Abstract
Backgroundgg:
Although current guidelines recommend ticagrelor to clopidogrel for patients with acute coronary syndrome, its benefit and risk are unclear for East Asians. This meta-analysis was performed to assess the efficacy and safety of ticagrelor in East Asian patients with acute coronary syndrome.
Methods:
Medline, EMBASE, and Cochrane Databases were searched from inception to July, 2021, for randomized controlled trials comparing ticagrelor with clopidogrel in East Asian patients with acute coronary syndrome. Major adverse cardiovascular events and bleeding events were assessed by using Mantel-Haenszel-pooled risk ratio and 95% confidence interval.
Results:
Five randomized controlled trials identified 2752 patients with acute coronary syndrome. Compared with clopidogrel, ticagrelor had no statistical difference of major adverse cardiovascular events (RR 0.87; 95% CI 0.52-1.45; P = .58), all cause death (RR 0.90, 95% CI 0.62-1.32; P = .60), cardiovascular death (RR 0.90, 95% CI 0.47-1.72; P = .74), myocardial infarction (RR 0.91, 95% CI 0.52-1.58; P = .73), and stroke (RR 0.87, 95% CI 0.48-1.57; P = .64). Despite ticagrelor did not increase the incidence of fatal bleeding (RR 2.49, 95% CI 0.79-7.87; P = 0.12), the risks of all bleeding (RR 1.71, 95% CI 1.36-2.16; P < .00001), major bleeding (RR 1.51, 95% CI 1.12-2.04; P = .007), non-coronary artery bypass grafting major bleeding (RR 1.83, 95% CI 1.23-2.71; P = .003), and minor bleeding (RR 1.92, 95% CI 1.40-2.64; P < .0001) were significantly higher.
Conclusions:
Although there was no significant difference in the incidence of fatal bleeding, ticagrelor displayed similar efficacy and dramatically increased the risk of other bleeding events.
Keywords: Ticagrelor, East Asian, acute coronary syndrome, randomized controlled trial, meta-analysis
Highlight
Five studies with 2752 East Asian patients with acute coronary syndrome were included.
We compared the efficacy and safety between ticagrelor and clopidogrel.
We did subgroup analyses according to patients’ baseline clinical presentations.
Ticagrelor increased the risk of bleeding without reduced ischemic events.
Introduction
A combination of aspirin and a kind of P2Y12 inhibitor as dual antiplatelet therapy (DAPT) is the cornerstone of secondary prevention in patients with acute coronary syndrome (ACS). Ticagrelor is a reversible non-thienopyridine oral P2Y12 inhibitor that provides faster, more potent, and consistent platelet inhibition than clopidogrel.1 Based on the Platelet Inhibition and Patient Outcomes (PLATO) trial,2 the European Society of Cardiology (ESC) guidelines3,4 consider to use clopidogrel only when ticagrelor is not available, cannot be tolerated, or is contraindicated (Ⅰ, C). Similarly, the American College of Cardiology (ACC)/American Heart Association (AHA) guideline5 recommends to use ticagrelor in preference to clopidogrel for maintenance of P2Y12 inhibitor therapy (IIa, B).
East Asians are the most populous race in the world with over 1.6 billion people. In the contemporary trials of antithrombotic treatment, East Asian patients with ACS show a similar or even lower rate of ischemic event and higher bleeding risk compared with Caucasian patients, which is referred to as the “East Asian Paradox.”6,7 Besides, different net clinical benefits have been observed between the races with P2Y12 inhibitors that may be related to racial differences in pharmacokinetic and pharmacodynamic profiles.8,9 Even so, the current DAPT recommendation in East Asia10-13 are mainly based on the American or European guidelines. In order to provide evidence for “race-tailored” DAPT in East Asian patients with ACS, we conducted a meta-analysis to assess the efficacy and safety of ticagrelor in the specific race.
Methods
Literature Search
We systematically searched Medline, EMBASE, and Cochrane Databases for all relevant articles comparing ticagrelor with clopidogrel in East Asian patients with ACS through July, 2021. Literature was searched with the following keywords: ticagrelor, clopidogrel, coronary disease, coronary artery disease, coronary heart disease, acute coronary syndrome, myocardial infarction, unstable angina, and random*. A comprehensive search of reference lists of all review articles and original studies retrieved by this method was performed to identify additional studies.
Selection Criteria
The inclusion criteria were the following: (1) trials designed as RCT; (2) trials based on East Asian patients with ACS; (3) trials compared ticagrelor with clopidogrel; (4) trials reported 6-month or longer outcomes; and (5) outcomes included ischemic events and/or bleeding events.
Data Abstraction
Two investigators independently assessed studies for possible inclusion by reading titles and/or abstracts, then viewed the full-texts of the remaining publications to pick up the ultimately available studies. Data extraction was done by 1 reviewer, and subsequently cross-checked by the other reviewer. Any divergences were discussed or determined by a third investigator. The following informations were abstracted: the first author and publication year, country, sample size, baseline features of patients, treatment features, follow-up time, efficacy outcomes, and/or safety outcomes and their definitions.
Bias Risk and Study Quality Assessment
The methodological quality of eligible studies was assessed by the Cochrane collaboration’s tool for assessing risk of bias including the following criteria: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other issues. The bias risk of each study was scored as low, unclear, or high in each section.
Statistical Analysis
Dichotomous data were expressed risk ratio (RR) with 95% CI. Heterogeneity of effect size across the studies was tested using Q statistics at the P < .10 level of significance. We also calculated the I 2 statistic with a quantitative measure of inconsistency across the studies. The data were pooled by random-effects model in case of significant heterogeneity (Cochran test with P < .10 or I 2 > 50%) was found. Otherwise, the fixed-effects model was used. Sensitivity analyses with fixed-effect models were performed to assess consistency among effect estimates that were obtained with random- and fixed-effects models. Potential publication bias was visually inspected by funnel plot if more than 10 studies. We conducted subgroup analyses according to sex (male and female), age (<65 years and ≥65 years), weight (<60 kg and ≥60 kg), body mass index (BMI) (<25 kg/m2 and ≥25 kg/m2), and clinical presentation [ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction–ACS (NSTEMI–ACS)]. Meta-analysis was performed with the software of Cochrane Review Manager 5.1.2 (Cochrane Library Software, Oxford, UK).
Results
Literature Retrieval, Information Extraction, and Bias Risk Assessment
Figure 1 shows a flow diagram for the selection process. A total of 5 RCTs14-18 that included 2752 patients (ticagrelor = 1379 vs. clopidogrel = 1373) were finally identified. Table 1 summarizes the characteristics of the selected studies. Among the 5 RCTs, two studies were based on patients from China,16,17 one study was based on patients from Korea,18 and the other two studies were based on patients from different East Asian countries.14,15 Four studies were multicenter trial14,15,17,18 and one was single center trial that only including patients older than 65 years.16 Four studies included three types of ACS patients using PLATO bleeding criteria and were followed up for 12 months,14-16,18 while one study included only STEMI patients using Thrombolysis in myocardial infarction (TIMI) bleeding criteria and was followed up for 6 months.17 The methodological quality of the included studies was good in general as shown in Table 1.
Figure 1.
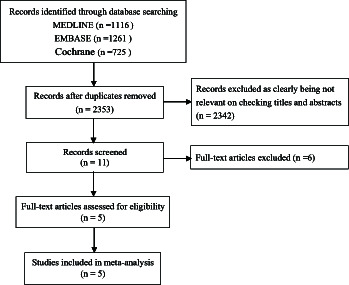
Study selection according to the PRISMA model.
Table 1.
Characteristics of the Included Studies
| Authors | Publication Year | Country (%) | Sample Size (I/C) | Follow-Up (months) | Age (I/C, Years) | Male (I/C, %) | BMI (I/C, kg/m2) | STEMI (I/C, %) | NSTEMI (I/C, %) | UA (I/C, %) | Smoker (I/C, %) | Hypertension (I/C, %) | Dyslipidemia (I/C, %) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kang et al14 | 2015 | Korea (NA)/China (NA) | 278/273 | 12 | 60/61* | 75/72* | 24/24* | 46/45* | 38/35* | 13/16* | 41/38* | 60/62* | 31/35* |
| Goto et al15 | 2015 | Japan (90)/China (4)/Korea (6) | 401/400 | 12 | 67/66 | 76/77 | 24/24 | 51/53 | 17/19 | 30/27 | 38/39 | 76/73 | 78/72 |
| Wang et al16 | 2016 | China (100) | 100/100 | 12 | 79/80 | 69/66 | NA | 37/32 | 44/47 | 19/21 | 37/41 | 79/82 | 84/79 |
| Tang et al17 | 2016 | China (100) | 200/200 | 6 | 64/64 | 71/73 | NA | 100/100 | 0/0 | 0/0 | 58/62 | 61/58 | 44/37 |
| Park et al18 | 2019 | Korea (100) | 400/400 | 12 | 63/62 | 74/76 | 25/25 | 43/39 | 37/39 | 21/22 | 37/35 | 56/48# | 52/49 |
Efficacy and Safety Analyses
Our pooled analysis indicated that ticagrelor did not reduce the incidence of MACE (RR 0.87, 95% CI 0.52-1.45; P =.58), all cause death (RR 0.90, 95%CI 0.62-1.32; P = .60), cardiovascular death (RR 0.90, 95% CI 0.47-1.72; P = 0.74), myocardial infarction (RR 0.91, 95% CI 0.52-1.58; P = .73), and stroke (RR 0.87, 95% CI 0.48-1.57; P = .64) (Figure 2). In terms of the safety outcomes, although ticagrelor did not increase the incidence of fatal bleeding (RR 2.49, 95% CI 0.79-7.87; P = .12), it significantly increased the risk of all bleeding (RR 1.71, 95% CI 1.36-2.16; P < .00001), major bleeding (RR 1.51, 95% CI 1.12-2.04; P = .007), non-CABG major bleeding (RR 1.83, 95% CI 1.23-2.71; P = .003), and minor bleeding (RR 1.92, 95%CI 1.40-2.64; P < .0001) (Figure 3).
Figure 2.
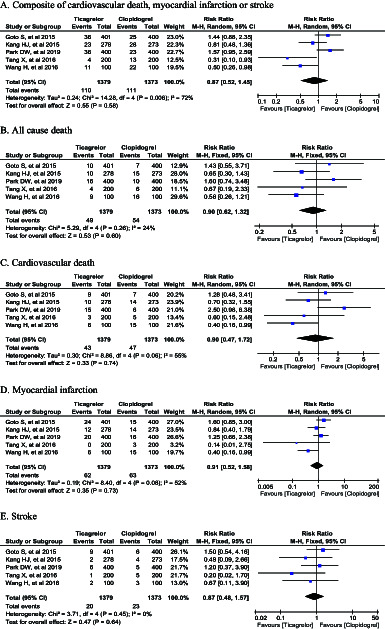
Forest plot of efficacy outcomes.
Figure 3.
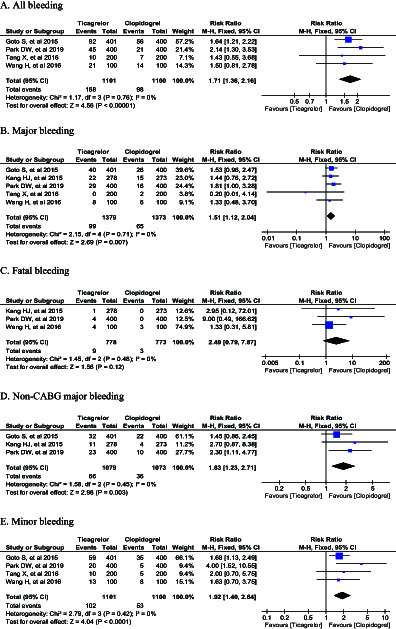
Forest plot of safety outcomes.
Subgroup Analyses
To explore the study heterogeneity, we further performed meta-analysis in subgroups based on several baseline clinical presentations (sex, age, weight, BMI, and clinical presentation). Table 2 shows a similar risk of MACE in females (RR 1.17, 95% CI 0.68-4.62; P = .64), in patients <65 years and ≥ 65 years (RR 0.64, 95% CI 0.27-1.54; P = .32 and RR 1.14, 95% CI 0.23-5.77; P = .87, respectively), in patients with BMI <25 kg/m2 and ≥25 kg/m2 (RR 1.56, 95% CI 0.99-2.44; P = .05 and RR 1.39, 95% CI 0.79-2.46; P = .26, respectively), and in patients with STEMI and NSTEMI-ACS (RR 0.94, 95% CI 0.37-2.40; P = .90 and RR 1.51, 95% CI 0.91-2.48; P = .11, respectively). Only in males’ subgroup, ticagrelor did significantly reduce the risk of MACE (RR 1.65, 95% CI 1.09-2.51; P = .02) (Table 2).
Table 1.
Characteristics of the Included Studies (continued)
| Authors | Diabetes (I/C, %) | History of stroke (I/C, %) | Prior MI (I/C, % | Prior PCI (I/C, %) | Prior CABG (I/C, %) | Treated with PCI (I/C, %) | β-Blocker (I/C, %) | CCB (I/C, %) | ACEI/ARB (I/C, %) | Statin (I/C, %) | Nitrates (I/C, %) | PPI (I/C, %) | Bleeding Criteria | Study Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kang et al14 | 29/30* | 7/6* | 12/18* | 8/8* | 1/2* | NA | 52/57* | 15/17* | 62/65* | 76/74* | NA | 36/34* | PLATO | Low risk |
| Goto et al15 | 38/31 | 7/7 | 8/8 | 11/11 | 1/0 | 85/85 | 10/11 | 29/27 | 42/40 | 54/51 | 88/86 | 42/44 | PLATO | Low risk |
| Wang et al16 | 42/39 | 11/10 | 17/15 | 3/6 | 0/0 | 75/71 | 69/74 | 69/63 | 61/67 | 83/79 | 90/89 | 31/33 | PLATO | Low risk |
| Tang et al17 | 29/21 | 16/17 | 8/5 | NA | NA | 100/100 | 41/48 | NA | 38/47 | 99/100 | 86/88 | NA | TIMI | Low risk |
| Park et al18 | 29/25 | 6/4 | 6/5 | 8/10 | 1/1 | 82/86 | 69/74 | 23/23 | 41/43 | 89/92 | NA | 3/2 | PLATO | Low risk |
I, intervention group; C, control group; BMI, body mass index; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; UA, unstable angina; MI, myocardial infarction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; CCB, calcium channel blocker; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; PPI, proton pump inhibitor; PLATO, platelet inhibition and patient outcomes trial; TIMI, thrombolysis in myocardial infarction; NA, not available; *data of all Asian population including East Asians and Southeast Asians; # P < .05.
Sensitivity Analyses
There was no difference in the results between the fixed-effect model and the random-effect model for the efficacy and safety outcomes.
Discussion
The major pathophysiological mechanism underlying ACS is atherosclerotic plaque rupture with resultant coronary thrombosis, and therefore, antiplatelet therapy is an important foundation in the treatment and prevention of recurrence of ACS.19 The optimal antiplatelet therapy aims to prevent thrombosis while avoiding hemorrhage. Since clopidogrel has substantial limitations in the management of ACS with a modest inhibition of platelet aggregation and a delayed onset and offset of action,20,21 ticagrelor is now preferred to clopidogrel as a first-line therapy in DAPT, as endorsed by both European and US guidelines.
However, a recent retrospective observational analysis of net adverse clinical events (NACE) in patients with ACS-indicated ticagrelor was not associated with significant difference in the risk of NACE but dramatically increased the risk of hemorrhagic events.22 Meanwhile, a recent network meta-analysis compared clopidogrel, ticagrelor, and prasugrel in patients with ACS-indicated ticagrelor significantly reduced the risk of ischemic events at the cost of increased major bleeding, but its direct pairwise meta-analysis showed there was no statistically significant differences in major bleeding risk between ticagrelor and clopidogrel.23 Thus, whether ticagrelor would increase the risk of major bleeding remains to be further discussed. In addition, there are significant differences between East Asian and Western patients in terms of physique, thrombogenicity, hemorrhagic diathesis, and on-treatment platelet reactivity.24 Although earlier meta-analysis had assessed ticagrelor and clopidogrel in East Asian patients with ACS, it included only 3 RCTs and fewer outcomes were synthesized.25 Therefore, whether the evidence and guidelines from Western countries can be generalized to East Asian patients remains largely unclear.
In this meta-analysis, we assessed the efficacy and safety of ticagrelor in East Asian patients with ACS, investigating the differences in treatment effects according to different baseline clinical presentations. The main findings of this meta-analysis were as follows: (1) ticagrelor displayed similar efficacies in MACE and its individual components; (2) although ticagrelor did not increase the incidence of fatal bleeding, the risks of other bleeding events were significantly higher; and (3) in males the benefit of ticagrelor appears to be favorable, while the risk of bleeding cannot be assessed due to lack of data. Earlier studies found a higher rate of MACE in females,26,27 which might be related to females more often present with atypical symptoms and signs.28 Recent study demonstrated that females after myocardial infarction had higher rates of cardiovascular mortality and all-cause mortality than male, even after adjustment for potential confounders, including baseline health status.29 (4) A similar risk of MACE was observed in females, in patients <65 years and ≥65 years, in patients with BMI < 25 kg/m2 and ≥25 kg/m2, and in patients with STEMI and NSTEMI-ACS.
Our findings were consistent with recent observational studies performed in East Asian. The Comparison of Efficacy and Safety Between TIcagrelor and Clopidogrel In Chinese (COSTIC) study showed the patients prescribed with ticagrelor had a similar incidence of MACE and a higher incidence of bleeding relative to those with clopidogrel at 6 months and 12 months.30 Another Korean nationwide cohort study of 70,715 patients with ACS demonstrated that, compared with clopidogrel, the use of ticagrelor was significantly associated with a higher incidence of bleeding at 2 years but no significant difference in composite ischemic events.31
We acknowledge that our meta-analysis had several limitations. First, despite we had systematically searched and reviewed relevant articles, the sample size was relatively small. Second, because of limited data, subgroup analysis of patients from different countries was not performed. Third, given the limited number of studies included in the analysis, our findings should be confirmed with future research.
Conclusions
Although there was no significant difference in the incidence of fatal bleeding, ticagrelor displayed similar efficacy and dramatically increased the risk of other bleeding events.
Declaration of Interests:
The authors declare that they have no competing interest.
Table 2.
Subgroup Analyses for Composite of Cardiovascular Death, Myocardial Infarction or Stroke (RR, 95% CI; P)
| Subgroup | Composite of Cardiovascular Death, Myocardial Infarction, or Stroke | |
|---|---|---|
| Sex | Male | 1.65 (1.09-2.51); 0.02 |
| Female | 1.17 (0.68-4.62); 0.64 | |
| Age | <65 years | 0.64 (0.27-1.54); 0.32 |
| ≥65 years | 1.14 (0.23-5.77); 0.87 | |
| Weight | <60 kg | 1.70 (0.94-3.06); 0.08 |
| ≥60 kg | 1.41 (0.91-2.19); 0.12 | |
| Body mass index | <25 kg/m2 | 1.56 (0.99-2.44); 0.05 |
| ≥25 kg/m2 | 1.39 (0.79-2.46); 0.26 | |
| Clinical presentation | STEMI | 0.94 (0.37-2.40); 0.90 |
| NSTEMI-ACS | 1.51 (0.91-2.48); 0.11 | |
STEMI, ST-segment elevation myocardial infarction; NSTEMI-ACS, non-ST-segment elevation myocardial infarction-acute coronary syndrome.
Footnotes
Ethics Committee Approval: Not applicable.
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – C.X., J.Z.; Design – C.X., J.Z.; Supervision – J.L., J.Z.; Fundings – None; Materials – X.C., Q.Q.; Data collection &/or processing – C.X., J.L., Q.Q.; Analysis &/or interpretation – J.L., Q.Q.; Literature search – C.X., Q.Q.; Writing – C.X., J.L.; Critical review – J.Z.
Funding: This study received no funding.
References
- 1. Storey RF, Angiolillo DJ, Patil SB, et al. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient outcomes) PLATELET substudy. J Am Coll Cardiol. 2010;56(18):1456 1462. 10.1016/j.jacc.2010.03.100) [DOI] [PubMed] [Google Scholar]
- 2. Wallentin L, Becker RC, Budaj A.et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045 1057. 10.1056/NEJMoa0904327) [DOI] [PubMed] [Google Scholar]
- 3. Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39(2):119 177. 10.1093/eurheartj/ehx393) [DOI] [PubMed] [Google Scholar]
- 4. Collet JP, Thiele H, Barbato E.et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020;29:ehaa575. [DOI] [PubMed] [Google Scholar]
- 5. Levine GN, Bates ER, Bittl JA, et al. ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2016;68(10):1082 1115. 10.1016/j.jacc.2016.03.513) [DOI] [PubMed] [Google Scholar]
- 6. Jeong YH. “East asian paradox”: challenge for the current antiplatelet strategy of “one-guideline-fits-all races” in acute coronary syndrome. Curr Cardiol Rep. 2014;16(5):485. 10.1007/s11886-014-0485-4) [DOI] [PubMed] [Google Scholar]
- 7. Bae JS, Ahn JH, Tantry US, Gurbel PA, Jeong YH. Should antithrombotic treatment strategies in East Asians differ from Caucasians? Curr Vasc Pharmacol. 2018;16(5):459 476. 10.2174/1570161116666180117103238) [DOI] [PubMed] [Google Scholar]
- 8. Lee JH, Ahn SG, Park B.et al. A pharmacodynamic study of the optimal P2Y12 inhibitor regimen for East Asian patients with acute coronary syndrome. Korean J Intern Med. 2015;30(5):620 628. 10.3904/kjim.2015.30.5.620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li H, Guo J, Carlson GF, Teng R. Pharmacodynamics, pharmacokinetics, and safety of ticagrelor in Chinese patients with stable coronary artery disease. Br J Clin Pharmacol. 2016;82(2):352 361. 10.1111/bcp.12950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levine GN, Jeong YH, Goto S.et al. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol. 2014;11(10):597 606. 10.1038/nrcardio.2014.104) [DOI] [PubMed] [Google Scholar]
- 11. Chinese Society of Cardiology of Chinese Medical Association. Chinese Society of Cardiology (CSC) guidelines for the diagnosis and management of patients with non-ST-segment elevation acute coronary syndrome. Chin J Cardiol. 2016;45(5):359 376. [Google Scholar]
- 12. Chinese Society of Cardiology of Chinese Medical Association. Chinese Society of Cardiology (CSC) guidelines for the diagnosis and management of patients with ST-segment elevation myocardial infarction. Chin J Cardiol. 2019;47(10):766 783. [DOI] [PubMed] [Google Scholar]
- 13. Kimura K, Kimura T, Ishihara M, et al. JCS 2018 guideline on diagnosis and treatment of acute coronary syndrome. Circ J. 2019;83(5):1085 1196. 10.1253/circj.CJ-19-0133) [DOI] [PubMed] [Google Scholar]
- 14. Kang HJ, Clare RM, Gao R, et al. Ticagrelor versus clopidogrel in Asian patients with acute coronary syndrome: a retrospective analysis from the Platelet Inhibition and Patient Outcomes (Plato) trial. Am Heart J. 2015;169(6):899 905.e1. 10.1016/j.ahj.2015.03.015) [DOI] [PubMed] [Google Scholar]
- 15. Goto S, Huang CH, Park SJ, Emanuelsson H, Kimura T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome -- randomized, double-blind, phase III PHILO study. Circ J. 2015;79(11):2452 2460. 10.1253/circj.CJ-15-0112) [DOI] [PubMed] [Google Scholar]
- 16. Wang H, Wang X. Efficacy and safety outcomes of ticagrelor compared with clopidogrel in elderly Chinese patients with acute coronary syndrome. Ther Clin Risk Manag. 2016;12:1101 1105. 10.2147/TCRM.S108965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang X, Li R, Jing Q.et al. Assessment of ticagrelor versus clopidogrel treatment in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Cardiovasc Pharmacol. 2016;68(2):115 120. 10.1097/FJC.0000000000000390) [DOI] [PubMed] [Google Scholar]
- 18. Park DW, Kwon O, Jang JS, et al. Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: a randomized clinical trial. Circulation. 2019;140(23):1865 1877. 10.1161/CIRCULATIONAHA.119.041766) [DOI] [PubMed] [Google Scholar]
- 19. Wiviott SD, Steg PG. Clinical evidence for oral antiplatelet therapy in acute coronary syndromes. Lancet. 2015;386(9990):292 302. 10.1016/S0140-6736(15)60213-6) [DOI] [PubMed] [Google Scholar]
- 20. Jiang XL, Samant S, Lesko LJ, Schmidt S. Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin Pharmacokinet. 2015;54(2):147 166. 10.1007/s40262-014-0230-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schilling U, Dingemanse J, Ufer M. Pharmacokinetics and pharmacodynamics of approved and investigational P2Y12 receptor antagonists. Clin Pharmacokinet. 2020;59(5):545 566. 10.1007/s40262-020-00864-4) [DOI] [PubMed] [Google Scholar]
- 22. You SC, Rho Y, Bikdeli B.et al. Association of ticagrelor vs clopidogrel with net adverse clinical events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. JAMA. 2020;324(16):1640 1650. 10.1001/jama.2020.16167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Navarese EP, Khan SU, Kołodziejczak M.et al. Comparative efficacy and safety of oral P2Y12 inhibitors in acute coronary syndrome: network meta-analysis of 52 816 patients from 12 randomized trials. Circulation. 2020;142(2):150 160. 10.1161/CIRCULATIONAHA.120.046786) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Numasawa Y, Sawano M, Fukuoka R.et al. Antithrombotic strategy for patients with acute coronary syndrome: a perspective from East Asia. J Clin Med. 2020;9(6):1963. 10.3390/jcm9061963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Misumida N, Aoi S, Kim SM, Ziada KM, Abdel-Latif A. Ticagrelor versus clopidogrel in East Asian patients with acute coronary syndrome: systematic review and meta-analysis. Cardiovasc Revasc Med. 2018;19(6):689 694. 10.1016/j.carrev.2018.01.009) [DOI] [PubMed] [Google Scholar]
- 26. Panagiotakos DB, Pitsavos C, Kourlaba G.et al. Sex-related characteristics in hospitalized patients with acute coronary syndromes--the Greek Study of Acute Coronary Syndromes (GREECS). Heart Vessels. 2007;22(1):9 15. 10.1007/s00380-006-0932-2) [DOI] [PubMed] [Google Scholar]
- 27. Ferrari R, Abergel H, Ford I.et al. Gender- and age-related differences in clinical presentation and management of outpatients with stable coronary artery disease. Int J Cardiol. 2013;167(6):2938 2943. 10.1016/j.ijcard.2012.08.013) [DOI] [PubMed] [Google Scholar]
- 28. Kouvari M, Yannakoulia M, Souliotis K, Panagiotakos DB. Challenges in sex- and gender-centered prevention and management of cardiovascular disease: implications of genetic, metabolic, and environmental paths. Angiology. 2018;69(10):843 853. 10.1177/0003319718756732) [DOI] [PubMed] [Google Scholar]
- 29. Dreyer RP, Zheng X, Xu X, et al. Sex differences in health outcomes at one year following acute myocardial infarction: a report from the China Patient-Centered evaluative assessment of cardiac events prospective acute myocardial infarction study. Eur Heart J Acute Cardiovasc Care. 2019;8(3):273 282. 10.1177/2048872618803726) [DOI] [PubMed] [Google Scholar]
- 30. Sun Y, Li C, Zhang L.et al. Clinical outcomes after ticagrelor and clopidogrel in Chinese post-stented patients. Atherosclerosis. 2019;290:52 58. 10.1016/j.atherosclerosis.2019.09.011) [DOI] [PubMed] [Google Scholar]
- 31. Yun JE, Kim YJ, Park JJ.et al. Safety and effectiveness of contemporary P2Y12 inhibitors in an East Asian population with acute coronary syndrome: a nationwide population-based cohort study. J Am Heart Assoc. 2019;8(14):e012078. 10.1161/JAHA.119.012078) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a