Abstract
Alcohol-associated liver disease (ALD) is a major driver of global liver related morbidity and mortality. There are 2.4 billion annual drinkers (950 million heavy drinkers) and the lifetime prevalence of any alcohol use disorder (AUD) is 5.1%-8.6%. In 2017, global prevalence of alcohol-associated compensated and decompensated cirrhosis was 23.6 million and 2.5 million. Combined, alcohol-associated cirrhosis and liver cancer account for 1% of all deaths worldwide with this burden expected to increase. Solutions for this growing epidemic must be multi-faceted and focused on both population and patient-level interventions. Reductions in ALD-related morbidity and mortality require solutions that focus on early identification and intervention, reducing alcohol consumption at the population level (taxation, reduced availability and restricted promotion), and solutions tailored to local socioeconomic realities (unrecorded alcohol consumption, focused youth education). Simple screening tools and algorithms can be applied at the population level to identify alcohol misuse, diagnose ALD using non-invasive serum and imaging markers, and risk-stratify higher-risk ALD/AUD patients. Novel methods of healthcare delivery and platforms are needed (telehealth, outreach, use of nonhealthcare providers, partnerships between primary and specialty care/tertiary hospitals) to proactively mitigate the global burden of ALD. An integrated approach that combines medical and AUD treatment is needed at the individual level to have the highest impact. Future needs include (1) improving quality of ALD data and standardizing care, (2) supporting innovative healthcare delivery platforms that can treat both ALD and AUD, (3) stronger and concerted advocacy by professional hepatology organizations, and (4) advancing implementation of digital interventions.
Keywords: morbidity, mortality, alcoholic cirrhosis, alcoholic hepatitis, epidemiology
Introduction
Alcohol-associated liver disease (ALD) is a major driver of global liver related morbidity and mortality. Around the world, in 2016, alcohol use was associated with 3 million deaths (5.3% of all deaths) surpassing hypertension and diabetes combined.(1, 2) Specifically, alcohol-attributable liver cirrhosis causes approximately 550,000-610,000 deaths and 22.2 million disability-adjusted life years and together with rising rates of liver cancer, caused 1% of worldwide mortality (2, 3). The purpose of our review is three-fold. First, we briefly review the global burden of ALD and place it in context of relevant changes anticipated to drive future trends. Second, we discuss population level screening for ALD and AUD for early recognition and management. Finally, we discuss strategies for mitigating the impact of ALD at the global level and offer focused solutions for delivering healthcare services at the regional and individual level.
A. Current and future burden of ALD
Alcohol use and misuse:
In 2016, 2.4 billion people consumed alcohol (1.5 billion male and 0.9 billion female) and approximately, 39.5% of these were heavy episodic drinkers (1, 2). Globally, per capita alcohol use rose from 5.7 L to 6.4 L over a 15-year period (2000-2016) (1, 4). Though the overall prevalence of heavy episodic drinking decreased, it rose in established drinkers, indicating that those who drink already are drinking more and more heavily than ever before (1). Drinking among youth is also increasing (23.5% among age 15-19 years) with higher rates of heavy episodic drinking (46%). The prevalence of current drinkers is lower among women than men across most regions except for South-East Asia and Western Pacific region. However, the absolute number of currently drinking women and amount of alcohol consumption has increased globally (1, 2).
The most severe form of alcohol misuse, alcohol use disorder (AUD), is characterized by the accumulation of multiple symptoms of alcohol use: increasing use of alcohol despite negative consequences and persistent, unsuccessful, attempts to stop (5). Globally, the diagnosis of AUD is highly prevalent. The lifetime prevalence of any AUD was 8.6% overall, ranging from a low of 0.7% in Iraq to a high of 22.7% in Australia (6). AUD symptoms persisted in 21-37% of those who reported a history of AUD in the pas.(6). Importantly, comorbid mental health disorders were frequently present alongside AUDs and often preceded the onset of alcohol use (7).
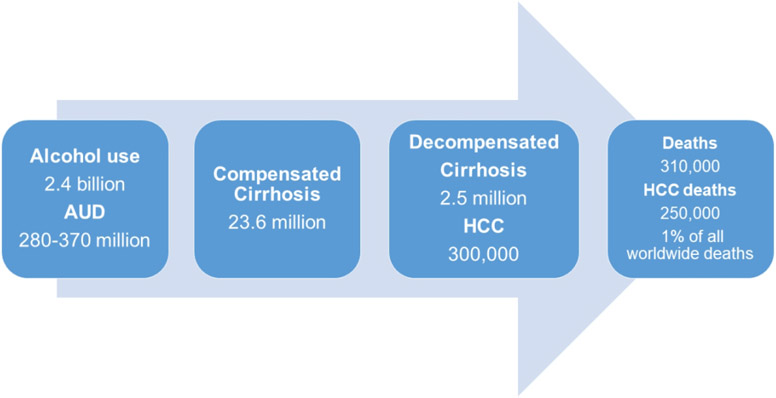
Alcohol-associated liver disease (Figure 1)
Figure 1:
Global burden of alcohol associated liver disease.
Recently commissioned works describe country and region-specific burden of liver disease in Europe (Hepahealth) as well as in Asia and Pacific regions (8-10).
Prevalence of Cirrhosis and hepatocellular carcinoma (HCC) (4):
Alcohol is responsible for about 21% of prevalent compensated cirrhosis cases. The global prevalence of alcohol associated compensated cirrhosis remained stable, 13.5 million (290/100,000 in 1990) to 23.6 million (288/100,000 in 2017) but decompensated cirrhosis increased from 1.1 million (25/100,000 in 1990) to 2.5 million (30 per 100,000 in 2017).(4) Regionally, the highest proportions are seen in Western Europe (42-43%) and Central Europe (43-46%). Alcohol was responsible for approximately 30% of HCC cases (11).
Mortality:
Cirrhosis due to alcohol use is responsible for 25% of all cirrhosis deaths (308K, 0.5% of total deaths worldwide) and ALD-cancer related deaths account for 30% of all liver cancer deaths (250K, 0.4% of deaths worldwide). Combined, alcohol related cirrhosis and liver cancer account for 1% of all deaths worldwide, but may be underestimated. As expected, the highest proportions were seen in central Europe (44.0%), Western Europe (41.7%) and Andean Latin America (38.1%). However, by absolute numbers, 45% of all ALD-related deaths occurred in 5 countries (India, US, Mexico, China and Russia) and 21.6% of all ALD deaths worldwide occurred in India (1, 4). Liver related deaths in the Asia-Pacific region accounted for under half of global ALD-related deaths (8).
The burden of ALD is expected to increase (12). There are several measured and unmeasured factors driving this change, specifically, changes in drinking patterns, socioeconomic factors as well as relevant comorbidities such as obesity may impact the future burden of ALD.
B. Screening and early diagnosis at the population level: A Blueprint for Early Action
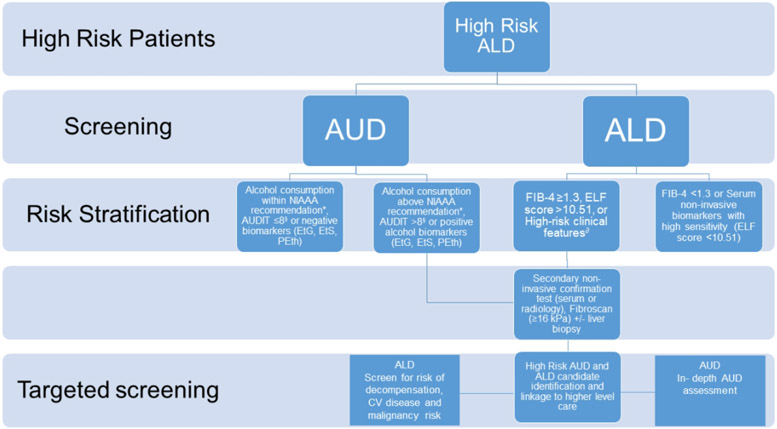
Screening at the population level requires a practical approach (Figure 2) as well as efficient care delivery methods that make use of appropriate triage of ALD and AUD patients (Figure 3). There are several guiding principles. First, early detection and screening for AUD and ALD is key for mitigating future burden. ALD is often detected at a late stage when patients are more likely to present with decompensated cirrhosis (13). Consequently, there is an unmet need for effective and economically reasonable pathways to screen for AUD and ALD-fibrosis before patients develop endstage liver disease. Second, easy to use tools for ALD and AUD screening and linkage to treatment are needed. Alcohol misuse needs to be widely screened for and linked directly to effective referrals to alcohol treatment personnel or pathways. Unfortunately, AUD treatment is limited by a shortage of providers, poor reimbursement, as well as patient attitudinal barriers that must be overcome. Third, technology should be leveraged to effectively screen at the population level. Currently, most alcohol screening is linked to healthcare environments, potentially leaving those who don’t interact with their health-care system undiagnosed. Novel “screening extenders” (smartphone apps and other mobile health innovations) may be one approach to achieve greater population-level screening. Smartphone apps to assess alcohol use are abundant, but evidence for their efficacy in the general population is varied and while many apps are targets to college-aged or younger adults, few, if any, have been tested in an ALD population (14). In resource rich areas, the use of electronic medical records (EMR) may be able to assist in screening, risk-stratifying and then triaging patients to a care pathway through the creation of automated early warning systems for liver disease. (15). Using feedback on the severity of alcohol-related liver damage coupled with AUDIT scores produced decreased drinking in a British primary care population (15, 16). Fourth, novel settings for screening need to be considered. Though screening may be easily accomplished if related to a healthcare visit (e.g. “for cause” consultation for elevated liver enzymes), unrelated screening opportunities need to be explored. For example, offering screening for alcohol misuse to all medical admissions to hospital with automatic referral to treatment services for those at the highest risk of dependency and risk for alcohol associated liver disease is feasible (15). Novel settings for alcohol use and ALD screening outside healthcare facilities should be pursued. Screening for hypertension at local barbershops frequented by African-American men was an effective screening approach (17). Finally, screening approaches need to be tailored to different regions of the world, especially in underserved areas. Smartphone technology is not simply an urban phenomenon; its penetration in rural areas is high and may be a potential medium for screening and intervention. Acceptance of smartphone and telehealth technology by cirrhosis patients is high, leaving a potential untapped area for intervention (18). Screening approaches that leverage risk of liver disease and participants concern for their liver health may have more success than generic screening approaches (16). Barriers to screening include social stigma, access to care, access to cost-effective screening tools, and attitudinal barriers (denial of alcohol use problems, lack of perceived need to alcohol use treatment).
Figure 2: Screening and risk stratification for alcohol associated liver disease.
*Daily and weekly doses within recommended standards of the National Institute of Alcohol Abuse and Alcoholism [NIAAA]: ≤4 drinks per day for men; $3 drinks per day for women; and ≤14 drinks per week for men; and ≤7 drinks per week for women.
§AUDIT-c (a shorter version of AUDIT) is considered positive screening with a result ≥3 for women and ≥4 for men.
∂High risk clinical features: aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio >2, elevated GGT, thrombocytopenia, jaundice, stigmas of advanced liver disease.
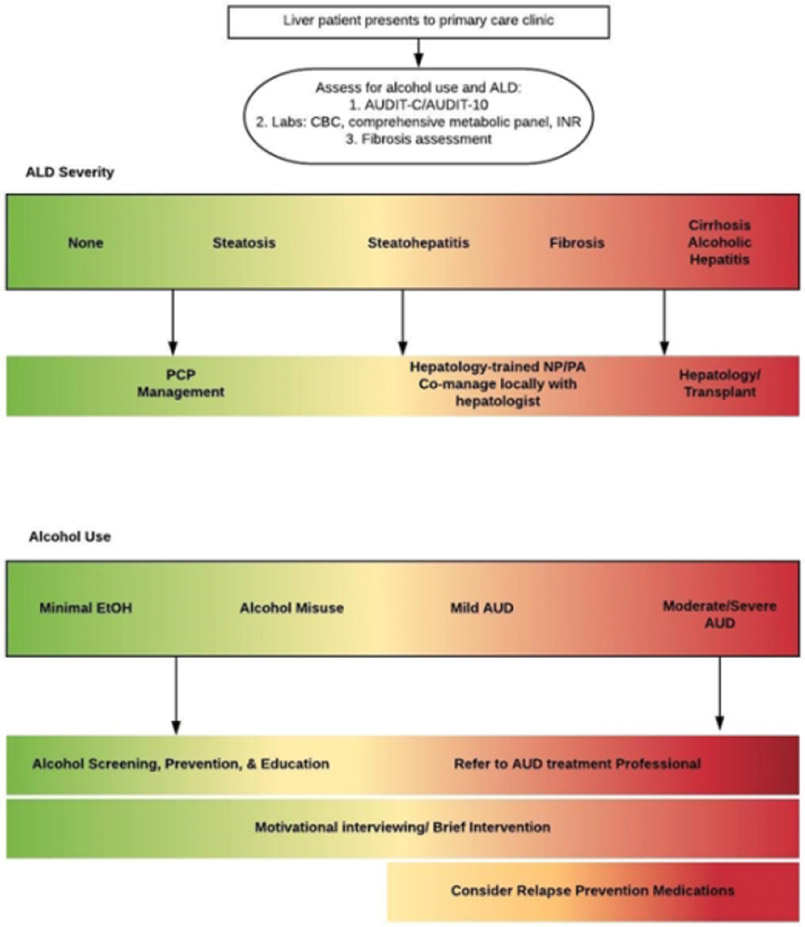
Figure 3:
Treatment paradigm for alcohol associated liver disease and alcohol use disorder
C. Diagnosing AUD: Building on the Screening Blueprint
The diagnosis of AUD is best made with an individual interview. Though adapting this at a population level is difficult, screening tests may identify the high-risk patient who would benefit from more in-depth questioning. A combination of consumption patterns, validated questionnaires for screening, and alcohol biomarkers may help providers know when to refer to an alcohol treatment professional for a more thorough diagnosis of a potential AUD and any underlying mental health disorder or comorbid substance use disorder. The Alcohol Use Disorders Identification Test (AUDIT) can differentiate between more severe alcohol dependence (mod/severe AUD) and less risky drinking. (19). A shorter version, AUDIT-C has 73% sensitivity and 91% specificity for AUD and 85% sensitivity and 89% specificity for detecting alcohol dependence (20). In addition, it has been adapted in regional languages and validated in several patient populations (21). Biomarkers may increase sensitivity for detection of alcohol use beyond self-report methods (22).
D. Diagnosis of liver disease: Expanding the Blueprint
Population level screening with a combination of non-invasive markers may help identify patients at highest risk (23). Patented and non-patented serum markers for non-invasive liver disease assessment (NILDA) may play a role in early diagnosis. As an example, the Enhanced Liver Fibrosis (ELF) score is predictive of clinical outcomes including liver-related mortality and may be useful as a screening test within primary care (24, 25). FIB-4, a widely used non patented index (age, aminotransferases and platelet count) may have a lower accuracy than patented markers but could have broad applications for screening at population level across different socio-demographic index (SDI) regions (26, 27).Other markers such as Fibrotest, APRI and Hepamet may also play a role (27).
Besides serum biomarkers, liver stiffness measurement (LSM) by transient elastography has good diagnostic performance to exclude significant fibrosis or cirrhosis in patients first assessed for ALD across Asian and European populations (26). Cutoffs values for presence of cirrhosis in ALD may be higher compared to other diseases. These tools may be more helpful at the population level to rule out liver disease (higher negative predictive value). Due to availability, radiology-based screening may be second-line for those at intermediate or high risk for ALD. Noninvasive screening for advanced alcohol-related fibrosis is a cost-effective intervention when a positive screening tool triggers the appropriate referral to more specialized care (MR elastography, hepatologists, transplant centers), and tailored to the prevalence of advanced fibrosis (25).
E. Delivering targeted interventions: Broadening the Blueprint to Policy and Populations
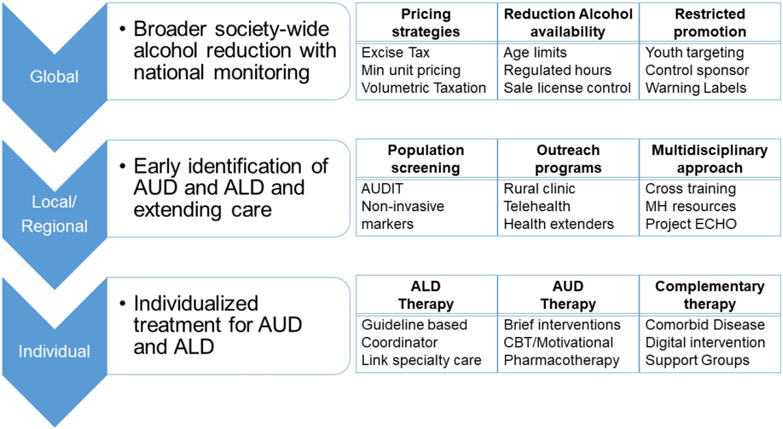
Though treatment needs to be focused on the individual, a combination of global and regional interventions is needed to decrease the number of potential people at risk (28) (Figure 4). Three broad goals are 1) society wide alcohol reduction (global solutions), 2) early identification of AUD and ALD (regional and local level), and 3) personalized treatment for AUD and ALD (individual level).
Figure 4:
Suggested strategies to mitigate impact of alcohol associated liver disease at the global, local/regional and individual level
Global solutions:
The World Health Organization (WHO) has led a number of initiatives at a global level to decrease the impact of alcohol (29). Of these, three are highlighted as “best buys” and include taxation, reduced availability, and restricted promotion to reduce per capital alcohol consumption.
1. Pricing strategies:
The price of alcohol purchase is the single strongest driver of alcohol consumption at a per capita population level. Strategies include a national alcohol policy, price regulation (minimum unit pricing of alcohol or a set price below which alcohol beverages cannot be sold), and taxation of alcohol beverages. Though most countries have alcohol excise taxes, less than half use alternate strategies such as adjusting taxes to keep up with inflation and income levels, imposing minimum pricing policies, volumetric taxes or banning below-cost selling or volume discounts (29). When pricing rises, alcohol consumption and alcohol related liver disease burden decreases and these gains are often felt most in those with the highest amount of alcohol use and in the lower socioeconomic status groups (30-32). Impact of pricing policies may however be variable, especially for low income moderate drinkers compared with high income heavy drinkers who can absorb pricing changes. Conversely, when prices are dropped through reduced taxation and other mechanisms, alcohol consumption and alcohol-related death rates increase (8-10). Blunting the impact of unrecorded alcohol use is another concern. In lower SDI countries, alcohol products may be homebrewed in an unregulated manner, leaving a large percentage of alcohol use unmeasured and “unseen”. This results in an inability to regulate by government measures and adds another dimension of health toxicity from products used in home-brewing that may have adverse health consequences (33). This translates into lower SDI regions having higher mortality for alcohol-attributable causes, despite reporting lower levels of recorded consumption (34). In Central America, for example, where homemade alcohol consumption is high, pricing-policies may not have the desired effect.
2. Reduction in availability of alcohol:
In addition to direct taxation, regulation of days and hours of alcohol sales, and control over the sale of liquor licenses and constraints on alcohol sales outlets may help (35-37). There is benefit in establishing a minimum legal purchasing age, but age restrictions vary globally and policy impact likewise varies (38). The impact of establishing a minimum legal drinking age on alcohol-related chronic disease mortality may be more pronounced in those that do not attend college (39). Although educational initiatives have been viewed as less effective in influencing per capita alcohol consumption at a national level compared to policies that regulate price of sale, there are some notable exceptions: Iceland was able to reduce its alcohol and drug use in younger individuals from 42% to 5% over a period of two decades with the implementation of several policy principles. This included (a) bringing in a curfew beyond which youth needed to be off public streets, (b) parents signing a pledge that they will not allow their teens to drink alcohol, (c) a purposeful increase in family time at night, (d) increasing after school activities including voucher programs to incentivize, (e) survey-based measurements and research aim to lower teen alcohol and illicit substance consumption, and (f) engaging politicians to assist in policy development and implementation to support these initiatives (40). The Icelandic experience shows that effective alcohol policy at a population level will likely need to be targeted to specific populations (in this case, youth) and involve a multi-faceted approach to achieve success.
3. Restricted promotion:
A focus on protecting children from alcohol marketing as well as targeting clinicians is advised (41). Government policies should regulate marketing promotions at the level of sports, standardize alcohol warning label messaging, and limit advertising targeting vulnerable populations, especially young individuals and, more recently, women (42, 43). Controls need to be placed on content and volume of marketing, decrease connections with youth related activities, limit use of digital media and increase oversight by public health at the country level (44, 45). Challenges to these government interventions exist including lobbying initiatives from contrarian groups (46).
Regional and local solutions: Changing the Healthcare Delivery Blueprint Through Digital Solutions
Innovative models of health care delivery that leverage novel telehealth initiatives (provider to patient and provider to provider) are needed in order expand the reach for screening and interventions at the state or health system level.
1. Identifying high risk populations:
Based on resource availability, infrastructure needs to be in place for implementing screening and linkage to treatment for AUD and ALD (Figure 3). Such models will need to be adapted to local circumstances and may include use of regional data to identify areas of highest risk within a system’s reach (e.g. use of hospitalization data for referral patterns or alcohol sales pattern in resource poor countries), and electronic medical records in resource rich countries for implementing screening. There has been some success at identifying cirrhosis patients in an electronic medical record for population health management using natural language processing or algorithm based identification of target population (47).
2. Linkage to treatment:
Critical to improving care for ALD patients is “knowledge extension”. Expanding the expertise of providers without having more providers will require digital solutions. Appropriate referral pathways tailored to regional and local characteristics are important for efficient and appropriate use of existing provider networks (48). For large health care systems, this may include development of networks of outreach clinics, with some areas able to implement integrated multidisciplinary ALD clinics incorporating both mental health and hepatology providers in caring for more advanced ALD patients (49). The establishment of acute liver services in district general hospitals linked with regional specialist centers for more complex investigations and treatment has been implemented successfully in the United Kingdom (50). Moreover, even advanced care (such as upper GI endoscopy, elastography or imaging) can be brought to the high-risk regions by coordinated outreach activities. For example, in India, the Rural Health Care Project, a network of modified buses with self-sufficient water and electric supply serve as mobile hospitals with diagnostic capabilities (inperson visits, testing, and procedures) and telemedicine capacities to provide free local healthcare to underserved populations (51). Other options are the implementation of provider-to-provider teleconsultation models, which allow access to specialist evaluation or co-management with other trained providers in the community (the Extension for Community Healthcare Outcomes (ECHO) model, which has been successfully used for substance use treatment as well as liver disease care (52, 53). Use of non-specialist informal health worker extenders in the community has also met with success (54).
3. Innovative clinics:
At the clinic level, cross training gastroenterology and hepatology to provide substance abuse care, primary care to assist with risk stratification of ALD and AUD, combined with telemedicine to deliver care across a wide geographic area and interface with networks of multidisciplinary providers may help extend the reach of ALD care through efficient use of existing resources (55). Dedicated multidisciplinary clinics may help to treat ALD and AUD given that integrated therapy with cognitive behavioral therapy and medical care increases abstinence (56). In the future, digital interventions via smartphone for reducing hazardous and harmful alcohol consumption, alcohol-related problems, or both, for people living in the community may be helpful (57). Slips and relapses to alcohol use occur outside of clinic and, as such, the time for intervention prior to these events frequently goes unnoticed. Mobile health interventions, particularly if they have a component of “just-in-time” access to alcohol use interventions during times of crisis, may be well-suited to this population. Though not developed specifically for ALD patients, there are many proprietary web and smartphone applications that assist in alcohol use treatment and recovery and have an evidence base to support their effectiveness which could be applied to ALD patients with AUD (58).
Deliver targeted interventions: A Blueprint for Individualized Patient-level Care
Treatment of ALD:
Recent guidelines (34, 59, 60) provide further recommendations for clinical management and will not be reviewed here.
Treatment of AUD:
Access to robust mental health care remains low for substance use disorders (11%) and AUD treatment (10%), with only 0.4% receiving any Food and Drug Administration (FDA) approved relapse prevention medication (61, 62). Barriers vary from attitudinal barriers (not feeling like they need treatment), to logistical barriers (lack of insurance coverage, distance from available AUD treatment) to concerns about anonymity and dislike of available AUD treatment modalities (63). AUD treatment is effective and reduces the risk of hepatic decompensation by 15% (62). Where possible, hepatologists should liaise with their local mental health and substance use providers to develop multidisciplinary clinics to treat advanced ALD patients, many of whom have moderate to severe AUD and comorbid mental health and substance use disorders. Integrated care, with mental health providers in the clinic, produced improved rates of alcohol abstinence, with cognitive behavior therapy and motivational enhancement therapy modalities providing benefit (56). Motivational interviewing is an evidence-based approach to assist ambivalent patients in moving towards changing unhealthy habits (64). Targeted, liver-focused feedback, where alcohol use and cessation is linked directly to changes in liver function and risk of developing worsening liver disease and coupled with motivational interviewing, can help patients decrease alcohol use in a primary care setting (16). Though Alcoholics Anonymous is a popular, free, and easily accessible means of support for alcohol cessation, many ALD patients dislike this modality (63). Clinic assessments of alcohol treatment preference, such as for group therapies versus one-on-one treatment, longer or shorter duration of treatment, or involvement of family members, may help give patients more agency in their choice of alcohol treatment and improve the likelihood of attendance. Treatment locators, such as the NIAAA Treatment navigator (https://alcoholtreatment.niaaa.nih.gov) and the SAMHSA Treatment Locator (www.findtreatment.samhsa.gov), can help patients and clinicians educate themselves about treatment options and find nearby substance use treatment resources. Likewise, regional resources need to be identified and highlighted in a synchronized manner. The use of relapse prevention medications is recommended by society guidelines (60, 65).
Conclusion and future directions
Future needs include the following:
1. Improving quality of ALD data and standardizing care:
Certain countries may have low rates of reporting potentially related to social stigma, low availability and quality of data, and poorly standardized definitions and diagnostic criteria. Standard drink sizes and recommendations differ worldwide from 8 grams of alcohol (United Kingdom), to 14 grams (United States), to 20 grams (Japan). A concerted effort is needed across regions to standardize definitions of alcohol use and alcohol content to facilitate generalizability of interventions and ease international research in alcohol use and ALD.
2. Support innovative healthcare delivery platforms that can treat both ALD and AUD.
At the present time, much of the focus is on boots on the ground interventions from impassioned mission-oriented physicians and other health care providers who decide to participate and contribute to care in these regions. There needs to be concerted effort to cross train local providers and non-medical extenders (see #4 below). In the future, populations lacking access to care may benefit from interventions which incorporate digital technologies that allow biomonitoring and connected care.
3. Stronger advocacy by liver societies:
Engagement and a stronger position taken by hepatology professional societies to eradicate infectious liver diseases such as Hepatitis C and Hepatitis B, including vaccination strategies, screening for infection and treatment has led to several benefits. It will be critical to begin to adapt some of the infrastructures used for these initiatives towards global alcohol consumption, although the tactics will vary given the distinctively different nature of alcohol as a precipitant of liver disease compared to viral etiologies. In this regard, the World Health Organization, non-governmental organizations and societies have provided varying levels of engagement for the global problem.
4. Advancing Implementation of digital Interventions:
Workforce limitations exist for both liver and substance use providers, and these limitations in numbers are unlikely to change rapidly. Implementing digital interventions to increase screening in healthcare systems, linkage to alcohol use and hepatology care (through e-consultations, ECHO-like models, remote monitoring) and digital interventions to decrease alcohol use are critical to improving care for ALD patients.
Given the increasing burden of ALD and AUD worldwide, strategies to screen and provide care must be implemented at multiple levels and will require robust communication and coordination at all levels. With efficient, smart targeting of tailored local solutions and broader adoption of society-level solutions through improved alcohol policies, the tide of ALD and AUD may begin to turn.
Funding:
SA: Baylor Foundation Grant (SA); JPA: Chilean government through the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1200227) and the Comisión Nacional de Investigación Científica y Tecnológica (grant CONICYT PIA/Basal PFB12, Basal Centre for Excellence in Science and Technology); JM: NIAAA K23 Career Development Award (K23 026333)
Authors had access to all the study data, take responsibility for the accuracy of the analysis, and had authority over manuscript preparation and the decision to submit the manuscript for publication. All authors approve the manuscript.
Abbreviations:
- ALD
alcohol associated liver disease
- AUD
alcohol use disorder
Footnotes
Disclosure: No relevant financial disclosures
Conflicts of interest: No personal or financial conflict of interest
REFERENCES
- 1.Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018;392:1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization WH. Global Report on Alcohol and Related Conditions. 2018.
- 3.World Health Organization. Global Status Report on Alcohol and Health. Geneva, Switzerland: World Health Organization.[cited in September 7, 2019]. Available in: http://www.who.int/substance_abuse/publications/global_alcohol_report/en/ 2018. [Google Scholar]
- 4.Collaborators GBDC. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Psychiatric Association., American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders : DSM-5. 5th ed. Arlington, VA: American Psychiatric Association, 2013: xliv, 947 p. [Google Scholar]
- 6.Glantz MD, Bharat C, Degenhardt L, Sampson NA, Scott KM, Lim CCW, Al-Hamzawi A, et al. The epidemiology of alcohol use disorders cross-nationally: Findings from the World Mental Health Surveys. Addict Behav 2020;102:106128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, et al. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 2015;72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarin SK, Kumar M, Eslam M, George J, Al Mahtab M, Akbar SMF, Jia J, et al. Liver diseases in the Asia-Pacific region: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol 2020;5:167–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon GR, Singh L, Sharma P, Yadav P, Sharma S, Kalaskar S, Singh H, et al. National Burden Estimates of healthy life lost in India, 2017: an analysis using direct mortality data and indirect disability data. Lancet Glob Health 2019;7:e1675–e1684. [DOI] [PubMed] [Google Scholar]
- 10.Pimpin L, Cortez-Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, Sheron N, et al. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J Hepatol 2018;69:718–735. [DOI] [PubMed] [Google Scholar]
- 11.Global Burden of Disease Liver Cancer C, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol 2017;3:1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guirguis J, Chhatwal J, Dasarathy J, Rivas J, McMichael D, Nagy LE, McCullough AJ, et al. Clinical impact of alcohol-related cirrhosis in the next decade: estimates based on current epidemiological trends in the United States. Alcohol Clin Exp Res 2015;39:2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellinger JL, Shedden K, Winder GS, Tapper E, Adams M, Fontana RJ, Volk ML, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology 2018;68:872–882. [DOI] [PubMed] [Google Scholar]
- 14.Colbert S, Thornton L, Richmond R. Smartphone apps for managing alcohol consumption: a literature review. Addict Sci Clin Pract 2020;15:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westwood G, Meredith P, Atkins S, Greengross P, Schmidt PE, Aspinall RJ. Universal screening for alcohol misuse in acute medical admissions is feasible and identifies patients at high risk of liver disease. J Hepatol 2017;67:559–567. [DOI] [PubMed] [Google Scholar]
- 16.Sheron N, Moore M, O'Brien W, Harris S, Roderick P. Feasibility of detection and intervention for alcohol-related liver disease in the community: the Alcohol and Liver Disease Detection study (ALDDeS). Br J Gen Pract 2013;63:e698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Victor RG, Lynch K, Li N, Blyler C, Muhammad E, Handler J, Brettler J, et al. A Cluster-Randomized Trial of Blood-Pressure Reduction in Black Barbershops. N Engl J Med 2018;378:1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louissaint J, Lok AS, Fortune BE, Tapper EB. Acceptance and Use of a Smartphone Application in Cirrhosis. Liver Int 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson JA, Lee A, Vinson D, Seale JP. Use of AUDIT-based measures to identify unhealthy alcohol use and alcohol dependence in primary care: a validation study. Alcohol Clin Exp Res 2013;37 Suppl 1:E253–259. [DOI] [PubMed] [Google Scholar]
- 20.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998;158:1789–1795. [DOI] [PubMed] [Google Scholar]
- 21.Pal HR, Jena R, Yadav D. Validation of the Alcohol Use Disorders Identification Test (AUDIT) in urban community outreach and de-addiction center samples in north India. J Stud Alcohol 2004;65:794–800. [DOI] [PubMed] [Google Scholar]
- 22.Fleming MF, Smith MJ, Oslakovic E, Lucey MR, Vue JX, Al-Saden P, Levitsky J. Phosphatidylethanol Detects Moderate-to-Heavy Alcohol Use in Liver Transplant Recipients. Alcohol Clin Exp Res 2017;41:857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bataller R, Gao B. Liver fibrosis in alcoholic liver disease. Semin Liver Dis 2015;35:146–156. [DOI] [PubMed] [Google Scholar]
- 24.Parkes J, Roderick P, Harris S, Day C, Mutimer D, Collier J, Lombard M, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut 2010;59:1245–1251. [DOI] [PubMed] [Google Scholar]
- 25.Asphaug L, Thiele M, Krag A, Melberg HO, Consortium G. Cost-Effectiveness of Noninvasive Screening for Alcohol-Related Liver Fibrosis. Hepatology 2019. [DOI] [PubMed] [Google Scholar]
- 26.Serra-Burriel M, Graupera I, Toran P, Thiele M, Roulot D, Wai-Sun Wong V, Neil Guha I, et al. Transient elastography for screening of liver fibrosis: Cost-effectiveness analysis from six prospective cohorts in Europe and Asia. J Hepatol 2019;71:1141–1151. [DOI] [PubMed] [Google Scholar]
- 27.Thiele M, Madsen BS, Hansen JF, Detlefsen S, Antonsen S, Krag A. Accuracy of the Enhanced Liver Fibrosis Test vs FibroTest, Elastography, and Indirect Markers in Detection of Advanced Fibrosis in Patients With Alcoholic Liver Disease. Gastroenterology 2018;154:1369–1379. [DOI] [PubMed] [Google Scholar]
- 28.Askgaard G, Kjaer MS, Tolstrup JS. Opportunities to Prevent Alcoholic Liver Cirrhosis in High-Risk Populations: A Systematic Review With Meta-Analysis. Am J Gastroenterol 2019;114:221–232. [DOI] [PubMed] [Google Scholar]
- 29.Rekve VPaD. Global status report on alcohol and health 2018: executive summary. Geneva: World Health Organization. 2018. [Google Scholar]
- 30.O'Donnell A, Anderson P, Jane-Llopis E, Manthey J, Kaner E, Rehm J. Immediate impact of minimum unit pricing on alcohol purchases in Scotland: controlled interrupted time series analysis for 2015-18. BMJ 2019;366:l5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang H, Livingston M, Room R, Callinan S, Marzan M, Brennan A, Doran C. Modelling the effects of alcohol pricing policies on alcohol consumption in subpopulations in Australia. Addiction 2020. [DOI] [PubMed] [Google Scholar]
- 32.Wagenaar AC, Tobler AL, Komro KA. Effects of alcohol tax and price policies on morbidity and mortality: a systematic review. Am J Public Health 2010;100:2270–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neufeld M, Rehm J. Effectiveness of policy changes to reduce harm from unrecorded alcohol in Russia between 2005 and now. Int J Drug Policy 2018;51:1–9. [DOI] [PubMed] [Google Scholar]
- 34.Arab JP, Roblero JP, Altamirano J, Bessone F, Chaves Araujo R, Higuera-De la Tijera F, Restrepo JC, et al. Alcohol-related liver disease: Clinical practice guidelines by the Latin American Association for the Study of the Liver (ALEH). Ann Hepatol 2019. [DOI] [PubMed] [Google Scholar]
- 35.Gray-Phillip G, Huckle T, Callinan S, Parry CDH, Chaiyasong S, Cuong PV, Mackintosh AM, et al. Availability of alcohol: Location, time and ease of purchase in high-and middle-income countries: Data from the International Alcohol Control Study. Drug Alcohol Rev 2018;37 Suppl 2:S36–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casswell S, Huckle T, Wall M, Parker K, Chaiyasong S, Parry CDH, Viet Cuong P, et al. Policy-relevant behaviours predict heavier drinking and mediate the relationship with age, gender and education status: Analysis from the International Alcohol Control Study. Drug Alcohol Rev 2018;37 Suppl 2:S86–S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White V, Azar D, Faulkner A, Coomber K, Durkin S, Livingston M, Chikritzhs T, et al. Adolescents' alcohol use and strength of policy relating to youth access, trading hours and driving under the influence: findings from Australia. Addiction 2018;113:1030–1042. [DOI] [PubMed] [Google Scholar]
- 38.Wagenaar AC, Toomey TL. Effects of minimum drinking age laws: review and analyses of the literature from 1960 to 2000. J Stud Alcohol Suppl 2002:206–225. [DOI] [PubMed] [Google Scholar]
- 39.Plunk AD, Krauss MJ, Syed-Mohammed H, Hur M, Cavzos-Rehg PA, Bierut LJ, Grucza RA. The Impact of the Minimum Legal Drinking Age on Alcohol-Related Chronic Disease Mortality. Alcohol Clin Exp Res 2016;40:1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young E. How Iceland Got Teens to Say not to Drugs. The Atlantic 2017. [Google Scholar]
- 41.Finan LJ, Lipperman-Kreda S, Grube JW, Balassone A, Kaner E. Alcohol Marketing and Adolescent and Young Adult Alcohol Use Behaviors: A Systematic Review of Cross-Sectional Studies. J Stud Alcohol Drugs Suppl 2020;Sup 19:42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibitoye M, Kaaya S, Parker R, Likindikoki S, Ngongi L, Sommer M. The influence of alcohol outlet density and advertising on youth drinking in urban Tanzania. Health Place 2019;58:102141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pettigrew S, Jongenelis MI, Glance D, Chikritzhs T, Pratt IS, Slevin T, Liang W, et al. The effect of cancer warning statements on alcohol consumption intentions. Health Educ Res 2016;31:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Bruijn A, Tanghe J, de Leeuw R, Engels R, Anderson P, Beccaria F, Bujalski M, et al. European longitudinal study on the relationship between adolescents' alcohol marketing exposure and alcohol use. Addiction 2016;111:1774–1783. [DOI] [PubMed] [Google Scholar]
- 45.Jernigan D, Noel J, Landon J, Thornton N, Lobstein T. Alcohol marketing and youth alcohol consumption: a systematic review of longitudinal studies published since 2008. Addiction 2017;112 Suppl 1:7–20. [DOI] [PubMed] [Google Scholar]
- 46.Maani Hessari N, Bertscher A, Critchlow N, Fitzgerald N, Knai C, Stead M, Petticrew M. Recruiting the "Heavy-Using Loyalists of Tomorrow": An Analysis of the Aims, Effects and Mechanisms of Alcohol Advertising, Based on Advertising Industry Evaluations. Int J Environ Res Public Health 2019;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanwal F, Mapaskhi S, Smith D, Taddei T, Hussain K, Madu S, Duong N, et al. Implementation of a Population-Based Cirrhosis Identification and Management System. Clin Gastroenterol Hepatol 2018;16:1182–1186 e1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gines P, Graupera I, Lammert F, Angeli P, Caballeria L, Krag A, Guha IN, et al. Screening for liver fibrosis in the general population: a call for action. Lancet Gastroenterol Hepatol 2016;1:256–260. [DOI] [PubMed] [Google Scholar]
- 49.Winder GS, Fernandez AC, Klevering K, Mellinger JL. Confronting the Crisis of Comorbid Alcohol Use Disorder and Alcohol-Related Liver Disease With a Novel Multidisciplinary Clinic. Psychosomatics 2020;61:238–253. [DOI] [PubMed] [Google Scholar]
- 50.Williams R, Alexander G, Armstrong I, Baker A, Bhala N, Camps-Walsh G, Cramp ME, et al. Disease burden and costs from excess alcohol consumption, obesity, and viral hepatitis: fourth report of the Lancet Standing Commission on Liver Disease in the UK. Lancet 2018;391:1097–1107. [DOI] [PubMed] [Google Scholar]
- 51.Talukdar R, Reddy DN. Making endoscopy mobile: a novel initiative for public healthcare. Endoscopy 2012;44:186–189. [DOI] [PubMed] [Google Scholar]
- 52.Serper M, Cubell AW, Deleener ME, Casher TK, Rosenberg DJ, Whitebloom D, Rosin RM. Telemedicine in Liver Disease and Beyond: Can the COVID-19 Crisis Lead to Action? Hepatology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arora S, Thornton K, Murata G, Deming P, Kalishman S, Dion D, Parish B, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med 2011;364:2199–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das J, Chowdhury A, Hussam R, Banerjee AV. The impact of training informal health care providers in India: A randomized controlled trial. Science 2016;354. [DOI] [PubMed] [Google Scholar]
- 55.van Ginneken N, Tharyan P, Lewin S, Rao GN, Meera SM, Pian J, Chandrashekar S, et al. Non-specialist health worker interventions for the care of mental, neurological and substance-abuse disorders in low- and middle-income countries. Cochrane Database Syst Rev 2013:CD009149. [DOI] [PubMed] [Google Scholar]
- 56.Khan A, Tansel A, White DL, Kayani WT, Bano S, Lindsay J, El-Serag HB, et al. Efficacy of Psychosocial Interventions in Inducing and Maintaining Alcohol Abstinence in Patients With Chronic Liver Disease: A Systematic Review. Clin Gastroenterol Hepatol 2016;14:191–202 e191-194; quiz e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaner EF, Beyer FR, Garnett C, Crane D, Brown J, Muirhead C, Redmore J, et al. Personalised digital interventions for reducing hazardous and harmful alcohol consumption in community-dwelling populations. Cochrane Database Syst Rev 2017;9:CD011479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Komaromy M, Duhigg D, Metcalf A, Carlson C, Kalishman S, Hayes L, Burke T, et al. Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Subst Abus 2016;37:20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and Treatment of Alcohol-Associated Liver Diseases: 2019 Practice Guidance From the American Association for the Study of Liver Diseases. Hepatology 2020;71:306–333. [DOI] [PubMed] [Google Scholar]
- 60.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol 2018;69:154–181. [DOI] [PubMed] [Google Scholar]
- 61.Quality. CfBHSa. Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50). Retrieved from http://www.samhsa.gov/data. 2015.
- 62.Mellinger JL, Fernandez A, Shedden K, Winder GS, Fontana RJ, Volk ML, Blow FC, et al. Gender Disparities in Alcohol Use Disorder Treatment Among Privately Insured Patients with Alcohol-Associated Cirrhosis. Alcohol Clin Exp Res 2019;43:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mellinger JL, Scott Winder G, DeJonckheere M, Fontana RJ, Volk ML, Lok ASF, Blow FC. Misconceptions, preferences and barriers to alcohol use disorder treatment in alcohol-related cirrhosis. J Subst Abuse Treat 2018;91:20–27. [DOI] [PubMed] [Google Scholar]
- 64.Colle I, Durand F, Pessione F, Rassiat E, Bernuau J, Barriere E, Lebrec D, et al. Clinical course, predictive factors and prognosis in patients with cirrhosis and type 1 hepatorenal syndrome treated with Terlipressin: a retrospective analysis. Journal of gastroenterology and hepatology 2002;17:882–888. [DOI] [PubMed] [Google Scholar]
- 65.Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and Treatment of Alcohol-Related Liver Diseases: 2019 Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2019. [DOI] [PubMed] [Google Scholar]