Abstract
Background
Physical activity (including exercise) may form an important part of regular care for people with cystic fibrosis (CF). This is an update of a previously published review.
Objectives
To assess the effects of physical activity interventions on exercise capacity by peak oxygen uptake, lung function by forced expiratory volume in one second (FEV1), health‐related quality of life (HRQoL) and further important patient‐relevant outcomes in people with cystic fibrosis (CF).
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register which comprises references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings. The most recent search was on 3 March 2022. We also searched two ongoing trials registers: clinicaltrials.gov, most recently on 4 March 2022; and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), most recently on 16 March 2022.
Selection criteria
We included all randomised controlled trials (RCTs) and quasi‐RCTs comparing physical activity interventions of any type and a minimum intervention duration of two weeks with conventional care (no physical activity intervention) in people with CF.
Data collection and analysis
Two review authors independently selected RCTs for inclusion, assessed methodological quality and extracted data. We assessed the certainty of the evidence using GRADE.
Main results
We included 24 parallel RCTs (875 participants). The number of participants in the studies ranged from nine to 117, with a wide range of disease severity. The studies' age demographics varied: in two studies, all participants were adults; in 13 studies, participants were 18 years and younger; in one study, participants were 15 years and older; in one study, participants were 12 years and older; and seven studies included all age ranges. The active training programme lasted up to and including six months in 14 studies, and longer than six months in the remaining 10 studies. Of the 24 included studies, seven implemented a follow‐up period (when supervision was withdrawn, but participants were still allowed to exercise) ranging from one to 12 months. Studies employed differing levels of supervision: in 12 studies, training was supervised; in 11 studies, it was partially supervised; and in one study, training was unsupervised. The quality of the included studies varied widely.
This Cochrane Review shows that, in studies with an active training programme lasting over six months in people with CF, physical activity probably has a positive effect on exercise capacity when compared to no physical activity (usual care) (mean difference (MD) 1.60, 95% confidence interval (CI) 0.16 to 3.05; 6 RCTs, 348 participants; moderate‐certainty evidence). The magnitude of improvement in exercise capacity is interpreted as small, although study results were heterogeneous. Physical activity interventions may have no effect on lung function (forced expiratory volume in one second (FEV1) % predicted) (MD 2.41, 95% CI ‒0.49 to 5.31; 6 RCTs, 367 participants), HRQoL physical functioning (MD 2.19, 95% CI ‒3.42 to 7.80; 4 RCTs, 247 participants) and HRQoL respiratory domain (MD ‒0.05, 95% CI ‒3.61 to 3.51; 4 RCTs, 251 participants) at six months and longer (low‐certainty evidence). One study (117 participants) reported no differences between the physical activity and control groups in the number of participants experiencing a pulmonary exacerbation by six months (incidence rate ratio 1.28, 95% CI 0.85 to 1.94) or in the time to first exacerbation over 12 months (hazard ratio 1.34, 95% CI 0.65 to 2.80) (both high‐certainty evidence); and no effects of physical activity on diabetic control (after 1 hour: MD ‒0.04 mmol/L, 95% CI ‒1.11 to 1.03; 67 participants; after 2 hours: MD ‒0.44 mmol/L, 95% CI ‒1.43 to 0.55; 81 participants; moderate‐certainty evidence). We found no difference between groups in the number of adverse events over six months (odds ratio 6.22, 95% CI 0.72 to 53.40; 2 RCTs, 156 participants; low‐certainty evidence).
For other time points (up to and including six months and during a follow‐up period with no active intervention), the effects of physical activity versus control were similar to those reported for the outcomes above. However, only three out of seven studies adding a follow‐up period with no active intervention (ranging between one and 12 months) reported on the primary outcomes of changes in exercise capacity and lung function, and one on HRQoL. These data must be interpreted with caution. Altogether, given the heterogeneity of effects across studies, the wide variation in study quality and lack of information on clinically meaningful changes for several outcome measures, we consider the overall certainty of evidence on the effects of physical activity interventions on exercise capacity, lung function and HRQoL to be low to moderate.
Authors' conclusions
Physical activity interventions for six months and longer likely improve exercise capacity when compared to no training (moderate‐certainty evidence). Current evidence shows little or no effect on lung function and HRQoL (low‐certainty evidence). Over recent decades, physical activity has gained increasing interest and is already part of multidisciplinary care offered to most people with CF. Adverse effects of physical activity appear rare and there is no reason to actively discourage regular physical activity and exercise. The benefits of including physical activity in an individual's regular care may be influenced by the type and duration of the activity programme as well as individual preferences for and barriers to physical activity. Further high‐quality and sufficiently‐sized studies are needed to comprehensively assess the benefits of physical activity and exercise in people with CF, particularly in the new era of CF medicine.
Plain language summary
Physical activity to improve exercise capacity in people with cystic fibrosis
Review question
We reviewed the evidence about whether physical activity interventions (including exercise) have any effect on exercise capacity, health‐related quality of life and lung function in people with cystic fibrosis (CF). This is an update of a previously published review.
Background
CF affects many systems in the body, but mainly the lungs. It causes shortness of breath and limits the amount of exercise people with CF can tolerate. The progress of lung disease leads to a low ability to exercise and to physical inactivity, which in turn affects health and health‐related quality of life. We looked for studies where people with CF engaged in a physical activity intervention (including endurance‐type activities such as walking, jogging, swimming and cycling; or resistance training; or combinations of both) compared to a control group with no intervention (usual care).
Search date
The evidence is current to 3 March 2022.
Study characteristics
We included 24 studies (875 participants) in this review. The number of people in each study ranged from nine to 117. Some studies included only children, others only adults, and some both children and adults. The studies included people with a wide range of disease severity. The studies used differing levels of supervision in their active training programmes: in 12 studies, participants were supervised; in 11 studies, participants were partially supervised; and in one study, participants were not supervised at all. The active training programme lasted up to and including six months in 14 studies, and longer than six months in the remaining 10 studies. Of the 24 included studies, seven added on a follow‐up period (when all participants reverted to usual care, but were still allowed to exercise if they wished). The quality of the included studies varied widely.
Key results
This systematic review shows that physical activity interventions for longer than six months probably improve exercise capacity in people with CF. When compared with no activity, physical activity interventions may make little or no difference to lung function and health‐related quality of life.
The largest study included in this review (117 participants) reported:
‐ no differences between the physical activity and control groups in the number of pulmonary exacerbations (a flare up of disease) (high‐certainty evidence);
‐ no differences in the time to the first flare up for 12 months (high‐certainty evidence);
‐ no beneficial effects of physical activity on diabetic control after nine months (moderate‐certainty evidence).
Two studies (156 participants) found no differences between groups in the number of reported adverse events (low‐certainty evidence).
For active training programmes lasting up to and including six months, the effects were similar to the longer programmes.
Only three studies which added a follow‐up period (of varying durations) reported data we could analyse on changes in exercise capacity and lung function; and only one reported on quality of life. These results must be interpreted with caution.
Overall and when compared to usual care (no intervention), physical activity and exercise training probably lead to slightly better exercise capacity, while they may have little or no effect on lung function and health‐related quality of life in people with CF.
Certainty of the evidence
We included 24 studies. Given the differences in effects across studies, the wide variation in study quality and the lack of information on clinically meaningful changes for several outcome measures, we consider the overall certainty of the evidence on the effects of physical activity interventions on exercise capacity, lung function and health‐related quality of life as low to moderate. We are uncertain about the effects we have seen and better‐quality studies will likely change these findings.
Factors affecting our certainty included that, in five studies, the characteristics of some of the people taking part were different between groups at the start of the studies, despite people being put into the different treatment groups at random.
Also, when comparing physical activity interventions to no intervention, people will always know which group they are in. However, we do not think the fact that people knew which treatment they were receiving would affect the results for lung function, as long as the assessments were done properly. In contrast, some bias may be introduced when investigators assessing a person's exercise capacity know to which group the person belongs. Investigators tried to prevent the outcome assessors from knowing to which groups the participants belonged in 10 included studies.
Selective reporting of results may also be an issue, especially as most of the included studies were not listed in trial registries, where details of the outcomes are reported.
Summary of findings
Summary of findings 1. Physical activity compared with no physical activity for cystic fibrosis.
| Physical activity compared with no physical activity for cystic fibrosis | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: at home or in hospital Intervention: physical activity Comparison: no physical activity (usual care) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Physical activity | |||||
|
Exercise capacity: change in VO2 peak (mL/min per kg bodyweight) Active intervention: > 6 months |
VO2 peak was 1.60 mL/min per kg bodyweight higher in the physical activity group than in the control group (0.16 mL/min per kg bodyweight higher to 3.05 mL/min per kg bodyweight higher). |
— | 348 (6) | ⊕⊕⊕⊝
Moderatea,b |
P = 0.005 Sensitivity analysis which removed 1 small outlying study did not alter the results. Other time points: Active intervention ≤ 6 months 8 studies reported the effect of physical activity for periods of up to and including 6 months (MD 2.10 mL/min per kg bodyweight, 95% CI 0.06 to 4.13; n = 323; P = 0.04). There was a high level of heterogeneity in the results. Follow‐up (no active intervention) This was reported by 3 out of 9 studies. VO2 peak was higher in the physical activity versus control groups (MD 3.27 mL/min per kg bodyweight, 95% CI 1.37 to 5.18; n = 125; P < 0.001). |
|
|
FEV1 % predicted (change from baseline) Active intervention: > 6 months |
The mean change in FEV1 % predicted was 2.41% higher in the physical activity group than in the control group (0.49% lower to 5.31% higher). | — | 367 (6) |
⊕⊕⊝⊝
Lowa,c |
P = 0.1 Sensitivity analysis which removed 1 small outlying study with wide CIs changed the effect slightly towards a beneficial effect of physical activity (MD 1.71 % predicted, 95% CI 0.15 to 3.26; P = 0.02). Other time points: Active intervention ≤ 6 months 8 studies found no difference between the physical activity group and control group (MD 1.30 % predicted, 95% CI ‒3.01 to 5.61; n = 356; P = 0.56). Follow‐up (no active intervention) 3/9 studies reported this outcome and found no difference between groups (MD 5.68 % predicted, 95% CI ‒1.88 to 13.23; n = 128; P = 0.14). |
|
|
HRQoL: change in CFQ‐R physical functioning domain score Active intervention: > 6 months |
The mean change in CFQ‐R score was 2.19 points higher in the physical activity group than in the control group (3.42 points lower to 7.80 points higher). | — | 247 (4) | ⊕⊕⊝⊝ Lowd | P = 0.44 Other time points: Active intervention ≤ 6 months 6 studies reported that there was no difference in HRQoL CFQ‐R scores between groups (MD 4.67, 95% CI ‒2.55 to 11.90; n = 217; P = 0.21). Follow‐up (no active intervention) No studies reported CFQ‐R after a period off training. |
|
|
HRQoL: change in CFQ‐R respiratory symptoms domain score Active intervention: > 6 months |
The mean change in CFQ‐R score was 0.05 points lower in the physical activity group than in the control group (3.61 points lower to 3.51 points higher). | — | 251 (4) |
⊕⊕⊝⊝ Lowd | P = 0.98 Other time points: Active intervention ≤ 6 months 5 studies reported that there was no difference in HRQoL CFQ‐R scores between groups (MD ‒1.87, 95% CI ‒5.66 to 1.92; n = 212; P = 0.33). Follow‐up (no active intervention) No studies reported CFQ‐R after a period off training. |
|
|
Pulmonary exacerbations: number of exacerbations occurring in the study period Active intervention: 12 months |
There was no difference in the number of pulmonary exacerbations between the physical activity and control group. The incidence rate ratio was 1.28 (95% CI 0.85 to 1.94). | — | 117 (1) |
⊕⊕⊕⊕
High |
P = 0.24 There was also no difference in the time to first exacerbation between the groups, HR 1.34 (95% CI 0.65 to 2.80). Other time points: Active intervention ≤ 6 months 1 study reported no difference in the number of exacerbations between groups at the 6‐month time point (incidence rate ratio 1.07, 95% CI 0.60 to 1.90), or in the time to first exacerbation (HR 1.34, 95% CI 0.65 to 2.80). Follow‐up (no active intervention) No studies reported this outcome after a period off training. |
|
|
Diabetic control: change in blood glucose levels at rest, at 60 and 120 minutes after a glucose ingestion (mmol/L) Active intervention: 9 months |
There were no differences between the physical activity and control groups with regard to blood glucose. At rest: MD ‒0.16 mmol/L (95% CI ‒0.44 to 0.12) After 60 minutes: MD ‒0.04 mmol/L (95% CI ‒1.11 to 1.03) After 120 minutes: MD ‒0.44 mmol/L (95% CI ‒1.43 to 0.55) |
— | 91 (1) |
⊕⊕⊕⊝ Moderatee | Participants included for this outcome did not have a diagnosis of CFRD on entry to the study. Other time points: Active intervention ≤ 6 months 1 study (n = 14, including 2 people with CFRD at study entry) assessed HbA1c, plasma glucose and insulin response to an oral glucose tolerance test. There was no difference in HbA1c (MD ‒0.00%, 95% CI ‒0.01 to 0.00). There was no difference in plasma glucose values between groups at any time point apart from at 120 minutes postglucose test when there was a significant difference favouring the exercise group (Beaudoin 2017). Follow‐up (no active intervention) No studies reported this outcome after a period off training. |
|
|
Adverse events: number of adverse events Active intervention: 12 months |
1 study reported no adverse events in either the physical activity or control group during the 12‐month study period (Kriemler 2013). A larger study reported no difference in the number of participants experiencing an adverse event or serious adverse event related to the intervention between the physical activity and no physical activity group (adverse events: OR 6.22, 95% CI 0.72 to 53.40; serious adverse events: OR 0.95, 95% CI 0.06 to 15.54) (Hebestreit 2022). |
— | 156 (2) |
⊕⊕⊝⊝ Lowf,g | Other time points: Active intervention ≤ 6 months 2 studies reported adverse events: in the first study there was muscle stiffness (common after active video games) and in the second study there was an ankle injury in the physical activity group and haemoptysis in 1 participant in the control group. 1 further study reported no adverse events during the 6‐week intervention period. Follow‐up (no active intervention) In 1 study it was not clear if the earlier reported muscle stiffness continued in the follow‐up period. The study that reported no adverse events in the 6‐week intervention period, also observed no adverse events in the follow‐up period. |
|
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CFQ‐R: Cystic Fibrosis Questionnaire – Revised; CFRD: cystic fibrosis‐related diabetes; FEV1: forced expiratory volume in 1 second; HbA1c: glycated haemoglobin; HR: hazard ratio; HRQoL: health‐related quality of life; MD: mean difference; n: number of participants; OR: odds ratio; VO2 peak: peak oxygen uptake. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
aDowngraded once due to high or unclear risk of bias across many of the domains for the included studies. Two studies contributing data to this outcome were at high risk of bias due to concerns around randomisation and allocation concealment. bThere was moderate heterogeneity in the results, but this was due to an outlying study (Kriemler 2013). When this study was removed from the analysis, the result remained significant and therefore we did not downgrade the certainty of evidence due to inconsistency. The outlying study included small numbers and had wide CIs around the effect. cThere was moderate heterogeneity in the results due to a small outlying study with wide CIs (Kriemler 2013); downgraded once. dDowngraded twice due to risk of bias across several domains in the studies included in this analysis. There were particular concerns around randomisation and allocation concealment in three of the four included studies. eDowngraded once due to imprecision caused by a small number of participants. fDowngraded once due to risk of bias in one of the two included studies for this outcome. gDowngraded once for imprecision (low event rates and wide CIs).
Background
Description of the condition
Cystic fibrosis (CF) is the most common life‐limiting, autosomal, recessively inherited disease in populations of Northern European descent. The worldwide incidence of CF has been estimated, on average, at between 1/3000 and 1/6000 live births, with great regional variation (Farrell 2008; Scotet 2020; Southern 2007). Life expectancy of people with CF has increased substantially over recent decades (MacKenzie 2014), with a large proportion of newborns expected to survive into their fifth decade and beyond (Keogh 2018). On the one hand, the changing demographics of CF lung disease and the growing population of older adults (Burgel 2015) with multiple chronic conditions and an increasing number of cardiovascular disease risk factors pose new challenges to healthcare professionals, including those providing and supervising physical activity and exercise training. On the other hand, a substantial proportion of people with CF can now benefit from highly effective drug therapies (Middleton 2019). However, their impact on individuals' daily physical activity and exercise behaviour is currently unknown and remains to be investigated. Reduced exercise capacity is still common among people with CF (Radtke 2018a), and is associated with reduced life expectancy (Hebestreit 2019; Nixon 1992; Pianosi 2005). Thus, healthcare professionals should encourage and support people with CF to live an active lifestyle early on, and, ideally, provide advice and guidance addressing individual barriers and facilitators to long‐term participation in physical activity.
Description of the intervention
Physical activity is defined as "any bodily movement produced by skeletal muscles that results in energy expenditure" (Caspersen 1985). Exercise training is a subcomponent of physical activity that is planned, structured and done repetitively, with the objective of improving or maintaining physical fitness (Caspersen 1985). It can be defined as participation in a programme of regular vigorous physical activity designed to improve physical performance, cardiovascular function, muscle strength or any combination of these three (Shephard 1994). There are basically two different types of exercise training: aerobic training or anaerobic training, but neither can be considered purely 'aerobic' or 'anaerobic' with respect to energy supply. Aerobic exercise usually involves periods of continuous and rhythmic training of large muscle groups (e.g. cycling or running) that rely predominantly on aerobic energy metabolism. Anaerobic exercise involves training (e.g. weight or resistance training, sprinting or high‐intensity interval training) at a high intensity for a very short duration (ACSM 2017). In this review, we use a broad categorisation of aerobic and anaerobic activities to characterise exercise training studies. However, unlike previous versions of this review, we no longer focus on comparisons between aerobic, anaerobic or a combination of aerobic and anaerobic training regimens versus no training.
Importantly, this review includes both physical activity and exercise training interventions. Exercise training refers to activities that are done for a certain purpose; for example, to improve fitness or to aid the clearance of secretions from the lungs. Since exercise training is a subcomponent of physical activity, we also include randomised controlled trials (RCTs) that focused on improving daily (vigorous) physical activity levels by using wearable technology, such as step counters and fitness trackers, using goal setting and providing motivational feedback throughout their intervention (Nuss 2021), telehealth interventions, or combinations of those. For the rest of this review, we will use the term 'physical activity' inclusive of formal exercise training for ease to the reader.
How the intervention might work
Physical activity has multiple beneficial effects, and is one of the five most important treatments, as rated by people with CF (Davies 2020). Physical activity contributes to the alleviation of exertional dyspnoea and improves exercise tolerance in people with CF (Cerny 2013). Regular physical activity slows the rate of decline in pulmonary function by improving sputum clearance (Cox 2016; Cox 2018; Schneiderman 2014), likely through a combination of hyperventilation, mechanical vibration, coughing and changes in sputum rheology, leading to facilitated and increased sputum expectoration (Dwyer 2011; Dwyer 2017; Hebestreit 2001).
Regular physical activity may also be an important part of the management of diabetes in CF, as it improves glycaemic control in type 1 diabetes mellitus by improving insulin sensitivity and reducing systemic inflammation (Galassetti 2013). Regular physical activity may also delay the onset of osteoporosis by preventing a reduction in bone mineral density (Tejero García 2011). Other postulated benefits of physical activity may be decreased anxiety and depression, and enhanced feelings of well‐being and health‐related quality of life (HRQoL) (Hebestreit 2014). Non‐adherence to prescribed physical activity may contribute to worsening signs and symptoms of respiratory disease, more frequent respiratory infections and a reduced ability to perform activities of daily living, and thus ultimately have a detrimental effect on the individual's prognosis. Side effects of physical activity are rare, so it can be considered safe in CF (Ruf 2010).
Why it is important to do this review
This review aims to provide evidence for the chronic effects of physical activity interventions on physiological, functional and patient‐reported outcomes in people with CF. Optimal physical activity programmes (e.g. duration, intensity, type of activity, level of supervision) for people with CF are unknown and have yet to be defined. Doing so would help to support healthcare professionals, who often lack confidence in providing individualised physical activity advice (Denford 2020). This is an update of previous versions of the review (Bradley 2002; Bradley 2008; Radtke 2015; Radtke 2017).
Objectives
To assess the effects of physical activity interventions on exercise capacity by peak oxygen uptake (VO2 peak), lung function by forced expiratory volume in one second (FEV1), HRQoL and further important patient‐relevant outcomes in people with CF.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs.
Types of participants
People with CF, of any age, and any degree of disease severity, diagnosed on the basis of clinical criteria and sweat testing or genotype analysis.
Types of interventions
Any type of prescribed physical activity intervention delivered to people with CF compared to usual care. We excluded studies which involved pure respiratory muscle training (exercise training specifically targeting the muscles that drive expansion or contraction of the chest, or both). In a post hoc change, we stipulated that studies must have an intervention duration of at least two weeks.
Types of outcome measures
For the 2022 review update, the review author team decided to reduce the number of secondary outcome measures to those that are most important to people living with CF, clinically relevant and patient‐centred. We removed outcomes that are rarely assessed, for which no standardised assessment is available, and outcomes that rely (mostly) on equations that are prone to measurement bias (e.g. fat‐free mass based on skinfold thickness). Please see more comprehensive details in the section Differences between protocol and review.
We assessed the following outcome measures at up to and including six months and longer than six months of active interventions and also for a follow‐up period where all participants received usual care.
Primary outcomes
Exercise capacity (VO2 peak reported either as L/min, mL/min and per kg bodyweight or kg fat‐free mass or as per cent (%) predicted)
Lung function measured as FEV1 (reported either as L or % predicted and as absolute values or change from baseline)
-
HRQoL (measured by generic or disease‐specific instruments, or both, using validated instruments or patient reports)
physical functioning
respiratory
other
Secondary outcomes
-
Additional indices of exercise capacity
peak work capacity (reported as either watt (W) absolute values, W per kg bodyweight, W % predicted or change from baseline)
submaximal exercise capacity (e.g. time to the limit of tolerance in constant work rate exercise tests or oxygen uptake or work rate at the anaerobic threshold, or both)
functional exercise capacity (i.e. 6‐minute walk test (6MWT) and shuttle tests)
-
Quadriceps muscle strength
isometric muscle strength, measured with strain gauges fixed to a medical bench/chair or using dynamometry (reported as either kg or newtons (N))
isokinetic muscle strength measured by isokinetic dynamometry (reported as newton‐metres (N.m))
Lung function measured as forced vital capacity (FVC) (reported either as L or % predicted and as absolute values or change from baseline)
-
Physical activity
subjective report (e.g. self‐reported diary or validated questionnaires of time spent in moderate‐to‐vigorous/intense activity)
objective report (e.g. pedometers (i.e. number of steps) or accelerometers (i.e. time spent in moderate‐to‐vigorous or vigorous physical activity, or both))
Body mass index (BMI) (reported as kg/m² or z‐scores)
-
Pulmonary exacerbations
number of exacerbations
time to first exacerbation
-
Hospitalisation
number of hospitalisations
number of days in hospital
Bone health, measured by dual x‐ray energy absorptiometry or peripheral quantitative computed tomography
Diabetic control, measured by fasting blood glucose levels (mmol/L or mg/dL), insulin levels (mmol/L or mg/dL) or homeostasis model assessment (HOMA) or oral glucose tolerance test (blood glucose in mmol/L or mg/dL)
Adverse events related to the physical activity intervention or exercise testing as part of intervention
Search methods for identification of studies
We searched for all relevant published and unpublished trials without restrictions on language, year or publication status.
Electronic searches
We identified relevant studies from the Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register using the term 'exercise'.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals – Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching through the abstract books of three major CF conferences: the International Cystic Fibrosis Conference, the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group website (cfgd.cochrane.org/our-specialised-trials-registers). Our most recent search of the Group's Cystic Fibrosis Trials Register was on 3 March 2022.
We also searched the following trials registers:
US National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov (www.clinicaltrials.gov; searched 4 March 2022);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (trialsearch.who.int/; searched 16 March 2022).
For details of our search strategies, please see Appendix 1.
Searching other resources
We searched the reference lists of each RCT and of review articles for additional publications that may contain RCTs. We contacted authors of studies included in this review and other experts in the field to request information on other published and unpublished studies.
Data collection and analysis
We used the following methods where possible.
Selection of studies
Two review authors (for the original review, JB and FM; for the 2015 and 2017 updates, SK and TR; for the 2022 update, TR and SS) independently assessed the titles and abstracts of identified citations and selected the studies to be included in the review. We excluded non‐RCTs, studies involving respiratory muscle training exclusively, studies which did not have a physical activity programme and those that did not meet the inclusion criteria, based on screening the abstracts or full‐text articles. If disagreement arose on the suitability of a study for inclusion in the review, we reached a consensus through discussion. We recorded any areas of disagreement. We excluded studies that did not fulfil all of the inclusion criteria, and listed their details with the reason for exclusion. A third review author resolved discrepancies where any disagreement or uncertainty between the two review authors persisted.
Data extraction and management
Two review authors (for the 2015 and 2017 updates, SK and TR, or for the included studies where SK and TR were authors, SS and SN; for the 2022 update, TR and SS), independently extracted data using a standard data acquisition form. We collected information about: study design (parallel versus multiarm; single‐centre versus multicentre; participants and study characteristics for baseline equality between groups; details on the number of participants screened for eligibility, randomised, analysed, excluded, lost to follow‐up and dropped out; method of randomisation and allocation concealment; blinding of personnel and outcome assessors; use of stratification; incomplete outcome data; selective reporting; use of intention‐to‐treat analysis); the detailed intervention (aerobic training, anaerobic training, or a combination of both training regimens; duration of physical activity intervention (either supervised, partially supervised or unsupervised, i.e. up to and including six months, over six months, and studies with a follow‐up period (where all participants received usual care)); and whether the intervention was supervised, partially supervised or not supervised, but still with access to resources for physical activity additional to usual care); and outcome measures (continuous and dichotomous). If disagreement arose about the quality of a study, we attempted to reach a consensus through discussion. If disagreement persisted, a third review author arbitrated. We recorded any areas of disagreement. One review author (for the original review, JB; from the 2015 update onwards, TR) entered the data into the Cochrane software Review Manager 5 (Review Manager 2014), and a second review author (for the 2015 and 2017 update, SK; for the 2021 update, SS) reviewed it. We contacted the authors of the included studies in case of unclear or missing data and information.
We pooled data comparing physical activity versus no activity. For all outcomes in this review, we combined the two active arms of the Kriemler 2013 and Selvadurai 2002 studies. For the meta‐analysis of the primary outcomes FEV1, VO2 peak and HRQoL, we chose the measurement time points with the longest duration of controlled intervention (i.e. the time point up to which control group participants were asked to maintain their baseline physical activity level).
We reported results from each category of physical activity intervention at the end of that specific category; we reported results from the follow‐up periods during which all participants received usual care in a separate category labelled 'Follow‐up (no active intervention)'. If a study reported multiple time points for a single category of intervention (supervised, partially supervised or unsupervised) within our predefined training period lengths (i.e. up to and including six months, and longer than six months), we reported the longest time point within the given category. For example, Kriemler 2013 included assessments at three months and six months (fully supervised), 12 months (during the second six months participants were not supervised but still had access to physical activity resources from the study) and 24 months (i.e. 12 months' follow‐up with no active intervention or provision of resources)). In this case, we reported the six‐month, 12‐month and 24‐month assessments and discarded the three‐month assessment.
Assessment of risk of bias in included studies
For the original review, two review authors judged the methodological quality of the review (JB, FM). For the review updates, two authors (2015 and 2017 update: SK and TR; 2022 update: SS and TR or SS and SN) independently assessed the risk of bias for each included study according to the Cochrane risk of bias tool (Higgins 2017). In particular, we examined details of the randomisation method with sequence generation, allocation concealment, degree of blinding, inclusion and exclusion criteria, dropouts or withdrawals, intention‐to‐treat and detailed statistical analysis. We also assessed the risk of selective reporting and any other potential sources of bias. For each domain, we judged the risk of bias as low, unclear or high. We considered unexplained dropouts or an unequal number of dropouts across treatment groups as a potential risk of bias. Likewise, we also considered a lack of important information (e.g. on adverse effects, missing data, statistical methods, etc.) as a potential risk of bias.
Measures of treatment effect
We reported continuous outcome data and calculated the mean differences (MDs) with 95% confidence intervals (CIs) where between‐group differences in the mean change from baseline were recorded. When data on the standard deviation (SD) for an individual group were not available, but instead standard error (SEM) of the difference was available, we used the calculator within the Review Manager 5 to compute the MD with 95% CIs (Review Manager 2014). Where possible, we used the published standard error of the mean (SEM), or alternatively, we used published CIs to estimate SEM. In this review update, we report the number of acute pulmonary exacerbations as incidence rate ratios (i.e. based on mixed Poisson regression models, as reported in Hebestreit 2022, which is the only included study that reported this outcome). We analysed the outcome of 'time to first pulmonary exacerbation' between the physical activity intervention and control groups as hazard ratios (HRs) with 95% CIs. We analysed the number of adverse events directly related to physical activity as odds ratios (ORs) with 95% CIs.
In future updates of this review, if trials use different measurement scales for an outcome, we plan to analyse the data using the standardised mean difference (SMD) with 95% CIs.
Unit of analysis issues
We have not included any cross‐over studies in this latest version of the review. If future versions of this review include cross‐over studies, and if data are presented in published papers from paired statistical analyses or if information is available to allow us to adjust for within‐patient correlation using the methods described by Elbourne 2002, we will use the generic inverse variance method for data analysis. If appropriate data are not presented to allow adjustment for within‐patient correlation, we will contact study investigators to request these data. If we are unable to make the necessary adjustments, we will describe data from cross‐over studies narratively in the review.
Dealing with missing data
We contacted the investigators of studies included in this review for further study details and data. Fourteen investigators responded. The investigators of four studies stated that the requested data were not available (Klijn 2004; Michel 1989; Schneiderman‐Walker 2000; Selvadurai 2002). The investigator of a one study confirmed that the extracted data were correct and that no further data were available (Cerny 1989). We also contacted the investigators of the Hebestreit study; additional data were provided and the paper has since been published. One investigator involved in the Phillips study, currently listed under Studies awaiting classification, confirmed that the study has been completed. We updated the information in the table (Phillips 2008). In both publications by Santana‐Sosa, the means and SEMs were reported for all variables; we contacted the investigators for additional data, which we received (Santana‐Sosa 2012; Santana‐Sosa 2014). The investigators of Carr 2018 responded to our initial request to provide additional raw data, but did not respond to further emails. We could not include the additional data.
Finally, investigators of eight studies provided additional raw data for this review update (Beaudoin 2017; Hebestreit 2010; Hebestreit 2022; Kriemler 2013; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020). We received raw data from the corresponding author of Beaudoin 2017 which allowed us to calculate MD and the corresponding SEM for various outcomes. For Hebestreit 2022, we extracted the MDs and their 95% CIs from the adjusted intention‐to‐treat models with imputation of missing data to compute the relevant SEM using the Review Manager 5 calculator (inverse variance analysis). For Kriemler 2013 study, we extracted mean changes and SDs from the adjusted models for each group and calculated the relevant SEMs (inverse variance analysis). The two studies by Santana‐Sosa reported means and SEM at baseline, post‐training and 'off training' (i.e. labelled as a 'detraining' period in their original publications and defined as a period during which no supervised exercise was offered to intervention group participants) (Santana‐Sosa 2012; Santana‐Sosa 2014), and we were unable to calculate the MD. We received incomplete raw data files from the authors. Due to inconsistencies in the data sets provided, we were unable to reproduce all data. Due to our concerns about data quality, we excluded both studies from the formal analysis in the review. Instead, we provided data from these studies in two additional tables (see Table 2; Table 3). Sawyer 2020 reported within‐group changes from baseline as medians (interquartile range) for various outcomes. We received raw data from the authors, checked the distribution of the data (and confirmed normal distribution of the majority of outcomes), and calculated MD and SEM for relevant outcomes.
1. Study results for Santana‐Sosa 2012.
| Variable | Group | Pretraining | Post‐training | Detraininga | P value | Comments |
| Age (mean (SEM)) years | Intervention | 11 (3) | — | — | — | — |
| Control | 10 (2) | — | — | — | ||
| Sex (% boys) | Intervention | 55 | — | — | — | |
| Control | 64 | — | — | — | ||
| VO2 peak (mean (95% CI)) mL/min per kg bodyweight | Intervention | N/A | 3.9 (1.8 to 6.1) | ‒3.4 (‒5.7 to 1.7) | 0.036 | Higher in controls at baseline (P = 0.023). Data were presented in a figure in the original publication. |
| Control | N/A | ‒2.2 (‒5.3 to 0.1) | ‒0.7 (‒4.4 to 5.9) | |||
| Leg press (mean (95% CI)) kg | Intervention | N/A | 24.9 (14.3 to 34.4) | ‒1.0 (‒4.1 to 3.3) | < 0.001 | Data are reported in a figure in the original publication. Significantly higher in controls at baseline (P = 0.014). |
| Control | N/A | N/A | N/A | |||
| Bench press (mean (95% CI)) kg | Intervention | N/A | 10.5 (7.0 to 14.0) | ‒1.2 (‒3.6 to 3.0) | < 0.001 | Significantly higher in controls at baseline (P = 0.007). Data presented in a figure in the original publication. |
| Control | N/A | N/A | N/A | |||
| Seated row (mean (95% CI)) kg | Intervention | N/A | 12.7 (9.2 to 16.0) | ‒0.2 (‒3.6 to 3.2) | < 0.001 | Significantly higher in controls at baseline (P = 0.009). Data presented in a figure in the original publication. |
| Control | N/A | N/A | N/A | |||
| Oxygen saturation at peak exercise (mean (SEM)) % | Intervention | 94.9 (0.9) | 95.6 (0.8) | 94.5 (1.2) | N/A | — |
| Control | 95.7 (0.5) | 96.4% (0.4) | 96.1 (0.5) | |||
| FEV1 (mean (SEM)) L | Intervention | 1.87 (0.24) | 1.94 (0.23) | 1.90 (0.25) | 0.769 | — |
| Control | 1.77 (0.17) | 1.87 (0.15) | 1.79 (0.19) | |||
| FVC (mean (SEM)) L | Intervention | 2.41 (0.24) | 2.49 (0.25) | 2.56 (0.29) | 0.920 | — |
| Control | 2.29 (0.19) | 2.36 (0.20) | 2.40 (0.24) | |||
| PImax (mean (SEM)) cmH2O | Intervention | 64.0 (5.5) | 69.8 (6.8) | 75.2 (6.2) | 0.797 | — |
| Control | 61.5 (6.9) | 72.2 (7.2) | 76.4 (7.5) | |||
| HRQoL score – children's report (median (range)) | Intervention | 696 (495–741) | 719 (550–734) | — | 0.257 | HRQoL was assessed before and after the intervention. P value for comparison pre versus post‐training. |
| Control | 649 (578–768) | 638 (461–791) | — | |||
| HRQoL score – parents' report (median (range)) | Intervention | 896 (688–1011) | 889 (811–973) | — | 0.143 | HRQoL was assessed before and after the intervention. |
| Control | 911 (842–1028) | 978 (684–1059) | — | |||
| Weight (mean (SEM)) kg | Intervention | 39.9 (3.5) | 40.5 (3.4) | 41.4 (3.4) | 0.723 | — |
| Control | 34.0 (2.6) | 35.1 (2.8) | 36.2 (3.0) | |||
| BMI (mean (SEM)) kg/m² | Intervention | 18.4 (1.0) | 18.3 (0.7) | 18.5 (0.7) | 0.959 | — |
| Control | 17.2 (0.8) | 17.1 (0.8) | 17.4 (0.9) | |||
| Fat‐free mass (mean (SEM)) % | Intervention | 78.1 (2.7) | 79.4 (2.8) | 78.8 (2.9) | 0.115 | — |
| Control | 81.1 (2.5) | 80.9 (2.1) | 81.1 (2.2) | |||
| Body fat (mean (SEM)) % | Intervention | 21.9 (2.7) | 20.6 (2.8) | 21.2 (2.9) | 0.115 | — |
| Control | 18.9 (2.5) | 19.1 (2.1) | 18.9 (2.2) | |||
| Compliance with physical training (mean (SEM)) % | Intervention | — | 95.1 (7.4) | — | — |
73% of children completed all training sessions. |
| Control | — | — | — | |||
| Adverse effects | Intervention | — | — | — | — | No adverse effects occurred during training or maximal exercise testing. |
| Control | — | — | — |
aDescribed in the original papers as "detraining" but corresponding to our definition of 'off training'.
BMI: body mass index; CI: confidence interval; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; HRQoL: health‐related quality of life; N/A: not applicable; PImax: maximum inspiratory mouth pressure; SEM: standard error of the mean; VO2 peak: peak oxygen consumption.
2. Study results for Santana‐Sosa 2014.
| Variable | Group | Pretraining | Post‐training | Detraininga | P value | Comments |
| Age (mean (SEM)) years | Intervention | 11 (1) | — | — | — | — |
| Control | 10 (1) | — | — | — | ||
| Sex (% boys) | Intervention | 60 | — | — | — | — |
| Control | 60 | — | — | — | ||
| VO2 peak (mean (95% CI) mL/min per kg bodyweight | Intervention | N/A | 6.9 (3.4 to 10.5) | ‒1.5 (‒2.7 to ‒0.4) | < 0.001 | Significantly higher in controls at baseline (P = 0.034). |
| Control | N/A | N/A | N/A | |||
| Leg press (mean (SEM)) kg | Intervention | 62.5 (6.5) | 89.5 (9.3) | 88.6 (9.2) | < 0.001 | Higher in controls at baseline (P = 0.046). |
| Control | 45.2 (4.7) | 43.9 (5.1) | 43.9 (5.4) | |||
| Bench press (mean (SEM)) kg | Intervention | 26.4 (2.7) | 38.4 (3.2) | 35.9 (2.9) | < 0.001 | — |
| Control | 23.2 (2.9) | 21.6 (3.2) | 21.7 (3.6) | |||
| Lateral row (mean (SEM)) kg | Intervention | 30.5 (3.6) | 43.0 (4.2) | 35.9 (2.9) | < 0.001 | — |
| Control | 23.2 (3.0) | 22.0 (3.1) | 21.7 (3.6) | |||
| Oxygen saturation at peak exercise (mean (SEM)) % | Intervention | 94.7 (0.7) | 94.5 (0.7) | 93.1 (0.8) | N/A | — |
| Control | 96.4 (0.4) | 96.2 (0.5) | 96.1 (0.6) | |||
| FEV1 (mean (SEM)) L | Intervention | 1.65 (0.19) | 1.74 (0.23) | 1.69 (0.24) | 0.486 | — |
| Control | 1.57 (0.26) | 1.55 (0.26) | 1.59 (0.26) | |||
| FVC (mean (SEM)) L | Intervention | 2.23 (0.27) | 2.34 (0.29) | 2.28 (0.28) | 0.156 | — |
| Control | 1.90 (0.33) | 1.85 (0.32) | 1.92 (0.32) | |||
| PImax (mean (SEM)) cmH2O | Intervention | 68.3 (6.3) | 107.6 (8.4) | 103.2 (8.1) | < 0.001 | — |
| Control | 69.5 (9.7) | 71.8 (10.0) | 66.7 (9.4) | |||
| HRQoL score (median (range)) | Intervention | 629 (505–701) | 688 (609–791) | — | 0.071 | HRQoL was assessed before and after the intervention. |
| Control | 636 (626–745) | 638 (626–737) | — | |||
| Weight (mean (SEM)) kg | Intervention | 36.4 (3.1) | 37.8 (3.2) | 38.3 (3.1) | 0.342 | — |
| Control | 31.5 (4.6) | 32.4 (4.7) | 32.7 (4.5) | |||
| Fat‐free mass (mean (SEM)) % of total | Intervention | 81.6 (1.3) | 82.6 (1.0) | 82.5 (1.0) | 0.001 | — |
| Control | 82.9 (1.8) | 82.8 (1.8) | 82.5 (1.9) | |||
| Body fat (mean (SEM)) % of total | Intervention | 18.4 (1.3) | 17.4 (1.2) | 17.5 (1.1) | 0.023 | — |
| Control | 17.1 (1.8) | 17.2 (1.8) | 17.5 (1.9) | |||
| Compliance with physical training (mean (SEM)) % | Intervention | — | 97.5 (1.7) | — | — | 70% of children completed all training sessions. |
| Control | — | — | — | |||
| Adverse effects | Intervention | — | — | — | — | No adverse effects occurred during training or exercise testing. |
| Control | — | — | — |
aDescribed in the original papers as "detraining" but corresponding to our definition of 'off training'.
CI: confidence interval; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; HRQoL: health‐related quality of life; N/A: not applicable; PImax: maximum inspiratory mouth pressure; SEM: standard error of the mean; VO2 peak: peak oxygen consumption.
Assessment of heterogeneity
We combined available data (extracted from published papers and calculated as previously stated) and conducted a meta‐analysis on the primary outcomes VO2 peak, FEV1 and HRQoL. We measured heterogeneity between studies using the Chi² test and the I² statistic (Higgins 2003). The Chi² test measures the deviation of observed effect sizes from the underlying overall effect. A low P value (or a large Chi² statistic relative to its degree of freedom) provides evidence of heterogeneity of intervention effects (variation in effect estimates beyond chance). We used a P value of 0.10, rather than the conventional level of 0.05, to determine statistical significance. The I² statistic, as defined by Higgins (Higgins 2017), measures heterogeneity as a percentage, where a value:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
The importance of the observed value of I² depends on: (i) magnitude and direction of effects; and (ii) strength of evidence for heterogeneity (e.g. P value from the Chi² test, or a CI for the I² statistic).
Assessment of reporting biases
We assessed relevant bias and selective reporting by comparing the 'methods' and 'results' sections from the included papers and trial registries, if available. We documented this information in the risk of bias tables for included studies (see Characteristics of included studies table), and in Figure 1 and Figure 2. If future updates of this review include and combine a sufficient number of studies (10 or more), we will assess publication bias, initially by visual inspection of a funnel plot. However, we are aware that an asymmetrical funnel plot is not necessarily due to publication bias.
1.

Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
2.

Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
Data synthesis
We used a fixed‐effect model for all outcome parameters using Review Manager 5 (Review Manager 2014). We used a random‐effects model for outcomes that were combined, and for which a meta‐analysis was performed (i.e. VO2 peak, FEV1, HRQoL). The random‐effects model incorporates any between‐study heterogeneity into a meta‐analysis. We selected the MD when we combined data and used forest plots to compare results across studies.
Subgroup analysis and investigation of heterogeneity
For future updates of this review, we plan to undertake subgroup analyses of children versus adults, (partially) supervised versus unsupervised training and according to disease severity, provided there is a sufficient number of studies (about 10) with at least moderate heterogeneity in the pooled analyses. Moreover, in the future, we plan to undertake subgroup analysis comparing studies performed in the 'new' era of CF medicine (i.e. after widespread availability of CF transmembrane conductance regulator modulator therapy) from 2020 onwards to those conducted before 2020.
Sensitivity analysis
We performed sensitivity analysis to investigate whether heterogeneity affected the overall pooled effects estimates by excluding from the pooled analysis individual studies that gave rise to methodological concerns. We restricted sensitivity analysis to primary outcomes. In future updates of this review (i.e. when more studies can be combined for meta‐analysis), we plan to perform two additional sensitivity analyses: with and without quasi‐randomised studies (not yet possible); and excluding studies with a high risk of bias from the analysis.
Summary of findings and assessment of the certainty of the evidence
We summarised the main findings of this review, including a grading of the certainty of evidence, in Table 1. We selected the following seven outcomes to report (chosen based on relevance to clinicians and consumers):
exercise capacity (VO2 peak);
Lung function measured as FEV1;
HRQoL: Cystic Fibrosis Questionnaire – Revised (CFQ‐R) physical functioning domain;
HRQoL: CFQ‐R respiratory symptoms;
pulmonary exacerbations;
diabetic control;
adverse events.
We determined the certainty of the evidence using the GRADE approach. We downgraded evidence in the presence of a high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, or high probability of publication bias. We downgraded evidence by one level if we considered the limitation to be serious, and by two levels if very serious.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; and Characteristics of ongoing studies tables.
Results of the search
The combined searches to date have identified 544 individual references. After initial screening to exclude those references which were obviously not eligible, 137 unique studies are listed in the review. We included 24 studies (61 references); excluded 95 studies (147 references; for further details, see Excluded studies); six studies (13 references) are currently awaiting classification; and 12 studies (16 references) are ongoing. Please see the study flow chart for details (Figure 3).
3.
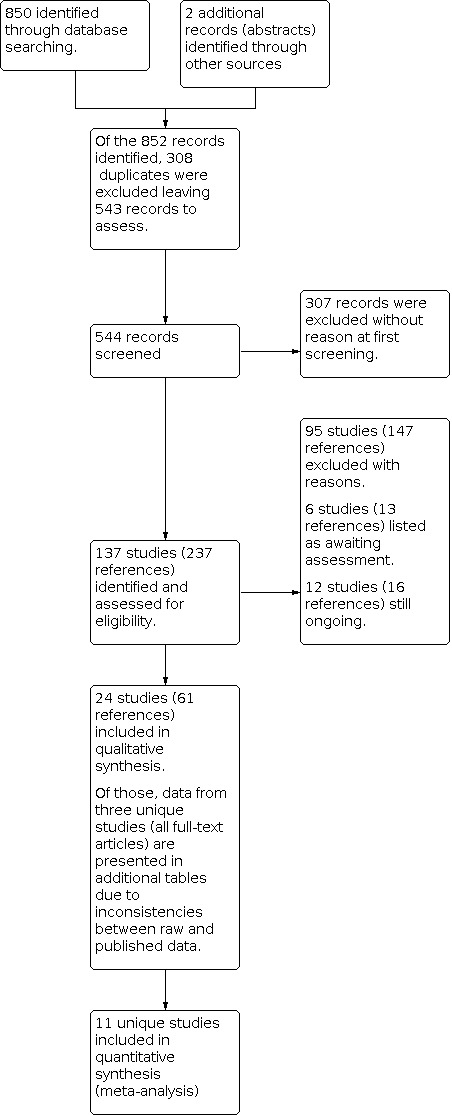
Study flow diagram.
Included studies
A total of 24 studies with 875 participants met the inclusion criteria (Alexander 2019; Beaudoin 2017; Carr 2018; Cerny 1989; Del Corral 2018; Donadio 2020; Douglas 2015; Güngör 2021; Gupta 2019; Hatziagorou 2019; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Klijn 2004; Kriemler 2013; Michel 1989; Moorcroft 2004; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020; Schneiderman‐Walker 2000; Selvadurai 2002; Turchetta 1991).
Three review authors (TR, HH and SK) were lead investigators of the ACTIVATE‐CF trial (Principal Investigator Helge Hebestreit), and had full access to the data before the publication of the main manuscript. The data were included in this review, and during the process of preparing this review update, the paper was accepted for publication and is appropriately cited (Hebestreit 2022). Two other review authors (SS and SN) conducted data extraction and management for this study.
Trial characteristics
All included studies were of a randomised parallel‐group design. The study by Beaudoin and colleagues was registered as a randomised cross‐over study (ClinicalTrials.gov), but results were reported as a randomised parallel‐group design in the final publication (Beaudoin 2017). There were 20 single‐centre studies (Alexander 2019; Beaudoin 2017; Carr 2018; Cerny 1989; Del Corral 2018; Donadio 2020; Douglas 2015; Güngör 2021; Gupta 2019; Hatziagorou 2019; Hommerding 2015; Klijn 2004; Michel 1989; Moorcroft 2004; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Schneiderman‐Walker 2000; Selvadurai 2002; Turchetta 1991). Three studies were national, multicentre studies: two were conducted in Germany and Switzerland (Hebestreit 2010; Kriemler 2013), and one in Australia (Sawyer 2020). One study was an international, multicentre study across eight countries in Europe and North America (Hebestreit 2022). The size of trials varied, from a minimum number of nine participants (Michel 1989), to a maximum of 117 participants (Hebestreit 2022). One study did not report the number of participants in each group and the MD between the treatment and control groups could not be calculated (Michel 1989).
There was wide heterogeneity in study designs, with 12 studies using a supervised training approach (Carr 2018; Cerny 1989; Donadio 2020; Douglas 2015; Güngör 2021; Klijn 2004; Michel 1989; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020; Selvadurai 2002; Turchetta 1991); 11 studies using a partially supervised approach (Alexander 2019; Beaudoin 2017; Del Corral 2018; Gupta 2019; Hatziagorou 2019; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Kriemler 2013; Rovedder 2014; Schneiderman‐Walker 2000); and one study using an unsupervised training approach (Moorcroft 2004).
The length of physical activity intervention varied substantially across the 24 studies. In 14 studies, the active intervention (either supervised, partially supervised or unsupervised but with access to study resources) lasted up to and including six months, while in 10 studies, it lasted longer than six months. Seven of the included studies implemented an additional follow‐up period (i.e. a period where supervision was withdrawn and participants received usual care and were not specifically discouraged from undertaking physical activity); these lasted from one to 12 months.
Four studies had active interventions of short duration (less than one month) and were carried out during hospitalisations (Cerny 1989; Michel 1989; Selvadurai 2002; Turchetta 1991). In Turchetta 1991, the hospital admission was for routine assessment; in Cerny 1989 and Selvadurai 2002, the hospital admission was due to an acute exacerbation requiring intravenous antibiotic treatment; and in Michel 1989, the reason for and the duration of admission were not reported. Four studies had active intervention periods lasting approximately eight weeks (Donadio 2020; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020). Both Santana‐Sosa studies had a two‐month active training period, plus a one‐month follow‐up period 'off training' during which the participants did not engage in supervised physical activity (described in the papers as "detraining") (Santana‐Sosa 2012; Santana‐Sosa 2014). Donadio 2020 and Sawyer 2020 were both fully supervised physical activity interventions. Five studies had an active intervention period of three months and were either home‐based (Alexander 2019; Beaudoin 2017; Hommerding 2015; Rovedder 2014), or performed at the hospital (Klijn 2004). Klijn 2004 also included a three‐month follow‐up. In the study by Güngör and colleagues, the active intervention lasted for six months. A therapist supervised the first six weeks. Afterwards, the families were encouraged with weekly telephone calls to continue their child's exercise programme until the six‐month study visit (Güngör 2021).
In 10 studies, the active interventions lasted longer than six months. Del Corral 2018 was a 12‐month study including a six‐week, home‐based, physical activity intervention with video games. After six weeks, the participants were encouraged to continue video gaming exercise, supervised by their parents or caregivers. Two studies were of 24 months' duration in total with 12 months of active interventions and 12 months of follow‐up (Hebestreit 2010; Kriemler 2013). After six months of supervised or partially supervised physical activity, the participants in the intervention groups were no longer supervised but were encouraged to maintain or increase their activity level while retaining access to the study resources, while participants in the control groups were told not to change their exercise behaviour during the first 12 months. After 12 months, all participants reverted to usual care for a follow‐up period (Hebestreit 2010; Kriemler 2013). In Hebestreit 2010, investigators combined the three‐ and six‐month study visits and six‐ to 12‐month follow‐up visits. In Kriemler 2013, all study visits were reported separately in the original publication (i.e. three, six, 12 and 24 months). For the purpose of this review, we included the data from six, 12 and 24 months (i.e. after 12 months' follow‐up). Carr 2018 was a nine‐month intervention study comparing face‐to‐face versus Internet‐delivered Tai Chi lessons. The Internet group started the intervention three months later than the face‐to‐face group; the Internet group served as the control group in this review and we reported data at the three‐month time point only up to which the control group received no active intervention. In four studies, the active intervention lasted 12 months (Gupta 2019; Hatziagorou 2019; Hebestreit 2022; Moorcroft 2004); in one study 24 months (Douglas 2015); and in one study three years (Schneiderman‐Walker 2000).
In total, seven studies undertook follow‐up periods where all participants reverted to usual care, with these lasting between one and 12 months (Hebestreit 2010; Klijn 2004; Kriemler 2013; Michel 1989; Santana‐Sosa 2012; Santana‐Sosa 2014; Selvadurai 2002).
Participants
Two studies included adults only (Beaudoin 2017; Moorcroft 2004); 12 studies included children and adolescents only (Del Corral 2018; Donadio 2020; Douglas 2015; Güngör 2021; Gupta 2019; Hatziagorou 2019; Hommerding 2015; Klijn 2004; Santana‐Sosa 2012; Santana‐Sosa 2014; Selvadurai 2002; Turchetta 1991), eight studies included both adults and children (Carr 2018; Cerny 1989; Hebestreit 2010; Hebestreit 2022; Kriemler 2013; Michel 1989; Rovedder 2014; Schneiderman‐Walker 2000); one study included prepubertal children (Alexander 2019); and one study included adolescents (15 years and older) and adults (Sawyer 2020). Overall, the studies included participants with a broad range of disease severity.
Most studies included participants of both sexes (Beaudoin 2017; Carr 2018; Del Corral 2018; Donadio 2020; Douglas 2015; Güngör 2021; Gupta 2019; Hatziagorou 2019; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Klijn 2004; Kriemler 2013; Moorcroft 2004; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020; Schneiderman‐Walker 2000; Selvadurai 2002; Turchetta 1991). However, no information was available for three studies (Alexander 2019; Cerny 1989, Michel 1989). A total of 17 studies provided information about the proportion of male and female participants at baseline (Beaudoin 2017; Carr 2018; Del Corral 2018; Donadio 2020; Güngör 2021; Gupta 2019; Hatziagorou 2019; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Kriemler 2013; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020; Selvadurai 2002; Turchetta 1991).
In 10/20 studies published as full‐text articles, FEV1 % predicted values were used as exclusion criteria (Beaudoin 2017; Güngör 2021; Gupta 2019; Hebestreit 2010; Hebestreit 2022; Klijn 2004; Kriemler 2013; Santana‐Sosa 2012; Santana‐Sosa 2014; Schneiderman‐Walker 2000); this was also true for the study available only in abstract form and trial register entry on ClinicalTrials.gov (Douglas 2015). The remaining eight studies published as full‐text articles did not specify disease severity based on FEV1 as an exclusion criterion (Carr 2018; Cerny 1989; Del Corral 2018; Hommerding 2015; Moorcroft 2004; Rovedder 2014; Sawyer 2020; Selvadurai 2002). No information was available from the remaining five studies, which were only published as abstracts (Alexander 2019; Donadio 2020; Hatziagorou 2019; Michel 1989; Turchetta 1991).
In four studies, the authors reported differences in baseline characteristics of the participants despite randomisation (Cerny 1989; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014). In Cerny 1989, lung function, measured as FEV1 and forced mid‐expiratory flow 25% to 75% (FEF25–75), was significantly lower in the control compared to the physical activity group at admission. In both Santana‐Sosa studies, the physical activity groups had a lower aerobic exercise capacity (VO2 peak) and lower muscle strength (most but not all strength measures) (Santana‐Sosa 2012; Santana‐Sosa 2014). In Rovedder 2014, there was a significantly lower BMI in the intervention group compared to the control group.
In Kriemler 2013, the control group experienced an unusual deterioration of physical health during the study, and the results should be interpreted with caution. In Del Corral 2018, mean modified shuttle walk test (MSWT) distance in the intervention group was 823.5 (SD 270.6) m and in the control group was 1085.5 (SD 255.6) m. The study did not report differences between groups in MSWT distance. Our own calculations using the Review Manager 5 software revealed a difference between groups at baseline (MD ‒262 m, 95% CI ‒425.1 to ‒98.86; P = 0.003). The authors adjusted for baseline values in their statistical analysis (Del Corral 2018). In Güngör 2021, the physical activity group appeared to have lower HRQoL (respiratory symptoms and physical functioning domain) compared to controls (MD of approximately 15 units to 20 units), but the authors reported that no significant difference existed in baseline characteristics between groups. This might be due to the small sample size and large SDs.
Interventions
As the aim of this review was to assess the efficacy of any type of physical activity intervention versus no physical activity intervention (usual care), we excluded studies which exclusively involved respiratory muscle training. All 24 studies included a control group which did not receive a prescribed physical activity programme.
Three studies had three study arms and compared different types of physical activity programmes (endurance training or resistance training or resistance training with neuromuscular electrical stimulation) with a control group (Donadio 2020; Kriemler 2013; Selvadurai 2002).
Five studies compared a training programme with short bouts of intense activity to a control group (Alexander 2019; Güngör 2021; Gupta 2019; Klijn 2004; Sawyer 2020). Alexander 2019 compared a 12‐week whole‐body vibration training programme to control; Gupta 2019 compared a 12‐month home‐based physical activity programme, including strengthening exercises and plyometric jumping exercises, to a control group; and Sawyer 2020 compared an eight‐week cycling‐based high‐intensity interval training programme to a control group (Sawyer 2020). Klijn 2004 compared a 12‐week exercise programme including short (20 seconds to 30 seconds) intense exercises to normal daily activities. Güngör 2021 investigated the effects of a six‐week pulmonary rehabilitation programme, including active cycle of breathing techniques and postural exercises, compared with an active cycle of breathing techniques but no additional physical activity programme. After the six‐week intervention, children and parents were encouraged with weekly telephone calls to continue with the exercises for the following six months (Güngör 2021).
Five studies compared endurance type activities alone to a control group (Cerny 1989; Hommerding 2015; Michel 1989; Schneiderman‐Walker 2000; Turchetta 1991).
In 10 studies, investigators compared the effects of a combined training programme (a mixture of endurance type and resistance training or strengthening activities) to a control group (Beaudoin 2017; Del Corral 2018; Douglas 2015; Hatziagorou 2019; Hebestreit 2010; Hebestreit 2022; Moorcroft 2004; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014). Beaudoin 2017 investigated a 12‐week combined endurance and resistance training programme compared to no training. Del Corral 2018 evaluated the efficacy of a six‐week video game programme, including a variety of physical activities such as running, squats, lunges and biceps curls; the intervention group participants were encouraged to continue the programme for 12 months. Douglas 2015 and Hatziagorou 2019 investigated individually tailored supervised or partially supervised physical activity programmes in children with CF over 12 months (Douglas 2015) and 24 months (Hatziagorou 2019). Hebestreit 2010 compared an individualised physical activity programme, including endurance‐type exercises, strengthening exercises or a combination of both regimens, with a control group over 24 months; the control group was simply encouraged to maintain their level of activity over 12 months. Hebestreit 2022 was a 12‐month individualised and partially supervised programme aimed at increasing vigorous activities using a combination of endurance‐type and strengthening exercises. Moorcroft and colleagues evaluated the effects of a 12‐month individualised, unsupervised physical activity training programme, including a combination of both endurance and resistance activities (Moorcroft 2004). Rovedder 2014 used unsupervised home‐based training with endurance and strengthening exercises over 12 weeks. Santana‐Sosa 2012, in hospitalised participants, compared supervised endurance and strengthening exercises, three times per week to a control group who were only informed of the benefits of exercise; both groups received the same chest physiotherapy during the entire study period. Santana‐Sosa 2014 compared an eight‐week combined programme (endurance and strength), including additional inspiratory muscle training, with a control group.
Carr 2018 compared Tai Chi programme to a control group for nine months.
In two studies, all participants additionally received intravenous antibiotic treatment (Cerny 1989; Selvadurai 2002).
Outcomes
The most commonly reported outcome measure was the change in FEV1, which all reported studies except reported (Alexander 2019; Del Corral 2018; Klijn 2004; Michel 1989). Fourteen studies documented the change in VO2 peak (Beaudoin 2017; Douglas 2015; Gupta 2019; Hatziagorou 2019; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Klijn 2004; Kriemler 2013; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020; Schneiderman‐Walker 2000; Selvadurai 2002). One study reported changes in VO2 at the anaerobic threshold following a physical activity intervention (Donadio 2020), and one study reported changes in VO2 during a submaximal constant work rate exercise test (Sawyer 2020). Sixteen studies reported change in HRQoL (Alexander 2019; Beaudoin 2017; Carr 2018; Del Corral 2018; Güngör 2021; Gupta 2019; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Klijn 2004; Kriemler 2013; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020; Selvadurai 2002), and 10 studies reported change in muscle strength (Beaudoin 2017; Del Corral 2018; Donadio 2020; Hebestreit 2010; Klijn 2004; Kriemler 2013; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Selvadurai 2002). Sixteen studies reported change in body composition (Alexander 2019; Beaudoin 2017; Carr 2018; Del Corral 2018; Hatziagorou 2019; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Klijn 2004; Kriemler 2013; Michel 1989; Moorcroft 2004; Santana‐Sosa 2012; Santana‐Sosa 2014; Schneiderman‐Walker 2000; Selvadurai 2002). Eight studies reported change in physical activity (Beaudoin 2017; Gupta 2019; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Kriemler 2013; Schneiderman‐Walker 2000; Selvadurai 2002), and five studies reported the change in other indices of exercise capacity (other than cardiopulmonary exercise testing) (Cerny 1989; Güngör 2021; Hommerding 2015; Moorcroft 2004; Rovedder 2014). Two studies reported changes in diabetic control (Beaudoin 2017; Hebestreit 2022), and two studies reported changes in bone health after the intervention (Alexander 2019; Gupta 2019). Six studies reported on adverse events (Del Corral 2018; Güngör 2021; Hebestreit 2022; Kriemler 2013; Sawyer 2020; Selvadurai 2002). Only one study reported the number of pulmonary exacerbations and time to first pulmonary exacerbation (Hebestreit 2022). No study reported hospitalisations.
Excluded studies
We excluded 95 studies for the following reasons.
A total of 24 studies were not RCTs (Andreasson 1987; Asher 1982; Balfour Lynn 1998; Barry 2001; Bongers 2015; Cantin 2005; de Jong 1994; Edlund 1986; Heijerman 1992; Hütler 2002; IRCT20161024030474N4; Moola 2017; NCT02277860; NCT02715921; NCT03117764; Orenstein 1981; Petrovic 2013; Pryor 1979; RBR‐34677v; Ruddy 2015; Salh 1989; Stanghelle 1998; Tuzin 1998; White 1997). Thirty‐eight studies did not include a physical activity programme according to our protocol (ACTRN12620001237976; Alarie 2012; Albinni 2004; Amelina 2006; Aquino 2006; Balestri 2004; Bellini 2018; Bieli 2017; Bilton 1992; Chang 2015; Chatham 1997; Combret 2018; Combret 2021; Cox 2013; Dwyer 2011; Falk 1988; Giacomodonato 2015; Happ 2013; Haynes 2016; Irons 2012; Kaak 2011; Lannefors 1992; Macleod 2008; Montero‐Ruiz 2020; NCT02199340; NCT02821130; NCT02875366; Ozaydin 2010; Patterson 2004; Rand 2012; Reix 2012; Salonini 2015; Spoletini 2020; Vallier 2016; Vivodtzev 2013; Ward 2018; Young 2019; Zeren 2019). There were 19 studies which did not use a control arm with 'no physical activity' (Bass 2019; Calik‐Kutukcu 2016; de Marchis 2017; del Corral Nunez‐Flores 2014; Gruber 1998; Gruet 2012; Kaltsakas 2021; Kuys 2011; Lang 2019; Lima 2014; Lowman 2012; Martinez Rodriguez 2017; NCT01759342; NCT04888767; NTR2092; Orenstein 2004; RBR‐5g9f6w; Reuveny 2020; Shaw 2016). Five studies were acute exercise studies and of insufficient duration (less than 14 days) to be included in this review (Dwyer 2017; Dwyer 2019; Kriemler 2016; Radtke 2018b; Wheatley 2015). Seven studies had a lack of information: the investigators of two studies informed us that no paper will be published and data were not available (Mandrusiak 2011; NCT00792194); an investigator of one study did not reply to our email request for more information about the study status and planned publication (Oliveira 2010); and for four studies, contact details could not be found online to contact study investigators (Almajan‐Guta 2011; Housinger 2015; Johnston 2004; Phillips 2008). Two studies were excluded for other reasons: one study focused on proprioceptive neuromuscular facilitation in children with chronic respiratory diseases (this type of training aims to improve flexibility and range of motion; it is not considered a type of physical activity intervention that is expected to elicit improvements in the outcomes listed in our review and therefore not relevant for this review) (NCT03420209); and for one study, the last status update on ClinicalTrials.gov was posted in 2005 (NCT00129350), and it is unlikely that this study will be published in the future (if published data are found in future literature searches, the study will be considered for inclusion in the review).
Studies awaiting classification
There are six studies awaiting classification (Bishay 2017; Cox 2019; IRCT20190407043190N1; NCT03100214; NCT04293926; Powers 2016).
Trial characteristics
All six studies awaiting classification were of a randomised parallel‐group design. Two studies were multicentre (Cox 2019; IRCT20190407043190N1), and four were single‐centre studies (Bishay 2017; NCT03100214; NCT04293926; Powers 2016). The study size (i.e. enrolment goal if actual number of participants was not available) ranged from 19 to 80 participants (Bishay 2017; Cox 2019; IRCT20190407043190N1; NCT03100214; NCT04293926; Powers 2016).
All studies reported inclusion and exclusion criteria (Bishay 2017; Cox 2019; IRCT20190407043190N1; NCT03100214; NCT04293926; Powers 2016). One study enrolled adults (aged 18 years and older) (Bishay 2017), two studies enrolled children and adolescents (IRCT20190407043190N1; NCT04293926), and three studies enrolled adolescents and adults (Cox 2019; NCT03100214; Powers 2016).
Interventions
There was great variety between studies with respect to physical activity modalities and approaches. One study employed a combined aerobic and anaerobic home‐based training programme (Powers 2016). One study investigated a four‐week combined aerobic and anaerobic training programme, but the setting was not entirely clear from the registry entry (IRCT20190407043190N1). One study was conducted with participants hospitalised for treatment of a pulmonary exacerbation (NCT03100214). One study investigated the efficacy of a 12‐week web‐based application for improving participation in physical activity compared to usual care following hospitalisation for a respiratory exacerbation (Cox 2019). In another study, participants received an activity monitor (Fitbit) to measure physical activity and were followed over one year, completing surveys and exercise tests. Participants in the control group received usual care and were offered Fitbits after the first year (Bishay 2017). One study aimed to assess the effects of an eight‐week resistance training programme on the variability in heart rate in children and adolescents with CF versus usual care (i.e. routine recommendations, including lifestyle recommendations) (NCT04293926).
Outcomes
Five studies defined changes in FEV1 after the physical activity intervention as a secondary study outcome (Bishay 2017; Cox 2019; NCT03100214; NCT04293926; Powers 2016). Four studies reported on functional exercise capacity using a graded exercise test (Bishay 2017), the 6MWT (NCT03100214), or shuttle test (Cox 2019; Powers 2016). Four studies reported changes in HRQoL (Bishay 2017; Cox 2019; IRCT20190407043190N1; Powers 2016). Two studies included physical activity (Cox 2019; Powers 2016).
Ongoing studies
We listed 12 studies as ongoing (Curran 2020; ISRCTN92573472; Monteiro 2019; NCT03273959; NCT03970369; NCT04249999; NCT04543929; NCT04683809; NCT04742049; NCT05147285; NCT05173194; NCT05239611).
Trial characteristics
All 12 ongoing studies are of a randomised parallel‐group design and registered with ClinicalTrials.gov or the ISRCTN registry. All but one of the studies are single‐centre studies; the exception is a multicentre study conducted across the UK (NCT04249999). The studies range in duration: the shortest being two weeks (NCT03273959), then six weeks (NCT04742049), eight weeks (Monteiro 2019; NCT05147285; NCT05173194), 12 weeks (ISRCTN92573472; NCT04249999; NCT04543929; NCT04683809; NCT05239611), and the longest lasting over six months (Curran 2020; NCT03970369). Three studies included a follow‐up period: of eight weeks (Monteiro 2019), three months (NCT03970369), and six months (NCT04249999). All 12 studies have specified their inclusion and exclusion criteria, and all 12 have included both sexes. Five studies focus on children, adolescents or both (Monteiro 2019; NCT03273959; NCT03970369; NCT04683809; NCT05147285), four on adults only (Curran 2020; ISRCTN92573472; NCT04543929; NCT05239611), one on people between 12 and 35 years of age (NCT04249999), and one on people 16 years and older (NCT05173194). In one study, participation in the intervention was not restricted by age (NCT04742049). In four studies, participation in the physical activity trial is restricted to participants with an FEV1 equal to or greater than 25% predicted (Curran 2020), or equal to or greater than 40% predicted (NCT04742049; NCT05147285; NCT05239611). The target sample size in the studies ranges from 20 to 94 study participants.
Interventions
There is a great variety in interventions with respect to the study designs.
In Curran 2020, participants receive a fitness tracker and personalised feedback via a text message every week about their physical activity levels. A physiotherapist discusses individual short‐ and long‐term goals with each participant. The control group also receives a fitness tracker, but will not receive individualised goals and feedback during the study.
In one study, participants in the partially supervised intervention group receive an exercise manual (hard copy) and access to an online exercise diary for 12 weeks. They also receive a fitness tracker, to measure daily steps and active minutes. The control group will receive usual care (ISRCTN92573472).
Monteiro 2019 aims to evaluate the effects of anaerobic interval training on glucose tolerance in children and adolescents with CF versus usual care (no exercise training).
In one study, participants receive routine physical therapy plus exercise training (non‐supervised) in the form of a booklet and are guided by a health professional during treatment for a pulmonary exacerbation (NCT03273959).
One pilot RCT investigates the effects of an individualised physical activity prescription plus activity monitoring (only intervention group) on retention to the trial, and feasibility and acceptability of activity monitoring in young people with CF (NCT03970369).
One multicentre study is investigating the effects of a physical activity intervention with an online platform to monitor daily activity compared to usual care (NCT04249999).
In one study, the effects of standard of care therapy plus exercise are being compared to standard of care only for improving cardiorespiratory fitness over 12 weeks (NCT04543929).
One study is evaluating the effects of a partially supervised telerehabilitation‐based physical activity programme versus usual care (no exercise prescription), including individuals that self‐isolated during the 2019 coronavirus pandemic (NCT04742049).
One study aims to assess the effects of rehabilitation sessions, including postural, breathing and high‐intensity interval training exercises, through online programmes for rehabilitation. The exercise programme is being applied three days a week for three months (NCT04683809).
One study is assessing the effects of different exercise training modalities on functional exercise capacity (primary outcome). One group takes part in online supervised stabilisation exercises, one group performs online supervised aerobic exercise training and stabilisation exercises, and one group receives physical activity recommendations (control) (NCT05147285).
Another study aims to assess the effects of a remotely supervised resistance exercise programme on lung function, muscle strength, body composition, quality of life and inflammatory markers in adults with CF (NCT05173194).
One study is evaluating the effects of a 12‐week home‐based telerehabilitation intervention compared to usual care (NCT05239611).
Outcomes
The primary outcome measures of the studies are: changes in (functional) exercise capacity measured with various tests (NCT03273959; ISRCTN92573472; NCT04742049); change in glucose tolerance (Monteiro 2019); change in HRQoL (NCT04683809); and the recruitment rates over a 10‐month period, retention to the trial, as well as feasibility and acceptability of physical activity monitoring (NCT03970369). Three registered studies include two different primary outcomes (NCT05147285; NCT05239611), or even four different primary outcomes (NCT05173194); this increases the risk of choosing an outcome with a 'statistically significant' result. Seven studies include FEV1 as an outcome (Curran 2020; Monteiro 2019; NCT04249999; NCT04543929; NCT05147285; NCT05173194; NCT05239611); two studies report including lung function as an outcome, but do not provide any further details (ISRCTN92573472; NCT03273959). Nine studies include quality of life (QoL) as an outcome (Curran 2020; ISRCTN92573472; Monteiro 2019; NCT04249999; NCT04543929; NCT04683809; NCT05147285; NCT05173194; NCT05239611), and five studies include objectively measured or self‐reported physical activity (Curran 2020; NCT03970369; NCT04249999; NCT04742049; NCT05147285). Several other secondary outcomes are being investigated; these are listed in the Characteristics of ongoing studies table.
Risk of bias in included studies
We assessed each study for risk of bias according to the Cochrane risk of bias tool, which categorises risk into low, high or unclear risk of bias (Higgins 2017). The results are displayed graphically in Figure 1 and Figure 2.
Allocation
Sequence generation
Nine studies described the methods used for generation of the randomisation sequence and were judged to have a low risk of bias (Carr 2018; Del Corral 2018; Douglas 2015; Gupta 2019; Hebestreit 2022; Hommerding 2015; Rovedder 2014; Sawyer 2020; Schneiderman‐Walker 2000). A total of 13 studies were described as randomised, but gave insufficient details of the randomisation methods used; we deemed these to have an unclear risk of bias (Alexander 2019; Beaudoin 2017; Cerny 1989; Donadio 2020; Güngör 2021; Hatziagorou 2019; Klijn 2004; Michel 1989; Moorcroft 2004; Santana‐Sosa 2012; Santana‐Sosa 2014; Selvadurai 2002; Turchetta 1991). The abstract for Hatziagorou 2019 states that participants were divided into two groups. We contacted the primary and corresponding author of this study and she confirmed that this is an RCT. However, no details of the method are available and we graded the risk of bias as unclear. In the remaining two studies, information on the generation of the random sequence was provided, but the method used in the studies can potentially introduce selection bias and lacks reproducibility (Hebestreit 2010; Kriemler 2013). We judged these as having a high risk of bias.
Allocation concealment
Only eight studies described how allocation was concealed. We judged six of these studies to have a low risk of bias (Del Corral 2018; Gupta 2019; Hebestreit 2022; Klijn 2004; Sawyer 2020; Selvadurai 2002), and the other two studies to have a high risk of bias (Hebestreit 2010; Kriemler 2013). In these studies, investigators drew lots from a bag to allocate participants. However, allocation concealment is no longer assured when an investigator is aware of the number of lots in the bag and is aware of which have already been drawn; for example, if, for one group, all available lots have already been drawn out. A total of 16 studies (six of which were published as abstracts only) did not give any details of the method of allocation concealment; we assessed these as having an unclear risk of bias (Alexander 2019; Beaudoin 2017; Carr 2018; Cerny 1989; Donadio 2020; Douglas 2015; Güngör 2021; Hatziagorou 2019; Hommerding 2015; Michel 1989; Moorcroft 2004; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Schneiderman‐Walker 2000; Turchetta 1991).
Blinding
None of the studies was blinded for group assignment, as it is impossible to blind physical activity and exercise training compared to no training.
Blinding of participants and personnel (performance bias)
In two studies, one researcher of the study team was blinded to the participants' group allocation (Klijn 2004; Rovedder 2014). Klijn and colleagues reported that the primary researcher was blinded to group allocation, but their role in the study was not clear (Klijn 2004). In Rovedder 2014, one researcher was blinded for randomisation, the intervention and was responsible for database entries. Furthermore, the study staff who administered the questionnaires and performed the tests to collect outcome data were blinded to the participants' treatment allocations. Nevertheless, we judged all included studies to have a high risk of bias for this domain, as blinding of participants to treatment allocation is not possible in exercise studies.
Blinding of outcome assessment (detection bias)
In 10 studies, outcome assessors were blinded to group allocation; we deemed these studies to have a low risk of bias (Del Corral 2018; Douglas 2015; Gupta 2019; Güngör 2021; Kriemler 2013; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020; Schneiderman‐Walker 2000). We deemed 14 studies to have an unclear risk of bias. It is unclear whether outcome measures were assessed by blinded investigators in 13 of the studies (Alexander 2019; Beaudoin 2017; Carr 2018; Cerny 1989; Donadio 2020; Douglas 2015; Hatziagorou 2019; Hebestreit 2010; Hommerding 2015; Michel 1989; Moorcroft 2004; Selvadurai 2002; Turchetta 1991); and one study reported that the primary researcher was blinded, but it is not clear whether this person was responsible for outcome assessment (Klijn 2004). Finally, in one study, the investigators explicitly stated that outcome assessors were not blinded, but we were not certain how this would influence the results for our outcomes (Hebestreit 2022).
Incomplete outcome data
We evaluated risk of bias for incomplete outcome data with respect to the use of an intention‐to‐treat analysis, including appropriate methods for imputing data and the dropout rate (balanced or unbalanced between groups), including a description of reasons for dropouts.
A total of 19 studies provided information about dropouts (Beaudoin 2017; Cerny 1989; Del Corral 2018; Douglas 2015; Gupta 2019; Güngör 2021; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Klijn 2004; Kriemler 2013; Moorcroft 2004; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020; Schneiderman‐Walker 2000; Selvadurai 2002). Five studies (published only in abstract form) did not give any details about dropouts (Alexander 2019; Donadio 2020; Douglas 2015; Hatziagorou 2019; Michel 1989; Turchetta 1991).
We assessed 11 studies as having a low risk of bias for incomplete outcome data (Cerny 1989; Del Corral 2018; Douglas 2015; Gupta 2019; Hebestreit 2022; Hommerding 2015; Klijn 2004; Kriemler 2013; Rovedder 2014; Sawyer 2020; Selvadurai 2002). Of these, four studies reported no dropouts (Cerny 1989; Gupta 2019; Hommerding 2015; Selvadurai 2002), and in six studies, the dropout rate was balanced among groups and reasons for dropout were reported (Del Corral 2018; Hebestreit 2022; Klijn 2004; Kriemler 2013, Rovedder 2014; Sawyer 2020). Additionally, Hebestreit 2022 and Rovedder 2014 used multiple imputation to account for missing data in their statistical analysis. In one study, there were few dropouts and the reasons were given (two of these withdrew for reasons not related to the intervention) (Douglas 2015).
We judged four studies as having a high risk of bias (Beaudoin 2017; Carr 2018; Santana‐Sosa 2012; Santana‐Sosa 2014). In Beaudoin 2017, the dropout rate (postrandomisation) was 18% (n = 3) and the group allocation of two study participants was not reported. This study was registered as a randomised cross‐over study (NCT02127957), but the results were only reported for the first phase and the original publication described it as a parallel design study (Beaudoin 2017). Carr randomised 51 participants; 21.6% dropped out with reasons that were reported in detail in the CONSORT flow diagram, but investigators did not perform an intention‐to‐treat analysis (Carr 2018). In the remaining two studies, dropout rates were high and unbalanced between groups (Santana‐Sosa 2012; Santana‐Sosa 2014). Both studies reported the use of intention‐to‐treat analysis, while one study used the 'last value carried forward' method (Santana‐Sosa 2012). In the other Santana‐Sosa study, the method used for data imputation was not reported (Santana‐Sosa 2014).
We rated the remaining studies at unclear risk of bias for incomplete outcome data (Alexander 2019; Donadio 2020; Güngör 2021; Hatziagorou 2019; Hebestreit 2010; Michel 1989; Moorcroft 2004; Schneiderman‐Walker 2000; Turchetta 1991). Five of these studies were published only in abstract form and did not give any details about dropouts (Alexander 2019; Donadio 2020; Hatziagorou 2019; Michel 1989; Turchetta 1991). In one study lasting longer than six months, dropouts were reported and balanced between groups, but reasons for dropouts were not described and intention‐to‐treat analysis was not used (Hebestreit 2010). Schneiderman‐Walker 2000 reported the reasons for participants dropping out and that an intention‐to‐treat analysis produced similar results for pulmonary function outcomes; however, data were only reported for 65 participants, excluding dropouts. Another study reported using an intention‐to‐treat analysis, but missing data were treated by omission rather than imputation and reasons for dropout were not clearly described (Moorcroft 2004). In one study, reasons for dropout were not reported for all individuals (Güngör 2021). Additionally, this study did not use intention‐to‐treat analysis, and was therefore rated at unclear risk of bias.
Selective reporting
We judged 10 studies to have a low risk of bias since they reported all outcomes detailed in their 'methods' sections for all time points in their 'Results' sections (Cerny 1989; Gupta 2019; Hebestreit 2022; Kriemler 2013; Moorcroft 2004; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020; Schneiderman‐Walker 2000). One of these studies mentioned in the original publication that data for HRQoL would be addressed separately (Kriemler 2013). Data from this study were published together with data from another study which used similar methods (Hebestreit 2010); the combined data are presented in a separate paper (Hebestreit 2014). In another study, Hebestreit 2022 reported in the publication that data for substudies are not included in the main report.
A total of 12 studies had an unclear risk of bias for selective outcome reporting (Alexander 2019; Del Corral 2018; Donadio 2020; Douglas 2015; Güngör 2021; Hatziagorou 2019; Hebestreit 2010; Hommerding 2015; Klijn 2004; Michel 1989; Selvadurai 2002; Turchetta 1991). Six studies were only available in abstract format and we could not assess selective reporting (Alexander 2019; Donadio 2020; Douglas 2015; Hatziagorou 2019; Michel 1989; Turchetta 1991). The remaining six studies did not report on all their stated outcomes. Five studies did not report all outcomes for HRQoL (Del Corral 2018; Güngör 2021; Klijn 2004; Hebestreit 2010; Hommerding 2015), and Hebestreit 2010 did not report all anaerobic exercise capacity outcomes. Two studies did not report all variables for cardiopulmonary exercise testing as mentioned in their 'methods' section (Hommerding 2015; Selvadurai 2002).
We judged Beaudoin 2017 at high risk of bias for selective reporting, because the study was registered as a randomised cross‐over study, but reported as a parallel‐design study. The second part of the study was not reported in the original publication.
Finally, we judged Carr 2018 at high risk of bias for selective reporting because data for HRQoL (primary endpoint) were not presented for all CFQ‐R domains for all time points.
Other potential sources of bias
Description of inclusion or exclusion criteria
Six studies were only available in abstract format and did not state inclusion or exclusion criteria (Alexander 2019; Donadio 2020; Douglas 2015; Hatziagorou 2019; Michel 1989; Turchetta 1991). The potential for bias was limited in the 15 studies which clearly stated inclusion and exclusion criteria (Beaudoin 2017; Carr 2018; Del Corral 2018; Gupta 2019; Güngör 2021; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Kriemler 2013; Moorcroft 2004; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020; Selvadurai 2002). Three studies described the inclusion criteria but not the exclusion criteria, which could be a potential source of bias (Cerny 1989; Klijn 2004; Schneiderman‐Walker 2000).
Statistical analysis
A total of 18 studies, published as full‐text articles, clearly described the methods of statistical analysis, thus eliminating a potential source of bias (Beaudoin 2017; Carr 2018; Cerny 1989; Del Corral 2018; Güngör 2021; Gupta 2019; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Klijn 2004; Kriemler 2013; Moorcroft 2004; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020; Schneiderman‐Walker 2000; Selvadurai 2002). Of the six studies published as abstracts (Alexander 2019; Donadio 2020; Douglas 2015; Hatziagorou 2019; Michel 1989; Turchetta 1991), one study reported details on the statistical analysis (Donadio 2020).
One study did not report the number of participants in each group so the MD between the treatment and control groups could not be calculated (Michel 1989).
In one study, information on sample size and recruitment goals differed between the information provided on the trial registry and the final publication (Beaudoin 2017). The study aimed to recruit 24 participants (12 in each group) but the recruitment goal was not achieved (18 were recruited and only 17 randomised). According to the power calculation provided in the original publication, 18 participants (nine per group) were required for the analysis. Only 14 participants actually completed the study (Beaudoin 2017). We judged this study to have a high risk of bias.
In two studies, the number of included participants was much lower than the enrolment goal: namely, 117/292 participants in Hebestreit 2022, and 17/32 participants in Sawyer 2020. Consequently, both studies were potentially underpowered for several outcomes. We judged these two studies to have a high risk of bias.
Group characteristics
In five studies, there were significant between‐group differences at baseline despite randomisation (Cerny 1989; Kriemler 2013; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014). In one study, FEV1 and FEF25–75 were significantly lower in the control group compared to the training group at admission (Cerny 1989). In a second study, differences in exercise capacity (peak power was higher in the strength training group compared to the control group) and in vigorous physical activity (lower in the aerobic training group compared to controls) were evident at baseline (Kriemler 2013). In both Santana‐Sosa studies, the training groups had a lower aerobic exercise capacity (VO2 peak) and lower muscle strength (most but not all strength measures) (Santana‐Sosa 2012; Santana‐Sosa 2014). In the fifth study, BMI was significantly lower in the intervention group compared to the control group (Rovedder 2014). In addition, in Güngör 2021, some HRQoL domains were lower in the intervention compared to the control group (Güngör 2021). The authors wrote that no statistically significant differences existed in baseline demographic characteristics between groups. It is uncertain whether these factors could be a potential source of bias so we judged the risk to be unclear for significant between‐group differences at baseline.
Ten of the 18 studies published as full‐text articles used FEV1 % predicted values as exclusion criteria (Beaudoin 2017; Güngör 2021; Gupta 2019; Hebestreit 2010; Hebestreit 2022; Klijn 2004; Kriemler 2013; Santana‐Sosa 2012; Santana‐Sosa 2014; Schneiderman‐Walker 2000); this was also true of one study where information on inclusion and exclusion criteria were available from ClinicalTrials.gov (Douglas 2015). The remaining eight studies that were published as full‐text articles did not specify disease severity based on FEV1 as an exclusion criterion (Carr 2018; Cerny 1989; Del Corral 2018; Hommerding 2015; Moorcroft 2004; Rovedder 2014; Sawyer 2020; Selvadurai 2002). There was no information available in the remaining five studies, published as abstracts (Alexander 2019; Donadio 2020; Hatziagorou 2019; Michel 1989; Turchetta 1991). We accept that studies which exclude participants on the basis of one of our outcomes may cause a risk of bias to the review. However, the risk of exercise‐induced adverse effects is likely to be higher in people with severe CF lung disease and many researchers tend to exclude those people because of this. In one study, financial support was provided to the physical activity group participants to foster the activity plan; this study was judged at unclear risk of bias (Hebestreit 2010).
Intervention
In the original publication by Beaudoin there was no information on the control intervention (Beaudoin 2017). We noticed discrepancies between the registered trial protocol (clinicaltrials.gov/ct2/show/NCT02127957) and published trial design (cross‐over versus parallel‐group design) (Beaudoin 2017).
Data discrepancies
We rated three studies as having a high risk of bias (Beaudoin 2017; Santana‐Sosa 2012; Santana‐Sosa 2014). Two studies for which we received some raw data from the authors were rated as high risk of bias due to inconsistencies between the raw data files and the data reported in the original publications (Santana‐Sosa 2012; Santana‐Sosa 2014). Furthermore, Beaudoin 2017 reported within‐group changes from baseline and not between‐group differences, as would be appropriate for an RCT. We calculated between‐group differences using raw data provided by the authors and our results suggest no between‐group differences for the primary endpoint. When considered alongside the fact that the stated power calculation requiring 18 participants to demonstrate a difference was not achieved (see 'Statistical analysis' above), there is a high risk of bias that the reported effects are not sound.
Effects of interventions
See: Table 1
We included 24 studies with 875 participants and pooled data comparing any type of physical activity intervention versus no physical activity intervention (Alexander 2019; Beaudoin 2017; Carr 2018; Cerny 1989; Del Corral 2018; Donadio 2020; Douglas 2015; Güngör 2021; Gupta 2019; Hatziagorou 2019; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Klijn 2004; Kriemler 2013; Michel 1989; Moorcroft 2004; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020; Schneiderman‐Walker 2000; Selvadurai 2002; Turchetta 1991). Two studies compared endurance training and resistance training versus no training and for all outcomes in this review, we combined the two active arms of each study (Kriemler 2013; Selvadurai 2002). Six studies were published as abstracts only (Alexander 2019; Donadio 2020; Douglas 2015; Hatziagorou 2019; Michel 1989; Turchetta 1991), and no information on outcomes relevant for this review was available from five of these (Alexander 2019; Donadio 2020; Hatziagorou 2019; Michel 1989; Turchetta 1991). See Table 1 for explanations of the judgements for the certainty of the evidence.
Where primary studies reported differences between groups but did not provide adequate data (means and SD) that could be presented in Review Manager 5 (Review Manager 2014), we included information from the primary (original) study in the results.
Within the results below, we present the effects of the active interventions (supervised, partially supervised, unsupervised but with access to study resources) at the end of each active intervention period at the time points of up to and including six months and more than six months and for follow‐up periods where all participants reverted to usual care. In total, 14 studies had active intervention periods up to and including six months (Alexander 2019; Beaudoin 2017; Cerny 1989; Donadio 2020; Güngör 2021; Hommerding 2015; Klijn 2004; Michel 1989; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014; Sawyer 2020; Selvadurai 2002; Turchetta 1991), and 10 studies had active intervention periods longer than six months (Carr 2018; Del Corral 2018; Douglas 2015; Gupta 2019; Hatziagorou 2019; Hebestreit 2010; Hebestreit 2022; Kriemler 2013; Moorcroft 2004; Schneiderman‐Walker 2000). Nine studies implemented an additional follow‐up period (Del Corral 2018; Güngör 2021; Hebestreit 2010; Klijn 2004; Kriemler 2013; Michel 1989; Santana‐Sosa 2012; Santana‐Sosa 2014; Selvadurai 2002).
If a study reported multiple time points for a category of activity (supervised, partially supervised, unsupervised but with access to study resources) within our predefined reporting periods (i.e. up to and including six months, and longer than six months), we reported the longest training period within the given category.
Due to our concerns about data quality from both studies by Santana‐Sosa (Santana‐Sosa 2012; Santana‐Sosa 2014), we excluded these from the formal analysis in the review. We provide their data in two additional tables (Table 2; Table 3).
In one small study (n = 19), data were not normally distributed and within‐group changes were reported as median (interquartile range (IQR)) (Güngör 2021). Mean or median differences were not available. We contacted the authors and requested raw data but did not receive feedback. We decided not to compute means (SDs) because of the small group sizes (nine and 10 study participants in the different groups) and skewed data distribution. We presented the data for this study descriptively.
Of note, when interpreting the results presented below, in Kriemler 2013, the control group experienced an unusual deterioration of physical health during the study; the results should be interpreted with caution. In Selvadurai 2002, all participants received intravenous antibiotic therapy during the in‐hospital physical activity training programme. In Klijn 2004, the pre‐exercise training values for the CFQ‐R physical functioning domain were substantially lower in the physical activity group compared to the control group, and the effects were large. For these reasons, we undertook a sensitivity analysis to determine the influence of these single studies to the overall pooled estimate. We evaluated the effect of Kriemler 2013 on the pooled estimate for VO2 peak; the effect of Kriemler 2013 and Selvadurai 2002 on the pooled effect estimate for FEV1, and the effect of Klijn 2004 on the pooled estimated for HRQoL.
Primary outcomes
1. Exercise capacity by peak oxygen uptake
Twelve studies reported VO2 peak (Beaudoin 2017; Douglas 2015; Gupta 2019; Hatziagorou 2019; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Klijn 2004; Kriemler 2013; Sawyer 2020; Selvadurai 2002; Schneiderman‐Walker 2000). See: Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4; Analysis 1.5.
1.1. Analysis.

Comparison 1: Physical activity versus control, Outcome 1: Change in VO2 peak (mL/min per kg bodyweight)
1.2. Analysis.

Comparison 1: Physical activity versus control, Outcome 2: Change in VO2 peak (mL/min per kg bodyweight): sensitivity analysis
1.3. Analysis.

Comparison 1: Physical activity versus control, Outcome 3: Change in VO2 peak (% predicted)
1.4. Analysis.

Comparison 1: Physical activity versus control, Outcome 4: Change in VO2 peak (mL/min per kg bodyweight): combined subgroups
1.5. Analysis.

Comparison 1: Physical activity versus control, Outcome 5: Change in VO2 peak (mL/min per kg bodyweight): combined subgroups – sensitivity analysis
Eight studies measured VO2 peak during an incremental cycling exercise test (Beaudoin 2017; Hatziagorou 2019; Hebestreit 2010; Hebestreit 2022; Klijn 2004; Kriemler 2013; Sawyer 2020; Schneiderman‐Walker 2000), and four studies used an incremental treadmill exercise test (Douglas 2015; Gupta 2019; Hommerding 2015; Selvadurai 2002). Data from Hatziagorou 2019 (published as abstract) could not be included in the analysis, and are reported descriptively.
Up to and including six months' active intervention
Eight studies reported VO2 peak at up to and including six months' active intervention (Beaudoin 2017; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Klijn 2004; Kriemler 2013; Sawyer 2020; Selvadurai 2002). The combined analyses (n = 323), in which data from the two active arms of the studies by Kriemler and Selvadurai were combined into a single arm, revealed a difference in VO2 peak between groups in favour of physical activity (MD 2.10 mL/min per kg bodyweight, 95% CI 0.06 to 4.13; I² = 76%; Analysis 1.1). Heterogeneity between these studies was substantial which was most likely due to the unusual deterioration of the control group in Kriemler 2013. In a sensitivity analysis excluding Kriemler 2013, the effect estimate changed towards null and between‐study heterogeneity decreased (MD 1.30 mL/min per kg bodyweight, 95% CI ‒0.17 to 2.78; I²= 56%; n = 287; Analysis 1.2).
One multicentre study reported changes in VO2 peak expressed as % predicted after six months of vigorous physical activity versus control (Hebestreit 2022). There was no evidence of a between‐group effect on VO2 peak after six months (MD 0.60% predicted, 95% CI ‒3.01 to 4.21; Analysis 1.3).
Over six months' active intervention
Six studies reported VO2 peak at over six months' active intervention (Douglas 2015; Gupta 2019; Hebestreit 2010; Hebestreit 2022; Kriemler 2013; Schneiderman‐Walker 2000) (Analysis 1.1). The combined analysis showed an effect on VO2 peak in favour of physical activity (MD 1.60 mL/min per kg bodyweight, 95% CI 0.16 to 3.05; I²= 59%; n = 348; Analysis 1.1). Certainty of evidence was moderate (Table 1). Heterogeneity between studies was substantial, most likely due to the heterogeneous study durations of 12 and 36 months (Analysis 1.1). In a sensitivity analysis (n = 318) excluding the Kriemler 2013 study, the effect estimate changed from 1.60 mL/min per kg bodyweight (95% CI 0.16 to 3.05) to 1.38 mL/min per kg bodyweight (95% CI 0.08 to 2.69), and between‐study heterogeneity changed minimally from 59% to 55% (Analysis 1.2).
Hebestreit 2022 reported a higher VO2 peak (% predicted) in the intervention compared to the control group (MD 4.53, 95% CI 1.07 to 7.99; n = 117; Analysis 1.3).
Hatziagorou 2019 (n = 30) reported an increase in VO2 peak of 23.8% in the intervention group after 12 months, while there was no change in the control group; however, the authors did not report the MD. At baseline, VO2 peak was 72.7% predicted in the intervention group, and 89.1% predicted in the control group.
Follow‐up (no active intervention)
Three studies reported VO2 peak at follow‐up periods ranging between one and 12 months (Hebestreit 2010; Kriemler 2013; Selvadurai 2002). In this comparison, VO2 peak was higher in the physical activity intervention versus control group (MD 3.27 mL/min per kg bodyweight, 95% CI 1.37 to 5.18; I² = 0%; n = 125; Analysis 1.1). In a sensitivity analysis excluding Kriemler 2013, the overall effect estimate and 95% CIs did not change substantially (MD 3.21 mL/min per kg bodyweight, 95% CI 1.27 to 5.14; I² = 0%; n = 99; Analysis 1.2). Between‐study heterogeneity remained unchanged.
Total results irrespective of duration of the active intervention
In a combined analysis of 11 studies, VO2 peak was higher in the physical activity group compared to the control group (MD 1.52 mL/min per kg bodyweight, 95% CI 0.31 to 2.73; I² = 60%; n = 496; Analysis 1.4). A sensitivity analysis excluding Kriemler 2013 resulted in a slightly lower effect of physical activity on VO2 peak, with little effect on between‐study heterogeneity (MD 1.38 mL/min per kg bodyweight, 95% CI 0.22 to 2.55; I² = 58%; n = 466; Analysis 1.5).
2. Lung function: forced expiratory volume in one second
A total of 18 studies reported FEV1 (Beaudoin 2017; Carr 2018; Cerny 1989; Donadio 2020; Douglas 2015; Güngör 2021; Gupta 2019; Hatziagorou 2019; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Klijn 2004; Kriemler 2013; Moorcroft 2004; Rovedder 2014; Sawyer 2020; Schneiderman‐Walker 2000; Selvadurai 2002).
We reported six studies descriptively. Three studies, two of which were published as abstracts only, reported on changes in FEV1 but did not present any data (Carr 2018; Donadio 2020; Hatziagorou 2019). Cerny 1989 reported no difference in the change in FEV1 % predicted, but data could not be extracted. Klijn 2004 reported that there were no differences between groups in lung function parameters, but no data were available for analysis. Güngör 2021 reported medians and IQRs, which we were unable to analyse in the review.
We analysed data from 12 studies. A total of 11 studies reported data on changes in FEV1 % predicted (Beaudoin 2017; Douglas 2015; Gupta 2019; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Kriemler 2013; Rovedder 2014; Sawyer 2020; Schneiderman‐Walker 2000; Selvadurai 2002; Analysis 1.6; Analysis 1.7; Analysis 1.10; Analysis 1.11), one study reported in z‐score units as well as FEV1 % predicted (Douglas 2015; Analysis 1.9), and one study reported in mL only (Moorcroft 2004; Analysis 1.8).
1.6. Analysis.

Comparison 1: Physical activity versus control, Outcome 6: Change in FEV1 (% predicted)
1.7. Analysis.

Comparison 1: Physical activity versus control, Outcome 7: Change in FEV1 (% predicted): sensitivity analysis
1.10. Analysis.

Comparison 1: Physical activity versus control, Outcome 10: Change in FEV1 (% predicted): combined subgroups
1.11. Analysis.

Comparison 1: Physical activity versus control, Outcome 11: Change in FEV1 (% predicted): sensitivity analysis
1.9. Analysis.

Comparison 1: Physical activity versus control, Outcome 9: Change in FEV1 (z‐score)
1.8. Analysis.

Comparison 1: Physical activity versus control, Outcome 8: Change in FEV1 (mL)
Up to and including six months' active intervention
Eight studies reported FEV1 up to and including six months' active intervention (Beaudoin 2017; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Kriemler 2013; Rovedder 2014; Sawyer 2020; Selvadurai 2002). In a combined analysis, there was no evidence of a between‐group difference for FEV1, but heterogeneity was substantial (MD 1.30% predicted, 95% CI ‒3.01 to 5.61; I² = 79%; n = 356; Analysis 1.6). A sensitivity analysis excluding Kriemler 2013 and Selvadurai 2002 changed the effect estimate for FEV1 to a beneficial effect in favour of control (MD ‒2.16 % predicted, 95% CI ‒4.14 to ‒0.17; n = 255; Analysis 1.7). In six of seven studies, the CIs included 0, and the summary estimate appeared to be mainly affected by Hebestreit 2022 (n = 117), as it received much weight in the combined analysis.
Cerny 1989 (n = 17), Carr 2018 (n = 40) and Donadio 2020 (n = 25) reported no differences in FEV1 after the intervention. Güngör 2021 reported no between‐group differences in median FEV1 % predicted after six weeks (intervention group: 90.5 (IQR 37.75) % predicted; n = 10; control group: 86 (IQR 19) % predicted; n = 9).
In Klijn 2004(n = 20), there were no differences between groups in lung function parameters, but no data were available for analysis.
Over six months' active intervention
Six studies reported FEV1 at over six months' active intervention (Douglas 2015; Gupta 2019; Hebestreit 2010; Hebestreit 2022; Kriemler 2013; Schneiderman‐Walker 2000). The combined analysis showed no between‐group differences in FEV1 % predicted and between‐study heterogeneity was moderate to substantial (MD 2.41 % predicted, 95% CI ‒0.49 to 5.31; I² = 52%; n = 367; Analysis 1.6). Certainty of the evidence was low (Table 1). In a sensitivity analysis excluding Kriemler 2013, FEV1 % predicted was higher in the physical activity intervention group compared to the control group (but there was an unexpected deterioration of lung health in the control group) (MD 1.71 % predicted, 95% CI 0.15 to 3.26; n = 333; Analysis 1.7). The exclusion of Kriemler 2013 changed the overall effect estimate favouring the physical activity intervention compared to no physical activity intervention, and increased the precision of the effect estimate (narrower 95% CIs). Of note, none of the individual studies included in the combined analysis showed an effect in change in FEV1 after the intervention (i.e. all 95% CIs included 0).
Moorcroft 2004 found no between physical activity and control on FEV1 after 12 months (MD 107 mL, 95% CI ‒73.98 to 287.98; Analysis 1.8). Douglas 2015 found no between‐group difference in FEV1 z‐score after 24 months (MD 0.12, 95% CI ‒0.37 to 0.61; Analysis 1.9).
The 12‐month intervention by Hatziagorou 2019 reported unchanged FEV1 in the physical activity group of 0.88% and no change in the control group (specific MD not reported; n = 30).
Follow‐up (no active intervention)
Three studies reported FEV1 with follow‐up periods ranging between one and 12 months (Hebestreit 2010; Kriemler 2013; Selvadurai 2002). There was no difference between physical activity and control groups (MD 5.68 % predicted, 95% CI ‒1.88 to 13.23; n = 128; Analysis 1.6). When two studies were excluded from this comparison in a sensitivity analysis (Kriemler 2013; Selvadurai 2002), Hebestreit 2010 found no difference between physical activity and no physical activity on FEV1 % predicted (MD ‒0.32, 95% CI ‒11.90 to 11.26; n = 31; Analysis 1.7).
Güngör 2021 reported no between‐group differences in FEV1 % predicted after six months' follow‐up (median: intervention group: 88.5 (IQR 8.75) % predicted; n = 10; control group: 95.5 (IQR 49.25) % predicted; n = 9).
Total results irrespective of duration of the active intervention
In a combined analysis of 11 studies, there was no evidence of a difference in FEV1 % predicted between groups and between‐study heterogeneity was moderate (MD 1.37, 95% CI ‒0.74 to 3.47; I² = 43%; n = 536; Analysis 1.10). A sensitivity analysis excluding Kriemler 2013 and Selvadurai 2002 reduced study heterogeneity but the overall effect did not change (MD 1.07, 95% CI ‒0.36 to 2.49; I² = 0%; n = 436; Analysis 1.11).
3. Health‐related quality of life
Eight studies reported HRQoL data (Beaudoin 2017; Douglas 2015; Hebestreit 2010; Hebestreit 2022; Klijn 2004; Kriemler 2013; Sawyer 2020; Selvadurai 2002). Seven studies reported on changes in the physical function domain of the CFQ‐R questionnaire (Beaudoin 2017; Douglas 2015; Hebestreit 2010; Hebestreit 2022; Klijn 2004; Kriemler 2013; Sawyer 2020), six studies reported on changes in respiratory symptoms (CFQ‐R) (Beaudoin 2017; Douglas 2015; Hebestreit 2010; Hebestreit 2022; Kriemler 2013; Sawyer 2020), and one study reported on changes in well‐being one month after completion of the study ('off training') (Selvadurai 2002). One study, published only as an abstract, reported no changes in HRQoL (using CFQ‐R) between the physical activity and control group after three months, but data were not reported in the abstract (Alexander 2019). Güngör 2021 assessed HRQoL with the CFQ‐R but postintervention data were not reported for the physical functioning and respiratory domains.
a. Physical functioning
Up to and including six months' active intervention
Six studies reported physical functioning up to and including six months' active intervention (Beaudoin 2017; Hebestreit 2010; Hebestreit 2022; Klijn 2004; Kriemler 2013; Sawyer 2020) (Analysis 1.12). In a combined analysis, the change in the CFQ‐R physical function domain was not different between the physical activity intervention and control groups, but between‐study heterogeneity was substantial (MD 4.67, 95% CI ‒2.55 to 11.90; I² = 65%; n = 217; Analysis 1.12). In Klijn 2004, the mean (SD) pre‐exercise training values for the CFQ‐R physical functioning domain were substantially lower in the physical activity group compared to the control group and the effects were large (70.3 (SD 13.8) with physical activity versus 83.2 (SD 18.5) with control; Analysis 1.12). In a sensitivity analysis excluding Klijn 2004, the effect estimate changed towards null and study heterogeneity decreased substantially (MD 0.10, 95% CI ‒4.05 to 4.25; I² = 0%; n = 197; Analysis 1.13).
1.12. Analysis.

Comparison 1: Physical activity versus control, Outcome 12: Change in HRQoL: CFQ‐R physical functioning domain
1.13. Analysis.

Comparison 1: Physical activity versus control, Outcome 13: Change in HRQoL: CFQ‐R physical functioning domain: sensitivity analysis
In Rovedder 2014, there were no differences in CFQ‐R physical function domain between the physical activity and control groups after three months. Data for HRQoL scales were reported in the original publication and presented as medians and IQRs so could not be analysed in the review. Data are presented in Table 4.
3. Health‐related quality of life (HRQoL) results for Rovedder 2014.
| Health‐related quality of life |
Exercise group (n = 19) (median (IQR)) |
Control group (n = 22) (median (IQR)) |
P value |
| HRQoL scale – physical | 6.1 (‒4 to 8) | 2.4 (‒10 to 13) | 0.742 |
| HRQoL scale – respiratory | 3.8 (0 to 11) | ‒4.7 (‒1 to 7) | 0.925 |
| SF‐36 – functional capacity | 2.8 (‒10 to 15) | 2.0 (‒11 to 10) | 0.916 |
| SF‐36 – physical aspects | 11.8 (‒25 to 50) | 6.8 (‒6 to 31) | 0.705 |
| SF‐36 – pain | ‒7.2 (‒28 to 11) | 8.0 (7 to 17) | 0.100 |
| SF‐36 – general health | 3.7 (‒5 to 10) | ‒3.5 (‒11 to 5) | 0.197 |
| SF‐36 – vitality | 1.2 (‒15 to 20) | 7.5 (‒1 to 21) | 0.416 |
| SF‐36 – social aspects | 15.2 (0 to 33) | 21.2 (0 to 66) | 0.989 |
| SF‐36 – emotional aspects | 4.7 (‒12 to 37) | 4.5 (‒12 to 25) | 0.914 |
| SF‐36 – mental health | ‒0.8 (‒12 to 12) | 0.9 (‒9 to 13) | 0.752 |
Pre–post changes in HRQoL measured using the CFQ and SF‐36. CFQ: Cystic Fibrosis Questionnaire; HRQoL: health‐related quality of life; IQR: interquartile range; n: number of participants; SF‐36: Medical Outcomes Study 36‐Item Short‐Form Health Survey.
Over six months' active intervention
Four studies reported physical functioning at over six months' active intervention (Douglas 2015; Hebestreit 2010; Hebestreit 2022; Kriemler 2013). In a combined analysis, there was no evidence of an effect comparing physical activity versus no physical activity intervention on change in the CFQ‐R physical function domain, and between‐study heterogeneity was moderate (MD 2.19, 95% CI ‒3.42 to 7.80; I² = 39%; n = 247; Analysis 1.12). Certainty of evidence was low (Table 1).
Total results irrespective of duration of the active intervention
In a combined analysis of seven studies, there were no between‐group effects for changes in the CFQ‐R physical functioning domain, and between‐study heterogeneity was substantial (MD 4.76, 95% CI ‒1.09 to 10.61; I² = 60%; n = 295; Analysis 1.14). A sensitivity analysis excluding Klijn 2004 changed the effect estimate towards null, and reduced between‐study heterogeneity (MD 2.44, 95% CI ‒1.43 to 6.30; I² = 0%; n = 275; I² = 0%; Analysis 1.15).
1.14. Analysis.

Comparison 1: Physical activity versus control, Outcome 14: Change in HRQoL: CFQ‐R physical functioning domain: combined subgroups
1.15. Analysis.

Comparison 1: Physical activity versus control, Outcome 15: Change in HRQoL: CFQ‐R physical functioning domain: combined subgroups – sensitivity analysis
b. Respiratory
Up to and including six months' active intervention
Five studies reported on changes in CFQ‐R respiratory symptoms up to and including six months' active intervention (Beaudoin 2017; Hebestreit 2010; Hebestreit 2022; Kriemler 2013; Sawyer 2020). In a combined analysis, there were no between‐group differences in CFQ‐R respiratory symptoms (MD ‒1.87, 95% CI ‒5.66 to 1.92; n = 212; Analysis 1.16).
1.16. Analysis.

Comparison 1: Physical activity versus control, Outcome 16: Change in HRQoL: CFQ‐R respiratory symptoms
In Rovedder 2014, there were no differences in CFQ‐R respiratory symptoms between the physical activity and control groups after three months. Data for HRQoL scales were reported in the original publication and presented as medians and IQRs so could not be analysed in the review. Data are presented in Table 4.
Over six months' active intervention
Four studies reported on changes in CFQ‐R respiratory symptoms at over six months' active intervention (Douglas 2015; Hebestreit 2010; Hebestreit 2022; Kriemler 2013). In a combined analysis, there were no differences in CFQ‐R respiratory symptoms between the intervention and control groups (MD ‒0.05, 95% CI ‒3.61 to 3.51; n = 251; Analysis 1.16). Certainty of evidence was low (Table 1).
Total results irrespective of duration of the active intervention
A combined analysis of six studies revealed no differences between physical activity versus no physical activity intervention on CFQ‐R respiratory symptoms (MD 0.22, 95% CI ‒3.15 to 3.58; n = 279; Analysis 1.17).
1.17. Analysis.

Comparison 1: Physical activity versus control, Outcome 17: Change in HRQoL: CFQ‐R respiratory symptoms: combined subgroups
c. Other
Up to and including six months' active intervention
In Rovedder 2014, there were no differences in the 36‐item Short Form Survey (SF‐36) (a frequently used self‐reported measure of health) between the physical activity and control group after three months. Data for HRQoL scales were reported in the original publication and presented as medians and IQRs so could not be analysed in the review. Data are presented in Table 4.
Follow‐up periods (no active intervention)
One study reported on changes in well‐being after the intervention had been completed using the Quality of Well‐Being questionnaire (Selvadurai 2002). The physical activity group reported higher well‐being compared to the control group one month after the study had been completed (MD 0.07, 95% CI 0.01 to 0.13; n = 66; Analysis 1.18).
1.18. Analysis.

Comparison 1: Physical activity versus control, Outcome 18: Change in HRQoL: Quality of Well‐Being scale
Secondary outcomes
1. Additional indices of exercise capacity
Eleven studies reported additional indices of exercise capacity (Cerny 1989; Del Corral 2018; Donadio 2020; Douglas 2015; Hebestreit 2010; Hebestreit 2022; Klijn 2004; Kriemler 2013; Rovedder 2014; Sawyer 2020; Schneiderman‐Walker 2000).
a. Peak work capacity
Seven studies reported on changes in peak work capacity during maximal exercise, expressed as either watt (W) absolute values, W/kg bodyweight, or W % predicted (Cerny 1989; Hebestreit 2010; Hebestreit 2022; Klijn 2004; Kriemler 2013; Sawyer 2020; Schneiderman‐Walker 2000). One study reported on changes in time to symptom limitation (Tlim) and VO2 peak (mL/min per kg bodyweight and % predicted) during a submaximal constant load cycling exercise test (Sawyer 2020), and one study (published as an abstract) reported on changes in VO2 at the anaerobic threshold (Donadio 2020).
Up to and including six months' active intervention
In a combined analysis of three studies, peak work capacity was higher in the physical activity intervention groups compared to the control groups (MD 0.32 W/kg bodyweight, 95% CI 0.12 to 0.51; I² = 69%; n = 164; Analysis 1.19). Between‐study heterogeneity was substantial and was likely explained by Kriemler 2013, which showed a large effect, probably due to the deterioration of lung health in the control group.
1.19. Analysis.

Comparison 1: Physical activity versus control, Outcome 19: Change in peak work capacity (W/kg bodyweight) during maximal exercise
In Klijn 2004, peak work capacity was higher in the training group compared to the control group after three months (MD 13.00 W, 95% CI 4.11 to 21.89; n = 20; Analysis 1.20).
1.20. Analysis.

Comparison 1: Physical activity versus control, Outcome 20: Change in peak work capacity (W) during maximal exercise
In a pooled analysis of Hebestreit 2022 and Sawyer 2020, peak work capacity was higher in the physical activity compared to the control groups after two to six months (MD 6.89% predicted, 95% CI 3.94 to 9.83; n = 117; Analysis 1.21).
1.21. Analysis.

Comparison 1: Physical activity versus control, Outcome 21: Change in peak work capacity (% predicted) during maximal exercise
Cerny 1989 presented results in figures, but raw data were not available. They reported no differences between groups in peak work capacity (W/kg bodyweight).
Over six months' active intervention
In a combined analysis of three studies, peak work capacity was higher in the intervention compared to the control groups (MD 0.18 W/kg bodyweight, 95% CI 0.07 to 0.29; n = 155; Analysis 1.19).
In a combined analysis of Hebestreit 2022 and Schneiderman‐Walker 2000, there was no evidence of a difference in peak exercise capacity between the physical activity intervention and control groups (MD 3.59% predicted, 95% CI ‒2.06 to 9.24; n = 168; Analysis 1.21).
Follow‐up (no active intervention)
Two studies reported peak work capacity after a 12‐month follow‐up (Hebestreit 2010; Kriemler 2013).
In a combined analysis of Hebestreit 2010 and Kriemler 2013, there was no evidence of a difference in peak exercise capacity between the physical activity and control groups were observed (MD 0.26 W/kg bodyweight, 95% CI ‒0.03 to 0.56; n = 51; Analysis 1.19).
b. Submaximal exercise capacity
Two studies reported on submaximal exercise capacity (Donadio 2020; Sawyer 2020).
Up to and including six months' active intervention
Donadio 2020 (n = 25) reported an increase in VO2 (% of max) at the anaerobic threshold within the group that was assigned to supervised resistance exercise with neuromuscular electrical stimulation (n = 6) and a decrease in VO2 (% of max) for the control group (n = 11). The mean values for VO2 (% of max) at baseline and end of treatment were 59.6 (SD 14.9) versus 68.9 (SD 10.8) (P = 0.05) in the group receiving exercise plus neuromuscular electrical stimulation, and 71.8 (SD 12.3) versus 62.1 (SD 11.6) (P = 0.01) in the control group. The paper did not provide the MD between groups and there was no information for the third group, which received supervised resistance training alone (n = 8) (Donadio 2020).
Sawyer 2020, in which the intervention group performed high‐intensity interval training, found improved Tlim during a constant work submaximal cycling test in the exercise compared to the control group after two months (MD 211.00 seconds, 95% CI 93.40 to 328.60; Analysis 1.22). However, there were no differences in VO2 (mL/min per kg bodyweight) or VO2 (% predicted) during a constant work submaximal exercise test on a cycle ergometer (MD 1.01 mL/min per kg bodyweight, 95% CI ‒0.89 to 2.91; MD 3.00% predicted, 95% CI ‒0.92 to 6.92; Analysis 1.23).
1.22. Analysis.

Comparison 1: Physical activity versus control, Outcome 22: Change in time to symptom limitation (Tlim in sec) during constant work submaximal exercise
1.23. Analysis.

Comparison 1: Physical activity versus control, Outcome 23: Change in VO2 (mL/min per kg bodyweight and % predicted) during constant work submaximal exercise
c. Functional exercise capacity
Two studies reported on changes in 6MWT distance (Del Corral 2018; Rovedder 2014). Three studies reported changes in MSWT performance (Del Corral 2018; Douglas 2015; Güngör 2021).
Up to and including six months' active intervention
In a combined analysis of two studies (Del Corral 2018; Rovedder 2014), 6MWT distance was higher in the intervention compared to the control groups (MD 25.32 m, 95% CI 11.56 to 39.08; n = 81; Analysis 1.24).
1.24. Analysis.

Comparison 1: Physical activity versus control, Outcome 24: Change in 6MWT distance (m)
In Del Corral 2018, the physical activity group had better performance in the MSWT compared to controls (MD 78.45 m, 95% CI 18.18 to 138.72; n = 40; Analysis 1.25).
1.25. Analysis.

Comparison 1: Physical activity versus control, Outcome 25: Change in modified shuttle walk distance (m)
Güngör 2021 reported no between‐group differences in MSWT performance after six weeks (median: intervention group: 990 (IQR 377.5) m; n = 10; control group: 760 (IQR 830) m; n = 9) and after six months (median: intervention group; 1235 (IQR 365) m; n = 10; control group: 960 (IQR 705) m; n = 10).
Over six months' active intervention
Two studies reported on changes in MSWT (Del Corral 2018; Douglas 2015); one study reported on changes in 6MWT distance (Del Corral 2018).
After 12 months, Del Corral 2018 found no difference between groups in the changes in 6MWT distance (MD ‒3.17 m, 95% CI ‒35.27 to 28.93; Analysis 1.24).
In a combined analysis of two studies, MSWT was higher in the intervention compared to the control group (MD 131.91 m, 95% CI 79.60 to 184.22; n = 107; Analysis 1.25).
2. Quadriceps muscle strength
One study reported quadriceps muscle strength (Selvadurai 2002).
a. Isometric muscle strength
No study reported this outcome.
b. Isokinetic muscle strength
Up to and including six months' active intervention
One study reported isokinetic muscle strength (Selvadurai 2002).
In Selvadurai 2002, the intervention group (n = 44) had higher quadriceps muscle strength compared to the control group (n = 22) at hospital discharge (MD 16.38 newton‐metre (N.m), 95% CI 12.34 to 20.42; n = 66; Analysis 1.26).
1.26. Analysis.

Comparison 1: Physical activity versus control, Outcome 26: Change in quadriceps muscle strength (Nm)
Follow‐up (no active intervention)
After one‐month follow‐up, Selvadurai 2002 found higher quadriceps muscle strength in the intervention group (n = 44) compared to control group (n = 22) (MD 12.68 N.m, 95% CI 8.88 to 16.48; n = 66; Analysis 1.26).
3. Lung function: forced vital capacity
Fifteen studies reported changes in FVC (Beaudoin 2017; Carr 2018; Cerny 1989; Güngör 2021; Gupta 2019; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Klijn 2004; Kriemler 2013; Moorcroft 2004; Rovedder 2014; Sawyer 2020; Schneiderman‐Walker 2000; Selvadurai 2002).
Up to and including six months' active intervention
Twelve studies FVC at up to and including six months' active intervention (Beaudoin 2017; Carr 2018; Cerny 1989; Güngör 2021; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Klijn 2004; Kriemler 2013; Rovedder 2014; Sawyer 2020; Selvadurai 2002).
In a combined analysis of eight studies, FVC % predicted there was no difference between groups, but between‐study heterogeneity was substantial (MD 1.70% predicted, 95% CI ‒1.95 to 5.35; I² = 80; n = 357; Analysis 1.27) (Beaudoin 2017; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Kriemler 2013; Rovedder 2014; Sawyer 2020; Selvadurai 2002). This was partly explained by issues with the control group in Kriemler 2013.
1.27. Analysis.

Comparison 1: Physical activity versus control, Outcome 27: Change in FVC (% predicted)
Güngör 2021 reported no between‐group differences in FVC % predicted after six weeks (median: intervention group 94 (IQR 19) % predicted; n = 10; control group 75.5 (IQR 54) % predicted; n = 9).
The remaining three studies reported this outcome, but did not provide data. Cerny 1989 reported that there was no difference in the change in FVC % predicted among groups; Klijn 2004 reported that there were no significant differences between groups in lung function parameters; and Carr 2018 reported no differences in FVC between groups after three months.
Over six months' active intervention
Five studies reported FVC % predicted at over six months' active intervention (Gupta 2019; Hebestreit 2010; Hebestreit 2022; Kriemler 2013; Schneiderman‐Walker 2000), and one study reported on FVC in mL (Moorcroft 2004).
A combined analysis of five studies lasting between 12 months and three years revealed a beneficial effect of physical activity on FVC (MD 2.51% predicted, 95% CI 0.24 to 4.78; n = 199; Analysis 1.27). However, the effect was overestimated due to inclusion of Kriemler 2013, in which the control group experienced a deterioration of lung health. Exclusion of this study changed the effect estimate towards null and reduced between‐study variability substantially.
In Moorcroft 2004, the intervention group had better FVC compared to the control group after 12 months (MD 213.00 mL, 95% CI 3.01 to 422.99; Analysis 1.28).
1.28. Analysis.

Comparison 1: Physical activity versus control, Outcome 28: Change in FVC (mL)
Follow‐up (no active intervention)
Three studies reported FVC % predicted at follow‐up (Hebestreit 2010; Kriemler 2013; Selvadurai 2002). A combined analysis revealed no between‐group difference in FVC following a period 'off training' of between one and 12 months, but between‐study heterogeneity was substantial (MD 5.37% predicted, 95% CI ‒1.69 to 12.43; I² = 82%; n = 125; Analysis 1.27).
Güngör 2021 reported no between‐group differences in median FVC % predicted after six months' follow‐up (intervention group: 93.5 (IQR 14) % predicted; n = 10; control group: 95 (IQR 41.5) % predicted; n = 9).
4. Physical activity
Five studies reported on physical activity, either subjectively measured (self‐reported) or objectively measured (Beaudoin 2017; Hebestreit 2010; Hebestreit 2022; Klijn 2004; Kriemler 2013).
a. Subjectively measured
Up to and including six months' active intervention
In a pooled analysis of two studies (Hebestreit 2010; Hebestreit 2022), self‐reported vigorous physical activity was higher in the physical activity intervention compared to the control groups after six months (MD 1.36 hours per week, 95% CI 0.86 to 1.86; n = 152; Analysis 1.32).
1.32. Analysis.

Comparison 1: Physical activity versus control, Outcome 32: Change in self‐reported vigorous physical activity (hours per week)
Klijn 2004 reported for a subgroup of participants (anaerobic group n = 18; controls n = 16) who completed an activity diary; there were no between‐group differences.
Over six months' active intervention
In a pooled analysis of two studies (Hebestreit 2010; Hebestreit 2022), self‐reported vigorous physical activity was higher in the intervention compared to the control groups after 12 months (MD 1.71 hours per week, 95% CI 1.13 to 2.29; n = 148; Analysis 1.32).
Follow‐up (no active intervention)
Hebestreit 2010 found higher self‐reported vigorous physical activity in the intervention group compared to the control group after six to 12 months' follow‐up (MD 1.63 hours per week, 95% CI 0.02 to 3.24; n = 18; Analysis 1.32).
b. Objectively measured
Up to and including six months' active intervention
Beaudoin 2017 reported no differences in the number of daily steps between the intervention and control groups after three months (MD ‒110.58 steps per day, 95% CI ‒2260.72 to 2037.56; n = 14; Analysis 1.29). After six months, Hebestreit 2022 also found no between‐group differences in the total daily number of steps (MD 584.00 steps per day, 95% CI ‒417.10 to 1585.10; 105 participants; Analysis 1.29) and 'aerobic steps' (i.e. steps that were counted when walking more than 60 steps per minute and more than 10 minutes continuously) (MD 330.00 steps per day, 95% CI ‒195.50 to 855.50; 101 participants; Analysis 1.30). We did not combine data on the number of daily steps from Beaudoin 2017 and Hebestreit 2022. These studies used different devices that were either worn around the hip or around the upper arm, and sensor placement is known to have a substantial impact on the number of daily steps.
1.29. Analysis.

Comparison 1: Physical activity versus control, Outcome 29: Change in objectively measured physical activity (steps per day)
1.30. Analysis.

Comparison 1: Physical activity versus control, Outcome 30: Change in objectively measured physical activity (aerobic steps per day)
Kriemler 2013 reported on objectively measured change in hours of moderate‐to‐vigorous physical activity per week and found no differences between the intervention (n = 25) and the control group (n = 9) after six months (MD ‒0.20 hours, 95% CI ‒1.38 to 1.78; Analysis 1.31).
1.31. Analysis.

Comparison 1: Physical activity versus control, Outcome 31: Change in objectively measured moderate‐to‐vigorous physical activity (hours per week)
Klijn 2004 reported for a subgroup of participants (anaerobic group n = 18; controls n = 16) who wore an activity accelerometer; there were no between‐group differences.
Over six months' active intervention
Two studies reported objectively measured physical activity at over six months' active intervention (Hebestreit 2022; Kriemler 2013).
Hebestreit 2022 found no between‐group differences in the daily number of steps between the physical activity and control group after 12 months (MD 806.00 steps per day, 95% CI ‒27.10 to 1639.10; n = 105; Analysis 1.29). In addition, there was no difference in total number of daily aerobic steps between groups after 12 months (MD 561.00, 95% CI 191.57 to 930.43; Analysis 1.30).
After 12 months, Kriemler 2013 found no differences in the time spent in moderate‐to‐vigorous physical activity undertaken each week for the intervention group compared to the control group (MD ‒0.14 hours per week, 95% CI ‒1.56 to 1.28; n = 32; Analysis 1.31).
Follow‐up (no active intervention)
Kriemler 2013 found no between‐group differences in the time spent in moderate‐to‐vigorous physical activity undertaken per week after 12 months 'off training' (MD 1.16 hours per week, 95% CI ‒0.57 to 2.89; n = 27; Analysis 1.31).
5. Body mass index
Seven studies reported changes in BMI (Beaudoin 2017; Del Corral 2018; Hebestreit 2010; Hebestreit 2022; Hommerding 2015; Kriemler 2013; Moorcroft 2004).
Up to and including six months' active intervention
In a pooled analysis of four studies (Beaudoin 2017; Hebestreit 2010; Hebestreit 2022; Kriemler 2013), there was no difference in BMI between the intervention and control groups (MD 0.02 kg/m²,95% CI ‒0.16 to 0.20; n = 203; Analysis 1.33). Hommerding 2015 reported no differences in BMI z‐scores between groups after three months (MD 0.10, 95% CI ‒0.16 to 0.36; n = 34; Analysis 1.34).
1.33. Analysis.

Comparison 1: Physical activity versus control, Outcome 33: Change in BMI (kg/m²)
1.34. Analysis.

Comparison 1: Physical activity versus control, Outcome 34: Change in BMI (z‐score)
Over six months' active intervention
In a pooled analysis of three studies (Hebestreit 2010; Hebestreit 2022; Moorcroft 2004), there were no differences in BMI between the intervention and control groups (MD 0.29 kg/m², 95% CI ‒0.04 to 0.62; n = 191; Analysis 1.33).
Follow‐up (no active intervention)
A pooled analysis of two studies (Hebestreit 2010; Kriemler 2013) revealed no between‐group differences in BMI after a period of six to 12 months' follow‐up (MD 0.61 kg/m², 95% CI ‒0.03 to 1.26; n = 60; Analysis 1.33).
6. Pulmonary exacerbations
One international multicentre study reported pulmonary exacerbations (Hebestreit 2022).
Up to and including six months' active intervention
In Hebestreit 2022, the number of pulmonary exacerbations, based on a mixed Poisson regression model, was not different between the physical activity and control group at the end of six months' partially supervised active intervention (incidence rate ratio 1.07, 95% CI 0.60 to 1.90; n = 117; Analysis 1.35). The time to first pulmonary exacerbation was calculated covering the entire study period of 12 months and is reported below.
1.35. Analysis.
Comparison 1: Physical activity versus control, Outcome 35: Number of pulmonary exacerbations
| Number of pulmonary exacerbations | |||||
| Study | Physical activity (n) | Control (n) | Incidence rate ratio | 95% CI | P value |
| Number of exacerbations at end of 6 months' partially supervised active intervention (mixed Poisson regression model) | |||||
| Hebestreit 2022 | 27 | 23 | 1.07 | 0.60 to 1.90 | 0.83 |
| Number of exacerbations after 12 months: 6 months' partially supervised activity followed by 6 months unsupervised activity with access to study resources (mixed Poisson regression model) | |||||
| Hebestreit 2022 | 61 | 53 | 1.28 | 0.85 to 1.94 | 0.24 |
Over six months' active intervention
Hebestreit 2022 reported no between‐group differences in the number of pulmonary exacerbations during the 12‐month study period (incidence rate ratio 1.28, 95% CI 0.85 to 1.94; n = 117; Analysis 1.35). There was no difference between groups in time to first pulmonary exacerbation, covering the entire study period of 12 months (hazard ratio (HR) 1.34, 95% CI 0.65 to 2.80; Analysis 1.36). Certainty of evidence was high (Table 1).
1.36. Analysis.
Comparison 1: Physical activity versus control, Outcome 36: Time to first pulmonary exacerbation
| Time to first pulmonary exacerbation | |||
| Study | Hazard ratio | 95% CI | P value |
| Hebestreit 2022 | 1.34 | 0.65 to 2.80 | 0.43 |
7. Hospitalisation
Two studies reported hospitalisation (Hebestreit 2022; Schneiderman‐Walker 2000). The principal investigator of a multicentre study provided raw data for this outcome (Hebestreit 2022).
In Hebestreit 2022, there was no difference between the intervention and the control groups in the number of participants being hospitalised over a period of 12 months (odds ratio (OR) 0.93, 95% CI 0.42 to 2.04; n = 117; Analysis 1.37).
1.37. Analysis.

Comparison 1: Physical activity versus control, Outcome 37: Number of hospitalisations
Schneiderman‐Walker 2000 (n = 65) reported no between‐group differences for the mean number of hospitalisations or mean number of days in hospital at years one, two and three.
8. Bone health
Two studies (n = 67) reported changes in bone health after three to 12 months (Alexander 2019; Gupta 2019).
Up to and including six months' active intervention
Alexander 2019 (n = 15, published as an abstract) reported increased bone mineral content (adjusted for height and lean body mass) in the intervention group compared to the control group after 12 weeks of whole body vibration training. The authors reported only P values and no other statistical data. It is unclear if the reported effects reflect between‐group comparisons.
Over six months' active intervention
After 12 months, Gupta 2019 found no between‐group differences in whole body bone mineral density and lumbar spine bone mineral density (whole body: MD ‒0.01 g/cm², 95% CI ‒0.04 to 0.03; n = 52; Analysis 1.38; lumbar spine: MD 0.00 g/cm², 95% CI ‒0.02 to 0.02; Analysis 1.39).
1.38. Analysis.

Comparison 1: Physical activity versus control, Outcome 38: Change in whole body bone mineral density (g/cm²)
1.39. Analysis.

Comparison 1: Physical activity versus control, Outcome 39: Change in lumbar spine bone mineral density (g/cm²)
9. Diabetic control
Two studies reported on changes in diabetic control after a physical activity intervention (Beaudoin 2017; Hebestreit 2022).
Up to and including six months' active intervention
Beaudoin 2017 reported on this outcome, and the investigators provided additional raw data from the study. The outcomes measured were glycated haemoglobin (HbA1c) and the plasma glucose and insulin response to a two‐hour oral glucose tolerance test before and after three months (Beaudoin 2017).
There were no differences in the change in HbA1c between the exercise and control groups (MD ‒0.00%, 95% CI ‒0.01 to 0.00; Analysis 1.40). In our analysis, this was also true for area under the curve for plasma glucose (MD ‒5.59, 95% CI ‒13.51 to 2.33; Analysis 1.41; in the original publication the authors reported a significant within‐group improvement in this outcome for the training group) and area under the curve for plasma insulin (MD ‒20.02, 95% CI ‒52.90 to 12.85; Analysis 1.42). However, after three months, the insulin sensitivity index was significantly higher in the exercise compared to the control group (MD 0.02, 95% CI 0.00 to 0.04; Analysis 1.43).
1.40. Analysis.

Comparison 1: Physical activity versus control, Outcome 40: Change in metabolic parameters (HbA1c (%))
1.41. Analysis.

Comparison 1: Physical activity versus control, Outcome 41: Change in metabolic parameters (glucose AUC)
1.42. Analysis.

Comparison 1: Physical activity versus control, Outcome 42: Change in metabolic parameters (total insulin AUC)
1.43. Analysis.

Comparison 1: Physical activity versus control, Outcome 43: Change in metabolic parameters (insulin sensitivity index)
Beaudoin and colleagues also reported data for plasma glucose and plasma insulin at different time points during the oral glucose tolerance test (i.e. at baseline and 30, 60, 90 and 120 minutes after the oral glucose load). The authors presented these data in figures in the original publication (Beaudoin 2017). For this review, we extracted data for the time points 0, 60 and 120 minutes after the oral glucose load.
There was no difference in plasma glucose values between groups at the time points of 0 and 60 minutes (0 minutes: MD 0.44 mmol/L, 95% CI ‒0.41 to 1.28; 60 minutes: MD ‒1.86 mmol/L, 95% CI ‒4.11 to 0.40). However, there was a differences in favour of the intervention group at 120 minutes after ingestion of the glucose solution (MD ‒3.24 mmol/L, 95% CI ‒6.41 to ‒0.06; Analysis 1.44). There was no difference in plasma insulin values between groups at 0 and 120 minutes (0 minutes: MD ‒2.10 µIU/mL, 95% CI ‒5.46 to 1.26; 120 minutes: MD 2.23 µIU/mL, 95% CI ‒13.98 to 18.45). However, there was a difference in plasma insulin in favour of the intervention group 60 minutes after the ingestion of the glucose solution (MD ‒12.39 µIU/mL, 95% CI ‒22.14 to ‒2.65; Analysis 1.45).
1.44. Analysis.

Comparison 1: Physical activity versus control, Outcome 44: Change in plasma glucose (mmol/L) during an oral glucose tolerance test: end of active intervention ≤ 6 months
1.45. Analysis.

Comparison 1: Physical activity versus control, Outcome 45: Change in plasma insulin (µIU/mL) during an oral glucose tolerance test: end of active intervention ≤ 6 months
The results presented here are different from the results reported in the original Beaudoin 2017 publication. Beaudoin and colleagues reported within‐group changes for plasma glucose and plasma insulin at different time points (Figure 1 A–D in the original publication) during the oral glucose tolerance test for the intervention and control groups separately (Beaudoin 2017). The results presented here should be interpreted with caution due to the low sample size and high chance of a type II error (failing to conclude there was an effect when there actually was).
Over six months' active intervention
Hebestreit 2022 (n = 91) reported changes in blood glucose during an oral glucose tolerance test in participants without a diagnosis of CF‐related diabetes at study entry. After nine months, there were no between‐group differences in blood glucose levels at rest, 60 or 120 minutes after glucose ingestion (at rest: MD ‒0.16 mmol/L, 95% CI ‒0.44 to 0.12; 60 minutes: MD ‒0.04 mmol/L, 95% CI ‒1.11 to 1.03; 120 minutes: MD ‒0.44 mmol/L, 95% CI ‒1.43 to 0.55; Analysis 1.46). Certainty of evidence was moderate (Table 1).
1.46. Analysis.

Comparison 1: Physical activity versus control, Outcome 46: Change in blood glucose (mmol/L) during an oral glucose tolerance test: end of active intervention > 6 months
10. Adverse events
Six studies reported adverse events (Del Corral 2018; Güngör 2021; Hebestreit 2022; Kriemler 2013; Sawyer 2020; Selvadurai 2002). Hebestreit 2022 provided additional data on adverse events. In the original publication, they reported the total number of adverse events per group; here, we focused on the number of people experiencing an adverse event.
Up to and including six months' active intervention
Del Corral 2018 reported that muscle stiffness was common during or after playing active video games and that no further adverse events occurred. They provided no further details.
Güngör 2021 reported that none of the study participants complained about pain before or during the intervention, and that there were no adverse events in either group during the supervised six‐week intervention.
The high‐intensity interval training study by Sawyer 2020 reported that no minor or major adverse event occurred during the two‐month study.
In Selvadurai 2002, one participant in the "aerobic" training group injured her ankle and missed two days of training. One participant from the control group developed haemoptysis and withdrew from the study.
Over six months' active intervention
Kriemler 2013, after 12 months (six months of partially supervised and six months of unsupervised training with access to resources but excluding the follow‐up period), reported that no adverse effects (e.g. injuries, pneumothorax, asthma attacks, hypoglycaemia) occurred.
Hebestreit 2022 reported adverse and serious adverse events for the intervention and control groups during the 12‐month active intervention period (six months of partially supervised and six months of unsupervised training with access to resources). There was no difference in the number of participants experiencing an adverse event directly related to physical activity between the physical activity intervention group and the control group (OR 6.22, 95% CI 0.72 to 53.40; n =117; Analysis 1.47). There was also no difference in the number of participants experiencing a serious adverse event directly related to physical activity between the physical activity intervention group and the control group (OR 0.95, 95% CI 0.06 to 15.54; Analysis 1.47). Reported adverse events were contusion of the right foot during football (n = 1, control group), knee pain (n = 2, intervention group), asthma attack while walking uphill (n = 1, intervention group), soft tissue injury to neck after a fall from the trampoline (n = 1 intervention group), pain in the foot (n = 2, intervention group). Serious adverse events were patella dislocation during football (n = 1, intervention group) and cruciate ligament fracture during skiing (n = 1, control group).
1.47. Analysis.

Comparison 1: Physical activity versus control, Outcome 47: Adverse events and serious adverse events
Certainty of evidence based on the results of Hebestreit 2022 and Kriemler 2013 was low (Table 1).
Follow‐up (no active intervention)
Kriemler 2013 and Selvadurai 2002 reported on adverse events during their active intervention periods but not during their follow‐up periods.
Del Corral 2018 reported that muscle stiffness was common during or after playing active video games, and that no further adverse events occurred. No further details were provided, and it is unclear if this was also true for the 12‐month follow‐up period.
Güngör 2021 reported that none of the study participants complained of pain before or during the intervention and that there were no adverse events during the follow‐up period.
Discussion
Summary of main results
In this systematic review, moderate‐certainty evidence indicates that physical activity interventions of longer than six months probably have a positive effect on aerobic exercise capacity in people with CF compared to no intervention. Low‐certainty evidence suggests that physical activity interventions may have no effect on lung function (specifically FEV1) and HRQoL. The effects were similar across studies of different durations of active intervention (i.e. up to and including six months and over six months) and during follow‐up periods where all participants reverted to usual care. The results for our primary outcomes during a follow‐up period should be interpreted with caution because only three studies with varying duration of follow‐up (ranging from one to 12 months) reported on the primary outcomes of VO2 peak and FEV1.
Primary outcomes
1. Exercise capacity
We considered this outcome in terms of VO2 peak. The improvement of VO2 peak may be considered clinically relevant as physical activity interventions address low aerobic exercise capacity, which is an important risk factor and strong predictor of mortality in CF (Hebestreit 2019; Nixon 1992; Pianosi 2005). In a meta‐analysis, we found moderate‐certainty evidence for an improvement in VO2 peak (mL/min per kg bodyweight) in favour of physical activity intervention compared to no intervention, irrespective of the duration of active intervention or follow‐up. The MD for the improvement in VO2 peak was approximately 2.10 mL/min per kg bodyweight for studies with an active intervention lasting up to and including six months; 1.60 mL/min per kg bodyweight for longer than six months; and 3.27 mL/min per kg bodyweight in studies after follow‐up (Analysis 1.1). Nevertheless, the effects were not consistent across all studies, and between‐study heterogeneity was substantial for studies with an active intervention lasting up to and including six months and over six months. The magnitude of improvement in VO2 peak was rather small but may still be considered clinically relevant (Saynor 2013; Wilkinson 2019); however, robust estimates for a meaningful change in VO2 peak over time in people with CF are yet to be determined. One study in adults with chronic kidney disease estimated a change in VO2 peak of 1.5 mL/min per kg bodyweight as clinically relevant (Wilkinson 2019). Pianosi and colleagues reported an annual decline in VO2 peak of 1.9 mL/min per kg bodyweight in children and adolescents with CF over five years (Pianosi 2005). In another longitudinal study in adolescents with CF, there was a mean annual decline in VO2 peak of 3.23% predicted (van de Weert‐Van Leeuwen 2012). Given the fact that VO2 peak is an independent predictor of mortality in CF (Hebestreit 2019; Nixon 1992; Pianosi 2005), regular physical activity (including structured exercise) should be promoted to maintain the highest possible aerobic fitness (VO2 peak).
2. Lung function: forced expiratory volume in one second
We found low‐certainty evidence that a physical activity intervention compared to control has little or no effect on FEV1 % predicted (MD 2.41 % predicted, 95% CI ‒0.49 to 5.31). These findings were based on a combined analysis of six studies (n = 367) with moderate to substantial between‐study heterogeneity (Analysis 1.6). In a sensitivity analysis of five studies lasting 12 to 24 months, FEV1 % predicted was higher in the physical activity intervention group compared to the control group (MD 1.71 % predicted, 95% CI 0.15 to 3.26; n = 333). This is in line with observational studies showing a slower annual rate of decline in FEV1 over time in physically active versus less active children and adults with CF (Cox 2016; Cox 2018; Elce 2018; Schneiderman 2014). It may well be the case that a physical activity programme needs to be performed over longer time periods to observe beneficial effects on lung function.
The largest international multicentre study to date (ACTIVATE‐CF; n = 117) provided data on the effects of vigorous physical activity, which we included in a meta‐analysis; results showed a beneficial effect in favour of the control group after six months (Hebestreit 2022). The effect was predominantly driven by an improvement in FEV1 in the control group, while FEV1 remained relatively unchanged in the intervention group. It was postulated that control group participants also started to do more physical activity, although less intensively (Hebestreit 2022). Interestingly, in a sensitivity analysis of six studies (excluding Kriemler 2013 and Selvadurai 2002) with physical activity interventions lasting up to and including six months, the MD in FEV1 between the intervention and control groups was ‒2.16 % predicted (95% CI ‒4.14 to ‒0.17; n = 255; Analysis 1.7). We can only speculate on the underlying reasons for these counterintuitive results. It may be that participants in the physical activity group perceived the intervention as an added stress on top of an already high treatment burden (Davies 2020), or that the interventions were too intensive with inadequate recovery time. It may also be the case that the physical activity programme induced inflammatory responses or increased infection susceptibility, or a combination of these (van de Weert‐Van Leeuwen 2013). Further studies are needed to better understand the immunological and physiological adaptations in response to repetitive bouts of acute physical activity on lung function in people with CF. This includes studying dose–response relationships on the effects of regular physical activity on lung function.
3. Health‐related quality of life
This review found that physical activity may make little or no difference to HRQoL measured with the CFQ‐R, which is a validated and responsive instrument to assess changes in HRQoL in people with CF (low‐certainty evidence) (Quittner 2009). However, the analysis was limited to four studies with physical activity interventions lasting longer than six months.
Secondary outcomes
With regard to the review's secondary outcomes presented in Table 1, one study with an active intervention period lasting longer than six months reported data on the number of pulmonary exacerbations and diabetic control (Hebestreit 2022), and two studies with active interventions lasting longer than six months reported data on adverse events (Hebestreit 2022; Kriemler 2013). In the multicentre Hebestreit 2022 study (n = 117), there were no differences in the number of pulmonary exacerbations between the physical activity and control group after 12 months (incidence rate ratio was 1.28, 95% CI 0.85 to 1.94; high‐certainty evidence; Table 1). This study also found no between‐group differences in diabetic control after nine months (moderate‐certainty evidence) (Table 1). During the 12‐month active intervention period in Kriemler 2013, investigators reported no adverse events in either the physical activity or control group, while low‐certainty evidence from Hebestreit 2022 suggests physical activity may or may not make a difference in the number of participants experiencing an adverse event (OR 6.22, 95% CI 0.72 to 53.40) or serious adverse event (OR 0.95, 95% CI 0.06 to 15.54) related to the intervention. Future studies are likely to change our confidence on the impact of regular physical activity and exercise on adverse events in people with CF. In Hebestreit 2022 the odds of experiencing an adverse event was six times higher in the intervention compared to the control group, but the lower CI was relatively close to one (statistically, if the CI crosses one, this implies there is no difference between arms of the study).
Overall completeness and applicability of evidence
In this review update, we included nine new studies, which almost doubled the number of included participants in comparison to the previous version (Radtke 2017). For the first time, we were able to perform meta‐analyses for the primary outcomes VO2 peak, FEV1 and HRQoL.
The studies included in this review were heterogeneous in terms of study quality, selection of study participants, sample size and intervention duration. The studies recruited mixed populations with regard to age, gender and disease severity, and may have some applicability to the general population of people living with CF. One important issue is participant selection based on predefined inclusion and exclusion criteria in the original studies. In a large number of the included full‐text articles (where inclusion and exclusion criteria were reported), participants were excluded based on disease severity expressed by FEV1, which is one of our primary outcome measures. We acknowledge that study investigators are ethically bound to keep potential exercise‐induced adverse reactions to a minimum; however, this limits the generalisability of the review's findings to people with mild‐to‐moderate CF lung disease. Moreover, it is important to note that most studies were conducted before the widespread availability of highly effective CFTR modulator therapies (Middleton 2019; Wainwright 2015), which clearly limits the generalisability of our findings to the current population of people living with CF. Moreover, this review includes a substantial number of small studies, known to overestimate effects of interventions compared to larger trials (Ioannidis 1998).
We choose aerobic exercise capacity (VO2 peak) and lung function (FEV1) as objectively measurable outcomes, and HRQoL as an important patient‐reported outcome. Overall out of 24 studies, 11 reported on changes in VO2 peak, 16 reported on changes in FEV1 and eight reported on changes in HRQoL. It may well be that the studies included in the meta‐analysis represented a selection of studies that did not cover the full spectrum of possible intervention effects that would have been observed if more of the included studies reported these outcomes.
With regard to changes in lung function, the sensitivity of FEV1 to detect change in response to a physical activity intervention needs to be discussed. This is particularly important in times of highly effective CFTR modulators (Middleton 2019), and in a population in which lung function is better than ever (Stanojevic 2016). There may be measurement methods more sensitive than FEV1 to document subtle, but clinically relevant, effects of regular physical activity on pulmonary function (Stanojevic 2016).
Since only a third of included studies reported on changes in HRQoL, the current evidence on the effects of physical activity interventions on changes in HRQoL is limited.
Quality of the evidence
Overall, there is moderate‐certainty evidence that physical activity interventions of longer than six months have a beneficial effect on VO2 peak, while there is low‐certainty evidence that physical activity interventions have no positive effect on FEV1 and HRQoL. Moreover, with regard to secondary outcomes, there were no differences in the risk for pulmonary exacerbations (high‐certainty evidence), change in diabetic control (blood glucose levels) (moderate‐certainty evidence) and adverse events (low‐certainty evidence) between physical activity interventions and usual care (no physical activity).
In general, several studies included in this review showed considerable methodological shortcomings based on the Cochrane risk of bias tool used to assess them (Higgins 2017). This may also reflect the inappropriate methodology of the current literature (i.e. insufficient power), in general. Most studies had small sample sizes, which puts them at risk of imprecision and lack of power, which can work in two ways: that is, underestimation or overestimation of intervention effects (Ellis 2010). This phenomenon might be explained by publication bias, as small studies are less likely be published if they present negative results (Hopewell 2009). Moreover, there were differences in baseline characteristics in five small studies (Cerny 1989; Del Corral 2018; Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014). Although testing for between‐group differences is not recommended in RCTs, some authors reported differences in participant characteristics at baseline. It is important to note that none of the studies reporting between‐group differences in baseline characteristics contributed data for the pooled analysis of the primary outcomes, which could have introduced bias.
In addition to interindividual differences in the course of CF lung disease and differences among studies in type, duration, level of supervision and implementation of the intervention, methodological differences in outcome measures may have contributed to the observed between‐study heterogeneity in the primary outcomes VO2 peak, FEV1 and HRQoL. Among the studies in the pooled analysis investigating change in VO2 peak, nine studies used a cycle ergometer, and three studies used a treadmill as testing modality. Different testing modalities (treadmill testing elicits higher VO2 peak values compared to cycle ergometry), testing protocols (e.g. ramp protocol versus minute‐by‐minute protocol versus three‐minute stages protocol), type of metabolic carts, and quality control procedures (e.g. calibration and verification methods, criteria to define a maximal effort) have likely contributed to the observed variability. Similar methodological challenges were recently addressed in an RCT investigating the effects of CFTR modulator treatment on VO2 peak (primary outcome) in children and adolescents with CF (Wilson 2021). This study highlighted several methodological challenges using cardiopulmonary exercise testing in a multicentre setting. However, several of those challenges can be addressed at design stage; for example, by implementing standard operating procedures and by using study‐specific equipment. The latter is often not possible in investigator‐initiated trials due to limited financial resources. Further, although the measurement of FEV1 is less complex than the measurement of VO2 peak, and all people with CF are used to performing spirometry measurements early in their lives, the same quality and standardisation principles apply. We noticed moderate‐to‐substantial between‐study heterogeneity in FEV1 that may partly be explained by several of the aforementioned factors, as well as by pretesting conditions, including withdrawal of beta 2‐mimetics or not. In addition, for this review update, we restricted HRQoL measurements with the CFQ‐R questionnaire to respiratory symptoms and the physical function domain of the CFQ‐R questionnaire. The pooled analysis revealed no effect of physical activity (versus usual care) on changes in respiratory symptoms and physical functioning. Between‐study heterogeneity was small to substantial, and the CIs of the effect estimates were wide. The large variability might be partly explained by inclusion of different groups of study participants (i.e. children, adolescents, adults), time and mode of administration, possible differences in languages and versions, few studies in the pooled analysis, and different study durations of 12 and 24 months covering a large observational period during which lung disease can substantially deteriorate.
Further research will likely have an important impact on our confidence in the estimate of effects of physical activity versus no physical activity intervention on the primary outcomes VO2 peak, FEV1 and HRQoL, and is (very) likely to change those estimates. We downgraded the certainty of evidence for studies with active interventions lasting longer than six months due to unclear or high risk of bias across several domains; in particular, due to concerns around randomisation and allocation concealment, and an outlying study.
A limited number of studies reported on secondary outcomes, such as bone health and diabetic control, suggesting that additional research will very likely add to the existing evidence and very likely change the estimate of effects. In general, a lack of efficacy does not necessarily mean that the intervention was ineffective: especially in longer‐term studies, poor adherence to physical activity, which requires precise monitoring, could be a reason for lack of intervention effects. Although the included studies used standard outcome measures to assess efficacy of physical activity and exercise training, robust estimates for a minimal clinically important difference of these outcome measures are often not available, thus limiting the interpretation of the magnitude of observed effects (e.g. VO2 peak).
RCTs are a powerful study design to assess interventional efficacy, assuming high‐quality standards for the randomisation and allocation concealment process aim to minimise confounding and selection bias. In this review, only nine studies had a low risk of bias due to clearly describing their randomisation procedures and six studies a low risk of bias for describing their allocation concealment. Two studies included in the meta‐analysis had a high risk of bias in both domains, leading us to downgrade the certainty of evidence. Research has shown that inadequate or unclear allocation concealment – compared to adequately reported concealment of random allocation – is associated with larger effects, introducing bias (Schulz 1995). The extent to which lack of methodological rigour has had an impact on our effects estimates is difficult to ascertain; the possibility cannot be ruled out.
In summary, this review includes a substantial number of small studies of low to moderate quality and predominantly unclear risk of bias.
Potential biases in the review process
Despite extensive searches, it is theoretically possible that we failed to identify studies. However, since the field of researchers publishing on physical activity interventions in CF is relatively small and close‐knit, we are quite confident that we did not miss any potentially relevant studies.
Two authors of this review (HH and SK) were Principal Investigators of three included studies (Hebestreit 2010; Hebestreit 2022; Kriemler 2013). Moreover, one review author (HH) was the Principal Investigator of the ACTIVATE‐CF trial, and two other authors (SK, TR) were core team members of the study (Hebestreit 2022). It is important to note that other review authors (SJN, SS) who were not members of the ACTIVATE‐CF study team performed the risk of bias assessment and data extraction for those studies.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, there are no other published systematic reviews on physical activity and exercise training interventions versus usual care in people with CF; in particular, reviews with a focus on RCTs.
Two systematic reviews focused on subjectively reported or objectively measured physical activity levels (or a combination of both) in children and adolescents with CF (Puppo 2020), or adults with CF (Shelley 2019). Both reviews included different types of study designs and were not restricted to RCTs.
We are aware of a study protocol for a systematic literature review that aims to summarise the effects of RCTs comparing physical activity and exercise interventions versus usual care, and which will assess fitness, physical activity, lung health, inflammation, body composition, glycaemic control, patient‐reported outcomes, adverse events and healthcare utilisation (Tomlinson 2021). In the study protocol, we noticed overlap with outcomes included in this review, but the planned review by Tomlinson and colleagues may extend the findings of this review, as the authors planned to include additional outcomes, such as inflammation and healthcare utilisation (Tomlinson 2021).
For the first time, since the original review in 2002 (Bradley 2002), we were able to combine data and perform a meta‐analysis. Despite a larger number of studies included in the current version of the review, the conclusions have not substantially changed compared to previous versions (Bradley 2002; Bradley 2008; Radtke 2015; Radtke 2017), but we extended our knowledge on clinically relevant and patient‐centred outcomes. Moreover, our certainty in the beneficial effects of regular physical activity and exercise training on aerobic exercise capacity has strengthened, while there were no beneficial effects on lung function and HRQoL (Bradley 2002; Bradley 2008; Radtke 2015; Radtke 2017).
Authors' conclusions
Implications for practice.
This review found moderate‐certainty evidence that physical activity interventions probably improve aerobic exercise capacity in people with cystic fibrosis (CF). From the evidence we have identified, physical activity interventions may have little or no effect on lung function or health‐related quality of life (HRQoL) at any time point (low‐certainty evidence). Between‐study heterogeneity ranged from low to substantial for the primary outcomes. Most studies included in this review are limited by their small size, insufficient duration and incomplete reporting.
Overall, the benefits obtained from including physical activity in a package of care may be influenced by the type and duration of training programme. Physical activity is already part of the regular care offered to most people with CF and there is no evidence to actively discourage this.
Implications for research.
Further research is needed to comprehensively assess the benefits of physical activity programmes in people with CF. There is a clear need for high‐quality studies with sufficient numbers of study participants and well‐chosen, objectively measurable, reproducible and sensitive primary outcome measures. Unfortunately, a substantial number of ongoing studies and those listed as awaiting classification in this review are of short duration and include a small sample size. We would argue that further small studies of short duration are unlikely to make a meaningful contribution, and we call for greater collaboration in designing studies in order to advance the field.
Below, we suggest how future study designs could be improved, including in terms of outcomes. Future well‐designed and well‐executed studies are very likely to change our confidence in the estimates for several outcomes included in this review.
The conduct of physical activity trials in a rare disease such as CF is extremely challenging for several reasons. First, participation rates in physical activity studies are often substantially lower than expected (Hebestreit 2022; Sawyer 2020), and researchers in the field should be encouraged to form study collaborations to achieve meaningful participation rates and statistical power. Second, contamination of control groups is a common issue in randomised controlled trials (RCTs), and could be overcome by offering control group participants an attractive alternative programme unrelated to the intervention, in order to avoid contamination and excess dropout rates. Third, participation rates and adherence to physical activity interventions have been shown to be suboptimal (Douglas 2015), and could potentially be improved by considering individual facilitators and barriers towards physical activity in order to build positive, long‐term physical activity behaviour (Gruet 2022). Fourth, training components (type, intensity, duration and frequency of physical activity) should be sufficient to elicit beneficial adaptations, but should also be tailored to an individual. In that context, a progressive increase in volume and intensity of physical activity over time should be considered and adapted to the individual participant. Finally, future studies should focus on clinically relevant outcomes such as bone health, diabetic control, exacerbations and HRQoL. Using forced expiratory volume in one second (FEV1) as a marker of lung disease severity may lack sensitivity to detect any changes within a given study period. Additional lung function parameters, such as the Lung Clearance Index, which is based on multiple washout techniques, might be considered.
Life expectancy for people with CF has substantially increased over recent decades (MacKenzie 2014), and new drug therapies have had a huge impact on the clinical course of people living with CF (Middleton 2019; Wainwright 2015). Changing demographics and increased life expectancy impact the clinical course of young people with CF, who are healthier than ever (Burgel 2015). This offers new opportunities for physical activity and exercise. It is expected that physical activity and exercise will gain more attention because of the health effects of cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies (e.g. substantially reduced sputum production), now available for the vast majority of people living with CF. A substantial proportion of people with CF already use exercise as a supplement to traditional chest physiotherapy (Rowbotham 2020), and one of the top 10 research priorities in CF lung disease is to investigate the effectiveness of exercise as a replacement airway clearance technique (Rowbotham 2018). Conversely, people with CF are getting older and experience multiple comorbidities, which may affect their physical activity levels and their ability to take part in structured physical activity programmes. The CF and exercise community should be open to developing and testing new training strategies to optimise the outcomes of physical activity interventions aimed at building positive long‐term physical activity behaviour (Gruet 2022). Future interventions should be targeted towards groups of individuals (e.g. children versus adults, mild versus severe CF lung disease and following lung transplantation) by considering different training modalities to maximise the benefit for these specific groups (Gruet 2022). This includes studies on dose–response relationships between physical activity and exercise stimuli and changes in lung function over time. Future studies should develop their intervention to incorporate elements of behaviour change theory. Wearable technology, such as fitness trackers and step counters to measure and monitor individual physical activity levels, in combination with motivational feedback and goal setting might be a promising approach for future physical activity interventions (Curran 2020).
Of note, RCTs are a powerful study design to establish causal relationships between an exposure and outcome. However, the successful conduct of RCTs in people with a rare disease is challenging. Healthcare professionals should think of alternatives such as the design of longitudinal multicentre studies using harmonised measurement techniques to study the role of a physically active lifestyle on health‐related and patient‐centred outcome measures. In this context, assessment of and control for confounders is of critical importance and should be carefully considered at the design stage. Ideally, such an effort is made with a multidisciplinary team involving clinicians, exercise scientists, physiotherapists, epidemiologists, methodologists and people with CF to cover their needs.
Finally, study investigators should carefully select the number and type of study outcomes. A high number of outcomes requiring time‐consuming assessments may decrease participants' adherence as well as increase the risk of false‐positive results by chance. Besides selecting clinically relevant and patient‐centred outcomes, testing of the inter‐relationships of outcome measures would ascertain whether, for instance, changes in HRQoL correlate with changes in exercise capacity (Hebestreit 2014).
What's new
| Date | Event | Description |
|---|---|---|
| 21 April 2022 | New citation required and conclusions have changed | Despite a larger number of studies included in the current version of the review, the conclusions have not substantially changed compared to previous versions (Bradley 2002; Bradley 2008; Radtke 2015; Radtke 2017). Nevertheless, the current review extends our knowledge of clinically relevant and patient‐centred outcomes, including adverse events and glycaemic control. Moreover, our certainty in the beneficial effects of regular physical activity and exercise training on aerobic exercise capacity has strengthened, while there were no beneficial effects on lung function and health‐related quality of life (Bradley 2002; Bradley 2008; Radtke 2015; Radtke 2017). |
| 21 April 2022 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register identified 113 references which were potentially eligible for inclusion in this updated review. Two references (abstracts) were identified through other sources. Additional searches of online databases identified a further 505 references (clinicaltrials.gov: 369 references; the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP): 136 references). After initial screening and the removal of duplicates, we assessed 100 new references. Included studies Four new references to three unique studies have been included (Alexander 2019; Donadio 2020; Hatziagorou 2019), and there were 10 new references for five already included studies (Carr 2018; Douglas 2015; Hebestreit 2010; Hommerding 2015; Santana‐Sosa 2014). There were six references for two studies previously listed as ongoing but now included (Gupta 2019; Sawyer 2020), and one reference for a further study previously listed as ongoing under the study ID Hebestreit 2016. This study has now been included and renamed Hebestreit 2022. We found two new references for studies listed as "awaiting classification" as clinical trials registry entries, which have now been included (Del Corral 2018; Güngör 2021). There were also three new references for a further study previously listed as "awaiting classification" under the study ID Lorenc 2015. This study has now been included and renamed Carr 2018. One study, previously listed as ongoing under the study ID Donadio 2017, has been confirmed to be related to the included study Hommerding 2015 and the 2017 reference has been added to the Hommerding 2015 study ID. Excluded studies 59 references to 35 new studies have been excluded with reasons (ACTRN12620001237976; Bass 2019; Bellini 2018; Cantin 2005; Combret 2018; Combret 2021; Cox 2013; de Marchis 2017; Dwyer 2019; Gruber 1998; Hütler 2002; IRCT20161024030474N4; Kaak 2011; Kaltsakas 2021; Lang 2019; Macleod 2008; Martinez Rodriguez 2017; Montero‐Ruiz 2020; Moola 2017; NCT00129350; NCT01759342; NCT02199340; NCT03420209; NCT04888767; NTR2092; Pryor 1979; Radtke 2018b; RBR‐34677v; RBR‐5g9f6w; Reuveny 2020; Ruddy 2015; Spoletini 2020; Ward 2018; White 1997; Young 2019; Zeren 2019). There were five new additional references to already excluded studies (Dwyer 2011; Falk 1988; Lima 2014; Reix 2012; Zeren 2019). Seven studies, previously listed as awaiting classification were excluded: investigators of three studies (with one new reference to one study) informed us that no paper will be published (Happ 2013; Mandrusiak 2011; NCT00792194); the investigators of one study did not reply to our email (Oliveira 2010); and for four studies, no contact details could be found online to contact study investigators (Almajan‐Guta 2011; Housinger 2015; Johnston 2004; Phillips 2008). Ongoing studies Two new studies (Curran 2020; Monteiro 2019), and 10 trial registry records have been added to "ongoing studies" (ISRCTN92573472; NCT03273959; NCT03970369; NCT04249999; NCT04543929; NCT04683809; NCT04742049; NCT05147285; NCT05173194; NCT05239611). Studies awaiting classification There were three new references for two studies already (and still) listed as "awaiting classification" (Cox 2019; Powers 2016), and two records were added from the trials registry searches (IRCT20190407043190N1; NCT04293926). We identified three new references to a further study that was previously listed as ongoing under the ID NCT02700243; this study has been completed and was added to "awaiting classification" (Bishay 2017). Online trials registry searches A search of Clinicaltrials.gov on 4 March 2022 identified 236 study records (after removal of 129 duplicates). Of these, 191 were physical activity interventions and were disregarded without further assessment (not listed in the review); 19 records had already been identified and listed in this review; 18 records were not randomised controlled trials (i.e. observational studies with single‐group assignment); in five studies the control group was not eligible for this review ('no physical activity intervention'); and three registered studies were prematurely terminated by the investigators. A search of the WHO International Clinical Trials Registry Platform (ICTRP) on 16 March 2022 identified 136 records to 134 unique studies, none of which were new studies. In summary, in this 2022 update we have included five new studies (Alexander 2019; Del Corral 2018; Donadio 2020; Güngör 2021; Hatziagorou 2019), one additional study previously listed as "awaiting classification" (Carr 2018), and three studies previously listed as ongoing (Gupta 2019; Hebestreit 2022; Sawyer 2020). We have also excluded 35 new studies (ACTRN12620001237976; Bass 2019; Bellini 2018; Cantin 2005; Combret 2018; Combret 2021; Cox 2013; de Marchis 2017; Dwyer 2019; Gruber 1998; Happ 2013; Hütler 2002; IRCT20161024030474N4; Kaak 2011; Kaltsakas 2021; Lang 2019; Macleod 2008; Martinez Rodriguez 2017; Montero‐Ruiz 2020; Moola 2017; NCT01759342; NCT02199340; NCT03420209; NCT04888767; NTR2092; Pryor 1979; Radtke 2018b; RBR‐34677v; RBR‐5g9f6w; Reuveny 2020; Ruddy 2015; Spoletini 2020; Ward 2018; White 1997; Young 2019; Zeren 2019), added 12 new studies to "ongoing studies" (Curran 2020; ISRCTN92573472; Monteiro 2019; NCT03273959; NCT03970369; NCT04249999; NCT04543929; NCT04742049; NCT04683809; NCT05147285; NCT05173194; NCT05239611), and added three studies to "awaiting classification" (Cox 2019; IRCT20190407043190N1; NCT04293926). |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 1 November 2017 | Amended | Formatting issues resolved |
| 19 October 2017 | New citation required but conclusions have not changed | Despite the inclusion of two new studies our conclusions remain the same. |
| 19 October 2017 | New search has been performed | A search of the Cystic Fibrosis and Genetic Disorders Review Group's Cystic Fibrosis Trials Register identified 38 new references which were potentially eligible for inclusion in the review. There was one additional reference to an already included study (Schneiderman‐Walker 2000) and six additional references to five already excluded studies (Amelina 2006; del Corral Nunez‐Flores 2014; Kuys 2011; Lima 2014; Salonini 2015). Six references to two new studies has been included (Beaudoin 2017; Douglas 2015) and seven references to five new studies are listed as 'Awaiting classification' (Housinger 2015; Johnston 2004; Carr 2018a; Mandrusiak 2011; Oliveira 2010). One study with two references is ongoing (Hebestreit 2022) and a total of 16 references to 13 new studies have been excluded (Bieli 2017; Bongers 2015; Calik‐Kutukcu 2016; Chang 2015; Dwyer 2017; Giacomodonato 2015; Haynes 2016; Kriemler 2016; Ozaydin 2010; Patterson 2004; Shaw 2016; Vallier 2016; Wheatley 2015). A search of clinicaltrials.gov identified 11 additional studies. Five studies were added to 'Awaiting classification' (NCT00609050; NCT00792194; NCT02552043; NCT03100214; Powers 2016), one study was added under ongoing studies (Bishay 2017a) and five studies were excluded (NCT02277860; NCT02715921; NCT02821130; NCT03117764; NCT02875366). A search of the WHO ICTRN identified three additional studies; one is listed as awaiting classification (ACTRN12617001009303) and two have been added under ongoing studies (Donadio 2017; Gupta 2017a). From this update we have stated a minimum duration of the intervention as being at least two weeks. |
| 15 June 2015 | New citation required but conclusions have not changed | Two authors from the original review have stepped down at this update and a new team of authors have taken on the review. The title of the review has been changed from 'Physical training for cystic fibrosis' to 'Physical exercise training for cystic fibrosis' as the new team felt this better reflected the content of the review. Despite the inclusion of new studies and data in this update of the review, the conclusions remain the same. |
| 15 June 2015 | New search has been performed | A search of the Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Register identified 32 new references which were potentially eligible for inclusion in this review. Three new studies (one reference each) were included (Rovedder 2014; Santana‐Sosa 2012; Santana‐Sosa 2014). Two studies previously listed as excluded have been reassessed and moved to included studies with two new references each (although one paper referred to both studies) (Hebestreit 2010; Kriemler 2013). One study has been moved from 'Awaiting classification' to included studies with an additional two references (Hommerding 2015). One was an additional reference to an already excluded study (Kuys 2011). A total of 14 new studies (20 references) were excluded (Alarie 2012; Amelina 2006; Asher 1982; Balfour Lynn 1998; del Corral Nunez‐Flores 2014; Dwyer 2011; Gruet 2012; Lima 2014; Lowman 2012; Petrovic 2013; Rand 2012; Reix 2012; Salonini 2015; Vivodtzev 2013). One study (one reference) has been listed as 'Awaiting classification' until we are able to obtain further information (Almajan‐Guta 2011). |
| 22 May 2012 | Amended | Contact details updated. |
| 7 March 2011 | New search has been performed | A total of two new references were identified in a search of the Group's CF Trials Register. One study was excluded as it compared Nintendo Wii exercise training to an existing exercise programme and hence did not meet the inclusion criteria (Kuys 2011). The other study did meet the inclusion criteria but outlined in its abstract that recruitment was ongoing and for this reason it has been listed as an ongoing study; results will be included in the review once the study has been completed (Phillips 2008a). In addition some amendments were made to the Background in order to incorporate updated guidelines and a relevant survey. |
| 19 January 2009 | Amended | The fourth primary outcome 'mortality' was moved to Secondary outcomes in line with Cochrane Collaboration guidance to limit the number of primary outcomes to three. |
| 5 January 2009 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register did not identify any references to trials which are potentially eligible for inclusion in this review. |
| 12 November 2008 | Amended | Converted to new review format. |
| 14 November 2007 | Amended | The generic inverse variance method has been used to analyse data which were previously not able to be presented in the 'Statistical Analysis'. The 'Synopsis' has been replaced by a new 'Plain Language Summary'. |
| 14 November 2007 | New search has been performed | The search identified 11 new references. Of these, two were additional references to already excluded studies (Albinni 2004; Edlund 1986). The remaining nine studies did not fulfil the inclusion criteria; four of these studies which seemed eligible from the title, have been excluded on the basis of trial design and are listed under 'Excluded studies' (Acquino 2006; Balestri 2004; Orenstein 2004; Stanghelle 1998). The study which was previously listed as 'Awaiting assessment' has been moved to the list of excluded studies after correspondence with the study authors (Hebestreit 2003). |
| 13 November 2007 | New citation required and conclusions have changed | Substantive amendment |
| 25 May 2005 | New search has been performed | A further article has been included (Klijn 2004). The full paper of the trial by Moorcroft (Moorcroft 2004) has also been included. Following publication of this paper, the details about the published abstracts of this trial, previously listed in the 'Characteristics of included studies' table, under Dodd 1998 and Moorcroft 2000 have been listed under Moorcroft 2004. We contacted authors of trials already included in the review regarding confirmation of data and requests for additional data. Their responses have been included in section detailing the search strategy. One trial has been moved from the 'Studies awaiting assessment' section to the 'Excluded studies' section of the review (Tuzin 1998). One trial has been added to the section 'Studies awaiting assessment' section (Hebestreit 2003). The authors have been contacted and have indicated that this study is in preparation for publication. |
| 31 July 2003 | Amended | The presentation of the data in MetaView has been re‐formatted. |
| 31 July 2003 | New search has been performed | The full paper of the Selvadurai trial has now been included, previously only the abstract of this trial was included in the review (Selvadurai 2002). A further two trials added to the 'Excluded studies' section of the review (Barry 2001; Kriemler 2001). |
Acknowledgements
We would like to thank Dr Judy Bradley and Dr Fidelma Moran, who stepped down at the 2015 update, for their previous contributions to the review, detailed below.
We would like to kindly thank Nikki Jahnke from the Cochrane Cystic Fibrosis and Genetics Disorders Group for her guidance and support during the process of writing this review.
We would like to kindly thank Christian Schindler who ran additional statistical analysis using data from the ACTIVATE‐CF trial to enable us to include additional raw data (Hebestreit 2022).
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed here are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Appendices
Appendix 1. Search methods – electronic searches
| Database or resource | Strategy |
| ClinicalTrials.gov | [BASIC SEARCH FORM] SEARCH 1 STATUS: All studies CONDITION OF DISEASE: cystic fibrosis OTHER TERMS: exercise training SEARCH 2 STATUS: All studies CONDITION OF DISEASE: cystic fibrosis OTHER TERMS: physical activity |
| WHO International Clinical Trials Registry Platform (ICTRP) | [BASIC SEARCH] SEARCH 1 exercise training AND cystic fibrosis SEARCH 2 physical activity AND cystic fibrosis |
Data and analyses
Comparison 1. Physical activity versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Change in VO2 peak (mL/min per kg bodyweight) | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 End of active intervention ≤ 6 months | 8 | 323 | Mean Difference (IV, Random, 95% CI) | 2.10 [0.06, 4.13] |
| 1.1.2 End of active intervention > 6 months | 6 | 348 | Mean Difference (IV, Random, 95% CI) | 1.60 [0.16, 3.05] |
| 1.1.3 Follow‐up (no active intervention) | 3 | 125 | Mean Difference (IV, Random, 95% CI) | 3.27 [1.37, 5.18] |
| 1.2 Change in VO2 peak (mL/min per kg bodyweight): sensitivity analysis | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 End of active intervention ≤ 6 months | 7 | 287 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐0.17, 2.78] |
| 1.2.2 End of active intervention > 6 months | 5 | 318 | Mean Difference (IV, Random, 95% CI) | 1.38 [0.08, 2.69] |
| 1.2.3 Follow‐up (no active intervention) | 2 | 99 | Mean Difference (IV, Random, 95% CI) | 3.21 [1.27, 5.14] |
| 1.3 Change in VO2 peak (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 End of active intervention ≤ 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.4 Change in VO2 peak (mL/min per kg bodyweight): combined subgroups | 11 | 496 | Mean Difference (IV, Random, 95% CI) | 1.52 [0.31, 2.73] |
| 1.5 Change in VO2 peak (mL/min per kg bodyweight): combined subgroups – sensitivity analysis | 10 | 466 | Mean Difference (IV, Random, 95% CI) | 1.38 [0.22, 2.55] |
| 1.6 Change in FEV1 (% predicted) | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.6.1 End of active intervention ≤ 6 months | 8 | 356 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐3.01, 5.61] |
| 1.6.2 End of active intervention > 6 months | 6 | 367 | Mean Difference (IV, Random, 95% CI) | 2.41 [‐0.49, 5.31] |
| 1.6.3 Follow‐up (no active intervention) | 3 | 128 | Mean Difference (IV, Random, 95% CI) | 5.68 [‐1.88, 13.23] |
| 1.7 Change in FEV1 (% predicted): sensitivity analysis | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.7.1 End of active intervention ≤ 6 months | 6 | 255 | Mean Difference (IV, Random, 95% CI) | ‐2.16 [‐4.14, ‐0.17] |
| 1.7.2 End of active intervention > 6 months | 5 | 333 | Mean Difference (IV, Random, 95% CI) | 1.71 [0.15, 3.26] |
| 1.7.3 Follow‐up (no active intervention) | 1 | 31 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐11.90, 11.26] |
| 1.8 Change in FEV1 (mL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.8.1 End of active intervention > 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.9 Change in FEV1 (z‐score) | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.37, 0.61] |
| 1.9.1 End of active intervention > 6 months | 1 | 67 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.37, 0.61] |
| 1.10 Change in FEV1 (% predicted): combined subgroups | 11 | 536 | Mean Difference (IV, Random, 95% CI) | 1.37 [‐0.74, 3.47] |
| 1.11 Change in FEV1 (% predicted): sensitivity analysis | 9 | 436 | Mean Difference (IV, Random, 95% CI) | 1.07 [‐0.36, 2.49] |
| 1.12 Change in HRQoL: CFQ‐R physical functioning domain | 7 | 464 | Mean Difference (IV, Random, 95% CI) | 3.57 [‐0.81, 7.95] |
| 1.12.1 End of active intervention ≤ 6 months | 6 | 217 | Mean Difference (IV, Random, 95% CI) | 4.67 [‐2.55, 11.90] |
| 1.12.2 End of active intervention > 6 months | 4 | 247 | Mean Difference (IV, Random, 95% CI) | 2.19 [‐3.42, 7.80] |
| 1.13 Change in HRQoL: CFQ‐R physical functioning domain: sensitivity analysis | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.13.1 End of active intervention ≤ 6 months | 5 | 197 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐4.05, 4.25] |
| 1.14 Change in HRQoL: CFQ‐R physical functioning domain: combined subgroups | 7 | 295 | Mean Difference (IV, Random, 95% CI) | 4.76 [‐1.09, 10.61] |
| 1.15 Change in HRQoL: CFQ‐R physical functioning domain: combined subgroups – sensitivity analysis | 6 | 275 | Mean Difference (IV, Random, 95% CI) | 2.44 [‐1.43, 6.30] |
| 1.16 Change in HRQoL: CFQ‐R respiratory symptoms | 6 | 463 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐3.50, 1.69] |
| 1.16.1 End of active intervention ≤ 6 months | 5 | 212 | Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐5.66, 1.92] |
| 1.16.2 End of active intervention > 6 months | 4 | 251 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐3.61, 3.51] |
| 1.17 Change in HRQoL: CFQ‐R respiratory symptoms: combined subgroups | 6 | 279 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐3.15, 3.58] |
| 1.18 Change in HRQoL: Quality of Well‐Being scale | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.18.1 Follow‐up (no active intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.19 Change in peak work capacity (W/kg bodyweight) during maximal exercise | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.19.1 End of active intervention ≤ 6 months | 3 | 164 | Mean Difference (IV, Random, 95% CI) | 0.32 [0.12, 0.51] |
| 1.19.2 End of active intervention > 6 months | 3 | 155 | Mean Difference (IV, Random, 95% CI) | 0.18 [0.07, 0.29] |
| 1.19.3 Follow‐up (no active intervention) | 2 | 51 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.03, 0.56] |
| 1.20 Change in peak work capacity (W) during maximal exercise | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.20.1 End of active intervention ≤ 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.21 Change in peak work capacity (% predicted) during maximal exercise | 3 | 285 | Mean Difference (IV, Random, 95% CI) | 5.12 [1.63, 8.61] |
| 1.21.1 End of active intervention ≤ 6 months | 2 | 117 | Mean Difference (IV, Random, 95% CI) | 6.89 [3.94, 9.83] |
| 1.21.2 End of active intervention > 6 months | 2 | 168 | Mean Difference (IV, Random, 95% CI) | 3.59 [‐2.06, 9.24] |
| 1.22 Change in time to symptom limitation (Tlim in sec) during constant work submaximal exercise | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.22.1 End of active intervention ≤ 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.23 Change in VO2 (mL/min per kg bodyweight and % predicted) during constant work submaximal exercise | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.23.1 End of active intervention ≤ 6 months ‐ VO2 peakexpressed as mL/min per kg bodyweight | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.23.2 Active intervention ≤ 6 months ‐ VO2 peakexpressed as % predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.24 Change in 6MWT distance (m) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.24.1 End of active intervention ≤ 6 months | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | 25.32 [11.56, 39.08] |
| 1.24.2 End of active intervention > 6 months | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐3.17 [‐35.27, 28.93] |
| 1.25 Change in modified shuttle walk distance (m) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.25.1 End of active intervention ≤ 6 months | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 78.45 [18.18, 138.72] |
| 1.25.2 End of active intervention > 6 months | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | 131.91 [79.60, 184.22] |
| 1.26 Change in quadriceps muscle strength (Nm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.26.1 End of active intervention ≤ 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.26.2 Follow‐up (no active intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.27 Change in FVC (% predicted) | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.27.1 End of active intervention ≤ 6 months | 8 | 357 | Mean Difference (IV, Random, 95% CI) | 1.70 [‐1.95, 5.35] |
| 1.27.2 End of active intervention > 6 months | 5 | 299 | Mean Difference (IV, Random, 95% CI) | 2.51 [0.24, 4.78] |
| 1.27.3 Follow‐up (no active intervention) | 3 | 125 | Mean Difference (IV, Random, 95% CI) | 5.37 [‐1.69, 12.43] |
| 1.28 Change in FVC (mL) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.28.1 End of active intervention > 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.29 Change in objectively measured physical activity (steps per day) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.29.1 End of active intervention ≤ 6 months | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.29.2 End of active intervention > 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.30 Change in objectively measured physical activity (aerobic steps per day) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.30.1 End of active intervention ≤ 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.30.2 End of active intervention > 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.31 Change in objectively measured moderate‐to‐vigorous physical activity (hours per week) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.31.1 End of active intervention ≤ 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.31.2 End of active intervention > 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.31.3 Follow‐up (no active intervention) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.32 Change in self‐reported vigorous physical activity (hours per week) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.32.1 End of active intervention ≤ 6 months | 2 | 152 | Mean Difference (IV, Fixed, 95% CI) | 1.36 [0.86, 1.86] |
| 1.32.2 End of active intervention > 6 months | 2 | 148 | Mean Difference (IV, Fixed, 95% CI) | 1.71 [1.13, 2.29] |
| 1.32.3 Follow‐up (no active intervention) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 1.63 [0.02, 3.24] |
| 1.33 Change in BMI (kg/m²) | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.33.1 End of active intervention ≤ 6 months | 4 | 203 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.16, 0.20] |
| 1.33.2 End of active intervention > 6 months | 3 | 191 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.04, 0.62] |
| 1.33.3 Follow‐up (no active intervention) | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.61 [‐0.03, 1.26] |
| 1.34 Change in BMI (z‐score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.34.1 End of active intervention ≤ 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.35 Number of pulmonary exacerbations | 1 | Other data | No numeric data | |
| 1.35.1 Number of exacerbations at end of 6 months' partially supervised active intervention (mixed Poisson regression model) | 1 | Other data | No numeric data | |
| 1.35.2 Number of exacerbations after 12 months: 6 months' partially supervised activity followed by 6 months unsupervised activity with access to study resources (mixed Poisson regression model) | 1 | Other data | No numeric data | |
| 1.36 Time to first pulmonary exacerbation | 1 | Other data | No numeric data | |
| 1.37 Number of hospitalisations | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.37.1 Hospitalisations during 12 months of active intervention | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.38 Change in whole body bone mineral density (g/cm²) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.38.1 End of active intervention > 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.39 Change in lumbar spine bone mineral density (g/cm²) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.39.1 End of active intervention > 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.40 Change in metabolic parameters (HbA1c (%)) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.40.1 End of active intervention ≤ 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.41 Change in metabolic parameters (glucose AUC) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.41.1 End of active intervention ≤ 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.42 Change in metabolic parameters (total insulin AUC) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.42.1 End of active intervention ≤ 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.43 Change in metabolic parameters (insulin sensitivity index) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.43.1 End of active intervention ≤ 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.44 Change in plasma glucose (mmol/L) during an oral glucose tolerance test: end of active intervention ≤ 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.44.1 Change in fasting plasma glucose (mmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.44.2 Change in 1‐hour plasma glucose (mmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.44.3 Change in 2‐hour plasma glucose (mmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.45 Change in plasma insulin (µIU/mL) during an oral glucose tolerance test: end of active intervention ≤ 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.45.1 Pretest measurement | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.45.2 After 60 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.45.3 After 120 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.46 Change in blood glucose (mmol/L) during an oral glucose tolerance test: end of active intervention > 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.46.1 Change in fasting blood glucose (mmol/L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.46.2 Change in blood glucose level (mmol/L) at 60 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.46.3 Change in blood glucose level (mmol/L) at 120 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.47 Adverse events and serious adverse events | 2 | Odds Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.47.1 Adverse events related to physical activity: end of active intervention > 6 months | 2 | 156 | Odds Ratio (IV, Fixed, 95% CI) | 6.22 [0.72, 53.40] |
| 1.47.2 Serious adverse events related to physical activity: end of active intervention > 6 months | 1 | 117 | Odds Ratio (IV, Fixed, 95% CI) | 0.95 [0.06, 15.54] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alexander 2019.
| Study characteristics | ||
| Methods |
Design: single‐centre, parallel RCT Location: no details given on hospital, city or country Inclusion criteria: not specified Exclusion criteria: not specified Duration: 12 weeks |
|
| Participants | 15 prepubertal children with CF, mean age 7.94 (SD 1.35) years Intervention group (n = 9): no further details available Control group (n = 6): no further details available |
|
| Interventions | 12‐week, home‐based, whole body vibration exercise training programme Intervention group: 12‐week, home‐based, whole body vibration training programme (5 times per week for 20 min) combined with their regular airway clearance therapy regimen. Control group: usual airway clearance therapy regimen. |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Study reported as an abstract and, therefore, the information was limited. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no details given for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Unclear whether personnel blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcomes assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details of dropouts or whether intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Unclear risk | Abstract so unable to assess if all outcomes used in methods were reported in results. |
| Other bias | Unclear risk | Did not state inclusion or exclusion criteria, neither did they describe the methods of statistical analysis used. |
Beaudoin 2017.
| Study characteristics | ||
| Methods |
Design: single‐centre, open‐label, parallel RCT (the record on clinicaltrials.gov states cross‐over design, but this is not evident from published paper) Location: Institut de Recherches Cliniques de Montreal, Canada Inclusion criteria: participants with CF; aged > 18 years; sedentary (< 100 min/week of structured exercise assessed by physical activity questionnaire and telephone interview; FEV1 > 40 % predicted; clinically stable for the last 6 weeks; IGT; CFRD without pharmacological treatment or elevated 1‐hour plasma glucose concentration during an OGTT (indeterminate 1‐hour glucose concentration > 11.0 but 2‐hour plasma glucose concentration < 7.8 mmol/L) Exclusion criteria: current pulmonary exacerbation; use of oral or IV corticosteroids; low SaO2 during exercise; history of haemoptysis in last 6 weeks Duration: 13 weeks |
|
| Participants | 14 participants with CF Group demographics Intervention group (n = 8): mean age 31.9 (range 24–41) years Control group (n = 6): mean age 35.5 (range 22–57) years |
|
| Interventions | 12‐week combined aerobic and resistance training study Intervention group: aerobic and resistance training exercises 3 times per week for about 20–40 min with a day off between the training sessions (in total 36 training sessions). Exercise intensity and volume were progressively increased. Participants recorded their training sessions in a diary. Once every 4 weeks, participants received a supervised training session and a telephone call on a weekly basis.
Control group: no information reported in the original publication. Detailed information on control intervention was available on ClinicalTrials.gov (NCT02127957). See 'Notes' below for further information. |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcomes measured at baseline and week 13. Further, inflammatory markers were measured in this study but inflammatory biomarkers are not outcomes relevant for this review. |
|
| Notes |
Study registration The study was registered as a cross‐over trial (ClinicalTrials.gov NCT02127957; clinicaltrials.gov/ct2/show/NCT02127957) but results were reported as parallel‐design study. The authors confirmed that they had to stop the study due to recruitment problems. The authors presented only results from the first study phase (12 weeks). Information provided on ClinicalTrials.gov "Intervention Model: Crossover Assignment" "Following the visit #6, patients in the control group will be invited to participate in a second study phase to participate in supervised exercise program. This participation will involve an additional 12 weeks of follow‐up, which included the same visit as Group 1 with exercises. In this case, to simplify participation and reduce the volume of blood collected, the final visit (#5) of the project will also be the first visit of exercises phase. This part of study involves 2 supervised training sessions and 8 follow up phone call. The exercises program will be performed three times per week for about one hour." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned open‐label study with 2 parallel arms. Randomisation was conducted in blocks by gender with a ratio of 2:2. No details given for generation of sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At screening, 1 participant could not be randomised due to an adverse event during CPET. 3 dropouts postrandomisation (18%).
The study was registered as a cross‐over study but results for the second study part were not presented. |
| Selective reporting (reporting bias) | High risk | Heart rate and SaO2 were measured during CPET, but results were not reported. The second study phase was not reported in the original publication. |
| Other bias | High risk |
Sample size Information on sample size and recruitment goals differed between the information provided under ClinicalTrials.gov and the final publication. This study aimed to recruit 24 participants (12 exercise group, 12 control group), see Clinicaltrials.gov, NCT02127957. The recruitment goal was not achieved (18 were recruited but only 17 randomised), but no information was provided in the final paper. According to the power calculation provided in the original publication, 18 participants (9 per group) were required for the analysis. Finally, 14 participants completed the study so the study is likely to be underpowered. Statistical analyses The authors reported pre–post within‐group changes and no between‐group differences as would be appropriate for an RCT. We received raw data from the authors and calculated between‐group differences for plasma glucose and plasma insulin values during the OGTT. Our results differed compared to the results reported in the original publication. The initial power analysis, aiming to demonstrate a difference of 1.5 mmol/L in plasma glucose levels 120 min after ingestion of the glucose solution after exercise training, required a study sample of 18 participants (9 per group). Finally, only 14 participants completed the study, reducing the statistical power to observe a difference between the interventions in the study. Control intervention In the original publication, there was no information on the control intervention. We noticed discrepancies between the registered (ClinicalTrials.gov) and published trial design (cross‐over versus parallel‐group design). |
Carr 2018.
| Study characteristics | ||
| Methods |
Design: parallel RCT; single‐centre comparative effectiveness Phase 2 trial Location: Royal Brompton Hospital, London, UK Inclusion criteria: participants with CF aged ≥ 6 years; no prior experience practicing Tai Chi; required to have the time to complete the study and be within reasonable distance of the centre for teachers to travel to lessons; have Internet access Exclusion criteria: taking part in any other interventional study or had participated in the pilot study Duration: 9 months |
|
| Participants | 40 participants with CF Group demographics Intervention group (n = 22): median age 22.8 (range 7.1–45.7) years, mean FEV1 69 (SD 21.6) % predicted Control group (n = 18): median age 22.8 (range 6.1–51.5) years, mean FEV1 77 (SD 21.8) % predicted |
|
| Interventions | Phase 2 study Intervention group: 8 × face‐to‐face Tai Chi sessions then given a DVD and a handout to use at home for 9 months and encouraged to practice up to 5 times per week Control group: no treatment (standard care) for the first 3 months (this is the control), then 8 × online Tai Chi sessions (e.g. via Skype) and given a DVD and a handout to use at home for 6 months and again encouraged to practice up to 5 times per week Programme evaluated at baseline and after 3, 6 and 9 months |
|
| Outcomes |
Primary endpoint
Secondary endpoints
Outcomes measured at baseline and after 3, 6 and 9 months. The outcomes for PSQI, FFMS and CAMM were not of interest for this review. |
|
| Notes | We contacted the corresponding author for additional raw data. The initial response was positive, but ultimately, we did not receive additional raw data. For the purpose of this review (i.e. comparison of exercise versus no exercise), we included changes from baseline to 3 months between the face‐to‐face Tai Chi group and the control group (i.e. starting with the Internet‐delivered intervention 3 months later). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors used random number tables to generate random sequencing for blocks of 6 participants in 3 groups according to participants' age (6–11 years; 12–16 years; > 16 years). |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 51 participants were randomised (27 in the face‐to‐face Tai Chi group and 24 in the Internet‐delivered Tai Chi group), of which 40 completed the study (22 in the face‐to‐face Tai Chi group and 18 in the Internet‐delivered Tai Chi group). Dropout rate was 21.6%. Reasons for study withdrawal were reported in detail in the CONSORT flow diagram. Intention‐to‐treat analysis was not performed. |
| Selective reporting (reporting bias) | High risk | The trial was registered with ClinicalTrials.gov (identifier: NCT02054377) and a study protocol was published (Lorenc et al. Chinese Journal of Integrative Medicine 2015 May 26. [Epub ahead of print]). HRQoL assessed with the CFQ‐R (9 quality‐of‐life domains) was defined as primary endpoint; i.e. change from baseline at 3 months, change from baseline at 6 months and change from baseline at 9 months. HRQoL was reported for 2/9 CFQ‐R domains (i.e. respiratory domain and digestion) at baseline (Table 1 in original publication). Individual responses to the CFQ‐R respiratory domain were visualised for all time points (Figure 2 in the original publication). Data for all other domains for the different time points were not reported. The authors noted that questionnaire returns at 9 months were low and no further analyses of the difference at this time were performed. Numbers (percentages) of available questionnaires were not reported. |
| Other bias | Low risk | Clearly stated inclusion and exclusion criteria and described method of statistical analysis used. |
Cerny 1989.
| Study characteristics | ||
| Methods |
Design: single‐centre, parallel RCT during hospital admission for acute exacerbation Location: no details given on hospital, city or country Inclusion criteria: participants with CF admitted to hospital for treatment of an acute exacerbation; able to perform a pulmonary function test and provided written informed consent (assumed patient or parental, depending on age) were included Exclusion criteria: not described Duration: mean 13 days |
|
| Participants | 17 participants with CF Group demographics Intervention group (n = 9): mean age 15.4 (SD 4.9) years Control group (n = 8): mean age 15.9 (SD 4.9) years |
|
| Interventions | Short‐term aerobic study Intervention group: 2 cycle ergometer sessions and 1 bronchial hygiene session per day during admission: mean 13 (SD 3) days Control group: 3 bronchial hygiene sessions per day during admission: mean 13 (SD 2.6) days |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no details of the method. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Unclear whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in methods were reported in results. Data reported for all time points. |
| Other bias | Unclear risk | Stated the inclusion criteria but not the exclusion criteria. Pulmonary function values FEV1 and FEF25–75 were lower in the control compared to the training group at admission. Clearly described statistical analysis methods. |
Del Corral 2018.
| Study characteristics | ||
| Methods |
Design: single‐centre, parallel RCT; simple randomisation (1:1 ratio); home‐based exercise training programme using active video games; blinding (outcome assessor) Location: Universidad Autónoma de Madrid, Madrid, Spain Inclusion criteria: diagnosis of CF; aged 7–18 years; clinically stable without exacerbation in the past 6 weeks prior to study start Exclusion criteria: evidence of cardiovascular, neuromuscular or osteoarticular comorbidities; lung transplant candidates; participation in a rehabilitation programme within the past 12 months prior to study start Duration: 12 months (6‐week intervention and 12‐month follow‐up period) |
|
| Participants | 40 participants with CF Group demographics Intervention group (n = 20): mean age 12.6 (SD 3.4) years; mean FEV1 82.7 (SD 21.7) % predicted Control group (n = 20): mean age 11 (SD 3) years; mean FEV1 86.2 (SD 20.5) % predicted |
|
| Interventions | Home‐based exercise training programme using active video games Intervention group: 6‐week, home‐based exercise training using the Nintendo Wii platform with the game EA SPORTS TM ACTIVE 2. The game involved exercises such as running, squats, lunges and biceps curls. Participants were instructed to exercise 5 times per week for 30–60 min per session and the training load was progressively increased over time. Participants were advised to perform all exercise at a fitness level of 3. A physiotherapist contacted the training group participants via telephone on a weekly basis. After the first 3‐month training period, the participants were instructed to continue with the exercise programme (minimum 2 days per week; 20 min duration). Control group: no exercise training programme (usual care) |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcomes were measured at baseline, after 6 weeks of training and at 12‐month follow‐up. aSee comment in the risk of bias table |
|
| Notes | HRQoL data were not included in this review. See detailed comment in risk of bias table ("domain selective reporting"). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers (GraphPad Software); simple randomisation (1:1) ratio. The randomisation sequence was generated by a person not involved in the study. |
| Allocation concealment (selection bias) | Low risk | Adequately sealed envelopes were used. An external person not involved in the study allocated participants to each group. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study staff who administered the questionnaires and collected outcome data were blinded to participants' group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear description and details about dropouts and loss to follow‐up: 1 participant in the intervention group did not finish the programme due to lack of time; 4 participants were lost to follow‐up (10%): 2 participants in the intervention group (1 = no response; 1 = died); 2 participants in the control group (no response). Intention‐to‐treat and per‐protocol analysis were performed. Intention‐to‐treat analysis was performed post hoc. |
| Selective reporting (reporting bias) | Unclear risk | The trial was registered with ClinicalTrials.gov (registration number: NCT02552043; clinicaltrials.gov/ct2/show/NCT02552043) and all outcomes were reported. Intention‐to‐treat analysis was performed post hoc. HRQoL data Data for HRQoL (CFQ‐R) for 3 different groups (children aged 6–11 years; parents of children aged 6–13 years; adolescents/adults aged > 14 years) were provided in 2 tables in the online supplements. The tables (1 for the per‐protocol analysis and 1 for the intention‐to‐treat analysis) contain mean (SD) values for all CFQ‐R domains, but mean differences and their 95% CIs and effect sizes were not reported, as the authors did for the other outcomes in their main publication (i.e. tables 2 and 3). The tables in the online supplement contained information on within‐group differences in HRQoL domains between baseline and end of intervention (i.e. 6 weeks) and baseline and follow‐up. Between‐group differences were only reported for the comparison between baseline and follow‐up values, not for baseline versus 6 weeks. It is not clear what type of statistical analysis was conducted and the numbers of participants in each group were not reported. Questionnaire response rates and potential missing data could not be evaluated. We decided not to use HRQoL data from the original publication. Data were not reported as change from baseline and we were not sufficiently confident to rely on final HRQoL scores for the analyses. This would assume that the different groups are comparable in their pre‐intervention HRQoL scores. However, this is not the case and we noticed some differences in pre‐intervention HRQoL domains between groups, e.g. respiratory symptom scale (mean values between 66 and 82 between the control groups). |
| Other bias | High risk |
Primary outcome The trial was registered with ClinicalTrials.gov (registration number: NCT02552043) and the 6MWT and MSWT were both listed as primary outcome measures. In the original publication, the MSWT was reported as a primary outcome measure and the 6MWT was reported as a secondary outcome measure. The sample size for this study was calculated to detect between‐group differences in 6MWT distance (see pages 3 and 4 in the original publication). In the intention‐to‐treat analysis (posthoc), effects of exercise training on 6MWT distance and MSWT distance were significant (based on P < 0.05) for the comparison between baseline versus 6 weeks, but not for the comparison between baseline and 12‐month follow‐up. In comparison, in the per‐protocol analysis, intervention effects were "significant" for the MSWT for the comparison between both measurement time points (i.e. baseline versus 6 weeks and baseline versus 12 months); whereas effects on 6MWT distance were "only" significant when baseline values were compared to the end of the training programme (i.e. 6 weeks). It appeared to the authors of this review that Del Corral and colleagues selected their primary outcome measure based on the results of the final statistical analysis, i.e. the outcome with the "more positive" result. |
Donadio 2020.
| Study characteristics | ||
| Methods |
Design: single‐centre, parallel RCT Location: Universidad Europea de Madrid, Madrid, Spain Inclusion criteria: diagnosis of CF; aged 6–18 years; signature of informed consent of legal guardian and patient Exclusion criteria: being a smoker; having had an exacerbation in last 3 months; having undergone gastric surgery; having enteral nutrition at present; attending the Hospital Infantil Universitario Niño Jesús of Madrid; currently taking CFTR modulators Duration: 8 weeks |
|
| Participants | 25 participants with CF (20 boys); mean age 12.7 (SD 2.9) years; mean FEV1 z‐score −1.5 (SD 1.5) Intervention group1 (n = 8): no additional information Intervention group2 (n = 6): no additional information Control group (n = 11): no additional information |
|
| Interventions | Study participants were randomised to 1 of 3 groups. Intervention group1: supervised resistance exercise training programme performed 3 times per week over 8 weeks. Intervention group2: supervised resistance exercise training programme using electrical stimulation of the lower limbs and posterior trunk muscles performed 3 times per week over 8 weeks. Control group: participants received standard exercise recommendations from the CF care team. |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcomes reported in abstract
|
|
| Notes | The full paper to this study was published during the process of finalising this review, i.e. after the last systematic literature search on 3 March 2022 (www.resmedjournal.com/article/S0954-6111(22)00063-4/fulltext). The results presented in the current review were drawn from a single abstract and therefore the information is currently limited. We will include results from this full paper at the next update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no details given for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Unclear if personnel blinded . |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcomes assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details of dropouts or whether intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Unclear risk | Did not state inclusion or exclusion criteria. |
| Other bias | Unclear risk | Did not state primary endpoint. No information on inclusion or exclusion criteria available. |
Douglas 2015.
| Study characteristics | ||
| Methods |
Design: single‐centre RCT (INSPIRE‐CF). Powered to show changes in primary outcome measure of FEV1 z‐score after 24 months (66 participants needed) Location: Great Ormond Street Hospital CF Unit, London, UK Inclusion criteria: participants with a documented diagnosis of CF; male or female aged 6 years or older at baseline and < 17 years old at the end of the 2‐year study; currently under the primary care of the Great Ormond Street Hospital CF Unit; able to perform spirometry with a baseline FEV1 percentage predicted of ≥ 40%, as measured on ≥ 3 occasions in the previous year, during times of clinical stability (i.e. not during an exacerbation, and not during or within 2 weeks of IV antibiotics); the participant's parent or legal guardian must have given informed consent; assent sought from all children. Exclusion criteria: people who had had lung transplantation or listed for lung transplantation; clinically significant disease or medical condition other than CF or CF‐related conditions that, in the opinion of the multidisciplinary clinical team, would compromise the safety of the individual; orthopaedic impairment that compromises exercise performance; mental impairment leading to inability to co‐operate; unable to understand verbal or written (or both) instructions in English; children unable to understand exactly what the physiotherapists were instructing them to do, for safe and effective exercise training sessions; unable to read information sheets and questionnaires available in English; participants, parents or legal guardians who are unwilling to sign consent to participate in the study. Quote: "The following criteria will not exclude a child from participating in the study, but based on the hospital's exercise laboratory's infection control protocol, may preclude the participant from Cardiopulmonary Exercise Testing.
Duration: 24 months |
|
| Participants | 71 participants with CF. Data from 67/71 participants available: mean age 10 (SD 3; range 6–15) years; mean FEV1 86.6 (SD 15.3) % predicted; mean FEV1 z‐score ‒1.10 (SD 1.23) Group demographics Intervention group (n = 34): mean FEV1 89.2% predicted; FEV1 z‐score ‒0.89; LCI 8.6; VO2 peak 36.1 mL/min per kg BW Control group (n = 33): mean FEV1 83.8% predicted; FEV1 z‐score ‒1.32; LCI 9.6; VO2 peak 36.9 mL/min per kg BW 67 participants completed study (4 dropouts: 1 from control; 3 from intervention). |
|
| Interventions |
Intervention group: standard specialist care including weekly exercise training Control group: standard specialist care without weekly exercise training |
|
| Outcomes |
At baseline, 12 and 24 months, measured the following outcomes.
Assessments at 6‐month study visit
|
|
| Notes | INSPIRE‐CF is a 24‐month exercise training study that investigates the effects of an individually tailored and supervised exercise training programme on lung function, exercise capacity and HRQoL for children with CF. The study has been completed. 5 abstracts have been published, but a full‐text article is not yet available. Data were extracted from the latest published abstract, presented at the 2017 World Confederation for Physical Activity Conference (Ledger et al. 2017; see under Douglas 2015) Study was powered to show changes after 24 months in primary outcome measure of FEV1 z‐score; required 66 participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised but no details of the method. Randomised by minimisation to 1 of the 2 groups (after baseline testing) by an independent blinded medical statistician using the SiMin software package (Wade 2006). |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators confirmed blinded outcome assessment for lung function (spirometry and multiple inert gas washout) and CPET. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants dropped out: 1 from the control group at 6 months (social concerns); 3 from the intervention group at 12 months (1 due to moving to a new area and changing hospitals; 2 because they no longer wished to exercise). |
| Selective reporting (reporting bias) | Unclear risk | Data published in abstract from, so unable to assess if all outcomes in methods were reported in results. Unable to assess if data were reported for all time points. |
| Other bias | Unclear risk | None identified based on limited information available. |
Güngör 2021.
| Study characteristics | ||
| Methods |
Design: parallel RCT; single‐centre study, triple blinding (study participant, care provider, outcome assessor) Location: Marmara University School of Medicine, Department of Physical Medicine and Rehabilitation, Istanbul, Turkey Duration: 6 weeks, including 3‐ and 6‐month follow‐up assessments |
|
| Participants | 22 participants with CF Inclusion criteria: boys and girls aged 6–14 years; ability to understand study aims Exclusion criteria: FEV1 < 30% predicted; cor pulmonale; advanced gastro‐oesophageal reflux; current hospital admission due to lung infection; neuromuscular disease |
|
| Interventions |
Intervention group: pulmonary rehabilitation programme including active cycle of breathing techniques (breathing control, chest expansion exercise, huff coughing) plus postural exercise programme, including thoracic vertebral mobilisation, pectoral stretching, scapular and thoracic extensor strengthening and core stability exercises. Breathing techniques and exercises performed once a week for 6 weeks Control group: pulmonary rehabilitation programme including active cycle of breathing techniques (once per week) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | Trial status: completed (last update 26 October 2018) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated that the study was randomised but generation of the code was not described. The only information they gave was that they used a sealed opaque envelope system with blocking. |
| Allocation concealment (selection bias) | Unclear risk | Used sealed opaque envelopes but there was little information to explain whether this meant that the allocation was concealed and from whom. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The paper stated that the trial was single‐blind (outcome assessors). However, the trial registration document (NCT03295201) stated that the trial was triple‐blind (participant, care provider, outcome assessor). Given that the participants had to do postural exercises if they were in the intervention arm, it is unclear to the review authors how they could be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All outcomes detailed in methods were reported in results. Data for HRQoL were not reported in detail; i.e. only for 2 selected subdomains. Data reported for all time points. 3 participants were lost to follow‐up. Reasons were reported for 1 participant (i.e. hospitalisation of control group participant). Intention‐to‐treat analysis was not performed. The study was registered with ClinicalTrials.gov (identifier: NCT03295201). |
| Selective reporting (reporting bias) | Unclear risk | HRQoL was assessed postintervention but data were only presented for the subdomains emotional function and treatment difficulties. Changes in emotional function and treatment difficulties improved in the intervention group, not in controls. Between‐group differences were not statistically significant. |
| Other bias | Unclear risk | It is not clear if the study participants were familiar with the MWST and if a practice test was done because of well‐known learning effects. Small sample size and lack of statistical power Remarkable differences in HRQoL domains at baseline (i.e. physical functioning and respiratory domain values were substantially lower in the intervention compared to the control group). |
Gupta 2019.
| Study characteristics | ||
| Methods |
Design: single‐centre, parallel RCT; stratified block randomisation, allocation concealed using sequentially numbered, sealed, opaque envelopes, open‐label Location: outpatient department of a tertiary care hospital in northern India Inclusion criteria: confirmed diagnosis of CF; aged 6–18 years; not having required IV antibiotics in the 1 month prior to enrolment into study; FEV1 ≥ 20% predicted Exclusion criteria: prior diagnosis of musculoskeletal disorder (e.g. rheumatoid arthritis, muscular dystrophy) or chronic renal failure Duration: 1 year |
|
| Participants | 52 participants with CF were included (30 males; 22 females). Group demographics Intervention group (n = 25): 15 females (60%); mean age 147.16 (SD 33.96) months; mean FEV1 61.44 (SD 24.72) % predicted; median BMI z‐score ‒2.46 (IQR ‒3.79 to ‒1.48) Control group (n = 27): 15 females (56%); mean age 152.22 (SD 40.01) months; mean FEV1 60.93 (SD 24.87) % predicted; median BMI z‐score ‒1.93 (IQR ‒3.59 to ‒0.91) |
|
| Interventions | Intervention group: home‐based exercise programme consisting of resistance exercises (e.g. squats, push‐ups, forward lunges) performed 3 times per week and plyometric jumping exercises (i.e. 3 types of jumps), each performed on a daily basis (20 times per day) over 1 year. Intensity of exercises was progressively increased over time. Intervention participants received a CD with animated demonstrations of exercises; they kept a diary and were contacted via telephone every 2 weeks. Control group: no exercise programme, continue with regular physical activity for 1 year. | |
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation used to randomly allocate participants to intervention and control groups using computer software. A person not involved in the study generated the list of random numbers. Stratification was based on pubertal status (prepubertal versus peri‐/postpubertal): children of both strata were further stratified based on their lung function (i.e. FEV1 ≥ 20% and ≤ 50% predicted versus > 50% predicted. |
| Allocation concealment (selection bias) | Low risk | Concealment of random allocation was done by enclosing assignments in sequentially numbered opaque, sealed envelopes for the 4 strata. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Personnel performing dual‐energy absorptiometry (primary outcome) scans and laboratory assays were blinded for group allocation. Personnel performing spirometry and exercise testing, etc. (secondary outcomes) were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study was retrospectively registered with Clinical Trials Registry‐India (Trial No: REF/2013/01/004447). All participants completed the trial. There were no reported dropouts during the study. |
| Selective reporting (reporting bias) | Low risk | No indication for selective reporting. |
| Other bias | Unclear risk | The primary outcome measure was defined as mean bone mineral density and not further specified. CPET: (quote): "Effort was considered to be at a maximal level when the participant showed clinical signs of intense effort or saturation fell below 90%". Arterial oxygen desaturation is common in people with CF lung disease during exercise testing. Oxygen saturation at peak exercise is independently related to FEV1 (Ruf 2009). Therefore, stopping an exercise test when SpO2 drops < 90% may significantly underestimate maximal exercise capacity. This is supported by the rather low end‐test heart rates achieved at maximal exercise on the treadmill (mean values about 160–167 bpm). |
Hatziagorou 2019.
| Study characteristics | ||
| Methods |
Design: single‐centre, partially supervised, parallel‐group RCT Location: Aristotle University of Thessaloniki, Greece Inclusion criteria: not stated Exclusion criteria: not stated Duration: 12 months |
|
| Participants | 30 participants with CF (50% male) Mean age 16.2 years; mean FEV1 91.2 (SD 20.1) % predicted; mean VO2 peak 80.9 (SD 17.6) % predicted Group demographics Intervention group (n = 15): mean FEV1 90.1% predicted; mean VO2 peak 72.7% predicted Control group (n = 15): mean FEV1 92.2% predicted; VO2 peak 89.1% predicted |
|
| Interventions |
Intervention group: individualised exercise training programme; supervised using accelerometry Control group: no exercise training |
|
| Outcomes |
|
|
| Notes | Limited information as published as abstract only. The abstract stated that participants were divided into 2 groups. We contacted the authors to clarify the study design. The first author of the abstract and principal investigator (Elpis Hatziagorou) confirmed that the study was an RCT. The Principal Investigator confirmed that the trial is a partially supervised intervention, in which a physiotherapist provides instructions and feedback regarding exercise training at outpatient follow‐up visits. Assessments were performed at 1, 3, 6 and 12 months after baseline assessments. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Unclear whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details of dropouts or whether it used an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Abstract, so unable to assess if all outcomes used in methods were reported in results. |
| Other bias | Unclear risk | Did not state inclusion or exclusion criteria, neither did they describe the methods of statistical analysis used. |
Hebestreit 2010.
| Study characteristics | ||
| Methods |
Design: multicentre parallel‐group RCT Location: different study sites (Frankfurt, Hanover, Würzburg) in Germany Inclusion criteria: participants with CF; aged ≥ 12 years; FEV1 ≥ 35% predicted; ability to perform physical activities Exclusion criteria: non CF‐related chronic diseases and CF‐related conditions posing an increased risk to the participant when exercising (e.g. oesophageal varicosis, pulmonary bullae, < 80% drop in SaO2 with exercise and signs of pulmonary hypertension on electrocardiogram or echocardiogram, or both). Duration: 24 months (6‐month intervention and long‐term, open follow‐up period) |
|
| Participants | 38 participants with CF Group demographics Intervention group (n = 23): mean age 19.5 (SD 6.4) years Control group (n = 15): mean age 19.4 (SD 5.3) years |
|
| Interventions | Long‐term, partially supervised conditioning programme Intervention group: exercise intervention with endurance‐type and strengthening exercises. Participants agreed to increase their vigorous physical activities by a minimum of 3 × 60 min per week in the first 6 months of the study. An individual exercise plan was devised for participants; activity counselling was stopped after the first 6 months and participants were encouraged to maintain or further increase their physical activity level. Control group: participants were kept their activity level constant during the first 12 months of study. During the second year (period from 12 to 24 months), they were free to change their activity behaviour. |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcomes measured at baseline and after 3, 6, 12, 18 and 24 months |
|
| Notes | Study is a full‐text article of the Hebestreit 2003 abstract (see under Hebestreit 2010). The author provided additional raw data for this review (e.g. data for RV/TLC, bodyweight, BMI, body fat, fat‐free mass and HRQoL) that were not reported in detail in the original paper. The control group in this study was also used in Kriemler 2013. Data from this control group were not used for any analysis in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 40 folded paper tickets were put into a bag with a 3:2 ratio, i.e. 24 tickets for the intervention group and 16 for the control group. Participants drew a ticket at random and the drawn ticket was then destroyed. Principal Investigator was aware of the number of lots in the bag. |
| Allocation concealment (selection bias) | High risk | Participants drew a folded paper ticket from an opaque bag with closed eyes. If all lots were drawn out by 1 study group, allocation concealment would no longer exist. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Unclear whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors were not blinded with respect to the participants' group allocation for VO2 peak and skinfold measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 participants dropped out during the first 12 months of the study: 3 gave no reason, 1 joined another study and 1 moved away. At 18 months dropout rate was 13% and at 24 months it was 26%. Dropouts were balanced between groups. Reasons for dropout were not recorded. Intervention group participants received financial support (maximum EUR 200) to foster the realisation of the exercise training plan, which potentially introduced bias. There was no indication for differential loss to follow‐up between the intervention and control group participants suggesting that the financial support did not influence attrition rates. Intention‐to‐treat was not performed. |
| Selective reporting (reporting bias) | Unclear risk | Anaerobic capacity (PP, MP) was only reported for 18–24 months' follow‐up (non‐significant) and results for HRQoL were only presented for the scale 'physical functioning'. There were no effects for all other HRQoL scales. |
| Other bias | Unclear risk | Financial support (maximum EUR 200) was offered for intervention group participants to foster the realisation of the exercise training plan. It is unclear if paying intervention group participants had an impact on attrition. Dropouts were balanced between groups suggesting that the financial incentive had no influence on attrition rates. |
Hebestreit 2022.
| Study characteristics | ||
| Methods |
Design: parallel‐group design; block randomisation stratified by FEV1 (< 70% predicted, ≥ 70% predicted) and country; computer‐generated list of random numbers; randomisation within the REDCap database at each study centre to allow complete allocation concealment Location: international multicentre RCT conducted in 27 centres across Europe and North America Inclusioncriteria: males and females aged 12 years and older with a confirmed diagnosis of CF; FEV1 ≥ 35% predicted and access to Internet Exclusioncriteria: participation in another clinical trial up to 4 weeks prior to the first baseline visit; pregnant or breastfeeding; inability to exercise; > 4 hours of reported vigorous physical activities per week currently or up to 3 months prior to baseline measurements and not already planned within the coming 6 months; unstable condition precluding exercise (major haemoptysis or pneumothorax within the last 3 months, acute exacerbation and IV antibiotics during the last 4 weeks, planned surgery, listed for lung transplantation, major musculoskeletal injuries such as fractures or sprains during the last 2 months, others according to the impression of the treating physician); cardiac arrhythmias with exercise; requiring additional oxygen with exercise; recent diagnosis of CF‐related diabetes 3 months prior to screening or at screening; recent changes in medication ≤ 1 month prior to screening (systemic steroids, ibuprofen, inhaled antibiotics, mannitol, dornase alfa, hypertonic saline); ≥ 1 G551D mutation and not on ivacaftor (VX770) yet but planned start or planned stop of ivacaftor during the trial and colonisation with Burkholderia cenocepacia. Duration: 12 months |
|
| Participants | 117 participants with CF Group demographics Intervention group (n = 60): 65 (56%) female; mean age 25.3 (SD 11.4) years; FEV1 74 (SD 22) % predicted; BMI 22.0 (SD 4.1) kg/m²; VO2 peak 71 (SD 17) % predicted Control group (n = 57): 52 (56%) female; mean age 22.8 (SD 10.8) years; FEV1 74 (SD 21) % predicted; BMI 20.8 (SD 3.5) kg/m²; VO2 peak 69 (SD 15) % predicted |
|
| Interventions |
Interventiongroup: participants were advised to add 3 hours of vigorous physical activities per week to baseline activities. Weekly exercises included ≥ 30 min of strength‐building activities and ≥ 2 hours of aerobic activities. Exercise bouts lasting ≥ 20 min were counted with respect to total weekly training time. Participants were given exercise counselling to boost motivation towards an active lifestyle, strategies included face‐to‐face information, motivational interviewing, goal setting, a written "activity contract" with specific information on which activities were scheduled for which day and for how long, a pedometer, a web‐based activity diary (www.activate-cf.org) providing feedback on missing time in vigorous activities to reach the weekly goal, and repeated counselling via telephone contacts and during clinic visits. A full manual describing the intervention and all intervention materials including the website was available in 4 languages: Dutch, English, French and German. Controlgroup: usual care. Group was advised to keep their physical activity level constant during the 12‐month study. |
|
| Outcomes |
Primary outcome
Secondary outcomes
Other outcomes
|
|
| Notes | The review authors Thomas Radtke, Helge Hebestreit and Susi Kriemler were lead investigators of the ACTIVATE‐CF trial and had full access to the data before the publication of the main manuscript. The data were included in this review, and during the process of preparing the review update, the paper was accepted for publication and appropriately cited. Author Sherie Smith and Sarah Nevitt performed data extraction and risk of bias assessment for this study. Data from substudies were not published in the main manuscript. The substudy on bone health and body composition using dual energy x‐ray absorptiometry was stopped due to insufficient recruitment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified block randomisation (1:1 ratio) within REDCap database, computer‐generated randomisation list generated by a statistician. Randomisation was stratified by country and lung disease severity (i.e. moderate‐to‐severe lung disease (FEV1 value < 70% predicted) or mild lung disease (FEV1 ≥ 70% predicted)). |
| Allocation concealment (selection bias) | Low risk | Randomisation within each study site conducted centrally via a database. Study investigators had no access to the randomisation list. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Unclear whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes detailed in methods were reported in results. Data reported for all time points. All randomised participants who received allocated intervention were included in a (modified) intention‐to‐treat analysis. Losses to follow‐up were reported with reasons. Data from substudies were not reported in the main publication (see comment in original report). The study was registered with ClinicalTrials.gov (identifier: NCT01744561) and a study protocol was published (Hebestreit et al. BMC Pulmonary Medicine 2018;18(1):31). |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. |
| Other bias | High risk | The estimated sample size of 292 participants was not achieved; 155 individuals were assessed for eligibility, and 117 individuals were randomised. Consequently, the analysis of the primary endpoint (i.e. change in FEV1 % predicted from baseline to 6 months) was underpowered and the unexpected finding of a significant difference favouring the control group might be due to chance. |
Hommerding 2015.
| Study characteristics | ||
| Methods |
Design: single‐centre parallel RCT Location: Centro Universitario Franciscano (UNIFRA), Santa Maria, Rio Grande do Sul, Brazil Inclusion criteria: participants with CF aged 7–20 years; stable disease, no signs of exacerbation of respiratory symptoms in last 15 days Exclusion criteria: cognitive impairment, non CF‐related bone and muscle abnormalities, heart disease with haemodynamic instability Duration: 3 months |
|
| Participants | 34 participants with CF (20 males, 14 females) Group demographics Intervention group (n = 17): mean age 13.4 (SD 2.8) years Control group (n = 17): mean age 12.7 (SD 3.3) years |
|
| Interventions | Aerobic exercise programme based on verbal and written guidelines. Intervention group: 3‐month aerobic exercise training programme based on verbal and written guidelines. Programme included exercises such as jogging, swimming, walking, ball games and stretching exercises. Participants were told to practice the exercises ≥ 2 × per week for ≥ 20 min. No recommendations provided regarding exercise intensity. Participants received telephone calls every 2 weeks and instructions were provided by 1 of the authors. Control group: participants were instructed about aerobic exercises once at baseline according to the CF centre routine. |
|
| Outcomes |
Outcomes measured at baseline and after 3 months. |
|
| Notes | Sample size estimated based on a mean change of 18.1 (SD 13.8) points in the physical score of the HRQoL questionnaire. Estimated sample size 15 participants in each group (95% power at a 5% level of significance). 2 more participants were included in each group to account for potential dropouts. Another study from the same group using the same aerobic exercise programme was published in 2015 (Schindel et al. Journal of Pediatrics 2015;166(3):710‐6). The responsible author of this publication confirmed that the most included participants were the same as in the Hommerding 2015. There were only marginal differences in lung function (FEV1, FVC and FEF25–75) compared to Hommerding 2015, for which reason we decided not to include lung function data in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated to the intervention or control group in blocks of 6. Used a computer‐based programme for randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Unclear whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts were reported during the study. |
| Selective reporting (reporting bias) | Unclear risk | Blood pressure was measured prior to and after CPET but not reported. Heart rate at rest and SaO2 at peak exercise were measured but results were not reported at baseline. |
| Other bias | Unclear risk | No validity criteria for maximal performance during CPET were reported in the methods. The mean peak heart rate reached during the exercise test was 157.1 (SD 38.5) bpm in the training group and 167.7 (SD 20.8) bpm in the control group, indicative of a submaximal effort. This likely underestimates the true VO2 peak of the study participants. |
Klijn 2004.
| Study characteristics | ||
| Methods |
Design: single‐centre, parallel RCT Location: Cystic Fibrosis Center at University Medical Center, Utrecht, Netherlands Inclusion criteria: participants with CF aged 9–18 years; stable clinical condition (i.e. no need for oral or IV antibiotic treatment in the 3 months prior to testing); absence of musculoskeletal disorders; and FEV1 > 30% predicted Exclusion criteria: not specified Duration: 12 weeks |
|
| Participants | 20 participants with CF (stable disease) completed study Group demographics Intervention group: (n = 11): mean age 13.6 (SD 1.3) years Control group: (n = 9): mean age 14.2 (SD 2.1) years 3 participants dropped out: 1 withdrew from the training group for practical reasons, and 2 from the control group as they did not complete assessments due to pulmonary exacerbations. |
|
| Interventions | Long‐term anaerobic study (12 weeks) Intervention group: anaerobic exercise (2 days per week for 30–45 min) Control group: normal daily activities |
|
| Outcomes |
Outcomes were measured at baseline and after 12 weeks. |
|
| Notes | To achieve a difference in PP per kg BW of 10% with an SD of 0.8 W/kg and a statistical power of 80%, it was calculated that 8 participants had to be included in each study group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no details of the method. |
| Allocation concealment (selection bias) | Low risk | Allocation concealed in opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Primary researcher was blinded but their role in the study was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Primary researcher was blinded, but it was unclear whether this researcher was responsible for outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear description and details about dropouts. 3 participants dropped out: 1 participant from the training group withdrew for practical reasons; 2 from the control group did not complete assessments due to pulmonary exacerbations. Intention‐to‐treat analysis was not performed. |
| Selective reporting (reporting bias) | Unclear risk | Results for HRQoL are only presented for the scale 'physical functioning', which was significantly higher in the training group after the 12‐week training period. There were no change in this HRQoL scale in the control group after 12 weeks. There were no significant effects for any other HRQoL scales. Data were not reported in detail. |
| Other bias | Unclear risk | Clearly stated inclusion criteria but exclusion criteria were not reported. Described statistical methods used in analysis. |
Kriemler 2013.
| Study characteristics | ||
| Methods |
Design: multicentre, parallel RCT with 3 arms Location: different study sites in Switzerland Inclusion criteria: diagnosis of CF; aged ≥ 12 years; FEV1 % predicted ≥ 35%; ability to perform physical activity without harm Exclusion criteria: non‐CF‐related chronic diseases and conditions posing an increased risk to the participant when exercising Duration: 24 months (6‐month intervention and long‐term, open follow‐up period) |
|
| Participants | 39 participants with CF split into 3 groups Group demographics Intervention group 1: (aerobic training) (n = 17): mean age 23.8 (95% CI 21.5 to 26.5) years Intervention group 2: (strength training) (n = 12): mean age 19.0 (95% CI 16.0 to 22.0) years Control group: (n = 10): mean age 20.3 (95% CI 17.0 to 23.6) years A separate control group from a parallel study (Hebestreit 2010) was added due to an unusual deterioration of physical health in the control group in this study (n = 15), mean age 19.5 (95% CI 16.8 to 22.2) years. Data from this control group were not used in this review. |
|
| Interventions | Long‐term exercise study Intervention group 1: participants consented to perform 3 aerobic training sessions per week of 30–45 min duration for the first 6 months and received support which was stopped thereafter. Intervention group 2: participants consented to perform 3 strength training sessions per week of 30–45 min duration for the first 6 months and received support which was stopped thereafter. Control group: participants in the control group were told to keep their activity level constant. Free access to a fitness centre for 1 year was offered after the first study year. |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcomes were measured at baseline and after 3, 6, 12 and 24 months. |
|
| Notes | Study was a full‐text article of the Kriemler 2001 and Hebestreit 2003 abstracts (see under Kriemler 2013 and Hebestreit 2010). Control group experienced a deterioration of physical health during the study. In the original paper, a second control group from a German study with similar design and methods (Hebestreit 2010) was used for comparisons. Data from this control group were not used in this review. The author provided additional raw data for this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomly assigned by a lot that was drawn with their eyes closed from an opaque bag. Investigator was aware of the number of lots in the bag. |
| Allocation concealment (selection bias) | High risk | Participants with their eyes closed drew a lot from an opaque bag. If all lots for 1 study group have been drawn out, allocation concealment would no longer exist. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Unclear whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded for pulmonary function testing (primary outcome FEV1). Outcome assessors were not involved in supervision and delivery of the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear description and details about excluded participants and dropouts. 3 participants were excluded at baseline due to FEV1 < 35% predicted. 8 participants dropped out at different time points (exacerbation n = 1; non‐compliance n = 2; death n = 2; unclear reasons n = 3). 2 participants who dropped out for unclear reasons were in the control group and 1 was in the aerobic training group. Dropout rate was 21%. Intention‐to‐treat analysis not performed. |
| Selective reporting (reporting bias) | Low risk | All outcome detailed in methods were reported in results except HRQoL (secondary outcome), which was mentioned to be reported separately. In the meantime, study was published as Hebestreit et al. BMC Pulmonary Medicine 2014;14:26. HRQoL data were pooled from 2 intervention studies (Hebestreit 2010; Kriemler 2013), and results were presented for baseline and 6‐month follow‐up. |
| Other bias | Unclear risk | Clearly stated inclusion and exclusion criteria and described statistical methods used in analysis. Due to the deterioration of physical health in the control group, the results of this study should be interpreted with caution. |
Michel 1989.
| Study characteristics | ||
| Methods |
Design: single‐centre, parallel RCT during hospital admission Location: no details given on hospital, city or country Inclusion criteria: not specified Exclusion criteria: not specified Duration: duration of hospital admission |
|
| Participants | 9 participants with CF; not stated how many allocated to each group Group demographics Intervention group: mean age 25.5 (SD 10.5) years Control group: mean age 21.5 (SD 3.2) years |
|
| Interventions | Short‐term aerobic study Intervention group: exercise and standardised CF protocol Control group: standardised CF protocol |
|
| Outcomes |
Outcomes were measured at 1‐month postdischarge. |
|
| Notes | Limited information as published as abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no details of method. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Unclear whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details of dropouts or whether intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Unclear risk | This was an abstract so unable to assess if all outcome used in methods were reported in results. Unable to assess if data were reported for all time points. |
| Other bias | Unclear risk | Did not state inclusion or exclusion criteria, neither did they describe the methods of statistical analysis used. |
Moorcroft 2004.
| Study characteristics | ||
| Methods |
Design: single‐centre, parallel RCT Location: adult CF centre in Manchester, UK Inclusion criteria: people with CF who were willing to participate were recruited from 150 people attending the adult CF centre in Manchester at time of study; all had documented CF based on clinical history plus either an increased sweat chloride or abnormal genetic testing Exclusion criteria: participation in another clinical trial, pregnancy, transplant listing, clinical cor pulmonale Duration: 1 year |
|
| Participants | 51 participants with CF were randomised; 42 completed the study Group demographics Intervention group (n = 30): mean age 23.5 (SD 6.4) years Control group (n = 18): 23.6 (SD 5.5) years 3 participants dropped out at the start of the programme: 1 from training group due to failure to attend on initial assessment; and 2 in the control group were withdrawn due to ill health. A further 6 participants dropped out during the 1‐year period |
|
| Interventions | Long‐term aerobic and anaerobic study over 1 year Intervention group: unsupervised exercise (based on individual preferences, general aerobic exercises for lower body and weight training for upper body) 3 times per week Control group: continue with usual activities |
|
| Outcomes |
Outcome were measured at baseline and after 1 year |
|
| Notes | Study was a full‐text article of Dodd 1998 and Moorcroft 2000 abstracts (see under Moorcroft 2004). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised to either active or control groups in a ratio of 3:2. A stratified randomisation in blocks (block size not stated) was used to balance the groups for FEV1, sputum colonisation by Burkholderia cepacia and gender. No details of method reported. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Unclear whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 participants dropped out at start of programme: 1 from training group due to failure to attend on initial assessment; and 2 in control group were withdrawn due to ill health. A further 6 participants dropped out during the 1‐year period. Reasons for dropout were not clearly reported. After 1 year, overall dropout rate was 18% and balanced between the groups (19% in the intervention and 15% in the control group). Intention‐to‐treat analysis was not performed. Missing data were treated by omission and only data for those who completed study presented. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in methods were reported in results. Data reported for all time points. |
| Other bias | Low risk | Clearly stated inclusion and exclusion criteria and described method of statistical analysis used. |
Rovedder 2014.
| Study characteristics | ||
| Methods |
Design: single‐centre, parallel RCT of a home‐based exercise programme Location: Porto Alegre Clinical Hospital, Porto Alegre, Brazil Inclusion criteria: participants diagnosed with CF in accordance with the criteria of the CF adult care consensus conference report by Yankaskas 2004; aged ≥ 16 years; ≥ 30 days of clinical respiratory disease stability Exclusion criteria: participants who refused to take part in the study; pregnant women; people with heart disease, orthopaedic or traumatological problems Duration: 3 months |
|
| Participants | 41 participants with CF Group demographics Intervention group (n = 22): mean age 23.8 (SD 8.3) years Control group (n = 19): mean age 25.4 (SD 6.9) years 2 study participants in the exercise group could not be assessed at the 3‐month follow‐up due to lung transplant assessment. |
|
| Interventions | 3‐month home‐based exercise programme Intervention group: participants received printed guidance for aerobic and muscle strengthening exercises and were advised to perform the programme on a daily basis. Weekly telephone contacts were performed during the 3‐month period. Control group: participants received standard programme without any specific exercise instructions. |
|
| Outcomes |
Outcomes were measured at baseline and after 3 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly allocated in blocks of 6 to exercise or control group. A computer programme was used to generate the randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. 1 researcher was blinded to the randomisation and intervention and was responsible for database entries. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants in the exercise group could not be assessed at the 3‐month visit due to submission to the lung transplant programme. Intention‐to‐treat analysis was used and imputations for missing data were performed for these 2 participants. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in methods were reported in results. Data reported for all time points |
| Other bias | Unclear risk | Clearly stated inclusion and exclusion criteria and described method of statistical analysis used. Baseline between‐group differences existed in BMI which could possibly impact on HRQoL (primary outcome). |
Santana‐Sosa 2012.
| Study characteristics | ||
| Methods |
Design: single‐centre, parallel RCT Location: Children's Hospital Infantil Universitario Niño Jesús in Madrid, Spain Inclusion criteria: potential participants included 111 children previously diagnosed using a genetic test for CF and treated at the Children's Hospital Niño Jesús in Madrid. Boys or girls aged 5–15 years and living in the Madrid area (able to attend training sessions) Exclusion criteria: severe lung deterioration, as defined by an FEV1 < 50% predicted; unstable clinical condition (i.e. hospitalisation within the previous 3 months); Burkholderia cepacia infection; musculoskeletal disease or any other disorder impairing exercise Duration: 3 months (8 weeks' training, 4 weeks' 'detraining') |
|
| Participants | 22 participants with CF Group demographics Intervention group (n = 11): mean age 11 (SEM 3) years; range 5–15 years Control group (n = 11): mean age 10.0 (SEM 2) years; range 6–14 years |
|
| Interventions | 8‐week intrahospital programme followed by a 4‐week detraining period. All participants received the same chest physiotherapy during the entire study period. Intervention group: supervised endurance and strengthening exercises, 3 times per week Control group: continue with standard therapy and instructed on the positive effects of regular physical activity |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes
Outcomes were measured at baseline, after 8 weeks of training and after 4 weeks of detraining. |
|
| Notes | Additional raw data for all included outcomes provided by the authors. The study authors used the term 'detraining', which is a time period during which no supervised exercise training was provided. The meaning of 'detraining' is consistent with our term 'off training', which also describes a period during which no (partially) supervised physical activity took place, but study participants were not explicitly discouraged from undertaking physical activity. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to exercise or control group (quote) "with a block on gender on the basis of a randomization sequence". No details about how randomisation sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Personnel involved in training not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to participants' group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Clear description of missing outcome data. 5 participants could not be assessed at different time points (1 postintervention and 4 after detraining) due to hospitalisations (n = 3), relocation (n = 1) and parents who declined further evaluation (n = 1). Dropout rate was unbalanced with 28% in the control group and 9% in the intervention group after the detraining period. Intention‐to‐treat analysis was used and missing outcome data (at post‐training or detraining visit) were replaced by baseline data. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in methods were reported in results. Data reported for all time points |
| Other bias | High risk | Some raw data were made available, but there were inconsistencies between raw data and data reported in the original publication. There were significant between‐group differences in primary (VO2 peak) and secondary (strength measures) outcome measures at baseline. |
Santana‐Sosa 2014.
| Study characteristics | ||
| Methods |
Design: single‐centre, parallel RCT Location: Children's Hospital Infantil Universitario Niño Jesús in Madrid, Spain Inclusion criteria: potential participants included 95 outpatient children previously diagnosed with CF by genetic testing and treated at the Children's Hospital Niño Jesús in Madrid. Males or females aged 6 to 17 years and living in the Madrid area (able to attend training sessions) Exclusion criteria: severe lung deterioration (FEV1 < 50% predicted); unstable clinical condition (i.e. hospitalisation within the previous 3 months); Burkholderia cepacia infection or any disorder (e.g. musculoskeletal) impairing exercise Duration: 3‐month study (8 weeks' training, 4 weeks' 'detraining') |
|
| Participants | 20 participants with CF Group demographics Intervention group (n = 10): mean age 11.1 (SEM 1.1) years Control group (n = 10): mean age 10.1 (SEM 1.1) years |
|
| Interventions | 8‐week programme followed by a 4‐week detraining period. All participants received the same standard chest physiotherapy Intervention group: whole body aerobic and weight training 3 times per week, plus 2 daily inspiratory muscle training sessions Control group: inspiratory muscle training only at a low intensity. |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes
Outcomes were measured at baseline, after 8 weeks of training and after 4 weeks of detraining. |
|
| Notes | Additional raw data for all included outcomes provided by the authors. Study authors used the term 'detraining', which is a time period during which no supervised exercise training was provided. The meaning of 'detraining' is consistent with our term 'off training', which also describes a period during which no (partially) supervised physical activity took place, but study participants were not explicitly discouraged from undertaking physical activity. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation to intervention or control group "with block on gender". No details given for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Personnel involved in training not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to participants' group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Clear description of missing outcome data. 3 participants from control group could not be assessed at different time points (1 at postintervention and detraining phase and 2 after detraining phase) due to hospitalisation for lung transplantation preparation (n = 1), infection with Burkholderia cepacia (n = 1) and refusal (n = 1). Unbalanced distribution of dropouts. Dropout rate in control group was 30% versus 0% in intervention group. Intention‐to‐treat analysis was reported, but it was not clear how missing data were handled. |
| Selective reporting (reporting bias) | Low risk | All outcomes detailed in methods were reported in results. Data reported for all time points. |
| Other bias | High risk | Some raw data were made available, but there were inconsistencies between raw data and data reported in the original publication. Significant between‐group differences in primary outcomes (VO2 peak and strength measures) existed at baseline. |
Sawyer 2020.
| Study characteristics | ||
| Methods |
Design: parallel‐design RCT (2 arms). Central randomisation (1:1 ratio); minimisation algorithm with stratification for study site, FEV1 (≥ 70% predicted, 40–69% predicted, ≤ 39% predicted) and ivacaftor treatment Location: Sir Charles Gairdner Hospital (adult service) and Perth Children's Hospital (paediatric service), Perth, Australia Inclusion criteria: males and females aged ≥ 15 years with BMI > 16 kg/m² Exclusion criteria: recent (within previous 4 weeks) pulmonary exacerbation requiring oral or IV antibiotics; comorbidity that would impact on the ability to undertake a maximal exercise test; poorly controlled diabetes; previous lung transplant or current listing for lung transplantation; participation in moderate‐intensity structured exercise ≥ 2 times per week for the previous 3 months, and inability to provide written informed consent Duration: 8 weeks |
|
| Participants | 14 participants with CF Group demographics Intervention group (n = 7): 4 females and 3 males; median age 31 (IQR 29–31) years; FEV1 66% predicted (IQR 45–83); BMI 23.2 (IQR 21.4–34.5) kg/m² Control group (n = 7): 5 females and 2 males; median age 31 (IQR 26–39) years; FEV1 57% predicted (IQR 39–80); BMI 24.6 (IQR 20.5–28.5) kg/m² |
|
| Interventions |
Intervention group: 8‐week low‐volume, high‐intensity interval training programme on a bicycle ergometer. 22 sessions were planned over 8 weeks. Each training session was composed of a 2‐min warm‐up phase (15–20 W), followed by a 30‐second work phase and 30‐second rest period, repeated 6 times. Total duration of each session was about 10 min. The training intensity increased progressively: first session at 60% of peak workload, aiming to achieve a training intensity of 80% of peak workload during the fourth training session. Thereafter, training intensity was increased as rapidly as symptoms of breathlessness and muscle fatigue permit. Each session was individually supervised by a physiotherapist. All sessions were audio recorded. Control group: usual care; no specific exercise programme; participants were contacted once per week (telephone calls, text messages or email) to monitor changes in symptoms, healthcare utilisation and participation in exercise over the preceding week. |
|
| Outcomes |
Primary outcome
Secondary outcomes
Other outcomes (intervention group only)
|
|
| Notes | Author provided raw data for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated on a 1:1 ratio via a central randomisation service (the National Health and Medical Research Council randomisation service). Used a minimisation algorithm to stratify for site of recruitment, lung disease severity (i.e. mild (FEV1 ≥ 70% predicted), moderate (FEV1 40–69% predicted) or severe (FEV1 ≤ 39% predicted)) and the use (or not) of ivacaftor. |
| Allocation concealment (selection bias) | Low risk | Central randomisation service (the National Health and Medical Research Council randomisation service). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Unclear whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to group allocation for follow‐up assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes were reported as planned. All participants completed the trial. No dropouts were reported during the study. Maximal exercise testing was completed by 10/14 participants and submaximal exercise testing by 11/14 participants at the 8‐week follow‐up assessment. Study was registered with the Australian and New Zealand Clinical Trials Registry (12617001271392) and a study protocol was published (Sawyer et al. BMC Sports Science, Medicine and Rehabilitation, 2018;10:19). |
| Selective reporting (reporting bias) | Low risk | No indication of selective reporting. |
| Other bias | High risk | The authors were able to include 14 participants. The sample size calculation defined a target sample size of 40 participants including a 20% loss to follow‐up (n = 32). The planned statistical analyses (i.e. linear models with adjustment for baseline values as covariates and group allocation as fixed effect) could not be realised. Non‐parametric test statistics were applied. Due to the small sample size, some variables of interest were not balanced between groups (e.g. sex, VO2 peak). |
Schneiderman‐Walker 2000.
| Study characteristics | ||
| Methods |
Design: single‐centre, parallel RCT Location: Hospital for Sick Children, Toronto, Canada Inclusion criteria: people with CF aged 7–19 years with a FEV1 > 40% predicted Exclusion criteria: not specified Duration: 3 years |
|
| Participants | 65 participants with CF; 2 groups similar at baseline. 7 dropouts Group demographics Intervention group (n = 30): mean age 13.4 (SD 3.9 years) Control group (n = 35): mean age 13.3 (SD 3.6) years |
|
| Interventions | Long‐term aerobic study Intervention group: minimum of 20 min aerobic activity plus 5 min warm up and cool down 3 times per week Control group: maintained regular activity (control) |
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Unclear whether personnel blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pulmonary function assessors were blinded to group assignment (primary outcome measure). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Clear description and details of 7 dropouts were recorded. Intention‐to‐treat analysis was reported to yield similar results for pulmonary function. Results were only reported for 65 participants who completed the 2‐year follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcome detailed in methods were reported in results. Data reported for all time points. |
| Other bias | Unclear risk | Groups similar at baseline. Stated the inclusion criteria but not the exclusion criteria. Described statistical methods used in analysis. |
Selvadurai 2002.
| Study characteristics | ||
| Methods |
Design: single‐centre, parallel RCT; hospital admission for recurrent chest infections Location: Royal Alexandra Hospital for Children, Sydney, Australia Inclusion criteria: children with CF, aged 8–16 years who were admitted to the Royal Alexandra Hospital for Children for the treatment of an infectious pulmonary exacerbation Exclusion criteria: children with known pulmonary hypertension, or who required daytime oxygen prior to the pulmonary exacerbation that led to the hospital admission |
|
| Participants | 66 children with CF (28 boys, 38 girls). No dropouts Group demographics Intervention group 1: aerobic exercise training (n = 22): mean age 13.2 (SD 2.0) years, 9 boys and 13 girls Intervention group 2: resistance exercise training (n = 22): mean age 13.1 (SD 2.1) years, 10 boys and 12 girls Control group (n = 22): mean age 13.2 (SD 2.0) years, 9 boys and 1 girl |
|
| Interventions | Short‐term aerobic and anaerobic/strength training study during hospital admission (mean duration 18.7 days, range 14–36 days).
Intervention group 1: 30‐min supervised aerobic exercise training 5 times per week Intervention group 2: 30‐min supervised resistance training 5 times per week Control group: no specific training |
|
| Outcomes |
Reported at hospital discharge and 1 month after hospital discharge. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation in sets of 6. No details given for generation of sequence. |
| Allocation concealment (selection bias) | Low risk | Concealed information inside opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Unclear whether personnel blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Stated no dropouts. |
| Selective reporting (reporting bias) | Unclear risk | Did not report on all secondary outcomes detailed in methods (e.g. VE, VCO2, respiratory quotient) in results. Data reported for all time points. |
| Other bias | Low risk | Clearly stated inclusion and exclusion criteria. Described statistical methods used to analyse data. |
Turchetta 1991.
| Study characteristics | ||
| Methods |
Design: single‐centre, parallel RCT, hospital admission for routine assessment of clinical condition Location: Ospedale Pediatrico Bambino Gesu, Rome, Italy Inclusion criteria: not specified Exclusion criteria: not specified Duration: 2 weeks |
|
| Participants | 12 children with CF, 8 boys, mean age 12.3 years No group demographics available |
|
| Interventions | Short‐term aerobic study Intervention group: 20 min running or treadmill per day for 2 weeks Control group: normal hospital treatment |
|
| Outcomes |
|
|
| Notes | This study was only reported in a single abstract and, therefore, information was limited. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no details given for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind participants to intervention. Unclear whether personnel blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details of dropouts or whether intention‐to‐treat analysis was used. |
| Selective reporting (reporting bias) | Unclear risk | This is an abstract so unable to assess if all outcome used in methods were reported in results. Data were reported for all time points. |
| Other bias | Unclear risk | Did not state inclusion or exclusion criteria, neither do they describe the methods of statistical analysis used. |
6MWT: 6‐minute walk test; Awe‐Score CF: Alfred Wellness Score for CF; BMI: body mass index; bpm: beats per minute; BW: bodyweight; CAMM: Child and Adolescent Mindfulness Measure; CF: cystic fibrosis; CFRD: cystic fibrosis‐related diabetes; CFTR: cystic fibrosis transmembrane conductance regulator; CFQ‐R: Cystic Fibrosis Questionnaire – Revised; CPET: cardiopulmonary exercise test; ERV: expiratory reserve volume; FEF25–75: forced mid‐expiratory flow between 25% and 75% of vital capacity; FEV1: forced expiratory volume in 1 second; FFMS: Five Facet Mindfulness Scale; FRC: functional residual capacity; FVC: forced vital capacity; HADS: Hospital Anxiety and Depression Scale; HAES: Habitual Activity Estimation Scale; HbA1c: glycated haemoglobin; HRQoL: health‐related quality of life; IC: inspiratory capacity; IGT: impaired glucose tolerance; IV: intravenous; LCI: Lung Clearance Index; min: minute; MP: mean power; MSWT: modified shuttle walk test; MVV: maximal voluntary ventilation; OGTT: oral glucose tolerance test; PACES: Physical Activity Enjoyment Scale; PEFR: peak expiratory flow rate; PFS: progression‐free survival; PImax: maximum inspiratory mouth pressure; PP: peak power; PSQI: Pittsburgh Sleep Quality Index; Raw: airways resistance; RCT: randomised controlled trial; RER: respiratory exchange ratio; RR: respiratory rate; RV: residual volume; SaO2: arterial oxygen saturation; SpO2: peripheral blood oxygen saturation; SD: standard deviation; SEM: standard error of the mean; sGAW: specific airways conductance; TLC: total lung capacity; VAS: Visual Analogue Scale; VE: minute ventilation; VE peak: peak minute ventilation; VO2 peak: peak oxygen uptake; VCO2: carbon dioxide production; VO2: oxygen uptake; W: watt; WAnT: Wingate Anaerobic Test.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12620001237976 | No control group with no physical activity. |
| Alarie 2012 | Study compared acute cardiovascular response in participants playing different active video games. No control group included. |
| Albinni 2004 | Study designed with the exercise group as the control group; therefore, we could not compare data with baseline; no physical exercise training according to our protocol. |
| Almajan‐Guta 2011 | No information available about whether a publication is planned for this study. We could not find contact details of the authors to get more information about the study status. |
| Amelina 2006 | IMT training and not physical exercise training according to our protocol. |
| Andreasson 1987 | Not a randomised controlled study. |
| Aquino 2006 | Study was designed to compare the effectiveness of a single treatment session of exercise and PEP on sputum clearance. Participants in this study did not undertake a programme of physical training. |
| Asher 1982 | IMT training and not physical exercise training according to our protocol. |
| Balestri 2004 | Study designed to compare the effectiveness of a single treatment session of exercise and PEP on sputum clearance. Participants in this study did not undertake a programme of physical training. |
| Balfour Lynn 1998 | Not a physical exercise training study; comparison of different tests for assessing exercise capacity. |
| Barry 2001 | Not a randomised controlled study. |
| Bass 2019 | No control group with no physical exercise training. |
| Bellini 2018 | Not a physical exercise training study. |
| Bieli 2017 | Study of respiratory muscle endurance training; not a physical exercise training study. |
| Bilton 1992 | Study designed to compare the effectiveness of a single treatment session of exercise or physiotherapy or exercise and physiotherapy on sputum clearance and lung function. Participants in this study did not undertake a programme of physical training. |
| Bongers 2015 | Study evaluating the clinical usefulness of the steep ramp test. Not a physical exercise training study. |
| Calik‐Kutukcu 2016 | No control group with no physical exercise training. |
| Cantin 2005 | Not a physical exercise training study. |
| Chang 2015 | Study of methods for evaluating muscle function and not a physical exercise training study. |
| Chatham 1997 | Study involved respiratory muscle training exclusively. This intervention did not constitute physical training as defined within our protocol. |
| Combret 2018 | Not a physical exercise training study. |
| Combret 2021 | Not a physical activity intervention. |
| Cox 2013 | Not a physical exercise training study. |
| de Jong 1994 | Not a randomised controlled study. |
| del Corral Nunez‐Flores 2014 | No control group with no physical training. |
| de Marchis 2017 | No control group with no physical exercise training. |
| Dwyer 2011 | Study duration insufficient. |
| Dwyer 2017 | Acute exercise study. Study duration insufficient. |
| Dwyer 2019 | This randomised cross‐over study evaluated the acute effects of airway clearance techniques and exercise on mucociliary clearance. Insufficient study duration. |
| Edlund 1986 | Not a randomised controlled study. |
| Falk 1988 | Study designed to compare the effectiveness of a single treatment session of exercise or PEP on lung function. Participants in this study did not undertake a programme of physical training. |
| Giacomodonato 2015 | Study of respiratory muscle endurance training and not a physical exercise training study. |
| Gruber 1998 | No control group with no physical exercise training. |
| Gruet 2012 | No control group with no physical exercise training. |
| Happ 2013 | Qualitative descriptive study nested within a randomised controlled trial of a self‐regulated, home‐based exercise programme. Outcomes of this study were not relevant for this review. |
| Haynes 2016 | Evaluation of the incremental step test, not a study of physical training. |
| Heijerman 1992 | Not a randomised controlled study. |
| Housinger 2015 | No contact details available online. Very unlikely that this study will be published. |
| Hütler 2002 | Not a physical exercise training study. |
| IRCT20161024030474N4 | Not a randomised controlled study. |
| Irons 2012 | Not a physical exercise training study; examined effect of a singing programme compared to no singing. |
| Johnston 2004 | No information available about whether a publication is planned for this study. We could not find contact details of the authors to obtain more information about the study status. |
| Kaak 2011 | Not a physical exercise training study. |
| Kaltsakas 2021 | Study compared interval versus continuous exercise training. No control group with no physical exercise training. |
| Kriemler 2016 | Study duration insufficient: only 3 single‐day interventions on non‐consecutive days of 1 week. |
| Kuys 2011 | Compared Nintendo Wii exercise training to an existing exercise programme; no control group with no physical training. |
| Lang 2019 | No control group with no physical exercise training. Study evaluated the efficacy of a telehealth physiotherapy intervention. Control group participants engaged in a home exercise programme and recorded their activities in a self‐reported paper‐based exercise diary. |
| Lannefors 1992 | Study designed to compare the effectiveness of a single treatment session of exercise and FET or PEP and FET or postural drainage, thoracic expansion exercises and FET on mucous clearance. Participants in this study did not undertake a programme of physical training. |
| Lima 2014 | No physical exercise training study; study looked at effect of non‐invasive ventilation on exercise capacity and lung function. |
| Lowman 2012 | No control group with no physical training. |
| Macleod 2008 | Not a physical exercise training study. |
| Mandrusiak 2011 | The first author of this study confirmed that the study will not be published. |
| Martinez Rodriguez 2017 | No control group with no physical training. |
| Montero‐Ruiz 2020 | Not a physical exercise training study. |
| Moola 2017 | Study assessed the feasibility of a parent‐mediated physical activity counselling programme for children with CF. The programme did not include supervised or partially supervised exercise sessions. |
| NCT00129350 | Study is unlikely to be published (last status update 2005). If study publications are identified in future searches, it will be considered for inclusion in this review. |
| NCT00792194 | The investigator informed us that the trial has been terminated prematurely due to recruitment problems and that no paper will be published. |
| NCT01759342 | No control group with no physical exercise training. |
| NCT02199340 | Not a physical exercise training study. |
| NCT02277860 | Not a randomised controlled study; single arm trial of physical exercise. |
| NCT02715921 | Not a randomised controlled study; single arm trial of physical exercise. |
| NCT02821130 | Study of CFTR potentiator therapy and effects on exercise capacity. |
| NCT02875366 | Study of CFTR potentiator therapy and effects on exercise capacity. |
| NCT03117764 | Not a randomised controlled study; study of the effect of antibiotics on muscular strength and not physical training. |
| NCT03420209 | Study focused on proprioceptive neuromuscular facilitation in children with chronic respiratory diseases. This type of training aims to improve flexibility and range of motion. It is not a classical exercise or physical intervention study according to our protocol. |
| NCT04888767 | No control group with no exercise training. Study compared high‐intensity interval training with moderate‐intensity continuous exercise training. |
| NTR2092 | IMT study. Not a physical exercise training study with a control group with no exercise. |
| Oliveira 2010 | We contacted 1 author to request more information about this study and discover whether a publication is planned. No response received. |
| Orenstein 1981 | Not a randomised controlled study. |
| Orenstein 2004 | Compared aerobic training to upper‐body strength training; no control group with no physical training. |
| Ozaydin 2010 | IMT training and not physical exercise training according to our protocol. |
| Patterson 2004 | Study evaluated the efficacy of the test of incremental respiratory endurance; not a physical training study. |
| Petrovic 2013 | Not a randomised controlled study. |
| Phillips 2008 | No contact details available online. Very unlikely that this study will be published. |
| Pryor 1979 | Not a physical exercise training study. |
| Radtke 2018b | Not a physical exercise training study. |
| Rand 2012 | Not a physical exercise training study. Study was designed to develop an incremental field exercise test for children with CF. |
| RBR‐34677v | Not a randomised controlled study. |
| RBR‐5g9f6w | No control group with no physical exercise training. |
| Reix 2012 | Acute study comparing exercise with expiratory breathing manoeuvres to breathing techniques for airway clearance. |
| Reuveny 2020 | No control group with no physical exercise training. |
| Ruddy 2015 | Study registered as a randomised controlled trial but study results were published without a control group. |
| Salh 1989 | Not a randomised controlled study. |
| Salonini 2015 | Comparison of 2 exercise interventions (Xbox Kinect versus stationary cycle). No control group with no physical training. |
| Shaw 2016 | No control group with no physical exercise training. |
| Spoletini 2020 | Not a physical exercise training study. |
| Stanghelle 1998 | Not a randomised controlled study. |
| Tuzin 1998 | Not a randomised controlled study. |
| Vallier 2016 | Study to evaluate MSWT and not a study of physical training. |
| Vivodtzev 2013 | Study evaluated neuromuscular electrical stimulation prior to endurance training in people with CF. No control group with no physical training. |
| Ward 2018 | Study investigated the use of exercise as a stand‐alone form of airway clearance in adults with CF. No control group with no physical exercise training. |
| Wheatley 2015 | Intervention only given on 3 single days; comparison of physical training and albuterol for airway clearance. |
| White 1997 | Not a physical exercise training study. |
| Young 2019 | Not a physical exercise training study. |
| Zeren 2019 | IMT training and not physical exercise training according to our protocol. |
CF: cystic fibrosis; CFTR: cystic fibrosis transmembrane conductance regulator; FET: forced expiration technique; IMT: inspiratory muscle training; MSWT: modified shuttle walk test; PEP: positive expiratory pressure.
Characteristics of studies awaiting classification [ordered by study ID]
Bishay 2017.
| Methods |
Design: parallel RCT, single‐centre study Location: Boston Children's Hospital, Boston, Massachusetts, USA Duration: 24 months |
| Participants |
Enrolment goal: 80 participants with CF Inclusioncriteria: men and women aged ≥ 18 years with confirmed diagnosis of CF; able to complete at least level 1 of the baseline exercise fitness test; participants must not have required IV antibiotics for a CF exacerbation within 30 days of starting the study Exclusioncriteria: pregnancy at enrolment; history of CF exacerbation requiring IV antibiotics within the last month; use of a fitness tracker or similar product within 6 months of enrolment |
| Interventions | Study evaluates whether use of a Fitbit device and an exercise prescription is associated with increased daily activity and, in turn, increased exercise tolerance in young adults with CF. Interventiongroup: given a Fitbit and followed for 1 year, completing surveys and exercise tests Controlgroup: usual care for 1 year, then offered a Fitbit in the 2nd year. Followed to assess use of Fitbit and health outcomes |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes |
Cox 2019.
| Methods |
Design: multicentre, parallel‐design RCT. Blinding: outcome assessor Location: 8 Australian sites (Alfred Health, Monash Health and Royal Children's Hospital, Victoria; Royal Hobart Hospital, Tasmania; Royal Prince Alfred Hospital, Westmead Hospital and Children's Hospital at Westmead, New South Wales; Royal Adelaide Hospital, South Australia) Duration: 12 weeks (with 3‐month and 12‐month follow‐up for different outcomes) |
| Participants |
Enrolment goal: 75 participants with CF Inclusion criteria: confirmed diagnosis of CF; age 12–35 years (inclusive); hospital inpatient admission (including hospital in the home) for IV antibiotic therapy for a respiratory cause; able to provide informed consent; able to access the Internet via computer or mobile device Exclusion criteria: severe comorbidity limiting mobilisation or physical activity participation (e.g. orthopaedic, cardiac or neurological condition); lung transplant recipients; pregnancy; participants (or parents) are unable to provide informed consent |
| Interventions | This trial investigates whether an Internet‐based application to improve physical activity participation is more effective than usual care in the period following hospitalisation for a respiratory exacerbation. All participants in both groups will be provided with standardised information regarding general physical activity recommendations for adolescents and young adults. Intervention group: in addition to usual care (see information for control group), participants will have access to an Internet‐based physical activity platform (ActivOnline: www.activonline.com.au) for the 12‐week intervention period. ActivOnline allows users to track their physical activity, set goals, and self‐monitor progress. When logging into ActivOnline, participants will be prompted to set weekly exercise and physical activity goals, as well as to record details of their physical activity or exercise sessions, including total time and step count. To support recording of daily step count, participants may use their own activity tracker or mobile telephone. A pedometer (Yamaxdigiwalker SW500, Yamasa Tokei Keiki Co, Ltd, Tokyo, Japan) will be provided to participants on request. Data entered into ActivOnline are displayed in numerical and graphical form to allow visualisation of progress over time. Participants can choose the frequency of use of ActivOnline, as data can be entered retrospectively. If no activity has been logged for 3 days, a standardised alert message will be issued by the ActivOnline program and emailed to the participant. Participants in the intervention group will also be able to communicate with research clinicians directly via the messaging system contained within ActivOnline about the trial or their clinical status, should they require (Cox et al. BMC Pulmonary Medicine 2019;19:253) Control group: usual care. Participants will be provided with age‐appropriate information regarding recommended guidelines for physical activity participation. Participants will be referred to a free online resource (www.nhs.uk/Livewell/fitness/Pages/physical-activity-guidelines-for-young-people.aspx) containing guidelines and information regarding amount and intensity of daily physical activity participation. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | Study characteristics were extracted from the information provided in the Australian New Zealand Clinical Trials Registry (ANZCTR) and the published protocol paper (Cox et al. BMC Pulmonary Medicine 2019;19:253). |
IRCT20190407043190N1.
| Methods |
Design: multicentre, parallel‐design, RCT Location: University of Medical Sciences, Ahvaz, Khouzestan, Iran and Aboozar Hospital, Ahvaz, Khouzestan, Iran Duration: 4 weeks |
| Participants |
Enrolment: 70 participants with CF Inclusion criteria: willingness to participate in the study (informed consent); understanding of Persian language; no acute and chronic psychological and physical illnesses; moderate disease severity based on physician's diagnosis (not further specified); ability to perform physical activity; absence of concomitant disease (not further specified) Exclusion criteria: age < 8 years and > 12 years; acute and chronic mental and physical disease |
| Interventions |
Intervention group: participants will receive, regularly according to their interest, aerobic physical exercises, such as cycling, swimming, walking, dancing, playing ball, rope skipping, jumping, and stretching guidelines for upper limbs, body and lower limbs (gymnastics) in 4 sessions of physical activity Control group: no training |
| Outcomes |
Primaryoutcome
Secondary outcomes: none |
| Notes | Description of study was unclear. Study was retrospectively registered. |
NCT03100214.
| Methods |
Design: parallel single‐centre RCT; outcome assessor (exercise supervisor) blinded Location: Hospital de Clínicas de Porto Alegre, Brazil Duration: up to 14 days |
| Participants |
Estimated enrolment: 68 participants with CF Inclusioncriteria: males and females age 16–50 years; diagnosed with CF according to consensus criteria (Yankaskas 2004) and regularly followed up in the Hospital de Clínicas de Porto Alegre Programme for Adolescents and Adults with CF; admitted to hospital (for ≥ 24 hours) due to exacerbation of lung disease Exclusioncriteria: cardiac, orthopaedic or trauma complications that make it impossible to perform the proposed exercises; pregnancy; haemodynamic instability, massive haemoptysis, pneumothorax and continuous use of non‐invasive ventilation |
| Interventions |
Interventiongroup: aerobic and anaerobic exercise 5 times a week during the hospitalisation period, with sessions lasting about 1 hour; programme beginning within 48 hours of admission Controlgroup: physiotherapeutic follow‐up (including respiratory physiotherapy, inhalation therapy and techniques for removal of secretions) performed by the physiotherapist of the programme for adults with CF during the hospitalisation period |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Notes | Study aims to evaluate the effects of an early rehabilitation programme based on aerobic training and muscle strength training in adolescents and adults with CF hospitalised at Hospital de Clinicas de Porto Alegre for exacerbation of lung disease. |
NCT04293926.
| Methods |
Design: single‐centre, parallel‐design, RCT Location: Universidad Europea de Madrid, Madrid, Spain Inclusion criteria: diagnosis of CF, age 6–18 years, mild‐to‐moderate lung function levels; written informed consent form by legal guardian and patient Exclusion criteria: active smoking, exacerbation in last 3 months, presence of gastrostomy, use of beta‐blocker drugs, diagnosed heart disease, alterations in the locomotor system Duration: 8 weeks |
| Participants | 19 participants were enrolled in this study (status: completed) |
| Interventions | Study aimed to assess the effects of a resistance exercise training programme on heart rate variability in children and adolescents with CF. Intervention group: 8‐week individualised and guided resistance exercise training programme (3 sessions per week, 60 min per session). Training prescription was individualised and based on the 5 repetition maximum test (60–80%). Upper‐ and lower‐limb exercises were performed, including seated bench press, seated lateral row and leg press. Control group: routine recommendations by the paediatrician, including specific lifestyle advice. No exercise training programme |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Notes | 6 primary outcomes were defined in this study, all of which are not relevant for this review. |
Powers 2016.
| Methods |
Design: parallel RCT ("Do More, B'More, Live Fit"), single‐centre study Location: Johns Hopkins University, Baltimore, Maryland, USA Duration: 6 months |
| Participants |
Enrolment goal: 60 participants with CF Inclusioncriteria: males and females aged 12–21 years with CF and cared for at Johns Hopkins; participants must have a smartphone or computer (or both) with universal serial bus (USB) to set‐up Fitbit Flex Exclusioncriteria: FEV1 < 40% predicted; individuals already participating in vigorous physical activity (as assessed by the study team) in year‐round organised sports or aerobic exercise for longer than 30 min more than 5 times per week (or organised sports and aerobic exercise) may or may not be included in this study at the discretion of the principal investigator and study team |
| Interventions |
Interventiongroup: at baseline assessment, participants given individualised exercise prescriptions with the aim of achieving 30 min of an endurance‐style exercise (team sports, walking, jump roping, stair climbing or more complex Tabata‐style workouts) 5 times per week for 6 months. At 4–6 weeks and 8–10 weeks after enrolment, participants attend a follow‐up 30‐min session which will vary based on initial assessment and previous exercise prescription success, but will include strength training for major muscles groups or flexibility exercises with yoga (or both), as well as reinforcement of previously learned techniques with additional individualised recommendations. Participants will also receive motivational messages starting 14 days after enrolment via preferred contact method (SMS, telephone call, email) every 3–4 days over the 6‐month study period. Participants also given access to "Do More, B'More, Live Fit" web page, which includes spotlighted exercises, instructional exercise photos and videos; also invited to join the "Do More, B'More, Live Fit" Activity Group via the Fitbit Dashboard and to friend the study team members and other exercise‐intervention participants in order to take part in Fitbit step‐goal challenges. Controlgroup: at baseline assessment, the Fitbit daily step goal is set at the manufacturer standard 10,000 steps. At routine clinic visits, baseline and follow‐up assessments (3‐ and 6‐month clinic visits) participants given generic, non‐personalised encouragement and recommendations (if requested by the participant) for physical activity. At the 3‐ and 6‐month visits, exercise is reinforced with generic encouragement, Fitbit data are exported and reviewed for any missing data due to equipment failure or user error. |
| Outcomes |
Primaryoutcomes
Secondaryoutcomes
|
| Notes | This study evaluates the "Do More, B'More, Live Fit", a 6‐month fitness programme designed to optimise exercise habits of people with CF through structured exercises with personalised coaching, exercise equipment including the Fitbit Flex, online support and motivational messages delivered electronically. The intervention incorporates fitness preferences and encompasses endurance, strength and flexibility exercises while adjusting to physical fitness needs. The hypothesis is that intervention participants will have increased and sustained engagement and better health outcomes compared to control group participants. |
6MWT: 6‐minute walk test; BREQ‐2: Behavioral Regulation in Exercise Questionnaire‐2; CF: cystic fibrosis; CFQ‐R: Cystic Fibrosis Questionnaire – Revised; FEF25–75: forced mid‐expiratory flow between 25% and 75% of vital capacity; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; HADS: Hospital Anxiety and Depression Scale; HAES: Habitual Activity Estimation Scale; HRQoL: health‐related quality of life; IV: intravenous; LCI: Lung Clearance Index; min: minute; MSWT: modified shuttle walk test; RCT: randomised controlled trial; R‐R intervals: intervals between successive heartbeats; SD: standard deviation.
Characteristics of ongoing studies [ordered by study ID]
Curran 2020.
| Study name | Steps Ahead: optimising physical activity in adults with cystic fibrosis: study protocol for a pilot randomised trial using wearable technology, goal setting and text message feedback ClinicalTrials.gov identifier: NCT03672058 Study protocol (version 3, 16 June 2021): hrbopenresearch.org/articles/3-21 |
| Methods |
Design: pilot, parallel‐design RCT; single centre Location: adult CF Unit, University Hospital Limerick, Ireland Duration: 24 weeks (12‐week intervention; 12‐week follow‐up) |
| Participants |
Enrolment goal: 50 participants with CF Inclusion criteria: age ≥ 18 years; confirmed diagnosis of CF (based on CF‐causing mutations or a sweat chloride concentration during 2 tests of > 60 mmol/L, or both); clinically stable individuals with CF attending University Hospital Limerick, determined by those who are not experiencing a pulmonary exacerbation. Pulmonary exacerbation is defined as acute or subacute worsening of respiratory symptoms which warrant change in treatment (i.e. new oral or intravenous antibiotics); access to a smartphone/tablet to access, and ability to upload, Fitbit application; capacity and willingness to give explicit informed consent Exclusion criteria: FEV1 < 25% predicted; on the waiting list for lung transplantation and have undergone lung transplantation; exacerbation in the 4 weeks prior to the study. Patients can undergo testing once they are finished their antibiotics and deemed clinically stable by the Respiratory Consultant; dependent on supplemental oxygen for exercise; pregnancy; any cardiac, neurological or musculoskeletal impairment that may impact on their ability to participate in the study; participation in another clinical trial up to 4 weeks prior to the first baseline visit |
| Interventions | The intervention consists of wearable technology, text message feedback and goal setting. Intervention group: participants are provided with wearable technology (Fitbit Charge 2), and educated on how to use it. It will be linked to an online monitoring system (Fitabase). Fitabase, the online monitoring system, enables the physiotherapists to access step count data remotely. The physiotherapist discusses the participant's physical activity levels (as measured at baseline by an accelerometer) and individual, patient‐centred physical activity goals are set with each participant. Participants are encouraged to write a minimum of 3 goals. Participants are asked to set a step count target for weeks 4, 8 and 12. Goals will be individualised to the participant, taking into account their preferences. Participants receive a weekly 1‐way personalised text message by their physiotherapist for 12 weeks. The text messages in this study are texts with positive reinforcement on step count attained by the participant. Control group: participants are provided with a Fitbit Charge 2 and educated on how to use it. It will be linked to "Fitabase" for data collection purposes. Participants receive no feedback on their physical activity levels throughout the study period. Follow‐up: at week 12, both groups will have outcome measures reassessed. Both groups will continue with the Fitbit Charge 2 only for the following 12 weeks. At the end of the 24 weeks, participants will have all outcome measures repeated. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Recruitment began in January 2019 |
| Contact information | Contact: Roisin Cahalan, PhD: telephone +353 61 202959 ext +35361202959; Email: roisin.cahalan@ul.ie Maire Curran, BSc: telephone +353 61 482151 ext +35361202959; Email: maire.curran@ul.ie |
| Notes |
ISRCTN92573472.
| Study name | The evaluation of a 12‐week partially supervised, self‐regulated exercise intervention in patients with cystic fibrosis (CF‐Ex) |
| Methods |
Design: single‐centre RCT Location: Dublin City University, Glasnevin, Dublin, UK Duration: 12 weeks |
| Participants |
Enrolment goal: 30 participants with CF Inclusion criteria: established diagnosis of CF (positive sweat chloride or genetic identification test); residing in Ireland; age ≥ 18 years; lung function ≥ 50% predicted Exclusion criteria: undergone lung transplantation; culturing MRSA, NTM or Burkholderia cepacia |
| Interventions | 12‐week partially supervised and self‐regulated exercise intervention Intervention group: exercise manual (hard copy) and access to an online exercise diary for a 12‐week period. Over this period, the exercise group will receive a Fitbit device to track daily steps and active min. Control group: usual care. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 9 September 2019 Trial end date: 9 July 2020 |
| Contact information | Miss Nicola Hurley: XB26, Dublin City University, Dublin 9, Ireland; telephone: +353 017008470; Email: nicola.hurley5@mail.dcu.ie |
| Notes | Study retrospectively registered |
Monteiro 2019.
| Study name | Effects of aerobic interval training on glucose tolerance in children and adolescents with cystic fibrosis: a randomized trial protocol |
| Methods |
Design: parallel design, RCT. Double blinded (investigator and outcome assessors) Location: 2 hospitals in the Brazilian states of Rio Grande do Norte and Paraíba Duration: 8‐week intervention with 8‐week follow‐up |
| Participants |
Enrolment goal: 20 participants with CF Inclusion criteria: diagnosis of CF, according to the Brazilian Guidelines for diagnosis and treatment of CF; age 6–18 years; boys males and females Exclusion criteria: inability to follow the study protocol (not further specified); exacerbation of the disease, with hospitalisation required during the intervention period; pregnancy |
| Interventions | The intervention aims to evaluate the effects of anaerobic interval training on glucose tolerance in children and adolescents with CF. Intervention group: participants will take part in an aerobic interval training programme conducted at home, 3 times a week on alternating days, and using a cycle ergometer for lower limbs (Altmayer Sport). Each session will start with 5 min warm‐up and end with 5 min cool‐down at 30–40% of the maximum heart rate. The training in the initial 2 weeks will be carried out in 6 sessions of 20 seconds, reaching 70–80% of maximum heart rate, interspersed by 2 min of active rest, and reaching 50–60% of maximum heart rate. Progression will be carried out every 2 weeks by adjusting the time and the number of exercise sessions. Participants will be given a diary before the start of the programme to record information about the disease exacerbation, training heart rates, modified Borg scale, and signs and symptoms observed during training. The diary will be returned to the research team after completion of the study for evaluation of adherence to the proposed intervention. 1 member of the research team will maintain weekly contact via mobile phone with the parent/guardian to stimulate the intervention and minimise possible deviations from the protocol. Control group: participants and their parents/caregivers will receive an educational intervention, which will be administered through an interactive presentation lasting 20 min. The presentation will address physiopathology, complications, treatments (medical and physiotherapeutic), physical exercises and prevention of exacerbation. Practical demonstrations of routine care such as the use of inhalation devices, bronchial hygiene techniques and medication intake will be performed. |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 4 February 2019 |
| Contact information | Karolinne Monteiro, MSc: telephone: +5584996387722; Email: karolsm@outlook.com.br Thayla Santino, MSc: telephone: +5583999424386; Email: thaylaamorim@gmail.com |
| Notes | Trial registration: ClinicalTrials.gov (NCT03653949) Outcomes that are not relevant for this review: change in functional exercise capacity measured with the 3‐min step test at baseline, after 8 and 16 weeks; and change in respiratory muscle strength (i.e. maximum expiratory pressure), measured at baseline, after 8 and 16 weeks. |
NCT03273959.
| Study name | Program of exercises during the hospitalization of children and adolescents with cystic fibrosis |
| Methods |
Design: parallel, single‐centre RCT; single blinded (outcome assessor) Location: Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil Duration: 14 days |
| Participants |
Estimated enrolment: 50 participants hospitalised for treatment of a pulmonary exacerbation Inclusion criteria: males and females with CF; age 6–18 years and followed by the Pediatric Pulmonology Team at Hospital de Clínicas de Porto Alegre; hospital admission defined as "a stay of 24 hours or more in any Hospital de Clinicas de Porto Alegre unit"; exacerbation of lung disease defined as the presence of ≥ 1 of the following: change in sputum volume and colour, new or enlarged haemoptysis, increased cough, increased dyspnoea, malaise, fatigue, lethargy, fever, anorexia or weight loss, headache or pain in the sinuses, alteration of the pulmonary auscultation, non‐FEV1 decrease of > 10%, radiological [sic], eradication of new bacteria Exclusion criteria: cardiac, orthopaedic or trauma complications that make it impossible to perform the proposed exercises; haemodynamic instability, massive haemoptysis, pneumothorax; continuous use of non‐invasive ventilation; pregnancy |
| Interventions |
Intervention group: routine physical therapy plus exercise programme in the form of a booklet and guided by a health professional. Programme includes exercises such as punching, climbing and descending stairs, sit and stand, push‐up on the wall, cycling and others, performed 5 times per week. Participants record their training in a diary. The programme is not supervised. Control group: routine physical therapy during hospitalisation |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 28 August 2017 |
| Contact information | Hospital de Clínicas de Porto Alegre, Brazil, 90035‐903 Bruna Ziegler: telephone: +55 51991221192; E‐mail: brunaziegler@yahoo.com.br Taiane Feiten: telephone: +55 51991539788; E‐mail: taifeiten@gmail.com |
| Notes | Recruitment status: unknown (latest update 6 September 2017); estimated study completion date: 28 March 2019 Contacted Ms Ziegler for more information about the status of the study (30 June 2021). |
NCT03970369.
| Study name | Motivated to move: a study to determine the feasibility of self‐monitoring physical activity in youth |
| Methods |
Design: pilot parallel, single‐centre RCT, no blinding Location: Exercise Medicine Clinic at McMaster Children's Hospital, Hamilton, Ontario, Canada Duration: 6 months (3 study visits over 6 months) |
| Participants |
Estimated enrolment: 30 participants Inclusion criteria: male and females with CF; aged 7–18 years; newly referred to the Exercise Medicine Clinic (i.e. either 1st or 2nd visit) Exclusion criteria: inability to communicate in English |
| Interventions | Participants at the Exercise Medicine Clinic receive individualised physical activity prescriptions to follow for the next 3 months. Intervention group: participants ("Monitor group") wear a step counter and receive personalised goals including feedback. Control group: participants receive the activity prescription including personalised goals ("Usual care"), but will not receive a step counter. Physical activity monitoring will be performed in all participants using an accelerometer which is worn around the waist for 7 days at baseline, 3‐ and 6‐month study visits. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 20 June 2019 |
| Contact information | McMaster University Exercise Medicine Clinic at McMaster Children's Hospital, Hamilton, Ontario, Canada Principal Investigator: Joyce Obeid, PhD; Contact: Clinical Research Co‐ordinator: telephone: 905‐521‐2100 ext 75620; E‐mail: proudfna@mcmaster.ca |
| Notes |
NCT04249999.
| Study name | ActivOnline: Physical Activity in Cystic Fibrosis Trial UK (ActiOnPACTUK) |
| Methods |
Design: parallel‐design, open‐label, RCT. Follow‐up assessments by blinded outcome assessors Location: University of Exeter, UK Duration: 12‐week intervention and 24‐week follow‐up |
| Participants |
Enrolment goal: 94 participants with CF Inclusion criteria: confirmed diagnosis of CF; aged 12–35 years (inclusive); able to provide informed consent/assent; able to access the Internet via computer or mobile device Exclusion criteria: presence of severe comorbidity limiting mobilisation or physical activity participation (e.g. orthopaedic, cardiac or neurological condition); previous lung transplantation; pregnancy; unable to provide informed consent/assent |
| Interventions | Physical activity intervention with an online platform to monitor daily activity Intervention group: access to online physical activity platform (www.activonline.com.au) in addition to usual care Control group: no access to online physical activity platform. Continue with usual care |
| Outcomes |
Primary outcomes
Secondary outcomes
Other outcomes
|
| Starting date | 7 May 2020 Recruitment status: active, not recruiting (30 June 2021) |
| Contact information | Professor Craig Williams: Director: Children's Health & Exercise Research Centre (CHERC) Sport and Health Sciences, University of Exeter, Exeter, UK; telephone: +44 (0)1392 724890; Email: C.A.Williams@exeter.ac.uk |
| Notes | The study team informed us about the following changes in the study design on 3 July 2021: in light of the ongoing COVID‐19 pandemic, the study team plans to perform the research activities online (including recruitment and consent), with data capture/measurements being performed by participants in their home, with questionnaires and monitors delivered by post (according to the original protocol). The intervention itself, and timelines for participation, remain the same as before. This has a 2‐fold objective, in that these changes will 1) minimise exposure risk to the target population (people with CF) who are still being advised to 'shield' at home, by removing visits to hospital; and 2) reduce burden on NHS staff and sites by removing the need to assist with recruiting/consenting participants and performing measures. These proposed changes will not adversely affect anyone already on the trial, as the COVID‐19 pandemic prevented recruitment throughout 2020 and therefore this project has yet to recruit its first participant. |
NCT04543929.
| Study name | Effects of innovative aerobic exercise training in cystic fibrosis |
| Methods |
Design: single‐centre, open‐label, parallel‐design RCT; no blinding Location: University of Kansas Medical Center, USA Duration: 12 weeks |
| Participants |
Enrolment goal: 9 participants with CF Inclusion criteria: diagnosis of CF; prescribed and taking for 28 days ivacaftor‐tezacaftor‐elexacaftor (Trikafta); aged ≥ 18 years Exclusion criteria: aged ≤ 17 years; not eligible for ivacaftor‐tezacaftor‐elexacaftor (Trikafta); inability to exercise; pregnancy; status after lung transplantation; already participating in > 150 min of aerobic exercise per week |
| Interventions | This study evaluates the effectiveness of standard of care therapy plus exercise compared to standard of care only for improving cardiorespiratory fitness. Intervention group: partially supervised and home‐based exercise training (exercise prescription plus standard of care) Control group: standard of care (no exercise prescription) |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | 11 February 2020 |
| Contact information | University of Kansas Medical Center, Kansas City, Kansas, USA Christine Morgan: telephone: 00 1 913‐588‐1572; Email: cmorgan6@kumc.edu Larry Scott: telephone: 00 1 913‐588‐1572; Email: lscott2@kumc.edu |
| Notes | The primary outcome is broadly defined and not clear to the authors of this review. |
NCT04683809.
| Study name | The effects of telerehabilitation on quality of life, anxiety and depression levels in children with cystic fibrosis and their caregivers |
| Methods |
Design: parallel RCT Location: Marmara University, Turkey Duration: 3 months |
| Participants |
Estimated sample size: 30 participants Inclusion criteria: diagnosed with CF; aged 6–13 years Exclusion criteria: current pulmonary exacerbation; musculoskeletal problems that hinder exercising; no Internet access; participants and parents do not consent to intervention |
| Interventions |
Intervention: rehabilitation sessions including postural, breathing and high‐intensity interval training exercises through online programmes for rehabilitation. Exercise programme will be applied 3 days a week for 3 months Control: routine follow‐up |
| Outcomes |
Primary outcome
|
| Starting date | January 2021 (estimated completion May 2021) |
| Contact information | Ozge Kenis‐Coskun: Email: ozgekenis@gmail.com |
| Notes | Sponsors and collaborators: Marmara University |
NCT04742049.
| Study name | The effects of telerehabilitation on muscle function, physical activity and sleep in cystic fibrosis during pandemic |
| Methods |
Design: single‐centre, parallel‐design, RCT Location: Hacettepe University, Ankara, Turkey Duration: 6 weeks |
| Participants |
Enrolment goal: 30 participants with CF Inclusion criteria: people diagnosed with CF; volunteering to participate in the study; in social isolation due to COVID‐19 pandemic; FEV1 > 40% at last pulmonary function test Exclusion criteria: acute pulmonary exacerbation at the time of study or within the last month (or both); diagnosis of COVID‐19 before or during study; being physically or perceptually competent to exercise [sic]; ABPA treated with systemic steroid therapy; inability to complete the exercise training; FEV1 < 40% predicted |
| Interventions | This study evaluates the effects of a telerehabilitation‐based exercise programme versus usual care in participants who are at home during the self‐isolation process due to the COVID‐19 pandemic. Intervention group: an online 6‐week training programme includes 30 min of exercise performed 3 days per week, supervised by a physiotherapist. Training will start with warm‐up and finish with cool‐down exercises. Control group: receives same exercise document including the same exercise protocol. Participants will be called by the physiotherapist once a week for follow‐up. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 28 December 2020 |
| Contact information | Kubra Kilic, PhD Student: Hacettepe University, Ankara, Turkey; telephone: +903123051576 (+903123051576 University); Email: fztktas@gmail.com |
| Notes | 5 different outcome measures were defined as primary endpoints. Single blinding of participants is mentioned on ClinicalTrials.gov. It is not clear to the review authors how participants can be blinded to exercise training/no exercise training in this study setting. |
NCT05147285.
| Study name | The effect of different exercise modalities applied by tele rehabilitation on functional capacity, oxidative stress and respiratory parameters in cystic fibrosis children |
| Methods |
Design: parallel RCT Location: Hacettepe University, Turkey Duration: 8 weeks |
| Participants |
Estimated sample size: 39 participants Inclusion criteria: aged 8–18 years with a diagnosis of CF; access to online exercise training; % predicted FEV1 > 40% Exclusion criteria: diagnosed with acute pulmonary exacerbation at the time of study or within the last month (or both); physically or perceptually competent to exercise [sic]; ABPA treated with systemic steroid therapy; FEV1 % < 40% |
| Interventions |
Intervention 1: online supervised stabilisation exercises 3 times a week for 8 weeks Intervention 2: online supervised aerobic exercise training and stabilisation exercises. Aerobic exercises will be performed for 8 weeks, for 30–45 min, at 65–75% of maximum heart rate, 3 days a week, on the days when stabilisation exercises are not performed. Control: the importance of physical activity will be explained to the participants and appropriate physical activity recommendations will be made. |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 22 October 2021 (estimated completion May 2022) |
| Contact information | Principal Investigator: Professor Deniz Dogru‐Ersoz, Hacettepe University; Contact: Kubra Kilic, MSc: telephone: +903123051576; Email: fztktas@gmail.com |
| Notes | Sponsors and collaborators: Hacettepe University |
NCT05173194.
| Study name | Effects of a remotely supervised exercise program on inflammatory markers, muscle strength and lung function in adult patients with cystic fibrosis |
| Methods |
Design: parallel RCT Location: Universidad Europea de Madrid, Madrid, Spain Duration: 8 weeks |
| Participants |
Estimated sample size: 48 participants Inclusion criteria: confirmed clinical and genetic diagnosis for CF; aged ≥ 16 years Exclusion criteria: musculoskeletal disorders that do not allow the performance of physical exercise; pregnancy; absence of registration of clinical required [sic] |
| Interventions |
Intervention: remotely supervised resistance exercise, 3 sessions of 60 min each per week. Training programme consisted of warm‐up and joint mobility, strength exercises for different muscle groups and cool down (stretching and breathing exercises) Control: routine recommendations from the multidisciplinary CF team |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 26 October 2021 (estimated completion December 2021) |
| Contact information | Margarita Perez Ruiz, PhD: telephone: +34912115200 ext 3010; Email: pruizmarga@gmail.com Rosa María Girón Moreno, PhD: telephone: +34915202200; Email: rmgiron@gmail.com |
| Notes | Sponsor and collaborator: Universidad Europea de Madrid |
NCT05239611.
| Study name | Feasibility of home‐based exercise program for adults with cystic fibrosis to improve patient‐centered outcomes, including a novel measure of ventilation |
| Methods |
Design: parallel RCT Location: University of Kansas Medical Center, Kansas City, USA Duration: 3 months |
| Participants |
Estimated sample size: 30 participants Inclusion criteria: people with a confirmed diagnosis of CF (2 CF mutations or sweat chloride > 60 mmol/L); aged ≥ 18 years; stable while either on/off CFTR modulator therapy and no plan to start/discontinue CFTR modulator therapy; clearance from their CF physician to participate in exercise; access to the Internet; not involved in an exercise intervention in the previous 6 months, and not performing structured exercise > 150 min per week Exclusion criteria: pregnancy; history of solid organ transplant; active treatment for mycobacterial infections; significant untreated hypoxaemia, oxygen dependent at rest or with exercise; FEV1 < 40% of predicted or clinical evidence of cor pulmonale; untreated arterial hypertension (resting systolic blood pressure > 140 mmHg, diastolic blood pressure > 90 mmHg); systolic blood pressure > 90 mmHg while standing; congestive heart failure; active treatment for ABPA; acute upper or lower respiratory infection or pulmonary exacerbation within 4 weeks prior to day 1; changes in therapy (including antibiotics) for pulmonary disease within 4 weeks prior to day 1; significant haemoptysis within 4 weeks prior to day 1 (≥ 5 mL of blood in 1 coughing episode or > 30 mL of blood in a 24‐hour period; ongoing participation in an investigational drug study within 60 days prior to day 1 |
| Interventions |
Intervention: each participant will be assigned a pulmonary rehabilitation coacha and receive a weekly exercise consulting session delivered by that coach during the 12‐week intervention Control: standard of care aIncluding a registered respiratory therapist and clinical registered dietitian who have been trained in exercise training as recommended by the American Association of Cardiovascular and Pulmonary Rehabilitation and American College of Sports Medicine |
| Outcomes |
Primary outcomes
Secondary outcomes
|
| Starting date | 14 February 2022 (estimated completion April 2023) |
| Contact information | Contact: Joel Mermis, MD: telephone: 9135886045; Email: jmermis@kumc.edu Contact: Dave Burnett, PhD: telephone: 913‐588‐9499; Email: dburnett@kumc.edu |
| Notes | Sponsors and Collaborators: University of Kansas Medical Center |
ABPA: allergic bronchopulmonary aspergillosis; BMI: body mass index; BREQ‐3: Behavioral Regulation in Exercise Questionnaire‐3; CES‐D scale: Centre for Epidemiological Studies – Depression scale; CF: cystic fibrosis; CFQ: Cystic Fibrosis Questionnaire; CFQ‐R: Cystic Fibrosis Questionnaire – Revised; CFTR: cystic fibrosis transmembrane conductance regulator; CPET: cardiopulmonary exercise test; DEXA: dual‐energy x‐ray absorptiometry; ECG: electrocardiogram; FEF25–75: forced mid‐expiratory flow between 25% and 75% of vital capacity; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; HADS: Hospital Anxiety and Depression Scale; HAES: Habitual Activity Estimation Scale; HbA1c: glycated haemoglobin; HRQoL: health‐related quality of life; IL: interleukin; min: minute; MRI: magnetic resonance imaging; MRSA: methicillin‐resistant Staphylococcus aureus; MSWT: modified shuttle walk test; NHS: National Health Service; NTM: non‐tuberculous mycobacteria; PEF: peak expiratory flow; PSQI: Pittsburgh Sleep Quality Index; RCT: randomised controlled trial; VE: minute ventilation; VO2 peak: peak oxygen uptake; VO2 max: maximum oxygen uptake; VO2 peak: peak oxygen uptake; Wpeak: peak work rate.
Differences between protocol and review
Post hoc changes for the 2022 update
Title of the review
We replaced the previous title of 'Physical exercise training for cystic fibrosis' with 'Physical activity and exercise training in cystic fibrosis'. This change was motivated by the fact that the review includes studies focusing on exercise training – a subcomponent of physical activity – as well as studies focusing more generally on physical activity interventions versus usual care. This is important considering the technological advances in measuring and monitoring daily physical activity with step counters and fitness trackers. So‐called 'wearables' are becoming popular tools in physical activity interventions and are used as motivational elements, for individual monitoring and as part of individual goal setting (see examples in Ongoing studies).
Structure of the review
We restructured the review with regard to type of physical activity (including exercise) and duration of the active physical activity programme. Studies in this review are now presented in three categories according to the duration of their active physical activity programme:
studies with an active intervention duration of up to and including six months;
studies with an active intervention duration longer than six months; and
studies implementing a follow‐up period (i.e. when participants revert to usual care).
Systematic reviews investigating the effects of behavioural change interventions on changes in physical activity revealed beneficial effects for various populations, and with some indication for differential effects of studies lasting six months and longer (Barrett 2021; Howlett 2019). However, the 'ideal' intervention duration for increasing physical activity and changing behaviour is not available in the literature, and may differ between different populations. We implemented the categories described above, and focused on long‐term studies (where the physical activity programme lasted longer than six months) to be able to capture the long‐term effects of physical activity and exercise on health outcomes, and to get an overview of the spectrum of potential adverse events and risks associated with physical activity. Moreover, effects on bone health are unlikely to be observed in studies with durations of up to six months. We prioritised the time period of longer than six months' duration to evaluate intervention effects in the meta‐analysis and the Table 1. Additionally, we were interested in the long‐term effects of physical activity interventions once the active intervention has ceased. Although we do not focus on behavioural change as an outcome, in a chronic disease such as CF maintaining a level of exercise capacity and physical activity may indicate a change in behaviour in the long term.
We removed the original comparisons of aerobic, anaerobic or a mix of combined aerobic and anaerobic exercise training compared to no exercise training from the review. In fact, no exercise can be considered solely aerobic or anaerobic in regard to energy supply. To date, the vast majority of research in the field of exercise in CF lung disease focuses on combinations of endurance‐type and strengthening exercises. Categorisation of physical activity and exercise training studies into aerobic, anaerobic or a combination of aerobic and anaerobic is often not possible and may lead to potential misclassification. Moreover, there is evidence to suggest that both endurance‐type and strengthening exercises elicit improvements in exercise capacity in people with CF (Kriemler 2013; Orenstein 2004). Consequently, the main comparison in this updated review is any type of exercise training compared to no exercise training (usual care). Finally, the measurement of physical activity has substantially improved over recent decades, and 'wearables' are more frequently used in exercise research to monitor physical activity and improve adherence. These developments also affect the design of studies, with increasingly more studies applying a partially supervised approach (coaching), including the use of online tools to motivate people with CF to increase their physical activity levels (i.e. irrespective of whether the training regimen is aerobic, anaerobic or a combination of both).
Changes to primary and secondary outcomes
We shortened the list of outcomes to focus on clinically relevant and patient‐centred outcomes.
Primary outcomes
With regard to HRQoL, the CFQ and CFQ‐R include different quality of life domains (e.g. physical functioning, role/school, vitality, emotion, treatment burden) and three symptoms scales (i.e. weight, respiratory and digestion). The CFQ‐R is the most widely used disease‐specific instrument to assess HRQoL in CF lung disease and is applied in many pharmacological and non‐pharmacological studies, including exercise interventions. We decided to restrict outcomes from this instrument to the respiratory symptom scale, for which a minimal important difference is available (Quittner 2009), and the physical functioning domain as an important patient‐reported outcome in physical activity and exercise training studies. The physical functioning domain and the respiratory symptoms scale from the CFQ‐R correlate with FEV1 and maximal exercise capacity measured by cardiopulmonary exercise testing (Hebestreit 2014), both of which are important health‐related markers in CF lung disease.
Secondary outcomes
We decided to remove the outcome mortality as it is very unlikely that 'classical' physical activity interventions and exercise training studies with durations of three to 12 months, for example, will report on mortality. People with end‐stage CF lung disease (i.e. at a higher risk for lung transplantation, mortality or both) are usually excluded from exercise trials, and FEV1 is frequently reported as an exclusion criterion (Radtke 2017). We also removed the outcomes anaerobic exercise capacity, antibiotic use, and compliance with physical activity and exercise training from the review.
We reduced outcome measures for body composition (now only reporting BMI) and additional indices of exercise capacity and lung function to the most important and frequently used outcomes in recently published and ongoing physical activity intervention studies. Additional indices of exercise capacity, such as peak heart rate, minute ventilation and lactate during exercise tests, are of limited value and not patient‐centred outcomes in exercise trials. Moreover, functional capacity tests such as the 12‐minute walk test or 3‐minute step test are rarely used in exercise research and we removed them from the list of outcomes. We reduced the outcome 'anaerobic exercise capacity and muscle strength' to quadriceps muscle strength (i.e. isometric strain gauge or dynamometry (or both) measurements and isokinetic dynamometry measurements) as it is feasible, reliable and applied in people with chronic respiratory disease (Maltais 2014). Further, we now only report FVC and have removed other additional indices of lung function because FEF25–75 of vital capacity, total lung capacity, functional residual capacity, residual volume, pulmonary diffusing capacity for carbon monoxide and pulmonary diffusing capacity for nitric oxide are less important outcomes for people with CF. Finally, we added hospitalisations (i.e. number of hospitalisations and number of days in hospital) to secondary outcomes as there is evidence that a higher level of physical activity is associated with reduced hospital admission (Cox 2016).
Risk of bias
For the domain 'blinding of participants and personnel (performance bias)', we changed the risk of bias from 'unclear' to 'high risk of bias' as blinding of participants to exercise is not possible.
Post hoc changes for the 2017 update
We added summary of findings tables, in line with Cochrane guidance.
We stipulated that the duration of each included study should be at least two weeks, which is the typical length of (drug) treatment for pulmonary exacerbations where people with CF may also take part in in‐hospital exercise training. Moreover, from an exercise physiology perspective, less than two weeks of structured exercise are unlikely to elicit meaningful changes in the chosen outcomes measures.
We added the Lung Clearance Index (LCI) derived from multiple‐breath washout to secondary outcome "4. Additional indices of pulmonary function and respiratory muscle strength". The LCI is a relatively new and much examined pulmonary function outcome measure and included in many clinical studies including exercise training interventions.
We also added the diffusing capacity for carbon monoxide (DLCO) and the diffusing capacity for nitric oxide (DLNO) to secondary outcome "4. Additional indices of pulmonary function and respiratory muscle strength". Non‐invasive measurement of the pulmonary diffusing capacity can provide novel physiological insights into the exercise training effects on pulmonary function beyond the much examined FEV1, derived from spirometry.
Post hoc changes for the 2015 update
The title of the review was changed from 'Physical training for cystic fibrosis' to 'Physical exercise training for cystic fibrosis' as the new team felt this better reflected the content of the review.
The fourth primary outcome 'mortality' was moved to secondary outcomes in line with Cochrane guidance to limit the number of primary outcomes to three. For this update, primary and secondary outcome measures were changed as follows:
Primary outcomes
We limited the primary outcome measures to:
Exercise capacity by peak oxygen uptake (VO2 peak);
Pulmonary function by forced expiratory volume in one second (FEV1);
Health‐related quality of life (HRQoL).
In CF, VO2 peak and FEV1 are strong predictors of mortality, objectively measurable and are often used as primary outcomes in studies of exercise training. The outcome measure HRQoL is an important participant‐reported outcome measure and is related to physical fitness in people with CF. None of the other primary outcomes from previous reviews has been shown to be of predictive value in CF and they should be considered explorative endpoints. All previous primary outcomes for pulmonary function are now integrated under the secondary outcome number 4 "Additional indices of pulmonary function and respiratory muscle strength", and exercise capacity variables, including effort, oxygenation and fatigue, are integrated into the secondary outcome number 3 " Additional indices of exercise capacity".
Secondary outcomes
We removed the secondary outcomes "Symptom scores", "Compliance with other treatment, such as chest physiotherapy, nutritional regimens" and "Cost evaluation". These outcomes are of unclear relevance, difficult to measure reliably and are rarely reported in physical training studies. We added the secondary outcome "Physical activity" because it is an important outcome in exercise training studies. The outcome "Measures of bone mineral density and diabetic control" was separated into "Bone health" and "Diabetic control" because these outcomes are unrelated and should be studied and reported separately. The outcome "Weight" was removed as a separate outcome and is now integrated within the outcome "Body composition" which comprises all measures of nutrition including bodyweight, body fat and fat‐free mass. The secondary outcome "Number of acute exacerbations, intravenous antibiotic courses and time off work or school" was separated as "Acute exacerbations (a) number of exacerbations; (b) time to first exacerbation" and "Antibiotic use (including oral, intravenous or inhaled antibiotics)".
Contributions of authors
The title for the protocol was conceived by the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Both Judy Bradley and Fidelma Moran designed and assisted in writing the protocol and produced the earlier versions of the full review.
For 2015 and 2017 updates, TR and SK were responsible for acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript.
For the 2022 update, TR, SS and SJN were responsible for acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript. SK and HH provided important methodological input during the preparation of this review.
SJNprovided statistical support for the 2015, 2017 and 2022 updates. All authors provided intellectual input, critically reviewed the manuscript and approved the final version of this updated review.
TR acts as guarantor for this review.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Institute for Health Research, UK
This systematic review was supported by the National Institute for Health Research, via Cochrane infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
HH has received financial compensation for travel and accommodation or free meeting participation (or both) at the European Cystic Fibrosis Society conference and the North American Cystic Fibrosis Conference for chairing or presenting at sessions focusing on exercise in cystic fibrosis. For writing an educational booklet on exercise in cystic fibrosis, HH has received money from Novartis. HH is also the lead investigator on one of the studies included in this review (Hebestreit 2010). As he is the lead investigator of the international multicentre trial ACTIVATE‐CF (Hebestreit 2022), his institution has received grants from the Mukoviszidose e.V. and a Vertex Innovation Award.
TR was a core study team member of the ACTIVATE‐CF trial (Hebestreit 2022). TR has received financial compensation for chairing and presenting at exercise sessions at the European Cystic Fibrosis Society conference. He has also received financial support (travel, accommodation) from Vifor Pharma Switzerland to participate at the European Cystic Fibrosis Society and European Respiratory Society conference.
SK is the lead investigator on one of the studies included in the review (Kriemler 2013), and was a core team member of the ACTIVATE‐CF trial (Hebestreit 2022)
SJN declares no known potential conflicts of interest.
SS declares no known potential conflicts of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Alexander 2019 {published data only}
- Alexander E, Wilson C, Kapur N, Brookes D. Is telehealth as effective as face to face therapy for delivery of whole body vibration training (WBVT) as an adjunct to physiotherapy in children and young people with cystic fibrosis (CF)? Respirology 2019;24:135. [CFGD REGISTER: MH178] [Google Scholar]
Beaudoin 2017 {published data only}
- Beaudoin N, Bouvet GF, Coriati A, Rabasa-Lhoret R, Berthiaume Y. Combined exercise training improves glycemic control in adults with cystic fibrosis. Medicine and Science in Sports and Exercise 2017;49(2):231-7. [CENTRAL: 1199954] [CFGD REGISTER: CO62b] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Beaudoin N, Bouvet GF, Coriati A, Rabasa-Lhoret R, Berthiaume Y. Combined exercise training improves glycemic control in adults with cystic fibrosis. Medicine and Science in Sports and Exercise 2017;49(2):231-7.Supplemental digital content. [CFGD REGISTER: CO62c] [DOI] [PubMed] [Google Scholar]
- Beaudoin N, Bouvet GF, Rabasa-Lhoret R, Berthiaume Y. Effects of a partially supervised combined exercise program on glycemic control in cystic fibrosis: pilot study. Pediatric Pulmonology 2015;50(Suppl 41):367. [ABSTRACT NO: 466] [CENTRAL: 1092169] [CFGD REGISTER: CO62a] [EMBASE: 72081745] [Google Scholar]
- NCT02127957. Effects of an exercise program among CF patients with dysglycemia (FKEX). clinicaltrials.gov/ct2/show/NCT02127957 (first received 1 May 2014).
Carr 2018 {published data only}
- Carr SB, Ronan P, Lorenc A, Mian A, Madge SL, Robinson N. Children and adults Tai Chi study (CF-CATS2): a randomised controlled feasibility study comparing internet-delivered with face-to-face Tai Chi lessons in cystic fibrosis. ERJ Open Research 2018;4(4):pii: 00042-2018. [CENTRAL: CN-01925903] [CFGD REGISTER: PE243f] [DOI: 10.1183/23120541.00042-2018] [EMBASE: 625801573] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr SB, Ronan P, Robinson N, Agent P, Mian A, Madge S. A randomised controlled trial of Tai Chi in cystic fibrosis. Pediatric Pulmonology 2016;51:376. [ABSTRACT NO: 480] [CENTRAL: 1212611] [CFGD REGISTER: PE243c] [EMBASE: 612358588] [Google Scholar]
- Lorenc A, Ronan P, Mian A, Madge S, Carr SB, Agent P, et al. Cystic fibrosis-Children and adults Tai Chi study (CF CATS2): can Tai Chi improve symptoms and quality of life for people with cystic fibrosis? Second phase study protocol. Chinese Journal of Integrative Medicine 2015 May 26 [Epub ahead of print]. [CENTRAL: 1343883] [CFGD REGISTER: PE243a] [DOI: 10.1007/s11655-015-2150-1] [PMID: ] [DOI] [PubMed]
- Madge S, Ronan P, Robinson N, Agent P, Mian A, Carr SB. Assessing the benefits of tai chi in people with CF and the feasibility of delivering Tai Chi over the internet (Skype®). Pediatric Pulmonology 2016;51(Suppl 45):370. [ABSTRACT NO: 465] [CENTRAL: 1343884] [CFGD REGISTER: PE243b] [Google Scholar]
- NCT02054377. Cystic fibrosis – children and adults Tai Chi study. clinicaltrials.gov/show/NCT02054377 (first posted 4 February 2014). [CFGD REGISTER: PE243h]
- Robinson N, Ronan P, Mian A, Madge S, Lorenc A, Agent P, et al. The potential of Tai Chi for long term health: exercise preferences for people with cystic fibrosis. BMC Complementary and Alternative Medicine 2017;17(Suppl 1):333. [ABSTRACT NO.: P324] [CENTRAL: CN-01442142] [CFGD REGISTER: PE243d] [DOI: 10.1186/s12906-017-1784-2] [EMBASE: 619710526] [DOI]
- Ronan P, Mian A, Carr SB, Madge SL, Lorenc A, Robinson N. Learning to breathe with Tai Chi online – qualitative data from a randomized controlled feasibility study of patients with cystic fibrosis. European Journal of Integrative Medicine 2020;40:101229. [CFGD REGISTER: PE243g] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronan P, Robinson N, Carr S, Mian A, Lorenc A, Agent P, et al. Benefits for people with cystic fibrosis learning Tai Chi: comparison of self-reported to objective data in a non-acute/community population. BMC Complementary and Alternative Medicine 2017;17(Suppl 1):333. [ABSTRACT NO.: P325] [CENTRAL: CN-01603089] [CFGD REGISTER: PE243e] [EMBASE: 619710531]
Cerny 1989 {published data only}
- Cerny FJ. Relative effects of bronchial drainage and exercise for in-hospital care of patients with cystic fibrosis. Physical Therapy 1989;69(8):633-9. [CFGD REGISTER: PE13] [DOI] [PubMed] [Google Scholar]
Del Corral 2018 {published data only}
- Del Corral T, Cebria I Iranzo MA, Lopez-de-Uralde-Villanueva I, Martinez-Alejos R, Blanco I, Vilaro J. Effectiveness of a home-based active video game programme in young cystic fibrosis patients. Respiration; International Review of Thoracic Diseases 2018;95(2):87-97. [CENTRAL: CN-01643331] [CFGD REGISTER: PE254] [EMBASE: 618880167] [PMID: ] [DOI] [PubMed] [Google Scholar]
- NCT02552043. Video game exercise effectiveness of a domiciliary pulmonary rehabilitation program in cystic fibrosis patients. clinicaltrials.gov/ct2/show/NCT02552043 (first received 15 September 2015).
Donadio 2020 {published data only}
- Donadio MV, Sanz V, Villa JR, Giron Moreno RM, Cobo F, Diez-Vega I, et al. Effects of exercise with neuromuscular electrical stimulation on peripheral muscle strength, lung function and aerobic fitness in patients with cystic fibrosis: a randomised controlled trial. Journal of Cystic Fibrosis 2020;19(Suppl):S38. [CFGD REGISTER: PE291a] [Google Scholar]
- NCT04153669. Exercise with or without electrical stimulation in cystic fibrosis (part I): effects on physical fitness (ECOMIRIN) [Effects of exercise with or without electrical stimulation in physical fitness in cystic fibrosis: an integrated vision (ECOMIRIN Study)]. clinicaltrials.gov/show/NCT04153669 (first posted 6 November 2019). [CFGD REGISTER: PE291b]
Douglas 2015 {published data only}
- Douglas H, Bryon M, Ledger S, Main E. My quality of life or yours? The discrepancies between parent and child reported quality of life scores. Journal of Cystic Fibrosis 2017;16(Suppl 1):S162. [CENTRAL: CN-01445959] [CFGD REGISTER: PE218d] [Google Scholar]
- Douglas H, Ledger S, Main E, Sarria Jaramillo L, Rayner P, Goldman A, et al. INSPIRE-CF: an interim review of participation of children with cystic fibrosis randomised to a weekly supervised exercise intervention. Journal of Cystic Fibrosis 2015;14(Suppl 1):S97. [ABSTRACT NO: 152] [CENTRAL: 1081484] [CFGD REGISTER: PE218a] [EMBASE: 71951805] [Google Scholar]
- Ledger S, Wade A, Douglas H, Sarria L, Rayner P, Goldman A, et al. Interim results for INSPIRE-CF: a 24-month RCT evaluating effects of weekly supervised exercise in children with CF. European Respiratory Journal 2016;48:OA 1493. [Google Scholar]
- Ledger SJ, Douglas H, Sarria Jaramillo L, Rayner P, Aurora P, Main E. INSPIRE-CF: levels of participation of children with cystic fibrosis randomised to a 24-month weekly supervised exercise intervention. Journal of Cystic Fibrosis 2016;15(Suppl 1):S44. [ABSTRACT NO: ePS04.5] [CFGD REGISTER: PE218b] [EMBASE: 614323554] [Google Scholar]
- Ledger SJ, Douglas H, Sarria-Jaramillo L, Rayner P, Goldman A, Giardini A, et al. INSPIRE-CF: a randomised trial evaluating the longitudinal effects of a weekly supervised exercise programme on children with cystic fibrosis. In: World Confederation of Physical Activity Congress; 2017 Jul 2-4; Cape Town, South Africa. Available online at www.abstractstosubmit.com/wcpt2017/abstracts/. 2017.
- Ledger SJ, Wade A, Douglas H, Sarria Jaramillo L, Rayner P, Goldman A, et al. Interim results for INSPIRE-CF: a 24-month randomised trial evaluating effects of a weekly exercise intervention for children with cystic fibrosis. Journal of Cystic Fibrosis 2016;15(Suppl 1):S88. [ABSTRACT NO: 144] [CENTRAL: 1171475] [CFGD REGISTER: PE218c] [EMBASE: 614323655] [Google Scholar]
- NCT01889927. INSPIRE-CF: an alternative physiotherapy model for children with cystic fibrosis [INSPIRE-CF: a randomised controlled trial investigating the clinical and economic benefits of an alternative model of physiotherapy care for children with cystic fibrosis]. clinicaltrials.gov/show/NCT01889927 (first received 1 July 2013). [CENTRAL: CN-01543155] [CFGD REGISTER: PE218e]
Güngör 2021 {published data only}
- Güngör S, Gencer-Atalay K, Bahar-Özdemir Y, Keniş-Coşkun O, Karadağ-Saygi E. The clinical effects of combining postural exercises with chest physiotherapy in cystic fibrosis: a single-blind, randomized-controlled trial. Turkish Journal of Physical Medicine and Rehabilitation 2021;67(1):91-8. [CFGD REGISTER: PE319b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03295201. Clinical effects of exercise program added to pulmonary rehabilitation in patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT03295201 (first received 27 September 2017). [CFGD REGISTER: PE319a]
Gupta 2019 {published data only}CTRI/2013/04/003531
- Gupta S, Mukherjee A, Lodha R, Kabra M, Deepak KK, Khadgawat R, et al. Effects of exercise intervention program on bone mineral accretion in children and adolescents with cystic fibrosis: a randomized controlled trial. Indian Journal of Pediatrics 2019;86(11):987-94. [CENTRAL: CN-01964575] [CFGD REGISTER: PE279a] [DOI: 10.1007/s12098-019-03019-x] [EMBASE: 2002279681] [PMID: ] [DOI] [PubMed]
- Gupta S, Mukherjee A, Lodha R, Kabra M, Deepak KK, Khadgawat R, et al. Effects of exercise intervention program on bone mineral accretion in children and adolescents with cystic fibrosis: a randomized controlled trial. Indian Journal of Pediatrics 2019;86(11):987-94. [CFGD REGISTER: PE279b] [DOI: 10.1007/s12098-019-03019-x] [DOI] [PubMed]
- Gupta S. Effects of exercise intervention program on bone mineral accretion in children and adolescents with cystic fibrosis. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2013/04/003531 (first received 4 April 2013).
Hatziagorou 2019 {published data only}
- Hatziagorou E, Giannakoulakos S, Mantsiou C, Kouroukli E, Nousia I, Kampouras A, et al. Does an individualised exercise program improve exercise capacity among young patients with cystic fibrosis? Journal of Cystic Fibrosis 2019;18(Suppl 1):S123. [ABSTRACT NO.: P236] [CENTRAL: CN-01984667] [CFGD REGISTER: PE283] [Google Scholar]
Hebestreit 2010 {published data only}
- Hebestreit A, Kriemler S, Kieser S, Junge S, Heilmann M, Ballmann M, et al. Effects of different conditioning programmes on aerobic capacity in CF. Pediatric Pulmonology 2003;36(Suppl 25):329. [CFGD REGISTER: PE145a] [Google Scholar]
- Hebestreit H, Kieser S, Junge S, Ballmann M, Hebestreit A, Schindler C, et al. Long-term effects of a partially supervised conditioning programme in cystic fibrosis. European Respiratory Journal 2010;35(3):578-83. [CFGD REGISTER: PE145b] [DOI] [PubMed] [Google Scholar]
- Hebestreit H, Schmid K, Kieser S, Junge S, Ballmann M, Roth K, et al. Quality of life is associated with physical activity and fitness in cystic fibrosis. BMC Pulmonary Medicine 2014;14:26. [CFGD REGISTER: PE145c//PE120c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00231686. Effects of a 6-months physical conditioning program in patients with cystic fibrosis [Effects of a 6-months physical conditioning program on health status and physical activity in youths and young adults with cystic fibrosis – MUKOTRAIN]. clinicaltrials.gov/show/NCT00231686 (first received 4 October 2005). [CFGD REGISTER: PE145d]
Hebestreit 2022 {published data only}
- Hebestreit H, Kriemler S, Schindler C, Stein L, Karila C, Urquhart DS, et al. Effects of a partially supervised conditioning program in cystic fibrosis: an international multicenter randomized controlled trial (ACTIVATE-CF). American Journal of Respiratory and Critical Care Medicine 2022;205(3):330-9. [DOI: 10.1164/rccm.202106-1419OC] [DOI] [PMC free article] [PubMed]
- Hebestreit H, Kriemler S, Schindler C, Stein L, Karila C, Urquhart DS, et al. Effects of a partially supervised conditioning program in cystic fibrosis: an international multi-centre, randomised controlled trial (ACTIVATE-CF). Journal of Cystic Fibrosis 2021;20(Suppl):S8. [CFGD REGISTER: PE244e] [Google Scholar]
- Hebestreit H, Lands LC, Alarie N, Schaeff J, Karila C, Orenstein DM, et al. Effects of a partially supervised conditioning programme in cystic fibrosis: an international multi-centre randomised controlled trial (ACTIVATE-CF): study protocol. BMC Pulmonary Medicine 2018;18(1):31. [CENTRAL: CN-01913839] [CFGD REGISTER: PE244b] [EMBASE: 620583585] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebestreit H. How to counsel patients regarding exercise and habitual physical activity? Journal of Cystic Fibrosis 2016;51(Suppl 45):188. [ABSTRACT NO: S20.4] [CFGD REGISTER: PE244a] [Google Scholar]
- NCT01744561. Effects of a partially supervised conditioning program in CF (ACTIVATE-CF) [Effects of a partially supervised conditioning program in CF: an international multi-centre, randomized controlled trial]. clinicaltrials.gov/show/NCT01744561 (first received 5 December 2012). [CENTRAL: 1343886] [CFGD REGISTER: PE244b]
Hommerding 2015 {published data only}
- Hommerding PX, Baptista RR, Makarewicz GT, Schindel CS, Donadio MV, Pinto LA, et al. Effects of an educational intervention of physical activity for children and adolescents with cystic fibrosis: a randomized controlled study. Respiratory Care 2015;60(1):81-7. [CFGD REGISTER: PE194b] [DOI] [PubMed] [Google Scholar]
- Marostica PJ, Hommerding P, Makarewicz G, Baptista R, Donadio MV. Efficacy of an educational intervention of physical activity for children and adolescents with cystic fibrosis. Journal of Cystic Fibrosis 2012;11(Suppl 1):S36. [ABSTRACT NO: WS16.6] [CFGD REGISTER: PE194a] [DOI] [PubMed] [Google Scholar]
- RBR-3r4h5s. Effect of exercise orientations in the posture and plantar pressure distribution in children and adolescents with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-3r4h5s (first received 9 July 2014). [CFGD REGISTER: PE194d]
- Schindel CS, Hommerding PX, Melo DA, Baptista RR, Marostica PJ, Donadio MV. Physical exercise recommendations improve postural changes found in children and adolescents with cystic fibrosis: a randomized controlled trial. Journal of Pediatrics 2015;166(3):710-6. [CFGD REGISTER: PE194c] [DOI] [PubMed] [Google Scholar]
Klijn 2004 {published data only}
- Klijn PH, Oudshoorn A, Ent CK, Net J, Helders PJ. Effects of anaerobic training in children with cystic fibrosis: a randomised controlled study. Chest 2004;125(4):1299-305. [CFGD REGISTER: PE147] [DOI] [PubMed] [Google Scholar]
Kriemler 2013 {published data only}
- Hebestreit H, Schmid K, Kieser S, Junge S, Ballmann M, Roth K, et al. Quality of life is associated with physical activity and fitness in cystic fibrosis. BMC Pulmonary Medicine 2014;14:26. [CFGD REGISTER: PE145c//PE120c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriemler S, Hebestreit A, Kieser S, Bachmann M, Hebestreit H. Six months of training improves lung function and aerobic performance in CF. In: 24th European Cystic Fibrosis Conference; 2001 Jun 6-9; Vienna, Austria. 2001:P62. [CFGD REGISTER: PE120a]
- Kriemler S, Kieser S, Junge S, Ballmann M, Hebestreit A, Schindler C, et al. Effect of supervised training on FEV1 in cystic fibrosis: a randomised controlled trial. Journal of Cystic Fibrosis 2013;12(6):714-20. [CFGD REGISTER: PE120b] [DOI] [PubMed] [Google Scholar]
Michel 1989 {published data only}
- Michel SH, Darbee JC, Pequignot E. Exercise, body composition and strength in cystic fibrosis. Pediatric Pulmonology 1989;4:116. [CFGD REGISTER: PE40] [Google Scholar]
Moorcroft 2004 {published data only}
- Dodd ME, Moorcroft AJ, Webb AK. Improved fitness and decreased symptoms of muscle fatigue for upper and lower body following an individualised unsupervised home exercise training programme in adults with cystic fibrosis. Pediatric Pulmonology 1998;17:347. [CFGD REGISTER: PE97a] [Google Scholar]
- Moorcroft AJ, Dodd ME, Morris J, Webb AK. Individualised unsupervised exercise training in adults with cystic fibrosis: a 1 year randomised controlled trial. Thorax 2004;59(12):1074-80. [CFGD REGISTER: PE97c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorcroft AJ, Dodd ME, Webb AK. Individualised home exercise training in CF – a one-year trial. In: XIIIth International Cystic Fibrosis Congress; 2000 Jun 4-8; Stockholm, Sweden. 2000:153. [CFGD REGISTER: PE97b]
Rovedder 2014 {published data only}
- Rovedder PM, Flores J, Ziegler B, Casarotto F, Jaques P, Baretto SS, et al. Exercise programme in patients with cystic fibrosis: a randomized controlled trial. Respiratory Medicine 2014;108(8):1134-40. [CFGD REGISTER: PE214] [DOI] [PubMed] [Google Scholar]
Santana‐Sosa 2012 {published data only}
- Santana-Sosa E, Groeneveld IF, Gonzalez-Saiz L, Lopez-Mojares LM, Villa-Asensi JR, Barrio Gonzalez MI, et al. Intrahospital weight and aerobic training in children with cystic fibrosis: a randomized controlled trial. Medicine and Science in Sports and Exercise 2012;44(1):2-11. [CFGD REGISTER: PE191] [DOI] [PubMed] [Google Scholar]
Santana‐Sosa 2014 {published data only}
- NCT01706445. Combined inspiratory muscle and 'whole muscle' training in children with cystic fibrosis. clinicaltrials.gov/show/NCT01706445 (first received 15 October 2012). [CFGD REGISTER: PE201b]
- Santana-Sosa E, Gonzalez-Saiz L, Groeneveld IF, Villa-Asensi JR, Barrio Gomez de Aguero MI, Fleck SJ, et al. Benefits of combining inspiratory muscle with 'whole muscle' training in children with cystic fibrosis: a randomised controlled trial. British Journal of Sports Medicine 2014;48(20):1513-7. [CENTRAL: 874806] [CFGD REGISTER: PE201] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sawyer 2020 {published data only}
- ACTRN12617001271392. Effects of high intensity interval training on exercise capacity in people with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617001271392 (first received 4 September 2017). [CFGD REGISTER: PE261c]
- Sawyer A, Cavalheri V, Jenkins S, Wood J, Cecins N, Bear N, et al. High-intensity interval training is effective at increasing exercise endurance capacity and is well tolerated by adults with cystic fibrosis. Journal of Clinical Medicine 2020;9(10):3098. [CFGD REGISTER: PE261d] [DOI: 10.3390/jcm9103098] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer A, Cavalheri V, Jenkins S, Wood J, Cecins N, Singh B, et al. Effects of high intensity interval training on exercise capacity in people with cystic fibrosis: study protocol for a randomised controlled trial. BMC Sports Science, Medicine & Rehabilitation 2018;10(1):19. [CENTRAL: CN-01935836] [CFGD REGISTER: PE261] [EMBASE: 627687202] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer A, Cavalheri V, Jenkins S, Wood J, Cecins N, Singh B, et al. High intensity interval-based cycle ergometry training is effective at increasing exercise endurance capacity and is well tolerated by adults with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2020;201(1):C107. [ABSTRACT NO.: A6118] [CFGD REGISTER: PE261b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer A, Cavalheri V, Jenkins S, Wood J, Cecins N, Singh B, et al. Low-volume high intensity interval training improves exercise endurance capacity and is well tolerated in people with cystic fibrosis: a randomised controlled trial. Respirology (Carlton, Vic.) 2020;25:34. [CFGD REGISTER: PE261e] [Google Scholar]
Schneiderman‐Walker 2000 {published data only}
- Papaioannou M. A randomized controlled trial of a 3-year home exercise program in cystic fibrosis. Pediatric Physical Therapy 2001;13(2):94-5. [CENTRAL: 1343880] [CFGD REGISTER: PE58c] [PMID: ] [PubMed] [Google Scholar]
- Reisman JJ, Schneiderman-Walker J, Corey M, Wilkes D, Pedder L, Levison H, et al. The role of an organized exercise program in cystic fibrosis – a three year study. Pediatric Pulmonology 1995;Suppl 12:261. [CENTRAL: 291537] [CFGD REGISTER: PE58a] [Google Scholar]
- Schneiderman-Walker J, Pollock SL, Corey M, Wilkes DD, Canny G, Pedder L, et al. A randomised controlled trial of a 3-year home exercise program in cystic fibrosis. Journal of Pediatrics 2000;136(3):304-10. [CFGD REGISTER: PE58b] [DOI] [PubMed] [Google Scholar]
Selvadurai 2002 {published data only}
- Selvadurai HC, Blimkie CJ, Meyers N, Mellis CM, Cooper PJ, Van Asperen PP. Randomized controlled study of in-hospital exercise training programs in children with cystic fibrosis. Pediatric Pulmonology 2002;33(3):194-200. [CFGD REGISTER: PE107b] [DOI] [PubMed] [Google Scholar]
- Selvadurai HC, Asperen PP, Cooper PJ, Mellis CM, Blimkie CJ. A randomised controlled study of in-hospital exercise training programs in children with cystic fibrosis (CF). Pediatric Pulmonology 1999;19:287-8. [CFGD REGISTER: PE107a] [DOI] [PubMed] [Google Scholar]
Turchetta 1991 {published data only}
- Turchetta A, Bella S, Calzolari A, Castro M, Ciuffetti C, Drago F, et al. Effect of controlled physical activity on lung function test of cystic fibrosis children. In: 17th European Cystic Fibrosis Conference; 1991 Jun 18-21; Copenhagen, Denmark. 1991:134. [CFGD REGISTER: PE45]
References to studies excluded from this review
ACTRN12620001237976 {published data only}
- ACTRN12620001237976. Virtual models for delivery of exercise training in cystic fibrosis (CF): an evaluation of patient engagement and feasibility. trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12620001237976 (first registered 18 November 2020). [CFGD REGISTER: PE336]
Alarie 2012 {published data only}
- Alarie N, Chan R, Thomas L, Marasco J, Amelie P, Nan W, et al. Cardiorespiratory responses to Nintendo Wii in children with cystic fibrosis: a pilot study. Pediatric Pulmonology 2012;47(S35):367. [ABSTRACT NO: 399] [CFGD REGISTER: PE196] [Google Scholar]
Albinni 2004 {published data only}
- Albinni S, Rath R, Renner S, Eichler I. Additional inspiratory muscle training intensifies the beneficial effects of cycle ergometer training in patients with cystic fibrosis. Journal of Cystic Fibrosis 2004;3(Suppl 1):S63. [CFGD REGISTER: PE148a] [Google Scholar]
- Eichler I, Renner S, Albinni S, Nachbaur E, Rath R. Inspiratory muscle training adds beneficial effects to cycle ergometer training in patients with cystic fibrosis. Pediatric Pulmonology 2005;40(Suppl 28):320. [CFGD REGISTER: PE148b] [Google Scholar]
Almajan‐Guta 2011 {published data only}
- Almajan-Guta B, Avram C, Almajan-Guta V, Rusu A, Ciuca I, Cluci O, et al. High motivation for playing sports in cystic fibrosis – what we play is life. Journal of Cystic Fibrosis 2011;10(Suppl 1):S64. [ABSTRACT NO.: 254] [CFGD REGISTER: PE189] [Google Scholar]
Amelina 2006 {published data only}
- Amelina E, Cherniak A, Chikina S, Krasovsky S, Appaeva A. Inspiratory muscle training (IMT) in cystic fibrosis adults. In: European Respiratory Society Annual Congress; 2006 Sep 2-6; Munich, Germany. 2006. [ABSTRACT NO: P4112] [CFGD REGISTER: PE177a]
- Cherniak A, Amelina E, Krasovsky S, Nekludova G, Chikina S. The effect of high intensity inspiratory muscle training in adults with cystic fibrosis (CF). European Respiratory Journal 2007;30(Suppl 51):767s. [ABSTRACT NO: E4514] [CENTRAL: 645383] [CFGD REGISTER: PE177b] [Google Scholar]
Andreasson 1987 {published data only}
- Andreasson B, Jonson B, Kornfalt R, Nordmark E, Sandstrom S. Long-term effects of physical exercise on working capacity and pulmonary function in cystic fibrosis. Acta Paediatrica Scandinavica 1987;76(1):70-5. [DOI] [PubMed] [Google Scholar]
Aquino 2006 {published data only}
- Aquino A, Balestri E, Dall Ara S, Lami I, Gobb F, Ambroni M, et al. Efficacy of physical exercise playing a video game for mucus clearance in patients with CF. Journal of Cystic Fibrosis 2006;5(Suppl):S83. [CFGD REGISTER: PE163] [Google Scholar]
Asher 1982 {published data only}
- Asher M, Pardy R, Coates A, Thomas E, Macklem PT. The effects of inspiratory muscle training in patients with cystic fibrosis. Australian and New Zealand Journal of Medicine 1983;13:204. [CFGD REGISTER: PE127a] [DOI] [PubMed] [Google Scholar]
- Asher MI, Pardy RL, Coates AL, Thomas E, Macklem PT. The effects of inspiratory muscle training in patients with cystic fibrosis. American Review of Respiratory Disease 1982;126(5):855-9. [CFGD REGISTER: PE127b] [DOI] [PubMed] [Google Scholar]
Balestri 2004 {published data only}
- Balestri E, Ambroni M, dall'Ara S, Miano A. Efficacy of physical exercise for mucus clearance in patients with cystic fibrosis (CF). Pediatric Pulmonology 2004;38(S27):316. [CFGD REGISTER: PE150] [Google Scholar]
Balfour Lynn 1998 {published data only}
- Balfour Lynn IM, Prasad SA, Laverty A, Whitehead BF, Dinwiddie R. A step in the right direction: assessing exercise tolerance in cystic fibrosis. Pediatric Pulmonology 1998;25(4):278-84. [CENTRAL: 462732] [CFGD REGISTER: PE69b] [DOI] [PubMed] [Google Scholar]
- Balfour-Lynn IM, Prasad SA, Laverty A, Whitehead BF, Dinwiddie R. Step-test vs 6-minute walk: assessing exercise tolerance in children with cystic fibrosis. Pediatric Pulmonology 1996;13:305. [CFGD REGISTER: PE69a] [DOI] [PubMed] [Google Scholar]
Barry 2001 {published data only}
- Barry S, Dodd J, Jensma M, Gallagher C. Benefits of high intensity strength training in adults with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2001;163(Suppl 5):A968. [CFGD REGISTER: PE133b] [Google Scholar]
- Barry S, Dodd J, Jensma M, Gallagher C. High intensity strength training improves fitness levels in adults with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A507. [CFGD REGISTER: PE133a] [Google Scholar]
Bass 2019 {published data only}
- Bass R, Bourke S, Doe S, Diego-Vicente L, Hope E, Smith A, et al. Is "Pactster" an acceptable tool to promote exercise participation in the adult cystic fibrosis community, with and without physiotherapy support? Journal of Cystic Fibrosis 2019;18(Suppl 1):S34. [CENTRAL: CN-01984666] [CFGD REGISTER: PE282] [Google Scholar]
Bellini 2018 {published data only}
- Bellini R, Cazzarolli C. Non-invasive ventilation during exercise in severe cystic fibrosis subjects: a preliminary study. European Respiratory Journal 2018;52(Suppl 62):PA1318. [CFGD REGISTER: OV37] [Google Scholar]
Bieli 2017 {published data only}
- Bieli C, Selina S, Demet I, Andreas J, Alexander M. Respiratory muscle endurance training in cystic fibrosis. European Respiratory Journal 2014;44(Suppl 58):P1972. [CFGD REGISTER: PE237b] [EMBASE: 71851116] [Google Scholar]
- Bieli C, Summermatter S, Boutellier U, Moeller A. Respiratory muscle training improves respiratory muscle endurance but not exercise tolerance in children with cystic fibrosis. Pediatric Pulmonology 2017;52(3):331-6. [CENTRAL: 1262285] [CFGD REGISTER: PE237a] [EMBASE: 614244451] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bilton 1992 {published data only}
- Bilton D, Dodd M, Webb AK. The benefits of exercise combined with physiotherapy in cystic fibrosis. Pediatric Pulmonology 1990;9(Suppl 5):238. [CFGD REGISTER: PE41a] [Google Scholar]
- Bilton D, Dodd ME, Abbot JV, Webb AK. The benefits of exercise combined with physiotherapy in the treatment of adults with cystic fibrosis. Respiratory Medicine 1992;86(6):507-11. [CFGD REGISTER: PE41b] [DOI] [PubMed] [Google Scholar]
Bongers 2015 {published data only}
- Bongers BC, Werkman MS, Arets HG, Takken T, Hulzebos HJ. A possible alternative exercise test for youths with cystic fibrosis: the steep ramp test. Medicine and Science in Sports and Exercise 2015;47(3):485-92. [CENTRAL: 1096730] [CFGD REGISTER: PE232] [PMID: ] [DOI] [PubMed] [Google Scholar]
Calik‐Kutukcu 2016 {published data only}
- Calik-Kutukcu E, Saglam M, Vardar-Yagli N, Cakmak A, Inal-Ince D, Bozdemir-Ozel C, et al. Listening to motivational music while walking elicits more positive affective response in patients with cystic fibrosis. Complementary Therapies in Clinical Practice 2016;23:52-8. [CENTRAL: 1199953] [CFGD REGISTER: PE234] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cantin 2005 {published data only}
- Cantin AM, Bacon M, Berthiaume Y. Mechanical airway clearance using the Frequencer electro-acoustical transducer in cystic fibrosis. Clinical and Investigative Medicine 2006;29(3):159-65. [CENTRAL: CN-00622988] [CFGD REGISTER: PE159b] [EMBASE: 43927076] [PubMed]
- Cantin AM, Berthiaume Y. Clearance of airway secretions with the Frequencer in patients with cystic fibrosis. Pediatric Pulmonology 2005;40(S28):322. [ABSTRACT NO.: 379] [CENTRAL: CN-00623756] [CFGD REGISTER: PE159a] [Google Scholar]
Chang 2015 {published data only}
- Chang K, Cotton J, Gashgarian S, Sheppard E, Slack D, Stephenson A, et al. Validation of clinical assessment tools for leg muscle strength and power in adults with cystic fibrosis. Pediatric Pulmonology 2015;50(Suppl 41):368. [ABSTRACT NO: 469] [CENTRAL: 1092168] [CFGD REGISTER: PE223] [Google Scholar]
Chatham 1997 {published data only}
- Chatham K, Ionescu A, Davis C, Baldwin J, Enright S, Shale DJ. Through range computer generated inspiratory muscle training in cystic fibrosis. Pediatric Pulmonology 1997;23(Suppl 14):299. [CFGD REGISTER: PE90] [Google Scholar]
Combret 2018 {published data only}
- Combret Y, Medrinal C, Prieur G, Quesada AR, Le Roux P, Reychler G. Comparative effect of backpack carrying on cystic fibrosis and healthy children: a randomized crossover controlled trial. European Respiratory Journal 2017;50(Suppl 61):PA1341. [ABSTRACT NO.: PA1341] [CENTRAL: CN-01793437] [CFGD REGISTER: PE256b] [DOI: 10.1183/1393003.congress-2017.PA1341 ] [EMBASE: 625792676] [Google Scholar]
- Combret Y, Medrinal C, Prieur G, Robledo Quesada A, Le Roux P, Reychler G. Effect of backpack carrying on forced vital capacity in cystic fibrosis: a randomized crossover-controlled trial. PloS One 2018;13(5):e0196750. [CENTRAL: CN-01621321] [CFGD REGISTER: PE256a] [EMBASE: 622059489] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Combret 2021 {published data only}
- Combret Y, Boujibar F, Gennari C, Medrinal C, Sicinski S, Bonnevie T, et al. Measurement properties of the one-minute sit-to-stand test in children and adolescents with cystic fibrosis: a multicenter randomized cross-over trial. PloS One 2021;16(2):e0246781. [CFGD REGISTER: PE295b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03069625. Sit-to-stand test in cystic fibrosis children and adolescents [Sit-to-stand test use in children and adolescents with cystic fibrosis: correlations with 6-minute walking test, quadriceps and respiratory muscle strength and health related quality of life]. clinicaltrials.gov/show/nct03069625 (first received 3 March 2017). [CENTRAL: CN-01577285]
Cox 2013 {published data only}
de Jong 1994 {published data only}
- De Jong W, Grevink RG, Roorda RJ, Kaptein AA, Schans CP. Effect of a home exercise training program in patients with cystic fibrosis. Chest 1994;105(2):463-8. [DOI] [PubMed] [Google Scholar]
del Corral Nunez‐Flores 2014 {published data only}
- Corral Nunez-Flores T, Percegona J, Seborga M, Trujillo N, Hernandez L, Rejas A, et al. Exercise physiologic response during three different video games in cystic fibrosis. Journal of Cystic Fibrosis 2011;10(Suppl 1):S64. [ABSTRACT NO.: 253] [CFGD REGISTER: PE190a] [Google Scholar]
- Corral Nunez-Flores T, Percegona J, Seborga M, Trujillo N, Hernandez L, Rejas A, et al. Exercise physiologic response during three different video games in cystic fibrosis. Pediatric Pulmonology 2011;46(S34):353. [ABSTRACT NO.: 389] [CFGD REGISTER: PE190b] [Google Scholar]
- Corral T, Percegona J, Seborga M, Rabinovich RA, Vilaro J. Physiological response during activity programs using Wii-based video games in patients with cystic fibrosis. Journal of Cystic Fibrosis 2014;13(6):706-11. [CFGD REGISTER: PE190c] [DOI] [PubMed] [Google Scholar]
- Corral T, Percegona J, Seborga M, Trujillo N, Hernandez L, Rejas A, et al. Exercise physiologic response during three different video games in cystic fibrosis patients. European Respiratory Journal 2011;38(55):878s. [ABSTRACT NO.: P4802] [CENTRAL: 1081933] [CFGD REGISTER: PE190d] [EMBASE: 72122296] [Google Scholar]
de Marchis 2017 {published data only}
- Marchis M, Graziano L, Alatri F, Perelli T, Giacomodonato B, Paris G, et al. Effectiveness of a psychomotor intervention in a group of paediatric patients with cystic fibrosis hospitalized for pulmonary exacerbation: a randomized controlled study. Journal of Cystic Fibrosis 2017;16(Suppl 1):S28-9. [CENTRAL: CN-01461866] [CFGD REGISTER: MH64] [EMBASE: 620749748] [Google Scholar]
Dwyer 2011 {published data only}
- Dwyer T, Alison J, McKeough Z, Daviskas E, Bye P. Exercise aids airway clearance by increasing respiratory flow rates and decreasing mucus viscoelasticity in CF. Pediatric Pulmonology 2008;43(Suppl 31):386. [ABSTRACT NO.: 513] [CFGD REGISTER: PE188a] [Google Scholar]
- Dwyer TJ, Alison JA, McKeough ZJ, Daviskas E, Bye PT. Effects of exercise on respiratory flow and sputum properties in patients with cystic fibrosis. Chest 2011;139(4):870-7. [CENTRAL: CN-00785593] [CFGD REGISTER: PE188b] [PMID: ] [DOI] [PubMed] [Google Scholar]
Dwyer 2017 {published data only}
- Dwyer TJ, Zainuldin R, Daviskas E, Bye PT, Alison JA. Effects of treadmill exercise versus Flutter(R) on respiratory flow and sputum properties in adults with cystic fibrosis: a randomised, controlled, cross-over trial. BMC Pulmonary Medicine 2017;17(1):14. [CFGD REGISTER: PE239] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwyer 2019 {published data only}
- ACTRN12608000287336. Does exercise enhance mucociliary clearance in adults with cystic fibrosis? www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12608000287336 (first received 4 June 2008). [CFGD REGISTER: PE274b]
- Dwyer TJ, Daviskas E, Zainuldin R, Verschuer J, Eberl S, Bye PT, et al. Effects of exercise and airway clearance (positive expiratory pressure) on mucus clearance in cystic fibrosis: a randomised crossover trial. European Respiratory Journal 2019;53(4):pii: 1801793. [CENTRAL: CN-01938004] [CFGD REGISTER: PE274a] [DOI: 10.1183/13993003.01793-2018] [EMBASE: 627483418] [PMID: ] [DOI] [PubMed] [Google Scholar]
Edlund 1986 {published data only}
- Adams TD, Edland LL, French RW, Herbst JJ, Ruttenberg HD, Ruhling RO. Effects of a swimming program on children with cystic fibrosis. International Journal of Sports Medicine 1984;5:156. [CFGD REGISTER: PE154a] [DOI] [PubMed] [Google Scholar]
- Edlund LD, French RW, Herbst JJ, Ruttenburg HD, Ruhling RO, Adams TD. Effects of a swimming program on children with cystic fibrosis. American Journal of Diseases of Children 1986;140(1):80-3. [CFGD REGISTER: PE154b] [DOI] [PubMed] [Google Scholar]
Falk 1988 {published data only}
- Falk M, Kelstrup M, Andersen JB, Pedersen SS, Rossing I, Dirksen H. PEP treatment or physical exercise. Effects on secretions expectorated and indices of central and peripheral airway function. A controlled study. In: 10th International Cystic Fibrosis Congress; 1988 Mar 5-10; Sydney, Australia. 1988:35. [CENTRAL: CN-00208301]
- Falk M, Kelstrup M, Andersen JB, Pedersen SS, Rossing I, Dirksen H. PEP treatment or physical exercise – effects on secretions expectorated and indices of central and peripheral airway function. A controlled study. Excerpta Medica, Asia Pacific Congress Series 1988;74:35. [CFGD REGISTER: PE36] [Google Scholar]
Giacomodonato 2015 {published data only}
- Giacomodonato B, Graziano L, Curzi M, Perelli T, De Sanctis S, Varchetta M, et al. Respiratory muscle endurance training with normocapnic hyperpnea in patients with cystic fibrosis. A randomized controlled study. Journal of Cystic Fibrosis 2015;14(Suppl 1):S41. [ABSTRACT NO: WS21.10] [CENTRAL: 1077187] [CFGD REGISTER: PE217] [Google Scholar]
Gruber 1998 {published data only}
- Gruber W, Kiosz D, Braumann KM, Kolbel R. Exercise and cystic fibrosis. A comparison of training programs with different frequency of training. International Journal of Sports Medicine 1998;19(Suppl):S16. [CENTRAL: CN-00493762] [CFGD REGISTER: PE285] [Google Scholar]
Gruet 2012 {published data only}
- Gruet M, Mely L, Brisswalter J, Vallier J-M. Neuromuscular electrical stimulation in cystic fibrosis. Journal of Cystic Fibrosis 2012;11(Suppl 1):S109. [ABSTRACT NO.: 208] [CFGD REGISTER: PE195] [Google Scholar]
Happ 2013 {published data only}
- Happ MB, Hoffman LA, Higgins LW, Divirgilio D, Orenstein DM. Parent and child perceptions of a self-regulated, home-based exercise program for children with cystic fibrosis. Nursing Research 2013;62(5):305-14. [CFGD REGISTER: PE307b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00609050. Exercise training study for patients with cystic fibrosis [Self-regulated exercise in CF: a randomized trial]. clinicaltrials.gov/ct2/show/NCT00609050 (first received 31 January 2008). [CFGD REGISTER: PE307a]
Haynes 2016 {published data only}
- Haynes F, Maddison L, Millward S, Hughes T, Dewar J. Evaluation of the incremental step test with adult CF patients. Journal of Cystic Fibrosis 2016;15(Suppl):S88. [ABSTRACT NO: 146] [CENTRAL: 1171474] [CFGD REGISTER: PE233] [Google Scholar]
Heijerman 1992 {published data only}
- Heijerman HG, Bakker W, Sterk PJ, Dijkman JH. Long-term effects of exercise training and hyperalimentation in adult cystic fibrosis patients with severe pulmonary dysfunction. International Journal of Rehabilitation Research 1992;15(3):252-7. [DOI] [PubMed] [Google Scholar]
Housinger 2015 {published data only}
- Housinger E, Sullivan M, Ruiz FE. Effects of an interdisciplinary motivational incentive-based walking program in pediatric pulmonary patients during hospitalization. Pediatric Pulmonology 2015;50(Suppl 41):369. [ABSTRACT NO: 471] [CENTRAL: 1092214] [CFGD REGISTER: PE222] [EMBASE: 72081750] [Google Scholar]
Hütler 2002 {published data only}
- Hütler M, Schnabel D, Staab D, Tacke A, Wahn U, Böning D, et al. Effect of growth hormone on exercise tolerance in children with cystic fibrosis. Medicine and Science in Sports and Exercise 2002;34(4):567-72. [CFGD REGISTER: GN125a] [DOI] [PubMed] [Google Scholar]
- Hütler M, Schnabel D, Staab D, Tacke A, Wahn U, Boning D. Growth hormone enhances peak performance in cystic fibrosis. International Journal of Sports Medicine 1998;19(Suppl):S17. [CENTRAL: CN-00494036] [CFGD REGISTER: GN125b] [Google Scholar]
IRCT20161024030474N4 {published data only}
- IRCT20161024030474N4. Effect of pulmonary rehabilitation on quality of life in children with cystic fibrosis and their mother's caregiver burden. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20161024030474N4 (first received 7 June 2020). [CFGD REGISTER: PE326]
Irons 2012 {published data only}
- ACTRN12609000471280. Let's sing out!: the effect of singing on quality of life and lung function of children and adolescents with cystic fibrosis. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ID=83944 (first received 16 June 2009). [CFGD REGISTER: PE185a]
- Irons JY, Kenny DT, McElrea M, Chang AB. Singing therapy for young people with cystic fibrosis: a randomized controlled pilot study. Music and Medicine 2012;4(3):136-45. [CENTRAL: 861294] [CFGD REGISTER: PE185b] [Google Scholar]
Johnston 2004 {published data only}
- Johnston K, Jenkins, Roberts C, Stick S. Improved attitude to exercise in overweight children with lung conditions after an exercise intervention. Respirology 2004;9(2 Suppl):A60. [CENTRAL: 475664] [CFGD REGISTER: PE231] [Google Scholar]
Kaak 2011 {published data only}
- Kaak I, Helbig I, Ankermann T. Didgeridoo playing as an adjunctive therapy to conventional physiotherapy for patients with cystic fibrosis. Zeitschrift fur Physiotherapeuten 2011;63(2):6-14. [CFGD REGISTER: PE292] [Google Scholar]
Kaltsakas 2021 {published data only}
- Kaltsakas G, Anastasopoulos N, Chynkiamis N, Zeliou P, Karapatoucha V, Kotsifas K, et al. Effect of high intensity interval exercise rehabilitation in cystic fibrosis. European Respiratory Journal 2017;50(Suppl 61):OA310. [CENTRAL: CN-01788028] [CFGD REGISTER: PE278b] [DOI: 10.1183/1393003.congress-2017] [EMBASE: 625788657] [DOI] [Google Scholar]
- Kaltsakas G, Anastasopoulos N, Chynkiamis N, Zeliou P, Karapatoucha V, Kotsifas K, et al. Functional capacity, peripheral muscle strength, and quality of life following interval versus continuous rehabilitative exercise training in cystic fibrosis. Thorax 2017;72(Suppl 3):A51. [CENTRAL: CN-01437830] [CFGD REGISTER: PE278a] [EMBASE: 619738976] [Google Scholar]
- Kaltsakas G, Chynkiamis N, Anastasopoulos N, Zeliou P, Karapatoucha V, Kotsifas K, et al. Interval versus constant-load exercise training in adults with cystic fibrosis. Respiratory Physiology & Neurobiology 2021;288:103643. [CFGD REGISTER: PE278c] [DOI: 10.1016/j.resp.2021.103643] [DOI] [PubMed] [Google Scholar]
Kriemler 2016 {published data only}
- Kriemler S, Radtke T, Christen G, Kerstan-Huber M, Hebestreit H. Short-term effect of different physical exercises and physiotherapy combinations on sputum expectoration, oxygen saturation, and lung function in young patients with cystic fibrosis. Lung 2016;194(4):659-64. [CFGD REGISTER: PE219b] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Radtke T, Christen G, Kerstan Huber M, Hebestreit H, Kriemler S. Short-term effect of different physical exercise–physiotherapy combinations on sputum production, oxygen saturation and lung function in young patients with cystic fibrosis. Journal of Cystic Fibrosis 2015;14(Suppl 1):S27. [ABSTRACT NO: WS14.2] [CENTRAL: 1081483] [CFGD REGISTER: PE219a] [EMBASE: 71951559] [DOI] [PubMed] [Google Scholar]
Kuys 2011 {published data only}
- Hall K, Peasey M, Wood M, Cobb R, Bell S, Kuys S. The effects of Nintendo-Wii exercise training in adults with cystic fibrosis. Physiotherapy (United Kingdom) 2011;97(Suppl 1):eS648. [CENTRAL: 1089274] [CFGD REGISTER: PE184c] [EMBASE: 71883056] [Google Scholar]
- Hall K, Peasey M, Wood M, Cobb R, Bell SC, Kuys S. The effects of Nintendo-Wii® exercise training in adults with cystic fibrosis. Respirology (Carlton, Vic.) 2011;16(Suppl 1):P56. [ABSTRACT NO: TP 077] [CFGD REGISTER: PE184d] [Google Scholar]
- Hall K, Peasey M, Wood M, Cobb R, Bell SC, Kuys S. The effects of Nintendo Wii exercise training in adults with cystic fibrosis. Journal of Cystic Fibrosis 2010;9(Suppl 1):A275. [CFGD REGISTER: PE184a] [Google Scholar]
- Kuys SS, Hall K, Peasey M, Wood M, Cobb R, Bell SC. Gaming console exercise and cycle or treadmill exercise provide similar cardiovascular demand in adults with cystic fibrosis: a randomised cross-over trial. Journal of Physiotherapy 2011;57(1):35-40. [CFGD REGISTER: PE184b] [DOI] [PubMed] [Google Scholar]
Lang 2019 {published data only}
- ACTRN12617001035314. CyFiT Telerehabilitation: technology based physiotherapy for peer driven participation in therapy, and quality of life. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617001035314 (first received 17 July 2017). [CFGD REGISTER: PE305a]
- Lang RL, Wilson C, Stockton K, Russell T, Johnston LM. CyFiT telehealth: protocol for a randomised controlled trial of an online outpatient physiotherapy service for children with cystic fibrosis. BMC Pulmonary Medicine 2019;19(1):21. [CFGD REGISTER: PE305b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lannefors 1992 {published data only}
- Lannefors L, Wollmer P. Mucus clearance in cystic fibrosis (CF) – a comparison between postural drainage, PEP-mask and physical exercise. In: 11th International Cystic Fibrosis Congress; 1992 Aug 22-27; Dubin, Ireland. 1992:AHP31. [CFGD REGISTER: PE46a] [PubMed]
- Lannefors L, Wollmerr P. Mucus clearance with three chest physiotherapy regimes in CF: a comparison between postural drainage, PEP and physical exercise. European Respiratory Journal 1992;5(6):748-53. [CFGD REGISTER: PE46b] [PubMed] [Google Scholar]
Lima 2014 {published data only}
- Lima C, De Andrade AD, Rattes C, Campos S, Brandao D, Aliverti A, et al. Effect of noninvasive ventilation on functional exercise capacity, lung function and compartmental chest wall volume in children with cystic fibrosis. European Respiratory Journal 2013;42:1075s. [ABSTRACT NO: P5065] [CENTRAL: 1099891] [CFGD REGISTER: PE216b] [EMBASE: 71843506] [Google Scholar]
- Lima CA, De Andrade AD, Campos SL, Brandao DC, Fregonezi G, Mourato IP, et al. Effects of noninvasive ventilation on treadmill 6-min walking distance and regional chest wall volumes in cystic fibrosis: randomized controlled trial. Respiratory Medicine 2014;108(10):1460-8. [CFGD REGISTER: PE216a] [DOI] [PubMed] [Google Scholar]
- NCT01987271. Effects of noninvasive ventilation on functional capacity of patients with cystic fibrosis [Effects of noninvasive ventilation during the treadmill walking test on cardiorespiratory system, walk distance, and thoracoabdominal kinematics of patients with cystic fibrosis: clinical randomized controlled trial]. clinicaltrials.gov/show/NCT01987271 (first received 19 November 2013). [CENTRAL: CN-01479093] [CFGD REGISTER: PE216c]
Lowman 2012 {published data only}
- Lowman JD, Britton LJ, Lee LS, Phillips AL, Hoover WC. Comprehensive exercise training during hospitalization for an acute CF exacerbation: a randomized controlled trial. Journal of Cystic Fibrosis 2012;11(Suppl 1):S23. [ABSTRACT NO.: WS10.5] [CFGD REGISTER: PE192] [Google Scholar]
Macleod 2008 {published data only}
- Horsley A, Ridley S, Beattie C, Macleod K, Greening A, Innes JA. Changes in lung gas mixing after physiotherapy in adults with cystic fibrosis. European Respiratory Journal 2007;30(Suppl 51):223s. [CENTRAL: CN-00645484] [CFGD REGISTER: PE252a] [Google Scholar]
- Macleod K, Dhouieb E, Riding K, Horsley A, Bell N, Alistair Innes J, et al. Acute changes in ventilation heterogeneity following routine physiotherapy in children with cystic fibrosis. In: European Respiratory Society Annual Congress; 2008 Oct 4-8; Berlin, Germany. 2008:P3903. [CENTRAL: CN-00679234] [CFGD REGISTER: PE252b]
Mandrusiak 2011 {published data only}
- ACTRN12607000612415. Effect of an exercise program on function, activity and participation of young people with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12607000612415 (first received 28 November 2007). [CFGD REGISTER: PE229b]
- Mandrusiak A, MacDonald J, Paratz J, Wilson C, Watter P. A novel exercise program for young people with cystic fibrosis: moving physiotherapy forward through targeted design. Physiotherapy 2011;97:eS1550. [CENTRAL: 1076180] [CFGD REGISTER: PE229] [EMBASE: 71884245] [POSTER NO.: SI-PO-210-22-Thu] [Google Scholar]
Martinez Rodriguez 2017 {published data only}
- Martinez Rodriguez ME, Suarez Cortina L, Maiz Carro L, Ruiz-De-Valbuena M, Jimenez Cosmes L. A pulmonary rehabilitation program to increase adherence to airway clearance techniques for children and adults with cystic fibrosis. Journal of Cystic Fibrosis 2017;16(Suppl 1):S58. [CENTRAL: CN-01461867] [CFGD REGISTER: PE248] [EMBASE: 620749723] [Google Scholar]
Montero‐Ruiz 2020 {published data only}ISRCTN11161411
- Frias JP, Montero-Ruiz A, Galvez LA, Perez-Ruiz E, Huelamo MP, Martin-Montanez E. A music therapy intervention as an adjunct to chest physiotherapy in children with cystic fibrosis. European Respiratory Journal 2018;52(Suppl 62):PA4626. [CFGD REGISTER: PE293b] [Google Scholar]
- Montero-Ruiz A, Fuentes LA, Perez Ruiz E, Garcia-Agua Soler N, Rius-Diaz F, Caro Aguilera P, et al. Effects of music therapy as an adjunct to chest physiotherapy in children with cystic fibrosis: a randomized controlled trial. PloS One 2020;15(10):e0241334. [CFGD REGISTER: PE293a] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moola 2017 {published data only}
- Moola FJ, Garcia E, Huynh E, Henry L, Penfound S, Consunji-Araneta R, et al. Physical activity counselling for children with cystic fibrosis. Respiratory Care 2017;62(11):1466-73. [CENTRAL: CN-01411945] [CFGD REGISTER: PE247] [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT00129350 {published data only}
- NCT00129350. Assessment of heart and heart-lung transplant patient outcomes following pulmonary rehabilitation. clinicaltrials.gov/show/NCT00129350 (first received 11 August 2005). [CFGD REGISTER: CO92]
NCT00792194 {published data only}
- NCT00792194. Improvement of aerobic capacity in cystic fibrosis patients with a one-year home training period. clinicaltrials.gov/ct2/show/NCT00792194 (first received 14 November 2008).
NCT01759342 {published data only}
- NCT01759342. Comprehensive exercise training program during hospitalization for an acute CF exacerbation. clinicaltrials.gov/show/NCT01759342 (first received 3 January 2013). [CFGD REGISTER: PE315]
NCT02199340 {published data only}
- NCT02199340. The iStep Study: development and validation of an incremental exercise step test for children with cystic fibrosis. clinicaltrials.gov/show/NCT02199340 (first received 24 July 2014). [CFGD REGISTER: PE313]
NCT02277860 {published data only}
- NCT02277860. Gaming console home-based exercise for adults with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT02277860 (first received 27 October 2014).
NCT02715921 {published data only}
- NCT02715921. Impact of telerehabilitation training on pediatric cystic fibrosis patients: an exploratory study. clinicaltrials.gov/ct2/show/NCT02715921 (first received 12 January 2016).
NCT02821130 {published data only}
- NCT02821130. Orkambi exercise study (Orkambi) [Effects of Orkambi on exertional dyspnea, exercise performance, and ventilatory responses in adults with cystic fibrosis]. clinicaltrials.gov/ct2/show/NCT02821130 (first received 6 June 2016).
NCT02875366 {published data only}
- NCT02875366. A study of the effects of lumacaftor/ivacaftor on exercise tolerance in subjects with cystic fibrosis, homozygous for the F508del-CFTR mutation [A phase 4, randomized, double-blind, placebo-controlled, parallel-design study of the effect of lumacaftor/ivacaftor combination therapy on exercise tolerance in subjects aged 12 years and older with cystic fibrosis, homozygous for the F508del-CFTR mutation]. clinicaltrials.gov/ct2/show/NCT02875366 (first received 15 August 2016).
NCT03117764 {published data only}
- NCT03117764. Evaluation of the impact of intravenous antibiotics on muscular strength in patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT03117764 (first received 31 March 2017).
NCT03420209 {published data only}
- NCT03420209. The effect of proprioceptive neuromuscular facilitation (PNF) technique for children with chronic pulmonary diseases. clinicaltrials.gov/show/nct03420209 (first received 05 February 2018). [CFGD REGISTER: PE339]
NCT04888767 {published data only}
- NCT04888767. Safety and feasibility of high-intensity interval training program in CF patients. clinicaltrials.gov/show/NCT04888767 (first received 17 May 2021). [CFGD REGISTER: PE338]
NTR2092 {published data only}
- NTR2092. Inspiratory muscle training prior to peripheral muscle training in children and adolescents with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR2092 (first received 4 November 2009). [CFGD REGISTER: PE314]
Oliveira 2010 {published data only}
- Oliveira AC, Jesus TA, Mesquita-Ferrari RA, Oliveira JC, Oliveira LV, Sampaio LM. Aerobic training and quality of life in cystic fibrosis. European Respiratory Journal 2010;36(Suppl 54):P2651. [CFGD REGISTER: PE245] [Google Scholar]
Orenstein 1981 {published data only}
- Orenstein DM, Franklin BA, Doershuk CF, Hellerstein HK, Germann KJ, Horowitz JG, et al. Exercise conditioning and cardiopulmonary fitness in CF. The effects of a three-month supervised running program. Chest 1981;80(4):392-8. [CFGD REGISTER: PE92] [DOI] [PubMed] [Google Scholar]
Orenstein 2004 {published data only}
- Orenstein DM, Hovell MF, Mulvihill M, Keating KK, Hofstetter CR, Kelsey S, et al. Strength vs aerobic training in children with cystic fibrosis: a randomised controlled trial. Chest 2004;126(4):1204-14. [CFGD REGISTER: PE165] [DOI] [PubMed] [Google Scholar]
Ozaydin 2010 {published data only}
- Ozaydin Z, Savci S, Saglam M, Arikan H, Inal-Ince D, Vardar-Yagli N, et al. Effects of inspiratory muscle training on functional capacity and muscle strength in patients with mild cystic fibrosis. In: European Respiratory Society Annual Congress; 2010 Sep 18-22; Barcelona, Spain. 2010. [ABSTRACT NO: 5134] [CENTRAL: 777297] [CFGD REGISTER: PE235]
Patterson 2004 {published data only}
- Patterson JE, Bradley JM. Inspiratory muscle training in adult patients with cystic fibrosis: a randomised controlled trial to evaluate the efficacy of the test of incremental respiratory endurance (TIRE). Thorax 2004;59(Suppl II):ii13. [ABSTRACT NO: S36] [CENTRAL: 518434] [CFGD REGISTER: PE236] [Google Scholar]
Petrovic 2013 {published data only}
- Petrovic M, Kaluza I, Pohl W. Effects of individualised aerobic exercise training in adults with cystic fibrosis: a 4 year controlled trial. Journal of Cystic Fibrosis 2013;12(Suppl 1):S28. [ABSTRACT NO.: WS14.4] [CENTRAL: 875002] [CFGD REGISTER: PE204] [Google Scholar]
Phillips 2008 {published data only}
- Phillips AL, Lee L, Britton LJ, Hoover W, Lowman JD. Efficacy of a standardised exercise protocol in inpatient care of patients with cystic fibrosis. Pediatric Pulmonology 2008;43(Suppl 31):385. [ABSTRACT NO: 510] [CFGD REGISTER: PE182] [Google Scholar]
Pryor 1979 {published data only}
- Hodson ME, Batten JC, Pryor JA, Webber BA. Evaluation of the forced expiration technique as an adjunct to postural drainage in the treatment of cystic fibrosis. In: 9th Meeting European Working Group for Cystic Fibrosis; 1979 Jun 12-13; Noordwijkerhout, Netherlands. 1979:57. [CFGD REGISTER: PE78b]
- Pryor JA, Webber BA, Hodson ME, Batten JC. Evaluation of the forced expiration technique as an adjunct to postural drainage in treatment of cystic fibrosis. British Medical Journal 1979;2(6187):417-8. [CENTRAL: CN-00208123] [CFGD REGISTER: PE78a] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor JA, Webber BA. An evaluation of the forced expiration technique as an adjunct to postural drainage. Physiotherapy 1979;65(10):304-7. [CENTRAL: CN-00208127] [CFGD REGISTER: PE78c] [PubMed] [Google Scholar]
Radtke 2018b {published data only}
- NCT02750722. Exercise and oscillatory positive expiratory pressure therapy in cystic fibrosis [Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusion capacity in cystic fibrosis: a randomized crossover trial]. clinicaltrials.gov/show/NCT02750722 (first received 25 April 2016). [CENTRAL: CN-01557680] [CFGD REGISTER: PE255d]
- Radtke T, Boeni L, Bohnacker P, Maggi-Bebba M, Fischer P, Kriemler S, et al. Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusing capacity in cystic fibrosis: a randomized, controlled, crossover trial. European Respiratory Journal 2018;52(Suppl 62):PA3418. [CENTRAL: CN-01915241] [CFGD REGISTER: PE255e] [EMBASE: 626624799] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke T, Boni L, Bohnacker P, Fischer P, Benden C, Dressel H. The many ways sputum flows – dealing with high within-subject variability in cystic fibrosis sputum rheology. Respiratory Physiology & Neurobiology 2018;254:36-9. [CENTRAL: CN-01607707] [CFGD REGISTER: PE255c] [EMBASE: 2000702039] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Radtke T, Boni L, Bohnacker P, Maggi-Beba M, Fischer P, Kriemler S, et al. Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusing capacity in cystic fibrosis: a randomized, controlled, crossover trial. BMC Pulmonary Medicine 2018;18(1):99. [CENTRAL: CN-01611277] [CFGD REGISTER: PE255a] [EMBASE: 622563058] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke T, Boni L, Bohnacker P, Maggi-Bebba M, Fischer P, Kriemler S, et al. Acute effects of combined exercise and oscillatory positive expiratory pressure therapy on sputum properties and lung diffusing capacity in cystic fibrosis: a randomised, controlled, crossover trial. Journal of Cystic Fibrosis 2018;17(Suppl 3):S15. [CENTRAL: CN-01746901] [CFGD REGISTER: PE255b] [EMBASE: 622931153] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rand 2012 {published data only}
- Rand S, Hill L, Prasad SA, Main E. Development of an incremental field exercise test for children with cystic fibrosis. Journal of Cystic Fibrosis 2012;11(Suppl 1):S36. [ABSTRACT NO.: WS16.7] [CFGD REGISTER: PE193] [Google Scholar]
RBR‐34677v {published data only}
- RBR-34677v. Use of videogames as physical training for children and adolescents with cystic fibrosis during hospitalization. www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-34677v (first received 9 June 2015). [CFGD REGISTER: PE316]
RBR‐5g9f6w {published data only}
- RBR-5g9f6w. Rehabilitation of children and adolescents with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-5g9f6w (first received 17 June 2020). [CFGD REGISTER: PE324]
Reix 2012 {published data only}
- NCT01509235. Self drainage in pediatric cystic fibrosis patients. clinicaltrials.gov/ct2/show/NCT01509235 (first received 12 January 2012). [CFGD REGISTER: PE183c]
- Reix P, Aubert F, Kassai B, Bige V, Bellon G. Better satisfaction of cystic fibrosis paediatric patients with autogenic drainage associated to exercise compared to conventional chest physiotherapy. Journal of Cystic Fibrosis 2009;8(Suppl 2):S73. [ABSTRACT NO.: 293] [CFGD REGISTER: PE183a] [Google Scholar]
- Reix P, Aubert F, Werck-Gallois MC, Toutain A, Mazzocchi C, Moreux N, et al. Exercise with incorporated expiratory manoeuvres was as effective as breathing techniques for airway clearance in children with cystic fibrosis: a randomised crossover trial. Journal of Physiotherapy 2012;58(4):241-7. [CENTRAL: 841658] [CFGD REGISTER: PE183b] [PMID: ] [DOI] [PubMed] [Google Scholar]
Reuveny 2020 {published data only}
- ISRCTN13864650. Using oxygen to improve exercise performance in patients with cystic fibrosis. trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN13864650 (first received 16 July 2019). [CFGD REGISTER: PE294b]
- Reuveny R, Dimenna FJ, Gunaratnam C, Arad AD, McElvaney GN, Susta D, et al. High-intensity interval training accelerates oxygen uptake kinetics and improves exercise tolerance for individuals with cystic fibrosis. BMC Sports Science, Medicine and Rehabilitation 2020;12(1):13. [CFGD REGISTER: PE294a] [DOI: 10.1186/s13102-020-0159-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruddy 2015 {published data only}
- NCT01325766. Study of yoga as a therapy for cystic fibrosis (CF) patients. clinicaltrials.gov/show/NCT01325766 (first received 30 March 2011). [CFGD REGISTER: PE337a]
- Ruddy J, Emerson J, Genatossio A, Breuner C, Weber T, Rosenfeld M. Yoga as a therapy for adolescents and young adults with cystic fibrosis: a pilot study. Global Advances in Health and Medicine 2015;4(6):32-6. [CFGD REGISTER: PE337b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salh 1989 {published data only}
- Salh W, Bilton D, Dodd M, Webb AK. Effect of exercise and physiotherapy in aiding sputum expectoration in adults with cystic fibrosis. Thorax 1989;44(12):1006-8. [CFGD REGISTER: PE63] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salonini 2015 {published data only}
- Salonini E, Gambazza S, Meneghelli I, Tridello G, Sanguanini M, Cazzarolli C, et al. Active video game playing in children and adolescents with cystic fibrosis: exercise or just fun? Respiratory Care 2015;60(8):1172-9. [CENTRAL: 1161387] [CFGD REGISTER: PE207b] [EMBASE: 2015465751] [DOI] [PubMed] [Google Scholar]
- Salonini E, Gambazza S, Tridello G, Sanguanini M, Cazzarolli C. Kinect active video game in cystic fibrosis: exercise or fun? Journal of Cystic Fibrosis 2013;12(Suppl 1):S108. [ABSTRACT NO.: 233] [CENTRAL: 875196] [CFGD REGISTER: PE207a] [Google Scholar]
Shaw 2016 {published data only}
- Shaw I, Kinsey JE, Richards R, Shaw BS. Individualized supervised resistance training during nebulization in adults with cystic fibrosis. Pakistan Journal of Medical Sciences 2016;32(5):1152-7. [CENTRAL: 1343881] [CFGD REGISTER: PE242] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spoletini 2020 {published data only}
- NCT03965832. HFNT during exercise in CF [A pilot study to evaluate the feasibility of using high-flow nasal therapy during exercise in patients with cystic fibrosis and severe lung disease]. clinicaltrials.gov/show/NCT03965832 (first received 29 May 2019). [CFGD REGISTER: PE308a]
- Spoletini G, Watson R, Pollard K, Lim WY, Etherington C, Clifton I, et al. Nasal high-flow therapy (NHFT) during exercise in patients with cystic fibrosis (CF): a randomized crossover trial. European Respiratory Journal 2020;56(Suppl 64):365. [CFGD REGISTER: PE308b] [Google Scholar]
Stanghelle 1998 {published data only}
- Stanghelle JK, Hjeltnes N, Bangstad HJ, Michalsen H. Effect of daily short bouts of trampoline exercise during 8 weeks on the pulmonary function and the maximal oxygen uptake of children with cystic fibrosis. International Journal of Sports Medicine 1988;9(Suppl 1):32-6. [CENTRAL: 568629] [CFGD REGISTER: PE155] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tuzin 1998 {published data only}
- Tuzin BJ, Mulvihill MM, Kilbourn KM, Bertran DA, Buono M, Hovell MF, et al. Increasing physical activity of children with cystic fibrosis: a home based family intervention. Pediatric Exercise Science 1998;10(1):57-68. [Google Scholar]
Vallier 2016 {published data only}
- Vallier JM, Rouissi M, Mely L, Gruet M. Physiological responses of the modified shuttle test in adults with cystic fibrosis. Journal of Cardiopulmonary Rehabilitation and Prevention 2016;36(4):288-92. [CENTRAL: 1343882] [CFGD REGISTER: PE241] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vivodtzev 2013 {published data only}
- NCT00391703. Assessment of quadriceps muscle electrostimulation used in patients suffering from cystic fibrosis. clinicaltrials.gov/show/NCT00391703 (first received 24 October 2006). [CFGD REGISTER: PE210c]
- Vivodtzev I, Decorte N, Wuyam B, Gonnet N, Durieu I, Levy P, et al. Benefits of neuromuscular electrical stimulation prior to endurance training in patients with cystic fibrosis and severe pulmonary dysfunction. Chest 2013;143(2):485-93. [CENTRAL: 980643] [CFGD REGISTER: PE201b] [DOI] [PubMed] [Google Scholar]
- Vivodtzev I, Decorte N, Wuyam B, Gonnet N, Durieu I, Levy P, et al. Benefits of neuromuscular electrical stimulation prior to endurance training in patients with cystic fibrosis and severe pulmonary dysfunction. Chest 2013;143(2):485-93. [CENTRAL: 906021] [CFGD REGISTER: PE201a] [EMBASE: 22911373] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ward 2018 {published data only}
- Ward N, Stiller K, Rowe H, Morrow S, Morton J, Greville H, et al. Airway clearance by exercising in mild cystic fibrosis: clinical outcomes. Respirology 2018;23(Suppl 1):140. [CENTRAL: CN-01607201] [CFGD REGISTER: PE257a] [EMBASE: 622091390] [DOI] [PubMed] [Google Scholar]
- Ward N, Stiller K, Rowe H, Morrow S, Morton J, Greville H, et al. Airway clearance by exercising in mild cystic fibrosis (ACE-CF): a feasibility study. Respiratory Medicine 2018;142:23-8. [CENTRAL: CN-01616954] [CFGD REGISTER: PE257b] [EMBASE: 2000968825] [DOI] [PubMed] [Google Scholar]
Wheatley 2015 {published data only}
- Wheatley CM, Baker SE, Morgan MA, Martinez MG, Liu B, Rowe SM, et al. Moderate intensity exercise mediates comparable increases in exhaled chloride as albuterol in individuals with cystic fibrosis. Respiratory Medicine 2015;109(8):1001-11. [CENTRAL: 1107333] [CFGD REGISTER: PE225b] [EMBASE: 2015118578] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley CM, Baker SE, Morgan MA, Martinez MG, Morgan WJ, Wong EC, et al. Effects of exercise intensity compared to albuterol in individuals with cystic fibrosis. Respiratory Medicine 2015;109(4):463-74. [CENTRAL: 1110247] [CFGD REGISTER: PE225a] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 1997 {published data only}
- Stiller K. Are thoracic expansion exercises necessary during the active cycle of breathing techniques for adult cystic fibrosis patients? Israel Journal of Medical Sciences 1996;32(Suppl):S275. [CFGD REGISTER: PE61a] [Google Scholar]
- White D, Stiller K, Willson K. The role of thoracic expansion exercises during the active cycle of breathing techniques. Physiotherapy Theory and Practice 1997;13(2):155-62. [CENTRAL: CN-00448712] [CFGD REGISTER: PE61b] [Google Scholar]
Young 2019 {published data only}
- Young R, Wilson P, Underhill B, Shafi N, Watson D. Comparison of the incremental Shuttle Walk Test for adult subjects with cystic fibrosis in two formats: hallway versus treadmill. Journal of Cystic Fibrosis 2019;18(Suppl 1):S161. [CENTRAL: CN-01984664] [CFGD REGISTER: PE280] [Google Scholar]
Zeren 2019 {published data only}
- NCT03375684. Effects of inspiratory muscle training on postural stability, balance, pulmonary function and functional capacity in children with cystic fibrosis. clinicaltrials.gov/show/NCT03375684 (first received 18 December 2017). [CFGD REGISTER: PE275c]
- Zeren M, Cakir E, Gurses HN. Effects of inspiratory muscle training on postural stability, pulmonary function and functional capacity in children with cystic fibrosis: a randomised controlled trial. Respiratory Medicine 2019;148:24-30. [CFGD REGISTER: PE275a] [DOI] [PubMed] [Google Scholar]
- Zeren M, Cakir E, Gurses HN. Effects of inspiratory muscle training on postural stability, pulmonary function and functional capacity in children with cystic fibrosis: a randomised controlled trial. Turkish Thoracic Journal 2019;20:S106. [CFGD REGISTER: PE275b] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bishay 2017 {published data only}
- Bishay LC, Gould A, Prushingskaya O, Hill K, Greenberg J, Ng J, et al. Baseline submaximal exercise tolerance as an outcome measure in a cystic fibrosis exercise study. American Journal of Respiratory and Critical Care Medicine 2017;195:C72. [ABSTRACT NO.: A6259] [CENTRAL: CN-01408842] [CFGD REGISTER: MH60c] [EMBASE: 617709675] [Google Scholar]
- Bishay LC, Gould A, Prushinskaya OV, Hill K, Greenberg J, Sawicki GS, et al. Acceptability of a fitness tracker for patients with CF: a qualitative analysis within a randomized trial. Pediatric Pulmonology 2017;52(S47):490. [ABSTRACT NO.: 707] [CENTRAL: CN-01430881] [CFGD REGISTER: MH60a] [EMBASE: 619069615] [Google Scholar]
- Bishay LC, Nelson E, Williams K, Greenberg J, Gould A, Bailey I, et al. Effect of a wearable fitness tracker on exercise tolerance for adults with cystic fibrosis: a pilot randomized clinical trial. Pediatric Pulmonology 2018;53(S2):345. [ABSTRACT NO.: 516] [CENTRAL: CN-01738935] [CFGD REGISTER: MH60b] [EMBASE: 624049022] [Google Scholar]
- NCT02700243. Increase tolerance for exercise and raise activity through connectedness trial (INTERACT) [Pilot RCT study using a Fitbit device to improve exercise tolerance in 80 patients with cystic fibrosis]. clinicaltrials.gov/ct2/show/NCT02700243 (first received 21 February 2016). [CFGD REGISTER: MH60d]
Cox 2019 {published data only}
- ACTRN12617001009303. Action: PACT. Be Active. Online. A trial to promote physical activity in young people with cystic fibrosis. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12617001009303 (first received 13 July 2017). [CFGD REGISTER: PE287b]
- Cox NS, Eldridge B, Rawlings S, Dreger J, Corda J, Hauser J, et al. A web-based intervention to promote physical activity in adolescents and young adults with cystic fibrosis: protocol for a randomized controlled trial. BMC Pulmonary Medicine 2019;19(1):253. [CFGD REGISTER: PE287a] [DOI] [PMC free article] [PubMed] [Google Scholar]
IRCT20190407043190N1 {published data only}
- IRCT20190407043190N1. Effect of the physical activity plan on the quality of life in children with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20190407043190N1 (first received 4 December 2019). [CFGD REGISTER: PE320]
NCT03100214 {published data only}
- NCT03100214. Effects of an early rehabilitation program during hospitalization in patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT03100214 (first received 31 January 2017). [CFGD REGISTER: PE317]
NCT04293926 {published data only}
- NCT04293926. Heart rate variability in children and adolescents with cystic fibrosis. clinicaltrials.gov/show/NCT04293926 (first received 3 March 2020). [CFGD REGISTER: PE321]
Powers 2016 {published data only}
- NCT03109912. Do More, B'More, Live Fit [Do More, B'More, Live Fit: an outpatient fitness-training pilot program designed to optimize the habit of exercise in adolescents and young adults with cystic fibrosis]. clinicaltrials.gov/ct2/show/NCT03109912 (first received 12 April 2017). [CENTRAL: CN-01380971] [CFGD REGISTER: PE246a]
- Powers KE, Herzog T, Loosen H, Berg K, Weir B, Riekert KA, et al. Step It Up: higher step count is significantly correlated with better exercise capacity in individuals with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2017;195(Abstract Issue):A6135. [CENTRAL: CN-01409315] [CFGD REGISTER: PE246d] [EMBASE: 617704603] [Google Scholar]
- Powers KE, Herzog TL, Loosen H, Berg K, Weir B, Riekert KA, et al. Self-reported physical activity does not correlate with ventilation inhomogeneity. Pediatric Pulmonology 2016;51(S45):370. [CENTRAL: CN-01212541] [CFGD REGISTER: PE246c] [EMBASE: 612359219] [Google Scholar]
- Powers KE, Loosen H, Berg K, Herzog TL, Weir B, Riekert KA, et al. Ventilation inhomogeneity and lung function are associated with exercise capacity. Pediatric Pulmonology 2016;51(S45):369-70. [CENTRAL: CN-01212542] [CFGD REGISTER: PE246b] [EMBASE: 612359207] [Google Scholar]
References to ongoing studies
Curran 2020 {published data only}
- Curran M, Tierney AC, Collins L, Kennedy L, McDonnell C, Jurascheck AJ, et al. Steps Ahead: optimising physical activity in adults with cystic fibrosis: study protocol for a pilot randomised trial using wearable technology, goal setting and text message feedback. HRB Open Research 2020;3:21. [CFGD REGISTER: PE309b] [DOI: 10.12688/hrbopenres.13025.3] [CLINICALTRIALS.GOV: NCT03672058] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03672058. Steps Ahead: optimising physical activity and health in adults with cystic fibrosis. clinicaltrials.gov/show/NCT03672058 (first received 14 September 2018). [CFGD REGISTER: PE309a]
ISRCTN92573472 {published data only}
- ISRCTN92573472. The evaluation of a 12-week partially supervised, self-regulated exercise intervention in patients with cystic fibrosis. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN92573472 (first received 24 February 2020). [CFGD REGISTER: PE323]
Monteiro 2019 {published data only}
- Monteiro KS, Azevedo MP, Jales LM, da Silva FE, Arrais RF, Mendonca KM. Effects of aerobic interval training on glucose tolerance in children and adolescents with cystic fibrosis: a randomized trial protocol. Trials 2019;20(1):768. [CFGD REGISTER: PE288a] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro KS, Azevedo MP, Jales LM, da Silva FE, Arrais RF, Mendonca KM. Effects of aerobic interval training on glucose tolerance in children and adolescents with cystic fibrosis: a randomized trial protocol. Trials 2019;20(1):768. Online supplementary information: additional file 1 SPIRIT 2013 checklist. [CFGD REGISTER: PE288c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro KS, Azevedo MP, Jales LM, da Silva FE, Arrais RF, Mendonca KM. Effects of aerobic interval training on glucose tolerance in children and adolescents with cystic fibrosis: a randomized trial protocol. Trials 2019;20(1):768. Online supplementary information: additional file 2 booklet. [CFGD REGISTER: PE288d] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro KS, Azevedo MP, Jales LM, da Silva FE, Arrais RF, Mendonca KM. Effects of aerobic interval training on glucose tolerance in children and adolescents with cystic fibrosis: a randomized trial protocol. Trials 2019;20(1):768. Online supplementary information: additional file 3 home data recording diary. [CFGD REGISTER: PE288e] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03653949. Effects of aerobic interval training on glucose tolerance in children and adolescents with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT03653949 (first received 31 August 2018). [CFGD REGISTER: PE288b]
NCT03273959 {published data only}
- NCT03273959. Program of exercises during the hospitalization of children and adolescents with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT03273959 (first received 6 September 2017). [CFGD: REGISTER: PI318]
NCT03970369 {published data only}
- NCT03970369. Motivated to move: a study to determine the feasibility of self-monitoring physical activity in youth. clinicaltrials.gov/ct2/show/NCT03970369 (first received 31 May 2019).
NCT04249999 {published data only}
- NCT04249999. ActivOnline: physical activity in cystic fibrosis trial UK (ActiOnPACTUK). clinicaltrials.gov/show/NCT04249999 (first received 31 January 2020). [CFGD REGISTER: PE303]
NCT04543929 {published data only}
- NCT04543929. Effects of innovative aerobic exercise training in cystic fibrosis. clinicaltrials.gov/show/NCT04543929 (first received 10 September 2020). [CFGD REGISTER: PE322]
NCT04683809 {published data only}
- NCT04683809. Effects of a telerehabilitation approach in children with cystic fibrosis [The effects of telerehabilitation on quality of life, anxiety and depression levels in children with cystic fibrosis and their caregivers]. clinicaltrials.gov/ct2/show/NCT04683809 (first received 24 December 2020).
NCT04742049 {published data only}
- NCT04742049. The effects of telerehabilitation on muscle function, physical activity and sleep in cystic fibrosis during pandemic. clinicaltrials.gov/show/NCT04742049 (first received 5 February 2021). [CFGD REGISTER: PE325]
NCT05147285 {published data only}
- NCT05147285. The effect of telerehabilitation on functional capacity, oxidative stress and respiratory parameters in cystic fibrosis [The effect of different exercise modalities applied by tele rehabilitation on functional capacity, oxidative stress and respiratory parameters in cystic fibrosis children]. clinicaltrials.gov/ct2/show/NCT05147285 (first received 7 December 2021).
NCT05173194 {published data only}
- NCT05173194. Remotely supervised exercise for adults with cystic fibrosis [Effects of a remotely supervised exercise program on inflammatory markers, muscle strength and lung function in adult patients with cystic fibrosis]. clinicaltrials.gov/ct2/show/NCT05173194 (first received 29 December 2021).
NCT05239611 {published data only}
- NCT05239611. Feasibility of home-based exercise program for adults with cystic fibrosis [Feasibility of home-based exercise program for adults with cystic fibrosis to improve patient-centered outcomes, including a novel measure of ventilation]. clinicaltrials.gov/ct2/show/NCT05239611 (first received 15 February 2022).
Additional references
ACSM 2017
- American College of Sports Medicine. ACSM's guidelines for exercise testing prescription. 10th edition. Philadelphia (PA): Lippincott Williams and Williams, 2017. [Google Scholar]
Barrett 2021
- Barrett S, Begg S, O'Halloran P, Howlett O, Lawrence J, Kingsley M. The effect of behaviour change interventions on changes in physical activity and anthropometrics in ambulatory hospital settings: a systematic review and meta-analysis. International Journal of Behavioral Nutrition and Physical Activity 2021;18(1):7. [DOI: 10.1186/s12966-020-01076-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burgel 2015
- Burgel PR, Bellis G, Olesen HV, Viviani L, Zolin A, Blasi F, et al, on behalf of the ERS/ECFS Task Force on the Provision of Care for Adults with Cystic Fibrosis in Europe. Future trends in cystic fibrosis demography in 34 European countries. European Respiratory Journal 2015;46:133-41. [DOI] [PubMed] [Google Scholar]
Caspersen 1985
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Reports 1985;100(2):126-31. [PMC free article] [PubMed] [Google Scholar]
Cerny 2013
- Cerny F. Exercise and cystic fibrosis (CF) 2.0. Pediatric Exercise Science 2013;25(4):616-23. [DOI] [PubMed] [Google Scholar]
Cox 2016
- Cox NS, Alison JA, Button BM, Wilson JW, Morton JM, Holland AE. Physical activity participation by adults with cystic fibrosis: an observational study. Respirology 2016;21(3):511-8. [DOI] [PubMed] [Google Scholar]
Cox 2018
- Cox NS, Alison JA, Button BM, Wilson JW, Morton JM, Holland AE. Accumulating physical activity in at least 10-minute bouts predicts better lung function after 3-years in adults with cystic fibrosis. ERJ Open Research 2018;4(2):00095-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2020
- Davies G, Rowbotham NJ, Smith S, Elliot ZC, Gathercole G, Rayner O, et al. Characterising burden of treatment in cystic fibrosis to identify priority areas for clinical trials. Journal of Cystic Fibrosis 2020;19:499-502. [DOI] [PubMed] [Google Scholar]
Denford 2020
- Denford S, Mackintosh KA, McNarry MA, Barker AR, Williams CA, Active Youth Unlimited Group. Promotion of physical activity for adolescents with cystic fibrosis: a qualitative study of UK multi disciplinary cystic fibrosis teams. Physiotherapy 2020;106:111-8. [DOI: 10.1016/j.physio.2019.01.012] [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Elce 2018
- Elce A, Nigro E, Gelzo M, Iacotucci P, Carnovale V, Liguori R, et al. Supervised physical exercise improves clinical, anthropometric and biochemical parameters in adult cystic fibrosis patients: a 2-year evaluation. Clinical Respiratory Journal 2018;12(7):2228-34. [DOI] [PubMed] [Google Scholar]
Ellis 2010
- Ellis PD. The Essential Guide to Effect Sizes: an Introduction to Statistical Power, Meta-analysis and the Interpretation of Research Results. Cambridge (UK): Cambridge University Press, 2010. [Google Scholar]
Farrell 2008
- Farrell PM. The prevalence of cystic fibrosis in the European Union. Journal of Cystic Fibrosis 2008;7(5):450-3. [DOI] [PubMed] [Google Scholar]
Galassetti 2013
- Galassetti P, Riddell MC. Exercise and type 1 diabetes (T1DM). Comprehensive Physiology 2013;3(3):1309-36. [DOI] [PubMed] [Google Scholar]
Gruet 2022
Hebestreit 2001
- Hebestreit A, Kersting U, Basler B, Jeschke R, Hebestreit H. Exercise inhibits epithelial sodium channels in patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2001;164(3):443-6. [DOI] [PubMed] [Google Scholar]
Hebestreit 2014
- Hebestreit H, Schmid K, Kieser S, Junge S, Ballmann M, Roth K, et al. Quality of life is associated with physical activity and fitness in cystic fibrosis. BMC Pulmonary Medicine 2014;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hebestreit 2019
- Hebestreit H, Hulzebos EH, Schneiderman JE, Karila C, Boas SR, Kriemler S, et al. Cardiopulmonary exercise testing provides additional prognostic information in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2019;199(8):987-95. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Hopewell 2009
- Hopewell S, Loudon K, Clarke MJ, Oxman AD, Dickersin K. Publication bias in clinical trials due to statistical significance or direction of trial results. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: MR000006. [DOI: 10.1002/14651858.MR000006.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howlett 2019
- Howlett N, Trivedi D, Troop NA, Chater AM. Are physical activity interventions for healthy inactive adults effective in promoting behavior change and maintenance, and which behavior change techniques are effective? A systematic review and meta-analysis. Translational Behavioral Medicine 2019;9(1):147-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ioannidis 1998
- Ioannidis JP, Cappelleri JC, Lau J. Issues in comparisons between meta-analyses and large trials. JAMA 1998;279(14):1089-93. [DOI] [PubMed] [Google Scholar]
Keogh 2018
- Keogh RH, Szszesniak R, Taylor-Robinson D, Bilton D. Up-to-date and projected estimates of survival for people with cystic fibrosis using baseline characteristics: a longitudinal study using UK patient registry data. Journal of Cystic Fibrosis 2018;17(2):218-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacKenzie 2014
- MacKenzie T, Gifford AH, Sabadosa KA, Quinton HB, Knapp EA, Goss CH, et al. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation patient registry. Annals of Internal Medicine 2014;161(4):233-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maltais 2014
- Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigaré R, et al. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2014;189(9):e15-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Middleton 2019
- Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. Elexacaftor–Tezacaftor–Ivacaftor for cystic fibrosis with a single Phe508del allele. New England Journal of Medicine 2019;381:1809-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nixon 1992
- Nixon PA, Orenstein DM, Kelsey SF, Doerschuk CF. The prognostic value of exercise testing in patients with cystic fibrosis. New England Journal of Medicine 1992;327(25):1785-8. [DOI] [PubMed] [Google Scholar]
Nuss 2021
- Nuss K, Moore K, Nelson T, Li K. Effects of motivational interviewing and wearable fitness trackers on motivation and physical activity: a systematic review. American Journal of Health Promotion 2021;35(2):226-35. [DOI] [PubMed] [Google Scholar]
Pianosi 2005
- Pianosi P, LeBlanc J, Almudevar A. Peak oxygen uptake and mortality in children with cystic fibrosis. Thorax 2005;60(1):50-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puppo 2020
- Puppo H, Torres-Castro R, Vasconcello-Castillo L, Acosta-Dighero R, Sepúlveda-Cáceres N, Quiroga-Marabolí P, et al. Physical activity in children and adolescents with cystic fibrosis: a systematic review and meta-analysis. Pediatric Pulmonology 2020;5(11):2863-76. [DOI] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire – Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;136(6):1610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Radtke 2018a
- Radtke T, Hebestreit H, Gallati S, Schneiderman JE, Braun J, Stevens D, et al. CFTR genotype and maximal exercise capacity in cystic fibrosis: a cross-sectional study. Annals of the American Thoracic Society 2018;15(2):209-16. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rowbotham 2018
- Rowbotham NJ, Smith S, Leighton PA, Rayner OC, Gathercole K, Elliott ZC, et al. The top 10 research priorities in cystic fibrosis developed by a partnership between people with CF and healthcare providers. Thorax 2018;73(4):388-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowbotham 2020
- Rowbotham NJ, Smith SJ, Davies G, Daniel T, Elliott ZC, Gathercole K, et al. Can exercise replace airway clearance techniques in cystic fibrosis? A survey of patients and healthcare professionals. Journal of Cystic Fibrosis 2020;19(4):e19-24. [DOI] [PubMed] [Google Scholar]
Ruf 2009
- Ruf K, Hebestreit H. Exercise-induced hypoxemia and cardiac arrhythmia in cystic fibrosis. Journal of Cystic Fibrosis 2009;8(2):83-90. [DOI: 10.1016/j.jcf.2008.09.008] [DOI] [PubMed] [Google Scholar]
Ruf 2010
- Ruf K, Winkler B, Hebestreit A, Gruber W, Hebestreit H. Risks associated with exercise testing and sports participation in cystic fibrosis. Journal of Cystic Fibrosis 2010;9(5):339-45. [DOI] [PubMed] [Google Scholar]
Saynor 2013
- Saynor ZL, Barker AR, Oades PJ, Williams CA. Reproducibility of maximal cardiopulmonary exercise testing for young cystic fibrosis patients. Journal of Cystic Fibrosis 2013;12(6):644-50. [DOI] [PubMed] [Google Scholar]
Schneiderman 2014
- Schneiderman JE, Wilkes DL, Atenafu EG, Nguyen T, Wells GD, Alarie N, et al. Longitudinal relationship between physical activity and lung health in patients with cystic fibrosis. European Respiratory Journal 2014;43(3):817-23. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
Scotet 2020
- Scotet V, Gutierrez H, Farrell PM. Newborn screening for CF across the globe – where is it worthwhile? International Journal of Neonatal Screening 2020;6(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shelley 2019
- Shelley J, Boddy LM, Knowles ZR, Stewart CE, Dawson EA. Physical activity and associations with clinical outcome measures in adults with cystic fibrosis: a systematic review. Journal of Cystic Fibrosis 2019;18(5):590-601. [DOI] [PubMed] [Google Scholar]
Shephard 1994
- Shephard RJ. Aerobic Fitness and Health. Leeds (UK): Human Kinetic Publishers, 1994. [Google Scholar]
Southern 2007
- Southern KW, Munck A, Pollitt R, Travert G, Zanolla L, Dankert-Roelse J, et al. A survey of newborn screening for cystic fibrosis in Europe. Journal of Cystic Fibrosis 2007;6 (1):57-65. [DOI] [PubMed] [Google Scholar]
Stanojevic 2016
- Stanojevic S, Ratjen F. Physiologic endpoints for clinical studies for cystic fibrosis. Journal of Cystic Fibrosis 2016;15(4):416-23. [DOI] [PubMed] [Google Scholar]
Tejero García 2011
- Tejero García S, Giráldez Sánchez MA, Cejudo P, Quintana Gallego E, Dapena J, García Jiménez R, et al. Bone health, daily physical activity, and exercise tolerance in patients with cystic fibrosis. Chest 2011;140(2):475-81. [DOI] [PubMed] [Google Scholar]
Tomlinson 2021
- Tomlinson OW, Denford S, Barker AR, Schneiderman JE, Campisi ES, Douglas H, et al. The impact of physical activity and exercise interventions for physical health in people with cystic fibrosis: protocol for a systematic review. Systematic Reviews 2021;26(10):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
van de Weert‐Van Leeuwen 2012
- de Weert-Van Leeuwen PB, Slieker MG, Hulzebos HJ, Kruitwagen CL, Van der Ent CK, Arets HG. Chronic infection and inflammation affect exercise capacity in cystic fibrosis. European Respiratory Journal 2012;39:893-8. [DOI] [PubMed] [Google Scholar]
van de Weert‐Van Leeuwen 2013
- de Weert-Van Leeuwen PB, Arets HG, Ent CK, Beekman JM. Infection, inflammation and exercise in cystic fibrosis. Respiratory Research 2013;14(1):32. [DOI: 10.1186/1465-9921-14-32] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wade 2006
- Wade A, Pan H, Eaton S, Ong E . An investigation of minimisation criteria. BMC Medical Research Methodology 2006;6:11. [DOI: 10.1186/1471-2288-6-11] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wainwright 2015
- Wainwright CE, Elborn S, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. Lumacaftor–Ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. New England Journal of Medicine 2015;373(3):220-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkinson 2019
- Wilkinson JW, Watson EL, Xenophontos S, Gould DW, Smith AC. The "minimum clinically important difference" in frequently reported objective physical function tests after a 12-week renal rehabilitation exercise intervention in nondialysis chronic kidney disease. American Journal of Physical Medicine & Rehabilitation 2019;98(6):431-7. [DOI] [PubMed] [Google Scholar]
Wilson 2021
- Wilson J, You X, Ellis M, Urquhart DS, Jha L, Duncan M, et al. VO 2max as an exercise tolerance endpoint in people with cystic fibrosis: lessons from a lumacaftor/ivacaftor trial. Journal of Cystic Fibrosis 2021;20(3):499-505. [DOI] [PubMed] [Google Scholar]
Yankaskas 2004
- Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest 2004;125(1 Suppl):1S-39S. [DOI: 10.1378/chest.125.1_suppl.1s] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bradley 2002
- Bradley JM, Moran F. Physical training for cystic fibrosis. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No: CD002768. [DOI: 10.1002/14651858.CD002768] [DOI] [PubMed] [Google Scholar]
Bradley 2008
- Bradley JM, Moran F. Physical training for cystic fibrosis. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD002768. [DOI: 10.1002/14651858.CD002768.pub2] [DOI] [PubMed] [Google Scholar]
Radtke 2015
- Radtke T, Nolan SJ, Hebestreit H, Kriemler S. Physical exercise training for cystic fibrosis. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD002768. [DOI: 10.1002/14651858.CD002768.pub3] [DOI] [PubMed] [Google Scholar]
Radtke 2017
- Radtke T, Nevitt SJ, Hebestreit H, Kriemler S. Physical exercise training for cystic fibrosis. Cochrane Database of Systematic Reviews 2017, Issue 11. Art. No: CD002768. [DOI: 10.1002/14651858.CD002768.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


