Abstract
Background:
Dopaminergic activity plays a role in mediating the rewarding aspects of abused drugs, including nicotine. Nicotine modulates the reinforcing properties of other motivational stimuli, yet the mechanisms of this interaction are poorly understood. This study aimed to ascertain the impact of nicotine exposure on neuronal activity associated with reinforcing outcomes in dependent smokers.
Methods:
Smokers (n = 28) and control subjects (n = 28) underwent functional imaging during performance of a monetary incentive delay task. Using a randomized, counterbalanced design, smokers completed scanning after placement of a nicotine or placebo patch; nonsmokers were scanned twice without nicotine manipulation. In regions along dopaminergic pathway trajectories, we considered event-related activity for valence (reward/gain vs. punishment/loss), magnitude (small, medium, large), and outcome (successful vs. unsuccessful).
Results:
Both nicotine and placebo patch conditions were associated with reduced activity in regions supporting anticipatory valence, including ventral striatum. In contrast, relative to controls, acute nicotine increased activity in dorsal striatum for anticipated magnitude. Across conditions, anticipatory valence-related activity in the striatum was negatively associated with plasma nicotine concentration, whereas the number of cigarettes daily correlated negatively with loss anticipation activity in the medial prefrontal cortex only during abstinence.
Conclusions:
These data suggest a partial dissociation in the state- and trait-specific effects of smoking and nicotine exposure on magnitude- and valence-dependent anticipatory activity within discrete reward processing brain regions. Such variability may help explain, in part, nicotine’s impact on the reinforcing properties of nondrug stimuli and speak to the continued motivation to smoke and cessation difficulty.
Introduction
Preclinical and human studies implicate brain regions along the mesocorticolimbic (MCL) and nigrostriatal (NS) dopamine (DA) pathways in processing reinforcing/rewarding stimuli, including drugs of abuse (1-9) (a complete list of abbreviations is also included in Supplement 1). It is hypothesized that DA’s role in reward processing involves the attribution of incentive salience to stimuli predicting rewards, rather than the hedonic experience of a reward and/or reward learning (10-12). Human functional imaging investigations partially support this postulation by highlighting the anatomic distinction in activation associated with hedonic versus motivational aspects of rewarding stimuli along MCL and NS DA pathways (13-22). Whereas reward anticipation appears to involve foci in the ventral striatum (VS), reward receipt is consistently associated with ventromedial prefrontal cortex activation (13-16, 23).
Nicotine’s central nervous effects are mediated via high-affinity nicotinic acetylcholine receptors (nAChRs) (24). That nAChRs are widely distributed throughout the brain, including in MCL and NS pathways, suggests that they play a role in modulating reward- related activity in dopaminergic (DArgic) pathway regions (25-27). Functional magnetic resonance imaging (fMRI) investigations in smokers support the involvement of these reward-related regions in a range of nicotine-related situational states (e.g., withdrawal, expectation, cue-induced reactivity, craving suppression) (28-35). Moreover, adult and adolescent smokers show reductions in anticipatory striatal activity for nondrug rewards (e.g. money) (36,37). Using a measure of reward learning for nondrug stimuli in a sub-sample of those included in the current study, we demonstrated nicotine-mediated reductions in learning-related striatal activity (38). Consistent with the notion that nicotine’s primary reinforcing properties include enhanced salience for motivational stimuli (39-42), these observations suggest that nicotine-dependent modulation of activity in reward-related regions in smokers likely extends to reinforcing stimuli beyond nicotine itself. However, although reduced anticipatory DArgic/MCL activity may be an antecedent to nicotine dependence (36), the relative impact of trait- and state- specific effects of smoking and nicotine exposure on the neural substrates of distinct reward processes has not been clearly delineated. Furthermore, the consequences of nicotine exposure may differ critically between nicotine-dependent and nondependent or nicotine-naive individuals.
Consequently, our aim was to determine whether trait (i.e., the combination of chronic nicotine exposure and potential risk factors for smoking) and state (i.e., acute nicotine administration) effects of nicotine would have a differential impact on the functional correlates of distinguishable reward processes in dependent smokers. Reward processing was assessed using a modified monetary incentive delay (MID) paradigm, which has been used to demonstrate regional specificity in DA pathway regions mediating reward anticipation and receipt (13-16). Because nicotine withdrawal is associated with state-specific changes in motivational processing, the relative differences between state- and trait-dependent aspects of nicotine use were considered in the absence of frank withdrawal. We hypothesized that being a chronically exposed, dependent smoker would engender reduced anticipatory DArgic/MCL activity (36-38). Following our earlier observation that acute nicotine did not have a differential impact on reward receipt (38), we further hypothesized that acute nicotine would affect motivational but not hedonic aspects of reward processing.
Methods and Materials
Participants
Adult dependent smokers (n = 28) and nonsmoking control subjects (n = 28) were recruited from the general population. Participants were matched for age, IQ, gender, and self-reported race (Table 1). Inclusion and exclusion criteria were as previously described (38).
Table 1.
Summary of Participant Demographics
| Smokers (n = 28) |
Control Subjects (n = 28) |
|
|---|---|---|
| Age, Years: Mean (SD) | 32.68 (10.02) | 30.11 (7.83) |
| Gender, Male:Female | 13:15 | 16:12 |
| IQ, WASI, Mean (SD) | 108.04 (11.63) | 107.65 (12.24) |
| Education, Years: Mean (SD) | 12.89 (2.49) | 14.00 (2.61) |
| Ethnicity, AA:C:As | 8:20:0 | 11:14:3 |
| Cigarettes/Day, Min-Max (mean) | 18–40 (22.80) | NA |
| Age at First Use, Years: Min-Max mean | 9–31 (15.46) | NA |
| Years of Use, Min-Max, (Mean) | 2.5–38 (16.48) | NA |
| FTND, Min-Max (Mean) | 3–9 (5.89) | NA |
There were no significant differences between groups on any demographic measure (see also Table S3 in the Supplement).
AA, African American; As, Asian/Asian American; C, Caucasian; FTND, Fagerström Test for Nicotine Dependence; Max, maximum; Min, minimum; NA, not applicable; WASI, Wechsler Adult Scale of Intelligence.
Procedure
This study was approved by the National Institute on Drug Abuse Intramural Research Program Institutional Review Board. Written informed consent was obtained from participants. Participation involved three visits: task/procedural training in a mock scanner and two MRI sessions. Each session also included a nicotine (Nicoderm; GlaxoSmithKline, Philadelphia, Pennsylvania) or placebo patch applied before scanning. Patch order was random and counterbalanced (n = 13 nicotine first). Participants refrained from consuming alcohol or over-the-counter medications for 24 hours and had no more than a half cup of caffeinated beverages before scanning. Prescanning monitoring of drug and alcohol use was as previously described (38) and included a urine drug test, alcohol breathlyzer, and expired carbon monoxide. Smokers completed a detailed smoking history, including the Fagerström Test for Nicotine Dependence (43) and time of last cigarette.
Patch Administration.
Patches were affixed to participants’ upper back 30 min after their last cigarette and 2 hours before scanning; a delay chosen as optimal for maximizing nicotine plasma concentrations in the nicotine condition and minimizing withdrawal in the placebo condition (44,45). Withdrawal, craving, and mood were queried pre- and postscanning using the Parrott Mood Questionnaire (46) and the Tobacco Craving Questionnaire (TCQ; 12-item short form) (47,48). Participants were debriefed regarding session order after study completion.
The Revised MID (MID-R).
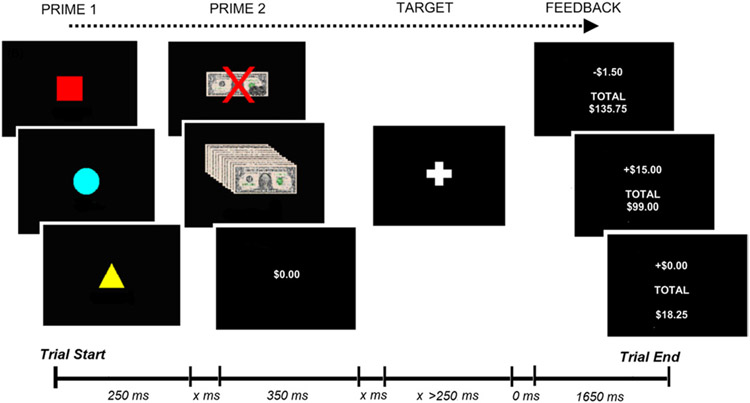
In the MID-R (Figure 1) participants attempted to press a button in response to a white cross (TARGET) during its visual presentation. The target’s initial duration was 250 msec. However, to ensure a “hit” on approximately two-thirds of trials, the duration was increased or decreased “online” in 25-msec intervals, depending on rate of success/failure.
Figure 1.
Revised monetary incentive delay task. x, variable presentation time (500 –3500 msec), where the two undefined intervals always summed to 4000 msec.
Trials included four sequential stimuli: PRIME-1, PRIME-2, TARGET, and FEEDBACK. PRIME-1 indicated trial valence: in Figure 1, the blue circle = gain, red square = loss, yellow triangle = neutral. Irrespective of performance, participants won $1 on gain trials and lost $.75 on loss trials. They neither won nor lost on neutral trials. PRIME-2 indicated the magnitude of potential monetary outcome, that is, one of three pseudo-randomly selected magnitudes (small, medium, large). To equate affective responding (22,49), potential losses were smaller than equivalent gains (gain = $2.50, $10, $15; loss = −$1.50, −$6, −$9). A “hit” on gain trials resulted in a monetary gain equivalent to PRIME-2 value, whereas only $1 was gained for a “miss.” On loss trials, a hit was associated with a $.75 loss, whereas a miss resulted in loss equal to PRIME-2. Trials concluded with FEEDBACK stimuli showing the trial outcome and current monetary total. Participants received a performance bonus equal to 10% of their winnings (up to $50/session).
Participants completed four, 10-min blocks per session, each consisting of 85 win, 85 loss and 28 neutral trials. Randomly selected rest periods (n = 64) were included to add temporal jitter.
Functional MRI.
Whole-brain echo planar images were acquired on a 3T Siemens Allegra scanner (Erlangen, Germany). Thirty-nine, 4-mm oblique axial (30° to anterior commissure-posterior commissure) slices were acquired with the following parameters: repetition time = 2000 msec; echo time = 27 msec; field of view = 220 X 220 mm at 64 X 64; flip angle = 78°. T1-weighted magnetization prepared rapid acquisition gradient echo structural images were also acquired in each session (voxel size = 1 mm3).
Blood Draw and Analysis.
Venous blood samples (5 mL) were collected from smokers immediately following each session (~40 min after MID-R) and analyzed as described previously (38,50).
Characterization.
Participants completed measures of attention and switching (51), memory (52), personality (53), exposure to environmental stressors (54,55), and current emotional state (56-59).
Data Analysis
Imaging data were analyzed using Analysis of Functional NeuroImages (60). Data quality control and motion correction procedures were as reported previously (38). Eight subjects were excluded for excessive motion. For remaining participants (n = 56), data time series were analyzed using voxelwise multiple regression. Regressors were expressed as a delta function convolved with a hemodynamic response function and its temporal derivative. Regressors included trial valence, magnitude, and feedback and motion parameters, which were included as regressors of no interest. A voxelwise average amplitude change equal to the percentage change from baseline (β) was calculated per participant, regressor, and session. The resultant activation maps were registered to a higher resolution (1 μL) standard stereotaxic space (61) and spatially blurred using a 4.2-mm full width at half maximum Gaussian isotropic kernel.
Random effects analyses considered group and drug condition effects on each task factor. Analyses included comparisons between control subjects and smokers (i.e., controls vs. smokers postplacebo [smokers+PBO] or postnicotine [smokers+NIC]) and between-conditions (i.e., smokers+PBO vs. smokers+NIC). To assess nicotine-mediated variability in reward-pathways and the potential influence of nicotine on other brain areas (62), we performed whole-brain analysis and a priori small volume correction (SVC) analyses. The SVC analyses considered activity in hypothesized DA pathway regions. Bilateral regions of interest (ROI) in the substantia nigra (SN), striatum (nucleus accumbens [NAcc], caudate, and putamen), and medial prefrontal cortex (mPFC; Brodmann’s area (BA) 10 and BA 32; Figure S1) were defined using a Talairach template. An ROI in the ventral tegmental area was defined as a 5-mm sphere at its anatomic locus (x, y, z = 0, −16, 7) (61). Voxelwise thresholds corrected for multiple comparisons were calculated using Monte Carlo simulations. Significance was determined as meeting or exceeding minimum cluster extent criteria at pCORRECTED < 0.05. Corrections for multiple comparisons were calculated separately for whole brain and SVC analyses. For SVC analyses, this correction accounted for the total ROI/SVC volume. The direction of significant results were confirmed with corrected (p < 0.05) predefined contrasts.
To account for the impact of the extent of nicotine exposure, post hoc linear regressions were used to explore the relationship between nicotine-related characteristics i.e., cigarettes per day [CPD], Fagerström, smoking duration (years), age at first cigarette, and plasma nicotine levels (PLASMA-NIC) and reward-related brain activity in smokers.
Behavioral data were analyzed in SPSS (IBM, Armonk, New York). Between-condition analyses of TCQ data considered four craving indices: emotionality, expectancy, compulsivity, and purposefulness. Mood rating analyses modeled changes across session (i.e., pre- vs. postscanning).
Results
Mood Ratings: Parrot
Controls Versus Smokers (Table S1 in Supplement).
As reported (38), irrespective of drug condition, session, or time (pre- vs. postsession), smokers were less relaxed and more alert than control subjects (p < 0.05). Furthermore, smokers were more irritated, dissatisfied, and distracted and less happy post- vs. presession (p < 0.05); there was no effect of time in control subjects.
Smokers+PBO Versus Smokers+NIC (Table S2 in Supplement).
Smokers+PBO were less relaxed, less calm, more irritated, and more distracted, compared with smokers+NIC (p < 0.05), suggesting the alleviation of mild withdrawal signs in the nicotine condition. There were no interactions of drug condition and time.
Mood Ratings: TCQ
See Table S2 in Supplement. Irrespective of drug condition, emotionality, expectancy, and purposefulness ratings were higher post- vs. presession (p < 0.05). Moreover, expectancy and purposefulness were higher in smokers+PBO vs. smokers+NIC (p < 0.05), supporting the notion of a mild deficit state in the placebo condition.
Characterization
Smokers and controls did not differ on any characterization measure (p > 0.05; Table S3 in Supplement).
Blood Plasma Analyses
Postsession plasma concentrations of nicotine, cotinine, and norcotinine were greater in smokers+NIC versus smokers+PBO (p < 0.005; Table S4 in Supplement).
Behavioral Data
Neither group nor drug condition influenced the final monetary outcome or reaction time on the MID-R. However, participants responded faster in the second session (p = 0.001; Table S5 in Supplement).
fMRI
Since our focus was nicotine’s impact on reward processing, control-only results are not discussed here. For completion, these data are provided in the Supplement (Table S9; Figures S2-S4).
Prime-1: Valence.
Prime-1 analyses considered the contrast of win and loss to neutral trials (i.e., [win - neutral] and [loss - neutral]), thus effectively limiting the influence of non-valence related activity. Whole-brain analyses suggested an effect of valence (i.e., gain > loss) bilaterally in the lingual gyrus and caudate. The same effect was seen in the right inferior frontal and left middle occipital gyri. SVC analyses confirmed the role of the caudate in processing trial valence (Table S6 in the Supplement).
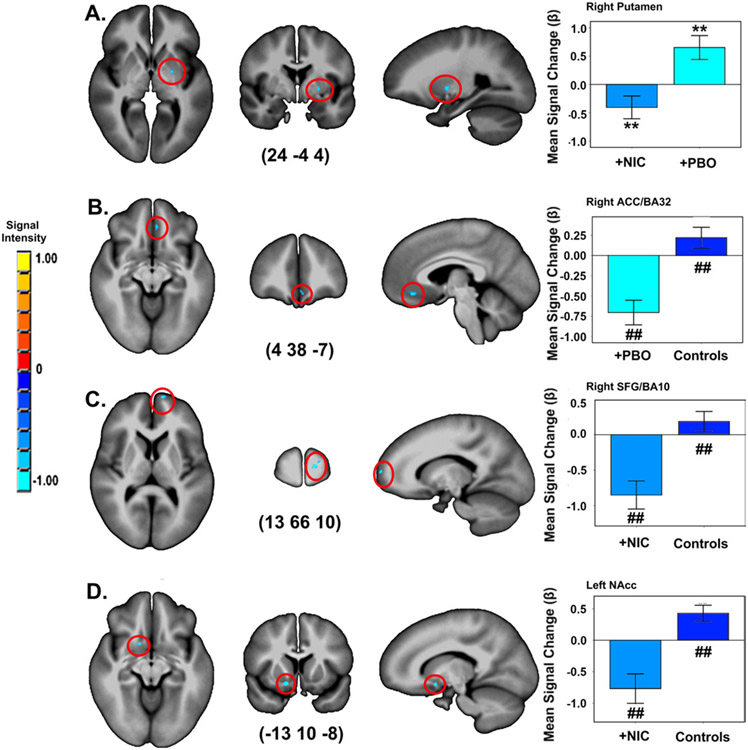
There were no effects of group or drug condition in whole-brain analyses. Conversely, SVC analyses revealed effects of both factors (Table 2, Figure 2). Valence-related function was reduced in the right putamen in smokers+NIC versus smokers+PBO. Similarly, smokers in both conditions exhibited lower valence-dependent activity compared with controls. Differences were seen in the right anterior cingulate cortex (ACC)/BA 32 (smokers+PBO < controls), right superior frontal gyrus (SFG)/BA10 (smokers+NIC < controls), and left NAcc (smokers+NIC < controls).
Table 2.
Group (Smokers vs. Control Subjects) and Drug Condition (Smokers+NIC vs. Smokers+PBO) Effects on Activity Associated with Trial Valence, Magnitude, and Outcome
| Talairach Coordinates |
||||||||
|---|---|---|---|---|---|---|---|---|
| Comparison (Drug/Group) |
Stimulus | Effect | Region | x | y | z | KE | Contrasta |
| Smokers+NIC vs. Smokers+PBO | Valence (Prime 1) | Drug conditionb | Right putamen | 24 | −4 | 4 | 38 | Nicotine < placebo |
| Outcome | Drug X outcomec | Left middle frontal gyrus | 28 | 15 | 53 | 249 | Nicotine: successful > unsuccessful Placebo: NS | |
| Controls vs. Smokers+NIC | Valence (Prime 1) | Groupb | Right SFG/BA 10 | 13 | 66 | 10 | 34 | Smokers < control subjects |
| Valence (Prime 1) | Groupb | Left NAcc | −13 | 10 | −8 | 28 | Smokers < control subjects | |
| Gain magnitude (Prime 2) | Groupb | Left caudate | −7 | 6 | 6 | 48 | Smokers > control subjects | |
| Loss magnitude (Prime 2) | Groupb | Left caudate | −10 | 1 | 14 | 55 | Smokers > control subjects | |
| Outcome | Group X outcomec | Left cingulate and BA 31 | −2 | −37 | 36 | 693 | Smokers: successful > unsuccessful control subjects: NS | |
| Controls vs. Smokers+PBO | Valence (Prime 1) | Groupb | Right ACC/BA 32 | 4 | 38 | −7 | 68 | Smokers < control subjects |
ACC, anterior cingulate cortex; BA, Brodmann’s area; KE, cluster extent (mm3); NAcc, nucleus accumbens; +NIC, postnicotine; NS, non significant; +PBO, postplacebo; SFG, superior frontal gyrus.
Significant at pCORRECTED < 0.05.
Small volume correction.
Whole-brain analysis.
Figure 2.
Impact of drug condition and group on valence-dependent activity in a priori regions of interest. (A) smokers postnicotine (smokers+NIC) vs. (B) postplacebo (smokers+PBO) vs. control subjects. (C, D) Smokers+NIC vs. control subjects. A priori contrasts p < 0.001/pCORRECTED < 0.05 **between conditions, ##between groups. Error bars show ± 1 SE. Activations are rendered on the ICBM452 T1 template from AFNI (60). ACC, anterior cingulate cortex; BA, Brodmann’s area; NAcc, nucleus accumbens; SFG, superior frontal gyrus.
Prime 2: Magnitude.
Gain magnitude was associated with activation in bilateral insula, left cingulate, caudate, BA 19, and right cuneus (Table S7 in Supplement). Magnitude-related changes in activity were also noted bilaterally in the cerebellum. Similarly, gain magnitude influenced activity in regions along the MCL DArgic pathway trajectory, including bilateral striatum (caudate and putamen), left ACC/BA 32, and SFG/BA 10. In these regions, activation was lowest for stimuli predicting small ($2.50) versus medium ($10) and/or large ($15) potential gains.
In contrast, loss magnitude influenced activity in a comparatively limited range of regions, including the right cuneus and lingual gyrus (Table S7 in Supplement). This effect was driven by reduced activity for primes signaling small (−$1.50) versus large impending losses (−$9).
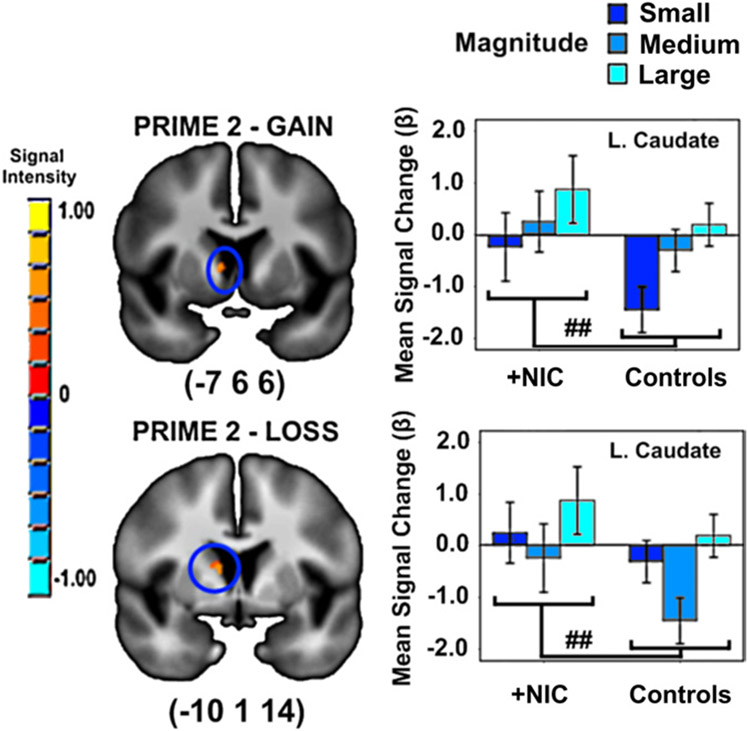
Drug condition did not alter activity associated with magnitude. However, there was an effect of group. Smokers+NIC exhibited greater loss and gain magnitude-related activity in left caudate compared with controls (Table 2, Figure 3).
Figure 3.
Impact of group (smokers postnicotine [+NIC] vs. control subjects) on magnitude-related activity in a priori regions of interest. ##A priori contrasts p < 0.001/pCORRECTED < 0.05 between groups. Error bars show ± 2 SE. Activations are rendered on the ICBM452 T1 template from AFNI (60).
Outcome.
Because of insufficient power to model all feedback levels, outcome analyses concentrated on two categories: successful (maximum gain or minimum loss) and unsuccessful (minimum gain or maximum loss). This distinction between categories was supported by exploratory analyses modeling all outcomes (Figure S5).
There was an effect of outcome (successful > unsuccessful) in all SVC regions (Table S8 in Supplement). Moreover, whole-brain analyses revealed identical outcome-related changes in a distributed set of brain areas, that is, left thalamus, right cuneus and bilateral precuneus, right superior and medial frontal gyri, right posterior cingulate, and the cerebellum.
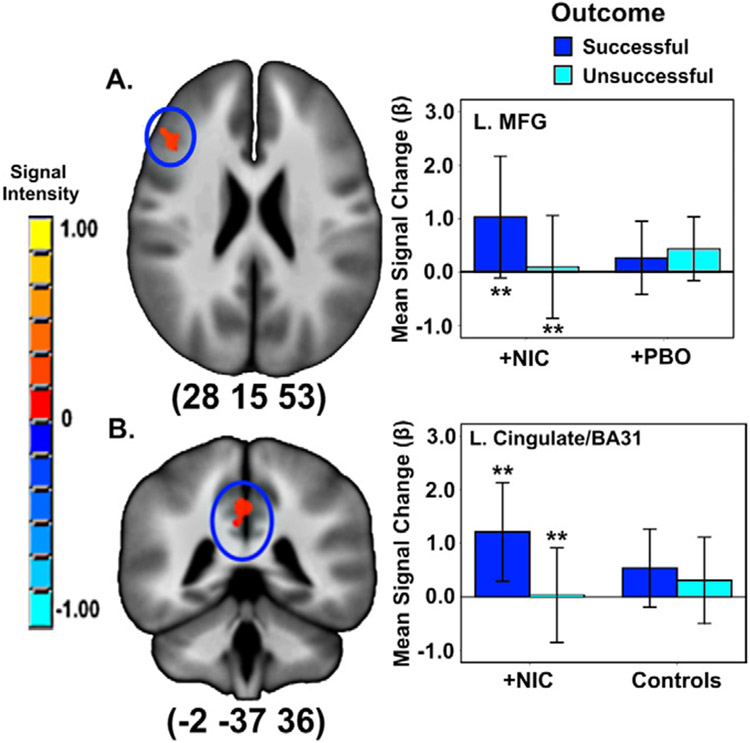
There was a drug condition X outcome interaction in the left middle frontal gyrus (MFG). Smokers+NIC showed an effect of outcome (successful > unsuccessful) in MFG that was absent in smokers+PBO (Figure 4). Similarly, there was a group X outcome interaction in left cingulate/BA 31. This was also due to an effect of outcome (successful > unsuccessful) in smokers+NIC only.
Figure 4.
Impact of (A) drug condition and (B) group (smokers postnicotine [+NIC] vs. controls) on outcome-related activity. **A priori contrasts p < 0.001/pCORRECTED < 0.05 between-conditions. Error bars show ± 2 SE. Activations are rendered on the ICBM452 T1 template from AFNI (60). BA, Brodmann’s area; L., left; MFG, middle frontal gyrus; +PBO, smokers postplacebo.
Nicotine-Related Characteristics.
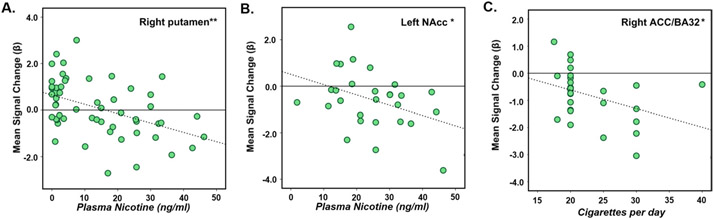
The impact of nicotine-related factors on activity in regions functionally defined by drug condition or group was explored in smokers. Only those regions showing changes in valence-related activity were influenced by these characteristics. For example, activity in the right putamen across trial types and conditions negatively correlated with PLASMA-NIC [F (1,26) = 13.79, p < 0.001; Figure 5A]. Similarly, gain-related activation in smokers in the left NAcc, where we noted an effect of group (smokers+NIC vs. controls), was also negatively associated with PLASMA-NIC [F (1,26) = 4.38, p < 0.05; Figure 5B]. Finally, activity related to loss anticipation negatively correlated with CPD in smokers+PBO in the right ACC/BA 32 [F (1,26) = 4.48, p < 0.05; Figure 5C].
Figure 5.
Significant linear regressions between valence-dependent anticipatory activity and nicotine/smoking characteristics. (A) Plasma nicotine (PLASMA-NIC) concentrations in smokers across conditions and trials types; R2 linear = 0.21; (B) PLASMA-NIC in smokers postnicotine (+NIC) and gain prime stimuli; R2 linear = 0.14; (C) cigarettes per day for smokers postplacebo (+PBO) and loss prime stimuli; R2 linear = 0.15. *p < 0.05. **p < 0.001. Partial overlap of data points in each of the plots did occur; however, the expected number of data points were included in each analysis and are represented in each plot (i.e., [A]: 56; [B]: 28; [C]: 28). ACC, anterior cingulate cortex; BA, Brodmann’s area; NAcc, nucleus accumbens.
Discussion
Using an incentive delay paradigm, we observed a partial dissociation in the trait- and state-related effects of smoking/nicotine exposure on activity related to discrete aspects of incentive motivation. Acute nicotine exposure in dependent smokers enhanced anticipatory magnitude-related function in the dorsal striatum (DS) and reward outcome sensitivity in mPFC. Moreover, both nicotine/ smoking conditions were associated with altered valence-dependent activity in the mPFC and VS. In smokers, valence-dependent activity in VS negatively correlated with PLASMA-NIC in both patch conditions. Conversely, mPFC activity was associated with CPD only in the placebo (i.e., trait) condition. Since the chronicity of nicotine exposure is related to the extent and duration of smoking, this latter observation may reflect chronicity-related changes in reward function.
Anticipatory Valence
Rewards generate robust increases in striatal DA release, particularly in the VS, which is associated with reward anticipation (13-14,63). In contrast, the perception of rewarding outcomes is commonly supported by mPFC activation (63). Since nicotine has an impact on function in these regions (25-27), the anatomic distinction for dissociable reward processes is important in delineating the motivational versus hedonic aspects of nicotine addiction.
Here nicotine and/or smoking were associated with reduced valence-dependent activity in DArgic reward pathway regions. Compared with control subjects, smokers showed lower activity for gain anticipation in mPFC (a region more synonymous with reward outcome), irrespective of patch type. Moreover, anticipatory valence was associated with reduced activity in VS in smokers+NIC compared with smokers+PBO and control subjects. Decreased incentive salience for nondrug rewards has been established in adult (37) and adolescent (36) abstinent smokers. Our data extend these observations by demonstrating the relative impact of state- and trait-related changes in motivational processing in smokers. Moreover, they suggest that decreased anticipatory valence-related activity for gains in mPFC in acutely abstinent smokers is not amenable to transdermal nicotine administration. Rather, acute nicotine may contribute to decreased anticipatory function related to valence in putative saliency regions. Nicotine’s acute impact on motivational processing may be critical to the drive to smoke and the likelihood of successful cessation. For example, reduced motivation for nondrug rewards in chronically dependent smokers may affect cessation strategies attempting to employ alternative rewards as a means of motivating smokers to abstain. If these changes are refractory to or exacerbated by acute nicotine, this may have important consequences for treatment strategy.
Nicotine-mediated changes in valence-dependent activity were affected by characteristics indexing acute (PLASMA-NIC) and chronic (CPD) nicotine exposure. These associations may involve nicotine’s influence on nAChRs. For example, chronic nicotine might affect brain activity via chronicity-related changes in the number and/or function of nAChRs in reward processing regions (64-68). Similarly, correlations between PLASMA-NIC and VS activity presumably reflect changes in nAChR occupancy following acute nicotine. Chronic nicotine exposure decreases DArgic function via selective upregulation of α4* receptors in striatal pathways and gamma-aminobutyric acid (GABA)ergic cells (65-67). Furthermore, nAChR subtypes that regulate cholinergic modulation of DA release (i.e., α4β2*) exhibit reduced availability following acute nicotine and smoking (51). Consequently, reduced valence-dependent activity and its relationship to PLASMA-NIC likely reflects selective up-regulation of α4β2* receptors in DArgic pathways coupled with high receptor occupancy levels in sated smokers, impacting cholinergic- and/or GABAergic-mediated DArgic activity.
Anticipatory Magnitude
In accordance with the notion that nicotine is a primary reinforcer and augments the motivational/reinforcing effects of accompanying stimuli (39-42,69), acute nicotine enhanced the saliency of stimuli indicating the magnitude of impending monetary outcomes. Magnitude-related activity for gains and losses was increased in the caudate in smokers+NIC versus controls. However, the absence of a condition-specific effect on magnitude suggests that this effect may potentially be due to some combination of the trait- and state-related effects of nicotine exposure.
Although VS sub-regions apparently code for valence and the magnitude of impending gains only (e.g., NAcc), the caudate mediates anticipated magnitude, irrespective of valence (63,70). Preclinical models of drug addiction suggest a shift from voluntary, goal-directed responding in the VS to more habitual, stimulus-driven activity in the DS via repeated cycles of drug exposure (71-74). Our data support DS sensitivity to nondrug stimuli. They also imply a shift from voluntary to habitual responding in dependent smokers, partially mediated by sensitivity to rewarding and punishing outcomes. Such dependency-mediated changes in caudate function appear to be driven by the saliency of anticipatory magnitude but not valence. Furthermore, these changes seem to be mediated by nicotine-related augmentation of function in DS, with a corresponding blunting in VS. This role of nicotine in DS is likely to have significant consequences for motivation and learning (75).
Outcome
In addition to anticipatory effects, there was dissociation in trait versus state effects of smoking/nicotine exposure in outcome-related activity. In smokers+NIC, there was a functional distinction in PFC regions between successful and unsuccessful outcomes that was not observed in smokers+PBO or controls (i.e., MFG/BA 6 and BA 31). Reduced subjective sensitivity to monetary gradients in other drug-dependent populations (e.g., cocaine) correlates with reduced activity in the MFG/BA 6 (76), an effect postulated to reflect reduced motivation (77). That the opposite pattern of activity was noted here (i.e., increased sensitivity to differential outcomes and increased MFG activity) may signal heightened motivational processing for nondrug rewards in dependent smokers under the acute influence of nicotine. This is in keeping with the notion of nicotine-mediated enhancement of reinforcing/motivational processing (39-42,69).
MID-R Versus MID
MID-R-related changes in activation were in accordance with previous MID studies (e.g., valence-dependent activation in VS and outcome-related activation in mPFC) (14-16). However, rewarding outcomes also increased activity in the striatum (VS and DS), an effect not seen in the original MID. This effect was independent of drug condition and consistent across striatal subregions. Thus, striatal activity was not specific to reward anticipation. Rather, it may be driven by saliency characteristics common to reward anticipation and receipt. Stimuli predicting rewards may have intrinsic salience that modifies behavior, whereas rewarding outcomes are salient because of their relevance in confirming the appropriateness of the preceding behavioral course. Striatal sensitivity to salience is in keeping with the postulations of Berridge and Robinson (10-12,78) and experimental observations of Zink and colleagues, who found striatal-mediated salience processing in the absence of reward (79-81).
Limitations
Our experimental design did not allow for disambiguation of acute nicotine effects and the prevention or alleviation of mild, early withdrawal. However, although some report that withdrawal may be detected as early as 2 hours (45) (i.e., consistent with the duration between patch placement and scanning here), others suggest that withdrawal symptoms manifest only after more extended periods (e.g., 6–12 hours) (82). Our results may also have been affected by the relative “heaviness” of chronic nicotine exposure in smokers, which could have contributed to the temporal trajectory of withdrawal/craving in abstinent smokers. Indeed, TCQ analyses indicated that smoking expectancy and intention to smoke were both greater in smokers+PBO. Thus, regardless of whether the results reflect acute effects of nicotine per se or alleviation of mild withdrawal, they speak to reward system functioning in smokers with or without nicotine at a time when they are ready for their next cigarette. However, because other symptoms of craving/withdrawal (i.e., emotionality and compulsivity) were not different between conditions, these differences may reflect a habitual tendency toward smoking. For example, smokers who normally smoke a pack or more per day would be conditioned to expect to smoke within 2 hours or less of their last cigarette in the absence of acute nicotine, irrespective of physiological symptoms of withdrawal. These issues warrant additional exploration in more varied groups of smokers. Other design approaches could consider reward-related activity at various time points since last cigarette, the inclusion of more substantially withdrawn participants, the administration of acute nicotine to nicotine-naive individuals or explicitly nondependent smokers may help to better clarify the nature of nicotine-dependent modulation of abstract reward processing.
Despite high levels of matching between groups, we are unable to delineate whether genetic risk for nicotine addiction or some interplay between genetic risk and environment (e.g., chronic nicotine) had an impact on our neurobiological outcomes. Addressing this would constitute an important extension of the work presented here and would require both the assessment of genetic variants that confer risk for smoking/nicotine addiction and substantially larger samples.
It is also important to consider individual differences in presession nicotine exposure or nicotine metabolism. To partially control for the former, times from last cigarette to patch and from patch to fMRI were tightly controlled. However, because of variability in CPD, these measures may have been insufficient to address this concern. Nonetheless, the within-subjects experimental design means that both factors would have been relatively consistent between drug conditions. For example, estimates of nicotine metabolism (i.e., ratio of plasma hydroxycotinine to cotinine) (83) were correlated across conditions [F (1,18) = 6.47, p < 0.05] and baseline levels of expired CO at the outset did not differ between conditions (p > 0.05).
Summary and Conclusions
Our data support the hypothesis that smoking and nicotine exposure have an impact on reward processing in DArgic pathway terminal regions that mediate motivational processing. They also extend previous observations regarding smoking-related reductions in anticipatory activity by delineating state- and trait-specific effects of nicotine/smoking on dissociable reward processes. Both conditions were associated with reductions in valence-dependent activity in VS and mPFC in smokers. However, following acute nicotine, smokers exhibited increased magnitude-dependent activity in the DS compared with control subjects only. Thus, by disentangling distinct aspects of saliency (i.e., valence and magnitude), we were able to observe dissociation in nicotine-mediated effects in anticipatory activity. That acute nicotine differentially affected magnitude- and valence-dependent activity in smokers indicates that reductions in neuronal activity associated with the motivational aspects of reward processing may be partially amendable to acute nicotine intake, but that this effect is stimulus specific. Moreover, this effect might be attributable to salience of the reinforcing stimuli rather than valence. Finally, there was no overall effect of nicotine on the hedonic aspects of monetary reward, yet we observed sensitivity to dissociable outcomes in PFC regions following acute nicotine that was absent in abstinent smokers and control subjects. This suggests nicotine-mediated changes in motivational processing and subjective sensitivity to rewarding versus punishing outcomes.
Delineating the influence of smoking trait-related and nicotine state-specific consequences on dissociable aspects of reward processing may inform smoking cessation strategies. For example, functional changes in DArgic reward pathways following chronic nicotine exposure are likely to be crucial to the maintenance of smoking and contribute to general dysfunction in reward processing. Moreover, acute nicotine’s impact on already altered reward processing likely contributes to the desire to smoke and/or the ability of nicotine replacement therapies to support cessation. Because the motivational aspects of reward processing appear to be supported by functions in anatomically distinct DArgic regions, the comparative influence of acute and/or chronic nicotine on activity in these areas is perhaps critical to the distinction between the desire to smoke (motivation) versus the pleasure derived from smoking (hedonia) in dependent smokers. These data may be usefully applied, potentially with genetic information, to individualize treatment choices and identify those who may be most receptive to nicotine replacement therapy versus perhaps, other classes of pharmacologic intervention.
Supplementary Material
Acknowledgements
This study was supported by the National Institute on Drug Abuse-Intramural Research Program. EJR is currently affiliated with the Transdisciplinary Science and Translational Prevention Program, Molecular Epidemiology, Genomics, Environment and Health, RTI International, Baltimore, Maryland.
We thank Loretta Spurgeon, Kimberley Slater, Eliscia Smith, and National Institute on Drug Abuse, Intramural Research Program nursing and recruitment staff for their invaluable assistance in running this study. We also thank Drs. J. Waltz and J. Gold (University of Maryland Psychiatric Research Center) and Dr. J. Schweitzer (University of California, Davis) for their contributions to the design of the revised monetary inventive delay task.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Ikemoto S (2007): Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56:27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikemoto S, Panksepp J (1996): Dissociations between appetitive and consummatory responses by pharmacological manipulations of reward-relevant brain regions. Behav Neurosci 110:331–345. [DOI] [PubMed] [Google Scholar]
- 3.Ikemoto S, Panksepp J (1999): The role of nucleus accumbens dopamine in motivated behavior: A unifying interpretation with special reference to reward-seeking. Brain Res Rev 31:6–41. [DOI] [PubMed] [Google Scholar]
- 4.McBride WJ, Murphy JM, Ikemoto S (1999): Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res 101:129–152. [DOI] [PubMed] [Google Scholar]
- 5.Diekhof EK, Falkai P, Gruber O (2008): Functional neuroimaging of reward processing and decision-making: A review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev 59:164–184. [DOI] [PubMed] [Google Scholar]
- 6.O’Doherty JP (2004): Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Curr Opin Neurobiol 14:769–776. [DOI] [PubMed] [Google Scholar]
- 7.McClure SM, York MK, Montague PR (2004): The neural substrates of reward processing in humans: The modern role of fMRI. Neuroscientist 10:260–268. [DOI] [PubMed] [Google Scholar]
- 8.Wise RA (2009): Roles for nigrostriatal—not just mesocorticolimbic— dopamine in reward and addiction. Trends Neurosci 32:517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koob GF, Nestler EJ (1997): The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci 9:482–497. [DOI] [PubMed] [Google Scholar]
- 10.Berridge KC (2007): The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology 191:391–431. [DOI] [PubMed] [Google Scholar]
- 11.Berridge KC, Robinson TE (1998): What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Rev 28:309–369. [DOI] [PubMed] [Google Scholar]
- 12.Berridge KC, Robinson TE (2003): Parsing reward. Trends Neurosci 26: 507–513. [DOI] [PubMed] [Google Scholar]
- 13.Knutson B, Adams CM, Fong GW, Hommer D (2001): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21:RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knutson B, Fong GW, Adams CM, Varner JL, Hommer D (2001): Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12:3683–3687. [DOI] [PubMed] [Google Scholar]
- 15.Knutson B, Fong GW, Bennett SM, Adams CM, Homme D (2003): A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event-related fMRI. NeuroImage 18: 263–272. [DOI] [PubMed] [Google Scholar]
- 16.Knutson B, Westdorp A, Kaiser E, Hommer D (2000): FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 12: 20–27. [DOI] [PubMed] [Google Scholar]
- 17.O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ (2002): Neural responses during anticipation of a primary taste reward. Neuron 33:815–826. [DOI] [PubMed] [Google Scholar]
- 18.Berns GS, McClure SM, Pagnoni G, Montague PR (2001): Predictability modulates human brain response to reward. J Neurosci 21:2793–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McClure SM, Berns GS, Montague PR (2003): Temporal prediction errors in a passive learning task activate human striatum. Neuron 38:339–346. [DOI] [PubMed] [Google Scholar]
- 20.O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ (2004): Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304:452–454. [DOI] [PubMed] [Google Scholar]
- 21.O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C (2001): Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci 4:95–102. [DOI] [PubMed] [Google Scholar]
- 22.Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P (2001): Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 30:619–639. [DOI] [PubMed] [Google Scholar]
- 23.Knutson B, Bjork JM, Fong GW, Hommer D, Mattay VS, Weinberger DR (2004): Amphetamine modulates human incentive processing. Neuron 43:261–269. [DOI] [PubMed] [Google Scholar]
- 24.Dani JA, Heinemann S (1996): Molecular and cellular aspects of nicotine abuse. Neuron 16:905–908. [DOI] [PubMed] [Google Scholar]
- 25.Clementi F, Fornasari D, Gotti C (2000): Neuronal nicotinic acetylcholine receptors: From structure to therapeutics. Trends Pharmacol Sci 21:35–37. [DOI] [PubMed] [Google Scholar]
- 26.Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, et al. (2010): Nicotinic acetylcholine receptors in the mesolimbic pathway: Primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci 30:5311–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C (2002): Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci 22:8785–8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azizian A, Monterosso J, O’Neill J, London ED (2009): Magnetic resonance imaging studies of cigarette smoking. In: Henningfield JE, London ED, Pogun S, editors. Nicotine Psychopharmacology. Berlin: Spinger-Verlag, 113–143. [Google Scholar]
- 29.Brody AL, Mandelkern MA, Olmstead RE, Jou J, Tiongson E, Allen V, et al. (2007): Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry 62:642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.David SP, Munafo MR, Johansen-Berg H, MacKillop J, Sweet LH, Cohen RA, et al. (2007): Effects of acute nicotine abstinence on cue-elicited ventral striatum/nucleus accumbens activation in female cigarette smokers: A functional magnetic resonance imaging study. Brain Imaging Behav 1:43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, et al. (2005): Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: A functional magnetic resonance imaging study. Biol Psychiatry 58:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Due DL, Huettel SA, Hall WG, Rubin DC (2002): Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: Evidence from functional magnetic resonance imaging. Am J Psychiatry 159:954–960. [DOI] [PubMed] [Google Scholar]
- 33.McBride D, Barrett SP, Kelly JT, Aw A, Dagher A (2006): Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: An fMRI study. Neuropsychopharmacology 31:2728–2738. [DOI] [PubMed] [Google Scholar]
- 34.Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, et al. (2006): Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psycho-pharmacology 184:577–588. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Salmeron BJ, Ross TJ, Gu H, Geng X, Yang Y, et al. (2011): Anatomical differences and network characteristics underlying smoking cue reactivity. Neuroimage 54:131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, et al. (2011): Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiatry 168:540–549. [DOI] [PubMed] [Google Scholar]
- 37.Buhler M, Vollstadt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF, et al. (2010): Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biol Psychiatry 67:745–752. [DOI] [PubMed] [Google Scholar]
- 38.Rose EJ, Ross TJ, Salmeron BJ, Lee M, Shakleya DM, Huestis M, et al. (2012): Chronic exposure to nicotine is associated with reduced reward-related activity in the striatum but not the midbrain. Biol Psychiatry 71:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donny EC, Caggiula AR, Weaver MT, Levin ME, Sved AF (2011): The reinforcement-enhancing effects of nicotine: Implications for the relationship between smoking, eating and weight. Physiol Behav 104:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, et al. (2003): Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: Implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 169: 68–76. [DOI] [PubMed] [Google Scholar]
- 41.Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, et al. (2006): Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 184:391–400. [DOI] [PubMed] [Google Scholar]
- 42.Palmatier MI, Liu X, Caggiula AR, Donny EC, Sved AF (2007): The role of nicotinic acetylcholine receptors in the primary reinforcing and reinforcement-enhancing effects of nicotine. Neuropsychopharmacology 32:1098–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991): The Fagerstrom Test for Nicotine Dependence—A revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 44.Gorsline J, Gupta SK, Dye D, Rolf CN (1993): Steady-state pharmacokinetics and dose relationship of nicotine delivered from Nicoderm (Nicotine Transdermal System). J Clin Pharmacol 33:161–168. [DOI] [PubMed] [Google Scholar]
- 45.Hendricks PS, Ditre JW, Drobes DJ, Brandon TJ (2006): The early time course of smoking withdrawal effects. Psychopharmacology 187:385–396. [DOI] [PubMed] [Google Scholar]
- 46.Parrott AC, Garnham NJ, Wesnes K, Pincock C (1996): Cigarette smoking and abstinence: Comparative effects upon cognitive task performance and mood state over 24 hours. Hum Psychopharmacol Clin Exp 11:391–400. [Google Scholar]
- 47.Heishman SJ, Singleton EG, Moolchan ET (2003): Tobacco Craving Questionnaire: Reliability and validity of a new multifactorial instrument. Nicotine Tob Res 5:645–654. [DOI] [PubMed] [Google Scholar]
- 48.Heishman SJ, Singleton EG, Pickworth WB (2007): Reliability and validity of a short form of the tobacco craving questionnaire. Nicotine Tob Res 10:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahneman D, Tversky A (1979): Prospect theory—Analysis of decision under risk. Econometrica 47:263–291. [Google Scholar]
- 50.Shakleya DM, Huestis MA (2009): Simultaneous and sensitive measurement of nicotine, cotinine, trans-3’-hydroxycotinine and norcotinine in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 877:3537–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reitan RM, Wolfson D (1993): The Halstead-Reitan Neuropsychological Test Battery. Tuscan, AZ: Neuropsychology Press. [Google Scholar]
- 52.Wechsler D (1987): Wecshler Memory Scale—Revised Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- 53.Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD (1994): The Temperament and Character Inventory (TCI): A Guide to Its Development and Use. St. Louis, MO: Washington University, Center for Psychobiology of Personality. [Google Scholar]
- 54.Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. (1994): Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry 151:1132–1136. [DOI] [PubMed] [Google Scholar]
- 55.Brugha TS, Bebbington PE, Stretch DD, MacCarthy B, Wykes T (1997): Predicting the short-term outcome of first episodes and recurrences of clinical depression: A prospective study of life events, difficulties, and social support networks. J Clin Psychiatry 58:298–306. [DOI] [PubMed] [Google Scholar]
- 56.Beck AT (1993): The Beck Anxiety Inventory. London: The Psychological Corporation. [Google Scholar]
- 57.Beck AT (1996): The Beck Depression Inventory—II. London: The Psychological Corporation. [Google Scholar]
- 58.Watson D, Clark LA, Tellegen A (1988): Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 54:1063–1070. [DOI] [PubMed] [Google Scholar]
- 59.Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, et al. (2002): Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med 32:959–976. [DOI] [PubMed] [Google Scholar]
- 60.Cox RW (1996): AFNI software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- 61.Talairach J, Tournoux P (1988): Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme. [Google Scholar]
- 62.Sharma A, Brody AL (2009): In vivo brain imaging of human exposure to nicotine and tobacco. Handb Exp Pharmacol (192):145–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haber SN, Knutson B (2010): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penton RE, Lester RA (2009): Cellular events in nicotine addiction. Semin Cell Dev Biol 20:418–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xiao C, Nashmi R, McKinney S, Cai H, McIntosh JM, Lester HA (2009): Chronic nicotine selectively enhances alpha4beta2* nicotinic acetylcholine receptors in the nigrostriatal dopamine pathway. J Neurosci 29:12428–12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, et al. (2007): Chronic nicotine cell specifically upregulates functional alpha 4*nicotinic receptors: Basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci 27:8202–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCallum SE, Parameswaran N, Bordia T, Fan H, Tyndale RF, Langston JW, et al. (2006): Increases in alpha4* but not alpha3*/alpha6* nicotinic receptor sites and function in the primate striatum following chronic oral nicotine treatment. J Neurochem 96:1028–1041. [DOI] [PubMed] [Google Scholar]
- 68.Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, et al. (2003): Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci 23:7820–7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glautier S, Clements K, White JA, Taylor C, Stolerman IP (1996): Alcohol and the reward value of cigarette smoking. Behav Pharmacol 7:144–154. [PubMed] [Google Scholar]
- 70.Delgado MR, Locke HM, Stenger VA, Fiez JA (2003): Dorsal striatum responds to reward and punishment: Effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci 3:27–38. [DOI] [PubMed] [Google Scholar]
- 71.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW (2008): Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Phil Trans R Soc B Biol Sci 363:3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Everitt BJ, Dickinson A, Robbins TW (2001): The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev 36:129–138. [DOI] [PubMed] [Google Scholar]
- 73.Robbins TW, Everitt BJ (1999): Drug addiction: Bad habits add up. Nature 398:567–570. [DOI] [PubMed] [Google Scholar]
- 74.Vanderschuren LJ, Di Ciano P, Everitt BJ (2005): Involvement of the dorsal striatum in cue–controlled cocaine seeking. J Neurosci 25:8665–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balleine BW, O’Doherty JP (2010): Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual control. Neuropsychopharmacology 35:48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldstein RZ, Tomasi D, Alia-Klein N, Cottone LA, Zhang L, Telang F, et al. (2007): Subjective sensitivity to monetary gradients is associated with frontolimbic activation to reward in cocaine abusers. Drug Alcohol Depend 87:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, et al. (2007): Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry 164:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robinson TE, Berridge KC (2008): The incentive sensitization theory of addiction: Some current issues. Phil Trans R Soc B Biol Sci 363:3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zink CF, Pagnoni G, Chappelow J, Martin-Skurski M, Berns GS (2006): Human striatal activation reflects degree of stimulus saliency. NeuroImage 29:977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zink CF, Pagnoni G, Martin ME, Dhamala M, Berns GS (2003): Human striatal response to salient nonrewarding stimuli. J Neurosci 23:8092–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS (2004): Human striatal responses to monetary reward depend on saliency. Neuron 42:509–517. [DOI] [PubMed] [Google Scholar]
- 82.Hughes JR, Higgins ST, Bickel WK (1994): Nicotine withdrawal versus other drug withdrawal syndromes: Similarities and dissimilarities. Addiction 89:1461–1470. [DOI] [PubMed] [Google Scholar]
- 83.Lerman C, Jepson C, Wileyto EP, Patterson F, Schnoll R, Mroziewicz M, et al. (2010): Genetic variation in nicotine metabolism predicts the efficacy of extended-duration transdermal nicotine therapy. Clin Pharmacol Ther 87:553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.