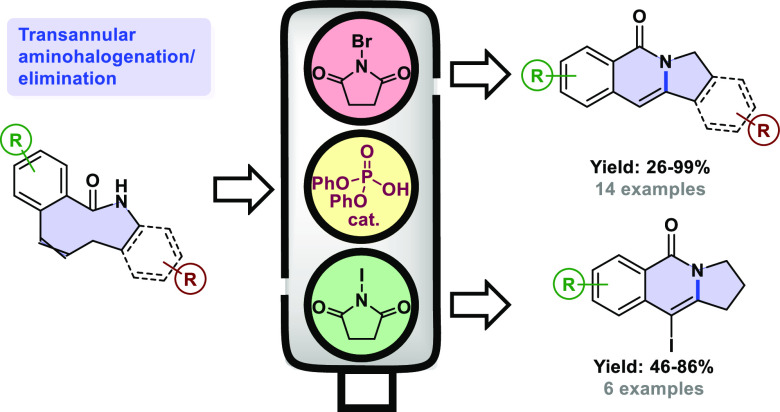
Abstract
A transannular approach has been developed for the construction of pyrrolo[1,2-b]isoquinolinones starting from benzo-fused nine-membered enelactams. This process takes place in the presence of a halogenating agent and under Brønsted acid catalysis and proceeds via a transannular amidohalogenation, followed by elimination. The reaction has been found to be wide in scope, enabling the formation of a variety of tricyclic products in good overall yield, regardless of the substitution pattern in the initial lactam substrate. The reaction has also been applied to the total synthesis of a reported topoisomerase I inhibitor and to the formal synthesis of rosettacin. Further extension of this methodology allows the preparation of 10-iodopyrrolo[1,2-b]isoquinolinones by using an excess of halogenating agent and these compounds can be further manipulated through standard Suzuki coupling chemistry into a variety of 10-aryl-substituted pyrrolo[1,2-b]isoquinolinones.
Introduction
The 2,3-dihydropyrrolo[1,2-b]isoquinolin-5(1H)-one framework constitutes the central core of several families of bioactive compounds, some of them with relevant therapeutic potential (see Scheme 1).1 In particular, this molecular architecture is the main structural feature of aromathecins, a family of topoisomerase I inhibitors that constitute promising chemotherapeutic agents against cancer, with some members already even approved for clinical uses.2 In addition, some other members of this family have been identified as highly active antiparasitic compounds which are able to selectively inhibit the phylogenetically unique topoisomerase IB present in the protozoan parasites Trypanosoma brucei, Trypanosoma cruzi, and the Leishmania species that cause African sleeping sickness (African trypanosomiasis) and Chagas disease (American trypanosomiasis). Moreover, isoindolo[2,1-b]isoquinolin-5(7H)-one, which also shares the same pyrroloisoquinoline motif has shown to exhibit similar topoisomerase I activity to camptothecin.3 Despite this promising activity, the use of aromathecin derivatives in clinical trials still suffers from poor solubility and dose-limiting toxicity,4 and consequently, there is still a constant need for the development of effective protocols that enable the construction of this scaffold with a special interest on those strategies that provide a diversity-oriented synthesis approach.
Scheme 1. Representative Examples of Bioactive Molecules Containing the 2,3-Dihydropyrrolo[1,2-b]isoquinolin-5(1H)-one Scaffold.
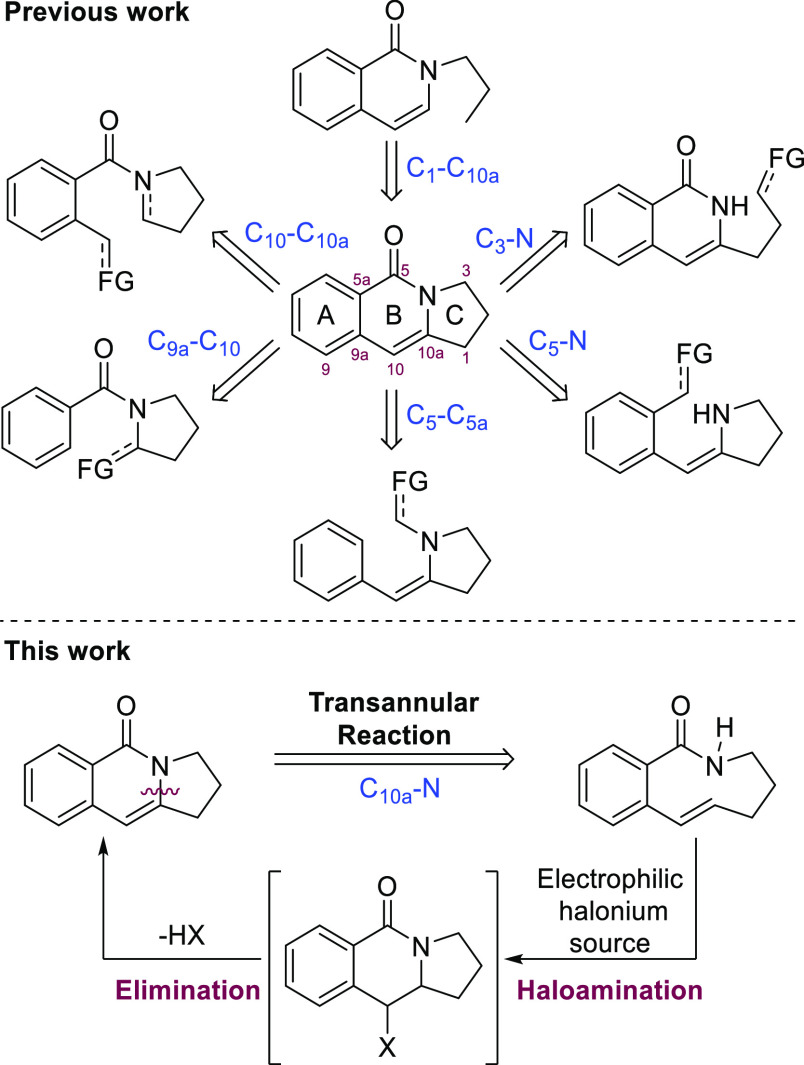
In general, the synthetic routes described up to date for the formation of the 2,3-dihydropyrrolo[1,2-b]isoquinolin-5(1H)-one scaffold involve the generation of the B and/or C rings through cyclization or cyclocondensation reactions. In all these cases, the retrosynthetic design involves disconnections of the C10–C10a,5 C3–N,6 C5–N,7 C5–C5a,8 C9a–C10,9 or C1–C10a10 (see Scheme 2), either individually or by combining different reactions in a cascade process that involves the simultaneous formation of more than one of these bonds at a time.11 Similar strategies have been taken for the synthesis of the isoindolo[2,1-b]isoquinolin-5(7H)-one core.12 As an alternative, we propose herein an unconventional and yet unexplored approach to this scaffold that comprises the generation of the C10a–N bond via formal oxidative transannular amido functionalization of a benzo-fused medium-sized unsaturated lactam, as shown in Scheme 2. In particular, we have directed our attention to study the transannular amidohalogenation reaction that should eventually provide a 10-halo-substituted pyrroloisoquinolin-5-one intermediate, this being a direct precursor of the target 2,3-dihydropyrrolo[1,2-b]isoquinolin-5(1H)-one upon the in situ elimination process. Transannular reactions, in which the two reacting sites are located within the cyclic structure of the starting material, have been widely used as a key step in the design of efficient syntheses of rather complex molecular scaffolds,13 including several examples of elegant total syntheses of natural products.14 In most cases, the transannular approach has demonstrated its performance as a key strategic decision that reduces the number of steps involved in the synthetic route and/or enables highly efficient stereocontrol due to the limited degree of conformational freedom associated with the medium- or large-size cyclic starting material, the latter effect also being exploited for the development of several enantioselective variants.15 Specifically, there are several precedents of highly effective transannular aminohalogenation or amidohalogenation reactions employed for the synthesis of bicyclic nitrogen-containing heterocycles such as pyrrolizidines,16 indolizidines,17 and related derivatives.18 Despite all progress in this field, the transannular approach has not been already employed for the construction of the isoquinoline core.
Scheme 2. Strategic Disconnections to the 2,3-Dihydropyrrolo[1,2-b]isoquinolin-5(1H)-one Core.
Results and Discussion
The synthesis of the starting benzo-fused lactam 5a required for the transannular reaction was accomplished by employing standard methodologies through the pathway shown in Table 1. Starting from methyl o-bromobenzoate (entry 1), Sonogashira coupling with 5-chloropent-1-yne delivered functionalized alkyne 1a in excellent yield, which was subsequently subjected to semihydrogenation under Lindlar catalysis, followed by standard Gabriel synthesis, leading to amine 4a, the latter undergoing lactamization upon treatment with a strong bulky base such as LiHMDS. This synthetic route provided the key lactam 5a in a very straightforward way (five steps from cheap and readily available starting materials) in good overall yield and could be implemented to the multigram scale. Next, we also proceeded to prepare several other lactam substrates through the same methodological approach, starting from o-bromobenzoates and incorporating other substituents in different positions. As it can be seen in Table 1, most compounds 5b-f could be obtained with good overall yields (entries 2–6).
Table 1. Synthesis of Lactam Precursors 5.
| entry | R1 | R2 | R3 | yield 1a–f (%)a | yield 2a–f (%)a | yield 3a–f (%)a | yield 4a–f (%)a | yield 5a–f (%)a |
|---|---|---|---|---|---|---|---|---|
| 1 | H | H | H | 93 (1a) | 91 (2a) | 87 (3a) | 74 (4a) | 50 (5a) |
| 2 | H | F | H | 97 (1b) | 94 (2b) | 82 (3b) | 76 (4b) | 73 (5b) |
| 3 | H | H | F | 94 (1c) | 81 (2c) | 79 (3c) | 67 (4c) | 44 (5c) |
| 4 | H | H | MeO | 91 (1d) | 55 (2d) | 94 (3d) | 71 (4d) | 43 (5d) |
| 5 | H | H | Me | 94 (1e) | 92 (2e) | 92 (3e) | 60 (4e) | 67 (5e) |
| 6 | Me | H | H | 76 (1f) | 98 (2f) | 77 (3f) | 48 (4f) | 86 (5f) |
Yield of pure products after flash column chromatography purification.
We next proceeded to evaluate the best conditions for the key transannular amidohalogenation/elimination process to take place with the highest possible yield and regioselectivity (Table 2). The initial reaction using N-bromosuccinimide (NBS) as the electrophilic halogenation reagent provided the desired transannular amidobromination/elimination product 6a after stirring for 1 h in toluene in an acceptable 78% yield (entry 1). Based on the known ability of Brønsted acids to accelerate this type of electrophilic aminohalogenation reaction, we surveyed the possibility of improving this result by using a Brønsted acid-catalyzed version of this transformation (entries 2–5). Indeed, the reaction in the presence of 10 mol % of trifluoroacetic acid took place faster (complete conversion of the starting material was observed after 30 min) but with a slightly lower yield (entry 2), but moving to the more acidic diphenylphosphoric acid resulted in a very clean and effective reaction (entry 3). Increasing the acidity of the catalyst to the corresponding triflamide was also effective, but the yield of the reaction was somewhat affected, observing the formation of several minor side products whose structure could not be elucidated (entry 4). Sulfonic acids result in lower yields for the same reaction (entry 5). We next evaluated other parameters of the reaction, such as the solvent or the temperature (entries 6–11). Carrying out the reaction in THF led to a rather sluggish reaction (entry 6), with a notable decrease in the yield of the process, but moving to acetonitrile resulted in a very high yield of the desired adduct 6a (entry 7). Other halogenated solvents were also tested (entries 8–10), observing that, in general, the reaction performed well in all cases, with the exceptional case of dichloromethane, which resulted in an almost quantitative formation of the transannular aminohalogenation/elimination product (entry 8). We tried to accelerate the reaction by working at a higher temperature (entry 11), but in this case, even though complete consumption of the starting material could be observed after 5 min, product 6a was isolated in poor yield as a result of the formation of many side products. Catalyst loading could be lowered down to 2.5 mol % without affecting the performance of the reaction (entry 12) and only requiring 1 h for the reaction to reach completion. When we attempted to work with 1 mol % of the Brønsted acid catalyst, the reaction was also very efficient, although it proceeded rather slowly (entry 13). Finally, we also checked the beneficial effect of the Brønsted acid catalyst under these conditions, observing that in the absence of diphenylphosphoric acid, the reaction required a very long time to reach completion and also provided a very low yield of adduct 6a (entry 14).
Table 2. Optimization of the Reaction Conditions for the Transannular Amidobromination/Elimination Process Using 5a as the Model Compound.
| entry | catalyst | solvent | time (min) | yield (%)a |
|---|---|---|---|---|
| 1 | none | toluene | 60 | 78 |
| 2 | TFA | toluene | 30 | 67 |
| 3 | (PhO)2P(O)OH | toluene | 30 | 90 |
| 4 | (PhO)2P(O)NHTf | toluene | 15 | 66 |
| 5 | (±)-CSA | toluene | 20 | 67 |
| 6 | (PhO)2P(O)OH | THF | 30 | 36 |
| 7 | (PhO)2P(O)OH | MeCN | 30 | 85 |
| 8 | (PhO)2P(O)OH | CH2Cl2 | 30 | 96 |
| 9 | (PhO)2P(O)OH | CHCl3 | 30 | 83 |
| 10 | (PhO)2P(O)OH | DCE | 30 | 73 |
| 11b | (PhO)2P(O)OH | CH2Cl2 | 5 | 54 |
| 12c | (PhO)2P(O)OH | CH2Cl2 | 60 | 98 |
| 13d | (PhO)2P(O)OH | CH2Cl2 | 120 | 83 |
| 14 | none | CH2Cl2 | 300 | 39 |
Yield of pure product 6a after flash column chromatography purification.
Reaction carried out at 50 °C.
Reaction carried out using 2.5 mol % of the catalyst.
Reaction carried out using 1 mol % of the catalyst.
Remarkably, when we tested these reaction conditions on substrate 5a but employing N-iodosuccinimide (NIS) as the halogenating reagent, product 6a was formed in low yield (Scheme 3) and the formation of iodinated adduct 7a was detected as the major product of the reaction, this being presumably generated after electrophilic iodination of the former. This was checked by treating isolated 6a with 1 equiv of NIS, observing the formation of 7a in a 76% yield after 24 h. This interesting new compound 7a was obtained in excellent yield from 5a by carrying out the reaction in the presence of 3 equiv of NIS. The reaction using N-chlorosuccinimide as the halogenating agent provided compound 8a in a 72% yield, which is the precursor to 6a in which elimination had not taken place.
Scheme 3. Transannular Amidoiodination/Elimination and Amidochlorination Reactions on Model Substrate 5a.
With an optimized protocol in hand, we decided to explore the potential of this methodology to prepare different families of 2,3-dihydropyrrolo[1,2-b]isoquinolin-5(1H)-one incorporating different substitution patterns (Table 3). As it can be seen in this table, the transannular amidobromination/elimination process leading to adducts 6 proceeded smoothly for all substrates regardless of the nature of the substituent placed at the 7 and 8 positions of the isoquinoline core (entries 1–5), only observing that the reaction required a longer time to reach to completion when electron-donating substituents were placed (entries 4 and 5). In fact, the reaction on substrate 5d containing a strongly electron-donating substituent such as a methoxy group provided only a 40% yield of the aminohalogenated precursor (together with other unidentified byproducts), and in this case, 1.2 equiv of a tertiary amine base such as DBU had to be added after consumption of the starting material in order to assist the elimination process (entry 4). Placing a substituent at the 9-position was much more challenging for the reaction, presumably because of the increased steric congestion, and adduct 6f could be only obtained in moderate yield (entry 6), observing the formation of several decomposition products after prolonged reaction times. It should be highlighted that it has been demonstrated that the amidobromination/elimination process could be carried out at a bigger scale, isolating the compound 6b with an excellent 98% yield starting from 1 mmol of 5b. Moreover, the applicability of this methodology is corroborated due to the fact that the total synthesis of rosettacin is described in the literature from adduct 6a.19 The amidoiodination/elimination/electrophilic iodination sequence leading to adducts 7a–f also proceeded efficiently, only observing a decrease in the yield of the reaction for the 9-substituted substrate 5f, although in this case, the unreacted starting material could be recovered intact (entry 6).
Table 3. Brønsted Acid-Catalyzed Transannular Aminohalogenation on Lactams 5a–f.
| entry | R1 | R2 | R3 | compd | yield 6a–f (%)a | compd | yield 7a–f (%)a |
|---|---|---|---|---|---|---|---|
| 1 | H | H | H | 6a | 98 (40 min) | 7a | 86 (16 h) |
| 2 | H | F | H | 6b | 98 (60 min) | 7b | 86 (16 h) |
| 3 | H | H | F | 6c | 68 (60 min) | 7c | 81 (16 h) |
| 4 | H | H | MeO | 6db | 41 (21 h) | 7d | 71 (23 h) |
| 5 | H | H | Me | 6e | 79 (3 h) | 7e | 85 (16 h) |
| 6 | Me | H | H | 6fb | 26 (72 h) | 7f | 46c (72 h) |
Yield of pure products after flash column chromatography purification.
1.2 equiv of DBU was added after complete conversion of the starting material had been observed.
98% yield based on the recovered starting material.
In addition, substrates 7 are suitable to be further diversified by capitalizing the vinyl iodide moiety present in their structure and their potential to undergo Suzuki coupling with aryl boronates under Pd-catalysis. In order to illustrate this possibility, several of these substrates 7 were reacted with phenylboronic acid under standard Suzuki coupling conditions, providing the corresponding 10-aryl-substituted 2,3-dihydropyrrolo[1,2-b]isoquinolin-5(1H)-ones 9a–d in excellent yields (Scheme 4).
Scheme 4. Suzuki Coupling on 3-Iodo-Substituted Substrates 7a–e.
On the other hand, we also faced the possibility of using this approach to the synthesis of isoindolo[2,1-b]isoquinolin-5(7H)-ones, which are well-known topoisomerase I inhibitors (see Scheme 1 for one example). We started with the synthesis of the key macrocyclic lactam precursor 12a, which was accomplished by initial amide formation between differently substituted o-halobenzylamines and o-vinylbenzoates to form compounds 10a–h in good overall yields after protection as the corresponding N-Boc derivatives. These were subjected subsequently to the intramolecular Pd-catalyzed Heck reaction that took place with complete diastereoselectivity to deliver (E)-configured lactams 12a–h after N-deprotection under standard conditions.20 This approach for the formation of the medium-sized lactam moiety was especially successful when arylamide 10-incorporated electron-donating substituents on any of the aryl moieties, providing significantly lower yields in the intramolecular Heck reactions in the cases in which fluorine or chlorine atoms were installed. Once the key lactams 12a–h had been prepared, these were submitted to the transannular amidobromination/elimination process under the standard conditions. However, in an initial attempt, the reaction provided the amidohalogenated product in which elimination had not taken place. This situation was solved by adding 1 equiv of a Brønsted base such as DBU together with NaI; the latter was required to promote a Finkelstein-type process that inverted the relative configuration of the amidohalogenation product to facilitate the elimination reaction through the E2 process.21 As it can be seen in Table 4, isoindolo[2,1-b]isoquinolin-5(7H)-one 13a, which had been reported to be a highly active topoisomerase I inhibitor was formed in excellent yield from lactam 12a under these conditions. In addition, the other lactams 12b–h prepared were also converted into isoindolo[2,1-b]isoquinolin-5(7H)-ones 13b–h through this transannular process in excellent yields regardless of the substitution patterns in any of the aryl moieties.
Table 4. Synthesis of Isoindolo[2,1-b]isoquinolin-5(7H)-ones.
| entry | R1 | R2 | R3 | R4 | yield 10a–f (%)a | yield 11a–f (%)a | yield 12a–f (%)a | yield 13a–f (%)a |
|---|---|---|---|---|---|---|---|---|
| 1 | H | H | H | H | 72 (10a) | 60 (11a) | 63 (12a) | 91 (13a) |
| 2 | F | H | H | H | 68 (10b) | 26 (11b) | 80 (12b) | 83 (13b) |
| 3 | H | F | H | H | 59 (10c) | 16 (11c) | 58 (12c) | 74 (13c) |
| 4 | Cl | H | H | H | 51 (10d) | 23 (11d) | 62 (12d) | 96 (13d) |
| 5 | Me | H | H | H | 67 (10e) | 68 (11e) | 59 (12e) | 92 (13e) |
| 6 | OMe | H | H | H | 62 (10f) | 64 (11f) | 69 (12f) | 45 (13f)b |
| 7 | H | H | H | F | 55 (10g) | 45 (11g) | 74 (12g) | 99 (13g) |
| 8 | H | H | Me | H | 75 (10h) | 92 (11h) | 66 (12h) | 82 (13h) |
Yield of pure products after flash column chromatography purification.
Addition of the NaI/DBU system was not necessary for performing the elimination step.
In conclusion, we have demonstrated that nine-membered enelactams can be used as useful starting materials for the preparation of differently substituted pyrrolo[1,2-b]isoquinolin-5(1H)-ones using a transannular approach as the key transformation for the construction of the tricyclic heterocyclic scaffold. This crucial transannular reaction consists of a Brønsted acid-catalyzed amidohalogenation process, followed by elimination, and provides the target products in good yields regardless of the substitution pattern in the cyclic lactam. Moreover, when the reaction is carried out in the presence of an excess of NIS as the electrophilic halogen source, a second halogenation reaction took place on the obtained adducts, furnishing the corresponding iodinated derivatives in good yields, with these compounds having a convenient functionality to be further functionalized through Suzuki coupling chemistry. The protocol described herein has been successfully applied to the preparation of the topoisomerase I inhibitor isoindolo[2,1-b]isoquinolin-5(7H)-one and several other related compounds, also including the formal total synthesis of rosettacin.
Experimental Section
General Methods and Materials
Analytical-grade solvents and commercially available reagents were purchased from commercial sources and used without further purification. Anhydrous solvents were purified and dried with activated molecular sieves prior to use.22 For reactions carried out under inert conditions, argon was previously dried through a column of P2O5 and a column of KOH and CaCl2. All the glassware was dried for 12 h prior to use in an oven at 140 °C and allowed to cool under a dehumidified atmosphere. Reactions at reduced temperatures were carried out using a Thermo Haake EK90 refrigerator. Reactions were monitored using analytical thin-layer chromatography (TLC) in precoated silica-backed plates (Merck Kiesegel 60 F254). These were visualized by ultraviolet irradiation, p-anisaldehyde, phosphomolybdic acid, or potassium permanganate dips.23 For flash chromatography, Silicycle 40–63 and 230–400 mesh silica gel was used.24 Monodimensional and/or bidimensional nuclear magnetic resonance proton and carbon spectra (1H NMR and 13C {1H} NMR) were acquired at 25 °C on a Bruker AC-300 spectrometer (300 MHz for 1H, 75.5 MHz for 13C and 282 MHz for 19F) and a Bruker AC-500 spectrometer (500 MHz for 1H and 125.7 MHz for 13C) at the indicated temperature. Chemical shifts (δ) are reported in parts per million relative to residual solvent signals (CHCl3, 7.26 ppm for 1H NMR, CDCl3, 77.16 ppm for 13C {1H} NMR) and coupling constants (J) in hertz (Hz). The following abbreviations are used to indicate the multiplicity in NMR spectra: s, singlet; d, doublet; t, triplet; q, quartet; p, pentet; app, apparent; m, multiplet; bs, broad signal. 13C {1H} NMR spectra were acquired on a broad-band decoupled mode using distortion-less enhancement by polarization transfer experiments for assigning different types of carbon environments. Assignments were made based upon the IUPAC numbering system. Mass spectra were recorded on an Agilent 7890 A gas chromatograph coupled to an Agilent 5975 C quadrupole mass spectrometer under electronic impact (EI) ionization at 70 eV. The obtained data is presented in mass units (m/z), and the values found in brackets belong to the relative intensities compared to the base peak (100%). High-resolution mass spectra were recorded on an Acquity UPLC system coupled to a quadrupole time-of-flight mass spectrometer (SYNAPT G2 HDMS) using electrospray ionization (ESI+). Infrared (IR) spectra were measured in a Jasco FT/IR 4100 (ATR), in the interval between 4000 and 400 cm–1 with a 4 cm–1 resolution. Only characteristic bands are given in each case. Melting points (mp) were measured using a Buchi B-540 apparatus in open capillary tubes and were uncorrected.
Procedure for the Synthesis of Methyl 2-(5-Chloropent-1-yn-1-yl)benzoate (1a)
In an oven-dried, two-necked round-bottom flask equipped with a condenser and stir bar with methyl 2-bromobenzoate (9 mL, 64 mmol), PdCl2(PPh3)2 (0.898 g, 1.28 mmol), PCy3 (0.718 g, 2.56 mmol), and CuI (0.49 g, 2.56 mmol) in freshly distilled Et3N (256 mL) under an Ar atmosphere, 5-chloropent-1-yne (10.6 mL, 95.5 mmol) was added. The mixture was heated using a heating plate to 80 °C over 18 h. Then, the reaction mixture was cooled to room temperature and was filtrated through a plug of Celite. Aq HCl 1 M (10 mL) was added to the filtrate, and the organic layer was extracted with EtOAc (3 × 10 mL). All the organic layers were washed with aq std. NaHCO3 (10 mL), dried over Na2SO4, and concentrated under vacuum. The crude was purified by silica gel flash chromatography (petroleum ether/EtOAc 19:1), obtaining 1a (13.3 g, 60 mmol, 93%) as a yellow oil. Rf: 0.6 (petroleum ether/EtOAc 9:1). 1H NMR (300 MHz, CDCl3): δ 7.88 (ddd, J = 7.8, 1.5, 0.6 Hz, 1H), 7.49 (dd, J = 7.8, 1.2 Hz, 1H), 7.41 (td, J = 7.5, 1.5 Hz, 1H), 7.31 (td, J = 7.6, 1.5 Hz, 1H), 3.90 (s, 3H), 3.77 (t, J = 6.4 Hz, 2H), 2.66 (t, J = 6.7 Hz, 2H), 2.07 (p, J = 6.6 Hz, 2H). 13C {1H} NMR (75 MHz, CDCl3): δ 166.9 (C), 134.3 (CH), 132.0 (C), 131.6 (CH), 130.3 (CH), 127.5 (CH), 124.1 (C), 93.7 (C), 80.3 (C), 52.2 (CH3), 43.8 (CH2), 31.5 (CH2), 17.3 (CH2). IR (ATR, cm–1): 2264 (C≡C), 1726 (C=O). MS (EI) m/z (%): 174 (100, M+ – CH3CH2Cl), 159 (21, M+ – CH3CH2CH2Cl), 115 (21, M+ – CO2Me–CH3CH2Cl). HRMS (ESI) m/z: [M + H]+ calcd for [C13H14ClO2]+, 237.0677; found, 237.0689 for compound: 1a.
Procedure for the Synthesis of Methyl (Z)-2-(2-(5-Chloropent-1-en-1-yl)benzoate) (2a)
To a two-necked round-bottom flask equipped with a stirring bar and H2 balloon with methyl 2-(5-chloropent-1-yn-1-yl)benzoate 1a (3 g, 12.7 mmol) in EtOAc (127 mL) and quinoline (0.12 mL, 1.02 mmol, 8 mol %), Pd on CaCO3 (1.35 g, 0.63 mmol, 5 mol %) was added. The air flask was evacuated under vacuum and backfilled with hydrogen three times, and the reaction mixture was allowed to stir at room temperature under a hydrogen atmosphere (balloon pressure) until full consumption of the starting material, 1 h as judged by TLC. The reaction mixture was filtered through a plug of Celite, and the filtrate was concentrated under vacuum. The crude was purified by silica gel flash chromatography (petroleum ether/EtOAc 19:1) obtaining 2a (3.0 g, 11.6 mmol, 91%) as a yellow oil. Rf: 0.62 (petroleum ether/EtOAc). 1H NMR (300 MHz, CDCl3): δ 7.95 (dd, J = 7.8, 1.2 Hz, 1H), 7.48 (td, J = 7.5, 1.3 Hz, 1H), 7.36–7.28 (m, 2H), 6.92 (d, J = 11.5 Hz, 1H), 5.67 (dt, J = 11.5, 7.4 Hz, 1H), 3.87 (s, 3H), 3.49 (t, J = 6.7 Hz, 2H), 2.27 (m, 2H), 1.92–1.78 (m, 2H). 13C {1H} NMR (75 MHz, CDCl3): δ 167.6 (C), 138.9 (C), 131.8 (CH), 130.8 (CH), 130.6 (CH), 130.5 (CH), 129.8 (CH), 129.4 (C), 127.0 (CH), 52.0 (CH3), 44.5 (CH2), 32.7 (CH2), 25.6 (CH2). IR (ATR, cm–1): 1720 (C=O). MS (EI) m/z (%): 238 (M+, 11), 161 (M+ – CH3CH2Cl–CH3, 100), 128 (M+ – CH3Cl–CO2Me, 35), 115 (M+ – CH3CH2Cl–CO2Me, 68). HRMS (ESI) m/z: [M + H]+ calcd for [C13H16ClO2]+, 239.0833; found, 239.0838 for compound: 2a.
Procedure for the Synthesis of Methyl (Z)-2-(5-(1,3-Dioxoisoindolin-2-yl)pent-1-en-1-yl)benzoate (3a)
An oven-dried two-necked round-bottom flask provided with a condenser and a magnetic bar with a suspension of methyl (Z)-2-(2-(5-chloropent-1-en-1-yl)benzoate) 2a (2.9 g, 12.2 mmol), Cs2CO3 (8.73 g, 26.8 mmol), phthalimide (2.69 g, 18.3 mmol), and KI (20.5 mg, 0.122 mmol) in DMF (70 mL) under an Ar atmosphere was heated using a heating plate at 100 °C for 2 h. Then, the reaction mixture was let to cool down to room temperature and water (100 mL) and EtOAc (50 mL) was added. The aqueous layer was extracted with EtOAc (3 × 25 mL), washed with H2O (2 × 75 mL), dried with Na2SO4, filtered and concentrated under vacuum. The crude was purified by silica gel flash chromatography (petroleum ether/EtOAc 9:1 to petroleum ether/EtOAc 8:2) in order to obtain 3a (3.7 g, 10.6 mmol, 87%) as a white solid. Rf: 0.5 (petroleum ether/EtOAc 8:2). mp 69–71 °C. 1H NMR (300 MHz, CDCl3): δ 7.89 (dd, J = 7.8, 1.0 Hz, 1H), 7.80 (dd, J = 5.5, 3.0 Hz, 2H), 7.68 (dd, J = 5.3, 3.2 Hz, 2H), 7.40 (td, J = 7.5, 1.2 Hz, 1H), 7.29–7.19 (m, 2H), 6.88 (d, J = 11.5 Hz, 1H), 5.72 (dt, J = 11.6, 7.4 Hz, 1H), 3.85 (s, 3H), 3.66–3.58 (m, 2H), 2.23–2.09 (m, 2H), 1.83–1.69 (m, 2H). 13C {1H} NMR (75 MHz, CDCl3): δ 168.5 (2xC), 167.8 (C), 138.8 (C), 134.0 (2xCH), 132.3 (2× C), 131.7 (CH), 130.7 (CH), 130.6 (CH), 130.4 (CH), 130.0 (CH), 129.5 (C), 126.9 (CH), 123.3 (2× CH), 52.1 (CH3), 37.8 (CH2), 28.7 (CH2), 25.8 (CH2). IR (ATR, cm–1): 1771 (O=C–N–C=O), 1708 (C=O). MS (EI) m/z (%): 317 (M+ – MeOH, 37), 170 (M+ – MeOH–Phth, 100). HRMS (ESI) m/z: [M + H]+ calcd for [C21H20NO4]+, 350.1387; found, 350.1390 for compound: 3a.
Procedure for the Synthesis of Methyl (Z)-2-(5-Aminopent-1-en-1-yl)benzoate (4a)
A round-bottom flask equipped with a magnetic bar was provided with (Z)-2-(5-(1,3-dioxoisoindolin-2-yl)pent-1-en-1-yl)benzoate 3a (3.55 g, 10.2 mmol) and hydrazine (50% w/w in water, 1.4 mL, 28.6 mmol) in EtOH (51 mL). The reaction mixture was heated using a heating plate at 50 °C for 2 h. Then, the reaction mixture was let to cool down to room temperature and was filtered through a plug of Celite The filtrate was concentrated under reduced pressure, and 1 M HCl (50mL) was added; the aqueous layer was washed with EtOAc (3 × 25 mL), basified with 4 M NaOH to pH = 9, extracted with CH2Cl2 (4 × 25 mL), dried with Na2SO4, filtered, and concentrated under vacuum to obtain 4a (1.66 g, 7.5 mmol, 74%) as a yellow oil. Rf: 0.2 (MeOH). 1H NMR (300 MHz, CDCl3): δ 7.96 (d, J = 7.8 Hz, 1H), 7.49 (t, J = 7.5 Hz, 1H), 7.37–7.28 (m, 2H), 6.90 (d, J = 11.6 Hz, 1H), 5.74 (dt, J = 11.6, 7.4 Hz, 1H), 3.90 (s, 3H), 2.68 (t, J = 6.5 Hz, 2H), 2.19 (q, J = 7.4 Hz, 2H), 1.57 (p, J = 7.2 Hz, 2H), 1.36 (br s, 2H). 13C {1H} NMR (75 MHz, CDCl3): δ 168.1 (C), 139.2 (C), 132.0 (CH), 131.4 (CH), 131.1 (CH), 130.8 (CH), 129.9 (C), 129.7 (CH), 127.2 (CH), 52.4 (CH3), 41.3 (CH2), 32.4 (CH2), 26.0 (CH2). IR (ATR, cm–1): 1719 (C=O), 1636 (N–H). MS (EI) m/z (%): 219 (M+, 10), 202 (M+ – NH2, 59), 161 (M+ – CH3–CH3CH2NH2, 47), 115 (M+ – CO2Me–CH3CH2NH2, 100). HRMS (ESI) m/z: [M + H]+ calcd for [C13H18NO2]+, 220.1332; found, 220.1340 for compound: 4a.
Procedure for the Synthesis of (Z)-2,3,4,5-Tetrahydro-1H-benzo[c]azonin-1-one (5a)
To an oven-dried two-necked round-bottom flask provided with a magnetic bar with methyl (Z)-2-(5-aminopent-1-en-1-yl)benzoate 4a (0.5 g, 2.3 mmol) in dry THF (115 mL) under an Ar atmosphere, LiHMDS 1 M in THF (6.9 mL, 6.9 mmol) was added dropwise at 0 °C. The reaction mixture was stirred at room temperature and monitored by TLC. Once the starting material (SM) was consumed, MeOH (1 mL) was added, and the solvent was removed under vacuum. The residue was dissolved in CH2Cl2 and filtrated through Celite. The filtrate was concentrated under vacuum and purified by silica gel flash chromatography (petroleum ether/EtOAc 8:2 to petroleum ether/EtOAc 7:3) to obtain 5a (0.21 g, 1.1 mmol, 50%) as a white solid. Rf: 0.4 (petroleum ether/EtOAc 7:3). mp 183–186 °C. 1H NMR (300 MHz, CDCl3): δ 7.42–7.27 (m, 3H), 7.17 (d, J = 7.0 Hz, 1H), 6.62 (d, J = 10.8 Hz, 1H), 6.30 (br s, 1H), 6.00 (dt, J = 10.6, 8.4 Hz, 1H), 3.41–2.99 (m, 2H), 2.30–1.78 (m, 2H), 1.51 (app p, J = 5.2 Hz, 2H). 13C {1H} NMR (75 MHz, CDCl3): δ 174.0 (C), 137.1 (C), 136.2 (C), 134.3 (CH), 128.7 (CH), 128.7 (CH), 128.3 (CH), 127.3 (CH), 125.2 (CH), 45.4 (CH2), 29.3 (CH2), 28.9 (CH2). IR (ATR, cm–1): 3287 (N–H), 1646 (N–C=O). MS (EI) m/z (%): 187 (M+, 60), 158 (M+ – CH3NH2, 100). HRMS (ESI) m/z: [M + H]+ calcd for [C13H17NO]+, 202.1232; found, 202.1235 for compound: 5a.
Procedure for the Synthesis of 2,3-Dihydropyrrolo[1,2-b]isoquinolin-5(1H)-one (6a)
To a reaction tube provided with a magnetic bar with (Z)-2,3,4,5-tetrahydro-1H-benzo[c]azonin-1-one 5a (20 mg, 0.107 mmol), a stock solution of diphenylphosphoric acid (0.7 mg, 0.003 mmol) in dry dichloromethane (220 μL) was added at 25 °C, followed by the addition of NBS (19 mg, 0.108 mmol). The reaction mixture was subjected to TLC, and when all starting material was consumed (typically 40 min), the solvent was evaporated under vacuum, and Et2O (1 mL) and a standard. aq solution of NaHCO3 (1 mL) were added. The layers were separated, and the aqueous phase was extracted with Et2O (3 × 1 mL). All organic layers were washed with water (3 × 1 mL) and brine (1 mL), dried with Na2SO4, filtered, and concentrated under vacuum. The crude was purified by silica gel flash chromatography (CH2Cl2 to CH2Cl2/MeOH 99:1) obtaining 6a (19 mg, 0.103 mmol, 98%) as a white solid. Rf: 0.3 (CH2Cl2/MeOH 99:1). mp 102–105 °C. 1H NMR (300 MHz, CDCl3): δ 8.31 (dq, J = 8.03, 0.61 Hz, 1H), 7.51 (ddd, J = 8.2, 7.0, 1.4 Hz, 1H), 7.38 (d, J = 7.8 Hz, 1H), 7.32 (dd, J = Hz, 1H), 6.34 (s, 1H), 4.15–4.06 (m, 2H), 3.02 (td, J = 7.7, 1.4 Hz, 2H), 2.20–2.04 (m, 2H). 13C {1H} NMR (75 MHz, CDCl3): δ 161.6 (C), 143.7 (C), 138.1 (C), 131.9 (CH), 127.3 (CH), 125.5 (CH), 125.5 (CH), 124.7 (C), 100.3 (CH), 47.9 (CH2), 31.3 (CH2), 22.0 (CH2). IR (ATR, cm–1): 2985 (C–H), 1657 (N–C=O), 1624 (C=C). MS (EI) m/z (%): 184.1 (M+, 100). HRMS (ESI) m/z: [M + H]+ calcd for [C12H12NO]+, 186.0919; found, 186.0919 for compound: 6a.
Procedure for the Synthesis of 10-Iodo-2,3-dihydropyrrolo[1,2-b]isoquinolin-5(1H)-one (7a)
To a reaction tube provided with a magnetic bar with (Z)-2,3,4,5-tetrahydro-1H-benzo[c]azonin-1-one 5a (27.4 mg, 0.145 mmol), a stock solution of diphenylphosphoric acid (0.9 mg, 0.004 mmol) in dry dichloromethane (290 μL) was added at 25 °C, followed by the addition of NIS (98 mg, 0.434 mmol). The reaction mixture was subjected to TLC, and when all starting material was consumed (typically 16 h), the solvent was evaporated under vacuum, and Et2O (1 mL) and a standard aq solution of Na2S2O3 (1 mL) were added; the mixture was stirred for 30 min at room temperature. Then, the layers were separated, and the aqueous phase was extracted with Et2O (3 × 1 mL). All organic layers were washed with water (3 × 1 mL), brine (1 mL), dried with Na2SO4, filtered, and concentrated under vacuum. The crude was purified by silica gel flash chromatography (CH2Cl2 to CH2Cl2/MeOH 99:1) in order to obtain 7a (38.6 mg, 0.124 mmol, 86%) as a pale-yellow solid. Rf: 0.3 (CH2Cl2/MeOH 99:1). mp 141–143 °C. 1H NMR (300 MHz, CDCl3): δ 8.37 (dd, J = 8.0, 0.9 Hz, 1H), 7.76 (dd, J = 8.1, 0.6 Hz, 1H), 7.69 (ddd, J = 8.3, 6.9, 1.14 Hz, 1H), 7.47 (ddd, J = 8.2, 7.0, 1.2 Hz, 1H), 4.36 (t, J = 7.4 Hz, 2H), 3.26 (t, J = 7.8 Hz, 2H), 2.25 (p, J = 7.5 Hz, 2H). 13C {1H} NMR (75 MHz, CDCl3): δ 160.9 (C), 146.7 (C), 138.0 (C), 133.1 (CH), 129.3 (CH), 127.7 (CH), 126.6 (CH), 124.9 (C), 68.7 (C), 50.2 (CH2), 37.5 (CH2), 20.8 (CH2). IR (ATR, cm–1): 1638 (N–C=O), 1609 (C=C). MS (EI) m/z (%): 311.0 (M+, 100). HRMS (ESI) m/z: [M + H]+ calcd for [C12H11INO]+, 311.9885; found, 311.9891 for compound: 7a.
Procedure for the Synthesis of 10-Phenyl-2,3-dihydropyrrolo[1,2-b]isoquinolin-5(1H)-one (9a)
An oven-dried 50 mL two-necked flask was charged with 10-iodo-2,3-dihydropyrrolo[1,2-b]isoquinolin-5(1H)-one 7a (31.2 mg, 0.1 mmol), phenyl boronic acid (15.9 mg, 0.13 mmol), Pd(PPh3)4 (5.7 mg, 0.005 mmol), and K2CO3 (69.1 mg, 0.5 mmol). Under an argon atmosphere, 1,4-dioxane (5 mL) and water (2 mL) were added, and the reaction mixture was subjected to vacuum and refilled with argon three times. The reaction mixture was heated using a heating plate up at 65 °C for 21 h. After completion (typically 24 h), the mixture was cooled to room temperature and diluted with EtOAc (10 mL). The mixture was filtered through a small pad of Celite. Afterward, the solvent was concentrated in vacuum. The resulting crude was purified by flash chromatography (petroleum ether/EtOAc 7:3 to 1:1) in order to obtain 9a (25.7 mg, 0.098 mmol, 98%) as a yellow solid. Rf: 0.27 (petroleum ether/EtOAc 1:1). mp 169–171 °C. 1H NMR (300 MHz, CDCl3): δ 8.48 (dd, J = 7.9, 0.8 Hz, 1H), 7.59–7.34 (m, 5H), 7.36–7.23 (m, 3H), 4.28 (t, J = 7.2 Hz, 2H), 2.93 (t, J = 7.6 Hz, 2H), 2.15 (p, J = 7.5 Hz, 2H). 13C {1H} NMR (75 MHz, CDCl3): δ 161.2 (C), 141.4 (C), 138.2 (C), 136.5 (C), 132.0 (CH), 130.7 (2× CH), 128.8 (2× CH), 127.7 (CH), 127.5 (CH), 125.7 (CH), 125.1 (C), 124.4 (CH), 113.8 (C), 48.6 (CH2), 31.2 (CH2), 22.0 (CH2). IR (ATR, cm–1): 1647 (C=O st), 1622 (CArom–CArom st), 1598 (CArom–CArom st). MS (EI) m/z (%): 261.0 (M+, 100), 260.1 (67), 165.0 (36), 163.0 (20), 76.9 (26). HRMS (ESI) m/z: [M + Hc+ Calcd for [C18H16NO]+, 262.1226; found, 262.1237 for compound: 9a.
Procedure for the Synthesis of tert-Butyl(2-iodobenzyl)(2-vinylbenzoyl)carbamate (10a)
In the first step, to an oven-dried 100 mL two-necked flask provided with the corresponding methyl 2-vinylbenzoate (1.20 g, 7.40 mmol) and (2-iodophenyl)methanamine (1.81 g, 7.80 mmol) under an Ar atmosphere, LiHMDS in THF 1 M (22.2 mL, 22.2 mmol) was added dropwise at room temperature to the stirring mixture, and it was allowed to stir for 16 h at this temperature. The solvent was evaporated under vacuum and purified by silica gel column chromatography (petroleum ether/EtOAc 8:2) to obtain N-(2-iodobenzyl)-2-vinylbenzylamide (2.21 g, 6.08 mmol, 82%) as a solid. Rf: 0.53 (petroleum ether/EtOAc 8:2). mp 136–138 °C. 1H NMR (300 MHz, CDCl3): δ 7.85 (dd, J = 7.9, 1.3 Hz, 1H), 7.61–7.23 (m, 6H), 6.28 (br s, 1H), 5.69 (dd, J = 17.5, 1.2 Hz, 1H), 5.33 (dd, J = 11.0, 1.2 Hz, 1H), 4.65 (d, J = 6.0 Hz, 2H). 13C {1H} NMR (75 MHz, CDCl3): δ 169.1 (C), 140.4 (C), 139.7 (CH), 136.3 (C), 135.0 (C), 134.7 (CH), 130.5 (CH), 130.2 (CH), 129.6 (CH), 128.8 (CH), 127.9 (CH), 127.7 (CH), 126.6 (CH), 117.1 (CH2), 99.3 (C), 48.9 (CH2). IR (ATR, cm–1): 3264 (N–H), 1640 (N–C=O), 1525 (C=C). MS (EI) m/z (%): 363.0 (M+, 15), 236.1 (M+ – I, 100). HRMS (ESI) m/z: [M + H]+ calcd for [C16H15INO]+, 364.0193; found, 364.0197. In the second step, to an oven-dried 50 mL two-necked flask, N-(2-iodobenzyl)-2-vinylbenzamide (1.92 g, 6.08 mmol) and di-tert-butyl dicarbonate (2.00 g, 9.12 mmol) under an Ar atmosphere were dissolved in dry CH2Cl2 (12 mL), and under stirring, DMAP (74 mg, 0.60 mmol) was added at room temperature. The reaction mixture was allowed to stir at room temperature for 16 h. Then, it was concentrated under vacuum and purified by silica gel column chromatography FC (petroleum ether/EtOAc 9:1 to 8:2) in order to obtain 10a (2.48 g, 5.35 mmol, 88%) (overall yield for the two steps = 72%) as a yellow solid. Rf: 0.47 (petroleum ether/EtOAc 8:2). mp 75–76 °C. 1H NMR (300 MHz, CDCl3): δ 7.76 (dd, J = 7.9, 1.3 Hz, 1H), 7.49 (d, J = 7.8 Hz, 1H), 7.35–7.16 (m, 4H), 7.10 (dd, J = 7.8, 1.6 Hz, 1H), 6.91–6.71 (m, 2H), 5.65 (dd, J = 17.4, 1.1 Hz, 1H), 5.28 (dd, J = 10.9, 1.1 Hz, 1H), 4.97 (s, 2H), 0.99 (s, 9H). 13C {1H} NMR (75 MHz, CDCl3): δ 172.1 (C), 152.3 (C), 139.5 (CH), 139.4 (C), 137.1 (C), 135.3 (C), 133.8 (CH), 129.7 (CH), 128.7 (CH), 128.4 (CH), 127.3 (CH), 126.3 (CH), 126.2 (CH), 125.8 (CH), 117.0 (CH2), 97.7 (C), 83.7 (C), 53.3 (CH2), 27.3 (3× CH3). IR (ATR, cm–1): 1733 (C=O), 1671 (N–C=O). MS (EI) m/z (%): 362.0 (M+, 15). HRMS (ESI) m/z: [M + Na]+ calcd for [C21H22INO3Na]+, 486.0537; found, 486.0538 for compound: 10a.
Procedure for the Synthesis of tert-Butyl(E)-5-oxo-5,7-dihydro-6H-dibenzo[c,g]azonine-6-carboxylate (11a)
To an oven-dried 250 mL flask provided with tert-butyl(2-iodobenzyl)(2-vinylbenzoyl)carbamate 10a (1.18 g, 2.55 mmol) in dry DMF (12.8 mL) under an Ar atmosphere, sodium acetate (418 mg, 5.10 mmol), triphenylphosphine (67 mg, 0.26 mmol), and palladium acetate (29 mg, 0.13 mmol) were added. The reaction mixture was stirred at 100 °C using a heating plate for 72 h. Then, it was cooled down and water (20 mL) and EtOAc (20 mL) were added; the mixture was filtered through Celite, the filtrate was extracted with EtOAc (4 × 20 mL), and the organic phase was washed with water (3 × 5 mL) and brine (10 mL), dried with Na2SO4 anhydrous, filtered, and concentrated under vacuum. The crude was purified by silica gel column chromatography FC (petroleum ether/EtOAc 9:1) in order to obtain 11a (513 mg, 1.53 mmol, 60%) as a yellow solid. Rf: 0.45 (petroleum ether/EtOAc 95:5). mp 74–77 °C. 1H NMR (300 MHz, CDCl3): δ 7.70 (dd, J = 7.5, 1.6 Hz, 1H), 7.53–7.41 (m, 2H), 7.40–7.25 (m, 5H), 6.59 (d, J = 16.6 Hz, 1H), 6.51 (d, J = 16.6 Hz, 1H), 5.41 (d, J = 15.8 Hz, 1H), 4.63 (d, J = 15.8 Hz, 1H), 1.00 (s, 9H). 13C {1H} NMR (75 MHz, CDCl3): δ 177.8 (C), 151.4 (C), 140.0 (C) 137.8 (C), 137.7 (C), 136.3 (CH), 133.0 (C), 130.1 (CH), 129.5 (CH), 128.3 (CH), 128.1 (CH), 128.0 (CH), 127.9 (CH), 127.5 (CH), 127.3 (CH), 125.1 (CH), 82.0 (C), 52.0 (CH2), 27.5 (3× CH3). IR (ATR, cm–1): 1722 (C=O), 1680 (N–C=O). MS (EI) m/z (%): 232.9 (M+ – Boc). HRMS (ESI) m/z: [M + Na]+ calcd for [C21H21NNaO3]+, 358.1414; found, 358.1420 for compound: 11a.
Procedure for the Synthesis of (E)-6,7-Dihydro-5H-dibenzo[c,g]azonin-5-one (12a)
tert-Butyl(E)-5-oxo-5,7-dihydro-6H-dibenzo[c,g]azonine-6-carboxylate 11a (67,1 mg, 0.200 mmol) was dissolved in CH2Cl2 (5 mL), and silica gel (230–400 mesh) (2.00 g) was added. The solvent was vacuumed, and the powdered solid obtained was irradiated in the microwave oven in an open Erlenmeyer flask at 540 W. The reaction was checked by TLC every 6 min until it was completed (typically 30 min). The reaction mixture was deadsorbed by thoroughly washing the silica gel with petroleum ether/EtOAC 1:1 with pressure in a column. The crude was purified by silica gel column chromatography (petroleum ether/EtOAc 8:2 to 7:3) in order to obtain 12a (29.6 mg, 0.126 mmol, 63%) as a white solid. Rf: 0.39 (petroleum ether/EtOAc 9:1). mp 205–208 °C. 1H NMR (300 MHz, CDCl3): δ 7.66–7.60 (m, 1H), 7.44–7.28 (m, 3H), 7.28–7.17 (m, 3H), 7.17–7.11 (m, 1H), 6.65 (d, J = 17.0 Hz, 1H), 6.41 (d, J = 17.0 Hz, 1H), 5.38 (dd, J = 16.1, 10.7 Hz, 1H), 4.82 (d, J = 10.7 Hz, 1H), 4.18 (d, J = 16.1 Hz, 1H). 13C {1H} NMR (75 MHz, CDCl3): δ 176.0 (C), 139.1 (C), 138.4 (C), 138.3 (C), 136.0 (CH), 132.6 (C), 130.4 (CH), 129.3 (CH), 129.0 (CH), 128.7 (CH), 128.1 (CH), 128.0 (CH), 127.9 (CH), 127.3 (CH), 125.7 (CH), 48.9 (CH2). IR (ATR, cm–1): 3280 (N–H), 1637 (N–C=O), 1521 (C=C). MS (EI) m/z (%): 235.1 (M+,100). HRMS (ESI) m/z: [M + H]+ calcd for [C16H14NO]+, 236.1075; found, 236.1074 for compound: 12a.
Procedure for the Synthesis of Isoindolo[2,1-b]isoquinolin-5(7H)-one (13a)
To a reaction tube provided with a magnetic bar with (E)-6,7-dihydro-5H-dibenzo[c,g]azonin-5-one 12a (23.5 mg, 0.100 mmol), a stock solution of diphenylphosphoric acid (0.6 mg, 0.003 mmol) in dry dichloromethane (200 μL) was added at 25 °C, followed by the addition of NBS (18 mg, 0.101 mmol). The reaction mixture was subjected to TLC (CH2Cl2/MeOH 98:2). Once the starting material was consumed (5h), DBU (18 μL, 0.120 mmol) and NaI (18 mg, 0.120 mmol) were added to the reaction mixture at 25 °C. The new reaction mixture was allowed to stir and was subjected to TLC until the intermediate was consumed (16 h). Then, a standard aq. solution of NH4Cl (1 mL) was added; the layers were separated, and the aqueous phase was extracted with CH2Cl2 (3 × 1 mL). All organic layers were washed with water (3 × 1 mL) and brine (1 mL), dried with Na2SO4, filtered, and concentrated under vacuum. The crude was purified by silica gel flash chromatography (CH2Cl2 to CH2Cl2/MeOH 99:1) to obtain 13a (21 mg, 0.09 mmol, 91%) as a pale-yellow solid. Rf: 0.49 (petroleum ether/EtOAc 1:1). mp 186–188 °C. 1H NMR (300 MHz, CDCl3): δ 8.52–8.44 (m, 1H), 7.82–7.75 (m, 1H), 7.70–7.60 (m, 2H), 7.58–7.52 (m, 1H), 7.53–7.41 (m, 3H), 7.01 (s, 1H), 5.18 (s, 2H). 13C {1H} NMR (75 MHz, CDCl3): δ 161.2 (C), 142.2 (C), 138.0 (C), 137.7 (C), 134.1 (C), 132.2 (CH), 129.9 (CH), 128.4 (CH), 127.5 (CH), 126.4 (CH), 126.2 (CH), 124.8 (C), 123.5 (CH), 121.1 (CH), 98.1 (CH), 52.1 (CH2). IR (ATR, cm–1): 1660 (N–C=O), 1627 (C=C). MS (EI) m/z (%): 233.0 (M+, 100). HRMS (ESI) m/z: [M + H]+ calcd for [C16H12NO]+, 234.0919; found, 234.0922 for compound: 13a.
Acknowledgments
The authors thank the Spanish Agencia Estatal de Investigación (FEDER-PID2020-118422-GB-I00 and FPI fellowship to A.S.), the Basque Government (IT908-16 and postdoctoral contract to J.L.B.), and UPV/EHU (fellowship to E.C.) for financial support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.2c01045.
Screening of reaction conditions, full experimental procedures, characterization data, and NMR spectra for new compounds prepared (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Matveeva M. D.; Purgatorio R.; Voskressensky G.; Altomare C. D. Pyrrolo[2,1-a]isoquinoline scaffold in drug discovery: advances in synthesis and medicinal chemistry. Future Med. Chem. 2019, 11, 2735–2755. 10.4155/fmc-2019-0136. [DOI] [PubMed] [Google Scholar]; b Nevskaya A. A.; Miftyakhova A. R. Current approaches to the synthesis of pyrrolo[2,1-a]isoquinolines (microreview). Chem. Heterocycl. Comp. 2019, 55, 193–195. 10.1007/s10593-019-02439-z. [DOI] [Google Scholar]
- a Cinelli M. A. Topoisomerase 1B poisons: Over a half-century of drug leads, clinical candidates, and serendipitous discoveries. Med. Res. Rev. 2019, 39, 1294–1337. 10.1002/med.21546. [DOI] [PubMed] [Google Scholar]; b Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- a Bodley A. L.; Shapiro T. A. Molecular and cytotoxic effects of camptothecin, a topoisomerase I inhibitor, on trypanosomes and Leishmania. Proc. Natl. Acad. Sci. U.S.A. 1995, 92, 3726–3730. 10.1073/pnas.92.9.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Bodley A. L.; Wani M. C.; Wall M. E.; Shapiro T. A. Antitrypanosomal activity of camptothecin analogs. Biochem. Pharmacol. 1995, 50, 937–942. 10.1016/0006-2952(95)00215-l. [DOI] [PubMed] [Google Scholar]; c Prada C. F.; Álvarez-Velilla R.; Balaña-Fouce R.; Prieto C.; Calvo-Álvarez E.; Escudero-Martínez J. M.; Requena J. M.; Ordóñez C.; Desideri A.; Pérez-Pertejo Y.; Reguera R. M. Gimatecan and other camptothecin derivatives poison Leishmania DNA-topoisomerase IB leading to a strong leishmanicidal effect. Biochem. Pharmacol. 2013, 85, 1433–1440. 10.1016/j.bcp.2013.02.024. [DOI] [PubMed] [Google Scholar]; d Nenortas N. P.; Cinelli M. A.; Morrell A. E.; Cushman M.; Shapiro T. A. Activity of aromathecins against African trypanosomes. Antimicrob. Agents Chemother. 2018, 62, e00786 10.1128/AAC.00786-18. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Hue T. M. V.; Cho W.-J. Structural modification of 3-arylisoquinolines to isoindolo[2,1-b]isoquinolinones for the development of novel topoisomerase I inhibitors with molecular docking study. Bioorg. Med. Chem. Lett. 2009, 19, 2551–2554. [DOI] [PubMed] [Google Scholar]
- Luzzio M. J.; Besterman J. M.; Emerson D. L.; Evans M. G.; Lackey K.; Leitner P. L.; McIntyre G.; Morton B.; Myers P. L.; Peel M.; Sisco J. M.; Sternbach D. D.; Tong W.; Truesdale A.; Uehling D. E.; Vuong A.; Yates J. Synthesis and antitumor activity of novel water soluble derivatives of camptothecin as specific inhibitors of topoisomerase I. J. Med. Chem. 1995, 38, 395–401. 10.1021/jm00003a001. [DOI] [PubMed] [Google Scholar]
- a Chen S.; Cai M.; Huang J.; Yao H.; Lin A. Cobalt-Catalyzed Dearomatization of Indoles via Transfer Hydrogenation To Afford Polycyclic Indolines. Org. Lett. 2021, 23, 2212–2216. 10.1021/acs.orglett.1c00354. [DOI] [PubMed] [Google Scholar]; b Yamada T.; Ozaki Y.; Yamawaki M.; Sugiura Y.; Nishino K.; Morita T.; Yoshimi Y. Reductive ipso-radical cyclization onto aromatic rings of five-membered alicyclic amino acids bearing N-(2-phenyl)benzoyl groups by photoinduced electron transfer promoted decarboxylation. Tetrahedron Lett. 2017, 58, 835–838. 10.1016/j.tetlet.2017.01.038. [DOI] [Google Scholar]; c Shinde M. H.; Ramana C. V. An Apparent Umpolung Reactivity of Indole through [Au]-Catalysed Cyclisation and Lewis-Acid-Mediated Allylation. Chem.—Eur. J. 2020, 26, 17171–17175. 10.1002/chem.202003441. [DOI] [PubMed] [Google Scholar]
- a Wahl M. H.; Jandl C.; Bach T. A. A [2 + 2] Photocycloaddition-Fragmentation Approach toward the Carbon Skeleton of cis-Fused Lycorine-type Alkaloids. Org. Lett. 2018, 20, 7674–7678. 10.1021/acs.orglett.8b03402. [DOI] [PubMed] [Google Scholar]; b Chen Y.-J.; Cai S.-L.; Wang C.-C.; Cheng J.-D.; Kramer S.; Sun X.-W. Asymmetric Total Syntheses of (−)-α-Lycorane, (−)-Zephyranthine, and Formal Synthesis of (+)-Clivonine. Chem.–Asian J. 2017, 12, 1309–1313. 10.1002/asia.201700555. [DOI] [PubMed] [Google Scholar]
- a Chen W.; Seidel D. α-C-H/N-H Annulation of Alicyclic Amines via Transient Imines: Preparation of Polycyclic Lactams. Org. Lett. 2021, 23, 3729–3734. 10.1021/acs.orglett.1c01125. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Doveston R. G.; Tosatti P.; Dow M.; Foley D. J.; Li H. Y.; Campbell A. J.; House D.; Churcher I.; Marsden S. P.; Nelson A. A unified lead-oriented synthesis of over fifty molecular scaffolds. Org. Biomol. Chem. 2015, 13, 859–865. 10.1039/c4ob02287d. [DOI] [PubMed] [Google Scholar]; c Chen F.; Su B.; Wang Q. Asymmetric synthesis of (S)-tylophorine and (S)-cryptopleurine via one-pot Curtius rearrangement and Friedel-Crafts reaction tandem sequence. Org. Chem. Front. 2014, 1, 674–677. 10.1039/c4qo00084f. [DOI] [Google Scholar]; d Su B.; Zhang H.; Deng M.; Wang Q. An enantioselective strategy for the total synthesis of (S)-tylophorine via catalytic asymmetric allylation and a one-pot DMAP-promoted isocyanate formation/Lewis acid catalyzed cyclization sequence. Org. Biomol. Chem. 2014, 12, 3616–3621. 10.1039/c4ob00200h. [DOI] [PubMed] [Google Scholar]; e Sun L.; Veith J. M.; Pera P.; Bernacki R. J.; Ojima I. Design and synthesis of de novo cytotoxic alkaloids by mimicking the bioactive conformation of paclitaxel. Bioorg. Med. Chem. 2010, 18, 7101–7112. 10.1016/j.bmc.2010.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Brenzovich W. E. Jr.; Lackner D.; Shunatona A. D.; Tkatchouk H. P.; Goddard E.; Toste W. A. III; Toste F. D. Gold-Catalyzed Intramolecular Aminoarylation of Alkenes: C–C Bond Formation through Bimolecular Reductive Elimination. Angew. Chem., Int. Ed. 2010, 49, 5519–5522. 10.1002/anie.201002739. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Jones M. T.; Schwartz B. D.; Willis A. C.; Banwell M. G. Rapid and Enantioselective Assembly of the Lycorine Framework Using Chemoenzymatic Techniques. Org. Lett. 2009, 11, 3506–3509. 10.1021/ol901364n. [DOI] [PubMed] [Google Scholar]; h Bertrand M. B.; Neukom J. D.; Wolfe J. P. Mild Conditions for Pd-Catalyzed Carboamination of N-Protected Hex-4-enylamines and 1-, 3-, and 4-Substituted Pent-4-enylamines. Scope, Limitations, and Mechanism of Pyrrolidine Formation. J. Org. Chem. 2008, 73, 8851–8860. 10.1021/jo801631v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Tang R.-S.; Chen L.-Y.; Lai C.-H.; Chuang T.-H. Palladium-Catalyzed Stereoselective Aza-Wacker-Heck Cyclization: One-Pot Stepwise Strategy toward Tetracyclic Fused Heterocycles. Org. Lett. 2020, 22, 9337–9341. 10.1021/acs.orglett.0c03552. [DOI] [PubMed] [Google Scholar]; b Liu G.-Q.; Reimann M.; Opatz T. Total Synthesis of Phenanthroindolizidine Alkaloids by Combining Iodoaminocyclization with Free Radical Cyclization. J. Org. Chem. 2016, 81, 6142–6148. 10.1021/acs.joc.6b01161. [DOI] [PubMed] [Google Scholar]; c Sibbald P. A.; Rosewall C. F.; Swartz R. D.; Michael F. E. Mechanism of N-Fluorobenzenesulfonimide Promoted Diamination and Carboamination Reactions: Divergent Reactivity of a Pd(IV) Species. J. Am. Chem. Soc. 2009, 131, 15945–15951. 10.1021/ja906915w. [DOI] [PubMed] [Google Scholar]; d Yamada K.; Yamashita M.; Sumiyoshi T.; Nishimura K.; Tomioka K. Total Synthesis of (−)-Lycorine and (−)-2-epi-Lycorine by Asymmetric Conjugate Addition Cascade. Org. Lett. 2009, 11, 1631–1633. 10.1021/ol9003564. [DOI] [PubMed] [Google Scholar]
- a Wang J.; Li J.; Shen X.; Dong C.; Lin J.; Wei K. Asymmetric total synthesis of (−)-δ-lycorane. Org. Chem. Front. 2017, 4, 1149–1152. 10.1039/c7qo00021a. [DOI] [Google Scholar]; b Tomooka K.; Suzuki M.; Uehara K.; Shimada M.; Akiyama T. Novel synthetic approach to nine-membered diallylic amides: stereochemical behavior and utility as chiral building block. Synlett 2008, 2518–2522. 10.1055/s-2008-1078235. [DOI] [Google Scholar]
- a El Blidi L.; Namoune A.; Bridoux A.; Nimbarte V. D.; Lawson A. M.; Comesse S.; Daich A. Expeditious Synthesis of the Topoisomerase I Inhibitors Isoindolo[2,1-b]isoquinolin-7(5H)-one and the Alkaloid Rosettacin Based on Aryl Radical Cyclization of Enamide Generated by Using N-Acyliminium Chemistry. Synthesis 2015, 47, 3583–3592. 10.1055/s-0034-1378811. [DOI] [Google Scholar]; b Beaulieu M.-A.; Ottenwaelder X.; Canesi S. Asymmetric synthesis of fortucine and reassignment of its absolute configuration. Chem.—Eur. J. 2014, 20, 7581–7584. 10.1002/chem.201402323. [DOI] [PubMed] [Google Scholar]; c Sun Z.; Zhou M.; Li X.; Meng X.; Peng F.; Zhang H.; Shao Z. Catalytic Asymmetric Assembly of Octahydroindolones: Divergent Synthesis of Lycorine-type Amaryllidaceae Alkaloids (+)-α-Lycorane and (+)-Lycorine. Chem.—Eur. J. 2014, 20, 6112–6119. 10.1002/chem.201400178. [DOI] [PubMed] [Google Scholar]; d Conner E. S.; Crocker K. E.; Fernando R. G.; Fronczek F. R.; Stanley G. G.; Ragains J. R. Visible-Light-Promoted Selenofunctionalization of Alkenes. Org. Lett. 2013, 15, 5558–5561. 10.1021/ol402753u. [DOI] [PubMed] [Google Scholar]; e Jung Y.-G.; Lee S.-C.; Cho H.-K.; Darvatkar N. B.; Song J.-Y.; Cho C.-G. Total Syntheses of (±)-α-Lycorane and (±)-1-Deoxylycorine. Org. Lett. 2013, 15, 132–135. 10.1021/ol303157b. [DOI] [PubMed] [Google Scholar]; f Aubert C.; Betschmann P.; Eichberg M. J.; Gandon V.; Heckrodt T. J.; Lehmann J.; Malacria M.; Masjost B.; Paredes E.; Vollhardt K. P. C.; Whitener G. D. Cobalt-mediated [2+2+2] cycloaddition versus C-H and N-H activation of pyridones and pyrazinones with alkynes: an experimental study. Chem.—Eur. J. 2007, 13, 7443–7465. 10.1002/chem.200601823. [DOI] [PubMed] [Google Scholar]
- a Chen Z.-Z.; Li S.-Q.; Zhang Y.-J.; Tang D.-Y.; Meng J.-P.; Lei J.; Li H.-Y.; Xu Z.-G. Synthesis of Pyridodiindoles with Anticancer Activity by a Three-Component Cascade Condensation. Org. Lett. 2018, 20, 7811–7815. 10.1021/acs.orglett.8b03245. [DOI] [PubMed] [Google Scholar]; b Yao T.; Zhang H.; Zhao Y. Synthesis of 9,10-Phenanthrenes via Palladium-Catalyzed Aryne Annulation by o-Halostyrenes and Formal Synthesis of (±)-Tylophorine. Org. Lett. 2016, 18, 2532–2535. 10.1021/acs.orglett.6b00558. [DOI] [PubMed] [Google Scholar]; and references herein; c Gu P.; Kang X.-Y.; Sun J.; Wang B.-J.; Yi M.; Li X.-Q.; Xue P.; Li R. Intramolecular Schmidt Reaction of Acyl Chlorides with Alkyl Azides: Rapid Access to Fused Polycyclic Nitrogen-Containing Heterocycles via a Multistep One-Pot Transformation. Org. Lett. 2012, 14, 5796–5799. 10.1021/ol302890a. [DOI] [PubMed] [Google Scholar]; d Fuller P. H.; Chemler S. R. Copper(II) Carboxylate-Promoted Intramolecular Carboamination of Alkenes for the Synthesis of Polycyclic Lactams. Org. Lett. 2007, 9, 5477–5480. 10.1021/ol702401w. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Kanchupalli V.; Shukla R. K.; Singh A.; Volla C. M. R. Rh(III)-Catalyzed Redox-Neutral Cascade Annulation of Benzamides with p -Quinone Methides. Eur. J. Org. Chem. 2020, 4494–4498. 10.1002/ejoc.202000863. [DOI] [Google Scholar]; f Raji Reddy C.; Mallesh K.; Bodasu S.; Donthiri R. R. Rh(III)-Catalyzed Domino [4 + 2] Annulation/Aza-Michael Addition of N-(Pivaloyloxy)benzamides with 1,5-Enynes via C-H Activation: Synthesis of Functionalized Aromathecins. J. Org. Chem. 2020, 85, 7905–7915. 10.1021/acs.joc.0c00615. [DOI] [PubMed] [Google Scholar]; g Song L.; Tian G.; Blanpain A.; Van Meervelt L.; Van der Eycken E. V. Diversification of peptidomimetics and oligopeptides through microwave-assisted Rhodium(III)-catalyzed intramolecular annulation. Adv. Synth. Catal. 2019, 361, 4442–4447. and references herein 10.1002/adsc.201900550. [DOI] [Google Scholar]; h Guo S.; Sun L.; Liu Y.; Ma N.; Zhang X.; Fan X. Rh(III)-Catalyzed Oxidative Spirocyclization of Isoquinolones with α-Diazo-1,3-indandiones. Org. Lett. 2019, 21, 4082–4086. 10.1021/acs.orglett.9b01263. [DOI] [PubMed] [Google Scholar]; and references herein; i Yan K.; Jin J.; Kong Y.; Li B.; Wang B. Palladium-Catalyzed Inert C–H Bond Activation and Cyclocarbonylation of Isoquinolones with Carbon Dioxide Leading to Isoindolo[2,1- b ]isoquinoline-5,7-Diones. Adv. Synth. Catal. 2019, 361, 3080–3085. 10.1002/adsc.201900305. [DOI] [Google Scholar]
- a Bansode A. H.; Suryavanshi G. Visible-Light-Induced Controlled Oxidation of N -Substituted 1,2,3,4-Tetrahydroisoquinolines for the Synthesis of 3,4-Dihydroisoquinolin-1(2 H)-ones and Isoquinolin-1(2 H -ones. Adv. Synth. Catal. 2021, 363, 1390–1400. 10.1002/adsc.202001266. [DOI] [Google Scholar]; and references herein; b Bai L.-G.; Zhou Y.; Zhuang X.; Zhang L.; Xue J.; Lin X.; Cai C.; Luo L.; Qun L. Base-promoted aerobic oxidation of N-alkyl iminium salts derived from isoquinolines and related heterocycles. Green Chem. 2020, 22, 197–203. 10.1039/c9gc03629f. [DOI] [Google Scholar]; c Song L.; Zhang X.; Tian G.; Robeyns K.; Van Meervelt L.; Harvey J. N.; Van der Eycken E. V. Intramolecular cascade annulation triggered by C H activation via rhodium hydride intermediate. Mol. Catal. 2019, 463, 30–36. 10.1016/j.mcat.2018.11.016. [DOI] [Google Scholar]; d Song L.; Tian G.; Van der Eycken E. V. Rhodium(III)-catalyzed intermolecular cascade annulation through C-H activation: Concise synthesis of rosettacin. Mol. Catal. 2018, 459, 129–134. 10.1016/j.mcat.2018.09.004. [DOI] [Google Scholar]; e Baguia H.; Deldaele C.; Romero E.; Michelet B.; Evano G. Copper-Catalyzed Photoinduced Radical Domino Cyclization of Ynamides and Cyanamides: A Unified Entry to Rosettacin, Luotonin A, and Deoxyvasicinone. Synthesis 2018, 50, 3022–3030. 10.1055/s-0037-1610134. [DOI] [Google Scholar]; f Raji Reddy C.; Mallesh K. Rh(III)-Catalyzed Cascade Annulations To Access Isoindolo[2,1-b]isoquinolin-5(7H)-ones via C-H Activation: Synthesis of Rosettacin. Org. Lett. 2018, 20, 150–153. 10.1021/acs.orglett.7b03509. [DOI] [PubMed] [Google Scholar]; g Luo W.-K.; Shi X.; Zhou W.; Yang L. Iodine-Catalyzed Oxidative Functionalization of Azaarenes with Benzylic C(sp3)-H Bonds via N-Alkylation/Amidation Cascade: Two-Step Synthesis of Isoindolo[2,1-b]isoquinolin-7(5H)-one. Org. Lett. 2016, 18, 2036–2039. 10.1021/acs.orglett.6b00646. [DOI] [PubMed] [Google Scholar]; h Van H. T. M.; Cho W.-J. Structural modification of 3-arylisoquinolines to isoindolo[2,1-b]isoquinolinones for the development of novel topoisomerase I inhibitors with molecular docking study. Bioorg. Med. Chem. Lett. 2009, 19, 2551–2554. 10.1016/j.bmcl.2009.03.042. [DOI] [PubMed] [Google Scholar]
- a Marsault E.; Toró A.; Nowak P.; Deslongchamps P. The transannular Diels-Alder strategy: applications to total synthesis. Tetrahedron 2001, 57, 4243–4260. 10.1016/s0040-4020(01)00121-1. [DOI] [Google Scholar]; b Handa S.; Pattenden G. Free radical-mediated macrocyclisations and transannular cyclisations in synthesis. Contemp. Org. Synth. 1997, 4, 196–215. 10.1039/co9970400196. [DOI] [Google Scholar]; c Montana A. M.; Batalla C.; Barcia J. A. Intramolecular Haloetherification and Transannular Hydroxycyclization of Alkenes. A Synthetic Methodology to Obtain Polycyclic Ethers and Amines. Curr. Org. Chem. 2009, 13, 919–938. 10.2174/138527209788452135. [DOI] [Google Scholar]; d Rizzo A.; Harutyunyan S. R. Azabicycles construction: the transannular ring contraction with N-protected nucleophiles. Org. Biomol. Chem. 2014, 12, 6570–6579. 10.1039/c4ob01311e. [DOI] [PubMed] [Google Scholar]
- For focused reviews, see; a Reyes E.; Uria U.; Carrillo L.; Vicario J. L. Transannular reactions in asymmetric total synthesis. Tetrahedron 2014, 70, 9461–9484. 10.1016/j.tet.2014.07.074. [DOI] [Google Scholar]; b Clarke P. A.; Reeder A. T.; Winn J. Transannulation Reactions in the Synthesis of Natural Products. Synthesis 2009, 691–709. 10.1055/s-0028-1083355. [DOI] [Google Scholar]
- a Knopff O.; Kuhne J.; Fehr C. Enantioselective Intramolecular Aldol Addition/Dehydration Reaction of a Macrocyclic Diketone: Synthesis of the Musk Odorants (R)-Muscone and (R,Z)-5-Muscenone. Angew. Chem., Int. Ed. 2007, 46, 1307–1310. 10.1002/anie.200604518. [DOI] [PubMed] [Google Scholar]; b Dermenci A.; Selig P. S.; Domaoal R. A.; Spasov K. A.; Anderson K. S.; Miller S. J. Quasi-biomimetic ring contraction promoted by a cysteine-based nucleophile: Total synthesis of Sch-642305, some analogs and their putative anti-HIV activities. Chem. Sci. 2011, 2, 1568–1572. 10.1039/c1sc00221j. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Balskus E. P.; Jacobsen E. N. Asymmetric catalysis of the transannular Diels-Alder reaction. Science 2007, 317, 1736–1740. 10.1126/science.1146939. [DOI] [PubMed] [Google Scholar]; d Rajapaksa N. S.; Jacobsen E. N. Enantioselective Catalytic Transannular Ketone-Ene Reactions. Org. Lett. 2013, 15, 4238–4241. 10.1021/ol401968m. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Jaschinski T.; Hiersemann M. {1,6}-Transannular Catalytic Asymmetric Gosteli–Claisen Rearrangement. Org. Lett. 2012, 14, 4114–4117. 10.1021/ol3017676. [DOI] [PubMed] [Google Scholar]; f Chandler C. L.; List B. Catalytic, asymmetric transannular aldolizations: total synthesis of (+)-hirsutene. J. Am. Chem. Soc. 2008, 130, 6737–6739. 10.1021/ja8024164. [DOI] [PubMed] [Google Scholar]; g Mato R.; Manzano R.; Reyes E.; Carrillo L.; Uria U.; Vicario J. L. Catalytic Enantioselective Transannular Morita–Baylis–Hillman Reaction. J. Am. Chem. Soc. 2019, 141, 9495–9499. 10.1021/jacs.9b03679. [DOI] [PubMed] [Google Scholar]; h Mato R.; Reyes E.; Carrillo L.; Uria U.; Prieto L.; Manzano R.; Vicario J. L. Catalytic enantioselective domino Michael/transannular aldol reaction under bifunctional catalysis. Chem. Commun. 2020, 56, 13149–13152. 10.1039/d0cc05981a. [DOI] [PubMed] [Google Scholar]; i Sendra J.; Manzano R.; Reyes E.; Vicario J. L.; Fernández E. Catalytic stereoselective borylative transannular reactions. Angew. Chem., Int. Ed. 2020, 59, 2100–2104. 10.1002/anie.201913438. [DOI] [PubMed] [Google Scholar]
- Amidohalogenation:; a Atmuri N. D. P.; Reilley D. J.; Lubell W. D. Peptidomimetic Synthesis by Way of Diastereoselective Iodoacetoxylation and Transannular Amidation of 7-9-Membered Lactams. Org. Lett. 2017, 19, 5066–5069. 10.1021/acs.orglett.7b02275. [DOI] [PubMed] [Google Scholar]; b Atmuri N. D. P.; Lubell W. D. Insight into Transannular Cyclization Reactions To Synthesize Azabicyclo[X.Y.Z]alkanone Amino Acid Derivatives from 8-, 9-, and 10-Membered Macrocyclic Dipeptide Lactams. J. Org. Chem. 2015, 80, 4904–4918. Aminohalogenation: 10.1021/acs.joc.5b00237. [DOI] [PubMed] [Google Scholar]; c Brock E. A.; Davies S. G.; Lee J. A.; Roberts P. M.; Thomson J. E. Polyhydroxylated pyrrolizidine alkaloids from transannular iodoaminations: application to the asymmetric syntheses of (−)-hyacinthacine A1, (−)-7a-epi-hyacinthacine A1, (−)-hyacinthacine A2, and (−)-1-epi-alexine. Org. Biomol. Chem. 2013, 11, 3187–3202. 10.1039/c3ob40205c. [DOI] [PubMed] [Google Scholar]; d Brock E. A.; Davies S. G.; Lee J. A.; Roberts P. M.; Thomson J. E. Asymmetric Synthesis of Polyhydroxylated Pyrrolizidines via Transannular Iodoamination with Concomitant N-Debenzylation. Org. Lett. 2011, 13, 1594–1597. 10.1021/ol103090z. [DOI] [PubMed] [Google Scholar]; e Cakmak M.; Mayer P.; Trauner D. An Efficient Synthesis of Loline alkaloids. Nat. Chem. 2011, 3, 543–545. 10.1038/nchem.1072. [DOI] [PubMed] [Google Scholar]; f White J. D.; Hrnciar P. Synthesis of Polyhydroxylated Pyrrolizidine Alkaloids of the Alexine Family by Tandem Ring-Closing Metathesis–Transannular Cyclization. (+)-Australine. J. Org. Chem. 2000, 65, 9129–9142. 10.1021/jo0012748. [DOI] [PubMed] [Google Scholar]; g White J. D.; Hrnciar P.; Yokochi A. F. T. Tandem Ring-Closing Metathesis Transannular Cyclization as a Route to Hydroxylated Pyrrolizidines. Asymmetric Synthesis of (+)-Australine. J. Am. Chem. Soc. 1998, 120, 7359–7360. 10.1021/ja9811400. [DOI] [Google Scholar]; h Lauritsen A.; Madsen R. Synthesis of naturally occurring iminosugars from d-fructose by the use of a zinc-mediated fragmentation reaction. Org. Biomol. Chem. 2006, 4, 2898–2905. 10.1039/b605818c. [DOI] [PubMed] [Google Scholar]; i Fürstner A.; Korte A. Total Synthesis of Epohelmin B and Its Analogues. Chem.—Asian J. 2008, 3, 310–318. 10.1002/asia.200700288. [DOI] [PubMed] [Google Scholar]
- Amidohalogenation:; a Atmuri N. D. P.; Lubell W. D. Stereo- and Regiochemical Transannular Cyclization of a Common Hexahydro-1H-azonine to Afford Three Different Indolizidinone Dipeptide Mimetics. J. Org. Chem. 2020, 85, 1340–1351. 10.1021/acs.joc.9b01861. [DOI] [PubMed] [Google Scholar]; b Surprenant S.; Lubell W. D. From Macrocycle Dipeptide Lactams To Azabicyclo[X.Y.0]alkanone Amino Acids: A Transannular Cyclization Route for Peptide Mimic Synthesis. Org. Lett. 2006, 8, 2851–2854. 10.1021/ol0609863. [DOI] [PubMed] [Google Scholar]; c Edstrom E. D. New Methodology for the Synthesis of Functionalized Indolizidine and Quinolizidine Ring Systems. J. Am. Chem. Soc. 1991, 113, 6690–6692. 10.1021/ja00017a060. [DOI] [Google Scholar]; d Yun H.; Kim J.; Sim J.; Lee S.; Han Y. T.; Chang D.-J.; Kim D.-D.; Suh Y.-G. Asymmetric Synthesis of 1-Deoxy-6,8a-di-epi-castanospermine and 1-Deoxy-6-epi-castanospermine. J. Org. Chem. 2012, 77, 5389–5393. 10.1021/jo300309z. [DOI] [PubMed] [Google Scholar]; e Sudau A.; Münch W.; Bats J.-W.; Nubbemeyer U. Synthesis of the Bicyclic Core of Pumiliotoxins. Eur. J. Org. Chem. 2002, 2002, 3304–3314. . [DOI] [Google Scholar]; f Sudau A.; Münch W.; Bats J.-W.; Nubbemeyer U. Planar Chirality: Cycloaddition and Transannular Reactions of Optically Active Azoninones that Contain (E)-Olefins. Chem.—Eur. J. 2001, 7, 611–621. . [DOI] [PubMed] [Google Scholar]; Aminohalogenation:; g Jensen T.; Mikkelsen M.; Lauritsen A.; Andresen T. L.; Gotfredsen C. H.; Madsen R. A Concise Synthesis of Castanospermine by the Use of a Transannular Cyclization. J. Org. Chem. 2009, 74, 8886–8889. 10.1021/jo9019495. [DOI] [PubMed] [Google Scholar]
- Amidohalogenation:; a Godina T. A.; Lubell W. D. Mimics of Peptide Turn Backbone and Side-Chain Geometry by a General Approach for Modifying Azabicyclo[5.3.0]alkanone Amino Acids. J. Org. Chem. 2011, 76, 5846–5849. 10.1021/jo2006363. [DOI] [PubMed] [Google Scholar]; b Boukanoun M. K.; Hou X.; Nikolajev L.; Ratni S.; Olson D.; Claing A.; Laporte S. A.; Chemtob S.; Lubell W. D. Investigation of the active turn geometry for the labour delaying activity of indolizidinone and azapeptide modulators of the prostaglandin F2α receptor. Org. Biomol. Chem. 2015, 13, 7750–7761. 10.1039/c5ob00962f. [DOI] [PubMed] [Google Scholar]
- Lerchen A.; Knecht T.; Koy M.; Daniliuc C. G.; Glorius F. A General Cp*CoIII-Catalyzed Intramolecular C–H Activation Approach for the Efficient Total Syntheses of Aromathecin, Protoberberine, and Tylophora Alkaloids. Chem.—Eur. J. 2017, 23, 12149–12152. 10.1002/chem.201702648. [DOI] [PubMed] [Google Scholar]
- Siro J. G.; Martín J.; García-Navío J. L.; Remuiñan M. J.; Vaquero J. J. Easy Microwave Assisted Deprotection of N-Boc Derivatives. Synlett 1998, 147–148. 10.1055/s-1998-1604. [DOI] [Google Scholar]
- This was confirmed by the results observed when using Z-12a: this substrate provides the transannular product 13a in 47% yield without any need for the incorporation of DBU.
- a Armarego W. L. F.; Chai C. L. L.. Purification of Laboratory Chemicals, 7th ed.; Elsevier: Oxford, 2012; [Google Scholar]; b Williams D. B. G.; Lawton M. Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants. J. Org. Chem. 2010, 75, 8351–8354. 10.1021/jo101589h. [DOI] [PubMed] [Google Scholar]
- Stahl E.Thin Layer Chromatography; Springer Verlag: Berlin, 1969. [Google Scholar]
- Still W. C.; Kahn H.; Mitra A. J. Rapid chromatographic technique for preparative separations with moderate resolution. J. Org. Chem. 1978, 43, 2923–2925. 10.1021/jo00408a041. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.