Abstract
Individuals addicted to most chemical substances present with hypoactive dopaminergic systems as well as altered prefrontal white matter structure. Prefrontal dopaminergic tone is under genetic control and is influenced by and modulates descending cortico-striatal glutamatergic pathways that in turn, regulate striatal dopamine release. The catechol-O-methyltransferase (COMT) gene contains an evolutionarily recent and common functional variant at codon 108/158 (rs4680) that plays an important role in modulating prefrontal dopaminergic tone. To determine if the COMT val158met genotype influences white matter integrity (i.e., fractional anisotropy (FA)) in substance users, 126 healthy controls and 146 substance users underwent genotyping and magnetic resonance imaging. A general linear model with two between-subjects factors (COMT genotype and addiction status) was performed using whole brain diffusion tensor imaging (DTI) to assess FA. A significant Genotype×Drug Use status interaction was found in the left prefrontal cortex. Post-hoc analysis showed reduced prefrontal FA only in Met/Met homozygotes who were also drug users. These data suggest that Met/Met homozygous individuals, in the context of addiction, have increased susceptibility to white matter structural alterations, which might contribute to previously identified structural and functional prefrontal cortical deficits in addiction.
Keywords: Imaging, genetics, nicotine, addiction, COMT, DTI
Introduction
Drug dependent individuals, regardless of their drug of choice, have marked decreases in striatal dopamine (DA) D2 receptors and attenuated striatal DA release (Volkow et al., 2001; Volkow et al., 2005; Volkow et al., 2007). This alteration in DA signaling, in addition to predicting relapse (Wang et al., in press), is associated with reduced metabolism in several frontal cortical areas including orbitofrontal, cingulate and dorsolateral prefrontal cortices (Volkow et al., 2009). These regions support behavioral and cognitive functions that are disrupted in addiction, including emotional regulation, self-control and executive function, respectively (Volkow et al., 2009). Dysregulated neuronal processing in these areas may thus contribute to compulsive, impulsive and impaired action regulation behaviors that characterize the addiction phenotype (Volkow et al., 2009; Volkow et al., 2005).
In addition to these DA-associated prefrontal functional changes, structural changes in prefrontal cortex (PFC) have also been found in drug-dependent individuals. For example, reduction in frontal grey matter volume is seen in cocaine dependent subjects (Matochik et al., 2003) that is inversely proportional to deficits in executive functioning (Fein et al., 2002; Franklin et al., 2002). Zhang et al. (2011) found reduced PFC gray matter density in smokers, with this reduction inversely related to the total lifetime cigarette use. They also reported a reduction in prefrontal white matter (WM) integrity that was negatively correlated with nicotine addiction severity as measured by the Fagerstrom index (FTND). Finally, Liu et al (2008) reported reduced medial frontal FA that correlated with months of heroin use. That these WM structural alterations may have functional significance is suggested by a recent study showing that the P3 event-related potential during a go/nogo task in alcoholics predicts FA in the bilateral cingulate bundle (Colrain et al., 2011).
Of course, both structural and functional alterations found in drug users may be either a consequence of or a cause for chronic drug use. Other factors not considered in the above studies, such as an individual’s genetic background, may amplify or protect against the effects of chronic drug exposure on brain structure and function. One such potential genetic polymorphism is the relatively common COMT val158met gene, a major determinate of dopaminergic tone in the brain, particularly in the PFC where the DA transporter is particularly sparse (Garris and Wightman, 1994). A valine (val) to methionine (met) substitution in a coding region of COMT is associated with a greater than two-fold decrease in COMT enzyme activity and DA catabolism (Chen et al., 2004; Lachman et al., 1996; Lotta et al., 1995) such that the Met allele confers a relative increase in local DA concentration (Tunbridge et al., 2004), especially in the PFC. Such elevated DA tone associated with the Met allele has been shown to confer an advantage for tasks involving sustained attention and memory and in contrast, a disadvantage for tasks involving cognitive flexibility (Bilder et al., 2004).
Dopamine also influences structural development of the PFC (Bhide, 2009). Indeed, COMTVal158Met, as a surrogate measure of prefrontal DA levels, has been shown to affect prefrontal WM pathways (Thomason et al., 2010), such that the Val allele, conferring low prefrontal dopaminergic tone, is associated with significantly elevated FA values and attenuated cortical thinning in a longitudinal study of children and young adults, suggesting a role for DA in myelination development (Bongarzone et al., 1998; Karadottir and Attwell, 2007).
Fractional anisotropy (FA), an MRI based metric of WM pathway microstructure integrity measured using diffusion tensor imaging (DTI), is based on the degree to which water preferentially diffuses along WM tracts (Basser, 1995). Higher FA is generally associated with developmental advancement (Barnea-Goraly et al., 2005; Eluvathingal et al., 2007; Lebel et al., 2008; McGraw et al., 2002; Morriss et al., 1999; Sakuma et al., 1991; Schneider et al., 2004; Snook et al., 2005), in part due to enhanced myelination during development. Indeed, myelination is thought to be one factor contributing to WM tract coherence measures (Mori and Zhang, 2006). Critically, with respect to the current study, in vitro data suggests that high levels of DA can inhibit myelination (Bongarzone et al., 1998; Karadottir and Attwell, 2007). In humans, Val homozygotes, i.e. those with the lowest levels of prefrontal DA, also exhibit the highest FA (Thomason et al., 2010).
Since WM structural differences resulting from genetically determined neurotransmitter (and other) microenvironments are antecedent to any structural or functional alterations that might occur in the context of substance use environmental factors, we investigated the interaction between prefrontal DA (as inferred by the COMT Val158Met polymorphism) and substance abuse on WM structural integrity. Met allele carriers show higher levels of prefrontal DA than Val allele carriers (Tunbridge et al., 2004); chronic drug users show reduced D1 receptor availability (Narendran et al., 2005) and reduced striatal D2 availability (Fehr et al., 2008). We, therefore, hypothesized that previously observed effects of reduced prefrontal FA in substance users (Liu et al., 2008; Zhang et al., 2011) will be amplified in Met allele carriers whose elevated DA tone (Turnbridge et al 2004) reduces WM integrity by impacting myelination (Bongarzone et al., 1998; Karadottir and Attwell, 2007). In addition, we hypothesized that WM integrity will be reduced in Val/Val homozygote drug users (vs. controls), though less so compared to the other two gene groups.
Methods and Materials
Participants:
Data were collected from 126 healthy controls and 146 drug users. The genotype distribution included 51 Met/Met homozygotes, 139 Met/Val heterozygotes, and 82 Val/Val homozygotes (see demographic details in Table 1 and Table S1 and S2 in supplementary materials), which was consistent with Hardy-Weinberg Equilibrium (X2=0.34, p=0.56). The drug-user group was assessed by the Structured Clinical Interview for DSM-IV (SCID), computerized self-report version, with follow-up clinical interview. Subjects in the drug-user group met DSM-IV abuse or dependence criteria for at least one of the following substances: nicotine (≥ 10 cigarettes per day or nicotine dependence), alcohol, cocaine, marijuana, or heroin/opiates; the distribution of substance use did not differ across the COMT genotype (nicotine: χ2 =0.194, p=0.907; alcohol: χ2=1.565, p=0.457; cocaine: χ2=1.178, p=0.555; marijuana: χ2=0.513, p=0.774; heroin/opiates: χ2=1.801, p=0.406). Of the 146 drug users, 30 used nicotine only and 90 used both nicotine and at least one other drug. The remaining 26 were nonsmoking illicit drug users. Participants in the control group had no history of abuse or dependence on any substance ever in their lifetime and were not former smokers. Data for total years of exposure to drug of choice was available for 141 out of 146 drug users. FTND scores were available for 113 out of .120 cigarette smokers
Table 1:
Participant demographic data
| Controls |
Drug-users |
||||||
|---|---|---|---|---|---|---|---|
| Met/Met | Met/Val | Val/Val | Met/Met | Met/Val | Val/Val | ||
| Allele | 19 | 66 | 41 | 32 | 73 | 41 | |
| Gender | M: 11; F: 8 | M: 28; F: 38 | M:24; F:17 | M: 17; F:15 | M: 51; F:22 | M:30; F:11 | |
| Age (mean±SD) | 31.4±9.5 | 29.8±8.5 | 30.6±7.9 | 34.1±10.1 | 34.3±9.0 | 35.0±10.3 | |
| Distribution of Drug use across COMT gene | Nicotine | - | - | - | 26 | 61 | 33 |
| Alcohol | - | - | - | 19 | 48 | 30 | |
| Cocaine | - | - | - | 8 | 26 | 14 | |
| Marijuana/THC | - | - | - | 14 | 33 | 21 | |
| Heroin/Opiates | - | - | - | 4 | 9 | 2 | |
| FTND | - | - | - | 6.08±1.98 | 4.80±2.24 | 4.50±1.80 | |
| Initial smoking age | - | - | - | 14.58±4.95 | 15.50±3.68 | 14.70±4.32 | |
|
| |||||||
| AIM score (i.e., Ethnic data) (mean±SD) |
Africa | 0.38±0.41 | 0.38±0.41 | 0.35±0.41 | 0.26±0.38 | 0.41±0.40 | 0.31±0.38 |
| Europe | 0.52±0.43 | 0.39±0.42 | 0.36±0.42 | 0.56±0.42 | 0.44±0.43 | 0.49±0.43 | |
| Middle East | 0.05±0.07 | 0.07±0.12 | 0.06±0.10 | 0.06±0.11 | 0.06±0.11 | 0.07±0.11 | |
| Asia | 0.03±0.03 | 0.09±0.19 | 0.12±0.23 | 0.08±0.16 | 0.05±0.10 | 0.05±0.08 | |
| Far East Asia | 0.00±0.00 | 0.06±0.21 | 0.09±0.26 | 0.02±0.09 | 0.02±0.11 | 0.03±0.15 | |
| Oceania | 0.01±0.02 | 0.01±0.01 | 0.01±0.02 | 0.01±0.01 | 0.01±0.02 | 0.00±0.00 | |
| America | 0.01±0.01 | 0.01±0.01 | 0.01±0.04 | 0.01±0.02 | 0.01±0.04 | 0.03±0.10 | |
Participants were recruited through newspaper advertisements, flyers, and referrals. After complete description of the study to the subjects, written informed consent was obtained from NIDA-IRP Institutional Review Board approved protocols. Screening procedures included a history and physical exam and a comprehensive laboratory panel (CBC, blood chemistries, liver function tests, thyroid function screening, erythrocyte sedimentation rate, HIV antibody, syphilis screening, urinalysis, pregnancy (females) and a comprehensive urine drug screen). Participants were excluded if they had any major medical, psychiatric or neurological disorders, were left-handed or if their T1 weighted images revealed gross structural abnormalities.
Data acquisition:
Experiments were performed on a Siemens 3T Allegra MRI scanner (Erlangen, Germany) at the NIDA-Intramural Research Program in Baltimore, MD. Subject head movement was minimized by use of foam padding and/or hardened polyurethane foam. A standard birdcage RF head coil was used to obtain whole-brain DTI using a single-shot, spin-echo echo-planar imaging technique (TR=5000ms, TE=87ms, BW=1700Hz/Pixel, FOV=220×220mm, matrix size=128×128, 35 slices, thickness=4mm, b value= 0 or 1000s/mm2). Thirteen unique volumes were collected to compute the tensor: a b=0s/mm2 image and 12 images with diffusion gradients applied in 12 non-collinear directions (Gx, Gy, Gz: [1.0, 0.0, 0.5], [0.0, 0.5, 1.0], [0.5, 1.0, 0.0], [1.0, 0.5, 0.0], [0.0, 1.0, 0.5], [0.5, 0.0, 1.0], [1.0, 0.0, −0.5], [0.0, −0.5, 1.0], [−0.5, 1.0, 0.0], [1.0, −0.5, 0.0], [0.0, 1.0, −0.5], [−0.5, 0.0, 1.0]).
Genotyping:
Genomic DNA was isolated from blood using standard protocols. The COMT Val158Met (rs4680) functional polymorphism was genotyped on a custom-designed ‘addictions array’ using the Illumina GoldenGate platform (Hodgkinson et al., 2008). A total of 186 ancestry informative markers (AIM) were genotyped in the study sample and in the HGDP-CEPH Human Genome Diversity Cell Line Panel (1051 individuals from 51 worldwide populations) (http://www.cephb.fr/HGDP-CEPH-Panel). PHASE Structure 2.2 (http://pritch.bsd.uchicago.edu/software.html) was run simultaneously using the AIM data from our sample and the 51 CEPH populations to identify population substructure and to compute individual ethnic factor scores. See Table 1 for AIM scores.
Data analysis
Participant characteristics: The seven AIM scores and age were not normally distributed in our study population (K-S test; p<0.05). Therefore, non-parametric Mann-Whitney (controls vs. drug users) and Kruskal-Wallis (3 genotypes: Met/Met, Met/Val, and Val/Val) tests were applied to age data and each of the AIM scores. Gender difference across gene groups and user groups was determined using a Chi-square test.
DTI alignment: We employed “unbiased” tract-based spatial statistics (TBSS) (Smith et al., 2006) with an implicit-reference based group-wise (IRG) registration method (Geng et al., 2009). FA images were first estimated by fitting the raw diffusion data to a tensor model using AFNI (Cox, 1996) and calculated according to Pierpaoli et al. (1996). Then, FA datasets were simultaneously registered onto an unbiased reference with IRG registration, a nonlinear registration procedure. Compared with conventional methods, which register images to a template reference, the IRG registration eliminates the bias associated with reference selection and produces smaller registration errors (Geng et al., 2009). The reference was then converted into standard space using an affine registration method and the transferred parameters were applied to all data sets. FSL 4.0 (Smith et al., 2004) was used to create a mean FA skeleton (Smith et al., 2006), which represents the centers of all tracts common to the group. Each participant’s aligned FA data were then projected onto this skeleton. A flowchart of this DTI data alignment process is shown in Figure 1.
Figure 1:
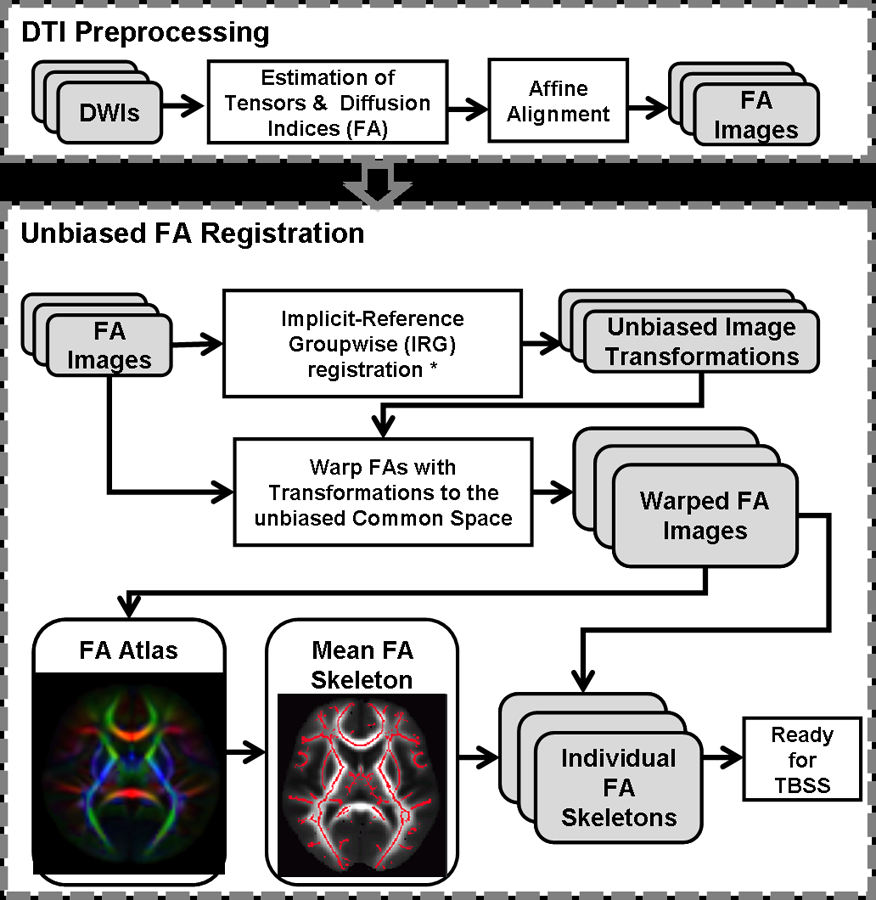
A flowchart of DTI data alignment and analysis pipeline
Whole brain analysis: Age, gender and genetic information (i.e., AIM scores) were first regressed out from the FA data using a non-parametric method (Quade, 1972). Then, a 3 (GENOTYPE) x 2 (DRUG USE GROUP) ANOVA was performed on the residuals with permutation-based testing (Nichols and Holmes, 2002). Additionally, since 120 of the 146 drug users were cigarette smokers, any association found between the COMT gene, addiction, and prefrontal WM may be driven solely by the cigarette smoking phenotype. Therefore, we also performed a separate 3 × 2 whole brain ANOVA in two sub-groups: smokers + other drug using smokers compared to controls, and nonsmoking drug users compared to controls. Whole brain comparisons between drug users and controls in each genotype group were applied to follow up on significant interactions. Significance was set to FWE corrected p < 0.05.
ROI analysis: Clusters showing a significant interaction or main effect in the total population, whole brain analysis, were set as regions of interest (ROI). Then, a Mann-Whitney U test was applied between drug users’ and controls’ data in each genotype group. Because FA in the ROIs was not normally distributed (K-S test, Z=1.421, p=0.035), a log transform was performed, resulting in FA values in both ROIs to be normally distributed (K-S test, PFC 1: Z=0.561, p=0.911; PFC 2: Z=0.909, p=0.380). A partial correlation was then performed between participant characteristics, including FTND score and total years of exposure to drug of choice, and FA. Finally, we tested whether the GENOTYPE X FTND (or total years of drug use) interaction on FA in each ROI was significant.
Results
Participants’ characteristics
The drug user group was significantly older (34.43±9.53 vs. 30.29±8.42; mean±SD than the control cohort (Z=3.672, p<0.001) and had more males (98 vs. 63) (χ2=8.210, p=0.004). However, age (χ2=0.312, p=0.855) and gender (χ2=2.215, p=0.330) did not differ across COMT genotypes. The drug user group had a higher European AIM score compared to the control group (Z=2.190, p=0.029). No other significant AIM score differences were found between drug users and controls or across the three genotypes. As such, age, gender, and seven AIM scores were used as covariates in subsequent whole brain and ROI analyses.
In our smoker sample, FTND showed a significant genotype effect (χ2=8.617, p=0.013), suggesting that FTND may be a highly heritable trait, which is consistent with previous studies (Feng et al., 2004; Kendler et al., 1999; Li, 2008; Vink et al., 2005). The Met/Met homozygotes showed significantly higher FTND scores (mean: 6.08, SD: 1.98) compared to the Met/Val heterozygotes (mean: 4.80, SD: 2.24; z=2.449, p=0.014) and Val/Val homozygotes (mean: 4.50, SD: 1.80; z=2.826, p=0.005). FTND score was not different between the Met/Val and Val/Val genotype groups (z=0.575, p=0.565). In contrast to FTND, total years of drug use did not show a significant genotype effect (χ2=0.098, p=0.952).
Whole Brain FA Analysis
Two WM regions demonstrated a significant GROUP x GENOTYPE interaction, both in the left PFC, specifically in anterior corona radiata (Figure 2A and B and Table 2). See also figure 4 showing the pattern of FA in drug users vs controls in each gene group. There were no significant WM regions showing a main effect of GENOTYPE or drug use GROUP status.
Figure 2:
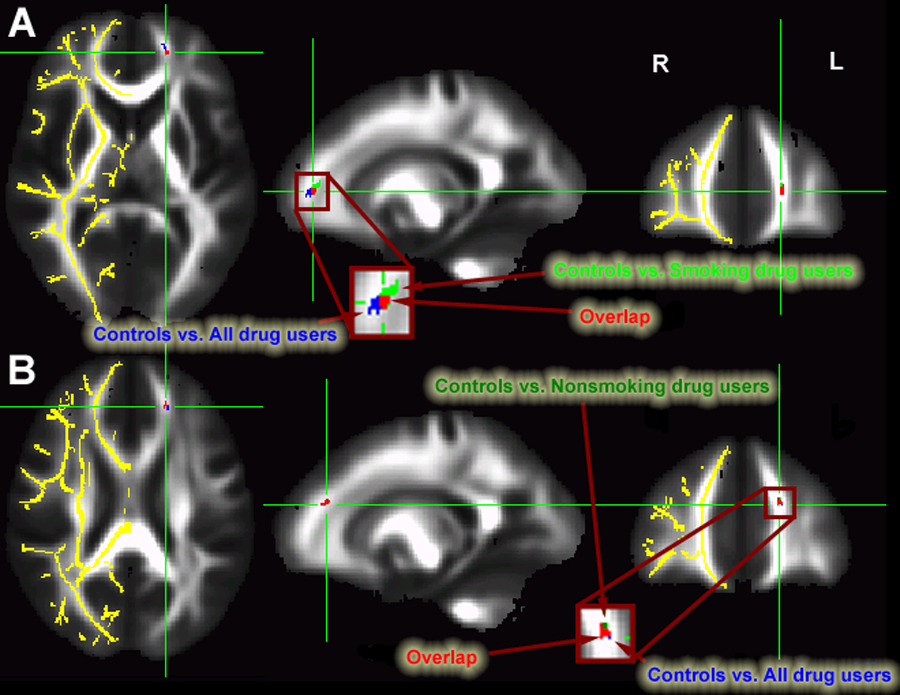
PFC regions (blue) showing significant interaction between COMT gene and addiction status A) PFC #1 showing overlap (red) between controls vs. all drug users (blue) and controls vs. smoking drug users (green); B) PFC #2 showing overlap (red) between controls vs. all drug users (blue) and controls vs. nonsmoking drug users (dark green). Localization of FA differences are projected onto a white matter skeleton (shown in YELLOW on the right hemisphere).
Table 2:
Coordinate and volume of each cluster showing significant DRUG x GENE interaction
| Volume (mm3) | FWE corrected p | MNI space * |
||||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Controls vs. all drug users | PFC 1 | 51 | 0.009 | −17.6 | 47.8 | 6.4 |
| PFC 2 | 31 | 0.036 | −17.6 | 37.8 | 23.3 | |
| Control vs. cigarette smokers | 29 | 0.043 | −17.6 | 37.7 | 23.5 | |
| Control vs. nonsmoking drug users | 29 | 0.044 | −18.3 | 44.6 | 9.1 | |
: (L-/R+, P-A+, I-S+)
Figure 4:
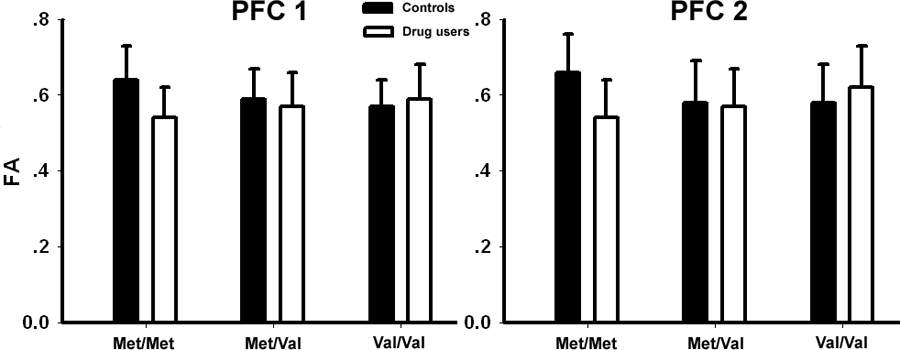
The mean FA value for each group in two prefrontal ROIs for each gene group. (Error bars show SD).
Since the majority of drug users in the cohort were cigarette smokers, we explored whether smoking alone could have driven the FA difference. As such, we performed separate whole brain analyses between controls and those drug users who smoked, and between controls and nonsmoking drug users. Intriguingly, each of the two PFC clusters identified in the above GROUP x GENOTYPE interaction were found in virtually the identical location when comparing just the smoking drug users and controls (Figure 2A) or nonsmoking drug abusers and controls (Figure 2B). The volume- corrected p value and the MNI coordinate of each WM cluster are listed in Table 2.
Post-hoc comparison between drug users and controls showed that only the Met/Met controls have significantly higher FA than the Met/Met drug users in both identified prefrontal ROIs (Figure 3). The location of these ROIs was very close to those showing significant GROUP x GENOTYPE interaction. Figure S1 illustrates several additional group differences in post-hoc comparison in the inferior and superior longitudinal fasciculus
Figure 3:
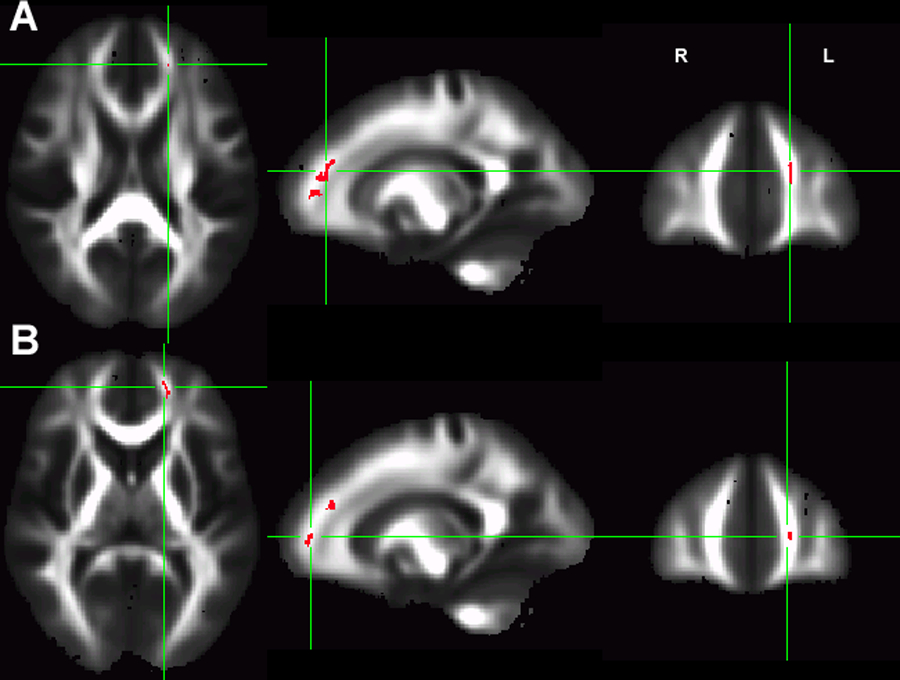
The whole brain comparison between drug users and controls in Met/Met alleles. The Met/Met drug users have significant lower FA than the Met/Met controls in two prefrontal clusters (A: volume=82mm3, MNI coordinates: L18.1, A39.7, S19.5, pcorrected=0.008; B: volume=55mm3, MNI coordinates: L17.5, A47.9, S6.7, corrected p=0.019).
ROI Analysis:
FTND score negatively predicted the two PFC FA alterations (Figure 5) such that greater nicotine addiction severity was associated with smaller FA (PFC 1: r[102]= −0.239, p=0.014; PFC 2: r[102]= −0.273, p=0.005). In contrast, total years of drug of choice exposure did not predict regional FA differences (PFC 1: r[130]=−0.123, p=0.159; PFC 2: r[130]=−0.155, p=0.076).
Figure 5:
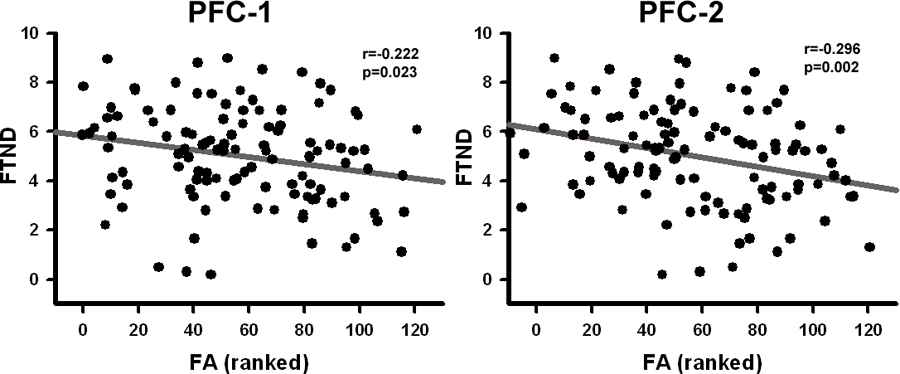
Significant correlation between FA in PFC #1 and PFC #2 clusters and FTND
Neither FTND score (PFC1: F=0.256, p=0.775; PFC2: F=1.086, p=0.342) nor total years of drug of choice exposure (PFC1: F=0.176, p=0.838; PFC2: F=1.466, p=0.235) showed a significant GENOTYPE X FTND (or total years of drug of choice exposure) interaction on FA in either ROI.
Discussion
The results of this study support a genetic vulnerability in prefrontal white matter microstructure following the environmental exposure to and addiction from chronic drug use, i.e., we found a significant interaction between GENOTYPE and DRUG USE. Only those smoking and nonsmoking drug users homozygous for the Met allele of the COMTVal158Met polymorphism had a significant regionally decreased left hemisphere prefrontal FA pathway compared to controls; there was no significant group difference in Val/Val homozygotes or Val/Met heterozygotes. Since there was no main effect of GENOTYPE, the COMT polymorphism does not appear in and of itself to engender structural changes in WM, at least those that are detectable using DTI. Rather, these results suggest that the Met allele confers susceptibility to developing WM changes within the context of drug use.
That WM structure is susceptible to a gene x environment interaction is plausible, as it has been shown that WM continues to develop and change throughout the lifespan (Thomason and Thompson, 2011). Further, regional WM changes have been previously noted in chronic drug using populations (Liu et al., 2008; Pfefferbaum et al., 2000). For example, cocaine and methamphetamine dependent individuals demonstrate altered prefrontal WM structure compared to controls (Chung et al., 2007; Lim et al., 2002). Further, both Arnone et al (2008) and our group (Zhang et al., 2011) have reported lower prefrontal FA in chronic marijuana and cigarette smokers, respectively, while Liu et al., (2008) reported decreased medial frontal FA in heroin dependence that correlated with years of drug use.
Importantly, such structural brain changes appear to reflect functional alterations. For example, reductions in frontal FA are related to impairment in decision-making and treatment outcome in cocaine dependence (Lane et al., 2010; Xu et al., 2010). Further, performance on tests of attention, working memory and visuospatial ability in alcohol dependent individuals is related to the regional microstructural integrity of the corpus callosum genu, the area where prefrontal WM tracts interconnect the hemispheres (Pfefferbaum et al., 2000).
Just as substance use has been shown to impact WM structure and cognitive function, the effect of the COMT Val158Met polymorphism itself can alter cognitive processing over the developmental trajectory, such that the working memory capacity advantage for Met allele carriers becomes evident only after age 10 (Dumontheil et al., 2011). White matter FA increases linearly over the first three decades of life then gradually declines with age, with the PFC being the latest brain region to develop (Thomason and Thompson, 2011). Given the effect of dopaminergic tone on myelination (Bongarzone et al., 1998; Karadottir and Attwell, 2007), it seems plausible that WM integrity in those with certain genetic polymorphisms, i.e. Met/Met homozygotes, may be uniquely susceptible, perhaps at certain critical points in development, to the profound changes in DA signaling that are known to occur in substance dependence (Volkow et al., 2009).
Might there be some vulnerability at the cellular level to changes in DA signaling in Met/Met homozygotes that is not apparent as a genetic effect alone, but that emerges with the onset of substance use wherein drug-induced changes in DA together with genetically-determined DAergic tone combine to perturb WM structure? Indeed, the PFC, the loci of our GENE x DRUG GROUP interaction, is where the COMT Val158Met polymorphism is thought to exert its major functional effect. Thus, it would seem plausible to find a relationship between years of exposure and reduced FA values. However, neither in this sample nor in our previous work (Zhang et al., 2011), did we find such a correlation between FA and burden of nicotine or other drug exposure. That notwithstanding, different trajectories of drug use have been identified in adolescence and these trajectories have been correlated with outcome measures like drug dependence development (Brook et al., 2011). It may be that these different trajectories during adolescence, something that our drug use questionnaires did not capture and which would be difficult to precisely quantify post hoc, rather than eventual levels of drug use at adulthood or cumulative burden of exposure, constitute a more critical environmental factor acting in concert with genetic factors to produce WM structural changes. Alternatively, the interaction of substance use with this genotype to alter WM might be sensitive to some low threshold of substance use rather than linearly affected with exposure magnitude.
The two PFC clusters showing a significant GENE x DRUG GROUP interaction appear to be regionally distinct, although both are located in the left prefrontal area. Intriguingly, the GENOTYPE x DRUG GROUP interaction using only the nonsmoking drug users and controls was very close to the first PFC region, while that between smoking drug users and controls overlapped with the second PFC ROI. That is, compared to controls, both cigarette smoking and nonsmoking drug users showed significant GENE x DRUG GROUP interaction in the left PFC, with FA in both regions negatively correlated with FTND in the smoking cohort. Of note, our previous study examining the effect of nicotine use on both gray and white matter structure (Zhang et al, 2011) also showed a negative correlation between FA and FTND in a very similar prefrontal region in a smaller cohort. In addition, similar prefrontal regional WM alterations have been reported in other addiction studies (Bell et al.; Bora et al.; Yeh et al., 2009; Zhang et al., 2011).
As noted above, previous studies have shown correlations between PFC WM alterations in addicts and addiction-related functional deficits on tasks involving executive functioning, including decision-making, memory as well as treatment outcome. Lane et al. (2010) report that compromised WM integrity in frontal and parietal regions in cocaine dependent subjects is associated with impaired performance on the Iowa Gambling Task, a task for which PFC function is critical. Yeh et al. (2009) found that lower FA in frontal and limbic fiber tracts correlated with lower visuospatial memory performance in alcoholics. Moreover, WM integrity predicts longer self-reported abstinence during treatment for cocaine dependence (Xu et al. 2010). These studies suggest frontal WM as a potential biomarker for behavioral deficits in addiction. That a genetic polymorphism interacts with drug exposure to manifest as WM structural alterations, as reported here, refines this potential intermediate phenotype. In the present study, areas of WM showing GENE x DRUG GROUP interaction (Table 2) lie in the forceps minor, a WM tract that connects left and right medial frontal gyri. Medial frontal gyrus has been shown to be activated during risky vs. safe decisions in the Iowa Gambling Task (Fukui et al., 2005). The latter has been shown to be sensitive to poor decision making among substance users (Buelow & Suhr, 2009) and these deficits in addicts are not necessarily due only to drug exposure (Bechara, 2007). That is, pre-existing factors may also contribute to abnormal behavior on this measure. Although our results do not include measures of decision making, the GENE x DRUG GROUP interaction yielding decreased FA in a WM tract connecting medial frontal cortices, an area critical for risky decision making on the Iowa Gambling Task suggests that impaired frontal communication via a WM structural abnormality might contribute to the observed deficit in addicts on this task of executive functioning. The results reported here highlight the complex interaction between drug exposure and genetic background. Some limitations of the current study merit consideration. First, only one SNP (COMT val158met) was assessed, so it is not possible to discuss the influence of other genetic factors influencing WM integrity in this population. However, our intent was not to study GENE x DRUG interactions on WM per se, but rather to use this SNP as a surrogate for the influence of drug-induced altered dopaminergic tone. A second limitation is that, in our sample, the drug user group was significantly older than the control cohort and had more males. Therefore, our prefrontal WM result may be influenced by age and/or gender difference between groups. Although we included age and gender as co-variables in the whole brain analysis, some residual influence of these factors may have remained. Likewise, there were differences in BDI and IQ scores between drug users and controls. When these were used as covariates (data available for 240 of 272 subjects), a trend (p<0.09) level was found in a cluster close to that reported above (See Fig S2), suggesting minimal effect of IQ and BDI on our main results. Nevertheless, to better address these potential limitations, a more closely matched replicate sample with more complete psychopathology and demographic information is needed.
An additional important limitation is that our sample of drug users is both ethnically diverse and abused multiple drugs. We were unable to balance specific drug use profiles across gene groups, although use of any one particular drug did not differ across the gene groups (see Table 1). However, our intent was to reflect the ‘reality on the ground’, wherein drug dependence is characterized by polydrug use within and between individuals and does not segregate by race. That said, most abused drugs, e.g. opiates, ethanol, nicotine, amphetamine and cocaine, are thought to act via a final common mechanism of increasing synaptic DA concentrations within the mesocorticolimbic system (Di Chiara and Imperato, 1988). Given this, the importance of studying the structural consequence of abusing a specific drug may not be of central importance, suggesting that studying the interaction between environmentally induced changes in dopaminergic signaling and genetically- determined prefrontal cortical dopaminergic tone is rational.
In conclusion, the GENOTYPE x DRUG USE interaction on PFC WM integrity reported herein warrants future exploration to further identify the relationship between genetic background and environmental exposure to drugs of abuse on structural and functional alterations, especially as a function of developmental trajectory, and whether genotype influences the potential reversal of some or all of these deficits during prolonged abstinence.
Supplementary Material
Acknowledgements
Supported by the Intramural Research Programs of NIDA and NIAAA. XZ is partially supported by the National Natural Science Foundation of China (31171083, 31230032), the Chinese Academy of Sciences the Hundred Talents Program (BJ2070000047), and the Fundamental Research Funds for the Central Universities of China (WK2070000007). We thank Kimberly Slater, Loretta Spurgeon and Catherine Demers for their assistance conducting this study.
Reference List
- Arnone D, Barrick TR, Chengappa S, Mackay CE, Clark CA, Abou-Saleh MT, 2008. Corpus callosum damage in heavy marijuana use: preliminary evidence from diffusion tensor tractography and tract-based spatial statistics. Neuroimage 41, 1067–1074. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, Dant CC, Reiss AL, 2005. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex 15, 1848–1854. [DOI] [PubMed] [Google Scholar]
- Basser PJ, 1995. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 8, 333–344. [DOI] [PubMed] [Google Scholar]
- Bechara A, 2007. Iowa Gambling Task Professional Manual Lutz: Psychological Asessment Resources. [Google Scholar]
- Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H, Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol Depend 114, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhide PG, 2009. Dopamine, cocaine and the development of cerebral cortical cytoarchitecture: a review of current concepts. Semin Cell Dev Biol 20, 395–402. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Lachman HM, Grace AA, 2004. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 29, 1943–1961. [DOI] [PubMed] [Google Scholar]
- Bongarzone ER, Howard SG, Schonmann V, Campagnoni AT, 1998. Identification of the dopamine D3 receptor in oligodendrocyte precursors: potential role in regulating differentiation and myelin formation. J Neurosci 18, 5344–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yucel M, Fornito A, Pantelis C, Harrison BJ, Cocchi L, Pell G, Lubman DI, White matter microstructure in opiate addiction. Addict Biol 17, 141–148. [DOI] [PubMed] [Google Scholar]
- Brook JS, Balka E, Zhang C, Pahl K, Brook DW, 2011. Adolescent Academic Adjustment Factors and the Trajectories of Cigarette Smoking from Adolescence to the Mid-thirties. Int J Ment Health 40, 7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buelow MT & Suhr JA, 2009. Construct validity of the iowa gambling task. Neuropsychological Reviews 19, 102–14. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR, 2004. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75, 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung A, Lyoo IK, Kim SJ, Hwang J, Bae SC, Sung YH, Sim ME, Song IC, Kim J, Chang KH, Renshaw PF, 2007. Decreased frontal white-matter integrity in abstinent methamphetamine abusers. Int J Neuropsychopharmacol 10, 765–775. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Sullivan EV, Ford JM, Mathalon DH, McPherson SL, Roach BJ, Crowley KE, Pfefferbaum A, 2011. Frontally mediated inhibitory processing and white matter microstructure: age and alcoholism effects. Psychopharmacology (Berl) 213, 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Roggeman C, Ziermans T, Peyrard-Janvid M, Matsson H, Kere J, Klingberg T, 2011. Influence of the COMT genotype on working memory and brain activity changes during development. Biol Psychiatry 70, 222–229. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Hasan KM, Kramer L, Fletcher JM, Ewing-Cobbs L, 2007. Quantitative diffusion tensor tractography of association and projection fibers in normally developing children and adolescents. Cereb Cortex 17, 2760–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, Eberhardt A, Klager M, Smolka MN, Scheurich A, Dielentheis T, Schmidt LG, Rosch F, Bartenstein P, Grunder G, Schreckenberger M, 2008. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry 165, 507–514. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ, 2002. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend 68, 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Niu T, Xing H, Xu X, Chen C, Peng S, Wang L, Laird N, 2004. A common haplotype of the nicotine acetylcholine receptor alpha 4 subunit gene is associated with vulnerability to nicotine addiction in men. Am J Hum Genet 75, 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR, 2002. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry 51, 134–142. [DOI] [PubMed] [Google Scholar]
- Fukui H, Murai T, Fukuyama H, Hayashi T, Hanakawa T, 2005. Functional activity treated torisk anticipation during performance fo the iowa gambling task. NeuroImage 24, 253–259. [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM, 1994. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci 14, 442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Christensen GE, Gu H, Ross TJ, Yang Y, 2009. Implicit reference-based group-wise image registration and its application to structural and functional MRI. Neuroimage 47, 1341–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, Shen PH, Heinz E, Lobos EA, Binder EB, Cubells J, Ehlers CL, Gelernter J, Mann J, Riley B, Roy A, Tabakoff B, Todd RD, Zhou Z, Goldman D, 2008. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol 43, 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadottir R, Attwell D, 2007. Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience 145, 1426–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA, 1999. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med 29, 299–308. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM, 1996. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 6, 243–250. [DOI] [PubMed] [Google Scholar]
- Lane SD, Steinberg JL, Ma L, Hasan KM, Kramer LA, Zuniga EA, Narayana PA, Moeller FG, 2010. Diffusion tensor imaging and decision making in cocaine dependence. PLoS One 5, e11591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C, 2008. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40, 1044–1055. [DOI] [PubMed] [Google Scholar]
- Li MD, 2008. Identifying susceptibility loci for nicotine dependence: 2008 update based on recent genome-wide linkage analyses. Hum Genet 123, 119–131. [DOI] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP, 2002. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry 51, 890–895. [DOI] [PubMed] [Google Scholar]
- Liu H, Li L, Hao Y, Cao D, Xu L, Rohrbaugh R, Xue Z, Hao W, Shan B, Liu Z, 2008. Disrupted white matter integrity in heroin dependence: a controlled study utilizing diffusion tensor imaging. Am J Drug Alcohol Abuse 34, 562–575. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J, 1995. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 34, 4202–4210. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI, 2003. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage 19, 1095–1102. [DOI] [PubMed] [Google Scholar]
- McGraw P, Liang L, Provenzale JM, 2002. Evaluation of normal age-related changes in anisotropy during infancy and childhood as shown by diffusion tensor imaging. AJR Am J Roentgenol 179, 1515–1522. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J, 2006. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51, 527–539. [DOI] [PubMed] [Google Scholar]
- Morriss MC, Zimmerman RA, Bilaniuk LT, Hunter JV, Haselgrove JC, 1999. Changes in brain water diffusion during childhood. Neuroradiology 41, 929–934. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG, Keefe R, Gil R, Martinez D, Slifstein M, Kegeles LS, Talbot PS, Huang Y, Hwang DR, Khenissi L, Cooper TB, Laruelle M, Abi-Dargham A, 2005. Altered prefrontal dopaminergic function in chronic recreational ketamine users. Am J Psychiatry 162, 2352–2359. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP, 2002. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M, 2000. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res 24, 1214–1221. [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G, 1996. Diffusion tensor MR imaging of the human brain. Radiology 201, 637–648. [DOI] [PubMed] [Google Scholar]
- Quade D, 1972. Nonparametric partial correlation Amsterdam. [Google Scholar]
- Sakuma H, Nomura Y, Takeda K, Tagami T, Nakagawa T, Tamagawa Y, Ishii Y, Tsukamoto T, 1991. Adult and neonatal human brain: diffusional anisotropy and myelination with diffusion-weighted MR imaging. Radiology 180, 229–233. [DOI] [PubMed] [Google Scholar]
- Schneider JF, Il’yasov KA, Hennig J, Martin E, 2004. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology 46, 258–266. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE, 2006. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 Suppl 1, S208–219. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C, 2005. Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage 26, 1164–1173. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Dougherty RF, Colich NL, Perry LM, Rykhlevskaia EI, Louro HM, Hallmayer JF, Waugh CE, Bammer R, Glover GH, Gotlib IH, 2010. COMT genotype affects prefrontal white matter pathways in children and adolescents. Neuroimage 53, 926–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason ME, Thompson PM, 2011. Diffusion imaging, white matter, and psychopathology. Annu Rev Clin Psychol 7, 63–85. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ, 2004. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci 24, 5331–5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink JM, Willemsen G, Boomsma DI, 2005. Heritability of smoking initiation and nicotine dependence. Behav Genet 35, 397–406. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N, 2001. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158, 2015–2021. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F, 2009. Imaging dopamine’s role in drug abuse and addiction. Neuropharmacology 56 Suppl 1, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P, 2005. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci 25, 3932–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, 2007. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci 27, 12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, Wong CT, Hoffman W, Jayne M, Alia-Klein N, Thanos P, Fowler JS, Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry 17, 918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN, 2010. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology 35, 1541–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh PH, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ, 2009. Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res 173, 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA, 2011. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage 54, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


