Abstract
In the manuscript, reductive and decarboxylative azaarylation of coumarin-3-carboxylic acids is described. It utilizes the photocatalytic activation of (cyano)azaarenes in the presence of fac-Ir(ppy)3 as a photocatalyst. The methodology is versatile and provides access to biologically relevant 4-substituted-chroman-2-ones. Visible light, photoredox catalyst, base, anhydrous solvent, and inert atmosphere constitute key parameters for the success of the described strategy. The developed methodology involves a wide range of coumarin-3-carboxylic acids as well as (cyano)azaarenes.
Introduction
Chroman-2-one, pyridine, and their derivatives constitute privileged structural motifs present in various natural products.1 Representative examples of both groups of compounds are shown in Scheme 1. Although these compounds are abundant in nature, synthetic methods for their preparation are of importance.2 In this context, it is worth noting that pyridine is the second most frequent nitrogen-containing heterocyclic scaffold that is present in 62 U.S. FDA approved drugs displaying a wide range of biological activities.3 Thus, the pyridine skeleton often serves as a “privileged” scaffold in drug design and discovery. Moreover, it is also a versatile building block utilized for the synthesis of chiral ligands applied in asymmetric catalysis.4 As a consequence, a lot of effort has been devoted toward the development of methods for the synthesis of pyridine derivatives.5 Recently, radical-based pyridylation reactions have attracted much attention, providing a flexible approach to pyridine derivatives by the application of photocatalysis. These strategies benefit from good functional group tolerance, procedural simplicity, and mild reaction conditions.6
Scheme 1. Importance of Chroman-2-one and Pyridine Derivatives.
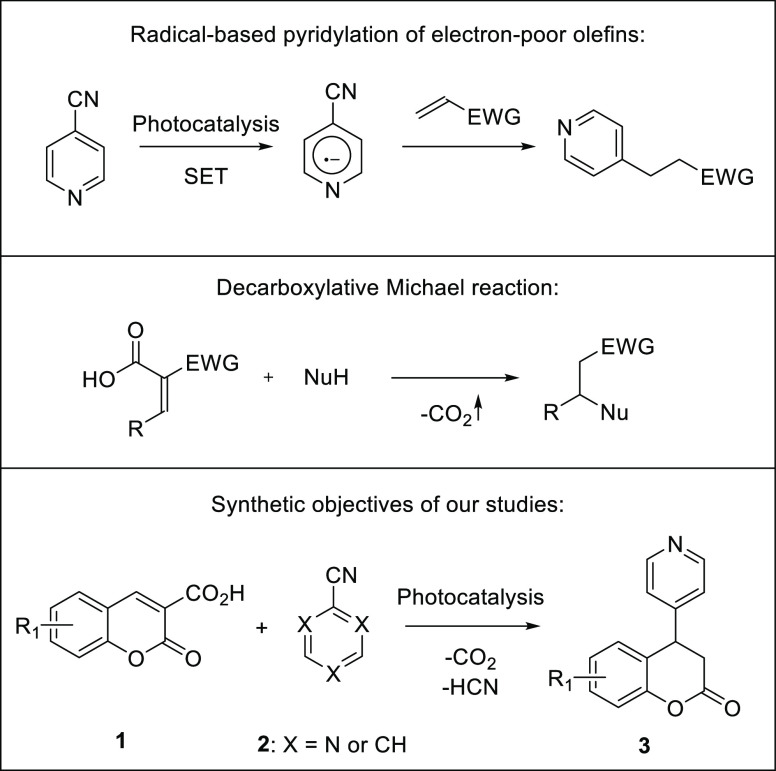
The addition of free radicals to electron-deficient olefins is known as Giese reaction (Scheme 2).7 Recent advancements in this field arise from the development of photo-mediated methods allowing for the free-radical formation under mild and nontoxic conditions.8
Scheme 2. Importance of Decarboxylative Approaches in Organic Synthesis and the Objectives of Our Study.
A decarboxylative Michael reaction based on nucleophilic addition to carboxylic-acid-activated olefins followed by a decarboxylation reaction constitutes a powerful synthetic tool.9 Recently, we described the first photocatalytic, doubly decarboxylative Giese reaction applicable to a wide range of carboxylic acids.10 Coumarin-3-carboxylic acids 1 constitute useful acceptors in this reaction, opening access to biologically relevant chroman-2-ones 3.11 Given the interesting properties of coumarin and pyridine derivatives, the task of development of synthetic routes leading to hybrid molecules bearing both structural motifs was undertaken. Notably, the synthesis of hybrid molecules containing more than one biologically active unit constitutes an important approach in modern drug design.12
Herein, we present our studies on the development of decarboxylative reductive arylation of coumarin-3-carboxylic acids. (Cyano)azaarenes were applied as nucleophiles in the Giese-type transformation. This methodology benefits from mild reaction conditions and a broad scope of substrates.
Results and Discussion
Initially, reactions between cyanopyridine 2a and coumarin derivatives 1 bearing either no or various activating groups in the 3-position were performed (Table 1, entries 1–4). Experiments were performed in acetonitrile in the presence of 4a as a photocatalyst and triethylamine as a base under irradiation with blue light and an inert atmosphere at room temperature. When simple coumarin 5a was used, no reaction was observed. Therefore, EWG-activated coumarin derivatives 1b–e were tested. Surprisingly, derivatives 5b–d displayed no reactivity under these conditions. To our delight, the incorporation of the carboxylic acid moiety into the structure of coumarin 1a resulted in the formation of the desired product 3aa, indicating the crucial role of the carboxylic-acid-group activation in the devised reactivity (Table 1, entry 5). Further optimization studies were performed using coumarin-3-carboxylic acid 1a and 4-cyanopyridine 2a as model substrates (Table 1, entries 6–22). In the first part of the optimization studies, the catalytic activity of six different photoredox catalysts was tested (with the irradiation with the light source of suitable wavelength) (Table 1, entries 5–10). When Eosin Y 4b was used, the formation of target product 3aa was not observed (Table 1, entry 6). Catalysts 4a and 4c–f provided the desired reactivity (Table 1, entries 5 and 7–10, respectively) with the best results obtained in the presence of catalysts 4a (Table 1, entry 5). In the course of further studies, the amount of 4-cyanopyridine 2a was tested. It was shown that the reaction with a 3-fold excess of 2a gave the product 3aa with 49% yield (Table 1, entry 11). Further increasing the amount of 4-cyanopyridine 2a did not improve the result. In the next step of optimization studies, the effect of the solvent on the reaction outcome was evaluated (Table 1, entries 11–16). The use of different solvents ensured the product formation; however, the best result was obtained when dimethyl sulfoxide was employed (Table 1, entry 13). During further investigations, the amount of catalyst 4a was studied (Table 1, entries 16–18). It proved possible to be lowered to 3 mol % without a significant change of the result (Table 1, entry 17). Furthermore, the effect of base on the reaction outcome was evaluated (Table 1, entries 19–21). When DABCO was used, product 3aa was not formed (Table 1, entry 20) and the application of DIPEA and N-methyl morpholine resulted in diminished yields (Table 1, entries 19 and 21). In the course of further studies, control experiments were performed (Table 1, entries 24–26). The use of stoichiometric amount of Et3N yielded the product 3aa with low yield (25%) (Table 1, entry 24). The reaction did not proceed in the absence of photoredox catalysts (Table 1, entry 25). A similar effect was observed when the transformation was attempted in the dark (Table 1, entry 26), thus confirming the crucial effect of photocatalyst and the source of light on the reaction outcome. Notably, the optimized reaction proved readily scalable to a 2 mmol scale and the product 3aa was obtained with a high yield (Table 1, entry 27). Finally, the experiment in the presence of TEMPO was carried out and no reaction was observed, thus confirming the radical nature of the developed reaction (Table 1, entry 28).
Table 1. Visible Light-Driven Reductive Azaarylation of Coumarin 1a and Its Derivatives 5a–d: Optimization studiesa.
| entry | catalyst | X | solvent | base | catalyst [mol %] | yield [%] |
|---|---|---|---|---|---|---|
| 1b | 4a | H (5a) | CH3CN | Et3N | 10 | |
| 2b | 4a | CN (5b) | CH3CN | Et3N | 10 | |
| 3b | 4a | CO2Et (5c) | CH3CN | Et3N | 10 | |
| 4b | 4a | C(O)Ph (5d) | CH3CN | Et3N | 10 | |
| 5b | 4a | CO2H (1a) | CH3CN | Et3N | 10 | 30 |
| 6c | 4b | CO2H (1a) | CH3CN | Et3N | 10 | |
| 7b | 4c | CO2H (1a) | CH3CN | Et3N | 10 | 21 |
| 8b | 4d | CO2H (1a) | CH3CN | Et3N | 10 | 14 |
| 9b | 4e | CO2H (1a) | CH3CN | Et3N | 10 | 24 |
| 10b | 4f | CO2H (1a) | CH3CN | Et3N | 10 | 12 |
| 11b,d | 4a | CO2H (1a) | CH3CN | Et3N | 10 | 49 |
| 12b,d | 4a | CO2H (1a) | CH2Cl2 | Et3N | 10 | 27 |
| 13b,d | 4a | CO2H (1a) | DMSO | Et3N | 10 | 61 |
| 14b,d | 4a | CO2H (1a) | DMF | Et3N | 10 | 15 |
| 15b,d | 4a | CO2H (1a) | CH3OH | Et3N | 10 | 26 |
| 16b,d | 4a | CO2H (1a) | DMSO | Et3N | 5 | 67 |
| 17b,d | 4a | CO2H (1a) | DMSO | Et3N | 3 | 68 |
| 18b,d | 4a | CO2H (1a) | DMSO | Et3N | 1 | 47 |
| 19b,d | 4a | CO2H (1a) | DMSO | DIPEA | 3 | 42 |
| 20b,d | 4a | CO2H (1a) | DMSO | DABCO | 3 | |
| 21b,d | 4a | CO2H (1a) | DMSO | NMM | 3 | 49 |
| 22b,d,e | 4a | CO2H (1a) | DMSO | Et3N | 3 | 81 |
| 23b,d,e,f | 4a | CO2H (1a) | DMSO | Et3N | 3 | 93 |
| 24b,d,e,f,g | 4a | CO2H (1a) | DMSO | Et3N | 3 | 25 |
| 25d,e,f | 4a | CO2H (1a) | DMSO | Et3N | ||
| 26b,d,e,f,h | 4a | CO2H (1a) | DMSO | Et3N | 3 | |
| 27b,d,e,f,i | 4a | CO2H (1a) | DMSO | Et3N | 3 | 74 (333 mg) |
| 28j | 4a | CO2H (1a) | DMSO | Et3N | 3 |
All reactions were performed in a 0.1 mmol scale using 1a or 5 (1.0 equiv) and 2a (2.0 equiv) in the presence of the corresponding photoredox catalyst 4 (10 mol %) and the corresponding base (2.5 equiv) in the solvent (1 mL) for 24 h at room temperature.
Reaction performed under irradiation with blue light.
Reaction performed under irradiation with green light.
Reaction performed using 2a (3 equiv).
Reaction performed for 48 h.
Reaction performed in DMSO (3 mL).
Reaction performed using Et3N (1 equiv).
Reaction performed in the dark.
Reaction performed at a 2 mmol scale.
Reaction performed in the presence of TEMPO (1 equiv).
With the optimized reaction conditions in hand (Table 1, entry 23), the scope of the developed methodology was evaluated (Schemes 3 and 4). Initially, various coumarin-3-carboxylic acids 1a–m were tested in the reaction (Scheme 3). Acids 1b–f bearing electron-donating groups on the aromatic ring provided products 3ab–af with very good yields. For the coumarin carboxylic acid 1a with a t-butyl substituent at the 6-position of the aromatic ring, the yield was the highest despite the presence of a bulky t-butyl substituent. In the course of further studies, it was found that substrates 1 bearing electron-withdrawing groups delivered products 3 in diminished yields. Short reoptimization studies indicated that modification of a previously developed procedure (involving dropwise addition of coumarin carboxylic acids 1g–m in dry DMSO (1 mL) over 2 h to the reaction mixture, see general procedure for details) enabled the improvement of the results. Dropwise addition of coumarin carboxylic acids 1g–m suppressed its decomposition over reaction time. Under these conditions, the reaction using coumarins 1g–k bearing fluorine, bromine, or chlorine atoms at various positions provided the corresponding products 3g–k in moderate to high yields. It is only in the case of coumarin 1k with a chlorine substituent in the 8-position of the aromatic ring that the yield of the reaction dropped to 34%. Similar results were observed for doubly substituted coumarin 1l. In this context, it is worth noting that coumarin 1l was not effective in the previous decarboxylative reactions performed by our group.9d,10 What is also worth emphasizing is that the reaction with doubly substituted coumarin 1m with two methoxy substituents in the aromatic ring provided the desired product 3am with very good yield.
Scheme 3. Visible Light-Driven Reductive Arylation of Coumarin-3-carboxylic Acids 1: Scope of Coumarin-3-carboxylic Acids 1.
Scheme 4. Visible Light-Driven Reductive Arylation of Coumarin-3-carboxylic Acids 1: Reaction Involving Cyanoheteroaromatic Derivatives 2a–2c.
Subsequently, the scope of the methodology with regard to different (cyano)azaarenes 2a–c was evaluated (Scheme 4). It was demonstrated that the developed protocol worked well for 4- and 2-substituted pyridines 2a and 2b as well as pyrimidine-2-carbonitrile 3c to give target products 3aa–3ca with very good yields. Disappointingly, no product formation was observed when cyanopyridines 2d and 2e were employed under optimized reaction conditions.
The postulated mechanism of the developed methodology begins with the blue light-driven excitation of the photocatalyst 4b (Scheme 5). Then, the electron transfer from the triethylamine to the photocatalyst takes place. Fluorescence quenching and cyclic voltammetry experiments confirmed the lack of quenching in the case of acids 1a as well as cyanopyridine 2a (for details, see the Supporting Information). Subsequently, the reduced Ir-catalyst acts as a reductant of the (cyano)azaarene 2a to give 7. The newly formed radical 7 undergoes the decarboxylative Giese-type reaction with the acceptor 8 to give 9 that undergoes hydrogen atom transfer to give 10. Two separate processes transform 10 into 3aa: (1) rearomatization of the pyridine ring via dehydrocyanation and (2) decarboxylative protonation to afford 3aa as the final product.
Scheme 5. Visible Light-Driven Reductive Arylation of Coumarin-3-carboxylic Acids 1: Reaction Mechanism.
Conclusions
In conclusion, we have developed a decarboxylative photocatalytic reductive arylation of coumarin-3-carboxylic acids 1 that represents a unique application of free-carboxylic-acid-activated olefins in radical transformations. The reactions between coumarin-3-carboxylic acids 1a–m and (cyano)azaarenes 2a–c were realized under photocatalytic activation in the presence of only 3 mol % of fac-Ir(ppy)3. The methodology proved versatile, leading to biologically relevant 4-substituted-chroman-2-ones 3aa–ca in good to high yields under mild reaction conditions.
Experimental Section
General Information
NMR spectra were acquired on a Bruker Ultra Shield 700 instrument, running at 700 MHz for 1H and 176 MHz for 13C. Chemical shifts (δ) are reported in ppm relative to residual solvent signals (CDCl3: 7.26 ppm for 1H NMR, 77.16 ppm for 13C NMR). Mass spectra were recorded on a Bruker Maxis Impact spectrometer using electrospray (ES+) ionization (referenced to the mass of the charged species). Analytical thin layer chromatography (TLC) was performed using pre-coated aluminum-backed plates (Merck Kieselgel 60 F254) and visualized by ultraviolet irradiation. Unless otherwise noted, analytical-grade solvents and commercially available reagents were used without further purification. For flash chromatography (FC), silica gel (w/ Ca, ∼0.1%, 230–400 mesh), green LED (50 W, λ = 525 nm), and blue LED (50 W, λ = 456 nm) were purchased from commercial supplier Kessil LED Photoreactor Lightning. Fluorescence measurements were performed using a Varian Cary Eclipse spectrofluorometer equipped with a thermostatted cell holder. Coumarine-3-carboxylic acids 1b–k were synthesized according to the literature procedure.13 Catalyst 4c was synthesized according to the literature procedure.14
6-Methyl-2-oxo-2H-chromene-3-carboxylic Acid (1b)
Compound 1b was synthesized according to the literature procedure13 as a white solid in 75% yield (153.0 mg). Analytical data were in accordance with the literature.
6-Methoxy-2-oxo-2H-chromene-3-carboxylic Acid (1c)
Compound 1c was synthesized according to the literature procedure13 as a white solid in 82% yield (180.4 mg). Analytical data were in accordance with the literature.
7-Methoxy-2-oxo-2H-chromene-3-carboxylic Acid (1d)
Compound 1d was synthesized according to the literature procedure13 as a white solid in 64% yield (140.8 mg). Analytical data were in accordance with the literature.
8-Methoxy-2-oxo-2H-chromene-3-carboxylic Acid (1e)
Compound 1e was synthesized according to the literature procedure13 as a white solid in 72% yield (158.4 mg). Analytical data were in accordance with the literature.
6-(tert-Butyl)-2-oxo-2H-chromene-3-carboxylic Acid (1f)
Compound 1f was synthesized according to the literature procedure13 as a white solid in 89% yield (218.9 mg). Analytical data were in accordance with the literature.
6-Fluoro-2-oxo-2H-chromene-3-carboxylic Acid (1g)
Compound 1g was synthesized according to the literature procedure13 as a white solid in 84% yield (174.7 mg). Analytical data were in accordance with the literature.
7-Bromo-2-oxo-2H-chromene-3-carboxylic Acid (1h)
Compound 1h was synthesized according to the literature procedure13 as a yellow solid in 62% yield (166.8 mg). Analytical data were in accordance with the literature.
8-Bromo-2-oxo-2H-chromene-3-carboxylic Acid (1i)
Compound 1i was synthesized according to the literature procedure13 as a yellow solid in 54% yield (145.3 mg). Analytical data were in accordance with the literature.
6-Chloro-2-oxo-2H-chromene-3-carboxylic Acid (1j)
Compound 1j was synthesized according to the literature procedure13 as a yellow solid in 89% yield (199.8 mg). Analytical data were in accordance with the literature.
8-Chloro-2-oxo-2H-chromene-3-carboxylic Acid (1k)
Compound 1k was synthesized according to the literature procedure13 as a yellow solid in 72% yield (161.6 mg). Analytical data were in accordance with the literature.
3-Oxo-3H-benzo[f]-chromene-2-carboxylic Acid (1l)
Compound 1l was synthesized according to the literature procedure13 as a yellow solid in 67% yield (160.9 mg). Analytical data were in accordance with the literature.
5,7-Dimethoxy-2-oxo-2H-chromene-3-carboxylic Acid (1m)
Compound 1m was synthesized according to the literature procedure13 as a white solid in 56% yield (140.1 mg). Analytical data were in accordance with the literature.
General Procedure for the Synthesis of Substituted 4-(Pyridin-4-yl)chroman-2-ones (3aa–3af)
In a 10 mL Schlenk tube, coumarin-3-carboxylic acids 1a–f (0.1 mmol, 1.0 equiv), 4-cyanopirydyne (0.3 mmol, 3.0 equiv), Et3N (0.25 mmol, 2.5 equiv), and catalyst fac-Ir(ppy)3 (3 mol %) were dissolved in dry DMSO (3 mL). The reaction mixture was degassed and filled three times with argon. Subsequently, the mixture was irradiated with blue LED for 48 h at room temperature. Next, the reaction was quenched with saturated solution of NaHCO3 (5 mL), extracted with CH2Cl2 (3 × 10 mL), and washed with brine (5 mL). The organic phase was dried over MgSO4 and concentrated under reduced pressure. The crude product was purified by silica gel chromatography (n-hexane:ethyl acetate, 2:1) to provide the desired products 3aa–af.
4-(Pyridin-4-yl)chroman-2-one (3aa)15
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 2:1) as a yellow oil in 93% yield (20.9 mg). 1H NMR (700 MHz, chloroform-d) δ 8.64–8.54 (m, 2H), 7.37–7.34 (m, 1H), 7.17 (dd, J = 8.2, 1.2 Hz, 1H), 7.14 (td, J = 7.5, 1.2 Hz, 1H), 7.10 (ddd, J = 4.4, 1.6, 0.6 Hz, 2H), 7.01 (dt, J = 7.5, 1.1 Hz, 1H), 4.34 (t, J = 6.5 Hz, 1H), 3.12 (dd, J = 16.0, 6.1 Hz, 1H), 3.04 (dd, J = 16.0, 6.8 Hz, 1H). 13C {1H} NMR (176 MHz, CDCl3) δ 166.7, 151.9, 150.7 (2C), 149.4, 129.6, 128.3, 125.1, 123.9, 122.7 (2C), 117.6, 40.2, 36.3. HRMS (ESI-TOF) m/z [M + H+] calculated for C14H12NO2+: 226.0863, found: 226.0864.
6-Methyl-4-(pyridin-4-yl)chroman-2-one (3ab)
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 2:1) as a brown oil in 85% yield (20.3 mg). 1H NMR (700 MHz, chloroform-d) δ 8.59–8.56 (m, 2H), 7.13 (dd, J = 8.4, 2.1 Hz, 1H), 7.09–7.07 (m, 2H), 7.04 (d, J = 8.4 Hz, 1H), 6.79 (d, J = 2.1 Hz, 1H), 4.28 (t, J = 6.4 Hz, 1H), 3.08 (dd, J = 16.0, 6.2 Hz, 1H), 3.00 (dd, J = 16.0, 6.5 Hz, 1H), 2.61 (s, 1H), 2.28 (s, 3H). 13C {1H} NMR (176 MHz, CDCl3) δ 167.0, 150.7 (2C), 149.8, 149.5, 134.8, 130.1, 128.6, 123.5, 122.7 (2C), 117.3, 40.3, 36.4, 20.9. HRMS (ESI-TOF) m/z [M + H+] calculated for C15H14NO2+: 240.1019, found: 240.1016.
6-Methoxy-4-(pyridin-4-yl)chroman-2-one (3ac)
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 2:1) as a yellow oil in 75% yield (19.1 mg). 1H NMR (700 MHz, chloroform-d) δ 8.60–8.55 (m, 2H), 7.11–7.08 (m, 3H), 6.87 (ddd, J = 8.9, 3.0, 0.5 Hz, 1H), 6.51 (dd, J = 3.0, 0.8 Hz, 1H), 4.28 (t, J = 6.4 Hz, 1H), 3.73 (s, 3H), 3.08 (dd, J = 16.0, 6.1 Hz, 1H), 3.00 (dd, J = 16.1, 6.7 Hz, 1H). 13C {1H} NMR (176 MHz, CDCl3) δ 166.9, 156.7, 150.6 (2C), 149.4, 145.8, 124.7, 122.8 (2C), 118.4, 114.5, 113.5, 55.8, 40.6, 36.3. HRMS (ESI-TOF) m/z [M + H+] calculated for C15H14NO3+: 256.0968, found: 256.0968.
7-Methoxy-4-(pyridin-4-yl)chroman-2-one (3ad)
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 2:1) as a pale yellow oil in 94% yield (24.0 mg). 1H NMR (700 MHz, chloroform-d) δ 8.57 (d, J = 5.0 Hz, 2H), 7.09–7.07 (m, 2H), 6.89 (dd, J = 8.4, 0.8 Hz, 1H), 6.70 (d, J = 2.5 Hz, 1H), 6.67 (dd, J = 8.4, 2.6 Hz, 1H), 4.27 (t, J = 6.4 Hz, 1H), 3.81 (s, 3H), 3.09 (dd, J = 15.9, 6.2 Hz, 1H), 3.00 (dd, J = 15.9, 6.7 Hz, 1H). 13C {1H} NMR (176 MHz, CDCl3) δ 166.7, 160.6, 152.7, 150.5 (2C), 150.1, 128.9, 122.8 (2C), 115.6, 111.3, 103.0, 55.8, 39.7, 36.6. HRMS (ESI-TOF) m/z [M + H+] calculated for C15H14NO3+: 256.0968, found: 256.0964.
8-Methoxy-4-(pyridin-4-yl)chroman-2-one (3ae)
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 2:1) as a pale yellow oil in 85% yield (21.7 mg). 1H NMR (700 MHz, chloroform-d) δ 8.57 (d, J = 5.0 Hz, 2H), 7.12–7.02 (m, 3H), 6.94 (dd, J = 8.2, 1.3 Hz, 1H), 6.59 (ddd, J = 7.7, 1.4, 0.8 Hz, 1H), 4.32 (t, J = 6.3 Hz, 1H), 3.92 (s, 3H), 3.14–3.07 (m, 1H), 3.03 (dd, J = 15.9, 6.3 Hz, 1H).13C {1H} NMR δ 166.1, 150.7 (2C), 149.3, 148.2, 141.2, 125.0, 124.9, 122.7 (2C), 119.6, 112.1, 56.3, 40.5, 36.1. HRMS (ESI-TOF) m/z [M + H+] calculated for C15H14NO3+: 256.0968, found: 256.0971.
6-tert-Butyl-4-(pyridin-4-yl)chroman-2-one (3af)
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 2:1) as a yellow oil in 95% yield (26.7 mg). 1H NMR (700 MHz, chloroform-d) δ 8.58 (d, J = 5.1 Hz, 2H), 7.36 (dd, J = 8.6, 2.4 Hz, 1H), 7.10–7.08 (m, 3H), 7.02 (d, J = 2.4 Hz, 1H), 4.31 (t, J = 6.1 Hz, 1H), 3.10 (dd, J = 15.9, 6.2 Hz, 1H), 3.02 (dd, J = 15.9, 6.0 Hz, 1H), 1.25 (s, 9H). 13C {1H} NMR (176 MHz, CDCl3) δ 167.0, 150.7 (2C), 149.7, 149.7, 148.3, 126.6, 125.2, 122.9, 122.7 (2C), 117.1, 40.6, 36.6, 34.6, 31.5 (3C). HRMS (ESI-TOF) m/z [M + H+] calculated for C18H20NO2+: 282.1489, found: 282.1491.
General Procedure for the Synthesis of Substituted 4-(Pyridin-4-yl)chroman-2-one 3ag–am
In a 10 mL Schlenk tube, 4-cyanopirydyne 2a (0.3 mmol, 3.0 equiv), Et3N (0.25 mmol, 2.5 equiv), and catalyst fac-Ir(ppy)3 (3 mol %) were dissolved in dry DMSO (2 mL). The reaction mixture was degassed and filled three times with argon. The mixture was irradiated with blue LED for 2 h at room temperature. Subsequently, coumarin-3-carboxylic acids 1g–m (0.1 mmol, 1.0 equiv) in dry DMSO (1 mL) was added dropwise over 2 h and stirred for additional 48 h. Next, the reaction was quenched with saturated solution of NaHCO3 (5 mL), extracted with CH2Cl2 (3 × 10 mL), and washed with brine (5 mL). The organic phase was dried over MgSO4 and concentrated under reduced pressure. The crude product was purified by silica gel chromatography (n-hexane:ethyl acetate, 2:1) to provide the desired products 3ag–am.
6-Fluoro-4-(pyridin-4-yl)chroman-2-one (3ag)
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 2:1) as a yellow oil in 56% yield (13.6 mg). 1H NMR (700 MHz, chloroform-d) δ 8.62–8.60 (m, 2H), 7.13 (dd, J = 9.0, 4.6 Hz, 1H), 7.10–7.08 (m, 2H), 7.04 (ddd, J = 9.0, 7.7, 2.9 Hz, 1H), 6.70 (ddd, J = 8.2, 2.9, 0.8 Hz, 1H), 4.31 (t, J = 6.7 Hz, 1H), 3.09 (dd, J = 16.0, 6.1 Hz, 1H), 3.02 (dd, J = 16.0, 7.3 Hz, 1H). 13C {1H} NMR (176 MHz, chloroform-d) δ 166.3, 159.3 (d, J = 245.2 Hz), 150.9 (2C), 148.5, 147.9 (d, J = 2.8 Hz), 125.6 (d, J = 7.6 Hz), 122.7 (2C), 119.0 (d, J = 8.3 Hz), 116.4 (d, J = 23.5 Hz), 114.9 (d, J = 24.4 Hz), 40.3, 35.9. HRMS (ESI-TOF) m/z [M + H+] calculated for C14H11NO2F +: 244.0768, found: 244.0769.
7-Bromo-4-(pyridin-4-yl)chroman-2-one (3ah)
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 2:1) as a pale yellow oil in 53% yield (16.1 mg). 1H NMR (700 MHz, chloroform-d) δ 8.61–8.60 (m, 2H), 7.34 (d, J = 2.0 Hz, 1H), 7.28–7.25 (m, 1H), 7.09–7.07 (m, 2H), 6.88 (d, J = 8.1 Hz, 1H), 4.29 (t, J = 6.6 Hz, 1H), 3.10 (dd, J = 16.0, 6.1 Hz, 1H), 3.02 (dd, J = 16.0, 7.0 Hz, 1H). 13C {1H} NMR (176 MHz, CDCl3) δ 165.8, 152.4, 150.9 (2C), 148.7, 129.5, 128.2, 123.0, 122.6 (2C), 122.6, 126.0, 77.2, 40.0, 36.1. HRMS (ESI-TOF) m/z [M + H+]calculated for C14H11BrNO2+: 303.9968, found: 303.9971.
8-Bromo-4-(pyridin-4-yl)chroman-2-one (3ai)
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 2:1) as a pale yellow oil in 61% yield (18.5 mg). 1H NMR (700 MHz, chloroform-d) δ 8.62–8.58 (m, 2H), 7.59 (ddd, J = 8.0, 1.6, 0.5 Hz, 1H), 7.08 (ddd, J = 4.4, 1.7, 0.6 Hz, 2H), 7.01 (t, J = 7.6 Hz, 1H), 6.95 (ddd, J = 7.6, 1.6, 0.8 Hz, 1H), 4.35 (t, J = 6.5 Hz, 1H), 3.12 (dd, J = 15.9, 6.1 Hz, 1H), 3.07 (dd, J = 15.9, 6.9 Hz, 1H). 13C {1H} NMR (176 MHz, CDCl3) δ 165.4, 150.9 (2C), 148.9, 148.6, 133.5, 127.4, 125.8, 125.7, 122.6 (2C), 111.5, 40.6, 36.0. HRMS (ESI-TOF) m/z [M + H+] calculated for C14H11NO2Br +: 303.9968, found: 303.9973.
6-Chloro-4-(pyridin-4-yl)chroman-2-one (3aj)
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 2:1) as a pale yellow oil in 96% yield (24.9 mg). 1H NMR (700 MHz, chloroform-d) δ 8.63–8.61 (m, 2H), 7.32 (ddd, J = 8.7, 2.5, 0.6 Hz, 1H), 7.11 (d, J = 8.7 Hz, 1H), 7.09 (ddd, J = 4.4, 1.6, 0.6 Hz, 2H), 6.98 (dd, J = 2.5, 0.8 Hz, 1H), 4.31 (t, J = 6.6 Hz, 1H), 3.10 (dd, J = 16.0, 6.1 Hz, 1H), 3.03 (dd, J = 16.0, 7.1 Hz, 1H). 13C {1H} NMR (176 MHz, CDCl3) δ 166.0, 150.9 (2C), 150.4, 148.4, 130.3, 129.7, 128.2, 125.6, 122.6 (2C), 119.0, 40.2, 35.9. HRMS (ESI-TOF) m/z [M + H+] calculated for C14H11ClNO2+: 260.0473, found: 260.0471.
8-Chloro-4-(pyridin-4-yl)chroman-2-one (3ak)
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 2:1) as a pale yellow oil in 34% yield (8.8 mg). 1H NMR (700 MHz, chloroform-d) δ 8.60 (d, J = 5.0 Hz, 2H), 7.42 (d, J = 8.0 Hz, 1H), 7.07 (dd, J = 21.2, 6.4 Hz, 3H), 6.91 (d, J = 7.7 Hz, 1H), 4.36 (t, J = 6.6 Hz, 1H), 3.12 (dd, J = 15.9, 6.1 Hz, 1H), 3.07 (dd, J = 15.9, 6.9 Hz, 1H). 13C {1H} NMR (176 MHz, CDCl3) δ 165.4, 150.8 (2C), 148.7, 147.8, 130.5, 126.6, 125.8, 125.2, 122.8, 122.7 (2C), 40.6, 36.0. HRMS (ESI-TOF) m/z [M + H+] calculated for C14H11ClNO2+: 260.0473, found: 260.0475.
1,2-Dihydro-3H-benzo[f]-1-(pyridin-4-yl)chromen-3-one (3al)16
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 2:1) as a pale yellow oil in 41% yield (11.3 mg). 1H NMR (700 MHz, chloroform-d) δ 8.53–8.51 (m, 2H), 7.92 (d, J = 9.1 Hz, 1H), 7.90–7.89 (m, 1H), 7.71 (dd, J = 8.5, 0.9 Hz, 1H), 7.51 (ddd, J = 8.5, 6.8, 1.4 Hz, 1H), 7.48 (ddd, J = 8.0, 6.8, 1.6 Hz, 1H), 7.37 (d, J = 9.0 Hz, 1H), 7.06 (ddd, J = 4.4, 1.6, 0.6 Hz, 2H), 4.94 (d, J = 6.7 Hz, 1H), 3.27 (dd, J = 16.0, 7.3 Hz, 1H), 3.18 (dd, J = 16.0, 1.8 Hz, 1H). 13C {1H} NMR (176 MHz, CDCl3) δ 166.3, 150.8 (2C), 150.1, 149.4, 131.3, 130.9, 130.7, 129.1, 128.0, 125.7, 122.7, 122.3 (2C), 117.7, 116.0, 37.1, 36.6. HRMS (ESI-TOF) m/z [M + H+] calculated for C18H14NO2+: 276.1019, found: 276.1023.
5,7-Dimethoxy-4-(pyridin-4-yl)chroman-2-one (3am)
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 2:1) as a pale yellow oil in 82% yield (23.4 mg). 1H NMR (700 MHz, chloroform-d) δ 8.63–8.29 (m, 2H), 7.17–6.90 (m, 2H), 6.31 (d, J = 2.3 Hz, 1H), 6.28 (d, J = 2.3 Hz, 1H), 4.52 (dd, J = 6.8, 2.4 Hz, 1H), 3.80 (s, 3H), 3.75 (s, 3H), 3.08–2.96 (m, 2H). 13C {1H} NMR (176 MHz, CDCl3) δ 166.9, 161.3, 157.5, 153.2, 150.6, 150.3 (3C), 122.2, 104.4, 95.3, 94.3, 56.0, 55.7, 36.0, 34.1. HRMS (ESI-TOF) m/z [M + H+] calculated for C16H16NO4+: 286.1074, found: 286.1068.
General Procedure for the Synthesis of 4-Substituted-chroman-2-one 3ba and 3ca
In a 10 mL Schlenk tube, Et3N (0.25 mmol, 2.5 equiv) and catalyst fac-Ir(ppy)3 (3 mol %) were dissolved in dry DMSO (1 mL). The reaction mixture was degassed and filled three times with argon. The mixture was irradiated with blue LED at room temperature. Coumarin-3-carboxylic acid 1a (0.1 mmol, 1.0 equiv) in dry DMSO (1 mL) and cyanoarenes 2a–c (0.3 mmol, 3.0 equiv) in dry DMSO (1 mL) were added dropwise over 2 h and stirred for additional 48 h. Next, the reaction was quenched with saturated solution of NaHCO3 (5 mL), extracted with CH2Cl2 (3 × 10 mL), and washed with brine (5 mL). The organic phase was dried over MgSO4 and concentrated under reduced pressure. The crude product was purified by silica gel chromatography (n-hexane:ethyl acetate, 5:1) to provide the desired products 3ba and 3ca.
4-(Pyridin-2-yl)chroman-2-one (3ba)
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 10:1) as a pale yellow oil in 87% yield (19.8 mg). 1H NMR (700 MHz, chloroform-d) δ 8.57 (ddd, J = 4.8, 1.9, 1.0 Hz, 1H), 7.62 (td, J = 7.7, 1.9 Hz, 1H), 7.29–7.27 (m, 1H), 7.17 (ddd, J = 7.6, 4.8, 1.1 Hz, 1H), 7.14–7.10 (m, 2H), 7.10–7.06 (m, 2H), 4.43 (dd, J = 6.3, 5.1 Hz, 1H), 3.31 (dd, J = 16.0, 5.1 Hz, 1H), 3.04 (dd, J = 16.0, 6.3 Hz, 1H). 13C {1H} NMR (176 MHz, CDCl3) δ 167.9, 160.1, 151.8, 150.2, 137.1, 129.1, 128.3, 124.6 (2C), 122.6, 122.0, 117.6, 43.0, 34.8. HRMS (ESI-TOF) m/z [M + H+] calculated for C14H12NO2+: 226.0863, found: 226.0864.
4-(Pyrimidin-2-yl)chroman-2-one (3ca)
The pure product was isolated by flash chromatography on silica gel (n-hexane/ethyl acetate, 10:1) as a yellow oil in 71% yield (16.0 mg). 1H NMR (700 MHz, chloroform-d) δ 8.67 (d, J = 4.9 Hz, 2H), 7.34 (ddd, J = 7.9, 1.6, 0.7 Hz, 1H), 7.28–7.25 (m, 1H), 7.16 (t, J = 4.9 Hz, 1H), 7.10–7.07 (m, 2H), 4.59 (dd, J = 6.5, 3.2 Hz, 1H), 3.29 (dd, J = 16.1, 3.2 Hz, 1H), 3.07 (dd, J = 16.1, 6.5 Hz, 1H). 13C {1H} NMR (176 MHz, CDCl3) δ 169.8, 167.7, 157.7 (2C), 151.6, 129.3, 128.7, 124.6, 123.3, 119.7, 117.6, 44.5, 33.6. HRMS (ESI-TOF) m/z [M + H+] calculated for C13H11N2O2+: 227.0815, found: 227.0818.
General Procedure for the Synthesis of 4-(Pyridin-4-yl)chroman-2-one (3aa) in a 2 mmol Scale15
In a 50 mL Schlenk tube, coumarin-3-carboxylic acid 1a (380.3 mg, 2.0 mmol, 1.0 equiv), 4-cyanopirydyne 2a (624.7 mg, 6.0 mmol, 3.0 equiv), Et3N (506.0 mg, 5.0 mmol, 2.5 equiv), and catalyst fac-Ir(ppy)3 (39.3 mg, 3 mol %) were dissolved in dry DMSO (20 mL). The reaction mixture was degassed and filled three times with argon. Subsequently, the mixture was irradiated with blue LED for 48 h at room temperature. Next, the reaction was quenched with saturated solution of NaHCO3 (50 mL), extracted with CH2Cl2 (3 × 75 mL), and washed with brine (50 mL). The organic phase was dried over MgSO4 and concentrated under reduced pressure. The crude product 3aa was purified by silica gel chromatography (n-hexane:ethyl acetate, 2:1) to provide the desired product 3aa as a yellow oil in 74% yield (333 mg).
Acknowledgments
Thanks are expressed to Adam Sikora (Faculty of Chemistry, Lodz University of Technology) for helping in performing fluorescence quenching experiments, Elżbieta Kuśmierek (Faculty of Chemistry, Lodz University of Technology) for performing cyclic voltammetry, and Marek Moczulski for fruitful discussions. This contribution has been completed while the first author (EK) was the Doctoral Candidate in the Interdisciplinary Doctoral School of Lodz University of Technology, Poland. E.K. thanks Lodz University of Technology for financial support within the “FU2N” programme, project number: W3/11D/2022.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.2c00683.
Cyclic voltammetry, fluorescence quenching, photochemical reaction setup, and copies of 1H and 13C NMR spectra (PDF)
Author Contributions
All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- a Kamat D. P.; Tilve S. G.; Kamat V. P.; Kirtany J. K. Syntheses and Biological Activities of Chroman-2-ones. Org. Prep. Proc. Int. 2015, 47, 1. 10.1080/00304948.2015.983805. [DOI] [Google Scholar]; b Okamoto T.; Kobayashi T.; Yoshida S. Chemical aspects of coumarin compounds for the prevention of hepatocellular carcinomas. Curr. Med. Chem. 2005, 5, 47. 10.2174/1568011053352622. [DOI] [PubMed] [Google Scholar]; c Asai F.; Iinuma M.; Tanaka T.; Mizuno M. Complex flavonoids in farinose exudate from Pityrogramma calomelanos. Phytochemistry 1991, 30, 3091. 10.1016/S0031-9422(00)98259-1. [DOI] [Google Scholar]; d Ee G. C. L. S.; Mah S. H.; Teh S. S.; Rahmani M.; Go R.; Taufiq-Yap Y. H. Soulamarin, a New Coumarin from Stem Bark of Calophyllum soulattri. Molecules 2011, 16, 9721. 10.3390/molecules16119721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Andersen Ø. M.; Markham K. R.. Flavonoids: Chemistry; Biochemistry and Applications: CRC, Taylor & Francis, Boca Raton, FL, 2006; [Google Scholar]; b Kamat D. P.; Tilve S. G.; Kamat V. P.; Kirtany J. K. Syntheses and Biological Activities of Chroman-2-ones. A Review. Org. Prep. Proc. Int. 2015, 47, 1–79. 10.1080/00304948.2015.983805. [DOI] [Google Scholar]; c Masters K.-S.; Bräse S. Xanthones from Fungi, Lichens, and Bacteria: The Natural Products and Their Synthesis. Chem. Rev. 2012, 112, 3717–3776. 10.1021/cr100446h. [DOI] [PubMed] [Google Scholar]; d Zhao D.-L.; Shao C.-L.; Gan L.-S.; Wang M.; Wang C.-Y. Chromone Derivatives from a Sponge-Derived Strain of the Fungus Corynespora cassiicola. J. Nat. Prod. 2015, 78, 286–293. 10.1021/np5009152. [DOI] [PubMed] [Google Scholar]; e Fu P.; Wang S.; Hong K.; Li X.; Liu P.; Wang Y.; Zhu W. Cytotoxic Bipyridines from the Marine-Derived Actinomycete Actinoalloteichus cyanogriseus WH1-2216-6. J. Nat. Prod. 2011, 74, 1751–1756. 10.1021/np200258h. [DOI] [PubMed] [Google Scholar]; f Olbe L.; Carlsson E.; Lindberg P. A proton-pump inhibitor expedition: the case histories of omeprazole and esomeprazole. Nat. Rev. Drug Discovery 2003, 2, 132–139. 10.1038/nrd1010. [DOI] [PubMed] [Google Scholar]; For selected reviews, see:; g Nibbs A. E.; Scheidt K. A. Asymmetric Methods for the Synthesis of Flavanones, Chromanones, and Azaflavanones. Eur. J. Org. Chem. 2012, 2012, 449. 10.1002/ejoc.201101228. [DOI] [PMC free article] [PubMed] [Google Scholar]; h McDonald B. R.; Scheidt K. A. Pyranone Natural Products as Inspirations for Catalytic Reaction Discovery and Development. Acc. Chem. Res. 2015, 48, 1172. 10.1021/ar5004576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Moffett R. B. Central Nervous System Depressants. VII.1pyridyl Coumarins. J. Med. Chem. 1964, 7, 446–449. 10.1021/jm00334a010. [DOI] [PubMed] [Google Scholar]; b Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]; c Shtyrlin N. V.; Pavelyev R. S.; Pugachev M. V.; Sysoeva L. P.; Musin R. Z.; Shtyrlin Y. G. Synthesis of novel 6-substituted sulfur-containing derivatives of pyridoxine. Tetrahedron Lett. 2012, 53, 3967–3970. 10.1016/j.tetlet.2012.05.086. [DOI] [Google Scholar]; d Reynolds R. D. Bioavailability of vitamin B-6 from plant foods. Am. J. Clin. Nutr. 1988, 48, 863–867. 10.1093/ajcn/48.3.863. [DOI] [PubMed] [Google Scholar]; e Gandhi P. T.; Athmarama T. N.; Arunkumar G. R. Novel nicotine analogues with potential anti-mycobacterial activity. Bioorg. Med. Chem. 2016, 24, 1637–1647. 10.1016/j.bmc.2016.02.035. [DOI] [PubMed] [Google Scholar]
- For pyridine ligands, see:; Chelucci G. Metal-complexes of optically active amino- and imino-based pyridine ligands in asymmetric catalysis. Coord. Chem. Rev. 2013, 257, 1887. 10.1016/j.ccr.2012.12.002. [DOI] [Google Scholar]
- a Minisci F.; Vismara E.; Fontana F. Recent developments of free-radical substitutions of heteroaromatic bases. Heterocycles 1989, 28, 489–519. 10.3987/REV-88-SR1. [DOI] [Google Scholar]; b Schlosser M.; Mongin F. Pyridine elaboration through organometallic intermediates: regiochemical control and completeness. Chem. Soc. Rev. 2007, 36, 1161–1172. 10.1039/b706241a. [DOI] [PubMed] [Google Scholar]; c Bull J. A.; Mousseau J. J.; Pelletier G.; Charette A. B. Synthesis of Pyridine and Dihydropyridine Derivatives by Regio- and Stereoselective Addition to N-Activated Pyridines. Chem. Rev. 2012, 112, 2642–2713. 10.1021/cr200251d. [DOI] [PubMed] [Google Scholar]
- For selected examples of the radical-based pyridylation reactions, see:; a Betori R. C.; Scheidt K. A. Reductive Arylation of Arylidene Malonates Using Photoredox Catalysis. ACS Catal. 2019, 9, 10350–10357. 10.1021/acscatal.9b03608. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Gao L.; Wang G.; Chen H.; Cao J.; Su X.; Liu X.; Yang M.; Cheng X.; Li S. Metal-free reductive coupling of aliphatic aldehydes/ketones with 4-cyanopyridines: expanded scope and mechanistic studies. Org. Chem. Front. 2020, 7, 2744–2751. 10.1039/D0QO00827C. [DOI] [Google Scholar]; c Seiple I. B.; Su S.; Rodriguez R. A.; Gianatassio R.; Fujiwara Y.; Sobel A. L.; Baran P. S. Direct C–H Arylation of Electron-Deficient Heterocycles with Arylboronic Acids. J. Am. Chem. Soc. 2010, 132, 13194–13196. 10.1021/ja1066459. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zhang S.; Li L.; Li J.; Shi J.; Xu K.; Gao W.; Zong L.; Li G.; Findlater M. Electrochemical Arylation of Aldehydes, Ketones, and Alcohols: from Cathodic Reduction to Convergent Paired Electrolysis. Angew. Chem., Int. Ed. 2021, 60, 7275–7282. 10.1002/anie.202015230. [DOI] [PubMed] [Google Scholar]; e Zhang S.; Li L.; Li X.; Zhang J.; Xu K.; Li G.; Findlater M. Electroreductive 4-Pyridylation of Electron-deficient Alkenes with Assistance of Ni(acac)2. Org. Lett. 2020, 22, 3570–3575. 10.1021/acs.orglett.0c01014. [DOI] [PubMed] [Google Scholar]; f Shen J.; Zhang Y.; Yua Y.; Wang M. Metal-free visible-light-induced photoredox-catalyzed intermolecular pyridylation/phosphinoylation of alkenes. Org. Chem. Front. 2021, 8, 901–907. 10.1039/D0QO01218A. [DOI] [Google Scholar]
- a Giese B.; González-Gómez J. A.; Witzel T. The Scope of Radical CC-Coupling by the “Tin Method”. Angew. Chem., Int. Ed. 1984, 23, 69. 10.1002/anie.198400691. [DOI] [Google Scholar]; b Rostoll-Berenguer J.; Blay G.; Pedro J. R.; Vila C. Photocatalytic Giese Addition of 1,4-Dihydroquinoxalin-2-ones to Electron-Poor Alkenes Using Visible Light. Org. Lett. 2020, 22, 8012. 10.1021/acs.orglett.0c02953. [DOI] [PubMed] [Google Scholar]
- a Parida S. K.; Mandal T.; Das S.; Hota S. K.; De Sarkar S.; Murarka S. Single Electron Transfer-Induced Redox Processes Involving N-(Acyloxy)phthalimides. ACS Catal. 2021, 11, 1640. 10.1021/acscatal.0c04756. [DOI] [Google Scholar]; b Dong Y.; Ji P.; Zhang Y.; Wang C.; Meng X.; Wang W. Organophotoredox-Catalyzed Formation of Alkyl-Aryl And -Alkyl C-S/Se Bonds from Coupling of Redox-Active Esters with Thio/Selenosulfonates. Org. Lett. 2020, 22, 9562. 10.1021/acs.orglett.0c03624. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Saget T.; König B. Photocatalytic Synthesis of Polycyclic Indolones. Chem. – Eur. J. 2020, 26, 7004. 10.1002/chem.202001324. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zheng Ch.; Wang G-Z.; Shang R. Catalyst-free Decarboxylation and Decarboxylative Giese Additions of Alkyl Carboxylates through Photoactivation of Electron Donor-Acceptor Complex. Adv. Synth. Catal. 2019, 361, 4500. [Google Scholar]; Buzzetti L.; Crisenza G. E. M.; Melchiorre P. Mechanistic Studies in Photocatalysis. Angew. Chem., Int. Ed. 2019, 58, 3730–3747. [DOI] [PubMed] [Google Scholar]; e Vega-Peñaloza A.; Javier Mateos J.; Companyó X.; Escudero-Casao M.; Dell’Amico L. A Rational Approach to Organo-Photocatalysis: Novel Designs and Structure-Property Relationships. Angew. Chem., Int. Ed. 2021, 133, 1096–1111. 10.1002/ange.202006416. [DOI] [PubMed] [Google Scholar]; f Bortolato T.; Cuadros S.; Simionato G.; Dell’Amico L. The advent and development of organophotoredox catalysis. Chem. Commun. 2022, 58, 1263–1283. 10.1039/D1CC05850A. [DOI] [PubMed] [Google Scholar]
- For reviews on decarboxylative strategies, see:; a Pan Y.; Tan C.-H. Catalytic Decarboxylative Reactions: Biomimetic Approaches Inspired by Polyketide Biosynthesis. Synthesis 2011, 2044. 10.1055/s-0030-1260607. [DOI] [Google Scholar]; b Wang Z.-L. Recent Advances in Catalytic Asymmetric Decarboxylative Addition Reactions. Adv. Synth. Catal. 2013, 355, 2745. 10.1002/adsc.201300375. [DOI] [Google Scholar]; c Nakamura S. Catalytic enantioselective decarboxylative reactions using organocatalysts. Org. Biomol. Chem. 2014, 12, 394. 10.1039/C3OB42161A. [DOI] [PubMed] [Google Scholar]; d Bojanowski J.; Albrecht A. Carboxylic-Acid-Activated Olefins in Decarboxylative Reactions. Asian J.Org. Chem. 2019, 8, 746. 10.1002/ajoc.201900166. [DOI] [Google Scholar]
- Moczulski M.; Kowalska E.; Kuśmierek E.; Albrecht Ł.; Albrecht A. Visible-light synthesis of 4-substituted-chroman-2-ones and 2-substituted-chroman-4-ones via doubly decarboxylative Giese reaction. RSC Adv. 2021, 11, 27782–27786. 10.1039/D1RA05914A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Xu L.; Shao Z.; Wang L.; Xiao J. Tandem sp3 C-H Functionalization/Decarboxylation of 2-Alkylazaarenes with Coumarin-3-carboxylic Acids. Org. Lett. 2014, 16, 796. 10.1021/ol403541g. [DOI] [PubMed] [Google Scholar]; b Han F.; Xun S.; Jia L.; Zhang Y.; Zou L.; Hu X. Traceless-Activation Strategy for Rh-Catalyzed Csp2 −H Arylation of Coumarins. Org. Lett. 2019, 21, 5907. 10.1021/acs.orglett.9b02040. [DOI] [PubMed] [Google Scholar]
- a Fayed E. A.; Sabour R.; Harras M. F.; Mehany A. B. M. Design, synthesis, biological evaluation and molecular modeling of new coumarin derivatives as potent anticancer agents. Med. Chem. Res. 2019, 28, 1284–1297. 10.1007/s00044-019-02373-x. [DOI] [Google Scholar]; b Feng Z.; Yu Y.; Yang X.; Zhong D.; Song D.; Yang H.; Chen X.; Zhou G.; Wu Z. Isomers of Coumarin-Based Cyclometalated Ir(III) Complexes with Easily Tuned Phosphorescent Color and Features for Highly Efficient Organic Light-Emitting Diodes. Inorg. Chem. 2019, 58, 7393–7408. 10.1021/acs.inorgchem.9b00534. [DOI] [PubMed] [Google Scholar]
- Song A.; Wang X.; Lam K. S. A convenient synthesis of coumarin-3-carboxylic acids via Knoevenagel condensation of Meldrum’s acid with ortho-hydroxyaryl aldehydes or ketones. Tetrahedron Lett. 2003, 44, 1755. 10.1016/S0040-4039(03)00108-4. [DOI] [Google Scholar]
- Luo J.; Zhang J. Donor–Acceptor Fluorophores for Visible-Light-Promoted Organic Synthesis: Photoredox/Ni Dual Catalytic C(sp3)–C(sp2) Cross-Coupling. ACS Catal. 2016, 6, 873–877. 10.1021/acscatal.5b02204. [DOI] [Google Scholar]
- Hoshikawa T.; Inoue M. Photoinduced direct 4-pyridination of C(sp3)–H Bonds. Chem. Sci. 2013, 4, 3118–3123. 10.1039/c3sc51080h. [DOI] [Google Scholar]
- Das P.; Ray S.; Bhanja P.; Bhaumik A.; Mukhopadhyay C. Serendipitous Observation of Liquid-Phase Size Selectivity inside a Mesoporous Silica Nanoreactor in the Reaction of Chromene with Formic Acid. ChemCatChem 2018, 10, 2260–2270. 10.1002/cctc.201701975. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.