous carcinoma is not infrequently misdiagnosed histopathologically, and among nonmelanomatous eyelid malignancies has the greatest potential for local recurrences and ultimately metastases.12
ICI drugs have been used off-label to treat small numbers of select patients with advanced ocular adnexal neoplasms with success,13–17 but more comprehensive studies embracing a range of disease severities have not yet been undertaken. A major barrier to the wider use of ICIs in the treatment of advanced ocular adnexal malignancies is the relative dearth of preclinical data obtained from ophthalmic tissues, although change in this area is rapid. Recent studies have found that PD-L1 and PD-L2 are expressed in high levels in ocular surface squamous carcinoma18,19 but at low levels in adenoid cystic carcinoma.20 At the inception of the current study, there were no published reports on PD-L1 or PD-L2 expression in sebaceous carcinoma. However, by the completion of this study and writing of this article, 4 other studies have reported the expression of these markers with varied results.21–24 In addition, 2 case reports have described the successful use of ICIs in patients with advanced ocular adnexal sebaceous carcinoma (OASC).13,14 Therefore, the goals of the current study are to assess the growing literature on PD-L1 and PD-L2 expression in OASC, and to place these results within an overall broader context in an effort to resolve selected conflicting results.
METHODS
THIS STUDY WAS APPROVED BY THE INSTITUTIONAL REVIEW board of the Massachusetts General Hospital and Partners Healthcare (IRB #2014P000478) and is compliant with the Declaration of Helsinki and Health Insurance Portability and Accountability Act regulations. A search of the Massachusetts General Hospital and Massachusetts Eye and Ear pathology files using the terms “sebaceous carcinoma,” “eye,” and “ocular” was performed for cases submitted between 1990 and 2017. Forty-five cases were identified. Pathology reports, existing histopathologic slides, and tissue blocks from the identified cases were reviewed. Twenty cases were found to have sufficient material in tissue blocks for immunohistochemical studies. All 20 were primary OACSs. Clinical charts were reviewed, and when possible, a tumor stage was assigned according to the 8th edition of the American Joint Committee on Cancer (AJCC)25 (Table 1). Some patients did not have clinical charts available for review because of a transition in our hospital’s medical record keeping system.
TABLE 1.
Patient and Tumor Clinical Characteristics and Immunostaining Results
| Patient No. | Age, Gender, Location | Maximum Tumor Dimension (mm) | TNM | AJCC | SLNB | Interventions Before Current Tissue/Source of Current Tissue | Area of Tumor Evaluated on Immunostained Slide (mm2)a | PD-L1 CPS | PD-L1 TPS | PD-L2 CPS | PD-L2 TPS | CD8 (0–3) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| 1 | 74/F/LMC | 21 | T3cN0M0 | IIa | Not done | Incisional biopsy and map biopsies/definitive excision | 190 | 14.7 | 0.3 | 18.5 | 0.0 | 2 |
| 2 | 58/F/LLL | 15 | T1bN1bM0 | IIIa | Attempted, tracer did not migrate | Incision and drainage, incisional biopsy, interferon/definitive excision | 140 | 75.1 | 69.7 | 27.0 | 12.9 | 3 |
| 3 | 86/F/RUL | 31 | T4aN2bM0 | IIIb | Performed, positive | Incomplete tumor excision and reconstruction/definitive excision (exenteration) | 180 | 16.9 | 10.7 | 13.9 | 7.0 | 2 |
| 4 | 44/F/RLL | 5 | T1bNxMx | Ia | Not done | Excision, self-excision/definitive excision | 10 | 9.4 | 3.5 | 12.7 | 10.2 | 1 |
| 5 | 87/F/RUL | 7 | T1bN0Mx | Ia | Not done | Incisional biopsy/definitive excision | 8 | 18.8 | 1.5 | 3.0 | 0.0 | 1 |
| 6 | 72/F/LUL | 4 | T1bNxMx | Ia | Attempted, tracer did not migrate | None/definitive excision | 12 | 54.3 | 7.9 | 23.0 | 7.6 | 2 |
| 7 | 92/M/LLL | 2 | T2bNxMx | IIa | Not done | Incisional biopsy, map biopsies/definitive excision | 30 | 33.5 | 15.7 | 0.0 | 0.0 | 1 |
| 8 | 46/F/LLL | 4 | T1cNxMx | Ia | Not done | 2 incision and drainage without pathology, incisional biopsy/definitive excision | 6 | 4.8 | 1.4 | 0.0 | 0.0 | 1 |
| 9 | 71/F/RUL | 3 | T1bNxMx | Ia | Not done | Excision without pathology, incisional biopsy/definitive excision | 32 | 83.3 | 3.3 | 37.3 | 0.0 | 2 |
| 10 | 73/F/RLL | Extensive orbital invasion; no measured dimensions | T4aN0M0 | IIb | Performed, negative | Incisional biopsy, incomplete excisions, mitomycin C, cryotherapy, interferon/definitive excision (exenteration) | 5 | 31.1 | 4.8 | 0.0 | 0.0 | 2 |
| 11 | 63/M/RUL | 28 | T3bN0M0 | IIa | Performed, negative | Incisional biopsy/definitive excision | 6 | 6.8 | 4.6 | 0.0 | 0.0 | 1 |
| 12 | 70/M/LUL | 13 | T2bN0M0 | IIa | Performed, negative | Incisional biopsy/definitive excision | 8 | 8.7 | 0.0 | 3.1 | 0.0 | 2 |
| 13 | 70/F/LLL | 6 | T1aN0M0 | Ia | Not done | 2 incisional biopsies/definitive excision | 2 | 1.3 | 0.0 | 0.0 | 0.0 | 1 |
| 14 | 74/M/LUL | Extensive pagetoid spread; no measured dimensions | TisN0M0 | 0 | Performed, negative | Incisional biopsy/definitive excision | 3 | 26.7 | 2.8 | 3.5 | 0.0 | 2 |
| 15 | 84/F/RUL | <10 | T1bNxMx | Ia | Not done | Tobramycin-dexamethasone, doxycycline, incisional biopsy/definitive excision | 2 | 7.3 | 0.8 | 0.0 | 0.0 | 2 |
| 16 | 56/F/RLL | 45 | T3bN0M0 | IIa | Not done | Incisional biopsy, map biopsies/attempted definitive excision (margin was positive) | 5 | 0.0 | 0.0 | 0.0 | 0.0 | 1 |
| 17 | 91/M/LUL | ≥4 | Unknown | Unknown | Unknown | None/incisional biopsy | 16 | 6.8 | 1.3 | 3.2 | 0.0 | 1 |
| 18 | 73/F/LUL, LLL, LMC | No data | Unknown | Unknown | Unknown | Unknown | 35 | 45.7 | 17.1 | 4.4 | 0.0 | 3 |
| 19 | 78/F/RLL | ≥8 | Unknown | Unknown | Unknown | Incisional biopsy/definitive excision | 12 | 11.5 | 2.9 | 0.7 | 0.0 | 2 |
| 20 | 82/M/LUL | No data | Unknown | Unknown | Unknown | Unknown | 119 | 101.5 | 95.8 | 6.9 | 0.0 | 3 |
AJCC = American Joint Committee on Cancer; CPS = combined positive score; F = female; LLL = left lower lid; LMC = left medial canthus; LUL = left upper lid; M = male; RLL = right lower lid; RUL = right upper lid; SLNB = sentinel lymph node biopsy; TNM = tumor, node, metastasis; TPS = tumor proportion score.
Excludes surrounding normal tissue on slide (only tumor area was measured).
Immunostaining was performed on 5-μm paraffin sections at the Massachusetts General Hospital’s Immunopathology Laboratory. The following antibodies were used: PD-L1 (rabbit monoclonal antibody clone E1L3N, 1:30 dilution; Cell Signaling Technology, Danvers, Massachusetts, USA), PD-L2 (rabbit monoclonal antibody clone D7U8C, 1:100 dilution; Cell Signaling Technology), and CD8 (rabbit polyclonal antibody, #ab4055, 1:200 dilution; Abcam, Cambridge, Massachusetts, USA). The area of the immunostained tumor within in the tissue sample on the slide was measured and was on average 41 mm2 (range 2–190 mm2). Most slides had normal tissue present surrounding the tumor, and the normal ocular tissue was not included in the area measurement.
Immunostained slides were scored independently by 2 pathologists. Expression of PD-L1 and of PD-L2 for each sample was scored with both the tumor proportion score (TPS) and combined positive score (CPS).26–32 For each sample, raw CPS and TPS scores were reported, as well as CPS and TPS ≥1 (Table 1 and Supplementary Table 1). Interobserver reliability scores between the 2 pathologists were calculated (Supplementary Table 1). Intraclass correlation (ICC) (agreement) scores were calculated for the raw CPS and TPS scores. For PD-L1 scored with CPS, the ICC (agreement) was 0.918; for PD-L1 scored with TPS, the ICC (agreement) was 0.961; for PD-L2 scored with CPS, the ICC (agreement) was 0.729; and for PD-L2 scored with TPS, the ICC (agreement) was 0.326. Agreement between the 2 pathologists in grading samples as having CPS or TPS ≥1 was also assessed. Agreement for CPS ≥1 for PD-L1 was 100%, agreement for TPS ≥1 for PD-L1 was 95%, agreement for CPS ≥1 for PD-L2 was 100%, and agreement for TPS ≥1 for PD-L2 was 95% (Supplementary Table 1).
Overall CD8 immunostaining was graded on a 0–3 scale, with 0 indicating no infiltration and 1, 2, and 3 indicating low, moderate, and high levels of infiltration, respectively.19,20
Results of immunohistochemical studies for PD-L1 and PD-L2 were analyzed with respect to CD8 expression, area of tumor immunostained on the slide, and clinical characteristics, including patient gender, age, eyelid involved, treatments before biopsy procedure, sentinel lymph node biopsy procedure, metastases or recurrences, and AJCC stage. Because of small sample sizes, certain statistical analyses could not be performed. Statistics were performed with TIBCO Statistica 13.5 (Palo Alto, California, USA). The Fisher exact test was used for cross tabulation when comparing categorical variables. Ordinal scores were compared between groups using 1-way analysis of variance. Pearson and Spearman correlations were used to test for relationships between ordinal variables. P < .05 was considered statistically significant.
RESULTS
PATIENT CLINICAL CHARACTERISTICS:
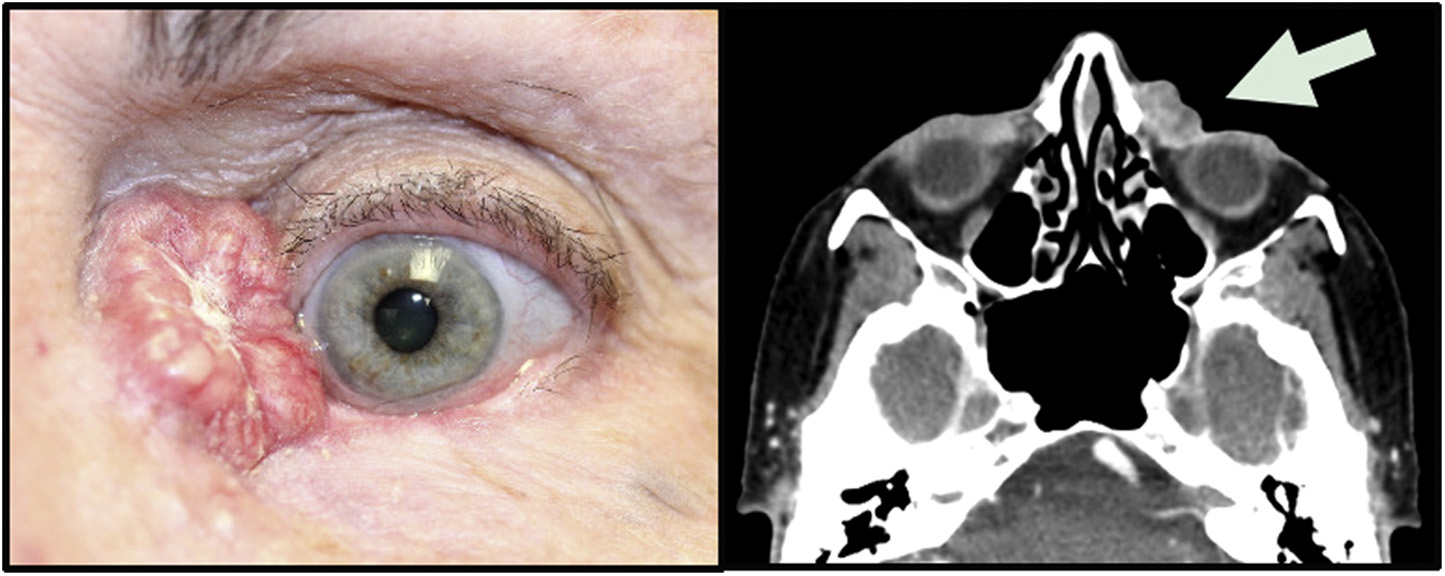
Twenty patients were included in this study, with 14 (70%) women and 6 (30%) men (Table 1). The mean age at diagnosis of sebaceous carcinoma diagnosis was 72.2 years (range 44–91 years). Sixteen (80%) patients were white, 1 (5%) was black, 2 (10%) were Asian, and 1 (5%) was of mixed ethnicity. Nine (45%) tumors were on the right side, while 11 (55%) were on the left. Ten (50%) involved the upper eyelid, 8 (40%) involved the lower eyelid, and 2 (10%) involved the medial canthus (Figure 1). Tumors ranged in size from 2 to >45 mm (Table 1). Tumor node metastases (TNM) and 8th edition AJCC stages were determined for 16 of 20 patients who had clinical records available for review. One tumor was intraepithelial and was AJCC stage 0, 7 tumors were AJCC stage Ia, 5 tumors were AJCC stage IIa, 1 tumor was AJCC stage IIb, 1 tumor was AJCC stage IIIa, and 1 was AJCC stage IIIb upon presentation (Table 1).
FIGURE 1. Clinical presentation of ocular adnexal sebaceous carcinoma. (Left) Clinical photograph of a sebaceous carcinoma in the left medial canthus (courtesy of Daniel R. Lefebvre). (Right) Axial computed tomography scan of the same patient. The left medial canthal mass is indicated by the arrow and does not extend deeper into the orbit. No bony erosion was detected.
Of the 16 patients with available medical records,12 (75%) had undergone a preceding surgical intervention (ranging from a small incisional biopsy procedure to a larger excision) before the surgical procedure that had produced the tissue that was immunostained in the current study, 1 (6%) patient had no previous surgical or medical interventions, 1 (6%) had undergone surgery and topical interferon therapy, 1 (6%) had been treated with surgery, cryotherapy, mitomycin C, and interferon, while 1 (6%) had been treated with surgery, topical tobramycin-dexamethasone, and systemic doxycycline because of initial suspicion for a chalazion rather than sebaceous carcinoma (Table 1).
Of these 16 patients, 7 (44%) elected to undergo a sentinel lymph node biopsy procedure, while 9 (56%) did not. Of the 7 who chose to undergo a sentinel lymph node biopsy procedure, in 2 (29%) patients the tracer did not migrate to an identifiable lymph node and the sentinel lymph node biopsy procedure could not be performed, in 4 (57%) patients the sentinel lymph node biopsy procedure was negative, and in 1 (14%) patient the biopsy specimen was positive. Of the 4 patients who had sentinel lymph node biopsy procedures with negative results, 1 subsequently developed metastases in the parotid gland. In total, 2 of 16 patients (12.5%) developed metastases, and in both cases metastases were in the parotid gland.
HISTOPATHOLOGIC FINDINGS:
Tumors from 20 patients were immunostained for PD-L1, PD-L2, and CD8. For many samples, the tissue on the slide included both normal eyelid structures and tumor. The area of tumor on the immunostained slide ranged from 2–190 mm2. PD-L1 and PD-L2 expression were graded with both the CPS and TPS systems.30–32
Nineteen samples (95%) expressed some degree of PD-L1 with the CPS, while 17 samples (85%) expressed some degree of PD-L1 with TPS (Figure 2). The difference in CPS and TPS confirmed that in 2 (10%) samples PD-L1 positivity was solely related to immune cell positivity rather than tumor cell positivity. For those samples exhibiting any positive PD-L1 expression, the mean PD-L1 CPS was 27.9 (range 1.3–101.5) while the mean PD-L1 TPS was 14.4 (range 0.3–95.8).
FIGURE 2. Programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) expression in ocular adnexal sebaceous carcinoma. (Top left) Photomicrograph of ocular adnexal sebaceous carcinoma. These variably vacuolated tumors were heterogeneously positive for PD-L1 and PD-L2 (hematoxylin and eosin, ×40). (Top right) Immunostaining for PD-L1 revealed a heterogeneous pattern of positivity, with some areas of the tumor weakly staining (left side), while other areas had diffuse PD-L1 positivity (right side; immunoperoxidase reaction, diaminobenzidine chromogen, hematoxylin counterstain, ×10). (Middle left) Some tumors had a predominance of PD-L1 positivity in the stromal (S) cells, with an absence of immunostaining in the adjacent tumor (T) cells (immunoperoxidase reaction, diaminobenzidine chromogen, hematoxylin counterstain, ×60). (Middle right) Other tumors displayed a predominance of membranous PD-L1 immunostaining in the tumor cells (immunoperoxidase reaction, diaminobenzidine chromogen, hematoxylin counterstain, ×60). (Bottom left) PD-L2 positivity in a membranous pattern was manifested by the tumor cells. There was also positivity on some intermixed stromal cells (immunoperoxidase reaction, diaminobenzidine chromogen, hematoxylin counterstain, ×60). (Bottom right) Many sebaceous carcinomas had a heavy infiltrate of associated CD8-positive T-lymphocytes (immunoperoxidase reaction, diaminobenzidine chromogen, hematoxylin counterstain, ×60).
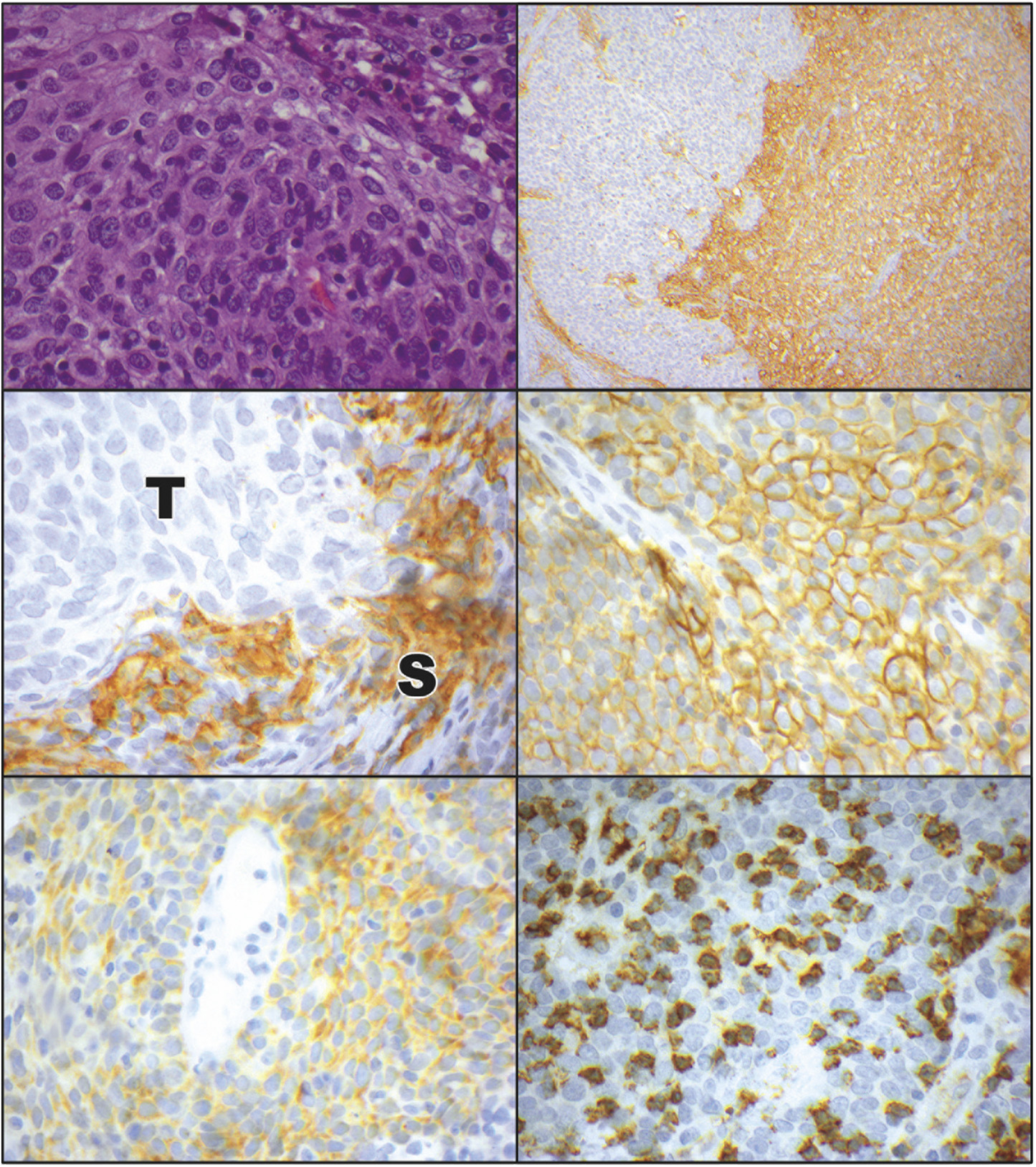
With regard to PD-L2, 13 samples (65%) expressed some degree of PD-L2 with the CPS, while only 4 samples (20%) expressed some degree of PD-L2 with TPS (Figure 2). As with PD-L1, PD-L2 expression was largely contributed by immune cells rather than tumor cells, with 9 tumors (45%) harboring PD-L2 expression solely on immune cells. The mean expression of PD-L2 with CPS in those samples that had any PD-L2 expression was 12.1 (range 3–37.3), while the mean expression of PD-L2 with TPS in those samples that had any PD-L2 expression was 9.4 (range 7–12.9).
PD-L1 and PD-L2 TPS and CPS were also scored as ≥1 or <1 because a score of ≥1 is often used as a cut-off in clinical trials or clinical practice for deciding whether a patient should be treated with immunotherapy. Nineteen samples (95%) had CPS ≥1 for PD-L1; 15 (75%) had TPS ≥1 for PD-L1; 12 (60%) had CPS ≥1 for PD-L2; and 4 (20%) had TPS ≥1 for PD-L2 (Table 1 and Supplementary Table 1). Concordance in scoring samples as ≥1 or <1 was 100% for PD-L1 CPS, 95% for PD-L1 TPS, 100% for PD-L2 CPS, and 95% for PD-L2 TPS for 2 pathologists.
All 20 tumors had associated CD8-positive T lymphocytic infiltrates (Figure 2). On a 0–3 scale, the mean ± standard deviation expression of CD8 was 1.75 ± 0.72.
Expression of PD-L1, PD-L2, and CD8 were analyzed to determine whether there were any relationships among the expression of these markers, and also between the expression of these markers and any clinical characteristics (Supplementary Tables 2–4). Certain analyses could not be performed because of the small sample size.
Relationships were identified between expression of PD-L1, PD-L2, and CD8 (Supplementary Tables 2–4). Samples with higher expression of PD-L1 tended to have a higher expression of PD-L2 based on CPS (P < .01). Samples with higher expression of PD-L1 also tended to have a higher expression of CD8 based on CPS (P = .01) and TPS (P < .01). Similarly, there appeared to be a trend for samples with higher expression of PD-L2 to have a higher expression of CD8 (P = .087 with the χ2 test, P = .18 with the Fisher exact test), but the overall number of samples expressing PD-L2 was lower than those expressing PD-L1, making statistical correlations less reliable.
No relationships were identified between PD-L1 and PD-L2 expression and patient age, gender, or tumor laterality. The sample size was deemed too small to determine if there were any significant relationships between PD-L1 or PD-L2 expression and interventions before biopsy procedure, tumor recurrence or metastases, sentinel lymph node biopsy procedure, and AJCC stage. For the most part, relationships were not found between expression of PD-L1 or PD-L2 and tumor location on the upper or lower eyelid, except for PD-L2 ≥1 CPS (P = .05). Of note, a relationship was identified between PD-L1 and PD-L2 expression and the area of tumor immunostained. Samples with larger immunostained tumor areas tended to have greater PD-L1 expression with CPS (P < 0.01) and TPS (P < 0.01). Similarly, samples with larger immunostained tumor areas tended to have greater PD-L2 expression with CPS (P < 0.03; Supplementary Tables 2–4).
DISCUSSION
THE CURRENT STUDY CONFIRMS THAT PD-L1 AND PD-L2 ARE expressed in a high percentage of OASCs, with PD-L1 positivity found in a greater percentage of tumors compared with PD-L2. In many tumors, PD-L1 and PD-L2 are expressed to a greater degree on the stromal cells that are intimately associated with the tumor rather than in the tumor cells themselves, although some tumors have a pronounced tumor-predominant PD-L1 and PD-L2 expression. The current study confirms that there is a correlation between high tissue expression levels of PD-L1, PD-L2, and CD8, with the caveat that statistical analyses on small numbers of patients may not accurately reflect findings revealed in larger groups. In addition, the current study suggests that sample size of the tissue immunoassayed may influence PD-L1 and PD-L2 expression results. The findings in the current study expand upon the results of recent comparable studies and support the premise that ICI drugs should be used on a trial basis for the treatment of advanced OASC.
To date, 5 studies, including the present study, have examined the expression of PD-L1 in sebaceous carcinoma (Table 2).21–24 Three of these studies have also looked at the expression of PD-1,21–23 while 2 (including this study) have investigated the expression of PD-L2.21 Although there are several similarities among the studies, there are also notable differences. For example, the degree of PD-L1 positivity varies substantially, ranging from 43%−95% (Table 2). These differences may stem from the variability that is inherent in studies with a low sample size, although the differences may also result from the use of different antibodies and different grading scales (Table 2); several antibodies currently exist for PD-L1 and PD-L2, as well as several grading scales.33–36 Among the 5 studies, 4 different PD-L1 antibodies were used, and 4 different scales were used to grade PD-L1 expression (Table 2). In some scales only positive tumor cells are counted, while in other scales both tumor and immune cell expression of the marker is scored; some scales use 1%, while others use 5% as a cut-off for positivity or a system combining many elements. Another confounding factor in scoring tumor positivity is the heterogeneity of PD-L1 expression within tumors,37,38 with our current data suggesting that sample area evaluated may influence positivity results.
TABLE 2.
Comparison of Studies of Programmed Cell Death Ligands 1 and 2 Expression in Ocular Adnexal Sebaceous Carcinoma
| Author, Year | Samples | Lymph Node Metastases: Positive Nodes (Nodes Tested) | Died of Disease | Antibodies Used | PD-L1 Scoring System | PD-L1 Positivity | PD-1 | PD-L2 |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Kandl and associates,23 2018 | 24 primary | 7 (24) | 3 | PD-L1 (Cell Signaling Technology 13684S), CD3 (DAKO A0452), CD8 (Life Sciences Technologies MS457s), FOXP3 (BioLegend 206D), and PD-1 (Abcam ab137132) | Positive: >1% membranous staining of tumor cells (selected areas evaluated; stromal cells not counted) | 12/24 (50%) with positive tumor cells in primary | Increased expression in CD8-T lymphocytes when tumor cells are PD-L1–positive | Not assessed |
| Xu and associates,24 2019 | 41 cases: 20 primary, 11 recurrent, and 10 lymph node metastases | 4 | 1 | PD-L1 (Gene Tech SP142) | Positive: >1% membranous staining of tumor cells; staining intensity graded from 1–3 (low-high) | 20/41 (49%): primary: 14/20 (70%); recurrent: 5/11 (45%); metastatic: 8/10 (80%) — adds up to 27/41 (66%) | Not assessed | Not assessed |
| Jayaraj and Sen,22 2019 | 30 cases (unknown if tissue was from primary or recurrences) | 7 | 1 | PD-L1 (Cell Signaling Technology E1L3N) and PD-1 (Cell Signaling Technology D4W2J) | Positive: combined IHC score of 3–6; negative: combined IHC score of 0–2; IHC score is the sum of percentage positivity and staining intensity scores — percentage positivity: 0 (<5%), 1+ (5–25%), 2+ (26–50%), or 3+ (51–100%); intensity: 0 (no stain), 1+ (weak), 2+ (medium), or 3+ (strong) | High PD-L1 13/30 (43%); unclear how many primary, how many recurrent | Increased expression in CD8-T lymphocytes when tumor cells are PD-L1–positive | Not assessed |
| Bowen and associates,21 2019 | 28 cases | 1 | 0 | PD-L1 (Cell Signaling Technology 405.9A11), PD-L2 (Cell Signaling Technology D7U8C), PD-1 (Cell Signaling Technology EH3), FOXP3 (Cell Signaling Technology D2WE), CD8 (Translational Research in Pathology Laboratory), CD4 (Translational Research in Pathology Laboratory), CD20 (Translational Research in Pathology Laboratory), and CD68 (Translational Research in Pathology Laboratory) | Positive: ≥5% of membranous tumor or infiltrating immune cell expression | 11/24 (46%) with positive stromal cells (but not tumor cells) | Positive if ≥5%; positive on T cells, not on tumor cells; PD-1 strongly associated with PD-L1 expression | Positive if ≥5% of cells; 13/28 (46%) with positive tumor membranous staining |
| Wolkow and associates, 2020 (current study) | 20 cases | 2 | 1 | PD-L1 (Cell Signaling Technology E1L3N), PD-L2 (Cell Signaling Technology D7U8C), and CD8 (Abcam ab4055) | Raw CPS reported and raw TPS reported; positive: ≥1 CPS (positive tumor and stromal cells per 100 tumor cells) or ≥1 TPS (positive tumor cells per 100 tumor cells) | 19/20 (95%) positive with CPS; 15/20 (75%) positive with TPS | Not assessed | Raw CPS reported and raw TPS reported; positive: ≥1 CPS (positive tumor and stromal cells per 100 tumor cells) or ≥1 TPS (positive tumor cells per 100 tumor cells); 12/20 (60%) positive with CPS and 4/20 (20%) positive with TPS |
CPS = combined positive score; PD-L1 = programmed cell death ligand 1; PD-L2 = programmed cell death ligand 2; TPS = tumor proportion score.
In clinical practice and in clinical trials in areas such as head and neck oncology, the 2 most commonly used scoring systems for PD-L1 expression are the CPS and the TPS.5,30–32,39,40 Similar scoring systems have not been generally agreed upon for PD-L2. In the current study, raw CPS and TPS numbers are reported for both PD-L1 and PD-L2 in order to facilitate comparisons with other studies in the literature (Table 1 and Supplementary Table 1) in addition to less refined ≥1 or <1 scores. The TPS differs from CPS in that it only considers tumor cells that immunostain positively for PD-L1, while the CPS also includes infiltrating immune cells that immunostain positively. Recently there has been increased appreciation of the importance of PD-L1 expression on immune cells.41 By providing both CPS and TPS in the current study, one fact that becomes apparent is that PD-L1 and PD-L2 positivity in a significant proportion of samples stems from positivity on immune cells rather than from tumor cells. While 95% of samples were positive (≥1) with CPS for PD-L1, only 75% of samples were positive (≥1) with TPS for PD-L1, confirming that in 20% of samples PD-L1 positivity was derived from immune cells. This difference was even greater with PD-L2, where 60% of samples were positive with CPS ≥1, but only 20% of samples were positive with TPS, confirming that 40% of samples derived their PD-L2 positivity from immune cells. The relative importance of PD-L1 and PD-L2 expression on tumor cells as opposed to immune cells is an area of ongoing investigation.41
Regarding preceding studies in the ophthalmic literature, Kandl and associates,23 from the University of Texas MD Anderson Cancer Center, examined the expression of PD-L1 in primary OASCs from 24 patients. Using the 13684S antibody and a scoring system that considered membranous staining in >1% of tumor cells as positive, but which discounted the stromal cells, 12 (50%) of the 24 samples in their study had positive PD-L1 expression. This scoring system of Kandl and associates23 is similar to the TPS. By directly comparing the results of Kandl and associates23 (50% positivity) to other studies such as the current one, where PD-L1 positivity with TPS ≥1 was 75%, and the results of Xu and associates,24 which are discussed below, PD-L1 positivity of OASC falls into a similar range. Kandl and associates’23 results, however, may underestimate PD-L1 positivity if compared with studies that use CPS. Kandl and associates23 also examined PD-1 expression on T-lymphocytes and the density of CD8-positive T-lymphocytes. PD-1-positive CD8 T-lymphocytes were found at the peripheries of tumors, and their densities were greater in tumors with a higher AJCC T category. Tumors expressing PD-L1 had denser infiltrates of PD-1–positive T-lymphocytes. These data support the concept that the PD-1/PD-L1 axis is active in some OASCs and that checkpoint blockade may be therapeutically beneficial.
Xu and associates24 from China examined the expression of PD-L1 in 41 samples: 20 primary OASCs, 11 recurrent OASCs, and 10 tumors metastatic to the lymph nodes. The SP142 antibody was used for PD-L1 detection, and samples were considered positive if ≥1% of tumor cells displayed membranous staining; stromal cells were not included in the count. This scoring approach, is again, similar to the TPS. Fourteen of 20 (70%) primary tumors, 5 of 11 (45%) recurrent tumors, and 8 of 10 (80%) metastatic tumors had positive PD-L1 expression. PD-1 and PD-L2 expression were not assessed. The expression levels of PD-L1 (70%) in primary OASC in Xu and associates’24 study are similar to those found in our current study with TPS (75%), although Xu and associates’24 study may again underestimate positivity compared with studies that have used the CPS scoring system.
A third study by Jayaraj and associates22 from India examined expression of PD-L1, PD-1, and CD8 in OASC. Although the same E1L3N antibody was used for PD-L1 detection as the one in our current study, a complex scoring system that incorporated both degree of tumor positivity and degree of staining intensity was applied, making it difficult to compare their results to those of our study. Tumor positivity was scored from 0–3; for example, tumors with <5% of positive cells were considered to have a score of 0 in the positivity score, while tumors with 5%−25% positivity were given a score of 1. Staining intensity was also graded from 0–3, and this score was added to the tumor positivity score. Tumors were overall positive if they had total scores of ≥3. Using this method, 13 of 30 (43%) samples were found to have a high PD-L1 expression level. When compared with TPS and CPS, this scoring system is significantly more complex. It is also significantly more stringent than the 1% cut-off used in most studies of OASC and may therefore underreport PD-L1 positivity. If reassessed with the TPS or CPS systems, positivity may be a great deal higher and comparable to the rates found in our study or the study by Xu and associates.24 As in Kandl and associates’ study,23 an association was found between high expression of PD-L1 and high expression of PD-1 on tumor-infiltrating immune cells.
The fourth of these studies by Bowen and associates21 differed from the current study and other preceding studies in that 1 mm2 core biopsy specimens were taken from each paraffin-embedded block to create tissue microarrays for immunohistochemical staining rather than using larger sections. The microarrays were immunostained for PD-1, PD-L1, and PD-L2, as well as for several inflammatory cell markers. The 405.9A11 antibody was used for PD-L1 detection; for PD-L2 detection the D7U8C antibody was used, the same antibody as in the current study. Tumor cells or infiltrating immune cells were considered positive if ≥5% had staining for PD-L1 or PD-L2 in the 1-mm2 tumor area. In essence, this scoring approach is most similar to CPS, but with a 5% cut-off rather than the more common 1%. Bowen and associates21 found that PD-L1 was not significantly expressed on the surfaces of tumor cells in the 28 tumors, but that it was expressed on the stromal cells of 11 of 24 (46%) tumors. PD-L2, on the other hand, was expressed on the surface membranes of tumor cells in 13 of the 28 (46%) cases and on tumor-associated stromal cells.
Bowen and associates’21 PD-L1 and PD-L2 results differ to some degree from results of other studies by having overall lower rates of PD-L1 and PD-L2 marker expression. In part this may be because only a small 1-mm2 area was immunostained from each sample; PD-L1 and PD-L2 expression is known to be highly variable throughout tissue samples, with several studies finding higher expression of markers near tumor peripheries.23,41 Therefore, if a core biopsy specimen is taken from the center of a tumor rather than the edge, the immunostaining result may underestimate the overall expression of the marker. Indeed, in our current study, we found that the area of the tumor immunostained appeared to have a statistically significant effect upon the degree of PD-L1 or PD-L2 expression, where larger samples tended to have higher PD-L1 and PD-L2 TPS and CPS. For example, in the current study the mean PD-L1 CPS for the 5 smallest tumors with an immunostained area ≤5 mm2 each was 13.3, while the mean CPS for the 5 tumors with the greatest immunostained areas was 50.8. The same holds true for PD-L2 in the current study, where the 5 tumors with the smallest immunostained areas had a mean CPS of 0.7, while the 5 tumors with the largest immunostained area had a mean CPS of 11.8. An additional factor that may have contributed to lower PD-L1 and PD-L2 scores in Bowen and associates’ study21 compared with other studies is that a 5% cut-off was used for positivity rather than the 1% cut-off used in other studies or the ≥1 often used for TPS and CPS. In addition, a PD-L1 antibody that was not used in any of the other studies was used. As tumor samples from ocular adnexal structures are often diminutive compared with tumor samples from other parts of the body, the potential effects of sample size and tumor heterogeneity on the expression of tumor markers are critical to keep in mind for future studies and clinical practice.
In summary, all 5 studies of PD-L1 expression in OASC have shown that PD-L1 is expressed at high levels on either tumor cells or on surrounding immune stromal cells. A few select patients with advanced OASC have now been treated off-label with ICIs with success. Domingo-Musibay and associates14 reported a patient with sebaceous carcinoma metastatic to the brain. Immunostaining of excised brain metastases revealed high (100%) PD-L1 staining. The patient was treated with the PD-1 inhibitor pembrolizumab with a decrease in tumor burden but subsequently developed adrenal insufficiency. Kodali and associates13 reported a 72-year-old woman who underwent orbital exenteration for advanced OASC. Two years after exenteration she developed cervical lymphadenopathy and a recurrent mass involving the superomedial orbit and ethmoid sinuses with intracranial extension through the skull base. The recurrence was considered inoperable and was treated with a combination of carboplatin and pembrolizumab. There was a favorable response with tumor regression. The patient remained tumor-free for ≥15 months after completion of combination therapy. Of interest, retrospective immunostaining for PD-L1 of this patient’s previously biopsied tumor displayed <1% positivity, which was not predictive of such a robust response. This phenomenon is not unique to sebaceous carcinoma. In a clinical trial in 23 patients treated with pembrolizumab for advanced Merkel cell carcinoma, of the 14 patients who responded to treatment only 8 (57%) had PD-L1–positive tumors, while 6 (42%) had PD-L1–negative tumors with <1% of tumor cells staining positively.42 Kandl and associates reported a third case of metastatic OASC that had responded to PD-L1 inhibition, but details are lacking because the manuscript is still under review.
The current and previous studies have minor methodologic differences and some unpredictable and inexplicable biologic behavioral aspects among them that do not vitiate the overall shared findings. A total 139 tumors have been tested in the 5 recent studies for PD-L1 expression with 75 (54%) deemed to have positive expression. Forty-eight tumors have been tested for PD-L2 expression, with 25 (52%) considered to be positive, again, taking account of differences in methodologies among the studies. Consequently, there is now a pressing and inescapable rationale for the implementation of ICI drugs in clinical trials particularly at advanced stages of OASC. Another insight supplied by recent experience in select patients with OASC is that even those with low expression of PD-L1 can, on occasion, benefit from ICIs.
Supplementary Material
Funding/Support:
Supported by an Austin L. Vickery Award from the Massachusetts General Hospital, United States Department of Pathology (Dr Faquin). Financial Disclosures: Dr Wolkow has been a consultant to Pykus Therapeutics. Drs Jakobiec, Afrogheh, Pai, and Faquin indicate no financial conflict of interest. We thank Martin Kidd from the Centre for Statistical Consultation, Department of Statistics and Actuarial Sciences, University of Stellenbosch, Stellenbosch, South Africa for his assistance with the statistical analyses. All authors attest that they meet the current ICMJE criteria for authorship.
Footnotes
ALL AUTHORS HAVE COMPLETED AND SUBMITTED THE ICMJE FORM FOR DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST.
Supplemental Material available at AJO.com
Contributor Information
NATALIE WOLKOW, David G. Cogan Ophthalmic Pathology Laboratory, Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, Massachusetts, USA; Ophthalmic Plastic and Reconstructive Surgery Service, Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, Massachusetts, USA.
FREDERICK A. JAKOBIEC, David G. Cogan Ophthalmic Pathology Laboratory, Department of Ophthalmology, Massachusetts Eye and Ear, Harvard Medical School, Boston, Massachusetts, USA
AMIR H. AFROGHEH, Department of Oral and Maxillofacial Pathology, National Health Laboratory Service, University of the Western Cape, Cape Town, South Africa.
SARA I. PAI, Division of Surgical Oncology, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA
WILLIAM C. FAQUIN, Department of Surgery and Division of Head and Neck Pathology, Department of Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA
REFERENCES
- 1.Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: an overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol 2018;62:29–39. [DOI] [PubMed] [Google Scholar]
- 2.Meng Y, Liang H, Hu J, et al. PD-L1 expression correlates with tumor infiltrating lymphocytes and response to neoadjuvant chemotherapy in cervical cancer. J Cancer 2018;9(16):2938–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukaigawa T, Hayashi R, Hashimoto K, Ugumori T, Hato N, Fujii S. Programmed death ligand-1 expression is associated with poor disease free survival in salivary gland carcinomas. J Surg Oncol 2016;114(1):36–43. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Teng F, Kong L, Yu J. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 2016;9:5023–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cramer JD, Burtness B, Ferris RL. Immunotherapy for head and neck cancer: recent advances and future directions. Oral Oncol 2019;99:104460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378(2):158–168. [DOI] [PubMed] [Google Scholar]
- 7.Conrady CD, Larochelle M, Pecen P, Palestine A, Shakoor A, Singh A. Checkpoint inhibitor-induced uveitis: a case series. Graefes Arch Clin Exp Ophthalmol 2018;256(1):187–191. [DOI] [PubMed] [Google Scholar]
- 8.Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA. Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina 2018;38(6):1063–1078. [DOI] [PubMed] [Google Scholar]
- 9.Noble CW, Gangaputra SS, Thompson IA, et al. Ocular adverse events following use of immune checkpoint inhibitors for metastatic malignancies. Ocul Immunol Inflamm 2019;28:854–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sagiv O, Kandl TJ, Thakar SD, et al. Extraocular muscle enlargement and thyroid eye disease-like orbital inflammation associated with immune checkpoint inhibitor therapy in cancer patients. Ophthalmic Plast Reconstr Surg 2019;35(1):50–52. [DOI] [PubMed] [Google Scholar]
- 11.Sun MM, Levinson RD, Filipowicz A, et al. Uveitis in patients treated with CTLA-4 and PD-1 checkpoint blockade inhibition. Ocul Immunol Inflamm 2020;28(2):217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakobiec FA, Mendoza PR. Eyelid sebaceous carcinoma: clinicopathologic and multiparametric immunohistochemical analysis that includes adipophilin. Am J Ophthalmol 2014;157(1):186–208.e2. [DOI] [PubMed] [Google Scholar]
- 13.Kodali S, Tipirneni E, Gibson PC, Cook D, Verschraegen C, Lane KA. Carboplatin and pembrolizumab chemoimmunotherapy achieves remission in recurrent, metastatic sebaceous carcinoma. Ophthalmic Plast Reconstr Surg 2018;34(5):e149–e151. [DOI] [PubMed] [Google Scholar]
- 14.Domingo-Musibay E, Murugan P, Giubellino A, et al. Near complete response to pembrolizumab in microsatellitestable metastatic sebaceous carcinoma. J Immunother Cancer 2018;6(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang M, Lally SE, Dalvin LA, Orloff MM, Shields CL. Conjunctival melanoma with orbital invasion and liver metastasis managed with systemic immune checkpoint inhibitor therapy. Indian J Ophthalmol 2019;67(12):2071–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finger PT, Pavlick AC. Checkpoint inhibition immunotherapy for advanced local and systemic conjunctival melanoma: a clinical case series. J Immunother Cancer 2019;7(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sagiv O, Thakar SD, Kandl TJ, et al. Immunotherapy with programmed cell death 1 inhibitors for 5 patients with conjunctival melanoma. JAMA Ophthalmol 2018;136(11):1236–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagarajan P, El-Hadad C, Gruschkus SK, et al. PD-L1/PD1 expression, composition of tumor-associated immune infiltrate, and HPV status in conjunctival squamous cell carcinoma. Invest Ophthalmol Vis Sci 2019;60(6):2388–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolkow N, Jakobiec FA, Afrogheh AH, Eagle RC Jr, Pai SI, Faquin WC. Programmed cell death 1 ligand 1 and programmed cell death 1 ligand 2 are expressed in conjunctival invasive squamous cell carcinoma: therapeutic implications. Am J Ophthalmol 2019;200:226–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolkow N, Jakobiec FA, Afrogheh AH, et al. PD-L1 and PD-L2 expression levels are low in primary and secondary adenoid cystic carcinomas of the orbit: therapeutic implications. Ophthalmic Plast Reconstr Surg 2020;36:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowen RC, Lawson BM, Jody NM, Potter HD, Lucarelli MJ. The programmed death pathway in ocular adnexal sebaceous carcinoma. Ophthalmic Plast Reconstr Surg 2020;36(1):74–79. [DOI] [PubMed] [Google Scholar]
- 22.Jayaraj P, Sen S. Evaluation of PD-L1 and PD-1 expression in aggressive eyelid sebaceous gland carcinoma and its clinical significance. Indian J Ophthalmol 2019;67(12):1983–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kandl TJ, Sagiv O, Curry JL, et al. High expression of PD-1 and PD-L1 in ocular adnexal sebaceous carcinoma. Oncoimmunology 2018;7(9):e1475874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu S, Yu H, Fu G, Fan X, Jia R. Programmed death receptor ligand 1 expression in eyelid sebaceous carcinoma: a consecutive case series of 41 patients. Acta Ophthalmol 2019;97(3):e390–e396. [DOI] [PubMed] [Google Scholar]
- 25.Esmaeli B, Dutton JJ, Graue GF, et al. Eyelid carcinoma. In: Amin MB, Edge S, Greene F, et al., eds. AJCC Cancer Staging Manual. 8th ed. Chicago, IL: American College of Surgeons; 2018:787–794 [Google Scholar]
- 26.Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med 2019;143(3):330–337. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita K, Iwatsuki M, Harada K, et al. Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand-1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer 2020;23(1):95–104. [DOI] [PubMed] [Google Scholar]
- 28.Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394(10212):1915–1928. [DOI] [PubMed] [Google Scholar]
- 29.Shitara K, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastrooesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392(10142):123–133. [DOI] [PubMed] [Google Scholar]
- 30.Agilent Technologies. Inc. PD-L1 IHC 22C3 pharmDx Interpretation Manual – NSCLC. Santa Clara, CA: Agilent Technologies, Inc; 2018. [Google Scholar]
- 31.Agilent Technologies. Inc. Interpretation Manual - Gastric or Gastroesophageal Junction Adenocarcinoma. Santa Clara, CA: Agilent Technologies, Inc; 2019. [Google Scholar]
- 32.Agilent Technologies, Inc.. PD-L1 IHC 22C3 pharmDx Interpretation Manual – Esophageal Squamous Cell Carcinoma (ESCC). Santa Clara, CA: Agilent Technologies, Inc; 2019. [Google Scholar]
- 33.Ma J, Li J, Qian M, et al. PD-L1 expression and the prognostic significance in gastric cancer: a retrospective comparison of three PD-L1 antibody clones (SP142, 28–8 and E1L3N). Diagn Pathol 2018;13(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol 2017;3(8):1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velcheti V, Patwardhan PD, Liu FX, Chen X, Cao X, Burke T. Real-world PD-L1 testing and distribution of PD-L1 tumor expression by immunohistochemistry assay type among patients with metastatic non-small cell lung cancer in the United States. PLoS One 2018;13(11):e0206370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Udall M, Rizzo M, Kenny J, et al. PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics. Diagn Pathol 2018;13(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmussen JH, Lelkaitis G, Hakansson K, et al. Intratumor heterogeneity of PD-L1 expression in head and neck squamous cell carcinoma. Br J Cancer 2019;120(10):1003–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paintal AS, Brockstein BE. PD-L1 CPS scoring accuracy in small biopsies and aspirate cell blocks from patients with head and neck squamous cell carcinoma. Head Neck Pathol 2020;14(3):657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 40.Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol 2016;24(6):392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mattox AK, Lee J, Westra WH, et al. PD-1 expression in head and neck squamous cell carcinomas derives primarily from functionally anergic CD4(+) TILs in the presence of PD-L1(+) TAMs. Cancer Res 2017;77(22):6365–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med 2016;374(26):2542–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


