Abstract
In Lactococcus lactis, the his operon contains all the genes necessary for histidine biosynthesis. It is transcribed from a unique promoter, localized 300 bp upstream of the first gene. The region corresponding to the untranslated 5′ end of the transcript, named the his leader region, displays the typical features of the T box transcriptional attenuation mechanism which is involved in the regulation of many amino acid biosynthetic operons and tRNA synthetase genes in gram-positive bacteria. Here we describe the regulation of transcription of the his operon by the level of histidine in the growth medium. In the absence of histidine, two transcripts are present. One covers the entire operon, while the other stops at a terminator situated about 250 bp downstream of the transcription start point. DNA sequences implicated in regulation of the his operon were identified by transcriptional fusion with luciferase genes and site-directed mutagenesis. In addition to the previously defined sequences necessary for effective T-box-mediated regulation, new essential regions were identified. Eighteen percent of the positions of the his leader region were found to differ in seven distantly related strains of L. lactis. Analysis of the variable positions supports the folding model of the central part of the his leader region. Lastly, in addition to the T-box-mediated regulation, the operon is regulated at the level of initiation of transcription, which is repressed in the presence of histidine. An operator site, necessary for full repression, overlaps the terminator involved in the T box attenuation mechanism. The functionality of the operator is altered on plasmids with low and high copy numbers, suggesting that supercoiling may play a role in the expression of the his operon. The extents of regulation at the levels of initiation and attenuation of transcription are 6- to 8-fold and 14-fold, respectively. Together, the two levels of control allow a 120-fold range of regulation of the L. lactis operon by histidine.
Extensive studies of amino acid biosynthetic genes in various bacteria have revealed a wide variety of models for gene organization and regulation. Together with the lac and trp operons, the his operon was used as a model system to study the mechanisms governing expression in enterobacteria and in many other microorganisms (reviewed in references 1, 2, and 47). As a result, the his operon may be considered a paradigm for the study of the evolution and the regulation of metabolic pathways. In this context, we have initiated the study of the histidine biosynthesis operon of Lactococcus lactis. This lactic acid bacterium is commonly used as a starter in the dairy industry and is becoming one of the best-characterized gram-positive bacteria. The his operon contains 12 open reading frames (ORFs), of which 8 encode enzymes known to be involved in histidine biosynthesis and 4 encode proteins of unknown function (6).
In addition to the goal of generating information on the genetic organization of this bacterium, the his operon was chosen because most dairy strains are histidine auxotrophs while strains isolated in the natural environment are prototrophs (7). Inactivation of the his operon is due to the accumulation of base substitutions and small deletions in several genes. Gene transfer in this region was also documented (8). The inactivation of the his operon probably confers a selective advantage to strains used in the industrial dairy processes and may be considered to promote adaptation of lactococci to this new environment. A preliminary study, conducted with L. lactis IL1403, which was obtained by plasmid curing of an industrial dairy strain, showed that transcription of the operon is also partially inactivated (7). This suggested that expression of the his operon may cause a selective disadvantage in L. lactis in milk and prompted further studies of the regulation of the his operon.
Based on sequence and potential mRNA secondary structure similarity, Grundy and Henkin (19) proposed that the L. lactis his operon was regulated by a mechanism of attenuation, involving a signature sequence named the T box, frequently found for genes encoding aminoacyl-tRNA synthetases and amino acid biosynthesis enzymes in gram-positive bacteria. In the presence of limiting amounts of the appropriate amino acid, transcription antitermination is mediated by uncharged tRNA, which acts as a positive regulator. Uncharged tRNA directly interacts with the leader mRNA at a specifier (the codon for the appropriate amino acid) and at the T box sequence to stabilize the antiterminator stem and promote transcription antitermination. This mechanism allows a 10- to 30-fold regulation in Bacillus subtilis (17, 21, 33). This range is far below the 6,000-fold potential level of regulation of the Escherichia coli his operon, resulting from the combination of the control of initiation and elongation of transcription. In this bacterium, transcription initiation is under the control of ppGpp, the effector of the stringent response, and its elongation is regulated by an attenuation mechanism. In addition, posttranscriptional regulation occurs by mRNA processing and the activity of the first enzyme of the pathway (HisG) could be stimulated or inhibited by several elements (1).
In this paper, we show that transcription of the L. lactis his operon is also controlled at two levels, initiation and elongation, leading to a 120-fold modulation of transcription in vivo. We confirmed that elongation of transcription is subject to an attenuation mechanism related to the T box system. Elements involved in this system were identified. The initiation of transcription was shown to be controlled by a repression mechanism depending on an operator site present 250 bp downstream of the mRNA start. In addition, we suggest that a more general control of the operon may be exerted by modification of the supercoiling, a factor which appears to interfere with the histidine-mediated repression.
MATERIALS AND METHODS
Bacterial strains, media, oligonucleotides, and DNA manipulation procedures.
The bacterial strains used in this study are listed in Table 1. E. coli TG1 and LLB1 were used for plasmid propagation (26). The pcn mutation of LLB1 reduces the copy number of pBS derivatives and was used to avoid the toxicity of some fragments when present at a high copy number (HC). L. lactis strains were grown at 30°C on M17 medium (44), on chemically defined medium (CDM) containing 0.1% histidine (CDM+H), or on CDM without histidine (CDM−H) (34). E. coli cells were grown in Luria-Bertani medium at 37°C (31). When needed, erythromycin (5 μg/ml for L. lactis and 100 μg/ml for E. coli) or ampicillin (50 μg/ml for E. coli) was added to the culture medium. Plasmids and total DNA were prepared as previously described (29, 31, 40). Procedures for DNA manipulations, transformation of E. coli cells, and cloning were essentially as described by Maniatis et al. (31). Electrotransformation of L. lactis was performed as described by Holo and Nes (23). All enzymes for DNA technology were used according to the manufacturer’s specifications. Oligonucleotides were synthesized on a DNA synthesizer oligo 1000M system (Beckman). The oligonucleotides used in this work are named as follows: the number indicates the position of the 5′ end of the oligonucleotides in the direct (D) or reverse (R) orientation compared to the sequence given in Fig. 1A. Oligonucleotides used for mutagenesis are listed in Table 1.
TABLE 1.
Bacterial strains, plasmids, and oligonucleotides
| Strain, plasmid, or oligonucleotide | Relevant markers and characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| TG1 | supE Dthi (lac-proAB) hsdD5 (F′+traD36 proAB lacIqZΔM15) | 13 |
| LLB1 | supE Dthi (lac-proAB) hsdD5 (F′+traD36 proAB lacIqZΔM15) pcnB | 26 |
| L. lactis | ||
| NCDO2118 | L. lactis subsp. lactis, isolated from frozen peas (1978), His+ | National Collection of Dairy Organisms |
| IL1403 | L. lactis subsp. lactis, plasmid free | 3 |
| NCDO763 | L. lactis subsp. cremoris | National Collection of Dairy Organisms |
| Co1 | L. lactis subsp. lactis isolated from maize (United States, 1993) | 38 |
| Co2 | L. lactis subsp. cremoris isolated from maize (United States, 1993) | 38 |
| Co4 | L. lactis subsp. cremoris isolated from maize (United States, 1993) | 38 |
| Co6 | L. lactis subsp. cremoris isolated from maize (United States, 1993) | 38 |
| JIM4458 | Cmr, NCDO2118 containing pVE6007 | This work |
| JIM5206 | Eryr, NCDO2118 Phis::pInt-1 | This work |
| Plasmids | ||
| pBS | Apr, M13ori pBR322ori | Stratagene |
| pVE6007 | Cmr, thermosensitive replicative plasmid in L. lactis | 30 |
| pIL253 | EmrL. lactis HC vector, derivative of pAMβ1 | 40 |
| pIL252 | EmrL. lactis LC vector, derivative of pAMβ1 | 40 |
| pJIM378 | 9-kb Sau3A fragment from NCDO2118 containing the L. lactis his operon cloned in pIL253 | 6 |
| pJIM2279 | Emr, HC vector derivative of pIL252 with copF:: linker | 35 |
| pJIM2530 | Emr, derivative of pJIM2279 carrying the luxAB genes from V. harveyi, terminator probe vector | 35 |
| pJIM2366 | Emr, derivative of pJIM2279 containing terminator upstream of the MCS and lux genes, promoter probe vector | 35 |
| pORI28 | Emr, integrative plasmid and derivative of pWV01 without RepA protein | 25 |
| pJIM2374 | Emr, derivative of pORI28 carrying luxAB gene from V. harveyi and the Em resistance gene from pIL253 | This work |
| pBS derivatives | ||
| pJIM702 | 1.4-kb EcoRI fragment from pJIM378 containing the promoter region of the his operon | This work |
| pBS-1 | 1.3-kb PCR-amplified fragment from pJIM702 cloned in EcoRI-BamHI | This work |
| pBS-1m1 | pBS-1 with mutations of 4 bp in T box sequence (nt 996–1000) made by PCR | This work |
| pBS-1m2 | pBS-1 with deletion of 46 bp (nt 632–677) | This work |
| pBS-1m3 | pBS-1 with deletion of 14 bp and mutation of 5 bp in terminator (nt 1034–1052) made by PCR | This work |
| pBS-1m4 | pBS-1 with mutation of 8 bp in terminator (nt 1034–1041) made by PCR | This work |
| pBS-2 | 388-bp PCR-amplified fragment of leader region (nt 904–1292) and cloned in SmaI | This work |
| pBS-3 | 413-bp PCR-amplified fragment of leader region (nt 879–1292) and cloned in SmaI | This work |
| pBS-4 | 294-bp PCR-amplified fragment of leader region (nt 791–1085) and cloned in SmaI | This work |
| pBS-4m1 | pBS-4 with deletion of 1 bp (nt 972) in the his leader region | This work |
| pBS-4m2 | pBS-4 with deletion of 8 bp (nt 972–979) in the his leader region | This work |
| pBS-6 | 830-bp fragment DNAse I of Phis from pJIM702 removing (nt 831–1292) terminator region | This work |
| pBS-7 | 948-bp fragment DNAse I of Phis from pJIM702 removing (nt 949–1292) terminator region | This work |
| Replicative plasmids with lux fusion | ||
| pTer-2a | 388-bp EcoRV/NotI insert from pBS-2 cloned in XbaI/NotI of pJIM2530d | This work |
| pTer-3a | 413-bp XbaI/EcoRV insert from pBS-3 cloned in XbaI/SacI of pJIM2530d | This work |
| pTer-4a | 294-bp XbaI/EcoRV insert from pBS-4 cloned in XbaI/NotI of pJIM2530d | This work |
| pTer-4m1a | 293-bp XbaI/EcoRV insert from pBS-4m1 cloned in XbaI/NotI of pJIM2530d | This work |
| pTer-4m2a | 286-bp XbaI/EcoRV insert from pBS-4m2 cloned in XbaI/NotI of pJIM2530d | This work |
| pProm-1b | 1.3-kb XbaI/EcoRV insert from pBS-1 cloned in XbaI/NotI of pJIM2366d | This work |
| pProm-1m3b | 1.3-kb XbaI/EcoRV insert from pBS-1m3 cloned in SmaI/XbaI of pJIM2366 | This work |
| pProm-7b | 948-bp XbaI/EcoRV insert from pBS-7 cloned in SmaI/XbaI of pJIM2366 | This work |
| Integrative plasmids with lux fusion (pInt series) | ||
| pInt-1 | 1.3-kb HindIII/XbaI insert from pBS-1 cloned in HindIII/XbaI of pJIM2374 | This work |
| pInt-1m1 | 1.3-kb HindIII/XbaI insert from pBS-1m1 cloned in HindIII/XbaI of pJIM2374 | This work |
| pInt-1m2 | 1.3-kb HindIII/XbaI insert from pBS-1m2 cloned in HindIII/XbaI of pJIM2374 | This work |
| pInt-1m3 | 1.3-kb HindIII/XbaI insert from pBS-1m3 cloned in HindIII/XbaI of pJIM2374 | This work |
| pInt-1m4 | 1.3-kb HindIII/XbaI insert from pBS-1m4 cloned in HindIII/XbaI of pJIM2374 | This work |
| pInt-5 | 650-bp KpnI/AccI fragment from pBS-1 cloned in SmaI of pJIM2374d | This work |
| pInt-6 | 830-bp HindIII/XbaI insert from pBS-6 cloned in HindIII/XbaI of pJIM2374 | This work |
| pInt-7 | 948-bp HindIII/XbaI insert from pBS-7 cloned in HindIII/XbaI of pJIM2374 | This work |
| Oligonucleotides with special featuresc | ||
| 995R | 5′-CCAATGCATTCCACCTTAATTTATCACTTTTTCAC-3′ | This work |
| 1001D | 5′-CCAATGCATATATTAAACCGTCCTTTAAGCC-3′ | This work |
| 1033R | 5′-CCAATGCATCCTAAAAGCACTTGGCTT-3′ | This work |
| 1042D | 5′-ATTATGCATCCTATATTATCTTTCTATTCTCGAAAG-3′ | This work |
| 1053D | 5′-ATTATGCATCTATTCTCGAAAGGAGAA-3′ | This work |
Terminator probe vector (pTer series HC) with his leader region insert. HC means that the vector is in a high-copy-number form, as described previously (35).
Promoter probe vector (pProm series LC) with Phis region insert. LC means that the vector is in a low-copy-number form, as described previously (35).
NsiI site is shown in italics.
Inserts and plasmids were blunted by T4 polymerase by standard procedure.
FIG. 1.
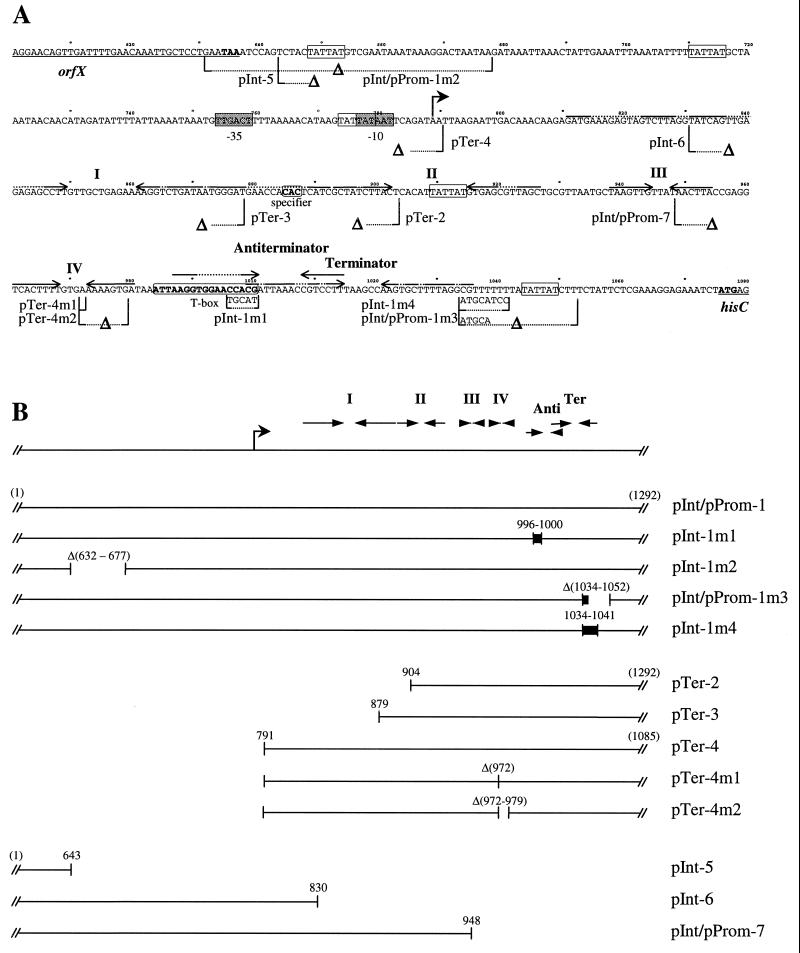
Organization and schematic representation of the histidine promoter region. (A) Nucleotide sequence and main features of the 5′ end of the his operon. The end of orfX and beginning of hisC (first gene of the his operon) are underlined, and their stop and initiation codons are shown in bold characters. The start of the his mRNA characterized by primer extension is shown by a bent arrow and the −35 and −10 promoter sequences are boxed in grey. Specifier and T box sequences are boxed and shown in bold characters. Stem-loop structures I, II, III, and IV, the antiterminator, and the terminator are represented as inverted arrows. TATTAT repeated sequences are boxed. Numbers indicate positions relative to the previously reported sequence (GenBank sequence, U92974) (6). Mutations made in this work are indicated below the sequence; Δ refers to deletion. (B) PCR-amplified DNA fragments used to analyze the regulation of the transcription. The bent arrow represents transcription start, and the horizontal arrows represent stem-loop structures of the his leader region. The numbers indicate the positions of the nucleotides at the 5′ and 3′ ends of the fragments and of the modifications. Δ are deletions (positions of deleted nucleotides are shown in parentheses), and black boxes denote substitutions.
Northern blot analysis of his transcripts.
RNA was isolated from L. lactis NCDO2118 grown in CDM to exponential phase (optical density at 600 nm [OD600] of 0.5), washed two times, and transferred for 20 min to CDM+H or CDM−H. Total RNA was prepared as previously described for B. subtilis (14). After extraction and treatment with phenol/chloroform, RNA was precipitated with ethanol; 50 μg of glyoxalated RNA was subjected to electrophoresis through a 1% agarose gel. Transfers and hybridizations were performed as described by Maniatis et al. (31). DNA probes were labeled with [α-32P]dCTP by using a random primed DNA labeling kit (Boehringer, Mannheim, Germany), and oligonucleotide DNA probes were labeled with [γ-32P]ATP with T4 polynucleotide kinase.
Primer extension and DNA sequencing.
Total RNA isolated from L. lactis NCDO2118 grown in CDM−H and CDM+H was used as the template for primer extension with the primer 848R. Fifty micrograms of RNA and 5 pmol of 32P-labeled oligonucleotide were heated at 85°C for 10 min and cooled slowly to 42°C in 15 μl of annealing mix (10mM Tris-HCl [pH 8], 1 mM EDTA, 1.25 M KCl). Elongation of the DNA strand was performed after addition of 19.2 μl of a mixture containing 0.2 mM deoxynucleoside triphosphates (0.2 mM each of dATP, dCTP, dTTP, and dGTP), RNase inhibitor (Gibco), and 1.2 U of avian myeloblastosis virus reverse transcriptase (Boehringer). Samples were incubated at 42°C for 1 h, precipitated, washed, dried, and suspended in 4 μl of Tris-EDTA (TE) and 6 μl of stop solution before loading on a denaturing 6% polyacrylamide gel. The control sequence was determined on plasmid pBS-1 as a template with the same primer. Quantification of the primer extension signals was performed on a PhosphorImager (Storm system; Molecular Dynamics). Double-stranded recombinant plasmid DNA was used as the template in dideoxy chain termination sequencing reactions (6, 39).
Determination of luciferase activity in L. lactis.
Luciferase assays were performed on a Bertold Lumat LB9501 apparatus. One milliliter of L. lactis culture was mixed with 5 μl of nonaldehyde, and the light emission was immediately measured. The value of the peak obtained was standardized to the OD600 of the culture. Luciferase activity was measured throughout the growth of the culture. Values reported in Tables 2 and 3 were measured at OD600 of 0.4.
TABLE 2.
Expression of Phis and his leader region-lux fusions on LC plasmids or integrated on the chromosome
| Plasmidsa | Promoter and leader regionb upstream of lux AB genes | Luciferase activities (lx/OD [103])
|
Strength of histidine regulation (fold)d | |
|---|---|---|---|---|
| CDM+H | CDM−H | |||
| pProm-1 | WTc | 12 ± 3 | 120 ± 40 | 10 |
| pInt-1 | 2.8 ± 0.9 | 335 ± 50 | 120 | |
| pInt-1m2 | Deletion of 46 bp upstream of Phis (nt 632 to 677) | 2.7 ± 0.3 | 290 ± 40 | 110 |
| pInt-6 | Deletion from nt 831 downstream of Phis | 230 ± 85 | 200 ± 30 | 0.9 |
| pProm-7 | Deletion from nt 948 downstream of Phis | 355 ± 25 | 390 ± 40 | 1.1 |
| pInt-7 | 265 ± 20 | 235 ± 60 | 0.9 | |
| Pprom-1m3 | Substitution of 14 bp; overlaps the terminator and TATTAT box | 150 ± 25 | 125 ± 35 | 0.8 |
| pInt-1m3 | 140 ± 40 | 270 ± 40 | 1.9 | |
| pInt-1m4 | Substitution of 8 bp; overlaps the terminator | 190 ± 60 | 330 ± 40 | 1.7 |
The lux fusions are on an LC plasmid for the pProm series or integrated on the chromosome for the pInt series.
See Fig. 1.
WT, the Phis and his leader region from wild-type fragment.
Obtained by the division of the values obtained in the presence of histidine by those obtained in the absence of histidine for the corresponding plasmid.
TABLE 3.
Mutational analysis of the his leader regiona
| Plasmid | Potential mRNA structure present upstream of lux AB genesb | Luciferase activities (lx/OD [103])
|
Strength of termination (fold)c | Antitermination factor (fold)d | |
|---|---|---|---|---|---|
| CDM+H | CDM−H | ||||
| pJIM2530 | 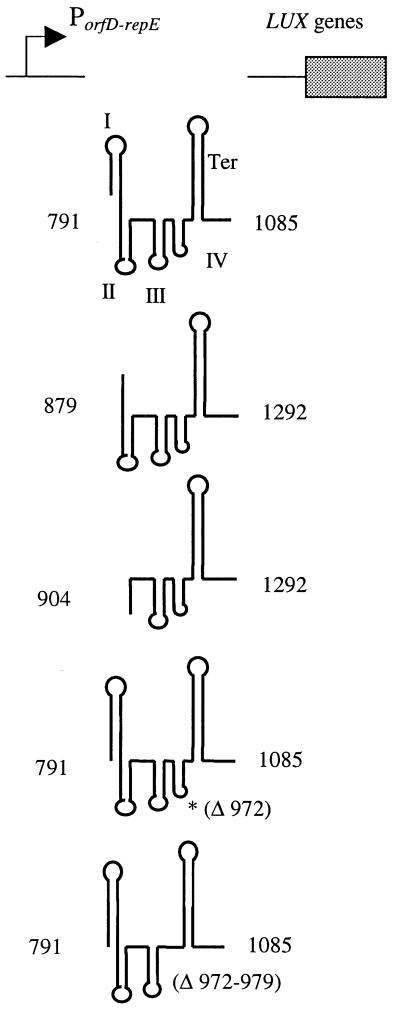 |
960 ± 190 | 930 ± 160 | 1 | 1 |
| pTer-4 | 23 ± 4 | 330 ± 40 | 42 | 14 | |
| pTer-3 | 10 ± 2 | 17 ± 0.6 | 96 | 1.7 | |
| pTer-2 | 5.2 ± 0.4 | 6.9 ± 0.3 | 185 | 1.3 | |
| pTer-4m1 | 10 ± 2 | 19 ± 3 | 96 | 1.9 | |
| pTer-4m2 | 12 ± 2 | 19 ± 3 | 80 | 1.6 | |
To study attenuation mechanism, the luciferase activity resulting from the insertion of various derivatives of the DNA segment carrying the his leader region between a constitutive promoter (PorfD-repE) and the lux genes on a plasmid was measured in L. lactis growing in CDM with or without histidine.
Fusions are under the control of constitutive promoter PorfD-repE from pJIM2530.
Quotient of the lux value of the control plasmid pJIM2530 and that of the indicated plasmid in the presence of histidine.
Quotient of the lux value obtained in the absence of histidine and that obtained in the presence of histidine for the same plasmid.
Histidinol dehydrogenase assay and protein determination.
In order to determine histidinol dehydrogenase activity, cells from 25-ml cultures at OD600 of 0.4 to 0.5 were harvested by centrifugation (5,000 × g; 15 min at 4°C) and stored at −20°C. The pellet was then resuspended in 300 μl of 0.05 M Tris buffer (pH 7.5) with 0.5 g of glass beads and shaken at maximum speed setting on a Biospec homogenizer at room temperature 3 times, for 2 min each time, with a 1-min pause between each pair of pulses. After centrifugation at 30,000 × g for 15 min at 4°C, the supernatant was passed through a G-50 Sephadex column that had been equilibrated and washed with 0.01 M Tris-HCl buffer (pH 7.3) at 4°C. Histidinol dehydrogenase from the crude bacterial extract was assayed by the spectrophotometric method of Martin et al. (32). One unit of enzyme yields an absorbance of 1.0 U at 520 nm after 20 min at 37°C. The protein contents of cell extracts were determined by using the Coomassie protein assay reagent (Pierce Chemical Co., Rockford, Ill.).
Construction of truncated and mutagenized derivatives of the his promoter region in E. coli.
Plasmids used for construction of the his promoter region derivatives in E. coli are listed in Table 1, and schematic drawings of the final constructs are presented in Fig. 1B. The mutagenized fragments were made in E. coli and recloned in promoter and terminator probe vectors in L. lactis. pJIM702 was used as the primary template to generate the constructions pBS-1, pBS-2, pBS-3, pBS-4, pBS-5, pBS-6, and pBS-7 presented in this work. It carries a 1.4-kb EcoRI fragment that includes the promoter and the 5′ end of the his operon in pBSKS+. Deletions and substitutions in the terminator and in the T box sequence were generated from plasmid pBS-1 by PCR techniques. This plasmid contains a 1.3-kb PCR fragment amplified from pJIM702 (with M13 primers [−47] [Promega] in pBS and 1292R) and cloned after double digestion with EcoRI and BamHI in pBSSK+. The oligonucleotide pairs 995R and 1001D, 1033R and 1053D, and 1033R and 1042D were used to amplify pBS-1, producing pBS-1m1, pBS-1m3, and pBS-1m4, respectively. The NsiI restriction site carried by the primers was used to generate cohesive ends prior to ligation and transformation. In pBS-1m3 and pBS-1m4, the terminator sequences were disabled at their 3′ ends (1034-CGT7ATATTATCTT-1052 was replaced with ATGCA and 1034-CGT6-1041 was replaced with ATGCATCC, respectively). In pBS-1m1, 5 bp in the T box sequence were changed (996-CCACG-1000 was replaced with TGCAT). In pBS-1m2, 46 bp (nucleotides [nt] 632 to 677) were deleted upstream of the promoter Phis. pBS-2, pBS-3, and pBS-4 contain 388-, 413-, and 294-bp PCR fragments obtained with oligonucleotide pairs 904D and 1292R, 879D and 1292R, and 791D and 1085R, respectively. These fragments were cloned in pBSSK+ digested with SmaI. pBS-4m1 and pBS-4m2 were constructed by PCR mutagenesis of pBS-4, with 1 bp (nt 972) and 8 bp (nt 972 to 979) deleted in the stem and loop of stem-loop IV of the his leader, respectively. pBS-6 and pBS-7 carry deletions, located at the 3′ end of the 1.4-kb fragment from pJIM702, that were produced by the action of DNase I. The cloned PCR products were sequenced to verify that no additional mutations had occurred.
Constructions of lux fusions in L. lactis replicative plasmid.
Two types of transcriptional fusions were constructed, one each in a terminator and a promoter probe vector, named pTer and pProm, respectively. The pTer series contains derivatives of the his leader region without the promoter cloned in pJIM2530 as the parental vector (35). In this plasmid, the fragments cloned in the multiple cloning sites (MCS) are followed by the luciferase reporter genes from Vibrio harveyi and transcribed from the constitutive promoter PorfD-repE present upstream of the MCS (27, 35). This plasmid is a convenient vector to estimate the strength of a terminator cloned in the MCS. It was used to study the regulation of the histidine operon by termination and antitermination. The resulting plasmids, pTer-2, pTer-3, pTer-4, pTer-4m1, and pTer-4m2, are maintained at HC (Table 1).
The pProm series contains the lux genes under the control of the Phis promoter followed by a modified his leader region. The promoter probe vector pJIM2366 (a derivative of pJIM2530) contains a terminator that prevents transcription of the lux genes from the PorfD-repE promoter (1.5 × 103 ± 0.5 × 103 lx/OD unit [35]). pProm-1 contains Phis and the complete and intact his leader region. pProm-1, pProm-1m3, and pProm-7 were constructed as described in Table 1. These plasmids were constructed to have HC and then switched to low copy number (LC) by KpnI restriction as described previously (35). The KpnI restriction deletes a linker present in copF, the product of which is a repressor that controls replication. After ligation, these plasmids were transformed in L. lactis NCDO2118, which contains an intact his operon.
Construction of lux transcriptional fusions in the chromosome.
The plasmids used to produce lux transcriptional fusions in the chromosome were constructed by subcloning various fragments containing the promoter into the L. lactis integrative vector pJIM2374. This vector contains the origin of replication of pWV01 but not the repA gene, the product of which is required for replication. This plasmid, a derivative of pORI28, is able to replicate if this protein is provided in trans (28) and contains the erythromycin resistance determinant of pIL253 as a marker, an MCS, and the lux genes as reporters. The construction of the integration plasmids pInt-1, pInt-1m1, pInt-1m2, pInt-1m3, pInt-1m4, pInt-5, pInt-6, and pInt-7 is described in Table 1. These plasmids were integrated at the his locus in the chromosome of L. lactis NCDO2118 by single crossover with pGhost4 as a helper as described by Godon et al. (15). The resulting strains contain the lux fusion with the modified promoters followed by the intact his operon.
RESULTS
Transcription of the his operon.
RNA from L. lactis NCDO2118 grown in CDM+H or CDM−H was analyzed by Northern blot, using as a probe the oligonucleotide 963R. No mRNA was detected in cells grown in the presence of histidine, suggesting that the initiation of transcription or mRNA stability is controlled by histidine (Fig. 2A). In cells grown in the absence of histidine, two transcripts, of 10 and 0.25 kb, were detected (Fig. 2A). The use of probes consisting of several fragments of the his operon and flanking regions showed that the longer transcript covers the entire his operon while the shorter is located in its promoter region (data not shown). The short transcript seems to be the result of an early transcription elongation stop.
FIG. 2.
Transcription analysis of the histidine operon. (A) Northern blot. Total RNA was extracted from L. lactis NCDO2118 cells grown with (+) and without (−) histidine and hybridized with oligonucleotide 963R. The size of the band is indicated. No transcript was detected in cells grown with histidine. (B) Primer extension analysis. Total RNA of L. lactis NCDO2118 cells grown with (+) and without (−) histidine was used as a template for primer extension with the 848R oligonucleotide. The same oligonucleotide was used for the sequencing reaction with plasmid pBS-1 as a template. The asterisk indicates the nucleotide corresponding to the 5′ end of the his mRNA found by primer extension.
Additional hybridization experiments with oligonucleotide probes were performed to localize the 5′ ends of the two transcripts and the 3′ end of the small one more precisely. Oligonucleotide 773R, upstream of the putative start point of transcription, did not hybridize with either of the two messengers (data not shown). Oligonucleotides 848R and 1036R, between the potential start point and the terminator, hybridized with the two transcripts (data not shown). Oligonucleotide 1085R, downstream of the potential terminator, hybridized only with the long transcript (data not shown). These observations indicate that both transcripts originate in a 75-bp region located between oligonucleotides 773R and 848R and that the short transcript ends in the 49-bp fragment defined by oligonucleotides 1036R and 1085R. This region contains a rho-independent terminator structure (Fig. 1A). The precise start point of transcription was mapped by primer extension at position 789 and is preceded by the −10 and −35 consensus promoter sequences (Fig. 1A and Fig. 2B).
The last step in histidine biosynthesis is carried out by the histidinol dehydrogenase encoded by the hisD gene, the fourth gene of the his operon. The measured HisD activity should reflect the expression of the his operon. HisD activity varied 26-fold depending on whether histidine was present, as it was 7.8 ± 1 and 0.3 ± 0.1 U/mg of protein in cells grown in CDM−H and in CDM+H, respectively. As the basal level of HisD activity is near the limit of detection, we investigated the regulation of the his operon using pInt-1. This is an integrative plasmid, carrying a transcriptional fusion of lux reporter genes with the entire his promoter region up to the first gene, hisC (Fig. 1B). The activities of the lux genes under the control of the his promoter were 335 × 103 and 2.8 × 103 activity lx/OD unit in the absence and in the presence of histidine, respectively, suggesting a 120-fold modulation of the transcription of the his operon (pInt-1, Table 2).
Structural features of the his leader region.
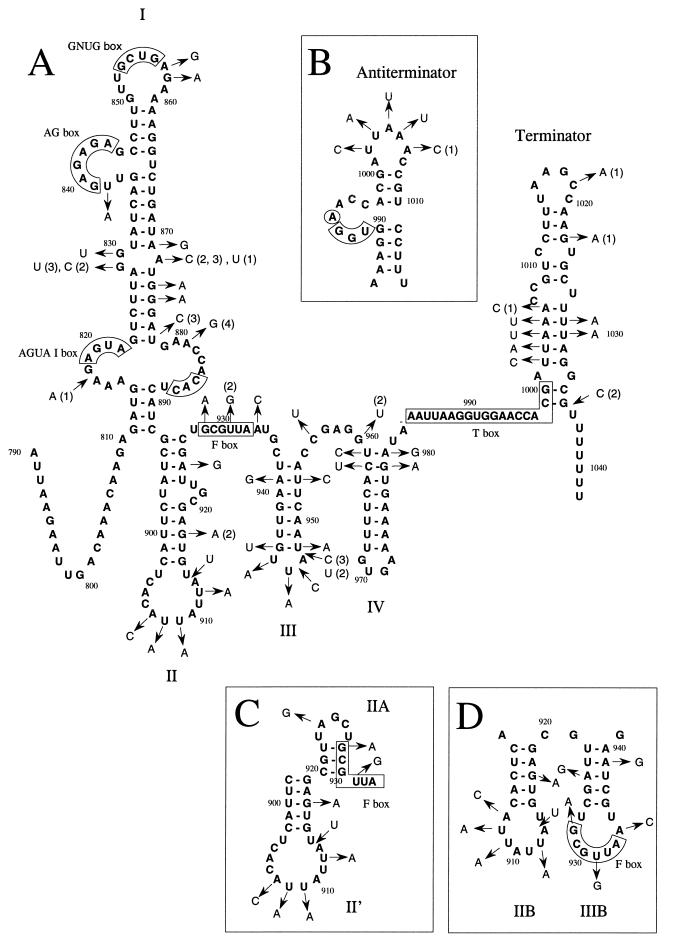
The 438-bp region upstream of the start codon of hisC contains the promoter Phis and a 287-bp noncoding sequence that was named the his leader region (Fig. 1A) (5). The his leader region has the sequence features of the tRNA-mediated antitermination systems described by Grundy and Henkin (19). It contains the typical T box sequence AAUUAAGGUGGAACCACG (nt 983 to 1000) (bases fitting with the consensus are underlined), a histidine specifier CAC (nt 885 to 887), and most of the other less conserved boxes (AGUA-I box [AGUA, nt 819 to 822], AG box [GAGAGA, nt 840 to 844], GNUG box [GCUG, nt 853 to 856], and F box [GCGUUA, nt 928 to 933]) (Fig. 1A and 3 [19, 30]). The AGUA-II and GAAC boxes are not present. In addition to these conserved sequences, the his leader region may be folded into three stem and loop structures (17). To examine the validity of the proposed model of folding and to detect sequences important for histidine regulation, we have assessed the genetic variability of this region in several L. lactis strains. Sequences important for the regulation as well as nucleotides involved in the pairing of stems should be conserved or complementary changes should maintain the proper folding of the leader. Forty-six variable nucleotides were found among the 252-bp sequences of three L. lactis subsp. lactis (IL1403, NCDO2118, and Co1) strains and four L. lactis subsp. cremoris (NCDO763, Co2, Co4, and Co6) strains (Fig. 3A).
FIG. 3.
Structural model of the L. lactis NCDO2118 his leader region mRNA. The specifier (CAC), the T-box sequence, and other conserved boxes are indicated. The arrows indicate the base changes found in several distantly related strains of L. lactis. The absence of additional indication means that the change was found in all L. lactis subsp. cremoris strains (NCDO763, Co2, Co3, Co4, and Co6). Numbers in parentheses specify changes that are present only in IL1403 (1), in NCDO763 (2), in Co2, Co3, and Co4 (3), or in Co3 (4). Sequences of L. lactis subsp. lactis Co1 and NCDO2118 are identical. Other numbers indicate positions relative to those shown in Fig. 1. (A) Terminator conformation; (B) antitermination conformation; (C) and (D) alternative conformations of stem II and stem III.
The sequences of the boxes described earlier are not subject to variation except for that of the F box, where two positions changed. The first change, G928A, is at a position which is conserved in less than 80% of the F boxes (36). The second, U931G, was found only in one strain and matches a position that was previously shown to be important for regulation of the tyrS gene (36). However, this base is not conserved in the leader of the L. lactis trp operon (45).
We also checked whether the changes would impair the folding of the three stem and loop structures described by Grundy and Henkin (19). Assuming that G-U base pairing is acceptable, the changes found do not affect the folding of the first and last stems (stems I and IV, Fig. 3), the terminator, and the antiterminator. However, some changes are not compatible with the formation of the second stem-loop structure previously proposed (19). A new model of folding yielded four stem-loop structures instead of three (Fig. 3A). In this model, most base changes occur between the stems or in the bulges of these structures (25 changes) and otherwise complementary changes maintain base pairing (12 changes) or the changes do not alter the pairing (G-C to G-U or G-U to A-U) (7 changes). Alternate structures for stems II and III might also be formed. The lower part of stem II could pair with the first bases of the F box to produce stem IIA as described by Rollins et al. (36) (Fig. 3C). Furthermore, these two stems can form stems IIB and IIIB (Fig. 3D). Stem IIB is similar to part of stem II proposed by Grundy and Henkin (19) but might be very unstable in the L. lactis subsp. cremoris strains because one change is present in the middle and two are present at the top of the stem. Interestingly, stem IIIB contains the F box in its loop (Fig. 3C).
In the bottom left bulge of stem I and the top bulges of stems II and III, additions of 1 or 2 nt are observed, suggesting that the size of these bulges is not important for regulation. The sequences of these three bulges were found to be highly variable. Changes occur in 4 of the 12 nt at the top bulge of stem II, in 2 of 3 in stem III, and in 5 of 7 in the antiterminator. Moreover, several changes were found in the other bulges of stem I close to the AGUA, AG, GNUC, and specifier boxes. This suggests that, outside of the conserved boxes, the primary sequences of the bulges are not essential for regulation.
Interestingly, the unpaired nucleotides that form the bulge present in the middle of stem I have all changed in a way that prevents any pairing in this region. This suggests that the flexibility due to the presence of this bulge is necessary for the functionality of stem I. In contrast, some unpaired regions are not extensively modified in different strains, such as the first 20 nt of the leader, the sequence between stems III and IV, and the bulges in the middle of stem I (between nt 881 and 892) and at the top of stem IV. This suggests that these sequences may be necessary for proper regulation of the operon.
Mutational study of the his attenuation mechanism.
Analysis of the mRNA and features of the his leader suggest that transcription of the his operon is regulated at two levels, initiation and elongation. In order to study the effect of attenuation on expression of the his genes, it was necessary to remove the native promoter which is controlled by histidine at the level of transcription initiation. We subcloned fragments containing the his leader region without Phis into the terminator probe vector pJIM2530; the resulting plasmids belong to the pTer series (Fig. 1B). Transcription of these fragments is under the control of the constitutive promoter PorfD-repE, and readthrough is measured with the lux reporter gene present downstream of the cloned fragment (35). In the presence of histidine, the his leader region in pTer-4 caused a 42-fold decrease of the expression of luciferase compared to that of the control pJIM2530 vector (Table 3). This confirmed the functionality of the terminator in the cloned fragment. In the absence of histidine, the efficiency of the termination was about 14-fold lower than in its presence, showing that the features necessary for antitermination are present on the cloned fragment. Deletions at the 5′ ends of the his leaders in pTer-3 and pTer-2 (deletions extending to nt 878 and 903, respectively) reduced significantly the control of the antitermination in the absence of histidine (Table 3). These results confirmed the importance of stem I in the attenuation mechanism. Moreover, these deletions increased termination activity in the presence of histidine two- and fourfold, respectively (Table 3). This result suggests an important role of the 5′-end sequence of the his leader region in termination of transcription, even in the presence of histidine. Similar results were also obtained with LC plasmids, suggesting that no factor was titrated by HC plasmids (data not shown).
Substitutions were introduced into the T box sequence to verify its importance in the attenuation mechanism of the his operon. In pInt-1m1, where part of the T box sequence 996-CCACG-1000 is replaced by TGCAT (Fig. 1B), the level of expression of luciferase is very low (<0.2 × 103 lx/OD unit in CDM) irrespective of the histidine content of the growth medium. This value is similar to the background level found in the absence of the reporter gene. We conclude that the antitermination is abolished by this change and that the attenuation mechanism is T box dependent.
T-box-dependent regulation has been studied extensively in B. subtilis, and the importance of several sequences in stems I and II has already been demonstrated (16, 36). Our analysis, based on the variability of the his leader sequences, shows that many changes that conserve base pairing can occur in the stem and loop structures, suggesting that structural features might be more important than the primary sequence. Strikingly, in all strains the bottom of stem-loop IV contains changes that maintain its structure, whereas no change occurred in the other 16 contiguous nucleotides, including the loop. This structure is thus a good candidate for involvement in the T box attenuation mechanism of the his operon. To verify the validity of this hypothesis, stem-loop IV was mutated by deletion of a single base at position 972 (pTer-4m1) or by an 8-bp deletion at positions 972 to 979 (pTer-4m2; Fig. 1B). In both cases, the range of regulation by histidine decreased dramatically to no more than twofold (Table 3). In the case of pTer-4m1, this difference cannot be explained by a decrease in the stability of stem-loop IV, as the free energy values are comparable in the wild-type and mutant strains (5.4 and 5.3 kJ, respectively). The latter results suggest that the sequence or integrity of stem-loop IV is required for the function of the attenuation mechanism. We did not find significant complementary sequence to stem-loop IV in the tRNAHis, the known diffusible partner that interacts with the mRNA leader, or in the his leader region. This suggests that another trans factor interacts with this region, and we decided to test for its presence by titration experiments conducted with mRNA from the leader region. The entire his leader region was overexpressed from the HC plasmid pProm-1 HC. The overexpression from pProm-1 HC was as much as 15-fold compared to the expression from pInt-1. The histidinol dehydrogenase activity produced from the native his operon in the NCDO2118 strain decreased 4.6-fold in cells containing pProm-1 HC (the activities were 7.8 ± 1 and 1.7 ± 0.8 U/mg of protein in NCDO2118 cells without and with pProm-1 HC, respectively). This observation supports the hypothesis that a diffusible factor partner, probably in addition to the tRNAHis, is required for attenuation of his transcription.
Transcription of the his operon is subject to repression of initiation.
Northern analysis, previously described, showed that control of transcription of the his operon by attenuation is strengthened by a second control at the level of initiation or of mRNA stability. The level of this control was quantified by reverse transcription of total RNA isolated after growth in the presence and in the absence of histidine. The signal of the primer extension product was sixfold lower under the former than under the latter condition (Fig. 2B). The expression of a fusion with the entire promoter and the his leader region on pInt-1 integrated on the chromosome should thus be controlled at two levels, initiation of transcription, or mRNA stability, and elongation of the transcription. Indeed, it is modulated 120-fold by histidine (Table 2). We demonstrated (see the preceding paragraph) that to the 120-fold modulation by histidine, attenuation appears to make a contribution responsible for a 14-fold modulation. A simple calculation suggests the presence of a second control level modulated eightfold by histidine. This calculated level accords with the data obtained by quantification in the reverse transcription experiment.
Absence of a terminator upstream histidine operon led us to check whether transcription starting upstream of the mapped Phis promoter did not influence expression of the his operon. The fusion upstream of Phis in pInt-5 (Fig. 1B) displayed a low activity (5.8 × 103 ± 1.3 × 103 lx/OD unit), similar to that of the repressed Phis promoter, and was independent of the presence of histidine.
To localize the minimal region necessary for the regulation of initiation, deletions were generated in the promoter region and fused with lux genes on an LC plasmid or integrated on the chromosome (Fig. 1). Deletion of 46 bp (nt 632 to 677) upstream of Phis on the integrated pInt-1m2 had no effect on regulation of Phis, indicating that the region upstream of nt 677 is not necessary for regulation (Table 2). Two other fusions, from which the 3′ end of the his leader region is deleted, pInt-6 and pInt-7, drive constitutive luciferase expression, indicating that the region downstream of nt 948 is necessary for the modulation of initiation (Table 2). As these deletions induce a high constitutive level of expression, these results suggest that initiation is regulated by a repressor. The requirement of sequences beyond nt 948 for the control of the initiation suggests that the operator site is distant from the promoter or is expanded over a large region. A search for repeated sequences in the promoter region led to the discovery of five TATTAT sequences separated by 62, 66, 135, and 135 bp (Fig. 1A). The fact that these repeats are regularly spaced and overlap the −10 box suggest that these sequences are operators that allow the binding of DNA binding proteins and eventually the formation of a DNA loop. To test the involvement of these sequences in regulation, we deleted the first and the last TATTAT boxes of the region on the integrative plasmids pInt-1m2 and pInt-1m3, respectively (Table 2 and Fig. 1A and B). The central sequences were not changed, since the his leader would have been modified, which could have interfered with the assessment of the role of the TATTAT boxes. The last TATTAT box and part of the terminator of the attenuation system were deleted in pInt-1m3, whereas the deletion was limited to the terminator in pInt-1m4, which was used as a control (Fig. 1B). Unexpectedly, the range of regulation by histidine in both of these fusions was found to be less than twofold, indicating that the two deletions in the terminator affect both the antitermination mechanism and initiation repression (Table 2). This result suggests that an essential operator site overlaps the terminator. The absence of a difference in the levels of expression between pInt-1m3 and pInt-1m4 excludes a predominant role for TATTAT sequences.
The experiments presented above were carried out with the lux fusions integrated in the chromosome. Similar experiments were also performed with fusions on LC plasmids. Interestingly, the activity of the entire promoter and leader region is modulated only 10-fold by histidine on an LC plasmid (five copies per chromosome; pProm-1) but is modulated 120-fold in the chromosome (pInt-1, Table 2). Similar levels of modulation were observed with plasmids with moderate copy number and HC (30 and 80 copies per chromosome, respectively; data not shown). This result suggests that one of the transcription mechanisms is not operative on the plasmid. As we have shown above that the his leader region present in this fragment was sufficient to exert the control by attenuation on a plasmid, it is likely that the control of initiation of transcription does not occur on plasmids.
DISCUSSION
Dual transcriptional control of the his operon.
As we have shown previously, a 10-kb transcript covering the L. lactis histidine operon is induced in response to histidine starvation (7). The levels of induction of the his operon as measured by an assay of histidinol dehydrogenase (hisD gene product) or by transcriptional fusion to lux reporter genes are 26- and 120-fold, respectively. We attribute this difference to the high level of background of the dehydrogenase assay. A short transcript of 250 nt starts at the same 5′ end as the 10-kb transcript and stops at a rho-independent terminator structure upstream of the first his gene. This accords with an attenuation mechanism of transcription control as suggested previously on the basis of the presence of conserved features in the his leader region (19). The dramatic decrease of the levels of all transcripts in the presence of histidine suggests that the his operon is also regulated at the level of initiation of transcription or of mRNA degradation and is thus subject to dual control.
Accuracy of the model of the secondary structure of the his leader.
Based on the presence of characteristic structural features in the his leader region, a general mechanism of transcription attenuation regulating the T box family gene was postulated to govern expression of the his operon (19). It involves the direct interaction of an uncharged tRNA with the leader region of the mRNA, and a model of the leader-region folding was proposed by Grundy and Henkin (19). To test whether this model holds for the his leader and to identify sequences important in the regulation, we first searched for the natural variation of the leader sequence among lactococci. Changes were observed for 18% of the nucleotides (46 of 252) of the his leader. Most changes are compatible with the secondary structure proposed previously, but a different model of folding of the central region of the his leader is presented here. It is based on the assumption that substitutions occur mainly in the loops or in the unpaired regions, whereas in paired regions complementary changes occur, conserving the pairing. The new model contains four stem-loop structures (Fig. 3) and some of them are different from those described in the recent model of T-box-regulated genes (36). Two previous reports also indicate that the secondary structure of this region may vary more than was described in the original model (19). Stem II is present upstream of stem I in the leader region of the T-box-regulated gene encoding the isoleucine-tRNA synthase of Staphylococcus aureus (18). The presence of an additional stem in the trp operon was also proposed (45). These changes do not challenge the general model but suggest that many variations can occur around common features. It remains unknown if these differences confer new properties to the T box systems.
The folding proposed here is compatible with the general model involving the interaction of tRNA with two bulges present in stem I and the antiterminator. The specifier sequence, a CAC triplet in the bulge region present on the right of stem I, could interact with the GUG anticodon of the his tRNA. However, the 5′UGGA3′ sequence in the bulge of the antiterminator is not completely complementary to the 5′(C73)-CCA3′ acceptor end of the L. lactis tRNAHis (unpublished data). The explanation of this divergence with the other T-box-regulated genes might be due to the special feature of the acceptor end of the tRNAHis, which has an additional base at its 5′ end that is paired with the C73. The absence of a discriminator would thus be specific to the tRNAHis. Lastly, the his leader region contains, in the proper location, conserved sequences that are important for regulation but which still have unknown functions in the attenuation mechanism (22). In stem I, AGUA-I, AG, and GNUG boxes are present in the bottom left, in the top left, and in the top bulges, respectively, with a single mismatch. The highly conserved sequence CCGUUA, named the F box, is located between stem-loop II and stem-loop III with a single mismatch. The AGUA-II and GAAC boxes are absent, as is the case in some other leaders (36).
In addition to the conservation of stems, our analysis revealed hypervariable regions in the loops of stem-loops II and III, suggesting they have no role in the formation of the folding of the his leader. Moreover, we have found a hypervariable unpaired region in the middle of stem I. In this region, most of the simple changes observed would allow additional pairing. Interestingly, compensatory changes that prevent pairing are always present. Such unpaired regions at the same location are present in most of the T-box-regulated leaders. They might confer increased flexibility to the upper part of stem I, which contains the AG and GNUG boxes.
Mutational analysis of the his leader region.
The his operon of L. lactis is effectively controlled by a T-box-dependent mechanism of attenuation. The region necessary for full regulation was demonstrated to be present on a 294-bp fragment, and this region induces a 14-fold modulation of the terminator readthrough (pTer-4; Table 3). It contains all the stem and loop structures described previously, including the attenuator and the terminator. The terminator, together with stems III and IV, terminates the transcription by a factor of about 185, independently of the presence of histidine (pTer-2; Table 3). The precise location of the terminator was determined by several mutations which abolish termination (e.g., pInt-1m4 and pInt-1m3, Table 2). The replacement of 5 bp in the T box sequence suppresses regulation by attenuation (pInt-1m1). This confirms that, in the his leader, the T box is involved in the formation of the antiterminator.
The role of stem I was assessed by deletions at the 5′ end of the his leader. The truncation of part of stem I, removing boxes AGUA, AG, and GNUG but not the specifier, results in a 10-fold decrease of the antitermination activity (pTer-3 and pTer-2, Table 3). These deletions also increase two- and fourfold, respectively, the basal level of termination in the his leader in the presence of histidine. Several elements of stem I were shown previously to be involved in the regulation of the ilv-leu operon and tyrS gene of B. subtilis (16, 36). Deletion of stem I of the ilv-leu operon and point mutations affecting GNUG and AG boxes in the tyrS gene dramatically increased the basal level of termination and abolished the tRNA control.
In addition to the features conserved among T box family genes, we have found that sequences specific for the his leader are necessary for the antitermination. Deletion of a single nucleotide in the loop of stem-loop IV, as well as complete deletion of the loop, dramatically decreased the histidine-dependent level of antitermination (pTer-4m1 and pTer-4m2, Table 3). These results show the importance of this region in the attenuation mechanism. However, we do not know whether the region interacts directly with the tRNAHis or plays another role. For example, it could allow secondary contacts important for the global conformation of the mRNA or interact with a trans-acting factor. The interactions of the anticodon and the acceptor stem of the tRNA with the leader are necessary but might not be sufficient to stabilize the antiterminator structure. The presence of trans-acting factors or extended contact between the leaders and their cognate tRNAs has been postulated but not demonstrated (22, 33). Although RNA-RNA interactions may be very complex and difficult to predict, the apparent lack of pairing longer than 4 bp between the tRNAHis and the his leader does not support the hypothesis of a simple extended contact between these two partners. In particular, the loop of stem-loop IV, where the deletion of a single nucleotide abolishes most of the histidine regulation, has no complementary sequence in the tRNAHis. This suggests the existence of a trans-acting activator different from the tRNA. Overexpression of the entire his leader decreases the expression of the his operon, suggesting that the excess of segments of the his leader mRNA in the cell titrates a positive effector involved in the attenuation mechanism. In the general model for the T box system, uncharged tRNA was demonstrated to act as a positive effector (12, 20, 33). However, during histidine starvation the titration of the uncharged tRNAHis by the overexpression of the his leader region is doubtful. These results suggest that an unknown factor, one different from the tRNAHis, may be involved in the antitermination mechanism.
Second transcription control of the his operon.
Histidine is one of the most energy-requiring amino acids to synthesize, but it is not abundant in the cell. Its biosynthesis should thus be tightly controlled. For example, the E. coli or Salmonella typhimurium his operon is controlled by a combination of metabolic regulation (range, 30-fold) and attenuation (range, 200-fold) which gives a potential range of regulation of 6,000-fold (46). However, the T box attenuation system allows no more than 14-fold modulation of the transcription of the his operon in L. lactis. This range is of the same order as the 10- to 30-fold modulation observed for the other T-box-regulated genes studied until now, including the regulation of tRNA synthetases and of the ilv-leu operon of B. subtilis (17, 21, 33). It could be expected that the transcription of synthetase genes is never completely repressed, as their products are necessary for the life of the cell independent of the environmental conditions. However, operons for amino acid biosynthesis (members of the T box gene family), like the ilv-leu operon of B. subtilis (17) and the trp operon of L. lactis (34), have multiple levels of control. In addition to the T-box-directed regulation of the transcription of the his operon, initiation of transcription or mRNA stability is modulated 6- to 8-fold in the presence of histidine, allowing a global range of control over 120-fold. Previous reports showed that the leaders of several mRNAs of the T box family genes are processed (4, 45). A processing event that might stabilize the mRNA of the thrS gene (4) or participate in the regulation of the trp operon (45) during amino acid starvation was demonstrated. However, we have not found a similar cleavage in the his leader region transcript. Moreover, the his leader luciferase fusion in the chromosome (pInt-1, Table 2) is subject to the two controls whereas in LC plasmid it is not (pProm-1, Table 2). mRNA stability is not likely to be dependent on the location of the gene, suggesting that the second control of the his operon is exerted at the level of initiation of transcription. Several mechanisms could regulate this step by altering the formation of the complex between the RNA polymerase and the promoter. In some cases repressors act by binding to operators which are adjacent to or overlap the promoter, resulting in a steric hindering of the binding of the RNA polymerase to the promoter. In E. coli, such sites are most frequently found between positions −50 and +30 of the respective promoters (4). In L. lactis, few combinations of regulatory proteins and their operator are known (24); however, the E. coli paradigm may apply.
Unexpectedly, deletions or substitutions in the terminator structure were found to abolish both the attenuation mechanism and most of the repression, which dropped from six- to eightfold to less than twofold (pInt-1m3 and pInt-1m4, Table 2). We have no evidence for a significant role of the TATTAT box in the regulation. The simplest way to explain this result is to assume that an operator site overlaps the terminator, the sequence of which is an imperfect palindrome. This feature is commonly found for the binding sites of homodimeric DNA-binding proteins. The difference in the control of transcription for the same fragment, 10-fold or 120-fold when present on a plasmid or integrated in the chromosome, shows that the activity of the repressor is dependent on the operator context (chromosome or plasmid) (wild type, Table 2). The absence of repression of the promoter carried on a plasmid does not depend on the copy number (4, 30, and 80 copies per chromosome tested) and the mechanism of plasmid replication (theta or sigma type). It is possible that the putative repressor is titrated, even by an LC plasmid. However, another possibility is that the initiation of the transcription or the repression mechanism is sensitive to DNA supercoiling. Alteration of the regulation of a promoter, due to a change in DNA supercoiling, has been widely described (9, 10, 43). In addition, expression from a promoter sensitive to supercoiling is also dependent on the location and therefore on the sequence context (11, 42). In particular, the degree of chromosomal superhelicity controls expression of the his-tRNA gene promoter, the product of which is an effector of the his operon regulation in E. coli (37).
Whatever the detailed mechanism involved, the total rate of regulation of the L. lactis his operon is only 120-fold, a factor which is more than one order of magnitude less than that in E. coli. This relatively low stringency of control could be a reason for the observed inactivation of the his operons of dairy strains (7). Indeed, in milk, where histidine is mostly provided by casein breakdown and the uptake of released oligopeptides, it could be advantageous to abolish completely the synthesis of this amino acid by introducing inactivating mutations.
ACKNOWLEDGMENTS
This work was supported by contract B104-CT96-0498 of the Commission of the European Communities.
We thank M. van de Guchte for critical reading of the manuscript. We acknowledge T. Henkin and H. Putzer for helpful discussions.
REFERENCES
- 1.Alifano P, Fani R, Lio P, Lazcano A, Bazzicalupo M, Carlomagno M S, Bruni C B. Histidine biosynthetic pathway and genes: structure, regulation and evolution. Microbiol Rev. 1996;60:44–69. doi: 10.1128/mr.60.1.44-69.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckwith J. The operon: an historical account. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 2. Washington, D.C: American Society for Microbiology; 1987. pp. 1439–1443. [Google Scholar]
- 3.Chopin A, Chopin M C, Moillo-Batt A, Langella P. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid. 1984;11:260–263. doi: 10.1016/0147-619x(84)90033-7. [DOI] [PubMed] [Google Scholar]
- 4.Collado-Vides J, Magasanik B, Gralla J D. Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev. 1991;55:371–394. doi: 10.1128/mr.55.3.371-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condon C, Putzer H, Grunberg-Manago M. Processing of the leader mRNA plays a major role in the induction of thrS expression following threonine starvation in Bacillus subtilis. Proc Natl Acad Sci USA. 1996;93:6992–6997. doi: 10.1073/pnas.93.14.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delorme C, Ehrlich D S, Renault P. Histidine biosynthesis genes in Lactococcus lactis subsp. lactis. J Bacteriol. 1992;174:6571–6579. doi: 10.1128/jb.174.20.6571-6579.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delorme C, Godon J J, Ehrlich S D, Renault P. Gene inactivation in Lactococcus lactis: histidine biosynthesis. J Bacteriol. 1993;175:4391–4399. doi: 10.1128/jb.175.14.4391-4399.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delorme C, Godon J J, Ehrlich S D, Renault P. Mosaic structure of large regions of the Lactococcus lactis subsp. cremoris chromosome. Microbiology. 1994;140:3053–3060. doi: 10.1099/13500872-140-11-3053. [DOI] [PubMed] [Google Scholar]
- 9.Drlica K. Control of bacterial DNA supercoiling. Mol Microbiol. 1992;6:425–433. doi: 10.1111/j.1365-2958.1992.tb01486.x. [DOI] [PubMed] [Google Scholar]
- 10.Eismann E R, Muller-Hill B. lac repressor forms stable loops in vitro with supercoiled wild-type lac DNA containing all three natural lac operators. J Mol Biol. 1990;213:763–775. doi: 10.1016/S0022-2836(05)80262-1. [DOI] [PubMed] [Google Scholar]
- 11.Fang M, Wu H Y. A promoter relay mechanism for sequential gene activation. J Bacteriol. 1998;180:626–633. doi: 10.1128/jb.180.3.626-633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrity D B, Zahler S A. Mutations in the gene for a tRNA that functions as a regulator of a transcriptional attenuator in Bacillus subtilis. Genetics. 1994;137:627–636. doi: 10.1093/genetics/137.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilson T J. Ph.D. thesis. Cambridge, England: University of Cambridge; 1984. [Google Scholar]
- 14.Glatron M F, Rapoport G. Biosynthesis of the parasporal inclusion of Bacillus thuringiensis: half-life of its corresponding messenger RNA. Biochimie. 1972;54:1291–1301. doi: 10.1016/s0300-9084(72)80070-1. [DOI] [PubMed] [Google Scholar]
- 15.Godon J J, Jury K, Shearman C A, Gasson M J. The Lactococcus lactis sex-factor aggregation gene cluA. Mol Microbiol. 1994;12:655–663. doi: 10.1111/j.1365-2958.1994.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 16.Grandoni J A, Fulmer S B, Brizzio V, Zahler S A, Calvo J M. Regions of the Bacillus subtilis ilv-leu operon involved in regulation by leucine. J Bacteriol. 1993;175:7581–7593. doi: 10.1128/jb.175.23.7581-7593.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grandoni J A, Zahler S A, Calvo J M. Transcriptional regulation of the ilv-leu operon of Bacillus subtilis. J Bacteriol. 1992;174:3212–3219. doi: 10.1128/jb.174.10.3212-3219.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grundy F J, Haldeman M T, Hornblow G M, Ward J M, Chalker A F, Henkin T M. The Staphylococcus aureus ileS gene, encoding isoleucyl-tRNA synthetase, is a member of the T-box family. J Bacteriol. 1997;179:3767–3772. doi: 10.1128/jb.179.11.3767-3772.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grundy F J, Henkin T M. Conservation of a transcription antitermination mechanism in aminoacyl-tRNA synthetase and amino acid biosynthesis genes in Gram-positive bacteria. J Mol Biol. 1994;235:798–804. doi: 10.1006/jmbi.1994.1038. [DOI] [PubMed] [Google Scholar]
- 20.Grundy F J, Henkin T M. tRNA as a positive regulator of transcription antitermination in B. subtilis. Cell. 1993;74:475–482. doi: 10.1016/0092-8674(93)80049-k. [DOI] [PubMed] [Google Scholar]
- 21.Grundy F J, Rollins S M, Henkin T M. Interaction between the acceptor end of tRNA and the T box stimulates antitermination in the Bacillus subtilis tyrS gene: a new role for the discriminator base. J Bacteriol. 1994;176:4518–4526. doi: 10.1128/jb.176.15.4518-4526.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henkin T M. tRNA-directed transcription antitermination. Mol Microbiol. 1994;13:381–387. doi: 10.1111/j.1365-2958.1994.tb00432.x. [DOI] [PubMed] [Google Scholar]
- 23.Holo H, Nes I F. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kok J. Inducible gene expression and environmentally regulated genes in lactic acid bacteria. Antonie Leeuwenhoek. 1996;70:129–145. doi: 10.1007/BF00395930. [DOI] [PubMed] [Google Scholar]
- 25.Law J, Buist G, Haandrikman A, Kok J, Venema G, Leenhouts K J. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J Bacteriol. 1995;177:7011–7018. doi: 10.1128/jb.177.24.7011-7018.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebourgeois P. Ph.D. thesis. Toulouse, France: Université Paul Sabatier; 1993. [Google Scholar]
- 27.Le Chatelier E, Ehrlich S D, Janniere L. The pAMβ1 CopF repressor regulates plasmid copy number by controlling transcription of the repE gene. Mol Microbiol. 1994;14:463–471. doi: 10.1111/j.1365-2958.1994.tb02181.x. [DOI] [PubMed] [Google Scholar]
- 28.Leenhouts K J, Buist G, Bolhuis A, ten Berge A, Kiel J, Mierau I, Dabrowska M, Venema G, Kok J. A general system for generating unlabelled gene replacements in bacterial chromosomes. Mol Gen Genet. 1996;253:217–224. doi: 10.1007/s004380050315. [DOI] [PubMed] [Google Scholar]
- 29.Loureiro Dos Santos A L, Chopin A. Shotgun cloning in Streptococcus lactis. FEMS Microbiol Lett. 1987;42:209–212. [Google Scholar]
- 30.Maguin E, Duwat P, Hege T, Ehrlich S D, Gruss A. New thermosensitive plasmid for gram-positive bacteria. J Bacteriol. 1992;174:5633–5638. doi: 10.1128/jb.174.17.5633-5638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 32.Martin R G, Berberich M A, Ames B N, Davis W W, Goldberger R F, Yourno J D. Enzymes and intermediates of histidine biosynthesis in Salmonella typhimurium. Methods Enzymol. 1961;147:1–44. [Google Scholar]
- 33.Putzer H, Laalami S, Brakhage A A, Condon C, Grunberg-Manago M. Aminoacyl-tRNA synthetase gene regulation in Bacillus subtilis: induction, repression and growth-rate regulation. Mol Microbiol. 1995;16:709–718. doi: 10.1111/j.1365-2958.1995.tb02432.x. [DOI] [PubMed] [Google Scholar]
- 34.Raya R, Bardowski J, Andersen P S, Ehrlich S D, Chopin A. Multiple transcriptional control of the Lactococcus lactis trp operon. J Bacteriol. 1998;180:3174–3180. doi: 10.1128/jb.180.12.3174-3180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renault P, Corthier G, Goupil N, Delorme C, Ehrlich S D. Plasmid vectors for Gram-positive bacteria switching from high to low copy number. Gene. 1996;183:175–182. doi: 10.1016/s0378-1119(96)00554-9. [DOI] [PubMed] [Google Scholar]
- 36.Rollins S M, Grundy F J, Henkin T M. Analysis of cis-acting sequence and structural elements required for antitermination of the Bacillus subtilis tyrS gene. Mol Microbiol. 1997;25:411–421. doi: 10.1046/j.1365-2958.1997.4851839.x. [DOI] [PubMed] [Google Scholar]
- 37.Rudd K E, Menzel R. his operons of Escherichia coli and Salmonella typhimurium are regulated by DNA supercoiling. Proc Natl Acad Sci USA. 1987;84:517–521. doi: 10.1073/pnas.84.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salama M, Sandine W, Giovannoni S. Development and application of oligonucleotide probes for identification of Lactococcus lactis subsp. cremoris. Appl Environ Microbiol. 1991;57:1313–1318. doi: 10.1128/aem.57.5.1313-1318.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanger F. Determination of nucleotide sequence in DNA. Science. 1981;214:1205–1210. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- 40.Simon D, Chopin A. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie. 1988;70:559–566. doi: 10.1016/0300-9084(88)90093-4. [DOI] [PubMed] [Google Scholar]
- 41.Smid E J, Konings W N. Relationship between utilization of proline and proline-containing peptides and growth of Lactococcus lactis. J Bacteriol. 1990;172:5286–5292. doi: 10.1128/jb.172.9.5286-5292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spirito F, Bossi L. Long-distance effect of downstream transcription on activity of the supercoiling-sensitive leu-500 promoter in a topA mutant of Salmonella typhimurium. J Bacteriol. 1996;178:7129–7137. doi: 10.1128/jb.178.24.7129-7137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun L, Fuchs J A. Regulation of the Escherichia coli nrd operon: role of DNA supercoiling. J Bacteriol. 1994;176:4617–4626. doi: 10.1128/jb.176.15.4617-4626.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terzaghi B, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van de Guchte M, Ehrlich S D, Chopin A. tRNATrp as a key element of antitermination in the Lactococcus lactis trp operon. Mol Microbiol. 1998;29:61–74. doi: 10.1046/j.1365-2958.1998.00903.x. [DOI] [PubMed] [Google Scholar]
- 46.Winkler M E. Biosynthesis of histidine. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 1. Washington, D.C: American Society for Microbiology; 1987. pp. 395–411. [Google Scholar]
- 47.Yanofsky C, Crawford I P. The tryptophan operon. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S>, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 2. Washington, D.C: American Society for Microbiology; 1987. pp. 1453–1472. [Google Scholar]