Abstract
Attachment experiences are thought to contribute to physical health across the lifespan. Evidence suggests that attachment style may serve as a protective factor for individuals’ physical health by mitigating the negative effects of social and environmental risk factors. In the present study, we evaluated how attachment styles may moderate the link between African American adolescents’ exposure to neighborhood poverty and accelerated cellular aging in young adulthood. Analyses revealed that allostatic load at age 19 mediated the association between neighborhood poverty in adolescence and changes in cellular aging from age 20 to 27. Notably, attachment avoidance (but not attachment anxiety) moderated this association, such that allostatic load was only associated with faster cellular aging for individuals who were high in avoidance. These findings suggest that allostatic load may give rise to faster cellular aging, but these detrimental effects of allostatic load can be offset by young adults’ effective use of attachment figures.
Although the primary focus of research on attachment has centered on how individual differences in attachment are associated with psychosocial functioning, a growing literature suggests that attachment-related experiences are also related to physiological systems that contribute to physical health (Ehrlich, 2019; Ehrlich et al., 2016; Pietromonaco & Beck, 2019). For example, a number of studies have linked attachment to neuroendocrine activity (e.g., Dozier et al., 2008; Gunnar et al., 1996; Hane & Fox, 2016), cardiovascular reactivity (e.g., Bourassa et al., 2019; Kim, 2006), and autonomic nervous system activity (e.g., Tabachnick et al., 2020). Collectively, these studies suggest that experiences within these close relationships may have cascading effects on physiological systems (Gouin et al., 2009; Pietromonaco & Powers, 2015), and if sustained, could give rise to individual differences in health trajectories.
In the present study, we explored how individuals’ attachment styles might mitigate the downstream effects associated with adolescents’ exposure to contextual risks in a sample of African Americans living in the rural South. Specifically, we examined how exposure to neighborhood poverty in adolescence might be associated with elevated allostatic load, a measure that reflects the chronic strain resulting from repeated stressors that activate the body’s regulatory controls across physiological systems (McEwen, 1998; Singer et al., 2004). Neighborhood poverty is thought to contribute to allostatic load through the difficult living conditions that often co-occur in disadvantaged communities, including a need to maintain vigilance, a lack of trust and support from neighbors, and limited access to resources, such as healthy food and safe places for recreation (Browning & Cagney, 2003). In addition, people living in underserved communities sometimes endure additional environmental risk factors, such as poor air quality and unsafe drinking water, that further add to both short- and long-term physical health risks (Henderson & Wells, 2021). Previous studies have found evidence to support the notion that neighborhood disadvantage is linked to allostatic load (e.g., Gustafsson et al., 2014; King et al., 2011; Schulz et al., 2013). Consistent with previous studies, we hypothesized that more years living in impoverished neighborhoods in adolescence would be associated with increased allostatic load in early adulthood.
Heightened allostatic load is of particular concern because this composite may forecast later health problems (Beckie, 2012). For example, among older adults, allostatic load was predictive of later cardiovascular disease and all-cause mortality (Seeman et al., 2001). Similarly, in a sample of adults in midlife, higher allostatic load predicted greater odds of mortality over an 11-year period (Castagné et al., 2018). Further, several studies have found that allostatic load composites predict physical health outcomes better than the individual biomarkers that are included in the composite (e.g., McEwen & Stellar, 1993; Seeman et al., 2004).
Although allostatic load has been shown to be predictive of chronic disease, frailty, and early mortality among older adults, less is known about the usefulness of allostatic load as a pre-disease warning sign among younger adults who may not yet show signs of chronic disease. As such, in the present study we explored the extent to which allostatic load may be linked to changes in cellular aging, which can be viewed as an intermediate marker of health that has prognostic significance for future disease diagnosis (Horvath et al., 2018). This marker of cellular aging, also known as an “epigenetic clock” (Horvath & Raj, 2018), evaluates DNA methylation patterns that reliably change with age. These epigenetic clocks work by comparing individuals’ chronological age to their biological age; higher values indicate faster aging relative to chronological age. Epigenetic clocks may provide useful information about the extent to which individuals are at increased risk for the onset of chronic disease and early mortality. Horvath’s clock, for example, has been used to predict a range of conditions, including obesity, frailty, and even some cancers (see Horvath & Raj, 2018). Thus, evidence of cellular aging may serve as a pre-disease warning sign that forecasts future chronic disease diagnosis and early mortality.
Although we expect that allostatic load will predict increases in accelerated cellular aging, we hypothesize that attachment styles could moderate the extent to which higher allostatic load translates into increased risk. In adults, attachment style reflects individuals’ expectations, emotion regulation strategies, and comfort in close relationships (Brennan et al., 1998; Shaver & Mikulincer, 2002). Individual differences in adult attachment style have been defined along two dimensions. Attachment avoidance captures individuals’ discomfort with intimacy and avoidance of relying on close relationship partners for support. In contrast, attachment anxiety reflects individuals’ worries about being rejected, abandoned, or unloved.
Individuals who have a close relationship partner who is reliable and effective in providing support may have better ways of managing their negative emotions, and this regulation might serve an important stress buffering role that could minimize strain on the body (Ehrlich et al., 2016; Simpson & Rholes, 2010). These benefits—or the lack thereof—may be particularly apparent in stressful contexts. For example, Gouin et al. (2009) found that attachment avoidance was positively associated with serum levels of interleukin (IL)-6, a marker of inflammation, after couples discussed sources of conflict in their relationship. Notably, however, attachment was unrelated to IL-6 produced following discussions where the same couples discussed personal concerns (i.e., something the individual would like to change about him/herself). In the present study, we examined the extent to which both attachment avoidance and attachment anxiety may offset the risks associated with allostatic load.
The present study leverages an existing sample of African American youth (now young adults) who were initially recruited for a randomized controlled trial of a family-centered intervention designed to prevent behavior problems and substance abuse (Brody et al., 2013). From ages 11 to 18, we assessed neighborhood poverty based on census tract information. At ages 19 and 20, young adults participated in two study visits, during which we collected biomarkers and clinical indicators to assess allostatic load. Cellular aging was measured from blood samples collected at ages 20 and 27. Finally, young adults’ attachment styles were measured by self-report at ages 25 and 27.
Method
Participants
Data for this study were drawn from the Strong African American Healthy Adults Project (SHAPE; Brody et al., 2013). Starting in 2001, SHAPE enrolled 667 Black children in fifth grade (Mage = 11.2 years, SD = 0.3) and each child’s primary caregiver (89.2% females). The families resided in nine rural counties in Georgia, where poverty rates are among the highest in the nation. Of the youth in the sample, 53% were female. At baseline, 80% of the caregivers had completed high school or earned a GED. Economically, these households can be characterized as working poor. The primary caregivers worked a mean of 30.6 hours per week and had a median household income of $1612 per month. Further, 42.3% of the families were living below federal poverty thresholds.
In 2009-2010, when the youth had reached ages 19 to 20, a subgroup of 500 was randomly selected for a substudy of allostatic load and DNA methylation. The selection of a random subsample was necessary because of financial constraints associated with the costs of assessing these biomarkers. Of these 500 participants, 399 (79.8%) provided an overnight urine sample and a blood sample. In 2017, when the participants’ mean age was 27 years, we re-assessed participants from the subgroup of 500 people assessed in 2009-2010 and obtained blood draws from 388 participants from which DNA methylation was assayed again. In 2015 and 2017, when participants’ mean ages were 25 and 27 years, young adults who were involved in a romantic relationship reported on their attachment style (n = 356). The sample for the present study was composed of 271 participants (98 men and 173 women) from whom blood was drawn at ages 20 and 27 and for whom data were available on the other study measures from ages 11 to 27. Compared with the original study cohort, the analytic sample had a higher percentage of female participants (63.8% vs. 52.8%), and during adolescence, their family experienced more years of poverty (M = 2.46 vs. M = 2.16), all ps < .05, but were otherwise similar on biomarkers collected at ages 19, 20, and 27 and all other study variables. The University of Georgia’s Institutional Review Board approved the protocol, and written consent was obtained from participants and their caregivers at all assessments.
Data Collection Procedures
All data were collected in participants’ homes using a standardized protocol. Interviews were conducted privately, with no other family members present or able to overhear the conversation. A Black field researcher, who was also a certified phlebotomist, went to each participant’s home to draw blood samples for later assessment of epigenetic aging at ages 20 and 27. Each family was paid $100 for each wave of data collection across adolescence. Young adult participants were paid $160 for blood draws and psychosocial assessments starting at age 19.
Measures
Adolescent neighborhood poverty.
The adolescent neighborhood poverty index was formed using data from when youths were 11, 12, 13, 16, 17, and 18 years of age. Neighborhood poverty was calculated as the percentage of residents in a neighborhood living below the federal poverty line. Adolescent neighborhood poverty scores across the six assessment waves were averaged.
Young adult allostatic load.
The protocol for measuring allostatic load (AL) when youth were 19 and 20 years of age was based on procedures that Evans (2003) developed for field studies involving children and adolescents. Resting blood pressure was monitored with a Critikon Dinamap Pro 100 (Critikon; Tampa, FL) while participants sat reading quietly. Three readings were taken every 2 minutes, and the average of the last two readings was used as the resting index. This procedure yields highly reliable indices of chronic resting blood pressure (Kamarck et al., 1992). Weight was measured using a standard home scale, and height was measured using a tape measure. The weight and height of each participant were recorded and used to calculate body mass index (BMI). Overnight urinary catecholamines and cortisol were assayed. Beginning on the evening of data collection, all urine that the participants voided from 8 p.m. to 8 a.m. was stored on ice in a container with metabisulfite as a preservative. Urine was delivered to the Emory University Hospital medical laboratory in Atlanta, Georgia, for assaying. Total unbound cortisol was assayed with a radioimmune assay (Contreras et al., 1986). Epinephrine and norepinephrine were assayed with high-pressure liquid chromatography with electrochemical detection (Riggin & Kissinger, 1977). Creatinine was assayed to control for differences in body size and incomplete urine voiding (Tietz, 1976).
The AL indicators included overnight cortisol, epinephrine, and norepinephrine; resting diastolic and systolic blood pressure; and BMI. All indicators were averaged across ages 19 and 20. AL was calculated by summing the number of physiological indicators on which each participant scored in the top quartile of risk. Possible scores ranged from 0 to 6.
Adult attachment style.
At ages 25 and 27, participants who were currently involved in a romantic relationship reported on their attachment avoidance and anxiety with respect to their romantic partners using the 10-item short form of the Experiences in Close Relationships Scale (Wei et al., 2007). The avoidance subscale (5 items; α = .63 and .65 at age 25 and 27, respectively) measures the extent to which a person is uncomfortable with closeness, intimacy, and emotional disclosure in close relationships (e.g., “I try to avoid getting too close to my partner”). The anxiety subscale (5 items, α = .69 and .66 at age 25 and 27, respectively) measures the extent to which a person worries about being rejected, abandoned, or unloved (e.g., “I find that my partner[s] don’t want to get as close as I would like”). For each item, participants rated the extent to which they agree with the statement using a seven-point Likert-type scale, with responses ranging from 1 (disagree strongly) to 7 (agree strongly).
Items from each subscale were summed such that higher scores reflect greater attachment anxiety and avoidance in close relationships. Because these dimensions were stable from age 25 to 27 (rs > .41, ps < .001), each dimension was averaged across the two time points.
Accelerated cellular aging.
When participants were ages 20 and 27 years, a certified phlebotomist went to each of their homes to draw blood draw. Peripheral blood mononuclear cells (PBMC) were isolated through density-gradient centrifugation, and DNA methylation was subsequently assessed with the Illumina Infinium (Sequenom, Inc., San Diego, CA, USA) HumanMethylation450 Beadchip at age 20 and HumanMethylationEPIC850 Beadchip at age 27. The beta value at each CpG locus was calculated as the ratio of the intensity of the methylated probe to the sum of intensities of the methylated and unmethylated probes. Quantile normalization methods were used, with separate normalization of Type I and Type II assays, because this approach has been found to produce marked improvement for the Illumina array in detection of relationships by correcting distributional problems inherent in the manufacturers default method for calculating the beta value. We then assessed cellular aging using Horvath et al.’s (2018) skin and blood clock. This estimates an individual’s biological age based on DNA methylation assessments at 391 CpGs scattered across the human genome; it was analyzed using the online “New Methylation Age Calculator” (https://dnamage.genetics.ucla.edu/) with the Advanced Analysis and the Normalize Data options. To transform Horvath’s skin and blood clock into a measure of accelerated cellular aging, we regressed the clock on chronological age, resulting in a measure of accelerated cellular aging that is adjusted to correlate with chronological age at 0. A positive value on this variable indicates accelerated cellular aging in years, whereas a negative value indicates decelerated aging in years. Because the PBMC pool contains multiple types of cells, whose distribution varies across people, we included estimates of cell type as covariates in regression models. These estimates were derived from DNA methylation profiles by the epigenetic age calculator (Horvath & Levine, 2015).
Covariates.
Sex was dummy coded such that males were coded 1 and females were coded 0. When participants were 11, 12, 13, 16, 17, and 18 years of age, caregivers provided data on their families’ income-to-needs ratios based on family size, which were used to compute household poverty (U.S. Census Bureau; Semega et al., 2020, p. 49). Poverty statuses at six assessment waves were summed to determine the number of years participants lived below federal poverty standards.
Plan of Analysis
We hypothesized that adolescent neighborhood poverty across ages 11 to 18 would forecast ages 19-20 allostatic load levels, which in turn, would interact with adult attachment styles to forecast change in participants’ cellular aging from age 20 to 27. We computed structural equation models (SEM) with latent difference scores (MacKinnon et al., 2002) to test study hypotheses. The models included the first structural path examining whether adolescent neighborhood poverty predicted elevated allostatic load during young adulthood and the second structural path examining whether the interaction of allostatic load and attachment styles predicted the change of accelerated cellular aging from age 20 to age 27. All interaction analyses were executed based on the conventions that Aiken and West (1991) prescribed, whereby the variables are first standardized and interactions are calculated as the product of the standardized variables.
The latent difference scores (LDS) were estimated to reflect the degree to which accelerated cellular aging changed from age 20 to 27. The LDS model decomposes the variance of the Time 2 (T2) variable into two components: variance associated with the Time 1 (T1) scores and variance associated with the absolute difference from T1. The LDS model includes the traditional statistical features of difference scores as model parameters: mean change, inter-individual differences change, and the covariance between T1 scores and change scores. In the current study, we used Mplus (Muthén & Muthén, 1998-2012) to test the LDS model. The change in accelerated cellular aging between age 20 and 27 was modeled with the following settings: (1) the accelerated cellular aging variable at age 27 was the single indicator of the latent difference scores (the loading was set to 1 without measurement error); (2) the accelerated cellular aging variable at age 27 was regressed on the accelerated cellular aging variable at age 20 and the path coefficient was set to be 1; and (3) the latent difference scores were regressed on the accelerated cellular aging variable at age 20 and the path coefficient was estimated. The setting was based on McArdle (2009) and Valente & MacKinnon (2017). Gender and family poverty at ages 11-18 were included as control variables in all the models.
We also calculated the conditional indirect effects of adolescent neighborhood poverty on youth change in cellular aging from age 20 to 27 through youth allostatic load at ages 19 and 20, by the levels of young adult attachment avoidance and anxiety at ages 25 and 27. First, the regression coefficient was estimated for the association between adolescent neighborhood poverty and young adult allostatic load (path A). Second, the regression coefficients were calculated for the association between allostatic load and changes in cellular aging for youth who had high (simple slope B1) vs. low (simple slope B2) attachment avoidance and anxiety. Third, the conditional indirect effect in which allostatic load serves as a link connecting neighborhood poverty to changes of cellular aging was quantified as the product of the two regression coefficients (A × Bj). Nonparametric bootstrapping was used to obtain the bias-corrected and accelerated confidence intervals of the coefficients and indirect effect (Preacher & Hayes, 2004). The coefficients and indirect effect were calculated 1000 times using random sampling with replacement to build a sampling distribution.
Results
Bivariate correlations and descriptive statistics for the study variables are presented in Table 1. The results of the SEM models are presented in Table 2. Overall model fits were good, with χ2(4) = 2.367, p = .669, comparative fit index = 1.000, and root mean square error of approximation = 0 (CI = 0, 0.072) for the model with attachment avoidance; and χ2(4) = 1.993, p = .737, comparative fit index = 1.000, and root mean square error of approximation = 0 (CI = 0, 0.065) for the model with attachment anxiety.
Table 1.
Correlations and Descriptive Statistics among Study Variables
| Mean (SD) or n (%) | Correlations |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 1. Gender, male | 98 (36.2%) | — | ||||||
| 2. Years living in poverty (ages 11-18) | 2.465 (1.910) | −.010 | — | |||||
| 3. Neighborhood poverty (ages 11 to 18) | 0.222 (0.073) | −.001 | .177** | — | ||||
| 4. Allostatic load (ages 19 and 20) | 1.472 (1.267) | .114 | .114 | .131* | — | |||
| 5. Attachment avoidance (ages 25 and 27) | 2.680 (1.029) | .168** | −.035 | .037 | −.001 | — | ||
| 6. Attachment anxiety (ages 25 and 27) | 2.899 (1.261) | .107 | −.079 | −.019 | .004 | .518*** | — | |
| 7. Cellular aging (age 20) | −0.312 (2.939) | .089 | .165** | −.037 | .046 | −.016 | −.061 | — |
| 8. Cellular aging (age 27) | 0.005 (2.364) | −.016 | .160** | −.041 | .125* | −.006 | −.078 | .472*** |
N = 271.
p < .05.
p < .01.
p < .001.
Table 2.
Indirect effects of neighborhood poverty at ages 11 to 18 on changes of cellular aging from age 20 to 27 through allostatic load at ages 19 and 20, moderated by attachment at ages 25-27
| Allostatic Load (ages 19&20) | Changes in Cellular Aging (from age 20 - 27) | Changes in Cellular Aging (from age 20 - 27) | ||||
|---|---|---|---|---|---|---|
| Predictors | b | [95% CI] | b | [95% CI] | b | [95% CI] |
| 1. Gender, male | 0.239 | [−0.007, 0.491] | −0.314 | [−0.826, 0.266] | −0.347 | [−0.849, 0.201] |
| 2. Family poverty (ages 11-18) | 0.050 | [−0.011, 0.107] | 0.091 | [−0.046, 0.243] | 0.095 | [−0.046, 0.252] |
| 3. Neighborhood poverty (ages 11-18) | 1.565* | [0.036, 3.094] | −1.950 | [−5.200, 1.858] | −1.840 | [−5.264, 1.991] |
| 4. Allostatic load (AL, ages 19 and 20) | - | - | 0.282* | [0.019, 0.502] | 0.264* | [0.007, 0.497] |
| 5. Attachment avoidance (ages 25 and 27) | - | - | 0.118 | [−0.136, 0.382] | 0.116 | [−0.133, 0.378] |
| 6. Attachment anxiety (ages 25 and 27) | - | - | −0.114 | [−0.333, 0.096] | −0.148 | [−0.430, 0.115] |
| 7. AL × Attachment avoidance | - | - | 0.241* | [0.010, 0.472] | - | - |
| 8. AL × Attachment anxiety | - | - | - | - | 0.033 | [−0.219, 0.301] |
N = 271; b = unstandardized regression coefficient; CI = confidence interval.
p < .05.
p < .01.
p < .001.
The results of the SEM models revealed that exposure to neighborhood poverty across ages 11 to 18 was associated with youth allostatic load at ages 19 and 20, b = 1.565, 95% CI [0.036, 3.094], p = .045. The model with attachment anxiety did not reveal a significant interactive effect. The model with attachment avoidance, however, revealed a significant interaction of allostatic load at ages 19 and 20 × attachment avoidance at ages 25 and 27 predicting the changes in epigenetic aging from age 20 to 27, b = .241, 95% CI [.010, .472], p = .040. This interaction is illustrated in Figure 1. Simple slopes were computed for estimated levels of changes in cellular aging by allostatic load for low (1 SD below the mean) and high (1 SD above the mean) levels of attachment avoidance. Allostatic load at ages 19 and 20 was associated with greater acceleration in cellular aging from age 20 to 27 for participants with high attachment avoidance (simple slope = .523, 95% CI [.121, .861], p = .006). In contrast, for young adults with low levels of attachment avoidance, allostatic load was not associated with changes in cellular aging (simple slope = .041, 95% CI [−.267, .336], p = .786).
Figure 1.
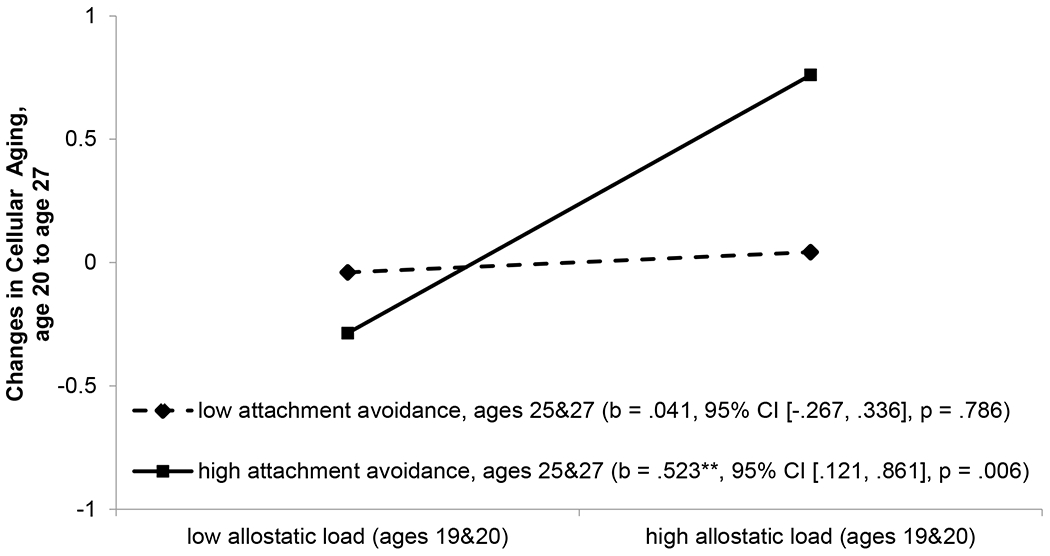
The effect of allostatic load at ages 19 and 20 on changes in cellular aging from age 20 to 27 by attachment avoidance at ages 25 and 27. Numbers in parentheses refer to slopes for low (1 SD below the mean) vs. high (1 SD above the mean) attachment avoidance. **p < .01.
The conditional indirect effects of neighborhood poverty across ages 11 to 18 on youth changes of cellular aging from age 20 to age 27 through allostatic load at ages 19 to 20 were calculated for low vs. high levels of attachment avoidance (see Figure 2). A significant indirect effect linking adolescent neighborhood poverty to adult change in cellular aging via young adult allostatic load only emerged when youth had high levels of avoidance (indirect effect = 0.819, 95% CI [0.057, 2.217]). No significant indirect effects emerged for youth who had low levels of avoidance (indirect effect = 0.064, 95% CI [−0.389, 0.804]).
Figure 2.
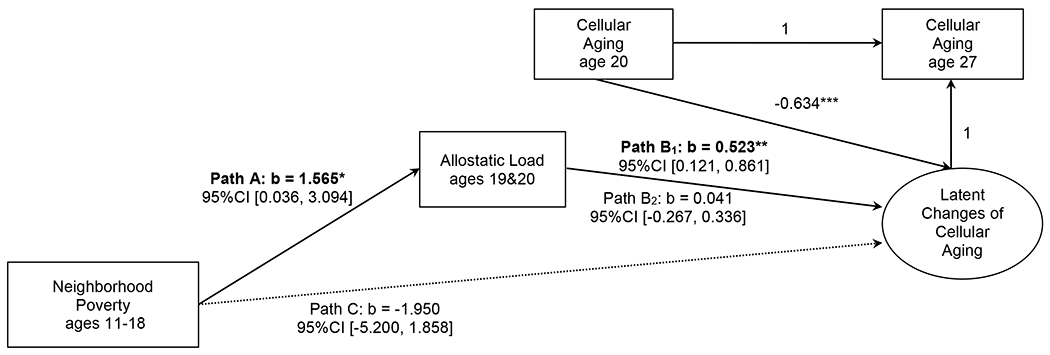
The conditional indirect effect of neighborhood poverty at ages 11 to 18 on changes of youths’ cellular aging from age 20 to 27 through allostatic load at ages 19 and 20, by attachment avoidance at ages 25 and 27. Path B1: simple slope for high (1 SD above the mean) attachment avoidance; Path B2: simple slope for low (1 SD below the mean) attachment avoidance. Statistically significant pathways indicated by boldface type. b = unstandardized regression coefficient; CI = confidence interval.
***p < .001. **p < .01. *p < .05.
Discussion
This study documents how the cascading effects of exposure to neighborhood poverty are mitigated for young adults who report low attachment avoidance in early adulthood. To the extent that African American young adults reported that they could depend on and trust their romantic partners (as indexed by low attachment avoidance), they were no longer at risk for accelerated cellular aging, despite their exposure to neighborhood poverty and emerging symptoms of higher allostatic load. In contrast, for young adults who were unable to rely on and avoided getting close to their romantic partners, exposure to neighborhood poverty in adolescence was associated with allostatic load at age 19, which was further associated with greater changes in cellular aging from age 20 to 27.
These findings raise some questions about why attachment avoidance, but not anxiety, moderated the association between allostatic load and changes in cellular aging. Notably, these findings are similar to those documented by Dagan et al. (2018), who found that adult attachment style moderated the association between early adversity in telomere length, another measure of cellular aging. In this study, early adversity was negatively associated with telomere length only for adults who were high in attachment avoidance. Similarly, in a sample of former prisoners of war, Ein-Dor et al. (2020) reported that attachment avoidance was negatively associated with telomere length. It may be that avoidant individuals are less able to seek social support, and as a consequence, they may continue to use ineffective strategies for coping and managing stressors. These findings call to mind studies of infants during stressful situations, such as the Strange Situation (Ainsworth et al., 1978). In one study, infants who were classified as avoidant had higher salivary alpha amylase and greater vagal withdrawal relative to secure infants (Hill-Soderlund et al., 2008). These markers reflect sympathetic and parasympathetic activity, respectively, and suggest that, while avoidant infants may appear unaffected by their mothers’ absence, their physiology indicates that they are struggling to regulate their distress. Such behavioral patterns can create a positive feedback loop across development, where avoidant individuals may not communicate with their attachment figures about their distress, leaving little opportunity for those relationship partners to provide comfort and support. Over time, this additional strain could lead to increased allostatic load and cellular aging, as observed in this sample.
In the present study, participants were asked to report about their attachment avoidance and anxiety with respect to their current romantic partner, and it will be important for future research to consider whether other attachment figures (notably parents) may confer similar protection in the face of contextual risks. It may be that the extent to which relationships with attachment figures provide stress-buffering support depends on the developmental period, with parents serving as important sources of support in childhood and adolescence (e.g., Brody et al., 2019) and romantic partners providing more support in adulthood (e.g., Ehrlich et al., 2019). These hypotheses, which await future investigation, suggest that there may be stability in the important role of attachment figures despite the changing identity of who that primary attachment figure may be.
This study adds to the growing evidence that attachment relationships play an important role for physical health. At the same time, questions remain about the extent to which attachment styles can disrupt the progression from childhood neighborhood poverty to allostatic load to increases in cellular aging. Our analytical model was complex and merits replication in other longitudinal samples, particularly with respect to testing hypotheses about the extent to which allostatic load can forecast changes in cellular aging. Additionally, several study limitations should be addressed in future research. First, only young adults from the larger study who reported that they were in a romantic relationship at age 25 or 27 were included in these analyses—a result of the attachment measure itself, which was framed to ask people specifically about how they feel about their current romantic relationships. Future studies should consider using the alternate version of the ECR, which asks about people about how they feel in close relationships generally, rather than romantic relationships specifically.
In addition, we assessed exposure to neighborhood poverty across adolescence, but it is possible that similar exposure during different developmental periods (e.g., early childhood, prenatally) could play a role in biological programming that sets the stage for physiological dysregulation and accelerated cellular aging later in life. Future studies that assess children’s exposure to neighborhood poverty across infancy, childhood, and adolescence could test hypotheses about whether there are particular sensitive periods in development during which youth are especially vulnerable to stressful neighborhood environments.
Finally, our study focused on youth from rural African American families, many of whom were living with considerable socioeconomic disadvantage and chronic stress. One question that remains is whether similar effects would emerge in studies focused on young adults with different demographic characteristics, or among Black Americans living under different conditions (e.g., with higher socioeconomic status or living in urban environments). Given the unique characteristics of this sample, we are hesitant to speculate about the generalizability of these findings, but we note that the benefits associated with reliable and caring attachment figures are widely observed in samples across race and cultural backgrounds (Mesman et al., 2016), and it seems reasonable to expect that these patterns would hold in samples with other racial and other demographic characteristics as well. One counterargument to this presumption, however, is that access to supportive attachment figures may be especially beneficial in challenging contexts. In the present study, participants were living in communities where they reported facing considerable racial discrimination and socioeconomic disadvantage. Thus, although the presence of dependable attachment figures may be valuable for all, the extent to which these benefits are observed in terms of tangible outcomes (such as slower cellular aging) could vary as a function of participants’ at-risk status.
In summary, the present study found that young adults’ exposure to neighborhood poverty across adolescence was associated with allostatic load in young adulthood, which in turn predicted accelerated cellular aging across a seven-year period. Notably, this association was disrupted when youth were able to use attachment figures effectively for support. These findings add to our current understanding about the possible protective role of attachment styles, which may reflect individuals’ abilities to rely on relationship partners for stress buffering, particularly within the context of challenging environments. Efforts to understand why low levels of attachment avoidance can mitigate risk for accelerated aging will be important to consider in future research.
Acknowledgments
This research was supported by grants R01 HD030588, P30 DA027827, P50 DA051361, DP2 MD013947, and R03 HD093918 from NIH, by the Jacobs Foundation (Early Career Research Fellowship 2018-1288-07), and by the Brain and Behavior Research Foundation (Young Investigator Grant #27302).
References
- Aiken LS, West SG (1991). Multiple regression: Testing and interpreting interactions. Sage. [Google Scholar]
- Beckie TM (2012). A systematic review of allostatic load, health, and health disparities. Biological Research for Nursing, 14, 311–346. [DOI] [PubMed] [Google Scholar]
- Bourassa KJ, Ruiz JM, & Sbarra DA (2019). The impact of physical proximity and attachment working models on cardiovascular reactivity: Comparing mental activation and romantic partner presence. Psychophysiology, 56, e13324. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Clark CL, & Shaver PR (1998). Self-report measurement of adult romantic attachment: An integrative overview. In Simpson JA & Rholes WS (Eds.), Attachment theory and close relationships (pp. 46–76). Guilford. [Google Scholar]
- Brody GH, Yu T, Chen E, Miller GE, Kogan SM, & Beach SR (2013). Is resilience only skin deep? Rural African Americans’ socioeconomic status-related risk and competence in preadolescence and psychological adjustment and allostatic load at age 19. Psychological Science, 24, 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning CR, & Cagney KA (2003). Moving beyond poverty: Neighborhood structure, social processes, and health. Journal of Health and Social Behavior, 44, 552–571. [PubMed] [Google Scholar]
- Castagné R, Garès V, Karimi M, Chadeau-Hyam M, Vineis P, Delpierre C, … & Lifepath Consortium. (2018). Allostatic load and subsequent all-cause mortality: Which biological markers drive the relationship? Findings from a UK birth cohort. European Journal of Epidemiology, 33, 441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras LN, Hane S, & Tyrrell JB (1986). Urinary cortisol in the assessment of pituitary-adrenal function: utility of 24-hour and spot determinations. The Journal of Clinical Endocrinology and Metabolism, 62, 965–969. [DOI] [PubMed] [Google Scholar]
- Dagan O, Asok A, Steele H, Steele M, & Bernard K (2018). Attachment security moderates the link between adverse childhood experiences and cellular aging. Development and Psychopathology, 30, 1211–1223. [DOI] [PubMed] [Google Scholar]
- Dozier M, Peloso E, Lewis E, Laurenceau JP, & Levine S (2008). Effects of an attachment-based intervention on the cortisol production of infants and toddlers in foster care. Development and Psychopathology, 20, 845–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich KB (2019). Attachment and psychoneuroimmunology. Current Opinion in Psychology, 25, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich KB, Miller GE, Jones JD, & Cassidy J (2016). Attachment and psychoneuroimmunology. In Cassidy J & Shaver PR (Eds.), Handbook of attachment: Theory, research, and clinical applications (3rd ed., pp. 180–201). Guilford. [Google Scholar]
- Ehrlich KB, Stern JA, Eccles J, Dinh JV, Hopper EA, Kemeny ME, Adam EK, & Cassidy J (2019). A preliminary investigation of attachment style and inflammation in African-American young adults. Attachment & Human Development, 21, 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW (2003). A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology, 39, 924–933. [DOI] [PubMed] [Google Scholar]
- Gouin J, Glaser R, Loving TJ, Malarkey WB, Stowell J, Houts C, & Kiecolt-Glaser JK (2009). Attachment avoidance predicts inflammatory responses to marital conflict. Brain, Behavior, and Immunity, 23, 898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M, Buss K, & Rigatuso J (1996). Stress reactivity and attachment security. Developmental Psychobiology, 29, 191–204. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, San Sebastian M, Janlert U, Theorell T, Westerlund H, & Hammarström A (2014). Life-course accumulation of neighborhood disadvantage and allostatic load: Empirical integration of three social determinants of health frameworks. American Journal of Public Health, 104, 904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane AA, & Fox NA (2016). Early caregiving and human biobehavioral development: a comparative physiology approach. Current Opinion in Behavioral Sciences, 7, 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S, & Wells R (2021). Environmental racism and the contamination of Black lives: A literature review. Journal of African American Studies. doi: 10.1007/s12111-020-09511-5 [DOI] [Google Scholar]
- Horvath S, Oshima J, Martin GM, Lu AT, Quach A, Cohen H, Felton S, Matsuyama M, Lowe D, Kabacik S, Wilson JG, Reiner AP, Maierhofer A, Flunkert J, Aviv A, Hou L, Baccarelli AA, Li Y, Stewart JD, Whitsel EA, … Raj K (2018). Epigenetic clock for skin and blood cells applied to Hutchinson Gilford Progeria Syndrome and ex vivo studies. Aging, 10, 1758–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, & Raj K (2018). DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics, 19, 371–384. [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Jennings JR, Debski TT, Glickman-Weiss E, Johnson PS, Eddy MJ, & Manuck SB (1992). Reliable measures of behaviorally-evoked cardiovascular reactivity from a PC-based test battery: Results from student and community samples. Psychophysiology, 29, 17–28. [DOI] [PubMed] [Google Scholar]
- Kim Y (2006). Gender, attachment, and relationship duration on cardiovascular reactivity to stress in a laboratory study of dating couples. Personal Relationships, 13, 103–114. [Google Scholar]
- King KE, Morenoff JD, & House JS (2011). Neighborhood context and social disparities in cumulative biological risk factors. Psychosomatic Medicine, 73, 572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffman JM, West SG, & Sheets V (2002). A comparison of methods to test mediation and other intervening variable effects. Psychological Methods, 7, 83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ (2009). Latent variable modeling of differences and changes with longitudinal data. Annual Review of Psychology, 60, 577–605. [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998). Protective and damaging effects of stress mediators. New England Journal of Medicine, 338, 171–179. [DOI] [PubMed] [Google Scholar]
- McEwen BS, & Stellar E (1993). Stress and the individual: Mechanisms leading to disease. Archives of Internal Medicine, 153, 2093–2101. [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998-2012). Mplus user’s guide: Statistical analysis with latent nariables (7th ed.). Muthén & Muthén. [Google Scholar]
- Pietromonaco PR, & Beck LA (2019). Adult attachment and physical health. Current Opinion in Psychology, 25, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietromonaco PR, & Powers SI (2015). Attachment and health-related physiological stress processes. Current Opinion in Psychology, 1, 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers, 36, 717–731. [DOI] [PubMed] [Google Scholar]
- Riggin RM, & Kissinger PT (1977). Determination of catecholamines in urine by reverse-phase liquid chromatography with electrochemical detection. Analytical Chemistry, 49, 2109–2111. [DOI] [PubMed] [Google Scholar]
- Schulz AJ, Mentz G, Lachance L, Zenk SN, Johnson J, Stokes C, & Mandell R (2013). Do observed or perceived characteristics of the neighborhood environment mediate associations between neighborhood poverty and cumulative biological risk? Health & Place, 24, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, Huang M, Singer BH, Bacur A, Gruenewald T, Berkman LF, & Reuben DB (2004). Cumulative biological and socioeconomic differences in mortality: MacArthur Studies of Successful Aging. Social Science and Medicine, 58, 1985–1997. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, & Singer BH (2001). Allostatic load as a marker of cumulative biological risk: MacArthur Studies of Successful Aging. Proceedings of the National Academy of Sciences, 98, 4770–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semega J, Kollar M, Creamer J, & Mohanty A (2019). Income and poverty in the United States: 2018. U.S. Census Bureau. [Google Scholar]
- Shaver PR, & Mikulincer M (2002). Attachment-related psychodynamics. Attachment & Human Development, 4, 133–161. [DOI] [PubMed] [Google Scholar]
- Tabachnick AR, Raby KL, Goldstein A, Zajac L, & Dozier M (2020). Attachment security in infancy predicts reduced parasympathetic reactivity in middle childhood. Attachment & Human Development. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tietz NW (1976). Fundamentals of clinical chemistry. Saunders. [Google Scholar]
- Valente MJ, & MacKinnon DP (2017). Comparing models of change to estimate the mediated effect in the pretest–posttest control group design. Structural Equation Modeling: A Multidisciplinary Journal, 24, 428–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Russell DW, Mallinckrodt B, & Vogel DL (2007). The Experiences in Close Relationship Scale (ECR)-short form: Reliability, validity, and factor structure. Journal of Personality Assessment, 88, 187–204. [DOI] [PubMed] [Google Scholar]


